User login
Used together, troponin and coronary calcium improve CV risk assessment
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Hot-off-the-press insights on heart failure
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
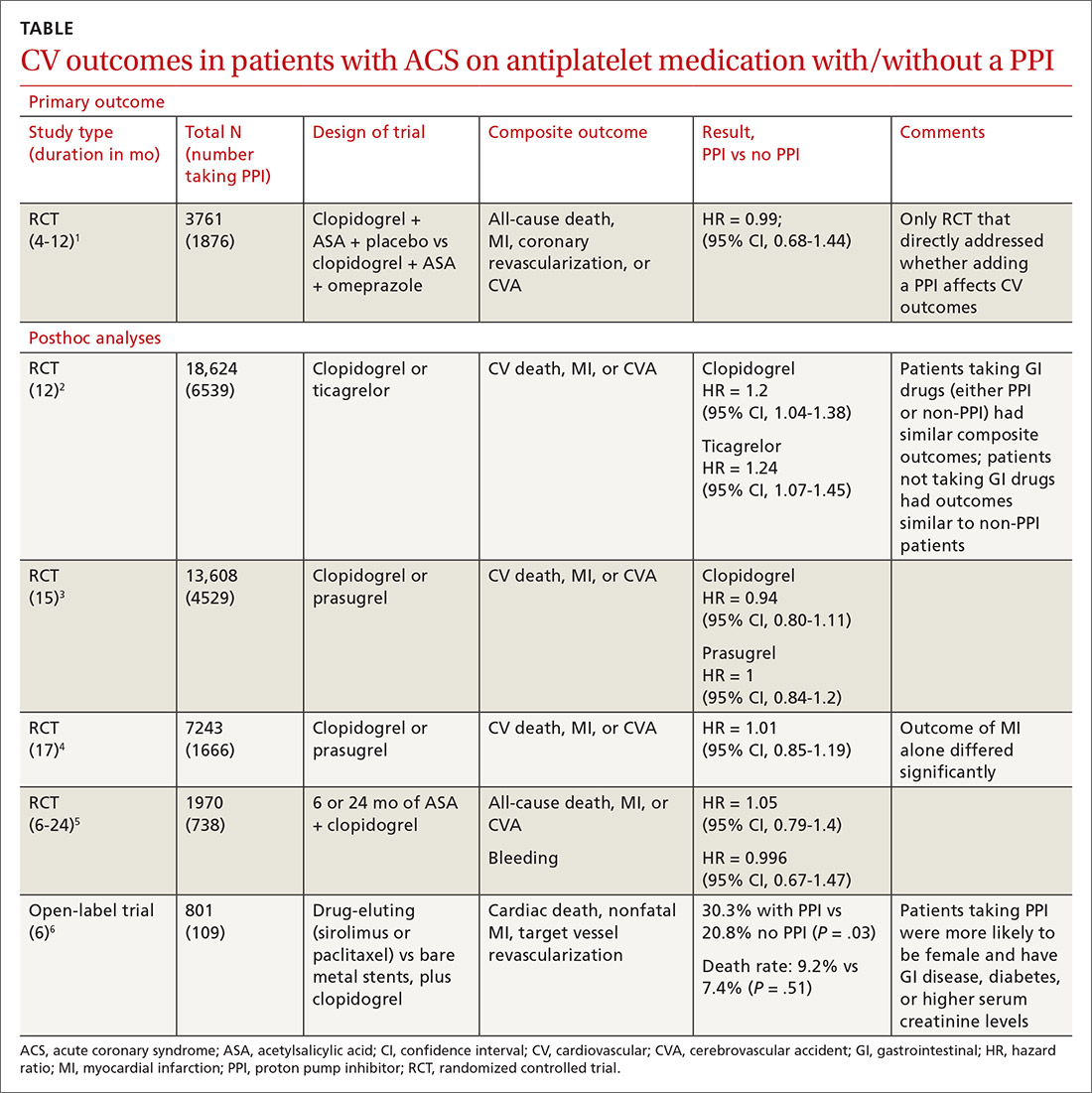
Does concurrent use of clopidogrel and PPIs increase CV risk in patients with ACS?
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE-BASED ANSWER:
No. Adding a proton pump inhibitor (PPI) in patients taking antiplatelet medications such as clopidogrel for acute coronary syndrome (ACS) doesn’t increase the composite risk of cardiovascular (CV) events: CV death, myocardial infarction (MI), and cerebrovascular accident (CVA) (strength of recommendation: B, randomized, controlled trial [RCT] and prepon-derance of posthoc analyses of large RCTs).
