User login
Weight bias affects views of kids’ obesity recommendations
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Novel resuscitation for patients with nonshockable rhythms in cardiac arrest
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
75 years: A look back on the fascinating history of methotrexate and folate antagonists
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
My patient chose quality of life over treatment
Several decades ago, a new patient came to my office with her family. She was elderly, in good health, spoke no English, and her extended family translated for her. Their request: “Don’t tell her that she has cancer.” Sharing her diagnosis with her would cause too much stress, they said. Their mother would not be able to tolerate the bad news, they said. She would “give up.”
I asked her (through her family and an interpreter) how much she wanted to know about what was going on, or would she prefer I confine my remarks to her family? It turns out that she did want to know her diagnosis and prognosis, and after a thorough discussion in front of her family about her treatment options, she decided she did not want to proceed with additional therapy. She wanted to focus on quality of life. I did not get the impression that this is what her family would have opted for.
The patient’s voice can take multiple directions, such as making informed decisions about their own care. When empowered, patients can and will express their wants, needs, feelings, and priorities to their providers, and they’ll participate in directing their own care. There is a growing body of evidence that shows patients who are more engaged and share decision-making with their health care professionals have better health outcomes and care experiences. Engaged patients feel more empowered and are more motivated to take action. They’re also more likely to follow treatment plans, take their medications, and heed their provider’s recommendations. By virtue of better treatments for lung cancer, many patients are living longer and better lives. Some of these patients even become “experts” on their own care, often bringing questions about research and clinical trials to the attention of their providers.
The patient’s voice in research and advocacy
The patient’s perspective is also key to a meaningful, successful clinical research project. Rather than being carried out to, about, or for the patient, patient involvement means research being carried out with or by patients. A patient and researcher may have different research goals. For example, patients may value being able to work, be with family, and live without pain, whereas a clinical researcher’s goal may be inducing responses. Patient involvement is important in both laboratory research and clinical research. The best-designed projects involve patient advocates from the beginning of the project to help make research relevant and meaningful to patients and include these perspectives through project completion.
More and more pharmaceutical companies are actively involving patients at all levels of protocol development, including protocol design and selection of relevant outcomes to patients. Benefits of engaging patients as partners in research include inclusion of real-world data, increased study enrollment, and translation of results to the cancer community in an understandable and accessible manner.
Accelerated research
Advocating for accelerated research is another area where the patient’s voice is important. Patients can and do identify research priorities for researchers, funding agencies, and pharma. Patients who support research advocacy are frequently part of meetings and panel discussions with researchers, the Food and Drug Administration, and the National Cancer Institute. And, they serve on advisory boards for pharmaceutical companies. They participate in grant reviews and institutional review boards, review manuscripts, and are active members of the cooperative groups and other professional societies. In fact, patient-led advocacy groups are raising money to help fund research they feel is most important to them. In lung cancer, for example, there are many groups organized around biomarkers, including the EGFR Resisters, ALK Positive, ROS1ders, MET Crusaders, and KRAS Kickers, who have raised hundreds of thousands of dollars to fund investigator-led translational research that would not have occurred without their involvement.
It is important to recognize that all patients are different and have different values and motivations that are important to them and influence their life decisions. Some patients want to know more about their condition and their preferences should be respected. Similarly, it’s critical to understand that not every patient is an advocate and not every advocate is a research advocate. Research advocates have more in-depth knowledge about the science of lung cancer and focus on representing the patient perspective for all lung cancer patients.
So, getting back to my original story: Did my patient “give up” by choosing palliative care without chemotherapy? Perhaps, but I don’t think she considered her decision “giving up.” Instead, she made the best decision possible for herself. What would have happened had she not been told of her diagnosis? She probably would not have spent extra quality time with her family, as they tried to ignore the obvious. And, after all, quality time with her family was all she wanted.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation. Ivy Elkins, cofounder of EGFR Resisters, a patient, survivor, and caregiver advocacy group, contributed to this article.
Several decades ago, a new patient came to my office with her family. She was elderly, in good health, spoke no English, and her extended family translated for her. Their request: “Don’t tell her that she has cancer.” Sharing her diagnosis with her would cause too much stress, they said. Their mother would not be able to tolerate the bad news, they said. She would “give up.”
I asked her (through her family and an interpreter) how much she wanted to know about what was going on, or would she prefer I confine my remarks to her family? It turns out that she did want to know her diagnosis and prognosis, and after a thorough discussion in front of her family about her treatment options, she decided she did not want to proceed with additional therapy. She wanted to focus on quality of life. I did not get the impression that this is what her family would have opted for.
The patient’s voice can take multiple directions, such as making informed decisions about their own care. When empowered, patients can and will express their wants, needs, feelings, and priorities to their providers, and they’ll participate in directing their own care. There is a growing body of evidence that shows patients who are more engaged and share decision-making with their health care professionals have better health outcomes and care experiences. Engaged patients feel more empowered and are more motivated to take action. They’re also more likely to follow treatment plans, take their medications, and heed their provider’s recommendations. By virtue of better treatments for lung cancer, many patients are living longer and better lives. Some of these patients even become “experts” on their own care, often bringing questions about research and clinical trials to the attention of their providers.
The patient’s voice in research and advocacy
The patient’s perspective is also key to a meaningful, successful clinical research project. Rather than being carried out to, about, or for the patient, patient involvement means research being carried out with or by patients. A patient and researcher may have different research goals. For example, patients may value being able to work, be with family, and live without pain, whereas a clinical researcher’s goal may be inducing responses. Patient involvement is important in both laboratory research and clinical research. The best-designed projects involve patient advocates from the beginning of the project to help make research relevant and meaningful to patients and include these perspectives through project completion.
More and more pharmaceutical companies are actively involving patients at all levels of protocol development, including protocol design and selection of relevant outcomes to patients. Benefits of engaging patients as partners in research include inclusion of real-world data, increased study enrollment, and translation of results to the cancer community in an understandable and accessible manner.
Accelerated research
Advocating for accelerated research is another area where the patient’s voice is important. Patients can and do identify research priorities for researchers, funding agencies, and pharma. Patients who support research advocacy are frequently part of meetings and panel discussions with researchers, the Food and Drug Administration, and the National Cancer Institute. And, they serve on advisory boards for pharmaceutical companies. They participate in grant reviews and institutional review boards, review manuscripts, and are active members of the cooperative groups and other professional societies. In fact, patient-led advocacy groups are raising money to help fund research they feel is most important to them. In lung cancer, for example, there are many groups organized around biomarkers, including the EGFR Resisters, ALK Positive, ROS1ders, MET Crusaders, and KRAS Kickers, who have raised hundreds of thousands of dollars to fund investigator-led translational research that would not have occurred without their involvement.
