User login
MDedge latest news is breaking news from medical conferences, journals, guidelines, the FDA and CDC.
Fibroids: Medical Therapy Not Hysterectomy Should Be First Treatment Choice Interventional Options Case Study
Although hysterectomy remains the most common procedure for treating fibroids and fibroids are the leading indication for hysterectomy, its long-term sequelae make less invasive alternatives the better choice for managing most of these myometrial masses, an invited clinical practice paper in the New England Journal of Medicine (NEJM) asserts.
The practice summary also calls for earlier identification and treatment of fibroid disease and may raise awareness among general gynecologists and primary care physicians less familiar with newer treatments.
Based on a review of evidence and existing formal guidelines, the paper urges wider use of uterus-sparing approaches such as hormone therapy, uterine-artery embolization, focused ultrasound ablation, and radiofrequency ablation. Authored by ob.gyns. Elizabeth A. Stewart, MD, and Shannon K. Laughlin-Tommaso, MD, MPH, of the Mayo Clinic in Rochester, Minnesota, the document also features textbook-style diagrams illustrating procedures.
“To clarify, this is not a new guidance but an invited clinical practice paper,” Laughlin-Tommaso told this news organization. “I believe NEJM recognized the gap in knowledge among all providers, for early diagnosis of uterine fibroids, especially in young patients and those presenting with anemia.”
The less invasive treatments highlighted in the paper can help women recover faster and resume their normal activities more quickly, said Laughlin-Tommaso. “Additionally, many studies have now shown that there are health benefits to keeping the uterus and the ovaries.”
Despite multiple uterine-sparing options, however, a recent study in a commercially insured population found nearly 60% of fibroid patients undergoing hysterectomy had never received a prior conservative treatment.
Why hysterectomy for a benign condition? Hysterectomy, which is universally available in ob.gyn. practices, makes decision-making easier for medical providers and patients, Laughlin-Tommaso explained. “It’s the only treatment that is definitive in that patients will not have bleeding or fibroids in the future and providers don’t have to determine which fibroids to treat or remove.”
More common in Black women, fibroids affect up to 80% of persons with a uterus during their lifetime and up to 50% have symptoms such as heavy and prolonged menstrual bleeding, anemia-associated fatigue, pelvic pressure, and menstrual and nonmenstrual pain, the authors noted. These lesions can also compress nearby structures causing painful intercourse, constipation, and urinary frequency, urgency, or retention.
In 2021 the American College of Obstetricians and Gynecologists issued a practice bulletin on the management of symptomatic uterine leiomyomas, similarly endorsing individualized care that accounts for the desire to preserve fertility or the uterus, increase quality of life, and reduce symptoms. It, too, recommended medical management as first-line treatment for symptomatic fibroids.
“This paper will be helpful for clinicians by covering some of the newer options such as gonadotropin-releasing hormone antagonists introduced in the past 5 years and tailoring treatment to patients depending on whether they still want to conceive,” Sandra M. Hurtado, MD, an ob.gyn. and an assistant professor at UTHealth Houston Medical Center and McGovern Medical School in Houston, Texas, said in an interview. “And the illustrations will be useful to doctors who are not gynecologists and will help to explain the interventional options to patients,” added Hurtado, who was not involved in the paper.
Offering another outside perspective on the paper, Charles J. Ascher-Walsh, MD, senior system vice chair for gynecology and division director of urogynecology in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology and Reproductive Science at Mount Sinai in New York City, called it a useful though not new summary. Reaching the wider audience of NEJM may raise awareness of newer fibroid therapies among general, nonspecialist ob.gyns., whose practices may concentrate largely on obstetrics, he added, “and the excellent illustrations clarify the treatment options.” In his view, broader awareness may increase much-needed funding for this neglected area of research.
Among the paper’s recommendations:
Diagnosis
Pelvic ultrasonography is the most cost-effective imaging method, providing information on size, location, and number of fibroids and ruling out adnexal masses. It is limited, however, by less-accurate resolution if the uterine volume is greater than 375 mL or if fibroids number more than four.
Medical Alternatives to Hysterectomy
Early diagnosis and first-line medical therapies are recommended.
Contraceptive hormones to control heavy menstrual bleeding are the first step in most algorithms for treating fibroid-related bleeding, despite low-quality evidence.
Nonsteroidal anti-inflammatory agents and tranexamic acid during menstruation also limit heavy menses but have more evidence of efficacy for idiopathic heavy menses.
Gonadotropin-releasing hormone (GnRH) agonists in depot form are approved for short-term preoperative therapy. While they cause amenorrhea in nearly 90% of patients and reduce uterine volume by 30%-60%, they have a high incidence of hypogonadal symptoms, including bone loss and hot flushes. They also cause a “steroidal flare” when the stored gonadotropins are released and cause subsequent heavy menstrual bleeding with the rapid decrease in estrogen levels.
Oral GnRH antagonist combinations are a major therapeutic advance, pairing a GnRH antagonist (such as elagolix or relugolix, which rapidly inhibit ovarian steroidogenesis) with estradiol and progestin at doses equivalent to systemic levels in the early follicular phase of the menstrual cycle.
In clinical trials these combinations decreased heavy menstrual bleeding by 50%-75%, pain by 40%-50%, and bulk-related symptoms through a 10% decrease in uterine volume. Side effects are few, with hot flushes, headaches, and nausea occurring in fewer than 20% of participants.
Smaller fibroids in the submucosal to intramural spaces can be treated transcervically, while larger lesions of any type or smaller subserosa fibroids are treated abdominally.
Uterine-artery embolization uses minimally invasive radiologically guided catheterization to release embolic particles directly into both uterine arteries. This process causes ischemic infarction of the fibroids and decreases bleeding, pain, and bulk-related symptoms.
Other procedures shrink individual fibroids with energy that creates coagulative necrosis. These include focused ultrasound ablation (with MRI or ultrasound guidance) and radiofrequency ablation (with laparoscopic or transcervical ultrasound guidance).
Unlike uterine-artery embolization, which treats all fibroids concurrently, these therapies require individual targeting of fibroids.
Radiofrequency ablation can be done concurrently with other surgical therapies, such as laparoscopic excision of endometriosis or hysteroscopic myomectomy.
Myomectomy, or the surgical removal of fibroids, is most often used in persons actively seeking pregnancy or having very large fibroids in whom shrinkage would be inadequate. Most guidelines recommend surgical excision rather than shrinking procedures to optimize fertility. However, myomectomy often commits patients to future cesarean section, which increases pregnancy-related morbidity.
Although myomectomy is seen as superior to uterine-artery embolization for improving quality of life, both approaches provide substantial symptom relief.
Recurrence
Incidence of recurring fibroids is high, with, for example, new fibroids developing in approximately 50% of persons within 5 years of myomectomy.
Earlier this year, a large cohort study reported that myomectomy was best for avoiding reintervention after surgical leiomyoma management.
Reintervention rates vary according to procedure, patient age, disease extent, and symptoms and can be as high as 33% up to 5 years after treatment, with lower percentages seen among persons older than 45 years of age.
Hysterectomy
Minimally invasive hysterectomy is recommended. Drawbacks to hysterectomy include perioperative risk and concomitant oophorectomy, which was common until the early 2000s when large cohort studies showed elevated risks of death, cardiovascular disease, dementia, and other illnesses compared with hysterectomy plus ovarian conservation. Oophorectomy but not hysterectomy rates have since decreased.
Still needing study, according to Laughlin-Tommaso are the underlying reasons for health disparities in fibroids, especially among Black and Latina individuals. “Some studies have found associations with vitamin D deficiency and with stress and racism,” she said.
Looking ahead, the authors stressed the need for a fibroid risk-prediction model, a staging system, and large randomized trials of treatment effectiveness. Also needed are methods for primary and secondary prevention. “Earlier screening and medical treatment in primary care settings could potentially minimize morbidity and the incidence of unnecessary hysterectomies, and primary care–based screening trials are warranted,” they wrote.
In addition to procedural illustrations the practice document includes a vignette of a 33-year-old never-pregnant Black woman (but desiring motherhood) with heavy menstrual bleeding, abdominal bloating, and non–iron deficiency anemia. Evaluation for thalassemia and sickle cell anemia is negative, but ultrasonography reveals an enlarged uterus with multiple fibroids and normal ovaries.
In line with the clinical review, the authors prescribe oral GnRH agonist combination therapy, plus iron and multivitamin supplementation, and recommend annual reassessment — earlier if pregnancy is desired or if symptoms escalate. Since the patient prioritizes fertility, hysterectomy would be appropriate only if she had biopsy-proven cancer.
The authors received no external funding for this practice paper, but both have funding from the National Institutes of Health for fibroid research. Laughlin-Tommaso reported royalties from UpToDate. Stewart reported research support from the Agency for Healthcare Research and Quality, and speaking, data-monitoring, and consulting fees for various private companies, including AbbVie, Anylam Pharmaceuticals, ASKA Pharma, and Myovant Sciences. She holds a patent on treatment for abnormal uterine bleeding and has been involved in CME for various medical educational agencies. Hurtado and Ascher-Walsh had no relevant conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
Although hysterectomy remains the most common procedure for treating fibroids and fibroids are the leading indication for hysterectomy, its long-term sequelae make less invasive alternatives the better choice for managing most of these myometrial masses, an invited clinical practice paper in the New England Journal of Medicine (NEJM) asserts.
The practice summary also calls for earlier identification and treatment of fibroid disease and may raise awareness among general gynecologists and primary care physicians less familiar with newer treatments.
Based on a review of evidence and existing formal guidelines, the paper urges wider use of uterus-sparing approaches such as hormone therapy, uterine-artery embolization, focused ultrasound ablation, and radiofrequency ablation. Authored by ob.gyns. Elizabeth A. Stewart, MD, and Shannon K. Laughlin-Tommaso, MD, MPH, of the Mayo Clinic in Rochester, Minnesota, the document also features textbook-style diagrams illustrating procedures.
“To clarify, this is not a new guidance but an invited clinical practice paper,” Laughlin-Tommaso told this news organization. “I believe NEJM recognized the gap in knowledge among all providers, for early diagnosis of uterine fibroids, especially in young patients and those presenting with anemia.”
The less invasive treatments highlighted in the paper can help women recover faster and resume their normal activities more quickly, said Laughlin-Tommaso. “Additionally, many studies have now shown that there are health benefits to keeping the uterus and the ovaries.”
Despite multiple uterine-sparing options, however, a recent study in a commercially insured population found nearly 60% of fibroid patients undergoing hysterectomy had never received a prior conservative treatment.
Why hysterectomy for a benign condition? Hysterectomy, which is universally available in ob.gyn. practices, makes decision-making easier for medical providers and patients, Laughlin-Tommaso explained. “It’s the only treatment that is definitive in that patients will not have bleeding or fibroids in the future and providers don’t have to determine which fibroids to treat or remove.”
More common in Black women, fibroids affect up to 80% of persons with a uterus during their lifetime and up to 50% have symptoms such as heavy and prolonged menstrual bleeding, anemia-associated fatigue, pelvic pressure, and menstrual and nonmenstrual pain, the authors noted. These lesions can also compress nearby structures causing painful intercourse, constipation, and urinary frequency, urgency, or retention.
In 2021 the American College of Obstetricians and Gynecologists issued a practice bulletin on the management of symptomatic uterine leiomyomas, similarly endorsing individualized care that accounts for the desire to preserve fertility or the uterus, increase quality of life, and reduce symptoms. It, too, recommended medical management as first-line treatment for symptomatic fibroids.
“This paper will be helpful for clinicians by covering some of the newer options such as gonadotropin-releasing hormone antagonists introduced in the past 5 years and tailoring treatment to patients depending on whether they still want to conceive,” Sandra M. Hurtado, MD, an ob.gyn. and an assistant professor at UTHealth Houston Medical Center and McGovern Medical School in Houston, Texas, said in an interview. “And the illustrations will be useful to doctors who are not gynecologists and will help to explain the interventional options to patients,” added Hurtado, who was not involved in the paper.
Offering another outside perspective on the paper, Charles J. Ascher-Walsh, MD, senior system vice chair for gynecology and division director of urogynecology in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology and Reproductive Science at Mount Sinai in New York City, called it a useful though not new summary. Reaching the wider audience of NEJM may raise awareness of newer fibroid therapies among general, nonspecialist ob.gyns., whose practices may concentrate largely on obstetrics, he added, “and the excellent illustrations clarify the treatment options.” In his view, broader awareness may increase much-needed funding for this neglected area of research.
Among the paper’s recommendations:
Diagnosis
Pelvic ultrasonography is the most cost-effective imaging method, providing information on size, location, and number of fibroids and ruling out adnexal masses. It is limited, however, by less-accurate resolution if the uterine volume is greater than 375 mL or if fibroids number more than four.
Medical Alternatives to Hysterectomy
Early diagnosis and first-line medical therapies are recommended.
Contraceptive hormones to control heavy menstrual bleeding are the first step in most algorithms for treating fibroid-related bleeding, despite low-quality evidence.
Nonsteroidal anti-inflammatory agents and tranexamic acid during menstruation also limit heavy menses but have more evidence of efficacy for idiopathic heavy menses.
Gonadotropin-releasing hormone (GnRH) agonists in depot form are approved for short-term preoperative therapy. While they cause amenorrhea in nearly 90% of patients and reduce uterine volume by 30%-60%, they have a high incidence of hypogonadal symptoms, including bone loss and hot flushes. They also cause a “steroidal flare” when the stored gonadotropins are released and cause subsequent heavy menstrual bleeding with the rapid decrease in estrogen levels.
Oral GnRH antagonist combinations are a major therapeutic advance, pairing a GnRH antagonist (such as elagolix or relugolix, which rapidly inhibit ovarian steroidogenesis) with estradiol and progestin at doses equivalent to systemic levels in the early follicular phase of the menstrual cycle.
In clinical trials these combinations decreased heavy menstrual bleeding by 50%-75%, pain by 40%-50%, and bulk-related symptoms through a 10% decrease in uterine volume. Side effects are few, with hot flushes, headaches, and nausea occurring in fewer than 20% of participants.
Smaller fibroids in the submucosal to intramural spaces can be treated transcervically, while larger lesions of any type or smaller subserosa fibroids are treated abdominally.
Uterine-artery embolization uses minimally invasive radiologically guided catheterization to release embolic particles directly into both uterine arteries. This process causes ischemic infarction of the fibroids and decreases bleeding, pain, and bulk-related symptoms.
Other procedures shrink individual fibroids with energy that creates coagulative necrosis. These include focused ultrasound ablation (with MRI or ultrasound guidance) and radiofrequency ablation (with laparoscopic or transcervical ultrasound guidance).
Unlike uterine-artery embolization, which treats all fibroids concurrently, these therapies require individual targeting of fibroids.
Radiofrequency ablation can be done concurrently with other surgical therapies, such as laparoscopic excision of endometriosis or hysteroscopic myomectomy.
Myomectomy, or the surgical removal of fibroids, is most often used in persons actively seeking pregnancy or having very large fibroids in whom shrinkage would be inadequate. Most guidelines recommend surgical excision rather than shrinking procedures to optimize fertility. However, myomectomy often commits patients to future cesarean section, which increases pregnancy-related morbidity.
Although myomectomy is seen as superior to uterine-artery embolization for improving quality of life, both approaches provide substantial symptom relief.
Recurrence
Incidence of recurring fibroids is high, with, for example, new fibroids developing in approximately 50% of persons within 5 years of myomectomy.
Earlier this year, a large cohort study reported that myomectomy was best for avoiding reintervention after surgical leiomyoma management.
Reintervention rates vary according to procedure, patient age, disease extent, and symptoms and can be as high as 33% up to 5 years after treatment, with lower percentages seen among persons older than 45 years of age.
Hysterectomy
Minimally invasive hysterectomy is recommended. Drawbacks to hysterectomy include perioperative risk and concomitant oophorectomy, which was common until the early 2000s when large cohort studies showed elevated risks of death, cardiovascular disease, dementia, and other illnesses compared with hysterectomy plus ovarian conservation. Oophorectomy but not hysterectomy rates have since decreased.
Still needing study, according to Laughlin-Tommaso are the underlying reasons for health disparities in fibroids, especially among Black and Latina individuals. “Some studies have found associations with vitamin D deficiency and with stress and racism,” she said.
Looking ahead, the authors stressed the need for a fibroid risk-prediction model, a staging system, and large randomized trials of treatment effectiveness. Also needed are methods for primary and secondary prevention. “Earlier screening and medical treatment in primary care settings could potentially minimize morbidity and the incidence of unnecessary hysterectomies, and primary care–based screening trials are warranted,” they wrote.
