User login
Study supports multigene panel testing for all breast cancer patients with second primary cancers
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
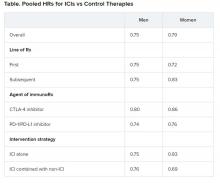
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
FROM JCO PRECISION ONCOLOGY
Atezolizumab TNBC indication ‘in jeopardy’ because of phase 3 results
The FDA said the phase 3 IMpassion131 trial showed that atezolizumab plus paclitaxel did not significantly reduce the risk of cancer progression and death, when compared with paclitaxel plus placebo, in programmed death-ligand 1 (PD-L1)–positive patients.
“Additionally, interim overall survival results favored paclitaxel plus placebo over paclitaxel plus atezolizumab in both the PD-L1-positive population and total population,” the FDA noted.
As a result, “health care professionals should not replace paclitaxel protein-bound (Abraxane) with paclitaxel in clinical practice,” the FDA advised.
Atezolizumab is approved for use in combination with protein-bound paclitaxel, also known as nanoparticle albumin–bound paclitaxel (nab-paclitaxel), to treat patients with unresectable, locally advanced, or metastatic TNBC whose tumors express PD-L1, as detected by an FDA-approved test. The combination was granted accelerated approval for this indication last year.
Atezolizumab plus nab-paclitaxel is the combination most often used in PD-L1-positive TNBC, as opposed to atezolizumab and unbound paclitaxel, said Melinda L. Telli, MD, an associate professor of medicine and director of the Stanford Cancer Institute Breast Cancer Program at Stanford (Calif.) University.
However, as the FDA noted, “continued approval of atezolizumab in combination with [nab-paclitaxel] may be contingent on proven benefit of the treatment in additional trials.”
Dr. Telli explained that atezolizumab was granted accelerated approval for the treatment of PD-L1-positive TNBC based on results of the phase 3 IMpassion130 trial, which compared nab-paclitaxel plus atezolizumab with nab-paclitaxel plus placebo.
“Additional data from IMpassion131 was hoped to be used to support the conversion of the accelerated approval to a full approval. Since IMpassion131 was negative, it unfortunately places the status of atezolizumab in [TNBC] in jeopardy as the benefits were not corroborated. The FDA may move to revoke the approval of atezolizumab for [TNBC],” Dr. Telli said.
In its alert, the FDA stated that it “will review the findings of IMpassion131 and will communicate new information regarding the IMpassion131 results and any potential changes to prescribing information.”
“We need to wait for full presentation and publication of the study results, but, in my assessment, the negative results in IMpassion131 are most likely due to differences in patient selection [from IMpassion130],” Dr. Telli said.
Results from IMpassion131 are scheduled to be presented at the ESMO Virtual Congress 2020.
The IMpassion trials were funded by Roche, maker of atezolizumab. Dr. Telli disclosed relationships with AbbVie, AstraZeneca, Merck, PharmaMar, Pfizer, and Tesaro.
The FDA said the phase 3 IMpassion131 trial showed that atezolizumab plus paclitaxel did not significantly reduce the risk of cancer progression and death, when compared with paclitaxel plus placebo, in programmed death-ligand 1 (PD-L1)–positive patients.
“Additionally, interim overall survival results favored paclitaxel plus placebo over paclitaxel plus atezolizumab in both the PD-L1-positive population and total population,” the FDA noted.
As a result, “health care professionals should not replace paclitaxel protein-bound (Abraxane) with paclitaxel in clinical practice,” the FDA advised.
Atezolizumab is approved for use in combination with protein-bound paclitaxel, also known as nanoparticle albumin–bound paclitaxel (nab-paclitaxel), to treat patients with unresectable, locally advanced, or metastatic TNBC whose tumors express PD-L1, as detected by an FDA-approved test. The combination was granted accelerated approval for this indication last year.
Atezolizumab plus nab-paclitaxel is the combination most often used in PD-L1-positive TNBC, as opposed to atezolizumab and unbound paclitaxel, said Melinda L. Telli, MD, an associate professor of medicine and director of the Stanford Cancer Institute Breast Cancer Program at Stanford (Calif.) University.
However, as the FDA noted, “continued approval of atezolizumab in combination with [nab-paclitaxel] may be contingent on proven benefit of the treatment in additional trials.”
Dr. Telli explained that atezolizumab was granted accelerated approval for the treatment of PD-L1-positive TNBC based on results of the phase 3 IMpassion130 trial, which compared nab-paclitaxel plus atezolizumab with nab-paclitaxel plus placebo.
“Additional data from IMpassion131 was hoped to be used to support the conversion of the accelerated approval to a full approval. Since IMpassion131 was negative, it unfortunately places the status of atezolizumab in [TNBC] in jeopardy as the benefits were not corroborated. The FDA may move to revoke the approval of atezolizumab for [TNBC],” Dr. Telli said.
In its alert, the FDA stated that it “will review the findings of IMpassion131 and will communicate new information regarding the IMpassion131 results and any potential changes to prescribing information.”
“We need to wait for full presentation and publication of the study results, but, in my assessment, the negative results in IMpassion131 are most likely due to differences in patient selection [from IMpassion130],” Dr. Telli said.
Results from IMpassion131 are scheduled to be presented at the ESMO Virtual Congress 2020.
The IMpassion trials were funded by Roche, maker of atezolizumab. Dr. Telli disclosed relationships with AbbVie, AstraZeneca, Merck, PharmaMar, Pfizer, and Tesaro.
The FDA said the phase 3 IMpassion131 trial showed that atezolizumab plus paclitaxel did not significantly reduce the risk of cancer progression and death, when compared with paclitaxel plus placebo, in programmed death-ligand 1 (PD-L1)–positive patients.
“Additionally, interim overall survival results favored paclitaxel plus placebo over paclitaxel plus atezolizumab in both the PD-L1-positive population and total population,” the FDA noted.
As a result, “health care professionals should not replace paclitaxel protein-bound (Abraxane) with paclitaxel in clinical practice,” the FDA advised.
Atezolizumab is approved for use in combination with protein-bound paclitaxel, also known as nanoparticle albumin–bound paclitaxel (nab-paclitaxel), to treat patients with unresectable, locally advanced, or metastatic TNBC whose tumors express PD-L1, as detected by an FDA-approved test. The combination was granted accelerated approval for this indication last year.
Atezolizumab plus nab-paclitaxel is the combination most often used in PD-L1-positive TNBC, as opposed to atezolizumab and unbound paclitaxel, said Melinda L. Telli, MD, an associate professor of medicine and director of the Stanford Cancer Institute Breast Cancer Program at Stanford (Calif.) University.
However, as the FDA noted, “continued approval of atezolizumab in combination with [nab-paclitaxel] may be contingent on proven benefit of the treatment in additional trials.”
Dr. Telli explained that atezolizumab was granted accelerated approval for the treatment of PD-L1-positive TNBC based on results of the phase 3 IMpassion130 trial, which compared nab-paclitaxel plus atezolizumab with nab-paclitaxel plus placebo.
