User login
Five reasons why medical meetings will never be the same
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
Identifying ovarian malignancy is not so easy
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
When an ovarian mass is anticipated or known, following evaluation of a patient’s history and physician examination, imaging via transvaginal and often abdominal ultrasound is the very next step. This evaluation likely will include both gray-scale and color Doppler examination. The initial concern always must be to identify ovarian malignancy.
Despite morphological scoring systems as well as the use of Doppler ultrasonography, there remains a lack of agreement and acceptance. In a 2008 multicenter study, Timmerman and colleagues evaluated 1,066 patients with 1,233 persistent adnexal tumors via transvaginal grayscale and Doppler ultrasound; 73% were benign tumors, and 27% were malignant tumors. Information on 42 gray-scale ultrasound variables and 6 Doppler variables was collected and evaluated to determine which variables had the highest positive predictive value for a malignant tumor and for a benign mass (Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365).
Five simple rules were selected that best predict malignancy (M-rules), as follows:
- Irregular solid tumor.
- Ascites.
- At least four papillary projections.
- Irregular multilocular-solid tumor with a greatest diameter greater than or equal to 10 cm.
- Very high color content on Doppler exam.
The following five simple rules suggested that a mass is benign (B-rules):
- Unilocular cyst.
- Largest solid component less than 7 mm.
- Acoustic shadows.
- Smooth multilocular tumor less than 10 cm.
- No detectable blood flow with Doppler exam.
Unfortunately, despite a sensitivity of 93% and specificity of 90%, and a positive and negative predictive value of 80% and 97%, these 10 simple rules were applicable to only 76% of tumors.
To assist those of us who are not gynecologic oncologists and who are often faced with having to determine whether surgery is recommended, I have elicited the expertise of Jubilee Brown, MD, professor and associate director of gynecologic oncology at the Levine Cancer Institute, Carolinas HealthCare System, in Charlotte, N.C., and the current president of the AAGL, to lead us in a review of evaluating an ovarian mass.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, Ill., and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
How to evaluate a suspicious ovarian mass
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Ovarian masses are common in women of all ages. It is important not to miss even one ovarian cancer, but we must also identify masses that will resolve on their own over time to avoid overtreatment. These concurrent goals of excluding malignancy while not overtreating patients are the basis for management of the pelvic mass. Additionally, fertility preservation is important when surgery is performed in a reproductive-aged woman.
An ovarian mass may be anything from a simple functional or physiologic cyst to an endometrioma to an epithelial carcinoma, a germ-cell tumor, or a stromal tumor (the latter three of which may metastasize). Across the general population, women have a 5%-10% lifetime risk of needing surgery for a suspected ovarian mass and a 1.4% (1 in 70) risk that this mass is cancerous. The majority of ovarian cysts or masses therefore are benign.
A thorough history – including family history – and physical examination with appropriate laboratory testing and directed imaging are important first steps for the ob.gyn. Fortunately, we have guidelines and criteria governing not only when observation or surgery is warranted but also when patients should be referred to a gynecologic oncologist. By following these guidelines,1 we are able to achieve the best outcomes.
Transvaginal ultrasound
A 2007 groundbreaking study led by Barbara Goff, MD, demonstrated that there are warning signs for ovarian cancer – symptoms that are significantly associated with malignancy. Dr. Goff and her coinvestigators evaluated the charts of hundreds of patients, including about 150 with ovarian cancer, and found that pelvic/abdominal pressure or pain, bloating, increase in abdominal size, and difficulty eating or feeling full were significantly and independently associated with cancer if these symptoms were present for less than a year and occurred at least 12 times per month.2
A pelvic examination is an integral part of evaluating every patient who has such concerns. That said, pelvic exams have limited ability to identify adnexal masses, especially in women who are obese – and that’s where imaging becomes especially important.
Masses generally can be considered simple or complex based on their appearance. A simple cyst is fluid-filled with thin, smooth walls and the absence of solid components or septations; it is significantly more likely to resolve on its own and is less likely to imply malignancy than a complex cyst, especially in a premenopausal woman. A complex cyst is multiseptated and/or solid – possibly with papillary projections – and is more concerning, especially if there is increased, new vascularity. Making this distinction helps us determine the risk of malignancy.
Transvaginal ultrasound (TVUS) is the preferred method for imaging, and our threshold for obtaining a TVUS should be very low. Women who have symptoms or concerns that can’t be attributed to a particular condition, and women in whom a mass can be palpated (even if asymptomatic) should have a TVUS. The imaging modality is cost effective and well tolerated by patients, does not expose the patient to ionizing radiation, and should generally be considered first-line imaging.3,4
Size is not predictive of malignancy, but it is important for determining whether surgery is warranted. In our experience, a mass of 8-10 cm or larger on TVUS is at risk of torsion and is unlikely to resolve on its own, even in a premenopausal woman. While large masses generally require surgery, patients of any age who have simple cysts smaller than 8-10 cm generally can be followed with serial exams and ultrasound; spontaneous regression is common.
Doppler ultrasonography is useful for evaluating blood flow in and around an ovarian mass and can be helpful for confirming suspected characteristics of a mass.
Recent studies from the radiology community have looked at the utility of the resistive index – a measure of the impedance and velocity of blood flow – as a predictor of ovarian malignancy. However, we caution against using Doppler to determine whether a mass is benign or malignant, or to determine the necessity of surgery. An abnormal ovary may have what is considered to be a normal resistive index, and the resistive index of a normal ovary may fall within the abnormal range. Doppler flow can be helpful, but it must be combined with other predictive features, like solid components with flow or papillary projections within a cyst, to define a decision about surgery.4,5
Magnetic resonance imaging can be useful in differentiating a fibroid from an ovarian mass, and a CT scan can be helpful in looking for disseminated disease when ovarian cancer is suspected based on ultrasound imaging, physical and history, and serum markers. A CT is useful, for instance, in a patient whose ovary is distended with ascites or who has upper abdominal complaints and a complex cyst. CT, PET, and MRI are not recommended in the initial evaluation of an ovarian mass.
The utility of serum biomarkers
Cancer antigen 125 (CA-125) testing may be helpful – in combination with other findings – for decision-making regarding the likelihood of malignancy and the need to refer patients. CA-125 is like Doppler in that a normal CA-125 cannot eliminate the possibility of cancer, and an abnormal CA-125 does not in and of itself imply malignancy. It’s far from a perfect cancer screening test.
CA-125 is a protein associated with epithelial ovarian malignancies, the type of ovarian cancer most commonly seen in postmenopausal women with genetic predispositions. Its specificity and positive predictive value are much higher in postmenopausal women than in average-risk premenopausal women (those without a family history or a known mutation that predisposes them to ovarian cancer). Levels of the marker are elevated in association with many nonmalignant conditions in premenopausal women – endometriosis, fibroids, and various inflammatory conditions, for instance – so the marker’s utility in this population is limited.
For women who have a family history of ovarian cancer or a known breast cancer gene 1 (BRCA1) or BRCA2 mutation, there are some data that suggest that monitoring with CA-125 measurements and TVUS may be a good approach to following patients prior to the age at which risk-reducing surgery can best be performed.
In an adolescent girl or a woman of reproductive age, we think less about epithelial cancer and more about germ-cell and stromal tumors. When a solid mass is palpated or visualized on imaging, we therefore will utilize a different set of markers; alpha-fetoprotein, L-lactate dehydrogenase, and beta-HCG, for instance, have much higher specificity than CA-125 does for germ-cell tumors in this age group and may be helpful in the evaluation. Similarly, in cases of a very large mass resembling a mucinous tumor, a carcinoembryonic antigen may be helpful.
A number of proprietary profiling technologies have been developed to determine the risk of a diagnosed mass being malignant. For instance, the OVA1 assay looks at five serum markers and scores the results, and the Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of three serum markers with menopausal status into a numerical score. Both have Food and Drug Administration approval for use in women in whom surgery has been deemed necessary. These panels can be fairly predictive of risk and may be helpful – especially in rural areas – in determining which women should be referred to a gynecologic oncologist for surgery.
It is important to appreciate that an ovarian cyst or mass should never be biopsied or aspirated lest a malignant tumor confined to one ovary be potentially spread to the peritoneum.
Referral to a gynecologic oncologist
Postmenopausal women with a CA-125 greater than 35 U/mL should be referred, as should postmenopausal women with ascites, those with a nodular or fixed pelvic mass, and those with suspected abdominal or distant metastases (per a CT scan, for instance).
In premenopausal women, ascites, a nodular or fixed mass, and evidence of metastases also are reasons for referral to a gynecologic oncologist. CA-125, again, is much more likely to be elevated for reasons other than malignancy and therefore is not as strong a driver for referral as in postmenopausal women. Patients with markedly elevated levels, however, should probably be referred – particularly when other clinical factors also suggest the need for consultation. While there is no evidence-based threshold for CA-125 in premenopausal women, a CA-125 greater than 200 U/mL is a good cutoff for referral.
For any patient, family history of breast and/or ovarian cancer – especially in a first-degree relative – raises the risk of malignancy and should figure prominently into decision-making regarding referral. Criteria for referral are among the points discussed in the ACOG 2016 Practice Bulletin on Evaluation and Management of Adnexal Masses.1
A note on BRCA mutations
As the American College of Obstetricians and Gynecologists says in its practice bulletin, the most important personal risk factor for ovarian cancer is a strong family history of breast or ovarian cancer. Women with such a family history can undergo genetic testing for BRCA mutations and have the opportunity to prevent ovarian cancers when mutations are detected. This simple blood test can save lives.
