User login
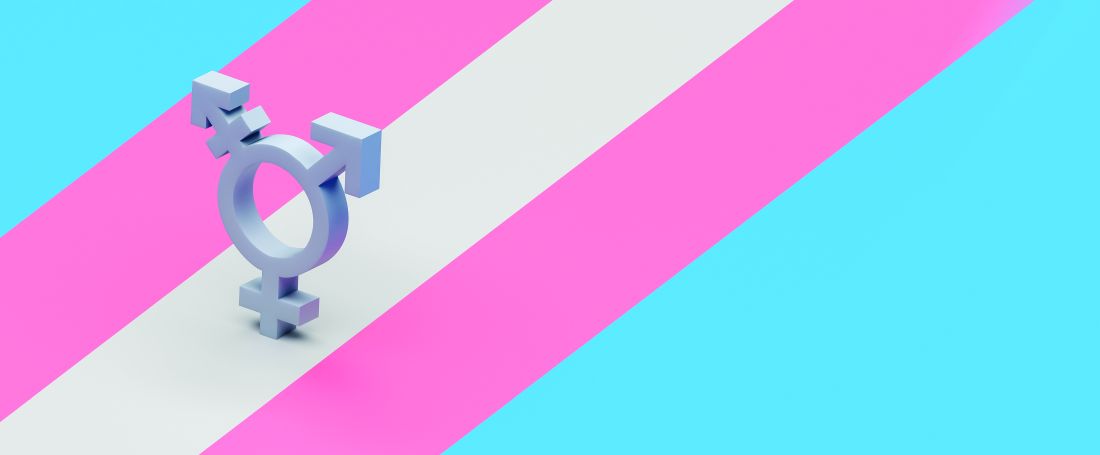
Why Texas Senate Bill 8 will negatively affect LGBTQ patients
On Sept. 1, Texas enacted astonishing legislation that effectively bans abortion after a fetal heartbeat is detected. In addition, it further empowers private citizens to sue anyone “aiding and abetting” patients who seek abortion services. Many organizations, including Planned Parenthood and the American College of Obstetricians and Gynecologists, have issued formalized statements condemning the bill. While we as obstetrician/gynecologists try to remain as nonpartisan as humanly possible in our patient care, unfortunately our specialty is inarguably one of the few in the medical field that is routinely significantly affected by federal and state politics.
It is no secret that Texas Senate Bill 8, otherwise referred to the “Texas Heartbeat Act,” will have devastating consequences for women, particularly women of color, but it will also have potentially catastrophic repercussions for patients who identify as LGBTQ. Overall, the LGBTQ population faces higher rates of poverty, unemployment, insurance coverage barriers, and provider discrimination because of their gender identity or sexual orientation, which can make access to abortion services challenging. Furthermore, they are more susceptible to hate-motivated violence and sexual assault and as a result, may seek to terminate pregnancies that result from these traumatic experiences.
A survey conducted by the Centers for Disease Control and Prevention examining rates of intimate partner violence and sexual violence found that 44% of lesbians and 61% of bisexual women experience rape and physical violence, compared with 35% of straight women.1 A separate survey revealed that 47% of transgender people are sexually assaulted at some point in their lifetime, with rates reaching as high as 65% among transgender people of color.2 Furthermore, many members of the LGBTQ population are misinformed or have misconceptions regarding their need for contraceptives and experience unintended pregnancies. As discussed in a previous column, one-third of pregnancies in transgender men were unplanned, and 20% of those patients were amenorrheic on testosterone at the time of conception.3
Current studies estimate that approximately 25% of all cisgender women will have an abortion. No corresponding data exist to describe the abortion rates of transgender and gender diverse patients.4,5 Bills such as Texas SB8 make accessing safe abortions for patients virtually impossible and interferes with the ability for physicians to provide patients with much needed health care services. It further delegitimizes rape and incest victims and is almost punitive in requiring such victims to carry the unintended pregnancies resulting from these heinous acts to term.
Regardless of a provider’s feelings toward abortion or even gender-affirming care, it is undeniable that access to these services is necessary and should be readily available to patients seeking them. As we all took an oath in medical school to “do no harm,” we must not only abide by that solemn decree in everyday patient interactions, but also live by those words to advocate for our patients when politics prohibit appropriate care. While discussions surrounding abortion are often limited to cisgender, heterosexual patients, providers must also be aware that abortion access spans across a wider spectrum that includes the LGBTQ community. Our patients, and all patients, deserve equal access to abortion. This harmful law sets a dangerous precedent that could continue to threaten these services with detrimental effects to our patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
3. Abern L, Maguire K. Obstet Gynecol 2018;131:65S.
4. Jones RK et al. Abortion incidence and service availability in the Unites States, 2017. New York, NY: Guttmacher Institute: 2019.
5. Moseson H et al. Am J Obstet Gynecol 2021;224:376.e1-11.
On Sept. 1, Texas enacted astonishing legislation that effectively bans abortion after a fetal heartbeat is detected. In addition, it further empowers private citizens to sue anyone “aiding and abetting” patients who seek abortion services. Many organizations, including Planned Parenthood and the American College of Obstetricians and Gynecologists, have issued formalized statements condemning the bill. While we as obstetrician/gynecologists try to remain as nonpartisan as humanly possible in our patient care, unfortunately our specialty is inarguably one of the few in the medical field that is routinely significantly affected by federal and state politics.
It is no secret that Texas Senate Bill 8, otherwise referred to the “Texas Heartbeat Act,” will have devastating consequences for women, particularly women of color, but it will also have potentially catastrophic repercussions for patients who identify as LGBTQ. Overall, the LGBTQ population faces higher rates of poverty, unemployment, insurance coverage barriers, and provider discrimination because of their gender identity or sexual orientation, which can make access to abortion services challenging. Furthermore, they are more susceptible to hate-motivated violence and sexual assault and as a result, may seek to terminate pregnancies that result from these traumatic experiences.
A survey conducted by the Centers for Disease Control and Prevention examining rates of intimate partner violence and sexual violence found that 44% of lesbians and 61% of bisexual women experience rape and physical violence, compared with 35% of straight women.1 A separate survey revealed that 47% of transgender people are sexually assaulted at some point in their lifetime, with rates reaching as high as 65% among transgender people of color.2 Furthermore, many members of the LGBTQ population are misinformed or have misconceptions regarding their need for contraceptives and experience unintended pregnancies. As discussed in a previous column, one-third of pregnancies in transgender men were unplanned, and 20% of those patients were amenorrheic on testosterone at the time of conception.3
Current studies estimate that approximately 25% of all cisgender women will have an abortion. No corresponding data exist to describe the abortion rates of transgender and gender diverse patients.4,5 Bills such as Texas SB8 make accessing safe abortions for patients virtually impossible and interferes with the ability for physicians to provide patients with much needed health care services. It further delegitimizes rape and incest victims and is almost punitive in requiring such victims to carry the unintended pregnancies resulting from these heinous acts to term.
Regardless of a provider’s feelings toward abortion or even gender-affirming care, it is undeniable that access to these services is necessary and should be readily available to patients seeking them. As we all took an oath in medical school to “do no harm,” we must not only abide by that solemn decree in everyday patient interactions, but also live by those words to advocate for our patients when politics prohibit appropriate care. While discussions surrounding abortion are often limited to cisgender, heterosexual patients, providers must also be aware that abortion access spans across a wider spectrum that includes the LGBTQ community. Our patients, and all patients, deserve equal access to abortion. This harmful law sets a dangerous precedent that could continue to threaten these services with detrimental effects to our patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
3. Abern L, Maguire K. Obstet Gynecol 2018;131:65S.
4. Jones RK et al. Abortion incidence and service availability in the Unites States, 2017. New York, NY: Guttmacher Institute: 2019.
5. Moseson H et al. Am J Obstet Gynecol 2021;224:376.e1-11.
On Sept. 1, Texas enacted astonishing legislation that effectively bans abortion after a fetal heartbeat is detected. In addition, it further empowers private citizens to sue anyone “aiding and abetting” patients who seek abortion services. Many organizations, including Planned Parenthood and the American College of Obstetricians and Gynecologists, have issued formalized statements condemning the bill. While we as obstetrician/gynecologists try to remain as nonpartisan as humanly possible in our patient care, unfortunately our specialty is inarguably one of the few in the medical field that is routinely significantly affected by federal and state politics.
It is no secret that Texas Senate Bill 8, otherwise referred to the “Texas Heartbeat Act,” will have devastating consequences for women, particularly women of color, but it will also have potentially catastrophic repercussions for patients who identify as LGBTQ. Overall, the LGBTQ population faces higher rates of poverty, unemployment, insurance coverage barriers, and provider discrimination because of their gender identity or sexual orientation, which can make access to abortion services challenging. Furthermore, they are more susceptible to hate-motivated violence and sexual assault and as a result, may seek to terminate pregnancies that result from these traumatic experiences.
A survey conducted by the Centers for Disease Control and Prevention examining rates of intimate partner violence and sexual violence found that 44% of lesbians and 61% of bisexual women experience rape and physical violence, compared with 35% of straight women.1 A separate survey revealed that 47% of transgender people are sexually assaulted at some point in their lifetime, with rates reaching as high as 65% among transgender people of color.2 Furthermore, many members of the LGBTQ population are misinformed or have misconceptions regarding their need for contraceptives and experience unintended pregnancies. As discussed in a previous column, one-third of pregnancies in transgender men were unplanned, and 20% of those patients were amenorrheic on testosterone at the time of conception.3
Current studies estimate that approximately 25% of all cisgender women will have an abortion. No corresponding data exist to describe the abortion rates of transgender and gender diverse patients.4,5 Bills such as Texas SB8 make accessing safe abortions for patients virtually impossible and interferes with the ability for physicians to provide patients with much needed health care services. It further delegitimizes rape and incest victims and is almost punitive in requiring such victims to carry the unintended pregnancies resulting from these heinous acts to term.
Regardless of a provider’s feelings toward abortion or even gender-affirming care, it is undeniable that access to these services is necessary and should be readily available to patients seeking them. As we all took an oath in medical school to “do no harm,” we must not only abide by that solemn decree in everyday patient interactions, but also live by those words to advocate for our patients when politics prohibit appropriate care. While discussions surrounding abortion are often limited to cisgender, heterosexual patients, providers must also be aware that abortion access spans across a wider spectrum that includes the LGBTQ community. Our patients, and all patients, deserve equal access to abortion. This harmful law sets a dangerous precedent that could continue to threaten these services with detrimental effects to our patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
3. Abern L, Maguire K. Obstet Gynecol 2018;131:65S.
4. Jones RK et al. Abortion incidence and service availability in the Unites States, 2017. New York, NY: Guttmacher Institute: 2019.
5. Moseson H et al. Am J Obstet Gynecol 2021;224:376.e1-11.
‘Locker room talk’ about death: Time for oncologists to stop
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
An appeal for equitable access to care for early pregnancy loss
Remarkable advances in care for early pregnancy loss (EPL) have occurred over the past several years. Misoprostol with mifepristone pretreatment is now the gold standard for medical management after recent research showed that this regimen improves both the efficacy and cost-effectiveness of medical management.1 Manual vacuum aspiration (MVA)’s portability, effectiveness, and safety ensure that providers can offer procedural EPL management in almost any clinical setting. Medication management and in-office uterine aspiration are two evidence-based options for EPL management that may increase access for the 25% of pregnant women who experience EPL. Unfortunately, many women do not have access to either option. Equitable access to early pregnancy loss management can be achieved by expanding access to mifepristone and office-based MVA.
