User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Deductibles a threat to more imaging after abnormal mammogram
CHICAGO – One in five women will skip further imaging after an abnormal mammogram if they have to pay out of pocket before their deductible is met, new data indicate.
“The ACA [Affordable Care Act] removed out-of-pocket costs for screening mammograms under most health plans to encourage women to partake in this important preventative health care measure,” Michael Ngo, MD, a radiology resident at Boston Medical Center and Boston University, said in a statement.
However, the screening mammogram is only the first step. If it’s abnormal, additional tests and a biopsy help determine whether the patient has cancer. The ACA does not mandate coverage for those, Dr. Ngo noted.
Dr. Ngo was lead author of the study presented at the annual meeting of the Radiological Society of North America.
Researchers collected 932 surveys. Asked whether they would skip follow-up imaging if they knew that they would have to pay a deductible, 151 of 714 (21.2%) said that they would skip the imaging; 424 (59.4%) said that they would not skip further imaging; and 139 (19.5%) were undecided. Responses differed by race, education level, household income, and insurance payer.
Groups most likely to forgo further tests
The groups with the highest percentage of persons who would skip additional imaging were Hispanic persons (33%); persons whose level of education was high school or less (31.0%); persons with a household income of less than $35,000 (27%); and those covered by Medicaid or who were uninsured (31.5%).
Wendie Berg, MD, PhD, professor of radiology at the University of Pittsburgh, who was not part of the study, said that because insurance companies had to cover initial mammograms fully under the ACA, “they generally increased deductibles. That resulted in more charges to patients when they came in for additional testing.
“It caught a lot of women by surprise,” she told this news organization.
The out-of-pocket charges can escalate with each step – more images, a biopsy, then more if they do have cancer, she said. This puts patients on the hook for hundreds, if not thousands, of dollars in medical bills.
However, Dr. Berg said, “The vast majority of women – 95% – who are called back for additional testing don’t have cancer. It is a problem that a lot of women will experience the cost and don’t have any benefit.”
Reducing false-positive recalls
The study highlights several things, she said. One is that “it’s incumbent on all of us to reduce false-positive recalls, which is one of the benefits of 3-D mammogram.”
Physicians who order additional tests must also consider the financial burden for patients, she said.
Some states have tackled the issue, she said. “Seven states do require insurance to cover diagnostic testing.” But those states differ in the extent of the coverage. DenseBreast-info.org, a website she helps with on a volunteer basis, explains the benefits by state.
Further compounding the problem is that not every insurer is subject to state law, she said.
Many states have programs that cover the cost for those who meet income requirements, although, she noted, some women make too much to qualify.
“It would be great to have a federal law that is inclusive,” she said.
Education efforts may help
Brian N. Dontchos, MD, with the University of Washington in Seattle, who was not part of the study, views the data another way. He told this news organization, “It is encouraging from the study that the majority of women would pursue additional imaging after an abnormal screening mammogram despite incurring more cost.”
He said that since direct patient education “has been shown to be effective in improving patient participation in screening programs, it is possible that, with more education of patients and providers, that advocacy could influence payers to support downstream imaging and biopsies that result from screening programs.”
Dr. Ngo, Dr. Berg, and Dr. Dontchos report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – One in five women will skip further imaging after an abnormal mammogram if they have to pay out of pocket before their deductible is met, new data indicate.
“The ACA [Affordable Care Act] removed out-of-pocket costs for screening mammograms under most health plans to encourage women to partake in this important preventative health care measure,” Michael Ngo, MD, a radiology resident at Boston Medical Center and Boston University, said in a statement.
However, the screening mammogram is only the first step. If it’s abnormal, additional tests and a biopsy help determine whether the patient has cancer. The ACA does not mandate coverage for those, Dr. Ngo noted.
Dr. Ngo was lead author of the study presented at the annual meeting of the Radiological Society of North America.
Researchers collected 932 surveys. Asked whether they would skip follow-up imaging if they knew that they would have to pay a deductible, 151 of 714 (21.2%) said that they would skip the imaging; 424 (59.4%) said that they would not skip further imaging; and 139 (19.5%) were undecided. Responses differed by race, education level, household income, and insurance payer.
Groups most likely to forgo further tests
The groups with the highest percentage of persons who would skip additional imaging were Hispanic persons (33%); persons whose level of education was high school or less (31.0%); persons with a household income of less than $35,000 (27%); and those covered by Medicaid or who were uninsured (31.5%).
Wendie Berg, MD, PhD, professor of radiology at the University of Pittsburgh, who was not part of the study, said that because insurance companies had to cover initial mammograms fully under the ACA, “they generally increased deductibles. That resulted in more charges to patients when they came in for additional testing.
“It caught a lot of women by surprise,” she told this news organization.
The out-of-pocket charges can escalate with each step – more images, a biopsy, then more if they do have cancer, she said. This puts patients on the hook for hundreds, if not thousands, of dollars in medical bills.
However, Dr. Berg said, “The vast majority of women – 95% – who are called back for additional testing don’t have cancer. It is a problem that a lot of women will experience the cost and don’t have any benefit.”
Reducing false-positive recalls
The study highlights several things, she said. One is that “it’s incumbent on all of us to reduce false-positive recalls, which is one of the benefits of 3-D mammogram.”
Physicians who order additional tests must also consider the financial burden for patients, she said.
Some states have tackled the issue, she said. “Seven states do require insurance to cover diagnostic testing.” But those states differ in the extent of the coverage. DenseBreast-info.org, a website she helps with on a volunteer basis, explains the benefits by state.
Further compounding the problem is that not every insurer is subject to state law, she said.
Many states have programs that cover the cost for those who meet income requirements, although, she noted, some women make too much to qualify.
“It would be great to have a federal law that is inclusive,” she said.
Education efforts may help
Brian N. Dontchos, MD, with the University of Washington in Seattle, who was not part of the study, views the data another way. He told this news organization, “It is encouraging from the study that the majority of women would pursue additional imaging after an abnormal screening mammogram despite incurring more cost.”
He said that since direct patient education “has been shown to be effective in improving patient participation in screening programs, it is possible that, with more education of patients and providers, that advocacy could influence payers to support downstream imaging and biopsies that result from screening programs.”
Dr. Ngo, Dr. Berg, and Dr. Dontchos report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – One in five women will skip further imaging after an abnormal mammogram if they have to pay out of pocket before their deductible is met, new data indicate.
“The ACA [Affordable Care Act] removed out-of-pocket costs for screening mammograms under most health plans to encourage women to partake in this important preventative health care measure,” Michael Ngo, MD, a radiology resident at Boston Medical Center and Boston University, said in a statement.
However, the screening mammogram is only the first step. If it’s abnormal, additional tests and a biopsy help determine whether the patient has cancer. The ACA does not mandate coverage for those, Dr. Ngo noted.
Dr. Ngo was lead author of the study presented at the annual meeting of the Radiological Society of North America.
Researchers collected 932 surveys. Asked whether they would skip follow-up imaging if they knew that they would have to pay a deductible, 151 of 714 (21.2%) said that they would skip the imaging; 424 (59.4%) said that they would not skip further imaging; and 139 (19.5%) were undecided. Responses differed by race, education level, household income, and insurance payer.
Groups most likely to forgo further tests
The groups with the highest percentage of persons who would skip additional imaging were Hispanic persons (33%); persons whose level of education was high school or less (31.0%); persons with a household income of less than $35,000 (27%); and those covered by Medicaid or who were uninsured (31.5%).
Wendie Berg, MD, PhD, professor of radiology at the University of Pittsburgh, who was not part of the study, said that because insurance companies had to cover initial mammograms fully under the ACA, “they generally increased deductibles. That resulted in more charges to patients when they came in for additional testing.
“It caught a lot of women by surprise,” she told this news organization.
The out-of-pocket charges can escalate with each step – more images, a biopsy, then more if they do have cancer, she said. This puts patients on the hook for hundreds, if not thousands, of dollars in medical bills.
However, Dr. Berg said, “The vast majority of women – 95% – who are called back for additional testing don’t have cancer. It is a problem that a lot of women will experience the cost and don’t have any benefit.”
Reducing false-positive recalls
The study highlights several things, she said. One is that “it’s incumbent on all of us to reduce false-positive recalls, which is one of the benefits of 3-D mammogram.”
Physicians who order additional tests must also consider the financial burden for patients, she said.
Some states have tackled the issue, she said. “Seven states do require insurance to cover diagnostic testing.” But those states differ in the extent of the coverage. DenseBreast-info.org, a website she helps with on a volunteer basis, explains the benefits by state.
Further compounding the problem is that not every insurer is subject to state law, she said.
Many states have programs that cover the cost for those who meet income requirements, although, she noted, some women make too much to qualify.
“It would be great to have a federal law that is inclusive,” she said.
Education efforts may help
Brian N. Dontchos, MD, with the University of Washington in Seattle, who was not part of the study, views the data another way. He told this news organization, “It is encouraging from the study that the majority of women would pursue additional imaging after an abnormal screening mammogram despite incurring more cost.”
He said that since direct patient education “has been shown to be effective in improving patient participation in screening programs, it is possible that, with more education of patients and providers, that advocacy could influence payers to support downstream imaging and biopsies that result from screening programs.”
Dr. Ngo, Dr. Berg, and Dr. Dontchos report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT RSNA 2022
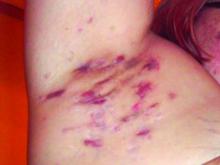
Consider quality of life, comorbidities in hidradenitis suppurativa
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Single chest x-ray could predict 10-year CVD risk
who presented the results of their deep-learning model at the annual meeting of the Radiological Society of North America.
Current American College of Cardiologists and American Heart Association guidelines recommend estimating 10-year risk of major adverse cardiovascular events (MACE) to determine whether a patient should receive statins to help prevent atherosclerotic cardiovascular disease (ASCVD). Statins are recommended for patients with a 10-year risk of 7.5% or higher, the authors noted.
The current ASCVD risk score is determined with nine factors: age, sex, race, systolic blood pressure, hypertension treatment, smoking, type 2 diabetes, and a lipid panel.
Not all data points available in EHR
But not all of those data points may be available through the electronic health record, “which makes novel and easier approaches for population-wide screening desirable,” said lead researcher Jakob Weiss, MD, a radiologist affiliated with the Cardiovascular Imaging Research Center at Massachusetts General Hospital and the AI in medicine program at the Brigham and Women’s Hospital in Boston.
Chest x-ray images, on the other hand, are commonly available. The images carry rich information beyond diagnostic data but have not been used in this type of prediction model because AI models have been lacking, Dr. Weiss said.
The researchers trained a deep-learning model with single chest x-rays only.
They used 147,497 chest x-rays from 40,643 participants in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial, a multicenter, randomized controlled trial designed and sponsored by the National Cancer Institute.
Dr. Weiss acknowledged that the population used to train the model was heavily White and that should be a consideration in validating the model.
They compared their model’s ability to predict 10-year ASCVD risk with the standard ACC/AHA model.
“Based on a single chest radiograph image, deep learning can predict the risk of future cardiovascular events independent of cardiovascular risk factors and with similar performance to the established and guideline-recommended ASCVD risk score,” Dr. Weiss said.
Tested against independent group
They tested the model against an independent group of 11,430 outpatients (average age, 60 years; 42.9% male) who underwent a routine outpatient chest x-ray at Mass General Brigham and were potentially eligible to receive statins.
Of those 11,430 patients, 1,096 (9.6%) had a major adverse cardiac event over the median follow-up of 10.3 years.
There was a significant association of CXR-CVD risk and MACE among patients eligible to receive statins, the researchers found (hazard ratio, 2.03; 95% confidence interval, 1.81-2.30; P < .001), which remained significant after adjusting for cardiovascular risk factors (adjusted HR, 1.63; 95% CI, 1.43-1.86; P < .001).
Some of the variables were missing in the standard model, but in a subgroup of 2,401 patients, all the variables were available.
They calculated ASCVD risk in that subgroup using the standard model and the CXR model and found that the performance was similar (c-statistic, 0.64 vs. 0.65; P = .48) to the ASCVD risk score (aHR, 1.58; 95% CI, 1.20-2.09; P = .001).
Ritu R. Gill MD, MPH, associate professor of radiology at Harvard Medical School in Boston, who was not part of the study, said in an interview that “the predictive algorithm is promising and potentially translatable and could enhance the annual medical checkup in a select population.
“The algorithm was developed using the PLCO cohort with radiographs, which are likely subjects in the lung cancer screening arm,” she said. “This cohort would be at high risk of cardiovascular diseases, as smoking is a known risk factor for atherosclerotic disease, and therefore the results are expected.
“The algorithm needs to be validated in an independent database with inclusion of subjects with younger age groups and adjusted for gender and racial diversity,” Gill said.
David Cho, MD, a cardiologist at the University of California, Los Angeles, who also was not part of the study, said in an interview that “this work is a great example of AI being able to detect clinically relevant outcomes with a widely used and low-cost screening test.
“The volume of data needed to train these models is already out there,” Dr. Cho said. “It just needs to be mined.”
He noted that this tool, if validated in randomized trials, could help determine risk among patients living in places where access to specialized cardiac care is limited.
Dr. Weiss and Dr. Cho disclosed no relevant financial relationships. Dr. Gill has received research support from Cannon Inc and consultant fees from Imbio and WorldCare.
A version of this article first appeared on Medscape.com.
who presented the results of their deep-learning model at the annual meeting of the Radiological Society of North America.