A modeling study we recently completed – not yet published – shows that it actually would be cost effective to do population screening with BRCA testing performed on every woman at age 30 years.
According to the National Cancer Institute website (last review: 2018), it is estimated that about 44% of women who inherit a BRCA1 mutation, and about 17% of those who inherit a BRAC2 mutation, will develop ovarian cancer by the age of 80 years. By identifying those mutations, women may undergo risk-reducing surgery at designated ages after childbearing is complete and bring their risk down to under 5%.
An international take on managing adnexal masses
- Pelvic ultrasound should include the transvaginal approach. Use Doppler imaging as indicated.
- Although simple ovarian cysts are not precursor lesions to a malignant ovarian cancer, perform a high-quality examination to make sure there are no solid/papillary structures before classifying a cyst as a simple cyst. The risk of progression to malignancy is extremely low, but some follow-up is prudent.
- The most accurate method of characterizing an ovarian mass currently is real-time pattern recognition sonography in the hands of an experienced imager.
- Pattern recognition sonography or a risk model such as the International Ovarian Tumor Analysis (IOTA) Simple Rules can be used to initially characterize an ovarian mass.
- When an ovarian lesion is classified as benign, the patient may be followed conservatively, or if indicated, surgery can be performed by a general gynecologist.
- Serial sonography can be beneficial, but there are limited prospective data to support an exact interval and duration.
- Fewer surgical interventions may result in an increase in sonographic surveillance.
- When an ovarian lesion is considered indeterminate on initial sonography, and after appropriate clinical evaluation, a “second-step” evaluation may include referral to an expert sonologist, serial sonography, application of established risk-prediction models, correlation with serum biomarkers, correlation with MRI, or referral to a gynecologic oncologist for further evaluation.
From the First International Consensus Report on Adnexal Masses: Management Recommendations
Source: Glanc P et al. J Ultrasound Med. 2017 May;36(5):849-63.
Dr. Brown reported that she had received an earlier grant from Aspira Labs, the company that developed the OVA1 assay. Dr. Miller reported that he has no relevant financial disclosures.
References
1. Obstet Gynecol. 2016 Nov. doi: 10.1097/AOG.0000000000001768.
2. Cancer. 2007 Jan 15. doi: 10.1002/cncr.22371.
3. Clin Obstet Gynecol. 2015 Mar. doi: 10.1097/GRF.0000000000000083.
4. Ultrasound Q. 2013 Mar. doi: 10.1097/RUQ.0b013e3182814d9b.
5. Ultrasound Obstet Gynecol. 2008 Jun. doi: 10.1002/uog.5365.
Molecular developments in treatment of UPSC
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
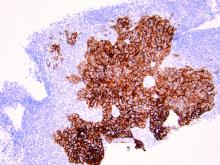
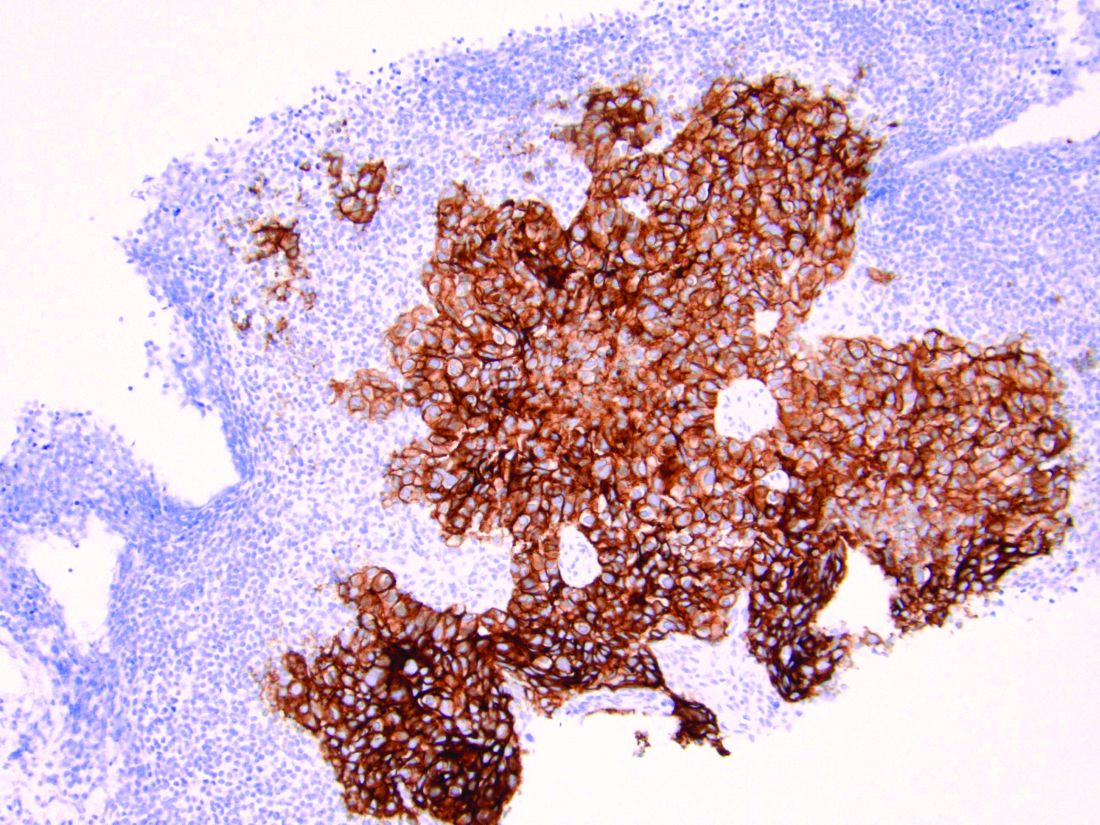
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Adolescent e-cigarette use: A public health crisis
The use of electronic cigarettes (e-cigarettes) in teenagers has been increasing rapidly in the United States, leading Surgeon General Jerome Adams, MD, MPH, to label it a public health concern.1 Easy accessibility and extensive marketing for e-cigarettes counteract public education campaigns and policies aimed at decreasing e-cigarette use in teenagers.
E-cigarettes are marketed to teenagers as small, easy-to-use pens or USB flash drive–like devices that can be hidden easily. Some devices can be used to smoke nicotine, delta-9-tetrahydrocannabinol (THC), cannabidiol, and butane hash oil. Some are sold with different nicotine flavors to increase their appeal. E-cigarette ads appear in retail stores, movies, magazines, newspapers, and on the internet.
According to the CDC, the number of middle and high school students using e-cigarettes increased from 3.6 million in 2018 to 5.4 million in 2019.2 Nicotine dependence from e-cigarette use can increase the risk of starting to smoke cigarettes. A 2015-2016 National Institute on Drug Abuse survey found a higher prevalence of e-cigarette use among 9th-, 10th-, and 12th-grade students compared with cigarette smoking (9.5%, 14%, 16.2% vs 3.6%, 6.2%, 11.4%, respectively).3 Due to the growing popularity of vaping among adolescents in the United States, Congress recently raised the legal age to purchase tobacco and vaping products to 21 years.
Evidence of adverse health effects associated with e-cigarette use continues to grow. In 2020, the Department of Health and Services in Wisconsin and the Department of Public Health in Illinois looked at e-cigarette use and pulmonary disease.4 Of 98 participants who reported e-cigarette use, 97% presented with respiratory symptoms, 77% had gastrointestinal symptoms, and 100% had constitutional symptoms. Chest imaging showed bilateral infiltrates in all patients. In addition, 95% were hospitalized, 26% underwent intubation and mechanical ventilation, and 1 patient died. Most participants (89%) reported using THC in their e-cigarette devices.4 Blount et al5 recently found a link between e-cigarette- or vaping-associated lung injury and vitamin E acetate, a toxicant found in bronchoalveolar lavage fluid of some patients who reported using e-cigarettes. Also, nicotine dependency from e-cigarettes may adversely affect brain development in children and adolescents.2
The first step in fighting this crisis is to educate children, parents, teachers, and health care professionals about e-cigarette use, including its prevalence, use compared with cigarette smoking, trends among teenagers, marketing techniques, and adverse effects. Fortunately, the US government and medical professionals and organizations have made ongoing efforts to discourage e-cigarette use. For example, the American Academy of Child and Adolescent Psychiatry supports the FDA’s regulation of e-cigarette use; encourages using evidence-based treatments for tobacco cessation; advocates for vigorous education regarding adolescent e-cigarette use; and endorses restrictions on e-cigarette advertisement.6 We strongly urge clinicians to be vigilant about e-cigarette use in their adolescent patients and to intervene in this public health crisis.
Immad A. Kiani, MD
PGY-3 Psychiatry Resident
Christiana Care Health Services
Department of Psychiatry
Wilmington, Delaware
Narpinder K. Malhi, MD
Child and Adolescent Psychiatrist
Christiana Care Health Services
Wilmington, Delaware
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Adams J. Surgeon General’s advisory on e-cigarette use among youth. US Department of Health & Human Services. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advisory-on-e-cigarette-use-among-youth-2018.pdf. Published 2018. Accessed August 7, 2020.