In addition to procedural illustrations the practice document includes a vignette of a 33-year-old never-pregnant Black woman (but desiring motherhood) with heavy menstrual bleeding, abdominal bloating, and non–iron deficiency anemia. Evaluation for thalassemia and sickle cell anemia is negative, but ultrasonography reveals an enlarged uterus with multiple fibroids and normal ovaries.
In line with the clinical review, the authors prescribe oral GnRH agonist combination therapy, plus iron and multivitamin supplementation, and recommend annual reassessment — earlier if pregnancy is desired or if symptoms escalate. Since the patient prioritizes fertility, hysterectomy would be appropriate only if she had biopsy-proven cancer.
The authors received no external funding for this practice paper, but both have funding from the National Institutes of Health for fibroid research. Laughlin-Tommaso reported royalties from UpToDate. Stewart reported research support from the Agency for Healthcare Research and Quality, and speaking, data-monitoring, and consulting fees for various private companies, including AbbVie, Anylam Pharmaceuticals, ASKA Pharma, and Myovant Sciences. She holds a patent on treatment for abnormal uterine bleeding and has been involved in CME for various medical educational agencies. Hurtado and Ascher-Walsh had no relevant conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
Although hysterectomy remains the most common procedure for treating fibroids and fibroids are the leading indication for hysterectomy, its long-term sequelae make less invasive alternatives the better choice for managing most of these myometrial masses, an invited clinical practice paper in the New England Journal of Medicine (NEJM) asserts.
The practice summary also calls for earlier identification and treatment of fibroid disease and may raise awareness among general gynecologists and primary care physicians less familiar with newer treatments.
Based on a review of evidence and existing formal guidelines, the paper urges wider use of uterus-sparing approaches such as hormone therapy, uterine-artery embolization, focused ultrasound ablation, and radiofrequency ablation. Authored by ob.gyns. Elizabeth A. Stewart, MD, and Shannon K. Laughlin-Tommaso, MD, MPH, of the Mayo Clinic in Rochester, Minnesota, the document also features textbook-style diagrams illustrating procedures.
“To clarify, this is not a new guidance but an invited clinical practice paper,” Laughlin-Tommaso told this news organization. “I believe NEJM recognized the gap in knowledge among all providers, for early diagnosis of uterine fibroids, especially in young patients and those presenting with anemia.”
The less invasive treatments highlighted in the paper can help women recover faster and resume their normal activities more quickly, said Laughlin-Tommaso. “Additionally, many studies have now shown that there are health benefits to keeping the uterus and the ovaries.”
Despite multiple uterine-sparing options, however, a recent study in a commercially insured population found nearly 60% of fibroid patients undergoing hysterectomy had never received a prior conservative treatment.
Why hysterectomy for a benign condition? Hysterectomy, which is universally available in ob.gyn. practices, makes decision-making easier for medical providers and patients, Laughlin-Tommaso explained. “It’s the only treatment that is definitive in that patients will not have bleeding or fibroids in the future and providers don’t have to determine which fibroids to treat or remove.”
More common in Black women, fibroids affect up to 80% of persons with a uterus during their lifetime and up to 50% have symptoms such as heavy and prolonged menstrual bleeding, anemia-associated fatigue, pelvic pressure, and menstrual and nonmenstrual pain, the authors noted. These lesions can also compress nearby structures causing painful intercourse, constipation, and urinary frequency, urgency, or retention.
In 2021 the American College of Obstetricians and Gynecologists issued a practice bulletin on the management of symptomatic uterine leiomyomas, similarly endorsing individualized care that accounts for the desire to preserve fertility or the uterus, increase quality of life, and reduce symptoms. It, too, recommended medical management as first-line treatment for symptomatic fibroids.
“This paper will be helpful for clinicians by covering some of the newer options such as gonadotropin-releasing hormone antagonists introduced in the past 5 years and tailoring treatment to patients depending on whether they still want to conceive,” Sandra M. Hurtado, MD, an ob.gyn. and an assistant professor at UTHealth Houston Medical Center and McGovern Medical School in Houston, Texas, said in an interview. “And the illustrations will be useful to doctors who are not gynecologists and will help to explain the interventional options to patients,” added Hurtado, who was not involved in the paper.
Offering another outside perspective on the paper, Charles J. Ascher-Walsh, MD, senior system vice chair for gynecology and division director of urogynecology in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology and Reproductive Science at Mount Sinai in New York City, called it a useful though not new summary. Reaching the wider audience of NEJM may raise awareness of newer fibroid therapies among general, nonspecialist ob.gyns., whose practices may concentrate largely on obstetrics, he added, “and the excellent illustrations clarify the treatment options.” In his view, broader awareness may increase much-needed funding for this neglected area of research.
Among the paper’s recommendations:
Diagnosis
Pelvic ultrasonography is the most cost-effective imaging method, providing information on size, location, and number of fibroids and ruling out adnexal masses. It is limited, however, by less-accurate resolution if the uterine volume is greater than 375 mL or if fibroids number more than four.
Medical Alternatives to Hysterectomy
Early diagnosis and first-line medical therapies are recommended.
Contraceptive hormones to control heavy menstrual bleeding are the first step in most algorithms for treating fibroid-related bleeding, despite low-quality evidence.
Nonsteroidal anti-inflammatory agents and tranexamic acid during menstruation also limit heavy menses but have more evidence of efficacy for idiopathic heavy menses.
Gonadotropin-releasing hormone (GnRH) agonists in depot form are approved for short-term preoperative therapy. While they cause amenorrhea in nearly 90% of patients and reduce uterine volume by 30%-60%, they have a high incidence of hypogonadal symptoms, including bone loss and hot flushes. They also cause a “steroidal flare” when the stored gonadotropins are released and cause subsequent heavy menstrual bleeding with the rapid decrease in estrogen levels.
Oral GnRH antagonist combinations are a major therapeutic advance, pairing a GnRH antagonist (such as elagolix or relugolix, which rapidly inhibit ovarian steroidogenesis) with estradiol and progestin at doses equivalent to systemic levels in the early follicular phase of the menstrual cycle.
In clinical trials these combinations decreased heavy menstrual bleeding by 50%-75%, pain by 40%-50%, and bulk-related symptoms through a 10% decrease in uterine volume. Side effects are few, with hot flushes, headaches, and nausea occurring in fewer than 20% of participants.
Smaller fibroids in the submucosal to intramural spaces can be treated transcervically, while larger lesions of any type or smaller subserosa fibroids are treated abdominally.
Uterine-artery embolization uses minimally invasive radiologically guided catheterization to release embolic particles directly into both uterine arteries. This process causes ischemic infarction of the fibroids and decreases bleeding, pain, and bulk-related symptoms.
Other procedures shrink individual fibroids with energy that creates coagulative necrosis. These include focused ultrasound ablation (with MRI or ultrasound guidance) and radiofrequency ablation (with laparoscopic or transcervical ultrasound guidance).
Unlike uterine-artery embolization, which treats all fibroids concurrently, these therapies require individual targeting of fibroids.
Radiofrequency ablation can be done concurrently with other surgical therapies, such as laparoscopic excision of endometriosis or hysteroscopic myomectomy.
Myomectomy, or the surgical removal of fibroids, is most often used in persons actively seeking pregnancy or having very large fibroids in whom shrinkage would be inadequate. Most guidelines recommend surgical excision rather than shrinking procedures to optimize fertility. However, myomectomy often commits patients to future cesarean section, which increases pregnancy-related morbidity.
Although myomectomy is seen as superior to uterine-artery embolization for improving quality of life, both approaches provide substantial symptom relief.
Recurrence
Incidence of recurring fibroids is high, with, for example, new fibroids developing in approximately 50% of persons within 5 years of myomectomy.
Earlier this year, a large cohort study reported that myomectomy was best for avoiding reintervention after surgical leiomyoma management.
Reintervention rates vary according to procedure, patient age, disease extent, and symptoms and can be as high as 33% up to 5 years after treatment, with lower percentages seen among persons older than 45 years of age.
Hysterectomy
Minimally invasive hysterectomy is recommended. Drawbacks to hysterectomy include perioperative risk and concomitant oophorectomy, which was common until the early 2000s when large cohort studies showed elevated risks of death, cardiovascular disease, dementia, and other illnesses compared with hysterectomy plus ovarian conservation. Oophorectomy but not hysterectomy rates have since decreased.
Still needing study, according to Laughlin-Tommaso are the underlying reasons for health disparities in fibroids, especially among Black and Latina individuals. “Some studies have found associations with vitamin D deficiency and with stress and racism,” she said.
Looking ahead, the authors stressed the need for a fibroid risk-prediction model, a staging system, and large randomized trials of treatment effectiveness. Also needed are methods for primary and secondary prevention. “Earlier screening and medical treatment in primary care settings could potentially minimize morbidity and the incidence of unnecessary hysterectomies, and primary care–based screening trials are warranted,” they wrote.
In addition to procedural illustrations the practice document includes a vignette of a 33-year-old never-pregnant Black woman (but desiring motherhood) with heavy menstrual bleeding, abdominal bloating, and non–iron deficiency anemia. Evaluation for thalassemia and sickle cell anemia is negative, but ultrasonography reveals an enlarged uterus with multiple fibroids and normal ovaries.
In line with the clinical review, the authors prescribe oral GnRH agonist combination therapy, plus iron and multivitamin supplementation, and recommend annual reassessment — earlier if pregnancy is desired or if symptoms escalate. Since the patient prioritizes fertility, hysterectomy would be appropriate only if she had biopsy-proven cancer.
The authors received no external funding for this practice paper, but both have funding from the National Institutes of Health for fibroid research. Laughlin-Tommaso reported royalties from UpToDate. Stewart reported research support from the Agency for Healthcare Research and Quality, and speaking, data-monitoring, and consulting fees for various private companies, including AbbVie, Anylam Pharmaceuticals, ASKA Pharma, and Myovant Sciences. She holds a patent on treatment for abnormal uterine bleeding and has been involved in CME for various medical educational agencies. Hurtado and Ascher-Walsh had no relevant conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Heat Waves Pose Significant Health Risks for Dually Eligible Older Individuals
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
‘Round Face’: A Viral Term’s Real Diagnostic Implications
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.
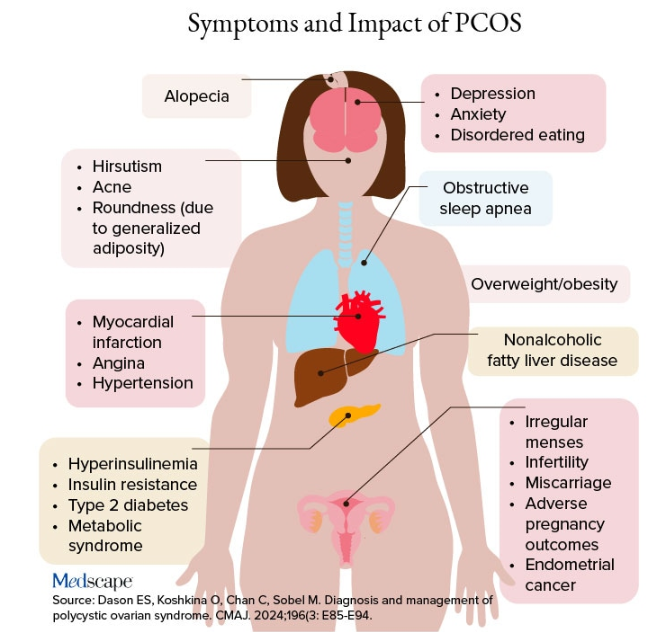
PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
Barzolvolimab Effective for CSU in Phase 2 Study
of an ongoing phase 2 study.
Moreover, in the study, barzolvolimab, an anti-KIT monoclonal antibody that inhibits the activation of and depletes mast cells, induced comparable responses in a subset of patients who had taken omalizumab, an anti–immunoglobulin E monoclonal antibody approved by the Food and Drug Administration for treating CSU.
The findings were presented at the annual European Academy of Dermatology and Venereology (EADV) 2024 Congress. Barzolvolimab is being developed by Celldex Therapeutics.
“Barzolvolimab treatment resulted in rapid, profound, and durable improvement in UAS7 [weekly Urticaria Activity Score 7],” said presenter Martin Metz, MD, professor of dermatology, Institute of Allergology, Charité – Universitätsmedizin Berlin in Germany, “with a deepening of response over 52 weeks in patients with antihistamine-refractory CSU.”
“Similar robust improvement was seen in patients previously treated with omalizumab, including refractory patients,” he added.
Because barzolvolimab was well tolerated over the course of the follow-up period, Metz said, it “has the potential to be an important new treatment option,” noting that patients are now being enrolled in global phase 3 studies of barzolvolimab.
Sustained Symptom Relief
Ana M. Giménez-Arnau, MD, PhD, associate professor of dermatology, Autonomous University and Pompeu Fabra University, Barcelona, Spain, told Medscape Medical News that the results are important, as they showed people who switched from placebo to the active drug also saw a long-term benefit.
What is “remarkable” about barzolvolimab, continued Giménez-Arnau, who was not involved in the study, is that it is the first drug to target the KIT receptor on mast cells and interfere with stimulating growth factors, thus making the cells that drive the development of CSU “disappear.”
The study included three different barzolvolimab regimens, with the 150-mg dose every 4 weeks and the 300-mg dose every 8 weeks achieving similar results, noted Giménez-Arnau.
For her, there are important questions to answer around the pharmacokinetic and pharmacodynamic profiles of the two regimens that remain, but she underlined that for the patient, the choice of regimen could have an impact on their quality of life.
“If we give 300 mg every 8 weeks,” she said, it appears “you can achieve disease control” while halving the frequency of subcutaneous injections.
She said that it would be “interesting to know” if 300 mg every 8 weeks is given as two 150-mg injections every 2 months or one 300-mg injection. If it is the former, Giménez Arnau said, “This is potentially an important benefit for the patient.”
Sustained Benefits at 1 Year
The study enrolled 208 patients with antihistamine-refractory CSU at sites in 10 countries, randomizing them to one of four arms: Subcutaneous injections of barzolvolimab 75 mg or 150 mg every 4 weeks, 300 mg every 8 weeks, or placebo every 4 weeks.
The mean age in each arm was between 42 and 47 years, and around 75% were women. Across the arms, 64%-76% had severe disease, as measured on the UAS7, at a mean score of 30.0-31.3. Around 20% had previously been treated with omalizumab.
Patients were treated for 16 weeks, during which time they completed daily and weekly diaries and attended six clinic visits at weeks 0, 2, 4, 8, 12, and 16. Results from the trial published earlier this year demonstrated that both the regimens (150 mg every 4 weeks and 300 mg every 8 weeks) achieved clinically meaningful and statistically significant improvement in UAS7, the primary endpoint, vs placebo at 12 weeks.
Participants in the barzolvolimab 75 mg and placebo arms were then randomized to receive barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, and those who had been in the 150-mg and 300-mg treatment arms continued with that treatment for a further 36 weeks. (The remaining patients have been continued on a further 24-week follow-up, but the data are not yet available.)
By the 52-week follow-up, 25% of patients who started in each of the barzolvolimab arms had discontinued treatment, as well as 16% first randomized to the placebo arm.
Metz reported that the improvements in UAS7 scores, observed as early as week 1, were sustained through week 52 in patients in both the ongoing 150-mg and 300-mg arms. Patients who initially started in the placebo and the barzolvolimab 75-mg groups caught up with those who had started on the higher doses, so that by week 52, there were no significant differences in urticaria activity, hives, or itch scores between the arms.
By week 52, the proportion of patients achieving well-controlled disease, defined as a UAS7 score ≤ 6, was 73.7% in the barzolvolimab 150 mg every 4-week arm and 68.2% in the 300 mg barzolvolimab every 8-week arm.
Notably, just 12.8% of patients in the placebo arm had achieved well-controlled CSU by week 16, but after switching to barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, 63% reached that target at week 52.
“Maybe even more striking and very interesting to look at,” said Metz, was the complete control of symptoms, meaning “not one single wheal and no itch.” By week 52, 52% of those on 300 mg every 8 weeks and 71.1% of those on 150 mg every 4 weeks had a complete response, with no itch/hives (UAS7 of 0).
Importantly, complete responses with barzolvolimab were observed early and were sustained or improved to week 52, Metz said, with, again, placebo and former barzolvolimab 75 mg patients catching up with those who started on 150 mg every 4 weeks and 300 mg every 8 weeks once they switched at week 16.
“This is the best data for chronic spontaneous urticaria that we have so far seen,” he said, adding that the responses were seen regardless of prior experience with omalizumab.