Psoriatic disease inflammation linked to heart failure
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
Empagliflozin failed to improve exercise capacity in heart failure
Empagliflozin showed favorable effects on diuretic use and congestion symptoms in patients with heart failure with reduced ejection fraction (HFrEF), but the oral sodium glucose cotransporter 2 (SGLT2) inhibitor did not improve the primary endpoint of improved exercise capacity in the EMPERIAL-Reduced trial, investigators reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
In the matching EMPERIAL-Preserved trial, conducted in patients with heart failure with preserved ejection fraction (HFpEF), empagliflozin (Jardiance) produced modest improvements in diuretic use, as well as a reduction in unscheduled outpatient visits, compared with placebo-treated controls, although these trends failed to achieve statistical significance. And as in the EMPERIAL-Reduced trial, the SGLT2 inhibitor didn’t move the needle at all on the primary endpoint of improved exercise capacity as measured by 6-minute hall walk distance.
EMPERIAL-Reduced and -Preserved were identically designed, concurrent, phase 3, double-blind, 12-week randomized trials of empagliflozin versus placebo in 312 patients with HFrEF and 315 with HFpEF, defined in EMPERIAL-preserved as a left ventricular ejection fraction above 40%. The majority of participants had type 2 diabetes.
From a baseline median 6-minute walk distance of about 300 meters, the 6-minute walk distance at week 12 was actually 4.0 meters worse in the empagliflozin-treated HFrEF patients than it was in controls and a mere 4.0 meters better than with placebo in empagliflozin-treated patients with HFpEF, reported William T. Abraham, MD, professor of medicine, director of the division of cardiovascular medicine, and associate dean at Ohio State University, Columbus.
He indicated that the audience shouldn’t make too much of the failure to achieve the primary endpoint in the two trials in light of the studies’ major limitations: namely, their relatively small size for purposes of evaluating clinical outcomes and the relatively short 12-week duration.
“In many ways, I would say it’s remarkable that we can observe a positive signal, a favorable signal, in outcomes around congestion. In the case of HFrEF it’s statistically significant, and in HFpEF it’s a trend towards improvement. Of course, there are larger trials ongoing that may confirm these observations. Hopefully the EMPERIAL trials predict a good outcome for those ongoing trials,” Dr. Abraham said.
Piotr Ponikowski, MD, presented the study results for the secondary outcomes of congestion symptoms, diuretic use, and utilization of health care resources. In EMPERIAL-Reduced, intensification of diuretic therapy – often a prelude to acute decompensation and a trip to the hospital – occurred at a rate of 4.5% with empagliflozin and 16.1% with placebo, for a highly significant 73% relative risk reduction. Intensification of loop diuretics occurred in 2.6% of the empagliflozin group and 14.2% of controls, for a 82% risk reduction.
“That’s a pretty significant effect,” observed Dr. Ponikowski, professor of cardiology and head of the department of heart diseases at the Medical University of Wroclaw (Poland).
Moreover, a congestion symptoms score comprising a summary of orthopnea, jugular veinous distention, and edema improved by 47% after 12 weeks on empagliflozin, a statistically significant and clinically meaningful improvement that grew in magnitude over time and at 12 weeks was twice as large, compared with the reduction in placebo group, he added.
There was a trend for fewer unscheduled outpatient visits in the empagliflozin arm of EMPERIAL-Reduced with a rate of 10.4%, compared with 25.8% in controls; however, this 26% reduction in relative risk did not achieve statistical significance.
Intensification of loop diuretics occurred in 9% of EMPERIAL-Preserved participants on empagliflozin and 13.5% on placebo, but this 34% reduction in risk didn’t reach significance.
Adverse events in the EMPERIAL trials were similar across the active treatment and placebo arms. The benign safety profile was similar to what was seen in the earlier major clinical trials of empagliflozin for treatment of type 2 diabetes.
Session chair Stephane Heymans, MD, PhD, of the University of Maastricht (the Netherlands) noted that a substantial minority of patients in EMPERIAL-Reduced were on the combined neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan (Entresto), whereas far fewer were in EMPERIAL-Preserved. He wondered if this greater use of background sacubitril/valsartan could explain empagliflozin’s greater efficacy in EMPERIAL-Reduced.
Highly unlikely, according to the investigators.