2. US Federal Drug and Drug Administration. Results from 2018 National Youth Tobacco Survey show dramatic increase in e-cigarette use among youth over past year. https://www.fda.gov/news-events/press-announcements/results-2018-national-youth-tobacco-survey-show-dramatic-increase-e-cigarette-use-among-youth-over. Published November 15, 2018. Accessed August 7, 2020.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the future national survey results on drug use, 1975-2016: overview, key findings on adolescent drug use. The University of Michigan Institute for Social Research. https://files.eric.ed.gov/fulltext/ED578534.pdf. Published January 2017. Accessed August 7, 2020.
4. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903-916.
5. Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705.
6. Electronic cigarettes. The American Academy of Child and Adolescent Psychiatry. https://www.aacap.org/AACAP/Policy_Statements/2015/Policy_Statement_on_Electronic_Cigarettes.aspx. Published June 2015. Accessed August 7, 2020.
The use of electronic cigarettes (e-cigarettes) in teenagers has been increasing rapidly in the United States, leading Surgeon General Jerome Adams, MD, MPH, to label it a public health concern.1 Easy accessibility and extensive marketing for e-cigarettes counteract public education campaigns and policies aimed at decreasing e-cigarette use in teenagers.
E-cigarettes are marketed to teenagers as small, easy-to-use pens or USB flash drive–like devices that can be hidden easily. Some devices can be used to smoke nicotine, delta-9-tetrahydrocannabinol (THC), cannabidiol, and butane hash oil. Some are sold with different nicotine flavors to increase their appeal. E-cigarette ads appear in retail stores, movies, magazines, newspapers, and on the internet.
According to the CDC, the number of middle and high school students using e-cigarettes increased from 3.6 million in 2018 to 5.4 million in 2019.2 Nicotine dependence from e-cigarette use can increase the risk of starting to smoke cigarettes. A 2015-2016 National Institute on Drug Abuse survey found a higher prevalence of e-cigarette use among 9th-, 10th-, and 12th-grade students compared with cigarette smoking (9.5%, 14%, 16.2% vs 3.6%, 6.2%, 11.4%, respectively).3 Due to the growing popularity of vaping among adolescents in the United States, Congress recently raised the legal age to purchase tobacco and vaping products to 21 years.
Evidence of adverse health effects associated with e-cigarette use continues to grow. In 2020, the Department of Health and Services in Wisconsin and the Department of Public Health in Illinois looked at e-cigarette use and pulmonary disease.4 Of 98 participants who reported e-cigarette use, 97% presented with respiratory symptoms, 77% had gastrointestinal symptoms, and 100% had constitutional symptoms. Chest imaging showed bilateral infiltrates in all patients. In addition, 95% were hospitalized, 26% underwent intubation and mechanical ventilation, and 1 patient died. Most participants (89%) reported using THC in their e-cigarette devices.4 Blount et al5 recently found a link between e-cigarette- or vaping-associated lung injury and vitamin E acetate, a toxicant found in bronchoalveolar lavage fluid of some patients who reported using e-cigarettes. Also, nicotine dependency from e-cigarettes may adversely affect brain development in children and adolescents.2
The first step in fighting this crisis is to educate children, parents, teachers, and health care professionals about e-cigarette use, including its prevalence, use compared with cigarette smoking, trends among teenagers, marketing techniques, and adverse effects. Fortunately, the US government and medical professionals and organizations have made ongoing efforts to discourage e-cigarette use. For example, the American Academy of Child and Adolescent Psychiatry supports the FDA’s regulation of e-cigarette use; encourages using evidence-based treatments for tobacco cessation; advocates for vigorous education regarding adolescent e-cigarette use; and endorses restrictions on e-cigarette advertisement.6 We strongly urge clinicians to be vigilant about e-cigarette use in their adolescent patients and to intervene in this public health crisis.
Immad A. Kiani, MD
PGY-3 Psychiatry Resident
Christiana Care Health Services
Department of Psychiatry
Wilmington, Delaware
Narpinder K. Malhi, MD
Child and Adolescent Psychiatrist
Christiana Care Health Services
Wilmington, Delaware
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The use of electronic cigarettes (e-cigarettes) in teenagers has been increasing rapidly in the United States, leading Surgeon General Jerome Adams, MD, MPH, to label it a public health concern.1 Easy accessibility and extensive marketing for e-cigarettes counteract public education campaigns and policies aimed at decreasing e-cigarette use in teenagers.
E-cigarettes are marketed to teenagers as small, easy-to-use pens or USB flash drive–like devices that can be hidden easily. Some devices can be used to smoke nicotine, delta-9-tetrahydrocannabinol (THC), cannabidiol, and butane hash oil. Some are sold with different nicotine flavors to increase their appeal. E-cigarette ads appear in retail stores, movies, magazines, newspapers, and on the internet.
According to the CDC, the number of middle and high school students using e-cigarettes increased from 3.6 million in 2018 to 5.4 million in 2019.2 Nicotine dependence from e-cigarette use can increase the risk of starting to smoke cigarettes. A 2015-2016 National Institute on Drug Abuse survey found a higher prevalence of e-cigarette use among 9th-, 10th-, and 12th-grade students compared with cigarette smoking (9.5%, 14%, 16.2% vs 3.6%, 6.2%, 11.4%, respectively).3 Due to the growing popularity of vaping among adolescents in the United States, Congress recently raised the legal age to purchase tobacco and vaping products to 21 years.
Evidence of adverse health effects associated with e-cigarette use continues to grow. In 2020, the Department of Health and Services in Wisconsin and the Department of Public Health in Illinois looked at e-cigarette use and pulmonary disease.4 Of 98 participants who reported e-cigarette use, 97% presented with respiratory symptoms, 77% had gastrointestinal symptoms, and 100% had constitutional symptoms. Chest imaging showed bilateral infiltrates in all patients. In addition, 95% were hospitalized, 26% underwent intubation and mechanical ventilation, and 1 patient died. Most participants (89%) reported using THC in their e-cigarette devices.4 Blount et al5 recently found a link between e-cigarette- or vaping-associated lung injury and vitamin E acetate, a toxicant found in bronchoalveolar lavage fluid of some patients who reported using e-cigarettes. Also, nicotine dependency from e-cigarettes may adversely affect brain development in children and adolescents.2
The first step in fighting this crisis is to educate children, parents, teachers, and health care professionals about e-cigarette use, including its prevalence, use compared with cigarette smoking, trends among teenagers, marketing techniques, and adverse effects. Fortunately, the US government and medical professionals and organizations have made ongoing efforts to discourage e-cigarette use. For example, the American Academy of Child and Adolescent Psychiatry supports the FDA’s regulation of e-cigarette use; encourages using evidence-based treatments for tobacco cessation; advocates for vigorous education regarding adolescent e-cigarette use; and endorses restrictions on e-cigarette advertisement.6 We strongly urge clinicians to be vigilant about e-cigarette use in their adolescent patients and to intervene in this public health crisis.
Immad A. Kiani, MD
PGY-3 Psychiatry Resident
Christiana Care Health Services
Department of Psychiatry
Wilmington, Delaware
Narpinder K. Malhi, MD
Child and Adolescent Psychiatrist
Christiana Care Health Services
Wilmington, Delaware
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Adams J. Surgeon General’s advisory on e-cigarette use among youth. US Department of Health & Human Services. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advisory-on-e-cigarette-use-among-youth-2018.pdf. Published 2018. Accessed August 7, 2020.
2. US Federal Drug and Drug Administration. Results from 2018 National Youth Tobacco Survey show dramatic increase in e-cigarette use among youth over past year. https://www.fda.gov/news-events/press-announcements/results-2018-national-youth-tobacco-survey-show-dramatic-increase-e-cigarette-use-among-youth-over. Published November 15, 2018. Accessed August 7, 2020.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the future national survey results on drug use, 1975-2016: overview, key findings on adolescent drug use. The University of Michigan Institute for Social Research. https://files.eric.ed.gov/fulltext/ED578534.pdf. Published January 2017. Accessed August 7, 2020.
4. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903-916.
5. Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705.
6. Electronic cigarettes. The American Academy of Child and Adolescent Psychiatry. https://www.aacap.org/AACAP/Policy_Statements/2015/Policy_Statement_on_Electronic_Cigarettes.aspx. Published June 2015. Accessed August 7, 2020.
1. Adams J. Surgeon General’s advisory on e-cigarette use among youth. US Department of Health & Human Services. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advisory-on-e-cigarette-use-among-youth-2018.pdf. Published 2018. Accessed August 7, 2020.
2. US Federal Drug and Drug Administration. Results from 2018 National Youth Tobacco Survey show dramatic increase in e-cigarette use among youth over past year. https://www.fda.gov/news-events/press-announcements/results-2018-national-youth-tobacco-survey-show-dramatic-increase-e-cigarette-use-among-youth-over. Published November 15, 2018. Accessed August 7, 2020.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the future national survey results on drug use, 1975-2016: overview, key findings on adolescent drug use. The University of Michigan Institute for Social Research. https://files.eric.ed.gov/fulltext/ED578534.pdf. Published January 2017. Accessed August 7, 2020.
4. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903-916.
5. Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705.