Changes in Hair Color, Skin Pigmentation
As for safety, during the first 16 weeks, 66% of those on active treatment and 39% on placebo experienced at least one adverse event. There were no treatment-related serious adverse events, compared with two among those who received treatment for the full 52 weeks.
The most common adverse events with active treatment were hair color changes (14% in the first 16 weeks and 26% among those treated for the full 52 weeks), neutropenia/reduced neutrophil count (9% in the first 16 weeks and 17% among those treated for the full 52 weeks), and skin hypopigmentation (1% in the first 16 weeks, 13% among those treated for the full 52 weeks, and 19% among those who switched from placebo to active treatment at 36 weeks). Urticaria was reported by 10% among patients on active treatment and 10% among those on placebo in the first 16 weeks, and by 15% of those treated for the full 52 weeks.
In the post-presentation discussion, Metz explained that the hypopigmentation appears to start around the hair follicle and is diffuse, so tends to look like vitiligo.
He suggested that the melanocytes around the hair follicle “seem to be the ones that are more stressed, maybe because of the hair follicle cycling,” adding that the effect is reversible and does not appear to be dose dependent.
The study was funded by Celldex Therapeutics. Metz declared relationships with AbbVie, ALK-Abelló, Almirall, Amgen, argenx, AstraZeneca, Astria, Attovia Therapeutics, Celldex, Celltrion, Escient Pharmaceuticals, Galen, Galderma, GSK, Incyte, Jasper, Lilly, Novartis, Pfizer, Pharvaris, Regeneron, Sanofi, Teva, Third Harmonic Bio, and Vifor.
A version of this article first appeared on Medscape.com.
of an ongoing phase 2 study.
Moreover, in the study, barzolvolimab, an anti-KIT monoclonal antibody that inhibits the activation of and depletes mast cells, induced comparable responses in a subset of patients who had taken omalizumab, an anti–immunoglobulin E monoclonal antibody approved by the Food and Drug Administration for treating CSU.
The findings were presented at the annual European Academy of Dermatology and Venereology (EADV) 2024 Congress. Barzolvolimab is being developed by Celldex Therapeutics.
“Barzolvolimab treatment resulted in rapid, profound, and durable improvement in UAS7 [weekly Urticaria Activity Score 7],” said presenter Martin Metz, MD, professor of dermatology, Institute of Allergology, Charité – Universitätsmedizin Berlin in Germany, “with a deepening of response over 52 weeks in patients with antihistamine-refractory CSU.”
“Similar robust improvement was seen in patients previously treated with omalizumab, including refractory patients,” he added.
Because barzolvolimab was well tolerated over the course of the follow-up period, Metz said, it “has the potential to be an important new treatment option,” noting that patients are now being enrolled in global phase 3 studies of barzolvolimab.
Sustained Symptom Relief
Ana M. Giménez-Arnau, MD, PhD, associate professor of dermatology, Autonomous University and Pompeu Fabra University, Barcelona, Spain, told Medscape Medical News that the results are important, as they showed people who switched from placebo to the active drug also saw a long-term benefit.
What is “remarkable” about barzolvolimab, continued Giménez-Arnau, who was not involved in the study, is that it is the first drug to target the KIT receptor on mast cells and interfere with stimulating growth factors, thus making the cells that drive the development of CSU “disappear.”
The study included three different barzolvolimab regimens, with the 150-mg dose every 4 weeks and the 300-mg dose every 8 weeks achieving similar results, noted Giménez-Arnau.
For her, there are important questions to answer around the pharmacokinetic and pharmacodynamic profiles of the two regimens that remain, but she underlined that for the patient, the choice of regimen could have an impact on their quality of life.
“If we give 300 mg every 8 weeks,” she said, it appears “you can achieve disease control” while halving the frequency of subcutaneous injections.
She said that it would be “interesting to know” if 300 mg every 8 weeks is given as two 150-mg injections every 2 months or one 300-mg injection. If it is the former, Giménez Arnau said, “This is potentially an important benefit for the patient.”
Sustained Benefits at 1 Year
The study enrolled 208 patients with antihistamine-refractory CSU at sites in 10 countries, randomizing them to one of four arms: Subcutaneous injections of barzolvolimab 75 mg or 150 mg every 4 weeks, 300 mg every 8 weeks, or placebo every 4 weeks.
The mean age in each arm was between 42 and 47 years, and around 75% were women. Across the arms, 64%-76% had severe disease, as measured on the UAS7, at a mean score of 30.0-31.3. Around 20% had previously been treated with omalizumab.
Patients were treated for 16 weeks, during which time they completed daily and weekly diaries and attended six clinic visits at weeks 0, 2, 4, 8, 12, and 16. Results from the trial published earlier this year demonstrated that both the regimens (150 mg every 4 weeks and 300 mg every 8 weeks) achieved clinically meaningful and statistically significant improvement in UAS7, the primary endpoint, vs placebo at 12 weeks.
Participants in the barzolvolimab 75 mg and placebo arms were then randomized to receive barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, and those who had been in the 150-mg and 300-mg treatment arms continued with that treatment for a further 36 weeks. (The remaining patients have been continued on a further 24-week follow-up, but the data are not yet available.)
By the 52-week follow-up, 25% of patients who started in each of the barzolvolimab arms had discontinued treatment, as well as 16% first randomized to the placebo arm.
Metz reported that the improvements in UAS7 scores, observed as early as week 1, were sustained through week 52 in patients in both the ongoing 150-mg and 300-mg arms. Patients who initially started in the placebo and the barzolvolimab 75-mg groups caught up with those who had started on the higher doses, so that by week 52, there were no significant differences in urticaria activity, hives, or itch scores between the arms.
By week 52, the proportion of patients achieving well-controlled disease, defined as a UAS7 score ≤ 6, was 73.7% in the barzolvolimab 150 mg every 4-week arm and 68.2% in the 300 mg barzolvolimab every 8-week arm.
Notably, just 12.8% of patients in the placebo arm had achieved well-controlled CSU by week 16, but after switching to barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, 63% reached that target at week 52.
“Maybe even more striking and very interesting to look at,” said Metz, was the complete control of symptoms, meaning “not one single wheal and no itch.” By week 52, 52% of those on 300 mg every 8 weeks and 71.1% of those on 150 mg every 4 weeks had a complete response, with no itch/hives (UAS7 of 0).
Importantly, complete responses with barzolvolimab were observed early and were sustained or improved to week 52, Metz said, with, again, placebo and former barzolvolimab 75 mg patients catching up with those who started on 150 mg every 4 weeks and 300 mg every 8 weeks once they switched at week 16.
“This is the best data for chronic spontaneous urticaria that we have so far seen,” he said, adding that the responses were seen regardless of prior experience with omalizumab.
Changes in Hair Color, Skin Pigmentation
As for safety, during the first 16 weeks, 66% of those on active treatment and 39% on placebo experienced at least one adverse event. There were no treatment-related serious adverse events, compared with two among those who received treatment for the full 52 weeks.
The most common adverse events with active treatment were hair color changes (14% in the first 16 weeks and 26% among those treated for the full 52 weeks), neutropenia/reduced neutrophil count (9% in the first 16 weeks and 17% among those treated for the full 52 weeks), and skin hypopigmentation (1% in the first 16 weeks, 13% among those treated for the full 52 weeks, and 19% among those who switched from placebo to active treatment at 36 weeks). Urticaria was reported by 10% among patients on active treatment and 10% among those on placebo in the first 16 weeks, and by 15% of those treated for the full 52 weeks.
In the post-presentation discussion, Metz explained that the hypopigmentation appears to start around the hair follicle and is diffuse, so tends to look like vitiligo.
He suggested that the melanocytes around the hair follicle “seem to be the ones that are more stressed, maybe because of the hair follicle cycling,” adding that the effect is reversible and does not appear to be dose dependent.
The study was funded by Celldex Therapeutics. Metz declared relationships with AbbVie, ALK-Abelló, Almirall, Amgen, argenx, AstraZeneca, Astria, Attovia Therapeutics, Celldex, Celltrion, Escient Pharmaceuticals, Galen, Galderma, GSK, Incyte, Jasper, Lilly, Novartis, Pfizer, Pharvaris, Regeneron, Sanofi, Teva, Third Harmonic Bio, and Vifor.
A version of this article first appeared on Medscape.com.
of an ongoing phase 2 study.
Moreover, in the study, barzolvolimab, an anti-KIT monoclonal antibody that inhibits the activation of and depletes mast cells, induced comparable responses in a subset of patients who had taken omalizumab, an anti–immunoglobulin E monoclonal antibody approved by the Food and Drug Administration for treating CSU.
The findings were presented at the annual European Academy of Dermatology and Venereology (EADV) 2024 Congress. Barzolvolimab is being developed by Celldex Therapeutics.
“Barzolvolimab treatment resulted in rapid, profound, and durable improvement in UAS7 [weekly Urticaria Activity Score 7],” said presenter Martin Metz, MD, professor of dermatology, Institute of Allergology, Charité – Universitätsmedizin Berlin in Germany, “with a deepening of response over 52 weeks in patients with antihistamine-refractory CSU.”
“Similar robust improvement was seen in patients previously treated with omalizumab, including refractory patients,” he added.
Because barzolvolimab was well tolerated over the course of the follow-up period, Metz said, it “has the potential to be an important new treatment option,” noting that patients are now being enrolled in global phase 3 studies of barzolvolimab.
Sustained Symptom Relief
Ana M. Giménez-Arnau, MD, PhD, associate professor of dermatology, Autonomous University and Pompeu Fabra University, Barcelona, Spain, told Medscape Medical News that the results are important, as they showed people who switched from placebo to the active drug also saw a long-term benefit.
What is “remarkable” about barzolvolimab, continued Giménez-Arnau, who was not involved in the study, is that it is the first drug to target the KIT receptor on mast cells and interfere with stimulating growth factors, thus making the cells that drive the development of CSU “disappear.”
The study included three different barzolvolimab regimens, with the 150-mg dose every 4 weeks and the 300-mg dose every 8 weeks achieving similar results, noted Giménez-Arnau.
For her, there are important questions to answer around the pharmacokinetic and pharmacodynamic profiles of the two regimens that remain, but she underlined that for the patient, the choice of regimen could have an impact on their quality of life.
“If we give 300 mg every 8 weeks,” she said, it appears “you can achieve disease control” while halving the frequency of subcutaneous injections.
She said that it would be “interesting to know” if 300 mg every 8 weeks is given as two 150-mg injections every 2 months or one 300-mg injection. If it is the former, Giménez Arnau said, “This is potentially an important benefit for the patient.”
Sustained Benefits at 1 Year
The study enrolled 208 patients with antihistamine-refractory CSU at sites in 10 countries, randomizing them to one of four arms: Subcutaneous injections of barzolvolimab 75 mg or 150 mg every 4 weeks, 300 mg every 8 weeks, or placebo every 4 weeks.
The mean age in each arm was between 42 and 47 years, and around 75% were women. Across the arms, 64%-76% had severe disease, as measured on the UAS7, at a mean score of 30.0-31.3. Around 20% had previously been treated with omalizumab.
Patients were treated for 16 weeks, during which time they completed daily and weekly diaries and attended six clinic visits at weeks 0, 2, 4, 8, 12, and 16. Results from the trial published earlier this year demonstrated that both the regimens (150 mg every 4 weeks and 300 mg every 8 weeks) achieved clinically meaningful and statistically significant improvement in UAS7, the primary endpoint, vs placebo at 12 weeks.
Participants in the barzolvolimab 75 mg and placebo arms were then randomized to receive barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, and those who had been in the 150-mg and 300-mg treatment arms continued with that treatment for a further 36 weeks. (The remaining patients have been continued on a further 24-week follow-up, but the data are not yet available.)
By the 52-week follow-up, 25% of patients who started in each of the barzolvolimab arms had discontinued treatment, as well as 16% first randomized to the placebo arm.
Metz reported that the improvements in UAS7 scores, observed as early as week 1, were sustained through week 52 in patients in both the ongoing 150-mg and 300-mg arms. Patients who initially started in the placebo and the barzolvolimab 75-mg groups caught up with those who had started on the higher doses, so that by week 52, there were no significant differences in urticaria activity, hives, or itch scores between the arms.
By week 52, the proportion of patients achieving well-controlled disease, defined as a UAS7 score ≤ 6, was 73.7% in the barzolvolimab 150 mg every 4-week arm and 68.2% in the 300 mg barzolvolimab every 8-week arm.
Notably, just 12.8% of patients in the placebo arm had achieved well-controlled CSU by week 16, but after switching to barzolvolimab 150 mg every 4 weeks or 300 mg every 8 weeks, 63% reached that target at week 52.
“Maybe even more striking and very interesting to look at,” said Metz, was the complete control of symptoms, meaning “not one single wheal and no itch.” By week 52, 52% of those on 300 mg every 8 weeks and 71.1% of those on 150 mg every 4 weeks had a complete response, with no itch/hives (UAS7 of 0).
Importantly, complete responses with barzolvolimab were observed early and were sustained or improved to week 52, Metz said, with, again, placebo and former barzolvolimab 75 mg patients catching up with those who started on 150 mg every 4 weeks and 300 mg every 8 weeks once they switched at week 16.
“This is the best data for chronic spontaneous urticaria that we have so far seen,” he said, adding that the responses were seen regardless of prior experience with omalizumab.
Changes in Hair Color, Skin Pigmentation
As for safety, during the first 16 weeks, 66% of those on active treatment and 39% on placebo experienced at least one adverse event. There were no treatment-related serious adverse events, compared with two among those who received treatment for the full 52 weeks.
The most common adverse events with active treatment were hair color changes (14% in the first 16 weeks and 26% among those treated for the full 52 weeks), neutropenia/reduced neutrophil count (9% in the first 16 weeks and 17% among those treated for the full 52 weeks), and skin hypopigmentation (1% in the first 16 weeks, 13% among those treated for the full 52 weeks, and 19% among those who switched from placebo to active treatment at 36 weeks). Urticaria was reported by 10% among patients on active treatment and 10% among those on placebo in the first 16 weeks, and by 15% of those treated for the full 52 weeks.
In the post-presentation discussion, Metz explained that the hypopigmentation appears to start around the hair follicle and is diffuse, so tends to look like vitiligo.
He suggested that the melanocytes around the hair follicle “seem to be the ones that are more stressed, maybe because of the hair follicle cycling,” adding that the effect is reversible and does not appear to be dose dependent.
The study was funded by Celldex Therapeutics. Metz declared relationships with AbbVie, ALK-Abelló, Almirall, Amgen, argenx, AstraZeneca, Astria, Attovia Therapeutics, Celldex, Celltrion, Escient Pharmaceuticals, Galen, Galderma, GSK, Incyte, Jasper, Lilly, Novartis, Pfizer, Pharvaris, Regeneron, Sanofi, Teva, Third Harmonic Bio, and Vifor.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Children With Severe Atopic Dermatitis Catch Up on Growth With Dupilumab
AMSTERDAM — , revealed a post hoc trial analysis.
The research was presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
The trial included a “rigorously selected … well-characterized, well-studied” population of children aged 6-11 years, said presenter Alan D. Irvine, MD, DSc, professor of dermatology, Trinity College Dublin, Ireland.
It showed that “severe atopic dermatitis does cause restriction of growth, as well as a higher weight, and therefore obviously a higher BMI [body mass index].”
He continued, however, that children at the lower percentiles of height receiving prompt treatment with dupilumab (Dupixent) “were able to rapidly move through the centiles over the 16 weeks of the study, and that may be the window for catch-up growth … when children are growing rapidly.”
Anna Yasmine Kirkorian, MD, chief of dermatology, Children’s National Hospital, Washington, DC, who was not involved in the study, said that she was “surprised” at the degree of growth achieved over the study period, as height is not something that jumps up “overnight.”
“On the other hand, it fits with my experience with children who’ve had the brakes on all of their life due to inflammation, whether it be height, going to school, sleeping — everything is sort of put on pause by this terrible inflammatory process,” she said.
“When you take the brakes off, they get to be who they are going to be,” Kirkorian added. “So I was surprised by the speed of it, but not by the fact that height was acquired.”
Her belief is that in the pre-dupilumab era, severe atopic dermatitis was often insufficiently controlled, so children were “smaller than you would predict from parental height,” and the treatment is “allowing them to reach their genetic potential.”
Post Hoc Analysis
In his presentation, Irvine emphasized that it has been clearly demonstrated that adolescents with moderate and severe atopic dermatitis have a significantly higher likelihood of being below the 25th percentile of height on growth reference charts.
Such children are also at a higher risk of having low bone mineral density and low serum alkaline phosphatase (ALP) levels . While data presented at the EADV 2023 Congress showed that dupilumab significantly increased serum levels of bone ALP compared with placebo, the underlying mechanism remains unclear.
