User login
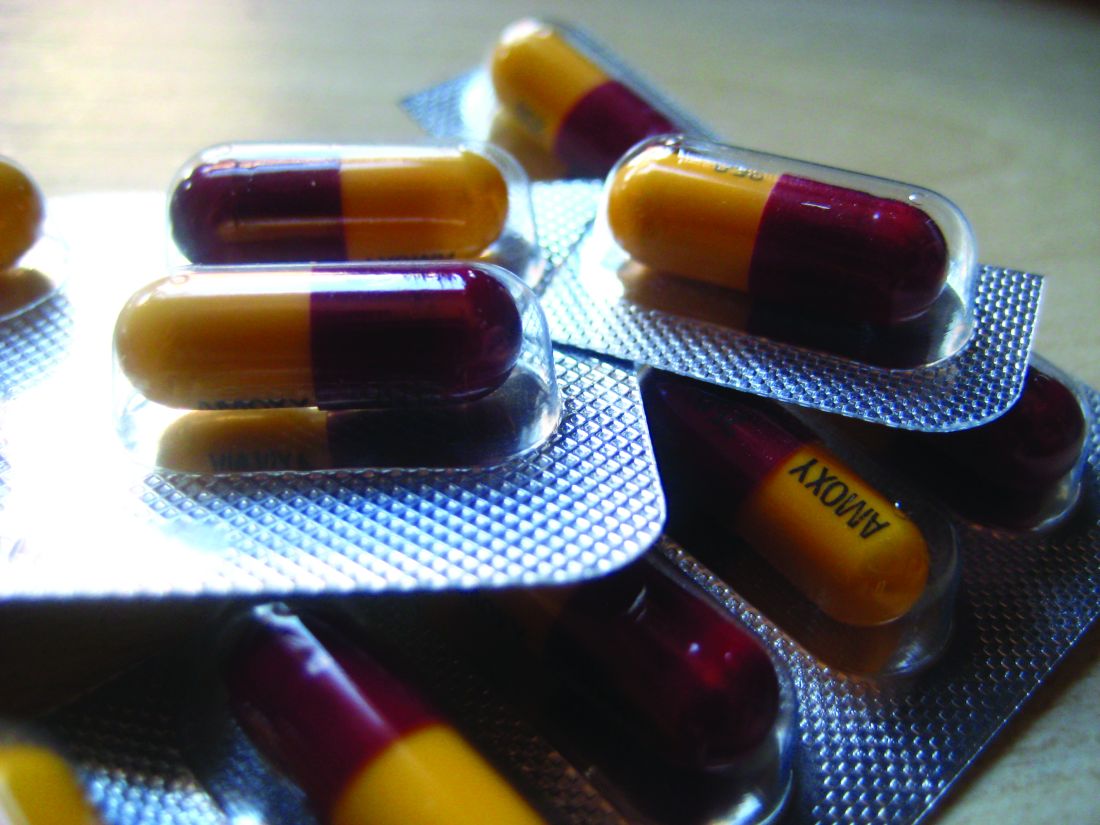
‘Alarming’ increase in fake pills laced with fentanyl, methamphetamine, DEA warns
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
Should Geriatric Veterans Get Immunotherapy?
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
FDA approves first oral drug for NSCLC with EGFR Exon 20 insertion
The drug is limited to use in patients whose disease has progressed on or after platinum-based chemotherapy and who have had the EGFR Exon 20 insertion mutation detected on an FDA-approved test.
Mobocertinib is the first oral tyrosine kinase inhibitor (TKI) specifically designed to target these mutations, which are less common than the more predominant EGFR mutations in this lung cancer.
“EGFR Exon 20 insertion+ NSCLC is an underserved cancer that we have been unable to target effectively with traditional EGFR TKIs,” said Pasi Jänne, MD, PhD, of the Dana Farber Cancer Institute, Boston, in a press statement from the maker, Takeda.
“The approval of [mobocertinib] marks another important step forward that provides physicians and their patients with a new targeted oral therapy specifically designed for this patient population that has shown clinically meaningful and sustained responses,” Dr. Jänne added.
According to the company, EGFR Exon 20 insertion+ NSCLC makes up approximately 1%-2% of patients with NSCLC and is more common in Asian populations compared with Western populations.