“Additional data from IMpassion131 was hoped to be used to support the conversion of the accelerated approval to a full approval. Since IMpassion131 was negative, it unfortunately places the status of atezolizumab in [TNBC] in jeopardy as the benefits were not corroborated. The FDA may move to revoke the approval of atezolizumab for [TNBC],” Dr. Telli said.
In its alert, the FDA stated that it “will review the findings of IMpassion131 and will communicate new information regarding the IMpassion131 results and any potential changes to prescribing information.”
“We need to wait for full presentation and publication of the study results, but, in my assessment, the negative results in IMpassion131 are most likely due to differences in patient selection [from IMpassion130],” Dr. Telli said.
Results from IMpassion131 are scheduled to be presented at the ESMO Virtual Congress 2020.
The IMpassion trials were funded by Roche, maker of atezolizumab. Dr. Telli disclosed relationships with AbbVie, AstraZeneca, Merck, PharmaMar, Pfizer, and Tesaro.
Hair dye and cancer study ‘offers some reassurance’
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Findings limited to White women in United States
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Chronicles of Cancer: A history of mammography, part 2
The push and pull of social forces
Science and technology emerge from and are shaped by social forces outside the laboratory and clinic. This is an essential fact of most new medical technology. In the Chronicles of Cancer series, part 1 of the story of mammography focused on the technological determinants of its development and use. Part 2 will focus on some of the social forces that shaped the development of mammography.
“Few medical issues have been as controversial – or as political, at least in the United States – as the role of mammographic screening for breast cancer,” according to Donald A. Berry, PhD, a biostatistician at the University of Texas MD Anderson Cancer Center, Houston.1
In fact, technology aside, the history of mammography has been and remains rife with controversy on the one side and vigorous promotion on the other, all enmeshed within the War on Cancer, corporate and professional interests, and the women’s rights movement’s growing issues with what was seen as a patriarchal medical establishment.
Today the issue of conflicts of interest are paramount in any discussion of new medical developments, from the early preclinical stages to ultimate deployment. Then, as now, professional and advocacy societies had a profound influence on government and social decision-making, but in that earlier, more trusting era, buoyed by the amazing changes that technology was bringing to everyday life and an unshakable commitment to and belief in “progress,” science and the medical community held a far more effective sway over the beliefs and behavior of the general population.
Women’s health observed
Although the main focus of the women’s movement with regard to breast cancer was a struggle against the common practice of routine radical mastectomies and a push toward breast-conserving surgeries, the issue of preventive care and screening with regard to women’s health was also a major concern.
Regarding mammography, early enthusiasm in the medical community and among the general public was profound. In 1969, Robert Egan described how mammography had a “certain magic appeal.” The patient, he continued, “feels something special is being done for her.” Women whose cancers had been discovered on a mammogram praised radiologists as heroes who had saved their lives.2
In that era, however, beyond the confines of the doctor’s office, mammography and breast cancer remained topics not discussed among the public at large, despite efforts by the American Cancer Society to change this.
ACS weighs in
Various groups had been promoting the benefits of breast self-examination since the 1930s, and in 1947, the American Cancer Society launched an awareness campaign, “Look for a Lump or Thickening in the Breast,” instructing women to perform a monthly breast self-exam. Between self-examination and clinical breast examinations in physicians’ offices, the ACS believed that smaller and more treatable breast cancers could be discovered.

In 1972, the ACS, working with the National Cancer Institute (NCI), inaugurated the Breast Cancer Detection Demonstration Project (BCDDP), which planned to screen over a quarter of a million American women for breast cancer. The initiative was a direct outgrowth of the National Cancer Act of 1971,3 the key legislation of the War on Cancer, promoted by President Richard Nixon in his State of the Union address in 1971 and responsible for the creation of the National Cancer Institute.
Arthur I. Holleb, MD, ACS senior vice president for medical affairs and research, announced that, “[T]he time has come for the American Cancer Society to mount a massive program on mammography just as we did with the Pap test,”2 according to Barron Lerner, MD, whose book “The Breast Cancer Wars” provides a history of the long-term controversies involved.4
The Pap test, widely promulgated in the 1950s and 1960s, had produced a decline in mortality from cervical cancer.
Regardless of the lack of data on effectiveness at earlier ages, the ACS chose to include women as young as 35 in the BCDDP in order “to inculcate them with ‘good health habits’ ” and “to make our screenee want to return periodically and to want to act as a missionary to bring other women into the screening process.”2
Celebrity status matters
All of the elements of a social revolution in the use of mammography were in place in the late 1960s, but the final triggers to raise social consciousness were the cases of several high-profile female celebrities. In 1973, beloved former child star Shirley Temple Black revealed her breast cancer diagnosis and mastectomy in an era when public discussion of cancer – especially breast cancer – was rare.4
But it wasn’t until 1974 that public awareness and media coverage exploded, sparked by the impact of First Lady Betty Ford’s outspokenness on her own experience of breast cancer. “In obituaries prior to the 1950s and 1960s, women who died from breast cancer were often listed as dying from ‘a prolonged disease’ or ‘a woman’s disease,’ ” according to Tasha Dubriwny, PhD, now an associate professor of communication and women’s and gender studies at Texas A&M University, College Station, when interviewed by the American Association for Cancer Research.5Betty Ford openly addressed her breast cancer diagnosis and treatment and became a prominent advocate for early screening, transforming the landscape of breast cancer awareness. And although Betty Ford’s diagnosis was based on clinical examination rather than mammography, its boost to overall screening was indisputable.
“Within weeks [after Betty Ford’s announcement] thousands of women who had been reluctant to examine their breasts inundated cancer screening centers,” according to a 1987 article in the New York Times.6 Among these women was Happy Rockefeller, the wife of Vice President Nelson A. Rockefeller. Happy Rockefeller also found that she had breast cancer upon screening, and with Betty Ford would become another icon thereafter for breast cancer screening.
“Ford’s lesson for other women was straightforward: Get a mammogram, which she had not done. The American Cancer Society and National Cancer Institute had recently mounted a demonstration project to promote the detection of breast cancer as early as possible, when it was presumed to be more curable. The degree to which women embraced Ford’s message became clear through the famous ‘Betty Ford blip.’ So many women got breast examinations and mammograms for the first time after Ford’s announcement that the actual incidence of breast cancer in the United States went up by 15 percent.”4
In a 1975 address to the American Cancer Society, Betty Ford said: “One day I appeared to be fine and the next day I was in the hospital for a mastectomy. It made me realize how many women in the country could be in the same situation. That realization made me decide to discuss my breast cancer operation openly, because I thought of all the lives in jeopardy. My experience and frank discussion of breast cancer did prompt many women to learn about self-examination, regular checkups, and such detection techniques as mammography. These are so important. I just cannot stress enough how necessary it is for women to take an active interest in their own health and body.”7
ACS guidelines evolve
It wasn’t until 1976 that the ACS issued its first major guidelines for mammography screening. The ACS suggested mammograms may be called for in women aged 35-39 if there was a personal history of breast cancer, and between ages 40 and 49 if their mother or sisters had a history of breast cancer. Women aged 50 years and older could have yearly screening. Thereafter, the use of mammography was encouraged more and more with each new set of recommendations.8
Between 1980 and 1982, these guidelines expanded to advising a baseline mammogram for women aged 35-39 years; that women consult with their physician between ages 40 and 49; and that women over 50 have a yearly mammogram.