For the current analysis, Irvine and colleagues determined that the proportion of children aged 6-11 years with severe atopic dermatitis and lower stature reach a ≥ 5 centile improvement in height following 16 weeks of dupilumab treatment.
They examined data from the LIBERTY AD PEDS trial, in which patients aged 6-11 years with severe atopic dermatitis were randomized to 300 mg dupilumab every 4 weeks or placebo along with a mild or moderately potent topical corticosteroid. The study found that, overall, dupilumab was associated with significant improvements in signs, symptoms, and quality of life compared with placebo.
Height measures at baseline revealed that “more boys and more girls were below the 50th centile than you would predict for a healthy, normal control population,” Irvine said. “If we look at weight, we see the opposite,” he continued, “with a disproportionate number of boys and girls who are above the 50th centile for weight at baseline.”
Consequently, “we’re seeing these children who are shorter and heavier than the predicted healthy weight range and, as a result, obviously have higher BMI,” Irvine noted, with 67% girls and 62% boys found to have a higher BMI than normal for their age.
After 16 weeks of treatment with dupilumab, there was a much greater gain in height than that seen among those on placebo, with the most pronounced effect seen in children who had the lowest height at baseline. Indeed, among children in the lowest 25% height percentile at baseline, 30.6% on dupilumab vs 11.9% on placebo experienced an increase in height of 5 centiles or more(P < .05).
“This reflects what we see in clinical practice,” Irvine said. “Children often grow dramatically on treatment for atopic dermatitis.”
Among patients with a baseline height below the 30th percentile, 31.9% treated with dupilumab vs 11.1% treated with placebo gained at least 5 centiles in height. The figures for children below the 40th height percentile at baseline were 31.3% vs 15.5% (P < .05 for both).
Although there remained a marked difference in the proportion of children below the 50th height percentile at baseline gaining 5 centiles or more in height, at 29.0% with dupilumab versus 15.7% with placebo, it was no longer significant.
“So the effect of catch-up growth, or growth through the centiles, is most marked in those who are in the 40th centile or below,” Irvine said, indicating that the “more growth restricted kids have much more potential to catch up.”
‘Convincing’ Data
Overall, Kirkorian said in the interview, the data are “convincing” and support her view that severe atopic dermatitis is a “terrible chronic disease that we really underappreciate.” Atopic dermatitis, she added, “should get the respect that any severe chronic illness would have, whether that be arthritis, diabetes, or cardiac disease, because it is a systemic disorder that … profoundly affects quality of life, every minute of every day.”
However, “we don’t get all the referrals we should, until the child has suffered for years and years, and the family has suffered,” as there is a bias that it can be outgrown — although not everybody does — and it “doesn’t look as conspicuous as other chronic skin disorders,” such as psoriasis.
“Now with this study,” Kirkorian said, “it gives us a really compelling point to make to parents, to the community, and to insurers that not only are we affecting the quality of life from the itch standpoint [with dupilumab] but we may have long profound effects on growth and bone health.”
The research was sponsored by Sanofi and Regeneron Pharmaceuticals. Irvine declared relationships with AbbVie, Arena Pharmaceuticals, BenevolentAI, Chugai Pharmaceutical, Dermavant, Eli Lily, Genentech, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, UCB, DS Biopharma, and Inflazome. Kirkorian declared relationships with Dermavant, Verrica Pharmaceuticals, Pfizer, and Incyte.
A version of this article first appeared on Medscape.com.
AMSTERDAM — , revealed a post hoc trial analysis.
The research was presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
The trial included a “rigorously selected … well-characterized, well-studied” population of children aged 6-11 years, said presenter Alan D. Irvine, MD, DSc, professor of dermatology, Trinity College Dublin, Ireland.
It showed that “severe atopic dermatitis does cause restriction of growth, as well as a higher weight, and therefore obviously a higher BMI [body mass index].”
He continued, however, that children at the lower percentiles of height receiving prompt treatment with dupilumab (Dupixent) “were able to rapidly move through the centiles over the 16 weeks of the study, and that may be the window for catch-up growth … when children are growing rapidly.”
Anna Yasmine Kirkorian, MD, chief of dermatology, Children’s National Hospital, Washington, DC, who was not involved in the study, said that she was “surprised” at the degree of growth achieved over the study period, as height is not something that jumps up “overnight.”
“On the other hand, it fits with my experience with children who’ve had the brakes on all of their life due to inflammation, whether it be height, going to school, sleeping — everything is sort of put on pause by this terrible inflammatory process,” she said.
“When you take the brakes off, they get to be who they are going to be,” Kirkorian added. “So I was surprised by the speed of it, but not by the fact that height was acquired.”
Her belief is that in the pre-dupilumab era, severe atopic dermatitis was often insufficiently controlled, so children were “smaller than you would predict from parental height,” and the treatment is “allowing them to reach their genetic potential.”
Post Hoc Analysis
In his presentation, Irvine emphasized that it has been clearly demonstrated that adolescents with moderate and severe atopic dermatitis have a significantly higher likelihood of being below the 25th percentile of height on growth reference charts.
Such children are also at a higher risk of having low bone mineral density and low serum alkaline phosphatase (ALP) levels . While data presented at the EADV 2023 Congress showed that dupilumab significantly increased serum levels of bone ALP compared with placebo, the underlying mechanism remains unclear.
For the current analysis, Irvine and colleagues determined that the proportion of children aged 6-11 years with severe atopic dermatitis and lower stature reach a ≥ 5 centile improvement in height following 16 weeks of dupilumab treatment.
They examined data from the LIBERTY AD PEDS trial, in which patients aged 6-11 years with severe atopic dermatitis were randomized to 300 mg dupilumab every 4 weeks or placebo along with a mild or moderately potent topical corticosteroid. The study found that, overall, dupilumab was associated with significant improvements in signs, symptoms, and quality of life compared with placebo.
Height measures at baseline revealed that “more boys and more girls were below the 50th centile than you would predict for a healthy, normal control population,” Irvine said. “If we look at weight, we see the opposite,” he continued, “with a disproportionate number of boys and girls who are above the 50th centile for weight at baseline.”
Consequently, “we’re seeing these children who are shorter and heavier than the predicted healthy weight range and, as a result, obviously have higher BMI,” Irvine noted, with 67% girls and 62% boys found to have a higher BMI than normal for their age.
After 16 weeks of treatment with dupilumab, there was a much greater gain in height than that seen among those on placebo, with the most pronounced effect seen in children who had the lowest height at baseline. Indeed, among children in the lowest 25% height percentile at baseline, 30.6% on dupilumab vs 11.9% on placebo experienced an increase in height of 5 centiles or more(P < .05).
“This reflects what we see in clinical practice,” Irvine said. “Children often grow dramatically on treatment for atopic dermatitis.”
Among patients with a baseline height below the 30th percentile, 31.9% treated with dupilumab vs 11.1% treated with placebo gained at least 5 centiles in height. The figures for children below the 40th height percentile at baseline were 31.3% vs 15.5% (P < .05 for both).
Although there remained a marked difference in the proportion of children below the 50th height percentile at baseline gaining 5 centiles or more in height, at 29.0% with dupilumab versus 15.7% with placebo, it was no longer significant.
“So the effect of catch-up growth, or growth through the centiles, is most marked in those who are in the 40th centile or below,” Irvine said, indicating that the “more growth restricted kids have much more potential to catch up.”
‘Convincing’ Data
Overall, Kirkorian said in the interview, the data are “convincing” and support her view that severe atopic dermatitis is a “terrible chronic disease that we really underappreciate.” Atopic dermatitis, she added, “should get the respect that any severe chronic illness would have, whether that be arthritis, diabetes, or cardiac disease, because it is a systemic disorder that … profoundly affects quality of life, every minute of every day.”
However, “we don’t get all the referrals we should, until the child has suffered for years and years, and the family has suffered,” as there is a bias that it can be outgrown — although not everybody does — and it “doesn’t look as conspicuous as other chronic skin disorders,” such as psoriasis.
“Now with this study,” Kirkorian said, “it gives us a really compelling point to make to parents, to the community, and to insurers that not only are we affecting the quality of life from the itch standpoint [with dupilumab] but we may have long profound effects on growth and bone health.”
The research was sponsored by Sanofi and Regeneron Pharmaceuticals. Irvine declared relationships with AbbVie, Arena Pharmaceuticals, BenevolentAI, Chugai Pharmaceutical, Dermavant, Eli Lily, Genentech, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, UCB, DS Biopharma, and Inflazome. Kirkorian declared relationships with Dermavant, Verrica Pharmaceuticals, Pfizer, and Incyte.
A version of this article first appeared on Medscape.com.
AMSTERDAM — , revealed a post hoc trial analysis.
The research was presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
The trial included a “rigorously selected … well-characterized, well-studied” population of children aged 6-11 years, said presenter Alan D. Irvine, MD, DSc, professor of dermatology, Trinity College Dublin, Ireland.
It showed that “severe atopic dermatitis does cause restriction of growth, as well as a higher weight, and therefore obviously a higher BMI [body mass index].”
He continued, however, that children at the lower percentiles of height receiving prompt treatment with dupilumab (Dupixent) “were able to rapidly move through the centiles over the 16 weeks of the study, and that may be the window for catch-up growth … when children are growing rapidly.”
Anna Yasmine Kirkorian, MD, chief of dermatology, Children’s National Hospital, Washington, DC, who was not involved in the study, said that she was “surprised” at the degree of growth achieved over the study period, as height is not something that jumps up “overnight.”
“On the other hand, it fits with my experience with children who’ve had the brakes on all of their life due to inflammation, whether it be height, going to school, sleeping — everything is sort of put on pause by this terrible inflammatory process,” she said.
“When you take the brakes off, they get to be who they are going to be,” Kirkorian added. “So I was surprised by the speed of it, but not by the fact that height was acquired.”
Her belief is that in the pre-dupilumab era, severe atopic dermatitis was often insufficiently controlled, so children were “smaller than you would predict from parental height,” and the treatment is “allowing them to reach their genetic potential.”
Post Hoc Analysis
In his presentation, Irvine emphasized that it has been clearly demonstrated that adolescents with moderate and severe atopic dermatitis have a significantly higher likelihood of being below the 25th percentile of height on growth reference charts.
Such children are also at a higher risk of having low bone mineral density and low serum alkaline phosphatase (ALP) levels . While data presented at the EADV 2023 Congress showed that dupilumab significantly increased serum levels of bone ALP compared with placebo, the underlying mechanism remains unclear.
For the current analysis, Irvine and colleagues determined that the proportion of children aged 6-11 years with severe atopic dermatitis and lower stature reach a ≥ 5 centile improvement in height following 16 weeks of dupilumab treatment.
They examined data from the LIBERTY AD PEDS trial, in which patients aged 6-11 years with severe atopic dermatitis were randomized to 300 mg dupilumab every 4 weeks or placebo along with a mild or moderately potent topical corticosteroid. The study found that, overall, dupilumab was associated with significant improvements in signs, symptoms, and quality of life compared with placebo.
Height measures at baseline revealed that “more boys and more girls were below the 50th centile than you would predict for a healthy, normal control population,” Irvine said. “If we look at weight, we see the opposite,” he continued, “with a disproportionate number of boys and girls who are above the 50th centile for weight at baseline.”
Consequently, “we’re seeing these children who are shorter and heavier than the predicted healthy weight range and, as a result, obviously have higher BMI,” Irvine noted, with 67% girls and 62% boys found to have a higher BMI than normal for their age.
After 16 weeks of treatment with dupilumab, there was a much greater gain in height than that seen among those on placebo, with the most pronounced effect seen in children who had the lowest height at baseline. Indeed, among children in the lowest 25% height percentile at baseline, 30.6% on dupilumab vs 11.9% on placebo experienced an increase in height of 5 centiles or more(P < .05).
“This reflects what we see in clinical practice,” Irvine said. “Children often grow dramatically on treatment for atopic dermatitis.”
Among patients with a baseline height below the 30th percentile, 31.9% treated with dupilumab vs 11.1% treated with placebo gained at least 5 centiles in height. The figures for children below the 40th height percentile at baseline were 31.3% vs 15.5% (P < .05 for both).
Although there remained a marked difference in the proportion of children below the 50th height percentile at baseline gaining 5 centiles or more in height, at 29.0% with dupilumab versus 15.7% with placebo, it was no longer significant.
“So the effect of catch-up growth, or growth through the centiles, is most marked in those who are in the 40th centile or below,” Irvine said, indicating that the “more growth restricted kids have much more potential to catch up.”
‘Convincing’ Data
Overall, Kirkorian said in the interview, the data are “convincing” and support her view that severe atopic dermatitis is a “terrible chronic disease that we really underappreciate.” Atopic dermatitis, she added, “should get the respect that any severe chronic illness would have, whether that be arthritis, diabetes, or cardiac disease, because it is a systemic disorder that … profoundly affects quality of life, every minute of every day.”
However, “we don’t get all the referrals we should, until the child has suffered for years and years, and the family has suffered,” as there is a bias that it can be outgrown — although not everybody does — and it “doesn’t look as conspicuous as other chronic skin disorders,” such as psoriasis.
“Now with this study,” Kirkorian said, “it gives us a really compelling point to make to parents, to the community, and to insurers that not only are we affecting the quality of life from the itch standpoint [with dupilumab] but we may have long profound effects on growth and bone health.”
The research was sponsored by Sanofi and Regeneron Pharmaceuticals. Irvine declared relationships with AbbVie, Arena Pharmaceuticals, BenevolentAI, Chugai Pharmaceutical, Dermavant, Eli Lily, Genentech, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, UCB, DS Biopharma, and Inflazome. Kirkorian declared relationships with Dermavant, Verrica Pharmaceuticals, Pfizer, and Incyte.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Nemolizumab Benefits for Atopic Dermatitis Maintained in Long-Term Follow-Up Study
(AD), revealed an interim analysis of the ARCADIA open-label extension study.
The research was presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
The results showed nemolizumab was associated with “ongoing clinically meaningful improvements in itch, skin lesions, and sleep disturbance,” said study presenter Diamant Thaçi, MD, PhD, of the Comprehensive Center for Inflammation Medicine, University of Lü̈beck in Germany.
Moreover, “patient-reported outcomes, including quality of life ... continued to improve over 56 weeks of treatment.” In addition, Thaçi added, the “safety data support the long-term use of nemolizumab for the treatment of adolescent and adult patients with moderate to severe atopic dermatitis.”
He explained that interleukin (IL) 31 is a key neuroimmune cytokine in AD, triggering itch, skin barrier disruption, and exacerbation of inflammation via its receptor. Nemolizumab inhibits IL-31 receptor binding and was shown in the ARCADIA 1 and ARCADIA 2 trials to provide, along with background topical corticosteroids, clinically meaningful improvements in itch, skin lesions, and sleep for up to weeks 48 of follow-up in adolescents and adults with moderate to severe AD.
The current open-label long-term extension study involved patients who were enrolled in both ARCADIA 1 and 2 trials, as well as those from four phase 2 and 2b studies, a phase 3b study, and adolescents who had not been included in a trial but who met the criteria for the extension study. All patients, whether they started on placebo plus background topical corticosteroids in a prior study, were treated with nemolizumab 30 mg subcutaneously every 4 weeks along with topical corticosteroids.
The interim analysis included all efficacy and safety data up to the cutoff of September 30, 2022, on 723 patients who had completed 56 weeks of treatment among the 1751 patients initially enrolled in the extension study.
The results showed that, regardless of whether patients were nemolizumab naive at enrollment or had previously taken the drug, there were increases in the proportion of patients with an Investigator Global Assessment (IGA) score of 0/1 and an Eczema Area and Severity Index (EASI) score of at least 75 (EASI-75) over the 56 weeks of the study.
In those naive to nemolizumab, the increase in the proportion with an IGA score of 0/1 increased from 17.7% at baseline to 49.0% at 56 weeks, while the proportion with an EASI-75 increased from 24.0% to 78.7%.
The increase in the proportion of patients with an IGA score 0/1 among those who had previously received nemolizumab increased from 28.5% at baseline to 47.1% at 56 weeks. The proportion with an EASI-75 was 38.1% at baseline, rising to 73.0% at 56 weeks.
Increases in the proportion of patients with an EASI score of at least 50 and at least 90 were also seen with nemolizumab, as were increases in the proportion of patients with an improvement of at least four points on the SCORing Atopic Dermatitis Pruritus visual analogue scale and Sleep loss scores.
Similarly, the proportion of patients with a reduction in Dermatology Life Quality Index of at least four points increased over the study period.
