User login
CDC panel delves into priorities for COVID vaccine distribution
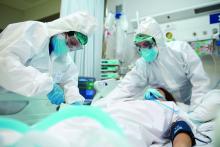
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
FDA expands Xofluza indication to include postexposure flu prophylaxis
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
Add delirium to checklist of COVID-19 symptoms in seniors
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Blood pressure treatment reduces bleeding in ICH
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ESO-WSO CONFERENCE 2020
Mortality rate of SARS-CoV-2 for similar patients is declining over time
Clinical question: Is the observed drop in COVID-19 mortality caused by changing demographics or improvements in patient care?
Background: At the start of the pandemic, COVID-19 had high mortality rates (6.9% in April according to the U.S. Centers for Disease Control and Prevention). More recently, the mortality rate had decreased to 1.9% of diagnosed cases at the end of September 2020. Concurrently, the median age of confirmed cases dropped as well, from 46 to 38 years, and availability of testing has expanded. It remains unclear whether the drop in mortality rate is because of affected patients with fewer comorbidities, less symptomatic patients, or improvements in clinical care.
Study design: Retrospective cohort study.
Setting: Large urban academic health system.
Synopsis: Researchers analyzed admissions from March 1 through Aug. 31, 2020, to NYU Langone Health System in New York of patients older than 18 years with laboratory-confirmed infection with SARS-CoV-2 during the hospitalization or in the preceding 2 weeks. In total, 5,118 patients qualified for analysis, of which 53% were hospitalized during March and April. Two separate multivariate logistic regression models for mortality were created based on patient demographics, comorbidities, and presenting vital signs and lab-result abnormalities. In the first model, the month of admission was not included, and the standardized mortality ratio (SMR) – the ratio of the sum of observed and expected deaths – for each month was obtained. In the second model, the month of admission was included as a covariate, and the average marginal effect (AME) – the difference in probability of death or discharge to hospice between March and a subsequent time period for equivalent patients – was calculated. The SMR declined progressively over time from 1.26 (95% confidence interval, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August. When accounting for demographic and clinical severity changes, the adjusted AME declined every subsequent month after March reaching a maximum of 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August.
Bottom line: Mortality from SARS-CoV-2 was significantly lower at the end of the 6-month period when adjusted for demographic and clinical factors for patients admitted to a single health care system in the United States.
Citation: Horwitz LI et al. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2020 Oct 23. doi: 10.12788/jhm.3552
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is the observed drop in COVID-19 mortality caused by changing demographics or improvements in patient care?
Background: At the start of the pandemic, COVID-19 had high mortality rates (6.9% in April according to the U.S. Centers for Disease Control and Prevention). More recently, the mortality rate had decreased to 1.9% of diagnosed cases at the end of September 2020. Concurrently, the median age of confirmed cases dropped as well, from 46 to 38 years, and availability of testing has expanded. It remains unclear whether the drop in mortality rate is because of affected patients with fewer comorbidities, less symptomatic patients, or improvements in clinical care.
Study design: Retrospective cohort study.
Setting: Large urban academic health system.
Synopsis: Researchers analyzed admissions from March 1 through Aug. 31, 2020, to NYU Langone Health System in New York of patients older than 18 years with laboratory-confirmed infection with SARS-CoV-2 during the hospitalization or in the preceding 2 weeks. In total, 5,118 patients qualified for analysis, of which 53% were hospitalized during March and April. Two separate multivariate logistic regression models for mortality were created based on patient demographics, comorbidities, and presenting vital signs and lab-result abnormalities. In the first model, the month of admission was not included, and the standardized mortality ratio (SMR) – the ratio of the sum of observed and expected deaths – for each month was obtained. In the second model, the month of admission was included as a covariate, and the average marginal effect (AME) – the difference in probability of death or discharge to hospice between March and a subsequent time period for equivalent patients – was calculated. The SMR declined progressively over time from 1.26 (95% confidence interval, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August. When accounting for demographic and clinical severity changes, the adjusted AME declined every subsequent month after March reaching a maximum of 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August.
Bottom line: Mortality from SARS-CoV-2 was significantly lower at the end of the 6-month period when adjusted for demographic and clinical factors for patients admitted to a single health care system in the United States.
Citation: Horwitz LI et al. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2020 Oct 23. doi: 10.12788/jhm.3552
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is the observed drop in COVID-19 mortality caused by changing demographics or improvements in patient care?
Background: At the start of the pandemic, COVID-19 had high mortality rates (6.9% in April according to the U.S. Centers for Disease Control and Prevention). More recently, the mortality rate had decreased to 1.9% of diagnosed cases at the end of September 2020. Concurrently, the median age of confirmed cases dropped as well, from 46 to 38 years, and availability of testing has expanded. It remains unclear whether the drop in mortality rate is because of affected patients with fewer comorbidities, less symptomatic patients, or improvements in clinical care.
