User login
Practice guidelines highlights from past year
Based on the most recent Infectious Diseases Society of America (IDSA) guidelines, what would be the preferred therapy?
A) Metronidazole
B) Fidaxomicin + bezlotoxumab
C) Vancomycin
D) Fecal microbiota transplant
The recommendations from the 2021 guidelines would be to treat with fidaxomicin and add bezlotoxumab.1 The guidelines highlight the following changes:
- In patients with an initial Clostridioides difficile infections (CDI) fidaxomicin is preferred over vancomycin.
- In patients with a recurrent CDI episode, fidaxomicin is favored over vancomycin. For patients with multiple recurrences, vancomycin in a tapered and pulsed regimen, vancomycin followed by rifaximin, and fecal microbiota transplantation are options in addition to fidaxomicin.
- Addition of bezlotoxumab to standard of care antibiotics is recommended for recurrence of CDI within the first 6 months over standard of care antibiotics alone
The feasibility of these recommendations is up for debate. The cost of a course of fidaxomicin is $2,800, and the cost of bezlotoxumab is about $4,500. Cost effectiveness studies that helped drive the recommendations show a savings by reducing future hospitalizations for C. diff.2 Unfortunately, this enthusiasm is not shared by many insurance companies for outpatient treatment.
Knee osteoarthritis
I will save you the excitement of the new acromegaly guidelines and focus on something we see all the time: knee osteoarthritis. The American Academy of Orthopedic Surgeons has released guidelines for this condition.3 The useful points I found were as follows:
- Topical application of nonsteroidal anti-inflammatory drugs (e.g., diclofenac) should be used to improve function and quality of life in patients with knee osteoarthritis.
- Exercise routines (i.e, supervised, unsupervised, and/or aquatic) are recommended versus no exercise for improving pain and function in patients with knee osteoarthritis.
- Not recommended is the use of oral narcotics (including tramadol), as they are not effective at improving pain or function, and their use results in a significant increased risk of adverse events.
- Not recommended for routine use in symptomatic knee osteoarthritis is intra-articular injection of hyaluronic acid.
I was happy to see topical NSAIDS recommended, as they are a much safer option in older patients than oral NSAIDS (which were also recommended). The recommendation against narcotics, including tramadol, is a shift from the recommendation of tramadol in the 2013 guidelines.4 Acetaminophen was enthusiastically recommended, and is still worth a try.
Sexually transmitted infections
- The dosing for the treatment of gonorrhea has increased to 500 mg of ceftriaxone (was 250 mg in 2015 guidelines), with a dose of 1 gram for patients who weigh more than 150 kg.
- Chlamydia infections should be treated with a 7-day course of doxycycline as the preferred antibiotic, except in pregnant women (where azithromycin is recommended).
- Herpes simplex virus 2 recurrences can be treated with twice-daily dosing of 800 mg of acyclovir for 5 days, or acyclovir 800 mg three times a day for 2 days. The shortest course for recurrence is famciclovir 1 gram twice a day for 1 day.
- The Centers for Disease Control and Prevention has removed the recommendation for avoidance of alcohol when taking metronidazole.
I hope these highlights of guidelines for common issues we see are helpful!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Johnson S et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused update guidelines on management of Clostridioides difficile Infection in adults. Clin Infect Dis. 2021 Sep 7;73(5):e1029-e1044.
2. Pabhu VS et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018 Feb 1;66(3):355-62.
3. American Academy of Orthopaedic Surgeons: Management of osteoarthritis of the knee (non-arthroplasty) – Evidence-based clinical practice guideline (2021 Aug 31. https://www.aaos.org/oak3cpg).
4. Jevsevar DS. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013: Sep;21(9):571-6.
5. Sexually transmitted infections treatment guidelines, 2021 recommendations and reports. MMWR 2021 Jul 23;70(4):1-187.
Based on the most recent Infectious Diseases Society of America (IDSA) guidelines, what would be the preferred therapy?
A) Metronidazole
B) Fidaxomicin + bezlotoxumab
C) Vancomycin
D) Fecal microbiota transplant
The recommendations from the 2021 guidelines would be to treat with fidaxomicin and add bezlotoxumab.1 The guidelines highlight the following changes:
- In patients with an initial Clostridioides difficile infections (CDI) fidaxomicin is preferred over vancomycin.
- In patients with a recurrent CDI episode, fidaxomicin is favored over vancomycin. For patients with multiple recurrences, vancomycin in a tapered and pulsed regimen, vancomycin followed by rifaximin, and fecal microbiota transplantation are options in addition to fidaxomicin.
- Addition of bezlotoxumab to standard of care antibiotics is recommended for recurrence of CDI within the first 6 months over standard of care antibiotics alone
The feasibility of these recommendations is up for debate. The cost of a course of fidaxomicin is $2,800, and the cost of bezlotoxumab is about $4,500. Cost effectiveness studies that helped drive the recommendations show a savings by reducing future hospitalizations for C. diff.2 Unfortunately, this enthusiasm is not shared by many insurance companies for outpatient treatment.
Knee osteoarthritis
I will save you the excitement of the new acromegaly guidelines and focus on something we see all the time: knee osteoarthritis. The American Academy of Orthopedic Surgeons has released guidelines for this condition.3 The useful points I found were as follows:
- Topical application of nonsteroidal anti-inflammatory drugs (e.g., diclofenac) should be used to improve function and quality of life in patients with knee osteoarthritis.
- Exercise routines (i.e, supervised, unsupervised, and/or aquatic) are recommended versus no exercise for improving pain and function in patients with knee osteoarthritis.
- Not recommended is the use of oral narcotics (including tramadol), as they are not effective at improving pain or function, and their use results in a significant increased risk of adverse events.
- Not recommended for routine use in symptomatic knee osteoarthritis is intra-articular injection of hyaluronic acid.
I was happy to see topical NSAIDS recommended, as they are a much safer option in older patients than oral NSAIDS (which were also recommended). The recommendation against narcotics, including tramadol, is a shift from the recommendation of tramadol in the 2013 guidelines.4 Acetaminophen was enthusiastically recommended, and is still worth a try.
Sexually transmitted infections
- The dosing for the treatment of gonorrhea has increased to 500 mg of ceftriaxone (was 250 mg in 2015 guidelines), with a dose of 1 gram for patients who weigh more than 150 kg.
- Chlamydia infections should be treated with a 7-day course of doxycycline as the preferred antibiotic, except in pregnant women (where azithromycin is recommended).
- Herpes simplex virus 2 recurrences can be treated with twice-daily dosing of 800 mg of acyclovir for 5 days, or acyclovir 800 mg three times a day for 2 days. The shortest course for recurrence is famciclovir 1 gram twice a day for 1 day.
- The Centers for Disease Control and Prevention has removed the recommendation for avoidance of alcohol when taking metronidazole.
I hope these highlights of guidelines for common issues we see are helpful!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Johnson S et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused update guidelines on management of Clostridioides difficile Infection in adults. Clin Infect Dis. 2021 Sep 7;73(5):e1029-e1044.
2. Pabhu VS et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018 Feb 1;66(3):355-62.
3. American Academy of Orthopaedic Surgeons: Management of osteoarthritis of the knee (non-arthroplasty) – Evidence-based clinical practice guideline (2021 Aug 31. https://www.aaos.org/oak3cpg).
4. Jevsevar DS. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013: Sep;21(9):571-6.
5. Sexually transmitted infections treatment guidelines, 2021 recommendations and reports. MMWR 2021 Jul 23;70(4):1-187.
Based on the most recent Infectious Diseases Society of America (IDSA) guidelines, what would be the preferred therapy?
A) Metronidazole
B) Fidaxomicin + bezlotoxumab
C) Vancomycin
D) Fecal microbiota transplant
The recommendations from the 2021 guidelines would be to treat with fidaxomicin and add bezlotoxumab.1 The guidelines highlight the following changes:
- In patients with an initial Clostridioides difficile infections (CDI) fidaxomicin is preferred over vancomycin.
- In patients with a recurrent CDI episode, fidaxomicin is favored over vancomycin. For patients with multiple recurrences, vancomycin in a tapered and pulsed regimen, vancomycin followed by rifaximin, and fecal microbiota transplantation are options in addition to fidaxomicin.
- Addition of bezlotoxumab to standard of care antibiotics is recommended for recurrence of CDI within the first 6 months over standard of care antibiotics alone
The feasibility of these recommendations is up for debate. The cost of a course of fidaxomicin is $2,800, and the cost of bezlotoxumab is about $4,500. Cost effectiveness studies that helped drive the recommendations show a savings by reducing future hospitalizations for C. diff.2 Unfortunately, this enthusiasm is not shared by many insurance companies for outpatient treatment.
Knee osteoarthritis
I will save you the excitement of the new acromegaly guidelines and focus on something we see all the time: knee osteoarthritis. The American Academy of Orthopedic Surgeons has released guidelines for this condition.3 The useful points I found were as follows:
- Topical application of nonsteroidal anti-inflammatory drugs (e.g., diclofenac) should be used to improve function and quality of life in patients with knee osteoarthritis.
- Exercise routines (i.e, supervised, unsupervised, and/or aquatic) are recommended versus no exercise for improving pain and function in patients with knee osteoarthritis.
- Not recommended is the use of oral narcotics (including tramadol), as they are not effective at improving pain or function, and their use results in a significant increased risk of adverse events.
- Not recommended for routine use in symptomatic knee osteoarthritis is intra-articular injection of hyaluronic acid.
I was happy to see topical NSAIDS recommended, as they are a much safer option in older patients than oral NSAIDS (which were also recommended). The recommendation against narcotics, including tramadol, is a shift from the recommendation of tramadol in the 2013 guidelines.4 Acetaminophen was enthusiastically recommended, and is still worth a try.
Sexually transmitted infections
- The dosing for the treatment of gonorrhea has increased to 500 mg of ceftriaxone (was 250 mg in 2015 guidelines), with a dose of 1 gram for patients who weigh more than 150 kg.
- Chlamydia infections should be treated with a 7-day course of doxycycline as the preferred antibiotic, except in pregnant women (where azithromycin is recommended).
- Herpes simplex virus 2 recurrences can be treated with twice-daily dosing of 800 mg of acyclovir for 5 days, or acyclovir 800 mg three times a day for 2 days. The shortest course for recurrence is famciclovir 1 gram twice a day for 1 day.
- The Centers for Disease Control and Prevention has removed the recommendation for avoidance of alcohol when taking metronidazole.
I hope these highlights of guidelines for common issues we see are helpful!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Johnson S et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused update guidelines on management of Clostridioides difficile Infection in adults. Clin Infect Dis. 2021 Sep 7;73(5):e1029-e1044.
2. Pabhu VS et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018 Feb 1;66(3):355-62.
3. American Academy of Orthopaedic Surgeons: Management of osteoarthritis of the knee (non-arthroplasty) – Evidence-based clinical practice guideline (2021 Aug 31. https://www.aaos.org/oak3cpg).
4. Jevsevar DS. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013: Sep;21(9):571-6.
5. Sexually transmitted infections treatment guidelines, 2021 recommendations and reports. MMWR 2021 Jul 23;70(4):1-187.
Practicing across state lines: A challenge for telemental health
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
Direct specialty care: Concierge service without the price tag
Four years ago, I was fully employed in a “traditional” rheumatology clinic. I met Alan, a 42-year-old gentleman who was a high school math teacher in my town. He was the first patient on my panel that day. Once I entered the examining room, Alan greeted me with: “You are the third rheumatologist who I have consulted for what everybody believes is fibromyalgia. I am paying out of pocket to see you as you are not on my insurance panel. I have researched your background, and I have high expectations of you.” He was cutting to the chase.
Alan had struggled with pain for about 1½ years. He insisted that he was very healthy before his symptoms started abruptly. In the past 2 years, his personal life had been under much stress as he was caring for a disabled child and facing an imminent divorce. While his symptoms were suggestive of an inflammatory arthritis, his workup was not. Unfortunately, the allocated time with Alan was 15 minutes – too short to cover both medical and personal struggles. Meanwhile, my nurses had to room in another two patients. I felt rushed and responsible for not letting the others wait. I asked Alan to keep a diary of his symptoms and come back in 2 weeks. A few minutes after discharging Alan, my nurse followed and asked me: “Where would you like me to add this patient, as you have no openings for 4 months ?”
“Overbook him!” I said.
This was happening almost every day. Scheduled patients, overbooked patients, tens of emails, calls to patients, and fights with insurance companies to approve tests and medications. Nearly every day I was getting home, preparing dinner, feeding my family, and going back to writing notes, as I would be financially penalized if my notes were not submitted in 24-48 hours. I had no time for my family and didn’t even think about any hobbies.
In 2 weeks, Alan came back for his visit. That day, I paid someone to take my kids to school and came to my office earlier. We had 1 hour to talk about his history. At the end of the visit, Alan said: “What kind of doctor are you? You looked into my eyes while I was talking, and you didn’t touch the computer keyboard?!” His remark was not uncommon for me. Most patients complain that physicians spend more time typing than looking at them. Maybe patients do not realize, but this is the only way that physicians get paid: writing the “proper notes” and placing the correct billing code.
Alan was diagnosed and treated successfully for seronegative rheumatoid arthritis. In 1 year, paying out of pocket to see me, he ended up spending many, many thousands of dollars. As you can imagine, I was not in control of those bills.
After 4 years in the traditional system, I decided to change something for my patients and for myself as their physician, and as a mother of three kids, a wife, a daughter, and a sister.
I decided to create a clinic where I am comfortable practicing “uncomplicated” medicine, as a friend of mine said. Today, insurance companies are restricting patients to limited panels of specialists. They dictate patients’ care, giving the false impression that they will save money. Insurance companies interfere with the physician’s medical judgment. They make algorithms to approve tests and have preferred lists of medication. They decide whether a test or a medication is appropriate for you. In addition, they don’t disclose how much they pay for your consultation, tests, and medication, and they ban the contracted parties from disclosing this information. They force patients to use their testing facilities and mailing pharmacies. Although patients and employers are the payers, they do not have access to their insurance companies’ “real” prices.
I decided that it was time to take control of my time spent with patients to make my services available when patients need me, without becoming a financial burden. I created a clinic where patients do not have copayments and will never receive a “surprise bill.” All costs are transparent to patients, including laboratory and imaging tests. Patients can talk to me on the phone, send a text, or email. A clinic where patients can talk to the physician on the phone or send a text or email? This is direct specialty care.
Is direct care a new concept? No, not at all. Is direct care the same as concierge medicine? I think it is a type of concierge service, but without the price tag.
Why?
Physicians practicing the traditional concierge medicine model here in the United States still bill patients’ insurance. In addition, to make their practice profitable, they charge a retainer fee that will allow them to keep a small patient panel. In contrast, direct care specialists do not have a contract with insurance companies.
I believe that both concierge medicine and direct care specialists offer exceptional care and better access to physicians. The difference is in costs: One is more expensive than the other. Traditional concierge medicine practices usually ask for high retainer fees in addition to copayments for visits. They do not offer any access to discounted pricing for laboratory or imaging tests. Patients continue to receive surprise bills from their insurance company.
