User login
Dangerous grandparents
Many decades ago I wrote a book I brazenly titled: “The Good Grandmother Handbook.” I had been a parent for a scant 7 or 8 years but based on my experiences in the office I felt I had accumulated enough wisdom to suggest to women in their fifth to seventh decades how they might conduct themselves around their grandchildren. Luckily, the book never got further than several hundred pages of crudely typed manuscript. This was before word processing programs had settled into the home computer industry, which was still in its infancy.
But I continue find the subject of grandparents interesting. Now, with grandchildren of my own (the oldest has just graduated from high school) and scores of peers knee deep in their own grandparenting adventures, I hope that my perspective now has a bit less of a holier-than-thou aroma.
My most recent muse-prodding event came when I stumbled across an article about the epidemiology of unintentional pediatric firearm fatalities. Looking at 10 years of data from the National Violent Death Reporting System, the investigators found that in 80% of the cases the firearm owner was a relative of the victim; in slightly more than 60% of the cases the event occurred in the victim’s home.
The data set was not granular enough to define the exact relationship between the child and relative who owned the gun. I suspect that most often the relative was a parent or an uncle or aunt. However, viewed through my septuagenarian prism, this paper prompted me to wonder in how many of these fatalities the firearm owner was a grandparent.
I have only anecdotal observations, but I can easily recall situations here in Maine in which a child has been injured by his or her grandfather’s gun. The data from the study show that pediatric fatalities are bimodal, with the majority occurring in the 1- to 5-year age group and a second peak in adolescence. The grandparent-involved cases I can recall were in the younger demographic.
Unfortunately, firearms aren’t the only threat that other grandparents and I pose to the health and safety of our grandchildren. I can remember before the development of, and the widespread use of, tamper-proof pill bottles, “grandma’s purse” overdoses were an unfortunately common occurrence.
More recently, at least here in Maine, we have been hearing more about motorized vehicle–related injuries and fatalities – grandparents backing over their grandchildren in the driveway or, more often, grandfathers (usually) taking their young grandchildren for rides on their snowmobiles, ATVs, lawn tractors, (fill in the blank). Whenever one of these events occurs, my mind quickly jumps beyond the tragic loss of life to imagining what terrible and long-lasting emotional chaos these incidents have spawned in those families.
During the pandemic, many parents and grandparents became aware of the threat that viral-spewing young children pose to the older and more vulnerable generation. On the other hand, many parents have been told that having a grandparent around can present a risk to the health and safety of their grandchildren. It can be a touchy subject in families, and grandparents may bristle at “being treated like a child” when they are reminded that children aren’t small adults and that their own behavior may be setting a bad example or putting their grandchildren at risk.
My generation had to learn how to buckle infants and toddlers into car seats because it was something that wasn’t done for our children. Fortunately, most new grandparents now already have those buckle-and-click skills and mindset. But,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Many decades ago I wrote a book I brazenly titled: “The Good Grandmother Handbook.” I had been a parent for a scant 7 or 8 years but based on my experiences in the office I felt I had accumulated enough wisdom to suggest to women in their fifth to seventh decades how they might conduct themselves around their grandchildren. Luckily, the book never got further than several hundred pages of crudely typed manuscript. This was before word processing programs had settled into the home computer industry, which was still in its infancy.
But I continue find the subject of grandparents interesting. Now, with grandchildren of my own (the oldest has just graduated from high school) and scores of peers knee deep in their own grandparenting adventures, I hope that my perspective now has a bit less of a holier-than-thou aroma.
My most recent muse-prodding event came when I stumbled across an article about the epidemiology of unintentional pediatric firearm fatalities. Looking at 10 years of data from the National Violent Death Reporting System, the investigators found that in 80% of the cases the firearm owner was a relative of the victim; in slightly more than 60% of the cases the event occurred in the victim’s home.
The data set was not granular enough to define the exact relationship between the child and relative who owned the gun. I suspect that most often the relative was a parent or an uncle or aunt. However, viewed through my septuagenarian prism, this paper prompted me to wonder in how many of these fatalities the firearm owner was a grandparent.
I have only anecdotal observations, but I can easily recall situations here in Maine in which a child has been injured by his or her grandfather’s gun. The data from the study show that pediatric fatalities are bimodal, with the majority occurring in the 1- to 5-year age group and a second peak in adolescence. The grandparent-involved cases I can recall were in the younger demographic.
Unfortunately, firearms aren’t the only threat that other grandparents and I pose to the health and safety of our grandchildren. I can remember before the development of, and the widespread use of, tamper-proof pill bottles, “grandma’s purse” overdoses were an unfortunately common occurrence.
More recently, at least here in Maine, we have been hearing more about motorized vehicle–related injuries and fatalities – grandparents backing over their grandchildren in the driveway or, more often, grandfathers (usually) taking their young grandchildren for rides on their snowmobiles, ATVs, lawn tractors, (fill in the blank). Whenever one of these events occurs, my mind quickly jumps beyond the tragic loss of life to imagining what terrible and long-lasting emotional chaos these incidents have spawned in those families.
During the pandemic, many parents and grandparents became aware of the threat that viral-spewing young children pose to the older and more vulnerable generation. On the other hand, many parents have been told that having a grandparent around can present a risk to the health and safety of their grandchildren. It can be a touchy subject in families, and grandparents may bristle at “being treated like a child” when they are reminded that children aren’t small adults and that their own behavior may be setting a bad example or putting their grandchildren at risk.
My generation had to learn how to buckle infants and toddlers into car seats because it was something that wasn’t done for our children. Fortunately, most new grandparents now already have those buckle-and-click skills and mindset. But,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Many decades ago I wrote a book I brazenly titled: “The Good Grandmother Handbook.” I had been a parent for a scant 7 or 8 years but based on my experiences in the office I felt I had accumulated enough wisdom to suggest to women in their fifth to seventh decades how they might conduct themselves around their grandchildren. Luckily, the book never got further than several hundred pages of crudely typed manuscript. This was before word processing programs had settled into the home computer industry, which was still in its infancy.
But I continue find the subject of grandparents interesting. Now, with grandchildren of my own (the oldest has just graduated from high school) and scores of peers knee deep in their own grandparenting adventures, I hope that my perspective now has a bit less of a holier-than-thou aroma.
My most recent muse-prodding event came when I stumbled across an article about the epidemiology of unintentional pediatric firearm fatalities. Looking at 10 years of data from the National Violent Death Reporting System, the investigators found that in 80% of the cases the firearm owner was a relative of the victim; in slightly more than 60% of the cases the event occurred in the victim’s home.
The data set was not granular enough to define the exact relationship between the child and relative who owned the gun. I suspect that most often the relative was a parent or an uncle or aunt. However, viewed through my septuagenarian prism, this paper prompted me to wonder in how many of these fatalities the firearm owner was a grandparent.
I have only anecdotal observations, but I can easily recall situations here in Maine in which a child has been injured by his or her grandfather’s gun. The data from the study show that pediatric fatalities are bimodal, with the majority occurring in the 1- to 5-year age group and a second peak in adolescence. The grandparent-involved cases I can recall were in the younger demographic.
Unfortunately, firearms aren’t the only threat that other grandparents and I pose to the health and safety of our grandchildren. I can remember before the development of, and the widespread use of, tamper-proof pill bottles, “grandma’s purse” overdoses were an unfortunately common occurrence.
More recently, at least here in Maine, we have been hearing more about motorized vehicle–related injuries and fatalities – grandparents backing over their grandchildren in the driveway or, more often, grandfathers (usually) taking their young grandchildren for rides on their snowmobiles, ATVs, lawn tractors, (fill in the blank). Whenever one of these events occurs, my mind quickly jumps beyond the tragic loss of life to imagining what terrible and long-lasting emotional chaos these incidents have spawned in those families.