The new approval is based on overall response rate (ORR) and duration of response (DoR) results from a phase 1/2 trial consisting of 114 patients with EGFR Exon 20 insertion+ NSCLC who received prior platinum-based therapy and were treated with the 160-mg dose.
Per an independent review committee, mobocertinib demonstrated a confirmed ORR of 28% and a median DoR of 17.5 months.
Median overall survival was 24 months and median progression-free survival was 7.3 months.
The FDA-approved next-generation sequencing (NGS) companion diagnostic for mobocertinib is Thermo Fisher Scientific’s Oncomine Dx Target Test, which identifies NSCLC patients with EGFR Exon 20 insertions.
“NGS testing is critical for these patients, as it can enable more accurate diagnoses compared to polymerase chain reaction (PCR) testing, which detects less than 50% of EGFR Exon 20 insertions,” according to the company.
Results from the phase 1/2 trial used in the FDA approval were presented at the 2021 American Society of Clinical Oncology Annual Meeting.
The most common adverse reactions (greater than 20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain, according to the company.
The prescribing information includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings and precautions for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
“Patients with EGFR Exon 20 insertion+ NSCLC have historically faced a unique set of challenges living with a very rare lung cancer that is not only underdiagnosed but also lacking targeted treatment options that can improve response rates,” said Marcia Horn, executive director, Exon 20 Group at the International Cancer Advocacy Network, in the press statement.
The FDA review was conducted under Project Orbis, an FDA initiative that enables concurrent submission and review of oncology products among international partners.
The new drug was also granted priority review and received breakthrough therapy, fast track, and orphan drug designations from the FDA.
A version of this article first appeared on Medscape.com.
The drug is limited to use in patients whose disease has progressed on or after platinum-based chemotherapy and who have had the EGFR Exon 20 insertion mutation detected on an FDA-approved test.
Mobocertinib is the first oral tyrosine kinase inhibitor (TKI) specifically designed to target these mutations, which are less common than the more predominant EGFR mutations in this lung cancer.
“EGFR Exon 20 insertion+ NSCLC is an underserved cancer that we have been unable to target effectively with traditional EGFR TKIs,” said Pasi Jänne, MD, PhD, of the Dana Farber Cancer Institute, Boston, in a press statement from the maker, Takeda.
“The approval of [mobocertinib] marks another important step forward that provides physicians and their patients with a new targeted oral therapy specifically designed for this patient population that has shown clinically meaningful and sustained responses,” Dr. Jänne added.
According to the company, EGFR Exon 20 insertion+ NSCLC makes up approximately 1%-2% of patients with NSCLC and is more common in Asian populations compared with Western populations.
The new approval is based on overall response rate (ORR) and duration of response (DoR) results from a phase 1/2 trial consisting of 114 patients with EGFR Exon 20 insertion+ NSCLC who received prior platinum-based therapy and were treated with the 160-mg dose.
Per an independent review committee, mobocertinib demonstrated a confirmed ORR of 28% and a median DoR of 17.5 months.
Median overall survival was 24 months and median progression-free survival was 7.3 months.
The FDA-approved next-generation sequencing (NGS) companion diagnostic for mobocertinib is Thermo Fisher Scientific’s Oncomine Dx Target Test, which identifies NSCLC patients with EGFR Exon 20 insertions.
“NGS testing is critical for these patients, as it can enable more accurate diagnoses compared to polymerase chain reaction (PCR) testing, which detects less than 50% of EGFR Exon 20 insertions,” according to the company.
Results from the phase 1/2 trial used in the FDA approval were presented at the 2021 American Society of Clinical Oncology Annual Meeting.
The most common adverse reactions (greater than 20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain, according to the company.
The prescribing information includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings and precautions for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
“Patients with EGFR Exon 20 insertion+ NSCLC have historically faced a unique set of challenges living with a very rare lung cancer that is not only underdiagnosed but also lacking targeted treatment options that can improve response rates,” said Marcia Horn, executive director, Exon 20 Group at the International Cancer Advocacy Network, in the press statement.
The FDA review was conducted under Project Orbis, an FDA initiative that enables concurrent submission and review of oncology products among international partners.