Why don’t direct specialty care practices contract with insurance companies? Contracting with insurance companies increases a practice’s overhead costs (as more money is spent on coding and billing services and more office staff). When practice overhead is lower, the cost of patient care can be significantly lower. Patients pay a monthly membership to become a direct specialty care practice member. The membership covers the cost of visits and access to the benefits of the practice. In addition, direct care specialists do not charge copayments or send surprise bills. They can contract directly with laboratory and imaging centers and offer discounted prices. Patients with insurance are welcome to use it to cover tests, imaging, and medication. The patient has the power to choose between paying a cash price versus a “covered” service.
Most young patients, like Alan, have a high-deductible plan. A few regular blood tests might cost a patient hundreds of dollars before meeting a deductible. One MRI scan costs $4,000-$6,000. Patients who join a direct specialty care practice pay $30-$40 for regular labs and $400-$500 for an MRI.
I am now 2 years into practicing medicine as a direct care specialist. It is not a dream anymore. Yes, you may call it “concierge medicine without the price tag.” I call it “direct specialty care.” My patients and I are both accountable to one another. Together, we make a plan, and we have the time to implement it.
I am not alone. Other specialists are embracing this model. That is why we created the Direct Specialty Care Alliance, a place where physicians are welcome to network and share with others what they have learned along their journeys.
After I started my company, Alan was one of the first patients to join. He embraced my practice model and became one of the ambassadors of the direct specialty care movement. He is back to a normal life of taking care of his family, getting his wife back, and teaching math to high school kids.
Dr Girnita is the CEO and founder of RheumatologistOnCall, actively seeing patients via telemedicine in 10 U.S. states. She is an advocate for digital health and telemedicine that will empower physicians and patients to take charge of their medical care. She is a cofounder of the Direct Specialty Care Alliance. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four years ago, I was fully employed in a “traditional” rheumatology clinic. I met Alan, a 42-year-old gentleman who was a high school math teacher in my town. He was the first patient on my panel that day. Once I entered the examining room, Alan greeted me with: “You are the third rheumatologist who I have consulted for what everybody believes is fibromyalgia. I am paying out of pocket to see you as you are not on my insurance panel. I have researched your background, and I have high expectations of you.” He was cutting to the chase.
Alan had struggled with pain for about 1½ years. He insisted that he was very healthy before his symptoms started abruptly. In the past 2 years, his personal life had been under much stress as he was caring for a disabled child and facing an imminent divorce. While his symptoms were suggestive of an inflammatory arthritis, his workup was not. Unfortunately, the allocated time with Alan was 15 minutes – too short to cover both medical and personal struggles. Meanwhile, my nurses had to room in another two patients. I felt rushed and responsible for not letting the others wait. I asked Alan to keep a diary of his symptoms and come back in 2 weeks. A few minutes after discharging Alan, my nurse followed and asked me: “Where would you like me to add this patient, as you have no openings for 4 months ?”
“Overbook him!” I said.
This was happening almost every day. Scheduled patients, overbooked patients, tens of emails, calls to patients, and fights with insurance companies to approve tests and medications. Nearly every day I was getting home, preparing dinner, feeding my family, and going back to writing notes, as I would be financially penalized if my notes were not submitted in 24-48 hours. I had no time for my family and didn’t even think about any hobbies.
In 2 weeks, Alan came back for his visit. That day, I paid someone to take my kids to school and came to my office earlier. We had 1 hour to talk about his history. At the end of the visit, Alan said: “What kind of doctor are you? You looked into my eyes while I was talking, and you didn’t touch the computer keyboard?!” His remark was not uncommon for me. Most patients complain that physicians spend more time typing than looking at them. Maybe patients do not realize, but this is the only way that physicians get paid: writing the “proper notes” and placing the correct billing code.
Alan was diagnosed and treated successfully for seronegative rheumatoid arthritis. In 1 year, paying out of pocket to see me, he ended up spending many, many thousands of dollars. As you can imagine, I was not in control of those bills.
After 4 years in the traditional system, I decided to change something for my patients and for myself as their physician, and as a mother of three kids, a wife, a daughter, and a sister.
I decided to create a clinic where I am comfortable practicing “uncomplicated” medicine, as a friend of mine said. Today, insurance companies are restricting patients to limited panels of specialists. They dictate patients’ care, giving the false impression that they will save money. Insurance companies interfere with the physician’s medical judgment. They make algorithms to approve tests and have preferred lists of medication. They decide whether a test or a medication is appropriate for you. In addition, they don’t disclose how much they pay for your consultation, tests, and medication, and they ban the contracted parties from disclosing this information. They force patients to use their testing facilities and mailing pharmacies. Although patients and employers are the payers, they do not have access to their insurance companies’ “real” prices.
I decided that it was time to take control of my time spent with patients to make my services available when patients need me, without becoming a financial burden. I created a clinic where patients do not have copayments and will never receive a “surprise bill.” All costs are transparent to patients, including laboratory and imaging tests. Patients can talk to me on the phone, send a text, or email. A clinic where patients can talk to the physician on the phone or send a text or email? This is direct specialty care.
Is direct care a new concept? No, not at all. Is direct care the same as concierge medicine? I think it is a type of concierge service, but without the price tag.
Why?
Physicians practicing the traditional concierge medicine model here in the United States still bill patients’ insurance. In addition, to make their practice profitable, they charge a retainer fee that will allow them to keep a small patient panel. In contrast, direct care specialists do not have a contract with insurance companies.
I believe that both concierge medicine and direct care specialists offer exceptional care and better access to physicians. The difference is in costs: One is more expensive than the other. Traditional concierge medicine practices usually ask for high retainer fees in addition to copayments for visits. They do not offer any access to discounted pricing for laboratory or imaging tests. Patients continue to receive surprise bills from their insurance company.
Why don’t direct specialty care practices contract with insurance companies? Contracting with insurance companies increases a practice’s overhead costs (as more money is spent on coding and billing services and more office staff). When practice overhead is lower, the cost of patient care can be significantly lower. Patients pay a monthly membership to become a direct specialty care practice member. The membership covers the cost of visits and access to the benefits of the practice. In addition, direct care specialists do not charge copayments or send surprise bills. They can contract directly with laboratory and imaging centers and offer discounted prices. Patients with insurance are welcome to use it to cover tests, imaging, and medication. The patient has the power to choose between paying a cash price versus a “covered” service.
Most young patients, like Alan, have a high-deductible plan. A few regular blood tests might cost a patient hundreds of dollars before meeting a deductible. One MRI scan costs $4,000-$6,000. Patients who join a direct specialty care practice pay $30-$40 for regular labs and $400-$500 for an MRI.
I am now 2 years into practicing medicine as a direct care specialist. It is not a dream anymore. Yes, you may call it “concierge medicine without the price tag.” I call it “direct specialty care.” My patients and I are both accountable to one another. Together, we make a plan, and we have the time to implement it.
I am not alone. Other specialists are embracing this model. That is why we created the Direct Specialty Care Alliance, a place where physicians are welcome to network and share with others what they have learned along their journeys.
After I started my company, Alan was one of the first patients to join. He embraced my practice model and became one of the ambassadors of the direct specialty care movement. He is back to a normal life of taking care of his family, getting his wife back, and teaching math to high school kids.
Dr Girnita is the CEO and founder of RheumatologistOnCall, actively seeing patients via telemedicine in 10 U.S. states. She is an advocate for digital health and telemedicine that will empower physicians and patients to take charge of their medical care. She is a cofounder of the Direct Specialty Care Alliance. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four years ago, I was fully employed in a “traditional” rheumatology clinic. I met Alan, a 42-year-old gentleman who was a high school math teacher in my town. He was the first patient on my panel that day. Once I entered the examining room, Alan greeted me with: “You are the third rheumatologist who I have consulted for what everybody believes is fibromyalgia. I am paying out of pocket to see you as you are not on my insurance panel. I have researched your background, and I have high expectations of you.” He was cutting to the chase.
Alan had struggled with pain for about 1½ years. He insisted that he was very healthy before his symptoms started abruptly. In the past 2 years, his personal life had been under much stress as he was caring for a disabled child and facing an imminent divorce. While his symptoms were suggestive of an inflammatory arthritis, his workup was not. Unfortunately, the allocated time with Alan was 15 minutes – too short to cover both medical and personal struggles. Meanwhile, my nurses had to room in another two patients. I felt rushed and responsible for not letting the others wait. I asked Alan to keep a diary of his symptoms and come back in 2 weeks. A few minutes after discharging Alan, my nurse followed and asked me: “Where would you like me to add this patient, as you have no openings for 4 months ?”
“Overbook him!” I said.
This was happening almost every day. Scheduled patients, overbooked patients, tens of emails, calls to patients, and fights with insurance companies to approve tests and medications. Nearly every day I was getting home, preparing dinner, feeding my family, and going back to writing notes, as I would be financially penalized if my notes were not submitted in 24-48 hours. I had no time for my family and didn’t even think about any hobbies.
In 2 weeks, Alan came back for his visit. That day, I paid someone to take my kids to school and came to my office earlier. We had 1 hour to talk about his history. At the end of the visit, Alan said: “What kind of doctor are you? You looked into my eyes while I was talking, and you didn’t touch the computer keyboard?!” His remark was not uncommon for me. Most patients complain that physicians spend more time typing than looking at them. Maybe patients do not realize, but this is the only way that physicians get paid: writing the “proper notes” and placing the correct billing code.
Alan was diagnosed and treated successfully for seronegative rheumatoid arthritis. In 1 year, paying out of pocket to see me, he ended up spending many, many thousands of dollars. As you can imagine, I was not in control of those bills.
After 4 years in the traditional system, I decided to change something for my patients and for myself as their physician, and as a mother of three kids, a wife, a daughter, and a sister.
I decided to create a clinic where I am comfortable practicing “uncomplicated” medicine, as a friend of mine said. Today, insurance companies are restricting patients to limited panels of specialists. They dictate patients’ care, giving the false impression that they will save money. Insurance companies interfere with the physician’s medical judgment. They make algorithms to approve tests and have preferred lists of medication. They decide whether a test or a medication is appropriate for you. In addition, they don’t disclose how much they pay for your consultation, tests, and medication, and they ban the contracted parties from disclosing this information. They force patients to use their testing facilities and mailing pharmacies. Although patients and employers are the payers, they do not have access to their insurance companies’ “real” prices.
I decided that it was time to take control of my time spent with patients to make my services available when patients need me, without becoming a financial burden. I created a clinic where patients do not have copayments and will never receive a “surprise bill.” All costs are transparent to patients, including laboratory and imaging tests. Patients can talk to me on the phone, send a text, or email. A clinic where patients can talk to the physician on the phone or send a text or email? This is direct specialty care.
Is direct care a new concept? No, not at all. Is direct care the same as concierge medicine? I think it is a type of concierge service, but without the price tag.
Why?
Physicians practicing the traditional concierge medicine model here in the United States still bill patients’ insurance. In addition, to make their practice profitable, they charge a retainer fee that will allow them to keep a small patient panel. In contrast, direct care specialists do not have a contract with insurance companies.
I believe that both concierge medicine and direct care specialists offer exceptional care and better access to physicians. The difference is in costs: One is more expensive than the other. Traditional concierge medicine practices usually ask for high retainer fees in addition to copayments for visits. They do not offer any access to discounted pricing for laboratory or imaging tests. Patients continue to receive surprise bills from their insurance company.
Why don’t direct specialty care practices contract with insurance companies? Contracting with insurance companies increases a practice’s overhead costs (as more money is spent on coding and billing services and more office staff). When practice overhead is lower, the cost of patient care can be significantly lower. Patients pay a monthly membership to become a direct specialty care practice member. The membership covers the cost of visits and access to the benefits of the practice. In addition, direct care specialists do not charge copayments or send surprise bills. They can contract directly with laboratory and imaging centers and offer discounted prices. Patients with insurance are welcome to use it to cover tests, imaging, and medication. The patient has the power to choose between paying a cash price versus a “covered” service.
Most young patients, like Alan, have a high-deductible plan. A few regular blood tests might cost a patient hundreds of dollars before meeting a deductible. One MRI scan costs $4,000-$6,000. Patients who join a direct specialty care practice pay $30-$40 for regular labs and $400-$500 for an MRI.
I am now 2 years into practicing medicine as a direct care specialist. It is not a dream anymore. Yes, you may call it “concierge medicine without the price tag.” I call it “direct specialty care.” My patients and I are both accountable to one another. Together, we make a plan, and we have the time to implement it.
I am not alone. Other specialists are embracing this model. That is why we created the Direct Specialty Care Alliance, a place where physicians are welcome to network and share with others what they have learned along their journeys.
After I started my company, Alan was one of the first patients to join. He embraced my practice model and became one of the ambassadors of the direct specialty care movement. He is back to a normal life of taking care of his family, getting his wife back, and teaching math to high school kids.
Dr Girnita is the CEO and founder of RheumatologistOnCall, actively seeing patients via telemedicine in 10 U.S. states. She is an advocate for digital health and telemedicine that will empower physicians and patients to take charge of their medical care. She is a cofounder of the Direct Specialty Care Alliance. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some physicians still lack access to COVID-19 vaccines
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at imnews@mdedge.com.
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at imnews@mdedge.com.
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at imnews@mdedge.com.
Is proactive TDM the way to go?
Dear colleagues,
We shift gears from discussing GI hospitalists to focusing on the treatment of inflammatory bowel disease. The introduction of anti-TNFs brought about a paradigm shift in IBD management. With the ability to measure drug and antibody levels, we are also able to alter dose and timing to increase the efficacy of these medications. Some experts have extended this reactive drug monitoring approach to a more proactive method with the expectation that this may prevent loss of efficacy and development of adverse events. Dr. Loren G. Rabinowitz and colleagues and Dr. Hans Herfarth describe these two approaches to anti-TNF management in IBD, drawing from the current data and their own experiences. I look forward to hearing your thoughts and experiences by email (ginews@gastro.org).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
Better outcomes than reactive TDM
By Loren G. Rabinowitz, MD; Konstantinos Papamichael, PhD, MD; and Adam S. Cheifetz, MD, AGAF
Therapeutic drug monitoring (TDM), or the practice of treatment optimization based on serum drug concentrations, is used in many settings, including solid organ transplantation, infection, and immune-mediated inflammatory diseases, including inflammatory bowel disease (IBD). In IBD, the use of TDM has been an area of keen research focus, and, in our view, should be standard practice for optimization of biologic therapy, particularly in the setting of anti–tumor necrosis factor (TNF) therapy. TDM has demonstrated utility in determining the correct timing and dosage of biologics and can provide the impetus for deescalating or discontinuing a biologic in favor of an alternative one. It also allows prescribers the ability to protect patients from severe infusion reactions if they have developed anti-drug antibodies (ADAs).