Current American College of Cardiologists and American Heart Association guidelines recommend estimating 10-year risk of major adverse cardiovascular events (MACE) to determine whether a patient should receive statins to help prevent atherosclerotic cardiovascular disease (ASCVD). Statins are recommended for patients with a 10-year risk of 7.5% or higher, the authors noted.
The current ASCVD risk score is determined with nine factors: age, sex, race, systolic blood pressure, hypertension treatment, smoking, type 2 diabetes, and a lipid panel.
Not all data points available in EHR
But not all of those data points may be available through the electronic health record, “which makes novel and easier approaches for population-wide screening desirable,” said lead researcher Jakob Weiss, MD, a radiologist affiliated with the Cardiovascular Imaging Research Center at Massachusetts General Hospital and the AI in medicine program at the Brigham and Women’s Hospital in Boston.
Chest x-ray images, on the other hand, are commonly available. The images carry rich information beyond diagnostic data but have not been used in this type of prediction model because AI models have been lacking, Dr. Weiss said.
The researchers trained a deep-learning model with single chest x-rays only.
They used 147,497 chest x-rays from 40,643 participants in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial, a multicenter, randomized controlled trial designed and sponsored by the National Cancer Institute.
Dr. Weiss acknowledged that the population used to train the model was heavily White and that should be a consideration in validating the model.
They compared their model’s ability to predict 10-year ASCVD risk with the standard ACC/AHA model.
“Based on a single chest radiograph image, deep learning can predict the risk of future cardiovascular events independent of cardiovascular risk factors and with similar performance to the established and guideline-recommended ASCVD risk score,” Dr. Weiss said.
Tested against independent group
They tested the model against an independent group of 11,430 outpatients (average age, 60 years; 42.9% male) who underwent a routine outpatient chest x-ray at Mass General Brigham and were potentially eligible to receive statins.
Of those 11,430 patients, 1,096 (9.6%) had a major adverse cardiac event over the median follow-up of 10.3 years.
There was a significant association of CXR-CVD risk and MACE among patients eligible to receive statins, the researchers found (hazard ratio, 2.03; 95% confidence interval, 1.81-2.30; P < .001), which remained significant after adjusting for cardiovascular risk factors (adjusted HR, 1.63; 95% CI, 1.43-1.86; P < .001).
Some of the variables were missing in the standard model, but in a subgroup of 2,401 patients, all the variables were available.
They calculated ASCVD risk in that subgroup using the standard model and the CXR model and found that the performance was similar (c-statistic, 0.64 vs. 0.65; P = .48) to the ASCVD risk score (aHR, 1.58; 95% CI, 1.20-2.09; P = .001).
Ritu R. Gill MD, MPH, associate professor of radiology at Harvard Medical School in Boston, who was not part of the study, said in an interview that “the predictive algorithm is promising and potentially translatable and could enhance the annual medical checkup in a select population.
“The algorithm was developed using the PLCO cohort with radiographs, which are likely subjects in the lung cancer screening arm,” she said. “This cohort would be at high risk of cardiovascular diseases, as smoking is a known risk factor for atherosclerotic disease, and therefore the results are expected.
“The algorithm needs to be validated in an independent database with inclusion of subjects with younger age groups and adjusted for gender and racial diversity,” Gill said.
David Cho, MD, a cardiologist at the University of California, Los Angeles, who also was not part of the study, said in an interview that “this work is a great example of AI being able to detect clinically relevant outcomes with a widely used and low-cost screening test.
“The volume of data needed to train these models is already out there,” Dr. Cho said. “It just needs to be mined.”
He noted that this tool, if validated in randomized trials, could help determine risk among patients living in places where access to specialized cardiac care is limited.
Dr. Weiss and Dr. Cho disclosed no relevant financial relationships. Dr. Gill has received research support from Cannon Inc and consultant fees from Imbio and WorldCare.
A version of this article first appeared on Medscape.com.
who presented the results of their deep-learning model at the annual meeting of the Radiological Society of North America.
Current American College of Cardiologists and American Heart Association guidelines recommend estimating 10-year risk of major adverse cardiovascular events (MACE) to determine whether a patient should receive statins to help prevent atherosclerotic cardiovascular disease (ASCVD). Statins are recommended for patients with a 10-year risk of 7.5% or higher, the authors noted.
The current ASCVD risk score is determined with nine factors: age, sex, race, systolic blood pressure, hypertension treatment, smoking, type 2 diabetes, and a lipid panel.
Not all data points available in EHR
But not all of those data points may be available through the electronic health record, “which makes novel and easier approaches for population-wide screening desirable,” said lead researcher Jakob Weiss, MD, a radiologist affiliated with the Cardiovascular Imaging Research Center at Massachusetts General Hospital and the AI in medicine program at the Brigham and Women’s Hospital in Boston.
Chest x-ray images, on the other hand, are commonly available. The images carry rich information beyond diagnostic data but have not been used in this type of prediction model because AI models have been lacking, Dr. Weiss said.
The researchers trained a deep-learning model with single chest x-rays only.
They used 147,497 chest x-rays from 40,643 participants in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial, a multicenter, randomized controlled trial designed and sponsored by the National Cancer Institute.
Dr. Weiss acknowledged that the population used to train the model was heavily White and that should be a consideration in validating the model.
They compared their model’s ability to predict 10-year ASCVD risk with the standard ACC/AHA model.
“Based on a single chest radiograph image, deep learning can predict the risk of future cardiovascular events independent of cardiovascular risk factors and with similar performance to the established and guideline-recommended ASCVD risk score,” Dr. Weiss said.
Tested against independent group
They tested the model against an independent group of 11,430 outpatients (average age, 60 years; 42.9% male) who underwent a routine outpatient chest x-ray at Mass General Brigham and were potentially eligible to receive statins.
Of those 11,430 patients, 1,096 (9.6%) had a major adverse cardiac event over the median follow-up of 10.3 years.
There was a significant association of CXR-CVD risk and MACE among patients eligible to receive statins, the researchers found (hazard ratio, 2.03; 95% confidence interval, 1.81-2.30; P < .001), which remained significant after adjusting for cardiovascular risk factors (adjusted HR, 1.63; 95% CI, 1.43-1.86; P < .001).
Some of the variables were missing in the standard model, but in a subgroup of 2,401 patients, all the variables were available.
They calculated ASCVD risk in that subgroup using the standard model and the CXR model and found that the performance was similar (c-statistic, 0.64 vs. 0.65; P = .48) to the ASCVD risk score (aHR, 1.58; 95% CI, 1.20-2.09; P = .001).
Ritu R. Gill MD, MPH, associate professor of radiology at Harvard Medical School in Boston, who was not part of the study, said in an interview that “the predictive algorithm is promising and potentially translatable and could enhance the annual medical checkup in a select population.
“The algorithm was developed using the PLCO cohort with radiographs, which are likely subjects in the lung cancer screening arm,” she said. “This cohort would be at high risk of cardiovascular diseases, as smoking is a known risk factor for atherosclerotic disease, and therefore the results are expected.
“The algorithm needs to be validated in an independent database with inclusion of subjects with younger age groups and adjusted for gender and racial diversity,” Gill said.
David Cho, MD, a cardiologist at the University of California, Los Angeles, who also was not part of the study, said in an interview that “this work is a great example of AI being able to detect clinically relevant outcomes with a widely used and low-cost screening test.
“The volume of data needed to train these models is already out there,” Dr. Cho said. “It just needs to be mined.”
He noted that this tool, if validated in randomized trials, could help determine risk among patients living in places where access to specialized cardiac care is limited.
Dr. Weiss and Dr. Cho disclosed no relevant financial relationships. Dr. Gill has received research support from Cannon Inc and consultant fees from Imbio and WorldCare.
A version of this article first appeared on Medscape.com.
AT RSNA 2022
Higher potency of fentanyl affects addiction treatment, screening
As fentanyl-related overdose deaths continue to increase, clinicians should take note of important differences that set the drug apart from the other drugs of misuse – and the troubling reality that fentanyl now contaminates most of them.
“It would be fair to tell patients, if you’re buying any illicit drugs – pills, powder, liquid, whatever it is, you’ve got to assume it’s either contaminated with or replaced by fentanyl,” said Edwin Salsitz, MD, an associate clinical professor at the Icahn School of Medicine at Mount Sinai, New York, during a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In many if not most cases, he noted, patients become addicted to fentanyl unknowingly. They assume they are ingesting oxycodone, cocaine, or another drug, and have no realization that they are even exposed to fentanyl until they test positive for it – or overdose.
Meanwhile, the high potency of fentanyl can overcome the opioid blockade of addiction treatment therapies – methadone and buprenorphine – that take away the high that users get from less potent drugs such as heroin.
“Fentanyl is overcoming this blockade that methadone and buprenorphine used to provide,” Dr. Salsitz said. “With fentanyl having such a higher potency, patients are saying ‘no, I still feel the fentanyl effects,’ and they continue feeling it even with 200 milligrams of methadone or 24 milligrams of buprenorphine.”
‘Wooden chest syndrome’
Among the lesser-known dangers of fentanyl is the possibility that some overdose deaths may occur as the result of a syndrome previously reported as a rare complication following the medical use of fentanyl in critically ill patients – fentanyl-induced chest-wall rigidity, or “wooden chest syndrome,” Dr. Salsitz explained.
In such cases, the muscles of respiration become rigid and paralyzed, causing suffocation within a matter of minutes – too soon to benefit from the overdose rescue medication naloxone.
In one recent study published in Clinical Toxicology , nearly half of fentanyl overdose deaths were found to have occurred even before the body had a chance to produce norfentanyl, a metabolite of fentanyl that takes only about 2-3 minutes to appear in the system, suggesting the deaths occurred rapidly.
In the study of 48 fentanyl deaths, no appreciable concentrations of norfentanyl could be detected in 20 of the 48 overdose deaths (42%), and concentrations were less than 1 ng/mL in 25 cases (52%).
“The lack of any measurable norfentanyl in half of our cases suggests a very rapid death, consistent with acute chest rigidity,” the authors reported.
“In several cases fentanyl concentrations were strikingly high (22 ng/mL and 20 ng/mL) with no norfentanyl detected,” they said.
Dr. Salsitz noted that the syndrome is not well known among the addiction treatment community.
“This is different than the usual respiratory opioid overdose where there’s a gradual decrease in the breathing rate and a gradual decrease in how much air is going in and out of the lungs,” Dr. Salsitz explained.
“With those cases, some may survive for an hour or longer, allowing time for someone to administer naloxone or to get the patient to the emergency room,” he said. “But with this, breathing stops and people can die within minutes.
“I think that this is one of the reasons that fentanyl deaths keep going up despite more and more naloxone availability out there,” he said.
Clearance may take longer
In toxicology testing for fentanyl, clinicians should also note the important difference between fentanyl and other opioids – that fentanyl, because of its high lipophilicity, may be detected in urine toxicology testing up to 3 weeks after last use. This is much longer than the 2- to 4-day clearance observed with other opioids, possibly causing patients to continue to test positive for the drug weeks after cessation.
This effect was observed in one recent study of 12 opioid use disorder patients in a residential treatment program who had previously been exposed to daily fentanyl.
The study showed the mean amount of time of fentanyl clearance was 2 weeks, with a range of 4-26 days after last use.
The authors pointed out that the findings “might explain recent reports of difficulty in buprenorphine inductions for persons who use fentanyl, and point to a need to better understand the pharmacokinetics of fentanyl in the context of opioid withdrawal in persons who regularly use fentanyl.”
Though the study was small, Dr. Salsitz said “that’s not a stumbling block to the important finding that, with regular use of fentanyl, the drug may stay in the urine for a long time.”
Dr. Salsitz noted that similar observations have been made at his center, with clinicians logically assuming that patients were still somehow getting fentanyl.
“When we initially found this in patients, we thought that they were using on the unit, perhaps that they brought in the fentanyl, because otherwise how could it stay in the urine that long,” he noted. “But fentanyl appears to be more lipophilic and gets into the fat; it’s then excreted very slowly and then stays in the urine.”
Dr. Salsitz said most practitioners think of fentanyl as a short-acting drug, so “it’s important to realize that people may continue to test positive and it should be thought of as a long-acting opioid.”
Opiate screening tests don’t work
Dr. Salsitz warned of another misconception in fentanyl testing – the common mistake of assuming that fentanyl should show up in a test for opiates – when in fact fentanyl is not, technically, an opiate.
“The word opiate only refers to morphine, codeine, heroin and sometimes hydrocodone,” he explained. “Other opioids are classified as semisynthetic, such as oxycodone, or synthetics, such as fentanyl and methadone, buprenorphine.”
“In order to detect the synthetics, you must have a separate strip for each one of those drugs. They will not show up positive on a screen for opiates,” he noted.
The belief that fentanyl and other synthetic and semisynthetic opioids will show positive on an opiate screen is a common misconception, he said. “The misunderstanding in toxicology interpretation is a problem for many practitioners, [but] it’s essential to understand because otherwise false assumptions about the patient will be considered.”
Another important testing misreading can occur with the antidepressant drug trazodone, which Dr. Salsitz cautioned may falsely test as positive for fentanyl on immunoassays.
“Trazodone is very commonly used in addiction treatment centers, but it can give a false positive on the fentanyl immunoassay and we’ve had a number of those cases,” he said.
Dr. Salsitz had no disclosures to report.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
As fentanyl-related overdose deaths continue to increase, clinicians should take note of important differences that set the drug apart from the other drugs of misuse – and the troubling reality that fentanyl now contaminates most of them.
