User login
Why is vitamin D hype so impervious to evidence?
The vitamin D story exudes teaching points: It offers a master class in critical appraisal, connecting the concepts of biologic plausibility, flawed surrogate markers, confounded observational studies, and slews of randomized controlled trials (RCTs) showing no benefits on health outcomes.
Yet despite the utter lack of benefit seen in trials, the hype continues. And the pandemic has only enhanced this hype as an onslaught of papers have reported the association of low vitamin D levels and COVID-19 disease.
My questions are simple: Why doesn’t the evidence persuade people? How many nonsignificant trials do we need before researchers stop studying vitamin D, doctors stop (routinely) measuring levels, and patients stop wasting money on the unhelpful supplement? What are the implications for this lack of persuasion?
Before exploring these questions, I want to set out that symptomatic vitamin deficiencies of any sort ought to be corrected.
Biologic plausibility and the pull of observational studies
It has long been known that vitamin D is crucial for bone health and that it can be produced in the skin with sun exposure. In the last decade, however, experts note that nearly every tissue and cell in our body has a vitamin D receptor. It then follows that if this many cells in the body can activate vitamin D, it must be vital for cardiovascular health, immune function, cancer prevention: basically, everything health related.
Oodles of observational studies have found that low serum levels of vitamin D correlate with higher mortality from all causes, cancer, cardiovascular disease, and now even COVID-19. Yet no matter the amount of statistical adjustment in these studies, we cannot know whether these associations are due to true causality.
The major issue is confounding: That is, people with low vitamin D levels have other conditions or diseases that lead to higher rates of ill health. Consider a patient with obesity, arthritis, and cognitive decline; this person is unlikely to do much exercise in the sun and may have low vitamin D levels. The low vitamin D level is simply a marker of overall poor health.
The randomized controlled trials tell a clear story
There are hundreds of vitamin D RCTs. The results simplify into one sentence: Vitamin D supplements do not improve health outcomes.
Here is a short summary of some recent studies.
VITAL, a massive (N > 25,000) RCT with 5 years of follow-up, compared vitamin D supplements to placebo and found no differences in the primary endpoints of cancer or cardiac events. Rates of death from any cause were nearly identical. Crucially, in subgroup analyses, the effects did not vary according to vitamin D levels at baseline.
The D-Health investigators randomly assigned more than 21,000 adults to vitamin D or placebo and after 5.7 years of follow-up reported no differences in the primary endpoint of overall mortality. There also were no differences in cardiovascular disease mortality.
Then you have the Mendelian randomized studies, which some have called nature’s RCT. These studies take advantage of the fact that some people are born with gene variations that predispose to low vitamin D levels. More than 60 Mendelian randomization studies have evaluated the consequences of lifelong genetically lowered vitamin D levels on various outcomes; most of these have found null effects.
Then there are the meta-analyses and systematic reviews. I loved the conclusion of this review of systematic reviews from the BMJ (emphasis mine):
“Despite a few hundred systematic reviews and meta-analyses, highly convincing evidence of a clear role of vitamin D does not exist for any outcome, but associations with a selection of outcomes are probable.”
The failure to persuade
My original plan was to emphasize the power of the RCT. Despite strong associations of low vitamin D levels with poor outcomes, the trials show no benefit to treatment. This strongly suggests (or nearly proves) that low vitamin D levels are akin to premature ventricular complexes after myocardial infarction: a marker for risk but not a target for therapy.
But I now see the more important issue as why scientists, funders, clinicians, and patients are not persuaded by clear evidence. Every day in clinic I see patients on vitamin D supplements; the journals keep publishing vitamin D studies. The proponents of vitamin D remain positive. And lately there is outsized attention and hope that vitamin D will mitigate SARS-CoV2 infection – based only on observational data.
You might argue against this point by saying vitamin D is natural and relatively innocuous, so who cares?
I offer three rebuttals to that point: Opportunity costs, distraction, and the insidious danger of poor critical appraisal skills. If you are burning money on vitamin D research, there is less available to study other important issues. If a patient is distracted by low vitamin D levels, she may pay less attention to her high body mass index or hypertension. And on the matter of critical appraisal, trust in medicine requires clinicians to be competent in critical appraisal. And these days, what could be more important than trust in medical professionals?
One major reason for the failure of persuasion of evidence is spin – or language that distracts from the primary endpoint. Here are two (of many) examples:
A meta-analysis of 50 vitamin D trials set out to study mortality. The authors found no significant difference in that primary endpoint. But the second sentence in their conclusion was that vitamin D supplements reduced the risk for cancer deaths by 15%. That’s a secondary endpoint in a study with nonsignificance in the primary endpoint. That is spin. This meta-analysis was completed before the Australian D-Health trial found that cancer deaths were 15% higher in the vitamin D arm, a difference that did not reach statistical significance.
The following example is worse: The authors of the VITAL trial, which found that vitamin D supplements had no effect on the primary endpoint of invasive cancer or cardiovascular disease, published a secondary analysis of the trial looking at a different endpoint: A composite incidence of metastatic and fatal invasive total cancer. They reported a 0.4% lower rate for the vitamin D group, a difference that barely made statistical significance at a P value of .04.
But everyone knows the dangers of reanalyzing data with a new endpoint after you have seen the data. What’s more, even if this were a reasonable post hoc analysis, the results are neither clinically meaningful nor statistically robust. Yet the fatally flawed paper has been viewed 60,000 times and picked up by 48 news outlets.
Another way to distract from nonsignificant primary outcomes is to nitpick the trials. The vitamin D dose wasn’t high enough, for instance. This might persuade me if there were one or two vitamin D trials, but there are hundreds of trials and meta-analyses, and their results are consistently null.
Conclusion: No, it is not hopeless
A nihilist would argue that fighting spin is futile. They would say you can’t fight incentives and business models. The incentive structure to publish is strong, and the journals and media know vitamin D studies garner attention – which is their currency.
I am not a nihilist and believe strongly that we must continue to teach critical appraisal and numerical literacy.
In fact, I would speculate that decades of poor critical appraisal by the medical profession have fostered outsized hope and created erroneous norms.