However, access to mifepristone and initiating office-based MVA is challenging. Mifepristone is one of several medications regulated under the Food and Drug Administration’s Risk Evaluation and Management Strategies (REMS) program.2
The REMS guidelines restrict clinicians in prescribing and dispensing mifepristone, including the key provision that mifepristone may be dispensed only in clinics, medical offices, and hospitals. Clinicians cannot write a prescription for mifepristone for a patient to pick up at the pharmacy. Efforts are underway to roll back the REMS. Barriers to office-based MVA include time, culture shift among staff, gathering equipment, and creating protocols. Clinicians can improve access to EPL management in a variety of ways:
- MVA training: Ob.gyns. who lack training in MVA use can take advantage of several programs designed to teach the skill to clinicians, including programs such as Training, Education, and Advocacy in Miscarriage Management (TEAMM).3,4 MVA is easy to learn for ob.gyns. and procedural complications are uncommon. In the office setting, complications requiring transfer to a higher level of care are rare.5 With adequate training, whether during residency or afterward, ob.gyns. can learn to safely and effectively use MVA for procedural EPL management in the office and in the emergency department.
- Partnerships with pharmacists to reduce barriers to mifepristone: Ob.gyns. working in a variety of clinical settings, including independent clinics, critical access hospitals, community hospitals, and academic medical centers, have worked closely with on-site pharmacists to place mifepristone on their practice sites’ formularies.6 These ob.gyn.–pharmacist collaborations often require explanations to institutional Pharmacy and Therapeutics (P&T) committees of the benefits of mifepristone to patients, detailed indications for mifepristone’s use, and methods to secure mifepristone on site.
- Partnerships with emergency department and outpatient nursing and administration to promote MVA: Provision of MVA is ideal for safe, effective, and cost-efficient procedural EPL management in both the emergency department and outpatient setting. However, access to MVA in emergency rooms and outpatient clinical settings is suboptimal. Some clinicians push back against MVA use in the emergency department, citing fears that performing the procedure in the emergency department unnecessarily uses staff and resources reserved for patients with more critical illnesses. Ob.gyns. should also work with emergency medicine physicians and emergency department nursing staff and hospital administrators in explaining that MVA in the emergency room is patient centered and cost effective.
Interdisciplinary collaboration and training are two strategies that can increase access to mifepristone and MVA for EPL management. Use of mifepristone/misoprostol and office/emergency department MVA for treatment of EPL is patient centered, evidence based, feasible, highly effective, and timely. These two health care interventions are practical in almost any setting, including rural and other low-resource settings. By using these strategies to overcome the logistical and institutional challenges, ob.gyns. can help countless women with EPL gain access to the best EPL care.
Dr. Espey is chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. Dr. Jackson is an obstetrician/gynecologist at Michigan State University in Flint. They have no disclosures to report.
References
1. Schreiber CA et al. N Engl J Med. 2018 Jun 7;378(23):2161-70.
2. Food and Drug Administration. Mifeprex (mifepristone) information.
3. The TEAMM (Training, Education, and Advocacy in Miscarriage Management) Project. Training interprofessional teams to manage miscarriage. Accessed March 15, 2021.
4. Quinley KE et al. Ann Emerg Med. 2019 Jul;72(1):86-92.
5. Milingos DS et al. BJOG. 2009 Aug;116(9):1268-71.
6. Calloway D et al. Contraception. 2021 Jul;104(1):24-8.
Remarkable advances in care for early pregnancy loss (EPL) have occurred over the past several years. Misoprostol with mifepristone pretreatment is now the gold standard for medical management after recent research showed that this regimen improves both the efficacy and cost-effectiveness of medical management.1 Manual vacuum aspiration (MVA)’s portability, effectiveness, and safety ensure that providers can offer procedural EPL management in almost any clinical setting. Medication management and in-office uterine aspiration are two evidence-based options for EPL management that may increase access for the 25% of pregnant women who experience EPL. Unfortunately, many women do not have access to either option. Equitable access to early pregnancy loss management can be achieved by expanding access to mifepristone and office-based MVA.
However, access to mifepristone and initiating office-based MVA is challenging. Mifepristone is one of several medications regulated under the Food and Drug Administration’s Risk Evaluation and Management Strategies (REMS) program.2
The REMS guidelines restrict clinicians in prescribing and dispensing mifepristone, including the key provision that mifepristone may be dispensed only in clinics, medical offices, and hospitals. Clinicians cannot write a prescription for mifepristone for a patient to pick up at the pharmacy. Efforts are underway to roll back the REMS. Barriers to office-based MVA include time, culture shift among staff, gathering equipment, and creating protocols. Clinicians can improve access to EPL management in a variety of ways:
- MVA training: Ob.gyns. who lack training in MVA use can take advantage of several programs designed to teach the skill to clinicians, including programs such as Training, Education, and Advocacy in Miscarriage Management (TEAMM).3,4 MVA is easy to learn for ob.gyns. and procedural complications are uncommon. In the office setting, complications requiring transfer to a higher level of care are rare.5 With adequate training, whether during residency or afterward, ob.gyns. can learn to safely and effectively use MVA for procedural EPL management in the office and in the emergency department.
- Partnerships with pharmacists to reduce barriers to mifepristone: Ob.gyns. working in a variety of clinical settings, including independent clinics, critical access hospitals, community hospitals, and academic medical centers, have worked closely with on-site pharmacists to place mifepristone on their practice sites’ formularies.6 These ob.gyn.–pharmacist collaborations often require explanations to institutional Pharmacy and Therapeutics (P&T) committees of the benefits of mifepristone to patients, detailed indications for mifepristone’s use, and methods to secure mifepristone on site.
- Partnerships with emergency department and outpatient nursing and administration to promote MVA: Provision of MVA is ideal for safe, effective, and cost-efficient procedural EPL management in both the emergency department and outpatient setting. However, access to MVA in emergency rooms and outpatient clinical settings is suboptimal. Some clinicians push back against MVA use in the emergency department, citing fears that performing the procedure in the emergency department unnecessarily uses staff and resources reserved for patients with more critical illnesses. Ob.gyns. should also work with emergency medicine physicians and emergency department nursing staff and hospital administrators in explaining that MVA in the emergency room is patient centered and cost effective.
Interdisciplinary collaboration and training are two strategies that can increase access to mifepristone and MVA for EPL management. Use of mifepristone/misoprostol and office/emergency department MVA for treatment of EPL is patient centered, evidence based, feasible, highly effective, and timely. These two health care interventions are practical in almost any setting, including rural and other low-resource settings. By using these strategies to overcome the logistical and institutional challenges, ob.gyns. can help countless women with EPL gain access to the best EPL care.
Dr. Espey is chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. Dr. Jackson is an obstetrician/gynecologist at Michigan State University in Flint. They have no disclosures to report.
References
1. Schreiber CA et al. N Engl J Med. 2018 Jun 7;378(23):2161-70.
2. Food and Drug Administration. Mifeprex (mifepristone) information.
3. The TEAMM (Training, Education, and Advocacy in Miscarriage Management) Project. Training interprofessional teams to manage miscarriage. Accessed March 15, 2021.
4. Quinley KE et al. Ann Emerg Med. 2019 Jul;72(1):86-92.
5. Milingos DS et al. BJOG. 2009 Aug;116(9):1268-71.
6. Calloway D et al. Contraception. 2021 Jul;104(1):24-8.
Remarkable advances in care for early pregnancy loss (EPL) have occurred over the past several years. Misoprostol with mifepristone pretreatment is now the gold standard for medical management after recent research showed that this regimen improves both the efficacy and cost-effectiveness of medical management.1 Manual vacuum aspiration (MVA)’s portability, effectiveness, and safety ensure that providers can offer procedural EPL management in almost any clinical setting. Medication management and in-office uterine aspiration are two evidence-based options for EPL management that may increase access for the 25% of pregnant women who experience EPL. Unfortunately, many women do not have access to either option. Equitable access to early pregnancy loss management can be achieved by expanding access to mifepristone and office-based MVA.
However, access to mifepristone and initiating office-based MVA is challenging. Mifepristone is one of several medications regulated under the Food and Drug Administration’s Risk Evaluation and Management Strategies (REMS) program.2
The REMS guidelines restrict clinicians in prescribing and dispensing mifepristone, including the key provision that mifepristone may be dispensed only in clinics, medical offices, and hospitals. Clinicians cannot write a prescription for mifepristone for a patient to pick up at the pharmacy. Efforts are underway to roll back the REMS. Barriers to office-based MVA include time, culture shift among staff, gathering equipment, and creating protocols. Clinicians can improve access to EPL management in a variety of ways:
- MVA training: Ob.gyns. who lack training in MVA use can take advantage of several programs designed to teach the skill to clinicians, including programs such as Training, Education, and Advocacy in Miscarriage Management (TEAMM).3,4 MVA is easy to learn for ob.gyns. and procedural complications are uncommon. In the office setting, complications requiring transfer to a higher level of care are rare.5 With adequate training, whether during residency or afterward, ob.gyns. can learn to safely and effectively use MVA for procedural EPL management in the office and in the emergency department.
- Partnerships with pharmacists to reduce barriers to mifepristone: Ob.gyns. working in a variety of clinical settings, including independent clinics, critical access hospitals, community hospitals, and academic medical centers, have worked closely with on-site pharmacists to place mifepristone on their practice sites’ formularies.6 These ob.gyn.–pharmacist collaborations often require explanations to institutional Pharmacy and Therapeutics (P&T) committees of the benefits of mifepristone to patients, detailed indications for mifepristone’s use, and methods to secure mifepristone on site.
- Partnerships with emergency department and outpatient nursing and administration to promote MVA: Provision of MVA is ideal for safe, effective, and cost-efficient procedural EPL management in both the emergency department and outpatient setting. However, access to MVA in emergency rooms and outpatient clinical settings is suboptimal. Some clinicians push back against MVA use in the emergency department, citing fears that performing the procedure in the emergency department unnecessarily uses staff and resources reserved for patients with more critical illnesses. Ob.gyns. should also work with emergency medicine physicians and emergency department nursing staff and hospital administrators in explaining that MVA in the emergency room is patient centered and cost effective.
Interdisciplinary collaboration and training are two strategies that can increase access to mifepristone and MVA for EPL management. Use of mifepristone/misoprostol and office/emergency department MVA for treatment of EPL is patient centered, evidence based, feasible, highly effective, and timely. These two health care interventions are practical in almost any setting, including rural and other low-resource settings. By using these strategies to overcome the logistical and institutional challenges, ob.gyns. can help countless women with EPL gain access to the best EPL care.
Dr. Espey is chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. Dr. Jackson is an obstetrician/gynecologist at Michigan State University in Flint. They have no disclosures to report.
References
1. Schreiber CA et al. N Engl J Med. 2018 Jun 7;378(23):2161-70.
2. Food and Drug Administration. Mifeprex (mifepristone) information.
3. The TEAMM (Training, Education, and Advocacy in Miscarriage Management) Project. Training interprofessional teams to manage miscarriage. Accessed March 15, 2021.