It is important to recognize that all patients are different and have different values and motivations that are important to them and influence their life decisions. Some patients want to know more about their condition and their preferences should be respected. Similarly, it’s critical to understand that not every patient is an advocate and not every advocate is a research advocate. Research advocates have more in-depth knowledge about the science of lung cancer and focus on representing the patient perspective for all lung cancer patients.
So, getting back to my original story: Did my patient “give up” by choosing palliative care without chemotherapy? Perhaps, but I don’t think she considered her decision “giving up.” Instead, she made the best decision possible for herself. What would have happened had she not been told of her diagnosis? She probably would not have spent extra quality time with her family, as they tried to ignore the obvious. And, after all, quality time with her family was all she wanted.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation. Ivy Elkins, cofounder of EGFR Resisters, a patient, survivor, and caregiver advocacy group, contributed to this article.
Several decades ago, a new patient came to my office with her family. She was elderly, in good health, spoke no English, and her extended family translated for her. Their request: “Don’t tell her that she has cancer.” Sharing her diagnosis with her would cause too much stress, they said. Their mother would not be able to tolerate the bad news, they said. She would “give up.”
I asked her (through her family and an interpreter) how much she wanted to know about what was going on, or would she prefer I confine my remarks to her family? It turns out that she did want to know her diagnosis and prognosis, and after a thorough discussion in front of her family about her treatment options, she decided she did not want to proceed with additional therapy. She wanted to focus on quality of life. I did not get the impression that this is what her family would have opted for.
The patient’s voice can take multiple directions, such as making informed decisions about their own care. When empowered, patients can and will express their wants, needs, feelings, and priorities to their providers, and they’ll participate in directing their own care. There is a growing body of evidence that shows patients who are more engaged and share decision-making with their health care professionals have better health outcomes and care experiences. Engaged patients feel more empowered and are more motivated to take action. They’re also more likely to follow treatment plans, take their medications, and heed their provider’s recommendations. By virtue of better treatments for lung cancer, many patients are living longer and better lives. Some of these patients even become “experts” on their own care, often bringing questions about research and clinical trials to the attention of their providers.
The patient’s voice in research and advocacy
The patient’s perspective is also key to a meaningful, successful clinical research project. Rather than being carried out to, about, or for the patient, patient involvement means research being carried out with or by patients. A patient and researcher may have different research goals. For example, patients may value being able to work, be with family, and live without pain, whereas a clinical researcher’s goal may be inducing responses. Patient involvement is important in both laboratory research and clinical research. The best-designed projects involve patient advocates from the beginning of the project to help make research relevant and meaningful to patients and include these perspectives through project completion.
More and more pharmaceutical companies are actively involving patients at all levels of protocol development, including protocol design and selection of relevant outcomes to patients. Benefits of engaging patients as partners in research include inclusion of real-world data, increased study enrollment, and translation of results to the cancer community in an understandable and accessible manner.
Accelerated research
Advocating for accelerated research is another area where the patient’s voice is important. Patients can and do identify research priorities for researchers, funding agencies, and pharma. Patients who support research advocacy are frequently part of meetings and panel discussions with researchers, the Food and Drug Administration, and the National Cancer Institute. And, they serve on advisory boards for pharmaceutical companies. They participate in grant reviews and institutional review boards, review manuscripts, and are active members of the cooperative groups and other professional societies. In fact, patient-led advocacy groups are raising money to help fund research they feel is most important to them. In lung cancer, for example, there are many groups organized around biomarkers, including the EGFR Resisters, ALK Positive, ROS1ders, MET Crusaders, and KRAS Kickers, who have raised hundreds of thousands of dollars to fund investigator-led translational research that would not have occurred without their involvement.
It is important to recognize that all patients are different and have different values and motivations that are important to them and influence their life decisions. Some patients want to know more about their condition and their preferences should be respected. Similarly, it’s critical to understand that not every patient is an advocate and not every advocate is a research advocate. Research advocates have more in-depth knowledge about the science of lung cancer and focus on representing the patient perspective for all lung cancer patients.
So, getting back to my original story: Did my patient “give up” by choosing palliative care without chemotherapy? Perhaps, but I don’t think she considered her decision “giving up.” Instead, she made the best decision possible for herself. What would have happened had she not been told of her diagnosis? She probably would not have spent extra quality time with her family, as they tried to ignore the obvious. And, after all, quality time with her family was all she wanted.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation. Ivy Elkins, cofounder of EGFR Resisters, a patient, survivor, and caregiver advocacy group, contributed to this article.
If we care about cancer patients, we must care about climate change
Because we care about our patients, we need to get involved in the climate change movement. If we want to help prevent cancer and deliver the best possible care to our patients, we need to stop burning fossil fuels. As addressed in an earlier version of this column, burning fossil fuels results in the release of particulate matter and particles measuring 2.5 micrometers in diameter (PM2.5), are classified as group 1 carcinogens by the International Association of Research and Cancer.
Fossil fuels also release greenhouse gases (carbon dioxide, methane, nitrous oxide, and fluorinated gases) which trap solar radiation that would otherwise have been reflected back into space after hitting the earth’s surface. Instead, it is redirected back to earth as infrared radiation warming the planet by 1.1° C since preindustrial times.
Climate change has a number of consequences, including more extreme weather events, rising sea levels, warming seas, environmental degradation, and affects water and food quality, supply, and production. A global increase of 1.5° C above the preindustrial average risks catastrophic harm to health that will be impossible to reverse, prompting the editors of over 260 health journals to call for emergency action to limit global temperature increases, restore biodiversity, and protect health.
In October, the 2022 version of the Lancet Countdown on health and climate change was issued and the findings are not good. “After 30 years of UNFCCC negotiations, the Lancet Countdown indicators show that countries and companies continue to make choices that threaten the health and survival of people in every part of the world. As countries devise ways to recover from the coexisting crises, the evidence is unequivocal. At this critical juncture, an immediate, health-centered response can still secure a future in which world populations can not only survive, but thrive,” the authors wrote. Governments and companies continue to prioritize fossil fuels over people’s health.