6. Electronic cigarettes. The American Academy of Child and Adolescent Psychiatry. https://www.aacap.org/AACAP/Policy_Statements/2015/Policy_Statement_on_Electronic_Cigarettes.aspx. Published June 2015. Accessed August 7, 2020.
Revamp the MOC
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
Reducing maternal mortality with prenatal care
As in its typical fashion, the question sprang out from a young Black patient after some meandering conversation during preconception counseling: “How do y’all prevent Black maternal mortality?” At the beginning of my career, I used to think preparing a patient for pregnancy involved recommending prenatal vitamins and rubella immunity screening. Now, having worked in a society with substantial racial health disparities for 14 years, there is greater awareness that pregnancy can be a matter of life or death that disproportionately affects people of color.
For Black patients in the United States, the maternal mortality ratio is almost four times higher than the ratio for White patients, 42 deaths versus 13 deaths per 100,000 live births, respectively.1 Georgia has the highest maternal mortality ratio in the United States at 67 maternal deaths per 100,000 live births. However, if you are a Black woman in Georgia, your chance of dying of pregnancy-related causes is 2.7 times that of a non-Hispanic White woman living in Georgia.2
Black patients often are not taken seriously, even when they are wealthy, have attained high levels of education, or are famous. Serena Williams, a Black woman and one of the most talented tennis players of all time, was ignored when complaining that she felt a blood clot had returned in her lungs post partum. As a recognition of this crisis, the Centers for Disease Control and Prevention has a new campaign to improve recognition of the warning signs of problems in pregnancy called the HEAR HER campaign. This issue is a pervasive problem in our lives that runs across the spectrum of Black experience. I have had Black friends, patients, and colleagues who have been ignored when complaining about labor pain, workplace discrimination, and even when trying to advocate for their patients. We need to uplift Black voices so they can be heard and support the initiatives and interventions they are asking for.
We practice standardized responses to emergencies and to health conditions. We use drills to practice our responses to life-threatening emergencies such as STAT cesarean delivery, shoulder dystocia, obstetrical hemorrhage, or treatment of preeclampsia and eclampsia. The Alliance for Innovation on Maternal Health has organized evidence-driven protocols called AIM bundles to reduce preventable maternal morbidity and mortality when implemented. Standardization is an important component of equitable treatment and reduction of disparities. The concept has been used across industries to reduce error and bias. The Alliance for Innovation on Maternal Health bundles even include a section on Reduction of Peripartum and Ethnic Disparities.
We admit that bias exists and that we need training to recognize and eliminate it. According to a study in the Proceedings of the National Academy of Sciences of the United States of America about racial bias in pain assessment more than 20% of White residents and medical students surveyed believed that Black people had less sensitive nerve endings than Whites.3 Studies show that this stereotype leads to inappropriate pain management in Black patients, a chief concern when considering how patients are treated on labor and delivery or after surgery.4 Additionally, unconscious bias can be addressed by hiring a diverse workforce at all levels. Familiarity with a diverse group can help us learn from one another in our day-to-day lives.
We need to offer the same high-quality preconception counseling to all of our patients. A patient’s perceived race or ethnicity is a poor indicator of their actual health needs. The amount of melanin in our skin is highly variable but our genetics are remarkably similar, therefore our health concerns are similar. All patients deserve a focus on prevention. Folic acid supplements in the form of prenatal vitamins should be recommended. Routine vaccinations and rubella immunity checks should be offered. Basic carrier screening for diseases of hemoglobin (which includes sickle cell trait), fragile X, spinomuscular atrophy, and cystic fibrosis should be offered. Finally, an emphasis on safety, mental health, and daily low-level exercise (i.e., walking) should be promoted to help prevent illness and injury in this age group. The leading causes of death for people of reproductive age are accidents, suicide, homicide, and heart disease – all preventable.
We treat the social determinants of health, not just the patient in front of us. When “race” is a risk factor for disease, it’s usually racism that’s the problem. As stated earlier, how much melanin is in our skin has little to do with our genetics – if we removed our skin, we’d have similar life expectancies and die of similar things. However, it has everything to do with how we navigate our society and access health care. The stress associated with being Black in America is the likely cause of preterm birth rates – leading to infant illness and death – and maternal mortality being higher in Black patients. This is referred to as “weathering” – the cumulative effects of stress as we age. It explains why Black women are more likely to die in pregnancy despite higher levels of education and increasing age – factors that are protective for other groups. Improving access to quality education, reforming the criminal justice system, affordable housing and child care, living wages, family planning, and universal basic health care exemplify the intersectionality of some of our greatest societal challenges. Addressing these root causes will reduce weathering and ultimately, save Black lives.
We strive to train more “underrepresented minorities” in medicine. According to the American Association of Medical Colleges, only 7.3% of medical students in 2019-2020 identified as Black or African American. This is way below their representation of 13% of the U.S. population. I’m proud that my division and department as a whole have hired and promoted diverse faculty with 30% of my generalist ob.gyn. colleagues being people of color. This shows that we have the input of diverse experiences as well as recognize the special concerns of patients of color. Underrepresented students interested in the health professions need us to do more to get their “foot in the door.” They are less likely to have connections to the field of medicine (family members, mentors), have access to prep courses or advisors, or have the finances to support the expensive application process. Reach out to your alma maters and ask how you can help mentor students at a young age and continue through adulthood, support scholarships, support unpaid internship recipients, and promote interconnectedness throughout this community.
I hope I answered my patient’s question in that moment, but I know what needs to be done is bigger that taking care of one patient. It will require small progress, by us, every single day. Until these interventions and others reshape our society, I’ll still have Black patients who say: “Don’t let me die, okay?” with a look right into my soul and a tight grip on my hand. And I’ll feel the immense weight of that trust, and squeeze the hand back.
Dr. Collins (she/her/hers) is assistant professor of obstetrics and gynecology, generalist division, at Emory University, Atlanta. She has no relevant financial disclosures. Email Dr. Collins at obnews@mdedge.com.
References
1. CDC Pregnancy Mortality Surveillance System, 2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
2. Maternal Mortality Fact Sheet, 2012-2015. https://dph.georgia.gov/maternal-mortality.
3. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4296-301.
4. Pain Med. 2012 Feb;13(2):150-74.
As in its typical fashion, the question sprang out from a young Black patient after some meandering conversation during preconception counseling: “How do y’all prevent Black maternal mortality?” At the beginning of my career, I used to think preparing a patient for pregnancy involved recommending prenatal vitamins and rubella immunity screening. Now, having worked in a society with substantial racial health disparities for 14 years, there is greater awareness that pregnancy can be a matter of life or death that disproportionately affects people of color.
For Black patients in the United States, the maternal mortality ratio is almost four times higher than the ratio for White patients, 42 deaths versus 13 deaths per 100,000 live births, respectively.1 Georgia has the highest maternal mortality ratio in the United States at 67 maternal deaths per 100,000 live births. However, if you are a Black woman in Georgia, your chance of dying of pregnancy-related causes is 2.7 times that of a non-Hispanic White woman living in Georgia.2
Black patients often are not taken seriously, even when they are wealthy, have attained high levels of education, or are famous. Serena Williams, a Black woman and one of the most talented tennis players of all time, was ignored when complaining that she felt a blood clot had returned in her lungs post partum. As a recognition of this crisis, the Centers for Disease Control and Prevention has a new campaign to improve recognition of the warning signs of problems in pregnancy called the HEAR HER campaign. This issue is a pervasive problem in our lives that runs across the spectrum of Black experience. I have had Black friends, patients, and colleagues who have been ignored when complaining about labor pain, workplace discrimination, and even when trying to advocate for their patients. We need to uplift Black voices so they can be heard and support the initiatives and interventions they are asking for.
We practice standardized responses to emergencies and to health conditions. We use drills to practice our responses to life-threatening emergencies such as STAT cesarean delivery, shoulder dystocia, obstetrical hemorrhage, or treatment of preeclampsia and eclampsia. The Alliance for Innovation on Maternal Health has organized evidence-driven protocols called AIM bundles to reduce preventable maternal morbidity and mortality when implemented. Standardization is an important component of equitable treatment and reduction of disparities. The concept has been used across industries to reduce error and bias. The Alliance for Innovation on Maternal Health bundles even include a section on Reduction of Peripartum and Ethnic Disparities.
We admit that bias exists and that we need training to recognize and eliminate it. According to a study in the Proceedings of the National Academy of Sciences of the United States of America about racial bias in pain assessment more than 20% of White residents and medical students surveyed believed that Black people had less sensitive nerve endings than Whites.3 Studies show that this stereotype leads to inappropriate pain management in Black patients, a chief concern when considering how patients are treated on labor and delivery or after surgery.4 Additionally, unconscious bias can be addressed by hiring a diverse workforce at all levels. Familiarity with a diverse group can help us learn from one another in our day-to-day lives.
We need to offer the same high-quality preconception counseling to all of our patients. A patient’s perceived race or ethnicity is a poor indicator of their actual health needs. The amount of melanin in our skin is highly variable but our genetics are remarkably similar, therefore our health concerns are similar. All patients deserve a focus on prevention. Folic acid supplements in the form of prenatal vitamins should be recommended. Routine vaccinations and rubella immunity checks should be offered. Basic carrier screening for diseases of hemoglobin (which includes sickle cell trait), fragile X, spinomuscular atrophy, and cystic fibrosis should be offered. Finally, an emphasis on safety, mental health, and daily low-level exercise (i.e., walking) should be promoted to help prevent illness and injury in this age group. The leading causes of death for people of reproductive age are accidents, suicide, homicide, and heart disease – all preventable.