Reactive TDM refers to a strategy of assessing drug concentration and presence of ADAs in the setting of primary nonresponse (PNR) and loss of response (LOR) to a biologic agent. In this context, TDM informs possible reasons for loss or lack of response to treatment – for example, insufficient drug concentration or the development of high-titer ADAs (immunogenicity) – thus better directing the management of these unwanted outcomes.1 Insufficient anti-TNF concentrations have been associated with PNR and lack of clinical remission at 1 year in patients with IBD,2 which underscores the need for a durable strategy to ensure appropriate drug concentrations from the induction through maintenance phases of biologic administration. Reactive TDM can also be used to inform the decision to abandon a particular therapy in favor of a different biologic and to guide the selection of the next biologic agent, and has been shown to be less expensive than empiric dose escalation.2 With regard to infliximab and adalimumab, it is our practice to continue dose escalation until drug concentrations are above 10-15 mcg/mL prior to abandoning therapy.1
For a significant number of patients, reactive TDM identifies at-risk patients too late, when ADAs have already formed. Because the number of medications to treat IBD remains limited, waiting for a patient to lose response to an agent, particularly anti-TNF therapies, increases the likelihood of immunogenicity, thus rendering an agent unusable. Proactive TDM or checking drug trough concentrations preemptively and at predetermined intervals, and dosing to an appropriate concentration, can improve patient outcomes. If drug concentration is determined to be not “at target,” dosage and timing of administration can be increased with or without the addition of an immunomodulator (thiopurines or methotrexate) to optimize the biologic’s efficacy and prevent immunogenicity. This approach allows the provider to anticipate and proactively guard against PNR and future LOR.
Proactive TDM of anti-TNF therapy has been associated with better patient outcomes in both pediatric and adult populations when compared with empiric dose optimization and/or reactive TDM.2,3 In patients with immune-mediated inflammatory diseases undergoing maintenance therapy with infliximab, proactive TDM was found to be more effective than treatment without TDM in sustaining disease control without disease worsening.4 Proactive TDM has been associated with better patient outcomes, including increased rates of clinical remission in both pediatric and adult populations and decreased rates of IBD-related surgery, hospitalization, serious infusion reactions, and development of ADAs, when compared with reactive TDM or empiric optimization. Preliminary data suggest that proactive TDM can also be used to efficiently guide dose deescalation in patients in remission with drug concentrations markedly above target and to allow for optimization of infliximab monotherapy so that combination therapy can be employed more judiciously (that is, in a patient who developed rapid ADA to a different anti-TNF).1 This could potentially attenuate the risks associated with long-term immunomodulator use, which include lymphomas and higher rates of serious and opportunistic infections. A recent study using a pharmacokinetic dashboard showed that the majority of patients with IBD will need accelerated dosing by the third infusion to maintain therapeutic infliximab concentrations during induction and maintenance therapy, highlighting the urgent need for widespread adoption of early proactive TDM.5 It is likely that proactive TDM is most important early in therapy when patients are most inflamed and have more rapid drug clearance. For this reason, proactive TDM should ideally be used for all patients during the induction phase. It is our practice to continue to follow drug concentrations one or two times per year once a patient has achieved remission. A recent literature review and consensus statement highlights the utility of TDM and what is known at this time.1
TDM should be standard of care for patients with IBD. At minimum, reactive TDM has rationalized the management of PNR, is associated with better outcomes, and is more cost effective than empiric dose escalation.1,2 In this setting, however, many patients have already developed ADAs that cannot be overcome. At present, anti-TNF therapy remains the most effective agent for our sickest patients with IBD. Given the as-yet limited armamentarium of medications available, particularly for patients with fistulizing perianal Crohn’s disease (CD) and severe ulcerative colitis (UC), proactive TDM, which allows for improved optimization and long-term durability of biologics, is essential to the care of any IBD patient requiring these medications. Proactive TDM should ideally be used for all patients during the induction phase and at least once during maintenance therapy. There is also the potential for TDM-driven dose de-escalation for patients in remission and optimization of infliximab monotherapy, thus avoiding combination therapy with an immunomodulator in some cases. Future perspectives for a more precise application of TDM include the use of pharmacokinetic modeling dashboards and pharmacogenetics toward achieving truly individualized medicine.3
Dr. Rabinowitz, Dr. Papamichael, and Dr. Cheifetz are with the department of medicine and division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Rabinowitz reports no conflicts of interest. Dr. Papamichael reports lecture fees from Mitsubishi Tanabe Pharma and Physicians’ Education Resource; consultancy fee from Prometheus Laboratories; and scientific advisory board fees from ProciseDx and Scipher Medicine Corporation. Dr. Cheifetz reports consulting for Janssen, AbbVie, Samsung, Arena Pharmaceuticals, Grifols, Prometheus, Bristol Myers Squibb, Artizan Biosciences, Artugan Therapeutics, and Equillium.
References
1. Cheifetz AS et al. Am J Gastroenterol. 2021 Oct 1;116(10):2014-25.
2. Kennedy NA et al. Lancet Gastroenterol Hepatol. 2019 May;4(5):341-53.
3. Papamichael K et al. Lancet Gastroenterol Hepatol. 2022;7(2):171-85.
4. Syversen SW et al. JAMA. 2021;326(23):2375-84.
5. Dubinsky MC et al. Inflamm Bowel Dis. 2022 Jan 3. doi: 10.1093/ibd/izab285.
Taking a closer look at the evidence
By Hans Herfarth, MD, PhD, AGAF
The debate of therapeutic drug monitoring (TDM) in the setting of anti–tumor necrosis factor (TNF) therapy has been ongoing for more than a decade. Reactive TDM, the measurement of drug concentrations in the context of loss of treatment response, is now generally accepted and recommended in multiple national and international inflammatory bowel disease (IBD) guidelines. Proactive TDM, defined as the systematic measurement of drug trough concentrations and anti-drug antibodies with dose adaptations to a predefined target drug concentration, seems to offer a possibility to stabilize drug levels and prevent anti-drug antibody formation due to low systemic drug levels, thus potentially preventing the well-known loss of response to anti-TNF therapy, which occurs in more than 50% of patients over time. However, proactive TDM is not endorsed by evidence-based guidelines, dividing IBD physicians into believers and nonbelievers and limiting uptake into clinical practice.
As with reactive TDM, one should assume that the framework for proactive TDM should have been reliably established based on factual data derived from prospective controlled studies and not rely on retrospective cohorts or “Expert Panel” consensus statements. And indeed, several prospective controlled studies with sizable IBD patient cohorts have been published. Of note, all TDM studies for IBD were conducted in patients on anti-TNF maintenance therapy, and currently no prospective studies in larger IBD populations are available for proactive TDM during induction therapy. Two prospective studies, the PRECISION and the NOR-DRUM trial, report that proactive TDM is better than no TDM at all.
However, in the comparison of proactive TDM and reactive TDM (including at least one drug adaptation in maintenance or drug escalation based on clinical symptoms or biomarkers), three studies have demonstrated no significant differences in drug persistence or overall maintenance of clinical remission. Only a fourth (the pediatric PAILOT study) reported a lower frequency of mild flares and less steroid exposure in the proactive TDM arm over 1 year, but it did not show differences in drug persistence or overall clinical remission compared with the reactive TDM arm. Interestingly, the differences in flare frequency were apparent only in patients on monotherapy but not in the subgroup on combination therapy with an immunomodulator, stressing the well-known beneficial effect of a combination therapy with thiopurines in CD first shown in the SONIC trial.
One problem, at least in the TDM trials with infliximab (IFX), may have been a delay in optimizing IFX levels until the next drug infusion because of the turnaround time of the drug assays. However, even the most recently published ultra-proactive TDM study with ad hoc dose adjustments based on
The value of proactive TDM in induction therapy remains an ongoing concern. There is no doubt that the severity of intestinal inflammation with subsequent loss of drug in the intestine can result in low drug serum concentrations correlating to lower clinical responses and higher rates of immunogenicity with the formation of anti-drug antibodies. A recent study including patients with chronic immune-mediated inflammatory diseases such as IBD, rheumatoid arthritis, and psoriasis did not find a value in proactive TDM of IFX in the induction phase, but more severe IBD may have been underrepresented in this study. Administration of significantly higher induction dosing of adalimumab (160 mg weeks 0, 1, 2, and 3) with significantly higher trough levels compared with a standard induction regimen in the SERENE study has not been shown to increase the short- or long-term remission rates in UC or CD patients. Therefore, higher trough levels in a patient population do not automatically result in better outcomes, but proactive TDM may still have found a few patients who may have benefited from an even higher induction regimen. The UC and CD SERENE maintenance studies also evaluated proactive TDM versus clinical adjustment based on clinical and biomarkers. After 1 year, no differences in the efficacy endpoints of clinical, endoscopic, and deep remission were found. These somewhat surprising results, which have been reported only in meeting abstracts, suggest that simply increasing trough levels to a higher target (one of the primary aims of proactive TDM) is not an effective universal approach for achieving higher remission rates in induction or better outcomes in maintenance. Instead, the SERENE data show that similar results can be achieved by regular clinical follow-up and monitoring of loss of response based on symptoms and/or biochemical markers followed by drug adaptation (which may then also be based on reactive TDM).
One unquestionable effect of proactive TDM is that the process of checking and controlling drug levels suggests for the treating physician better control over the anti-TNF therapy and for the patient reassurance that the treatment is in the intended target range. Proactive TDM also may be cost effective in the group of patients whose anti-TNF treatment regimen can be deescalated because of high drug levels. Despite the increased number of studies showing no clinical advantage of proactive TDM of every patient on anti-TNF therapy, there may be benefits for subgroups. Proactive TDM with point-of-care testing of drug levels may be helpful during induction therapy in patients with a high inflammatory burden, which results in uncontrolled drug loss via the intestine. Proactive TDM during maintenance therapy (for example, every 6-12 months) may be beneficial in subgroups of patients at risk for developing low anti-TNF levels or anti-drug antibodies, such as patients with a genetic predisposition to anti-TNF anti-drug antibody formation (such as the HLA-DQ1*05 allele), patients on a second anti-TNF therapy after loss of response to the first one, and patients on anti-TNF therapy in combination with thiopurines or methotrexate who deescalate to anti-TNF monotherapy.
In summary, there is no doubt that proactive TDM is better than no TDM (meaning no drug adjustments at all). However, nearly all controlled prospective studies show no significant benefit of proactive TDM versus reactive TDM or drug escalation based on clinical symptoms or biomarkers. Future studies should target clearly defined patient groups at risk of losing response to anti-TNF to clarify if proactive TDM is a valuable tool to achieving better therapeutic results in clinical practice.
Dr. Herfarth is professor of medicine and codirector of the UNC Multidisciplinary IBD Center at University of North Carolina at Chapel Hill. He reports serving as a consultant to Alivio Therapeutics, AMAG, Bristol Myers Squibb, Boehringer Ingleheim, ExeGi Pharma, Finch, Gilead, Janssen, Lycera, Merck, Otsuka, Pfizer, PureTech, and Seres and receiving research support from Allakos, Artizan Biosciences, and Pfizer.
Relevant resources
- Syversen SW et al. JAMA. 2021;326:2375-84.
- Strik AS et al. Scand J Gastroenterol. 2021 Feb;56(2):145-54.
- Bossuyt P et al. J Crohns Colitis. 2022 Feb 23;16(2):199-206.
- D’Haens G et al. Gastroenterology. 2018;154:1343-51.e1.
Dear colleagues,
We shift gears from discussing GI hospitalists to focusing on the treatment of inflammatory bowel disease. The introduction of anti-TNFs brought about a paradigm shift in IBD management. With the ability to measure drug and antibody levels, we are also able to alter dose and timing to increase the efficacy of these medications. Some experts have extended this reactive drug monitoring approach to a more proactive method with the expectation that this may prevent loss of efficacy and development of adverse events. Dr. Loren G. Rabinowitz and colleagues and Dr. Hans Herfarth describe these two approaches to anti-TNF management in IBD, drawing from the current data and their own experiences. I look forward to hearing your thoughts and experiences by email (ginews@gastro.org).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
Better outcomes than reactive TDM
By Loren G. Rabinowitz, MD; Konstantinos Papamichael, PhD, MD; and Adam S. Cheifetz, MD, AGAF
Therapeutic drug monitoring (TDM), or the practice of treatment optimization based on serum drug concentrations, is used in many settings, including solid organ transplantation, infection, and immune-mediated inflammatory diseases, including inflammatory bowel disease (IBD). In IBD, the use of TDM has been an area of keen research focus, and, in our view, should be standard practice for optimization of biologic therapy, particularly in the setting of anti–tumor necrosis factor (TNF) therapy. TDM has demonstrated utility in determining the correct timing and dosage of biologics and can provide the impetus for deescalating or discontinuing a biologic in favor of an alternative one. It also allows prescribers the ability to protect patients from severe infusion reactions if they have developed anti-drug antibodies (ADAs).
Reactive TDM refers to a strategy of assessing drug concentration and presence of ADAs in the setting of primary nonresponse (PNR) and loss of response (LOR) to a biologic agent. In this context, TDM informs possible reasons for loss or lack of response to treatment – for example, insufficient drug concentration or the development of high-titer ADAs (immunogenicity) – thus better directing the management of these unwanted outcomes.1 Insufficient anti-TNF concentrations have been associated with PNR and lack of clinical remission at 1 year in patients with IBD,2 which underscores the need for a durable strategy to ensure appropriate drug concentrations from the induction through maintenance phases of biologic administration. Reactive TDM can also be used to inform the decision to abandon a particular therapy in favor of a different biologic and to guide the selection of the next biologic agent, and has been shown to be less expensive than empiric dose escalation.2 With regard to infliximab and adalimumab, it is our practice to continue dose escalation until drug concentrations are above 10-15 mcg/mL prior to abandoning therapy.1
For a significant number of patients, reactive TDM identifies at-risk patients too late, when ADAs have already formed. Because the number of medications to treat IBD remains limited, waiting for a patient to lose response to an agent, particularly anti-TNF therapies, increases the likelihood of immunogenicity, thus rendering an agent unusable. Proactive TDM or checking drug trough concentrations preemptively and at predetermined intervals, and dosing to an appropriate concentration, can improve patient outcomes. If drug concentration is determined to be not “at target,” dosage and timing of administration can be increased with or without the addition of an immunomodulator (thiopurines or methotrexate) to optimize the biologic’s efficacy and prevent immunogenicity. This approach allows the provider to anticipate and proactively guard against PNR and future LOR.