Between 1983 and 1991, the recommendations were for a baseline mammogram for women aged 35-39 years; a mammogram every 1-2 years for women aged 40-49; and yearly mammograms for women aged 50 and up. The baseline mammogram recommendation was dropped in 1992.
Between 1997 and 2015, the stakes were upped, and women aged 40-49 years were now recommended to have yearly mammograms, as were still all women aged 50 years and older.
In October 2015, the ACS changed their recommendation to say that women aged 40-44 years should have the choice of initiating mammogram screening, and that the risks and benefits of doing so should be discussed with their physicians. Women aged 45 years and older were still recommended for yearly mammogram screening. That recommendation stands today.
Controversies arise over risk/benefit
The technology was not, however, universally embraced. “By the late 1970s, mammography had diffused much more widely but had become a source of tremendous controversy. On the one hand, advocates of the technology enthusiastically touted its ability to detect smaller, more curable cancers. On the other hand, critics asked whether breast x-rays, particularly for women aged 50 and younger, actually caused more harm than benefit.”2
In addition, meta-analyses of the nine major screening trials conducted between 1965 and 1991 indicated that the reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials.
“Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60=69 years,” according to a review by the U.S. Preventive Services Task Force.9
The estimates for the group aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
Age has continued to be a major factor in determining the cost/benefit of routine mammography screening, with the American College of Physicians stating in its 2019 guidelines, “The potential harms outweigh the benefits in most women aged 40 to 49 years,” and adding, “In average-risk women aged 75 years or older or in women with a life expectancy of 10 years or less, clinicians should discontinue screening for breast cancer.”10
A Cochrane Report from 2013 was equally critical: “If we assume that screening reduces breast cancer mortality by 15% after 13 years of follow-up and that overdiagnosis and overtreatment is at 30%, it means that for every 2,000 women invited for screening throughout 10 years, one will avoid dying of breast cancer and 10 healthy women, who would not have been diagnosed if there had not been screening, will be treated unnecessarily. Furthermore, more than 200 women will experience important psychological distress including anxiety and uncertainty for years because of false positive findings.”11
Conflicting voices exist
These reports advising a more nuanced evaluation of the benefits of mammography, however, were received with skepticism from doctors committed to the vision of breast cancer screening and convinced by anecdotal evidence in their own practices.
These reports were also in direct contradiction to recommendations made in 1997 by the National Cancer Institute, which recommended screening mammograms every 3 years for women aged 40-49 years at average risk of breast cancer.
Such scientific vacillation has contributed to a love/hate relationship with mammography in the mainstream media, fueling new controversies with regard to breast cancer screening, sometimes as much driven by public suspicion and political advocacy as by scientific evolution.
Vocal opponents of universal mammography screening arose throughout the years, and even the cases of Betty Ford and Happy Rockefeller have been called into question as iconic demonstrations of the effectiveness of screening. And although not directly linked to the issue of screening, the rebellion against the routine use of radical mastectomies, a technique pioneered by Halsted in 1894 and in continuing use into the modern era, sparked outrage in women’s rights activists who saw it as evidence of a patriarchal medical establishment making arbitrary decisions concerning women’s bodies. For example, feminist and breast cancer activist Rose Kushner argued against the unnecessary disfigurement of women’s bodies and urged the use and development of less drastic techniques, including partial mastectomies and lumpectomies as viable choices. And these choices were increasingly supported by the medical community as safe and effective alternatives for many patients.12
A 2015 paper in the Journal of the Royal Society of Medicine was bluntly titled “Mammography screening is harmful and should be abandoned.”13 According to the author, who was the editor of the 2013 Cochrane Report, “I believe that if screening had been a drug, it would have been withdrawn from the market long ago.” And the popular press has not been shy at weighing in on the controversy, driven, in part, by the lack of consensus and continually changing guidelines, with major publications such as U.S. News and World Report, the Washington Post, and others addressing the issue over the years. And even public advocacy groups such as the Susan G. Komen organization14 are supporting the more modern professional guidelines in taking a more nuanced approach to the discussion of risks and benefits for individual women.
In 2014, the Swiss Medical Board, a nationally appointed body, recommended that new mammography screening programs should not be instituted in that country and that limits be placed on current programs because of the imbalance between risks and benefits of mammography screening.15 And a study done in Australia in 2020 agreed, stating, “Using data of 30% overdiagnosis of women aged 50 to 69 years in the NSW [New South Wales] BreastScreen program in 2012, we calculated an Australian ratio of harm of overdiagnosis to benefit (breast cancer deaths avoided) of 15:1 and recommended stopping the invitation to screening.”16
Conclusion
If nothing else, the history of mammography shows that the interconnection of social factors with the rise of a medical technology can have profound impacts on patient care. Technology developed by men for women became a touchstone of resentment in a world ever more aware of sex and gender biases in everything from the conduct of clinical trials to the care (or lack thereof) of women with heart disease. Tied for so many years to a radically disfiguring and drastic form of surgery that affected what many felt to be a hallmark and representation of womanhood1,17 mammography also carried the weight of both the real and imaginary fears of radiation exposure.
Well into its development, the technology still found itself under intense public scrutiny, and was enmeshed in a continual media circus, with ping-ponging discussions of risk/benefit in the scientific literature fueling complaints by many of the dominance of a patriarchal medical community over women’s bodies.
With guidelines for mammography still evolving, questions still remaining, and new technologies such as digital imaging falling short in their hoped-for promise, the story remains unfinished, and the future still uncertain. One thing remains clear, however: In the right circumstances, with the right patient population, and properly executed, mammography has saved lives when tied to effective, early treatment, whatever its flaws and failings. This truth goes hand in hand with another reality: It may have also contributed to considerable unanticipated harm through overdiagnosis and overtreatment.
Overall, the history of mammography is a cautionary tale for the entire medical community and for the development of new medical technologies. The push-pull of the demand for progress to save lives and the slowness and often inconclusiveness of scientific studies that validate new technologies create gray areas, where social determinants and professional interests vie in an information vacuum for control of the narrative of risks vs. benefits.
The story of mammography is not yet concluded, and may never be, especially given the unlikelihood of conducting the massive randomized clinical trials that would be needed to settle the issue. It is more likely to remain controversial, at least until the technology of mammography becomes obsolete, replaced by something new and different, which will likely start the push-pull cycle all over again.
And regardless of the risks and benefits of mammography screening, the issue of treatment once breast cancer is identified is perhaps one of more overwhelming import.
References
1. Berry, DA. The Breast. 2013;22[Supplement 2]:S73-S76.
2. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography.” Background paper for the Institute of Medicine report Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer. 2001.
3. NCI website. The National Cancer Act of 1971. www.cancer.gov/about-nci/overview/history/national-cancer-act-1971.
4. Lerner BH. The Huffington Post, Sep. 26, 2014.
5. Wu C. Cancer Today. 2012;2(3): Sep. 27.
6. “”The New York Times. Oct. 17, 1987.
7. Ford B. Remarks to the American Cancer Society. 1975.
8. The American Cancer Society website. History of ACS Recommendations for the Early Detection of Cancer in People Without Symptoms.