We treat the social determinants of health, not just the patient in front of us. When “race” is a risk factor for disease, it’s usually racism that’s the problem. As stated earlier, how much melanin is in our skin has little to do with our genetics – if we removed our skin, we’d have similar life expectancies and die of similar things. However, it has everything to do with how we navigate our society and access health care. The stress associated with being Black in America is the likely cause of preterm birth rates – leading to infant illness and death – and maternal mortality being higher in Black patients. This is referred to as “weathering” – the cumulative effects of stress as we age. It explains why Black women are more likely to die in pregnancy despite higher levels of education and increasing age – factors that are protective for other groups. Improving access to quality education, reforming the criminal justice system, affordable housing and child care, living wages, family planning, and universal basic health care exemplify the intersectionality of some of our greatest societal challenges. Addressing these root causes will reduce weathering and ultimately, save Black lives.
We strive to train more “underrepresented minorities” in medicine. According to the American Association of Medical Colleges, only 7.3% of medical students in 2019-2020 identified as Black or African American. This is way below their representation of 13% of the U.S. population. I’m proud that my division and department as a whole have hired and promoted diverse faculty with 30% of my generalist ob.gyn. colleagues being people of color. This shows that we have the input of diverse experiences as well as recognize the special concerns of patients of color. Underrepresented students interested in the health professions need us to do more to get their “foot in the door.” They are less likely to have connections to the field of medicine (family members, mentors), have access to prep courses or advisors, or have the finances to support the expensive application process. Reach out to your alma maters and ask how you can help mentor students at a young age and continue through adulthood, support scholarships, support unpaid internship recipients, and promote interconnectedness throughout this community.
I hope I answered my patient’s question in that moment, but I know what needs to be done is bigger that taking care of one patient. It will require small progress, by us, every single day. Until these interventions and others reshape our society, I’ll still have Black patients who say: “Don’t let me die, okay?” with a look right into my soul and a tight grip on my hand. And I’ll feel the immense weight of that trust, and squeeze the hand back.
Dr. Collins (she/her/hers) is assistant professor of obstetrics and gynecology, generalist division, at Emory University, Atlanta. She has no relevant financial disclosures. Email Dr. Collins at obnews@mdedge.com.
References
1. CDC Pregnancy Mortality Surveillance System, 2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
2. Maternal Mortality Fact Sheet, 2012-2015. https://dph.georgia.gov/maternal-mortality.
3. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4296-301.
4. Pain Med. 2012 Feb;13(2):150-74.
As in its typical fashion, the question sprang out from a young Black patient after some meandering conversation during preconception counseling: “How do y’all prevent Black maternal mortality?” At the beginning of my career, I used to think preparing a patient for pregnancy involved recommending prenatal vitamins and rubella immunity screening. Now, having worked in a society with substantial racial health disparities for 14 years, there is greater awareness that pregnancy can be a matter of life or death that disproportionately affects people of color.
For Black patients in the United States, the maternal mortality ratio is almost four times higher than the ratio for White patients, 42 deaths versus 13 deaths per 100,000 live births, respectively.1 Georgia has the highest maternal mortality ratio in the United States at 67 maternal deaths per 100,000 live births. However, if you are a Black woman in Georgia, your chance of dying of pregnancy-related causes is 2.7 times that of a non-Hispanic White woman living in Georgia.2
Black patients often are not taken seriously, even when they are wealthy, have attained high levels of education, or are famous. Serena Williams, a Black woman and one of the most talented tennis players of all time, was ignored when complaining that she felt a blood clot had returned in her lungs post partum. As a recognition of this crisis, the Centers for Disease Control and Prevention has a new campaign to improve recognition of the warning signs of problems in pregnancy called the HEAR HER campaign. This issue is a pervasive problem in our lives that runs across the spectrum of Black experience. I have had Black friends, patients, and colleagues who have been ignored when complaining about labor pain, workplace discrimination, and even when trying to advocate for their patients. We need to uplift Black voices so they can be heard and support the initiatives and interventions they are asking for.
We practice standardized responses to emergencies and to health conditions. We use drills to practice our responses to life-threatening emergencies such as STAT cesarean delivery, shoulder dystocia, obstetrical hemorrhage, or treatment of preeclampsia and eclampsia. The Alliance for Innovation on Maternal Health has organized evidence-driven protocols called AIM bundles to reduce preventable maternal morbidity and mortality when implemented. Standardization is an important component of equitable treatment and reduction of disparities. The concept has been used across industries to reduce error and bias. The Alliance for Innovation on Maternal Health bundles even include a section on Reduction of Peripartum and Ethnic Disparities.
We admit that bias exists and that we need training to recognize and eliminate it. According to a study in the Proceedings of the National Academy of Sciences of the United States of America about racial bias in pain assessment more than 20% of White residents and medical students surveyed believed that Black people had less sensitive nerve endings than Whites.3 Studies show that this stereotype leads to inappropriate pain management in Black patients, a chief concern when considering how patients are treated on labor and delivery or after surgery.4 Additionally, unconscious bias can be addressed by hiring a diverse workforce at all levels. Familiarity with a diverse group can help us learn from one another in our day-to-day lives.
We need to offer the same high-quality preconception counseling to all of our patients. A patient’s perceived race or ethnicity is a poor indicator of their actual health needs. The amount of melanin in our skin is highly variable but our genetics are remarkably similar, therefore our health concerns are similar. All patients deserve a focus on prevention. Folic acid supplements in the form of prenatal vitamins should be recommended. Routine vaccinations and rubella immunity checks should be offered. Basic carrier screening for diseases of hemoglobin (which includes sickle cell trait), fragile X, spinomuscular atrophy, and cystic fibrosis should be offered. Finally, an emphasis on safety, mental health, and daily low-level exercise (i.e., walking) should be promoted to help prevent illness and injury in this age group. The leading causes of death for people of reproductive age are accidents, suicide, homicide, and heart disease – all preventable.
We treat the social determinants of health, not just the patient in front of us. When “race” is a risk factor for disease, it’s usually racism that’s the problem. As stated earlier, how much melanin is in our skin has little to do with our genetics – if we removed our skin, we’d have similar life expectancies and die of similar things. However, it has everything to do with how we navigate our society and access health care. The stress associated with being Black in America is the likely cause of preterm birth rates – leading to infant illness and death – and maternal mortality being higher in Black patients. This is referred to as “weathering” – the cumulative effects of stress as we age. It explains why Black women are more likely to die in pregnancy despite higher levels of education and increasing age – factors that are protective for other groups. Improving access to quality education, reforming the criminal justice system, affordable housing and child care, living wages, family planning, and universal basic health care exemplify the intersectionality of some of our greatest societal challenges. Addressing these root causes will reduce weathering and ultimately, save Black lives.
We strive to train more “underrepresented minorities” in medicine. According to the American Association of Medical Colleges, only 7.3% of medical students in 2019-2020 identified as Black or African American. This is way below their representation of 13% of the U.S. population. I’m proud that my division and department as a whole have hired and promoted diverse faculty with 30% of my generalist ob.gyn. colleagues being people of color. This shows that we have the input of diverse experiences as well as recognize the special concerns of patients of color. Underrepresented students interested in the health professions need us to do more to get their “foot in the door.” They are less likely to have connections to the field of medicine (family members, mentors), have access to prep courses or advisors, or have the finances to support the expensive application process. Reach out to your alma maters and ask how you can help mentor students at a young age and continue through adulthood, support scholarships, support unpaid internship recipients, and promote interconnectedness throughout this community.
I hope I answered my patient’s question in that moment, but I know what needs to be done is bigger that taking care of one patient. It will require small progress, by us, every single day. Until these interventions and others reshape our society, I’ll still have Black patients who say: “Don’t let me die, okay?” with a look right into my soul and a tight grip on my hand. And I’ll feel the immense weight of that trust, and squeeze the hand back.
Dr. Collins (she/her/hers) is assistant professor of obstetrics and gynecology, generalist division, at Emory University, Atlanta. She has no relevant financial disclosures. Email Dr. Collins at obnews@mdedge.com.
References
1. CDC Pregnancy Mortality Surveillance System, 2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
2. Maternal Mortality Fact Sheet, 2012-2015. https://dph.georgia.gov/maternal-mortality.
3. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4296-301.
4. Pain Med. 2012 Feb;13(2):150-74.
Rooting out systemic racism: Equal access to maternal, prenatal care
Against the backdrop of the COVID-19 pandemic and ongoing national conversations around racial injustice, it is more important than ever that we identify and root out systemic discrimination – including in our health care system. As an ob.gyn., I’ve spent my entire career making sure that women receive the best care, and have witnessed firsthand the results of a system that provides differing levels of care based on one’s socioeconomic level, race, or ethnicity.
This disparity is borne out in this country’s maternal health outcomes. For example, the latest figures from the Centers for Disease Control and Prevention indicate that the maternal mortality rate for black women, 37.1 deaths per 100,000 live births, is more than double the rate for white women at 14.7. In addition, the black infant mortality rate, at 11.4 per 1,000 live births, is also more than double the white infant mortality rate, 4.9. While many of these differences stem from discriminatory levels of coverage and care after delivery, just as important is the coverage and care before delivery: prenatal care.