The new drug was also granted priority review and received breakthrough therapy, fast track, and orphan drug designations from the FDA.
A version of this article first appeared on Medscape.com.
The drug is limited to use in patients whose disease has progressed on or after platinum-based chemotherapy and who have had the EGFR Exon 20 insertion mutation detected on an FDA-approved test.
Mobocertinib is the first oral tyrosine kinase inhibitor (TKI) specifically designed to target these mutations, which are less common than the more predominant EGFR mutations in this lung cancer.
“EGFR Exon 20 insertion+ NSCLC is an underserved cancer that we have been unable to target effectively with traditional EGFR TKIs,” said Pasi Jänne, MD, PhD, of the Dana Farber Cancer Institute, Boston, in a press statement from the maker, Takeda.
“The approval of [mobocertinib] marks another important step forward that provides physicians and their patients with a new targeted oral therapy specifically designed for this patient population that has shown clinically meaningful and sustained responses,” Dr. Jänne added.
According to the company, EGFR Exon 20 insertion+ NSCLC makes up approximately 1%-2% of patients with NSCLC and is more common in Asian populations compared with Western populations.
The new approval is based on overall response rate (ORR) and duration of response (DoR) results from a phase 1/2 trial consisting of 114 patients with EGFR Exon 20 insertion+ NSCLC who received prior platinum-based therapy and were treated with the 160-mg dose.
Per an independent review committee, mobocertinib demonstrated a confirmed ORR of 28% and a median DoR of 17.5 months.
Median overall survival was 24 months and median progression-free survival was 7.3 months.
The FDA-approved next-generation sequencing (NGS) companion diagnostic for mobocertinib is Thermo Fisher Scientific’s Oncomine Dx Target Test, which identifies NSCLC patients with EGFR Exon 20 insertions.
“NGS testing is critical for these patients, as it can enable more accurate diagnoses compared to polymerase chain reaction (PCR) testing, which detects less than 50% of EGFR Exon 20 insertions,” according to the company.
Results from the phase 1/2 trial used in the FDA approval were presented at the 2021 American Society of Clinical Oncology Annual Meeting.
The most common adverse reactions (greater than 20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain, according to the company.
The prescribing information includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings and precautions for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
“Patients with EGFR Exon 20 insertion+ NSCLC have historically faced a unique set of challenges living with a very rare lung cancer that is not only underdiagnosed but also lacking targeted treatment options that can improve response rates,” said Marcia Horn, executive director, Exon 20 Group at the International Cancer Advocacy Network, in the press statement.
The FDA review was conducted under Project Orbis, an FDA initiative that enables concurrent submission and review of oncology products among international partners.
The new drug was also granted priority review and received breakthrough therapy, fast track, and orphan drug designations from the FDA.
A version of this article first appeared on Medscape.com.
Moderna vaccine more effective than Pfizer and J&J
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
Nonopioid med promising for neuropathic pain
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Low RA flare rate reported after Pfizer COVID vaccination
Patients with rheumatoid arthritis in remission had a rate of flare following vaccination with the Pfizer/BioNtech COVID-19 vaccine that appears to be on par with rates seen with other vaccines in patients with RA, according to results from a small Italian cohort study.
“Our data show a very low flare rate [7.8% (6 of 77)] after the BNT162b2 COVID-19 vaccine in patients with RA in remission and are consistent with previous findings about varicella-zoster virus (6.7%) and hepatitis B virus (2.2%) vaccinations,” Riccardo Bixio, MD, and colleagues from University of Verona (Italy) Hospital Trust wrote in ACR Open Rheumatology. “Because remission is not commonly obtained in the real world, we are aware that our findings may not be generalizable to all patients with RA receiving COVID-19 vaccination.”
Other studies of flare rate after COVID-19 vaccination in patients with a variety of rheumatic and musculoskeletal diseases have reported rates ranging from 5% to 17%, they said.
The 77 consecutive patients from the University of Verona center that conducted the study were all in clinical remission in the 3 months before vaccination based on a 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP) of less than 2.6, and all had discontinued antirheumatic therapies according to American College of Rheumatology COVID-19 recommendations. The researchers defined flares as agreement between patient and rheumatologist assessments and a DAS28-CRP increase of more than 1.2.
Five of the six people with a flare had it occur after the second dose at a mean of 2.6 days later, and all flares were resolved within 2 weeks using glucocorticoids with or without anti-inflammatory drugs. One flare was called severe. The overall disease activity of the cohort after 3 months was not significantly changed after vaccination.
In noting that five out of the six patients with flares had withdrawn or delayed antirheumatic therapies around the time of vaccination according to ACR recommendations, the authors wrote that “Even if there is no direct evidence that holding therapies could occur in a higher proportion of disease flares, we suggest that clinicians consider this possibility when counseling patients about COVID-19 vaccination.”
The authors had no outside funding for the study and had no potential conflicts of interest to disclose.
Patients with rheumatoid arthritis in remission had a rate of flare following vaccination with the Pfizer/BioNtech COVID-19 vaccine that appears to be on par with rates seen with other vaccines in patients with RA, according to results from a small Italian cohort study.
“Our data show a very low flare rate [7.8% (6 of 77)] after the BNT162b2 COVID-19 vaccine in patients with RA in remission and are consistent with previous findings about varicella-zoster virus (6.7%) and hepatitis B virus (2.2%) vaccinations,” Riccardo Bixio, MD, and colleagues from University of Verona (Italy) Hospital Trust wrote in ACR Open Rheumatology. “Because remission is not commonly obtained in the real world, we are aware that our findings may not be generalizable to all patients with RA receiving COVID-19 vaccination.”
Other studies of flare rate after COVID-19 vaccination in patients with a variety of rheumatic and musculoskeletal diseases have reported rates ranging from 5% to 17%, they said.
The 77 consecutive patients from the University of Verona center that conducted the study were all in clinical remission in the 3 months before vaccination based on a 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP) of less than 2.6, and all had discontinued antirheumatic therapies according to American College of Rheumatology COVID-19 recommendations. The researchers defined flares as agreement between patient and rheumatologist assessments and a DAS28-CRP increase of more than 1.2.
Five of the six people with a flare had it occur after the second dose at a mean of 2.6 days later, and all flares were resolved within 2 weeks using glucocorticoids with or without anti-inflammatory drugs. One flare was called severe. The overall disease activity of the cohort after 3 months was not significantly changed after vaccination.
In noting that five out of the six patients with flares had withdrawn or delayed antirheumatic therapies around the time of vaccination according to ACR recommendations, the authors wrote that “Even if there is no direct evidence that holding therapies could occur in a higher proportion of disease flares, we suggest that clinicians consider this possibility when counseling patients about COVID-19 vaccination.”
The authors had no outside funding for the study and had no potential conflicts of interest to disclose.
Patients with rheumatoid arthritis in remission had a rate of flare following vaccination with the Pfizer/BioNtech COVID-19 vaccine that appears to be on par with rates seen with other vaccines in patients with RA, according to results from a small Italian cohort study.
“Our data show a very low flare rate [7.8% (6 of 77)] after the BNT162b2 COVID-19 vaccine in patients with RA in remission and are consistent with previous findings about varicella-zoster virus (6.7%) and hepatitis B virus (2.2%) vaccinations,” Riccardo Bixio, MD, and colleagues from University of Verona (Italy) Hospital Trust wrote in ACR Open Rheumatology. “Because remission is not commonly obtained in the real world, we are aware that our findings may not be generalizable to all patients with RA receiving COVID-19 vaccination.”
Other studies of flare rate after COVID-19 vaccination in patients with a variety of rheumatic and musculoskeletal diseases have reported rates ranging from 5% to 17%, they said.
The 77 consecutive patients from the University of Verona center that conducted the study were all in clinical remission in the 3 months before vaccination based on a 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP) of less than 2.6, and all had discontinued antirheumatic therapies according to American College of Rheumatology COVID-19 recommendations. The researchers defined flares as agreement between patient and rheumatologist assessments and a DAS28-CRP increase of more than 1.2.
Five of the six people with a flare had it occur after the second dose at a mean of 2.6 days later, and all flares were resolved within 2 weeks using glucocorticoids with or without anti-inflammatory drugs. One flare was called severe. The overall disease activity of the cohort after 3 months was not significantly changed after vaccination.
In noting that five out of the six patients with flares had withdrawn or delayed antirheumatic therapies around the time of vaccination according to ACR recommendations, the authors wrote that “Even if there is no direct evidence that holding therapies could occur in a higher proportion of disease flares, we suggest that clinicians consider this possibility when counseling patients about COVID-19 vaccination.”
The authors had no outside funding for the study and had no potential conflicts of interest to disclose.
FROM ACR OPEN RHEUMATOLOGY
Antibiotic use and colon cancer: More evidence of link
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
Will interchangeable insulin be more affordable in the U.S.?
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
Anakinra improved survival in hospitalized COVID-19 patients
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
FROM NATURE MEDICINE
‘Dawn of a new era’ in the treatment of renal cell carcinoma
according to expert opinion.
The high hopes have been generated by results from the randomized, phase 3 KEYNOTE-564 trial, showing that monotherapy with pembrolizumab (Keytruda, Merck) was associated with significantly longer disease-free survival (DFS) after nephrectomy than placebo (77.3% vs. 68.1%, respectively). Median follow-up was 24 months.
The results come from the trial’s first interim analysis of data from 994 patients with clear-cell renal cell carcinoma (RCC) at high risk of recurrence.
For the pembrolizumab group, the estimated percentage alive at 24 months was 96.6%, compared with 93.5% in the placebo group (hazard ratio for death, 0.54), said Toni Choueiri, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
However, grade 3 or higher adverse events (any cause) occurred at almost twice the rate in the pembrolizumab versus the placebo group (32.4% vs. 17.7%). The new study was published online Aug. 18, 2021, in the New England Journal of Medicine.
The study results were first presented at the 2021 American Society of Clinical Oncology annual meeting and described as likely to be practice changing in this setting, as reported by this news organization.
Currently, this patient population has “no options for adjuvant therapy to reduce the risk of recurrence that have high levels of supporting evidence,” observed the authors.
That’s about to change, as the trial results “herald the dawn of a new era in the treatment of renal cell carcinoma,” Rana McKay, MD, University of California San Diego Health, wrote in an accompanying editorial.
Multiple studies have investigated potential adjuvant therapies in RCC since the 1980s, she observed.
“For the first time, we now have an effective adjuvant immunotherapy option for patients with resected renal cell carcinoma at high risk of recurrence,” Dr. McKay said in an interview.
To date, the lack of clinically beneficial adjuvant therapy options in RCC has been “humbling,” Dr. Choueiri said in an interview. “We hope we can push the envelope further and get more patients with RCC some good options that make them live longer and better.”
Although the standard of care for patients diagnosed with locoregional RCC is partial or total nephrectomy, nearly half of patients eventually experience disease recurrence following surgery, Dr. Choueiri noted.
“No standard, globally approved adjuvant therapy options are currently available for this population,” he said. Clinical guidelines recommend patients at high risk of disease recurrence after surgery be entered into a clinical trial or undergo active surveillance.
Researchers will continue to follow the results for overall survival, a secondary endpoint. “The very early look suggests encouraging results [in overall survival] with an HR of 0.54,” Dr. Choueiri noted.
In the meantime, the prolongation of DFS represents a clear clinical benefit, said Dr. McKay, “given the magnitude of the increase” and “the limited incidence of toxic effects.”
KEYNOTE-564 will alter the adjuvant treatment landscape for RCC as a positive phase 3 trial of adjuvant immunotherapy for the disease, she added.
A number of earlier studies have investigated the use of adjuvant vascular endothelial growth factor–targeting agents in RCC. Only the 2016 Sunitinib Treatment of Renal Adjuvant Cancer (S-TRAC) trial showed improved DFS with sunitinib, compared with placebo (6.8 vs. 5.6 years). Subsequently, sunitinib was approved for adjuvant use in the United States. However, the S-TRAC trial also showed that sunitinib therapy was associated with an increased incidence of toxic effects and lower quality of life scores, and researchers did not observe any benefit in overall survival.
“Despite regulatory approval in the U.S., sunitinib is not approved for adjuvant use by the European Medicines Agency and has limited utilization in clinical practice given the low benefit-risk ratio,” Dr. McKay pointed out.
Study details
KEYNOTE-564 involved 996 patients with clear-cell RCC at high risk for recurrence after nephrectomy, with or without metastasectomy. They were randomly assigned in a 1:1 ratio to receive a 200-mg dose of adjuvant pembrolizumab or placebo given intravenously once every 3 weeks for up to 17 cycles for approximately 1 year.
The vast majority of patients enrolled in the study had localized disease with no evidence of metastases (M0) and intermediate to high or high risk of disease recurrence after partial or complete nephrectomy. However, 5.8% of patients in both the pembrolizumab and placebo groups had M1 NED (metastatic stage 1, no evidence of disease) status after nephrectomy and resection of metastatic lesions. These patients were also at intermediate to high or high risk of recurrence.
The benefit of pembrolizumab, compared with placebo, was maintained in this subgroup, said the investigators. “At this point, we continue to look at the data, but we know that there was a benefit for DFS in the population we included,” said Dr. Choueiri. “When we looked at several subgroups such as PD-L1 status, geography, gender, performance status, M0/M1, all HRs were less than 1 suggesting benefit from pembrolizumab over placebo.”
“Subset analyses by stage are going to be important to determine which group of patients will derive the most benefit,” asserted Dr. McKay. “While those with M1 NED appear to derive benefit with HR for DFS of 0.29, those with M1 NED comprise a small percentage of patient enrolled in the trial.”
Studies exploring tissue- and blood-based biomarkers, including circulating tumor DNA, will be key to identify patients at highest risk for recurrence or adjuvant treatment, Dr. McKay emphasized. “The adoption of adjuvant immune checkpoint inhibitors brings along new questions regarding patient selection, therapeutic use in patients with non–clear-cell renal cell carcinoma, and systemic treatment after recurrence during or after the receipt of adjuvant therapy.”
KEYNOTE-564 was funded by Merck. Multiple study authors including Dr. Choueiri have financial ties to the pharmaceutical industry, including Merck.
A version of this article first appeared on Medscape.com.
according to expert opinion.
The high hopes have been generated by results from the randomized, phase 3 KEYNOTE-564 trial, showing that monotherapy with pembrolizumab (Keytruda, Merck) was associated with significantly longer disease-free survival (DFS) after nephrectomy than placebo (77.3% vs. 68.1%, respectively). Median follow-up was 24 months.
The results come from the trial’s first interim analysis of data from 994 patients with clear-cell renal cell carcinoma (RCC) at high risk of recurrence.
For the pembrolizumab group, the estimated percentage alive at 24 months was 96.6%, compared with 93.5% in the placebo group (hazard ratio for death, 0.54), said Toni Choueiri, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
However, grade 3 or higher adverse events (any cause) occurred at almost twice the rate in the pembrolizumab versus the placebo group (32.4% vs. 17.7%). The new study was published online Aug. 18, 2021, in the New England Journal of Medicine.
The study results were first presented at the 2021 American Society of Clinical Oncology annual meeting and described as likely to be practice changing in this setting, as reported by this news organization.
Currently, this patient population has “no options for adjuvant therapy to reduce the risk of recurrence that have high levels of supporting evidence,” observed the authors.
That’s about to change, as the trial results “herald the dawn of a new era in the treatment of renal cell carcinoma,” Rana McKay, MD, University of California San Diego Health, wrote in an accompanying editorial.
Multiple studies have investigated potential adjuvant therapies in RCC since the 1980s, she observed.
“For the first time, we now have an effective adjuvant immunotherapy option for patients with resected renal cell carcinoma at high risk of recurrence,” Dr. McKay said in an interview.
To date, the lack of clinically beneficial adjuvant therapy options in RCC has been “humbling,” Dr. Choueiri said in an interview. “We hope we can push the envelope further and get more patients with RCC some good options that make them live longer and better.”
Although the standard of care for patients diagnosed with locoregional RCC is partial or total nephrectomy, nearly half of patients eventually experience disease recurrence following surgery, Dr. Choueiri noted.
“No standard, globally approved adjuvant therapy options are currently available for this population,” he said. Clinical guidelines recommend patients at high risk of disease recurrence after surgery be entered into a clinical trial or undergo active surveillance.
Researchers will continue to follow the results for overall survival, a secondary endpoint. “The very early look suggests encouraging results [in overall survival] with an HR of 0.54,” Dr. Choueiri noted.
In the meantime, the prolongation of DFS represents a clear clinical benefit, said Dr. McKay, “given the magnitude of the increase” and “the limited incidence of toxic effects.”
KEYNOTE-564 will alter the adjuvant treatment landscape for RCC as a positive phase 3 trial of adjuvant immunotherapy for the disease, she added.
A number of earlier studies have investigated the use of adjuvant vascular endothelial growth factor–targeting agents in RCC. Only the 2016 Sunitinib Treatment of Renal Adjuvant Cancer (S-TRAC) trial showed improved DFS with sunitinib, compared with placebo (6.8 vs. 5.6 years). Subsequently, sunitinib was approved for adjuvant use in the United States. However, the S-TRAC trial also showed that sunitinib therapy was associated with an increased incidence of toxic effects and lower quality of life scores, and researchers did not observe any benefit in overall survival.
“Despite regulatory approval in the U.S., sunitinib is not approved for adjuvant use by the European Medicines Agency and has limited utilization in clinical practice given the low benefit-risk ratio,” Dr. McKay pointed out.
Study details
KEYNOTE-564 involved 996 patients with clear-cell RCC at high risk for recurrence after nephrectomy, with or without metastasectomy. They were randomly assigned in a 1:1 ratio to receive a 200-mg dose of adjuvant pembrolizumab or placebo given intravenously once every 3 weeks for up to 17 cycles for approximately 1 year.
The vast majority of patients enrolled in the study had localized disease with no evidence of metastases (M0) and intermediate to high or high risk of disease recurrence after partial or complete nephrectomy. However, 5.8% of patients in both the pembrolizumab and placebo groups had M1 NED (metastatic stage 1, no evidence of disease) status after nephrectomy and resection of metastatic lesions. These patients were also at intermediate to high or high risk of recurrence.
The benefit of pembrolizumab, compared with placebo, was maintained in this subgroup, said the investigators. “At this point, we continue to look at the data, but we know that there was a benefit for DFS in the population we included,” said Dr. Choueiri. “When we looked at several subgroups such as PD-L1 status, geography, gender, performance status, M0/M1, all HRs were less than 1 suggesting benefit from pembrolizumab over placebo.”
“Subset analyses by stage are going to be important to determine which group of patients will derive the most benefit,” asserted Dr. McKay. “While those with M1 NED appear to derive benefit with HR for DFS of 0.29, those with M1 NED comprise a small percentage of patient enrolled in the trial.”
Studies exploring tissue- and blood-based biomarkers, including circulating tumor DNA, will be key to identify patients at highest risk for recurrence or adjuvant treatment, Dr. McKay emphasized. “The adoption of adjuvant immune checkpoint inhibitors brings along new questions regarding patient selection, therapeutic use in patients with non–clear-cell renal cell carcinoma, and systemic treatment after recurrence during or after the receipt of adjuvant therapy.”
KEYNOTE-564 was funded by Merck. Multiple study authors including Dr. Choueiri have financial ties to the pharmaceutical industry, including Merck.
A version of this article first appeared on Medscape.com.
according to expert opinion.
The high hopes have been generated by results from the randomized, phase 3 KEYNOTE-564 trial, showing that monotherapy with pembrolizumab (Keytruda, Merck) was associated with significantly longer disease-free survival (DFS) after nephrectomy than placebo (77.3% vs. 68.1%, respectively). Median follow-up was 24 months.
The results come from the trial’s first interim analysis of data from 994 patients with clear-cell renal cell carcinoma (RCC) at high risk of recurrence.
For the pembrolizumab group, the estimated percentage alive at 24 months was 96.6%, compared with 93.5% in the placebo group (hazard ratio for death, 0.54), said Toni Choueiri, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
However, grade 3 or higher adverse events (any cause) occurred at almost twice the rate in the pembrolizumab versus the placebo group (32.4% vs. 17.7%). The new study was published online Aug. 18, 2021, in the New England Journal of Medicine.
The study results were first presented at the 2021 American Society of Clinical Oncology annual meeting and described as likely to be practice changing in this setting, as reported by this news organization.
Currently, this patient population has “no options for adjuvant therapy to reduce the risk of recurrence that have high levels of supporting evidence,” observed the authors.