“It looks like, as is the case with most of our heart failure therapies, that we do see incremental value here. If you met the criteria for these trials, it appears you derived benefit from empagliflozin regardless of whether you were on an angiotensin receptor neprilysin inhibitor or not. I think that speaks to the incremental benefit of SGLT2 inhibitors on top of current guideline-directed medical therapy,” Dr. Abraham said.
Dr. Ponikowski observed that the same point was underscored in the DAPA-HF trial of the SGLT2 inhibitor dapagliflozin (Farxiga) in patients with heart failure (DAPA-HF: N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“You’ll see that the mortality and morbidity and quality-of-life benefit is in those treated with dapagliflozin with or without angiotensin receptor neprilysin inhibition; so, regardless of background therapy. And the effect is especially clear in patients on both therapies,” the cardiologist said.
The EMPERIAL trials were sponsored by Boehringer Ingelheim. Dr. Abraham and Dr. Ponikowksi reported receiving consultant fees from the company for serving on the trials’ executive committee.
Empagliflozin showed favorable effects on diuretic use and congestion symptoms in patients with heart failure with reduced ejection fraction (HFrEF), but the oral sodium glucose cotransporter 2 (SGLT2) inhibitor did not improve the primary endpoint of improved exercise capacity in the EMPERIAL-Reduced trial, investigators reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
In the matching EMPERIAL-Preserved trial, conducted in patients with heart failure with preserved ejection fraction (HFpEF), empagliflozin (Jardiance) produced modest improvements in diuretic use, as well as a reduction in unscheduled outpatient visits, compared with placebo-treated controls, although these trends failed to achieve statistical significance. And as in the EMPERIAL-Reduced trial, the SGLT2 inhibitor didn’t move the needle at all on the primary endpoint of improved exercise capacity as measured by 6-minute hall walk distance.
EMPERIAL-Reduced and -Preserved were identically designed, concurrent, phase 3, double-blind, 12-week randomized trials of empagliflozin versus placebo in 312 patients with HFrEF and 315 with HFpEF, defined in EMPERIAL-preserved as a left ventricular ejection fraction above 40%. The majority of participants had type 2 diabetes.
From a baseline median 6-minute walk distance of about 300 meters, the 6-minute walk distance at week 12 was actually 4.0 meters worse in the empagliflozin-treated HFrEF patients than it was in controls and a mere 4.0 meters better than with placebo in empagliflozin-treated patients with HFpEF, reported William T. Abraham, MD, professor of medicine, director of the division of cardiovascular medicine, and associate dean at Ohio State University, Columbus.
He indicated that the audience shouldn’t make too much of the failure to achieve the primary endpoint in the two trials in light of the studies’ major limitations: namely, their relatively small size for purposes of evaluating clinical outcomes and the relatively short 12-week duration.
“In many ways, I would say it’s remarkable that we can observe a positive signal, a favorable signal, in outcomes around congestion. In the case of HFrEF it’s statistically significant, and in HFpEF it’s a trend towards improvement. Of course, there are larger trials ongoing that may confirm these observations. Hopefully the EMPERIAL trials predict a good outcome for those ongoing trials,” Dr. Abraham said.
Piotr Ponikowski, MD, presented the study results for the secondary outcomes of congestion symptoms, diuretic use, and utilization of health care resources. In EMPERIAL-Reduced, intensification of diuretic therapy – often a prelude to acute decompensation and a trip to the hospital – occurred at a rate of 4.5% with empagliflozin and 16.1% with placebo, for a highly significant 73% relative risk reduction. Intensification of loop diuretics occurred in 2.6% of the empagliflozin group and 14.2% of controls, for a 82% risk reduction.
“That’s a pretty significant effect,” observed Dr. Ponikowski, professor of cardiology and head of the department of heart diseases at the Medical University of Wroclaw (Poland).
Moreover, a congestion symptoms score comprising a summary of orthopnea, jugular veinous distention, and edema improved by 47% after 12 weeks on empagliflozin, a statistically significant and clinically meaningful improvement that grew in magnitude over time and at 12 weeks was twice as large, compared with the reduction in placebo group, he added.
There was a trend for fewer unscheduled outpatient visits in the empagliflozin arm of EMPERIAL-Reduced with a rate of 10.4%, compared with 25.8% in controls; however, this 26% reduction in relative risk did not achieve statistical significance.
Intensification of loop diuretics occurred in 9% of EMPERIAL-Preserved participants on empagliflozin and 13.5% on placebo, but this 34% reduction in risk didn’t reach significance.
Adverse events in the EMPERIAL trials were similar across the active treatment and placebo arms. The benign safety profile was similar to what was seen in the earlier major clinical trials of empagliflozin for treatment of type 2 diabetes.