Prenatal care includes a variety of screening tests, including those that can help expecting mothers identify whether the baby is more or less likely to have certain genetic disorders. These tests include traditional and less accurate options like serum or combined screening, and newer noninvasive prenatal testing (NIPT) options that use blood samples from the mother to analyze the baby’s DNA.
Research already has demonstrated that NIPT is the most accurate and effective screening option for common chromosomal abnormalities (Ont Health Technol Assess Ser. 2019;19[4]:1-166). A 2015 New England Journal of Medicine study showed that, without access to NIPT, 22% of pregnancies with Down syndrome were missed. With older screening methods, 5.4% of positive results for Down syndrome were false, compared with 0.06% of the NIPT tests (N Engl J Med 2015;372:1589-97). Older, less accurate screening tests cause unnecessary referrals to specialists for possible invasive testing, resulting in additional costs and undue stress on women and their families.
And yet, troubling new data have shown that black and Hispanic women have less access to NIPT than white women. Currently, NIPT is available to all California women through the state-funded prenatal screening program as a second-tier test. Many women, however, decide to opt out or go outside of the state program to have NIPT as a first-tier test, choosing private payer or other plans instead. New data shared by the California Department of Public Health with ob.gyns. and maternal-fetal medicine physicians in California showed that white women who opted out of California’s state-funded prenatal screening program were more than twice as likely to gain access to NIPT as black and Hispanic women (39%-17%). We can assume this to be true of women outside California as well – women who have no access to a state-funded program like California’s and depend solely on private payer or other health care plans. In fact, although some commercial insurance companies do cover NIPT, noninvasive prenatal screening is not covered by large insurance companies like Aetna and UnitedHealthcare.
As ob.gyns., physicians, and health professionals, we need to ask ourselves: Why is there such a great disparity in the access of superior and more effective NIPT options for black and Hispanic women?
Many reasons are apparent. There are significant differences by ethnic and racial groups in their knowledge of the availability of prenatal testing. Furthermore, there are higher levels of mistrust along certain racial and ethnic lines of the medical system in this country; specific religious or ethnic beliefs also may obviate the use of prenatal testing or diagnosis. Significant differences also exist in the types of health coverage by race and ethnicity, ultimately impacting the ability to have prenatal testing. Finally, there are different physician group recommendations. While medical societies such as the American College of Medical Genetics, International Society for Prenatal Diagnosis, and the American Society of Human Genetics all have long endorsed newer NIPT option for all pregnant women, two of the national physician groups that make recommendations about what care pregnant women should be able to access – the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – only recently have recommended broad access to NIPT. As a result, some state Medicaid programs have not made NIPT available to patients.
We know that racial disparities are a public health crisis in America. The recent data from the California Department of Public Health, paired with COVID-19’s disproportionate impact on blacks and Hispanics, only further illustrate the existing health disparities experienced by this country’s communities of color.
We need to root out systemic discrimination in health care and we can begin with our maternal health care. Providing equitable access to the most accurate and consistent prenatal screenings, including NIPT options, regardless of insurance plan, socioeconomic level, race, or ethnicity is paramount in starting this work.
Dr. Gaither is a double board–certified physician in ob.gyn. and maternal-fetal medicine. She is director of perinatal services for NYC Health+Hospitals/Lincoln. She reports no conflicts of interest. Email her at obnews@mdedge.com.
Against the backdrop of the COVID-19 pandemic and ongoing national conversations around racial injustice, it is more important than ever that we identify and root out systemic discrimination – including in our health care system. As an ob.gyn., I’ve spent my entire career making sure that women receive the best care, and have witnessed firsthand the results of a system that provides differing levels of care based on one’s socioeconomic level, race, or ethnicity.
This disparity is borne out in this country’s maternal health outcomes. For example, the latest figures from the Centers for Disease Control and Prevention indicate that the maternal mortality rate for black women, 37.1 deaths per 100,000 live births, is more than double the rate for white women at 14.7. In addition, the black infant mortality rate, at 11.4 per 1,000 live births, is also more than double the white infant mortality rate, 4.9. While many of these differences stem from discriminatory levels of coverage and care after delivery, just as important is the coverage and care before delivery: prenatal care.
Prenatal care includes a variety of screening tests, including those that can help expecting mothers identify whether the baby is more or less likely to have certain genetic disorders. These tests include traditional and less accurate options like serum or combined screening, and newer noninvasive prenatal testing (NIPT) options that use blood samples from the mother to analyze the baby’s DNA.
Research already has demonstrated that NIPT is the most accurate and effective screening option for common chromosomal abnormalities (Ont Health Technol Assess Ser. 2019;19[4]:1-166). A 2015 New England Journal of Medicine study showed that, without access to NIPT, 22% of pregnancies with Down syndrome were missed. With older screening methods, 5.4% of positive results for Down syndrome were false, compared with 0.06% of the NIPT tests (N Engl J Med 2015;372:1589-97). Older, less accurate screening tests cause unnecessary referrals to specialists for possible invasive testing, resulting in additional costs and undue stress on women and their families.
And yet, troubling new data have shown that black and Hispanic women have less access to NIPT than white women. Currently, NIPT is available to all California women through the state-funded prenatal screening program as a second-tier test. Many women, however, decide to opt out or go outside of the state program to have NIPT as a first-tier test, choosing private payer or other plans instead. New data shared by the California Department of Public Health with ob.gyns. and maternal-fetal medicine physicians in California showed that white women who opted out of California’s state-funded prenatal screening program were more than twice as likely to gain access to NIPT as black and Hispanic women (39%-17%). We can assume this to be true of women outside California as well – women who have no access to a state-funded program like California’s and depend solely on private payer or other health care plans. In fact, although some commercial insurance companies do cover NIPT, noninvasive prenatal screening is not covered by large insurance companies like Aetna and UnitedHealthcare.
As ob.gyns., physicians, and health professionals, we need to ask ourselves: Why is there such a great disparity in the access of superior and more effective NIPT options for black and Hispanic women?
Many reasons are apparent. There are significant differences by ethnic and racial groups in their knowledge of the availability of prenatal testing. Furthermore, there are higher levels of mistrust along certain racial and ethnic lines of the medical system in this country; specific religious or ethnic beliefs also may obviate the use of prenatal testing or diagnosis. Significant differences also exist in the types of health coverage by race and ethnicity, ultimately impacting the ability to have prenatal testing. Finally, there are different physician group recommendations. While medical societies such as the American College of Medical Genetics, International Society for Prenatal Diagnosis, and the American Society of Human Genetics all have long endorsed newer NIPT option for all pregnant women, two of the national physician groups that make recommendations about what care pregnant women should be able to access – the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – only recently have recommended broad access to NIPT. As a result, some state Medicaid programs have not made NIPT available to patients.
We know that racial disparities are a public health crisis in America. The recent data from the California Department of Public Health, paired with COVID-19’s disproportionate impact on blacks and Hispanics, only further illustrate the existing health disparities experienced by this country’s communities of color.
We need to root out systemic discrimination in health care and we can begin with our maternal health care. Providing equitable access to the most accurate and consistent prenatal screenings, including NIPT options, regardless of insurance plan, socioeconomic level, race, or ethnicity is paramount in starting this work.
Dr. Gaither is a double board–certified physician in ob.gyn. and maternal-fetal medicine. She is director of perinatal services for NYC Health+Hospitals/Lincoln. She reports no conflicts of interest. Email her at obnews@mdedge.com.
Against the backdrop of the COVID-19 pandemic and ongoing national conversations around racial injustice, it is more important than ever that we identify and root out systemic discrimination – including in our health care system. As an ob.gyn., I’ve spent my entire career making sure that women receive the best care, and have witnessed firsthand the results of a system that provides differing levels of care based on one’s socioeconomic level, race, or ethnicity.
This disparity is borne out in this country’s maternal health outcomes. For example, the latest figures from the Centers for Disease Control and Prevention indicate that the maternal mortality rate for black women, 37.1 deaths per 100,000 live births, is more than double the rate for white women at 14.7. In addition, the black infant mortality rate, at 11.4 per 1,000 live births, is also more than double the white infant mortality rate, 4.9. While many of these differences stem from discriminatory levels of coverage and care after delivery, just as important is the coverage and care before delivery: prenatal care.
Prenatal care includes a variety of screening tests, including those that can help expecting mothers identify whether the baby is more or less likely to have certain genetic disorders. These tests include traditional and less accurate options like serum or combined screening, and newer noninvasive prenatal testing (NIPT) options that use blood samples from the mother to analyze the baby’s DNA.
Research already has demonstrated that NIPT is the most accurate and effective screening option for common chromosomal abnormalities (Ont Health Technol Assess Ser. 2019;19[4]:1-166). A 2015 New England Journal of Medicine study showed that, without access to NIPT, 22% of pregnancies with Down syndrome were missed. With older screening methods, 5.4% of positive results for Down syndrome were false, compared with 0.06% of the NIPT tests (N Engl J Med 2015;372:1589-97). Older, less accurate screening tests cause unnecessary referrals to specialists for possible invasive testing, resulting in additional costs and undue stress on women and their families.