Imagine a counter-factual world in which clinicians have taught society that the human body is unlike an engine that can be repaired by fixing one part (i.e., the vitamin D level), that magic bullets (insulin) are rare, that most treatments fail, or that you can’t rely on association studies to prove efficacy.
In this world, people would be immune from spin and hype.
The norm would be that pills, supplements, and procedures are not what delivers good health. What delivers health is an amalgam of good luck, healthy habits, and lots of time spent outside playing in the sun.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The vitamin D story exudes teaching points: It offers a master class in critical appraisal, connecting the concepts of biologic plausibility, flawed surrogate markers, confounded observational studies, and slews of randomized controlled trials (RCTs) showing no benefits on health outcomes.
Yet despite the utter lack of benefit seen in trials, the hype continues. And the pandemic has only enhanced this hype as an onslaught of papers have reported the association of low vitamin D levels and COVID-19 disease.
My questions are simple: Why doesn’t the evidence persuade people? How many nonsignificant trials do we need before researchers stop studying vitamin D, doctors stop (routinely) measuring levels, and patients stop wasting money on the unhelpful supplement? What are the implications for this lack of persuasion?
Before exploring these questions, I want to set out that symptomatic vitamin deficiencies of any sort ought to be corrected.
Biologic plausibility and the pull of observational studies
It has long been known that vitamin D is crucial for bone health and that it can be produced in the skin with sun exposure. In the last decade, however, experts note that nearly every tissue and cell in our body has a vitamin D receptor. It then follows that if this many cells in the body can activate vitamin D, it must be vital for cardiovascular health, immune function, cancer prevention: basically, everything health related.
Oodles of observational studies have found that low serum levels of vitamin D correlate with higher mortality from all causes, cancer, cardiovascular disease, and now even COVID-19. Yet no matter the amount of statistical adjustment in these studies, we cannot know whether these associations are due to true causality.
The major issue is confounding: That is, people with low vitamin D levels have other conditions or diseases that lead to higher rates of ill health. Consider a patient with obesity, arthritis, and cognitive decline; this person is unlikely to do much exercise in the sun and may have low vitamin D levels. The low vitamin D level is simply a marker of overall poor health.
The randomized controlled trials tell a clear story
There are hundreds of vitamin D RCTs. The results simplify into one sentence: Vitamin D supplements do not improve health outcomes.
Here is a short summary of some recent studies.
VITAL, a massive (N > 25,000) RCT with 5 years of follow-up, compared vitamin D supplements to placebo and found no differences in the primary endpoints of cancer or cardiac events. Rates of death from any cause were nearly identical. Crucially, in subgroup analyses, the effects did not vary according to vitamin D levels at baseline.
The D-Health investigators randomly assigned more than 21,000 adults to vitamin D or placebo and after 5.7 years of follow-up reported no differences in the primary endpoint of overall mortality. There also were no differences in cardiovascular disease mortality.
Then you have the Mendelian randomized studies, which some have called nature’s RCT. These studies take advantage of the fact that some people are born with gene variations that predispose to low vitamin D levels. More than 60 Mendelian randomization studies have evaluated the consequences of lifelong genetically lowered vitamin D levels on various outcomes; most of these have found null effects.
Then there are the meta-analyses and systematic reviews. I loved the conclusion of this review of systematic reviews from the BMJ (emphasis mine):
“Despite a few hundred systematic reviews and meta-analyses, highly convincing evidence of a clear role of vitamin D does not exist for any outcome, but associations with a selection of outcomes are probable.”
The failure to persuade
My original plan was to emphasize the power of the RCT. Despite strong associations of low vitamin D levels with poor outcomes, the trials show no benefit to treatment. This strongly suggests (or nearly proves) that low vitamin D levels are akin to premature ventricular complexes after myocardial infarction: a marker for risk but not a target for therapy.
But I now see the more important issue as why scientists, funders, clinicians, and patients are not persuaded by clear evidence. Every day in clinic I see patients on vitamin D supplements; the journals keep publishing vitamin D studies. The proponents of vitamin D remain positive. And lately there is outsized attention and hope that vitamin D will mitigate SARS-CoV2 infection – based only on observational data.
You might argue against this point by saying vitamin D is natural and relatively innocuous, so who cares?
I offer three rebuttals to that point: Opportunity costs, distraction, and the insidious danger of poor critical appraisal skills. If you are burning money on vitamin D research, there is less available to study other important issues. If a patient is distracted by low vitamin D levels, she may pay less attention to her high body mass index or hypertension. And on the matter of critical appraisal, trust in medicine requires clinicians to be competent in critical appraisal. And these days, what could be more important than trust in medical professionals?
One major reason for the failure of persuasion of evidence is spin – or language that distracts from the primary endpoint. Here are two (of many) examples:
A meta-analysis of 50 vitamin D trials set out to study mortality. The authors found no significant difference in that primary endpoint. But the second sentence in their conclusion was that vitamin D supplements reduced the risk for cancer deaths by 15%. That’s a secondary endpoint in a study with nonsignificance in the primary endpoint. That is spin. This meta-analysis was completed before the Australian D-Health trial found that cancer deaths were 15% higher in the vitamin D arm, a difference that did not reach statistical significance.
The following example is worse: The authors of the VITAL trial, which found that vitamin D supplements had no effect on the primary endpoint of invasive cancer or cardiovascular disease, published a secondary analysis of the trial looking at a different endpoint: A composite incidence of metastatic and fatal invasive total cancer. They reported a 0.4% lower rate for the vitamin D group, a difference that barely made statistical significance at a P value of .04.
But everyone knows the dangers of reanalyzing data with a new endpoint after you have seen the data. What’s more, even if this were a reasonable post hoc analysis, the results are neither clinically meaningful nor statistically robust. Yet the fatally flawed paper has been viewed 60,000 times and picked up by 48 news outlets.
Another way to distract from nonsignificant primary outcomes is to nitpick the trials. The vitamin D dose wasn’t high enough, for instance. This might persuade me if there were one or two vitamin D trials, but there are hundreds of trials and meta-analyses, and their results are consistently null.
Conclusion: No, it is not hopeless
A nihilist would argue that fighting spin is futile. They would say you can’t fight incentives and business models. The incentive structure to publish is strong, and the journals and media know vitamin D studies garner attention – which is their currency.