Among the key findings from the report, Marina Romanello, PhD, of the Institute for Global Health at University College London, and her colleagues, call for “A health-centered response to the coexisting climate, energy, and cost-of-living crises provides an opportunity to deliver a healthy, low-carbon future. The associated reduction in the burden of disease will in turn reduce the strain on overwhelmed health care providers, and enable better care.”
The authors also state that “Well-prepared health systems are essential to protect populations from the health impacts of climate change. However, global health systems have been drastically weakened by the effects of the COVID-19 pandemic, and the funds available for climate action decreased in 239 (30%) of 798 cities, with health systems increasingly being affected by extreme weather events and supply chain disruptions.”
And, the authors are concerned that health systems have left themselves vulnerable to climate change–related health hazards because they have not adapted their operations for climate-related changes. “Only 48 of 95 countries have assessed their climate change adaptation needs and only 63% of countries reported high to very high implementation status for health emergency management in 2021. Increasing adaptation to climate change has the potential to simultaneously improve the capacity of health systems to manage both future infectious disease outbreaks and other health emergencies.”
There is roughly a 50% chance that the 1.5° C threshold proposed in the Paris Agreement will be exceeded within 5 years. The carbon intensity of the global energy system has been reduced by less than 1% from 1992 levels, when the United Nations Framework Convention on Climate Change was adopted. At our current pace, global emissions could be 13.7% above 2010 levels by 2030 and fully decarbonizing the energy system would take 150 years. Clearly, we are nowhere near meeting the goals of the Paris Agreement signed in 2015 by 192 countries and the European Union. Participants pledged to decrease their carbon footprint by 50% by 2030, and net zero by the end of the century.
The effect of increasing greenhouse gases in our atmosphere will have a massive impact on the prevention and care of cancer patients. Air pollution is responsible for about 14% of lung cancer deaths throughout the world. Rising temperatures lead to extreme weather events which disrupts infrastructure and the ability to access health care, leading to delays in treatment, increased morbidity, and death. Screening rates for cancer go down, which leads to more patients presenting with advanced cancer in the future.
As oncologists who care deeply about their patients, we need to get actively involved. It is our responsibility to our current and future patients to do whatever we can to prevent cancer and reduce its complications.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Because we care about our patients, we need to get involved in the climate change movement. If we want to help prevent cancer and deliver the best possible care to our patients, we need to stop burning fossil fuels. As addressed in an earlier version of this column, burning fossil fuels results in the release of particulate matter and particles measuring 2.5 micrometers in diameter (PM2.5), are classified as group 1 carcinogens by the International Association of Research and Cancer.
Fossil fuels also release greenhouse gases (carbon dioxide, methane, nitrous oxide, and fluorinated gases) which trap solar radiation that would otherwise have been reflected back into space after hitting the earth’s surface. Instead, it is redirected back to earth as infrared radiation warming the planet by 1.1° C since preindustrial times.
Climate change has a number of consequences, including more extreme weather events, rising sea levels, warming seas, environmental degradation, and affects water and food quality, supply, and production. A global increase of 1.5° C above the preindustrial average risks catastrophic harm to health that will be impossible to reverse, prompting the editors of over 260 health journals to call for emergency action to limit global temperature increases, restore biodiversity, and protect health.
In October, the 2022 version of the Lancet Countdown on health and climate change was issued and the findings are not good. “After 30 years of UNFCCC negotiations, the Lancet Countdown indicators show that countries and companies continue to make choices that threaten the health and survival of people in every part of the world. As countries devise ways to recover from the coexisting crises, the evidence is unequivocal. At this critical juncture, an immediate, health-centered response can still secure a future in which world populations can not only survive, but thrive,” the authors wrote. Governments and companies continue to prioritize fossil fuels over people’s health.
Among the key findings from the report, Marina Romanello, PhD, of the Institute for Global Health at University College London, and her colleagues, call for “A health-centered response to the coexisting climate, energy, and cost-of-living crises provides an opportunity to deliver a healthy, low-carbon future. The associated reduction in the burden of disease will in turn reduce the strain on overwhelmed health care providers, and enable better care.”
The authors also state that “Well-prepared health systems are essential to protect populations from the health impacts of climate change. However, global health systems have been drastically weakened by the effects of the COVID-19 pandemic, and the funds available for climate action decreased in 239 (30%) of 798 cities, with health systems increasingly being affected by extreme weather events and supply chain disruptions.”
And, the authors are concerned that health systems have left themselves vulnerable to climate change–related health hazards because they have not adapted their operations for climate-related changes. “Only 48 of 95 countries have assessed their climate change adaptation needs and only 63% of countries reported high to very high implementation status for health emergency management in 2021. Increasing adaptation to climate change has the potential to simultaneously improve the capacity of health systems to manage both future infectious disease outbreaks and other health emergencies.”
There is roughly a 50% chance that the 1.5° C threshold proposed in the Paris Agreement will be exceeded within 5 years. The carbon intensity of the global energy system has been reduced by less than 1% from 1992 levels, when the United Nations Framework Convention on Climate Change was adopted. At our current pace, global emissions could be 13.7% above 2010 levels by 2030 and fully decarbonizing the energy system would take 150 years. Clearly, we are nowhere near meeting the goals of the Paris Agreement signed in 2015 by 192 countries and the European Union. Participants pledged to decrease their carbon footprint by 50% by 2030, and net zero by the end of the century.
The effect of increasing greenhouse gases in our atmosphere will have a massive impact on the prevention and care of cancer patients. Air pollution is responsible for about 14% of lung cancer deaths throughout the world. Rising temperatures lead to extreme weather events which disrupts infrastructure and the ability to access health care, leading to delays in treatment, increased morbidity, and death. Screening rates for cancer go down, which leads to more patients presenting with advanced cancer in the future.
As oncologists who care deeply about their patients, we need to get actively involved. It is our responsibility to our current and future patients to do whatever we can to prevent cancer and reduce its complications.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Because we care about our patients, we need to get involved in the climate change movement. If we want to help prevent cancer and deliver the best possible care to our patients, we need to stop burning fossil fuels. As addressed in an earlier version of this column, burning fossil fuels results in the release of particulate matter and particles measuring 2.5 micrometers in diameter (PM2.5), are classified as group 1 carcinogens by the International Association of Research and Cancer.
Fossil fuels also release greenhouse gases (carbon dioxide, methane, nitrous oxide, and fluorinated gases) which trap solar radiation that would otherwise have been reflected back into space after hitting the earth’s surface. Instead, it is redirected back to earth as infrared radiation warming the planet by 1.1° C since preindustrial times.