That’s about to change, as the trial results “herald the dawn of a new era in the treatment of renal cell carcinoma,” Rana McKay, MD, University of California San Diego Health, wrote in an accompanying editorial.
Multiple studies have investigated potential adjuvant therapies in RCC since the 1980s, she observed.
“For the first time, we now have an effective adjuvant immunotherapy option for patients with resected renal cell carcinoma at high risk of recurrence,” Dr. McKay said in an interview.
To date, the lack of clinically beneficial adjuvant therapy options in RCC has been “humbling,” Dr. Choueiri said in an interview. “We hope we can push the envelope further and get more patients with RCC some good options that make them live longer and better.”
Although the standard of care for patients diagnosed with locoregional RCC is partial or total nephrectomy, nearly half of patients eventually experience disease recurrence following surgery, Dr. Choueiri noted.
“No standard, globally approved adjuvant therapy options are currently available for this population,” he said. Clinical guidelines recommend patients at high risk of disease recurrence after surgery be entered into a clinical trial or undergo active surveillance.
Researchers will continue to follow the results for overall survival, a secondary endpoint. “The very early look suggests encouraging results [in overall survival] with an HR of 0.54,” Dr. Choueiri noted.
In the meantime, the prolongation of DFS represents a clear clinical benefit, said Dr. McKay, “given the magnitude of the increase” and “the limited incidence of toxic effects.”
KEYNOTE-564 will alter the adjuvant treatment landscape for RCC as a positive phase 3 trial of adjuvant immunotherapy for the disease, she added.
A number of earlier studies have investigated the use of adjuvant vascular endothelial growth factor–targeting agents in RCC. Only the 2016 Sunitinib Treatment of Renal Adjuvant Cancer (S-TRAC) trial showed improved DFS with sunitinib, compared with placebo (6.8 vs. 5.6 years). Subsequently, sunitinib was approved for adjuvant use in the United States. However, the S-TRAC trial also showed that sunitinib therapy was associated with an increased incidence of toxic effects and lower quality of life scores, and researchers did not observe any benefit in overall survival.
“Despite regulatory approval in the U.S., sunitinib is not approved for adjuvant use by the European Medicines Agency and has limited utilization in clinical practice given the low benefit-risk ratio,” Dr. McKay pointed out.
Study details
KEYNOTE-564 involved 996 patients with clear-cell RCC at high risk for recurrence after nephrectomy, with or without metastasectomy. They were randomly assigned in a 1:1 ratio to receive a 200-mg dose of adjuvant pembrolizumab or placebo given intravenously once every 3 weeks for up to 17 cycles for approximately 1 year.
The vast majority of patients enrolled in the study had localized disease with no evidence of metastases (M0) and intermediate to high or high risk of disease recurrence after partial or complete nephrectomy. However, 5.8% of patients in both the pembrolizumab and placebo groups had M1 NED (metastatic stage 1, no evidence of disease) status after nephrectomy and resection of metastatic lesions. These patients were also at intermediate to high or high risk of recurrence.
The benefit of pembrolizumab, compared with placebo, was maintained in this subgroup, said the investigators. “At this point, we continue to look at the data, but we know that there was a benefit for DFS in the population we included,” said Dr. Choueiri. “When we looked at several subgroups such as PD-L1 status, geography, gender, performance status, M0/M1, all HRs were less than 1 suggesting benefit from pembrolizumab over placebo.”
“Subset analyses by stage are going to be important to determine which group of patients will derive the most benefit,” asserted Dr. McKay. “While those with M1 NED appear to derive benefit with HR for DFS of 0.29, those with M1 NED comprise a small percentage of patient enrolled in the trial.”
Studies exploring tissue- and blood-based biomarkers, including circulating tumor DNA, will be key to identify patients at highest risk for recurrence or adjuvant treatment, Dr. McKay emphasized. “The adoption of adjuvant immune checkpoint inhibitors brings along new questions regarding patient selection, therapeutic use in patients with non–clear-cell renal cell carcinoma, and systemic treatment after recurrence during or after the receipt of adjuvant therapy.”
KEYNOTE-564 was funded by Merck. Multiple study authors including Dr. Choueiri have financial ties to the pharmaceutical industry, including Merck.
A version of this article first appeared on Medscape.com.