9. Nelson HD et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. 2016; Evidence Syntheses, No. 124; pp.29-49.
10. Qasseem A et al. Annals of Internal Medicine. 2019;170(8):547-60.
11. Gotzsche PC et al. Cochrane Report 2013.
12. Lerner, BH. West J Med. May 2001;174(5):362-5.
13. Gotzsche PC. J R Soc Med. 2015;108(9): 341-5.
14. Susan G. Komen website. Weighing the Benefits and Risks of Mammography.
15. Biller-Andorno N et al. N Engl J Med 2014;370:1965-7.
16. Burton R et al. JAMA Netw Open. 2020;3(6):e208249.
17. Webb C et al. Plast Surg. 2019;27(1):49-53.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
The push and pull of social forces
The push and pull of social forces
Science and technology emerge from and are shaped by social forces outside the laboratory and clinic. This is an essential fact of most new medical technology. In the Chronicles of Cancer series, part 1 of the story of mammography focused on the technological determinants of its development and use. Part 2 will focus on some of the social forces that shaped the development of mammography.
“Few medical issues have been as controversial – or as political, at least in the United States – as the role of mammographic screening for breast cancer,” according to Donald A. Berry, PhD, a biostatistician at the University of Texas MD Anderson Cancer Center, Houston.1
In fact, technology aside, the history of mammography has been and remains rife with controversy on the one side and vigorous promotion on the other, all enmeshed within the War on Cancer, corporate and professional interests, and the women’s rights movement’s growing issues with what was seen as a patriarchal medical establishment.
Today the issue of conflicts of interest are paramount in any discussion of new medical developments, from the early preclinical stages to ultimate deployment. Then, as now, professional and advocacy societies had a profound influence on government and social decision-making, but in that earlier, more trusting era, buoyed by the amazing changes that technology was bringing to everyday life and an unshakable commitment to and belief in “progress,” science and the medical community held a far more effective sway over the beliefs and behavior of the general population.
Women’s health observed
Although the main focus of the women’s movement with regard to breast cancer was a struggle against the common practice of routine radical mastectomies and a push toward breast-conserving surgeries, the issue of preventive care and screening with regard to women’s health was also a major concern.
Regarding mammography, early enthusiasm in the medical community and among the general public was profound. In 1969, Robert Egan described how mammography had a “certain magic appeal.” The patient, he continued, “feels something special is being done for her.” Women whose cancers had been discovered on a mammogram praised radiologists as heroes who had saved their lives.2
In that era, however, beyond the confines of the doctor’s office, mammography and breast cancer remained topics not discussed among the public at large, despite efforts by the American Cancer Society to change this.
ACS weighs in
Various groups had been promoting the benefits of breast self-examination since the 1930s, and in 1947, the American Cancer Society launched an awareness campaign, “Look for a Lump or Thickening in the Breast,” instructing women to perform a monthly breast self-exam. Between self-examination and clinical breast examinations in physicians’ offices, the ACS believed that smaller and more treatable breast cancers could be discovered.

In 1972, the ACS, working with the National Cancer Institute (NCI), inaugurated the Breast Cancer Detection Demonstration Project (BCDDP), which planned to screen over a quarter of a million American women for breast cancer. The initiative was a direct outgrowth of the National Cancer Act of 1971,3 the key legislation of the War on Cancer, promoted by President Richard Nixon in his State of the Union address in 1971 and responsible for the creation of the National Cancer Institute.
Arthur I. Holleb, MD, ACS senior vice president for medical affairs and research, announced that, “[T]he time has come for the American Cancer Society to mount a massive program on mammography just as we did with the Pap test,”2 according to Barron Lerner, MD, whose book “The Breast Cancer Wars” provides a history of the long-term controversies involved.4
The Pap test, widely promulgated in the 1950s and 1960s, had produced a decline in mortality from cervical cancer.
Regardless of the lack of data on effectiveness at earlier ages, the ACS chose to include women as young as 35 in the BCDDP in order “to inculcate them with ‘good health habits’ ” and “to make our screenee want to return periodically and to want to act as a missionary to bring other women into the screening process.”2
Celebrity status matters
All of the elements of a social revolution in the use of mammography were in place in the late 1960s, but the final triggers to raise social consciousness were the cases of several high-profile female celebrities. In 1973, beloved former child star Shirley Temple Black revealed her breast cancer diagnosis and mastectomy in an era when public discussion of cancer – especially breast cancer – was rare.4
But it wasn’t until 1974 that public awareness and media coverage exploded, sparked by the impact of First Lady Betty Ford’s outspokenness on her own experience of breast cancer. “In obituaries prior to the 1950s and 1960s, women who died from breast cancer were often listed as dying from ‘a prolonged disease’ or ‘a woman’s disease,’ ” according to Tasha Dubriwny, PhD, now an associate professor of communication and women’s and gender studies at Texas A&M University, College Station, when interviewed by the American Association for Cancer Research.5Betty Ford openly addressed her breast cancer diagnosis and treatment and became a prominent advocate for early screening, transforming the landscape of breast cancer awareness. And although Betty Ford’s diagnosis was based on clinical examination rather than mammography, its boost to overall screening was indisputable.
“Within weeks [after Betty Ford’s announcement] thousands of women who had been reluctant to examine their breasts inundated cancer screening centers,” according to a 1987 article in the New York Times.6 Among these women was Happy Rockefeller, the wife of Vice President Nelson A. Rockefeller. Happy Rockefeller also found that she had breast cancer upon screening, and with Betty Ford would become another icon thereafter for breast cancer screening.
“Ford’s lesson for other women was straightforward: Get a mammogram, which she had not done. The American Cancer Society and National Cancer Institute had recently mounted a demonstration project to promote the detection of breast cancer as early as possible, when it was presumed to be more curable. The degree to which women embraced Ford’s message became clear through the famous ‘Betty Ford blip.’ So many women got breast examinations and mammograms for the first time after Ford’s announcement that the actual incidence of breast cancer in the United States went up by 15 percent.”4
In a 1975 address to the American Cancer Society, Betty Ford said: “One day I appeared to be fine and the next day I was in the hospital for a mastectomy. It made me realize how many women in the country could be in the same situation. That realization made me decide to discuss my breast cancer operation openly, because I thought of all the lives in jeopardy. My experience and frank discussion of breast cancer did prompt many women to learn about self-examination, regular checkups, and such detection techniques as mammography. These are so important. I just cannot stress enough how necessary it is for women to take an active interest in their own health and body.”7
ACS guidelines evolve
It wasn’t until 1976 that the ACS issued its first major guidelines for mammography screening. The ACS suggested mammograms may be called for in women aged 35-39 if there was a personal history of breast cancer, and between ages 40 and 49 if their mother or sisters had a history of breast cancer. Women aged 50 years and older could have yearly screening. Thereafter, the use of mammography was encouraged more and more with each new set of recommendations.8
Between 1980 and 1982, these guidelines expanded to advising a baseline mammogram for women aged 35-39 years; that women consult with their physician between ages 40 and 49; and that women over 50 have a yearly mammogram.