4. Quinley KE et al. Ann Emerg Med. 2019 Jul;72(1):86-92.
5. Milingos DS et al. BJOG. 2009 Aug;116(9):1268-71.
6. Calloway D et al. Contraception. 2021 Jul;104(1):24-8.
Dopamine and reward: The story of social media
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
Authors’ response
My co-authors and I appreciate the excellent comments regarding our Photo Rounds column, “Foot rash and joint pain,” and would like to provide some additional detail.
After our patient’s 27-day hospital stay, he was admitted to a rehabilitation center for continued inpatient physical therapy for 14 days due to weakness and deconditioning. Following his discharge from the rehabilitation center, the patient was still confined to a wheelchair. He was prescribed an oral prednisone taper (as mentioned in our article) and celecoxib 200 mg bid and referred for outpatient physical therapy. At a follow-up appointment with the rheumatologist, he received adalimumab 80 mg followed by 40 mg every other week, which led to improvement in his range of motion and pain. Two months after outpatient physical therapy, the patient was lost to follow-up.
We agree with Dr. Hahn et al that many of these patients with chlamydia-associated ReA become “long-haulers.” In medicine—especially when rare diseases are considered—we must often make decisions without perfect science. The studies referenced by Dr. Hahn et al suggest that combinations of doxycycline and rifampin or azithromycin and rifampin may treat not only chlamydial infection, but ReA and associated cutaneous disease, as well.1,2 While these studies are small in size, larger studies may never be funded. We agree that combination therapy should be considered in this population of patients.
Hannah R. Badon, MD
Ross L. Pearlman, MD
Robert T. Brodell, MD
Jackson, MS
1. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
2. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
My co-authors and I appreciate the excellent comments regarding our Photo Rounds column, “Foot rash and joint pain,” and would like to provide some additional detail.
After our patient’s 27-day hospital stay, he was admitted to a rehabilitation center for continued inpatient physical therapy for 14 days due to weakness and deconditioning. Following his discharge from the rehabilitation center, the patient was still confined to a wheelchair. He was prescribed an oral prednisone taper (as mentioned in our article) and celecoxib 200 mg bid and referred for outpatient physical therapy. At a follow-up appointment with the rheumatologist, he received adalimumab 80 mg followed by 40 mg every other week, which led to improvement in his range of motion and pain. Two months after outpatient physical therapy, the patient was lost to follow-up.
We agree with Dr. Hahn et al that many of these patients with chlamydia-associated ReA become “long-haulers.” In medicine—especially when rare diseases are considered—we must often make decisions without perfect science. The studies referenced by Dr. Hahn et al suggest that combinations of doxycycline and rifampin or azithromycin and rifampin may treat not only chlamydial infection, but ReA and associated cutaneous disease, as well.1,2 While these studies are small in size, larger studies may never be funded. We agree that combination therapy should be considered in this population of patients.
Hannah R. Badon, MD
Ross L. Pearlman, MD
Robert T. Brodell, MD
Jackson, MS
My co-authors and I appreciate the excellent comments regarding our Photo Rounds column, “Foot rash and joint pain,” and would like to provide some additional detail.
After our patient’s 27-day hospital stay, he was admitted to a rehabilitation center for continued inpatient physical therapy for 14 days due to weakness and deconditioning. Following his discharge from the rehabilitation center, the patient was still confined to a wheelchair. He was prescribed an oral prednisone taper (as mentioned in our article) and celecoxib 200 mg bid and referred for outpatient physical therapy. At a follow-up appointment with the rheumatologist, he received adalimumab 80 mg followed by 40 mg every other week, which led to improvement in his range of motion and pain. Two months after outpatient physical therapy, the patient was lost to follow-up.
We agree with Dr. Hahn et al that many of these patients with chlamydia-associated ReA become “long-haulers.” In medicine—especially when rare diseases are considered—we must often make decisions without perfect science. The studies referenced by Dr. Hahn et al suggest that combinations of doxycycline and rifampin or azithromycin and rifampin may treat not only chlamydial infection, but ReA and associated cutaneous disease, as well.1,2 While these studies are small in size, larger studies may never be funded. We agree that combination therapy should be considered in this population of patients.
Hannah R. Badon, MD
Ross L. Pearlman, MD
Robert T. Brodell, MD
Jackson, MS
1. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
2. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
1. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
2. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
How best to treat “long-haulers” with reactive arthritis?
In the June Photo Rounds column, “Foot rash and joint pain” (J Fam Pract. 2021;70:249-251), Badon et al presented a case of chlamydia-associated reactive arthritis (ReA), formerly called Reiter syndrome, in a 21-year-old man following Chlamydia trachomatis urethritis. We would like to point out that, contrary to the conventional definition of ReA, in which the causative pathogen can’t be cultured from the affected joints,1 chlamydia-associated ReA is associated with evidence of chronic joint infection that, while not cultivable, can be confirmed by real-time polymerase chain reaction testing of metabolically active pathogens in synovial tissue and/or fluid.2
C trachomatis and C pneumoniae are the most frequent causative pathogens to elicit ReA.3 Short-course antibiotics and anti-inflammatory treatments can palliate ReA, but these treatments often do not provide a cure.3 Two controlled clinical trials demonstrated that chlamydia-associated ReA can be treated successfully with longer-term combination antibiotic therapy.4,5 ReA is usually diagnosed in the acute stage (first 6 months) and can become chronic in 30% of cases.6 It would be interesting to know the long-term treatment and outcome data for the case patient.
David L. Hahn, MD, MS
Alan P. Hudson, PhD
Charles Stratton, MD
Wilmore Webley, PhD
Judith Whittum-Hudson, PhD
1. Yu D, van Tubergenm A. Reactive arthritis. UpToDate. Updated 2021. Accessed August 10, 2021. www.uptodate.com/contents/reactive-arthritis
2. Gérard HC, Carter JD, Hudson AP. Chlamydia trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic chlamydia-induced reactive arthritis. Am J Med Sci. 2013;346:22-25. doi: 10.1097/MAJ.0b013e3182648740
3. Zeidler H, Hudson AP. New insights into chlamydia and arthritis. Promise of a cure? Ann Rheum Dis. 2014;73:637-644. doi: 10.1136/annrheumdis-2013-204110
4. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
5. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
6. Carter JD, Inman RD, Whittum-Hudson J, et al. Chlamydia and chronic arthritis. Ann Med. 2012;44:784-792. doi: 10.3109/07853890.2011.606830
In the June Photo Rounds column, “Foot rash and joint pain” (J Fam Pract. 2021;70:249-251), Badon et al presented a case of chlamydia-associated reactive arthritis (ReA), formerly called Reiter syndrome, in a 21-year-old man following Chlamydia trachomatis urethritis. We would like to point out that, contrary to the conventional definition of ReA, in which the causative pathogen can’t be cultured from the affected joints,1 chlamydia-associated ReA is associated with evidence of chronic joint infection that, while not cultivable, can be confirmed by real-time polymerase chain reaction testing of metabolically active pathogens in synovial tissue and/or fluid.2
C trachomatis and C pneumoniae are the most frequent causative pathogens to elicit ReA.3 Short-course antibiotics and anti-inflammatory treatments can palliate ReA, but these treatments often do not provide a cure.3 Two controlled clinical trials demonstrated that chlamydia-associated ReA can be treated successfully with longer-term combination antibiotic therapy.4,5 ReA is usually diagnosed in the acute stage (first 6 months) and can become chronic in 30% of cases.6 It would be interesting to know the long-term treatment and outcome data for the case patient.
David L. Hahn, MD, MS
Alan P. Hudson, PhD
Charles Stratton, MD
Wilmore Webley, PhD
Judith Whittum-Hudson, PhD
In the June Photo Rounds column, “Foot rash and joint pain” (J Fam Pract. 2021;70:249-251), Badon et al presented a case of chlamydia-associated reactive arthritis (ReA), formerly called Reiter syndrome, in a 21-year-old man following Chlamydia trachomatis urethritis. We would like to point out that, contrary to the conventional definition of ReA, in which the causative pathogen can’t be cultured from the affected joints,1 chlamydia-associated ReA is associated with evidence of chronic joint infection that, while not cultivable, can be confirmed by real-time polymerase chain reaction testing of metabolically active pathogens in synovial tissue and/or fluid.2
C trachomatis and C pneumoniae are the most frequent causative pathogens to elicit ReA.3 Short-course antibiotics and anti-inflammatory treatments can palliate ReA, but these treatments often do not provide a cure.3 Two controlled clinical trials demonstrated that chlamydia-associated ReA can be treated successfully with longer-term combination antibiotic therapy.4,5 ReA is usually diagnosed in the acute stage (first 6 months) and can become chronic in 30% of cases.6 It would be interesting to know the long-term treatment and outcome data for the case patient.
David L. Hahn, MD, MS
Alan P. Hudson, PhD
Charles Stratton, MD
Wilmore Webley, PhD
Judith Whittum-Hudson, PhD
1. Yu D, van Tubergenm A. Reactive arthritis. UpToDate. Updated 2021. Accessed August 10, 2021. www.uptodate.com/contents/reactive-arthritis
2. Gérard HC, Carter JD, Hudson AP. Chlamydia trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic chlamydia-induced reactive arthritis. Am J Med Sci. 2013;346:22-25. doi: 10.1097/MAJ.0b013e3182648740
3. Zeidler H, Hudson AP. New insights into chlamydia and arthritis. Promise of a cure? Ann Rheum Dis. 2014;73:637-644. doi: 10.1136/annrheumdis-2013-204110
4. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
5. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
6. Carter JD, Inman RD, Whittum-Hudson J, et al. Chlamydia and chronic arthritis. Ann Med. 2012;44:784-792. doi: 10.3109/07853890.2011.606830
1. Yu D, van Tubergenm A. Reactive arthritis. UpToDate. Updated 2021. Accessed August 10, 2021. www.uptodate.com/contents/reactive-arthritis
2. Gérard HC, Carter JD, Hudson AP. Chlamydia trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic chlamydia-induced reactive arthritis. Am J Med Sci. 2013;346:22-25. doi: 10.1097/MAJ.0b013e3182648740
3. Zeidler H, Hudson AP. New insights into chlamydia and arthritis. Promise of a cure? Ann Rheum Dis. 2014;73:637-644. doi: 10.1136/annrheumdis-2013-204110
4. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
5. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
6. Carter JD, Inman RD, Whittum-Hudson J, et al. Chlamydia and chronic arthritis. Ann Med. 2012;44:784-792. doi: 10.3109/07853890.2011.606830
Buprenorphine offers a way to rise from the ashes of addiction
One of the most rewarding aspects of being a physician is having a direct impact on alleviating patient suffering. On the other hand, one of the more difficult elements is a confrontational patient with unreasonable expectations or inappropriate demands. I have experienced both ends of the spectrum while engaging with patients who have opioid use disorder (OUD).
An untreated patient with OUD might provide an untruthful history, attempt to falsify exam findings, or even become threatening or abusive in an attempt to secure opiate pain medication. Managing a patient with OUD by providing buprenorphine treatment, however, is a completely different experience.