“It would be fair to tell patients, if you’re buying any illicit drugs – pills, powder, liquid, whatever it is, you’ve got to assume it’s either contaminated with or replaced by fentanyl,” said Edwin Salsitz, MD, an associate clinical professor at the Icahn School of Medicine at Mount Sinai, New York, during a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In many if not most cases, he noted, patients become addicted to fentanyl unknowingly. They assume they are ingesting oxycodone, cocaine, or another drug, and have no realization that they are even exposed to fentanyl until they test positive for it – or overdose.
Meanwhile, the high potency of fentanyl can overcome the opioid blockade of addiction treatment therapies – methadone and buprenorphine – that take away the high that users get from less potent drugs such as heroin.
“Fentanyl is overcoming this blockade that methadone and buprenorphine used to provide,” Dr. Salsitz said. “With fentanyl having such a higher potency, patients are saying ‘no, I still feel the fentanyl effects,’ and they continue feeling it even with 200 milligrams of methadone or 24 milligrams of buprenorphine.”
‘Wooden chest syndrome’
Among the lesser-known dangers of fentanyl is the possibility that some overdose deaths may occur as the result of a syndrome previously reported as a rare complication following the medical use of fentanyl in critically ill patients – fentanyl-induced chest-wall rigidity, or “wooden chest syndrome,” Dr. Salsitz explained.
In such cases, the muscles of respiration become rigid and paralyzed, causing suffocation within a matter of minutes – too soon to benefit from the overdose rescue medication naloxone.
In one recent study published in Clinical Toxicology , nearly half of fentanyl overdose deaths were found to have occurred even before the body had a chance to produce norfentanyl, a metabolite of fentanyl that takes only about 2-3 minutes to appear in the system, suggesting the deaths occurred rapidly.
In the study of 48 fentanyl deaths, no appreciable concentrations of norfentanyl could be detected in 20 of the 48 overdose deaths (42%), and concentrations were less than 1 ng/mL in 25 cases (52%).
“The lack of any measurable norfentanyl in half of our cases suggests a very rapid death, consistent with acute chest rigidity,” the authors reported.
“In several cases fentanyl concentrations were strikingly high (22 ng/mL and 20 ng/mL) with no norfentanyl detected,” they said.
Dr. Salsitz noted that the syndrome is not well known among the addiction treatment community.
“This is different than the usual respiratory opioid overdose where there’s a gradual decrease in the breathing rate and a gradual decrease in how much air is going in and out of the lungs,” Dr. Salsitz explained.
“With those cases, some may survive for an hour or longer, allowing time for someone to administer naloxone or to get the patient to the emergency room,” he said. “But with this, breathing stops and people can die within minutes.
“I think that this is one of the reasons that fentanyl deaths keep going up despite more and more naloxone availability out there,” he said.
Clearance may take longer
In toxicology testing for fentanyl, clinicians should also note the important difference between fentanyl and other opioids – that fentanyl, because of its high lipophilicity, may be detected in urine toxicology testing up to 3 weeks after last use. This is much longer than the 2- to 4-day clearance observed with other opioids, possibly causing patients to continue to test positive for the drug weeks after cessation.
This effect was observed in one recent study of 12 opioid use disorder patients in a residential treatment program who had previously been exposed to daily fentanyl.
The study showed the mean amount of time of fentanyl clearance was 2 weeks, with a range of 4-26 days after last use.
The authors pointed out that the findings “might explain recent reports of difficulty in buprenorphine inductions for persons who use fentanyl, and point to a need to better understand the pharmacokinetics of fentanyl in the context of opioid withdrawal in persons who regularly use fentanyl.”
Though the study was small, Dr. Salsitz said “that’s not a stumbling block to the important finding that, with regular use of fentanyl, the drug may stay in the urine for a long time.”
Dr. Salsitz noted that similar observations have been made at his center, with clinicians logically assuming that patients were still somehow getting fentanyl.
“When we initially found this in patients, we thought that they were using on the unit, perhaps that they brought in the fentanyl, because otherwise how could it stay in the urine that long,” he noted. “But fentanyl appears to be more lipophilic and gets into the fat; it’s then excreted very slowly and then stays in the urine.”
Dr. Salsitz said most practitioners think of fentanyl as a short-acting drug, so “it’s important to realize that people may continue to test positive and it should be thought of as a long-acting opioid.”
Opiate screening tests don’t work
Dr. Salsitz warned of another misconception in fentanyl testing – the common mistake of assuming that fentanyl should show up in a test for opiates – when in fact fentanyl is not, technically, an opiate.
“The word opiate only refers to morphine, codeine, heroin and sometimes hydrocodone,” he explained. “Other opioids are classified as semisynthetic, such as oxycodone, or synthetics, such as fentanyl and methadone, buprenorphine.”
“In order to detect the synthetics, you must have a separate strip for each one of those drugs. They will not show up positive on a screen for opiates,” he noted.
The belief that fentanyl and other synthetic and semisynthetic opioids will show positive on an opiate screen is a common misconception, he said. “The misunderstanding in toxicology interpretation is a problem for many practitioners, [but] it’s essential to understand because otherwise false assumptions about the patient will be considered.”
Another important testing misreading can occur with the antidepressant drug trazodone, which Dr. Salsitz cautioned may falsely test as positive for fentanyl on immunoassays.
“Trazodone is very commonly used in addiction treatment centers, but it can give a false positive on the fentanyl immunoassay and we’ve had a number of those cases,” he said.
Dr. Salsitz had no disclosures to report.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
As fentanyl-related overdose deaths continue to increase, clinicians should take note of important differences that set the drug apart from the other drugs of misuse – and the troubling reality that fentanyl now contaminates most of them.
“It would be fair to tell patients, if you’re buying any illicit drugs – pills, powder, liquid, whatever it is, you’ve got to assume it’s either contaminated with or replaced by fentanyl,” said Edwin Salsitz, MD, an associate clinical professor at the Icahn School of Medicine at Mount Sinai, New York, during a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In many if not most cases, he noted, patients become addicted to fentanyl unknowingly. They assume they are ingesting oxycodone, cocaine, or another drug, and have no realization that they are even exposed to fentanyl until they test positive for it – or overdose.
Meanwhile, the high potency of fentanyl can overcome the opioid blockade of addiction treatment therapies – methadone and buprenorphine – that take away the high that users get from less potent drugs such as heroin.
“Fentanyl is overcoming this blockade that methadone and buprenorphine used to provide,” Dr. Salsitz said. “With fentanyl having such a higher potency, patients are saying ‘no, I still feel the fentanyl effects,’ and they continue feeling it even with 200 milligrams of methadone or 24 milligrams of buprenorphine.”
‘Wooden chest syndrome’
Among the lesser-known dangers of fentanyl is the possibility that some overdose deaths may occur as the result of a syndrome previously reported as a rare complication following the medical use of fentanyl in critically ill patients – fentanyl-induced chest-wall rigidity, or “wooden chest syndrome,” Dr. Salsitz explained.
In such cases, the muscles of respiration become rigid and paralyzed, causing suffocation within a matter of minutes – too soon to benefit from the overdose rescue medication naloxone.
In one recent study published in Clinical Toxicology , nearly half of fentanyl overdose deaths were found to have occurred even before the body had a chance to produce norfentanyl, a metabolite of fentanyl that takes only about 2-3 minutes to appear in the system, suggesting the deaths occurred rapidly.
In the study of 48 fentanyl deaths, no appreciable concentrations of norfentanyl could be detected in 20 of the 48 overdose deaths (42%), and concentrations were less than 1 ng/mL in 25 cases (52%).
“The lack of any measurable norfentanyl in half of our cases suggests a very rapid death, consistent with acute chest rigidity,” the authors reported.
“In several cases fentanyl concentrations were strikingly high (22 ng/mL and 20 ng/mL) with no norfentanyl detected,” they said.
Dr. Salsitz noted that the syndrome is not well known among the addiction treatment community.
“This is different than the usual respiratory opioid overdose where there’s a gradual decrease in the breathing rate and a gradual decrease in how much air is going in and out of the lungs,” Dr. Salsitz explained.
“With those cases, some may survive for an hour or longer, allowing time for someone to administer naloxone or to get the patient to the emergency room,” he said. “But with this, breathing stops and people can die within minutes.
“I think that this is one of the reasons that fentanyl deaths keep going up despite more and more naloxone availability out there,” he said.
Clearance may take longer
In toxicology testing for fentanyl, clinicians should also note the important difference between fentanyl and other opioids – that fentanyl, because of its high lipophilicity, may be detected in urine toxicology testing up to 3 weeks after last use. This is much longer than the 2- to 4-day clearance observed with other opioids, possibly causing patients to continue to test positive for the drug weeks after cessation.
This effect was observed in one recent study of 12 opioid use disorder patients in a residential treatment program who had previously been exposed to daily fentanyl.
The study showed the mean amount of time of fentanyl clearance was 2 weeks, with a range of 4-26 days after last use.
The authors pointed out that the findings “might explain recent reports of difficulty in buprenorphine inductions for persons who use fentanyl, and point to a need to better understand the pharmacokinetics of fentanyl in the context of opioid withdrawal in persons who regularly use fentanyl.”
Though the study was small, Dr. Salsitz said “that’s not a stumbling block to the important finding that, with regular use of fentanyl, the drug may stay in the urine for a long time.”
Dr. Salsitz noted that similar observations have been made at his center, with clinicians logically assuming that patients were still somehow getting fentanyl.
“When we initially found this in patients, we thought that they were using on the unit, perhaps that they brought in the fentanyl, because otherwise how could it stay in the urine that long,” he noted. “But fentanyl appears to be more lipophilic and gets into the fat; it’s then excreted very slowly and then stays in the urine.”
Dr. Salsitz said most practitioners think of fentanyl as a short-acting drug, so “it’s important to realize that people may continue to test positive and it should be thought of as a long-acting opioid.”
Opiate screening tests don’t work
Dr. Salsitz warned of another misconception in fentanyl testing – the common mistake of assuming that fentanyl should show up in a test for opiates – when in fact fentanyl is not, technically, an opiate.
“The word opiate only refers to morphine, codeine, heroin and sometimes hydrocodone,” he explained. “Other opioids are classified as semisynthetic, such as oxycodone, or synthetics, such as fentanyl and methadone, buprenorphine.”
“In order to detect the synthetics, you must have a separate strip for each one of those drugs. They will not show up positive on a screen for opiates,” he noted.
The belief that fentanyl and other synthetic and semisynthetic opioids will show positive on an opiate screen is a common misconception, he said. “The misunderstanding in toxicology interpretation is a problem for many practitioners, [but] it’s essential to understand because otherwise false assumptions about the patient will be considered.”
Another important testing misreading can occur with the antidepressant drug trazodone, which Dr. Salsitz cautioned may falsely test as positive for fentanyl on immunoassays.
“Trazodone is very commonly used in addiction treatment centers, but it can give a false positive on the fentanyl immunoassay and we’ve had a number of those cases,” he said.
Dr. Salsitz had no disclosures to report.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Lung cancer screening pushes 20-year survival rate to 80%
CHICAGO – , findings from a 20-year international study indicate.
Claudia Henschke, MD, PhD, professor of radiology and director of the Early Lung and Cardiac Action Program at the Icahn School of Medicine at Mount Sinai, New York, presented research results at the annual meeting of the Radiological Society of North America.
The researchers studied lung-cancer–specific survival (LCS) of 87,416 participants enrolled in an international, prospective study named the International Early Lung Cancer Action Program.
Lung cancer is the leading cause of cancer death. The American Lung Association states the average 5-year survival rate is 18.6%. Only 16% of the cancers are caught early and more than half of people with lung cancer die within a year of diagnosis.
Participants’ 20-year survival rate 80%
Results of this large international study showed the overall 20-year survival rate for the 1,285 screening participants diagnosed with early-stage cancer was 80% (95% confidence interval, 77%-83%). Among the 1,285 diagnosed, 83% had stage 1 cancer, Dr. Henschke said.
Lung cancer survival (LCS) was 100% for the 139 participants with nonsolid nodule consistency and for the 155 participants with part-solid consistency. LCS was 73% (95% CI, 69%-77%) for the 991 with solid consistency, and for clinical stage IA participants LCS was 86% (95% CI, 83%-89%), regardless of consistency.
For participants with pathologic stage IA lung cancer 10 mm or less in average diameter, the 20-year survival rate with identification and resection was 92% (95% CI, 87%-96%).
No lung cancer deaths were identified in the part-solid and nonsolid cancers, the researchers report.
These results show the 10-year findings from 2006 published in the New England Journal of Medicine, which also showed 80% survival rates with low-dose CT, have persisted, she said.
At the time of the 2006 paper, 95% of Americans diagnosed with lung cancer died from it, Dr. Henschke said.
Dr. Henschke notes that by the time symptoms appear, lung cancer is often advanced, so the best tool for detecting early-stage lung cancer is enrolling in an annual screening program.
When cancer is small enough and can be surgically removed, patients can be effectively cured long-term, she said.
“In the future, perhaps blood markers will allow us to detect it in the first half of the life cycle of lung cancer instead of CT at the beginning of the second half of the life cycle,” Dr. Henschke said.
“The study raises the power of prospective data collection in the context of clinical care as recommended by the Institute of Medicine long ago,” she said.
Findings “very promising”
Ernest Hawk, MD, MPH, head of the division of cancer prevention and population sciences at the University of Texas MD Anderson Cancer, Houston, told this news organization the findings look “very promising.” Dr. Hawk was not involved in the study.
“This was one of the earliest studies to evaluate low-dose CT scanning. Their report that the initial benefits seem to be holding up over a longer period of observation is great,” he said.
“This bolsters the data that lung cancer screening is beneficial over a longer period of observation,” he said, noting that most of the randomized controlled trials have been shorter.