Regarding safety, Thaçi said, there appeared to be fewer adverse events than had been previously reported with nemolizumab. “We don’t see any signs of conjunctivitis,” he continued, or significant risk of infection apart from for COVID-19, but he pointed out that the study was conducted during the pandemic, which was “a very difficult time.”
The most common treatment-related adverse events were, aside from COVID-19, nasopharyngitis in about 10%-11% of patients, upper respiratory tract infection in about 6% to almost 7%, and headache in about 5%.
Among the adverse events of special interest, newly diagnosed asthma or worsening of asthma occurred in 4.7%-4.8% of patients, while peripheral edema was seen in 0.8%-1.7%.
“Besides this, the study results are really looking very good,” he said, adding: “It means, in a long-term study, we can say today that nemolizumab has revealed the [same] safety profile that was shown in the ARCADIA 1 and 2 trials.”
Alan D. Irvine, MD, DSc, professor of dermatology, Trinity College Dublin in Ireland, who was not involved in the study, underlined that the current interim assessment does not represent the complete dataset and is based on observed cases rather than a more rigorous methodology, such as net reclassification improvement analysis.
“So it makes it a little harder to interpret when you don’t know how many people are dropping out and why they’re dropping out,” he told this news organization. “That said, those who remain on drug out to 56 weeks do experience ongoing improvement in disease control.”
Consequently, “the most reliable message you can take from this interim analysis of long-term data is that there were no new safety signals,” and nemolizumab looks “safe and well-tolerated.”
Where nemolizumab would fit into the treatment pathway for moderate to severe AD remains an open question, Irvine said, although he believes that IL-13 pathway inhibitors such as dupilumab, tralokinumab, and lebrikizumab “will remain the treatment of choice for the immediate future due to prescriber familiarity and good efficacy data.”
However, for patients who are unsuitable for IL-13 inhibitors and/or Janus kinase inhibitors such as abrocitinib and upadacitinib, nemolizumab “could be an interesting alternative.”
“That’s probably where it is going to start,” Irvine said, “and then obviously that will change over time and as the data mature and prescribers become more familiar with the drug in the real world.”
Nemolizumab (Nemluvio) is approved for treating prurigo nodularis (PN) in the United States and in Japan and is under Food and Drug Administration review for treating AD. It is also under review for PN and AD in Europe, Canada, the United Kingdom, and several other countries, according to Galderma. It is also approved for treating pruritus associated with AD in pediatric, adolescent, and adult patients in Japan.
The study was funded by Galderma. Thaçi declared relationships with AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Galderma, Janssen-Cilag, Kyowa Kirin, LEO Pharma, L’Oréal, Eli Lilly, Novartis, Pfizer, Regeneron, Sanofi, Target RWE, and UCB. Irvine declared relationships with AbbVie, Arena Pharmaceuticals, BenevolentAl, Chugai Pharmaceutical, Dermavant, Eli Lily, Genentech, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, UCB, DS Biopharma, and Inflazome.
A version of this article first appeared on Medscape.com.
(AD), revealed an interim analysis of the ARCADIA open-label extension study.
The research was presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
The results showed nemolizumab was associated with “ongoing clinically meaningful improvements in itch, skin lesions, and sleep disturbance,” said study presenter Diamant Thaçi, MD, PhD, of the Comprehensive Center for Inflammation Medicine, University of Lü̈beck in Germany.
Moreover, “patient-reported outcomes, including quality of life ... continued to improve over 56 weeks of treatment.” In addition, Thaçi added, the “safety data support the long-term use of nemolizumab for the treatment of adolescent and adult patients with moderate to severe atopic dermatitis.”
He explained that interleukin (IL) 31 is a key neuroimmune cytokine in AD, triggering itch, skin barrier disruption, and exacerbation of inflammation via its receptor. Nemolizumab inhibits IL-31 receptor binding and was shown in the ARCADIA 1 and ARCADIA 2 trials to provide, along with background topical corticosteroids, clinically meaningful improvements in itch, skin lesions, and sleep for up to weeks 48 of follow-up in adolescents and adults with moderate to severe AD.
The current open-label long-term extension study involved patients who were enrolled in both ARCADIA 1 and 2 trials, as well as those from four phase 2 and 2b studies, a phase 3b study, and adolescents who had not been included in a trial but who met the criteria for the extension study. All patients, whether they started on placebo plus background topical corticosteroids in a prior study, were treated with nemolizumab 30 mg subcutaneously every 4 weeks along with topical corticosteroids.
The interim analysis included all efficacy and safety data up to the cutoff of September 30, 2022, on 723 patients who had completed 56 weeks of treatment among the 1751 patients initially enrolled in the extension study.
The results showed that, regardless of whether patients were nemolizumab naive at enrollment or had previously taken the drug, there were increases in the proportion of patients with an Investigator Global Assessment (IGA) score of 0/1 and an Eczema Area and Severity Index (EASI) score of at least 75 (EASI-75) over the 56 weeks of the study.
In those naive to nemolizumab, the increase in the proportion with an IGA score of 0/1 increased from 17.7% at baseline to 49.0% at 56 weeks, while the proportion with an EASI-75 increased from 24.0% to 78.7%.
The increase in the proportion of patients with an IGA score 0/1 among those who had previously received nemolizumab increased from 28.5% at baseline to 47.1% at 56 weeks. The proportion with an EASI-75 was 38.1% at baseline, rising to 73.0% at 56 weeks.
Increases in the proportion of patients with an EASI score of at least 50 and at least 90 were also seen with nemolizumab, as were increases in the proportion of patients with an improvement of at least four points on the SCORing Atopic Dermatitis Pruritus visual analogue scale and Sleep loss scores.
Similarly, the proportion of patients with a reduction in Dermatology Life Quality Index of at least four points increased over the study period.
Regarding safety, Thaçi said, there appeared to be fewer adverse events than had been previously reported with nemolizumab. “We don’t see any signs of conjunctivitis,” he continued, or significant risk of infection apart from for COVID-19, but he pointed out that the study was conducted during the pandemic, which was “a very difficult time.”
The most common treatment-related adverse events were, aside from COVID-19, nasopharyngitis in about 10%-11% of patients, upper respiratory tract infection in about 6% to almost 7%, and headache in about 5%.
Among the adverse events of special interest, newly diagnosed asthma or worsening of asthma occurred in 4.7%-4.8% of patients, while peripheral edema was seen in 0.8%-1.7%.
“Besides this, the study results are really looking very good,” he said, adding: “It means, in a long-term study, we can say today that nemolizumab has revealed the [same] safety profile that was shown in the ARCADIA 1 and 2 trials.”
Alan D. Irvine, MD, DSc, professor of dermatology, Trinity College Dublin in Ireland, who was not involved in the study, underlined that the current interim assessment does not represent the complete dataset and is based on observed cases rather than a more rigorous methodology, such as net reclassification improvement analysis.
“So it makes it a little harder to interpret when you don’t know how many people are dropping out and why they’re dropping out,” he told this news organization. “That said, those who remain on drug out to 56 weeks do experience ongoing improvement in disease control.”
Consequently, “the most reliable message you can take from this interim analysis of long-term data is that there were no new safety signals,” and nemolizumab looks “safe and well-tolerated.”
Where nemolizumab would fit into the treatment pathway for moderate to severe AD remains an open question, Irvine said, although he believes that IL-13 pathway inhibitors such as dupilumab, tralokinumab, and lebrikizumab “will remain the treatment of choice for the immediate future due to prescriber familiarity and good efficacy data.”
However, for patients who are unsuitable for IL-13 inhibitors and/or Janus kinase inhibitors such as abrocitinib and upadacitinib, nemolizumab “could be an interesting alternative.”
“That’s probably where it is going to start,” Irvine said, “and then obviously that will change over time and as the data mature and prescribers become more familiar with the drug in the real world.”
Nemolizumab (Nemluvio) is approved for treating prurigo nodularis (PN) in the United States and in Japan and is under Food and Drug Administration review for treating AD. It is also under review for PN and AD in Europe, Canada, the United Kingdom, and several other countries, according to Galderma. It is also approved for treating pruritus associated with AD in pediatric, adolescent, and adult patients in Japan.
The study was funded by Galderma. Thaçi declared relationships with AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Galderma, Janssen-Cilag, Kyowa Kirin, LEO Pharma, L’Oréal, Eli Lilly, Novartis, Pfizer, Regeneron, Sanofi, Target RWE, and UCB. Irvine declared relationships with AbbVie, Arena Pharmaceuticals, BenevolentAl, Chugai Pharmaceutical, Dermavant, Eli Lily, Genentech, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, UCB, DS Biopharma, and Inflazome.
A version of this article first appeared on Medscape.com.
(AD), revealed an interim analysis of the ARCADIA open-label extension study.
The research was presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
The results showed nemolizumab was associated with “ongoing clinically meaningful improvements in itch, skin lesions, and sleep disturbance,” said study presenter Diamant Thaçi, MD, PhD, of the Comprehensive Center for Inflammation Medicine, University of Lü̈beck in Germany.
Moreover, “patient-reported outcomes, including quality of life ... continued to improve over 56 weeks of treatment.” In addition, Thaçi added, the “safety data support the long-term use of nemolizumab for the treatment of adolescent and adult patients with moderate to severe atopic dermatitis.”
He explained that interleukin (IL) 31 is a key neuroimmune cytokine in AD, triggering itch, skin barrier disruption, and exacerbation of inflammation via its receptor. Nemolizumab inhibits IL-31 receptor binding and was shown in the ARCADIA 1 and ARCADIA 2 trials to provide, along with background topical corticosteroids, clinically meaningful improvements in itch, skin lesions, and sleep for up to weeks 48 of follow-up in adolescents and adults with moderate to severe AD.
The current open-label long-term extension study involved patients who were enrolled in both ARCADIA 1 and 2 trials, as well as those from four phase 2 and 2b studies, a phase 3b study, and adolescents who had not been included in a trial but who met the criteria for the extension study. All patients, whether they started on placebo plus background topical corticosteroids in a prior study, were treated with nemolizumab 30 mg subcutaneously every 4 weeks along with topical corticosteroids.
The interim analysis included all efficacy and safety data up to the cutoff of September 30, 2022, on 723 patients who had completed 56 weeks of treatment among the 1751 patients initially enrolled in the extension study.
The results showed that, regardless of whether patients were nemolizumab naive at enrollment or had previously taken the drug, there were increases in the proportion of patients with an Investigator Global Assessment (IGA) score of 0/1 and an Eczema Area and Severity Index (EASI) score of at least 75 (EASI-75) over the 56 weeks of the study.
In those naive to nemolizumab, the increase in the proportion with an IGA score of 0/1 increased from 17.7% at baseline to 49.0% at 56 weeks, while the proportion with an EASI-75 increased from 24.0% to 78.7%.
The increase in the proportion of patients with an IGA score 0/1 among those who had previously received nemolizumab increased from 28.5% at baseline to 47.1% at 56 weeks. The proportion with an EASI-75 was 38.1% at baseline, rising to 73.0% at 56 weeks.
Increases in the proportion of patients with an EASI score of at least 50 and at least 90 were also seen with nemolizumab, as were increases in the proportion of patients with an improvement of at least four points on the SCORing Atopic Dermatitis Pruritus visual analogue scale and Sleep loss scores.
Similarly, the proportion of patients with a reduction in Dermatology Life Quality Index of at least four points increased over the study period.
Regarding safety, Thaçi said, there appeared to be fewer adverse events than had been previously reported with nemolizumab. “We don’t see any signs of conjunctivitis,” he continued, or significant risk of infection apart from for COVID-19, but he pointed out that the study was conducted during the pandemic, which was “a very difficult time.”
The most common treatment-related adverse events were, aside from COVID-19, nasopharyngitis in about 10%-11% of patients, upper respiratory tract infection in about 6% to almost 7%, and headache in about 5%.
Among the adverse events of special interest, newly diagnosed asthma or worsening of asthma occurred in 4.7%-4.8% of patients, while peripheral edema was seen in 0.8%-1.7%.
“Besides this, the study results are really looking very good,” he said, adding: “It means, in a long-term study, we can say today that nemolizumab has revealed the [same] safety profile that was shown in the ARCADIA 1 and 2 trials.”
Alan D. Irvine, MD, DSc, professor of dermatology, Trinity College Dublin in Ireland, who was not involved in the study, underlined that the current interim assessment does not represent the complete dataset and is based on observed cases rather than a more rigorous methodology, such as net reclassification improvement analysis.
“So it makes it a little harder to interpret when you don’t know how many people are dropping out and why they’re dropping out,” he told this news organization. “That said, those who remain on drug out to 56 weeks do experience ongoing improvement in disease control.”
Consequently, “the most reliable message you can take from this interim analysis of long-term data is that there were no new safety signals,” and nemolizumab looks “safe and well-tolerated.”
Where nemolizumab would fit into the treatment pathway for moderate to severe AD remains an open question, Irvine said, although he believes that IL-13 pathway inhibitors such as dupilumab, tralokinumab, and lebrikizumab “will remain the treatment of choice for the immediate future due to prescriber familiarity and good efficacy data.”
However, for patients who are unsuitable for IL-13 inhibitors and/or Janus kinase inhibitors such as abrocitinib and upadacitinib, nemolizumab “could be an interesting alternative.”
“That’s probably where it is going to start,” Irvine said, “and then obviously that will change over time and as the data mature and prescribers become more familiar with the drug in the real world.”
Nemolizumab (Nemluvio) is approved for treating prurigo nodularis (PN) in the United States and in Japan and is under Food and Drug Administration review for treating AD. It is also under review for PN and AD in Europe, Canada, the United Kingdom, and several other countries, according to Galderma. It is also approved for treating pruritus associated with AD in pediatric, adolescent, and adult patients in Japan.
The study was funded by Galderma. Thaçi declared relationships with AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Galderma, Janssen-Cilag, Kyowa Kirin, LEO Pharma, L’Oréal, Eli Lilly, Novartis, Pfizer, Regeneron, Sanofi, Target RWE, and UCB. Irvine declared relationships with AbbVie, Arena Pharmaceuticals, BenevolentAl, Chugai Pharmaceutical, Dermavant, Eli Lily, Genentech, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, UCB, DS Biopharma, and Inflazome.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Lichen Planus Responds to Treatment with Topical Ruxolitinib in Phase 2 Study
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Study Finds No Increased MACE Risk for JAK Inhibitors in Patients With Atopic Dermatitis
, suggested the results of a large, US-based, retrospective cohort study.
This holds true even in individuals aged 50 years or older, whose age puts them at increased cardiovascular (CV) risk, said Amina El Ayadi, PhD, of the University of Texas Medical Branch at Galveston. He presented the findings at the recent European Academy of Dermatology and Venereology (EADV) 2024 Congress.
Specifically, the analysis looked at treatment with the oral JAK1 inhibitors upadacitinib (Rinvoq) and abrocitinib (Cibinqo), both approved for treating AD in the United States, and found that the relative risk for MACE, such as acute myocardial infarction, cardiac arrest, stroke, or acute deep vein thrombosis, was ≤ 1.0 compared with those not treated with a JAKi.
Similarly, the relative risk for other CV safety endpoints, such as having an abnormal ECG or pericardial effusion, was also around 1.0. There was a slight increase in the relative risk for arrhythmias, peripheral edema, angina pectoris, or heart failure, but no value went > 1.6 and CIs spanned 1.0, indicating the results lack statistical significance.
Reassurance for Dermatologists?
“This suggests that oral administration of these drugs to the patient with atopic dermatitis does not increase the risk of major adverse cardiac events, and dermatologists, based on our data, can safely consider JAK inhibitors for treating moderate to severe dermatitis, even in patients with high risk for these diseases,” El Ayadi said during a late-breaking news session at the meeting.
Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Department at Miguel Servet University Hospital in Zaragoza, Spain, who was one of the chairs for the session, said that this was “very good news for us.”
Gilaberte Calzada, president of the Spanish Academy of Dermatology and Venereology, asked if there were any data on the duration of treatment with the two JAKis included in the analysis. El Ayadi said that this was something that would be looked at in future data analyses.
Gilaberte Calzada also observed that because the CIs were wide, with more time, “we will have more defined data.”
Analyses Overview
For the two analyses — one in the overall population of patients with AD and the other in those aged 50 years or older — electronic medical record (EMR) data from the TriNetX Research Network were used. This is a global, federated health research network that contains EMRs for more than 275 million patients from over 120 healthcare organizations, El Ayadi explained.
To perform the analyses, the research team queried the TriNetX database to find all patients diagnosed with AD via the International Classification of Diseases, Tenth Revision code L20. They then determined if patients had been treated with JAKi or not, and specifically, with upadacitinib or abrocitinib. Those who had not received any JAKi treatment were the control population.
