User login
Is the tide turning on the ‘grubby’ affair of EXCEL and the European guidelines?
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
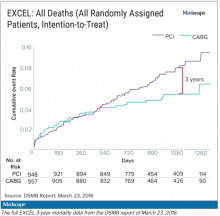
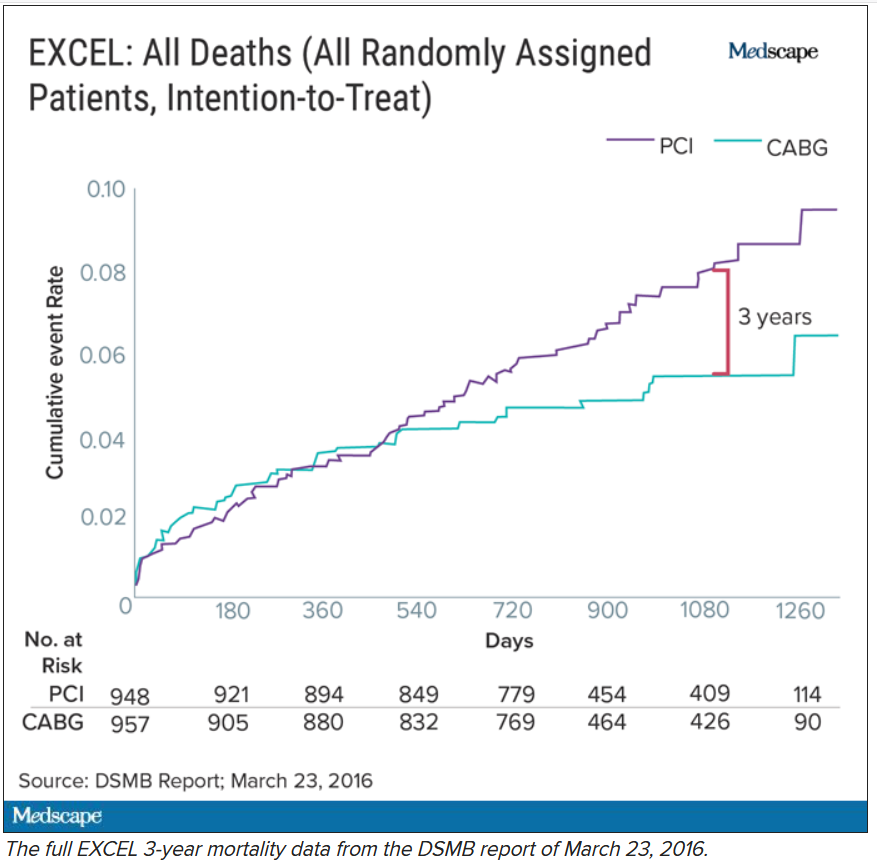
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
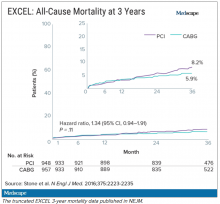
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
Echocardiography in AMI not associated with improved outcomes
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
'Cardio-obstetrics' tied to better outcome in pregnancy with CVD
A multidisciplinary cardio-obstetrics team-based care model may help improve cardiovascular care for pregnant women with cardiovascular disease (CVD), according to a recent study.
“We sought to describe clinical characteristics, maternal and fetal outcomes, and cardiovascular readmissions in a cohort of pregnant women with underlying CVD followed by a cardio-obstetrics team,” wrote Ella Magun, MD, of Columbia University, New York, and coauthors. Their report is in the Journal of the American College of Cardiology.
The researchers reported the outcomes of a retrospective cohort analysis involving 306 pregnant women with CVD, who were treated at a quaternary care hospital in New York City.
They defined cardio-obstetrics as a team-based collaborative approach to maternal care that includes maternal fetal medicine, cardiology, anesthesiology, neonatology, nursing, social work, and pharmacy.
More than half of the women in the cohort (53%) were Hispanic and Latino, and 74% were receiving Medicaid, suggesting low socioeconomic status. Key outcomes of interest were cardiovascular readmissions at 30 days, 90 days, and 1 year. Secondary endpoints included maternal death, need for a left ventricular assist device or heart transplantation, and fetal demise.
The most frequently observed forms of CVD were arrhythmias (29%), cardiomyopathy (24%), congenital heart disease (24%), valvular disease (16%), and coronary artery disease (4%). The median Cardiac Disease in Pregnancy (CARPREG II) score was 3, and 43% of women had a CARPREG II score of 4 or higher.