During the pandemic, many parents and grandparents became aware of the threat that viral-spewing young children pose to the older and more vulnerable generation. On the other hand, many parents have been told that having a grandparent around can present a risk to the health and safety of their grandchildren. It can be a touchy subject in families, and grandparents may bristle at “being treated like a child” when they are reminded that children aren’t small adults and that their own behavior may be setting a bad example or putting their grandchildren at risk.
My generation had to learn how to buckle infants and toddlers into car seats because it was something that wasn’t done for our children. Fortunately, most new grandparents now already have those buckle-and-click skills and mindset. But,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Thoughts on primary care in 2023
As we all face remarkable challenges in giving great care to our patients and maintaining great care for ourselves, I wanted to share a few thoughts I have had regarding difficult things I have seen in the past few months.
- Call centers: Yikes! I think this is an overlooked stress on the primary care system. In a cost-cutting effort, organizations have gone to call centers to handle incoming calls, and hold times can be enormous. My own organization often has wait times longer than 30 minutes. I recently called another organization and had a wait time more than 30 minutes. Patients become frustrated and will message their primary care team to intervene for scheduling issues then will arrive at their appointments frustrated by all the hassles.
- Difficult encounters: We all have visits that we know will be challenging. I think it is even more difficult when we enter the visit stressed and tired. I have always found that, when I am in a calm place, even the most difficult visits go much better. Our patients arrive at clinic visits more stressed and tired too, as they face the challenge of a stretched and overwhelmed primary care system.
- Limited availability of specialists: My organization has had a sharp increase in wait times for specialty care over the past few years. Waits for some specialties can be almost a year. A study by Reddy and colleagues found a wait time of 3 months for patients referred to gastroenterologists.1 The lack of timely access to specialists adds to the stress and burden of primary care professionals. Managing problems deemed in need of subspecialty care as patients wait for appointments is difficult.
- Patient portals: Some practices are starting to figure this out this problem, others aren’t. Budd reviewed all the factors with the EHR that contribute to physician burnout.2 Portals have added another source of patient care outside face-to-face visits that adds to physician work load; for many practices is not appropriately accounted for in effort or productivity measures. Some practices are now starting to charge for patient messaging, but this may require even more physician time in documentation and billing. Unless this directly helps the physician reduce work hours or improve compensation, then it may make the problem worse.
There is little mystery why it seems so hard ... it is! Many things have been added to the plate of primary care professionals (increased messaging, calming patients frustrated with the medical system, and increased need for bridging care while patients wait for specialty appointments). Our patients need us now more than ever to give excellent, compassionate care in a poorly functioning system. We need to be emotionally and physically healthy enough to be there for our patients. Prioritize your own needs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Reddy K et al. Health Equity. 2018 Jun 1;2(1):103-8.
2. Budd J. J Prim Care Community Health. 2023 Apr 19.
As we all face remarkable challenges in giving great care to our patients and maintaining great care for ourselves, I wanted to share a few thoughts I have had regarding difficult things I have seen in the past few months.
- Call centers: Yikes! I think this is an overlooked stress on the primary care system. In a cost-cutting effort, organizations have gone to call centers to handle incoming calls, and hold times can be enormous. My own organization often has wait times longer than 30 minutes. I recently called another organization and had a wait time more than 30 minutes. Patients become frustrated and will message their primary care team to intervene for scheduling issues then will arrive at their appointments frustrated by all the hassles.
- Difficult encounters: We all have visits that we know will be challenging. I think it is even more difficult when we enter the visit stressed and tired. I have always found that, when I am in a calm place, even the most difficult visits go much better. Our patients arrive at clinic visits more stressed and tired too, as they face the challenge of a stretched and overwhelmed primary care system.
- Limited availability of specialists: My organization has had a sharp increase in wait times for specialty care over the past few years. Waits for some specialties can be almost a year. A study by Reddy and colleagues found a wait time of 3 months for patients referred to gastroenterologists.1 The lack of timely access to specialists adds to the stress and burden of primary care professionals. Managing problems deemed in need of subspecialty care as patients wait for appointments is difficult.
- Patient portals: Some practices are starting to figure this out this problem, others aren’t. Budd reviewed all the factors with the EHR that contribute to physician burnout.2 Portals have added another source of patient care outside face-to-face visits that adds to physician work load; for many practices is not appropriately accounted for in effort or productivity measures. Some practices are now starting to charge for patient messaging, but this may require even more physician time in documentation and billing. Unless this directly helps the physician reduce work hours or improve compensation, then it may make the problem worse.
There is little mystery why it seems so hard ... it is! Many things have been added to the plate of primary care professionals (increased messaging, calming patients frustrated with the medical system, and increased need for bridging care while patients wait for specialty appointments). Our patients need us now more than ever to give excellent, compassionate care in a poorly functioning system. We need to be emotionally and physically healthy enough to be there for our patients. Prioritize your own needs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Reddy K et al. Health Equity. 2018 Jun 1;2(1):103-8.
2. Budd J. J Prim Care Community Health. 2023 Apr 19.
As we all face remarkable challenges in giving great care to our patients and maintaining great care for ourselves, I wanted to share a few thoughts I have had regarding difficult things I have seen in the past few months.
- Call centers: Yikes! I think this is an overlooked stress on the primary care system. In a cost-cutting effort, organizations have gone to call centers to handle incoming calls, and hold times can be enormous. My own organization often has wait times longer than 30 minutes. I recently called another organization and had a wait time more than 30 minutes. Patients become frustrated and will message their primary care team to intervene for scheduling issues then will arrive at their appointments frustrated by all the hassles.
- Difficult encounters: We all have visits that we know will be challenging. I think it is even more difficult when we enter the visit stressed and tired. I have always found that, when I am in a calm place, even the most difficult visits go much better. Our patients arrive at clinic visits more stressed and tired too, as they face the challenge of a stretched and overwhelmed primary care system.
- Limited availability of specialists: My organization has had a sharp increase in wait times for specialty care over the past few years. Waits for some specialties can be almost a year. A study by Reddy and colleagues found a wait time of 3 months for patients referred to gastroenterologists.1 The lack of timely access to specialists adds to the stress and burden of primary care professionals. Managing problems deemed in need of subspecialty care as patients wait for appointments is difficult.
- Patient portals: Some practices are starting to figure this out this problem, others aren’t. Budd reviewed all the factors with the EHR that contribute to physician burnout.2 Portals have added another source of patient care outside face-to-face visits that adds to physician work load; for many practices is not appropriately accounted for in effort or productivity measures. Some practices are now starting to charge for patient messaging, but this may require even more physician time in documentation and billing. Unless this directly helps the physician reduce work hours or improve compensation, then it may make the problem worse.
There is little mystery why it seems so hard ... it is! Many things have been added to the plate of primary care professionals (increased messaging, calming patients frustrated with the medical system, and increased need for bridging care while patients wait for specialty appointments). Our patients need us now more than ever to give excellent, compassionate care in a poorly functioning system. We need to be emotionally and physically healthy enough to be there for our patients. Prioritize your own needs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Reddy K et al. Health Equity. 2018 Jun 1;2(1):103-8.
2. Budd J. J Prim Care Community Health. 2023 Apr 19.
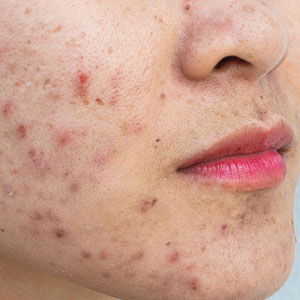
The Growing Pains of Changing Times for Acne and Rosacea Pathophysiology: Where Will It All End Up?
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
Breakthroughs and challenges in hepatology
CHICAGO – The most notable is the progress from discovery of the hepatitis C virus (previously non-A, non-B hepatitis) in 1989 to a near 100% cure with 8-12 weeks of oral medications in just 30 years, culminating in the The Nobel Prize in Physiology or Medicine in 2020.