For the first analysis, no age-specific filter was applied. The investigators identified 1674 people with AD who had been treated with the JAKis and around 1.2 million who had not. Propensity score matching, based on age at diagnosis, biologic sex, and CV comorbidities, was performed to give a total of 1674 patients who had and 1674 who had not been treated with these medications.
In the second analysis, only those aged 50 years or older were considered; 875 patients who had received JAKi treatment were identified and around 250,000 who had not. Propensity score matching based on the same variables gave two groups of 875 people who had or had not taken a JAKi.
Queried over the age cutoff used, El Ayadi noted, “We did an analysis looking at patients 65 and older. However, we came up with lower patient numbers. … We do have this data, and we did not see any significant risk.”
The study was independently supported. El Ayadi and Gilaberte Calzada reported no conflicts of interest in relation to the presented findings.
A version of this article first appeared on Medscape.com.
, suggested the results of a large, US-based, retrospective cohort study.
This holds true even in individuals aged 50 years or older, whose age puts them at increased cardiovascular (CV) risk, said Amina El Ayadi, PhD, of the University of Texas Medical Branch at Galveston. He presented the findings at the recent European Academy of Dermatology and Venereology (EADV) 2024 Congress.
Specifically, the analysis looked at treatment with the oral JAK1 inhibitors upadacitinib (Rinvoq) and abrocitinib (Cibinqo), both approved for treating AD in the United States, and found that the relative risk for MACE, such as acute myocardial infarction, cardiac arrest, stroke, or acute deep vein thrombosis, was ≤ 1.0 compared with those not treated with a JAKi.
Similarly, the relative risk for other CV safety endpoints, such as having an abnormal ECG or pericardial effusion, was also around 1.0. There was a slight increase in the relative risk for arrhythmias, peripheral edema, angina pectoris, or heart failure, but no value went > 1.6 and CIs spanned 1.0, indicating the results lack statistical significance.
Reassurance for Dermatologists?
“This suggests that oral administration of these drugs to the patient with atopic dermatitis does not increase the risk of major adverse cardiac events, and dermatologists, based on our data, can safely consider JAK inhibitors for treating moderate to severe dermatitis, even in patients with high risk for these diseases,” El Ayadi said during a late-breaking news session at the meeting.
Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Department at Miguel Servet University Hospital in Zaragoza, Spain, who was one of the chairs for the session, said that this was “very good news for us.”
Gilaberte Calzada, president of the Spanish Academy of Dermatology and Venereology, asked if there were any data on the duration of treatment with the two JAKis included in the analysis. El Ayadi said that this was something that would be looked at in future data analyses.
Gilaberte Calzada also observed that because the CIs were wide, with more time, “we will have more defined data.”
Analyses Overview
For the two analyses — one in the overall population of patients with AD and the other in those aged 50 years or older — electronic medical record (EMR) data from the TriNetX Research Network were used. This is a global, federated health research network that contains EMRs for more than 275 million patients from over 120 healthcare organizations, El Ayadi explained.
To perform the analyses, the research team queried the TriNetX database to find all patients diagnosed with AD via the International Classification of Diseases, Tenth Revision code L20. They then determined if patients had been treated with JAKi or not, and specifically, with upadacitinib or abrocitinib. Those who had not received any JAKi treatment were the control population.
For the first analysis, no age-specific filter was applied. The investigators identified 1674 people with AD who had been treated with the JAKis and around 1.2 million who had not. Propensity score matching, based on age at diagnosis, biologic sex, and CV comorbidities, was performed to give a total of 1674 patients who had and 1674 who had not been treated with these medications.
In the second analysis, only those aged 50 years or older were considered; 875 patients who had received JAKi treatment were identified and around 250,000 who had not. Propensity score matching based on the same variables gave two groups of 875 people who had or had not taken a JAKi.
Queried over the age cutoff used, El Ayadi noted, “We did an analysis looking at patients 65 and older. However, we came up with lower patient numbers. … We do have this data, and we did not see any significant risk.”
The study was independently supported. El Ayadi and Gilaberte Calzada reported no conflicts of interest in relation to the presented findings.
A version of this article first appeared on Medscape.com.
, suggested the results of a large, US-based, retrospective cohort study.
This holds true even in individuals aged 50 years or older, whose age puts them at increased cardiovascular (CV) risk, said Amina El Ayadi, PhD, of the University of Texas Medical Branch at Galveston. He presented the findings at the recent European Academy of Dermatology and Venereology (EADV) 2024 Congress.
Specifically, the analysis looked at treatment with the oral JAK1 inhibitors upadacitinib (Rinvoq) and abrocitinib (Cibinqo), both approved for treating AD in the United States, and found that the relative risk for MACE, such as acute myocardial infarction, cardiac arrest, stroke, or acute deep vein thrombosis, was ≤ 1.0 compared with those not treated with a JAKi.
Similarly, the relative risk for other CV safety endpoints, such as having an abnormal ECG or pericardial effusion, was also around 1.0. There was a slight increase in the relative risk for arrhythmias, peripheral edema, angina pectoris, or heart failure, but no value went > 1.6 and CIs spanned 1.0, indicating the results lack statistical significance.
Reassurance for Dermatologists?
“This suggests that oral administration of these drugs to the patient with atopic dermatitis does not increase the risk of major adverse cardiac events, and dermatologists, based on our data, can safely consider JAK inhibitors for treating moderate to severe dermatitis, even in patients with high risk for these diseases,” El Ayadi said during a late-breaking news session at the meeting.
Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Department at Miguel Servet University Hospital in Zaragoza, Spain, who was one of the chairs for the session, said that this was “very good news for us.”
Gilaberte Calzada, president of the Spanish Academy of Dermatology and Venereology, asked if there were any data on the duration of treatment with the two JAKis included in the analysis. El Ayadi said that this was something that would be looked at in future data analyses.
Gilaberte Calzada also observed that because the CIs were wide, with more time, “we will have more defined data.”
Analyses Overview
For the two analyses — one in the overall population of patients with AD and the other in those aged 50 years or older — electronic medical record (EMR) data from the TriNetX Research Network were used. This is a global, federated health research network that contains EMRs for more than 275 million patients from over 120 healthcare organizations, El Ayadi explained.
To perform the analyses, the research team queried the TriNetX database to find all patients diagnosed with AD via the International Classification of Diseases, Tenth Revision code L20. They then determined if patients had been treated with JAKi or not, and specifically, with upadacitinib or abrocitinib. Those who had not received any JAKi treatment were the control population.
For the first analysis, no age-specific filter was applied. The investigators identified 1674 people with AD who had been treated with the JAKis and around 1.2 million who had not. Propensity score matching, based on age at diagnosis, biologic sex, and CV comorbidities, was performed to give a total of 1674 patients who had and 1674 who had not been treated with these medications.
In the second analysis, only those aged 50 years or older were considered; 875 patients who had received JAKi treatment were identified and around 250,000 who had not. Propensity score matching based on the same variables gave two groups of 875 people who had or had not taken a JAKi.
Queried over the age cutoff used, El Ayadi noted, “We did an analysis looking at patients 65 and older. However, we came up with lower patient numbers. … We do have this data, and we did not see any significant risk.”
The study was independently supported. El Ayadi and Gilaberte Calzada reported no conflicts of interest in relation to the presented findings.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Topical JAK Inhibitor Effective for Hand Eczema, Two Studies Suggest
suggested the results of two separate studies presented during the late-breaking sessions at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
In the 24-week, phase 3 DELTA FORCE trial, topical delgocitinib was compared head to head with oral alitretinoin for managing chronic hand eczema (CHE). Results showed that greater improvements from baseline to week 12 in Hand Eczema Severity Index (HECSI) scores could be achieved with delgocitinib cream than with alitretinoin capsules.
And in another analysis, which involved patients with the atopic subtype of CHE only, the application of topical delgocitinib was found to be as good as treatment with subcutaneous dupilumab (Dupixent) at improving both HECSI scores and the Investigator Global Assessment for CHE response (IGA-CHE).
Potentially a ‘Highly Impactful’ Therapy
“Chronic hand eczema is a common yet burdensome skin condition that poses a considerable challenge for dermatologists. Diversity in morphologic presentation and clinical etiology has been a key limitation for the development of a safe, targeted, one-size-fits-all therapeutic approach,” Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University of Medicine and Science, and the founder and director of the Center for Medical Dermatology and Immunology Research in Chicago, Illinois, said in an interview.
“These data show that delgocitinib cream is poised to be a novel and highly impactful topical therapy for the treatment of CHE,” said Chovatiya.
DELTA FORCE showed that the efficacy and safety of delgocitinib cream was “superior to alitretinoin, the only approved oral option for CHE,” he said. And the other study, a comparative analysis, showed that delgocitinib’s efficacy was “comparable to the biologic dupilumab specifically for the treatment of atopic hand eczema,” said Chovatiya, one of the authors of that study. He was not an author of the DELTA FORCE study.
DELTA FORCE
While it remains an investigational drug in the United States, where it is under Food and Drug Administration review for CHE, delgocitinib cream (Anzupgo) was recently approved by the European Commission for use in adults with moderate to severe hand eczema who do not respond to or who are unable to use topical corticosteroids. Approval was based on data from two phase 3 studies , DELTA 1 and DELTA 2, which compared delgocitinib cream against a cream vehicle, as well as an open-label, long-term extension study, DELTA 3.
In the DELTA FORCE study, 513 adults with severe CHE (IGA-CHE score of 4) were recruited at 102 clinical centers in Europe and North America and randomly allocated to topical treatment with delgocitinib cream, 20 mg/g applied twice daily, or alitretinoin capsules, 30 mg once daily. Treatment with delgocitinib was for 16 weeks, and treatment with alitretinoin was for 12 weeks. The latter’s dose could be reduced to 10 mg in the event of intolerability, and both treatments could be reintroduced if necessary, with a final follow-up at 24 weeks.
Study investigator Ana Maria Giménez-Arnau, MD, PhD, of the Hospital del Mar Research Institute, Pompeu Fabra University, Barcelona, Spain, who presented the findings, noted that alitretinoin (Toctino) is an oral systemic retinoid approved in a few European countries, Canada, Israel, and South Korea for the treatment of severe CHE.
The mean age of the participants was 45 years, almost two thirds were women, and the majority (93%) were White; 90% of patients had been recruited in Europe. The median duration of CHE was 4 years.
At baseline, the median HECSI score was recorded as 79.5 in the delgocitinib arm and 80.0 in the alitretinoin arm. At 12 weeks, the least squares mean change in HECSI score from baseline was –67.6 in the delgocitinib arm and –51.5 in the alitretinoin arm, giving a significant difference of –16.1 between the two groups (P < .001).
Giménez-Arnau reported that delgocitinib also outperformed alitretinoin for all other endpoints assessed, including the following: ≥ 90% improvement in HECSI (HECSI-90), IGA-CHE treatment success (defined as a score of 0/1 indicating clear/almost clear skin), changes in Hand Eczema Symptom Diary (HESD) itch and HESD pain scores, area under the curve for HECSI-90, change in Dermatology Life Quality Index score — which were all assessed at 12 weeks — and change in HECSI from baseline to week 24.
There was “significant improvement in the reduction of the HECSI from the first week” of treatment, Giménez-Arnau said at the meeting. Notably, that the effect increased to 12 weeks and then was sustained. A similar pattern was seen for IGA-CHE treatment success and for HESD pain. This is important as “chronic hand eczema is really painful,” she said.
As for safety, 49.4% of patients in the delgocitinib arm vs 76.1% of patients in the alitretinoin arm experienced any type of adverse event (AE). Serious AEs occurred in 2% and 4.9% of patients in each group, respectively, with fewer AEs leading to trial drug discontinuation observed in the delgocitinib arm (1.2% vs 10.1%). The proportion of AEs “probably or possibly” related to the trial drug was 9.5% in the delgocitinib group vs 54.3% in the alitretinoin group.
Comparison With Dupilumab in Another Trial
Delgocitinib is no longer just an investigational medication, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said during a separate late-breaking presentation at the EADV 2024 meeting. “I think it’s big news because now we have an important topical option for our patients with chronic hand eczema.”
Armstrong presented a matched-adjusted indirect comparison (MAIC) of delgocitinib vs dupilumab for the treatment of moderate to severe atopic hand eczema, which she described as “the next best thing” to a head-to-head trial.
MAICs are where patient level data from one or more clinical trials evaluating drug “A” are compared with aggregate data from one or more clinical trials evaluating drug “B.” In this case, individual patient data from the DELTA 1 and DELTA 2 trials of delgocitinib were compared with published aggregate data from the phase 3 LIBERTY-AD-HAFT trial of dupilumab.
A total of 201 patients with atopic hand eczema in the DELTA 1 and DELTA 2 trials were matched to 133 patients in the LIBERTY-AD-HAFT trial. Of these, 128 had been treated with delgocitinib cream, 73 with a cream vehicle, 67 with subcutaneous dupilumab, and 66 with a subcutaneous placebo.
“We’re trying to compare as much as possible apples to apples here in terms of the etiology of hand eczema,” Armstrong said. She noted that after matching and weighting based on age, sex, race, and baseline HECSI score, baseline characteristics in the two groups of patients were similar. The mean age was about 35.8 years in the two active treatment arms and 33.4 years in the two placebo arms, and mean baseline HESCI scores were about 79-80.
The endpoints compared were ≥ 75% improvement in HECSI; HECSI-90, HECSI percentage improvement, and IGA-CHE in the DELTA 1 and DELTA 2 trials; or a Hand and Foot IGA score of 0/1.
“The key message to take away from this is that there were no statistically significant differences between topical delgocitinib twice daily vs subcutaneous injection of dupilumab by week 16 in the treatment of patients with atopic hand dermatitis,” Armstrong reported. Odds ratios varied between 1.1 and 1.3 for the various endpoints.
Menno de Rie, MD, PhD, professor of dermatology and immunology at Amsterdam University Medical Center in the Netherlands, who cochaired the session, said that “I appreciate very much that you took the effort to compare these totally different compounds and showed us the methodology that you did. It’s really very impressive.”
Topical, Systemic, or Both?
Armstrong was questioned on how to manage someone with atopic hand dermatitis who developed lesions elsewhere on the body.
“I would take a really individualized approach to this patient,” she responded. If the eczema has been limited to the hands and has been there for a while, then perhaps delgocitinib would be her choice, but if they developed lesions elsewhere on the body, then a systemic treatment such as dupilumab may be preferable.
“The good thing is that this study shows that you can offer the patient either of those options and really engage the patient in a shared decision-making process.”
And with regards to whether the two might possibly be used together, Armstrong acknowledged insurance coverage restrictions could be a limiting factor in the United States, but elsewhere — and from a scientific point of view — this could make sense.
“If we have a patient, for example, who has moderate to severe atopic dermatitis involving the body, but also very severe hand eczema as well, one may possibly consider a combination of a systemic medication that’s helpful for the extensive area of involvement on the body ... and now you have a topical therapy, delgocitinib, where you can use it locally, have very deep efficacy locally, to kind of help augment that disease phenotype in that patient population.”
The studies were funded by Leo Pharma. Chovatiya, Giménez-Arnau, and Armstrong acknowledged ties to LEO Pharma, among other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
suggested the results of two separate studies presented during the late-breaking sessions at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
In the 24-week, phase 3 DELTA FORCE trial, topical delgocitinib was compared head to head with oral alitretinoin for managing chronic hand eczema (CHE). Results showed that greater improvements from baseline to week 12 in Hand Eczema Severity Index (HECSI) scores could be achieved with delgocitinib cream than with alitretinoin capsules.
And in another analysis, which involved patients with the atopic subtype of CHE only, the application of topical delgocitinib was found to be as good as treatment with subcutaneous dupilumab (Dupixent) at improving both HECSI scores and the Investigator Global Assessment for CHE response (IGA-CHE).
Potentially a ‘Highly Impactful’ Therapy
“Chronic hand eczema is a common yet burdensome skin condition that poses a considerable challenge for dermatologists. Diversity in morphologic presentation and clinical etiology has been a key limitation for the development of a safe, targeted, one-size-fits-all therapeutic approach,” Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University of Medicine and Science, and the founder and director of the Center for Medical Dermatology and Immunology Research in Chicago, Illinois, said in an interview.
“These data show that delgocitinib cream is poised to be a novel and highly impactful topical therapy for the treatment of CHE,” said Chovatiya.