Between 1983 and 1991, the recommendations were for a baseline mammogram for women aged 35-39 years; a mammogram every 1-2 years for women aged 40-49; and yearly mammograms for women aged 50 and up. The baseline mammogram recommendation was dropped in 1992.
Between 1997 and 2015, the stakes were upped, and women aged 40-49 years were now recommended to have yearly mammograms, as were still all women aged 50 years and older.
In October 2015, the ACS changed their recommendation to say that women aged 40-44 years should have the choice of initiating mammogram screening, and that the risks and benefits of doing so should be discussed with their physicians. Women aged 45 years and older were still recommended for yearly mammogram screening. That recommendation stands today.
Controversies arise over risk/benefit
The technology was not, however, universally embraced. “By the late 1970s, mammography had diffused much more widely but had become a source of tremendous controversy. On the one hand, advocates of the technology enthusiastically touted its ability to detect smaller, more curable cancers. On the other hand, critics asked whether breast x-rays, particularly for women aged 50 and younger, actually caused more harm than benefit.”2
In addition, meta-analyses of the nine major screening trials conducted between 1965 and 1991 indicated that the reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials.
“Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60=69 years,” according to a review by the U.S. Preventive Services Task Force.9
The estimates for the group aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
Age has continued to be a major factor in determining the cost/benefit of routine mammography screening, with the American College of Physicians stating in its 2019 guidelines, “The potential harms outweigh the benefits in most women aged 40 to 49 years,” and adding, “In average-risk women aged 75 years or older or in women with a life expectancy of 10 years or less, clinicians should discontinue screening for breast cancer.”10
A Cochrane Report from 2013 was equally critical: “If we assume that screening reduces breast cancer mortality by 15% after 13 years of follow-up and that overdiagnosis and overtreatment is at 30%, it means that for every 2,000 women invited for screening throughout 10 years, one will avoid dying of breast cancer and 10 healthy women, who would not have been diagnosed if there had not been screening, will be treated unnecessarily. Furthermore, more than 200 women will experience important psychological distress including anxiety and uncertainty for years because of false positive findings.”11
Conflicting voices exist
These reports advising a more nuanced evaluation of the benefits of mammography, however, were received with skepticism from doctors committed to the vision of breast cancer screening and convinced by anecdotal evidence in their own practices.
These reports were also in direct contradiction to recommendations made in 1997 by the National Cancer Institute, which recommended screening mammograms every 3 years for women aged 40-49 years at average risk of breast cancer.
Such scientific vacillation has contributed to a love/hate relationship with mammography in the mainstream media, fueling new controversies with regard to breast cancer screening, sometimes as much driven by public suspicion and political advocacy as by scientific evolution.
Vocal opponents of universal mammography screening arose throughout the years, and even the cases of Betty Ford and Happy Rockefeller have been called into question as iconic demonstrations of the effectiveness of screening. And although not directly linked to the issue of screening, the rebellion against the routine use of radical mastectomies, a technique pioneered by Halsted in 1894 and in continuing use into the modern era, sparked outrage in women’s rights activists who saw it as evidence of a patriarchal medical establishment making arbitrary decisions concerning women’s bodies. For example, feminist and breast cancer activist Rose Kushner argued against the unnecessary disfigurement of women’s bodies and urged the use and development of less drastic techniques, including partial mastectomies and lumpectomies as viable choices. And these choices were increasingly supported by the medical community as safe and effective alternatives for many patients.12
A 2015 paper in the Journal of the Royal Society of Medicine was bluntly titled “Mammography screening is harmful and should be abandoned.”13 According to the author, who was the editor of the 2013 Cochrane Report, “I believe that if screening had been a drug, it would have been withdrawn from the market long ago.” And the popular press has not been shy at weighing in on the controversy, driven, in part, by the lack of consensus and continually changing guidelines, with major publications such as U.S. News and World Report, the Washington Post, and others addressing the issue over the years. And even public advocacy groups such as the Susan G. Komen organization14 are supporting the more modern professional guidelines in taking a more nuanced approach to the discussion of risks and benefits for individual women.
In 2014, the Swiss Medical Board, a nationally appointed body, recommended that new mammography screening programs should not be instituted in that country and that limits be placed on current programs because of the imbalance between risks and benefits of mammography screening.15 And a study done in Australia in 2020 agreed, stating, “Using data of 30% overdiagnosis of women aged 50 to 69 years in the NSW [New South Wales] BreastScreen program in 2012, we calculated an Australian ratio of harm of overdiagnosis to benefit (breast cancer deaths avoided) of 15:1 and recommended stopping the invitation to screening.”16
Conclusion
If nothing else, the history of mammography shows that the interconnection of social factors with the rise of a medical technology can have profound impacts on patient care. Technology developed by men for women became a touchstone of resentment in a world ever more aware of sex and gender biases in everything from the conduct of clinical trials to the care (or lack thereof) of women with heart disease. Tied for so many years to a radically disfiguring and drastic form of surgery that affected what many felt to be a hallmark and representation of womanhood1,17 mammography also carried the weight of both the real and imaginary fears of radiation exposure.
Well into its development, the technology still found itself under intense public scrutiny, and was enmeshed in a continual media circus, with ping-ponging discussions of risk/benefit in the scientific literature fueling complaints by many of the dominance of a patriarchal medical community over women’s bodies.
With guidelines for mammography still evolving, questions still remaining, and new technologies such as digital imaging falling short in their hoped-for promise, the story remains unfinished, and the future still uncertain. One thing remains clear, however: In the right circumstances, with the right patient population, and properly executed, mammography has saved lives when tied to effective, early treatment, whatever its flaws and failings. This truth goes hand in hand with another reality: It may have also contributed to considerable unanticipated harm through overdiagnosis and overtreatment.
Overall, the history of mammography is a cautionary tale for the entire medical community and for the development of new medical technologies. The push-pull of the demand for progress to save lives and the slowness and often inconclusiveness of scientific studies that validate new technologies create gray areas, where social determinants and professional interests vie in an information vacuum for control of the narrative of risks vs. benefits.
The story of mammography is not yet concluded, and may never be, especially given the unlikelihood of conducting the massive randomized clinical trials that would be needed to settle the issue. It is more likely to remain controversial, at least until the technology of mammography becomes obsolete, replaced by something new and different, which will likely start the push-pull cycle all over again.
And regardless of the risks and benefits of mammography screening, the issue of treatment once breast cancer is identified is perhaps one of more overwhelming import.
References
1. Berry, DA. The Breast. 2013;22[Supplement 2]:S73-S76.
2. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography.” Background paper for the Institute of Medicine report Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer. 2001.
3. NCI website. The National Cancer Act of 1971. www.cancer.gov/about-nci/overview/history/national-cancer-act-1971.
4. Lerner BH. The Huffington Post, Sep. 26, 2014.
5. Wu C. Cancer Today. 2012;2(3): Sep. 27.
6. “”The New York Times. Oct. 17, 1987.
7. Ford B. Remarks to the American Cancer Society. 1975.
8. The American Cancer Society website. History of ACS Recommendations for the Early Detection of Cancer in People Without Symptoms.