I am not a nihilist and believe strongly that we must continue to teach critical appraisal and numerical literacy.
In fact, I would speculate that decades of poor critical appraisal by the medical profession have fostered outsized hope and created erroneous norms.
Imagine a counter-factual world in which clinicians have taught society that the human body is unlike an engine that can be repaired by fixing one part (i.e., the vitamin D level), that magic bullets (insulin) are rare, that most treatments fail, or that you can’t rely on association studies to prove efficacy.
In this world, people would be immune from spin and hype.
The norm would be that pills, supplements, and procedures are not what delivers good health. What delivers health is an amalgam of good luck, healthy habits, and lots of time spent outside playing in the sun.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The vitamin D story exudes teaching points: It offers a master class in critical appraisal, connecting the concepts of biologic plausibility, flawed surrogate markers, confounded observational studies, and slews of randomized controlled trials (RCTs) showing no benefits on health outcomes.
Yet despite the utter lack of benefit seen in trials, the hype continues. And the pandemic has only enhanced this hype as an onslaught of papers have reported the association of low vitamin D levels and COVID-19 disease.
My questions are simple: Why doesn’t the evidence persuade people? How many nonsignificant trials do we need before researchers stop studying vitamin D, doctors stop (routinely) measuring levels, and patients stop wasting money on the unhelpful supplement? What are the implications for this lack of persuasion?
Before exploring these questions, I want to set out that symptomatic vitamin deficiencies of any sort ought to be corrected.
Biologic plausibility and the pull of observational studies
It has long been known that vitamin D is crucial for bone health and that it can be produced in the skin with sun exposure. In the last decade, however, experts note that nearly every tissue and cell in our body has a vitamin D receptor. It then follows that if this many cells in the body can activate vitamin D, it must be vital for cardiovascular health, immune function, cancer prevention: basically, everything health related.
Oodles of observational studies have found that low serum levels of vitamin D correlate with higher mortality from all causes, cancer, cardiovascular disease, and now even COVID-19. Yet no matter the amount of statistical adjustment in these studies, we cannot know whether these associations are due to true causality.
The major issue is confounding: That is, people with low vitamin D levels have other conditions or diseases that lead to higher rates of ill health. Consider a patient with obesity, arthritis, and cognitive decline; this person is unlikely to do much exercise in the sun and may have low vitamin D levels. The low vitamin D level is simply a marker of overall poor health.
The randomized controlled trials tell a clear story
There are hundreds of vitamin D RCTs. The results simplify into one sentence: Vitamin D supplements do not improve health outcomes.
Here is a short summary of some recent studies.
VITAL, a massive (N > 25,000) RCT with 5 years of follow-up, compared vitamin D supplements to placebo and found no differences in the primary endpoints of cancer or cardiac events. Rates of death from any cause were nearly identical. Crucially, in subgroup analyses, the effects did not vary according to vitamin D levels at baseline.
The D-Health investigators randomly assigned more than 21,000 adults to vitamin D or placebo and after 5.7 years of follow-up reported no differences in the primary endpoint of overall mortality. There also were no differences in cardiovascular disease mortality.
Then you have the Mendelian randomized studies, which some have called nature’s RCT. These studies take advantage of the fact that some people are born with gene variations that predispose to low vitamin D levels. More than 60 Mendelian randomization studies have evaluated the consequences of lifelong genetically lowered vitamin D levels on various outcomes; most of these have found null effects.
Then there are the meta-analyses and systematic reviews. I loved the conclusion of this review of systematic reviews from the BMJ (emphasis mine):
“Despite a few hundred systematic reviews and meta-analyses, highly convincing evidence of a clear role of vitamin D does not exist for any outcome, but associations with a selection of outcomes are probable.”
The failure to persuade
My original plan was to emphasize the power of the RCT. Despite strong associations of low vitamin D levels with poor outcomes, the trials show no benefit to treatment. This strongly suggests (or nearly proves) that low vitamin D levels are akin to premature ventricular complexes after myocardial infarction: a marker for risk but not a target for therapy.
But I now see the more important issue as why scientists, funders, clinicians, and patients are not persuaded by clear evidence. Every day in clinic I see patients on vitamin D supplements; the journals keep publishing vitamin D studies. The proponents of vitamin D remain positive. And lately there is outsized attention and hope that vitamin D will mitigate SARS-CoV2 infection – based only on observational data.
You might argue against this point by saying vitamin D is natural and relatively innocuous, so who cares?
I offer three rebuttals to that point: Opportunity costs, distraction, and the insidious danger of poor critical appraisal skills. If you are burning money on vitamin D research, there is less available to study other important issues. If a patient is distracted by low vitamin D levels, she may pay less attention to her high body mass index or hypertension. And on the matter of critical appraisal, trust in medicine requires clinicians to be competent in critical appraisal. And these days, what could be more important than trust in medical professionals?
One major reason for the failure of persuasion of evidence is spin – or language that distracts from the primary endpoint. Here are two (of many) examples:
A meta-analysis of 50 vitamin D trials set out to study mortality. The authors found no significant difference in that primary endpoint. But the second sentence in their conclusion was that vitamin D supplements reduced the risk for cancer deaths by 15%. That’s a secondary endpoint in a study with nonsignificance in the primary endpoint. That is spin. This meta-analysis was completed before the Australian D-Health trial found that cancer deaths were 15% higher in the vitamin D arm, a difference that did not reach statistical significance.
The following example is worse: The authors of the VITAL trial, which found that vitamin D supplements had no effect on the primary endpoint of invasive cancer or cardiovascular disease, published a secondary analysis of the trial looking at a different endpoint: A composite incidence of metastatic and fatal invasive total cancer. They reported a 0.4% lower rate for the vitamin D group, a difference that barely made statistical significance at a P value of .04.
But everyone knows the dangers of reanalyzing data with a new endpoint after you have seen the data. What’s more, even if this were a reasonable post hoc analysis, the results are neither clinically meaningful nor statistically robust. Yet the fatally flawed paper has been viewed 60,000 times and picked up by 48 news outlets.