Proactive TDM of anti-TNF therapy has been associated with better patient outcomes in both pediatric and adult populations when compared with empiric dose optimization and/or reactive TDM.2,3 In patients with immune-mediated inflammatory diseases undergoing maintenance therapy with infliximab, proactive TDM was found to be more effective than treatment without TDM in sustaining disease control without disease worsening.4 Proactive TDM has been associated with better patient outcomes, including increased rates of clinical remission in both pediatric and adult populations and decreased rates of IBD-related surgery, hospitalization, serious infusion reactions, and development of ADAs, when compared with reactive TDM or empiric optimization. Preliminary data suggest that proactive TDM can also be used to efficiently guide dose deescalation in patients in remission with drug concentrations markedly above target and to allow for optimization of infliximab monotherapy so that combination therapy can be employed more judiciously (that is, in a patient who developed rapid ADA to a different anti-TNF).1 This could potentially attenuate the risks associated with long-term immunomodulator use, which include lymphomas and higher rates of serious and opportunistic infections. A recent study using a pharmacokinetic dashboard showed that the majority of patients with IBD will need accelerated dosing by the third infusion to maintain therapeutic infliximab concentrations during induction and maintenance therapy, highlighting the urgent need for widespread adoption of early proactive TDM.5 It is likely that proactive TDM is most important early in therapy when patients are most inflamed and have more rapid drug clearance. For this reason, proactive TDM should ideally be used for all patients during the induction phase. It is our practice to continue to follow drug concentrations one or two times per year once a patient has achieved remission. A recent literature review and consensus statement highlights the utility of TDM and what is known at this time.1
TDM should be standard of care for patients with IBD. At minimum, reactive TDM has rationalized the management of PNR, is associated with better outcomes, and is more cost effective than empiric dose escalation.1,2 In this setting, however, many patients have already developed ADAs that cannot be overcome. At present, anti-TNF therapy remains the most effective agent for our sickest patients with IBD. Given the as-yet limited armamentarium of medications available, particularly for patients with fistulizing perianal Crohn’s disease (CD) and severe ulcerative colitis (UC), proactive TDM, which allows for improved optimization and long-term durability of biologics, is essential to the care of any IBD patient requiring these medications. Proactive TDM should ideally be used for all patients during the induction phase and at least once during maintenance therapy. There is also the potential for TDM-driven dose de-escalation for patients in remission and optimization of infliximab monotherapy, thus avoiding combination therapy with an immunomodulator in some cases. Future perspectives for a more precise application of TDM include the use of pharmacokinetic modeling dashboards and pharmacogenetics toward achieving truly individualized medicine.3
Dr. Rabinowitz, Dr. Papamichael, and Dr. Cheifetz are with the department of medicine and division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Rabinowitz reports no conflicts of interest. Dr. Papamichael reports lecture fees from Mitsubishi Tanabe Pharma and Physicians’ Education Resource; consultancy fee from Prometheus Laboratories; and scientific advisory board fees from ProciseDx and Scipher Medicine Corporation. Dr. Cheifetz reports consulting for Janssen, AbbVie, Samsung, Arena Pharmaceuticals, Grifols, Prometheus, Bristol Myers Squibb, Artizan Biosciences, Artugan Therapeutics, and Equillium.
References
1. Cheifetz AS et al. Am J Gastroenterol. 2021 Oct 1;116(10):2014-25.
2. Kennedy NA et al. Lancet Gastroenterol Hepatol. 2019 May;4(5):341-53.
3. Papamichael K et al. Lancet Gastroenterol Hepatol. 2022;7(2):171-85.
4. Syversen SW et al. JAMA. 2021;326(23):2375-84.
5. Dubinsky MC et al. Inflamm Bowel Dis. 2022 Jan 3. doi: 10.1093/ibd/izab285.
Taking a closer look at the evidence
By Hans Herfarth, MD, PhD, AGAF
The debate of therapeutic drug monitoring (TDM) in the setting of anti–tumor necrosis factor (TNF) therapy has been ongoing for more than a decade. Reactive TDM, the measurement of drug concentrations in the context of loss of treatment response, is now generally accepted and recommended in multiple national and international inflammatory bowel disease (IBD) guidelines. Proactive TDM, defined as the systematic measurement of drug trough concentrations and anti-drug antibodies with dose adaptations to a predefined target drug concentration, seems to offer a possibility to stabilize drug levels and prevent anti-drug antibody formation due to low systemic drug levels, thus potentially preventing the well-known loss of response to anti-TNF therapy, which occurs in more than 50% of patients over time. However, proactive TDM is not endorsed by evidence-based guidelines, dividing IBD physicians into believers and nonbelievers and limiting uptake into clinical practice.
As with reactive TDM, one should assume that the framework for proactive TDM should have been reliably established based on factual data derived from prospective controlled studies and not rely on retrospective cohorts or “Expert Panel” consensus statements. And indeed, several prospective controlled studies with sizable IBD patient cohorts have been published. Of note, all TDM studies for IBD were conducted in patients on anti-TNF maintenance therapy, and currently no prospective studies in larger IBD populations are available for proactive TDM during induction therapy. Two prospective studies, the PRECISION and the NOR-DRUM trial, report that proactive TDM is better than no TDM at all.
However, in the comparison of proactive TDM and reactive TDM (including at least one drug adaptation in maintenance or drug escalation based on clinical symptoms or biomarkers), three studies have demonstrated no significant differences in drug persistence or overall maintenance of clinical remission. Only a fourth (the pediatric PAILOT study) reported a lower frequency of mild flares and less steroid exposure in the proactive TDM arm over 1 year, but it did not show differences in drug persistence or overall clinical remission compared with the reactive TDM arm. Interestingly, the differences in flare frequency were apparent only in patients on monotherapy but not in the subgroup on combination therapy with an immunomodulator, stressing the well-known beneficial effect of a combination therapy with thiopurines in CD first shown in the SONIC trial.
One problem, at least in the TDM trials with infliximab (IFX), may have been a delay in optimizing IFX levels until the next drug infusion because of the turnaround time of the drug assays. However, even the most recently published ultra-proactive TDM study with ad hoc dose adjustments based on
The value of proactive TDM in induction therapy remains an ongoing concern. There is no doubt that the severity of intestinal inflammation with subsequent loss of drug in the intestine can result in low drug serum concentrations correlating to lower clinical responses and higher rates of immunogenicity with the formation of anti-drug antibodies. A recent study including patients with chronic immune-mediated inflammatory diseases such as IBD, rheumatoid arthritis, and psoriasis did not find a value in proactive TDM of IFX in the induction phase, but more severe IBD may have been underrepresented in this study. Administration of significantly higher induction dosing of adalimumab (160 mg weeks 0, 1, 2, and 3) with significantly higher trough levels compared with a standard induction regimen in the SERENE study has not been shown to increase the short- or long-term remission rates in UC or CD patients. Therefore, higher trough levels in a patient population do not automatically result in better outcomes, but proactive TDM may still have found a few patients who may have benefited from an even higher induction regimen. The UC and CD SERENE maintenance studies also evaluated proactive TDM versus clinical adjustment based on clinical and biomarkers. After 1 year, no differences in the efficacy endpoints of clinical, endoscopic, and deep remission were found. These somewhat surprising results, which have been reported only in meeting abstracts, suggest that simply increasing trough levels to a higher target (one of the primary aims of proactive TDM) is not an effective universal approach for achieving higher remission rates in induction or better outcomes in maintenance. Instead, the SERENE data show that similar results can be achieved by regular clinical follow-up and monitoring of loss of response based on symptoms and/or biochemical markers followed by drug adaptation (which may then also be based on reactive TDM).
One unquestionable effect of proactive TDM is that the process of checking and controlling drug levels suggests for the treating physician better control over the anti-TNF therapy and for the patient reassurance that the treatment is in the intended target range. Proactive TDM also may be cost effective in the group of patients whose anti-TNF treatment regimen can be deescalated because of high drug levels. Despite the increased number of studies showing no clinical advantage of proactive TDM of every patient on anti-TNF therapy, there may be benefits for subgroups. Proactive TDM with point-of-care testing of drug levels may be helpful during induction therapy in patients with a high inflammatory burden, which results in uncontrolled drug loss via the intestine. Proactive TDM during maintenance therapy (for example, every 6-12 months) may be beneficial in subgroups of patients at risk for developing low anti-TNF levels or anti-drug antibodies, such as patients with a genetic predisposition to anti-TNF anti-drug antibody formation (such as the HLA-DQ1*05 allele), patients on a second anti-TNF therapy after loss of response to the first one, and patients on anti-TNF therapy in combination with thiopurines or methotrexate who deescalate to anti-TNF monotherapy.
In summary, there is no doubt that proactive TDM is better than no TDM (meaning no drug adjustments at all). However, nearly all controlled prospective studies show no significant benefit of proactive TDM versus reactive TDM or drug escalation based on clinical symptoms or biomarkers. Future studies should target clearly defined patient groups at risk of losing response to anti-TNF to clarify if proactive TDM is a valuable tool to achieving better therapeutic results in clinical practice.
Dr. Herfarth is professor of medicine and codirector of the UNC Multidisciplinary IBD Center at University of North Carolina at Chapel Hill. He reports serving as a consultant to Alivio Therapeutics, AMAG, Bristol Myers Squibb, Boehringer Ingleheim, ExeGi Pharma, Finch, Gilead, Janssen, Lycera, Merck, Otsuka, Pfizer, PureTech, and Seres and receiving research support from Allakos, Artizan Biosciences, and Pfizer.
Relevant resources
- Syversen SW et al. JAMA. 2021;326:2375-84.
- Strik AS et al. Scand J Gastroenterol. 2021 Feb;56(2):145-54.
- Bossuyt P et al. J Crohns Colitis. 2022 Feb 23;16(2):199-206.
- D’Haens G et al. Gastroenterology. 2018;154:1343-51.e1.
Dear colleagues,
We shift gears from discussing GI hospitalists to focusing on the treatment of inflammatory bowel disease. The introduction of anti-TNFs brought about a paradigm shift in IBD management. With the ability to measure drug and antibody levels, we are also able to alter dose and timing to increase the efficacy of these medications. Some experts have extended this reactive drug monitoring approach to a more proactive method with the expectation that this may prevent loss of efficacy and development of adverse events. Dr. Loren G. Rabinowitz and colleagues and Dr. Hans Herfarth describe these two approaches to anti-TNF management in IBD, drawing from the current data and their own experiences. I look forward to hearing your thoughts and experiences by email (ginews@gastro.org).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
Better outcomes than reactive TDM
By Loren G. Rabinowitz, MD; Konstantinos Papamichael, PhD, MD; and Adam S. Cheifetz, MD, AGAF
Therapeutic drug monitoring (TDM), or the practice of treatment optimization based on serum drug concentrations, is used in many settings, including solid organ transplantation, infection, and immune-mediated inflammatory diseases, including inflammatory bowel disease (IBD). In IBD, the use of TDM has been an area of keen research focus, and, in our view, should be standard practice for optimization of biologic therapy, particularly in the setting of anti–tumor necrosis factor (TNF) therapy. TDM has demonstrated utility in determining the correct timing and dosage of biologics and can provide the impetus for deescalating or discontinuing a biologic in favor of an alternative one. It also allows prescribers the ability to protect patients from severe infusion reactions if they have developed anti-drug antibodies (ADAs).
Reactive TDM refers to a strategy of assessing drug concentration and presence of ADAs in the setting of primary nonresponse (PNR) and loss of response (LOR) to a biologic agent. In this context, TDM informs possible reasons for loss or lack of response to treatment – for example, insufficient drug concentration or the development of high-titer ADAs (immunogenicity) – thus better directing the management of these unwanted outcomes.1 Insufficient anti-TNF concentrations have been associated with PNR and lack of clinical remission at 1 year in patients with IBD,2 which underscores the need for a durable strategy to ensure appropriate drug concentrations from the induction through maintenance phases of biologic administration. Reactive TDM can also be used to inform the decision to abandon a particular therapy in favor of a different biologic and to guide the selection of the next biologic agent, and has been shown to be less expensive than empiric dose escalation.2 With regard to infliximab and adalimumab, it is our practice to continue dose escalation until drug concentrations are above 10-15 mcg/mL prior to abandoning therapy.1
For a significant number of patients, reactive TDM identifies at-risk patients too late, when ADAs have already formed. Because the number of medications to treat IBD remains limited, waiting for a patient to lose response to an agent, particularly anti-TNF therapies, increases the likelihood of immunogenicity, thus rendering an agent unusable. Proactive TDM or checking drug trough concentrations preemptively and at predetermined intervals, and dosing to an appropriate concentration, can improve patient outcomes. If drug concentration is determined to be not “at target,” dosage and timing of administration can be increased with or without the addition of an immunomodulator (thiopurines or methotrexate) to optimize the biologic’s efficacy and prevent immunogenicity. This approach allows the provider to anticipate and proactively guard against PNR and future LOR.
Proactive TDM of anti-TNF therapy has been associated with better patient outcomes in both pediatric and adult populations when compared with empiric dose optimization and/or reactive TDM.2,3 In patients with immune-mediated inflammatory diseases undergoing maintenance therapy with infliximab, proactive TDM was found to be more effective than treatment without TDM in sustaining disease control without disease worsening.4 Proactive TDM has been associated with better patient outcomes, including increased rates of clinical remission in both pediatric and adult populations and decreased rates of IBD-related surgery, hospitalization, serious infusion reactions, and development of ADAs, when compared with reactive TDM or empiric optimization. Preliminary data suggest that proactive TDM can also be used to efficiently guide dose deescalation in patients in remission with drug concentrations markedly above target and to allow for optimization of infliximab monotherapy so that combination therapy can be employed more judiciously (that is, in a patient who developed rapid ADA to a different anti-TNF).1 This could potentially attenuate the risks associated with long-term immunomodulator use, which include lymphomas and higher rates of serious and opportunistic infections. A recent study using a pharmacokinetic dashboard showed that the majority of patients with IBD will need accelerated dosing by the third infusion to maintain therapeutic infliximab concentrations during induction and maintenance therapy, highlighting the urgent need for widespread adoption of early proactive TDM.5 It is likely that proactive TDM is most important early in therapy when patients are most inflamed and have more rapid drug clearance. For this reason, proactive TDM should ideally be used for all patients during the induction phase. It is our practice to continue to follow drug concentrations one or two times per year once a patient has achieved remission. A recent literature review and consensus statement highlights the utility of TDM and what is known at this time.1
TDM should be standard of care for patients with IBD. At minimum, reactive TDM has rationalized the management of PNR, is associated with better outcomes, and is more cost effective than empiric dose escalation.1,2 In this setting, however, many patients have already developed ADAs that cannot be overcome. At present, anti-TNF therapy remains the most effective agent for our sickest patients with IBD. Given the as-yet limited armamentarium of medications available, particularly for patients with fistulizing perianal Crohn’s disease (CD) and severe ulcerative colitis (UC), proactive TDM, which allows for improved optimization and long-term durability of biologics, is essential to the care of any IBD patient requiring these medications. Proactive TDM should ideally be used for all patients during the induction phase and at least once during maintenance therapy. There is also the potential for TDM-driven dose de-escalation for patients in remission and optimization of infliximab monotherapy, thus avoiding combination therapy with an immunomodulator in some cases. Future perspectives for a more precise application of TDM include the use of pharmacokinetic modeling dashboards and pharmacogenetics toward achieving truly individualized medicine.3
Dr. Rabinowitz, Dr. Papamichael, and Dr. Cheifetz are with the department of medicine and division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Rabinowitz reports no conflicts of interest. Dr. Papamichael reports lecture fees from Mitsubishi Tanabe Pharma and Physicians’ Education Resource; consultancy fee from Prometheus Laboratories; and scientific advisory board fees from ProciseDx and Scipher Medicine Corporation. Dr. Cheifetz reports consulting for Janssen, AbbVie, Samsung, Arena Pharmaceuticals, Grifols, Prometheus, Bristol Myers Squibb, Artizan Biosciences, Artugan Therapeutics, and Equillium.