9. Nelson HD et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. 2016; Evidence Syntheses, No. 124; pp.29-49.
10. Qasseem A et al. Annals of Internal Medicine. 2019;170(8):547-60.
11. Gotzsche PC et al. Cochrane Report 2013.
12. Lerner, BH. West J Med. May 2001;174(5):362-5.
13. Gotzsche PC. J R Soc Med. 2015;108(9): 341-5.
14. Susan G. Komen website. Weighing the Benefits and Risks of Mammography.
15. Biller-Andorno N et al. N Engl J Med 2014;370:1965-7.
16. Burton R et al. JAMA Netw Open. 2020;3(6):e208249.
17. Webb C et al. Plast Surg. 2019;27(1):49-53.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Science and technology emerge from and are shaped by social forces outside the laboratory and clinic. This is an essential fact of most new medical technology. In the Chronicles of Cancer series, part 1 of the story of mammography focused on the technological determinants of its development and use. Part 2 will focus on some of the social forces that shaped the development of mammography.
“Few medical issues have been as controversial – or as political, at least in the United States – as the role of mammographic screening for breast cancer,” according to Donald A. Berry, PhD, a biostatistician at the University of Texas MD Anderson Cancer Center, Houston.1
In fact, technology aside, the history of mammography has been and remains rife with controversy on the one side and vigorous promotion on the other, all enmeshed within the War on Cancer, corporate and professional interests, and the women’s rights movement’s growing issues with what was seen as a patriarchal medical establishment.
Today the issue of conflicts of interest are paramount in any discussion of new medical developments, from the early preclinical stages to ultimate deployment. Then, as now, professional and advocacy societies had a profound influence on government and social decision-making, but in that earlier, more trusting era, buoyed by the amazing changes that technology was bringing to everyday life and an unshakable commitment to and belief in “progress,” science and the medical community held a far more effective sway over the beliefs and behavior of the general population.
Women’s health observed
Although the main focus of the women’s movement with regard to breast cancer was a struggle against the common practice of routine radical mastectomies and a push toward breast-conserving surgeries, the issue of preventive care and screening with regard to women’s health was also a major concern.
Regarding mammography, early enthusiasm in the medical community and among the general public was profound. In 1969, Robert Egan described how mammography had a “certain magic appeal.” The patient, he continued, “feels something special is being done for her.” Women whose cancers had been discovered on a mammogram praised radiologists as heroes who had saved their lives.2
In that era, however, beyond the confines of the doctor’s office, mammography and breast cancer remained topics not discussed among the public at large, despite efforts by the American Cancer Society to change this.
ACS weighs in
Various groups had been promoting the benefits of breast self-examination since the 1930s, and in 1947, the American Cancer Society launched an awareness campaign, “Look for a Lump or Thickening in the Breast,” instructing women to perform a monthly breast self-exam. Between self-examination and clinical breast examinations in physicians’ offices, the ACS believed that smaller and more treatable breast cancers could be discovered.

In 1972, the ACS, working with the National Cancer Institute (NCI), inaugurated the Breast Cancer Detection Demonstration Project (BCDDP), which planned to screen over a quarter of a million American women for breast cancer. The initiative was a direct outgrowth of the National Cancer Act of 1971,3 the key legislation of the War on Cancer, promoted by President Richard Nixon in his State of the Union address in 1971 and responsible for the creation of the National Cancer Institute.
Arthur I. Holleb, MD, ACS senior vice president for medical affairs and research, announced that, “[T]he time has come for the American Cancer Society to mount a massive program on mammography just as we did with the Pap test,”2 according to Barron Lerner, MD, whose book “The Breast Cancer Wars” provides a history of the long-term controversies involved.4
The Pap test, widely promulgated in the 1950s and 1960s, had produced a decline in mortality from cervical cancer.
Regardless of the lack of data on effectiveness at earlier ages, the ACS chose to include women as young as 35 in the BCDDP in order “to inculcate them with ‘good health habits’ ” and “to make our screenee want to return periodically and to want to act as a missionary to bring other women into the screening process.”2
Celebrity status matters
All of the elements of a social revolution in the use of mammography were in place in the late 1960s, but the final triggers to raise social consciousness were the cases of several high-profile female celebrities. In 1973, beloved former child star Shirley Temple Black revealed her breast cancer diagnosis and mastectomy in an era when public discussion of cancer – especially breast cancer – was rare.4
But it wasn’t until 1974 that public awareness and media coverage exploded, sparked by the impact of First Lady Betty Ford’s outspokenness on her own experience of breast cancer. “In obituaries prior to the 1950s and 1960s, women who died from breast cancer were often listed as dying from ‘a prolonged disease’ or ‘a woman’s disease,’ ” according to Tasha Dubriwny, PhD, now an associate professor of communication and women’s and gender studies at Texas A&M University, College Station, when interviewed by the American Association for Cancer Research.5Betty Ford openly addressed her breast cancer diagnosis and treatment and became a prominent advocate for early screening, transforming the landscape of breast cancer awareness. And although Betty Ford’s diagnosis was based on clinical examination rather than mammography, its boost to overall screening was indisputable.
“Within weeks [after Betty Ford’s announcement] thousands of women who had been reluctant to examine their breasts inundated cancer screening centers,” according to a 1987 article in the New York Times.6 Among these women was Happy Rockefeller, the wife of Vice President Nelson A. Rockefeller. Happy Rockefeller also found that she had breast cancer upon screening, and with Betty Ford would become another icon thereafter for breast cancer screening.
“Ford’s lesson for other women was straightforward: Get a mammogram, which she had not done. The American Cancer Society and National Cancer Institute had recently mounted a demonstration project to promote the detection of breast cancer as early as possible, when it was presumed to be more curable. The degree to which women embraced Ford’s message became clear through the famous ‘Betty Ford blip.’ So many women got breast examinations and mammograms for the first time after Ford’s announcement that the actual incidence of breast cancer in the United States went up by 15 percent.”4
In a 1975 address to the American Cancer Society, Betty Ford said: “One day I appeared to be fine and the next day I was in the hospital for a mastectomy. It made me realize how many women in the country could be in the same situation. That realization made me decide to discuss my breast cancer operation openly, because I thought of all the lives in jeopardy. My experience and frank discussion of breast cancer did prompt many women to learn about self-examination, regular checkups, and such detection techniques as mammography. These are so important. I just cannot stress enough how necessary it is for women to take an active interest in their own health and body.”7
ACS guidelines evolve
It wasn’t until 1976 that the ACS issued its first major guidelines for mammography screening. The ACS suggested mammograms may be called for in women aged 35-39 if there was a personal history of breast cancer, and between ages 40 and 49 if their mother or sisters had a history of breast cancer. Women aged 50 years and older could have yearly screening. Thereafter, the use of mammography was encouraged more and more with each new set of recommendations.8
Between 1980 and 1982, these guidelines expanded to advising a baseline mammogram for women aged 35-39 years; that women consult with their physician between ages 40 and 49; and that women over 50 have a yearly mammogram.
Between 1983 and 1991, the recommendations were for a baseline mammogram for women aged 35-39 years; a mammogram every 1-2 years for women aged 40-49; and yearly mammograms for women aged 50 and up. The baseline mammogram recommendation was dropped in 1992.