Another way to distract from nonsignificant primary outcomes is to nitpick the trials. The vitamin D dose wasn’t high enough, for instance. This might persuade me if there were one or two vitamin D trials, but there are hundreds of trials and meta-analyses, and their results are consistently null.
Conclusion: No, it is not hopeless
A nihilist would argue that fighting spin is futile. They would say you can’t fight incentives and business models. The incentive structure to publish is strong, and the journals and media know vitamin D studies garner attention – which is their currency.
I am not a nihilist and believe strongly that we must continue to teach critical appraisal and numerical literacy.
In fact, I would speculate that decades of poor critical appraisal by the medical profession have fostered outsized hope and created erroneous norms.
Imagine a counter-factual world in which clinicians have taught society that the human body is unlike an engine that can be repaired by fixing one part (i.e., the vitamin D level), that magic bullets (insulin) are rare, that most treatments fail, or that you can’t rely on association studies to prove efficacy.
In this world, people would be immune from spin and hype.
The norm would be that pills, supplements, and procedures are not what delivers good health. What delivers health is an amalgam of good luck, healthy habits, and lots of time spent outside playing in the sun.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Practice valuation
.
Too often, physicians are not receiving a fair return on the equity they have worked so hard to build over several decades, either because they have waited too long and must accept what is offered, or because they simply take the buyer’s word for their practice’s value. Don’t put yourself in either of those positions, and don’t entertain any offers until you obtain an objective appraisal from a neutral party.
Of course, a medical practice is trickier to value than an ordinary business, and usually requires the services of an experienced professional appraiser. Entire books have been written about the process, so I can’t hope to cover it completely in 750 words; but three basic yardsticks are essential for determining the equity, or book value, of a practice:
- Tangible assets. Equipment, cash, accounts receivable, and other property owned by the practice.
- Liabilities. Accounts payable, outstanding loans, and anything else owed to others.
- Intangible assets. Sometimes called “good will” – the reputation of the physicians, the location and name recognition of the practice, the loyalty and volume of patients, and other, well, intangibles.
Valuing tangible assets is comparatively straightforward, but there are several ways to do it, and when reviewing a practice appraisal you should ask which of them was used. Depreciated value is the book value of equipment and supplies as determined by their purchase price, less the amount their value has decreased since purchase. Remaining useful life value estimates how long the equipment can be expected to last. Market (or replacement) value is the amount it would cost on the open market to replace all equipment and supplies.
Intangible assets are more difficult to value. Many components are analyzed, including location, interior and exterior decor, accessibility to patients, age and functional status of equipment, systems in place to promote efficiency, reasons why patients come back (if in fact they do), and the overall reputation of the practice in the community. Other important factors include the “payer mix” (what percentage pays cash, how many third-party contracts are in place and how well they pay, etcetera), the extent and strength of the referral base, and the presence of supplemental income streams, such as clinical research.
It is also important to determine to what extent intangible assets are transferable. For example, unique skills with a laser, neurotoxins, or filler substances, or extraordinary personal charisma, may increase your practice’s value to you, but they are worthless to the next owner, and he or she will be unwilling to pay for them unless your services become part of the deal.
Once again there are many ways to estimate intangible asset value, and once again you should ask which were used. Cash flow analysis works on the assumption that cash flow is a measure of intangible value. Capitalization of earnings puts a value, or capitalization, on the practice’s income streams using a variety of assumptions. Guideline comparison uses various databases to compare your practice with other, similar ones that have changed hands in the past.
Two newer techniques that some consider a better estimate of intangible assets are the replacement method, which estimates the costs of starting the practice over again in the current market; and the excess earnings method, which measures how far above average your practice’s earnings (and thus its overall value) are.
Asset-based valuation is the most popular, but by no means the only method available. Income-based valuation looks at the source and strength of a practice’s income stream as a creator of value, as well as whether or not its income stream under a different owner would mirror its present one. This in turn becomes the basis for an understanding of the fair market value of both tangible and intangible assets. Market valuation combines the asset-based and income-based approaches, along with an analysis of sales and mergers of comparable practices in the community, to determine the value of a practice in its local market.
Whatever methods are used, it is important that the appraisal be done by an experienced and independent financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation is supplied to support the conclusions reached. This is especially important if the appraisal will be relied upon in the sale or merger of the practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
.
Too often, physicians are not receiving a fair return on the equity they have worked so hard to build over several decades, either because they have waited too long and must accept what is offered, or because they simply take the buyer’s word for their practice’s value. Don’t put yourself in either of those positions, and don’t entertain any offers until you obtain an objective appraisal from a neutral party.
Of course, a medical practice is trickier to value than an ordinary business, and usually requires the services of an experienced professional appraiser. Entire books have been written about the process, so I can’t hope to cover it completely in 750 words; but three basic yardsticks are essential for determining the equity, or book value, of a practice:
- Tangible assets. Equipment, cash, accounts receivable, and other property owned by the practice.
- Liabilities. Accounts payable, outstanding loans, and anything else owed to others.
- Intangible assets. Sometimes called “good will” – the reputation of the physicians, the location and name recognition of the practice, the loyalty and volume of patients, and other, well, intangibles.
Valuing tangible assets is comparatively straightforward, but there are several ways to do it, and when reviewing a practice appraisal you should ask which of them was used. Depreciated value is the book value of equipment and supplies as determined by their purchase price, less the amount their value has decreased since purchase. Remaining useful life value estimates how long the equipment can be expected to last. Market (or replacement) value is the amount it would cost on the open market to replace all equipment and supplies.
Intangible assets are more difficult to value. Many components are analyzed, including location, interior and exterior decor, accessibility to patients, age and functional status of equipment, systems in place to promote efficiency, reasons why patients come back (if in fact they do), and the overall reputation of the practice in the community. Other important factors include the “payer mix” (what percentage pays cash, how many third-party contracts are in place and how well they pay, etcetera), the extent and strength of the referral base, and the presence of supplemental income streams, such as clinical research.
It is also important to determine to what extent intangible assets are transferable. For example, unique skills with a laser, neurotoxins, or filler substances, or extraordinary personal charisma, may increase your practice’s value to you, but they are worthless to the next owner, and he or she will be unwilling to pay for them unless your services become part of the deal.