Lung cancer screening is now recommended for high-risk individuals – those with at least a 20-pack-year history of tobacco use who are between 50 and 80 years old.
So far, screening is still limited to people at high risk, Dr. Hawk said, though there’s discussion about whether benefit would extend to people exposed to asbestos, for instance, or secondhand smoke.
“The biggest challenge right now is getting the screening to those who actually meet the criteria,” Dr. Hawk said.
Medscape reported earlier this month that less than 6% of high-risk smokers have the recommended annual lung cancer screening, according to a new report from the American Lung Association.
Dr. Henschke is on the Advisory Board for LungLifeAI and is on the board for the Early Diagnosis and Treatment Research Foundation. Dr. Hawk reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , findings from a 20-year international study indicate.
Claudia Henschke, MD, PhD, professor of radiology and director of the Early Lung and Cardiac Action Program at the Icahn School of Medicine at Mount Sinai, New York, presented research results at the annual meeting of the Radiological Society of North America.
The researchers studied lung-cancer–specific survival (LCS) of 87,416 participants enrolled in an international, prospective study named the International Early Lung Cancer Action Program.
Lung cancer is the leading cause of cancer death. The American Lung Association states the average 5-year survival rate is 18.6%. Only 16% of the cancers are caught early and more than half of people with lung cancer die within a year of diagnosis.
Participants’ 20-year survival rate 80%
Results of this large international study showed the overall 20-year survival rate for the 1,285 screening participants diagnosed with early-stage cancer was 80% (95% confidence interval, 77%-83%). Among the 1,285 diagnosed, 83% had stage 1 cancer, Dr. Henschke said.
Lung cancer survival (LCS) was 100% for the 139 participants with nonsolid nodule consistency and for the 155 participants with part-solid consistency. LCS was 73% (95% CI, 69%-77%) for the 991 with solid consistency, and for clinical stage IA participants LCS was 86% (95% CI, 83%-89%), regardless of consistency.
For participants with pathologic stage IA lung cancer 10 mm or less in average diameter, the 20-year survival rate with identification and resection was 92% (95% CI, 87%-96%).
No lung cancer deaths were identified in the part-solid and nonsolid cancers, the researchers report.
These results show the 10-year findings from 2006 published in the New England Journal of Medicine, which also showed 80% survival rates with low-dose CT, have persisted, she said.
At the time of the 2006 paper, 95% of Americans diagnosed with lung cancer died from it, Dr. Henschke said.
Dr. Henschke notes that by the time symptoms appear, lung cancer is often advanced, so the best tool for detecting early-stage lung cancer is enrolling in an annual screening program.
When cancer is small enough and can be surgically removed, patients can be effectively cured long-term, she said.
“In the future, perhaps blood markers will allow us to detect it in the first half of the life cycle of lung cancer instead of CT at the beginning of the second half of the life cycle,” Dr. Henschke said.
“The study raises the power of prospective data collection in the context of clinical care as recommended by the Institute of Medicine long ago,” she said.
Findings “very promising”
Ernest Hawk, MD, MPH, head of the division of cancer prevention and population sciences at the University of Texas MD Anderson Cancer, Houston, told this news organization the findings look “very promising.” Dr. Hawk was not involved in the study.
“This was one of the earliest studies to evaluate low-dose CT scanning. Their report that the initial benefits seem to be holding up over a longer period of observation is great,” he said.
“This bolsters the data that lung cancer screening is beneficial over a longer period of observation,” he said, noting that most of the randomized controlled trials have been shorter.
Lung cancer screening is now recommended for high-risk individuals – those with at least a 20-pack-year history of tobacco use who are between 50 and 80 years old.
So far, screening is still limited to people at high risk, Dr. Hawk said, though there’s discussion about whether benefit would extend to people exposed to asbestos, for instance, or secondhand smoke.
“The biggest challenge right now is getting the screening to those who actually meet the criteria,” Dr. Hawk said.
Medscape reported earlier this month that less than 6% of high-risk smokers have the recommended annual lung cancer screening, according to a new report from the American Lung Association.
Dr. Henschke is on the Advisory Board for LungLifeAI and is on the board for the Early Diagnosis and Treatment Research Foundation. Dr. Hawk reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , findings from a 20-year international study indicate.
Claudia Henschke, MD, PhD, professor of radiology and director of the Early Lung and Cardiac Action Program at the Icahn School of Medicine at Mount Sinai, New York, presented research results at the annual meeting of the Radiological Society of North America.
The researchers studied lung-cancer–specific survival (LCS) of 87,416 participants enrolled in an international, prospective study named the International Early Lung Cancer Action Program.
Lung cancer is the leading cause of cancer death. The American Lung Association states the average 5-year survival rate is 18.6%. Only 16% of the cancers are caught early and more than half of people with lung cancer die within a year of diagnosis.
Participants’ 20-year survival rate 80%
Results of this large international study showed the overall 20-year survival rate for the 1,285 screening participants diagnosed with early-stage cancer was 80% (95% confidence interval, 77%-83%). Among the 1,285 diagnosed, 83% had stage 1 cancer, Dr. Henschke said.
Lung cancer survival (LCS) was 100% for the 139 participants with nonsolid nodule consistency and for the 155 participants with part-solid consistency. LCS was 73% (95% CI, 69%-77%) for the 991 with solid consistency, and for clinical stage IA participants LCS was 86% (95% CI, 83%-89%), regardless of consistency.
For participants with pathologic stage IA lung cancer 10 mm or less in average diameter, the 20-year survival rate with identification and resection was 92% (95% CI, 87%-96%).
No lung cancer deaths were identified in the part-solid and nonsolid cancers, the researchers report.
These results show the 10-year findings from 2006 published in the New England Journal of Medicine, which also showed 80% survival rates with low-dose CT, have persisted, she said.
At the time of the 2006 paper, 95% of Americans diagnosed with lung cancer died from it, Dr. Henschke said.
Dr. Henschke notes that by the time symptoms appear, lung cancer is often advanced, so the best tool for detecting early-stage lung cancer is enrolling in an annual screening program.
When cancer is small enough and can be surgically removed, patients can be effectively cured long-term, she said.
“In the future, perhaps blood markers will allow us to detect it in the first half of the life cycle of lung cancer instead of CT at the beginning of the second half of the life cycle,” Dr. Henschke said.
“The study raises the power of prospective data collection in the context of clinical care as recommended by the Institute of Medicine long ago,” she said.
Findings “very promising”
Ernest Hawk, MD, MPH, head of the division of cancer prevention and population sciences at the University of Texas MD Anderson Cancer, Houston, told this news organization the findings look “very promising.” Dr. Hawk was not involved in the study.
“This was one of the earliest studies to evaluate low-dose CT scanning. Their report that the initial benefits seem to be holding up over a longer period of observation is great,” he said.
“This bolsters the data that lung cancer screening is beneficial over a longer period of observation,” he said, noting that most of the randomized controlled trials have been shorter.
Lung cancer screening is now recommended for high-risk individuals – those with at least a 20-pack-year history of tobacco use who are between 50 and 80 years old.
So far, screening is still limited to people at high risk, Dr. Hawk said, though there’s discussion about whether benefit would extend to people exposed to asbestos, for instance, or secondhand smoke.
“The biggest challenge right now is getting the screening to those who actually meet the criteria,” Dr. Hawk said.
Medscape reported earlier this month that less than 6% of high-risk smokers have the recommended annual lung cancer screening, according to a new report from the American Lung Association.
Dr. Henschke is on the Advisory Board for LungLifeAI and is on the board for the Early Diagnosis and Treatment Research Foundation. Dr. Hawk reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT RSNA 2022
Whole breast radiation for breast cancer shown to be safe and effective
The findings from a phase 3 clinical trial are a boon to patient convenience.
“These findings are indeed practice changing. This was a well-designed trial that looked at shortening treatment from 6 weeks down to 3 weeks. And, they showed equivalent local control and importantly, a good cosmetic outcome over time,” said Kathleen Horst, MD, who served as a discussant at a press conference held at the annual meeting of the American Society for Radiation Oncology where the findings were presented.
“This is substantially more convenient. It is cost effective, both for the health care system and for individual patients. Importantly, our patients come in for treatment every day. They’re taking time off of work, they have to arrange for childcare, and they have to arrange for transportation. So this makes a big difference for these patients,” said Dr. Horst, who is a professor of radiation oncology at Stanford (Calif.) Medicine and director of well-being in the radiation department at Stanford Medicine.
The study was presented by Frank A. Vicini, MD, FASTRO, a radiation oncologist with GenesisCare, Farmington Hills, Mich.
“One of the things I think that was surprising is I think all of us were thinking that this might be a more toxic regimen, but as Dr. Vincini showed, over time it was equally effective and with minimal toxicity, and cosmesis over time was stable, and that’s important. Importantly, that included patient-reported outcomes, not just the physician-reported outcomes. Broadly, I think these findings are applicable for many patients, all patients who are receiving whole breast radiotherapy with an added boost. I think over time this is going to improve the quality of life of our patients. It is an innovative change that everyone is going to be excited to embrace,” Dr. Horst said.
Previous randomized, controlled trials showed that an additional radiation dose to the tumor bed following lumpectomy and whole breast irradiation reduces the relative risk of local recurrence by about 35%. However, this increases treatment time for patients who have already endured an extensive regimen. For whole breast irradiation, hypofractionated radiation is in 15-16 fractions over 3 weeks has comparable recurrence rates as a 5-week regimen, but the relevant trials did not examine the effect hypofractionation may have on a radiation boost to the tumor bed of high-risk patients. Because of this lack of evidence, current practice is for the boost to remain sequential in five to eight fractions after completion of whole breast irradiation, which adds a week to a week and a half to treatment length.
The study included 2,262 patients who were randomized to receive a sequential boost or a concomitant boost. After a median follow-up of 7.4 years, there were 54 ipsilateral breast recurrence (IBR) events. The estimated 7-year risk of IBR was 2.2% in the sequential boost and 2.6% in the concurrent risk group (hazard ratio, 1.32; noninferiority test P = .039). Approximately 60% of patients received adjuvant chemotherapy.
Grade 3 or higher adverse events were similar, with a frequency of 3.3% in the sequential group and 3.5% in the concurrent group (P = .79). The researchers used the Global Cosmetic Score to assess outcomes from the perspective of both physicians and patients; 86% of physicians rated the outcome as excellent/good in the sequential group versus 82% in the concurrent group (P = .33).
“For high-risk early-stage breast cancer patients undergoing breast conservation, a concurrent boost with hypofractionated whole breast irradiation as compared to a sequential boost, results in noninferior local recurrence rates with no significant difference in toxicity, noninferior patient-rated cosmesis, no significant difference in physician rated cosmesis, and delivering the entire treatment even at high risk patients in 3 weeks. Just as critical, the use of target volume–based radiation planning for 3-D [three-dimensional] conformal or [intensity-modulated radiation therapy] whole breast irradiation assessed by dose volume analysis is feasible, and resulted in very low toxicity in the treatment arms, regardless of the fractionation schedule, or the boost delivery,” said Dr. Vincini during the press conference.
The study was grant funded. Neither Dr. Vincini nor Dr. Horst had relevant financial disclosures.
The findings from a phase 3 clinical trial are a boon to patient convenience.
“These findings are indeed practice changing. This was a well-designed trial that looked at shortening treatment from 6 weeks down to 3 weeks. And, they showed equivalent local control and importantly, a good cosmetic outcome over time,” said Kathleen Horst, MD, who served as a discussant at a press conference held at the annual meeting of the American Society for Radiation Oncology where the findings were presented.
“This is substantially more convenient. It is cost effective, both for the health care system and for individual patients. Importantly, our patients come in for treatment every day. They’re taking time off of work, they have to arrange for childcare, and they have to arrange for transportation. So this makes a big difference for these patients,” said Dr. Horst, who is a professor of radiation oncology at Stanford (Calif.) Medicine and director of well-being in the radiation department at Stanford Medicine.
The study was presented by Frank A. Vicini, MD, FASTRO, a radiation oncologist with GenesisCare, Farmington Hills, Mich.
“One of the things I think that was surprising is I think all of us were thinking that this might be a more toxic regimen, but as Dr. Vincini showed, over time it was equally effective and with minimal toxicity, and cosmesis over time was stable, and that’s important. Importantly, that included patient-reported outcomes, not just the physician-reported outcomes. Broadly, I think these findings are applicable for many patients, all patients who are receiving whole breast radiotherapy with an added boost. I think over time this is going to improve the quality of life of our patients. It is an innovative change that everyone is going to be excited to embrace,” Dr. Horst said.
Previous randomized, controlled trials showed that an additional radiation dose to the tumor bed following lumpectomy and whole breast irradiation reduces the relative risk of local recurrence by about 35%. However, this increases treatment time for patients who have already endured an extensive regimen. For whole breast irradiation, hypofractionated radiation is in 15-16 fractions over 3 weeks has comparable recurrence rates as a 5-week regimen, but the relevant trials did not examine the effect hypofractionation may have on a radiation boost to the tumor bed of high-risk patients. Because of this lack of evidence, current practice is for the boost to remain sequential in five to eight fractions after completion of whole breast irradiation, which adds a week to a week and a half to treatment length.
The study included 2,262 patients who were randomized to receive a sequential boost or a concomitant boost. After a median follow-up of 7.4 years, there were 54 ipsilateral breast recurrence (IBR) events. The estimated 7-year risk of IBR was 2.2% in the sequential boost and 2.6% in the concurrent risk group (hazard ratio, 1.32; noninferiority test P = .039). Approximately 60% of patients received adjuvant chemotherapy.