Session chair Stephane Heymans, MD, PhD, of the University of Maastricht (the Netherlands) noted that a substantial minority of patients in EMPERIAL-Reduced were on the combined neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan (Entresto), whereas far fewer were in EMPERIAL-Preserved. He wondered if this greater use of background sacubitril/valsartan could explain empagliflozin’s greater efficacy in EMPERIAL-Reduced.
Highly unlikely, according to the investigators.
“It looks like, as is the case with most of our heart failure therapies, that we do see incremental value here. If you met the criteria for these trials, it appears you derived benefit from empagliflozin regardless of whether you were on an angiotensin receptor neprilysin inhibitor or not. I think that speaks to the incremental benefit of SGLT2 inhibitors on top of current guideline-directed medical therapy,” Dr. Abraham said.
Dr. Ponikowski observed that the same point was underscored in the DAPA-HF trial of the SGLT2 inhibitor dapagliflozin (Farxiga) in patients with heart failure (DAPA-HF: N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“You’ll see that the mortality and morbidity and quality-of-life benefit is in those treated with dapagliflozin with or without angiotensin receptor neprilysin inhibition; so, regardless of background therapy. And the effect is especially clear in patients on both therapies,” the cardiologist said.
The EMPERIAL trials were sponsored by Boehringer Ingelheim. Dr. Abraham and Dr. Ponikowksi reported receiving consultant fees from the company for serving on the trials’ executive committee.
Empagliflozin showed favorable effects on diuretic use and congestion symptoms in patients with heart failure with reduced ejection fraction (HFrEF), but the oral sodium glucose cotransporter 2 (SGLT2) inhibitor did not improve the primary endpoint of improved exercise capacity in the EMPERIAL-Reduced trial, investigators reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
In the matching EMPERIAL-Preserved trial, conducted in patients with heart failure with preserved ejection fraction (HFpEF), empagliflozin (Jardiance) produced modest improvements in diuretic use, as well as a reduction in unscheduled outpatient visits, compared with placebo-treated controls, although these trends failed to achieve statistical significance. And as in the EMPERIAL-Reduced trial, the SGLT2 inhibitor didn’t move the needle at all on the primary endpoint of improved exercise capacity as measured by 6-minute hall walk distance.
EMPERIAL-Reduced and -Preserved were identically designed, concurrent, phase 3, double-blind, 12-week randomized trials of empagliflozin versus placebo in 312 patients with HFrEF and 315 with HFpEF, defined in EMPERIAL-preserved as a left ventricular ejection fraction above 40%. The majority of participants had type 2 diabetes.
From a baseline median 6-minute walk distance of about 300 meters, the 6-minute walk distance at week 12 was actually 4.0 meters worse in the empagliflozin-treated HFrEF patients than it was in controls and a mere 4.0 meters better than with placebo in empagliflozin-treated patients with HFpEF, reported William T. Abraham, MD, professor of medicine, director of the division of cardiovascular medicine, and associate dean at Ohio State University, Columbus.
He indicated that the audience shouldn’t make too much of the failure to achieve the primary endpoint in the two trials in light of the studies’ major limitations: namely, their relatively small size for purposes of evaluating clinical outcomes and the relatively short 12-week duration.
“In many ways, I would say it’s remarkable that we can observe a positive signal, a favorable signal, in outcomes around congestion. In the case of HFrEF it’s statistically significant, and in HFpEF it’s a trend towards improvement. Of course, there are larger trials ongoing that may confirm these observations. Hopefully the EMPERIAL trials predict a good outcome for those ongoing trials,” Dr. Abraham said.
Piotr Ponikowski, MD, presented the study results for the secondary outcomes of congestion symptoms, diuretic use, and utilization of health care resources. In EMPERIAL-Reduced, intensification of diuretic therapy – often a prelude to acute decompensation and a trip to the hospital – occurred at a rate of 4.5% with empagliflozin and 16.1% with placebo, for a highly significant 73% relative risk reduction. Intensification of loop diuretics occurred in 2.6% of the empagliflozin group and 14.2% of controls, for a 82% risk reduction.
“That’s a pretty significant effect,” observed Dr. Ponikowski, professor of cardiology and head of the department of heart diseases at the Medical University of Wroclaw (Poland).
Moreover, a congestion symptoms score comprising a summary of orthopnea, jugular veinous distention, and edema improved by 47% after 12 weeks on empagliflozin, a statistically significant and clinically meaningful improvement that grew in magnitude over time and at 12 weeks was twice as large, compared with the reduction in placebo group, he added.
There was a trend for fewer unscheduled outpatient visits in the empagliflozin arm of EMPERIAL-Reduced with a rate of 10.4%, compared with 25.8% in controls; however, this 26% reduction in relative risk did not achieve statistical significance.
Intensification of loop diuretics occurred in 9% of EMPERIAL-Preserved participants on empagliflozin and 13.5% on placebo, but this 34% reduction in risk didn’t reach significance.
Adverse events in the EMPERIAL trials were similar across the active treatment and placebo arms. The benign safety profile was similar to what was seen in the earlier major clinical trials of empagliflozin for treatment of type 2 diabetes.
Session chair Stephane Heymans, MD, PhD, of the University of Maastricht (the Netherlands) noted that a substantial minority of patients in EMPERIAL-Reduced were on the combined neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan (Entresto), whereas far fewer were in EMPERIAL-Preserved. He wondered if this greater use of background sacubitril/valsartan could explain empagliflozin’s greater efficacy in EMPERIAL-Reduced.
Highly unlikely, according to the investigators.
“It looks like, as is the case with most of our heart failure therapies, that we do see incremental value here. If you met the criteria for these trials, it appears you derived benefit from empagliflozin regardless of whether you were on an angiotensin receptor neprilysin inhibitor or not. I think that speaks to the incremental benefit of SGLT2 inhibitors on top of current guideline-directed medical therapy,” Dr. Abraham said.
Dr. Ponikowski observed that the same point was underscored in the DAPA-HF trial of the SGLT2 inhibitor dapagliflozin (Farxiga) in patients with heart failure (DAPA-HF: N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“You’ll see that the mortality and morbidity and quality-of-life benefit is in those treated with dapagliflozin with or without angiotensin receptor neprilysin inhibition; so, regardless of background therapy. And the effect is especially clear in patients on both therapies,” the cardiologist said.
The EMPERIAL trials were sponsored by Boehringer Ingelheim. Dr. Abraham and Dr. Ponikowksi reported receiving consultant fees from the company for serving on the trials’ executive committee.
FROM ESC HEART FAILURE 2020
Why doctors keep monitoring kids who recover from mysterious COVID-linked illness
He’s a 5-year-old boy and would much rather talk about cartoons or the ideas for inventions that constantly pop into his head.
“Hold your horses, I think I know what I’m gonna make,” he said, holding up a finger in the middle of a conversation. “I’m gonna make something that lights up and attaches to things with glue, so if you don’t have a flashlight, you can just use it!”
In New York, at least 237 kids, including Israel, appear to have Multisystem Inflammatory Syndrome in Children (MIS-C). And state officials continue to track the syndrome, but the Centers for Disease Control and Prevention did not respond to repeated requests for information on how many children nationwide have been diagnosed so far with MIS-C.
A study published June 29 in the New England Journal of Medicine reported on 186 patients in 26 states who had been diagnosed with MIS-C. A researcher writing in the same issue added reports from other countries, finding that about 1,000 children worldwide have been diagnosed with MIS-C.
Tracking the long-term health effects of MIS-C
Israel is friendly and energetic, but he’s also really good at sitting still. During a recent checkup at the Children’s Hospital at Montefiore, New York, he had no complaints about all the stickers and wires a health aide attached to him for an EKG. And when Marc Foca, MD, an infectious disease specialist, came by to listen to his heart and lungs, and prod his abdomen, Israel barely seemed to notice.
There were still some tests pending, but overall, Dr. Foca said, “Israel looks like a totally healthy 5-year-old.”
“Stay safe!” Israel called out, as Dr. Foca left. It’s his new sign-off, instead of goodbye. His mother, Janelle Moholland, explained Israel came up with it himself. And she’s also hoping that, after a harrowing couple of weeks in early May, Israel himself will “stay safe.”
That’s why they’ve been returning to Montefiore for the periodic checkups, even though Israel seems to have recovered fully from both COVID-19 and MIS-C.
MIS-C is relatively rare, and it apparently responds well to treatment, but it is new enough – and mysterious enough – that doctors here want to make sure the children who recover don’t experience any related health complications in the future.
“We’ve seen these kids get really sick, and get better and recover and go home, yet we don’t know what the long-term outcomes are,” said Nadine Choueiter, MD, a pediatric cardiologist at Montefiore. “So that’s why we will be seeing them.”
When Israel first got sick at the end of April, his illness didn’t exactly look like COVID-19. He had persistent high fevers, with his temperature reaching 104° F – but no problems breathing. He wasn’t eating. He was barely drinking. He wasn’t using the bathroom. He had abdominal pains. His eyes were red.
They went to the ED a couple of times and visited an urgent care center, but the doctors sent them home without testing him for the coronavirus. Ms. Moholland, 29, said she felt powerless.
“There was nothing I could do but make him comfortable,” she said. “I literally had to just trust in a higher power and just hope that He would come through for us. It taught me a lot about patience and faith.”
As Israel grew sicker, and they still had no answers, Ms. Moholland grew frustrated. “I wish his pediatrician and [the ED and urgent care staff] had done what they were supposed to do and given him a test” when Israel first got sick, Ms. Moholland said. “What harm would it have done? He suffered for about 10 or 11 days that could have been avoided.”
In a later interview, she talked with NPR about how COVID-19 has disproportionately affected the African American community because of a combination of underlying health conditions and lack of access to good health care. She said she felt she, too, had fallen victim to those disparities.
“It affects me, personally, because I am African American, but you just never know,” she said. “It’s hard. We’re living in uncertain times – very uncertain times.”
Finally, the Children’s Hospital at Montefiore admitted Israel – and the test she’d been trying to get for days confirmed he had the virus.
“I was literally in tears, like begging them not to discharge me because I knew he was not fine,” she recalled.
Israel was in shock, and by the time he got to the hospital, doctors were on the lookout for MIS-C, so they recognized his symptoms – which were distinct from most people with COVID-19.
Doctors gave Israel fluids and intravenous immunoglobulin, a substance obtained from donated human plasma, which is used to treat deficiencies in the immune system.
Immunoglobulin has been effective in children like Israel because MIS-C appears to be caused by an immune overreaction to the initial coronavirus infection, according to Dr. Choueiter.
“The immune system starts attacking the body itself, including the arteries of the heart,” she said.
In some MIS-C cases – though not Israel’s – the attack occurs in the coronary arteries, inflaming and dilating them. That also happens in a different syndrome affecting children, Kawasaki disease. About 5% of Kawasaki patients experience aneurysms – which can fatally rupture blood vessels – after the initial condition subsides.
Dr. Choueiter and colleagues want to make sure MIS-C patients don’t face similar risks. So far, they’re cautiously optimistic.
“We have not seen any new decrease in heart function or any new coronary artery dilations,” she said. “When we check their blood, their inflammatory markers are back to normal. For the parents, the child is back to baseline, and it’s as if this illness is a nightmare that’s long gone.”
For a Pennsylvania teen, the MIS-C diagnosis came much later
Not every child who develops MIS-C tests positive for the coronavirus, though many will test positive for antibodies to the coronavirus, indicating they had been infected previously. That was the case with Andrew Lis, a boy from Pennsylvania who was the first MIS-C patient seen at the Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del.
Andrew had been a healthy 14-year-old boy before he got sick. He and his twin brother love sports and video games. He said the first symptom was a bad headache. He developed a fever the next day, then constipation and intense stomach pain.
“It was terrible,” Andrew said. “It was unbearable. I couldn’t really move a lot.”
His mother, Ingrid Lis, said they were thinking appendicitis, not coronavirus, at first. In fact, she hesitated to take Andrew to the hospital, for fear of exposing him to the virus. But after Andrew stopped eating because of his headache and stomach discomfort, “I knew I couldn’t keep him home anymore,” Mrs. Lis said.
Andrew was admitted to the hospital April 12, but that was before reports of the mysterious syndrome had started trickling out of Europe.
Over about 5 days in the pediatric ICU, Andrew’s condition deteriorated rapidly, as doctors struggled to figure out what was wrong. Puzzled, they tried treatments for scarlet fever, strep throat, and toxic shock syndrome. Andrew’s body broke out in rashes, then his heart began failing and he was put on a ventilator. Andrew’s father, Ed Lis, said doctors told the family to brace for the worst: “We’ve got a healthy kid who a few days ago was just having these sort of strange symptoms. And now they’re telling us that we could lose him.”
Though Andrew’s symptoms were atypical for Kawasaki disease, doctors decided to give him the standard treatment for that condition – administering intravenous immunoglobulin, the same treatment Israel Shippy received.
“Within the 24 hours of the infusion, he was a different person,” Mrs. Lis said. Andrew was removed from the ventilator, and his appetite eventually returned. “That’s when we knew that we had turned that corner.”
It wasn’t until after Andrew’s discharge that his doctors learned about MIS-C from colleagues in Europe. They recommended the whole family be tested for antibodies to the coronavirus. Although Andrew tested positive, the rest of the family – both parents, Andrew’s twin brother and two older siblings – all tested negative. Andrew’s mother is still not sure how he was exposed since the family had been observing a strict lockdown since mid-March. Both she and her husband were working remotely from home, and she says they all wore masks and were conscientious about hand-washing when they ventured out for groceries. She thinks Andrew must have been exposed at least a month before his illness began.
And she’s puzzled why the rest of her close-knit family wasn’t infected as well. “We are a Latino family,” Mrs. Lis said. “We are very used to being together, clustering in the same room.” Even when Andrew was sick, she says, all six of them huddled in his bedroom to comfort him.
Meanwhile, Andrew has made a quick recovery. Not long after his discharge in April, he turned 15 and resumed an exercise routine involving running, push-ups, and sit-ups. A few weeks later, an ECG showed Andrew’s heart was “perfect,” Mr. Lis said. Still, doctors have asked Andrew to follow up with a cardiologist every 3 months.
An eye on the long-term effects
The medical team at Montefiore is tracking the 40 children they have already treated and discharged. With kids showing few symptoms in the immediate aftermath, Dr. Choueiter hopes the long-term trajectory after MIS-C will be similar to what happens after Kawasaki disease.
“Usually children who have had coronary artery dilations [from Kawasaki disease] that have resolved within the first 6 weeks of the illness do well long-term,” said Dr. Choueiter, who runs the Kawasaki disease program at Montefiore.
The Montefiore team is asking patients affected by MIS-C to return for a checkup 1 week after discharge, then after 1 month, 3 months, 6 months, and a year. They will be evaluated by pediatric cardiologists, hematologists, rheumatologists and infectious disease specialists.
Montefiore and other children’s hospitals around the country are sharing information. Dr. Choueiter wants to establish an even longer-term monitoring program for MIS-C, comparable with registries that exist for other diseases.
Ms. Moholland is glad the hospital is being vigilant.
“The uncertainty of not knowing whether it could come back in his future is a little unsettling,” she said. “But I am hopeful.”
This story is part of a partnership that includes WNYC, NPR, and Kaiser Health News. A version of this article originally appeared on Kaiser Health News.
He’s a 5-year-old boy and would much rather talk about cartoons or the ideas for inventions that constantly pop into his head.
“Hold your horses, I think I know what I’m gonna make,” he said, holding up a finger in the middle of a conversation. “I’m gonna make something that lights up and attaches to things with glue, so if you don’t have a flashlight, you can just use it!”
In New York, at least 237 kids, including Israel, appear to have Multisystem Inflammatory Syndrome in Children (MIS-C). And state officials continue to track the syndrome, but the Centers for Disease Control and Prevention did not respond to repeated requests for information on how many children nationwide have been diagnosed so far with MIS-C.
A study published June 29 in the New England Journal of Medicine reported on 186 patients in 26 states who had been diagnosed with MIS-C. A researcher writing in the same issue added reports from other countries, finding that about 1,000 children worldwide have been diagnosed with MIS-C.
Tracking the long-term health effects of MIS-C
Israel is friendly and energetic, but he’s also really good at sitting still. During a recent checkup at the Children’s Hospital at Montefiore, New York, he had no complaints about all the stickers and wires a health aide attached to him for an EKG. And when Marc Foca, MD, an infectious disease specialist, came by to listen to his heart and lungs, and prod his abdomen, Israel barely seemed to notice.
There were still some tests pending, but overall, Dr. Foca said, “Israel looks like a totally healthy 5-year-old.”
“Stay safe!” Israel called out, as Dr. Foca left. It’s his new sign-off, instead of goodbye. His mother, Janelle Moholland, explained Israel came up with it himself. And she’s also hoping that, after a harrowing couple of weeks in early May, Israel himself will “stay safe.”
That’s why they’ve been returning to Montefiore for the periodic checkups, even though Israel seems to have recovered fully from both COVID-19 and MIS-C.
MIS-C is relatively rare, and it apparently responds well to treatment, but it is new enough – and mysterious enough – that doctors here want to make sure the children who recover don’t experience any related health complications in the future.
“We’ve seen these kids get really sick, and get better and recover and go home, yet we don’t know what the long-term outcomes are,” said Nadine Choueiter, MD, a pediatric cardiologist at Montefiore. “So that’s why we will be seeing them.”