There is no controversy about the effectiveness of buprenorphine treatment for OUD. Patients seeking it are not looking for inappropriate care but rather a treatment that is established as an unequivocal standard with proven results for better treatment outcomes1-3 and reduced mortality.4 Personally, I’ve found offering buprenorphine treatment to be one of the most rewarding aspects of practicing medicine. It is a real joy to witness people turn their lives around with meaningful outcomes such as gainful employment, eradication of hepatitis C, reconciliation of broken relationships, resolution of legal troubles, and long-term sobriety. Being a part of lives that are practically resurrected from the ashes of addiction by prescribing medicine is indeed an exceptional experience.
On April 28, 2021, the Department of Health and Human Services provided notice for immediate action allowing for any DEA-licensed provider to obtain an X-waiver to treat 30 active patients without educational prerequisite or certification of behavioral health referral capacity.5 The X-waiver requirements were reduced, as outlined by SAMSHA,6 to a simple online notice of intent7 that can be completed in less than 5 minutes.
I encourage my colleagues to obtain the X-waiver by the simplified process, start prescribing buprenorphine, and be a part of the solution to the opioid epidemic. Of course, there will be struggles and lessons learned, but these can most certainly be eclipsed by a focus on the rewarding experience of restoring wholeness to the lives of many patients.
Aaron Newcomb, DO
Carbondale, IL
1. Norton BL, Beitin A, Glenn M, et al. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38-42. doi: 10.1016/j.jsat.2017.01.015
2. Evans EA, Zhu Y, Yoo C, et al. Criminal justice outcomes over 5 years after randomization to buprenorphine-naloxone or methadone treatment for opioid use disorder. Addiction. 2019;114:1396-1404. doi: 10.1111/add.14620
3. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane. Published February 6, 2014. Accessed August 10, 2021. www.cochrane.org/CD002207/ADDICTN_buprenorphine-maintenance-versus-placebo-or-methadone-maintenance-for-opioid-dependence
4. Methadone and buprenorphine reduce risk of death after opioid overdose. National Institutes of Health. Published June 19, 2018. Accessed August 10, 2021. www.nih.gov/news-events/news-releases/methadone-buprenorphine-reduce-risk-death-after-opioid-overdose
5. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Department of Health and Human Services; 2021. Accessed August 10, 2021. www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
6. US Department of Health & Human Services. Become a buprenorphine waivered practitioner. SAMHSA. Updated May 14, 2021. Accessed August 10, 2021. www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner
7. Buprenorphine waiver notification. SAMHSA. Accessed August 10, 2021. https://buprenorphine.samhsa.gov/forms/select-practitioner-type.php
One of the most rewarding aspects of being a physician is having a direct impact on alleviating patient suffering. On the other hand, one of the more difficult elements is a confrontational patient with unreasonable expectations or inappropriate demands. I have experienced both ends of the spectrum while engaging with patients who have opioid use disorder (OUD).
An untreated patient with OUD might provide an untruthful history, attempt to falsify exam findings, or even become threatening or abusive in an attempt to secure opiate pain medication. Managing a patient with OUD by providing buprenorphine treatment, however, is a completely different experience.
There is no controversy about the effectiveness of buprenorphine treatment for OUD. Patients seeking it are not looking for inappropriate care but rather a treatment that is established as an unequivocal standard with proven results for better treatment outcomes1-3 and reduced mortality.4 Personally, I’ve found offering buprenorphine treatment to be one of the most rewarding aspects of practicing medicine. It is a real joy to witness people turn their lives around with meaningful outcomes such as gainful employment, eradication of hepatitis C, reconciliation of broken relationships, resolution of legal troubles, and long-term sobriety. Being a part of lives that are practically resurrected from the ashes of addiction by prescribing medicine is indeed an exceptional experience.
On April 28, 2021, the Department of Health and Human Services provided notice for immediate action allowing for any DEA-licensed provider to obtain an X-waiver to treat 30 active patients without educational prerequisite or certification of behavioral health referral capacity.5 The X-waiver requirements were reduced, as outlined by SAMSHA,6 to a simple online notice of intent7 that can be completed in less than 5 minutes.
I encourage my colleagues to obtain the X-waiver by the simplified process, start prescribing buprenorphine, and be a part of the solution to the opioid epidemic. Of course, there will be struggles and lessons learned, but these can most certainly be eclipsed by a focus on the rewarding experience of restoring wholeness to the lives of many patients.
Aaron Newcomb, DO
Carbondale, IL
One of the most rewarding aspects of being a physician is having a direct impact on alleviating patient suffering. On the other hand, one of the more difficult elements is a confrontational patient with unreasonable expectations or inappropriate demands. I have experienced both ends of the spectrum while engaging with patients who have opioid use disorder (OUD).
An untreated patient with OUD might provide an untruthful history, attempt to falsify exam findings, or even become threatening or abusive in an attempt to secure opiate pain medication. Managing a patient with OUD by providing buprenorphine treatment, however, is a completely different experience.
There is no controversy about the effectiveness of buprenorphine treatment for OUD. Patients seeking it are not looking for inappropriate care but rather a treatment that is established as an unequivocal standard with proven results for better treatment outcomes1-3 and reduced mortality.4 Personally, I’ve found offering buprenorphine treatment to be one of the most rewarding aspects of practicing medicine. It is a real joy to witness people turn their lives around with meaningful outcomes such as gainful employment, eradication of hepatitis C, reconciliation of broken relationships, resolution of legal troubles, and long-term sobriety. Being a part of lives that are practically resurrected from the ashes of addiction by prescribing medicine is indeed an exceptional experience.
On April 28, 2021, the Department of Health and Human Services provided notice for immediate action allowing for any DEA-licensed provider to obtain an X-waiver to treat 30 active patients without educational prerequisite or certification of behavioral health referral capacity.5 The X-waiver requirements were reduced, as outlined by SAMSHA,6 to a simple online notice of intent7 that can be completed in less than 5 minutes.
I encourage my colleagues to obtain the X-waiver by the simplified process, start prescribing buprenorphine, and be a part of the solution to the opioid epidemic. Of course, there will be struggles and lessons learned, but these can most certainly be eclipsed by a focus on the rewarding experience of restoring wholeness to the lives of many patients.
Aaron Newcomb, DO
Carbondale, IL
1. Norton BL, Beitin A, Glenn M, et al. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38-42. doi: 10.1016/j.jsat.2017.01.015
2. Evans EA, Zhu Y, Yoo C, et al. Criminal justice outcomes over 5 years after randomization to buprenorphine-naloxone or methadone treatment for opioid use disorder. Addiction. 2019;114:1396-1404. doi: 10.1111/add.14620
3. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane. Published February 6, 2014. Accessed August 10, 2021. www.cochrane.org/CD002207/ADDICTN_buprenorphine-maintenance-versus-placebo-or-methadone-maintenance-for-opioid-dependence
4. Methadone and buprenorphine reduce risk of death after opioid overdose. National Institutes of Health. Published June 19, 2018. Accessed August 10, 2021. www.nih.gov/news-events/news-releases/methadone-buprenorphine-reduce-risk-death-after-opioid-overdose
5. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Department of Health and Human Services; 2021. Accessed August 10, 2021. www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
6. US Department of Health & Human Services. Become a buprenorphine waivered practitioner. SAMHSA. Updated May 14, 2021. Accessed August 10, 2021. www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner
7. Buprenorphine waiver notification. SAMHSA. Accessed August 10, 2021. https://buprenorphine.samhsa.gov/forms/select-practitioner-type.php
1. Norton BL, Beitin A, Glenn M, et al. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38-42. doi: 10.1016/j.jsat.2017.01.015
2. Evans EA, Zhu Y, Yoo C, et al. Criminal justice outcomes over 5 years after randomization to buprenorphine-naloxone or methadone treatment for opioid use disorder. Addiction. 2019;114:1396-1404. doi: 10.1111/add.14620
3. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane. Published February 6, 2014. Accessed August 10, 2021. www.cochrane.org/CD002207/ADDICTN_buprenorphine-maintenance-versus-placebo-or-methadone-maintenance-for-opioid-dependence
4. Methadone and buprenorphine reduce risk of death after opioid overdose. National Institutes of Health. Published June 19, 2018. Accessed August 10, 2021. www.nih.gov/news-events/news-releases/methadone-buprenorphine-reduce-risk-death-after-opioid-overdose
5. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Department of Health and Human Services; 2021. Accessed August 10, 2021. www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
6. US Department of Health & Human Services. Become a buprenorphine waivered practitioner. SAMHSA. Updated May 14, 2021. Accessed August 10, 2021. www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner
7. Buprenorphine waiver notification. SAMHSA. Accessed August 10, 2021. https://buprenorphine.samhsa.gov/forms/select-practitioner-type.php
Smart watch glucose monitoring on the horizon
Earlier this year, technology news sites reported that the Apple Watch Series 7 and the Samsung Galaxy Watch 4 were going to have integrated optical sensors for checking interstitial fluid glucose levels with no blood sampling needed. By the summer, new articles indicated that the glucose sensing watches would not be released this year for either Apple or Samsung.
For now, the newest technology available for monitoring glucose is continuous glucose monitoring (CGM), which involves a tiny sensor being inserted under the skin. The sensor tests glucose every few minutes, and a transmitter wirelessly sends the information to a monitor, which may be part of an insulin pump or a separate device. Some CGMs send information directly to a smartphone or tablet, according to the National Institutes of Health.
In 1999 the Food and Drug Administration approved the first CGM, which was only approved for downloading 3 days of data at a doctor’s office. Interestingly, the first real-time CGM device for patients to use on their own was a watch, the Glucowatch Biographer. Because of irritation and other issues, that watch never caught on. In 2006 and 2008, Dexcom and then Abbott released the first real-time CGMs that allowed patients to frequently check their own blood sugars.1,2
How CGM has advanced diabetes management
The advent of CGM has advanced the field of diabetes management in many ways.
It has allowed patients to get real time feedback on how their behavior affects their blood sugar. The use of CGM along with the ensuing behavioral changes actually leads to a decrease in hemoglobin A1c, along with a lower risk of hypoglycemia. CGM has also resulted in patients having a better understanding of several aspects of glucose control, including glucose variability and nocturnal hypoglycemia.
Affordable, readily accessible CGM monitors that allow patients to intermittently use CGM have become available over the last 3 years.
In the United States alone, 34.2 million people have diabetes – nearly 1 in every 10 people. Many do not do self-monitoring of blood glucose and most do not use CGM. The current alternative to CGM – self monitoring of blood glucose – is cumbersome, and, since it requires regular finger sticks, is painful. Also, there is significant cost to each test strip that is used to self-monitor, and most insurance limits the number of times a day a patient can check their blood sugar. CGM used to be reserved only for patients who use multiple doses of insulin daily, and only began being approved for use for patients on basal insulin alone in June 2021.3
Most primary care doctors are just beginning to learn how to interpret CGM data.
Smart watch glucose monitoring predictions
When smart watch glucose monitoring arrives, it will suddenly change the playing field for patients with diabetes and their doctors alike.
We expect it to bring down the price of CGM and make it readily available to any patient who owns a smart watch with that function.
For doctors, the new technology will result in them suddenly being asked to advise their patients on how to use the data generated by watch-based CGM.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece. Dr. Persampiere is a second-year resident in the family medicine residency program at Abington Jefferson Health. You can contact them at fpnews@mdedge.com.