This remarkable feat is matched by the progress from discovery of the hepatitis B virus (initially coined Australia antigen) and a 1976 Nobel Prize to an effective vaccine in 1981, to a list of antiviral drugs approved beginning in 1992 (with currently available nucleos(t)ide analogues associated with near zero risk of antiviral drug resistance even when used as monotherapy), to demonstration that both hepatitis B vaccine and antivirals can prevent liver cancer. Other major breakthroughs include blood-based and image-based noninvasive assessment of liver fibrosis obviating the need for liver biopsy to stage liver disease, and multiple systemic therapies for advanced liver cancer.
However, there remain many challenges. While we have the tools to eliminate hepatitis B and hepatitis C, resources and coordinated efforts are needed to realize this feasible goal. Development of a vaccine for hepatitis C and a cure for hepatitis B will facilitate this goal.
The biggest present-day challenges in hepatology are the epidemics of nonalcoholic fatty liver disease (NAFLD) and alcohol-associated liver disease (ALD), particularly since both are increasingly impacting young people, socially disadvantaged populations, and those with mental health problems. Social isolation and mental and financial stressors associated with the COVID pandemic have aggravated both NAFLD and ALD, which have now become the leading indications for liver transplantation. Multi-pronged approaches, including public policies and education, destigmatization, easy access to mental health care, provider training, and provision of multi-disciplinary care, are needed to curb this tsunami. NAFLD affects more than 30% of the global population, and screening with simple biomarker(s) followed by liver stiffness measurement using elastography has been proposed to identify patients with or at high risk of advanced fibrosis or cirrhosis for specialist care. NAFLD is a heterogeneous disease and the requirement for paired liver biopsies to demonstrate benefit have made drug development challenging. Better phenotyping of disease and surrogates for outcomes are needed. Liver cancer is one of the top cancer killers globally, but it is also a preventable cancer making prevention and early treatment of liver disease a top public health priority.
Dr. Lok is director of clinical hepatology and assistant dean for clinical research, University of Michigan Medical School, Ann Arbor. She disclosed research grants with AstraZeneca, Kowa, and Target. She has served as a consultant/adviser to Abbott, Chroma, GlaxoSmithKline, Roche, Virion, and Novo Nordisk. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023. DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The most notable is the progress from discovery of the hepatitis C virus (previously non-A, non-B hepatitis) in 1989 to a near 100% cure with 8-12 weeks of oral medications in just 30 years, culminating in the The Nobel Prize in Physiology or Medicine in 2020.
This remarkable feat is matched by the progress from discovery of the hepatitis B virus (initially coined Australia antigen) and a 1976 Nobel Prize to an effective vaccine in 1981, to a list of antiviral drugs approved beginning in 1992 (with currently available nucleos(t)ide analogues associated with near zero risk of antiviral drug resistance even when used as monotherapy), to demonstration that both hepatitis B vaccine and antivirals can prevent liver cancer. Other major breakthroughs include blood-based and image-based noninvasive assessment of liver fibrosis obviating the need for liver biopsy to stage liver disease, and multiple systemic therapies for advanced liver cancer.
However, there remain many challenges. While we have the tools to eliminate hepatitis B and hepatitis C, resources and coordinated efforts are needed to realize this feasible goal. Development of a vaccine for hepatitis C and a cure for hepatitis B will facilitate this goal.
The biggest present-day challenges in hepatology are the epidemics of nonalcoholic fatty liver disease (NAFLD) and alcohol-associated liver disease (ALD), particularly since both are increasingly impacting young people, socially disadvantaged populations, and those with mental health problems. Social isolation and mental and financial stressors associated with the COVID pandemic have aggravated both NAFLD and ALD, which have now become the leading indications for liver transplantation. Multi-pronged approaches, including public policies and education, destigmatization, easy access to mental health care, provider training, and provision of multi-disciplinary care, are needed to curb this tsunami. NAFLD affects more than 30% of the global population, and screening with simple biomarker(s) followed by liver stiffness measurement using elastography has been proposed to identify patients with or at high risk of advanced fibrosis or cirrhosis for specialist care. NAFLD is a heterogeneous disease and the requirement for paired liver biopsies to demonstrate benefit have made drug development challenging. Better phenotyping of disease and surrogates for outcomes are needed. Liver cancer is one of the top cancer killers globally, but it is also a preventable cancer making prevention and early treatment of liver disease a top public health priority.
Dr. Lok is director of clinical hepatology and assistant dean for clinical research, University of Michigan Medical School, Ann Arbor. She disclosed research grants with AstraZeneca, Kowa, and Target. She has served as a consultant/adviser to Abbott, Chroma, GlaxoSmithKline, Roche, Virion, and Novo Nordisk. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023. DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The most notable is the progress from discovery of the hepatitis C virus (previously non-A, non-B hepatitis) in 1989 to a near 100% cure with 8-12 weeks of oral medications in just 30 years, culminating in the The Nobel Prize in Physiology or Medicine in 2020.
This remarkable feat is matched by the progress from discovery of the hepatitis B virus (initially coined Australia antigen) and a 1976 Nobel Prize to an effective vaccine in 1981, to a list of antiviral drugs approved beginning in 1992 (with currently available nucleos(t)ide analogues associated with near zero risk of antiviral drug resistance even when used as monotherapy), to demonstration that both hepatitis B vaccine and antivirals can prevent liver cancer. Other major breakthroughs include blood-based and image-based noninvasive assessment of liver fibrosis obviating the need for liver biopsy to stage liver disease, and multiple systemic therapies for advanced liver cancer.
However, there remain many challenges. While we have the tools to eliminate hepatitis B and hepatitis C, resources and coordinated efforts are needed to realize this feasible goal. Development of a vaccine for hepatitis C and a cure for hepatitis B will facilitate this goal.
The biggest present-day challenges in hepatology are the epidemics of nonalcoholic fatty liver disease (NAFLD) and alcohol-associated liver disease (ALD), particularly since both are increasingly impacting young people, socially disadvantaged populations, and those with mental health problems. Social isolation and mental and financial stressors associated with the COVID pandemic have aggravated both NAFLD and ALD, which have now become the leading indications for liver transplantation. Multi-pronged approaches, including public policies and education, destigmatization, easy access to mental health care, provider training, and provision of multi-disciplinary care, are needed to curb this tsunami. NAFLD affects more than 30% of the global population, and screening with simple biomarker(s) followed by liver stiffness measurement using elastography has been proposed to identify patients with or at high risk of advanced fibrosis or cirrhosis for specialist care. NAFLD is a heterogeneous disease and the requirement for paired liver biopsies to demonstrate benefit have made drug development challenging. Better phenotyping of disease and surrogates for outcomes are needed. Liver cancer is one of the top cancer killers globally, but it is also a preventable cancer making prevention and early treatment of liver disease a top public health priority.
Dr. Lok is director of clinical hepatology and assistant dean for clinical research, University of Michigan Medical School, Ann Arbor. She disclosed research grants with AstraZeneca, Kowa, and Target. She has served as a consultant/adviser to Abbott, Chroma, GlaxoSmithKline, Roche, Virion, and Novo Nordisk. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023. DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
AT DDW 2023
Disconnecting to reconnect
I recently returned from a bucket list trip rafting the full length of the Grand Canyon via the Colorado River. It was a spectacular trip, filled with thrilling rapids, awe-inspiring hikes through slot canyons, and swimming in the turquoise waters of Havasu Falls.