Once again there are many ways to estimate intangible asset value, and once again you should ask which were used. Cash flow analysis works on the assumption that cash flow is a measure of intangible value. Capitalization of earnings puts a value, or capitalization, on the practice’s income streams using a variety of assumptions. Guideline comparison uses various databases to compare your practice with other, similar ones that have changed hands in the past.
Two newer techniques that some consider a better estimate of intangible assets are the replacement method, which estimates the costs of starting the practice over again in the current market; and the excess earnings method, which measures how far above average your practice’s earnings (and thus its overall value) are.
Asset-based valuation is the most popular, but by no means the only method available. Income-based valuation looks at the source and strength of a practice’s income stream as a creator of value, as well as whether or not its income stream under a different owner would mirror its present one. This in turn becomes the basis for an understanding of the fair market value of both tangible and intangible assets. Market valuation combines the asset-based and income-based approaches, along with an analysis of sales and mergers of comparable practices in the community, to determine the value of a practice in its local market.
Whatever methods are used, it is important that the appraisal be done by an experienced and independent financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation is supplied to support the conclusions reached. This is especially important if the appraisal will be relied upon in the sale or merger of the practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
.
Too often, physicians are not receiving a fair return on the equity they have worked so hard to build over several decades, either because they have waited too long and must accept what is offered, or because they simply take the buyer’s word for their practice’s value. Don’t put yourself in either of those positions, and don’t entertain any offers until you obtain an objective appraisal from a neutral party.
Of course, a medical practice is trickier to value than an ordinary business, and usually requires the services of an experienced professional appraiser. Entire books have been written about the process, so I can’t hope to cover it completely in 750 words; but three basic yardsticks are essential for determining the equity, or book value, of a practice:
- Tangible assets. Equipment, cash, accounts receivable, and other property owned by the practice.
- Liabilities. Accounts payable, outstanding loans, and anything else owed to others.
- Intangible assets. Sometimes called “good will” – the reputation of the physicians, the location and name recognition of the practice, the loyalty and volume of patients, and other, well, intangibles.
Valuing tangible assets is comparatively straightforward, but there are several ways to do it, and when reviewing a practice appraisal you should ask which of them was used. Depreciated value is the book value of equipment and supplies as determined by their purchase price, less the amount their value has decreased since purchase. Remaining useful life value estimates how long the equipment can be expected to last. Market (or replacement) value is the amount it would cost on the open market to replace all equipment and supplies.
Intangible assets are more difficult to value. Many components are analyzed, including location, interior and exterior decor, accessibility to patients, age and functional status of equipment, systems in place to promote efficiency, reasons why patients come back (if in fact they do), and the overall reputation of the practice in the community. Other important factors include the “payer mix” (what percentage pays cash, how many third-party contracts are in place and how well they pay, etcetera), the extent and strength of the referral base, and the presence of supplemental income streams, such as clinical research.
It is also important to determine to what extent intangible assets are transferable. For example, unique skills with a laser, neurotoxins, or filler substances, or extraordinary personal charisma, may increase your practice’s value to you, but they are worthless to the next owner, and he or she will be unwilling to pay for them unless your services become part of the deal.
Once again there are many ways to estimate intangible asset value, and once again you should ask which were used. Cash flow analysis works on the assumption that cash flow is a measure of intangible value. Capitalization of earnings puts a value, or capitalization, on the practice’s income streams using a variety of assumptions. Guideline comparison uses various databases to compare your practice with other, similar ones that have changed hands in the past.
Two newer techniques that some consider a better estimate of intangible assets are the replacement method, which estimates the costs of starting the practice over again in the current market; and the excess earnings method, which measures how far above average your practice’s earnings (and thus its overall value) are.
Asset-based valuation is the most popular, but by no means the only method available. Income-based valuation looks at the source and strength of a practice’s income stream as a creator of value, as well as whether or not its income stream under a different owner would mirror its present one. This in turn becomes the basis for an understanding of the fair market value of both tangible and intangible assets. Market valuation combines the asset-based and income-based approaches, along with an analysis of sales and mergers of comparable practices in the community, to determine the value of a practice in its local market.