Climate change has a number of consequences, including more extreme weather events, rising sea levels, warming seas, environmental degradation, and affects water and food quality, supply, and production. A global increase of 1.5° C above the preindustrial average risks catastrophic harm to health that will be impossible to reverse, prompting the editors of over 260 health journals to call for emergency action to limit global temperature increases, restore biodiversity, and protect health.
In October, the 2022 version of the Lancet Countdown on health and climate change was issued and the findings are not good. “After 30 years of UNFCCC negotiations, the Lancet Countdown indicators show that countries and companies continue to make choices that threaten the health and survival of people in every part of the world. As countries devise ways to recover from the coexisting crises, the evidence is unequivocal. At this critical juncture, an immediate, health-centered response can still secure a future in which world populations can not only survive, but thrive,” the authors wrote. Governments and companies continue to prioritize fossil fuels over people’s health.
Among the key findings from the report, Marina Romanello, PhD, of the Institute for Global Health at University College London, and her colleagues, call for “A health-centered response to the coexisting climate, energy, and cost-of-living crises provides an opportunity to deliver a healthy, low-carbon future. The associated reduction in the burden of disease will in turn reduce the strain on overwhelmed health care providers, and enable better care.”
The authors also state that “Well-prepared health systems are essential to protect populations from the health impacts of climate change. However, global health systems have been drastically weakened by the effects of the COVID-19 pandemic, and the funds available for climate action decreased in 239 (30%) of 798 cities, with health systems increasingly being affected by extreme weather events and supply chain disruptions.”
And, the authors are concerned that health systems have left themselves vulnerable to climate change–related health hazards because they have not adapted their operations for climate-related changes. “Only 48 of 95 countries have assessed their climate change adaptation needs and only 63% of countries reported high to very high implementation status for health emergency management in 2021. Increasing adaptation to climate change has the potential to simultaneously improve the capacity of health systems to manage both future infectious disease outbreaks and other health emergencies.”
There is roughly a 50% chance that the 1.5° C threshold proposed in the Paris Agreement will be exceeded within 5 years. The carbon intensity of the global energy system has been reduced by less than 1% from 1992 levels, when the United Nations Framework Convention on Climate Change was adopted. At our current pace, global emissions could be 13.7% above 2010 levels by 2030 and fully decarbonizing the energy system would take 150 years. Clearly, we are nowhere near meeting the goals of the Paris Agreement signed in 2015 by 192 countries and the European Union. Participants pledged to decrease their carbon footprint by 50% by 2030, and net zero by the end of the century.
The effect of increasing greenhouse gases in our atmosphere will have a massive impact on the prevention and care of cancer patients. Air pollution is responsible for about 14% of lung cancer deaths throughout the world. Rising temperatures lead to extreme weather events which disrupts infrastructure and the ability to access health care, leading to delays in treatment, increased morbidity, and death. Screening rates for cancer go down, which leads to more patients presenting with advanced cancer in the future.
As oncologists who care deeply about their patients, we need to get actively involved. It is our responsibility to our current and future patients to do whatever we can to prevent cancer and reduce its complications.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Shared decision-making (when you’re wearing the paper gown)
I offer screening mammograms to my patients starting at age 40. I have developed a little script to explain that I recommend them routinely by age 50, but at younger ages, individual decision-making is required because the science to support breast cancer screening has more tradeoffs in younger patients.1 Some patients have questions; many immediately know their preferences.
For me, personally, I felt comfortable waiting until sometime after age 40 to start screening. I have a reassuring family history; my mother has 5 sisters, without any breast or ovarian cancer among them. When, in my mid-40s, I told a doctor that I preferred to wait until I was closer to age 50 to get a mammogram, she urged me to begin screening immediately. Even as a physician, the drive to be a “good patient” was strong. I made the mammogram appointment.
Like many patients, my first mammogram was not normal.2,3 After a second round of tests, and then a third, the radiologist gave me the results: Everything is fine. It is just normal breast tissue. To be on the safe side, you should do a follow-up mammogram and ultrasound in 6 months.
I asked why I needed to do follow-up imaging if the only thing that multiple diagnostic tests had shown was normal tissue—not a cyst, nor a fibroadenoma or any other abnormality.
“Well, do it, don’t do it, but I recommend it,” the radiologist said. The conversation was over.
My experience as a patient came to mind when I read this month’s article on shared decision-making by Mackwood et al.4 The authors discuss principles and techniques for shared decision-making in practice, which include enlisting the patient as the expert in their own values, and putting forth the health care professional as a source of reliable information when the evidence supports more than one reasonable strategy in a health care decision.
Aligning values, science, and action can be challenging, to be sure. It can be made easier through long-term relationships, such as the ones that family physicians have with their patients. One of the benefits of longitudinal practice is coming to know what our patients prefer instead of having to start from scratch with each visit. The belief that our values will be mutually respected is part of what builds trust in a doctor–patient relationship. We can use tools to support information delivery at the patient’s health literacy level to make the science more understandable. This in turn makes it easier for patients to integrate the science into their own value system.
Continue to: One of the most critical...
One of the most critical aspects of shared decision-making is also one of the hardest. As physicians, we need to be comfortable with a patient making a choice that we might not make ourselves. Perhaps we would choose to observe an otitis media in our own afebrile 6-year-old, or maybe we would not opt for semaglutide to treat our own obesity. Patients can have a different set of values and experiences driving their decision-making. The principles of shared decision-making teach us that our training and experience are not the priority in every situation.
In my case, the radiologist may have assumed that because I had gone through all of the testing, I believed that screening did far more good than harm and that I would be back in 6 months. From my point of view, I saw the screening as more of a mixed bag; it was possibly doing good, but at the risk of doing harm with false-positives and the possibility of overdiagnosis. She also may have been pressed for time and not had any available point-of-care tools to help explain her decision-making process. I left without understanding what the evidence was for close-interval follow-up, let alone having a chance to share in the decision-making process.
Shared decision-making and evidence-based medicine are closely connected concepts; the decision rests on the evidence, and the evidence cannot be applied to patients without asking their perspectives.5 Mackwood et al4 point out that shared decision-making can be implemented with little to no increase in the time we spend with patients, and at no substantial increase in costs of care.
Shared decision-making is a skill. Like any skill, the more we practice, the more capable we will become with it. And frankly, it doesn’t hurt to remember how we’ve felt when we’ve been the patient wearing that paper gown.