After a median follow-up of 2.6 years, the 30-day and 90-day cardiovascular readmission rates were 1.9% and 4.6%, which was lower than the national 30-day postpartum rate of readmission (3.6%). One maternal death (0.3%) occurred within a year of delivery (woman with Eisenmenger syndrome).
“Despite high CARPREG II scores in this patient population, we found low rates of maternal and fetal complications with a low rate of 30- and 90-day readmissions following delivery,” the researchers wrote.
Experts weigh in
“We’re seeing widely increasing interest in the implementation of cardio-obstetrics models for multidisciplinary collaborative care and initial studies suggest these team-based models improve pregnancy and postpartum outcomes for women with cardiac disease,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and professor at Baylor College of Medicine in Houston.
Dr. Magun and colleagues acknowledged that a key limitation of the present study was the retrospective, single-center design.
“With program expansions over the next 2-3 years, I expect to see an increasing number of prospective studies with larger sample sizes evaluating the impact of cardio-obstetrics teams on maternal morbidity and mortality,” Dr. Hollier said.
“These findings suggest that our cardio-obstetrics program may help provide improved cardiovascular care to an otherwise underserved population,” the authors concluded.
In an editorial accompanying the reports, Pamela Ouyang, MBBS, and Garima Sharma, MD, wrote that, although this study wasn’t designed to assess the benefit of cardio-obstetric teams relative to standard of care, its implementation of a multidisciplinary team-based care model showed excellent long-term outcomes.
The importance of coordinated postpartum follow-up with both cardiologists and obstetricians is becoming increasingly recognized, especially for women with poor pregnancy outcomes and with CVD that arises during pregnancy, such as pregnancy-associated spontaneous coronary artery dissection and peripartum cardiomyopathy, wrote Dr. Ouyang and Dr. Sharma, both with Johns Hopkins University in Baltimore.
“I’m very excited about the growing recognition of the importance of cardio-obstetrics and the emergence of many of these models of care at various institutions,” Melinda Davis, MD, of the University of Michigan in Ann Arbor, said in an interview.
“Over the next few years, I expect we will see several studies that show the benefits of the cardio-obstetrics model of care,” she explained. “Multicenter collaboration will be very important for learning about the optimal way to manage high-risk conditions during pregnancy.”
No funding sources were reported. The authors of this paper disclosed no conflicts of interest.
SOURCE: Magun E et al. JACC. 2020 Nov 3. doi: 10.1016/j.jacc.2020.08.071.
A multidisciplinary cardio-obstetrics team-based care model may help improve cardiovascular care for pregnant women with cardiovascular disease (CVD), according to a recent study.
“We sought to describe clinical characteristics, maternal and fetal outcomes, and cardiovascular readmissions in a cohort of pregnant women with underlying CVD followed by a cardio-obstetrics team,” wrote Ella Magun, MD, of Columbia University, New York, and coauthors. Their report is in the Journal of the American College of Cardiology.
The researchers reported the outcomes of a retrospective cohort analysis involving 306 pregnant women with CVD, who were treated at a quaternary care hospital in New York City.
They defined cardio-obstetrics as a team-based collaborative approach to maternal care that includes maternal fetal medicine, cardiology, anesthesiology, neonatology, nursing, social work, and pharmacy.
More than half of the women in the cohort (53%) were Hispanic and Latino, and 74% were receiving Medicaid, suggesting low socioeconomic status. Key outcomes of interest were cardiovascular readmissions at 30 days, 90 days, and 1 year. Secondary endpoints included maternal death, need for a left ventricular assist device or heart transplantation, and fetal demise.
The most frequently observed forms of CVD were arrhythmias (29%), cardiomyopathy (24%), congenital heart disease (24%), valvular disease (16%), and coronary artery disease (4%). The median Cardiac Disease in Pregnancy (CARPREG II) score was 3, and 43% of women had a CARPREG II score of 4 or higher.
After a median follow-up of 2.6 years, the 30-day and 90-day cardiovascular readmission rates were 1.9% and 4.6%, which was lower than the national 30-day postpartum rate of readmission (3.6%). One maternal death (0.3%) occurred within a year of delivery (woman with Eisenmenger syndrome).
“Despite high CARPREG II scores in this patient population, we found low rates of maternal and fetal complications with a low rate of 30- and 90-day readmissions following delivery,” the researchers wrote.
Experts weigh in
“We’re seeing widely increasing interest in the implementation of cardio-obstetrics models for multidisciplinary collaborative care and initial studies suggest these team-based models improve pregnancy and postpartum outcomes for women with cardiac disease,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and professor at Baylor College of Medicine in Houston.
Dr. Magun and colleagues acknowledged that a key limitation of the present study was the retrospective, single-center design.