Whatever methods are used, it is important that the appraisal be done by an experienced and independent financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation is supplied to support the conclusions reached. This is especially important if the appraisal will be relied upon in the sale or merger of the practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Tips for connecting with your patients
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
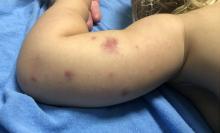
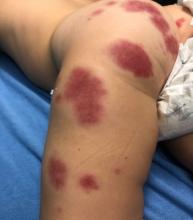
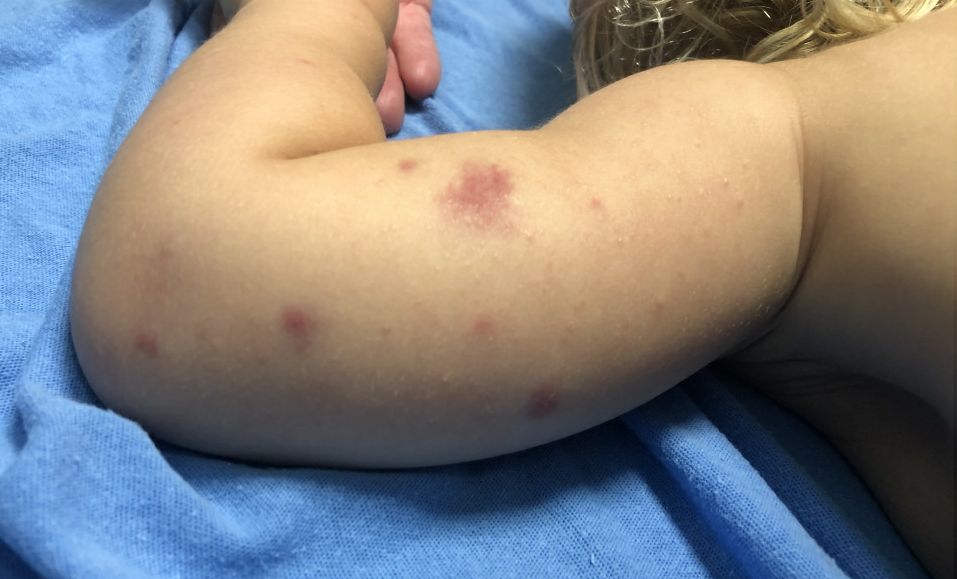
A 19-month-old vaccinated female with 2 days of rash
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Treatment duration for acute otitis media – so many choices
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.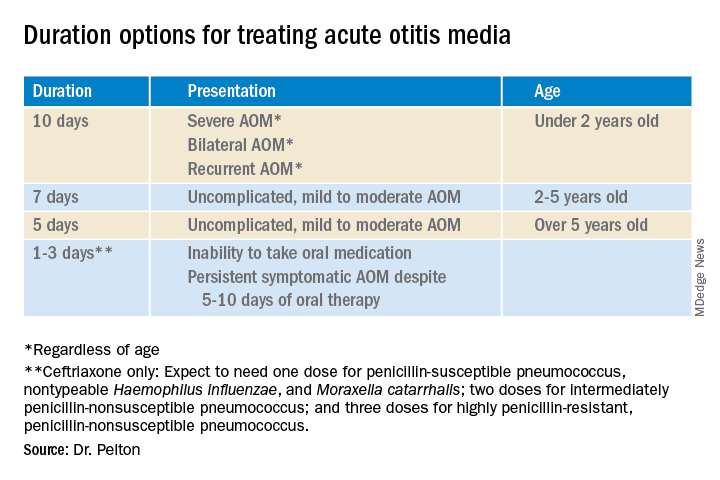
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.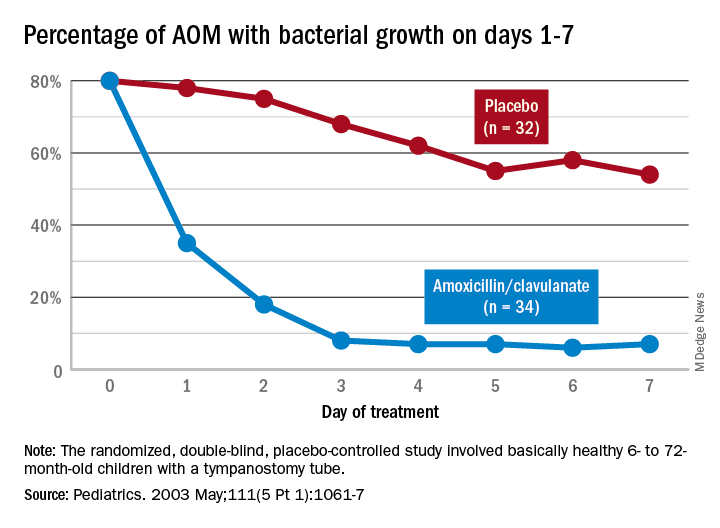
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at pdnews@mdedge.com.
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at pdnews@mdedge.com.
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at pdnews@mdedge.com.
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
ctDNA shows promise for assessing lung cancer treatment response
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
Caring for suicidal youth: An approach for pediatricians
This month’s column is driven by the recent increase of youth in crisis, and COVID-19–related limitations of higher-level services. Suicide is the second leading cause of death among youth1 and populations who face discrimination are at increased risk.2,3
A pediatrician colleague recently asked me about how to support patients who may be at risk. With inpatient units and emergency departments over capacity, properly allocating resources to patients with the most acute needs is crucial. When appropriate, providing preventive suicide care in primary care similarly saves lives.
Case summary
Cassandra is a 16-year-old Black girl who told a friend on Snapchat that she did not want to be alive. The friend told her parents and Cassandra’s parents brought their daughter to an urgent primary care appointment. Cassandra has had a history of difficulty with large transitions like a family move when she was 13. She spent more time in her room for several months before joining the volleyball team and making new friends. She has always done well academically in school but struggled with insomnia and classwork when her high school shifted to remote learning for the 2020-2021 school year because of the pandemic. This year she attends school in person but is unable to play volleyball because of COVID-19 restrictions. Her parents report that she is again spending more time online in her room. She is passing her classes and doing well in math, but overall, her grades have fallen since the pandemic began. She reports recent difficulties with friends and notes feeling hopeless about a changing climate and race relations in the United States.
Discussion
This case example illustrates some factors pediatricians can consider in determining how to proceed in similar circumstances. What are Cassandra’s immediate risk and treatment needs? In cases like Cassandra’s, the American Academy of Pediatrics recommends the ABCD (Assess, Build hope, Connect, Develop a safety plan) approach.4 Preparing practices to deliver this best possible preventive suicide care is essential.
1. Is this patient at imminent risk of harming herself?
Assess: Screen for suicide risk and assess risk level. Several standardized screening tools exist for gauging a patient’s risk. The Ask Suicide Screening Questionnaire (asQ) is a straightforward screening tool (not to be confused with the ASQ Ages and Stages developmental screening). These questionnaires take only a few minutes and next steps are suggested depending on the score (low, moderate, or high risk) and clinical judgment. What matters most is using a standardized screener to directly ask questions about suicide and then follow up appropriately based on risk.
2. What can be done during the visit to promote a good outcome?
Build hope/reasons for living. Validate that people sometimes feel suicidal when things are difficult, but that the feelings come and go and people go on to live meaningful lives. Tell the patients that you care about keeping them safe when the feelings come up. Motivational interviewing can be helpful to reflect back patient-identified reasons for living. Genuinely tell the patients how much you care about their wellbeing.
3. What can be done outside the visit to promote a good outcome?
Connect: Strengthen connections with protective adults. Make a plan to have the patient connect regularly with parents/trusted adults. She could engage in social action, or connect one-on-one. With more structured social opportunities, she will spend less time online. Medical practices can reach out with postcards and phone calls to show that they care about the patient, an intervention called “Caring Contacts” that has been shown to decrease suicide.
4. Once suicide risk is identified, what are specific tools to use during the visit to keep her safe?
Develop a plan for staying safe: Restrict access to lethal means, develop a safety plan and healthy ways of coping. There is a free 2-hour CALM (Counseling on Access to Lethal Means) training to help providers feel competent in restricting access to lethal means prior to increased risk. This resource provides safety plan templates that help identify triggers, specific ways to stay safe, people to talk to, and suicide prevention resources including lifelines (988) and chat options (text 2 letter state to 741741).