References
1. Cheifetz AS et al. Am J Gastroenterol. 2021 Oct 1;116(10):2014-25.
2. Kennedy NA et al. Lancet Gastroenterol Hepatol. 2019 May;4(5):341-53.
3. Papamichael K et al. Lancet Gastroenterol Hepatol. 2022;7(2):171-85.
4. Syversen SW et al. JAMA. 2021;326(23):2375-84.
5. Dubinsky MC et al. Inflamm Bowel Dis. 2022 Jan 3. doi: 10.1093/ibd/izab285.
Taking a closer look at the evidence
By Hans Herfarth, MD, PhD, AGAF
The debate of therapeutic drug monitoring (TDM) in the setting of anti–tumor necrosis factor (TNF) therapy has been ongoing for more than a decade. Reactive TDM, the measurement of drug concentrations in the context of loss of treatment response, is now generally accepted and recommended in multiple national and international inflammatory bowel disease (IBD) guidelines. Proactive TDM, defined as the systematic measurement of drug trough concentrations and anti-drug antibodies with dose adaptations to a predefined target drug concentration, seems to offer a possibility to stabilize drug levels and prevent anti-drug antibody formation due to low systemic drug levels, thus potentially preventing the well-known loss of response to anti-TNF therapy, which occurs in more than 50% of patients over time. However, proactive TDM is not endorsed by evidence-based guidelines, dividing IBD physicians into believers and nonbelievers and limiting uptake into clinical practice.
As with reactive TDM, one should assume that the framework for proactive TDM should have been reliably established based on factual data derived from prospective controlled studies and not rely on retrospective cohorts or “Expert Panel” consensus statements. And indeed, several prospective controlled studies with sizable IBD patient cohorts have been published. Of note, all TDM studies for IBD were conducted in patients on anti-TNF maintenance therapy, and currently no prospective studies in larger IBD populations are available for proactive TDM during induction therapy. Two prospective studies, the PRECISION and the NOR-DRUM trial, report that proactive TDM is better than no TDM at all.
However, in the comparison of proactive TDM and reactive TDM (including at least one drug adaptation in maintenance or drug escalation based on clinical symptoms or biomarkers), three studies have demonstrated no significant differences in drug persistence or overall maintenance of clinical remission. Only a fourth (the pediatric PAILOT study) reported a lower frequency of mild flares and less steroid exposure in the proactive TDM arm over 1 year, but it did not show differences in drug persistence or overall clinical remission compared with the reactive TDM arm. Interestingly, the differences in flare frequency were apparent only in patients on monotherapy but not in the subgroup on combination therapy with an immunomodulator, stressing the well-known beneficial effect of a combination therapy with thiopurines in CD first shown in the SONIC trial.
One problem, at least in the TDM trials with infliximab (IFX), may have been a delay in optimizing IFX levels until the next drug infusion because of the turnaround time of the drug assays. However, even the most recently published ultra-proactive TDM study with ad hoc dose adjustments based on
The value of proactive TDM in induction therapy remains an ongoing concern. There is no doubt that the severity of intestinal inflammation with subsequent loss of drug in the intestine can result in low drug serum concentrations correlating to lower clinical responses and higher rates of immunogenicity with the formation of anti-drug antibodies. A recent study including patients with chronic immune-mediated inflammatory diseases such as IBD, rheumatoid arthritis, and psoriasis did not find a value in proactive TDM of IFX in the induction phase, but more severe IBD may have been underrepresented in this study. Administration of significantly higher induction dosing of adalimumab (160 mg weeks 0, 1, 2, and 3) with significantly higher trough levels compared with a standard induction regimen in the SERENE study has not been shown to increase the short- or long-term remission rates in UC or CD patients. Therefore, higher trough levels in a patient population do not automatically result in better outcomes, but proactive TDM may still have found a few patients who may have benefited from an even higher induction regimen. The UC and CD SERENE maintenance studies also evaluated proactive TDM versus clinical adjustment based on clinical and biomarkers. After 1 year, no differences in the efficacy endpoints of clinical, endoscopic, and deep remission were found. These somewhat surprising results, which have been reported only in meeting abstracts, suggest that simply increasing trough levels to a higher target (one of the primary aims of proactive TDM) is not an effective universal approach for achieving higher remission rates in induction or better outcomes in maintenance. Instead, the SERENE data show that similar results can be achieved by regular clinical follow-up and monitoring of loss of response based on symptoms and/or biochemical markers followed by drug adaptation (which may then also be based on reactive TDM).
One unquestionable effect of proactive TDM is that the process of checking and controlling drug levels suggests for the treating physician better control over the anti-TNF therapy and for the patient reassurance that the treatment is in the intended target range. Proactive TDM also may be cost effective in the group of patients whose anti-TNF treatment regimen can be deescalated because of high drug levels. Despite the increased number of studies showing no clinical advantage of proactive TDM of every patient on anti-TNF therapy, there may be benefits for subgroups. Proactive TDM with point-of-care testing of drug levels may be helpful during induction therapy in patients with a high inflammatory burden, which results in uncontrolled drug loss via the intestine. Proactive TDM during maintenance therapy (for example, every 6-12 months) may be beneficial in subgroups of patients at risk for developing low anti-TNF levels or anti-drug antibodies, such as patients with a genetic predisposition to anti-TNF anti-drug antibody formation (such as the HLA-DQ1*05 allele), patients on a second anti-TNF therapy after loss of response to the first one, and patients on anti-TNF therapy in combination with thiopurines or methotrexate who deescalate to anti-TNF monotherapy.
In summary, there is no doubt that proactive TDM is better than no TDM (meaning no drug adjustments at all). However, nearly all controlled prospective studies show no significant benefit of proactive TDM versus reactive TDM or drug escalation based on clinical symptoms or biomarkers. Future studies should target clearly defined patient groups at risk of losing response to anti-TNF to clarify if proactive TDM is a valuable tool to achieving better therapeutic results in clinical practice.
Dr. Herfarth is professor of medicine and codirector of the UNC Multidisciplinary IBD Center at University of North Carolina at Chapel Hill. He reports serving as a consultant to Alivio Therapeutics, AMAG, Bristol Myers Squibb, Boehringer Ingleheim, ExeGi Pharma, Finch, Gilead, Janssen, Lycera, Merck, Otsuka, Pfizer, PureTech, and Seres and receiving research support from Allakos, Artizan Biosciences, and Pfizer.
Relevant resources
- Syversen SW et al. JAMA. 2021;326:2375-84.
- Strik AS et al. Scand J Gastroenterol. 2021 Feb;56(2):145-54.
- Bossuyt P et al. J Crohns Colitis. 2022 Feb 23;16(2):199-206.
- D’Haens G et al. Gastroenterology. 2018;154:1343-51.e1.
Stress and infertility – is it a proven cause and effect?
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.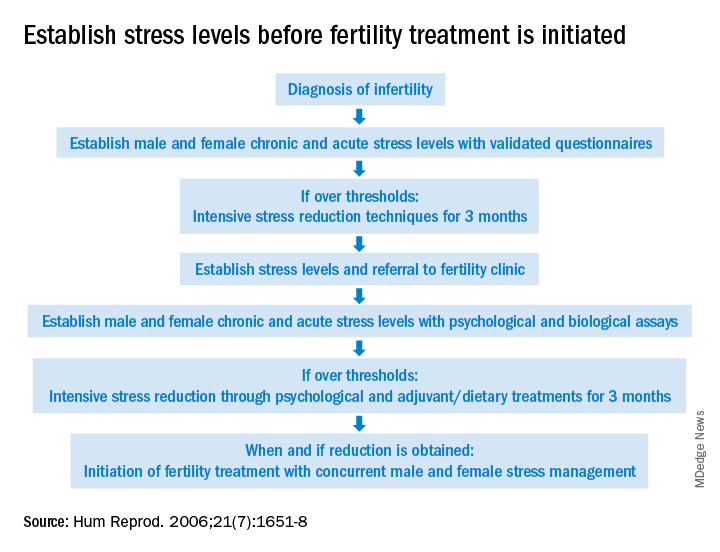
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
ACIP 2022 child/adolescent immunization schedule: What’s new?
On Feb. 17, 2022, the updated Recommended Childhood and Adolescent Immunization Schedule was released by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention. Pediatric providers across the country eagerly await this annual update to learn what changes lie in store for recommended immunization practices. During the week that has gone by since the 2022 release, I’ve had a chance to reflect on some of the highlights that are worth noting.
The SARS-CoV-2 (COVID-19) vaccines are not on the schedule yet, undoubtedly because of the preliminary nature of the vaccine data for children and the emergency use authorization vaccine status. We currently have interim recommendations for childhood COVID-19 vaccines.
Brand new in 2022
Two new items in the 2022 schedules are worth reviewing. The first is an entirely new recommendation to administer dengue vaccine to children aged 9-16 years living in endemic areas, but only if they already have laboratory-confirmed past dengue infection. For U.S. practitioners, the endemic areas to remember are Puerto Rico and the U.S. Virgin islands in the Caribbean, as well as Pacific island areas, such as the Marshall Islands, Palau, and the Federated States of Micronesia. There is a link in the document to additional recommendations.
The second totally new item is the combination preparation, Vaxelis, which contains DTaP, inactivated poliovirus, Haemophilus influenzae b conjugate, and hepatitis B vaccines. There are extensive recommendations for how to work it into the vaccine schedule, including some situations when it should not be used.
Selected reminders in childhood immunization
I’ll start with some key reminders about what not to do. Remember that the live inactivated influenza virus vaccine (LAIV) is recommended to begin only at age 2 years and older, compared with the inactivated influenza vaccine, which begins at 6 months. In addition, LAIV is contraindicated in patients aged 2-4 years who have a history of asthma or wheezing. Remember to avoid live virus vaccines, such as LAIV, MMR, and varicella, during pregnancy but be ready to administer those vaccines right after delivery. Similarly, HPV vaccine should be delayed until after pregnancy.
There are many special situation recommendations; I’ll highlight only a few here. One reminder is that although MMR and hepatitis A are both recommended to begin at 12 months, infants aged 6-11 months who are undergoing international travel to high-risk areas can begin with one dose before departure and then receive a two-dose series after turning 12 months of age.
Pneumococcal vaccination. Some children should receive both the pneumococcal conjugate vaccine (PCV13) and the pneumococcal polysaccharide vaccine (PPSV23). Those groups include children with chronic heart disease, chronic lung disease, diabetes, cerebral spinal fluid leaks or cochlear implants, and sickle cell disease, as well as many other immunocompromising conditions. Kids who need both preparations should receive the conjugate vaccine first, but they should never receive the conjugate vaccine and the polysaccharide vaccine at the same visit.
Meningococcal vaccination. Meningococcal vaccine special situations can be quite complicated. For meningococcus A,C,W,Y (MenACWY) vaccination, children with immunocompromising conditions should receive different schedules from those of typical children, but the recommendations vary by preparation.
For adolescents aged 16-23 years, the decision whether to administer the meningococcal serogroup B (MenB) vaccine is based on shared clinical decision-making, a recommendation that began in 2020. Patients with certain immunocompromising conditions are considered at higher risk and should more routinely receive MenB vaccination, with recommendations varying depending on the preparation utilized. The MenB preparations are not interchangeable. In addition, patients may receive both MenACWY and MenB vaccines on the same day, but they should be given at different body sites.
A few final reminders
In certain cases, you might avoid administering what would otherwise be routine vaccinations. For example, the rotavirus series should not begin if the infant is aged 15 weeks or older. Only one dose of Haemophilus influenzae b vaccine is indicated after age 15 months and none at 60 months or older if the child does not have high-risk conditions.
Finally, the total number of doses for some vaccines, such as pneumococcus and polio, vary depending on how old the child is if not already fully vaccinated. For example, for pneumococcal conjugate vaccine catch-up in a healthy child, one dose after age 24 months would bring the child up to date. For inactivated poliovirus in children aged 4 years or older, a third dose given at least 6 months after the second dose would bring that child up to date.
The tables can be a challenge to interpret, but fortunately simpler tables for parents are available. These make excellent handouts to have available in the office!
Dr. Basco is professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
On Feb. 17, 2022, the updated Recommended Childhood and Adolescent Immunization Schedule was released by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention. Pediatric providers across the country eagerly await this annual update to learn what changes lie in store for recommended immunization practices. During the week that has gone by since the 2022 release, I’ve had a chance to reflect on some of the highlights that are worth noting.
The SARS-CoV-2 (COVID-19) vaccines are not on the schedule yet, undoubtedly because of the preliminary nature of the vaccine data for children and the emergency use authorization vaccine status. We currently have interim recommendations for childhood COVID-19 vaccines.
Brand new in 2022
Two new items in the 2022 schedules are worth reviewing. The first is an entirely new recommendation to administer dengue vaccine to children aged 9-16 years living in endemic areas, but only if they already have laboratory-confirmed past dengue infection. For U.S. practitioners, the endemic areas to remember are Puerto Rico and the U.S. Virgin islands in the Caribbean, as well as Pacific island areas, such as the Marshall Islands, Palau, and the Federated States of Micronesia. There is a link in the document to additional recommendations.
The second totally new item is the combination preparation, Vaxelis, which contains DTaP, inactivated poliovirus, Haemophilus influenzae b conjugate, and hepatitis B vaccines. There are extensive recommendations for how to work it into the vaccine schedule, including some situations when it should not be used.
Selected reminders in childhood immunization
I’ll start with some key reminders about what not to do. Remember that the live inactivated influenza virus vaccine (LAIV) is recommended to begin only at age 2 years and older, compared with the inactivated influenza vaccine, which begins at 6 months. In addition, LAIV is contraindicated in patients aged 2-4 years who have a history of asthma or wheezing. Remember to avoid live virus vaccines, such as LAIV, MMR, and varicella, during pregnancy but be ready to administer those vaccines right after delivery. Similarly, HPV vaccine should be delayed until after pregnancy.
There are many special situation recommendations; I’ll highlight only a few here. One reminder is that although MMR and hepatitis A are both recommended to begin at 12 months, infants aged 6-11 months who are undergoing international travel to high-risk areas can begin with one dose before departure and then receive a two-dose series after turning 12 months of age.
Pneumococcal vaccination. Some children should receive both the pneumococcal conjugate vaccine (PCV13) and the pneumococcal polysaccharide vaccine (PPSV23). Those groups include children with chronic heart disease, chronic lung disease, diabetes, cerebral spinal fluid leaks or cochlear implants, and sickle cell disease, as well as many other immunocompromising conditions. Kids who need both preparations should receive the conjugate vaccine first, but they should never receive the conjugate vaccine and the polysaccharide vaccine at the same visit.
Meningococcal vaccination. Meningococcal vaccine special situations can be quite complicated. For meningococcus A,C,W,Y (MenACWY) vaccination, children with immunocompromising conditions should receive different schedules from those of typical children, but the recommendations vary by preparation.
For adolescents aged 16-23 years, the decision whether to administer the meningococcal serogroup B (MenB) vaccine is based on shared clinical decision-making, a recommendation that began in 2020. Patients with certain immunocompromising conditions are considered at higher risk and should more routinely receive MenB vaccination, with recommendations varying depending on the preparation utilized. The MenB preparations are not interchangeable. In addition, patients may receive both MenACWY and MenB vaccines on the same day, but they should be given at different body sites.