“With program expansions over the next 2-3 years, I expect to see an increasing number of prospective studies with larger sample sizes evaluating the impact of cardio-obstetrics teams on maternal morbidity and mortality,” Dr. Hollier said.
“These findings suggest that our cardio-obstetrics program may help provide improved cardiovascular care to an otherwise underserved population,” the authors concluded.
In an editorial accompanying the reports, Pamela Ouyang, MBBS, and Garima Sharma, MD, wrote that, although this study wasn’t designed to assess the benefit of cardio-obstetric teams relative to standard of care, its implementation of a multidisciplinary team-based care model showed excellent long-term outcomes.
The importance of coordinated postpartum follow-up with both cardiologists and obstetricians is becoming increasingly recognized, especially for women with poor pregnancy outcomes and with CVD that arises during pregnancy, such as pregnancy-associated spontaneous coronary artery dissection and peripartum cardiomyopathy, wrote Dr. Ouyang and Dr. Sharma, both with Johns Hopkins University in Baltimore.
“I’m very excited about the growing recognition of the importance of cardio-obstetrics and the emergence of many of these models of care at various institutions,” Melinda Davis, MD, of the University of Michigan in Ann Arbor, said in an interview.
“Over the next few years, I expect we will see several studies that show the benefits of the cardio-obstetrics model of care,” she explained. “Multicenter collaboration will be very important for learning about the optimal way to manage high-risk conditions during pregnancy.”
No funding sources were reported. The authors of this paper disclosed no conflicts of interest.
SOURCE: Magun E et al. JACC. 2020 Nov 3. doi: 10.1016/j.jacc.2020.08.071.
A multidisciplinary cardio-obstetrics team-based care model may help improve cardiovascular care for pregnant women with cardiovascular disease (CVD), according to a recent study.
“We sought to describe clinical characteristics, maternal and fetal outcomes, and cardiovascular readmissions in a cohort of pregnant women with underlying CVD followed by a cardio-obstetrics team,” wrote Ella Magun, MD, of Columbia University, New York, and coauthors. Their report is in the Journal of the American College of Cardiology.
The researchers reported the outcomes of a retrospective cohort analysis involving 306 pregnant women with CVD, who were treated at a quaternary care hospital in New York City.
They defined cardio-obstetrics as a team-based collaborative approach to maternal care that includes maternal fetal medicine, cardiology, anesthesiology, neonatology, nursing, social work, and pharmacy.
More than half of the women in the cohort (53%) were Hispanic and Latino, and 74% were receiving Medicaid, suggesting low socioeconomic status. Key outcomes of interest were cardiovascular readmissions at 30 days, 90 days, and 1 year. Secondary endpoints included maternal death, need for a left ventricular assist device or heart transplantation, and fetal demise.
The most frequently observed forms of CVD were arrhythmias (29%), cardiomyopathy (24%), congenital heart disease (24%), valvular disease (16%), and coronary artery disease (4%). The median Cardiac Disease in Pregnancy (CARPREG II) score was 3, and 43% of women had a CARPREG II score of 4 or higher.
After a median follow-up of 2.6 years, the 30-day and 90-day cardiovascular readmission rates were 1.9% and 4.6%, which was lower than the national 30-day postpartum rate of readmission (3.6%). One maternal death (0.3%) occurred within a year of delivery (woman with Eisenmenger syndrome).
“Despite high CARPREG II scores in this patient population, we found low rates of maternal and fetal complications with a low rate of 30- and 90-day readmissions following delivery,” the researchers wrote.
Experts weigh in
“We’re seeing widely increasing interest in the implementation of cardio-obstetrics models for multidisciplinary collaborative care and initial studies suggest these team-based models improve pregnancy and postpartum outcomes for women with cardiac disease,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and professor at Baylor College of Medicine in Houston.
Dr. Magun and colleagues acknowledged that a key limitation of the present study was the retrospective, single-center design.
“With program expansions over the next 2-3 years, I expect to see an increasing number of prospective studies with larger sample sizes evaluating the impact of cardio-obstetrics teams on maternal morbidity and mortality,” Dr. Hollier said.
“These findings suggest that our cardio-obstetrics program may help provide improved cardiovascular care to an otherwise underserved population,” the authors concluded.
In an editorial accompanying the reports, Pamela Ouyang, MBBS, and Garima Sharma, MD, wrote that, although this study wasn’t designed to assess the benefit of cardio-obstetric teams relative to standard of care, its implementation of a multidisciplinary team-based care model showed excellent long-term outcomes.