Grade 3 or higher adverse events were similar, with a frequency of 3.3% in the sequential group and 3.5% in the concurrent group (P = .79). The researchers used the Global Cosmetic Score to assess outcomes from the perspective of both physicians and patients; 86% of physicians rated the outcome as excellent/good in the sequential group versus 82% in the concurrent group (P = .33).
“For high-risk early-stage breast cancer patients undergoing breast conservation, a concurrent boost with hypofractionated whole breast irradiation as compared to a sequential boost, results in noninferior local recurrence rates with no significant difference in toxicity, noninferior patient-rated cosmesis, no significant difference in physician rated cosmesis, and delivering the entire treatment even at high risk patients in 3 weeks. Just as critical, the use of target volume–based radiation planning for 3-D [three-dimensional] conformal or [intensity-modulated radiation therapy] whole breast irradiation assessed by dose volume analysis is feasible, and resulted in very low toxicity in the treatment arms, regardless of the fractionation schedule, or the boost delivery,” said Dr. Vincini during the press conference.
The study was grant funded. Neither Dr. Vincini nor Dr. Horst had relevant financial disclosures.
The findings from a phase 3 clinical trial are a boon to patient convenience.
“These findings are indeed practice changing. This was a well-designed trial that looked at shortening treatment from 6 weeks down to 3 weeks. And, they showed equivalent local control and importantly, a good cosmetic outcome over time,” said Kathleen Horst, MD, who served as a discussant at a press conference held at the annual meeting of the American Society for Radiation Oncology where the findings were presented.
“This is substantially more convenient. It is cost effective, both for the health care system and for individual patients. Importantly, our patients come in for treatment every day. They’re taking time off of work, they have to arrange for childcare, and they have to arrange for transportation. So this makes a big difference for these patients,” said Dr. Horst, who is a professor of radiation oncology at Stanford (Calif.) Medicine and director of well-being in the radiation department at Stanford Medicine.
The study was presented by Frank A. Vicini, MD, FASTRO, a radiation oncologist with GenesisCare, Farmington Hills, Mich.
“One of the things I think that was surprising is I think all of us were thinking that this might be a more toxic regimen, but as Dr. Vincini showed, over time it was equally effective and with minimal toxicity, and cosmesis over time was stable, and that’s important. Importantly, that included patient-reported outcomes, not just the physician-reported outcomes. Broadly, I think these findings are applicable for many patients, all patients who are receiving whole breast radiotherapy with an added boost. I think over time this is going to improve the quality of life of our patients. It is an innovative change that everyone is going to be excited to embrace,” Dr. Horst said.
Previous randomized, controlled trials showed that an additional radiation dose to the tumor bed following lumpectomy and whole breast irradiation reduces the relative risk of local recurrence by about 35%. However, this increases treatment time for patients who have already endured an extensive regimen. For whole breast irradiation, hypofractionated radiation is in 15-16 fractions over 3 weeks has comparable recurrence rates as a 5-week regimen, but the relevant trials did not examine the effect hypofractionation may have on a radiation boost to the tumor bed of high-risk patients. Because of this lack of evidence, current practice is for the boost to remain sequential in five to eight fractions after completion of whole breast irradiation, which adds a week to a week and a half to treatment length.
The study included 2,262 patients who were randomized to receive a sequential boost or a concomitant boost. After a median follow-up of 7.4 years, there were 54 ipsilateral breast recurrence (IBR) events. The estimated 7-year risk of IBR was 2.2% in the sequential boost and 2.6% in the concurrent risk group (hazard ratio, 1.32; noninferiority test P = .039). Approximately 60% of patients received adjuvant chemotherapy.
Grade 3 or higher adverse events were similar, with a frequency of 3.3% in the sequential group and 3.5% in the concurrent group (P = .79). The researchers used the Global Cosmetic Score to assess outcomes from the perspective of both physicians and patients; 86% of physicians rated the outcome as excellent/good in the sequential group versus 82% in the concurrent group (P = .33).
“For high-risk early-stage breast cancer patients undergoing breast conservation, a concurrent boost with hypofractionated whole breast irradiation as compared to a sequential boost, results in noninferior local recurrence rates with no significant difference in toxicity, noninferior patient-rated cosmesis, no significant difference in physician rated cosmesis, and delivering the entire treatment even at high risk patients in 3 weeks. Just as critical, the use of target volume–based radiation planning for 3-D [three-dimensional] conformal or [intensity-modulated radiation therapy] whole breast irradiation assessed by dose volume analysis is feasible, and resulted in very low toxicity in the treatment arms, regardless of the fractionation schedule, or the boost delivery,” said Dr. Vincini during the press conference.
The study was grant funded. Neither Dr. Vincini nor Dr. Horst had relevant financial disclosures.
FROM ASTRO 2022
Newer agents for nosocomial pneumonia: The right drug for the right bug
“The right drug at the right time with the right dose for the right bug for the right duration.” That, said professor Kristina Crothers, MD, is the general guidance for optimizing antibiotic use (while awaiting an infectious disease consult). In her oral presentation at the annual meeting of the American College of Chest Physicians, “Choosing newer antibiotics for nosocomial pneumonia,” Dr. Crothers asked the question: “Beyond the guidelines: When should novel antimicrobials be used?”
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) are the most common nosocomial infections at 22%, and are the leading cause of death attributable to hospital-acquired infections. They increase mortality by 20%-50%, with an economic burden of about $40,000 per patient. The incidence of multidrug-resistant (MDR) organism infections varies widely by locality, but several factors increase the likelihood: prior broad-spectrum antibiotic exposure within the past 90 days; longer hospitalization; indwelling vascular devices; tracheostomy; and ventilator dependence. The Centers for Disease Control and Prevention lists as “Serious Threat” the HAP/VAP MDR organisms methicillin resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa (PSA) with difficult-to-treat-resistance, and beta-lactamase producing Enterobacterales (ESBL). In the category of “Urgent Threat” the CDC lists: carbapenamase-resistant Enterobacterales (CRE) (carbapenamase producing or non–carbapenemase producing), and carbapenem-resistant Acinetobacter (CRAB), according to Dr. Crothers who is at the University of Washington Veterans Affairs Puget Sound Health Care System, Seattle.
Newer antibiotics for HAP/VAP that are still beyond the guidelines include telavancin and tedizolid as gram-positive agents, and as gram-negative ones: ceftazidime-avibactam, ceftolozane-tazobactam, cefiderocol, imipenem-cilastatin-relebactam and meropenem-vaborbactam, she added.
Tedizolid, Dr. Crothers stated, is a novel oxazolidinone, and is an alternative to vancomycin and linezolid for gram-positive HAP/VAP. In the VITAL noninferiority study versus linezolid with 726 patients, it was noninferior to linezolid for 28-day all-cause mortality (28% vs. 26%), but did not achieve noninferiority for investigator-assessed clinical cure (56% vs. 64%).
Televancin, a semisynthetic derivative of vancomycin, in the ATTAIN studies vs. vancomycin had overall similar cure rates. It is FDA-approved for S. aureus HAP/VAP but not other bacterial causes. It should be reserved for those who cannot receive vancomycin or linezolid, with normal renal function, according to Dr. Crothers. Excluded from first-line treatment of gram-positive HAP/VAP are daptomycin, ceftaroline, ceftobiprole, and tigecycline.
Ceftazidime-avibactam, a third-generation cephalosporin-plus novel beta-lactamase inhibitor has wide activity (Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, PSA and Haemophilus influenzae. It is also active against some extended-spectrum beta-lactamases (ESBLs), ampC beta-lactamases (AmpCs), and K. pneumoniae carbapenemase (KPC)–producing Enterobacterales, but not with metallo-beta-lactamases). Ceftazidime-avibactam is also indicated for HAP/VAP, and has a toxicity profile including nausea, vomiting, and diarrhea.
In the REPROVE trial of ceftazidime-avibactam vs. meropenem for 7-14 days with 527 clinically evaluable patients (37% K. pneumoniae, 30% P. aeruginosa, and 33%-35% VAP), the clinical cure at 21-25 days post randomization was 69% vs. 73%, respectively, with similar adverse events.
Ceftolozane-tazobactam, a novel fifth-generation cephalosporin plus a beta-lactamase inhibitor has activity against PSA including extensively drug-resistant PSA, AmpC, and ESBL-E, but it has limited activity against Acinetobacter and Stenotrophomonas. It is indicated for HAP/VAP, has reduced efficacy with creatine clearance of 50 mL/min or less, increases transaminases and renal impairment, and causes diarrhea. In ASPECT-NP (n = 726) ceftolozane-tazobactam versus meropenem for 8-14 days (HAP/VAP), showed a 28 day-mortality of 24% vs. 25%, respectively, with test of cure at 54% vs. 53% at 7-14 days post therapy. Adverse events were similar between groups.
Imipenem-cilastatin-relebactam, a novel beta-lactamase inhibitor plus carbapenem, is indicated for HAP/VAP and has activity against ESBL, CRE: KPC-producing Enterobacterales, PSA including AmpC. It can cause seizures (requires caution with central nervous system disorders and renal impairment). It increases transaminases, anemia, diarrhea, and reduces potassium and sodium. In RESTORE-IMI 2 (n = 537 with HAP/VAP) it was noninferior for 28-day all-cause mortality vs. piperacillin and tazobactam (16% vs. 21%), with similar adverse events.
Cefiderocol, a siderophore cephalosporin, is indicated for HAP/VAP. It has a wide spectrum of activity: ESBL, CRE, CR PSA, Stenotrophomonas maltophilia, Acinetobacter baumanii, Streptococcus.) It increases transaminases, diarrhea, and atrial fibrillation, and it reduces potassium and magnesium. In APEKS-NP versus linezolid plus cefiderocol or extended meropenem infusion (HAP/VAP n = 292; gram-negative pneumonia = 251; 60% invasive mechanical ventilation) it was noninferior for 14-day all-cause mortality (12.4% vs. 11.6%) with similar adverse events. In CREDIBLE-CR vs. best available therapy for carbapenem-resistant gram-negative infections, clinical cure rates were similar (50% vs. 53% in 59 HAP/VAP patients at 7 days), but with more deaths in the cefiderocol arm. Adverse events were > 90% in both groups and 34% vs. 19% died, mostly with Acinetobacter.
Meropenem-vaborbactam, a novel beta-lactamase inhibitor plus carbapenem, is approved and indicated for HAP/VAP in Europe. It has activity against MDR, Enterobacterales including CRE. Its toxicities include headache, phlebitis/infusion-site reactions and diarrhea. In TANGO-2 versus best available treatment for carbapenem-resistant Enterobacteriaceae (CRE) (n = 77, 47 with confirmed CRE), clinical cure was increased and mortality decreased compared with best available therapy. Treatment- and renal-related adverse events were lower for meropenem-vaborbactam.
In closing, Dr. Crothers cited advice from the paper by Tamma et al. (“Rethinking how Antibiotics are Prescribed” JAMA. 2018) about the need to review findings after therapy has been initiated to confirm the pneumonia diagnosis: Novel agents should be kept in reserve in the absence of MDR risk factors for MRSA and gram-negative bacilli; therapy should be deescalated after 48-72 hours if MDR organisms are not detected; and therapy should be directed to the specific organism detected. Most HAP and VAP in adults can be treated for 7 days, she added.
“Know indications for new therapeutic agents approved for nosocomial pneumonia,” she concluded.
Dr. Crothers reported having no disclosures.
“The right drug at the right time with the right dose for the right bug for the right duration.” That, said professor Kristina Crothers, MD, is the general guidance for optimizing antibiotic use (while awaiting an infectious disease consult). In her oral presentation at the annual meeting of the American College of Chest Physicians, “Choosing newer antibiotics for nosocomial pneumonia,” Dr. Crothers asked the question: “Beyond the guidelines: When should novel antimicrobials be used?”
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) are the most common nosocomial infections at 22%, and are the leading cause of death attributable to hospital-acquired infections. They increase mortality by 20%-50%, with an economic burden of about $40,000 per patient. The incidence of multidrug-resistant (MDR) organism infections varies widely by locality, but several factors increase the likelihood: prior broad-spectrum antibiotic exposure within the past 90 days; longer hospitalization; indwelling vascular devices; tracheostomy; and ventilator dependence. The Centers for Disease Control and Prevention lists as “Serious Threat” the HAP/VAP MDR organisms methicillin resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa (PSA) with difficult-to-treat-resistance, and beta-lactamase producing Enterobacterales (ESBL). In the category of “Urgent Threat” the CDC lists: carbapenamase-resistant Enterobacterales (CRE) (carbapenamase producing or non–carbapenemase producing), and carbapenem-resistant Acinetobacter (CRAB), according to Dr. Crothers who is at the University of Washington Veterans Affairs Puget Sound Health Care System, Seattle.
Newer antibiotics for HAP/VAP that are still beyond the guidelines include telavancin and tedizolid as gram-positive agents, and as gram-negative ones: ceftazidime-avibactam, ceftolozane-tazobactam, cefiderocol, imipenem-cilastatin-relebactam and meropenem-vaborbactam, she added.
Tedizolid, Dr. Crothers stated, is a novel oxazolidinone, and is an alternative to vancomycin and linezolid for gram-positive HAP/VAP. In the VITAL noninferiority study versus linezolid with 726 patients, it was noninferior to linezolid for 28-day all-cause mortality (28% vs. 26%), but did not achieve noninferiority for investigator-assessed clinical cure (56% vs. 64%).