1. USPSTF. Breast cancer screening. Accessed January 6, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
2. Rauscher GH, Murphy AM, Qiu Q, et al. The “sweet spot” revisited: optimal recall rates for cancer detection with 2D and 3D digital screening mammography in the Metro Chicago Breast Cancer Registry. AJR Am J Roentgenol. 2021;216:894-902. doi: 10.2214/AJR.19.22429
3. Sumkin JH, Ganott MA, Chough DM, et al. Recall rate reduction with tomosynthesis during baseline screening examinations: an assessment from a prospective trial. Acad Radiol. 2015;22:1477-1482. doi: 10.1016/j.acra.2015.08.015
4. Mackwood MB, Imset I, Morrow C. How to integrate shared decision-making into your practice. J Fam Pract. 2023;72:7-17. doi: 10.12788/jfp.0536
5. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312:1295-1296. doi: 10.1001/jama.2014.10186
I offer screening mammograms to my patients starting at age 40. I have developed a little script to explain that I recommend them routinely by age 50, but at younger ages, individual decision-making is required because the science to support breast cancer screening has more tradeoffs in younger patients.1 Some patients have questions; many immediately know their preferences.
For me, personally, I felt comfortable waiting until sometime after age 40 to start screening. I have a reassuring family history; my mother has 5 sisters, without any breast or ovarian cancer among them. When, in my mid-40s, I told a doctor that I preferred to wait until I was closer to age 50 to get a mammogram, she urged me to begin screening immediately. Even as a physician, the drive to be a “good patient” was strong. I made the mammogram appointment.
Like many patients, my first mammogram was not normal.2,3 After a second round of tests, and then a third, the radiologist gave me the results: Everything is fine. It is just normal breast tissue. To be on the safe side, you should do a follow-up mammogram and ultrasound in 6 months.
I asked why I needed to do follow-up imaging if the only thing that multiple diagnostic tests had shown was normal tissue—not a cyst, nor a fibroadenoma or any other abnormality.
“Well, do it, don’t do it, but I recommend it,” the radiologist said. The conversation was over.
My experience as a patient came to mind when I read this month’s article on shared decision-making by Mackwood et al.4 The authors discuss principles and techniques for shared decision-making in practice, which include enlisting the patient as the expert in their own values, and putting forth the health care professional as a source of reliable information when the evidence supports more than one reasonable strategy in a health care decision.
Aligning values, science, and action can be challenging, to be sure. It can be made easier through long-term relationships, such as the ones that family physicians have with their patients. One of the benefits of longitudinal practice is coming to know what our patients prefer instead of having to start from scratch with each visit. The belief that our values will be mutually respected is part of what builds trust in a doctor–patient relationship. We can use tools to support information delivery at the patient’s health literacy level to make the science more understandable. This in turn makes it easier for patients to integrate the science into their own value system.
Continue to: One of the most critical...
One of the most critical aspects of shared decision-making is also one of the hardest. As physicians, we need to be comfortable with a patient making a choice that we might not make ourselves. Perhaps we would choose to observe an otitis media in our own afebrile 6-year-old, or maybe we would not opt for semaglutide to treat our own obesity. Patients can have a different set of values and experiences driving their decision-making. The principles of shared decision-making teach us that our training and experience are not the priority in every situation.
In my case, the radiologist may have assumed that because I had gone through all of the testing, I believed that screening did far more good than harm and that I would be back in 6 months. From my point of view, I saw the screening as more of a mixed bag; it was possibly doing good, but at the risk of doing harm with false-positives and the possibility of overdiagnosis. She also may have been pressed for time and not had any available point-of-care tools to help explain her decision-making process. I left without understanding what the evidence was for close-interval follow-up, let alone having a chance to share in the decision-making process.
Shared decision-making and evidence-based medicine are closely connected concepts; the decision rests on the evidence, and the evidence cannot be applied to patients without asking their perspectives.5 Mackwood et al4 point out that shared decision-making can be implemented with little to no increase in the time we spend with patients, and at no substantial increase in costs of care.
Shared decision-making is a skill. Like any skill, the more we practice, the more capable we will become with it. And frankly, it doesn’t hurt to remember how we’ve felt when we’ve been the patient wearing that paper gown.
I offer screening mammograms to my patients starting at age 40. I have developed a little script to explain that I recommend them routinely by age 50, but at younger ages, individual decision-making is required because the science to support breast cancer screening has more tradeoffs in younger patients.1 Some patients have questions; many immediately know their preferences.
For me, personally, I felt comfortable waiting until sometime after age 40 to start screening. I have a reassuring family history; my mother has 5 sisters, without any breast or ovarian cancer among them. When, in my mid-40s, I told a doctor that I preferred to wait until I was closer to age 50 to get a mammogram, she urged me to begin screening immediately. Even as a physician, the drive to be a “good patient” was strong. I made the mammogram appointment.
Like many patients, my first mammogram was not normal.2,3 After a second round of tests, and then a third, the radiologist gave me the results: Everything is fine. It is just normal breast tissue. To be on the safe side, you should do a follow-up mammogram and ultrasound in 6 months.
I asked why I needed to do follow-up imaging if the only thing that multiple diagnostic tests had shown was normal tissue—not a cyst, nor a fibroadenoma or any other abnormality.
“Well, do it, don’t do it, but I recommend it,” the radiologist said. The conversation was over.
My experience as a patient came to mind when I read this month’s article on shared decision-making by Mackwood et al.4 The authors discuss principles and techniques for shared decision-making in practice, which include enlisting the patient as the expert in their own values, and putting forth the health care professional as a source of reliable information when the evidence supports more than one reasonable strategy in a health care decision.
Aligning values, science, and action can be challenging, to be sure. It can be made easier through long-term relationships, such as the ones that family physicians have with their patients. One of the benefits of longitudinal practice is coming to know what our patients prefer instead of having to start from scratch with each visit. The belief that our values will be mutually respected is part of what builds trust in a doctor–patient relationship. We can use tools to support information delivery at the patient’s health literacy level to make the science more understandable. This in turn makes it easier for patients to integrate the science into their own value system.
Continue to: One of the most critical...
One of the most critical aspects of shared decision-making is also one of the hardest. As physicians, we need to be comfortable with a patient making a choice that we might not make ourselves. Perhaps we would choose to observe an otitis media in our own afebrile 6-year-old, or maybe we would not opt for semaglutide to treat our own obesity. Patients can have a different set of values and experiences driving their decision-making. The principles of shared decision-making teach us that our training and experience are not the priority in every situation.