References
1. Hirsh I. Introduction: History of Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association. 2018.
2. Peters A. The Evidence Base for Continuous Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association 2018.
3. “Medicare Loosening Restrictions for Continuous Glucose Monitor (CGM) Coverage,” Healthline. 2021 Jul 13.
Earlier this year, technology news sites reported that the Apple Watch Series 7 and the Samsung Galaxy Watch 4 were going to have integrated optical sensors for checking interstitial fluid glucose levels with no blood sampling needed. By the summer, new articles indicated that the glucose sensing watches would not be released this year for either Apple or Samsung.
For now, the newest technology available for monitoring glucose is continuous glucose monitoring (CGM), which involves a tiny sensor being inserted under the skin. The sensor tests glucose every few minutes, and a transmitter wirelessly sends the information to a monitor, which may be part of an insulin pump or a separate device. Some CGMs send information directly to a smartphone or tablet, according to the National Institutes of Health.
In 1999 the Food and Drug Administration approved the first CGM, which was only approved for downloading 3 days of data at a doctor’s office. Interestingly, the first real-time CGM device for patients to use on their own was a watch, the Glucowatch Biographer. Because of irritation and other issues, that watch never caught on. In 2006 and 2008, Dexcom and then Abbott released the first real-time CGMs that allowed patients to frequently check their own blood sugars.1,2
How CGM has advanced diabetes management
The advent of CGM has advanced the field of diabetes management in many ways.
It has allowed patients to get real time feedback on how their behavior affects their blood sugar. The use of CGM along with the ensuing behavioral changes actually leads to a decrease in hemoglobin A1c, along with a lower risk of hypoglycemia. CGM has also resulted in patients having a better understanding of several aspects of glucose control, including glucose variability and nocturnal hypoglycemia.
Affordable, readily accessible CGM monitors that allow patients to intermittently use CGM have become available over the last 3 years.
In the United States alone, 34.2 million people have diabetes – nearly 1 in every 10 people. Many do not do self-monitoring of blood glucose and most do not use CGM. The current alternative to CGM – self monitoring of blood glucose – is cumbersome, and, since it requires regular finger sticks, is painful. Also, there is significant cost to each test strip that is used to self-monitor, and most insurance limits the number of times a day a patient can check their blood sugar. CGM used to be reserved only for patients who use multiple doses of insulin daily, and only began being approved for use for patients on basal insulin alone in June 2021.3
Most primary care doctors are just beginning to learn how to interpret CGM data.
Smart watch glucose monitoring predictions
When smart watch glucose monitoring arrives, it will suddenly change the playing field for patients with diabetes and their doctors alike.
We expect it to bring down the price of CGM and make it readily available to any patient who owns a smart watch with that function.
For doctors, the new technology will result in them suddenly being asked to advise their patients on how to use the data generated by watch-based CGM.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece. Dr. Persampiere is a second-year resident in the family medicine residency program at Abington Jefferson Health. You can contact them at fpnews@mdedge.com.
References
1. Hirsh I. Introduction: History of Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association. 2018.
2. Peters A. The Evidence Base for Continuous Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association 2018.
3. “Medicare Loosening Restrictions for Continuous Glucose Monitor (CGM) Coverage,” Healthline. 2021 Jul 13.
Earlier this year, technology news sites reported that the Apple Watch Series 7 and the Samsung Galaxy Watch 4 were going to have integrated optical sensors for checking interstitial fluid glucose levels with no blood sampling needed. By the summer, new articles indicated that the glucose sensing watches would not be released this year for either Apple or Samsung.
For now, the newest technology available for monitoring glucose is continuous glucose monitoring (CGM), which involves a tiny sensor being inserted under the skin. The sensor tests glucose every few minutes, and a transmitter wirelessly sends the information to a monitor, which may be part of an insulin pump or a separate device. Some CGMs send information directly to a smartphone or tablet, according to the National Institutes of Health.
In 1999 the Food and Drug Administration approved the first CGM, which was only approved for downloading 3 days of data at a doctor’s office. Interestingly, the first real-time CGM device for patients to use on their own was a watch, the Glucowatch Biographer. Because of irritation and other issues, that watch never caught on. In 2006 and 2008, Dexcom and then Abbott released the first real-time CGMs that allowed patients to frequently check their own blood sugars.1,2
How CGM has advanced diabetes management
The advent of CGM has advanced the field of diabetes management in many ways.
It has allowed patients to get real time feedback on how their behavior affects their blood sugar. The use of CGM along with the ensuing behavioral changes actually leads to a decrease in hemoglobin A1c, along with a lower risk of hypoglycemia. CGM has also resulted in patients having a better understanding of several aspects of glucose control, including glucose variability and nocturnal hypoglycemia.
Affordable, readily accessible CGM monitors that allow patients to intermittently use CGM have become available over the last 3 years.
In the United States alone, 34.2 million people have diabetes – nearly 1 in every 10 people. Many do not do self-monitoring of blood glucose and most do not use CGM. The current alternative to CGM – self monitoring of blood glucose – is cumbersome, and, since it requires regular finger sticks, is painful. Also, there is significant cost to each test strip that is used to self-monitor, and most insurance limits the number of times a day a patient can check their blood sugar. CGM used to be reserved only for patients who use multiple doses of insulin daily, and only began being approved for use for patients on basal insulin alone in June 2021.3
Most primary care doctors are just beginning to learn how to interpret CGM data.
Smart watch glucose monitoring predictions
When smart watch glucose monitoring arrives, it will suddenly change the playing field for patients with diabetes and their doctors alike.
We expect it to bring down the price of CGM and make it readily available to any patient who owns a smart watch with that function.
For doctors, the new technology will result in them suddenly being asked to advise their patients on how to use the data generated by watch-based CGM.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece. Dr. Persampiere is a second-year resident in the family medicine residency program at Abington Jefferson Health. You can contact them at fpnews@mdedge.com.
References
1. Hirsh I. Introduction: History of Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association. 2018.
2. Peters A. The Evidence Base for Continuous Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association 2018.
3. “Medicare Loosening Restrictions for Continuous Glucose Monitor (CGM) Coverage,” Healthline. 2021 Jul 13.
Time to hit pause on ‘pausing’ puberty in gender-dysphoric youth
Teens are identifying as transgender in record numbers. In 2017, 3-4 in 100 teens in the United States reported that they are or may be transgender. A more recent 2021 study suggests that the rate of transgender identification among America’s youth may be as high as 9 in 100. All of the major gender centers in the world have reported a several-thousand-percent increase in youth presenting with gender distress.
How do we reconcile these numbers with 2013 data reporting the prevalence of adult gender dysphoria to be a rare 2-14 in 100,000? Reflection is warranted because many U.S. medical societies support providing youth who have transgender identification (over 1 million children and adolescents, using the latest estimates) with access to powerful endocrine interventions.
GnRH analogues (colloquially known as “puberty blockers”) are now available at Tanner stage 2 of puberty – a threshold crossed by females as young as 8-9 years old. Cross-sex hormones and surgeries follow, and mastectomies are now available to children as young as 13. Genital-altering surgeries, as well as the removal of the ovaries, uterus, and testes, can be obtained as soon as a patient turns 18.
What’s driving this massive increase in trans-identified youth? What are the risks, benefits, and uncertainties associated with hormonal and surgical interventions? Do such interventions improve the long-term psychological health of gender-dysphoric youth? How many will regret the irreversible changes made to their bodies during what may have been a temporary phase in their development?
We don’t know the answers to these questions, but we need to figure them out before offering such interventions. Frontline clinicians – especially those working with youth – will not be able to remain on the sidelines of this issue for much longer. Each clinician considering writing a prescription for puberty blockers or cross-sex hormones, or generating a referral for surgery, will need to answer for themselves: Just because I can, does it mean I should?
What’s contributing to the rapid rise of gender-dysphoric youth?
The etiology of the rapid rise of transgender identifications in young people is vigorously debated. Proponents of hormonal and surgical interventions for youth argue that the several-thousand-percent increase in the numbers of youth seeking gender reassignment is a reflection of more social acceptance of transgender identities, allowing more young people to “come out.” But closer examination of this claim reveals several inconsistencies.
Because adolescent and young adult females now account for 6-8 in 10 of the presenting cases (previously, prepubertal males were more common), one would expect a commensurate increase in the rate of transgender identification in older females. This has not occurred. In addition, more than three-quarters of currently presenting cases have significant mental health problems or suffer from neurocognitive comorbidities such as autism spectrum disorder or attention-deficit/hyperactivity disorder – a much higher burden of mental health comorbidities than the historical cohort with gender dysphoria.
There is legitimate concern that these comorbid mental health conditions, as well as the influence of social groups and online immersion into transgender topics, may be playing a role in the rapidly growing rate of transgender identification among these particularly vulnerable youth.
The initial study positing the theory that social influence is playing a role in the increased incidence of “late” or adolescent-onset (vs. childhood-onset) transgender-identified youth was harshly attacked by proponents of medical transitioning of youth, despite the fact that the study utilized methods similar to those used in other areas of health research. The study underwent an unprecedented second peer review and emerged with largely unchanged conclusions.
Since the study’s publication, a number of mental health clinicians working directly with gender-distressed youth have corroborated a rapid onset of transgender identification among teens with previously gender-normative childhoods.
Pioneers in gender dysphoria treatment are changing course
Several European countries that were pioneers in pediatric medical transition are now reversing course toward far more caution after their own evidence evaluations failed to show that medically transitioning gender-distressed youth improves mental health outcomes. In Sweden, following Karolinska Hospital’s announcement that it will no longer transition people under 18 outside of strictly regulated clinical trials, a number of other pediatric gender clinics followed suit and made the same decision.
In the United Kingdom, Keira Bell – a young woman who was treated with “affirmative” hormonal and surgical interventions before detransitioning – brought a challenge against the national gender clinic. Her landmark case and the UK High Court’s original judgement against the clinic have highlighted the urgency to reassess treatment approaches for the increasingly varied presentations of gender dysphoria in young people. As this article went to press, the UK’s national gender clinic won its appeal against Keira Bell, meaning that doctors there will once again be able to decide whether their patients under 16 can properly consent to puberty blockers. Keira Bell said she is disappointed with this decision and will be seeking permission to appeal to the Supreme Court. She said the medical service had become “politicized,” and added: “A global conversation has begun and has been shaped by this case. It has shone a light into the dark corners of a medical scandal that is harming children and harmed me. There is more to be done.”
And the UK National Health Service (NHS) has already commissioned an independent systematic review of data, which concluded that the evidence of benefit of hormonal interventions in gender dysphoric youth is of very low certainty and must be carefully weighed against the risks. An independent taskforce has also been convened to reassess the country’s approach to treating gender dysphoric youth.
Finland has arguably undertaken the biggest change of all. An early adopter of pediatric medical transition, researchers there noticed that adolescents who had mental health struggles at baseline failed to improve after transition. The Finnish national Gender Identity Development services issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
Leaders of America’s medical societies have been slower to respond. Recently, the Society for Evidence-Based Gender Medicine applied to share information about youth gender transitions at the yearly meeting of the American Academy of Pediatrics (AAP). The application was denied without explanation, despite the fact that 80% of rank-and-file pediatricians who voted on AAP resolutions days earlier endorsed a resolution calling for a reassessment of the evidence and more caution regarding gender transitions of minors.