Televancin, a semisynthetic derivative of vancomycin, in the ATTAIN studies vs. vancomycin had overall similar cure rates. It is FDA-approved for S. aureus HAP/VAP but not other bacterial causes. It should be reserved for those who cannot receive vancomycin or linezolid, with normal renal function, according to Dr. Crothers. Excluded from first-line treatment of gram-positive HAP/VAP are daptomycin, ceftaroline, ceftobiprole, and tigecycline.
Ceftazidime-avibactam, a third-generation cephalosporin-plus novel beta-lactamase inhibitor has wide activity (Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, PSA and Haemophilus influenzae. It is also active against some extended-spectrum beta-lactamases (ESBLs), ampC beta-lactamases (AmpCs), and K. pneumoniae carbapenemase (KPC)–producing Enterobacterales, but not with metallo-beta-lactamases). Ceftazidime-avibactam is also indicated for HAP/VAP, and has a toxicity profile including nausea, vomiting, and diarrhea.
In the REPROVE trial of ceftazidime-avibactam vs. meropenem for 7-14 days with 527 clinically evaluable patients (37% K. pneumoniae, 30% P. aeruginosa, and 33%-35% VAP), the clinical cure at 21-25 days post randomization was 69% vs. 73%, respectively, with similar adverse events.
Ceftolozane-tazobactam, a novel fifth-generation cephalosporin plus a beta-lactamase inhibitor has activity against PSA including extensively drug-resistant PSA, AmpC, and ESBL-E, but it has limited activity against Acinetobacter and Stenotrophomonas. It is indicated for HAP/VAP, has reduced efficacy with creatine clearance of 50 mL/min or less, increases transaminases and renal impairment, and causes diarrhea. In ASPECT-NP (n = 726) ceftolozane-tazobactam versus meropenem for 8-14 days (HAP/VAP), showed a 28 day-mortality of 24% vs. 25%, respectively, with test of cure at 54% vs. 53% at 7-14 days post therapy. Adverse events were similar between groups.
Imipenem-cilastatin-relebactam, a novel beta-lactamase inhibitor plus carbapenem, is indicated for HAP/VAP and has activity against ESBL, CRE: KPC-producing Enterobacterales, PSA including AmpC. It can cause seizures (requires caution with central nervous system disorders and renal impairment). It increases transaminases, anemia, diarrhea, and reduces potassium and sodium. In RESTORE-IMI 2 (n = 537 with HAP/VAP) it was noninferior for 28-day all-cause mortality vs. piperacillin and tazobactam (16% vs. 21%), with similar adverse events.
Cefiderocol, a siderophore cephalosporin, is indicated for HAP/VAP. It has a wide spectrum of activity: ESBL, CRE, CR PSA, Stenotrophomonas maltophilia, Acinetobacter baumanii, Streptococcus.) It increases transaminases, diarrhea, and atrial fibrillation, and it reduces potassium and magnesium. In APEKS-NP versus linezolid plus cefiderocol or extended meropenem infusion (HAP/VAP n = 292; gram-negative pneumonia = 251; 60% invasive mechanical ventilation) it was noninferior for 14-day all-cause mortality (12.4% vs. 11.6%) with similar adverse events. In CREDIBLE-CR vs. best available therapy for carbapenem-resistant gram-negative infections, clinical cure rates were similar (50% vs. 53% in 59 HAP/VAP patients at 7 days), but with more deaths in the cefiderocol arm. Adverse events were > 90% in both groups and 34% vs. 19% died, mostly with Acinetobacter.
Meropenem-vaborbactam, a novel beta-lactamase inhibitor plus carbapenem, is approved and indicated for HAP/VAP in Europe. It has activity against MDR, Enterobacterales including CRE. Its toxicities include headache, phlebitis/infusion-site reactions and diarrhea. In TANGO-2 versus best available treatment for carbapenem-resistant Enterobacteriaceae (CRE) (n = 77, 47 with confirmed CRE), clinical cure was increased and mortality decreased compared with best available therapy. Treatment- and renal-related adverse events were lower for meropenem-vaborbactam.
In closing, Dr. Crothers cited advice from the paper by Tamma et al. (“Rethinking how Antibiotics are Prescribed” JAMA. 2018) about the need to review findings after therapy has been initiated to confirm the pneumonia diagnosis: Novel agents should be kept in reserve in the absence of MDR risk factors for MRSA and gram-negative bacilli; therapy should be deescalated after 48-72 hours if MDR organisms are not detected; and therapy should be directed to the specific organism detected. Most HAP and VAP in adults can be treated for 7 days, she added.
“Know indications for new therapeutic agents approved for nosocomial pneumonia,” she concluded.
Dr. Crothers reported having no disclosures.
“The right drug at the right time with the right dose for the right bug for the right duration.” That, said professor Kristina Crothers, MD, is the general guidance for optimizing antibiotic use (while awaiting an infectious disease consult). In her oral presentation at the annual meeting of the American College of Chest Physicians, “Choosing newer antibiotics for nosocomial pneumonia,” Dr. Crothers asked the question: “Beyond the guidelines: When should novel antimicrobials be used?”
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) are the most common nosocomial infections at 22%, and are the leading cause of death attributable to hospital-acquired infections. They increase mortality by 20%-50%, with an economic burden of about $40,000 per patient. The incidence of multidrug-resistant (MDR) organism infections varies widely by locality, but several factors increase the likelihood: prior broad-spectrum antibiotic exposure within the past 90 days; longer hospitalization; indwelling vascular devices; tracheostomy; and ventilator dependence. The Centers for Disease Control and Prevention lists as “Serious Threat” the HAP/VAP MDR organisms methicillin resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa (PSA) with difficult-to-treat-resistance, and beta-lactamase producing Enterobacterales (ESBL). In the category of “Urgent Threat” the CDC lists: carbapenamase-resistant Enterobacterales (CRE) (carbapenamase producing or non–carbapenemase producing), and carbapenem-resistant Acinetobacter (CRAB), according to Dr. Crothers who is at the University of Washington Veterans Affairs Puget Sound Health Care System, Seattle.
Newer antibiotics for HAP/VAP that are still beyond the guidelines include telavancin and tedizolid as gram-positive agents, and as gram-negative ones: ceftazidime-avibactam, ceftolozane-tazobactam, cefiderocol, imipenem-cilastatin-relebactam and meropenem-vaborbactam, she added.
Tedizolid, Dr. Crothers stated, is a novel oxazolidinone, and is an alternative to vancomycin and linezolid for gram-positive HAP/VAP. In the VITAL noninferiority study versus linezolid with 726 patients, it was noninferior to linezolid for 28-day all-cause mortality (28% vs. 26%), but did not achieve noninferiority for investigator-assessed clinical cure (56% vs. 64%).
Televancin, a semisynthetic derivative of vancomycin, in the ATTAIN studies vs. vancomycin had overall similar cure rates. It is FDA-approved for S. aureus HAP/VAP but not other bacterial causes. It should be reserved for those who cannot receive vancomycin or linezolid, with normal renal function, according to Dr. Crothers. Excluded from first-line treatment of gram-positive HAP/VAP are daptomycin, ceftaroline, ceftobiprole, and tigecycline.
Ceftazidime-avibactam, a third-generation cephalosporin-plus novel beta-lactamase inhibitor has wide activity (Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, PSA and Haemophilus influenzae. It is also active against some extended-spectrum beta-lactamases (ESBLs), ampC beta-lactamases (AmpCs), and K. pneumoniae carbapenemase (KPC)–producing Enterobacterales, but not with metallo-beta-lactamases). Ceftazidime-avibactam is also indicated for HAP/VAP, and has a toxicity profile including nausea, vomiting, and diarrhea.
In the REPROVE trial of ceftazidime-avibactam vs. meropenem for 7-14 days with 527 clinically evaluable patients (37% K. pneumoniae, 30% P. aeruginosa, and 33%-35% VAP), the clinical cure at 21-25 days post randomization was 69% vs. 73%, respectively, with similar adverse events.
Ceftolozane-tazobactam, a novel fifth-generation cephalosporin plus a beta-lactamase inhibitor has activity against PSA including extensively drug-resistant PSA, AmpC, and ESBL-E, but it has limited activity against Acinetobacter and Stenotrophomonas. It is indicated for HAP/VAP, has reduced efficacy with creatine clearance of 50 mL/min or less, increases transaminases and renal impairment, and causes diarrhea. In ASPECT-NP (n = 726) ceftolozane-tazobactam versus meropenem for 8-14 days (HAP/VAP), showed a 28 day-mortality of 24% vs. 25%, respectively, with test of cure at 54% vs. 53% at 7-14 days post therapy. Adverse events were similar between groups.
Imipenem-cilastatin-relebactam, a novel beta-lactamase inhibitor plus carbapenem, is indicated for HAP/VAP and has activity against ESBL, CRE: KPC-producing Enterobacterales, PSA including AmpC. It can cause seizures (requires caution with central nervous system disorders and renal impairment). It increases transaminases, anemia, diarrhea, and reduces potassium and sodium. In RESTORE-IMI 2 (n = 537 with HAP/VAP) it was noninferior for 28-day all-cause mortality vs. piperacillin and tazobactam (16% vs. 21%), with similar adverse events.
Cefiderocol, a siderophore cephalosporin, is indicated for HAP/VAP. It has a wide spectrum of activity: ESBL, CRE, CR PSA, Stenotrophomonas maltophilia, Acinetobacter baumanii, Streptococcus.) It increases transaminases, diarrhea, and atrial fibrillation, and it reduces potassium and magnesium. In APEKS-NP versus linezolid plus cefiderocol or extended meropenem infusion (HAP/VAP n = 292; gram-negative pneumonia = 251; 60% invasive mechanical ventilation) it was noninferior for 14-day all-cause mortality (12.4% vs. 11.6%) with similar adverse events. In CREDIBLE-CR vs. best available therapy for carbapenem-resistant gram-negative infections, clinical cure rates were similar (50% vs. 53% in 59 HAP/VAP patients at 7 days), but with more deaths in the cefiderocol arm. Adverse events were > 90% in both groups and 34% vs. 19% died, mostly with Acinetobacter.
Meropenem-vaborbactam, a novel beta-lactamase inhibitor plus carbapenem, is approved and indicated for HAP/VAP in Europe. It has activity against MDR, Enterobacterales including CRE. Its toxicities include headache, phlebitis/infusion-site reactions and diarrhea. In TANGO-2 versus best available treatment for carbapenem-resistant Enterobacteriaceae (CRE) (n = 77, 47 with confirmed CRE), clinical cure was increased and mortality decreased compared with best available therapy. Treatment- and renal-related adverse events were lower for meropenem-vaborbactam.
In closing, Dr. Crothers cited advice from the paper by Tamma et al. (“Rethinking how Antibiotics are Prescribed” JAMA. 2018) about the need to review findings after therapy has been initiated to confirm the pneumonia diagnosis: Novel agents should be kept in reserve in the absence of MDR risk factors for MRSA and gram-negative bacilli; therapy should be deescalated after 48-72 hours if MDR organisms are not detected; and therapy should be directed to the specific organism detected. Most HAP and VAP in adults can be treated for 7 days, she added.
“Know indications for new therapeutic agents approved for nosocomial pneumonia,” she concluded.
Dr. Crothers reported having no disclosures.
FROM CHEST 2022
‘Modest’ benefit for lecanemab in Alzheimer’s disease, but adverse events are common
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
AT CTAD 2022
NSAIDs for knee osteoarthritis may worsen pain over time
CHICAGO – Taking NSAIDs for knee osteoarthritis may worsen inflammation and pain over time, suggest new data revealed at the annual meeting of the Radiological Society of North America.
Johanna Luitjens, MD, a postdoctoral scholar in the department of radiology and biomedical Imaging at the University of California, San Francisco, told this news organization that NSAIDs are frequently used to treat OA pain because inflammation is one of the main drivers of OA, but whether they actually help outcomes has been unclear. Her study suggests that they don’t help – and may actually worsen – outcomes.
In particular, this study looked at the impact of NSAIDs on synovitis – the inflammation of the membrane lining the knee joint – by using MRI-based structural biomarkers.
OA, the most common form of arthritis, affects more than 32 million adults in the United States and more than 500 million people worldwide.
No approved therapy to reduce OA progression
Little is known of the long-term effects of NSAIDs on OA progression. Currently, there’s no approved therapy to cure OA or to reduce its advance.
Dr. Luitjens noted, however, that the synovial membrane mediates development and progression of OA and may be a good therapeutic target.
Researchers studied participants from the Osteoarthritis Initiative (OAI) cohort with moderate to severe OA who used NSAIDs regularly for at least 1 year between baseline and 4-year follow-up. All participants had high-quality 3T MRI of the knee at baseline and after 4 years. Images were scored for biomarkers of inflammation, including cartilage thickness and composition.
Dr. Luitjens and associates studied 721 participants who matched the inclusion criteria (129 with and 592 participants without regular NSAID use). The available data did not further specify amounts of NSAIDs used.
At baseline, significantly higher signal intensity in the infrapatellar fat pad (IFP) was seen in patients who used NSAID, compared with controls (adjusted difference in score, 0.26; 95% confidence interval, –0.5 to –0.129; P = .039).
In addition, at the end of the study period, there was a significantly greater increase in signal intensity of IFP (adjusted difference in score, 0.46; 95% CI, 0.2-0.72; P < .001) and higher increase in effusion synovitis (adjusted difference in score, 0.27; 95% CI, 0.06-0.47; P = .01) in NSAID users, compared with controls.
IFP size and synovial proliferation score did not different significantly between groups at the start of the study and showed no significant change over time.