In my case, the radiologist may have assumed that because I had gone through all of the testing, I believed that screening did far more good than harm and that I would be back in 6 months. From my point of view, I saw the screening as more of a mixed bag; it was possibly doing good, but at the risk of doing harm with false-positives and the possibility of overdiagnosis. She also may have been pressed for time and not had any available point-of-care tools to help explain her decision-making process. I left without understanding what the evidence was for close-interval follow-up, let alone having a chance to share in the decision-making process.
Shared decision-making and evidence-based medicine are closely connected concepts; the decision rests on the evidence, and the evidence cannot be applied to patients without asking their perspectives.5 Mackwood et al4 point out that shared decision-making can be implemented with little to no increase in the time we spend with patients, and at no substantial increase in costs of care.
Shared decision-making is a skill. Like any skill, the more we practice, the more capable we will become with it. And frankly, it doesn’t hurt to remember how we’ve felt when we’ve been the patient wearing that paper gown.
1. USPSTF. Breast cancer screening. Accessed January 6, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
2. Rauscher GH, Murphy AM, Qiu Q, et al. The “sweet spot” revisited: optimal recall rates for cancer detection with 2D and 3D digital screening mammography in the Metro Chicago Breast Cancer Registry. AJR Am J Roentgenol. 2021;216:894-902. doi: 10.2214/AJR.19.22429
3. Sumkin JH, Ganott MA, Chough DM, et al. Recall rate reduction with tomosynthesis during baseline screening examinations: an assessment from a prospective trial. Acad Radiol. 2015;22:1477-1482. doi: 10.1016/j.acra.2015.08.015
4. Mackwood MB, Imset I, Morrow C. How to integrate shared decision-making into your practice. J Fam Pract. 2023;72:7-17. doi: 10.12788/jfp.0536
5. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312:1295-1296. doi: 10.1001/jama.2014.10186
1. USPSTF. Breast cancer screening. Accessed January 6, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
2. Rauscher GH, Murphy AM, Qiu Q, et al. The “sweet spot” revisited: optimal recall rates for cancer detection with 2D and 3D digital screening mammography in the Metro Chicago Breast Cancer Registry. AJR Am J Roentgenol. 2021;216:894-902. doi: 10.2214/AJR.19.22429
3. Sumkin JH, Ganott MA, Chough DM, et al. Recall rate reduction with tomosynthesis during baseline screening examinations: an assessment from a prospective trial. Acad Radiol. 2015;22:1477-1482. doi: 10.1016/j.acra.2015.08.015
4. Mackwood MB, Imset I, Morrow C. How to integrate shared decision-making into your practice. J Fam Pract. 2023;72:7-17. doi: 10.12788/jfp.0536
5. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312:1295-1296. doi: 10.1001/jama.2014.10186
Preoperative preparation for gender-affirming vaginoplasty surgery
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
The field of gender-affirming surgery is one of the fastest growing surgical specialties in the country. Within the last few years, the number of procedures has increased markedly – with a total of 16,353 performed in 2020 compared with 8,304 in 2017.1,2 As the number of surgeries increases, so does the need for a standardized approach to preoperative evaluation and patient preparation.
Gender-affirming genital surgery for transfeminine individuals encompasses a spectrum of procedures that includes removal of the testicles (orchiectomy), creation of a neovaginal canal (full-depth vaginoplasty), and creation of external vulvar structures without a vaginal canal (zero-depth vaginoplasty). Each of these requires different levels of preoperative preparedness and medical optimization, and has unique postoperative challenges. Often, these postoperative complications can be mitigated with adequate patient education.
Many centers that offer genital gender-affirming surgery have a multidisciplinary team composed of a social worker, mental health providers, care coordinators, primary care providers, and surgeons. This team is essential to providing supportive services within their respective scope of practices.
The role of the mental health provider cannot be understated. While the updated standards of care from the World Professional Association for Transgender Health no longer require two letters from mental health providers prior to genital surgery, it is important to recognize that many insurance companies have not yet updated their policies and still require two letters. Even when insurance companies adjust their policies to reflect current standards, a mental health assessment is still necessary to determine if patients have any mental health issues that could negatively affect their surgical outcome.3 Furthermore, a continued relationship with a mental health provider is beneficial for patients as they go through a stressful and life-changing procedure.4
As with any surgery, understanding patient goals and expectations is a key element in achieving optimal patient satisfaction. Patients with high esthetic or functional expectations experience higher rates of disappointment after surgery and have more difficulty coping with complications.5
Decisions about proceeding with a particular type of genital surgery should consider a patient’s desire to have vaginal-receptive intercourse, their commitment to dilation, financial stability, a safe environment for recovery, a support network, and the ability to understand and cope with potential complications.4 Patients will present with a wide variety of educational backgrounds and medical literacy, and will have differing intellectual capabilities.4 Consultations should take into account potential challenges these factors may play in patients’ ability to understand this complex surgery.
An adequate amount of time should be allotted to addressing these challenges. In my practice, a consultation for a gender-affirming genital surgery takes approximately 60 minutes. A preoperative packet with information is mailed to the patient ahead of time that will be reviewed at the time of the visit. During the consultation, I utilize a visual presentation that details the preoperative requirements and different types of surgical procedures, shows preoperative and postoperative surgical results, and discusses potential complications. Before the consultation, I advise that patients bring a support person (ideally the person who will assist in postoperative care) and a list of questions that they may have.
Both full- and shallow-depth procedures are reviewed at the time of initial consultation. For patients who seek a full-depth vaginoplasty procedure, it is important to determine whether patients are committed to dilation and have a safe, supportive environment to do so. Patients may have physical limitations, such as obesity or mobility issues, that could make dilation difficult or even impossible. Patients may not have stable housing, may experience financial restrictions that would impede their ability to purchase necessary supplies, and lack a support person who can care for them in the immediate postoperative period. Many patients are unaware of the importance these social factors play in a successful outcome. Social workers and care coordinators are important resources when these challenges are encountered.
Medical optimization is not unlike other gynecologic procedures with a few exceptions. Obesity, diabetes, and smoking play larger roles in surgical complications than in other surgeries as vaginoplasty techniques use pedicled flaps that rely on adequate blood supply. Obesity, poorly controlled diabetes, and smoking are associated with increased rates of wound infection, poor wound healing, and graft loss. Smoking cessation for 8 weeks prior to surgery and for 4 weeks afterward is mandatory.