A few final reminders
In certain cases, you might avoid administering what would otherwise be routine vaccinations. For example, the rotavirus series should not begin if the infant is aged 15 weeks or older. Only one dose of Haemophilus influenzae b vaccine is indicated after age 15 months and none at 60 months or older if the child does not have high-risk conditions.
Finally, the total number of doses for some vaccines, such as pneumococcus and polio, vary depending on how old the child is if not already fully vaccinated. For example, for pneumococcal conjugate vaccine catch-up in a healthy child, one dose after age 24 months would bring the child up to date. For inactivated poliovirus in children aged 4 years or older, a third dose given at least 6 months after the second dose would bring that child up to date.
The tables can be a challenge to interpret, but fortunately simpler tables for parents are available. These make excellent handouts to have available in the office!
Dr. Basco is professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
On Feb. 17, 2022, the updated Recommended Childhood and Adolescent Immunization Schedule was released by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention. Pediatric providers across the country eagerly await this annual update to learn what changes lie in store for recommended immunization practices. During the week that has gone by since the 2022 release, I’ve had a chance to reflect on some of the highlights that are worth noting.
The SARS-CoV-2 (COVID-19) vaccines are not on the schedule yet, undoubtedly because of the preliminary nature of the vaccine data for children and the emergency use authorization vaccine status. We currently have interim recommendations for childhood COVID-19 vaccines.
Brand new in 2022
Two new items in the 2022 schedules are worth reviewing. The first is an entirely new recommendation to administer dengue vaccine to children aged 9-16 years living in endemic areas, but only if they already have laboratory-confirmed past dengue infection. For U.S. practitioners, the endemic areas to remember are Puerto Rico and the U.S. Virgin islands in the Caribbean, as well as Pacific island areas, such as the Marshall Islands, Palau, and the Federated States of Micronesia. There is a link in the document to additional recommendations.
The second totally new item is the combination preparation, Vaxelis, which contains DTaP, inactivated poliovirus, Haemophilus influenzae b conjugate, and hepatitis B vaccines. There are extensive recommendations for how to work it into the vaccine schedule, including some situations when it should not be used.
Selected reminders in childhood immunization
I’ll start with some key reminders about what not to do. Remember that the live inactivated influenza virus vaccine (LAIV) is recommended to begin only at age 2 years and older, compared with the inactivated influenza vaccine, which begins at 6 months. In addition, LAIV is contraindicated in patients aged 2-4 years who have a history of asthma or wheezing. Remember to avoid live virus vaccines, such as LAIV, MMR, and varicella, during pregnancy but be ready to administer those vaccines right after delivery. Similarly, HPV vaccine should be delayed until after pregnancy.
There are many special situation recommendations; I’ll highlight only a few here. One reminder is that although MMR and hepatitis A are both recommended to begin at 12 months, infants aged 6-11 months who are undergoing international travel to high-risk areas can begin with one dose before departure and then receive a two-dose series after turning 12 months of age.
Pneumococcal vaccination. Some children should receive both the pneumococcal conjugate vaccine (PCV13) and the pneumococcal polysaccharide vaccine (PPSV23). Those groups include children with chronic heart disease, chronic lung disease, diabetes, cerebral spinal fluid leaks or cochlear implants, and sickle cell disease, as well as many other immunocompromising conditions. Kids who need both preparations should receive the conjugate vaccine first, but they should never receive the conjugate vaccine and the polysaccharide vaccine at the same visit.
Meningococcal vaccination. Meningococcal vaccine special situations can be quite complicated. For meningococcus A,C,W,Y (MenACWY) vaccination, children with immunocompromising conditions should receive different schedules from those of typical children, but the recommendations vary by preparation.
For adolescents aged 16-23 years, the decision whether to administer the meningococcal serogroup B (MenB) vaccine is based on shared clinical decision-making, a recommendation that began in 2020. Patients with certain immunocompromising conditions are considered at higher risk and should more routinely receive MenB vaccination, with recommendations varying depending on the preparation utilized. The MenB preparations are not interchangeable. In addition, patients may receive both MenACWY and MenB vaccines on the same day, but they should be given at different body sites.
A few final reminders
In certain cases, you might avoid administering what would otherwise be routine vaccinations. For example, the rotavirus series should not begin if the infant is aged 15 weeks or older. Only one dose of Haemophilus influenzae b vaccine is indicated after age 15 months and none at 60 months or older if the child does not have high-risk conditions.
Finally, the total number of doses for some vaccines, such as pneumococcus and polio, vary depending on how old the child is if not already fully vaccinated. For example, for pneumococcal conjugate vaccine catch-up in a healthy child, one dose after age 24 months would bring the child up to date. For inactivated poliovirus in children aged 4 years or older, a third dose given at least 6 months after the second dose would bring that child up to date.
The tables can be a challenge to interpret, but fortunately simpler tables for parents are available. These make excellent handouts to have available in the office!
Dr. Basco is professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The best crystalloid for the critically ill
Hemodynamic instability is rewarded with a sojourn in the intensive care unit (ICU). When the intensivists see it, they’re going to throw fluids at it. Most likely a crystalloid of some type. This has been true for decades, centuries even. When I was a medical student, which was decades but not centuries ago, I used crystalloids every day on the surgical wards, in the operating room, in the emergency department, or on the medicine wards. Medicine docs preferred normal saline (NS) and surgeons used lactated Ringer’s solution (LR). I never gave this a second thought.
During medical school, I was drawn to internal medicine by the heavy emphasis on evidence-based medicine in the field. Prior to 2015 though, there wasn’t much data to support using one crystalloid formulation over another. Pre-2010, we had an American Thoracic Society (ATS) consensus statement on using crystalloid vs. colloid, making recommendations largely drawn from the SAFE trial. The ATS statement also suggested starches may be harmful, a view that was confirmed in a series of articles published in 2012 and 2013. There was less discussion about what type of crystalloid was best.
In 2014, I finally read a paper that compared crystalloid formulations. It was a network meta-analysis, which is “statistician speak” for combining disparate trials to make indirect comparisons. In the absence of large, randomized trials, this approach was a welcome addition to the data we had at the time. The authors concluded that “balanced” (typically LR or Plasma-Lyte) are superior to “unbalanced” (another term for NS) crystalloids. Balanced fluids typically have acetate or lactate and have a higher pH and lower chloride than NS. I found the signal for balanced fluids interesting at the time but promptly forgot about it.
Since 2015, the critical care community has rallied to produce a bevy of large trials comparing balanced vs. unbalanced crystalloids. The first was the SPLIT trial, which showed equivalence. Then came the SMART trial in 2018, which showed balanced fluids were better. Of note, another trial with an identical design (SALT-ED) was published in the same issue of The New England Journal of Medicine as SMART. SALT-ED enrolled patients in the emergency department, not the ICU, but also found benefit to using balanced fluids, albeit not for their primary outcome. I admit, after SMART and SALT-ED were published, I made the switch to LR. A secondary analysis of patients with sepsis pushed me further toward LR, while others withheld judgment.
Then we saw publication of the BaSICs trial, another large, randomized study evaluating crystalloid composition. I was hoping this one might put the issue to rest. That nephrologist who perseverated on every patient’s chloride during morning report would be vindicated. NS would prove to be too unbalanced and would finally be retired. No such luck. This is critical care medicine, where the initial signal is rarely confirmed in the follow-up trials. BaSICs found no difference between crystalloids for most important outcomes. The study did find balanced fluids may worsen outcomes for patients with head injuries.
Finally, there’s the PLUS trial, a large, multicenter randomized controlled trial comparing Plasma-Lyte vs. NS in the ICU. I could make the argument that this trial was the best of the bunch, and it was negative. The researchers did an excellent job of showing that serum pH and chloride levels did vary by fluid composition, but despite this, mortality and renal outcomes did not differ. Case closed? Crystalloid composition doesn’t matter, right?
An editorial that accompanies the BaSICs trial does an outstanding job of reviewing SPLIT, SMART, and BaSICs. The authors discuss design and population differences that may have led to differing results, and there are many. They conclude for most patients in the ICU, there’s no compelling reason to choose one crystalloid over another. Perhaps they’re right.
An updated meta-analysis that included all the studies I’ve mentioned concluded there was an 89% probability that balanced fluid reduces mortality for ICU patients. How could the meta-analysis authors reach this conclusion given all the negative trials? It has to do with their statistical methods – they performed both standard, frequentist (if statistical significance isn’t reached the study, is considered negative) and Bayesian analyses (posterior probability of benefit is calculated, regardless of P value). The frequentist approach was negative, but the posterior probability for benefit remained high.
Personally, I see no reason not to favor LR when resuscitating ICU patients without head injuries. In particular, it seems that medical patients (who made up almost 80% of those in the SMART trial) and those with sepsis may benefit. The critical care community has again outdone itself by performing large, well-designed trials to address important questions. Despite not having a definitive answer on crystalloid resuscitation, we know a lot more than we did when I was a medical student.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He reported receiving research grant from: Fisher-Paykel and receiving income from the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
Hemodynamic instability is rewarded with a sojourn in the intensive care unit (ICU). When the intensivists see it, they’re going to throw fluids at it. Most likely a crystalloid of some type. This has been true for decades, centuries even. When I was a medical student, which was decades but not centuries ago, I used crystalloids every day on the surgical wards, in the operating room, in the emergency department, or on the medicine wards. Medicine docs preferred normal saline (NS) and surgeons used lactated Ringer’s solution (LR). I never gave this a second thought.
During medical school, I was drawn to internal medicine by the heavy emphasis on evidence-based medicine in the field. Prior to 2015 though, there wasn’t much data to support using one crystalloid formulation over another. Pre-2010, we had an American Thoracic Society (ATS) consensus statement on using crystalloid vs. colloid, making recommendations largely drawn from the SAFE trial. The ATS statement also suggested starches may be harmful, a view that was confirmed in a series of articles published in 2012 and 2013. There was less discussion about what type of crystalloid was best.
In 2014, I finally read a paper that compared crystalloid formulations. It was a network meta-analysis, which is “statistician speak” for combining disparate trials to make indirect comparisons. In the absence of large, randomized trials, this approach was a welcome addition to the data we had at the time. The authors concluded that “balanced” (typically LR or Plasma-Lyte) are superior to “unbalanced” (another term for NS) crystalloids. Balanced fluids typically have acetate or lactate and have a higher pH and lower chloride than NS. I found the signal for balanced fluids interesting at the time but promptly forgot about it.
Since 2015, the critical care community has rallied to produce a bevy of large trials comparing balanced vs. unbalanced crystalloids. The first was the SPLIT trial, which showed equivalence. Then came the SMART trial in 2018, which showed balanced fluids were better. Of note, another trial with an identical design (SALT-ED) was published in the same issue of The New England Journal of Medicine as SMART. SALT-ED enrolled patients in the emergency department, not the ICU, but also found benefit to using balanced fluids, albeit not for their primary outcome. I admit, after SMART and SALT-ED were published, I made the switch to LR. A secondary analysis of patients with sepsis pushed me further toward LR, while others withheld judgment.
Then we saw publication of the BaSICs trial, another large, randomized study evaluating crystalloid composition. I was hoping this one might put the issue to rest. That nephrologist who perseverated on every patient’s chloride during morning report would be vindicated. NS would prove to be too unbalanced and would finally be retired. No such luck. This is critical care medicine, where the initial signal is rarely confirmed in the follow-up trials. BaSICs found no difference between crystalloids for most important outcomes. The study did find balanced fluids may worsen outcomes for patients with head injuries.
Finally, there’s the PLUS trial, a large, multicenter randomized controlled trial comparing Plasma-Lyte vs. NS in the ICU. I could make the argument that this trial was the best of the bunch, and it was negative. The researchers did an excellent job of showing that serum pH and chloride levels did vary by fluid composition, but despite this, mortality and renal outcomes did not differ. Case closed? Crystalloid composition doesn’t matter, right?
An editorial that accompanies the BaSICs trial does an outstanding job of reviewing SPLIT, SMART, and BaSICs. The authors discuss design and population differences that may have led to differing results, and there are many. They conclude for most patients in the ICU, there’s no compelling reason to choose one crystalloid over another. Perhaps they’re right.
An updated meta-analysis that included all the studies I’ve mentioned concluded there was an 89% probability that balanced fluid reduces mortality for ICU patients. How could the meta-analysis authors reach this conclusion given all the negative trials? It has to do with their statistical methods – they performed both standard, frequentist (if statistical significance isn’t reached the study, is considered negative) and Bayesian analyses (posterior probability of benefit is calculated, regardless of P value). The frequentist approach was negative, but the posterior probability for benefit remained high.
Personally, I see no reason not to favor LR when resuscitating ICU patients without head injuries. In particular, it seems that medical patients (who made up almost 80% of those in the SMART trial) and those with sepsis may benefit. The critical care community has again outdone itself by performing large, well-designed trials to address important questions. Despite not having a definitive answer on crystalloid resuscitation, we know a lot more than we did when I was a medical student.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He reported receiving research grant from: Fisher-Paykel and receiving income from the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
Hemodynamic instability is rewarded with a sojourn in the intensive care unit (ICU). When the intensivists see it, they’re going to throw fluids at it. Most likely a crystalloid of some type. This has been true for decades, centuries even. When I was a medical student, which was decades but not centuries ago, I used crystalloids every day on the surgical wards, in the operating room, in the emergency department, or on the medicine wards. Medicine docs preferred normal saline (NS) and surgeons used lactated Ringer’s solution (LR). I never gave this a second thought.
During medical school, I was drawn to internal medicine by the heavy emphasis on evidence-based medicine in the field. Prior to 2015 though, there wasn’t much data to support using one crystalloid formulation over another. Pre-2010, we had an American Thoracic Society (ATS) consensus statement on using crystalloid vs. colloid, making recommendations largely drawn from the SAFE trial. The ATS statement also suggested starches may be harmful, a view that was confirmed in a series of articles published in 2012 and 2013. There was less discussion about what type of crystalloid was best.
In 2014, I finally read a paper that compared crystalloid formulations. It was a network meta-analysis, which is “statistician speak” for combining disparate trials to make indirect comparisons. In the absence of large, randomized trials, this approach was a welcome addition to the data we had at the time. The authors concluded that “balanced” (typically LR or Plasma-Lyte) are superior to “unbalanced” (another term for NS) crystalloids. Balanced fluids typically have acetate or lactate and have a higher pH and lower chloride than NS. I found the signal for balanced fluids interesting at the time but promptly forgot about it.
Since 2015, the critical care community has rallied to produce a bevy of large trials comparing balanced vs. unbalanced crystalloids. The first was the SPLIT trial, which showed equivalence. Then came the SMART trial in 2018, which showed balanced fluids were better. Of note, another trial with an identical design (SALT-ED) was published in the same issue of The New England Journal of Medicine as SMART. SALT-ED enrolled patients in the emergency department, not the ICU, but also found benefit to using balanced fluids, albeit not for their primary outcome. I admit, after SMART and SALT-ED were published, I made the switch to LR. A secondary analysis of patients with sepsis pushed me further toward LR, while others withheld judgment.