Enacting suicide prevention requires practice readiness and workflow changes. Providers should assess mental health supports in and out of the office, and then rehearse workflow around suicide prevention care. Increasingly, there are embedded case managers or behavioral health providers available. Sometimes local mental health crisis services are the best option. A practice introductory letter to community mental health practitioners can improve later coordination efforts when caring for suicidal youth. Having practice-level support for provider well-being can improve outcomes.
Case follow-up
After interviewing the girl separately, and performing a PHQ-A and an asQ, followed by the Brief Suicide Safety Assessment to screen for acuity, the pediatrician felt confident that Cassandra was suffering from moderate depression and had moderate but not imminent risk of suicide. Options to treat her depression were discussed with Cassandra and her parents, and a referral to therapy was made.
The provider knew that depression care is complementary but not sufficient as standalone suicide prevention. The provider used the asQ pathway to determine next steps. He made a safety plan, and referred her to an outpatient mental health clinician with whom the practice had an established relationship for an urgent mental health evaluation. A follow-up primary care appointment was scheduled within 72 hours to re-check safety and ensure that she had an appointment scheduled to start therapy. A nurse contacted the patient and her family regularly to check on her wellbeing. Her parents made a plan with her volleyball coach to organize outdoor off-season conditioning to help with exercise and socializing. The family removed screens prior to bedtime and sleep improved. At a 3-month follow-up, Cassandra had only mild depressive symptoms and the frequency and intensity of her suicidal ideation had decreased.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont, a Federally Qualified Health Center. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. National Institute of Mental Health: Suicide.
2. Hottes TS et al. Am J Public Health. 2016 May;106(5):e1-12.
3. Bridge JA et al. JAMA Pediatr. 2018;172(7):697-9.
4. Asarnow JR. SAMHSA Center for Adolescent Suicide and Self-Harm..
This month’s column is driven by the recent increase of youth in crisis, and COVID-19–related limitations of higher-level services. Suicide is the second leading cause of death among youth1 and populations who face discrimination are at increased risk.2,3
A pediatrician colleague recently asked me about how to support patients who may be at risk. With inpatient units and emergency departments over capacity, properly allocating resources to patients with the most acute needs is crucial. When appropriate, providing preventive suicide care in primary care similarly saves lives.
Case summary
Cassandra is a 16-year-old Black girl who told a friend on Snapchat that she did not want to be alive. The friend told her parents and Cassandra’s parents brought their daughter to an urgent primary care appointment. Cassandra has had a history of difficulty with large transitions like a family move when she was 13. She spent more time in her room for several months before joining the volleyball team and making new friends. She has always done well academically in school but struggled with insomnia and classwork when her high school shifted to remote learning for the 2020-2021 school year because of the pandemic. This year she attends school in person but is unable to play volleyball because of COVID-19 restrictions. Her parents report that she is again spending more time online in her room. She is passing her classes and doing well in math, but overall, her grades have fallen since the pandemic began. She reports recent difficulties with friends and notes feeling hopeless about a changing climate and race relations in the United States.
Discussion
This case example illustrates some factors pediatricians can consider in determining how to proceed in similar circumstances. What are Cassandra’s immediate risk and treatment needs? In cases like Cassandra’s, the American Academy of Pediatrics recommends the ABCD (Assess, Build hope, Connect, Develop a safety plan) approach.4 Preparing practices to deliver this best possible preventive suicide care is essential.
1. Is this patient at imminent risk of harming herself?
Assess: Screen for suicide risk and assess risk level. Several standardized screening tools exist for gauging a patient’s risk. The Ask Suicide Screening Questionnaire (asQ) is a straightforward screening tool (not to be confused with the ASQ Ages and Stages developmental screening). These questionnaires take only a few minutes and next steps are suggested depending on the score (low, moderate, or high risk) and clinical judgment. What matters most is using a standardized screener to directly ask questions about suicide and then follow up appropriately based on risk.
2. What can be done during the visit to promote a good outcome?
Build hope/reasons for living. Validate that people sometimes feel suicidal when things are difficult, but that the feelings come and go and people go on to live meaningful lives. Tell the patients that you care about keeping them safe when the feelings come up. Motivational interviewing can be helpful to reflect back patient-identified reasons for living. Genuinely tell the patients how much you care about their wellbeing.
3. What can be done outside the visit to promote a good outcome?
Connect: Strengthen connections with protective adults. Make a plan to have the patient connect regularly with parents/trusted adults. She could engage in social action, or connect one-on-one. With more structured social opportunities, she will spend less time online. Medical practices can reach out with postcards and phone calls to show that they care about the patient, an intervention called “Caring Contacts” that has been shown to decrease suicide.
4. Once suicide risk is identified, what are specific tools to use during the visit to keep her safe?
Develop a plan for staying safe: Restrict access to lethal means, develop a safety plan and healthy ways of coping. There is a free 2-hour CALM (Counseling on Access to Lethal Means) training to help providers feel competent in restricting access to lethal means prior to increased risk. This resource provides safety plan templates that help identify triggers, specific ways to stay safe, people to talk to, and suicide prevention resources including lifelines (988) and chat options (text 2 letter state to 741741).
Enacting suicide prevention requires practice readiness and workflow changes. Providers should assess mental health supports in and out of the office, and then rehearse workflow around suicide prevention care. Increasingly, there are embedded case managers or behavioral health providers available. Sometimes local mental health crisis services are the best option. A practice introductory letter to community mental health practitioners can improve later coordination efforts when caring for suicidal youth. Having practice-level support for provider well-being can improve outcomes.
Case follow-up
After interviewing the girl separately, and performing a PHQ-A and an asQ, followed by the Brief Suicide Safety Assessment to screen for acuity, the pediatrician felt confident that Cassandra was suffering from moderate depression and had moderate but not imminent risk of suicide. Options to treat her depression were discussed with Cassandra and her parents, and a referral to therapy was made.