For those of you who are fortunate to have experienced a similar adventure, I think you’ll agree one of the best things about the trip (aside from the breathtaking scenery) was the ability to completely unplug. Not only did I travel without my trusty laptop, but cell service was nonexistent. The effect of this forced digital detox was magical. By mentally disconnecting from work without the constant ping of my email and EHR inbox, our group had deeper conversations and formed genuine connections without the distractions of technology. In the frenetically paced world of modern health care where clinicians are reachable wherever they are in the world (even on vacation) as the boundaries between work and life blur, there are increasingly fewer times like this when we can fully disconnect. Yet, periodically disconnecting from work is critical, particularly for the clinician community, which is grappling with increasing levels of burnout and its consequences. As you embark on your well-deserved summer vacations, I hope you have an opportunity to set aside your devices to reconnect more fully with your family and friends, but also yourself.
In this month’s issue of GI&Hepatology News, we update you on AGA’s ongoing advocacy efforts to challenge UnitedHealthcare’s plans to impose increased administrative burdens on GI practices relating to routine GI procedures. We also highlight a landmark clinical trial in pediatric Crohn’s disease recently published in Gastroenterology. In our quarterly Perspectives column, Dr. Mariam Naveed and Dr. Petr Protiva outline important considerations regarding when to stop surveillance for colorectal neoplasia in elderly patients. Finally, our July Member Spotlight features gastroenterologist Dr. Russ Arjal, who shares his experiences launching Telebelly Health, an entirely virtual GI practice.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
I recently returned from a bucket list trip rafting the full length of the Grand Canyon via the Colorado River. It was a spectacular trip, filled with thrilling rapids, awe-inspiring hikes through slot canyons, and swimming in the turquoise waters of Havasu Falls.
For those of you who are fortunate to have experienced a similar adventure, I think you’ll agree one of the best things about the trip (aside from the breathtaking scenery) was the ability to completely unplug. Not only did I travel without my trusty laptop, but cell service was nonexistent. The effect of this forced digital detox was magical. By mentally disconnecting from work without the constant ping of my email and EHR inbox, our group had deeper conversations and formed genuine connections without the distractions of technology. In the frenetically paced world of modern health care where clinicians are reachable wherever they are in the world (even on vacation) as the boundaries between work and life blur, there are increasingly fewer times like this when we can fully disconnect. Yet, periodically disconnecting from work is critical, particularly for the clinician community, which is grappling with increasing levels of burnout and its consequences. As you embark on your well-deserved summer vacations, I hope you have an opportunity to set aside your devices to reconnect more fully with your family and friends, but also yourself.
In this month’s issue of GI&Hepatology News, we update you on AGA’s ongoing advocacy efforts to challenge UnitedHealthcare’s plans to impose increased administrative burdens on GI practices relating to routine GI procedures. We also highlight a landmark clinical trial in pediatric Crohn’s disease recently published in Gastroenterology. In our quarterly Perspectives column, Dr. Mariam Naveed and Dr. Petr Protiva outline important considerations regarding when to stop surveillance for colorectal neoplasia in elderly patients. Finally, our July Member Spotlight features gastroenterologist Dr. Russ Arjal, who shares his experiences launching Telebelly Health, an entirely virtual GI practice.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
I recently returned from a bucket list trip rafting the full length of the Grand Canyon via the Colorado River. It was a spectacular trip, filled with thrilling rapids, awe-inspiring hikes through slot canyons, and swimming in the turquoise waters of Havasu Falls.
For those of you who are fortunate to have experienced a similar adventure, I think you’ll agree one of the best things about the trip (aside from the breathtaking scenery) was the ability to completely unplug. Not only did I travel without my trusty laptop, but cell service was nonexistent. The effect of this forced digital detox was magical. By mentally disconnecting from work without the constant ping of my email and EHR inbox, our group had deeper conversations and formed genuine connections without the distractions of technology. In the frenetically paced world of modern health care where clinicians are reachable wherever they are in the world (even on vacation) as the boundaries between work and life blur, there are increasingly fewer times like this when we can fully disconnect. Yet, periodically disconnecting from work is critical, particularly for the clinician community, which is grappling with increasing levels of burnout and its consequences. As you embark on your well-deserved summer vacations, I hope you have an opportunity to set aside your devices to reconnect more fully with your family and friends, but also yourself.
In this month’s issue of GI&Hepatology News, we update you on AGA’s ongoing advocacy efforts to challenge UnitedHealthcare’s plans to impose increased administrative burdens on GI practices relating to routine GI procedures. We also highlight a landmark clinical trial in pediatric Crohn’s disease recently published in Gastroenterology. In our quarterly Perspectives column, Dr. Mariam Naveed and Dr. Petr Protiva outline important considerations regarding when to stop surveillance for colorectal neoplasia in elderly patients. Finally, our July Member Spotlight features gastroenterologist Dr. Russ Arjal, who shares his experiences launching Telebelly Health, an entirely virtual GI practice.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
MD rushes in after lightning strikes four people at White House
It was one of those dog days of August where the humidity is palpable and the pressure is so hot and thick you can almost feel the ions in the air. At the time (2022), I was a White House fellow and senior adviser in the West Wing Office of Public Engagement and in the Office of the Vice President.
I was leaving the White House around 7:00 p.m. through the front gate on Lafayette Square. I had a dinner reservation with a friend, so I was in a rush. It was super overcast. Lo and behold, three steps after I closed the gate behind me, it started pouring. Rain came down so hard I had to take shelter.
There’s a stone building in front of the White House with archways, so I took cover underneath one of them, hoping that in a couple of minutes the rain would pass. Behind the archways are these thick, black, iron gates.
Just as I was about to make a run for it, I heard: BOOM!
It was like a bomb had gone off. In one moment, I saw the lightning bolt, heard the thunder, and felt the heat. It was all one rush of sensation. I couldn’t remember having been that scared in a long time.
I thought, “I definitely have to get out of here. In a couple of minutes there might be another strike, and I’m sitting next to iron gates!” I saw a little bit of a window in the downpour, so I started booking it. I knew there was a sheltered Secret Service area around the corner where they park their cars. A much safer place to be.
I was sprinting on the sidewalk and spotted a bunch of Secret Service agents on their bikes riding in the opposite direction, back toward the park. I knew they wouldn’t be out on bikes in this mess without a reason. As they reached me, one agent said, “Clear the sidewalk! We’re coming through with a bunch of equipment.”
I yelled, “What’s going on?”
“Four people were just struck by lightning,” he said as he zoomed past.
I thought: “Sh*t. I have to go back.”
It was like two different parts of my brain were active at the exact same time. My subcortical brain at the level of the amygdala was like: “You just ran from there, idiot. Why are you running back?” And another part of my brain was like: “This is who you are.”
The lightning had struck one of the largest trees in the park. Four bodies splayed out in one direction from the tree. They’d been taking shelter underneath it when they were hit and were blown off to one side. By the time I got there, two Secret Service agents were on the scene doing CPR. Some bystanders had started to run over.
I did a quick round of pulse checks to see everyone’s status, and all four were apneic and pulseless. I told the two Secret Service agents to keep doing compressions on the first person. Two bystanders also began compressions on another person, an older man.
More Secret Service agents arrived, and I said, “We need to do compressions on this other person right now.” One of the agents took a moment to question who I could be and why I was there. I said, “I’m a doctor. I know I’m not dressed like one, but I’m a physician.”
I told some agents to go find an AED, because these people needed to be shocked.
After they left, I was effectively trying to triage which of these four people would get the AED first. Initially, I spent more of my time on the young man, and we began to get some response from him. I then spent some time with the young woman.
It turned out there were AEDs in the pouches on the Secret Service bikes, but they were very small, dinky AEDs. We tried to apply the pads, but it was downpouring so much that the adhesive wouldn’t stick. I told one of the agents we needed a towel.
Through all this I was concerned we were going to be struck again. I mean, the metal statue of Lafayette was right there! They say lighting doesn’t strike in the same place twice, but who knows if that’s really true?
The towel arrived, and we were able to get the chests of the younger people dry enough for the AED pads. We applied two shocks first to the woman, then the young man. We got his pulse back quickly. The woman’s came back as well, but it felt much weaker.
EMS arrived shortly thereafter. We got all four patients on the transport, and they were transferred to the hospital.