The importance of coordinated postpartum follow-up with both cardiologists and obstetricians is becoming increasingly recognized, especially for women with poor pregnancy outcomes and with CVD that arises during pregnancy, such as pregnancy-associated spontaneous coronary artery dissection and peripartum cardiomyopathy, wrote Dr. Ouyang and Dr. Sharma, both with Johns Hopkins University in Baltimore.
“I’m very excited about the growing recognition of the importance of cardio-obstetrics and the emergence of many of these models of care at various institutions,” Melinda Davis, MD, of the University of Michigan in Ann Arbor, said in an interview.
“Over the next few years, I expect we will see several studies that show the benefits of the cardio-obstetrics model of care,” she explained. “Multicenter collaboration will be very important for learning about the optimal way to manage high-risk conditions during pregnancy.”
No funding sources were reported. The authors of this paper disclosed no conflicts of interest.
SOURCE: Magun E et al. JACC. 2020 Nov 3. doi: 10.1016/j.jacc.2020.08.071.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Novel drug slows progression of diabetic kidney disease
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM KIDNEY WEEK
ACC expert consensus on post-TAVR arrhythmias
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology (ACC) has released a new Expert Consensus Decision Pathway (ECDP) on the management of conduction disturbances after transcatheter aortic valve replacement (TAVR).
The document provides guidance to clinicians in identifying and managing this common complication of TAVR, covering the pre-TAVR, periprocedural and post-TAVR periods.
“Conduction disturbances after TAVR are common and there is currently heterogeneity in how they’re managed, ranging from a casual observational approach to invasive electrophysiological studies and preemptive pacemaker implantation,” said writing committee chair Scott Lilly, MD, PhD, from the Ohio State Wexner Medical Center in Columbus.
“We felt this kind of collaborative effort to review what little research there is on this topic and come to [an] expert consensus was long overdue,” he added.
The document was published online Oct. 21 in the Journal of the American College of Cardiology.
Dr. Lilly stressed in an interview that this effort is an ECDP and not a guideline “because there is not data out there to solidly stand on and say, ‘This is the way we should do things.’ “
His hope is that this document will generate more discussion on this topic and spur some (probably National Institutes of Health–sponsored) clinical trials to better guide practice.
Not uncommon and not decreasing
Complete heart block requiring permanent pacemaker (PPM) implantation is seen in about 15% of patients within 30 days after TAVR. While this is a clear indication for PPM, there is no consensus on the management of less severe conduction disturbances such as new bundle branch or transient complete atrioventricular (AV) heart block.
Unlike the rates of bleeding, vascular injury, and stroke, which have decreased over time, the rates of in-hospital PPM implantation after TAVR have not changed significantly since commercialization in 2012. This is a concern because TAVR is increasingly used in younger, lower-risk patients.
“The pacemaker rate really hasn’t improved at a clip we would like to see if it was going to be a durable technology,” Dr. Lilly said.
Consensus regarding a reasonable strategy to manage cardiac conduction disturbances after TAVR has been elusive. This is a result of several things: a dearth of adequately powered, randomized controlled trials; the often transient nature of the conduction disturbances; evolving technologies; and the interplay of cardiology subspecialties involved.
The 2013 European Society of Cardiology guidelines address pacing post-TAVR, but do not provide in-depth discussion on the topic. This is the first effort sponsored by a cardiovascular society in the United States to review the existing data and experience and propose evidence-based expert guidance.
Pre-TAVR assessment
Pre-TAVR assessment should consider the patient’s risk for postprocedure conduction disturbances, the authors said. Since bradyarrythmias and aortic stenosis may present similarly (fatigue, lightheadedness, and syncope being hallmarks of both), a careful history is needed to determine if bradyarrhythmia is present.
An electrocardiogram (ECG) or ambulatory rhythm monitoring may identify baseline conduction abnormalities and help predict the need for post-TAVR PPM.
“In this section, we underscored some of the literature that has raised awareness about the presence of preexisting arrhythmias in TAVR patients and suggest that monitoring in selected patients before the procedure is reasonable, particularly those presenting with syncope or lightheadedness,” said Dr. Lilly.
Intraprocedural management
On the day of the procedure, patients determined to have elevated risk for complete AV heart block require careful perioperative ECG and hemodynamic monitoring. Regardless of preexisting risk, said the authors that all patients should be monitored on a telemetry unit during the procedure with ability to do emergency pacing if necessary.
“In the periprocedural section, we address the role of electrophysiological studies for identifying patients at high-risk of subsequent heart block,” said Dr. Lilly. “That’s a practice that’s occurring at a number of centers, but the data out there is insufficient to establish it as a pacemaker indication. Routine EP testing for patients deemed at risk for conduction disturbances after TAVR is not guideline-based and more research is needed.”