The provider knew that depression care is complementary but not sufficient as standalone suicide prevention. The provider used the asQ pathway to determine next steps. He made a safety plan, and referred her to an outpatient mental health clinician with whom the practice had an established relationship for an urgent mental health evaluation. A follow-up primary care appointment was scheduled within 72 hours to re-check safety and ensure that she had an appointment scheduled to start therapy. A nurse contacted the patient and her family regularly to check on her wellbeing. Her parents made a plan with her volleyball coach to organize outdoor off-season conditioning to help with exercise and socializing. The family removed screens prior to bedtime and sleep improved. At a 3-month follow-up, Cassandra had only mild depressive symptoms and the frequency and intensity of her suicidal ideation had decreased.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont, a Federally Qualified Health Center. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. National Institute of Mental Health: Suicide.
2. Hottes TS et al. Am J Public Health. 2016 May;106(5):e1-12.
3. Bridge JA et al. JAMA Pediatr. 2018;172(7):697-9.
4. Asarnow JR. SAMHSA Center for Adolescent Suicide and Self-Harm..
This month’s column is driven by the recent increase of youth in crisis, and COVID-19–related limitations of higher-level services. Suicide is the second leading cause of death among youth1 and populations who face discrimination are at increased risk.2,3
A pediatrician colleague recently asked me about how to support patients who may be at risk. With inpatient units and emergency departments over capacity, properly allocating resources to patients with the most acute needs is crucial. When appropriate, providing preventive suicide care in primary care similarly saves lives.
Case summary
Cassandra is a 16-year-old Black girl who told a friend on Snapchat that she did not want to be alive. The friend told her parents and Cassandra’s parents brought their daughter to an urgent primary care appointment. Cassandra has had a history of difficulty with large transitions like a family move when she was 13. She spent more time in her room for several months before joining the volleyball team and making new friends. She has always done well academically in school but struggled with insomnia and classwork when her high school shifted to remote learning for the 2020-2021 school year because of the pandemic. This year she attends school in person but is unable to play volleyball because of COVID-19 restrictions. Her parents report that she is again spending more time online in her room. She is passing her classes and doing well in math, but overall, her grades have fallen since the pandemic began. She reports recent difficulties with friends and notes feeling hopeless about a changing climate and race relations in the United States.
Discussion
This case example illustrates some factors pediatricians can consider in determining how to proceed in similar circumstances. What are Cassandra’s immediate risk and treatment needs? In cases like Cassandra’s, the American Academy of Pediatrics recommends the ABCD (Assess, Build hope, Connect, Develop a safety plan) approach.4 Preparing practices to deliver this best possible preventive suicide care is essential.
1. Is this patient at imminent risk of harming herself?
Assess: Screen for suicide risk and assess risk level. Several standardized screening tools exist for gauging a patient’s risk. The Ask Suicide Screening Questionnaire (asQ) is a straightforward screening tool (not to be confused with the ASQ Ages and Stages developmental screening). These questionnaires take only a few minutes and next steps are suggested depending on the score (low, moderate, or high risk) and clinical judgment. What matters most is using a standardized screener to directly ask questions about suicide and then follow up appropriately based on risk.
2. What can be done during the visit to promote a good outcome?
Build hope/reasons for living. Validate that people sometimes feel suicidal when things are difficult, but that the feelings come and go and people go on to live meaningful lives. Tell the patients that you care about keeping them safe when the feelings come up. Motivational interviewing can be helpful to reflect back patient-identified reasons for living. Genuinely tell the patients how much you care about their wellbeing.
3. What can be done outside the visit to promote a good outcome?
Connect: Strengthen connections with protective adults. Make a plan to have the patient connect regularly with parents/trusted adults. She could engage in social action, or connect one-on-one. With more structured social opportunities, she will spend less time online. Medical practices can reach out with postcards and phone calls to show that they care about the patient, an intervention called “Caring Contacts” that has been shown to decrease suicide.
4. Once suicide risk is identified, what are specific tools to use during the visit to keep her safe?
Develop a plan for staying safe: Restrict access to lethal means, develop a safety plan and healthy ways of coping. There is a free 2-hour CALM (Counseling on Access to Lethal Means) training to help providers feel competent in restricting access to lethal means prior to increased risk. This resource provides safety plan templates that help identify triggers, specific ways to stay safe, people to talk to, and suicide prevention resources including lifelines (988) and chat options (text 2 letter state to 741741).
Enacting suicide prevention requires practice readiness and workflow changes. Providers should assess mental health supports in and out of the office, and then rehearse workflow around suicide prevention care. Increasingly, there are embedded case managers or behavioral health providers available. Sometimes local mental health crisis services are the best option. A practice introductory letter to community mental health practitioners can improve later coordination efforts when caring for suicidal youth. Having practice-level support for provider well-being can improve outcomes.
Case follow-up
After interviewing the girl separately, and performing a PHQ-A and an asQ, followed by the Brief Suicide Safety Assessment to screen for acuity, the pediatrician felt confident that Cassandra was suffering from moderate depression and had moderate but not imminent risk of suicide. Options to treat her depression were discussed with Cassandra and her parents, and a referral to therapy was made.
The provider knew that depression care is complementary but not sufficient as standalone suicide prevention. The provider used the asQ pathway to determine next steps. He made a safety plan, and referred her to an outpatient mental health clinician with whom the practice had an established relationship for an urgent mental health evaluation. A follow-up primary care appointment was scheduled within 72 hours to re-check safety and ensure that she had an appointment scheduled to start therapy. A nurse contacted the patient and her family regularly to check on her wellbeing. Her parents made a plan with her volleyball coach to organize outdoor off-season conditioning to help with exercise and socializing. The family removed screens prior to bedtime and sleep improved. At a 3-month follow-up, Cassandra had only mild depressive symptoms and the frequency and intensity of her suicidal ideation had decreased.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont, a Federally Qualified Health Center. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. National Institute of Mental Health: Suicide.
2. Hottes TS et al. Am J Public Health. 2016 May;106(5):e1-12.
3. Bridge JA et al. JAMA Pediatr. 2018;172(7):697-9.
4. Asarnow JR. SAMHSA Center for Adolescent Suicide and Self-Harm..
Did you know these things about nicotine? Your patients don’t
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
A test for cannabis-caused impairment
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
“I didn’t want to meet you.” Dispelling myths about palliative care
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.