The whole experience had taken 14 minutes.
At the time, I felt confident that the young man was going to survive. We’re taught that lightning bolt strikes are survivable if you can shock someone quickly. He also got pretty good CPR. But the next day I was watching the news and learned that he had passed away. So, of course I was thinking the worst about the others as well.
But a week and a half later, I learned that the young woman had been discharged from the ICU. She was the only one who made it. Her name is Amber, and we got connected through a reporter. About 2 weeks later, I invited her to the White House. I took her to the Oval Office. I met her mom and dad and husband, and we had dinner. We’ve been in touch ever since.
I remember the first time we talked on the phone, Amber said something along the lines of, “This sucks. Obviously, I was not planning for any of this to happen. But I also think there’s something good that could come from this.”
I was so surprised and happy to hear her say that. I had something similar happen to me when I was a teenager – caught in the wrong place at the wrong time. I tried to intervene in a gang fight in my neighborhood. I thought a kid was going to get killed, so I jumped in, imagining I could save the day. I didn’t. They broke a bunch of my bones and I was in the hospital for a bit.
I remember thinking then that my life was over. But after some time, I found a new perspective, which was: Maybe that life is over. But maybe this could be the beginning of a new one. And maybe those things that I’ve been afraid of doing, the dreams that I have, maybe now I’m actually free to go after them.
I told Amber, if there are things that you have been waiting to do, this could be the time. She wants to be an international human rights activist, and she is kicking butt in a graduate school program to begin on that pathway. It’s been really cool to watch her chase this dream with way more vigor than she had before.
I think we bonded because we’ve gone through – obviously not the same thing, but a similar moment of being confronted with your own mortality. Realizing that life can just shatter. And so, while we’re here, we might as well go for it with all the force of a person who knows this could all disappear in an instant.
It was an extremely humbling moment. It reaffirmed that my life is not about me. I have to use the time that I’ve got on behalf of other people as much as I can. What is my life about if not being useful?
Dr. Martin is an emergency medicine physician and faculty member at the MGH Center for Social Justice and Health Equity at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
It was one of those dog days of August where the humidity is palpable and the pressure is so hot and thick you can almost feel the ions in the air. At the time (2022), I was a White House fellow and senior adviser in the West Wing Office of Public Engagement and in the Office of the Vice President.
I was leaving the White House around 7:00 p.m. through the front gate on Lafayette Square. I had a dinner reservation with a friend, so I was in a rush. It was super overcast. Lo and behold, three steps after I closed the gate behind me, it started pouring. Rain came down so hard I had to take shelter.
There’s a stone building in front of the White House with archways, so I took cover underneath one of them, hoping that in a couple of minutes the rain would pass. Behind the archways are these thick, black, iron gates.
Just as I was about to make a run for it, I heard: BOOM!
It was like a bomb had gone off. In one moment, I saw the lightning bolt, heard the thunder, and felt the heat. It was all one rush of sensation. I couldn’t remember having been that scared in a long time.
I thought, “I definitely have to get out of here. In a couple of minutes there might be another strike, and I’m sitting next to iron gates!” I saw a little bit of a window in the downpour, so I started booking it. I knew there was a sheltered Secret Service area around the corner where they park their cars. A much safer place to be.
I was sprinting on the sidewalk and spotted a bunch of Secret Service agents on their bikes riding in the opposite direction, back toward the park. I knew they wouldn’t be out on bikes in this mess without a reason. As they reached me, one agent said, “Clear the sidewalk! We’re coming through with a bunch of equipment.”
I yelled, “What’s going on?”
“Four people were just struck by lightning,” he said as he zoomed past.
I thought: “Sh*t. I have to go back.”
It was like two different parts of my brain were active at the exact same time. My subcortical brain at the level of the amygdala was like: “You just ran from there, idiot. Why are you running back?” And another part of my brain was like: “This is who you are.”
The lightning had struck one of the largest trees in the park. Four bodies splayed out in one direction from the tree. They’d been taking shelter underneath it when they were hit and were blown off to one side. By the time I got there, two Secret Service agents were on the scene doing CPR. Some bystanders had started to run over.
I did a quick round of pulse checks to see everyone’s status, and all four were apneic and pulseless. I told the two Secret Service agents to keep doing compressions on the first person. Two bystanders also began compressions on another person, an older man.
More Secret Service agents arrived, and I said, “We need to do compressions on this other person right now.” One of the agents took a moment to question who I could be and why I was there. I said, “I’m a doctor. I know I’m not dressed like one, but I’m a physician.”
I told some agents to go find an AED, because these people needed to be shocked.
After they left, I was effectively trying to triage which of these four people would get the AED first. Initially, I spent more of my time on the young man, and we began to get some response from him. I then spent some time with the young woman.
It turned out there were AEDs in the pouches on the Secret Service bikes, but they were very small, dinky AEDs. We tried to apply the pads, but it was downpouring so much that the adhesive wouldn’t stick. I told one of the agents we needed a towel.
Through all this I was concerned we were going to be struck again. I mean, the metal statue of Lafayette was right there! They say lighting doesn’t strike in the same place twice, but who knows if that’s really true?
The towel arrived, and we were able to get the chests of the younger people dry enough for the AED pads. We applied two shocks first to the woman, then the young man. We got his pulse back quickly. The woman’s came back as well, but it felt much weaker.
EMS arrived shortly thereafter. We got all four patients on the transport, and they were transferred to the hospital.
The whole experience had taken 14 minutes.
At the time, I felt confident that the young man was going to survive. We’re taught that lightning bolt strikes are survivable if you can shock someone quickly. He also got pretty good CPR. But the next day I was watching the news and learned that he had passed away. So, of course I was thinking the worst about the others as well.
But a week and a half later, I learned that the young woman had been discharged from the ICU. She was the only one who made it. Her name is Amber, and we got connected through a reporter. About 2 weeks later, I invited her to the White House. I took her to the Oval Office. I met her mom and dad and husband, and we had dinner. We’ve been in touch ever since.
I remember the first time we talked on the phone, Amber said something along the lines of, “This sucks. Obviously, I was not planning for any of this to happen. But I also think there’s something good that could come from this.”
I was so surprised and happy to hear her say that. I had something similar happen to me when I was a teenager – caught in the wrong place at the wrong time. I tried to intervene in a gang fight in my neighborhood. I thought a kid was going to get killed, so I jumped in, imagining I could save the day. I didn’t. They broke a bunch of my bones and I was in the hospital for a bit.
I remember thinking then that my life was over. But after some time, I found a new perspective, which was: Maybe that life is over. But maybe this could be the beginning of a new one. And maybe those things that I’ve been afraid of doing, the dreams that I have, maybe now I’m actually free to go after them.
I told Amber, if there are things that you have been waiting to do, this could be the time. She wants to be an international human rights activist, and she is kicking butt in a graduate school program to begin on that pathway. It’s been really cool to watch her chase this dream with way more vigor than she had before.
I think we bonded because we’ve gone through – obviously not the same thing, but a similar moment of being confronted with your own mortality. Realizing that life can just shatter. And so, while we’re here, we might as well go for it with all the force of a person who knows this could all disappear in an instant.
It was an extremely humbling moment. It reaffirmed that my life is not about me. I have to use the time that I’ve got on behalf of other people as much as I can. What is my life about if not being useful?
Dr. Martin is an emergency medicine physician and faculty member at the MGH Center for Social Justice and Health Equity at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
It was one of those dog days of August where the humidity is palpable and the pressure is so hot and thick you can almost feel the ions in the air. At the time (2022), I was a White House fellow and senior adviser in the West Wing Office of Public Engagement and in the Office of the Vice President.
I was leaving the White House around 7:00 p.m. through the front gate on Lafayette Square. I had a dinner reservation with a friend, so I was in a rush. It was super overcast. Lo and behold, three steps after I closed the gate behind me, it started pouring. Rain came down so hard I had to take shelter.