The AAP leadership apparently ignored the resounding support for this resolution, but the clear message from that vote is that frontline pediatricians do not agree with the “one size fits all” approach of automatically affirming gender-distressed youth as transgender and proceeding to gender reassignment.
What we know and don’t know
There is now growing evidence that the “gender-affirming” model, based on the unproven assumption that gender reassignment is the best way to help gender-distressed youth, is not living up to its promise. This should not be surprising. Despite more than 50 years of experience with mature adult gender transitions, there is a lack of convincing evidence that transitions improve the psychological functioning of those with gender dysphoria, and studies on regret have been plagued by high dropout rates that prevent meaningful conclusions for practitioners and patients alike. Pediatric transitions are a much more recent phenomenon, with little to no quality data to guide decision-making.
We are witnessing a growing number of vocal regretters who underwent gender reassignment as teens and young adults under “gender-affirming” care protocols in recent years. A review of stories on the subreddit r/detrans, which counts over 20,000 members (not all are detransitioners, as the forum is open to those fully detransitioned, partially detransitioned, desisted [those who identified as transgender for a period of time in their youth but no longer do], and questioning their transition) is flush with first-hand accounts of regret and should be mandatory reading for any clinician who is considering becoming a prescriber of gender-affirmative care.
Here is a brief outline of what we know – and more importantly, what we don’t know – about the practice of medically transitioning minors.
Most cases of early childhood-onset gender dysphoria self-resolve. Eleven out of 11 studies that followed the trajectory of gender-variant youth show that the most common outcome is natural resolution of gender dysphoria around or after puberty. Among those diagnosed as having gender identity disorder, 67% no longer met the diagnostic criteria as adults; among those subthreshold for diagnosis, 93% were not gender dysphoric as adults. Gender dysphoria in childhood is a far better predictor of future homosexuality than of future trans identity.
The future trajectory of people whose transgender identity emerged during or after puberty is entirely unknown. No one has studied future trajectories of patients whose transgender identity emerged for the first time after the onset of puberty – a previously rare but now increasingly common presentation. Growing numbers of young detransitioners and desisters are precisely from this demographic, suggesting that a transgender identity that emerges in adolescence may not be durable.
Social transition does not improve mental health outcomes. Recent studies show that while socially transitioned children can thrive in the short term, they do not fare any better than their non–socially transitioned dysphoric peers. It appears that peer relations, not the social transition status, predict mental health in gender-dysphoric children. We don’t yet know the long-term trajectories of socially transitioned minors, but emerging evidence suggests that they may be more likely to persist with gender-related distress rather than outgrow it, as previously observed. This in turn necessitates decades of invasive and risky medical interventions. In fact, the Dutch researchers who pioneered the protocol used to medically transition minors explicitly and strongly discouraged social transition of children and early adolescents.
Nearly 100% of children who begin puberty blockers will proceed to cross-sex hormones and surgeries. The two main studies that have evaluated the effects of puberty blockers on mental health found no improvements or improvements of marginal clinical significance. Both studies are also at critical risk of bias due to the absence of control groups.
Four additional studies looking at the mental health effects of puberty blockers were plagued by design limitations and also failed to show any convincing positive effects on psychological health. However, one effect of puberty blockers has been consistently replicated: At least four studies show that virtually all of the children who start puberty blockers proceed to cross-sex hormones. This suggests that rather than being a pause button, puberty blockers may serve as the “gas pedal” for gender transition.
Most of the long-term health risks are largely unknown. No long-term studies exist of patients who underwent medical transition as teens or young adults. Therefore, our ability to assess risks vs. benefits is limited. Puberty blockers have been demonstrated to significantly impair bone health, and it is not clear whether this will result in future osteoporosis. Cross-sex hormones are associated with roughly 3-5 times the risk for heart attacks and strokes, though long-term studies are of insufficient quality for accurate risk assessments. Other risks associated with these endocrine interventions will come to light as the practice continues to scale and as young people spend years and decades on these interventions. The risks to fertility are largely unknown, but it is almost certain that if puberty blockers are given at the early stages of puberty and followed by cross-sex hormones, sterility will result.
The medical pathway of “affirmative care” rests on a single Dutch study that is not applicable to the current populations of gender-dysphoric youth. Most of the youth presenting for care today would have been explicitly disqualified by the original Dutch protocol, as most have significant mental health comorbidities and post-puberty onset of trans identities. This fact has been recognized by the principal investigators of the Dutch protocol itself, who have recently begun to sound the alarm about the potential misapplication of their protocol and who suggest that psychotherapy – rather than gender reassignment – is more appropriate for many of the currently presenting cases.
On suicidality
The urgency to put gender-dysphoric youth through gender reassignment despite the dearth of evidence appears to stem from the notion that if we don’t intervene medically and in short order, these youth will commit suicide. However, studies using quality data reveal a markedly different reality.
While gender-dysphoric youth do have elevated rates of suicidality, it’s not uniquely high. In fact, it’s roughly similar to the rate of suicidality found in populations of youth referred for other mental health conditions. Quality long-term studies that explored whether transition leads to reduced suicidality have not been able to demonstrate a reduction.
Medicine has a pattern of enthusiastically embracing unproven medical interventions, only to find out years or decades later that the harms from those interventions outweigh the benefits. We owe it to our patients to be transparent about the limits of our knowledge and the fact that the “affirmative care” pathway is largely irreversible.
When the benefits of an intervention have not been shown to outweigh the risks, medical ethics dictate that such interventions should not occur outside of clinical trials. We must not conflate medical care for gender-dysphoric youth with experimental and risky interventions that are based on low-quality evidence. It’s time to hit pause on gender transitions for youth.
A brief history of the Dutch protocol
Before the mid-1990s, medical transition was primarily reserved for mature adults. However, noting the “never-disappearing masculine appearance” of many adult male transitioners, a team of Dutch researchers hypothesized that it might be appropriate to provide early intervention to a carefully selected group of adolescents before the irreversible physical changes of puberty occur.
To differentiate the majority of gender-dysphoric children who would outgrow their cross-sex identification by adulthood from the few who would probably not have resolution and would wish to transition later in life, the Dutch gender clinic designed a rigorous screening protocol, with multidisciplinary teams closely following prospective candidates for several years.
To qualify for early intervention, the adolescents had to have had persistent and severe cross-sex identification from early childhood (cases of adolescent-onset trans identity were disqualified); the distress had to worsen during puberty; and the adolescents had to be free from any other significant mental health conditions. For qualifying adolescents, puberty blockers were initiated no earlier than 12 years of age, cross-sex hormones at 16, and surgeries upon turning 18. Ongoing psychotherapy was provided through the entire assessment and intervention period.
The Dutch team published the final results of their research in 2014. The authors reported that at the average age of 21 (approximately 1.5 years post surgery), the young people were free from gender dysphoria and functioning well. Despite a postsurgical death from infection, several new diagnoses of metabolic illness, and multiple dropouts, the Western world enthusiastically embraced the early-intervention model. Concerningly, the only attempt to replicate the Dutch protocol outside of the Netherlands failed to show any psychological improvements, and to date, no long-term outcome data are available for the cohort of the 55 treated Dutch adolescents.
These progressively irreversible interventions form the basis of the “Dutch Protocol.” Currently, this protocol is being scaled in ways it was never designed for. For example, it strongly discouraged childhood social transition and did not transition adolescents with postpubertal onset of transgender identity or those with significant mental health comorbidities. Yet, treating such cases with the interventions outlined in the Dutch protocol is now common, and the age of eligibility for hormonal and surgical interventions has progressively lowered, with children as young as 8 now eligible to begin puberty blockers.
William Malone, MD, is an assistant professor of endocrinology practicing in Southern Idaho and an adviser to the Society for Evidence-Based Gender Medicine. A version of this article first appeared on Medscape.com.
Teens are identifying as transgender in record numbers. In 2017, 3-4 in 100 teens in the United States reported that they are or may be transgender. A more recent 2021 study suggests that the rate of transgender identification among America’s youth may be as high as 9 in 100. All of the major gender centers in the world have reported a several-thousand-percent increase in youth presenting with gender distress.
How do we reconcile these numbers with 2013 data reporting the prevalence of adult gender dysphoria to be a rare 2-14 in 100,000? Reflection is warranted because many U.S. medical societies support providing youth who have transgender identification (over 1 million children and adolescents, using the latest estimates) with access to powerful endocrine interventions.
GnRH analogues (colloquially known as “puberty blockers”) are now available at Tanner stage 2 of puberty – a threshold crossed by females as young as 8-9 years old. Cross-sex hormones and surgeries follow, and mastectomies are now available to children as young as 13. Genital-altering surgeries, as well as the removal of the ovaries, uterus, and testes, can be obtained as soon as a patient turns 18.
What’s driving this massive increase in trans-identified youth? What are the risks, benefits, and uncertainties associated with hormonal and surgical interventions? Do such interventions improve the long-term psychological health of gender-dysphoric youth? How many will regret the irreversible changes made to their bodies during what may have been a temporary phase in their development?
We don’t know the answers to these questions, but we need to figure them out before offering such interventions. Frontline clinicians – especially those working with youth – will not be able to remain on the sidelines of this issue for much longer. Each clinician considering writing a prescription for puberty blockers or cross-sex hormones, or generating a referral for surgery, will need to answer for themselves: Just because I can, does it mean I should?
What’s contributing to the rapid rise of gender-dysphoric youth?
The etiology of the rapid rise of transgender identifications in young people is vigorously debated. Proponents of hormonal and surgical interventions for youth argue that the several-thousand-percent increase in the numbers of youth seeking gender reassignment is a reflection of more social acceptance of transgender identities, allowing more young people to “come out.” But closer examination of this claim reveals several inconsistencies.
Because adolescent and young adult females now account for 6-8 in 10 of the presenting cases (previously, prepubertal males were more common), one would expect a commensurate increase in the rate of transgender identification in older females. This has not occurred. In addition, more than three-quarters of currently presenting cases have significant mental health problems or suffer from neurocognitive comorbidities such as autism spectrum disorder or attention-deficit/hyperactivity disorder – a much higher burden of mental health comorbidities than the historical cohort with gender dysphoria.
There is legitimate concern that these comorbid mental health conditions, as well as the influence of social groups and online immersion into transgender topics, may be playing a role in the rapidly growing rate of transgender identification among these particularly vulnerable youth.
The initial study positing the theory that social influence is playing a role in the increased incidence of “late” or adolescent-onset (vs. childhood-onset) transgender-identified youth was harshly attacked by proponents of medical transitioning of youth, despite the fact that the study utilized methods similar to those used in other areas of health research. The study underwent an unprecedented second peer review and emerged with largely unchanged conclusions.
Since the study’s publication, a number of mental health clinicians working directly with gender-distressed youth have corroborated a rapid onset of transgender identification among teens with previously gender-normative childhoods.
Pioneers in gender dysphoria treatment are changing course
Several European countries that were pioneers in pediatric medical transition are now reversing course toward far more caution after their own evidence evaluations failed to show that medically transitioning gender-distressed youth improves mental health outcomes. In Sweden, following Karolinska Hospital’s announcement that it will no longer transition people under 18 outside of strictly regulated clinical trials, a number of other pediatric gender clinics followed suit and made the same decision.
In the United Kingdom, Keira Bell – a young woman who was treated with “affirmative” hormonal and surgical interventions before detransitioning – brought a challenge against the national gender clinic. Her landmark case and the UK High Court’s original judgement against the clinic have highlighted the urgency to reassess treatment approaches for the increasingly varied presentations of gender dysphoria in young people. As this article went to press, the UK’s national gender clinic won its appeal against Keira Bell, meaning that doctors there will once again be able to decide whether their patients under 16 can properly consent to puberty blockers. Keira Bell said she is disappointed with this decision and will be seeking permission to appeal to the Supreme Court. She said the medical service had become “politicized,” and added: “A global conversation has begun and has been shaped by this case. It has shone a light into the dark corners of a medical scandal that is harming children and harmed me. There is more to be done.”
And the UK National Health Service (NHS) has already commissioned an independent systematic review of data, which concluded that the evidence of benefit of hormonal interventions in gender dysphoric youth is of very low certainty and must be carefully weighed against the risks. An independent taskforce has also been convened to reassess the country’s approach to treating gender dysphoric youth.
Finland has arguably undertaken the biggest change of all. An early adopter of pediatric medical transition, researchers there noticed that adolescents who had mental health struggles at baseline failed to improve after transition. The Finnish national Gender Identity Development services issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
Leaders of America’s medical societies have been slower to respond. Recently, the Society for Evidence-Based Gender Medicine applied to share information about youth gender transitions at the yearly meeting of the American Academy of Pediatrics (AAP). The application was denied without explanation, despite the fact that 80% of rank-and-file pediatricians who voted on AAP resolutions days earlier endorsed a resolution calling for a reassessment of the evidence and more caution regarding gender transitions of minors.
The AAP leadership apparently ignored the resounding support for this resolution, but the clear message from that vote is that frontline pediatricians do not agree with the “one size fits all” approach of automatically affirming gender-distressed youth as transgender and proceeding to gender reassignment.
What we know and don’t know
There is now growing evidence that the “gender-affirming” model, based on the unproven assumption that gender reassignment is the best way to help gender-distressed youth, is not living up to its promise. This should not be surprising. Despite more than 50 years of experience with mature adult gender transitions, there is a lack of convincing evidence that transitions improve the psychological functioning of those with gender dysphoria, and studies on regret have been plagued by high dropout rates that prevent meaningful conclusions for practitioners and patients alike. Pediatric transitions are a much more recent phenomenon, with little to no quality data to guide decision-making.
We are witnessing a growing number of vocal regretters who underwent gender reassignment as teens and young adults under “gender-affirming” care protocols in recent years. A review of stories on the subreddit r/detrans, which counts over 20,000 members (not all are detransitioners, as the forum is open to those fully detransitioned, partially detransitioned, desisted [those who identified as transgender for a period of time in their youth but no longer do], and questioning their transition) is flush with first-hand accounts of regret and should be mandatory reading for any clinician who is considering becoming a prescriber of gender-affirmative care.
Here is a brief outline of what we know – and more importantly, what we don’t know – about the practice of medically transitioning minors.
Most cases of early childhood-onset gender dysphoria self-resolve. Eleven out of 11 studies that followed the trajectory of gender-variant youth show that the most common outcome is natural resolution of gender dysphoria around or after puberty. Among those diagnosed as having gender identity disorder, 67% no longer met the diagnostic criteria as adults; among those subthreshold for diagnosis, 93% were not gender dysphoric as adults. Gender dysphoria in childhood is a far better predictor of future homosexuality than of future trans identity.
The future trajectory of people whose transgender identity emerged during or after puberty is entirely unknown. No one has studied future trajectories of patients whose transgender identity emerged for the first time after the onset of puberty – a previously rare but now increasingly common presentation. Growing numbers of young detransitioners and desisters are precisely from this demographic, suggesting that a transgender identity that emerges in adolescence may not be durable.
Social transition does not improve mental health outcomes. Recent studies show that while socially transitioned children can thrive in the short term, they do not fare any better than their non–socially transitioned dysphoric peers. It appears that peer relations, not the social transition status, predict mental health in gender-dysphoric children. We don’t yet know the long-term trajectories of socially transitioned minors, but emerging evidence suggests that they may be more likely to persist with gender-related distress rather than outgrow it, as previously observed. This in turn necessitates decades of invasive and risky medical interventions. In fact, the Dutch researchers who pioneered the protocol used to medically transition minors explicitly and strongly discouraged social transition of children and early adolescents.
Nearly 100% of children who begin puberty blockers will proceed to cross-sex hormones and surgeries. The two main studies that have evaluated the effects of puberty blockers on mental health found no improvements or improvements of marginal clinical significance. Both studies are also at critical risk of bias due to the absence of control groups.
Four additional studies looking at the mental health effects of puberty blockers were plagued by design limitations and also failed to show any convincing positive effects on psychological health. However, one effect of puberty blockers has been consistently replicated: At least four studies show that virtually all of the children who start puberty blockers proceed to cross-sex hormones. This suggests that rather than being a pause button, puberty blockers may serve as the “gas pedal” for gender transition.
Most of the long-term health risks are largely unknown. No long-term studies exist of patients who underwent medical transition as teens or young adults. Therefore, our ability to assess risks vs. benefits is limited. Puberty blockers have been demonstrated to significantly impair bone health, and it is not clear whether this will result in future osteoporosis. Cross-sex hormones are associated with roughly 3-5 times the risk for heart attacks and strokes, though long-term studies are of insufficient quality for accurate risk assessments. Other risks associated with these endocrine interventions will come to light as the practice continues to scale and as young people spend years and decades on these interventions. The risks to fertility are largely unknown, but it is almost certain that if puberty blockers are given at the early stages of puberty and followed by cross-sex hormones, sterility will result.
The medical pathway of “affirmative care” rests on a single Dutch study that is not applicable to the current populations of gender-dysphoric youth. Most of the youth presenting for care today would have been explicitly disqualified by the original Dutch protocol, as most have significant mental health comorbidities and post-puberty onset of trans identities. This fact has been recognized by the principal investigators of the Dutch protocol itself, who have recently begun to sound the alarm about the potential misapplication of their protocol and who suggest that psychotherapy – rather than gender reassignment – is more appropriate for many of the currently presenting cases.
On suicidality
The urgency to put gender-dysphoric youth through gender reassignment despite the dearth of evidence appears to stem from the notion that if we don’t intervene medically and in short order, these youth will commit suicide. However, studies using quality data reveal a markedly different reality.
While gender-dysphoric youth do have elevated rates of suicidality, it’s not uniquely high. In fact, it’s roughly similar to the rate of suicidality found in populations of youth referred for other mental health conditions. Quality long-term studies that explored whether transition leads to reduced suicidality have not been able to demonstrate a reduction.
Medicine has a pattern of enthusiastically embracing unproven medical interventions, only to find out years or decades later that the harms from those interventions outweigh the benefits. We owe it to our patients to be transparent about the limits of our knowledge and the fact that the “affirmative care” pathway is largely irreversible.
When the benefits of an intervention have not been shown to outweigh the risks, medical ethics dictate that such interventions should not occur outside of clinical trials. We must not conflate medical care for gender-dysphoric youth with experimental and risky interventions that are based on low-quality evidence. It’s time to hit pause on gender transitions for youth.
A brief history of the Dutch protocol
Before the mid-1990s, medical transition was primarily reserved for mature adults. However, noting the “never-disappearing masculine appearance” of many adult male transitioners, a team of Dutch researchers hypothesized that it might be appropriate to provide early intervention to a carefully selected group of adolescents before the irreversible physical changes of puberty occur.
To differentiate the majority of gender-dysphoric children who would outgrow their cross-sex identification by adulthood from the few who would probably not have resolution and would wish to transition later in life, the Dutch gender clinic designed a rigorous screening protocol, with multidisciplinary teams closely following prospective candidates for several years.
To qualify for early intervention, the adolescents had to have had persistent and severe cross-sex identification from early childhood (cases of adolescent-onset trans identity were disqualified); the distress had to worsen during puberty; and the adolescents had to be free from any other significant mental health conditions. For qualifying adolescents, puberty blockers were initiated no earlier than 12 years of age, cross-sex hormones at 16, and surgeries upon turning 18. Ongoing psychotherapy was provided through the entire assessment and intervention period.
The Dutch team published the final results of their research in 2014. The authors reported that at the average age of 21 (approximately 1.5 years post surgery), the young people were free from gender dysphoria and functioning well. Despite a postsurgical death from infection, several new diagnoses of metabolic illness, and multiple dropouts, the Western world enthusiastically embraced the early-intervention model. Concerningly, the only attempt to replicate the Dutch protocol outside of the Netherlands failed to show any psychological improvements, and to date, no long-term outcome data are available for the cohort of the 55 treated Dutch adolescents.
These progressively irreversible interventions form the basis of the “Dutch Protocol.” Currently, this protocol is being scaled in ways it was never designed for. For example, it strongly discouraged childhood social transition and did not transition adolescents with postpubertal onset of transgender identity or those with significant mental health comorbidities. Yet, treating such cases with the interventions outlined in the Dutch protocol is now common, and the age of eligibility for hormonal and surgical interventions has progressively lowered, with children as young as 8 now eligible to begin puberty blockers.
William Malone, MD, is an assistant professor of endocrinology practicing in Southern Idaho and an adviser to the Society for Evidence-Based Gender Medicine. A version of this article first appeared on Medscape.com.
Teens are identifying as transgender in record numbers. In 2017, 3-4 in 100 teens in the United States reported that they are or may be transgender. A more recent 2021 study suggests that the rate of transgender identification among America’s youth may be as high as 9 in 100. All of the major gender centers in the world have reported a several-thousand-percent increase in youth presenting with gender distress.
How do we reconcile these numbers with 2013 data reporting the prevalence of adult gender dysphoria to be a rare 2-14 in 100,000? Reflection is warranted because many U.S. medical societies support providing youth who have transgender identification (over 1 million children and adolescents, using the latest estimates) with access to powerful endocrine interventions.
GnRH analogues (colloquially known as “puberty blockers”) are now available at Tanner stage 2 of puberty – a threshold crossed by females as young as 8-9 years old. Cross-sex hormones and surgeries follow, and mastectomies are now available to children as young as 13. Genital-altering surgeries, as well as the removal of the ovaries, uterus, and testes, can be obtained as soon as a patient turns 18.
What’s driving this massive increase in trans-identified youth? What are the risks, benefits, and uncertainties associated with hormonal and surgical interventions? Do such interventions improve the long-term psychological health of gender-dysphoric youth? How many will regret the irreversible changes made to their bodies during what may have been a temporary phase in their development?
We don’t know the answers to these questions, but we need to figure them out before offering such interventions. Frontline clinicians – especially those working with youth – will not be able to remain on the sidelines of this issue for much longer. Each clinician considering writing a prescription for puberty blockers or cross-sex hormones, or generating a referral for surgery, will need to answer for themselves: Just because I can, does it mean I should?
What’s contributing to the rapid rise of gender-dysphoric youth?
The etiology of the rapid rise of transgender identifications in young people is vigorously debated. Proponents of hormonal and surgical interventions for youth argue that the several-thousand-percent increase in the numbers of youth seeking gender reassignment is a reflection of more social acceptance of transgender identities, allowing more young people to “come out.” But closer examination of this claim reveals several inconsistencies.
Because adolescent and young adult females now account for 6-8 in 10 of the presenting cases (previously, prepubertal males were more common), one would expect a commensurate increase in the rate of transgender identification in older females. This has not occurred. In addition, more than three-quarters of currently presenting cases have significant mental health problems or suffer from neurocognitive comorbidities such as autism spectrum disorder or attention-deficit/hyperactivity disorder – a much higher burden of mental health comorbidities than the historical cohort with gender dysphoria.
There is legitimate concern that these comorbid mental health conditions, as well as the influence of social groups and online immersion into transgender topics, may be playing a role in the rapidly growing rate of transgender identification among these particularly vulnerable youth.
The initial study positing the theory that social influence is playing a role in the increased incidence of “late” or adolescent-onset (vs. childhood-onset) transgender-identified youth was harshly attacked by proponents of medical transitioning of youth, despite the fact that the study utilized methods similar to those used in other areas of health research. The study underwent an unprecedented second peer review and emerged with largely unchanged conclusions.
Since the study’s publication, a number of mental health clinicians working directly with gender-distressed youth have corroborated a rapid onset of transgender identification among teens with previously gender-normative childhoods.
Pioneers in gender dysphoria treatment are changing course
Several European countries that were pioneers in pediatric medical transition are now reversing course toward far more caution after their own evidence evaluations failed to show that medically transitioning gender-distressed youth improves mental health outcomes. In Sweden, following Karolinska Hospital’s announcement that it will no longer transition people under 18 outside of strictly regulated clinical trials, a number of other pediatric gender clinics followed suit and made the same decision.
In the United Kingdom, Keira Bell – a young woman who was treated with “affirmative” hormonal and surgical interventions before detransitioning – brought a challenge against the national gender clinic. Her landmark case and the UK High Court’s original judgement against the clinic have highlighted the urgency to reassess treatment approaches for the increasingly varied presentations of gender dysphoria in young people. As this article went to press, the UK’s national gender clinic won its appeal against Keira Bell, meaning that doctors there will once again be able to decide whether their patients under 16 can properly consent to puberty blockers. Keira Bell said she is disappointed with this decision and will be seeking permission to appeal to the Supreme Court. She said the medical service had become “politicized,” and added: “A global conversation has begun and has been shaped by this case. It has shone a light into the dark corners of a medical scandal that is harming children and harmed me. There is more to be done.”
And the UK National Health Service (NHS) has already commissioned an independent systematic review of data, which concluded that the evidence of benefit of hormonal interventions in gender dysphoric youth is of very low certainty and must be carefully weighed against the risks. An independent taskforce has also been convened to reassess the country’s approach to treating gender dysphoric youth.
Finland has arguably undertaken the biggest change of all. An early adopter of pediatric medical transition, researchers there noticed that adolescents who had mental health struggles at baseline failed to improve after transition. The Finnish national Gender Identity Development services issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
Leaders of America’s medical societies have been slower to respond. Recently, the Society for Evidence-Based Gender Medicine applied to share information about youth gender transitions at the yearly meeting of the American Academy of Pediatrics (AAP). The application was denied without explanation, despite the fact that 80% of rank-and-file pediatricians who voted on AAP resolutions days earlier endorsed a resolution calling for a reassessment of the evidence and more caution regarding gender transitions of minors.
The AAP leadership apparently ignored the resounding support for this resolution, but the clear message from that vote is that frontline pediatricians do not agree with the “one size fits all” approach of automatically affirming gender-distressed youth as transgender and proceeding to gender reassignment.
What we know and don’t know
There is now growing evidence that the “gender-affirming” model, based on the unproven assumption that gender reassignment is the best way to help gender-distressed youth, is not living up to its promise. This should not be surprising. Despite more than 50 years of experience with mature adult gender transitions, there is a lack of convincing evidence that transitions improve the psychological functioning of those with gender dysphoria, and studies on regret have been plagued by high dropout rates that prevent meaningful conclusions for practitioners and patients alike. Pediatric transitions are a much more recent phenomenon, with little to no quality data to guide decision-making.
We are witnessing a growing number of vocal regretters who underwent gender reassignment as teens and young adults under “gender-affirming” care protocols in recent years. A review of stories on the subreddit r/detrans, which counts over 20,000 members (not all are detransitioners, as the forum is open to those fully detransitioned, partially detransitioned, desisted [those who identified as transgender for a period of time in their youth but no longer do], and questioning their transition) is flush with first-hand accounts of regret and should be mandatory reading for any clinician who is considering becoming a prescriber of gender-affirmative care.
Here is a brief outline of what we know – and more importantly, what we don’t know – about the practice of medically transitioning minors.
Most cases of early childhood-onset gender dysphoria self-resolve. Eleven out of 11 studies that followed the trajectory of gender-variant youth show that the most common outcome is natural resolution of gender dysphoria around or after puberty. Among those diagnosed as having gender identity disorder, 67% no longer met the diagnostic criteria as adults; among those subthreshold for diagnosis, 93% were not gender dysphoric as adults. Gender dysphoria in childhood is a far better predictor of future homosexuality than of future trans identity.
The future trajectory of people whose transgender identity emerged during or after puberty is entirely unknown. No one has studied future trajectories of patients whose transgender identity emerged for the first time after the onset of puberty – a previously rare but now increasingly common presentation. Growing numbers of young detransitioners and desisters are precisely from this demographic, suggesting that a transgender identity that emerges in adolescence may not be durable.
Social transition does not improve mental health outcomes. Recent studies show that while socially transitioned children can thrive in the short term, they do not fare any better than their non–socially transitioned dysphoric peers. It appears that peer relations, not the social transition status, predict mental health in gender-dysphoric children. We don’t yet know the long-term trajectories of socially transitioned minors, but emerging evidence suggests that they may be more likely to persist with gender-related distress rather than outgrow it, as previously observed. This in turn necessitates decades of invasive and risky medical interventions. In fact, the Dutch researchers who pioneered the protocol used to medically transition minors explicitly and strongly discouraged social transition of children and early adolescents.
Nearly 100% of children who begin puberty blockers will proceed to cross-sex hormones and surgeries. The two main studies that have evaluated the effects of puberty blockers on mental health found no improvements or improvements of marginal clinical significance. Both studies are also at critical risk of bias due to the absence of control groups.
Four additional studies looking at the mental health effects of puberty blockers were plagued by design limitations and also failed to show any convincing positive effects on psychological health. However, one effect of puberty blockers has been consistently replicated: At least four studies show that virtually all of the children who start puberty blockers proceed to cross-sex hormones. This suggests that rather than being a pause button, puberty blockers may serve as the “gas pedal” for gender transition.
Most of the long-term health risks are largely unknown. No long-term studies exist of patients who underwent medical transition as teens or young adults. Therefore, our ability to assess risks vs. benefits is limited. Puberty blockers have been demonstrated to significantly impair bone health, and it is not clear whether this will result in future osteoporosis. Cross-sex hormones are associated with roughly 3-5 times the risk for heart attacks and strokes, though long-term studies are of insufficient quality for accurate risk assessments. Other risks associated with these endocrine interventions will come to light as the practice continues to scale and as young people spend years and decades on these interventions. The risks to fertility are largely unknown, but it is almost certain that if puberty blockers are given at the early stages of puberty and followed by cross-sex hormones, sterility will result.
The medical pathway of “affirmative care” rests on a single Dutch study that is not applicable to the current populations of gender-dysphoric youth. Most of the youth presenting for care today would have been explicitly disqualified by the original Dutch protocol, as most have significant mental health comorbidities and post-puberty onset of trans identities. This fact has been recognized by the principal investigators of the Dutch protocol itself, who have recently begun to sound the alarm about the potential misapplication of their protocol and who suggest that psychotherapy – rather than gender reassignment – is more appropriate for many of the currently presenting cases.
On suicidality
The urgency to put gender-dysphoric youth through gender reassignment despite the dearth of evidence appears to stem from the notion that if we don’t intervene medically and in short order, these youth will commit suicide. However, studies using quality data reveal a markedly different reality.
While gender-dysphoric youth do have elevated rates of suicidality, it’s not uniquely high. In fact, it’s roughly similar to the rate of suicidality found in populations of youth referred for other mental health conditions. Quality long-term studies that explored whether transition leads to reduced suicidality have not been able to demonstrate a reduction.
Medicine has a pattern of enthusiastically embracing unproven medical interventions, only to find out years or decades later that the harms from those interventions outweigh the benefits. We owe it to our patients to be transparent about the limits of our knowledge and the fact that the “affirmative care” pathway is largely irreversible.
When the benefits of an intervention have not been shown to outweigh the risks, medical ethics dictate that such interventions should not occur outside of clinical trials. We must not conflate medical care for gender-dysphoric youth with experimental and risky interventions that are based on low-quality evidence. It’s time to hit pause on gender transitions for youth.
A brief history of the Dutch protocol
Before the mid-1990s, medical transition was primarily reserved for mature adults. However, noting the “never-disappearing masculine appearance” of many adult male transitioners, a team of Dutch researchers hypothesized that it might be appropriate to provide early intervention to a carefully selected group of adolescents before the irreversible physical changes of puberty occur.
To differentiate the majority of gender-dysphoric children who would outgrow their cross-sex identification by adulthood from the few who would probably not have resolution and would wish to transition later in life, the Dutch gender clinic designed a rigorous screening protocol, with multidisciplinary teams closely following prospective candidates for several years.
To qualify for early intervention, the adolescents had to have had persistent and severe cross-sex identification from early childhood (cases of adolescent-onset trans identity were disqualified); the distress had to worsen during puberty; and the adolescents had to be free from any other significant mental health conditions. For qualifying adolescents, puberty blockers were initiated no earlier than 12 years of age, cross-sex hormones at 16, and surgeries upon turning 18. Ongoing psychotherapy was provided through the entire assessment and intervention period.
The Dutch team published the final results of their research in 2014. The authors reported that at the average age of 21 (approximately 1.5 years post surgery), the young people were free from gender dysphoria and functioning well. Despite a postsurgical death from infection, several new diagnoses of metabolic illness, and multiple dropouts, the Western world enthusiastically embraced the early-intervention model. Concerningly, the only attempt to replicate the Dutch protocol outside of the Netherlands failed to show any psychological improvements, and to date, no long-term outcome data are available for the cohort of the 55 treated Dutch adolescents.
These progressively irreversible interventions form the basis of the “Dutch Protocol.” Currently, this protocol is being scaled in ways it was never designed for. For example, it strongly discouraged childhood social transition and did not transition adolescents with postpubertal onset of transgender identity or those with significant mental health comorbidities. Yet, treating such cases with the interventions outlined in the Dutch protocol is now common, and the age of eligibility for hormonal and surgical interventions has progressively lowered, with children as young as 8 now eligible to begin puberty blockers.
William Malone, MD, is an assistant professor of endocrinology practicing in Southern Idaho and an adviser to the Society for Evidence-Based Gender Medicine. A version of this article first appeared on Medscape.com.