Study design: Retrospective cohort study.
Setting: Large urban academic health system.
Synopsis: Researchers analyzed admissions from March 1 through Aug. 31, 2020, to NYU Langone Health System in New York of patients older than 18 years with laboratory-confirmed infection with SARS-CoV-2 during the hospitalization or in the preceding 2 weeks. In total, 5,118 patients qualified for analysis, of which 53% were hospitalized during March and April. Two separate multivariate logistic regression models for mortality were created based on patient demographics, comorbidities, and presenting vital signs and lab-result abnormalities. In the first model, the month of admission was not included, and the standardized mortality ratio (SMR) – the ratio of the sum of observed and expected deaths – for each month was obtained. In the second model, the month of admission was included as a covariate, and the average marginal effect (AME) – the difference in probability of death or discharge to hospice between March and a subsequent time period for equivalent patients – was calculated. The SMR declined progressively over time from 1.26 (95% confidence interval, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August. When accounting for demographic and clinical severity changes, the adjusted AME declined every subsequent month after March reaching a maximum of 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August.
Bottom line: Mortality from SARS-CoV-2 was significantly lower at the end of the 6-month period when adjusted for demographic and clinical factors for patients admitted to a single health care system in the United States.
Citation: Horwitz LI et al. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2020 Oct 23. doi: 10.12788/jhm.3552
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
FROM THE JOURNAL OF HOSPITAL MEDICINE
IDSA updates COVID guidelines for antibodies, antivirals, other drugs
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
Antidepressant shows early promise for mild COVID-19
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
FDA authorizes baricitinib combo for COVID-19
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The pandemic experience through the eyes of APPs
The evolution of hospitalist advanced practice providers
Throughout the chaos of the COVID-19 pandemic, advanced practice providers (APPs) – physician assistants (PAs) and nurse practitioners (NPs) – have become an integral component of the hospitalist response. As many physicians began shifting into telemedicine and away from direct patient care, APPs have been eagerly jumping in to fill the gaps. Their work has been changing almost as dramatically and quickly as the pandemic itself, bringing with it expected challenges but bestowing hugely satisfying, often unanticipated, rewards.
APPs on the rise
As the coronavirus pandemic evolves, the role of APPs is evolving right alongside it. With the current relaxation of hospital bylaw restrictions on APPs, their utilization has increased, said Tracy Cardin, ACNP-BC, SFHM, a nurse practitioner and vice president of advanced practice providers at Sound Physicians. “We have not really furloughed any advanced practice providers,” Ms. Cardin said. “In fact, I consider them to be, within hospital medicine, a key lever to finding more cost-effective care delivery models.”
Ms. Cardin said APPs have been working more independently since COVID-19 started, seeing patients on their own and using physician consultation and backup via telemedicine or telephone as needed. With the reduction in elective surgeries and patient volumes at many hospitals, APP-led care also saves money. Because one of the biggest costs is labor, Ms. Cardin said, offering this high-quality care delivery model using APPs in collaboration with physician providers helps defray some of that cost. “We’re hoping that advanced practice providers are really a solution to some of these financial pressures in a lot of different ways,” she said.
“COVID … forced us to expedite conversations about how to maximize caseloads using APPs,” said Alicia Sheffer, AGAC-AGPC NP, a nurse practitioner and Great Lakes regional director of advanced practice providers at Sound Physicians in Cincinnati. Some of those staffing model changes have included using APPs while transitioning ICUs and med-surg units to COVID cohort units, APP-led COVID cohorts, and APP-led ICUs.
“At first the hospital system had ideas about bringing in telemedicine as an alternative to seeing patients, rather than just putting APPs on the front lines and having them go in and see patients,” said Jessica Drane, APRN, PhD, DNP FNP-C, a nurse practitioner and regional director of advanced practice provider services and hospital medicine at Sound Physicians in San Antonio. In Texas at the beginning of the pandemic, hospital numbers were so low that Dr. Drane did not work at all in April. “We were all afraid we were going to lose our jobs,” she said. Then the state got slammed and APPs have been desperately needed.
Ilaria Gadalla, DMSc, PA-C, a PA at Treasure Coast Hospitalists in Port St. Lucie, Fla., and the PA program director at South University, West Palm Beach, Fla., noted that many of her APP colleagues have pivoted fluidly from other specialties to the hospitalist realm as the need for frontline workers has increased. “Hospitalists have shined through this and their value has been recognized even more than previously as a result of COVID-19,” Dr. Gadalla said.
“I don’t think it’s any surprise that hospitalists became a pillar of the COVID pandemic,” said Bridget McGrath, PA-C, a physician assistant and director of the NP/PAs service line for the section of hospital medicine at the University of Chicago. “There are just some innate traits that hospitalists have, such as the ability to be flexible, to problem solve, and to be the solution to the problem.”