And yet, troubling new data have shown that black and Hispanic women have less access to NIPT than white women. Currently, NIPT is available to all California women through the state-funded prenatal screening program as a second-tier test. Many women, however, decide to opt out or go outside of the state program to have NIPT as a first-tier test, choosing private payer or other plans instead. New data shared by the California Department of Public Health with ob.gyns. and maternal-fetal medicine physicians in California showed that white women who opted out of California’s state-funded prenatal screening program were more than twice as likely to gain access to NIPT as black and Hispanic women (39%-17%). We can assume this to be true of women outside California as well – women who have no access to a state-funded program like California’s and depend solely on private payer or other health care plans. In fact, although some commercial insurance companies do cover NIPT, noninvasive prenatal screening is not covered by large insurance companies like Aetna and UnitedHealthcare.
As ob.gyns., physicians, and health professionals, we need to ask ourselves: Why is there such a great disparity in the access of superior and more effective NIPT options for black and Hispanic women?
Many reasons are apparent. There are significant differences by ethnic and racial groups in their knowledge of the availability of prenatal testing. Furthermore, there are higher levels of mistrust along certain racial and ethnic lines of the medical system in this country; specific religious or ethnic beliefs also may obviate the use of prenatal testing or diagnosis. Significant differences also exist in the types of health coverage by race and ethnicity, ultimately impacting the ability to have prenatal testing. Finally, there are different physician group recommendations. While medical societies such as the American College of Medical Genetics, International Society for Prenatal Diagnosis, and the American Society of Human Genetics all have long endorsed newer NIPT option for all pregnant women, two of the national physician groups that make recommendations about what care pregnant women should be able to access – the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – only recently have recommended broad access to NIPT. As a result, some state Medicaid programs have not made NIPT available to patients.
We know that racial disparities are a public health crisis in America. The recent data from the California Department of Public Health, paired with COVID-19’s disproportionate impact on blacks and Hispanics, only further illustrate the existing health disparities experienced by this country’s communities of color.
We need to root out systemic discrimination in health care and we can begin with our maternal health care. Providing equitable access to the most accurate and consistent prenatal screenings, including NIPT options, regardless of insurance plan, socioeconomic level, race, or ethnicity is paramount in starting this work.
Dr. Gaither is a double board–certified physician in ob.gyn. and maternal-fetal medicine. She is director of perinatal services for NYC Health+Hospitals/Lincoln. She reports no conflicts of interest. Email her at obnews@mdedge.com.
Vitamin D pearls
Case: A 56-year-old man with a history of type 2 diabetes, hypertension, hyperlipidemia, and obesity calls clinic to discuss concerns about COVID-19, stating: “I want to do everything I can to reduce my risk of infection.” In addition to physical distancing, mask wearing, hand hygiene, and control of chronic conditions, which of the following supplements would you recommend for this patient?
1. Coenzyme Q10 160 mg twice a day
2. Vitamin D 2,000 IU daily
3. Vitamin E 400 IU daily
4. Vitamin B12 1,000 mcg daily
Of these choices, vitamin D supplementation is likely the best option, based on the limited data that is available.
Risk factors for worse COVID-19 outcome, such as older age, obesity, and more pigmented skin are also risk factors for vitamin D deficiency. This makes the study of vitamin D and COVID-19 both challenging and relevant.
In a recent study of 7,807 people living in Israel, Merzon and colleagues found that low plasma vitamin D level was an independent risk factor for COVID-19 infection. Mean plasma vitamin D level was significantly lower among those who tested positive for COVID-19 (19.00 ng/mL) than negative (20.55 ng/ mL). After controlling for demographic variables and several medical conditions, the adjusted odds ratio of COVID-19 infection in those with lower vitamin D was 1.45 (95% confidence interval, 1.08-1.95; P < .001). However, the odds of hospitalization for COVID-19 was not significantly associated with vitamin D level.1
Prior studies have also looked at vitamin D and respiratory infection. Martineau and colleagues analyzed 25 randomized, controlled trials with a pooled number of 11,321 individuals, including healthy ones and those with comorbidities, and found that oral vitamin D supplementation in daily or weekly doses had a protective effect against acute respiratory infection (adjusted odds ratio, 0.88; 95% CI, 0.81-0.96; P < .001). Patients with vitamin D deficiency (less than 25 nmol/L) experienced the most protective benefit. Vitamin D did not influence respiratory infection outcome.2
These studies suggest an adequate vitamin D level may be protective against infection with COVID-19, but who will benefit from vitamin D supplementation, and in what dose? Per U.S. Preventive Services Task Force guidelines, there is insufficient evidence to recommend screening for vitamin D deficiency in asymptomatic adults. Regarding daily dietary intake, the Institute of Medicine recommends 600 IU for persons aged 1-70, and 800 IU for those aged over 70 years. Salmon (447 IU per 3 oz serving), tuna (154 IU), and fortified milk (116 IU) are among the most vitamin D–rich foods.3 The recommended upper level of intake is 4,000 IU/day.
Too much of a good thing?
Extra vitamin D is stored in adipose tissue. If it builds up over time, storage sites may be overwhelmed, causing a rise in serum D level. While one might expect a subsequent rise in calcium levels, studies have shown this happens inconsistently, and at very high vitamin D levels, over 120 ng/mL.4 Most people would have to take at least 50,000 IU daily for several months to see an effect. The main adverse outcome of vitamin D toxicity is kidney stones, mediated by increased calcium in the blood and urine.
Several animal models have demonstrated hypervitaminosis D–induced aortic and coronary artery calcification. Like with kidney stones, the mechanism appears to be through increased calcium and phosphate levels. Shroff and colleagues studied serum vitamin D levels and vascular disease in children with renal disease on dialysis and found a U-shaped distribution: Children with both low and high vitamin D levels had significantly increased carotid artery intima-media thickness and calcification.5 Given the specialized nature of this population, it’s unclear whether these results can be generalized to most people. More studies are warranted on this topic.
Other benefits
Vitamin D is perhaps most famous for helping to build strong bones. Avenell and colleagues performed a Cochrane meta-analysis of vitamin D supplementation in older adults and found that vitamin D alone did not significantly reduce the risk of hip or other new fracture. Vitamin D plus calcium supplementation did reduce the risk of hip fracture (nine trials, pooled number of individuals was 49,853; relative risk, 0.84; P = .01).6
A lesser-known benefit of vitamin D is muscle protection. A prospective study out of the Jewish Hospital of Cincinnati followed 146 adults who were intolerant to two or more statins because of muscle side effects and found to have a vitamin D level below 32 ng per mL. Subjects were given vitamin D replacement (50,000 units weekly) and followed for 2 years. On statin rechallenge, 88-95% tolerated a statin with vitamin D levels 53-55 ng/mL.7
Pearl
Vitamin D supplementation may protect against COVID-19 infection and has very low chance of harm at daily doses at or below 4,000 IU. Other benefits of taking vitamin D include bone protection and reduction in statin-induced myopathy. The main adverse effect is kidney stones.
Ms. Sharninghausen is a medical student at the University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Merzon E et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: An Israeli population‐based study. FEBS J. 2020. doi: 10.1111/febs.15495.
2. Martineau AR et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
3. “How to Get More Vitamin D From Your Food,” Cleveland Clinic. 2019 Oct 23. https://health.clevelandclinic.org/how-to-get-more-vitamin-d-from-your-food/.
4. Galior K et al. Development of vitamin d toxicity from overcorrection of vitamin D Deficiency: A review of case reports. Nutrients. 2018;10(8):953. doi: 10.3390/nu10080953
5. Shroff R et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19(6):1239-46. doi: 10.1681/ASN.2007090993.
6. Avenell A et al. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014 Apr 14;2014(4):CD000227. doi: 10.1002/14651858.CD000227.pub4.
7. Khayznikov M et al. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N Am J Med Sci. 2015;7(3):86-93. doi:10.4103/1947-2714.153919
Case: A 56-year-old man with a history of type 2 diabetes, hypertension, hyperlipidemia, and obesity calls clinic to discuss concerns about COVID-19, stating: “I want to do everything I can to reduce my risk of infection.” In addition to physical distancing, mask wearing, hand hygiene, and control of chronic conditions, which of the following supplements would you recommend for this patient?
1. Coenzyme Q10 160 mg twice a day
2. Vitamin D 2,000 IU daily
3. Vitamin E 400 IU daily
4. Vitamin B12 1,000 mcg daily
Of these choices, vitamin D supplementation is likely the best option, based on the limited data that is available.
Risk factors for worse COVID-19 outcome, such as older age, obesity, and more pigmented skin are also risk factors for vitamin D deficiency. This makes the study of vitamin D and COVID-19 both challenging and relevant.