The document also outlines the effects of medications and anesthesia on postprocedure conduction abnormalities.
Post-TAVR management
The authors define post-TAVR management as continuing through 30-days after discharge.
The ECDP carefully outlines which patients can be discharged without monitoring and those for whom outpatient monitoring can be considered.
“If I’m going to pick one thing from this section, it’s the monitoring piece. A lot of patients that have a conduction disturbance right after TAVR – but you’re not sure if it’s going to progress and require a pacemaker – might stay in the hospital for an extended time waiting to see if the heart holds up,” reported Dr. Lilly.
“But a number of centers are now discharging people at 1 or 2 days, which begs the question: What do you do with these folks? Our group has published data showing that 30-day monitoring in select patients is a safe approach,” said Dr. Lilly.
There are shortcomings, however, in existing data, and recommendations will likely change as more data are collected, he explained.
As well, there remains uncertainty in how conduction block should be managed after TAVR, and clinical judgment is “foundational” in this, wrote the authors.
“This document is meant to help programs deal with these situations right now, acknowledging full and well, that really good randomized clinical data is not available,” said Dr. Lilly.
Dr. Lilly has disclosed no relevant financial relationships. The work of the writing committee was supported exclusively by the American College of Cardiology without commercial support.
A version of this article originally appeared on Medscape.com.
AACE issues ‘cookbook’ algorithm to manage dyslipidemia
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).
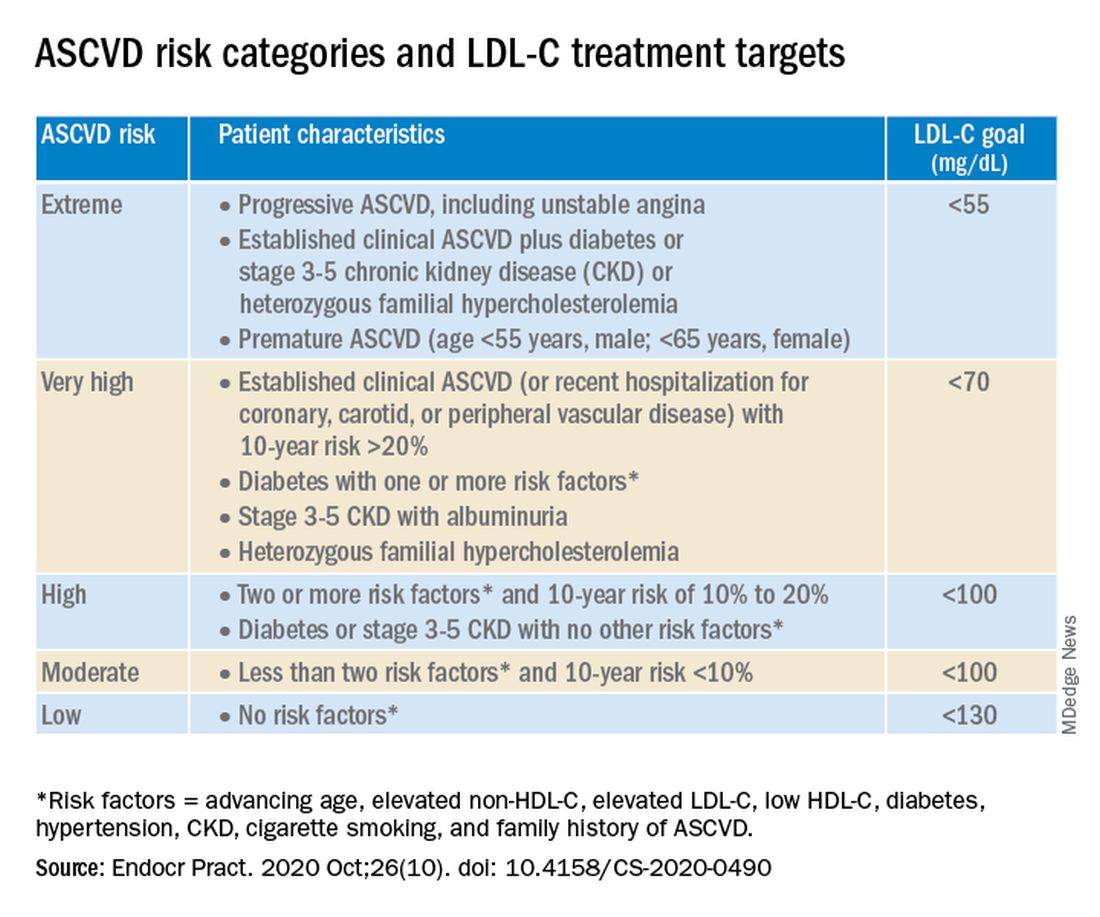
“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
Higher serum omega-3 tied to better outcome after STEMI
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Valvular disease and COVID-19 are a deadly mix; don’t delay intervention
Danny Dvir, MD, has a message for physicians who have patients with severe valvular heart disease who are deferring valve replacement or repair until after the COVID-19 pandemic: Urge them not to wait.