Building team camaraderie
Ms. Cardin says that the need for APPs has led to an evolving integration between physicians and APPs. The growing teamwork and bonding between colleagues have been some of the most rewarding aspects of the pandemic for Dr. Gadalla. “We rely even more on each other and there isn’t really a line of, ‘I’m a physician versus an NP or PA or nurse.’ We’re all working together with the same goal,” she said.
Ms. McGrath said she has been learning what it means to lead a team during a challenging time. It has been gratifying for her to watch mentors get down to the bare bones of patient care and see everyone unify, putting aside roles and titles and coming together to care for their patients in innovative ways.
“This pandemic has really opened up a lot of doors for us because up until now, we were used almost like scribes for physicians,” Dr. Drane said. She has seen even the most resistant hospital systems beginning to rely on APPs as the pandemic has progressed. “They have become pleasantly surprised at what an APP can do.”
Work challenges
Obviously, challenges abound. Dr. Gadalla listed hers as visiting restrictions that invariably lead to slower patient visits thanks to obligatory phone calls, constantly fluctuating patient censuses, sporadic elective surgeries, watching colleagues become furloughed, and trying to balance external perceptions with what’s actually happening in the hospital.
Overall, though, “There have been a lot more rewards than barriers,” added Dr. Drane.
One of the biggest obstacles for health care workers navigating a pandemic is balancing work and home life, not to mention having time to unwind while working long hours. “Finding time for my family has been very limited. My kids feel really neglected,” said Dr. Gadalla. Some days, she gets up extra early to exercise to help clear her head, but other times she’s just too exhausted to even move.
Dr. Drane agreed that the work can get overwhelming. “We’re changing the way we practice almost every week, which can make you doubt yourself as an educator, as a practitioner. You constantly feel like you’re not sure what you’re doing, and people trust you to heal them,” she said. “Today is my first day off in 24 days. I only got it off because I said I needed a moment.”
Ms. Sheffer’s crazy days were at the beginning of the pandemic when she had to self-quarantine from her family and was working nonstop. “I would come home and sleep and work and wake up in the middle of the night and double check and triple check and go back to sleep and work, and that consumed me for several months,” she said.
The biggest challenge for Ms. Sheffer has been coping with public fear. “No matter how logical our medical approach has been, I think the constant feeling of the public threat of COVID has had this insidious effect on how patients approach their health,” she said. “We’re spending a lot more time shaping our approach to best address their fears first and not to politicize COVID so we can actually deal with the health issue at hand.”
Complications of COVID
With all the restrictions, caring for patients these days has meant learning to interact with them in different ways that aren’t as personal, Ms. McGrath said. It has been difficult to lose “that humanity of medicine, the usual ways that you interact with your patients that are going through a vulnerable time,” she noted.
Additionally, students in the medical field are being held back from graduation because they cannot participate in direct patient care. This is particularly problematic for PAs and medical students who must touch patients to graduate, Dr. Gadalla said. “All of this is slowing down future providers. We’re going to have trouble catching up. Who’s going to relieve us? That’s a huge problem and no one is finding solutions for that yet,” she said.
At the University of Chicago, Ms. McGrath explained, they created virtual rotations so that PA students could continue to do them at the university. Not only has the experience reminded Ms. McGrath how much she loves being a medical educator and fighting for the education of PA students, but she was surprised to find that her patients came to appreciate the time they spent with her students on the virtual platform as well.
“It’s isolating for patients to be in the hospital in a vulnerable state and with no support system,” she said. “I think being a part of [the PA students’] education gave some meaning to their hospitalization and highlighted that collaboration and connection is a human need.”
Despite everything, there’s a noticeable emphasis on the flowering buds of hope, unity, compassion, and pride that have been quietly blooming from the daily hardships. As Ms. Cardin puts it, “It’s so cliché to say that there’s a crisis. The other word is ‘opportunity,’ and it’s true, there are opportunities here.”
Taking care of each other
Creating resources for providers has been a priority at the University of Chicago, according to Ms. McGrath. “As hospitalists, we’re used to taking care of a variety of patients, but our section leadership and providers on the front lines quickly realized that COVID patients are more akin to trauma patients with their quick changes in health, as well as their isolation, fear, and unexpected deterioration,” she said. Her facility has implemented wellness initiatives to help prevent burnout and mental health problems in COVID providers so they can continue to give the best care to their patients.
Both Ms. Sheffer and Dr. Drane say that they have a peer network of APPs at Sound Physicians to call on for questions and support. And it’s encouraging to know you’re not alone and to keep tabs on how colleagues in other states are doing, Ms. Sheffer noted.