For patients with a history of smoking, a nicotine test is performed within 4 weeks of surgery. Many surgeons have body mass index requirements, typically ranging between 20 and 30 kg/m2, despite limited data. This paradigm is shifting to consider body fat distribution rather than BMI alone. Extensive body fat in the mons or groin area can increase the difficulty of pelvic floor dissection during surgery and impede visualization for dilation in the postoperative period. There are reports of patients dilating into their rectum or neourethra, which can have catastrophic consequences. For these patients, a zero-depth vaginoplasty or orchiectomy may initially be a safer option.
Many patients are justifiably excited to undergo the procedures as quality of life is typically improved after surgery. However, even with adequate counseling, many patients often underestimate the extensive recovery process. This surgical procedure requires extensive planning and adequate resources.4 Patients must be able to take off from work for prolonged periods of time (typically 6 weeks), which can serve as a source of financial stress. To maintain the integrity of suture lines in the genital region, prolonged or limited mobilization is recommended. This can create boredom and forces patients to rely on a caregiver for activities of daily living, such as household chores, cooking meals, and transportation.
Gender-affirming genital surgery is not only a complex surgical procedure but also requires extensive preoperative education and postoperative support. As this field continues to grow, patients, providers, and caregivers should work toward further developing a collaborative care model to optimize surgical outcomes and patient satisfaction.
Dr. Brandt is an ob.gyn. and fellowship-trained gender affirming surgeon in West Reading, Pa.
References
1. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2020.
2. American Society of Plastic Surgeons. Plastic Surgery Statistics Report–2017.
3. Coleman E et al. Standards of care for the health of transgender and gender diverse people. Version 8. Int J Transgender Health. 23(S1):S1-S258. doi :10.1080/26895269.2022.2100644.
4. Penkin A et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:37-44.
5. Waljee J et al. Surgery. 2014;155:799-808.
Is it time to unionize?
According to an article in the Wall Street Journal (Mosbergen D. 2023 Jan 16), physicians-in-training in several parts of the country are attempting to unionize. The Committee of Interns and Residents (CIR), a union representing about 15% of the 140,000 residents and fellows in the United States reports that it has been adding chapters at an accelerated rate since the pandemic began.
Most of the 1,400 residents at Palo Alto–based Stanford Medicine recently voted to unionize seeking better compensation and improved working conditions including more accommodations for residents with disabilities or who are breastfeeding. At the University of Illinois at Chicago, house officers are also exploring an association with CIR hoping that collective bargaining might help them get “better pay and working conditions that could alleviate some burnout and stress.”
Although physicians have been hesitant to organize themselves around workplace concerns, nurses have a more robust history of unionizing and taking action. Recently, the nurses at two of New York’s largest medical centers went on a strike for 3 days that ended after the medical centers agreed to their primary demand of hiring more nurses and committing to set more workable nurse to patient ratios.
In an unusual but historic incident of workplace activism, the residents and interns at the then notoriously decrepit Boston City Hospital staged a “heal-in” in 1967 during which they admitted more patients (all with legitimate conditions for admission) than the hospital could handle. While more pay was included in their demands (interns were being paid $3,600/year and senior residents $7,500/year), the primary complaint of the house officers focused on patient health and safety issues. The crisis this work action triggered finally brought into sharp focus the city’s failure to care for its most needy citizens and over time, changes have been made (TIME Magazine. U.S. edition. Jun 21;91:25).
Having spent some time at the Boston City Hospital as a medical student in the 1960s I can attest to the deplorable conditions. While you might not be washing your hands and instruments in rusty sinks or having to brush flaking paint off your patients’ cribs, you may be experiencing working conditions that are threatening the health and safety of you, your coworkers, and not least of all your patients. Staffing shortages, clunky electronic health record systems that are adding hours of work to your day, screen after screen of data entry tasks that prevent you from meeting your patients eye-to-eye, and piles of prior authorization requests clogging your inbox to name just a few.
Who can you complain to, other than your coworkers? The patients brought their problems to you; it doesn’t seem fair to add to their burden by sharing your own. Maybe it’s time to think about joining a union to strengthen your voice and create some change.
But “union” and “strike” don’t sound very professional and certainly not coming from the mouths of folks who have chosen to be caregivers. However, things have changed. Most of us are employees now. We need to finally accept that role and begin acting like employees working under stressful and unhealthy conditions. Does the word “burnout” make the notion of unionizing any more palatable?
The American Medical Association’s code of ethics wisely discourages physicians from engaging in actions that withhold medical care. However, the Boston City Hospital house officers provided just one example of a work action that can draw attention to the problem while still providing care to the patients in our trust.
Simply, joining our voices can be a powerful force in the war of words and images. Patients don’t like the impersonalization that has come with the current crop of electronic health record systems and the tortuous phone trees they must navigate just to talk to a human voice any more than we do. Instead of complaining to the patients, we should explain to them that the working conditions we must endure have the same roots as the things they don’t like about coming to see us.
I hope your situation still allows you to have an effective voice. If it doesn’t maybe it’s time to consider unionizing. However, if asking for more pay is anywhere near the top of your grievance list, I don’t want to join your union because you are doomed to failure on the public relations battlefield.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
According to an article in the Wall Street Journal (Mosbergen D. 2023 Jan 16), physicians-in-training in several parts of the country are attempting to unionize. The Committee of Interns and Residents (CIR), a union representing about 15% of the 140,000 residents and fellows in the United States reports that it has been adding chapters at an accelerated rate since the pandemic began.
Most of the 1,400 residents at Palo Alto–based Stanford Medicine recently voted to unionize seeking better compensation and improved working conditions including more accommodations for residents with disabilities or who are breastfeeding. At the University of Illinois at Chicago, house officers are also exploring an association with CIR hoping that collective bargaining might help them get “better pay and working conditions that could alleviate some burnout and stress.”
Although physicians have been hesitant to organize themselves around workplace concerns, nurses have a more robust history of unionizing and taking action. Recently, the nurses at two of New York’s largest medical centers went on a strike for 3 days that ended after the medical centers agreed to their primary demand of hiring more nurses and committing to set more workable nurse to patient ratios.