DELTA FORCE showed that the efficacy and safety of delgocitinib cream was “superior to alitretinoin, the only approved oral option for CHE,” he said. And the other study, a comparative analysis, showed that delgocitinib’s efficacy was “comparable to the biologic dupilumab specifically for the treatment of atopic hand eczema,” said Chovatiya, one of the authors of that study. He was not an author of the DELTA FORCE study.
DELTA FORCE
While it remains an investigational drug in the United States, where it is under Food and Drug Administration review for CHE, delgocitinib cream (Anzupgo) was recently approved by the European Commission for use in adults with moderate to severe hand eczema who do not respond to or who are unable to use topical corticosteroids. Approval was based on data from two phase 3 studies , DELTA 1 and DELTA 2, which compared delgocitinib cream against a cream vehicle, as well as an open-label, long-term extension study, DELTA 3.
In the DELTA FORCE study, 513 adults with severe CHE (IGA-CHE score of 4) were recruited at 102 clinical centers in Europe and North America and randomly allocated to topical treatment with delgocitinib cream, 20 mg/g applied twice daily, or alitretinoin capsules, 30 mg once daily. Treatment with delgocitinib was for 16 weeks, and treatment with alitretinoin was for 12 weeks. The latter’s dose could be reduced to 10 mg in the event of intolerability, and both treatments could be reintroduced if necessary, with a final follow-up at 24 weeks.
Study investigator Ana Maria Giménez-Arnau, MD, PhD, of the Hospital del Mar Research Institute, Pompeu Fabra University, Barcelona, Spain, who presented the findings, noted that alitretinoin (Toctino) is an oral systemic retinoid approved in a few European countries, Canada, Israel, and South Korea for the treatment of severe CHE.
The mean age of the participants was 45 years, almost two thirds were women, and the majority (93%) were White; 90% of patients had been recruited in Europe. The median duration of CHE was 4 years.
At baseline, the median HECSI score was recorded as 79.5 in the delgocitinib arm and 80.0 in the alitretinoin arm. At 12 weeks, the least squares mean change in HECSI score from baseline was –67.6 in the delgocitinib arm and –51.5 in the alitretinoin arm, giving a significant difference of –16.1 between the two groups (P < .001).
Giménez-Arnau reported that delgocitinib also outperformed alitretinoin for all other endpoints assessed, including the following: ≥ 90% improvement in HECSI (HECSI-90), IGA-CHE treatment success (defined as a score of 0/1 indicating clear/almost clear skin), changes in Hand Eczema Symptom Diary (HESD) itch and HESD pain scores, area under the curve for HECSI-90, change in Dermatology Life Quality Index score — which were all assessed at 12 weeks — and change in HECSI from baseline to week 24.
There was “significant improvement in the reduction of the HECSI from the first week” of treatment, Giménez-Arnau said at the meeting. Notably, that the effect increased to 12 weeks and then was sustained. A similar pattern was seen for IGA-CHE treatment success and for HESD pain. This is important as “chronic hand eczema is really painful,” she said.
As for safety, 49.4% of patients in the delgocitinib arm vs 76.1% of patients in the alitretinoin arm experienced any type of adverse event (AE). Serious AEs occurred in 2% and 4.9% of patients in each group, respectively, with fewer AEs leading to trial drug discontinuation observed in the delgocitinib arm (1.2% vs 10.1%). The proportion of AEs “probably or possibly” related to the trial drug was 9.5% in the delgocitinib group vs 54.3% in the alitretinoin group.
Comparison With Dupilumab in Another Trial
Delgocitinib is no longer just an investigational medication, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said during a separate late-breaking presentation at the EADV 2024 meeting. “I think it’s big news because now we have an important topical option for our patients with chronic hand eczema.”
Armstrong presented a matched-adjusted indirect comparison (MAIC) of delgocitinib vs dupilumab for the treatment of moderate to severe atopic hand eczema, which she described as “the next best thing” to a head-to-head trial.
MAICs are where patient level data from one or more clinical trials evaluating drug “A” are compared with aggregate data from one or more clinical trials evaluating drug “B.” In this case, individual patient data from the DELTA 1 and DELTA 2 trials of delgocitinib were compared with published aggregate data from the phase 3 LIBERTY-AD-HAFT trial of dupilumab.
A total of 201 patients with atopic hand eczema in the DELTA 1 and DELTA 2 trials were matched to 133 patients in the LIBERTY-AD-HAFT trial. Of these, 128 had been treated with delgocitinib cream, 73 with a cream vehicle, 67 with subcutaneous dupilumab, and 66 with a subcutaneous placebo.
“We’re trying to compare as much as possible apples to apples here in terms of the etiology of hand eczema,” Armstrong said. She noted that after matching and weighting based on age, sex, race, and baseline HECSI score, baseline characteristics in the two groups of patients were similar. The mean age was about 35.8 years in the two active treatment arms and 33.4 years in the two placebo arms, and mean baseline HESCI scores were about 79-80.
The endpoints compared were ≥ 75% improvement in HECSI; HECSI-90, HECSI percentage improvement, and IGA-CHE in the DELTA 1 and DELTA 2 trials; or a Hand and Foot IGA score of 0/1.
“The key message to take away from this is that there were no statistically significant differences between topical delgocitinib twice daily vs subcutaneous injection of dupilumab by week 16 in the treatment of patients with atopic hand dermatitis,” Armstrong reported. Odds ratios varied between 1.1 and 1.3 for the various endpoints.
Menno de Rie, MD, PhD, professor of dermatology and immunology at Amsterdam University Medical Center in the Netherlands, who cochaired the session, said that “I appreciate very much that you took the effort to compare these totally different compounds and showed us the methodology that you did. It’s really very impressive.”
Topical, Systemic, or Both?
Armstrong was questioned on how to manage someone with atopic hand dermatitis who developed lesions elsewhere on the body.
“I would take a really individualized approach to this patient,” she responded. If the eczema has been limited to the hands and has been there for a while, then perhaps delgocitinib would be her choice, but if they developed lesions elsewhere on the body, then a systemic treatment such as dupilumab may be preferable.
“The good thing is that this study shows that you can offer the patient either of those options and really engage the patient in a shared decision-making process.”
And with regards to whether the two might possibly be used together, Armstrong acknowledged insurance coverage restrictions could be a limiting factor in the United States, but elsewhere — and from a scientific point of view — this could make sense.
“If we have a patient, for example, who has moderate to severe atopic dermatitis involving the body, but also very severe hand eczema as well, one may possibly consider a combination of a systemic medication that’s helpful for the extensive area of involvement on the body ... and now you have a topical therapy, delgocitinib, where you can use it locally, have very deep efficacy locally, to kind of help augment that disease phenotype in that patient population.”
The studies were funded by Leo Pharma. Chovatiya, Giménez-Arnau, and Armstrong acknowledged ties to LEO Pharma, among other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
suggested the results of two separate studies presented during the late-breaking sessions at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
In the 24-week, phase 3 DELTA FORCE trial, topical delgocitinib was compared head to head with oral alitretinoin for managing chronic hand eczema (CHE). Results showed that greater improvements from baseline to week 12 in Hand Eczema Severity Index (HECSI) scores could be achieved with delgocitinib cream than with alitretinoin capsules.
And in another analysis, which involved patients with the atopic subtype of CHE only, the application of topical delgocitinib was found to be as good as treatment with subcutaneous dupilumab (Dupixent) at improving both HECSI scores and the Investigator Global Assessment for CHE response (IGA-CHE).
Potentially a ‘Highly Impactful’ Therapy
“Chronic hand eczema is a common yet burdensome skin condition that poses a considerable challenge for dermatologists. Diversity in morphologic presentation and clinical etiology has been a key limitation for the development of a safe, targeted, one-size-fits-all therapeutic approach,” Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University of Medicine and Science, and the founder and director of the Center for Medical Dermatology and Immunology Research in Chicago, Illinois, said in an interview.
“These data show that delgocitinib cream is poised to be a novel and highly impactful topical therapy for the treatment of CHE,” said Chovatiya.
DELTA FORCE showed that the efficacy and safety of delgocitinib cream was “superior to alitretinoin, the only approved oral option for CHE,” he said. And the other study, a comparative analysis, showed that delgocitinib’s efficacy was “comparable to the biologic dupilumab specifically for the treatment of atopic hand eczema,” said Chovatiya, one of the authors of that study. He was not an author of the DELTA FORCE study.
DELTA FORCE
While it remains an investigational drug in the United States, where it is under Food and Drug Administration review for CHE, delgocitinib cream (Anzupgo) was recently approved by the European Commission for use in adults with moderate to severe hand eczema who do not respond to or who are unable to use topical corticosteroids. Approval was based on data from two phase 3 studies , DELTA 1 and DELTA 2, which compared delgocitinib cream against a cream vehicle, as well as an open-label, long-term extension study, DELTA 3.
In the DELTA FORCE study, 513 adults with severe CHE (IGA-CHE score of 4) were recruited at 102 clinical centers in Europe and North America and randomly allocated to topical treatment with delgocitinib cream, 20 mg/g applied twice daily, or alitretinoin capsules, 30 mg once daily. Treatment with delgocitinib was for 16 weeks, and treatment with alitretinoin was for 12 weeks. The latter’s dose could be reduced to 10 mg in the event of intolerability, and both treatments could be reintroduced if necessary, with a final follow-up at 24 weeks.
Study investigator Ana Maria Giménez-Arnau, MD, PhD, of the Hospital del Mar Research Institute, Pompeu Fabra University, Barcelona, Spain, who presented the findings, noted that alitretinoin (Toctino) is an oral systemic retinoid approved in a few European countries, Canada, Israel, and South Korea for the treatment of severe CHE.
The mean age of the participants was 45 years, almost two thirds were women, and the majority (93%) were White; 90% of patients had been recruited in Europe. The median duration of CHE was 4 years.
At baseline, the median HECSI score was recorded as 79.5 in the delgocitinib arm and 80.0 in the alitretinoin arm. At 12 weeks, the least squares mean change in HECSI score from baseline was –67.6 in the delgocitinib arm and –51.5 in the alitretinoin arm, giving a significant difference of –16.1 between the two groups (P < .001).
Giménez-Arnau reported that delgocitinib also outperformed alitretinoin for all other endpoints assessed, including the following: ≥ 90% improvement in HECSI (HECSI-90), IGA-CHE treatment success (defined as a score of 0/1 indicating clear/almost clear skin), changes in Hand Eczema Symptom Diary (HESD) itch and HESD pain scores, area under the curve for HECSI-90, change in Dermatology Life Quality Index score — which were all assessed at 12 weeks — and change in HECSI from baseline to week 24.
There was “significant improvement in the reduction of the HECSI from the first week” of treatment, Giménez-Arnau said at the meeting. Notably, that the effect increased to 12 weeks and then was sustained. A similar pattern was seen for IGA-CHE treatment success and for HESD pain. This is important as “chronic hand eczema is really painful,” she said.
As for safety, 49.4% of patients in the delgocitinib arm vs 76.1% of patients in the alitretinoin arm experienced any type of adverse event (AE). Serious AEs occurred in 2% and 4.9% of patients in each group, respectively, with fewer AEs leading to trial drug discontinuation observed in the delgocitinib arm (1.2% vs 10.1%). The proportion of AEs “probably or possibly” related to the trial drug was 9.5% in the delgocitinib group vs 54.3% in the alitretinoin group.
Comparison With Dupilumab in Another Trial
Delgocitinib is no longer just an investigational medication, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said during a separate late-breaking presentation at the EADV 2024 meeting. “I think it’s big news because now we have an important topical option for our patients with chronic hand eczema.”
Armstrong presented a matched-adjusted indirect comparison (MAIC) of delgocitinib vs dupilumab for the treatment of moderate to severe atopic hand eczema, which she described as “the next best thing” to a head-to-head trial.
MAICs are where patient level data from one or more clinical trials evaluating drug “A” are compared with aggregate data from one or more clinical trials evaluating drug “B.” In this case, individual patient data from the DELTA 1 and DELTA 2 trials of delgocitinib were compared with published aggregate data from the phase 3 LIBERTY-AD-HAFT trial of dupilumab.
A total of 201 patients with atopic hand eczema in the DELTA 1 and DELTA 2 trials were matched to 133 patients in the LIBERTY-AD-HAFT trial. Of these, 128 had been treated with delgocitinib cream, 73 with a cream vehicle, 67 with subcutaneous dupilumab, and 66 with a subcutaneous placebo.
“We’re trying to compare as much as possible apples to apples here in terms of the etiology of hand eczema,” Armstrong said. She noted that after matching and weighting based on age, sex, race, and baseline HECSI score, baseline characteristics in the two groups of patients were similar. The mean age was about 35.8 years in the two active treatment arms and 33.4 years in the two placebo arms, and mean baseline HESCI scores were about 79-80.
The endpoints compared were ≥ 75% improvement in HECSI; HECSI-90, HECSI percentage improvement, and IGA-CHE in the DELTA 1 and DELTA 2 trials; or a Hand and Foot IGA score of 0/1.
“The key message to take away from this is that there were no statistically significant differences between topical delgocitinib twice daily vs subcutaneous injection of dupilumab by week 16 in the treatment of patients with atopic hand dermatitis,” Armstrong reported. Odds ratios varied between 1.1 and 1.3 for the various endpoints.
Menno de Rie, MD, PhD, professor of dermatology and immunology at Amsterdam University Medical Center in the Netherlands, who cochaired the session, said that “I appreciate very much that you took the effort to compare these totally different compounds and showed us the methodology that you did. It’s really very impressive.”
Topical, Systemic, or Both?
Armstrong was questioned on how to manage someone with atopic hand dermatitis who developed lesions elsewhere on the body.
“I would take a really individualized approach to this patient,” she responded. If the eczema has been limited to the hands and has been there for a while, then perhaps delgocitinib would be her choice, but if they developed lesions elsewhere on the body, then a systemic treatment such as dupilumab may be preferable.
“The good thing is that this study shows that you can offer the patient either of those options and really engage the patient in a shared decision-making process.”
And with regards to whether the two might possibly be used together, Armstrong acknowledged insurance coverage restrictions could be a limiting factor in the United States, but elsewhere — and from a scientific point of view — this could make sense.
“If we have a patient, for example, who has moderate to severe atopic dermatitis involving the body, but also very severe hand eczema as well, one may possibly consider a combination of a systemic medication that’s helpful for the extensive area of involvement on the body ... and now you have a topical therapy, delgocitinib, where you can use it locally, have very deep efficacy locally, to kind of help augment that disease phenotype in that patient population.”
The studies were funded by Leo Pharma. Chovatiya, Giménez-Arnau, and Armstrong acknowledged ties to LEO Pharma, among other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Triple P
Podcasts, websites, and large “Parenting” sections in bookstores testify to the large demand for parent guidance and support, but also to the fact that there is no one universally accepted guidebook, such as Benjamin Spock provided for parents almost 80 years ago with Baby and Child Care.
We will describe the basic components of this curriculum so that you may determine whether it might be useful to the families in your practice. Then we will expand upon the domains that have proven essential for parents to nurture healthy development in their children. Even if you do not have the time or resources to provide the full Triple P curriculum, you can offer these principles directly to parents and decide when to refer them to access more formal parent training and coaching.
Triple P was developed by psychologist Matthew Sanders, to “promote positive, caring relationships between parents and their children and to help parents develop effective management strategies for dealing with a variety of childhood behavior problems and common developmental issues” as his doctoral project in Australia in the 1980s. Research in the 1990s suggested substantial efficacy, and it was packaged for broader adoption in the early 2000s. It is a tiered approach, meaning there is content for universal education (level 1), up through more intensive, specialized, and individualized content to be delivered in group or individual settings focused on building specific skills or addressing select problems. It was originally developed for the parents of 0- to 11-year-old children, with additional curricula for parents of teenagers created later. It always is delivered to parents only, through a mix of video and reading, or in-person groups or individual coaching. While the universal education resources are available for free to families of children under 12 in Australia, resources and training are available for a fee in the United states (triplep.net). Research has demonstrated considerable efficacy at reducing some of the common behavioral problems of childhood, improving parental confidence and family harmony, and decreasing rates of parental depression. It has even demonstrated efficacy in reducing the incidence of child maltreatment.
Triple P focuses on what Sanders calls the five key principles of positive parenting:
- 1. Creating a safe and engaging environment for children
- 2. Providing a positive learning environment for children
- 3. Assertive discipline
- 4. Having realistic expectations
- 5. Parental self-care.
The educational materials and more intensive parent trainings are all focused on developing knowledge and skills in the parents that will promote a positive relationship with their children, teach the children new skills while encouraging desirable behaviors, and managing problematic behaviors. The training happens with written or video scenarios, up through individualized skill coaching with homework and direct feedback from trained clinicians. While information about the universally helpful knowledge and skills can be found online or accessed through some local programs in the United States, the higher levels of intervention are less consistently available. You should explore what is available in your community, but even if you don’t have the resources for your own training, you are already offering parent guidance at every visit.