“The peer support system has been helpful,” Dr. Drane said. “This job, right now, takes pieces of you every day. Sometimes it’s so emotional that you can’t put it into words. You just have to cry and get it out so that you can go be with your family.”
Getting back to basics
The changes in patient care have turned into something Ms. McGrath said she appreciates. “This pandemic has really stripped away the extra fluff of medicine and brought us back to the reason why many of us have gotten into the field, because it became about the patients again,” she says. “You quickly learn your strengths and weaknesses as a provider and as a leader, and that flows into the decisions you’re making for your team and for your patients.”
Ms. Sheffer acknowledged that it is difficult to deal with patients’ family members who don’t understand that they can’t visit their sick relatives, but she said the flip side is that frontline workers become surrogate family members, an outcome she considers to be an honor.
“You step into the emotion with the family or with the patient because you’re all they have. That is a beautiful, honorable role, but it’s also tremendously emotional and sometimes devastating,” she said. “But to me, it’s one of the most beautiful things I’ve been able to offer in a time where we don’t even know what to do with COVID.”
Limited resources mixed with a healthy dose of fear can stifle creativity, Dr. Drane said. Right away, she noticed that despite the abundance of incentive spirometers at her hospital, they were not being utilized. She came in 2 hours early for 3 days to pass one out to every patient under investigation or COVID-positive patient and enlisted the help of her chief nursing officer, CEO, and regional medical director to get everyone on board.
Dr. Drane’s out-of-the-box thinking has enabled people to go home without oxygen 2 days earlier and cut the hospital’s length of stay by 5%. “It’s something so small, but it has such a great end reward,” she said. “I’m proud of this project because it didn’t take money; it was getting creative with what we already have.”
Renewed pride and passion
Dr. Drane is intensely proud of being an NP and working on the front lines. She sees that the pandemic has encouraged her and other APPs to expand their horizons.
“For me, it’s made me work to get dual certified,” she said. “APPs can be all-inclusive. I feel like I’m doing what I was meant to do and it’s not just a job anymore.”
Ms. McGrath is even more passionate about being a hospitalist now, as she has realized how valuable their unique skill sets are. “I think other people have also been able to realize that our ability to see the patient as a whole has allowed us to take care of this pandemic, because this disease impacts all organ systems and has a trickle-down effect that we as hospitalists are well versed to manage,” she said.
Ms. Cardin’s work involves communicating with APPs all around the country. Recently she had a phone exchange with an APP who needed to vent.
“She was weeping, and I thought she was going to say, ‘I can’t do this anymore, I need to go home,’ ” said Ms. Cardin. “Instead, she said, ‘I just want to make a difference in one of these people’s lives.’ And that is who the advanced practice providers are. They’re willing to go into those COVID units. They’re willing to be in the front lines. They are dedicated. They’re just intensely inspirational to me.”
The evolution of hospitalist advanced practice providers
The evolution of hospitalist advanced practice providers
Throughout the chaos of the COVID-19 pandemic, advanced practice providers (APPs) – physician assistants (PAs) and nurse practitioners (NPs) – have become an integral component of the hospitalist response. As many physicians began shifting into telemedicine and away from direct patient care, APPs have been eagerly jumping in to fill the gaps. Their work has been changing almost as dramatically and quickly as the pandemic itself, bringing with it expected challenges but bestowing hugely satisfying, often unanticipated, rewards.
APPs on the rise
As the coronavirus pandemic evolves, the role of APPs is evolving right alongside it. With the current relaxation of hospital bylaw restrictions on APPs, their utilization has increased, said Tracy Cardin, ACNP-BC, SFHM, a nurse practitioner and vice president of advanced practice providers at Sound Physicians. “We have not really furloughed any advanced practice providers,” Ms. Cardin said. “In fact, I consider them to be, within hospital medicine, a key lever to finding more cost-effective care delivery models.”
Ms. Cardin said APPs have been working more independently since COVID-19 started, seeing patients on their own and using physician consultation and backup via telemedicine or telephone as needed. With the reduction in elective surgeries and patient volumes at many hospitals, APP-led care also saves money. Because one of the biggest costs is labor, Ms. Cardin said, offering this high-quality care delivery model using APPs in collaboration with physician providers helps defray some of that cost. “We’re hoping that advanced practice providers are really a solution to some of these financial pressures in a lot of different ways,” she said.
“COVID … forced us to expedite conversations about how to maximize caseloads using APPs,” said Alicia Sheffer, AGAC-AGPC NP, a nurse practitioner and Great Lakes regional director of advanced practice providers at Sound Physicians in Cincinnati. Some of those staffing model changes have included using APPs while transitioning ICUs and med-surg units to COVID cohort units, APP-led COVID cohorts, and APP-led ICUs.
“At first the hospital system had ideas about bringing in telemedicine as an alternative to seeing patients, rather than just putting APPs on the front lines and having them go in and see patients,” said Jessica Drane, APRN, PhD, DNP FNP-C, a nurse practitioner and regional director of advanced practice provider services and hospital medicine at Sound Physicians in San Antonio. In Texas at the beginning of the pandemic, hospital numbers were so low that Dr. Drane did not work at all in April. “We were all afraid we were going to lose our jobs,” she said. Then the state got slammed and APPs have been desperately needed.
Ilaria Gadalla, DMSc, PA-C, a PA at Treasure Coast Hospitalists in Port St. Lucie, Fla., and the PA program director at South University, West Palm Beach, Fla., noted that many of her APP colleagues have pivoted fluidly from other specialties to the hospitalist realm as the need for frontline workers has increased. “Hospitalists have shined through this and their value has been recognized even more than previously as a result of COVID-19,” Dr. Gadalla said.
“I don’t think it’s any surprise that hospitalists became a pillar of the COVID pandemic,” said Bridget McGrath, PA-C, a physician assistant and director of the NP/PAs service line for the section of hospital medicine at the University of Chicago. “There are just some innate traits that hospitalists have, such as the ability to be flexible, to problem solve, and to be the solution to the problem.”
Building team camaraderie
Ms. Cardin says that the need for APPs has led to an evolving integration between physicians and APPs. The growing teamwork and bonding between colleagues have been some of the most rewarding aspects of the pandemic for Dr. Gadalla. “We rely even more on each other and there isn’t really a line of, ‘I’m a physician versus an NP or PA or nurse.’ We’re all working together with the same goal,” she said.
Ms. McGrath said she has been learning what it means to lead a team during a challenging time. It has been gratifying for her to watch mentors get down to the bare bones of patient care and see everyone unify, putting aside roles and titles and coming together to care for their patients in innovative ways.
“This pandemic has really opened up a lot of doors for us because up until now, we were used almost like scribes for physicians,” Dr. Drane said. She has seen even the most resistant hospital systems beginning to rely on APPs as the pandemic has progressed. “They have become pleasantly surprised at what an APP can do.”
Work challenges
Obviously, challenges abound. Dr. Gadalla listed hers as visiting restrictions that invariably lead to slower patient visits thanks to obligatory phone calls, constantly fluctuating patient censuses, sporadic elective surgeries, watching colleagues become furloughed, and trying to balance external perceptions with what’s actually happening in the hospital.
Overall, though, “There have been a lot more rewards than barriers,” added Dr. Drane.
One of the biggest obstacles for health care workers navigating a pandemic is balancing work and home life, not to mention having time to unwind while working long hours. “Finding time for my family has been very limited. My kids feel really neglected,” said Dr. Gadalla. Some days, she gets up extra early to exercise to help clear her head, but other times she’s just too exhausted to even move.
Dr. Drane agreed that the work can get overwhelming. “We’re changing the way we practice almost every week, which can make you doubt yourself as an educator, as a practitioner. You constantly feel like you’re not sure what you’re doing, and people trust you to heal them,” she said. “Today is my first day off in 24 days. I only got it off because I said I needed a moment.”
Ms. Sheffer’s crazy days were at the beginning of the pandemic when she had to self-quarantine from her family and was working nonstop. “I would come home and sleep and work and wake up in the middle of the night and double check and triple check and go back to sleep and work, and that consumed me for several months,” she said.
The biggest challenge for Ms. Sheffer has been coping with public fear. “No matter how logical our medical approach has been, I think the constant feeling of the public threat of COVID has had this insidious effect on how patients approach their health,” she said. “We’re spending a lot more time shaping our approach to best address their fears first and not to politicize COVID so we can actually deal with the health issue at hand.”
Complications of COVID
With all the restrictions, caring for patients these days has meant learning to interact with them in different ways that aren’t as personal, Ms. McGrath said. It has been difficult to lose “that humanity of medicine, the usual ways that you interact with your patients that are going through a vulnerable time,” she noted.
Additionally, students in the medical field are being held back from graduation because they cannot participate in direct patient care. This is particularly problematic for PAs and medical students who must touch patients to graduate, Dr. Gadalla said. “All of this is slowing down future providers. We’re going to have trouble catching up. Who’s going to relieve us? That’s a huge problem and no one is finding solutions for that yet,” she said.
At the University of Chicago, Ms. McGrath explained, they created virtual rotations so that PA students could continue to do them at the university. Not only has the experience reminded Ms. McGrath how much she loves being a medical educator and fighting for the education of PA students, but she was surprised to find that her patients came to appreciate the time they spent with her students on the virtual platform as well.
“It’s isolating for patients to be in the hospital in a vulnerable state and with no support system,” she said. “I think being a part of [the PA students’] education gave some meaning to their hospitalization and highlighted that collaboration and connection is a human need.”
Despite everything, there’s a noticeable emphasis on the flowering buds of hope, unity, compassion, and pride that have been quietly blooming from the daily hardships. As Ms. Cardin puts it, “It’s so cliché to say that there’s a crisis. The other word is ‘opportunity,’ and it’s true, there are opportunities here.”
Taking care of each other
Creating resources for providers has been a priority at the University of Chicago, according to Ms. McGrath. “As hospitalists, we’re used to taking care of a variety of patients, but our section leadership and providers on the front lines quickly realized that COVID patients are more akin to trauma patients with their quick changes in health, as well as their isolation, fear, and unexpected deterioration,” she said. Her facility has implemented wellness initiatives to help prevent burnout and mental health problems in COVID providers so they can continue to give the best care to their patients.
Both Ms. Sheffer and Dr. Drane say that they have a peer network of APPs at Sound Physicians to call on for questions and support. And it’s encouraging to know you’re not alone and to keep tabs on how colleagues in other states are doing, Ms. Sheffer noted.
“The peer support system has been helpful,” Dr. Drane said. “This job, right now, takes pieces of you every day. Sometimes it’s so emotional that you can’t put it into words. You just have to cry and get it out so that you can go be with your family.”
Getting back to basics
The changes in patient care have turned into something Ms. McGrath said she appreciates. “This pandemic has really stripped away the extra fluff of medicine and brought us back to the reason why many of us have gotten into the field, because it became about the patients again,” she says. “You quickly learn your strengths and weaknesses as a provider and as a leader, and that flows into the decisions you’re making for your team and for your patients.”
Ms. Sheffer acknowledged that it is difficult to deal with patients’ family members who don’t understand that they can’t visit their sick relatives, but she said the flip side is that frontline workers become surrogate family members, an outcome she considers to be an honor.
“You step into the emotion with the family or with the patient because you’re all they have. That is a beautiful, honorable role, but it’s also tremendously emotional and sometimes devastating,” she said. “But to me, it’s one of the most beautiful things I’ve been able to offer in a time where we don’t even know what to do with COVID.”
Limited resources mixed with a healthy dose of fear can stifle creativity, Dr. Drane said. Right away, she noticed that despite the abundance of incentive spirometers at her hospital, they were not being utilized. She came in 2 hours early for 3 days to pass one out to every patient under investigation or COVID-positive patient and enlisted the help of her chief nursing officer, CEO, and regional medical director to get everyone on board.
Dr. Drane’s out-of-the-box thinking has enabled people to go home without oxygen 2 days earlier and cut the hospital’s length of stay by 5%. “It’s something so small, but it has such a great end reward,” she said. “I’m proud of this project because it didn’t take money; it was getting creative with what we already have.”
Renewed pride and passion
Dr. Drane is intensely proud of being an NP and working on the front lines. She sees that the pandemic has encouraged her and other APPs to expand their horizons.
“For me, it’s made me work to get dual certified,” she said. “APPs can be all-inclusive. I feel like I’m doing what I was meant to do and it’s not just a job anymore.”
Ms. McGrath is even more passionate about being a hospitalist now, as she has realized how valuable their unique skill sets are. “I think other people have also been able to realize that our ability to see the patient as a whole has allowed us to take care of this pandemic, because this disease impacts all organ systems and has a trickle-down effect that we as hospitalists are well versed to manage,” she said.
Ms. Cardin’s work involves communicating with APPs all around the country. Recently she had a phone exchange with an APP who needed to vent.
“She was weeping, and I thought she was going to say, ‘I can’t do this anymore, I need to go home,’ ” said Ms. Cardin. “Instead, she said, ‘I just want to make a difference in one of these people’s lives.’ And that is who the advanced practice providers are. They’re willing to go into those COVID units. They’re willing to be in the front lines. They are dedicated. They’re just intensely inspirational to me.”
Throughout the chaos of the COVID-19 pandemic, advanced practice providers (APPs) – physician assistants (PAs) and nurse practitioners (NPs) – have become an integral component of the hospitalist response. As many physicians began shifting into telemedicine and away from direct patient care, APPs have been eagerly jumping in to fill the gaps. Their work has been changing almost as dramatically and quickly as the pandemic itself, bringing with it expected challenges but bestowing hugely satisfying, often unanticipated, rewards.
APPs on the rise
As the coronavirus pandemic evolves, the role of APPs is evolving right alongside it. With the current relaxation of hospital bylaw restrictions on APPs, their utilization has increased, said Tracy Cardin, ACNP-BC, SFHM, a nurse practitioner and vice president of advanced practice providers at Sound Physicians. “We have not really furloughed any advanced practice providers,” Ms. Cardin said. “In fact, I consider them to be, within hospital medicine, a key lever to finding more cost-effective care delivery models.”
Ms. Cardin said APPs have been working more independently since COVID-19 started, seeing patients on their own and using physician consultation and backup via telemedicine or telephone as needed. With the reduction in elective surgeries and patient volumes at many hospitals, APP-led care also saves money. Because one of the biggest costs is labor, Ms. Cardin said, offering this high-quality care delivery model using APPs in collaboration with physician providers helps defray some of that cost. “We’re hoping that advanced practice providers are really a solution to some of these financial pressures in a lot of different ways,” she said.
“COVID … forced us to expedite conversations about how to maximize caseloads using APPs,” said Alicia Sheffer, AGAC-AGPC NP, a nurse practitioner and Great Lakes regional director of advanced practice providers at Sound Physicians in Cincinnati. Some of those staffing model changes have included using APPs while transitioning ICUs and med-surg units to COVID cohort units, APP-led COVID cohorts, and APP-led ICUs.
“At first the hospital system had ideas about bringing in telemedicine as an alternative to seeing patients, rather than just putting APPs on the front lines and having them go in and see patients,” said Jessica Drane, APRN, PhD, DNP FNP-C, a nurse practitioner and regional director of advanced practice provider services and hospital medicine at Sound Physicians in San Antonio. In Texas at the beginning of the pandemic, hospital numbers were so low that Dr. Drane did not work at all in April. “We were all afraid we were going to lose our jobs,” she said. Then the state got slammed and APPs have been desperately needed.
Ilaria Gadalla, DMSc, PA-C, a PA at Treasure Coast Hospitalists in Port St. Lucie, Fla., and the PA program director at South University, West Palm Beach, Fla., noted that many of her APP colleagues have pivoted fluidly from other specialties to the hospitalist realm as the need for frontline workers has increased. “Hospitalists have shined through this and their value has been recognized even more than previously as a result of COVID-19,” Dr. Gadalla said.
“I don’t think it’s any surprise that hospitalists became a pillar of the COVID pandemic,” said Bridget McGrath, PA-C, a physician assistant and director of the NP/PAs service line for the section of hospital medicine at the University of Chicago. “There are just some innate traits that hospitalists have, such as the ability to be flexible, to problem solve, and to be the solution to the problem.”
Building team camaraderie
Ms. Cardin says that the need for APPs has led to an evolving integration between physicians and APPs. The growing teamwork and bonding between colleagues have been some of the most rewarding aspects of the pandemic for Dr. Gadalla. “We rely even more on each other and there isn’t really a line of, ‘I’m a physician versus an NP or PA or nurse.’ We’re all working together with the same goal,” she said.
Ms. McGrath said she has been learning what it means to lead a team during a challenging time. It has been gratifying for her to watch mentors get down to the bare bones of patient care and see everyone unify, putting aside roles and titles and coming together to care for their patients in innovative ways.
“This pandemic has really opened up a lot of doors for us because up until now, we were used almost like scribes for physicians,” Dr. Drane said. She has seen even the most resistant hospital systems beginning to rely on APPs as the pandemic has progressed. “They have become pleasantly surprised at what an APP can do.”
Work challenges
Obviously, challenges abound. Dr. Gadalla listed hers as visiting restrictions that invariably lead to slower patient visits thanks to obligatory phone calls, constantly fluctuating patient censuses, sporadic elective surgeries, watching colleagues become furloughed, and trying to balance external perceptions with what’s actually happening in the hospital.
Overall, though, “There have been a lot more rewards than barriers,” added Dr. Drane.
One of the biggest obstacles for health care workers navigating a pandemic is balancing work and home life, not to mention having time to unwind while working long hours. “Finding time for my family has been very limited. My kids feel really neglected,” said Dr. Gadalla. Some days, she gets up extra early to exercise to help clear her head, but other times she’s just too exhausted to even move.
Dr. Drane agreed that the work can get overwhelming. “We’re changing the way we practice almost every week, which can make you doubt yourself as an educator, as a practitioner. You constantly feel like you’re not sure what you’re doing, and people trust you to heal them,” she said. “Today is my first day off in 24 days. I only got it off because I said I needed a moment.”
Ms. Sheffer’s crazy days were at the beginning of the pandemic when she had to self-quarantine from her family and was working nonstop. “I would come home and sleep and work and wake up in the middle of the night and double check and triple check and go back to sleep and work, and that consumed me for several months,” she said.
The biggest challenge for Ms. Sheffer has been coping with public fear. “No matter how logical our medical approach has been, I think the constant feeling of the public threat of COVID has had this insidious effect on how patients approach their health,” she said. “We’re spending a lot more time shaping our approach to best address their fears first and not to politicize COVID so we can actually deal with the health issue at hand.”
Complications of COVID
With all the restrictions, caring for patients these days has meant learning to interact with them in different ways that aren’t as personal, Ms. McGrath said. It has been difficult to lose “that humanity of medicine, the usual ways that you interact with your patients that are going through a vulnerable time,” she noted.
Additionally, students in the medical field are being held back from graduation because they cannot participate in direct patient care. This is particularly problematic for PAs and medical students who must touch patients to graduate, Dr. Gadalla said. “All of this is slowing down future providers. We’re going to have trouble catching up. Who’s going to relieve us? That’s a huge problem and no one is finding solutions for that yet,” she said.
At the University of Chicago, Ms. McGrath explained, they created virtual rotations so that PA students could continue to do them at the university. Not only has the experience reminded Ms. McGrath how much she loves being a medical educator and fighting for the education of PA students, but she was surprised to find that her patients came to appreciate the time they spent with her students on the virtual platform as well.
“It’s isolating for patients to be in the hospital in a vulnerable state and with no support system,” she said. “I think being a part of [the PA students’] education gave some meaning to their hospitalization and highlighted that collaboration and connection is a human need.”
Despite everything, there’s a noticeable emphasis on the flowering buds of hope, unity, compassion, and pride that have been quietly blooming from the daily hardships. As Ms. Cardin puts it, “It’s so cliché to say that there’s a crisis. The other word is ‘opportunity,’ and it’s true, there are opportunities here.”
Taking care of each other
Creating resources for providers has been a priority at the University of Chicago, according to Ms. McGrath. “As hospitalists, we’re used to taking care of a variety of patients, but our section leadership and providers on the front lines quickly realized that COVID patients are more akin to trauma patients with their quick changes in health, as well as their isolation, fear, and unexpected deterioration,” she said. Her facility has implemented wellness initiatives to help prevent burnout and mental health problems in COVID providers so they can continue to give the best care to their patients.
Both Ms. Sheffer and Dr. Drane say that they have a peer network of APPs at Sound Physicians to call on for questions and support. And it’s encouraging to know you’re not alone and to keep tabs on how colleagues in other states are doing, Ms. Sheffer noted.
“The peer support system has been helpful,” Dr. Drane said. “This job, right now, takes pieces of you every day. Sometimes it’s so emotional that you can’t put it into words. You just have to cry and get it out so that you can go be with your family.”
Getting back to basics
The changes in patient care have turned into something Ms. McGrath said she appreciates. “This pandemic has really stripped away the extra fluff of medicine and brought us back to the reason why many of us have gotten into the field, because it became about the patients again,” she says. “You quickly learn your strengths and weaknesses as a provider and as a leader, and that flows into the decisions you’re making for your team and for your patients.”
Ms. Sheffer acknowledged that it is difficult to deal with patients’ family members who don’t understand that they can’t visit their sick relatives, but she said the flip side is that frontline workers become surrogate family members, an outcome she considers to be an honor.
“You step into the emotion with the family or with the patient because you’re all they have. That is a beautiful, honorable role, but it’s also tremendously emotional and sometimes devastating,” she said. “But to me, it’s one of the most beautiful things I’ve been able to offer in a time where we don’t even know what to do with COVID.”
Limited resources mixed with a healthy dose of fear can stifle creativity, Dr. Drane said. Right away, she noticed that despite the abundance of incentive spirometers at her hospital, they were not being utilized. She came in 2 hours early for 3 days to pass one out to every patient under investigation or COVID-positive patient and enlisted the help of her chief nursing officer, CEO, and regional medical director to get everyone on board.
Dr. Drane’s out-of-the-box thinking has enabled people to go home without oxygen 2 days earlier and cut the hospital’s length of stay by 5%. “It’s something so small, but it has such a great end reward,” she said. “I’m proud of this project because it didn’t take money; it was getting creative with what we already have.”
Renewed pride and passion
Dr. Drane is intensely proud of being an NP and working on the front lines. She sees that the pandemic has encouraged her and other APPs to expand their horizons.
“For me, it’s made me work to get dual certified,” she said. “APPs can be all-inclusive. I feel like I’m doing what I was meant to do and it’s not just a job anymore.”
Ms. McGrath is even more passionate about being a hospitalist now, as she has realized how valuable their unique skill sets are. “I think other people have also been able to realize that our ability to see the patient as a whole has allowed us to take care of this pandemic, because this disease impacts all organ systems and has a trickle-down effect that we as hospitalists are well versed to manage,” she said.
Ms. Cardin’s work involves communicating with APPs all around the country. Recently she had a phone exchange with an APP who needed to vent.
“She was weeping, and I thought she was going to say, ‘I can’t do this anymore, I need to go home,’ ” said Ms. Cardin. “Instead, she said, ‘I just want to make a difference in one of these people’s lives.’ And that is who the advanced practice providers are. They’re willing to go into those COVID units. They’re willing to be in the front lines. They are dedicated. They’re just intensely inspirational to me.”
FDA approves first at-home COVID-19 test kit
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.