The results showed no long-term benefit of NSAID use. Joint inflammation and cartilage quality were worse at baseline in the participants taking NSAIDs, compared with the control group, and worsened at 4-year follow-up.
Design limits strength
Amanda E. Nelson, MD, associate professor of medicine, division of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill, cautioned against assuming causality, pointing out that the OAI is an observational cohort study. (Dr. Nelson was not involved in the OAI or Dr. Luitjens’ analysis.)
“[The OAI is] large and well known, but it wasn’t designed to compare these groups, and this was a small subset,” she said in an interview. Without randomization, it’s hard to judge the results.
“It may be that people on NSAIDs for the duration of the study had more pain and had more disease to begin with, or had more symptoms or had failed other treatments,” she said, adding that the effect sizes were small.
Measures such as the IFP are ranked 0-3, so “the clinical difference of a 0.26 difference on a 0-3 scale is a bit uncertain,” she said.
Dr. Luitjens said that the researchers tried to adjust for potential confounders but agreed that randomized controlled trials are needed to better advise physicians and patients on the benefits or harms of using NSAIDs for OA.
Weighing the risks in older adults
Una Makris, MD, associate professor of internal medicine in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, noted that NSAIDs are “not always the safest option.”
“We are still in desperate need of disease-modifying drugs in OA with rigorous randomized trials to show efficacy for outcomes that are most meaningful to patients,” Dr. Makris, who was not involved in the study, told this news organization.
“OA is most common in older adults, those often with multiple comorbidities, so we must always weigh the risks – including known adverse effects which can be amplified in older adults – and benefits with the goal of improved function and less pain,” Dr. Makris said.
NSAID use also should be considered in the context of body mass index, cardiovascular risk, prior trauma or injury, other medication use, and behavioral factors, including physical activity, she said.
Dr. Luitjens, Dr. Nelson, and Dr. Makris reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Taking NSAIDs for knee osteoarthritis may worsen inflammation and pain over time, suggest new data revealed at the annual meeting of the Radiological Society of North America.
Johanna Luitjens, MD, a postdoctoral scholar in the department of radiology and biomedical Imaging at the University of California, San Francisco, told this news organization that NSAIDs are frequently used to treat OA pain because inflammation is one of the main drivers of OA, but whether they actually help outcomes has been unclear. Her study suggests that they don’t help – and may actually worsen – outcomes.
In particular, this study looked at the impact of NSAIDs on synovitis – the inflammation of the membrane lining the knee joint – by using MRI-based structural biomarkers.
OA, the most common form of arthritis, affects more than 32 million adults in the United States and more than 500 million people worldwide.
No approved therapy to reduce OA progression
Little is known of the long-term effects of NSAIDs on OA progression. Currently, there’s no approved therapy to cure OA or to reduce its advance.
Dr. Luitjens noted, however, that the synovial membrane mediates development and progression of OA and may be a good therapeutic target.
Researchers studied participants from the Osteoarthritis Initiative (OAI) cohort with moderate to severe OA who used NSAIDs regularly for at least 1 year between baseline and 4-year follow-up. All participants had high-quality 3T MRI of the knee at baseline and after 4 years. Images were scored for biomarkers of inflammation, including cartilage thickness and composition.
Dr. Luitjens and associates studied 721 participants who matched the inclusion criteria (129 with and 592 participants without regular NSAID use). The available data did not further specify amounts of NSAIDs used.
At baseline, significantly higher signal intensity in the infrapatellar fat pad (IFP) was seen in patients who used NSAID, compared with controls (adjusted difference in score, 0.26; 95% confidence interval, –0.5 to –0.129; P = .039).
In addition, at the end of the study period, there was a significantly greater increase in signal intensity of IFP (adjusted difference in score, 0.46; 95% CI, 0.2-0.72; P < .001) and higher increase in effusion synovitis (adjusted difference in score, 0.27; 95% CI, 0.06-0.47; P = .01) in NSAID users, compared with controls.
IFP size and synovial proliferation score did not different significantly between groups at the start of the study and showed no significant change over time.
The results showed no long-term benefit of NSAID use. Joint inflammation and cartilage quality were worse at baseline in the participants taking NSAIDs, compared with the control group, and worsened at 4-year follow-up.
Design limits strength
Amanda E. Nelson, MD, associate professor of medicine, division of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill, cautioned against assuming causality, pointing out that the OAI is an observational cohort study. (Dr. Nelson was not involved in the OAI or Dr. Luitjens’ analysis.)
“[The OAI is] large and well known, but it wasn’t designed to compare these groups, and this was a small subset,” she said in an interview. Without randomization, it’s hard to judge the results.
“It may be that people on NSAIDs for the duration of the study had more pain and had more disease to begin with, or had more symptoms or had failed other treatments,” she said, adding that the effect sizes were small.
Measures such as the IFP are ranked 0-3, so “the clinical difference of a 0.26 difference on a 0-3 scale is a bit uncertain,” she said.
Dr. Luitjens said that the researchers tried to adjust for potential confounders but agreed that randomized controlled trials are needed to better advise physicians and patients on the benefits or harms of using NSAIDs for OA.
Weighing the risks in older adults
Una Makris, MD, associate professor of internal medicine in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, noted that NSAIDs are “not always the safest option.”
“We are still in desperate need of disease-modifying drugs in OA with rigorous randomized trials to show efficacy for outcomes that are most meaningful to patients,” Dr. Makris, who was not involved in the study, told this news organization.
“OA is most common in older adults, those often with multiple comorbidities, so we must always weigh the risks – including known adverse effects which can be amplified in older adults – and benefits with the goal of improved function and less pain,” Dr. Makris said.
NSAID use also should be considered in the context of body mass index, cardiovascular risk, prior trauma or injury, other medication use, and behavioral factors, including physical activity, she said.
Dr. Luitjens, Dr. Nelson, and Dr. Makris reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Taking NSAIDs for knee osteoarthritis may worsen inflammation and pain over time, suggest new data revealed at the annual meeting of the Radiological Society of North America.
Johanna Luitjens, MD, a postdoctoral scholar in the department of radiology and biomedical Imaging at the University of California, San Francisco, told this news organization that NSAIDs are frequently used to treat OA pain because inflammation is one of the main drivers of OA, but whether they actually help outcomes has been unclear. Her study suggests that they don’t help – and may actually worsen – outcomes.
In particular, this study looked at the impact of NSAIDs on synovitis – the inflammation of the membrane lining the knee joint – by using MRI-based structural biomarkers.
OA, the most common form of arthritis, affects more than 32 million adults in the United States and more than 500 million people worldwide.
No approved therapy to reduce OA progression
Little is known of the long-term effects of NSAIDs on OA progression. Currently, there’s no approved therapy to cure OA or to reduce its advance.
Dr. Luitjens noted, however, that the synovial membrane mediates development and progression of OA and may be a good therapeutic target.
Researchers studied participants from the Osteoarthritis Initiative (OAI) cohort with moderate to severe OA who used NSAIDs regularly for at least 1 year between baseline and 4-year follow-up. All participants had high-quality 3T MRI of the knee at baseline and after 4 years. Images were scored for biomarkers of inflammation, including cartilage thickness and composition.
Dr. Luitjens and associates studied 721 participants who matched the inclusion criteria (129 with and 592 participants without regular NSAID use). The available data did not further specify amounts of NSAIDs used.
At baseline, significantly higher signal intensity in the infrapatellar fat pad (IFP) was seen in patients who used NSAID, compared with controls (adjusted difference in score, 0.26; 95% confidence interval, –0.5 to –0.129; P = .039).
In addition, at the end of the study period, there was a significantly greater increase in signal intensity of IFP (adjusted difference in score, 0.46; 95% CI, 0.2-0.72; P < .001) and higher increase in effusion synovitis (adjusted difference in score, 0.27; 95% CI, 0.06-0.47; P = .01) in NSAID users, compared with controls.
IFP size and synovial proliferation score did not different significantly between groups at the start of the study and showed no significant change over time.
The results showed no long-term benefit of NSAID use. Joint inflammation and cartilage quality were worse at baseline in the participants taking NSAIDs, compared with the control group, and worsened at 4-year follow-up.
Design limits strength
Amanda E. Nelson, MD, associate professor of medicine, division of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill, cautioned against assuming causality, pointing out that the OAI is an observational cohort study. (Dr. Nelson was not involved in the OAI or Dr. Luitjens’ analysis.)
“[The OAI is] large and well known, but it wasn’t designed to compare these groups, and this was a small subset,” she said in an interview. Without randomization, it’s hard to judge the results.
“It may be that people on NSAIDs for the duration of the study had more pain and had more disease to begin with, or had more symptoms or had failed other treatments,” she said, adding that the effect sizes were small.
Measures such as the IFP are ranked 0-3, so “the clinical difference of a 0.26 difference on a 0-3 scale is a bit uncertain,” she said.
Dr. Luitjens said that the researchers tried to adjust for potential confounders but agreed that randomized controlled trials are needed to better advise physicians and patients on the benefits or harms of using NSAIDs for OA.
Weighing the risks in older adults
Una Makris, MD, associate professor of internal medicine in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, noted that NSAIDs are “not always the safest option.”
“We are still in desperate need of disease-modifying drugs in OA with rigorous randomized trials to show efficacy for outcomes that are most meaningful to patients,” Dr. Makris, who was not involved in the study, told this news organization.
“OA is most common in older adults, those often with multiple comorbidities, so we must always weigh the risks – including known adverse effects which can be amplified in older adults – and benefits with the goal of improved function and less pain,” Dr. Makris said.
NSAID use also should be considered in the context of body mass index, cardiovascular risk, prior trauma or injury, other medication use, and behavioral factors, including physical activity, she said.
Dr. Luitjens, Dr. Nelson, and Dr. Makris reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT RSNA 2022
Consider radiologic imaging for high-risk cutaneous SCC, expert advises
DENVER –
In a study published in 2020, Emily Ruiz, MD, MPH, and colleagues identified 87 CSCC tumors in 83 patients who underwent baseline or surveillance imaging primary at the Brigham and Women’s Hospital Mohs Surgery Clinic and the Dana-Farber Cancer Institute High-Risk Skin Cancer Clinic, both in Boston, from Jan. 1, 2017, to June 1, 2019. Of the 87 primary CSCCs, 48 (58%) underwent surveillance imaging. The researchers found that imaging detected additional disease in 26 patients, or 30% of cases, “whether that be nodal metastasis, local invasion beyond what was clinically accepted, or in-transit disease,” Dr. Ruiz, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s, said during the annual meeting of the American Society for Dermatologic Surgery. “But if you look at the 16 nodal metastases in this cohort, all were picked up on imaging and not on clinical exam.”
Since publication of these results, Dr. Ruiz routinely considers baseline radiologic imaging in T2b and T3 tumors; borderline T2a tumors (which she said they are now calling “T2a high,” for those who have one risk factor plus another intermediate risk factor),” and T2a tumors in patients who are profoundly immunosuppressed.
“My preference is to always do [the imaging] before treatment unless I’m up-staging them during surgery,” said Dr. Ruiz, who also directs the High-Risk Skin Cancer Clinic at Dana Farber. “We have picked up nodal metastases before surgery, which enables us to create a good therapeutic plan for our patients before we start operating. Then we image them every 6 months or so for about 2 years. Sometimes we will extend that out to 3 years.”
Some clinicians use sentinel lymph node biopsy (SLNB) as a diagnostic test, but there are mixed results about its prognostic significance. A retrospective observational study of 720 patients with CSCC found that SLNB provided no benefit regarding further metastasis or tumor-specific survival, compared with those who received routine observation and follow-up, “but head and neck surgeons in the U.S. are putting together some prospective data from multiple centers,” Dr. Ruiz said. “I think in the coming years, you will have more multicenter data to inform us as to whether to do SLNB or not.”
Surgery may be the mainstay of treatment for resectable SCC, but the emerging role of neoadjuvant therapeutics is changing the way oncologists treat these tumors. For example, in a phase 2 trial recently published in the New England Journal of Medicine, 79 patients with stage II-IV CSCC received up to four doses of immunotherapy with the programmed death receptor–1 (PD-1) blocker cemiplimab administered every 3 weeks. The primary endpoint was a pathologic complete response, defined as the absence of viable tumor cells in the surgical specimen at a central laboratory. The researchers observed that 68% of patients had an objective response.
“These were patients with localized tumors that were either very aggressive or had nodal metastases,” said Dr, Ruiz, who was the site primary investigator at Dana Farber and a coauthor of the NEJM study. “This has altered the way we approach treating our larger tumors that could be resectable but have a lot of disease either locally or in the nodal basin. We think that we can shrink down the tumor and make it easier to resect, but also there is the possibility or improving outcomes.”
At Brigham and Women’s and the Dana Farber, she and her colleagues consider immunotherapy for multiple recurrent tumors that have been previously irradiated; cases of large tumor burden locally or in the nodal basin; tumors that have a complex surgical plan; cases where there is a low likelihood of achieving clear surgical margins; and cases of in-transit disease.
“We use two to four doses of immunotherapy prior to surgery and assess the tumor response after two doses both clinically and radiologically,” she said. “If the tumor continues to grow, we would do surgery sooner.”
The side-effect profile of immunotherapy is another consideration. “Some patients are not appropriate for a neoadjuvant immunotherapy approach, such as transplant patients,” she said.
According to the latest National Comprehensive Cancer Network guidelines, surgery with or without adjuvant radiation is the current standard of care for treating CSCC. These guidelines were developed without much data to support the use of radiation, but a 20-year retrospective cohort study at Brigham and Women’s Hospital and the Cleveland Clinic Foundation found that adjuvant radiation following margin resection in high T-stage CSCC cut the risk of local and locoregional recurrence in half.