Practical Strategies
Below are practical strategies to offer parents the knowledge and support that are essential to “positive parenting,” so they may nurture their children’s healthiest development.
Attunement: Attunement is simply a parent’s ability to know who their child is and where their child is at any given time. This covers an appreciation of the child’s temperament, style, interests, strengths, and vulnerabilities. Where their child is at includes being able to read that particular child’s cues: Are they hungry? Sleepy? Sick? Frustrated? Parents are the experts on their children, but their children are also always changing. You can help the parents in your practice be intentional about being attuned to their children, so they can always be deepening their understanding of who their children are (becoming) and where they are at in any given moment. This requires protecting regular, unstructured time when they can give their children their full attention: reading, doing an art project, practicing music, or basketball. Schedules are often packed with work and school, driving between many structured activities. Reassure the parents in your practice that time spent in play is just as important. When a parent is present, attentive, and curious, asking questions, learning about the child’s thoughts, feelings and ideas, they are doing some of the most essential (and delightful) work of raising children.
Positive Environment: A “positive environment” is child-centered, with access to age-appropriate activities of a wide range. Offering first-time parents written resources about child development and age-appropriate games, books, and activities is an easy way to support positive parenting. A positive environment also has structure and routines, so children can play and explore with the comfort of knowing what to expect and what is expected of them. Do they have a regular bedtime and bedtime routines? Do they consistently eat dinner together and clean up as a family? Do they have reliable unstructured time together, maybe playing board games or kickball after dinner? These varying but predictable routines provide opportunities for children to practice helping, following through, sharing, and tolerating frustration or failure, and they give parents low-stakes opportunities to offer praise for their effort, compassion when they struggle, and affection for no reason at all. They lower the chances of parent-child interactions being predominantly reactive, demanding, pleading, or angry.
Effective Discipline: A positive environment includes reasonable and consistent consequences for rule breaking and poor behavior, and an essential part of predictability includes clear ground rules for what is expected of children at home, around chores, getting ready for school and bedtime, and their behavior. Parents need to agree on and children should understand what the consequences will be for breaking rules. Parents should also have a clear strategy for consistently and calmly enforcing rules. This is not easy, but is just as important as affection and play. If parents are struggling with discipline, it is worth asking for a specific example to learn about where the trouble lies. Are parents not on the same page? Are they worried about their children’s distress? Do they lose their temper and the matter escalates? Clear ground rules and a game plan can help them to stay calm instead of resorting to pleading and yelling. Speaking with them about the value of planning and communicating about these expectations and rules during a quiet time, not in the midst of conflict, might be enough to help them with effective discipline. Others may need more support. Books like 123 Magic with more detail on how to manage time outs can be helpful. For those parents who are managing greater difficulty, a referral to parent coaching (with a modality such as Triple P, Parent-Child Interaction Training or Collaborative Problem Solving) may be needed.
Parental Well-Being: Being aligned with one’s spouse (or other caregiver) in how to manage challenging child behaviors is essential to a healthy relationship, and overall well-being is an essential ingredient in creating a nurturing, positive environment at home. How is the parents’ communication with each other overall? Do they have time together that is not focused on the children? Does each parent have time for outside interests or hobbies? How about other important relationships? Do they prioritize their own sleep, regular exercise, and good nutrition? It can be powerful if they plan family activities that are centered on their own passions and interests as well as their children’s. It is powerful for parents to hear from you that when they protect some of their time and energy to simply care for their own health and well-being, they are building a positive environment for their children, both in how they will show up for their family and in what they model.
Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
Suggested Reading
Sanders MR et al. The Development and Dissemination of the Triple P – Positive Parenting Program: A Multilevel Evidence-Based System of Parenting and Family Support. Prev Sci. 2002 Sep;3(3):173-89. doi: 10.1023/a:1019942516231.
Sanders MR. The Triple P System of Evidence-Based Parenting Support: Past, Present, and Future Directions. Clin Child Fam Psychol Rev. 2023 Dec;26(4):880-903. doi: 10.1007/s10567-023-00441-8.
Podcasts, websites, and large “Parenting” sections in bookstores testify to the large demand for parent guidance and support, but also to the fact that there is no one universally accepted guidebook, such as Benjamin Spock provided for parents almost 80 years ago with Baby and Child Care.
We will describe the basic components of this curriculum so that you may determine whether it might be useful to the families in your practice. Then we will expand upon the domains that have proven essential for parents to nurture healthy development in their children. Even if you do not have the time or resources to provide the full Triple P curriculum, you can offer these principles directly to parents and decide when to refer them to access more formal parent training and coaching.
Triple P was developed by psychologist Matthew Sanders, to “promote positive, caring relationships between parents and their children and to help parents develop effective management strategies for dealing with a variety of childhood behavior problems and common developmental issues” as his doctoral project in Australia in the 1980s. Research in the 1990s suggested substantial efficacy, and it was packaged for broader adoption in the early 2000s. It is a tiered approach, meaning there is content for universal education (level 1), up through more intensive, specialized, and individualized content to be delivered in group or individual settings focused on building specific skills or addressing select problems. It was originally developed for the parents of 0- to 11-year-old children, with additional curricula for parents of teenagers created later. It always is delivered to parents only, through a mix of video and reading, or in-person groups or individual coaching. While the universal education resources are available for free to families of children under 12 in Australia, resources and training are available for a fee in the United states (triplep.net). Research has demonstrated considerable efficacy at reducing some of the common behavioral problems of childhood, improving parental confidence and family harmony, and decreasing rates of parental depression. It has even demonstrated efficacy in reducing the incidence of child maltreatment.
Triple P focuses on what Sanders calls the five key principles of positive parenting:
- 1. Creating a safe and engaging environment for children
- 2. Providing a positive learning environment for children
- 3. Assertive discipline
- 4. Having realistic expectations
- 5. Parental self-care.
The educational materials and more intensive parent trainings are all focused on developing knowledge and skills in the parents that will promote a positive relationship with their children, teach the children new skills while encouraging desirable behaviors, and managing problematic behaviors. The training happens with written or video scenarios, up through individualized skill coaching with homework and direct feedback from trained clinicians. While information about the universally helpful knowledge and skills can be found online or accessed through some local programs in the United States, the higher levels of intervention are less consistently available. You should explore what is available in your community, but even if you don’t have the resources for your own training, you are already offering parent guidance at every visit.
Practical Strategies
Below are practical strategies to offer parents the knowledge and support that are essential to “positive parenting,” so they may nurture their children’s healthiest development.
Attunement: Attunement is simply a parent’s ability to know who their child is and where their child is at any given time. This covers an appreciation of the child’s temperament, style, interests, strengths, and vulnerabilities. Where their child is at includes being able to read that particular child’s cues: Are they hungry? Sleepy? Sick? Frustrated? Parents are the experts on their children, but their children are also always changing. You can help the parents in your practice be intentional about being attuned to their children, so they can always be deepening their understanding of who their children are (becoming) and where they are at in any given moment. This requires protecting regular, unstructured time when they can give their children their full attention: reading, doing an art project, practicing music, or basketball. Schedules are often packed with work and school, driving between many structured activities. Reassure the parents in your practice that time spent in play is just as important. When a parent is present, attentive, and curious, asking questions, learning about the child’s thoughts, feelings and ideas, they are doing some of the most essential (and delightful) work of raising children.
Positive Environment: A “positive environment” is child-centered, with access to age-appropriate activities of a wide range. Offering first-time parents written resources about child development and age-appropriate games, books, and activities is an easy way to support positive parenting. A positive environment also has structure and routines, so children can play and explore with the comfort of knowing what to expect and what is expected of them. Do they have a regular bedtime and bedtime routines? Do they consistently eat dinner together and clean up as a family? Do they have reliable unstructured time together, maybe playing board games or kickball after dinner? These varying but predictable routines provide opportunities for children to practice helping, following through, sharing, and tolerating frustration or failure, and they give parents low-stakes opportunities to offer praise for their effort, compassion when they struggle, and affection for no reason at all. They lower the chances of parent-child interactions being predominantly reactive, demanding, pleading, or angry.
Effective Discipline: A positive environment includes reasonable and consistent consequences for rule breaking and poor behavior, and an essential part of predictability includes clear ground rules for what is expected of children at home, around chores, getting ready for school and bedtime, and their behavior. Parents need to agree on and children should understand what the consequences will be for breaking rules. Parents should also have a clear strategy for consistently and calmly enforcing rules. This is not easy, but is just as important as affection and play. If parents are struggling with discipline, it is worth asking for a specific example to learn about where the trouble lies. Are parents not on the same page? Are they worried about their children’s distress? Do they lose their temper and the matter escalates? Clear ground rules and a game plan can help them to stay calm instead of resorting to pleading and yelling. Speaking with them about the value of planning and communicating about these expectations and rules during a quiet time, not in the midst of conflict, might be enough to help them with effective discipline. Others may need more support. Books like 123 Magic with more detail on how to manage time outs can be helpful. For those parents who are managing greater difficulty, a referral to parent coaching (with a modality such as Triple P, Parent-Child Interaction Training or Collaborative Problem Solving) may be needed.
Parental Well-Being: Being aligned with one’s spouse (or other caregiver) in how to manage challenging child behaviors is essential to a healthy relationship, and overall well-being is an essential ingredient in creating a nurturing, positive environment at home. How is the parents’ communication with each other overall? Do they have time together that is not focused on the children? Does each parent have time for outside interests or hobbies? How about other important relationships? Do they prioritize their own sleep, regular exercise, and good nutrition? It can be powerful if they plan family activities that are centered on their own passions and interests as well as their children’s. It is powerful for parents to hear from you that when they protect some of their time and energy to simply care for their own health and well-being, they are building a positive environment for their children, both in how they will show up for their family and in what they model.
Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
Suggested Reading
Sanders MR et al. The Development and Dissemination of the Triple P – Positive Parenting Program: A Multilevel Evidence-Based System of Parenting and Family Support. Prev Sci. 2002 Sep;3(3):173-89. doi: 10.1023/a:1019942516231.
Sanders MR. The Triple P System of Evidence-Based Parenting Support: Past, Present, and Future Directions. Clin Child Fam Psychol Rev. 2023 Dec;26(4):880-903. doi: 10.1007/s10567-023-00441-8.
Podcasts, websites, and large “Parenting” sections in bookstores testify to the large demand for parent guidance and support, but also to the fact that there is no one universally accepted guidebook, such as Benjamin Spock provided for parents almost 80 years ago with Baby and Child Care.
We will describe the basic components of this curriculum so that you may determine whether it might be useful to the families in your practice. Then we will expand upon the domains that have proven essential for parents to nurture healthy development in their children. Even if you do not have the time or resources to provide the full Triple P curriculum, you can offer these principles directly to parents and decide when to refer them to access more formal parent training and coaching.
Triple P was developed by psychologist Matthew Sanders, to “promote positive, caring relationships between parents and their children and to help parents develop effective management strategies for dealing with a variety of childhood behavior problems and common developmental issues” as his doctoral project in Australia in the 1980s. Research in the 1990s suggested substantial efficacy, and it was packaged for broader adoption in the early 2000s. It is a tiered approach, meaning there is content for universal education (level 1), up through more intensive, specialized, and individualized content to be delivered in group or individual settings focused on building specific skills or addressing select problems. It was originally developed for the parents of 0- to 11-year-old children, with additional curricula for parents of teenagers created later. It always is delivered to parents only, through a mix of video and reading, or in-person groups or individual coaching. While the universal education resources are available for free to families of children under 12 in Australia, resources and training are available for a fee in the United states (triplep.net). Research has demonstrated considerable efficacy at reducing some of the common behavioral problems of childhood, improving parental confidence and family harmony, and decreasing rates of parental depression. It has even demonstrated efficacy in reducing the incidence of child maltreatment.
Triple P focuses on what Sanders calls the five key principles of positive parenting:
- 1. Creating a safe and engaging environment for children
- 2. Providing a positive learning environment for children
- 3. Assertive discipline
- 4. Having realistic expectations
- 5. Parental self-care.
The educational materials and more intensive parent trainings are all focused on developing knowledge and skills in the parents that will promote a positive relationship with their children, teach the children new skills while encouraging desirable behaviors, and managing problematic behaviors. The training happens with written or video scenarios, up through individualized skill coaching with homework and direct feedback from trained clinicians. While information about the universally helpful knowledge and skills can be found online or accessed through some local programs in the United States, the higher levels of intervention are less consistently available. You should explore what is available in your community, but even if you don’t have the resources for your own training, you are already offering parent guidance at every visit.
Practical Strategies
Below are practical strategies to offer parents the knowledge and support that are essential to “positive parenting,” so they may nurture their children’s healthiest development.
Attunement: Attunement is simply a parent’s ability to know who their child is and where their child is at any given time. This covers an appreciation of the child’s temperament, style, interests, strengths, and vulnerabilities. Where their child is at includes being able to read that particular child’s cues: Are they hungry? Sleepy? Sick? Frustrated? Parents are the experts on their children, but their children are also always changing. You can help the parents in your practice be intentional about being attuned to their children, so they can always be deepening their understanding of who their children are (becoming) and where they are at in any given moment. This requires protecting regular, unstructured time when they can give their children their full attention: reading, doing an art project, practicing music, or basketball. Schedules are often packed with work and school, driving between many structured activities. Reassure the parents in your practice that time spent in play is just as important. When a parent is present, attentive, and curious, asking questions, learning about the child’s thoughts, feelings and ideas, they are doing some of the most essential (and delightful) work of raising children.
Positive Environment: A “positive environment” is child-centered, with access to age-appropriate activities of a wide range. Offering first-time parents written resources about child development and age-appropriate games, books, and activities is an easy way to support positive parenting. A positive environment also has structure and routines, so children can play and explore with the comfort of knowing what to expect and what is expected of them. Do they have a regular bedtime and bedtime routines? Do they consistently eat dinner together and clean up as a family? Do they have reliable unstructured time together, maybe playing board games or kickball after dinner? These varying but predictable routines provide opportunities for children to practice helping, following through, sharing, and tolerating frustration or failure, and they give parents low-stakes opportunities to offer praise for their effort, compassion when they struggle, and affection for no reason at all. They lower the chances of parent-child interactions being predominantly reactive, demanding, pleading, or angry.
Effective Discipline: A positive environment includes reasonable and consistent consequences for rule breaking and poor behavior, and an essential part of predictability includes clear ground rules for what is expected of children at home, around chores, getting ready for school and bedtime, and their behavior. Parents need to agree on and children should understand what the consequences will be for breaking rules. Parents should also have a clear strategy for consistently and calmly enforcing rules. This is not easy, but is just as important as affection and play. If parents are struggling with discipline, it is worth asking for a specific example to learn about where the trouble lies. Are parents not on the same page? Are they worried about their children’s distress? Do they lose their temper and the matter escalates? Clear ground rules and a game plan can help them to stay calm instead of resorting to pleading and yelling. Speaking with them about the value of planning and communicating about these expectations and rules during a quiet time, not in the midst of conflict, might be enough to help them with effective discipline. Others may need more support. Books like 123 Magic with more detail on how to manage time outs can be helpful. For those parents who are managing greater difficulty, a referral to parent coaching (with a modality such as Triple P, Parent-Child Interaction Training or Collaborative Problem Solving) may be needed.
Parental Well-Being: Being aligned with one’s spouse (or other caregiver) in how to manage challenging child behaviors is essential to a healthy relationship, and overall well-being is an essential ingredient in creating a nurturing, positive environment at home. How is the parents’ communication with each other overall? Do they have time together that is not focused on the children? Does each parent have time for outside interests or hobbies? How about other important relationships? Do they prioritize their own sleep, regular exercise, and good nutrition? It can be powerful if they plan family activities that are centered on their own passions and interests as well as their children’s. It is powerful for parents to hear from you that when they protect some of their time and energy to simply care for their own health and well-being, they are building a positive environment for their children, both in how they will show up for their family and in what they model.
Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
Suggested Reading
Sanders MR et al. The Development and Dissemination of the Triple P – Positive Parenting Program: A Multilevel Evidence-Based System of Parenting and Family Support. Prev Sci. 2002 Sep;3(3):173-89. doi: 10.1023/a:1019942516231.
Sanders MR. The Triple P System of Evidence-Based Parenting Support: Past, Present, and Future Directions. Clin Child Fam Psychol Rev. 2023 Dec;26(4):880-903. doi: 10.1007/s10567-023-00441-8.