Data from the Multicenter International Valve Disease Registry vividly demonstrate that clinical outcomes are poor in patients with uncorrected valve disease who become hospitalized with COVID-19. Indeed, the mortality rate within 30 days after hospital admission in 136 such patients enrolled in the registry from centers in Europe, North America, and Israel was 42%, Dr. Dvir reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
“That’s dramatically higher than for an age-matched population infected with COVID-19 without valvular heart disease, which is 10%-15%,” he noted at the meeting sponsored by the Cardiovascular Research Foundation.
The bright spot was that, in the small subgroup of 15 registry participants who underwent transcatheter or, much less frequently, surgical treatment of their failing valve while COVID-19 infected, 30-day mortality was far lower. In fact, it was comparable with the background rate in hospitalized COVID-19 patients without valve disease, according to Dr. Dvir, an interventional cardiologist at Shaare Zedek Medical Center, Hebrew University, Jerusalem.
He personally did several of the transcatheter aortic valve replacements.
“It’s doable. I truly believe that when you get a severe aortic stenosis patient who’s infected with the coronavirus, they get very unstable, but we can treat them. We can treat them even during the infection,” Dr. Dvir said.
The majority of patients in the registry had severe aortic stenosis. In the 42 such patients aged 80 years or more who didn’t undergo transcatheter aortic valve replacement (TAVR) or surgical valve replacement, 30-day mortality was 60%. In contrast, only one of the six patients in this advanced-age category who underwent valve replacement while infected died. Similarly, 30-day mortality was 24% among those younger than age 80 who valve remained untreated, but it dropped to 11% in those who received a prosthetic valve.
“We try our best to protect our patients through social distancing, but we have a treatment that can potentially reduce their mortality risk if they get infected later on. So I say to my patients: ‘Don’t wait at home. Do not wait! If you get infected when you have severe aortic stenosis, the clinical outcome is bad.’ But it seems reasonable that if they get infected when they’ve already been treated for their aortic stenosis or mitral regurgitation, they will do better.”
Dr. Dvir noted that, although the case numbers in the registry series were small and subject to potential bias, the data suggest this treatment approach may be lifesaving.
Session comoderator Timothy D. Henry, MD, commented that this registry study contains a great take-home point: “This is really consistent with what see in a lot of the other areas of COVID, that what we know to be best clinical care, we should do it, with or without the COVID.”
He asked Dr. Dvir about any special measures he takes while doing TAVR in this extreme setting. In the United States, for example, interventionalists are increasingly using transesophageal echocardiography to guide their procedures using conscious sedation, without intubation, noted Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research at the Christ Hospital, Cincinnati.
“We try to minimize the procedure time; that’s one of the important things,” Dr. Dvir replied. “And you need to be protected during the procedure in a very cautious and meticulous way. You need many fans in the room because you sweat a lot.”
Discussant Renu Virmani, MD, president of the CVPath Institute in Gaithersburg, Md., commented: “The main thing I get from this presentation is the need for patients to be educated that if you’ve got valve disease, you’re better off getting it treated before you’ve got COVID. Obviously, try to prevent getting COVID – that’s the best thing you can do – but you can’t always control that.”
Discussant Mamas Mamas, MD, professor of cardiology at Keele University, Staffordshire, England, said deferred treatment of severe valvular heart disease during the pandemic has created a looming public health crisis in the United Kingdom.
“We’ve analyzed the U.K. management of aortic stenosis, and what we’ve found is that during the COVID pandemic there have been 2,500 fewer cases of aortic stenosis that have been treated. We’ve got 2,500 patients on the waiting list, and we’ve got to work out how we’re going to treat them. We estimate with simulations that about 300 of them are going to die before we can get them treated for their aortic stenosis,” according to Dr. Mamas.
Dr. Henry commented that deferral of valve procedures is “really challenging” for a couple of reasons: Not only are patients scared to come into the hospital because they fear getting COVID, but they don’t want to be hospitalized during the pandemic because their family can’t visit them there.