In a recent study of 7,807 people living in Israel, Merzon and colleagues found that low plasma vitamin D level was an independent risk factor for COVID-19 infection. Mean plasma vitamin D level was significantly lower among those who tested positive for COVID-19 (19.00 ng/mL) than negative (20.55 ng/ mL). After controlling for demographic variables and several medical conditions, the adjusted odds ratio of COVID-19 infection in those with lower vitamin D was 1.45 (95% confidence interval, 1.08-1.95; P < .001). However, the odds of hospitalization for COVID-19 was not significantly associated with vitamin D level.1
Prior studies have also looked at vitamin D and respiratory infection. Martineau and colleagues analyzed 25 randomized, controlled trials with a pooled number of 11,321 individuals, including healthy ones and those with comorbidities, and found that oral vitamin D supplementation in daily or weekly doses had a protective effect against acute respiratory infection (adjusted odds ratio, 0.88; 95% CI, 0.81-0.96; P < .001). Patients with vitamin D deficiency (less than 25 nmol/L) experienced the most protective benefit. Vitamin D did not influence respiratory infection outcome.2
These studies suggest an adequate vitamin D level may be protective against infection with COVID-19, but who will benefit from vitamin D supplementation, and in what dose? Per U.S. Preventive Services Task Force guidelines, there is insufficient evidence to recommend screening for vitamin D deficiency in asymptomatic adults. Regarding daily dietary intake, the Institute of Medicine recommends 600 IU for persons aged 1-70, and 800 IU for those aged over 70 years. Salmon (447 IU per 3 oz serving), tuna (154 IU), and fortified milk (116 IU) are among the most vitamin D–rich foods.3 The recommended upper level of intake is 4,000 IU/day.
Too much of a good thing?
Extra vitamin D is stored in adipose tissue. If it builds up over time, storage sites may be overwhelmed, causing a rise in serum D level. While one might expect a subsequent rise in calcium levels, studies have shown this happens inconsistently, and at very high vitamin D levels, over 120 ng/mL.4 Most people would have to take at least 50,000 IU daily for several months to see an effect. The main adverse outcome of vitamin D toxicity is kidney stones, mediated by increased calcium in the blood and urine.
Several animal models have demonstrated hypervitaminosis D–induced aortic and coronary artery calcification. Like with kidney stones, the mechanism appears to be through increased calcium and phosphate levels. Shroff and colleagues studied serum vitamin D levels and vascular disease in children with renal disease on dialysis and found a U-shaped distribution: Children with both low and high vitamin D levels had significantly increased carotid artery intima-media thickness and calcification.5 Given the specialized nature of this population, it’s unclear whether these results can be generalized to most people. More studies are warranted on this topic.
Other benefits
Vitamin D is perhaps most famous for helping to build strong bones. Avenell and colleagues performed a Cochrane meta-analysis of vitamin D supplementation in older adults and found that vitamin D alone did not significantly reduce the risk of hip or other new fracture. Vitamin D plus calcium supplementation did reduce the risk of hip fracture (nine trials, pooled number of individuals was 49,853; relative risk, 0.84; P = .01).6
A lesser-known benefit of vitamin D is muscle protection. A prospective study out of the Jewish Hospital of Cincinnati followed 146 adults who were intolerant to two or more statins because of muscle side effects and found to have a vitamin D level below 32 ng per mL. Subjects were given vitamin D replacement (50,000 units weekly) and followed for 2 years. On statin rechallenge, 88-95% tolerated a statin with vitamin D levels 53-55 ng/mL.7
Pearl
Vitamin D supplementation may protect against COVID-19 infection and has very low chance of harm at daily doses at or below 4,000 IU. Other benefits of taking vitamin D include bone protection and reduction in statin-induced myopathy. The main adverse effect is kidney stones.
Ms. Sharninghausen is a medical student at the University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Merzon E et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: An Israeli population‐based study. FEBS J. 2020. doi: 10.1111/febs.15495.
2. Martineau AR et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
3. “How to Get More Vitamin D From Your Food,” Cleveland Clinic. 2019 Oct 23. https://health.clevelandclinic.org/how-to-get-more-vitamin-d-from-your-food/.
4. Galior K et al. Development of vitamin d toxicity from overcorrection of vitamin D Deficiency: A review of case reports. Nutrients. 2018;10(8):953. doi: 10.3390/nu10080953
5. Shroff R et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19(6):1239-46. doi: 10.1681/ASN.2007090993.
6. Avenell A et al. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014 Apr 14;2014(4):CD000227. doi: 10.1002/14651858.CD000227.pub4.
7. Khayznikov M et al. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N Am J Med Sci. 2015;7(3):86-93. doi:10.4103/1947-2714.153919
Case: A 56-year-old man with a history of type 2 diabetes, hypertension, hyperlipidemia, and obesity calls clinic to discuss concerns about COVID-19, stating: “I want to do everything I can to reduce my risk of infection.” In addition to physical distancing, mask wearing, hand hygiene, and control of chronic conditions, which of the following supplements would you recommend for this patient?
1. Coenzyme Q10 160 mg twice a day
2. Vitamin D 2,000 IU daily
3. Vitamin E 400 IU daily
4. Vitamin B12 1,000 mcg daily
Of these choices, vitamin D supplementation is likely the best option, based on the limited data that is available.
Risk factors for worse COVID-19 outcome, such as older age, obesity, and more pigmented skin are also risk factors for vitamin D deficiency. This makes the study of vitamin D and COVID-19 both challenging and relevant.
In a recent study of 7,807 people living in Israel, Merzon and colleagues found that low plasma vitamin D level was an independent risk factor for COVID-19 infection. Mean plasma vitamin D level was significantly lower among those who tested positive for COVID-19 (19.00 ng/mL) than negative (20.55 ng/ mL). After controlling for demographic variables and several medical conditions, the adjusted odds ratio of COVID-19 infection in those with lower vitamin D was 1.45 (95% confidence interval, 1.08-1.95; P < .001). However, the odds of hospitalization for COVID-19 was not significantly associated with vitamin D level.1
Prior studies have also looked at vitamin D and respiratory infection. Martineau and colleagues analyzed 25 randomized, controlled trials with a pooled number of 11,321 individuals, including healthy ones and those with comorbidities, and found that oral vitamin D supplementation in daily or weekly doses had a protective effect against acute respiratory infection (adjusted odds ratio, 0.88; 95% CI, 0.81-0.96; P < .001). Patients with vitamin D deficiency (less than 25 nmol/L) experienced the most protective benefit. Vitamin D did not influence respiratory infection outcome.2
These studies suggest an adequate vitamin D level may be protective against infection with COVID-19, but who will benefit from vitamin D supplementation, and in what dose? Per U.S. Preventive Services Task Force guidelines, there is insufficient evidence to recommend screening for vitamin D deficiency in asymptomatic adults. Regarding daily dietary intake, the Institute of Medicine recommends 600 IU for persons aged 1-70, and 800 IU for those aged over 70 years. Salmon (447 IU per 3 oz serving), tuna (154 IU), and fortified milk (116 IU) are among the most vitamin D–rich foods.3 The recommended upper level of intake is 4,000 IU/day.
Too much of a good thing?
Extra vitamin D is stored in adipose tissue. If it builds up over time, storage sites may be overwhelmed, causing a rise in serum D level. While one might expect a subsequent rise in calcium levels, studies have shown this happens inconsistently, and at very high vitamin D levels, over 120 ng/mL.4 Most people would have to take at least 50,000 IU daily for several months to see an effect. The main adverse outcome of vitamin D toxicity is kidney stones, mediated by increased calcium in the blood and urine.
Several animal models have demonstrated hypervitaminosis D–induced aortic and coronary artery calcification. Like with kidney stones, the mechanism appears to be through increased calcium and phosphate levels. Shroff and colleagues studied serum vitamin D levels and vascular disease in children with renal disease on dialysis and found a U-shaped distribution: Children with both low and high vitamin D levels had significantly increased carotid artery intima-media thickness and calcification.5 Given the specialized nature of this population, it’s unclear whether these results can be generalized to most people. More studies are warranted on this topic.
Other benefits
Vitamin D is perhaps most famous for helping to build strong bones. Avenell and colleagues performed a Cochrane meta-analysis of vitamin D supplementation in older adults and found that vitamin D alone did not significantly reduce the risk of hip or other new fracture. Vitamin D plus calcium supplementation did reduce the risk of hip fracture (nine trials, pooled number of individuals was 49,853; relative risk, 0.84; P = .01).6
A lesser-known benefit of vitamin D is muscle protection. A prospective study out of the Jewish Hospital of Cincinnati followed 146 adults who were intolerant to two or more statins because of muscle side effects and found to have a vitamin D level below 32 ng per mL. Subjects were given vitamin D replacement (50,000 units weekly) and followed for 2 years. On statin rechallenge, 88-95% tolerated a statin with vitamin D levels 53-55 ng/mL.7
Pearl
Vitamin D supplementation may protect against COVID-19 infection and has very low chance of harm at daily doses at or below 4,000 IU. Other benefits of taking vitamin D include bone protection and reduction in statin-induced myopathy. The main adverse effect is kidney stones.
Ms. Sharninghausen is a medical student at the University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Merzon E et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: An Israeli population‐based study. FEBS J. 2020. doi: 10.1111/febs.15495.
2. Martineau AR et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
3. “How to Get More Vitamin D From Your Food,” Cleveland Clinic. 2019 Oct 23. https://health.clevelandclinic.org/how-to-get-more-vitamin-d-from-your-food/.
4. Galior K et al. Development of vitamin d toxicity from overcorrection of vitamin D Deficiency: A review of case reports. Nutrients. 2018;10(8):953. doi: 10.3390/nu10080953
5. Shroff R et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19(6):1239-46. doi: 10.1681/ASN.2007090993.
6. Avenell A et al. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014 Apr 14;2014(4):CD000227. doi: 10.1002/14651858.CD000227.pub4.
7. Khayznikov M et al. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N Am J Med Sci. 2015;7(3):86-93. doi:10.4103/1947-2714.153919
Mitigating psychiatric disorder relapse in pregnancy during pandemic
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.