There’s a stone building in front of the White House with archways, so I took cover underneath one of them, hoping that in a couple of minutes the rain would pass. Behind the archways are these thick, black, iron gates.
Just as I was about to make a run for it, I heard: BOOM!
It was like a bomb had gone off. In one moment, I saw the lightning bolt, heard the thunder, and felt the heat. It was all one rush of sensation. I couldn’t remember having been that scared in a long time.
I thought, “I definitely have to get out of here. In a couple of minutes there might be another strike, and I’m sitting next to iron gates!” I saw a little bit of a window in the downpour, so I started booking it. I knew there was a sheltered Secret Service area around the corner where they park their cars. A much safer place to be.
I was sprinting on the sidewalk and spotted a bunch of Secret Service agents on their bikes riding in the opposite direction, back toward the park. I knew they wouldn’t be out on bikes in this mess without a reason. As they reached me, one agent said, “Clear the sidewalk! We’re coming through with a bunch of equipment.”
I yelled, “What’s going on?”
“Four people were just struck by lightning,” he said as he zoomed past.
I thought: “Sh*t. I have to go back.”
It was like two different parts of my brain were active at the exact same time. My subcortical brain at the level of the amygdala was like: “You just ran from there, idiot. Why are you running back?” And another part of my brain was like: “This is who you are.”
The lightning had struck one of the largest trees in the park. Four bodies splayed out in one direction from the tree. They’d been taking shelter underneath it when they were hit and were blown off to one side. By the time I got there, two Secret Service agents were on the scene doing CPR. Some bystanders had started to run over.
I did a quick round of pulse checks to see everyone’s status, and all four were apneic and pulseless. I told the two Secret Service agents to keep doing compressions on the first person. Two bystanders also began compressions on another person, an older man.
More Secret Service agents arrived, and I said, “We need to do compressions on this other person right now.” One of the agents took a moment to question who I could be and why I was there. I said, “I’m a doctor. I know I’m not dressed like one, but I’m a physician.”
I told some agents to go find an AED, because these people needed to be shocked.
After they left, I was effectively trying to triage which of these four people would get the AED first. Initially, I spent more of my time on the young man, and we began to get some response from him. I then spent some time with the young woman.
It turned out there were AEDs in the pouches on the Secret Service bikes, but they were very small, dinky AEDs. We tried to apply the pads, but it was downpouring so much that the adhesive wouldn’t stick. I told one of the agents we needed a towel.
Through all this I was concerned we were going to be struck again. I mean, the metal statue of Lafayette was right there! They say lighting doesn’t strike in the same place twice, but who knows if that’s really true?
The towel arrived, and we were able to get the chests of the younger people dry enough for the AED pads. We applied two shocks first to the woman, then the young man. We got his pulse back quickly. The woman’s came back as well, but it felt much weaker.
EMS arrived shortly thereafter. We got all four patients on the transport, and they were transferred to the hospital.
The whole experience had taken 14 minutes.
At the time, I felt confident that the young man was going to survive. We’re taught that lightning bolt strikes are survivable if you can shock someone quickly. He also got pretty good CPR. But the next day I was watching the news and learned that he had passed away. So, of course I was thinking the worst about the others as well.
But a week and a half later, I learned that the young woman had been discharged from the ICU. She was the only one who made it. Her name is Amber, and we got connected through a reporter. About 2 weeks later, I invited her to the White House. I took her to the Oval Office. I met her mom and dad and husband, and we had dinner. We’ve been in touch ever since.
I remember the first time we talked on the phone, Amber said something along the lines of, “This sucks. Obviously, I was not planning for any of this to happen. But I also think there’s something good that could come from this.”
I was so surprised and happy to hear her say that. I had something similar happen to me when I was a teenager – caught in the wrong place at the wrong time. I tried to intervene in a gang fight in my neighborhood. I thought a kid was going to get killed, so I jumped in, imagining I could save the day. I didn’t. They broke a bunch of my bones and I was in the hospital for a bit.
I remember thinking then that my life was over. But after some time, I found a new perspective, which was: Maybe that life is over. But maybe this could be the beginning of a new one. And maybe those things that I’ve been afraid of doing, the dreams that I have, maybe now I’m actually free to go after them.
I told Amber, if there are things that you have been waiting to do, this could be the time. She wants to be an international human rights activist, and she is kicking butt in a graduate school program to begin on that pathway. It’s been really cool to watch her chase this dream with way more vigor than she had before.
I think we bonded because we’ve gone through – obviously not the same thing, but a similar moment of being confronted with your own mortality. Realizing that life can just shatter. And so, while we’re here, we might as well go for it with all the force of a person who knows this could all disappear in an instant.
It was an extremely humbling moment. It reaffirmed that my life is not about me. I have to use the time that I’ve got on behalf of other people as much as I can. What is my life about if not being useful?
Dr. Martin is an emergency medicine physician and faculty member at the MGH Center for Social Justice and Health Equity at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
What’s new in the new jaundice guidelines?
More than 15 years in the making, the revised AAP Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation was released in 2022. A key driving force for this revision was the expanded evidence base regarding monitoring and treatment of newborns 35 or more weeks’ gestation to prevent bilirubin encephalopathy and kernicterus.
Here, we summarize the highlights of the new guidelines and point out practical ways to incorporate these guidelines into daily practice.
What has changed?
If you are familiar with the previous guidelines (2004 or the 2009 update) for the management of newborn jaundice, you’ll note that the treatment graphs for phototherapy and exchange transfusion have been updated with new, slightly higher thresholds.
Bilirubin thresholds for starting phototherapy are about 2 mg/dL higher overall than indicated in previous iterations of the guidelines.
This change reflects new evidence that infants don’t typically develop bilirubin neurotoxicity until the total serum bilirubin (TSB) reaches levels well above the previous exchange transfusion threshold, justifying a narrow increase in the bilirubin level for starting phototherapy. Also, phototherapy treatment thresholds are now risk-adjusted, with separate curves for each gestational age from 35 weeks to > 38 weeks.
To find the applicable phototherapy threshold, use the infant’s gestational age (rounding down) and determine whether the infant has even a single neurotoxicity risk factor other than prematurity. Neurotoxicity risk factors include a low albumin level, isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other hemolytic conditions; sepsis; or any significant clinical instability in the previous 24 hours.
For example, a 384/7 weeks’ gestation newborn has a TSB of 12 mg/dL at 48 hours of age but no neurotoxicity risk factors. Using the graph Phototherapy Thresholds: No Hyperbilirubinemia Neurotoxicity Risk Factors, should the infant be placed under phototherapy at this time? (Answer: No. The threshold for starting phototherapy on this infant is approximately 16 mg/dL.)
When hyperbilirubinemia becomes a medical emergency
A new term, “escalation of care,” has been adopted to describe actions to take when the newborn’s TSB climbs to within 2 mg/dL of the exchange transfusion threshold – a medical emergency. Instructions on how to ensure intensive phototherapy, and when to initiate an urgent exchange transfusion, are given, including the critical need to maintain intensive phototherapy continuously during infant transport and admission to another facility.
Transcutaneous vs. serum bilirubin
Either a serum TSB or a transcutaneous bilirubin (TcB) should be measured in all infants between 24 and 48 hours after birth or before discharge if that occurs earlier. TcB measurements are valid and reliable when used as a screening test to identify infants who require a TSB measurement. Although the two tests are generally correlated, they are not identical, and treatment decisions should be based on TSB levels. A TSB should be obtained if the TcB exceeds or is within 3 mg/dL of the phototherapy treatment threshold, or if the TcB is ≥ 15 mg/dL.