“These patients are mostly over 80 years old. No one wants to come in the hospital when the family won’t be around, especially when you’re 90 years old,” the interventional cardiologist said.
Dr. Dvir reported serving as a consultant to Medtronic, Edwards Lifesciences, Abbott, and Jena.
Danny Dvir, MD, has a message for physicians who have patients with severe valvular heart disease who are deferring valve replacement or repair until after the COVID-19 pandemic: Urge them not to wait.
Data from the Multicenter International Valve Disease Registry vividly demonstrate that clinical outcomes are poor in patients with uncorrected valve disease who become hospitalized with COVID-19. Indeed, the mortality rate within 30 days after hospital admission in 136 such patients enrolled in the registry from centers in Europe, North America, and Israel was 42%, Dr. Dvir reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
“That’s dramatically higher than for an age-matched population infected with COVID-19 without valvular heart disease, which is 10%-15%,” he noted at the meeting sponsored by the Cardiovascular Research Foundation.
The bright spot was that, in the small subgroup of 15 registry participants who underwent transcatheter or, much less frequently, surgical treatment of their failing valve while COVID-19 infected, 30-day mortality was far lower. In fact, it was comparable with the background rate in hospitalized COVID-19 patients without valve disease, according to Dr. Dvir, an interventional cardiologist at Shaare Zedek Medical Center, Hebrew University, Jerusalem.
He personally did several of the transcatheter aortic valve replacements.
“It’s doable. I truly believe that when you get a severe aortic stenosis patient who’s infected with the coronavirus, they get very unstable, but we can treat them. We can treat them even during the infection,” Dr. Dvir said.
The majority of patients in the registry had severe aortic stenosis. In the 42 such patients aged 80 years or more who didn’t undergo transcatheter aortic valve replacement (TAVR) or surgical valve replacement, 30-day mortality was 60%. In contrast, only one of the six patients in this advanced-age category who underwent valve replacement while infected died. Similarly, 30-day mortality was 24% among those younger than age 80 who valve remained untreated, but it dropped to 11% in those who received a prosthetic valve.
“We try our best to protect our patients through social distancing, but we have a treatment that can potentially reduce their mortality risk if they get infected later on. So I say to my patients: ‘Don’t wait at home. Do not wait! If you get infected when you have severe aortic stenosis, the clinical outcome is bad.’ But it seems reasonable that if they get infected when they’ve already been treated for their aortic stenosis or mitral regurgitation, they will do better.”
Dr. Dvir noted that, although the case numbers in the registry series were small and subject to potential bias, the data suggest this treatment approach may be lifesaving.
Session comoderator Timothy D. Henry, MD, commented that this registry study contains a great take-home point: “This is really consistent with what see in a lot of the other areas of COVID, that what we know to be best clinical care, we should do it, with or without the COVID.”
He asked Dr. Dvir about any special measures he takes while doing TAVR in this extreme setting. In the United States, for example, interventionalists are increasingly using transesophageal echocardiography to guide their procedures using conscious sedation, without intubation, noted Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research at the Christ Hospital, Cincinnati.
“We try to minimize the procedure time; that’s one of the important things,” Dr. Dvir replied. “And you need to be protected during the procedure in a very cautious and meticulous way. You need many fans in the room because you sweat a lot.”
Discussant Renu Virmani, MD, president of the CVPath Institute in Gaithersburg, Md., commented: “The main thing I get from this presentation is the need for patients to be educated that if you’ve got valve disease, you’re better off getting it treated before you’ve got COVID. Obviously, try to prevent getting COVID – that’s the best thing you can do – but you can’t always control that.”
Discussant Mamas Mamas, MD, professor of cardiology at Keele University, Staffordshire, England, said deferred treatment of severe valvular heart disease during the pandemic has created a looming public health crisis in the United Kingdom.
“We’ve analyzed the U.K. management of aortic stenosis, and what we’ve found is that during the COVID pandemic there have been 2,500 fewer cases of aortic stenosis that have been treated. We’ve got 2,500 patients on the waiting list, and we’ve got to work out how we’re going to treat them. We estimate with simulations that about 300 of them are going to die before we can get them treated for their aortic stenosis,” according to Dr. Mamas.
Dr. Henry commented that deferral of valve procedures is “really challenging” for a couple of reasons: Not only are patients scared to come into the hospital because they fear getting COVID, but they don’t want to be hospitalized during the pandemic because their family can’t visit them there.
“These patients are mostly over 80 years old. No one wants to come in the hospital when the family won’t be around, especially when you’re 90 years old,” the interventional cardiologist said.
Dr. Dvir reported serving as a consultant to Medtronic, Edwards Lifesciences, Abbott, and Jena.