In an unusual but historic incident of workplace activism, the residents and interns at the then notoriously decrepit Boston City Hospital staged a “heal-in” in 1967 during which they admitted more patients (all with legitimate conditions for admission) than the hospital could handle. While more pay was included in their demands (interns were being paid $3,600/year and senior residents $7,500/year), the primary complaint of the house officers focused on patient health and safety issues. The crisis this work action triggered finally brought into sharp focus the city’s failure to care for its most needy citizens and over time, changes have been made (TIME Magazine. U.S. edition. Jun 21;91:25).
Having spent some time at the Boston City Hospital as a medical student in the 1960s I can attest to the deplorable conditions. While you might not be washing your hands and instruments in rusty sinks or having to brush flaking paint off your patients’ cribs, you may be experiencing working conditions that are threatening the health and safety of you, your coworkers, and not least of all your patients. Staffing shortages, clunky electronic health record systems that are adding hours of work to your day, screen after screen of data entry tasks that prevent you from meeting your patients eye-to-eye, and piles of prior authorization requests clogging your inbox to name just a few.
Who can you complain to, other than your coworkers? The patients brought their problems to you; it doesn’t seem fair to add to their burden by sharing your own. Maybe it’s time to think about joining a union to strengthen your voice and create some change.
But “union” and “strike” don’t sound very professional and certainly not coming from the mouths of folks who have chosen to be caregivers. However, things have changed. Most of us are employees now. We need to finally accept that role and begin acting like employees working under stressful and unhealthy conditions. Does the word “burnout” make the notion of unionizing any more palatable?
The American Medical Association’s code of ethics wisely discourages physicians from engaging in actions that withhold medical care. However, the Boston City Hospital house officers provided just one example of a work action that can draw attention to the problem while still providing care to the patients in our trust.
Simply, joining our voices can be a powerful force in the war of words and images. Patients don’t like the impersonalization that has come with the current crop of electronic health record systems and the tortuous phone trees they must navigate just to talk to a human voice any more than we do. Instead of complaining to the patients, we should explain to them that the working conditions we must endure have the same roots as the things they don’t like about coming to see us.
I hope your situation still allows you to have an effective voice. If it doesn’t maybe it’s time to consider unionizing. However, if asking for more pay is anywhere near the top of your grievance list, I don’t want to join your union because you are doomed to failure on the public relations battlefield.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
According to an article in the Wall Street Journal (Mosbergen D. 2023 Jan 16), physicians-in-training in several parts of the country are attempting to unionize. The Committee of Interns and Residents (CIR), a union representing about 15% of the 140,000 residents and fellows in the United States reports that it has been adding chapters at an accelerated rate since the pandemic began.
Most of the 1,400 residents at Palo Alto–based Stanford Medicine recently voted to unionize seeking better compensation and improved working conditions including more accommodations for residents with disabilities or who are breastfeeding. At the University of Illinois at Chicago, house officers are also exploring an association with CIR hoping that collective bargaining might help them get “better pay and working conditions that could alleviate some burnout and stress.”
Although physicians have been hesitant to organize themselves around workplace concerns, nurses have a more robust history of unionizing and taking action. Recently, the nurses at two of New York’s largest medical centers went on a strike for 3 days that ended after the medical centers agreed to their primary demand of hiring more nurses and committing to set more workable nurse to patient ratios.
In an unusual but historic incident of workplace activism, the residents and interns at the then notoriously decrepit Boston City Hospital staged a “heal-in” in 1967 during which they admitted more patients (all with legitimate conditions for admission) than the hospital could handle. While more pay was included in their demands (interns were being paid $3,600/year and senior residents $7,500/year), the primary complaint of the house officers focused on patient health and safety issues. The crisis this work action triggered finally brought into sharp focus the city’s failure to care for its most needy citizens and over time, changes have been made (TIME Magazine. U.S. edition. Jun 21;91:25).
Having spent some time at the Boston City Hospital as a medical student in the 1960s I can attest to the deplorable conditions. While you might not be washing your hands and instruments in rusty sinks or having to brush flaking paint off your patients’ cribs, you may be experiencing working conditions that are threatening the health and safety of you, your coworkers, and not least of all your patients. Staffing shortages, clunky electronic health record systems that are adding hours of work to your day, screen after screen of data entry tasks that prevent you from meeting your patients eye-to-eye, and piles of prior authorization requests clogging your inbox to name just a few.
Who can you complain to, other than your coworkers? The patients brought their problems to you; it doesn’t seem fair to add to their burden by sharing your own. Maybe it’s time to think about joining a union to strengthen your voice and create some change.
But “union” and “strike” don’t sound very professional and certainly not coming from the mouths of folks who have chosen to be caregivers. However, things have changed. Most of us are employees now. We need to finally accept that role and begin acting like employees working under stressful and unhealthy conditions. Does the word “burnout” make the notion of unionizing any more palatable?
The American Medical Association’s code of ethics wisely discourages physicians from engaging in actions that withhold medical care. However, the Boston City Hospital house officers provided just one example of a work action that can draw attention to the problem while still providing care to the patients in our trust.
Simply, joining our voices can be a powerful force in the war of words and images. Patients don’t like the impersonalization that has come with the current crop of electronic health record systems and the tortuous phone trees they must navigate just to talk to a human voice any more than we do. Instead of complaining to the patients, we should explain to them that the working conditions we must endure have the same roots as the things they don’t like about coming to see us.
I hope your situation still allows you to have an effective voice. If it doesn’t maybe it’s time to consider unionizing. However, if asking for more pay is anywhere near the top of your grievance list, I don’t want to join your union because you are doomed to failure on the public relations battlefield.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Update on secondary cytoreduction in recurrent ovarian cancer
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.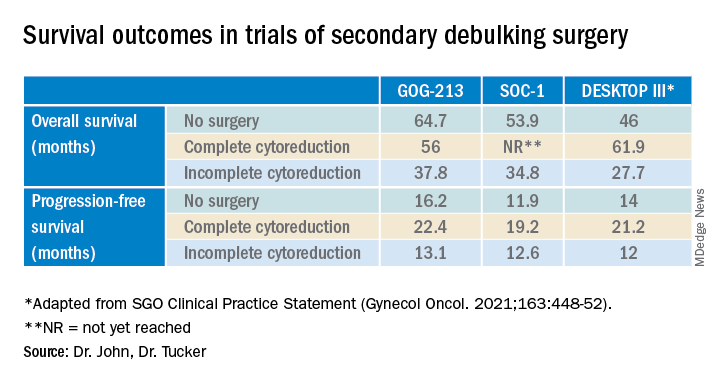
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Disability in medicine: My experience
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.