“This is something that radiation oncologists have told us for years, but there was no data to support it, so it was nice to see that borne out in clinical data,” said Dr. Ruiz, the study’s lead author. The 10% risk of local recurrence observed in the study “may not be high enough for some of our older patients, so we wanted to see if we could identify a group of high tumors that had higher risk of local recurrence,” she said. They found that patients who had a greater than 20% risk of poor outcome were those with recurrent tumors, those with tumors 6 cm or greater in size, and those with all four BWH risk factors (tumor diameter ≥ 2 cm, poorly differentiated histology, perineural invasion ≥ 0.1 mm, or tumor invasion beyond fat excluding bone invasion).
“Those risks were also cut in half if you added radiation,” she said. “So, the way I now approach counseling patients is, I try to estimate their baseline risk as best I can based on the tumor itself. I tell them that if they want to do adjuvant radiation it would cut the risk in half. Some patients are too frail and want to pass on it, while others are very interested.”
Of patients who did not receive radiation but had a disease recurrence, just under half of tumors were salvageable, about 25% died of their disease, and 23% had persistent disease. “I think this does support using radiation earlier on for the appropriate patient,” Dr. Ruiz said. “I consider the baseline risks [and] balance that with the patient’s comorbidities.”
Limited data exists on adjuvant immunotherapy for CSCC, but two ongoing randomized prospective clinical trials underway are studying the PD-1 inhibitors cemiplimab and pembrolizumab versus placebo. “We don’t have data yet, but prior to randomization, patients undergo surgery with macroscopic gross resection of all disease,” Dr. Ruiz said. “All tumors receive ART [adjuvant radiation therapy] prior to randomization”
Dr. Ruiz disclosed that she is a consultant for Sanofi, Regeneron, Genentech, and Jaunce Therapeutics. She is also a member of the advisory board for Checkpoint Therapeutics and is an investigator for Merck, Sanofi, and Regeneron.
DENVER –
In a study published in 2020, Emily Ruiz, MD, MPH, and colleagues identified 87 CSCC tumors in 83 patients who underwent baseline or surveillance imaging primary at the Brigham and Women’s Hospital Mohs Surgery Clinic and the Dana-Farber Cancer Institute High-Risk Skin Cancer Clinic, both in Boston, from Jan. 1, 2017, to June 1, 2019. Of the 87 primary CSCCs, 48 (58%) underwent surveillance imaging. The researchers found that imaging detected additional disease in 26 patients, or 30% of cases, “whether that be nodal metastasis, local invasion beyond what was clinically accepted, or in-transit disease,” Dr. Ruiz, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s, said during the annual meeting of the American Society for Dermatologic Surgery. “But if you look at the 16 nodal metastases in this cohort, all were picked up on imaging and not on clinical exam.”
Since publication of these results, Dr. Ruiz routinely considers baseline radiologic imaging in T2b and T3 tumors; borderline T2a tumors (which she said they are now calling “T2a high,” for those who have one risk factor plus another intermediate risk factor),” and T2a tumors in patients who are profoundly immunosuppressed.
“My preference is to always do [the imaging] before treatment unless I’m up-staging them during surgery,” said Dr. Ruiz, who also directs the High-Risk Skin Cancer Clinic at Dana Farber. “We have picked up nodal metastases before surgery, which enables us to create a good therapeutic plan for our patients before we start operating. Then we image them every 6 months or so for about 2 years. Sometimes we will extend that out to 3 years.”
Some clinicians use sentinel lymph node biopsy (SLNB) as a diagnostic test, but there are mixed results about its prognostic significance. A retrospective observational study of 720 patients with CSCC found that SLNB provided no benefit regarding further metastasis or tumor-specific survival, compared with those who received routine observation and follow-up, “but head and neck surgeons in the U.S. are putting together some prospective data from multiple centers,” Dr. Ruiz said. “I think in the coming years, you will have more multicenter data to inform us as to whether to do SLNB or not.”
Surgery may be the mainstay of treatment for resectable SCC, but the emerging role of neoadjuvant therapeutics is changing the way oncologists treat these tumors. For example, in a phase 2 trial recently published in the New England Journal of Medicine, 79 patients with stage II-IV CSCC received up to four doses of immunotherapy with the programmed death receptor–1 (PD-1) blocker cemiplimab administered every 3 weeks. The primary endpoint was a pathologic complete response, defined as the absence of viable tumor cells in the surgical specimen at a central laboratory. The researchers observed that 68% of patients had an objective response.
“These were patients with localized tumors that were either very aggressive or had nodal metastases,” said Dr, Ruiz, who was the site primary investigator at Dana Farber and a coauthor of the NEJM study. “This has altered the way we approach treating our larger tumors that could be resectable but have a lot of disease either locally or in the nodal basin. We think that we can shrink down the tumor and make it easier to resect, but also there is the possibility or improving outcomes.”
At Brigham and Women’s and the Dana Farber, she and her colleagues consider immunotherapy for multiple recurrent tumors that have been previously irradiated; cases of large tumor burden locally or in the nodal basin; tumors that have a complex surgical plan; cases where there is a low likelihood of achieving clear surgical margins; and cases of in-transit disease.
“We use two to four doses of immunotherapy prior to surgery and assess the tumor response after two doses both clinically and radiologically,” she said. “If the tumor continues to grow, we would do surgery sooner.”
The side-effect profile of immunotherapy is another consideration. “Some patients are not appropriate for a neoadjuvant immunotherapy approach, such as transplant patients,” she said.
According to the latest National Comprehensive Cancer Network guidelines, surgery with or without adjuvant radiation is the current standard of care for treating CSCC. These guidelines were developed without much data to support the use of radiation, but a 20-year retrospective cohort study at Brigham and Women’s Hospital and the Cleveland Clinic Foundation found that adjuvant radiation following margin resection in high T-stage CSCC cut the risk of local and locoregional recurrence in half.
“This is something that radiation oncologists have told us for years, but there was no data to support it, so it was nice to see that borne out in clinical data,” said Dr. Ruiz, the study’s lead author. The 10% risk of local recurrence observed in the study “may not be high enough for some of our older patients, so we wanted to see if we could identify a group of high tumors that had higher risk of local recurrence,” she said. They found that patients who had a greater than 20% risk of poor outcome were those with recurrent tumors, those with tumors 6 cm or greater in size, and those with all four BWH risk factors (tumor diameter ≥ 2 cm, poorly differentiated histology, perineural invasion ≥ 0.1 mm, or tumor invasion beyond fat excluding bone invasion).
“Those risks were also cut in half if you added radiation,” she said. “So, the way I now approach counseling patients is, I try to estimate their baseline risk as best I can based on the tumor itself. I tell them that if they want to do adjuvant radiation it would cut the risk in half. Some patients are too frail and want to pass on it, while others are very interested.”
Of patients who did not receive radiation but had a disease recurrence, just under half of tumors were salvageable, about 25% died of their disease, and 23% had persistent disease. “I think this does support using radiation earlier on for the appropriate patient,” Dr. Ruiz said. “I consider the baseline risks [and] balance that with the patient’s comorbidities.”
Limited data exists on adjuvant immunotherapy for CSCC, but two ongoing randomized prospective clinical trials underway are studying the PD-1 inhibitors cemiplimab and pembrolizumab versus placebo. “We don’t have data yet, but prior to randomization, patients undergo surgery with macroscopic gross resection of all disease,” Dr. Ruiz said. “All tumors receive ART [adjuvant radiation therapy] prior to randomization”
Dr. Ruiz disclosed that she is a consultant for Sanofi, Regeneron, Genentech, and Jaunce Therapeutics. She is also a member of the advisory board for Checkpoint Therapeutics and is an investigator for Merck, Sanofi, and Regeneron.
DENVER –
In a study published in 2020, Emily Ruiz, MD, MPH, and colleagues identified 87 CSCC tumors in 83 patients who underwent baseline or surveillance imaging primary at the Brigham and Women’s Hospital Mohs Surgery Clinic and the Dana-Farber Cancer Institute High-Risk Skin Cancer Clinic, both in Boston, from Jan. 1, 2017, to June 1, 2019. Of the 87 primary CSCCs, 48 (58%) underwent surveillance imaging. The researchers found that imaging detected additional disease in 26 patients, or 30% of cases, “whether that be nodal metastasis, local invasion beyond what was clinically accepted, or in-transit disease,” Dr. Ruiz, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s, said during the annual meeting of the American Society for Dermatologic Surgery. “But if you look at the 16 nodal metastases in this cohort, all were picked up on imaging and not on clinical exam.”
Since publication of these results, Dr. Ruiz routinely considers baseline radiologic imaging in T2b and T3 tumors; borderline T2a tumors (which she said they are now calling “T2a high,” for those who have one risk factor plus another intermediate risk factor),” and T2a tumors in patients who are profoundly immunosuppressed.
“My preference is to always do [the imaging] before treatment unless I’m up-staging them during surgery,” said Dr. Ruiz, who also directs the High-Risk Skin Cancer Clinic at Dana Farber. “We have picked up nodal metastases before surgery, which enables us to create a good therapeutic plan for our patients before we start operating. Then we image them every 6 months or so for about 2 years. Sometimes we will extend that out to 3 years.”
Some clinicians use sentinel lymph node biopsy (SLNB) as a diagnostic test, but there are mixed results about its prognostic significance. A retrospective observational study of 720 patients with CSCC found that SLNB provided no benefit regarding further metastasis or tumor-specific survival, compared with those who received routine observation and follow-up, “but head and neck surgeons in the U.S. are putting together some prospective data from multiple centers,” Dr. Ruiz said. “I think in the coming years, you will have more multicenter data to inform us as to whether to do SLNB or not.”
Surgery may be the mainstay of treatment for resectable SCC, but the emerging role of neoadjuvant therapeutics is changing the way oncologists treat these tumors. For example, in a phase 2 trial recently published in the New England Journal of Medicine, 79 patients with stage II-IV CSCC received up to four doses of immunotherapy with the programmed death receptor–1 (PD-1) blocker cemiplimab administered every 3 weeks. The primary endpoint was a pathologic complete response, defined as the absence of viable tumor cells in the surgical specimen at a central laboratory. The researchers observed that 68% of patients had an objective response.
“These were patients with localized tumors that were either very aggressive or had nodal metastases,” said Dr, Ruiz, who was the site primary investigator at Dana Farber and a coauthor of the NEJM study. “This has altered the way we approach treating our larger tumors that could be resectable but have a lot of disease either locally or in the nodal basin. We think that we can shrink down the tumor and make it easier to resect, but also there is the possibility or improving outcomes.”
At Brigham and Women’s and the Dana Farber, she and her colleagues consider immunotherapy for multiple recurrent tumors that have been previously irradiated; cases of large tumor burden locally or in the nodal basin; tumors that have a complex surgical plan; cases where there is a low likelihood of achieving clear surgical margins; and cases of in-transit disease.
“We use two to four doses of immunotherapy prior to surgery and assess the tumor response after two doses both clinically and radiologically,” she said. “If the tumor continues to grow, we would do surgery sooner.”
The side-effect profile of immunotherapy is another consideration. “Some patients are not appropriate for a neoadjuvant immunotherapy approach, such as transplant patients,” she said.
According to the latest National Comprehensive Cancer Network guidelines, surgery with or without adjuvant radiation is the current standard of care for treating CSCC. These guidelines were developed without much data to support the use of radiation, but a 20-year retrospective cohort study at Brigham and Women’s Hospital and the Cleveland Clinic Foundation found that adjuvant radiation following margin resection in high T-stage CSCC cut the risk of local and locoregional recurrence in half.
“This is something that radiation oncologists have told us for years, but there was no data to support it, so it was nice to see that borne out in clinical data,” said Dr. Ruiz, the study’s lead author. The 10% risk of local recurrence observed in the study “may not be high enough for some of our older patients, so we wanted to see if we could identify a group of high tumors that had higher risk of local recurrence,” she said. They found that patients who had a greater than 20% risk of poor outcome were those with recurrent tumors, those with tumors 6 cm or greater in size, and those with all four BWH risk factors (tumor diameter ≥ 2 cm, poorly differentiated histology, perineural invasion ≥ 0.1 mm, or tumor invasion beyond fat excluding bone invasion).
“Those risks were also cut in half if you added radiation,” she said. “So, the way I now approach counseling patients is, I try to estimate their baseline risk as best I can based on the tumor itself. I tell them that if they want to do adjuvant radiation it would cut the risk in half. Some patients are too frail and want to pass on it, while others are very interested.”
Of patients who did not receive radiation but had a disease recurrence, just under half of tumors were salvageable, about 25% died of their disease, and 23% had persistent disease. “I think this does support using radiation earlier on for the appropriate patient,” Dr. Ruiz said. “I consider the baseline risks [and] balance that with the patient’s comorbidities.”
Limited data exists on adjuvant immunotherapy for CSCC, but two ongoing randomized prospective clinical trials underway are studying the PD-1 inhibitors cemiplimab and pembrolizumab versus placebo. “We don’t have data yet, but prior to randomization, patients undergo surgery with macroscopic gross resection of all disease,” Dr. Ruiz said. “All tumors receive ART [adjuvant radiation therapy] prior to randomization”
Dr. Ruiz disclosed that she is a consultant for Sanofi, Regeneron, Genentech, and Jaunce Therapeutics. She is also a member of the advisory board for Checkpoint Therapeutics and is an investigator for Merck, Sanofi, and Regeneron.
AT ASDS 2022