Following up: When to check another bilirubin level
Prior to these new guidelines, the question of when to get the next bilirubin level was based on Vinod Bhutani, MD’s risk nomogram, which classified newborn bilirubin levels within high-, intermediate-, or low-risk zones for needing phototherapy. A bilirubin level in the high-risk zone indicated the need for earlier follow-up. These risk zones have been replaced with a more specific table that provides recommended postdischarge follow-up based on how close the newborn’s bilirubin level is to the hour-specific threshold for treatment. The closer the latest TSB or TcB level is to the newborn’s risk-based phototherapy threshold, the sooner the follow-up to check another bilirubin level will need to be.
Most infants discharged before 72 hours of age will need follow-up within 2 days. Newborns with TSB levels nearing the level for phototherapy (within 2 mg/dL or less) should remain in the hospital.
Five tips for using the new guidelines
Bilitool.org, a popular and useful app, has already been updated to reflect the changes in the new guidelines, making it easy to apply the new thresholds and create a follow-up plan for each patient.
The guidelines provide recommendations for when to check rebound bilirubin levels after stopping phototherapy (hint: babies with neurotoxic risk factors). A TcB device should not be used while the infant is being treated with phototherapy. However, a TcB can be measured once the baby has been off phototherapy for at least 24 hours.
If you have at least two bilirubin measurements, you can calculate the “rate of rise” in bilirubin level. A rapid rate of rise, which serves as a clinical indicator of hemolysis, is defined as ≥ 0.3 mg/dL per hour in the first 24 hours or ≥ 0.2 mg/dL per hour after the first 24 hours of life. This is especially helpful when hemolysis is suspected even if the newborn’s direct antibody test (DAT) is negative. In this scenario, the infant is considered to have a neurotoxic risk factor.
When you initiate phototherapy, be aware of the infant’s bilirubin level threshold for stopping phototherapy (2 mg/dL below the starting phototherapy threshold), as well as the threshold for escalation of care (2 mg/dL below the exchange transfusion threshold).
Because the thresholds for starting phototherapy and initiating exchange transfusion are slightly higher and specific to gestational age, clinicians can more confidently use less phototherapy.
Other guideline highlights
The neurotoxic risk factors and corresponding thresholds are important. If the newborn has one or more neurotoxic risk factors other than prematurity, the neurotoxic risk threshold graph should be used when assessing the need for treatment. Neurotoxic risk thresholds should also be used for newborns whose bilirubin levels continue rising on phototherapy.
The guidelines emphasize that G6PD is one of the most important causes of hazardous hyperbilirubinemia leading to kernicterus in the United States and worldwide. Overall, 13% of African American males and about 4% of African American females have G6PD deficiency.
Finally, the guidelines remind clinicians that an important way to reduce the chances that phototherapy will be needed is to encourage early and frequent feeding (8-12 times in 24 hours).
The AAP Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation contains a great deal more information, but these basic principles should allow practitioners to begin to incorporate these guidelines into daily practice.
Dr. Amaya is associate professor, department of pediatrics, Medical University of South Carolina, Charleston, and medical director, level 1 nursery, department of pediatrics, MUSC general academic pediatrics. She disclosed ties with Medical University of South Carolina. Dr. Balog is clinical associate professor of pediatrics, Medical University of South Carolina, Charleston. She has no relevant financial relationships. Dr. Basco is professor, department of pediatrics, Medical University of South Carolina, Charleston; director, division of general pediatrics, department of pediatrics, MUSC Children’s Hospital. He has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
More than 15 years in the making, the revised AAP Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation was released in 2022. A key driving force for this revision was the expanded evidence base regarding monitoring and treatment of newborns 35 or more weeks’ gestation to prevent bilirubin encephalopathy and kernicterus.
Here, we summarize the highlights of the new guidelines and point out practical ways to incorporate these guidelines into daily practice.
What has changed?
If you are familiar with the previous guidelines (2004 or the 2009 update) for the management of newborn jaundice, you’ll note that the treatment graphs for phototherapy and exchange transfusion have been updated with new, slightly higher thresholds.
Bilirubin thresholds for starting phototherapy are about 2 mg/dL higher overall than indicated in previous iterations of the guidelines.
This change reflects new evidence that infants don’t typically develop bilirubin neurotoxicity until the total serum bilirubin (TSB) reaches levels well above the previous exchange transfusion threshold, justifying a narrow increase in the bilirubin level for starting phototherapy. Also, phototherapy treatment thresholds are now risk-adjusted, with separate curves for each gestational age from 35 weeks to > 38 weeks.
To find the applicable phototherapy threshold, use the infant’s gestational age (rounding down) and determine whether the infant has even a single neurotoxicity risk factor other than prematurity. Neurotoxicity risk factors include a low albumin level, isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other hemolytic conditions; sepsis; or any significant clinical instability in the previous 24 hours.
For example, a 384/7 weeks’ gestation newborn has a TSB of 12 mg/dL at 48 hours of age but no neurotoxicity risk factors. Using the graph Phototherapy Thresholds: No Hyperbilirubinemia Neurotoxicity Risk Factors, should the infant be placed under phototherapy at this time? (Answer: No. The threshold for starting phototherapy on this infant is approximately 16 mg/dL.)
When hyperbilirubinemia becomes a medical emergency
A new term, “escalation of care,” has been adopted to describe actions to take when the newborn’s TSB climbs to within 2 mg/dL of the exchange transfusion threshold – a medical emergency. Instructions on how to ensure intensive phototherapy, and when to initiate an urgent exchange transfusion, are given, including the critical need to maintain intensive phototherapy continuously during infant transport and admission to another facility.
Transcutaneous vs. serum bilirubin
Either a serum TSB or a transcutaneous bilirubin (TcB) should be measured in all infants between 24 and 48 hours after birth or before discharge if that occurs earlier. TcB measurements are valid and reliable when used as a screening test to identify infants who require a TSB measurement. Although the two tests are generally correlated, they are not identical, and treatment decisions should be based on TSB levels. A TSB should be obtained if the TcB exceeds or is within 3 mg/dL of the phototherapy treatment threshold, or if the TcB is ≥ 15 mg/dL.
Following up: When to check another bilirubin level
Prior to these new guidelines, the question of when to get the next bilirubin level was based on Vinod Bhutani, MD’s risk nomogram, which classified newborn bilirubin levels within high-, intermediate-, or low-risk zones for needing phototherapy. A bilirubin level in the high-risk zone indicated the need for earlier follow-up. These risk zones have been replaced with a more specific table that provides recommended postdischarge follow-up based on how close the newborn’s bilirubin level is to the hour-specific threshold for treatment. The closer the latest TSB or TcB level is to the newborn’s risk-based phototherapy threshold, the sooner the follow-up to check another bilirubin level will need to be.
Most infants discharged before 72 hours of age will need follow-up within 2 days. Newborns with TSB levels nearing the level for phototherapy (within 2 mg/dL or less) should remain in the hospital.
Five tips for using the new guidelines
Bilitool.org, a popular and useful app, has already been updated to reflect the changes in the new guidelines, making it easy to apply the new thresholds and create a follow-up plan for each patient.
The guidelines provide recommendations for when to check rebound bilirubin levels after stopping phototherapy (hint: babies with neurotoxic risk factors). A TcB device should not be used while the infant is being treated with phototherapy. However, a TcB can be measured once the baby has been off phototherapy for at least 24 hours.
If you have at least two bilirubin measurements, you can calculate the “rate of rise” in bilirubin level. A rapid rate of rise, which serves as a clinical indicator of hemolysis, is defined as ≥ 0.3 mg/dL per hour in the first 24 hours or ≥ 0.2 mg/dL per hour after the first 24 hours of life. This is especially helpful when hemolysis is suspected even if the newborn’s direct antibody test (DAT) is negative. In this scenario, the infant is considered to have a neurotoxic risk factor.
When you initiate phototherapy, be aware of the infant’s bilirubin level threshold for stopping phototherapy (2 mg/dL below the starting phototherapy threshold), as well as the threshold for escalation of care (2 mg/dL below the exchange transfusion threshold).