Danny Dvir, MD, has a message for physicians who have patients with severe valvular heart disease who are deferring valve replacement or repair until after the COVID-19 pandemic: Urge them not to wait.
Data from the Multicenter International Valve Disease Registry vividly demonstrate that clinical outcomes are poor in patients with uncorrected valve disease who become hospitalized with COVID-19. Indeed, the mortality rate within 30 days after hospital admission in 136 such patients enrolled in the registry from centers in Europe, North America, and Israel was 42%, Dr. Dvir reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
“That’s dramatically higher than for an age-matched population infected with COVID-19 without valvular heart disease, which is 10%-15%,” he noted at the meeting sponsored by the Cardiovascular Research Foundation.
The bright spot was that, in the small subgroup of 15 registry participants who underwent transcatheter or, much less frequently, surgical treatment of their failing valve while COVID-19 infected, 30-day mortality was far lower. In fact, it was comparable with the background rate in hospitalized COVID-19 patients without valve disease, according to Dr. Dvir, an interventional cardiologist at Shaare Zedek Medical Center, Hebrew University, Jerusalem.
He personally did several of the transcatheter aortic valve replacements.
“It’s doable. I truly believe that when you get a severe aortic stenosis patient who’s infected with the coronavirus, they get very unstable, but we can treat them. We can treat them even during the infection,” Dr. Dvir said.
The majority of patients in the registry had severe aortic stenosis. In the 42 such patients aged 80 years or more who didn’t undergo transcatheter aortic valve replacement (TAVR) or surgical valve replacement, 30-day mortality was 60%. In contrast, only one of the six patients in this advanced-age category who underwent valve replacement while infected died. Similarly, 30-day mortality was 24% among those younger than age 80 who valve remained untreated, but it dropped to 11% in those who received a prosthetic valve.
“We try our best to protect our patients through social distancing, but we have a treatment that can potentially reduce their mortality risk if they get infected later on. So I say to my patients: ‘Don’t wait at home. Do not wait! If you get infected when you have severe aortic stenosis, the clinical outcome is bad.’ But it seems reasonable that if they get infected when they’ve already been treated for their aortic stenosis or mitral regurgitation, they will do better.”
Dr. Dvir noted that, although the case numbers in the registry series were small and subject to potential bias, the data suggest this treatment approach may be lifesaving.
Session comoderator Timothy D. Henry, MD, commented that this registry study contains a great take-home point: “This is really consistent with what see in a lot of the other areas of COVID, that what we know to be best clinical care, we should do it, with or without the COVID.”
He asked Dr. Dvir about any special measures he takes while doing TAVR in this extreme setting. In the United States, for example, interventionalists are increasingly using transesophageal echocardiography to guide their procedures using conscious sedation, without intubation, noted Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research at the Christ Hospital, Cincinnati.
“We try to minimize the procedure time; that’s one of the important things,” Dr. Dvir replied. “And you need to be protected during the procedure in a very cautious and meticulous way. You need many fans in the room because you sweat a lot.”
Discussant Renu Virmani, MD, president of the CVPath Institute in Gaithersburg, Md., commented: “The main thing I get from this presentation is the need for patients to be educated that if you’ve got valve disease, you’re better off getting it treated before you’ve got COVID. Obviously, try to prevent getting COVID – that’s the best thing you can do – but you can’t always control that.”
Discussant Mamas Mamas, MD, professor of cardiology at Keele University, Staffordshire, England, said deferred treatment of severe valvular heart disease during the pandemic has created a looming public health crisis in the United Kingdom.
“We’ve analyzed the U.K. management of aortic stenosis, and what we’ve found is that during the COVID pandemic there have been 2,500 fewer cases of aortic stenosis that have been treated. We’ve got 2,500 patients on the waiting list, and we’ve got to work out how we’re going to treat them. We estimate with simulations that about 300 of them are going to die before we can get them treated for their aortic stenosis,” according to Dr. Mamas.
Dr. Henry commented that deferral of valve procedures is “really challenging” for a couple of reasons: Not only are patients scared to come into the hospital because they fear getting COVID, but they don’t want to be hospitalized during the pandemic because their family can’t visit them there.
“These patients are mostly over 80 years old. No one wants to come in the hospital when the family won’t be around, especially when you’re 90 years old,” the interventional cardiologist said.
Dr. Dvir reported serving as a consultant to Medtronic, Edwards Lifesciences, Abbott, and Jena.
FROM TCT 2020
AHA adds recovery, emotional support to CPR guidelines
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
FROM CIRCULATION
Artificially sweetened drinks add to CVD risk
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY