User login
Adult-Onset Still Disease: Persistent Pruritic Papular Rash With Unique Histopathologic Findings
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
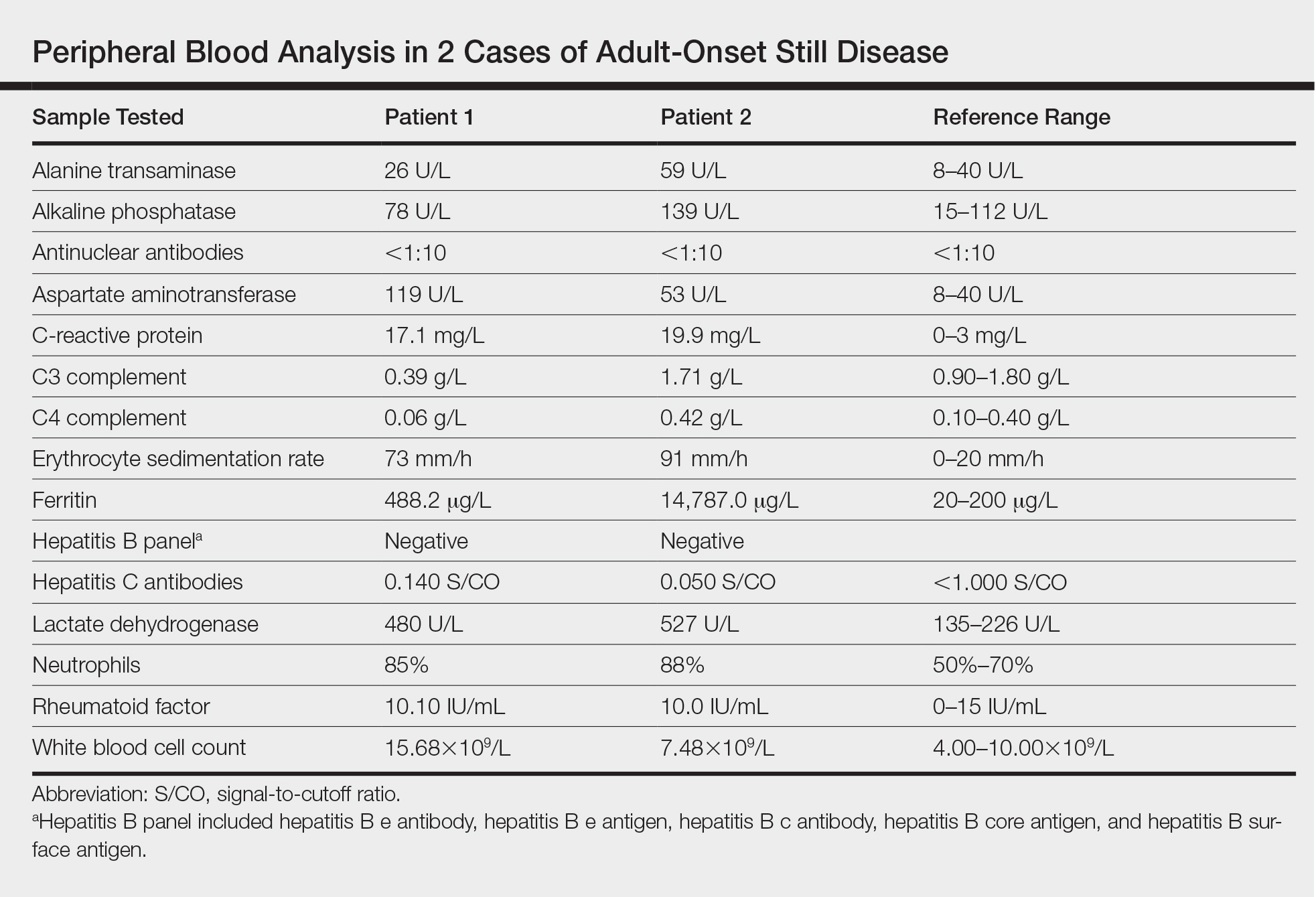
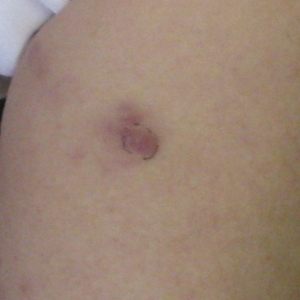
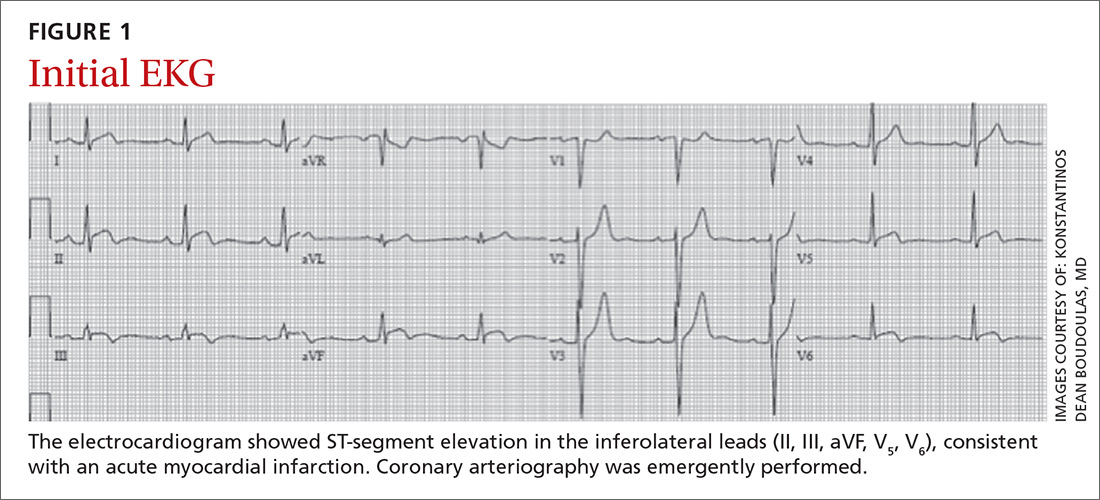
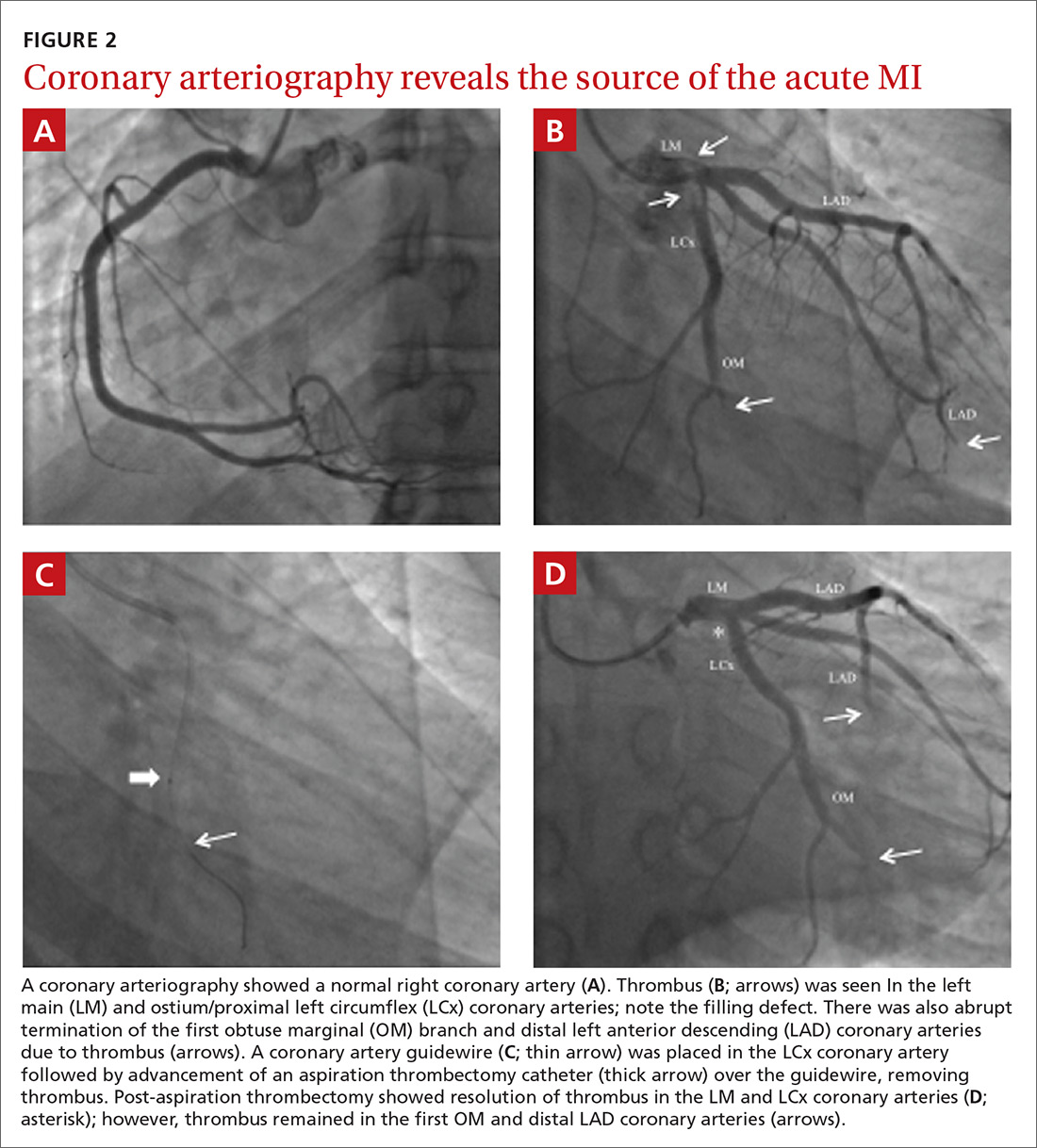
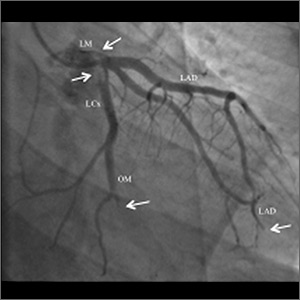
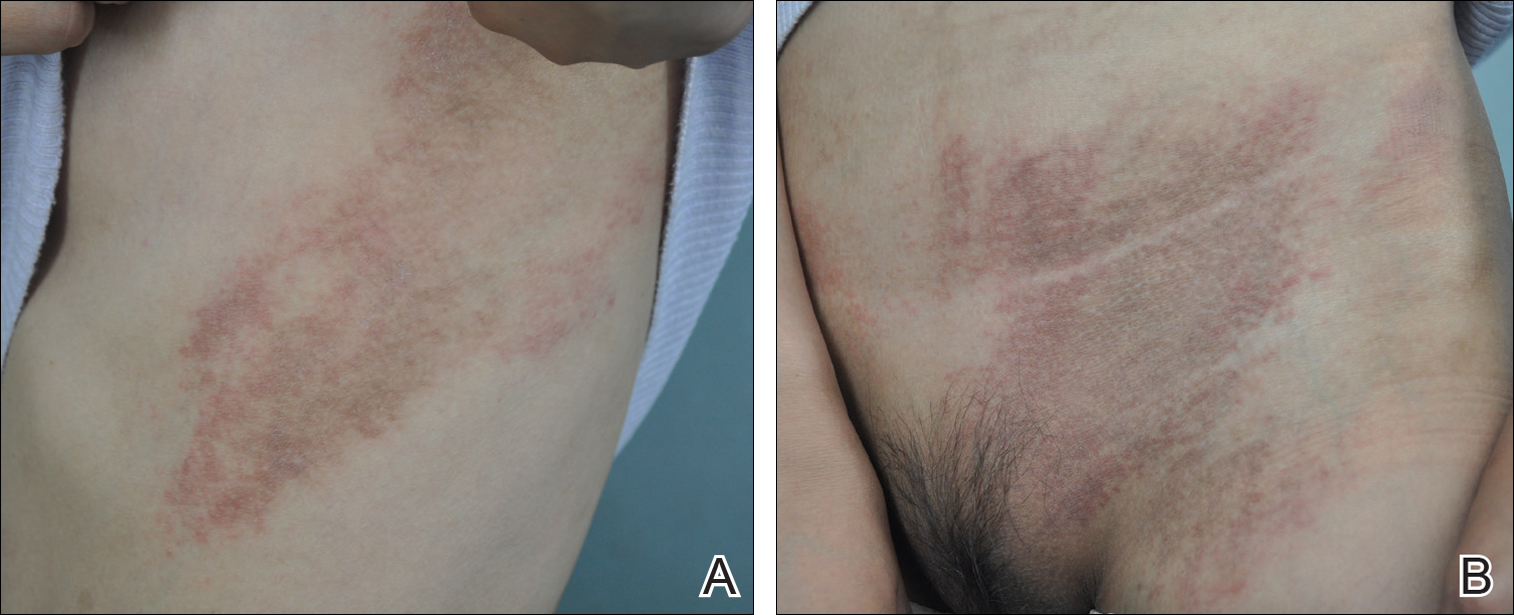
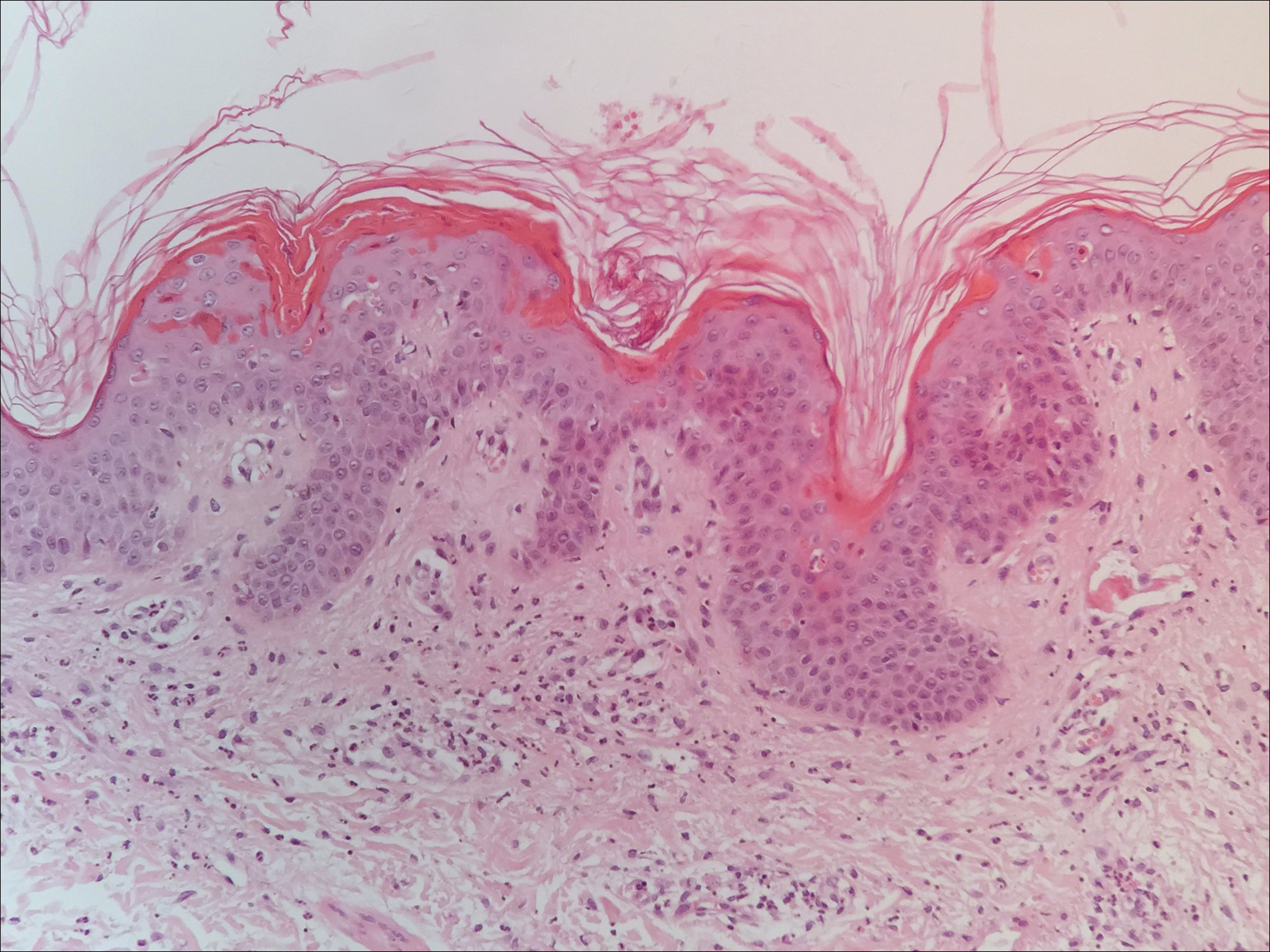
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).
The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
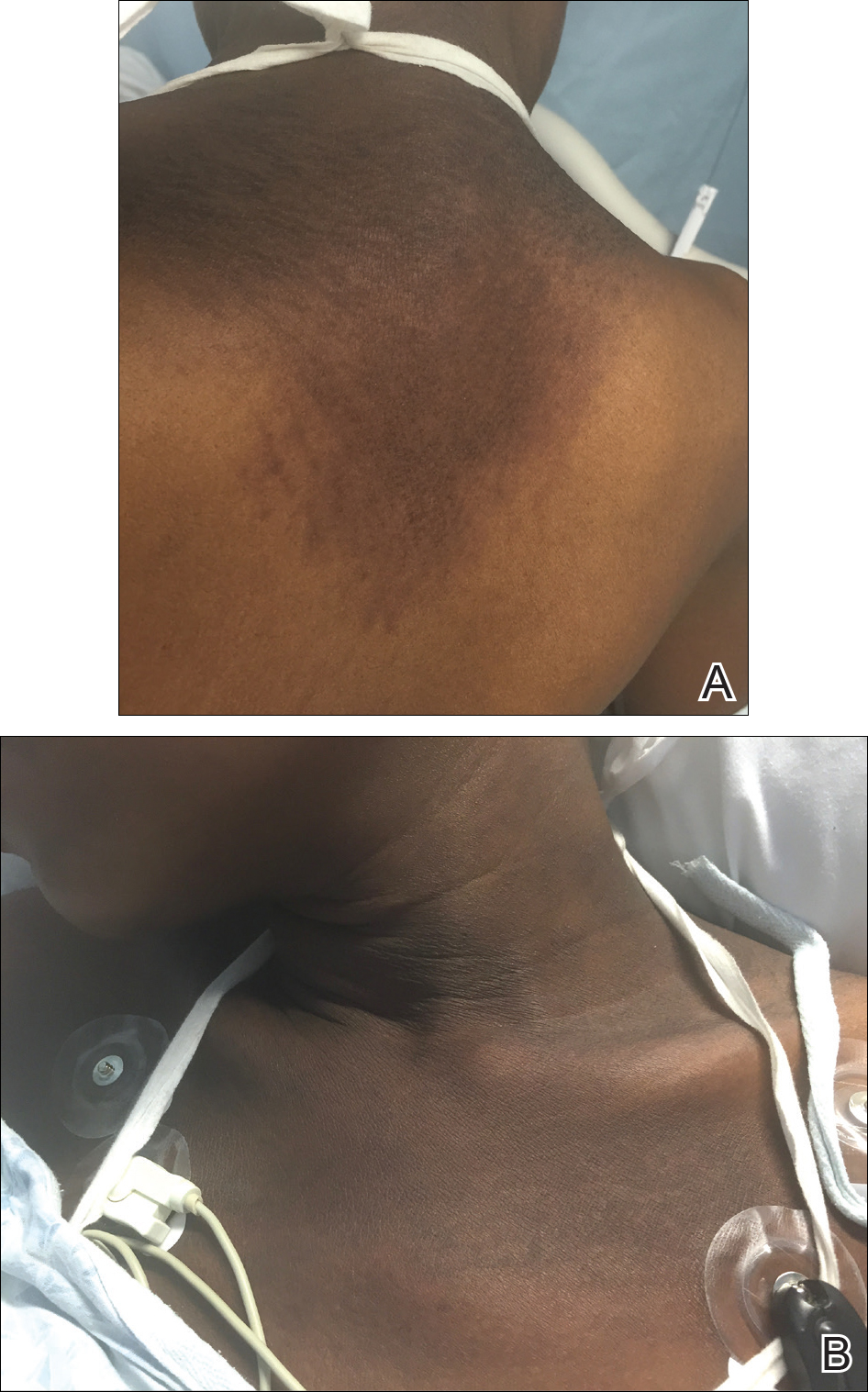
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.
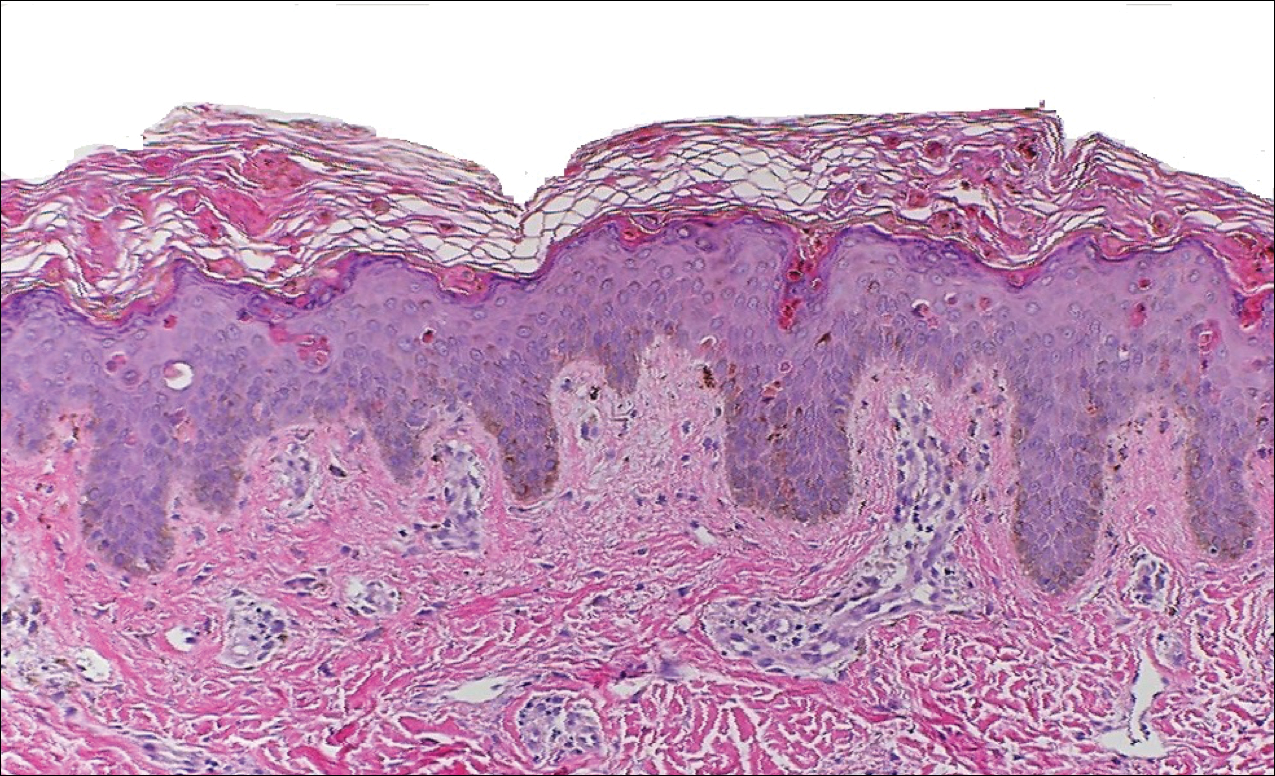
Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).
The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).


The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.

Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).

The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).


The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.

Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).

The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
Practice Points
- Serologic testing and skin biopsy are necessary in the timely and appropriate diagnosis of adult-onset Still disease (AOSD).
- In patients with a persistent pruritic papular rash, consider AOSD if there is a supporting history.
- Skin biopsy is diagnostic of AOSD with the unique histopathologic findings of dyskeratotic keratinocytes in the cornified layers as well as in the epidermis and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis.
Acral Cutaneous Metastasis From a Primary Breast Carcinoma Following Chemotherapy With Bevacizumab and Paclitaxel
Cutaneous metastasis of internal malignancy is a relatively uncommon phenomenon, with an overall incidence of 5.3% in cancer patients.1 Cutaneous involvement typically occurs late in the course of disease but can occasionally be the first extranodal sign of metastatic disease. Breast cancer has the highest rate of cutaneous metastasis, most often involving the chest wall1; however, cutaneous metastasis to the acral sites is exceedingly rare. The hand is the site of 0.1% of all metastatic lesions, with only 10% of these being cutaneous lesions and the remaining 90% being osseous metastases.2 Herein, we report a case of multiple cutaneous metastases to acral sites involving the palmar and plantar surfaces of the hands and feet.
Case Report
A 54-year-old black woman with a history of stage IV carcinoma of the breast was admitted to the university medical center with exquisitely painful cutaneous nodules on the hands and feet of 5 weeks’ duration that had started to cause difficulty with walking and daily activities. The patient reported that the breast carcinoma had initially been diagnosed in Nigeria 2 years prior, but she did not receive treatment until moving to the United States. She received a total of 4 cycles of chemotherapy with paclitaxel and bevacizumab, which was discontinued 6 weeks prior to admission due to pain in the lower extremities that was thought to be secondary to neuropathy. One week after discontinuation of chemotherapy, the patient reported increasing pain in the extremities and new-onset painful nodules on the hands and feet. Treatment with gabapentin as well as several courses of antibiotics failed to improve the condition.
She was admitted for symptomatic pain control and a dermatology consultation. Physical examination revealed multiple firm, tender, subcutaneous nodules on the volar surfaces of the soles, toes, palms, and fingertips (Figure 1). A nodule also was noted on the scalp. A punch biopsy of a nodule on the right fourth finger revealed a dermal carcinoma (Figure 2). On immunohistochemistry, the tumor stained positive for cytokeratin 5/6, cytokeratin 7, and gross cystic disease fluid protein 15. It did not demonstrate connection to the epidermis or adnexal structures. Although the tumor did not express estrogen or progesterone receptors, the findings were compatible with metastasis from the patient’s primary breast carcinoma with poor differentiation. A biopsy of the primary breast carcinoma was not available for review from Nigeria.
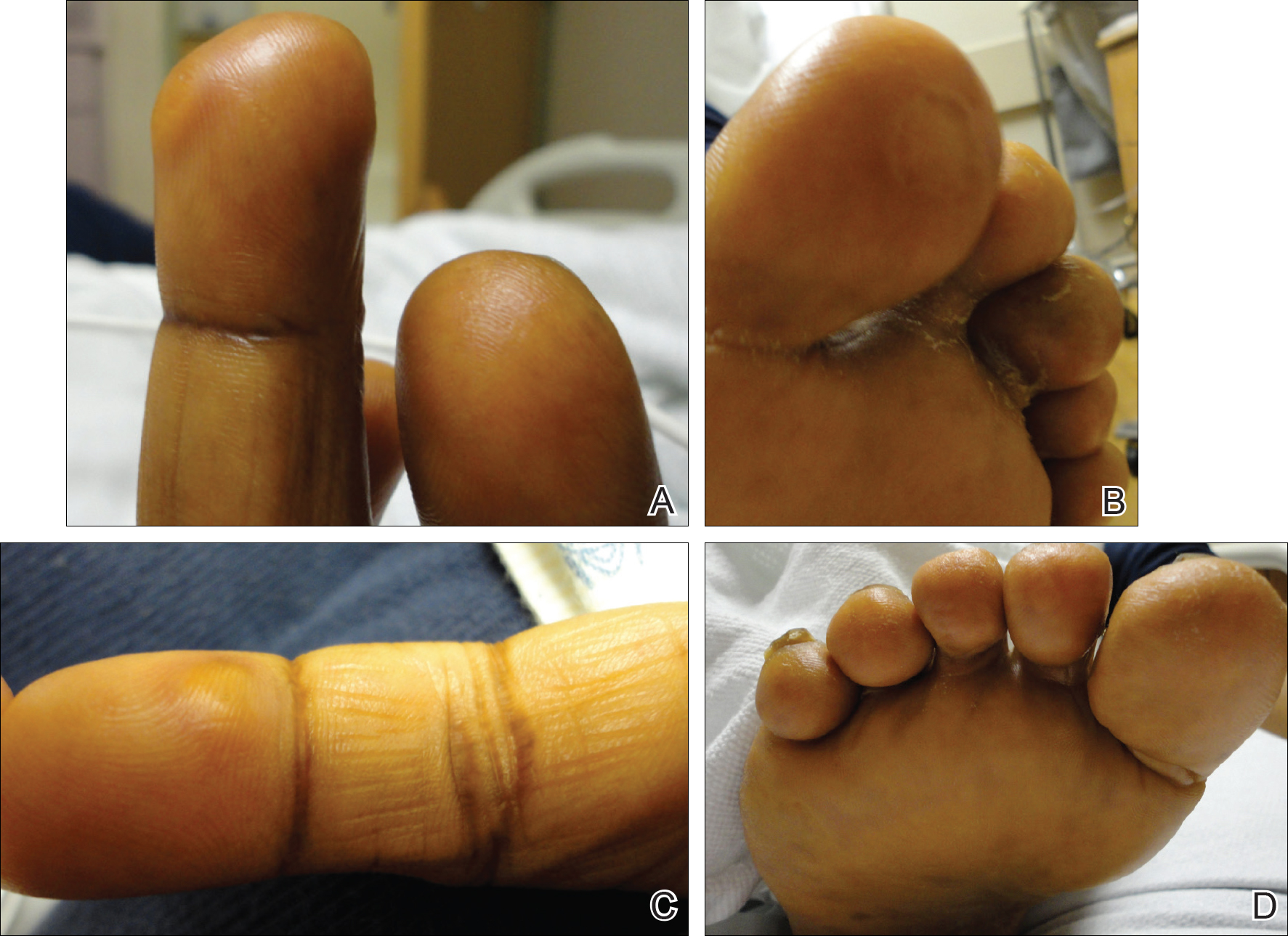
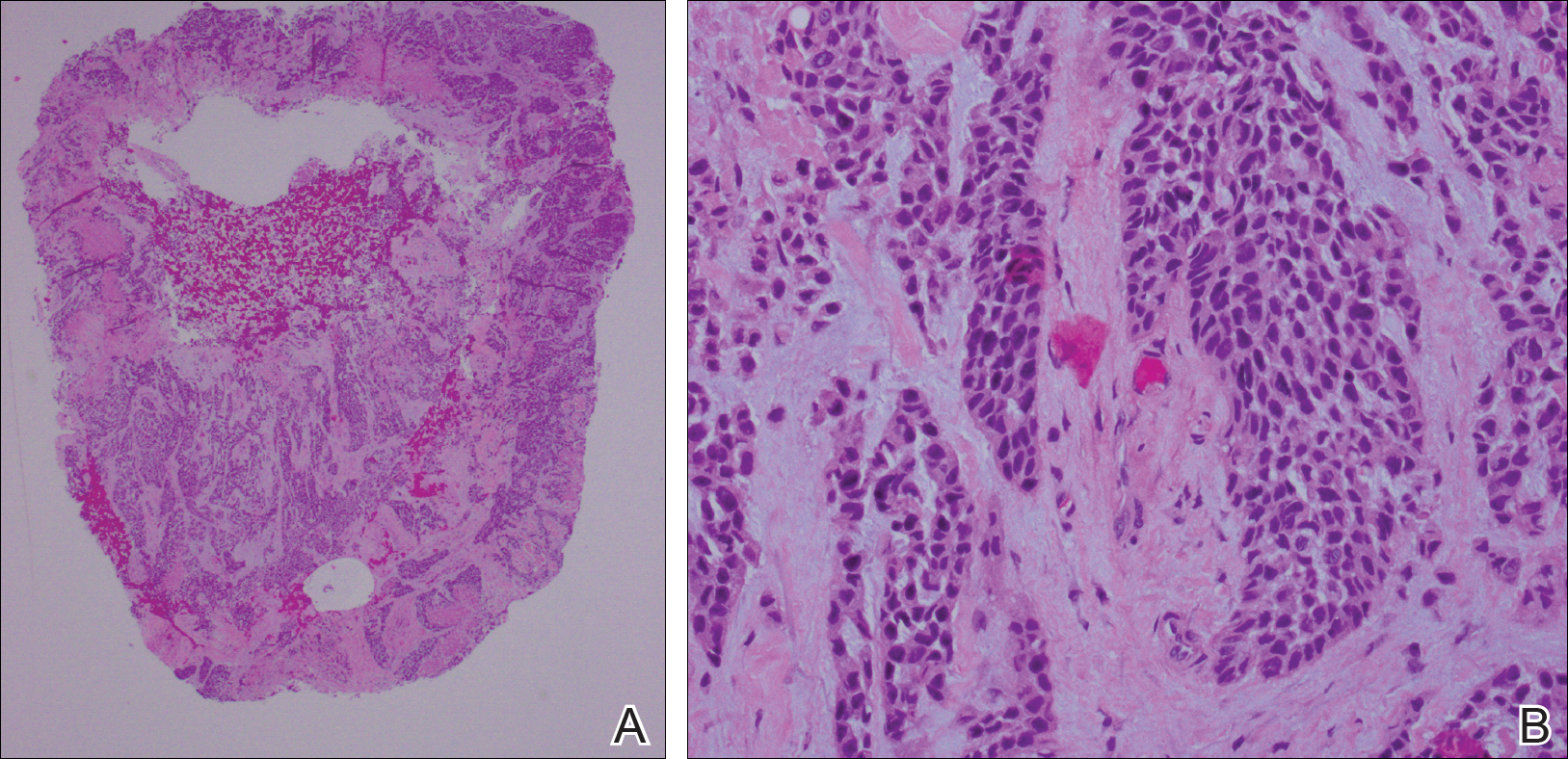
Comment
The majority of cases reporting acral cutaneous metastasis from internal malignancies are unilateral, involving only one extremity. Several hypotheses have been provided, including spread from localized trauma, which causes disruption of blood vessels and consequent extravasation and localization of tumor cells into the extravascular space.3 The distal extremities are particularly vulnerable to trauma, making this hypothesis plausible.
Considering the overall rarity of metastases to acral sites, it is interesting that our patient developed multiple distal nodules on both the hands and feet. The rapid onset of cutaneous nodules shortly after a course of chemotherapy led the team to consider the physiologic effects of paclitaxel and bevacizumab in the etiology of the acral cutaneous metastases. Karamouzis et al3 described a similar case of multiple cutaneous metastases with a bilateral acral distribution. This case also was associated with chemotherapy in the treatment of breast cancer. The authors proposed hand-foot syndrome, a chemotherapy-related eruption localized to acral skin, as a possible mechanism for hematogenous spread of malignant cells.3 The pathogenesis of hand-foot syndrome is not well understood, but the unique anatomy and physiology of acral skin including temperature gradients, rapidly dividing epidermal cells, absence of hair follicles and sebaceous glands, wide dermal papillae, and exposure to high pressures from carrying body weight and repetitive minor trauma may contribute to the localization of signs and symptoms.3,4 Our case supports a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer; however, our patient did not have apparent signs or symptoms of hand-foot syndrome during the course of treatment. We propose that effects of bevacizumab on acral skin may have contributed to the development of our patient’s metastatic pattern.
Bevacizumab, a monoclonal antibody to vascular endothelial growth factor A, has well-known vascular side effects. Unlike the inhibition of vascular endothelial growth factor A provided by the receptor tyrosine kinase inhibitors sorafenib and sunitinib, bevacizumab typically is not associated with hand-foot syndrome.5 However, several cases have been reported with chemotherapy-associated palmoplantar eruptions that resolved after withholding bevacizumab while continuing other chemotherapeutic agents, suggesting that bevacizumab-induced changes in acral skin contributed to the eruption.6 Specific factors that could contribute to acral metastasis in patients taking bevacizumab are endothelial dysfunction and capillary rarefaction of the acral skin, as well as hemorrhage, decreased wound healing, and changes in vascular permeability.5,7
We present a rare case of acral cutaneous metastasis associated with bevacizumab, one of few reported cases associated with a taxane chemotherapeutic agent.3 More cases need to be identified and reported to establish a causative association, if indeed existent, between acral cutaneous metastasis of breast carcinoma and the use of bevacizumab as well as other chemotherapeutic drugs.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:409-410.
- Karamouzis MV, Ardavanis A, Alexopoulos A, et al. Multiple cutaneous acral metastases in a woman with breast adenocarcinoma treated with pegylated liposomal doxorubicin: incidental or aetiological association? Eur J Cancer Care (Engl). 2005;14:267-271.
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. incidence, recognition and management. Am J Clin Dermatol. 2000;1:225-234.
- Wozel G, Sticherling M, Schon MP. Cutaneous side effects of inhibition of VEGF signal transduction. J Dtsch Dermatol Ges. 2010;8:243-249.
- Munehiro A, Yoneda K, Nakai K, et al. Bevacizumab-induced hand-foot syndrome: circumscribed type. Br J Dermatol. 2010;162:1411-1413.
- Mourad JJ, des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. a crucial role for microcirculation. Ann Oncol. 2008;19:927-934.
Cutaneous metastasis of internal malignancy is a relatively uncommon phenomenon, with an overall incidence of 5.3% in cancer patients.1 Cutaneous involvement typically occurs late in the course of disease but can occasionally be the first extranodal sign of metastatic disease. Breast cancer has the highest rate of cutaneous metastasis, most often involving the chest wall1; however, cutaneous metastasis to the acral sites is exceedingly rare. The hand is the site of 0.1% of all metastatic lesions, with only 10% of these being cutaneous lesions and the remaining 90% being osseous metastases.2 Herein, we report a case of multiple cutaneous metastases to acral sites involving the palmar and plantar surfaces of the hands and feet.
Case Report
A 54-year-old black woman with a history of stage IV carcinoma of the breast was admitted to the university medical center with exquisitely painful cutaneous nodules on the hands and feet of 5 weeks’ duration that had started to cause difficulty with walking and daily activities. The patient reported that the breast carcinoma had initially been diagnosed in Nigeria 2 years prior, but she did not receive treatment until moving to the United States. She received a total of 4 cycles of chemotherapy with paclitaxel and bevacizumab, which was discontinued 6 weeks prior to admission due to pain in the lower extremities that was thought to be secondary to neuropathy. One week after discontinuation of chemotherapy, the patient reported increasing pain in the extremities and new-onset painful nodules on the hands and feet. Treatment with gabapentin as well as several courses of antibiotics failed to improve the condition.
She was admitted for symptomatic pain control and a dermatology consultation. Physical examination revealed multiple firm, tender, subcutaneous nodules on the volar surfaces of the soles, toes, palms, and fingertips (Figure 1). A nodule also was noted on the scalp. A punch biopsy of a nodule on the right fourth finger revealed a dermal carcinoma (Figure 2). On immunohistochemistry, the tumor stained positive for cytokeratin 5/6, cytokeratin 7, and gross cystic disease fluid protein 15. It did not demonstrate connection to the epidermis or adnexal structures. Although the tumor did not express estrogen or progesterone receptors, the findings were compatible with metastasis from the patient’s primary breast carcinoma with poor differentiation. A biopsy of the primary breast carcinoma was not available for review from Nigeria.


Comment
The majority of cases reporting acral cutaneous metastasis from internal malignancies are unilateral, involving only one extremity. Several hypotheses have been provided, including spread from localized trauma, which causes disruption of blood vessels and consequent extravasation and localization of tumor cells into the extravascular space.3 The distal extremities are particularly vulnerable to trauma, making this hypothesis plausible.
Considering the overall rarity of metastases to acral sites, it is interesting that our patient developed multiple distal nodules on both the hands and feet. The rapid onset of cutaneous nodules shortly after a course of chemotherapy led the team to consider the physiologic effects of paclitaxel and bevacizumab in the etiology of the acral cutaneous metastases. Karamouzis et al3 described a similar case of multiple cutaneous metastases with a bilateral acral distribution. This case also was associated with chemotherapy in the treatment of breast cancer. The authors proposed hand-foot syndrome, a chemotherapy-related eruption localized to acral skin, as a possible mechanism for hematogenous spread of malignant cells.3 The pathogenesis of hand-foot syndrome is not well understood, but the unique anatomy and physiology of acral skin including temperature gradients, rapidly dividing epidermal cells, absence of hair follicles and sebaceous glands, wide dermal papillae, and exposure to high pressures from carrying body weight and repetitive minor trauma may contribute to the localization of signs and symptoms.3,4 Our case supports a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer; however, our patient did not have apparent signs or symptoms of hand-foot syndrome during the course of treatment. We propose that effects of bevacizumab on acral skin may have contributed to the development of our patient’s metastatic pattern.
Bevacizumab, a monoclonal antibody to vascular endothelial growth factor A, has well-known vascular side effects. Unlike the inhibition of vascular endothelial growth factor A provided by the receptor tyrosine kinase inhibitors sorafenib and sunitinib, bevacizumab typically is not associated with hand-foot syndrome.5 However, several cases have been reported with chemotherapy-associated palmoplantar eruptions that resolved after withholding bevacizumab while continuing other chemotherapeutic agents, suggesting that bevacizumab-induced changes in acral skin contributed to the eruption.6 Specific factors that could contribute to acral metastasis in patients taking bevacizumab are endothelial dysfunction and capillary rarefaction of the acral skin, as well as hemorrhage, decreased wound healing, and changes in vascular permeability.5,7
We present a rare case of acral cutaneous metastasis associated with bevacizumab, one of few reported cases associated with a taxane chemotherapeutic agent.3 More cases need to be identified and reported to establish a causative association, if indeed existent, between acral cutaneous metastasis of breast carcinoma and the use of bevacizumab as well as other chemotherapeutic drugs.
Cutaneous metastasis of internal malignancy is a relatively uncommon phenomenon, with an overall incidence of 5.3% in cancer patients.1 Cutaneous involvement typically occurs late in the course of disease but can occasionally be the first extranodal sign of metastatic disease. Breast cancer has the highest rate of cutaneous metastasis, most often involving the chest wall1; however, cutaneous metastasis to the acral sites is exceedingly rare. The hand is the site of 0.1% of all metastatic lesions, with only 10% of these being cutaneous lesions and the remaining 90% being osseous metastases.2 Herein, we report a case of multiple cutaneous metastases to acral sites involving the palmar and plantar surfaces of the hands and feet.
Case Report
A 54-year-old black woman with a history of stage IV carcinoma of the breast was admitted to the university medical center with exquisitely painful cutaneous nodules on the hands and feet of 5 weeks’ duration that had started to cause difficulty with walking and daily activities. The patient reported that the breast carcinoma had initially been diagnosed in Nigeria 2 years prior, but she did not receive treatment until moving to the United States. She received a total of 4 cycles of chemotherapy with paclitaxel and bevacizumab, which was discontinued 6 weeks prior to admission due to pain in the lower extremities that was thought to be secondary to neuropathy. One week after discontinuation of chemotherapy, the patient reported increasing pain in the extremities and new-onset painful nodules on the hands and feet. Treatment with gabapentin as well as several courses of antibiotics failed to improve the condition.
She was admitted for symptomatic pain control and a dermatology consultation. Physical examination revealed multiple firm, tender, subcutaneous nodules on the volar surfaces of the soles, toes, palms, and fingertips (Figure 1). A nodule also was noted on the scalp. A punch biopsy of a nodule on the right fourth finger revealed a dermal carcinoma (Figure 2). On immunohistochemistry, the tumor stained positive for cytokeratin 5/6, cytokeratin 7, and gross cystic disease fluid protein 15. It did not demonstrate connection to the epidermis or adnexal structures. Although the tumor did not express estrogen or progesterone receptors, the findings were compatible with metastasis from the patient’s primary breast carcinoma with poor differentiation. A biopsy of the primary breast carcinoma was not available for review from Nigeria.


Comment
The majority of cases reporting acral cutaneous metastasis from internal malignancies are unilateral, involving only one extremity. Several hypotheses have been provided, including spread from localized trauma, which causes disruption of blood vessels and consequent extravasation and localization of tumor cells into the extravascular space.3 The distal extremities are particularly vulnerable to trauma, making this hypothesis plausible.
Considering the overall rarity of metastases to acral sites, it is interesting that our patient developed multiple distal nodules on both the hands and feet. The rapid onset of cutaneous nodules shortly after a course of chemotherapy led the team to consider the physiologic effects of paclitaxel and bevacizumab in the etiology of the acral cutaneous metastases. Karamouzis et al3 described a similar case of multiple cutaneous metastases with a bilateral acral distribution. This case also was associated with chemotherapy in the treatment of breast cancer. The authors proposed hand-foot syndrome, a chemotherapy-related eruption localized to acral skin, as a possible mechanism for hematogenous spread of malignant cells.3 The pathogenesis of hand-foot syndrome is not well understood, but the unique anatomy and physiology of acral skin including temperature gradients, rapidly dividing epidermal cells, absence of hair follicles and sebaceous glands, wide dermal papillae, and exposure to high pressures from carrying body weight and repetitive minor trauma may contribute to the localization of signs and symptoms.3,4 Our case supports a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer; however, our patient did not have apparent signs or symptoms of hand-foot syndrome during the course of treatment. We propose that effects of bevacizumab on acral skin may have contributed to the development of our patient’s metastatic pattern.
Bevacizumab, a monoclonal antibody to vascular endothelial growth factor A, has well-known vascular side effects. Unlike the inhibition of vascular endothelial growth factor A provided by the receptor tyrosine kinase inhibitors sorafenib and sunitinib, bevacizumab typically is not associated with hand-foot syndrome.5 However, several cases have been reported with chemotherapy-associated palmoplantar eruptions that resolved after withholding bevacizumab while continuing other chemotherapeutic agents, suggesting that bevacizumab-induced changes in acral skin contributed to the eruption.6 Specific factors that could contribute to acral metastasis in patients taking bevacizumab are endothelial dysfunction and capillary rarefaction of the acral skin, as well as hemorrhage, decreased wound healing, and changes in vascular permeability.5,7
We present a rare case of acral cutaneous metastasis associated with bevacizumab, one of few reported cases associated with a taxane chemotherapeutic agent.3 More cases need to be identified and reported to establish a causative association, if indeed existent, between acral cutaneous metastasis of breast carcinoma and the use of bevacizumab as well as other chemotherapeutic drugs.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:409-410.
- Karamouzis MV, Ardavanis A, Alexopoulos A, et al. Multiple cutaneous acral metastases in a woman with breast adenocarcinoma treated with pegylated liposomal doxorubicin: incidental or aetiological association? Eur J Cancer Care (Engl). 2005;14:267-271.
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. incidence, recognition and management. Am J Clin Dermatol. 2000;1:225-234.
- Wozel G, Sticherling M, Schon MP. Cutaneous side effects of inhibition of VEGF signal transduction. J Dtsch Dermatol Ges. 2010;8:243-249.
- Munehiro A, Yoneda K, Nakai K, et al. Bevacizumab-induced hand-foot syndrome: circumscribed type. Br J Dermatol. 2010;162:1411-1413.
- Mourad JJ, des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. a crucial role for microcirculation. Ann Oncol. 2008;19:927-934.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:409-410.
- Karamouzis MV, Ardavanis A, Alexopoulos A, et al. Multiple cutaneous acral metastases in a woman with breast adenocarcinoma treated with pegylated liposomal doxorubicin: incidental or aetiological association? Eur J Cancer Care (Engl). 2005;14:267-271.
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. incidence, recognition and management. Am J Clin Dermatol. 2000;1:225-234.
- Wozel G, Sticherling M, Schon MP. Cutaneous side effects of inhibition of VEGF signal transduction. J Dtsch Dermatol Ges. 2010;8:243-249.
- Munehiro A, Yoneda K, Nakai K, et al. Bevacizumab-induced hand-foot syndrome: circumscribed type. Br J Dermatol. 2010;162:1411-1413.
- Mourad JJ, des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. a crucial role for microcirculation. Ann Oncol. 2008;19:927-934.
Practice Points
- Cutaneous involvement of internal malignancy typically occurs late in the disease course but can occasionally be the first extranodal sign of metastatic disease.
- Acral cutaneous metastasis from internal malignancies typically is unilateral, involving only one extremity; however, this case demonstrates involvement on both the hands and feet.
- This case support a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer.
Mycobacterium abscessus: A Rare Cause of Periprosthetic Knee Joint Infection
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
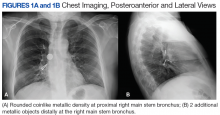
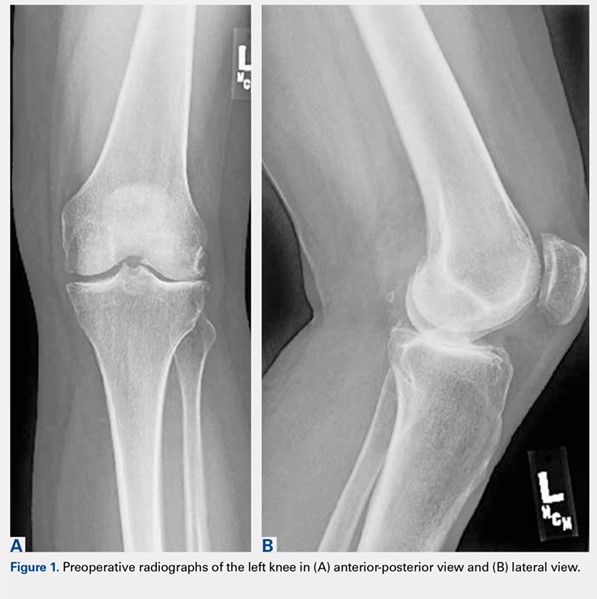
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).
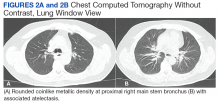
She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).
Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
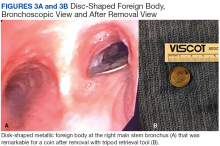
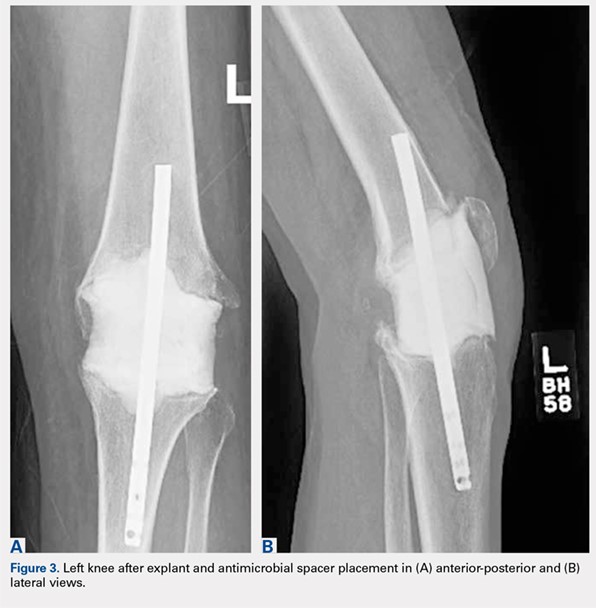
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.
Continue to: One month postoperatively...
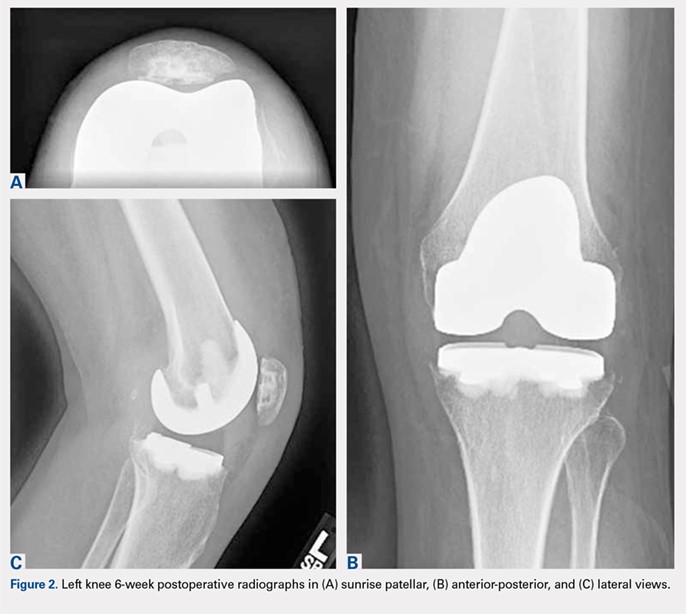
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
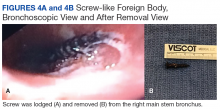
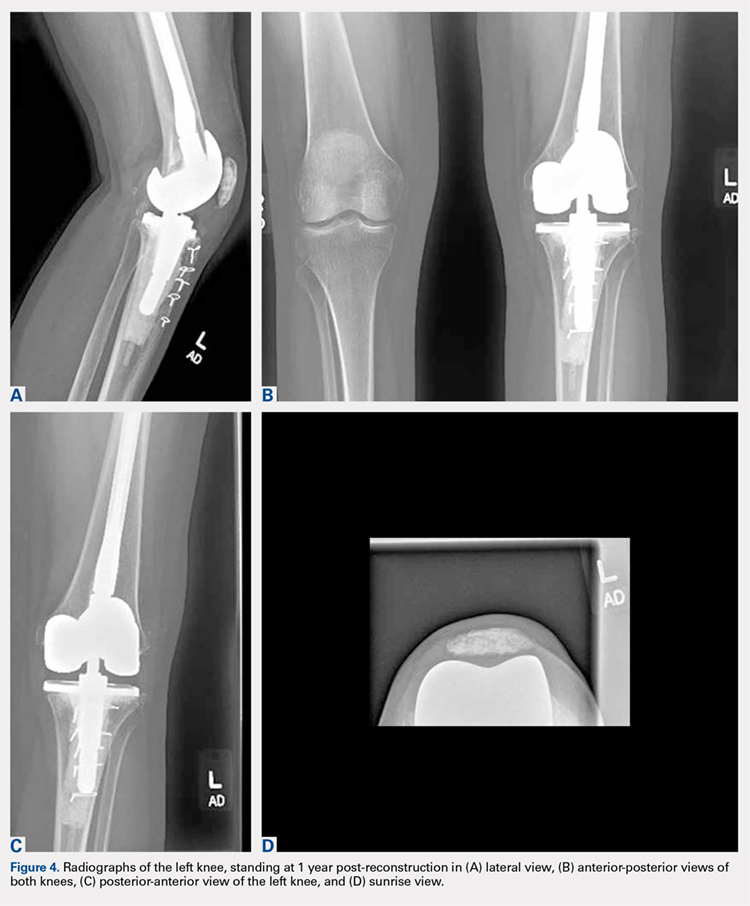
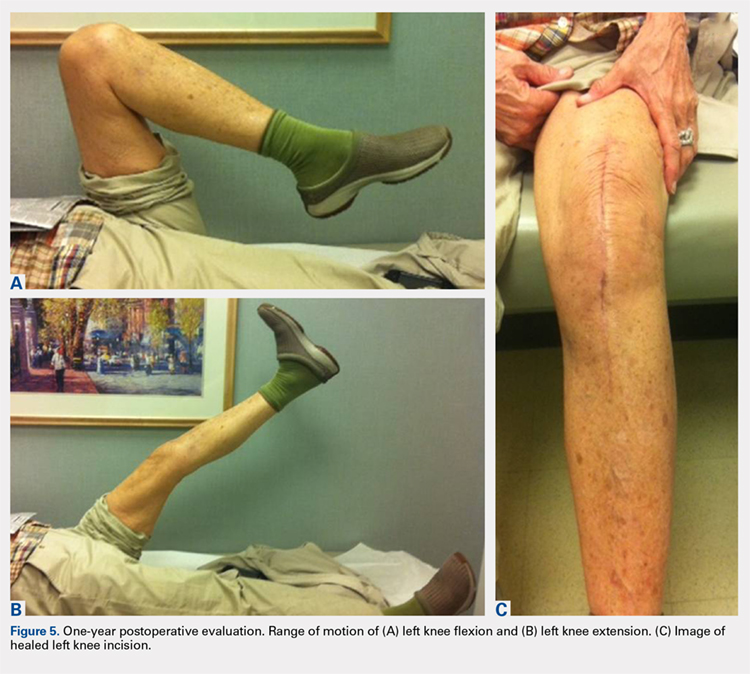
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.
Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.
CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).

She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).

Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.

Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.


Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.

CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).

She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).

Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.

Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.


Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.

CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
TAKE-HOME POINTS:
- Periprosthetic joint infections due to Mycobacterium abscess have been rarely reported, and no specific guidlines exist to inform treatment.
- Medical management alone was not successful in our clinical case and cannot be recommended.
- Combination medical and surgical management may provide the best opportunity for clincal cure of periprosthetic infections.
- In complicated periprosthetic joint infections involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach likley represents the preferred path to clinical cure.
- Successful erradiation of periprosthetic infection with M. abscessus may not preclude acceptable outcomes after revision TKA.
Arthroscopically-Guided, Cannulated, Headless Compression Screw Fixation of the Symptomatic Os Acromiale
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
TAKE-HOME POINTS
- Os acromiale is a failure of acromial ossification centers to fuse, and occurs in 8% of the population.
- Symptomatic os acromiale can be treated with repair, or sometimes excision or acromioplasty.
- Repair preserves the anterior deltoid origin and can result in less pain than excision of the fragment.
- Repair of larger fragments can be completed with cannulated screws to reliably achieve union.
- The arthroscope-assisted repair technique described in this article preserves vascularity and can reduce the risk of hardware-related complaints.
Amantadine-Induced Livedo Reticularis in a Child Treated Off Label for Neurobehavioral Disorders
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).
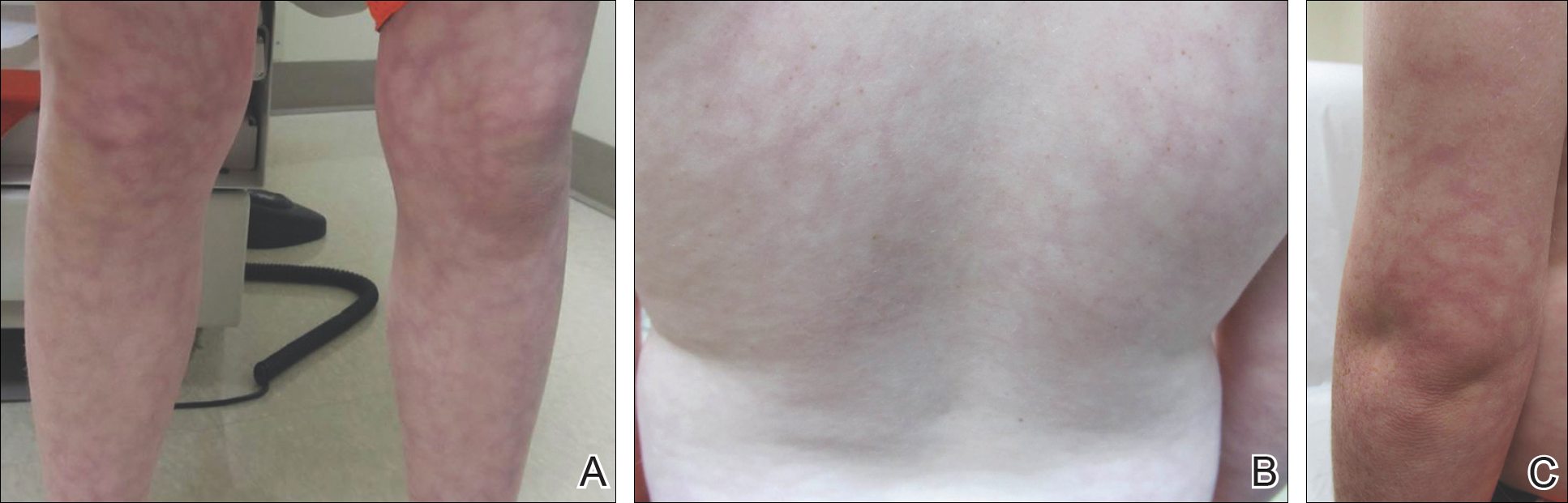
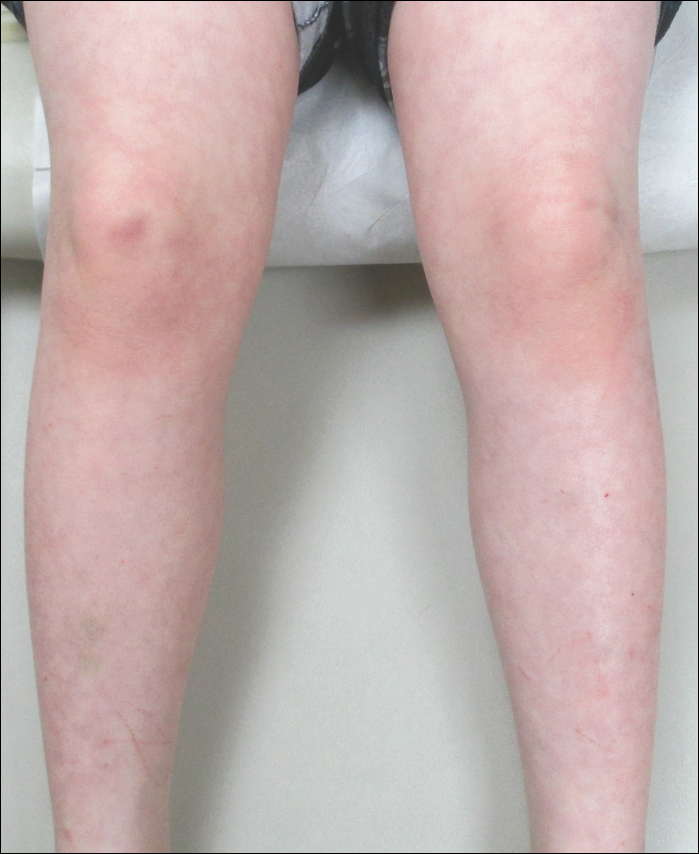
Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Practice Points
- Amantadine is a generally well-tolerated medication that is more commonly used for off-label treatment of several pediatric neurobehavioral conditions such as attention deficit hyperactivity disorder, autism spectrum disorders, obsessive compulsive disorder, depression, and others.
- Livedo reticularis has known associations with several medications and diseases; however, the most common presentation is cutis marmorata, a benign condition that typically affects newborns.
Nevus of Ota Associated With a Primary Uveal Melanoma and Intracranial Melanoma Metastasis
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.
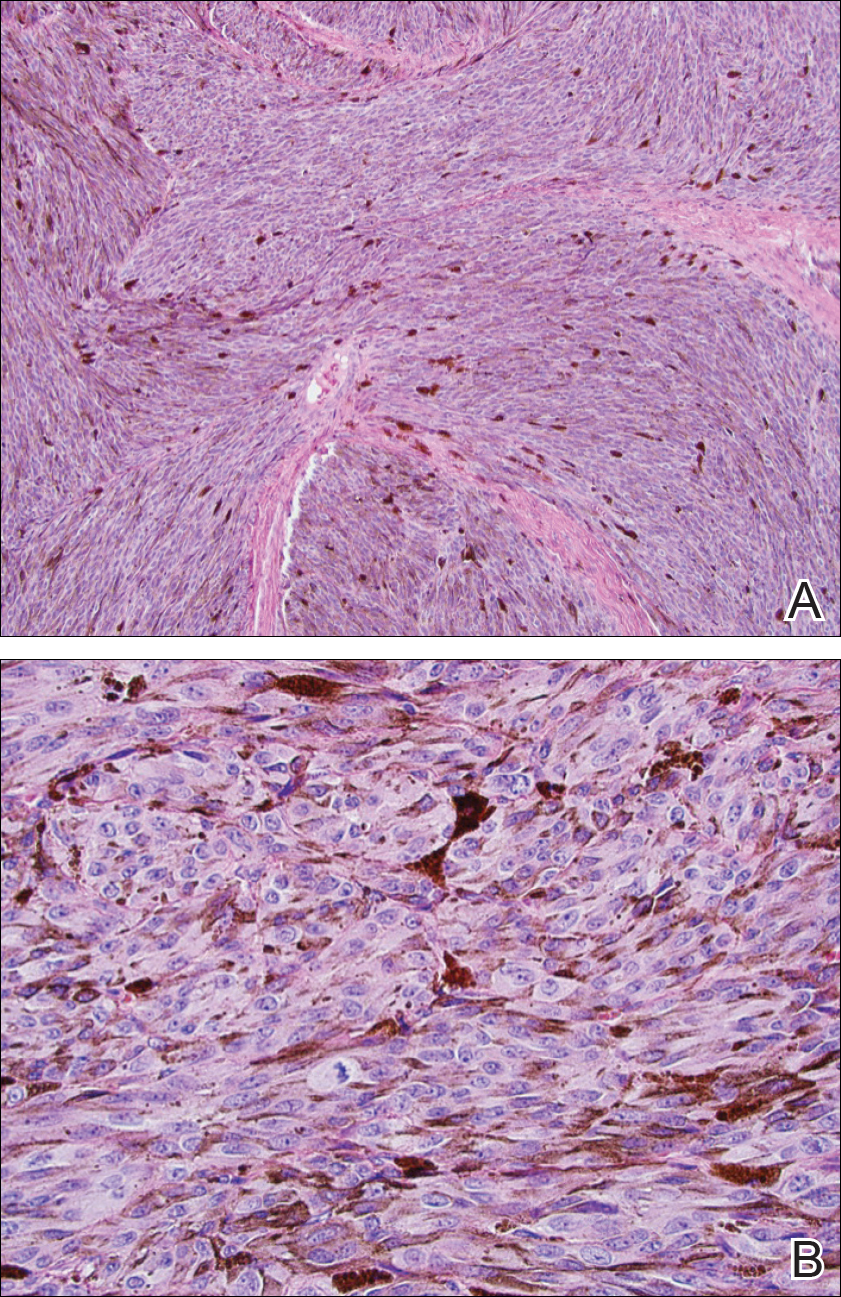
The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.

The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Practice Points
- Nevus of Ota is a hyperpigmented dermatosis that typically is distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.
- GNAQ and BAP1 mutations in patients with nevus of Ota confer a greater risk for malignant melanoma and metastatic progression.
- Ongoing ophthalmologic screening is paramount in patients with nevus of Ota and may prevent devastating sequelae.
When Rodents Attack: A Review of Rabies and Post-Exposure Prophylaxis
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
Flexible Bronchoscopic Removal of 3 Foreign Objects
Consider flexible bronchoscopy as an option to retrieve aspirated foreign bodies in the airway.
Airway foreign-body aspiration may cause no symptoms, although it can produce acute and life-threatening central airway obstruction.1 In the US, at least 2,700 people, including more than 300 children, die of foreign-body aspiration each year.2 Most foreign-body aspirations occur in children and elderly patients.3 In adults, dementia, drug intoxication, strokes, seizures, and neurologic disorders may predispose patients to aspiration.3 Some of the consequences of an aspirated object are complete or partial airway obstruction, respiratory distress and failure, pneumothorax, and hemorrhage.2 In addition, inadvertent aspiration of foreign objects in asymptomatic patients may not be evident for months, resulting in late complications as postobstructive pneumonia, bronchiectasis, or lung abscess.2
We present a case of a patient with documented schizophrenia with nonadherence to his antipsychotic medications who aspirated different objects. Flexible bronchoscopy was performed since rigid bronchoscopy is not available at our institution. Several bronchoscopy tools were required to successfully remove the objects and avoid further invasive interventions, such as cardiothoracic surgery.
Case Presentation
A 55-year-old man with schizophrenia on antipsychotics developed cough, shortness of breath, and dysphagia of 1-month of evolution. Because his symptoms worsened, his mother brought him to the emergency department. Peripheral oxygen saturation was 97% at room air. Lung auscultation was remarkable for bilateral scattered rhonchi and wheezes.
Laboratory results showed leukocytosis with neutrophilia and hypotonic hypovolemic hyponatremia.
The patient stated that he did not remember swallowing any objects, although his mother confirmed that he was not adherent with his antipsychotic medications, which could have predisposed him to aspiration secondary to possible psychotic episodes.
Piperacillin/tazobactam 4.5 g every 8 hours was started to cover anaerobic bacterial organisms causing abscess, and IV fluids were given for hypovolemia. Flexible bronchoscopy (rigid bronchoscopy is superior although not available at our institution) was planned to be performed in the operating room (OR) because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects.
A bronchoscopy was performed, showing a disk-shaped metallic foreign body at the right main stem bronchus. After multiple attempts using the tripod retrieval tool, a coin was removed (Figures 3A and 3B).
The patient was reintubated without any complications. A postprocedure chest radiograph showed the absence of foreign bodies and no pneumothorax. The patient completed IV antibiotic with piperacillin/tazobactam and supportive therapy with clinical improvement and successful extubation within 2 days. Cardiothoracic surgery was not required. Psychiatry service recommended to continue the same antipsychotic medications, administered only by his mother to assure adherence and to avoid similar future events. The patient was discharged home without any immediate complications despite having had a coin, nail, and screw aspiration (Figure 6).
Discussion
More than 50% of foreign bodies lodge at the right main stem bronchus due to the trachea’s anatomical position.2,4 In adults, foreign-body aspiration may present with nonspecific symptoms, such as cough and dyspnea.4 Other symptoms might include wheezes, chest discomfort, and sputum production. A chest radiograph is helpful as part of the initial diagnostic workup. A chest CT scan without contrast should be performed to confirm the diagnosis and to plan possible foreign-body retrieval.
Bronchoscopy is the gold standard for diagnosis and management of foreign-body aspiration.1 Rigid bronchoscopy is superior to flexible bronchoscopy in removal of large airway foreign bodies.1 The rigid bronchoscopy provides the ability to function as an endotracheal tube, thus allowing control of the airway and a conduit through which foreign bodies can be removed.1 Nonetheless, sometimes retrieval of foreign bodies deeper into the subsegmental bronchi cannot be achieved.1 Moreover, the required equipment or knowledgeable staff is not always available.1 Therefore, flexible bronchoscopy is an option to retrieve airway foreign bodies especially those located distal in the airway and for those medical centers without rigid bronchoscopy as is the case in our institution.
In our case, flexible bronchoscopy was performed in the OR because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects. Anesthesia Service assistance was requested anticipating need for patient sedation and intubation. We used the tripod and snare retrieval tools to remove 3 foreign objects located at the right main stem bronchus. Even though multiple attempts were made and endotracheal intubation was required, a successful retrieval with flexible bronchoscopy was performed. Moreover, cardiothoracic surgery was not required avoiding more invasive interventions with subsequent morbidity and mortality.
Conclusion
Flexible bronchoscopy is an important tool within the arsenal of the Pulmonology Service. The management of the underlying etiology also should be performed. In our case, the Psychiatry Service recommended that the patient’s medications should be administered by his mother to avoid similar events in the future. Flexible bronchoscopy can be a valuable option for foreign objects removal, especially those distally located in the lung segments as well as in those medical centers where rigid bronchoscopy is not available.
1. Mehta D, Mehta C, Bansal S, Singla S, Tangri N. Flexible bronchoscopic removal of a three piece foreign body from a child’s bronchus. Australas Med J. 2012;5(4):227-230.
2. Mercado JA, Rodríguez W. Occult aspiration of a chicken wishbone as a cause of hemoptysis. P R Health Sci J. 1999;18(1):71-73.
3. Robles-Arias CM, Campos-Santiago Z, Vega MT, Rosa-Cruz F, Rodríguez-Cintrón W. Aspiration of a dental tool during a crown placement procedure. Fed Pract. 2014;31(6):12-14.
4. Blanco-Ramos M, Botana-Rial M, García-Fontán E, Fernández-Villar A, Gallas-Torreira M. Update in the extraction of airway foreign bodies in adults. J Thorac Dis. 2016;8(11):3452-3456.
Consider flexible bronchoscopy as an option to retrieve aspirated foreign bodies in the airway.
Consider flexible bronchoscopy as an option to retrieve aspirated foreign bodies in the airway.
Airway foreign-body aspiration may cause no symptoms, although it can produce acute and life-threatening central airway obstruction.1 In the US, at least 2,700 people, including more than 300 children, die of foreign-body aspiration each year.2 Most foreign-body aspirations occur in children and elderly patients.3 In adults, dementia, drug intoxication, strokes, seizures, and neurologic disorders may predispose patients to aspiration.3 Some of the consequences of an aspirated object are complete or partial airway obstruction, respiratory distress and failure, pneumothorax, and hemorrhage.2 In addition, inadvertent aspiration of foreign objects in asymptomatic patients may not be evident for months, resulting in late complications as postobstructive pneumonia, bronchiectasis, or lung abscess.2
We present a case of a patient with documented schizophrenia with nonadherence to his antipsychotic medications who aspirated different objects. Flexible bronchoscopy was performed since rigid bronchoscopy is not available at our institution. Several bronchoscopy tools were required to successfully remove the objects and avoid further invasive interventions, such as cardiothoracic surgery.
Case Presentation
A 55-year-old man with schizophrenia on antipsychotics developed cough, shortness of breath, and dysphagia of 1-month of evolution. Because his symptoms worsened, his mother brought him to the emergency department. Peripheral oxygen saturation was 97% at room air. Lung auscultation was remarkable for bilateral scattered rhonchi and wheezes.
Laboratory results showed leukocytosis with neutrophilia and hypotonic hypovolemic hyponatremia.
The patient stated that he did not remember swallowing any objects, although his mother confirmed that he was not adherent with his antipsychotic medications, which could have predisposed him to aspiration secondary to possible psychotic episodes.
Piperacillin/tazobactam 4.5 g every 8 hours was started to cover anaerobic bacterial organisms causing abscess, and IV fluids were given for hypovolemia. Flexible bronchoscopy (rigid bronchoscopy is superior although not available at our institution) was planned to be performed in the operating room (OR) because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects.
A bronchoscopy was performed, showing a disk-shaped metallic foreign body at the right main stem bronchus. After multiple attempts using the tripod retrieval tool, a coin was removed (Figures 3A and 3B).
The patient was reintubated without any complications. A postprocedure chest radiograph showed the absence of foreign bodies and no pneumothorax. The patient completed IV antibiotic with piperacillin/tazobactam and supportive therapy with clinical improvement and successful extubation within 2 days. Cardiothoracic surgery was not required. Psychiatry service recommended to continue the same antipsychotic medications, administered only by his mother to assure adherence and to avoid similar future events. The patient was discharged home without any immediate complications despite having had a coin, nail, and screw aspiration (Figure 6).
Discussion
More than 50% of foreign bodies lodge at the right main stem bronchus due to the trachea’s anatomical position.2,4 In adults, foreign-body aspiration may present with nonspecific symptoms, such as cough and dyspnea.4 Other symptoms might include wheezes, chest discomfort, and sputum production. A chest radiograph is helpful as part of the initial diagnostic workup. A chest CT scan without contrast should be performed to confirm the diagnosis and to plan possible foreign-body retrieval.
Bronchoscopy is the gold standard for diagnosis and management of foreign-body aspiration.1 Rigid bronchoscopy is superior to flexible bronchoscopy in removal of large airway foreign bodies.1 The rigid bronchoscopy provides the ability to function as an endotracheal tube, thus allowing control of the airway and a conduit through which foreign bodies can be removed.1 Nonetheless, sometimes retrieval of foreign bodies deeper into the subsegmental bronchi cannot be achieved.1 Moreover, the required equipment or knowledgeable staff is not always available.1 Therefore, flexible bronchoscopy is an option to retrieve airway foreign bodies especially those located distal in the airway and for those medical centers without rigid bronchoscopy as is the case in our institution.
In our case, flexible bronchoscopy was performed in the OR because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects. Anesthesia Service assistance was requested anticipating need for patient sedation and intubation. We used the tripod and snare retrieval tools to remove 3 foreign objects located at the right main stem bronchus. Even though multiple attempts were made and endotracheal intubation was required, a successful retrieval with flexible bronchoscopy was performed. Moreover, cardiothoracic surgery was not required avoiding more invasive interventions with subsequent morbidity and mortality.
Conclusion
Flexible bronchoscopy is an important tool within the arsenal of the Pulmonology Service. The management of the underlying etiology also should be performed. In our case, the Psychiatry Service recommended that the patient’s medications should be administered by his mother to avoid similar events in the future. Flexible bronchoscopy can be a valuable option for foreign objects removal, especially those distally located in the lung segments as well as in those medical centers where rigid bronchoscopy is not available.
Airway foreign-body aspiration may cause no symptoms, although it can produce acute and life-threatening central airway obstruction.1 In the US, at least 2,700 people, including more than 300 children, die of foreign-body aspiration each year.2 Most foreign-body aspirations occur in children and elderly patients.3 In adults, dementia, drug intoxication, strokes, seizures, and neurologic disorders may predispose patients to aspiration.3 Some of the consequences of an aspirated object are complete or partial airway obstruction, respiratory distress and failure, pneumothorax, and hemorrhage.2 In addition, inadvertent aspiration of foreign objects in asymptomatic patients may not be evident for months, resulting in late complications as postobstructive pneumonia, bronchiectasis, or lung abscess.2
We present a case of a patient with documented schizophrenia with nonadherence to his antipsychotic medications who aspirated different objects. Flexible bronchoscopy was performed since rigid bronchoscopy is not available at our institution. Several bronchoscopy tools were required to successfully remove the objects and avoid further invasive interventions, such as cardiothoracic surgery.
Case Presentation
A 55-year-old man with schizophrenia on antipsychotics developed cough, shortness of breath, and dysphagia of 1-month of evolution. Because his symptoms worsened, his mother brought him to the emergency department. Peripheral oxygen saturation was 97% at room air. Lung auscultation was remarkable for bilateral scattered rhonchi and wheezes.
Laboratory results showed leukocytosis with neutrophilia and hypotonic hypovolemic hyponatremia.
The patient stated that he did not remember swallowing any objects, although his mother confirmed that he was not adherent with his antipsychotic medications, which could have predisposed him to aspiration secondary to possible psychotic episodes.
Piperacillin/tazobactam 4.5 g every 8 hours was started to cover anaerobic bacterial organisms causing abscess, and IV fluids were given for hypovolemia. Flexible bronchoscopy (rigid bronchoscopy is superior although not available at our institution) was planned to be performed in the operating room (OR) because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects.
A bronchoscopy was performed, showing a disk-shaped metallic foreign body at the right main stem bronchus. After multiple attempts using the tripod retrieval tool, a coin was removed (Figures 3A and 3B).
The patient was reintubated without any complications. A postprocedure chest radiograph showed the absence of foreign bodies and no pneumothorax. The patient completed IV antibiotic with piperacillin/tazobactam and supportive therapy with clinical improvement and successful extubation within 2 days. Cardiothoracic surgery was not required. Psychiatry service recommended to continue the same antipsychotic medications, administered only by his mother to assure adherence and to avoid similar future events. The patient was discharged home without any immediate complications despite having had a coin, nail, and screw aspiration (Figure 6).
Discussion
More than 50% of foreign bodies lodge at the right main stem bronchus due to the trachea’s anatomical position.2,4 In adults, foreign-body aspiration may present with nonspecific symptoms, such as cough and dyspnea.4 Other symptoms might include wheezes, chest discomfort, and sputum production. A chest radiograph is helpful as part of the initial diagnostic workup. A chest CT scan without contrast should be performed to confirm the diagnosis and to plan possible foreign-body retrieval.
Bronchoscopy is the gold standard for diagnosis and management of foreign-body aspiration.1 Rigid bronchoscopy is superior to flexible bronchoscopy in removal of large airway foreign bodies.1 The rigid bronchoscopy provides the ability to function as an endotracheal tube, thus allowing control of the airway and a conduit through which foreign bodies can be removed.1 Nonetheless, sometimes retrieval of foreign bodies deeper into the subsegmental bronchi cannot be achieved.1 Moreover, the required equipment or knowledgeable staff is not always available.1 Therefore, flexible bronchoscopy is an option to retrieve airway foreign bodies especially those located distal in the airway and for those medical centers without rigid bronchoscopy as is the case in our institution.
In our case, flexible bronchoscopy was performed in the OR because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects. Anesthesia Service assistance was requested anticipating need for patient sedation and intubation. We used the tripod and snare retrieval tools to remove 3 foreign objects located at the right main stem bronchus. Even though multiple attempts were made and endotracheal intubation was required, a successful retrieval with flexible bronchoscopy was performed. Moreover, cardiothoracic surgery was not required avoiding more invasive interventions with subsequent morbidity and mortality.
Conclusion
Flexible bronchoscopy is an important tool within the arsenal of the Pulmonology Service. The management of the underlying etiology also should be performed. In our case, the Psychiatry Service recommended that the patient’s medications should be administered by his mother to avoid similar events in the future. Flexible bronchoscopy can be a valuable option for foreign objects removal, especially those distally located in the lung segments as well as in those medical centers where rigid bronchoscopy is not available.
1. Mehta D, Mehta C, Bansal S, Singla S, Tangri N. Flexible bronchoscopic removal of a three piece foreign body from a child’s bronchus. Australas Med J. 2012;5(4):227-230.
2. Mercado JA, Rodríguez W. Occult aspiration of a chicken wishbone as a cause of hemoptysis. P R Health Sci J. 1999;18(1):71-73.
3. Robles-Arias CM, Campos-Santiago Z, Vega MT, Rosa-Cruz F, Rodríguez-Cintrón W. Aspiration of a dental tool during a crown placement procedure. Fed Pract. 2014;31(6):12-14.
4. Blanco-Ramos M, Botana-Rial M, García-Fontán E, Fernández-Villar A, Gallas-Torreira M. Update in the extraction of airway foreign bodies in adults. J Thorac Dis. 2016;8(11):3452-3456.
1. Mehta D, Mehta C, Bansal S, Singla S, Tangri N. Flexible bronchoscopic removal of a three piece foreign body from a child’s bronchus. Australas Med J. 2012;5(4):227-230.
2. Mercado JA, Rodríguez W. Occult aspiration of a chicken wishbone as a cause of hemoptysis. P R Health Sci J. 1999;18(1):71-73.
3. Robles-Arias CM, Campos-Santiago Z, Vega MT, Rosa-Cruz F, Rodríguez-Cintrón W. Aspiration of a dental tool during a crown placement procedure. Fed Pract. 2014;31(6):12-14.
4. Blanco-Ramos M, Botana-Rial M, García-Fontán E, Fernández-Villar A, Gallas-Torreira M. Update in the extraction of airway foreign bodies in adults. J Thorac Dis. 2016;8(11):3452-3456.
A Rare Case of Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type
CASE REPORT
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.
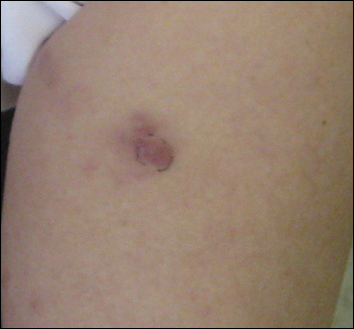
An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.
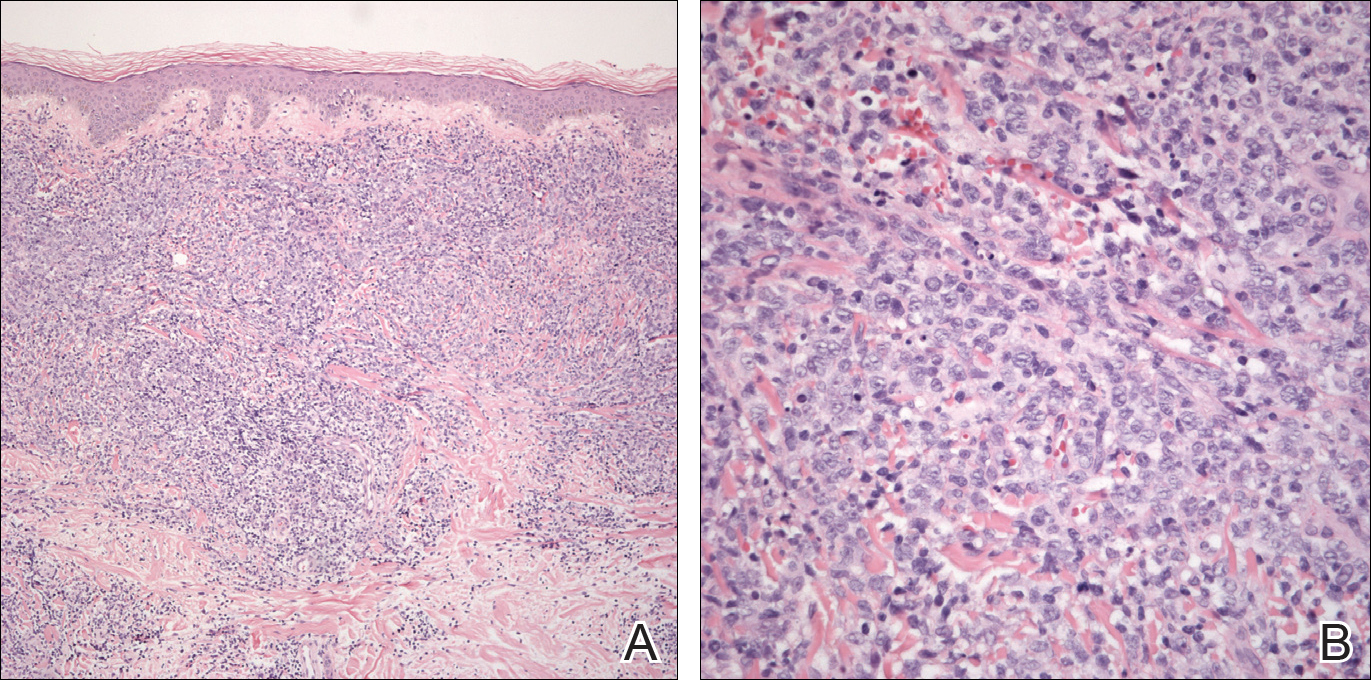
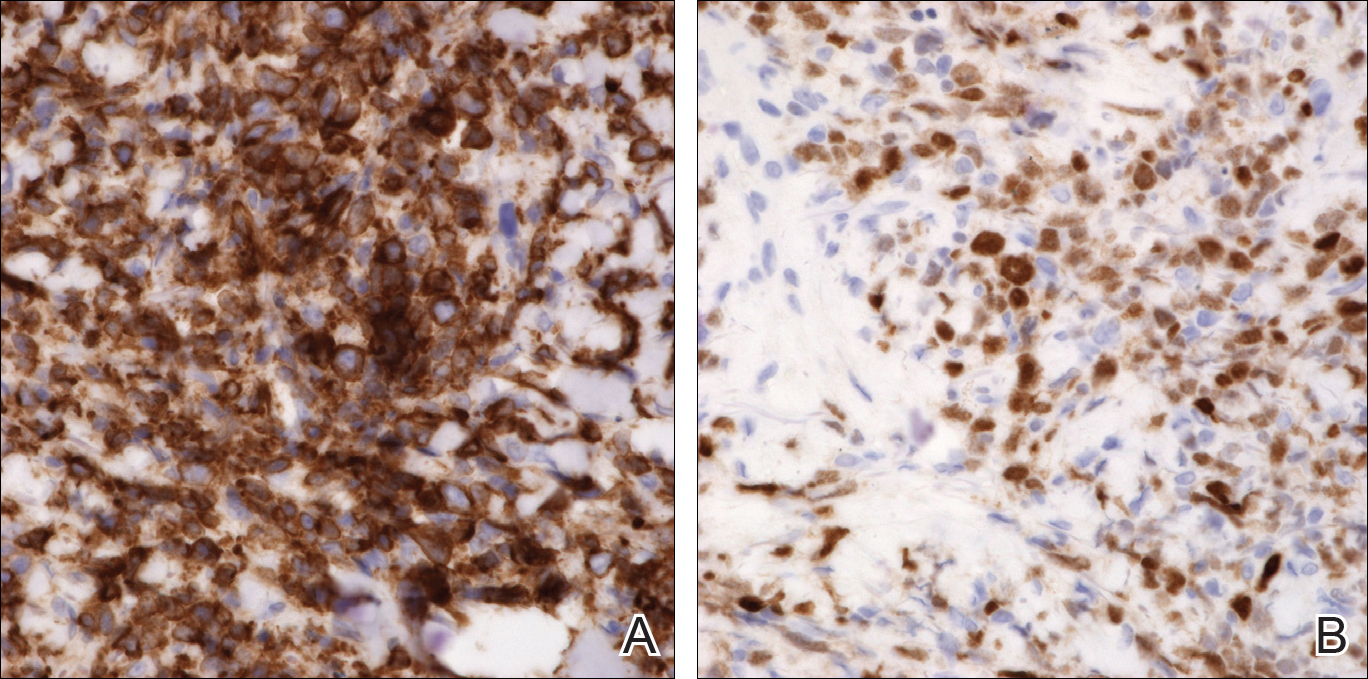
COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
CASE REPORT
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.

An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.


COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
CASE REPORT
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.

An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.


COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
Practice Points
- Primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) is characterized by the presence of large round cells on histopathology.
- There are potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
24-year-old with history of smoking tobacco and cannabis • dyspnea • chest tightness
THE CASE
A 24-year-old man with a history of smoking tobacco presented to the hospital with acute-onset chest tightness and dyspnea shortly after smoking cannabis. He was otherwise healthy and hemodynamically stable upon arrival to the emergency department. An electrocardiogram (EKG) was obtained.
THE DIAGNOSIS
The EKG showed ST-segment elevation in the inferolateral leads, consistent with an acute myocardial infarction (AMI) (FIGURE 1). The patient was immediately transported to the cardiac catheterization laboratory, where coronary arteriography demonstrated a normal right coronary artery (FIGURE 2A). Diffuse thrombosis without atherosclerosis was seen throughout the left coronary arteries, including the left main artery, distal left anterior descending (LAD) artery, first diagonal branch of the LAD artery, ostial and proximal left circumflex (LCx) arteries, and first obtuse marginal (OM) branch of the LCx artery (FIGURE 2B).
DISCUSSION
The most common cause of AMI is underlying coronary atherosclerosis;1 however, AMI may occur due to in-situ thrombosis, thromboembolism, or coronary artery vasospasm, especially due to cocaine or other substance abuse. Occasionally, coronary arteries may be damaged due to viral myocarditis, autoimmune vasculitis, dissection of the ascending aorta, or dissection of a coronary artery, especially during pregnancy and postpartum.2,3
Cannabis and tobacco increase cardiovascular events
Smoking cannabis has been shown to increase adrenergic activity, resulting in an increased heart rate and elevated arterial pressure.4,5 These changes may increase myocardial oxygen demand and may result in a decrease in myocardial oxygen supply due to a decrease in the diastolic time.6 Smoking cannabis can also increase carboxyhemoglobin levels, which may compromise tissue oxygenation.
The risk for AMI has been shown to increase within the first hour of smoking cannabis.5 A few reports have documented cases of acute coronary syndrome following cannabis use; the majority of affected patients presented with chest pain within hours of smoking cannabis and were found to have a thrombus in a coronary artery, which was then treated medically or with percutaneous coronary intervention.7 Rare cases of cardiovascular death following cannabis use have also been reported.7,8
It has been suggested that coronary artery vasospasm may occur from cannabis use, which may precipitate thrombosis; however, this is not well defined.9 It is not clear if vasospasm was the inciting factor for thrombus formation in this case, as there was extensive and diffuse thrombus far greater than that expected solely from coronary artery vasospasm.
Continue to: AMI without atherosclerosis? Consider thrombosis
AMI without atherosclerosis? Consider thrombosis
In-situ coronary thrombosis should be considered in the differential diagnosis of a patient with an AMI without evidence of coronary atherosclerosis. Further, smoking cannabis immediately prior to symptom onset should heighten awareness for potential coronary thrombosis. Lifelong anticoagulation therapy may be indicated in these patients due to the catastrophic nature of the condition and limited data on this particular situation.
What’s recommended. Cessation of cannabis and tobacco smoking is recommended, as the use of these substances may contribute to the development of coronary thrombosis.3-7,9 A registry of patients with coronary thrombosis without coronary atherosclerosis, especially in states where cannabis is legal, would be useful to gauge the potential increase in such events. In addition, information related to the cardiovascular effects of using alternate routes of drug delivery, such as vaping devices, are limited; therefore, this practice should be closely monitored, as well.
Our patient’s outcome
Thrombus removal. In the cardiac catheterization laboratory, the majority of the thrombus was removed and coronary blood flow was improved using a thrombectomy catheter (FIGURE 2C). Residual thrombus remained in the very distal coronary arteries (FIGURE 2D), so heparin infusion was continued.
Imaging studies. Following the procedure, an echocardiogram demonstrated left ventricular (LV) regional wall motion abnormalities with moderately reduced LV systolic function and an ejection fraction (EF) of 35%. Troponin I levels peaked at 35 ng/mL. No LV apical thrombus or intracardiac defects (eg, patent foramen ovale, atrial septal defect, ventricular septal defect) that might have contributed to thromboembolism or paradoxical embolus were seen on echocardiogram or cardiac magnetic resonance imaging. In addition, ultrasound of the lower extremities did not demonstrate deep venous thrombosis.
Continue to: A toxicology screen
A toxicology screen was positive for tetrahydrocannabinol (THC) and negative for other substances. Hypercoagulable laboratory studies were normal, including anticardiolipin antibody IgG and IgM, factor V Leiden, prothrombin G20210A mutation, thrombin time, antithrombin III, and protein C and S activity. It was therefore believed that the AMI was due to in-situ coronary artery thrombus formation precipitated acutely by smoking cannabis—possibly with an underlying hypercoagulable state, even though no laboratory abnormalities were detected.
Our patient was discharged on lifelong aspirin 81 mg/d and oral rivaroxaban (maintenance dose of 20 mg/d). Metoprolol succinate 12.5 mg/d and lisinopril 2.5 mg/d were also initiated due
THE TAKEWAY
Coronary thrombosis can result in an AMI, even without underlying coronary atherosclerosis. Smoking cannabis may predispose individuals to in-situ coronary thrombosis and subsequent AMI. Although not often encountered in clinical practice, providers must be aware of this phenomenon in the differential diagnosis for AMI—particularly in young patients without traditional risk factors.
CORRESPONDENCE
Konstantinos Dean Boudoulas, MD, The Ohio State University Davis Lung and Heart Research Institute, 473 W. 12th Avenue, Suite 200, Columbus, Ohio 43210; kdboudoulas@osumc.edu
1. Davies MJ, Woolf N, Robertson WB. Pathology of acute myocardial infarction with particular reference to occlusive coronary thrombi. Br Heart J. 1976;38:659-664.
2. Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861-870.
3. Sharifi M, Frolich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995;107:36-40.
4. Tatli E, Yilmaztepe M, Altun G, et al. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. Int J Cardiol. 2007;120:420-422.
5. Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805-2809.
6. Boudoulas KD, Borer JS, Boudoulas H. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology. 2015;132:199-212.
7. Yurtdas M, Aydın MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J. 2012;42:641-645.
8. Jouanjus E, Lapeyre-Mestre M, Micallef J; French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc. 2014;3:e000638.
9. Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med. 2014;47:277-281.
THE CASE
A 24-year-old man with a history of smoking tobacco presented to the hospital with acute-onset chest tightness and dyspnea shortly after smoking cannabis. He was otherwise healthy and hemodynamically stable upon arrival to the emergency department. An electrocardiogram (EKG) was obtained.
THE DIAGNOSIS
The EKG showed ST-segment elevation in the inferolateral leads, consistent with an acute myocardial infarction (AMI) (FIGURE 1). The patient was immediately transported to the cardiac catheterization laboratory, where coronary arteriography demonstrated a normal right coronary artery (FIGURE 2A). Diffuse thrombosis without atherosclerosis was seen throughout the left coronary arteries, including the left main artery, distal left anterior descending (LAD) artery, first diagonal branch of the LAD artery, ostial and proximal left circumflex (LCx) arteries, and first obtuse marginal (OM) branch of the LCx artery (FIGURE 2B).
DISCUSSION
The most common cause of AMI is underlying coronary atherosclerosis;1 however, AMI may occur due to in-situ thrombosis, thromboembolism, or coronary artery vasospasm, especially due to cocaine or other substance abuse. Occasionally, coronary arteries may be damaged due to viral myocarditis, autoimmune vasculitis, dissection of the ascending aorta, or dissection of a coronary artery, especially during pregnancy and postpartum.2,3
Cannabis and tobacco increase cardiovascular events
Smoking cannabis has been shown to increase adrenergic activity, resulting in an increased heart rate and elevated arterial pressure.4,5 These changes may increase myocardial oxygen demand and may result in a decrease in myocardial oxygen supply due to a decrease in the diastolic time.6 Smoking cannabis can also increase carboxyhemoglobin levels, which may compromise tissue oxygenation.
The risk for AMI has been shown to increase within the first hour of smoking cannabis.5 A few reports have documented cases of acute coronary syndrome following cannabis use; the majority of affected patients presented with chest pain within hours of smoking cannabis and were found to have a thrombus in a coronary artery, which was then treated medically or with percutaneous coronary intervention.7 Rare cases of cardiovascular death following cannabis use have also been reported.7,8
It has been suggested that coronary artery vasospasm may occur from cannabis use, which may precipitate thrombosis; however, this is not well defined.9 It is not clear if vasospasm was the inciting factor for thrombus formation in this case, as there was extensive and diffuse thrombus far greater than that expected solely from coronary artery vasospasm.
Continue to: AMI without atherosclerosis? Consider thrombosis
AMI without atherosclerosis? Consider thrombosis
In-situ coronary thrombosis should be considered in the differential diagnosis of a patient with an AMI without evidence of coronary atherosclerosis. Further, smoking cannabis immediately prior to symptom onset should heighten awareness for potential coronary thrombosis. Lifelong anticoagulation therapy may be indicated in these patients due to the catastrophic nature of the condition and limited data on this particular situation.
What’s recommended. Cessation of cannabis and tobacco smoking is recommended, as the use of these substances may contribute to the development of coronary thrombosis.3-7,9 A registry of patients with coronary thrombosis without coronary atherosclerosis, especially in states where cannabis is legal, would be useful to gauge the potential increase in such events. In addition, information related to the cardiovascular effects of using alternate routes of drug delivery, such as vaping devices, are limited; therefore, this practice should be closely monitored, as well.
Our patient’s outcome
Thrombus removal. In the cardiac catheterization laboratory, the majority of the thrombus was removed and coronary blood flow was improved using a thrombectomy catheter (FIGURE 2C). Residual thrombus remained in the very distal coronary arteries (FIGURE 2D), so heparin infusion was continued.
Imaging studies. Following the procedure, an echocardiogram demonstrated left ventricular (LV) regional wall motion abnormalities with moderately reduced LV systolic function and an ejection fraction (EF) of 35%. Troponin I levels peaked at 35 ng/mL. No LV apical thrombus or intracardiac defects (eg, patent foramen ovale, atrial septal defect, ventricular septal defect) that might have contributed to thromboembolism or paradoxical embolus were seen on echocardiogram or cardiac magnetic resonance imaging. In addition, ultrasound of the lower extremities did not demonstrate deep venous thrombosis.
Continue to: A toxicology screen
A toxicology screen was positive for tetrahydrocannabinol (THC) and negative for other substances. Hypercoagulable laboratory studies were normal, including anticardiolipin antibody IgG and IgM, factor V Leiden, prothrombin G20210A mutation, thrombin time, antithrombin III, and protein C and S activity. It was therefore believed that the AMI was due to in-situ coronary artery thrombus formation precipitated acutely by smoking cannabis—possibly with an underlying hypercoagulable state, even though no laboratory abnormalities were detected.
Our patient was discharged on lifelong aspirin 81 mg/d and oral rivaroxaban (maintenance dose of 20 mg/d). Metoprolol succinate 12.5 mg/d and lisinopril 2.5 mg/d were also initiated due
THE TAKEWAY
Coronary thrombosis can result in an AMI, even without underlying coronary atherosclerosis. Smoking cannabis may predispose individuals to in-situ coronary thrombosis and subsequent AMI. Although not often encountered in clinical practice, providers must be aware of this phenomenon in the differential diagnosis for AMI—particularly in young patients without traditional risk factors.
CORRESPONDENCE
Konstantinos Dean Boudoulas, MD, The Ohio State University Davis Lung and Heart Research Institute, 473 W. 12th Avenue, Suite 200, Columbus, Ohio 43210; kdboudoulas@osumc.edu
THE CASE
A 24-year-old man with a history of smoking tobacco presented to the hospital with acute-onset chest tightness and dyspnea shortly after smoking cannabis. He was otherwise healthy and hemodynamically stable upon arrival to the emergency department. An electrocardiogram (EKG) was obtained.
THE DIAGNOSIS
The EKG showed ST-segment elevation in the inferolateral leads, consistent with an acute myocardial infarction (AMI) (FIGURE 1). The patient was immediately transported to the cardiac catheterization laboratory, where coronary arteriography demonstrated a normal right coronary artery (FIGURE 2A). Diffuse thrombosis without atherosclerosis was seen throughout the left coronary arteries, including the left main artery, distal left anterior descending (LAD) artery, first diagonal branch of the LAD artery, ostial and proximal left circumflex (LCx) arteries, and first obtuse marginal (OM) branch of the LCx artery (FIGURE 2B).
DISCUSSION
The most common cause of AMI is underlying coronary atherosclerosis;1 however, AMI may occur due to in-situ thrombosis, thromboembolism, or coronary artery vasospasm, especially due to cocaine or other substance abuse. Occasionally, coronary arteries may be damaged due to viral myocarditis, autoimmune vasculitis, dissection of the ascending aorta, or dissection of a coronary artery, especially during pregnancy and postpartum.2,3
Cannabis and tobacco increase cardiovascular events
Smoking cannabis has been shown to increase adrenergic activity, resulting in an increased heart rate and elevated arterial pressure.4,5 These changes may increase myocardial oxygen demand and may result in a decrease in myocardial oxygen supply due to a decrease in the diastolic time.6 Smoking cannabis can also increase carboxyhemoglobin levels, which may compromise tissue oxygenation.
The risk for AMI has been shown to increase within the first hour of smoking cannabis.5 A few reports have documented cases of acute coronary syndrome following cannabis use; the majority of affected patients presented with chest pain within hours of smoking cannabis and were found to have a thrombus in a coronary artery, which was then treated medically or with percutaneous coronary intervention.7 Rare cases of cardiovascular death following cannabis use have also been reported.7,8
It has been suggested that coronary artery vasospasm may occur from cannabis use, which may precipitate thrombosis; however, this is not well defined.9 It is not clear if vasospasm was the inciting factor for thrombus formation in this case, as there was extensive and diffuse thrombus far greater than that expected solely from coronary artery vasospasm.
Continue to: AMI without atherosclerosis? Consider thrombosis
AMI without atherosclerosis? Consider thrombosis
In-situ coronary thrombosis should be considered in the differential diagnosis of a patient with an AMI without evidence of coronary atherosclerosis. Further, smoking cannabis immediately prior to symptom onset should heighten awareness for potential coronary thrombosis. Lifelong anticoagulation therapy may be indicated in these patients due to the catastrophic nature of the condition and limited data on this particular situation.
What’s recommended. Cessation of cannabis and tobacco smoking is recommended, as the use of these substances may contribute to the development of coronary thrombosis.3-7,9 A registry of patients with coronary thrombosis without coronary atherosclerosis, especially in states where cannabis is legal, would be useful to gauge the potential increase in such events. In addition, information related to the cardiovascular effects of using alternate routes of drug delivery, such as vaping devices, are limited; therefore, this practice should be closely monitored, as well.
Our patient’s outcome
Thrombus removal. In the cardiac catheterization laboratory, the majority of the thrombus was removed and coronary blood flow was improved using a thrombectomy catheter (FIGURE 2C). Residual thrombus remained in the very distal coronary arteries (FIGURE 2D), so heparin infusion was continued.
Imaging studies. Following the procedure, an echocardiogram demonstrated left ventricular (LV) regional wall motion abnormalities with moderately reduced LV systolic function and an ejection fraction (EF) of 35%. Troponin I levels peaked at 35 ng/mL. No LV apical thrombus or intracardiac defects (eg, patent foramen ovale, atrial septal defect, ventricular septal defect) that might have contributed to thromboembolism or paradoxical embolus were seen on echocardiogram or cardiac magnetic resonance imaging. In addition, ultrasound of the lower extremities did not demonstrate deep venous thrombosis.
Continue to: A toxicology screen
A toxicology screen was positive for tetrahydrocannabinol (THC) and negative for other substances. Hypercoagulable laboratory studies were normal, including anticardiolipin antibody IgG and IgM, factor V Leiden, prothrombin G20210A mutation, thrombin time, antithrombin III, and protein C and S activity. It was therefore believed that the AMI was due to in-situ coronary artery thrombus formation precipitated acutely by smoking cannabis—possibly with an underlying hypercoagulable state, even though no laboratory abnormalities were detected.
Our patient was discharged on lifelong aspirin 81 mg/d and oral rivaroxaban (maintenance dose of 20 mg/d). Metoprolol succinate 12.5 mg/d and lisinopril 2.5 mg/d were also initiated due
THE TAKEWAY
Coronary thrombosis can result in an AMI, even without underlying coronary atherosclerosis. Smoking cannabis may predispose individuals to in-situ coronary thrombosis and subsequent AMI. Although not often encountered in clinical practice, providers must be aware of this phenomenon in the differential diagnosis for AMI—particularly in young patients without traditional risk factors.
CORRESPONDENCE
Konstantinos Dean Boudoulas, MD, The Ohio State University Davis Lung and Heart Research Institute, 473 W. 12th Avenue, Suite 200, Columbus, Ohio 43210; kdboudoulas@osumc.edu
1. Davies MJ, Woolf N, Robertson WB. Pathology of acute myocardial infarction with particular reference to occlusive coronary thrombi. Br Heart J. 1976;38:659-664.
2. Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861-870.
3. Sharifi M, Frolich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995;107:36-40.
4. Tatli E, Yilmaztepe M, Altun G, et al. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. Int J Cardiol. 2007;120:420-422.
5. Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805-2809.
6. Boudoulas KD, Borer JS, Boudoulas H. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology. 2015;132:199-212.
7. Yurtdas M, Aydın MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J. 2012;42:641-645.
8. Jouanjus E, Lapeyre-Mestre M, Micallef J; French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc. 2014;3:e000638.
9. Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med. 2014;47:277-281.
1. Davies MJ, Woolf N, Robertson WB. Pathology of acute myocardial infarction with particular reference to occlusive coronary thrombi. Br Heart J. 1976;38:659-664.
2. Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861-870.
3. Sharifi M, Frolich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995;107:36-40.
4. Tatli E, Yilmaztepe M, Altun G, et al. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. Int J Cardiol. 2007;120:420-422.
5. Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805-2809.
6. Boudoulas KD, Borer JS, Boudoulas H. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology. 2015;132:199-212.
7. Yurtdas M, Aydın MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J. 2012;42:641-645.
8. Jouanjus E, Lapeyre-Mestre M, Micallef J; French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc. 2014;3:e000638.
9. Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med. 2014;47:277-281.