Between 1997 and 2015, the stakes were upped, and women aged 40-49 years were now recommended to have yearly mammograms, as were still all women aged 50 years and older.
In October 2015, the ACS changed their recommendation to say that women aged 40-44 years should have the choice of initiating mammogram screening, and that the risks and benefits of doing so should be discussed with their physicians. Women aged 45 years and older were still recommended for yearly mammogram screening. That recommendation stands today.
Controversies arise over risk/benefit
The technology was not, however, universally embraced. “By the late 1970s, mammography had diffused much more widely but had become a source of tremendous controversy. On the one hand, advocates of the technology enthusiastically touted its ability to detect smaller, more curable cancers. On the other hand, critics asked whether breast x-rays, particularly for women aged 50 and younger, actually caused more harm than benefit.”2
In addition, meta-analyses of the nine major screening trials conducted between 1965 and 1991 indicated that the reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials.
“Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60=69 years,” according to a review by the U.S. Preventive Services Task Force.9
The estimates for the group aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
Age has continued to be a major factor in determining the cost/benefit of routine mammography screening, with the American College of Physicians stating in its 2019 guidelines, “The potential harms outweigh the benefits in most women aged 40 to 49 years,” and adding, “In average-risk women aged 75 years or older or in women with a life expectancy of 10 years or less, clinicians should discontinue screening for breast cancer.”10
A Cochrane Report from 2013 was equally critical: “If we assume that screening reduces breast cancer mortality by 15% after 13 years of follow-up and that overdiagnosis and overtreatment is at 30%, it means that for every 2,000 women invited for screening throughout 10 years, one will avoid dying of breast cancer and 10 healthy women, who would not have been diagnosed if there had not been screening, will be treated unnecessarily. Furthermore, more than 200 women will experience important psychological distress including anxiety and uncertainty for years because of false positive findings.”11
Conflicting voices exist
These reports advising a more nuanced evaluation of the benefits of mammography, however, were received with skepticism from doctors committed to the vision of breast cancer screening and convinced by anecdotal evidence in their own practices.
These reports were also in direct contradiction to recommendations made in 1997 by the National Cancer Institute, which recommended screening mammograms every 3 years for women aged 40-49 years at average risk of breast cancer.
Such scientific vacillation has contributed to a love/hate relationship with mammography in the mainstream media, fueling new controversies with regard to breast cancer screening, sometimes as much driven by public suspicion and political advocacy as by scientific evolution.
Vocal opponents of universal mammography screening arose throughout the years, and even the cases of Betty Ford and Happy Rockefeller have been called into question as iconic demonstrations of the effectiveness of screening. And although not directly linked to the issue of screening, the rebellion against the routine use of radical mastectomies, a technique pioneered by Halsted in 1894 and in continuing use into the modern era, sparked outrage in women’s rights activists who saw it as evidence of a patriarchal medical establishment making arbitrary decisions concerning women’s bodies. For example, feminist and breast cancer activist Rose Kushner argued against the unnecessary disfigurement of women’s bodies and urged the use and development of less drastic techniques, including partial mastectomies and lumpectomies as viable choices. And these choices were increasingly supported by the medical community as safe and effective alternatives for many patients.12
A 2015 paper in the Journal of the Royal Society of Medicine was bluntly titled “Mammography screening is harmful and should be abandoned.”13 According to the author, who was the editor of the 2013 Cochrane Report, “I believe that if screening had been a drug, it would have been withdrawn from the market long ago.” And the popular press has not been shy at weighing in on the controversy, driven, in part, by the lack of consensus and continually changing guidelines, with major publications such as U.S. News and World Report, the Washington Post, and others addressing the issue over the years. And even public advocacy groups such as the Susan G. Komen organization14 are supporting the more modern professional guidelines in taking a more nuanced approach to the discussion of risks and benefits for individual women.
In 2014, the Swiss Medical Board, a nationally appointed body, recommended that new mammography screening programs should not be instituted in that country and that limits be placed on current programs because of the imbalance between risks and benefits of mammography screening.15 And a study done in Australia in 2020 agreed, stating, “Using data of 30% overdiagnosis of women aged 50 to 69 years in the NSW [New South Wales] BreastScreen program in 2012, we calculated an Australian ratio of harm of overdiagnosis to benefit (breast cancer deaths avoided) of 15:1 and recommended stopping the invitation to screening.”16
Conclusion
If nothing else, the history of mammography shows that the interconnection of social factors with the rise of a medical technology can have profound impacts on patient care. Technology developed by men for women became a touchstone of resentment in a world ever more aware of sex and gender biases in everything from the conduct of clinical trials to the care (or lack thereof) of women with heart disease. Tied for so many years to a radically disfiguring and drastic form of surgery that affected what many felt to be a hallmark and representation of womanhood1,17 mammography also carried the weight of both the real and imaginary fears of radiation exposure.
Well into its development, the technology still found itself under intense public scrutiny, and was enmeshed in a continual media circus, with ping-ponging discussions of risk/benefit in the scientific literature fueling complaints by many of the dominance of a patriarchal medical community over women’s bodies.
With guidelines for mammography still evolving, questions still remaining, and new technologies such as digital imaging falling short in their hoped-for promise, the story remains unfinished, and the future still uncertain. One thing remains clear, however: In the right circumstances, with the right patient population, and properly executed, mammography has saved lives when tied to effective, early treatment, whatever its flaws and failings. This truth goes hand in hand with another reality: It may have also contributed to considerable unanticipated harm through overdiagnosis and overtreatment.
Overall, the history of mammography is a cautionary tale for the entire medical community and for the development of new medical technologies. The push-pull of the demand for progress to save lives and the slowness and often inconclusiveness of scientific studies that validate new technologies create gray areas, where social determinants and professional interests vie in an information vacuum for control of the narrative of risks vs. benefits.
The story of mammography is not yet concluded, and may never be, especially given the unlikelihood of conducting the massive randomized clinical trials that would be needed to settle the issue. It is more likely to remain controversial, at least until the technology of mammography becomes obsolete, replaced by something new and different, which will likely start the push-pull cycle all over again.
And regardless of the risks and benefits of mammography screening, the issue of treatment once breast cancer is identified is perhaps one of more overwhelming import.
References
1. Berry, DA. The Breast. 2013;22[Supplement 2]:S73-S76.
2. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography.” Background paper for the Institute of Medicine report Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer. 2001.
3. NCI website. The National Cancer Act of 1971. www.cancer.gov/about-nci/overview/history/national-cancer-act-1971.
4. Lerner BH. The Huffington Post, Sep. 26, 2014.
5. Wu C. Cancer Today. 2012;2(3): Sep. 27.
6. “”The New York Times. Oct. 17, 1987.
7. Ford B. Remarks to the American Cancer Society. 1975.
8. The American Cancer Society website. History of ACS Recommendations for the Early Detection of Cancer in People Without Symptoms.
9. Nelson HD et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. 2016; Evidence Syntheses, No. 124; pp.29-49.
10. Qasseem A et al. Annals of Internal Medicine. 2019;170(8):547-60.
11. Gotzsche PC et al. Cochrane Report 2013.
12. Lerner, BH. West J Med. May 2001;174(5):362-5.
13. Gotzsche PC. J R Soc Med. 2015;108(9): 341-5.
14. Susan G. Komen website. Weighing the Benefits and Risks of Mammography.
15. Biller-Andorno N et al. N Engl J Med 2014;370:1965-7.
16. Burton R et al. JAMA Netw Open. 2020;3(6):e208249.
17. Webb C et al. Plast Surg. 2019;27(1):49-53.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
A Rare Case of Triple Positive Inflammatory Breast Cancer in An Elderly Male
BACKGROUND: An 84-year-old male presented with a rapidly growing left breast mass associated with warmth, erythema, and serous discharge from left nipple for 2.5 months. Physical exam revealed ‘peau d’orange’ appearance of skin and a 3×7 cm, firm, irregular, fixed mass in left breast. Core needle biopsy of left breast revealed invasive ductal carcinoma and a computed tomography scan of chest showed multiple small pulmonary nodules. Patient was diagnosed with inflammatory breast carcinoma (Stage IV, cT4d cN1 cM1), ER/ PR positive, HER-2 positive. BRCA testing was negative. After a normal MUGA scan, patient was started on weekly paclitaxel and trastuzumab. After 4 cycles patient developed diarrhea and elected to stop paclitaxel. After 10 cycles of trastuzumab, patient developed signs of heart failure and a MUGA showed depressed left ventricular ejection fraction (LVEF). Trastuzumab was held and patient was started on tamoxifen. Patient had progression of primary mass into a fungating lesion and evidence of new pulmonary metastatic disease on tamoxifen. The primary lesion was treated with palliative radiation and after a subsequent MUGA scan showed normalization of LVEF; trastuzumab was resumed. Patient had stable disease on trastuzumab and continued to follow with oncology.
DISCUSSION: Male breast cancer is < 1% of all breast cancer but incidence is rising in the US. Risk factors include family history, BRCA2 > BRCA1, obesity, cirrhosis, and radiation exposure. Inflammatory breast cancer (IBC) is a rapidly progressive malignancy with a clinicopathological diagnosis. There are paucity of data of IBC in men due to rarity of the disease. Many patients initially are misdiagnosed with mastitis, unresponsive to antibiotics. At diagnosis, most patients have a higher age compared with females (by 5-10 years), and advanced stage, though have a similar prognosis by stage. Prognostic factors and treatment principles are same as females with multimodal approach of chemotherapy, radiation therapy, and hormone therapy.
CONCLUSIONS: IBC in men is very rare and awareness of its risk factors and presentation can lead to early diagnosis and better survival. Urgent referral to oncology is needed if index of suspicion is high. Further research is needed for defining best treatment modalities in elderly males.”
BACKGROUND: An 84-year-old male presented with a rapidly growing left breast mass associated with warmth, erythema, and serous discharge from left nipple for 2.5 months. Physical exam revealed ‘peau d’orange’ appearance of skin and a 3×7 cm, firm, irregular, fixed mass in left breast. Core needle biopsy of left breast revealed invasive ductal carcinoma and a computed tomography scan of chest showed multiple small pulmonary nodules. Patient was diagnosed with inflammatory breast carcinoma (Stage IV, cT4d cN1 cM1), ER/ PR positive, HER-2 positive. BRCA testing was negative. After a normal MUGA scan, patient was started on weekly paclitaxel and trastuzumab. After 4 cycles patient developed diarrhea and elected to stop paclitaxel. After 10 cycles of trastuzumab, patient developed signs of heart failure and a MUGA showed depressed left ventricular ejection fraction (LVEF). Trastuzumab was held and patient was started on tamoxifen. Patient had progression of primary mass into a fungating lesion and evidence of new pulmonary metastatic disease on tamoxifen. The primary lesion was treated with palliative radiation and after a subsequent MUGA scan showed normalization of LVEF; trastuzumab was resumed. Patient had stable disease on trastuzumab and continued to follow with oncology.
DISCUSSION: Male breast cancer is < 1% of all breast cancer but incidence is rising in the US. Risk factors include family history, BRCA2 > BRCA1, obesity, cirrhosis, and radiation exposure. Inflammatory breast cancer (IBC) is a rapidly progressive malignancy with a clinicopathological diagnosis. There are paucity of data of IBC in men due to rarity of the disease. Many patients initially are misdiagnosed with mastitis, unresponsive to antibiotics. At diagnosis, most patients have a higher age compared with females (by 5-10 years), and advanced stage, though have a similar prognosis by stage. Prognostic factors and treatment principles are same as females with multimodal approach of chemotherapy, radiation therapy, and hormone therapy.
CONCLUSIONS: IBC in men is very rare and awareness of its risk factors and presentation can lead to early diagnosis and better survival. Urgent referral to oncology is needed if index of suspicion is high. Further research is needed for defining best treatment modalities in elderly males.”
BACKGROUND: An 84-year-old male presented with a rapidly growing left breast mass associated with warmth, erythema, and serous discharge from left nipple for 2.5 months. Physical exam revealed ‘peau d’orange’ appearance of skin and a 3×7 cm, firm, irregular, fixed mass in left breast. Core needle biopsy of left breast revealed invasive ductal carcinoma and a computed tomography scan of chest showed multiple small pulmonary nodules. Patient was diagnosed with inflammatory breast carcinoma (Stage IV, cT4d cN1 cM1), ER/ PR positive, HER-2 positive. BRCA testing was negative. After a normal MUGA scan, patient was started on weekly paclitaxel and trastuzumab. After 4 cycles patient developed diarrhea and elected to stop paclitaxel. After 10 cycles of trastuzumab, patient developed signs of heart failure and a MUGA showed depressed left ventricular ejection fraction (LVEF). Trastuzumab was held and patient was started on tamoxifen. Patient had progression of primary mass into a fungating lesion and evidence of new pulmonary metastatic disease on tamoxifen. The primary lesion was treated with palliative radiation and after a subsequent MUGA scan showed normalization of LVEF; trastuzumab was resumed. Patient had stable disease on trastuzumab and continued to follow with oncology.
DISCUSSION: Male breast cancer is < 1% of all breast cancer but incidence is rising in the US. Risk factors include family history, BRCA2 > BRCA1, obesity, cirrhosis, and radiation exposure. Inflammatory breast cancer (IBC) is a rapidly progressive malignancy with a clinicopathological diagnosis. There are paucity of data of IBC in men due to rarity of the disease. Many patients initially are misdiagnosed with mastitis, unresponsive to antibiotics. At diagnosis, most patients have a higher age compared with females (by 5-10 years), and advanced stage, though have a similar prognosis by stage. Prognostic factors and treatment principles are same as females with multimodal approach of chemotherapy, radiation therapy, and hormone therapy.
CONCLUSIONS: IBC in men is very rare and awareness of its risk factors and presentation can lead to early diagnosis and better survival. Urgent referral to oncology is needed if index of suspicion is high. Further research is needed for defining best treatment modalities in elderly males.”
VTE, sepsis risk increased among COVID-19 patients with cancer
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
FROM AACR: COVID-19 AND CANCER
First guideline on NGS testing in cancer, from ESMO
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
One-off blast of RT, rather than weeks, for early breast cancer
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.