Then we saw publication of the BaSICs trial, another large, randomized study evaluating crystalloid composition. I was hoping this one might put the issue to rest. That nephrologist who perseverated on every patient’s chloride during morning report would be vindicated. NS would prove to be too unbalanced and would finally be retired. No such luck. This is critical care medicine, where the initial signal is rarely confirmed in the follow-up trials. BaSICs found no difference between crystalloids for most important outcomes. The study did find balanced fluids may worsen outcomes for patients with head injuries.
Finally, there’s the PLUS trial, a large, multicenter randomized controlled trial comparing Plasma-Lyte vs. NS in the ICU. I could make the argument that this trial was the best of the bunch, and it was negative. The researchers did an excellent job of showing that serum pH and chloride levels did vary by fluid composition, but despite this, mortality and renal outcomes did not differ. Case closed? Crystalloid composition doesn’t matter, right?
An editorial that accompanies the BaSICs trial does an outstanding job of reviewing SPLIT, SMART, and BaSICs. The authors discuss design and population differences that may have led to differing results, and there are many. They conclude for most patients in the ICU, there’s no compelling reason to choose one crystalloid over another. Perhaps they’re right.
An updated meta-analysis that included all the studies I’ve mentioned concluded there was an 89% probability that balanced fluid reduces mortality for ICU patients. How could the meta-analysis authors reach this conclusion given all the negative trials? It has to do with their statistical methods – they performed both standard, frequentist (if statistical significance isn’t reached the study, is considered negative) and Bayesian analyses (posterior probability of benefit is calculated, regardless of P value). The frequentist approach was negative, but the posterior probability for benefit remained high.
Personally, I see no reason not to favor LR when resuscitating ICU patients without head injuries. In particular, it seems that medical patients (who made up almost 80% of those in the SMART trial) and those with sepsis may benefit. The critical care community has again outdone itself by performing large, well-designed trials to address important questions. Despite not having a definitive answer on crystalloid resuscitation, we know a lot more than we did when I was a medical student.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He reported receiving research grant from: Fisher-Paykel and receiving income from the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
Exploring the relationship of COVID-19 vaccines and fertility
Introduction
Amidst an aggressive vaccination campaign for COVID-19, misinformation has spread over the Internet, affecting public perception and making some people hesitant to participate in ongoing immunization campaigns. Of chief concern are issues pertaining to fertility or viability of sperm – information circulating on social networks posits that the coronavirus vaccine may influence infertility in men, which, according to physicians, is not grounded in reality. From the perspective of evidence-based medicine, there is a dearth of information suggesting an untoward effect of the vaccine on male fertility. The risk of adverse reactions arising from approved vaccines is negligible, with mild, albeit controllable, side effects demonstrated by patients in clinical trials. Therefore, there is no plausible reason for the general public to avoid vaccinations.1
Infertility following vaccination
The source of confusion can be traced back to a study conducted by researchers at the University of Miami Miller School of Medicine; the general public has conflated a side effect of the virus, namely, infertility and erectile dysfunction, with that of the vaccine.2 According to Ranjith Ramasamy, MD, director of the urology program at Miller, “We were the first to demonstrate that the COVID virus, itself, can affect male fertility and be a potential cause for erectile dysfunction. We are now the first to examine if there is any impact of the COVID vaccine on male fertility potential, which we did not find.”3
Coronavirus can indeed cause significant damage to the testicular tissue of infected men by means of mediating ACE2 expression on Leydig and Sertoli cells of the testis. It should be noted that COVID-19 may potentially attack any type of cell in the body that expresses the enzyme ACE2. However, it is particularly harmful to cells with high levels of expression of this enzyme, such as testicular cells. The spermatogenesis process can be affected, thereby posing a risk to male fertility.4
Expanding on the theme of fertility during the pandemic, a number of false claims5-7 about the vaccine and its overall effect on the placenta and fertility have also emerged as a contentious topic for debate on social media; doctors continue to explain why the theories are not reasonable or a cause for concern. The World Health Organization (WHO) provides recommendations on COVID-19 vaccinations for pregnant and/or lactating women and encourages a shared decision process involving risk/benefit assessment with the prescribing physician.5 Pregnant women, especially those with underlying comorbid conditions, are susceptible to developing severe symptom manifestations of COVID-19 with the disease also being associated with an increased likelihood of premature birth. As far as lactating women are concerned, the evidence thus far has indicated that the risk of side effects of the vaccine is very low, suggesting that these women could be vaccinated.5
The vaccine is the best option
While more studies are needed to ascertain the relationship between COVID-19 and male infertility, the vaccine is currently the best option for those who are concerned about their fertility from exposure to the coronavirus. Because of delayed wholesale acceptance of vaccines by the general population, clinicians should continue to emphasize the importance of preventive care with respect to disease exposure.6
In addition, those who are concerned with fertility can opt for ways to preserve their reproductive capacity, such as the removal of semen for freezing sperm, albeit with adherence to sperm-washing procedures to preclude cross-contamination from viruses.8,9 For the preservation of sperm, the noninvasive method is often performed, preferably collected in several samples. Then, the semen is cryopreserved.8 In some instances, the sperm can also be removed directly from the testicles with a simple needle or by means of a minor surgical procedure.
A wait and try approach is advocated by clinicians for individuals who have already experienced COVID-19 symptoms and are therefore concerned about the prospect of childbearing.10 If the couple is unable to conceive after a year of trying, it is recommended that they consult a reproductive specialist; the clinician can carry out a comprehensive evaluation and order a series of tests to identify the source of the problem, indicating whether there are alternative methods for helping the couple to start a family (addressing the underlying factors involved in infertility, or treating via assisted reproduction procedures, such as in vitro fertilization).11
Dr. Aman is faculty member at the biology department of City Colleges of Chicago, and a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF). She disclosed no relevant financial relationships. Dr. Islam is a medical writer for the IMCHF, Montreal, is based in New York, and disclosed no relevant financial relationships. Mr. Choudhry is a research assistant at the IMCHF and he has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. Berry SD et al. J Am Geriatr Soc. 2021 May;69(5):1140-6.
2. Achua JK et al. World J Men’s Health. 2021 Jan;39(1):65-74.
3. Broderick JM. Urology Times. 2021 June.
4. Huang C et al. Andrology. 2021 Jan;9(1):80-7.
5. Sajjadi NB et al. J Osteopath Med. 2021 Apr 12;121(6):583-7.
6. Sallam M et al. Vaccines. 2021 Jan;9(1):42.
7. Islam MS et al. PloS One. 2021 May 12;16(5):e0251605.
8. Tesarik J. J Fertil Preserv. 2021;2:art246111.
9. Adiga SK et al. Reprod BioMed Online. 2020 Dec;41(6):991-7.
10. FAQs related to COVID-19. Q: If I get sick or test positive for COVID-19, when is it safe to become pregnant? American Society for Reproductive Medicine.
11. Cross C. Wellness and Prevention: Why can’t I get pregnant? John Hopkins Medicine.
Introduction
Amidst an aggressive vaccination campaign for COVID-19, misinformation has spread over the Internet, affecting public perception and making some people hesitant to participate in ongoing immunization campaigns. Of chief concern are issues pertaining to fertility or viability of sperm – information circulating on social networks posits that the coronavirus vaccine may influence infertility in men, which, according to physicians, is not grounded in reality. From the perspective of evidence-based medicine, there is a dearth of information suggesting an untoward effect of the vaccine on male fertility. The risk of adverse reactions arising from approved vaccines is negligible, with mild, albeit controllable, side effects demonstrated by patients in clinical trials. Therefore, there is no plausible reason for the general public to avoid vaccinations.1
Infertility following vaccination
The source of confusion can be traced back to a study conducted by researchers at the University of Miami Miller School of Medicine; the general public has conflated a side effect of the virus, namely, infertility and erectile dysfunction, with that of the vaccine.2 According to Ranjith Ramasamy, MD, director of the urology program at Miller, “We were the first to demonstrate that the COVID virus, itself, can affect male fertility and be a potential cause for erectile dysfunction. We are now the first to examine if there is any impact of the COVID vaccine on male fertility potential, which we did not find.”3
Coronavirus can indeed cause significant damage to the testicular tissue of infected men by means of mediating ACE2 expression on Leydig and Sertoli cells of the testis. It should be noted that COVID-19 may potentially attack any type of cell in the body that expresses the enzyme ACE2. However, it is particularly harmful to cells with high levels of expression of this enzyme, such as testicular cells. The spermatogenesis process can be affected, thereby posing a risk to male fertility.4
Expanding on the theme of fertility during the pandemic, a number of false claims5-7 about the vaccine and its overall effect on the placenta and fertility have also emerged as a contentious topic for debate on social media; doctors continue to explain why the theories are not reasonable or a cause for concern. The World Health Organization (WHO) provides recommendations on COVID-19 vaccinations for pregnant and/or lactating women and encourages a shared decision process involving risk/benefit assessment with the prescribing physician.5 Pregnant women, especially those with underlying comorbid conditions, are susceptible to developing severe symptom manifestations of COVID-19 with the disease also being associated with an increased likelihood of premature birth. As far as lactating women are concerned, the evidence thus far has indicated that the risk of side effects of the vaccine is very low, suggesting that these women could be vaccinated.5
The vaccine is the best option
While more studies are needed to ascertain the relationship between COVID-19 and male infertility, the vaccine is currently the best option for those who are concerned about their fertility from exposure to the coronavirus. Because of delayed wholesale acceptance of vaccines by the general population, clinicians should continue to emphasize the importance of preventive care with respect to disease exposure.6
In addition, those who are concerned with fertility can opt for ways to preserve their reproductive capacity, such as the removal of semen for freezing sperm, albeit with adherence to sperm-washing procedures to preclude cross-contamination from viruses.8,9 For the preservation of sperm, the noninvasive method is often performed, preferably collected in several samples. Then, the semen is cryopreserved.8 In some instances, the sperm can also be removed directly from the testicles with a simple needle or by means of a minor surgical procedure.
A wait and try approach is advocated by clinicians for individuals who have already experienced COVID-19 symptoms and are therefore concerned about the prospect of childbearing.10 If the couple is unable to conceive after a year of trying, it is recommended that they consult a reproductive specialist; the clinician can carry out a comprehensive evaluation and order a series of tests to identify the source of the problem, indicating whether there are alternative methods for helping the couple to start a family (addressing the underlying factors involved in infertility, or treating via assisted reproduction procedures, such as in vitro fertilization).11
Dr. Aman is faculty member at the biology department of City Colleges of Chicago, and a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF). She disclosed no relevant financial relationships. Dr. Islam is a medical writer for the IMCHF, Montreal, is based in New York, and disclosed no relevant financial relationships. Mr. Choudhry is a research assistant at the IMCHF and he has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. Berry SD et al. J Am Geriatr Soc. 2021 May;69(5):1140-6.
2. Achua JK et al. World J Men’s Health. 2021 Jan;39(1):65-74.
3. Broderick JM. Urology Times. 2021 June.
4. Huang C et al. Andrology. 2021 Jan;9(1):80-7.
5. Sajjadi NB et al. J Osteopath Med. 2021 Apr 12;121(6):583-7.
6. Sallam M et al. Vaccines. 2021 Jan;9(1):42.
7. Islam MS et al. PloS One. 2021 May 12;16(5):e0251605.
8. Tesarik J. J Fertil Preserv. 2021;2:art246111.
9. Adiga SK et al. Reprod BioMed Online. 2020 Dec;41(6):991-7.
10. FAQs related to COVID-19. Q: If I get sick or test positive for COVID-19, when is it safe to become pregnant? American Society for Reproductive Medicine.
11. Cross C. Wellness and Prevention: Why can’t I get pregnant? John Hopkins Medicine.
Introduction
Amidst an aggressive vaccination campaign for COVID-19, misinformation has spread over the Internet, affecting public perception and making some people hesitant to participate in ongoing immunization campaigns. Of chief concern are issues pertaining to fertility or viability of sperm – information circulating on social networks posits that the coronavirus vaccine may influence infertility in men, which, according to physicians, is not grounded in reality. From the perspective of evidence-based medicine, there is a dearth of information suggesting an untoward effect of the vaccine on male fertility. The risk of adverse reactions arising from approved vaccines is negligible, with mild, albeit controllable, side effects demonstrated by patients in clinical trials. Therefore, there is no plausible reason for the general public to avoid vaccinations.1
Infertility following vaccination
The source of confusion can be traced back to a study conducted by researchers at the University of Miami Miller School of Medicine; the general public has conflated a side effect of the virus, namely, infertility and erectile dysfunction, with that of the vaccine.2 According to Ranjith Ramasamy, MD, director of the urology program at Miller, “We were the first to demonstrate that the COVID virus, itself, can affect male fertility and be a potential cause for erectile dysfunction. We are now the first to examine if there is any impact of the COVID vaccine on male fertility potential, which we did not find.”3
Coronavirus can indeed cause significant damage to the testicular tissue of infected men by means of mediating ACE2 expression on Leydig and Sertoli cells of the testis. It should be noted that COVID-19 may potentially attack any type of cell in the body that expresses the enzyme ACE2. However, it is particularly harmful to cells with high levels of expression of this enzyme, such as testicular cells. The spermatogenesis process can be affected, thereby posing a risk to male fertility.4
Expanding on the theme of fertility during the pandemic, a number of false claims5-7 about the vaccine and its overall effect on the placenta and fertility have also emerged as a contentious topic for debate on social media; doctors continue to explain why the theories are not reasonable or a cause for concern. The World Health Organization (WHO) provides recommendations on COVID-19 vaccinations for pregnant and/or lactating women and encourages a shared decision process involving risk/benefit assessment with the prescribing physician.5 Pregnant women, especially those with underlying comorbid conditions, are susceptible to developing severe symptom manifestations of COVID-19 with the disease also being associated with an increased likelihood of premature birth. As far as lactating women are concerned, the evidence thus far has indicated that the risk of side effects of the vaccine is very low, suggesting that these women could be vaccinated.5
The vaccine is the best option
While more studies are needed to ascertain the relationship between COVID-19 and male infertility, the vaccine is currently the best option for those who are concerned about their fertility from exposure to the coronavirus. Because of delayed wholesale acceptance of vaccines by the general population, clinicians should continue to emphasize the importance of preventive care with respect to disease exposure.6
In addition, those who are concerned with fertility can opt for ways to preserve their reproductive capacity, such as the removal of semen for freezing sperm, albeit with adherence to sperm-washing procedures to preclude cross-contamination from viruses.8,9 For the preservation of sperm, the noninvasive method is often performed, preferably collected in several samples. Then, the semen is cryopreserved.8 In some instances, the sperm can also be removed directly from the testicles with a simple needle or by means of a minor surgical procedure.
A wait and try approach is advocated by clinicians for individuals who have already experienced COVID-19 symptoms and are therefore concerned about the prospect of childbearing.10 If the couple is unable to conceive after a year of trying, it is recommended that they consult a reproductive specialist; the clinician can carry out a comprehensive evaluation and order a series of tests to identify the source of the problem, indicating whether there are alternative methods for helping the couple to start a family (addressing the underlying factors involved in infertility, or treating via assisted reproduction procedures, such as in vitro fertilization).11
Dr. Aman is faculty member at the biology department of City Colleges of Chicago, and a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF). She disclosed no relevant financial relationships. Dr. Islam is a medical writer for the IMCHF, Montreal, is based in New York, and disclosed no relevant financial relationships. Mr. Choudhry is a research assistant at the IMCHF and he has no disclosures. Dr. Zia Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. Berry SD et al. J Am Geriatr Soc. 2021 May;69(5):1140-6.
2. Achua JK et al. World J Men’s Health. 2021 Jan;39(1):65-74.
3. Broderick JM. Urology Times. 2021 June.
4. Huang C et al. Andrology. 2021 Jan;9(1):80-7.
5. Sajjadi NB et al. J Osteopath Med. 2021 Apr 12;121(6):583-7.
6. Sallam M et al. Vaccines. 2021 Jan;9(1):42.
7. Islam MS et al. PloS One. 2021 May 12;16(5):e0251605.
8. Tesarik J. J Fertil Preserv. 2021;2:art246111.
9. Adiga SK et al. Reprod BioMed Online. 2020 Dec;41(6):991-7.
10. FAQs related to COVID-19. Q: If I get sick or test positive for COVID-19, when is it safe to become pregnant? American Society for Reproductive Medicine.
11. Cross C. Wellness and Prevention: Why can’t I get pregnant? John Hopkins Medicine.
Obstetrical care for gender diverse patients: A summary from the SMFM annual meeting
The purpose of this commentary is to provide a brief summary of discussions centering around reproductive health experiences and obstetrical care for gender-diverse patients from the recent Society of Maternal & Fetal Medicine meeting. Two presentations featured patient perspectives combined with physician lectures to provide a comprehensive outlook on unique reproductive care needs for this growing population.
One of the speakers, Trystan Reese, is a transgender activist, educator, and transgender male who chose to carry his own pregnancy and subsequently delivered his son in 2017. During the summit, he described many barriers that he faced during his pregnancy and offered providers suggestions on how to improve the care for members of the gender-diverse community seeking to start a family.
We often think of conception and pregnancy as experiences unique to one gender. This is simply not the case. In discussing preconceptual care and pregnancy, it is paramount for providers to make the distinction between gender identity and natal sex. Gender identity is an internal sense of self in relation to natal sex. Depending on this intrinsic feeling, people may identify as cisgender, transgender, or as a gender outside of the standard binary. Natal sex describes biologic characteristics such as chromosomal makeup, reproductive anatomy, and secondary sexual changes. In keeping these distinctions in mind, pregnancy is therefore exclusive to a person’s natal sex, not gender identity. One of the biggest challenges in caring for transgender patients who desire pregnancy, is the psychological distress related to the gendered notions surrounding this experience.1
There are many ways in which patients encounter unintentional marginalization within the medical system. For example, many electronic medical record systems don’t allow for pronouns or give error messages if the patient’s gender identity is different from their sex assigned at birth. Patients who attend prenatal appointments or birth classes are given documents that center around cisgender women and heterosexual relationships. The labor and delivery wards themselves typically include language such as “maternity,” and birth certificates have distinct “mother” and “father” denotations.1 Insurance coverage for prenatal care and delivery can be problematic if a patient who is assigned female at birth has changed their gender marker to “male” on their insurance card.
Many of these roadblocks can be ameliorated by utilizing more inclusive terminology. Terms such as “maternal” can be replaced with “pregnant patients, parent, or patients giving birth.” Names of maternity wards can be altered to perinatal units, which is more inclusive and more descriptive of the wide variety of patients that may experience childbirth and parenthood.1 Introducing “you-centered” language can also be helpful. Instead of saying “women may find ...” providers can try saying “patients may find ...” or “individuals may find.”1
Most of the medical and obstetrical care of gender-diverse patients is routine. Prenatal labs, aneuploidy screening, ultrasounds, and fetal surveillance do not differ between transgender and cisgender patients. However, the experience of pregnancy itself can significantly heighten feelings of dysphoria as it inherently leads to patients confronting aspects of their biological sex.2 Because of the teratogenic nature of testosterone, patients are required to stop taking testosterone prior to conception and for the duration of pregnancy. This can also heighten dysphoria and lead to increased rates of anxiety and depression.3
Many transgender patients can safely achieve a normal vaginal birth.4 A small survey of 41 people demonstrated that more transgender men who had taken testosterone were delivered by cesarean section (36% vs. 19%).3 Staff training is an important aspect of caring for a transgender patient in labor to ensure that all members of the labor unit are cognizant of appropriate name and pronoun usage. Another interesting, although unsurprising, fact is that many transgender gestational parents chose a community-based (out-of-hospital) birth according to a 2014 study.1 This is predominantly because of the discrimination patients face when delivering within a hospital setting.
Postpartum depression screening should be conducted prior to patients leaving the hospital and individualized during postpartum appointments. Reinitiation of testosterone can occur 4-6 weeks after delivery.1
While pregnancy can pose some unique challenges to gender-diverse individuals, these intricacies are not insurmountable. The result of pregnancy, regardless of one’s gender identity, is the same – parenthood. One patient’s description of his experience was particularly poignant: “Pregnancy and childbirth were very male experiences for me. When I birthed my children, I was born into fatherhood.”1 It is up to all providers to modify clinical settings, as well as our patient interactions and use of language, if we are to provide inclusion in obstetrics.1,5
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Brandt JS et al. “Understanding intersections: Care for transgender and gender diverse patient populations.” SMFM 2022 annual meeting. 2022 Feb 2.
2. Hoffkling A et al. BMC Pregnancy Childbirth. 2017 Nov 8;17(Suppl 2):332.
3. Light AD et al. Obstet Gynecol. 2014;124:1120-7.
4. Moseson H et al. Int J Transgend Health. 2021 Nov 17;22(1-2):30-41.
5. Brandt JS et al. Obstetrical care for trans*person, in “Trans*gynecology: Managing transgender patients in obstetrics and gynecology practice.” (Cambridge, England: Cambridge University Press, 2022).
The purpose of this commentary is to provide a brief summary of discussions centering around reproductive health experiences and obstetrical care for gender-diverse patients from the recent Society of Maternal & Fetal Medicine meeting. Two presentations featured patient perspectives combined with physician lectures to provide a comprehensive outlook on unique reproductive care needs for this growing population.
One of the speakers, Trystan Reese, is a transgender activist, educator, and transgender male who chose to carry his own pregnancy and subsequently delivered his son in 2017. During the summit, he described many barriers that he faced during his pregnancy and offered providers suggestions on how to improve the care for members of the gender-diverse community seeking to start a family.
We often think of conception and pregnancy as experiences unique to one gender. This is simply not the case. In discussing preconceptual care and pregnancy, it is paramount for providers to make the distinction between gender identity and natal sex. Gender identity is an internal sense of self in relation to natal sex. Depending on this intrinsic feeling, people may identify as cisgender, transgender, or as a gender outside of the standard binary. Natal sex describes biologic characteristics such as chromosomal makeup, reproductive anatomy, and secondary sexual changes. In keeping these distinctions in mind, pregnancy is therefore exclusive to a person’s natal sex, not gender identity. One of the biggest challenges in caring for transgender patients who desire pregnancy, is the psychological distress related to the gendered notions surrounding this experience.1
There are many ways in which patients encounter unintentional marginalization within the medical system. For example, many electronic medical record systems don’t allow for pronouns or give error messages if the patient’s gender identity is different from their sex assigned at birth. Patients who attend prenatal appointments or birth classes are given documents that center around cisgender women and heterosexual relationships. The labor and delivery wards themselves typically include language such as “maternity,” and birth certificates have distinct “mother” and “father” denotations.1 Insurance coverage for prenatal care and delivery can be problematic if a patient who is assigned female at birth has changed their gender marker to “male” on their insurance card.
Many of these roadblocks can be ameliorated by utilizing more inclusive terminology. Terms such as “maternal” can be replaced with “pregnant patients, parent, or patients giving birth.” Names of maternity wards can be altered to perinatal units, which is more inclusive and more descriptive of the wide variety of patients that may experience childbirth and parenthood.1 Introducing “you-centered” language can also be helpful. Instead of saying “women may find ...” providers can try saying “patients may find ...” or “individuals may find.”1
Most of the medical and obstetrical care of gender-diverse patients is routine. Prenatal labs, aneuploidy screening, ultrasounds, and fetal surveillance do not differ between transgender and cisgender patients. However, the experience of pregnancy itself can significantly heighten feelings of dysphoria as it inherently leads to patients confronting aspects of their biological sex.2 Because of the teratogenic nature of testosterone, patients are required to stop taking testosterone prior to conception and for the duration of pregnancy. This can also heighten dysphoria and lead to increased rates of anxiety and depression.3
Many transgender patients can safely achieve a normal vaginal birth.4 A small survey of 41 people demonstrated that more transgender men who had taken testosterone were delivered by cesarean section (36% vs. 19%).3 Staff training is an important aspect of caring for a transgender patient in labor to ensure that all members of the labor unit are cognizant of appropriate name and pronoun usage. Another interesting, although unsurprising, fact is that many transgender gestational parents chose a community-based (out-of-hospital) birth according to a 2014 study.1 This is predominantly because of the discrimination patients face when delivering within a hospital setting.
Postpartum depression screening should be conducted prior to patients leaving the hospital and individualized during postpartum appointments. Reinitiation of testosterone can occur 4-6 weeks after delivery.1
While pregnancy can pose some unique challenges to gender-diverse individuals, these intricacies are not insurmountable. The result of pregnancy, regardless of one’s gender identity, is the same – parenthood. One patient’s description of his experience was particularly poignant: “Pregnancy and childbirth were very male experiences for me. When I birthed my children, I was born into fatherhood.”1 It is up to all providers to modify clinical settings, as well as our patient interactions and use of language, if we are to provide inclusion in obstetrics.1,5
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Brandt JS et al. “Understanding intersections: Care for transgender and gender diverse patient populations.” SMFM 2022 annual meeting. 2022 Feb 2.
2. Hoffkling A et al. BMC Pregnancy Childbirth. 2017 Nov 8;17(Suppl 2):332.
3. Light AD et al. Obstet Gynecol. 2014;124:1120-7.
4. Moseson H et al. Int J Transgend Health. 2021 Nov 17;22(1-2):30-41.
5. Brandt JS et al. Obstetrical care for trans*person, in “Trans*gynecology: Managing transgender patients in obstetrics and gynecology practice.” (Cambridge, England: Cambridge University Press, 2022).
The purpose of this commentary is to provide a brief summary of discussions centering around reproductive health experiences and obstetrical care for gender-diverse patients from the recent Society of Maternal & Fetal Medicine meeting. Two presentations featured patient perspectives combined with physician lectures to provide a comprehensive outlook on unique reproductive care needs for this growing population.
One of the speakers, Trystan Reese, is a transgender activist, educator, and transgender male who chose to carry his own pregnancy and subsequently delivered his son in 2017. During the summit, he described many barriers that he faced during his pregnancy and offered providers suggestions on how to improve the care for members of the gender-diverse community seeking to start a family.
We often think of conception and pregnancy as experiences unique to one gender. This is simply not the case. In discussing preconceptual care and pregnancy, it is paramount for providers to make the distinction between gender identity and natal sex. Gender identity is an internal sense of self in relation to natal sex. Depending on this intrinsic feeling, people may identify as cisgender, transgender, or as a gender outside of the standard binary. Natal sex describes biologic characteristics such as chromosomal makeup, reproductive anatomy, and secondary sexual changes. In keeping these distinctions in mind, pregnancy is therefore exclusive to a person’s natal sex, not gender identity. One of the biggest challenges in caring for transgender patients who desire pregnancy, is the psychological distress related to the gendered notions surrounding this experience.1
There are many ways in which patients encounter unintentional marginalization within the medical system. For example, many electronic medical record systems don’t allow for pronouns or give error messages if the patient’s gender identity is different from their sex assigned at birth. Patients who attend prenatal appointments or birth classes are given documents that center around cisgender women and heterosexual relationships. The labor and delivery wards themselves typically include language such as “maternity,” and birth certificates have distinct “mother” and “father” denotations.1 Insurance coverage for prenatal care and delivery can be problematic if a patient who is assigned female at birth has changed their gender marker to “male” on their insurance card.
Many of these roadblocks can be ameliorated by utilizing more inclusive terminology. Terms such as “maternal” can be replaced with “pregnant patients, parent, or patients giving birth.” Names of maternity wards can be altered to perinatal units, which is more inclusive and more descriptive of the wide variety of patients that may experience childbirth and parenthood.1 Introducing “you-centered” language can also be helpful. Instead of saying “women may find ...” providers can try saying “patients may find ...” or “individuals may find.”1
Most of the medical and obstetrical care of gender-diverse patients is routine. Prenatal labs, aneuploidy screening, ultrasounds, and fetal surveillance do not differ between transgender and cisgender patients. However, the experience of pregnancy itself can significantly heighten feelings of dysphoria as it inherently leads to patients confronting aspects of their biological sex.2 Because of the teratogenic nature of testosterone, patients are required to stop taking testosterone prior to conception and for the duration of pregnancy. This can also heighten dysphoria and lead to increased rates of anxiety and depression.3
Many transgender patients can safely achieve a normal vaginal birth.4 A small survey of 41 people demonstrated that more transgender men who had taken testosterone were delivered by cesarean section (36% vs. 19%).3 Staff training is an important aspect of caring for a transgender patient in labor to ensure that all members of the labor unit are cognizant of appropriate name and pronoun usage. Another interesting, although unsurprising, fact is that many transgender gestational parents chose a community-based (out-of-hospital) birth according to a 2014 study.1 This is predominantly because of the discrimination patients face when delivering within a hospital setting.
Postpartum depression screening should be conducted prior to patients leaving the hospital and individualized during postpartum appointments. Reinitiation of testosterone can occur 4-6 weeks after delivery.1
While pregnancy can pose some unique challenges to gender-diverse individuals, these intricacies are not insurmountable. The result of pregnancy, regardless of one’s gender identity, is the same – parenthood. One patient’s description of his experience was particularly poignant: “Pregnancy and childbirth were very male experiences for me. When I birthed my children, I was born into fatherhood.”1 It is up to all providers to modify clinical settings, as well as our patient interactions and use of language, if we are to provide inclusion in obstetrics.1,5
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Brandt JS et al. “Understanding intersections: Care for transgender and gender diverse patient populations.” SMFM 2022 annual meeting. 2022 Feb 2.
2. Hoffkling A et al. BMC Pregnancy Childbirth. 2017 Nov 8;17(Suppl 2):332.
3. Light AD et al. Obstet Gynecol. 2014;124:1120-7.
4. Moseson H et al. Int J Transgend Health. 2021 Nov 17;22(1-2):30-41.
5. Brandt JS et al. Obstetrical care for trans*person, in “Trans*gynecology: Managing transgender patients in obstetrics and gynecology practice.” (Cambridge, England: Cambridge University Press, 2022).