Because the thresholds for starting phototherapy and initiating exchange transfusion are slightly higher and specific to gestational age, clinicians can more confidently use less phototherapy.
Other guideline highlights
The neurotoxic risk factors and corresponding thresholds are important. If the newborn has one or more neurotoxic risk factors other than prematurity, the neurotoxic risk threshold graph should be used when assessing the need for treatment. Neurotoxic risk thresholds should also be used for newborns whose bilirubin levels continue rising on phototherapy.
The guidelines emphasize that G6PD is one of the most important causes of hazardous hyperbilirubinemia leading to kernicterus in the United States and worldwide. Overall, 13% of African American males and about 4% of African American females have G6PD deficiency.
Finally, the guidelines remind clinicians that an important way to reduce the chances that phototherapy will be needed is to encourage early and frequent feeding (8-12 times in 24 hours).
The AAP Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation contains a great deal more information, but these basic principles should allow practitioners to begin to incorporate these guidelines into daily practice.
Dr. Amaya is associate professor, department of pediatrics, Medical University of South Carolina, Charleston, and medical director, level 1 nursery, department of pediatrics, MUSC general academic pediatrics. She disclosed ties with Medical University of South Carolina. Dr. Balog is clinical associate professor of pediatrics, Medical University of South Carolina, Charleston. She has no relevant financial relationships. Dr. Basco is professor, department of pediatrics, Medical University of South Carolina, Charleston; director, division of general pediatrics, department of pediatrics, MUSC Children’s Hospital. He has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
More than 15 years in the making, the revised AAP Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation was released in 2022. A key driving force for this revision was the expanded evidence base regarding monitoring and treatment of newborns 35 or more weeks’ gestation to prevent bilirubin encephalopathy and kernicterus.
Here, we summarize the highlights of the new guidelines and point out practical ways to incorporate these guidelines into daily practice.
What has changed?
If you are familiar with the previous guidelines (2004 or the 2009 update) for the management of newborn jaundice, you’ll note that the treatment graphs for phototherapy and exchange transfusion have been updated with new, slightly higher thresholds.
Bilirubin thresholds for starting phototherapy are about 2 mg/dL higher overall than indicated in previous iterations of the guidelines.
This change reflects new evidence that infants don’t typically develop bilirubin neurotoxicity until the total serum bilirubin (TSB) reaches levels well above the previous exchange transfusion threshold, justifying a narrow increase in the bilirubin level for starting phototherapy. Also, phototherapy treatment thresholds are now risk-adjusted, with separate curves for each gestational age from 35 weeks to > 38 weeks.
To find the applicable phototherapy threshold, use the infant’s gestational age (rounding down) and determine whether the infant has even a single neurotoxicity risk factor other than prematurity. Neurotoxicity risk factors include a low albumin level, isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other hemolytic conditions; sepsis; or any significant clinical instability in the previous 24 hours.
For example, a 384/7 weeks’ gestation newborn has a TSB of 12 mg/dL at 48 hours of age but no neurotoxicity risk factors. Using the graph Phototherapy Thresholds: No Hyperbilirubinemia Neurotoxicity Risk Factors, should the infant be placed under phototherapy at this time? (Answer: No. The threshold for starting phototherapy on this infant is approximately 16 mg/dL.)
When hyperbilirubinemia becomes a medical emergency
A new term, “escalation of care,” has been adopted to describe actions to take when the newborn’s TSB climbs to within 2 mg/dL of the exchange transfusion threshold – a medical emergency. Instructions on how to ensure intensive phototherapy, and when to initiate an urgent exchange transfusion, are given, including the critical need to maintain intensive phototherapy continuously during infant transport and admission to another facility.
Transcutaneous vs. serum bilirubin
Either a serum TSB or a transcutaneous bilirubin (TcB) should be measured in all infants between 24 and 48 hours after birth or before discharge if that occurs earlier. TcB measurements are valid and reliable when used as a screening test to identify infants who require a TSB measurement. Although the two tests are generally correlated, they are not identical, and treatment decisions should be based on TSB levels. A TSB should be obtained if the TcB exceeds or is within 3 mg/dL of the phototherapy treatment threshold, or if the TcB is ≥ 15 mg/dL.
Following up: When to check another bilirubin level
Prior to these new guidelines, the question of when to get the next bilirubin level was based on Vinod Bhutani, MD’s risk nomogram, which classified newborn bilirubin levels within high-, intermediate-, or low-risk zones for needing phototherapy. A bilirubin level in the high-risk zone indicated the need for earlier follow-up. These risk zones have been replaced with a more specific table that provides recommended postdischarge follow-up based on how close the newborn’s bilirubin level is to the hour-specific threshold for treatment. The closer the latest TSB or TcB level is to the newborn’s risk-based phototherapy threshold, the sooner the follow-up to check another bilirubin level will need to be.
Most infants discharged before 72 hours of age will need follow-up within 2 days. Newborns with TSB levels nearing the level for phototherapy (within 2 mg/dL or less) should remain in the hospital.
Five tips for using the new guidelines
Bilitool.org, a popular and useful app, has already been updated to reflect the changes in the new guidelines, making it easy to apply the new thresholds and create a follow-up plan for each patient.
The guidelines provide recommendations for when to check rebound bilirubin levels after stopping phototherapy (hint: babies with neurotoxic risk factors). A TcB device should not be used while the infant is being treated with phototherapy. However, a TcB can be measured once the baby has been off phototherapy for at least 24 hours.
If you have at least two bilirubin measurements, you can calculate the “rate of rise” in bilirubin level. A rapid rate of rise, which serves as a clinical indicator of hemolysis, is defined as ≥ 0.3 mg/dL per hour in the first 24 hours or ≥ 0.2 mg/dL per hour after the first 24 hours of life. This is especially helpful when hemolysis is suspected even if the newborn’s direct antibody test (DAT) is negative. In this scenario, the infant is considered to have a neurotoxic risk factor.
When you initiate phototherapy, be aware of the infant’s bilirubin level threshold for stopping phototherapy (2 mg/dL below the starting phototherapy threshold), as well as the threshold for escalation of care (2 mg/dL below the exchange transfusion threshold).
Because the thresholds for starting phototherapy and initiating exchange transfusion are slightly higher and specific to gestational age, clinicians can more confidently use less phototherapy.
Other guideline highlights
The neurotoxic risk factors and corresponding thresholds are important. If the newborn has one or more neurotoxic risk factors other than prematurity, the neurotoxic risk threshold graph should be used when assessing the need for treatment. Neurotoxic risk thresholds should also be used for newborns whose bilirubin levels continue rising on phototherapy.
The guidelines emphasize that G6PD is one of the most important causes of hazardous hyperbilirubinemia leading to kernicterus in the United States and worldwide. Overall, 13% of African American males and about 4% of African American females have G6PD deficiency.
Finally, the guidelines remind clinicians that an important way to reduce the chances that phototherapy will be needed is to encourage early and frequent feeding (8-12 times in 24 hours).
The AAP Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation contains a great deal more information, but these basic principles should allow practitioners to begin to incorporate these guidelines into daily practice.
Dr. Amaya is associate professor, department of pediatrics, Medical University of South Carolina, Charleston, and medical director, level 1 nursery, department of pediatrics, MUSC general academic pediatrics. She disclosed ties with Medical University of South Carolina. Dr. Balog is clinical associate professor of pediatrics, Medical University of South Carolina, Charleston. She has no relevant financial relationships. Dr. Basco is professor, department of pediatrics, Medical University of South Carolina, Charleston; director, division of general pediatrics, department of pediatrics, MUSC Children’s Hospital. He has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Interventional psychiatry: What are the next steps?
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
Homelessness in urban areas: The role of mental illness and need for collaboration
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
More on an asymmetric life, transient global amnesia
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d