When Israel first got sick at the end of April, his illness didn’t exactly look like COVID-19. He had persistent high fevers, with his temperature reaching 104° F – but no problems breathing. He wasn’t eating. He was barely drinking. He wasn’t using the bathroom. He had abdominal pains. His eyes were red.
They went to the ED a couple of times and visited an urgent care center, but the doctors sent them home without testing him for the coronavirus. Ms. Moholland, 29, said she felt powerless.
“There was nothing I could do but make him comfortable,” she said. “I literally had to just trust in a higher power and just hope that He would come through for us. It taught me a lot about patience and faith.”
As Israel grew sicker, and they still had no answers, Ms. Moholland grew frustrated. “I wish his pediatrician and [the ED and urgent care staff] had done what they were supposed to do and given him a test” when Israel first got sick, Ms. Moholland said. “What harm would it have done? He suffered for about 10 or 11 days that could have been avoided.”
In a later interview, she talked with NPR about how COVID-19 has disproportionately affected the African American community because of a combination of underlying health conditions and lack of access to good health care. She said she felt she, too, had fallen victim to those disparities.
“It affects me, personally, because I am African American, but you just never know,” she said. “It’s hard. We’re living in uncertain times – very uncertain times.”
Finally, the Children’s Hospital at Montefiore admitted Israel – and the test she’d been trying to get for days confirmed he had the virus.
“I was literally in tears, like begging them not to discharge me because I knew he was not fine,” she recalled.
Israel was in shock, and by the time he got to the hospital, doctors were on the lookout for MIS-C, so they recognized his symptoms – which were distinct from most people with COVID-19.
Doctors gave Israel fluids and intravenous immunoglobulin, a substance obtained from donated human plasma, which is used to treat deficiencies in the immune system.
Immunoglobulin has been effective in children like Israel because MIS-C appears to be caused by an immune overreaction to the initial coronavirus infection, according to Dr. Choueiter.
“The immune system starts attacking the body itself, including the arteries of the heart,” she said.
In some MIS-C cases – though not Israel’s – the attack occurs in the coronary arteries, inflaming and dilating them. That also happens in a different syndrome affecting children, Kawasaki disease. About 5% of Kawasaki patients experience aneurysms – which can fatally rupture blood vessels – after the initial condition subsides.
Dr. Choueiter and colleagues want to make sure MIS-C patients don’t face similar risks. So far, they’re cautiously optimistic.
“We have not seen any new decrease in heart function or any new coronary artery dilations,” she said. “When we check their blood, their inflammatory markers are back to normal. For the parents, the child is back to baseline, and it’s as if this illness is a nightmare that’s long gone.”
For a Pennsylvania teen, the MIS-C diagnosis came much later
Not every child who develops MIS-C tests positive for the coronavirus, though many will test positive for antibodies to the coronavirus, indicating they had been infected previously. That was the case with Andrew Lis, a boy from Pennsylvania who was the first MIS-C patient seen at the Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del.
Andrew had been a healthy 14-year-old boy before he got sick. He and his twin brother love sports and video games. He said the first symptom was a bad headache. He developed a fever the next day, then constipation and intense stomach pain.
“It was terrible,” Andrew said. “It was unbearable. I couldn’t really move a lot.”
His mother, Ingrid Lis, said they were thinking appendicitis, not coronavirus, at first. In fact, she hesitated to take Andrew to the hospital, for fear of exposing him to the virus. But after Andrew stopped eating because of his headache and stomach discomfort, “I knew I couldn’t keep him home anymore,” Mrs. Lis said.
Andrew was admitted to the hospital April 12, but that was before reports of the mysterious syndrome had started trickling out of Europe.
Over about 5 days in the pediatric ICU, Andrew’s condition deteriorated rapidly, as doctors struggled to figure out what was wrong. Puzzled, they tried treatments for scarlet fever, strep throat, and toxic shock syndrome. Andrew’s body broke out in rashes, then his heart began failing and he was put on a ventilator. Andrew’s father, Ed Lis, said doctors told the family to brace for the worst: “We’ve got a healthy kid who a few days ago was just having these sort of strange symptoms. And now they’re telling us that we could lose him.”
Though Andrew’s symptoms were atypical for Kawasaki disease, doctors decided to give him the standard treatment for that condition – administering intravenous immunoglobulin, the same treatment Israel Shippy received.
“Within the 24 hours of the infusion, he was a different person,” Mrs. Lis said. Andrew was removed from the ventilator, and his appetite eventually returned. “That’s when we knew that we had turned that corner.”
It wasn’t until after Andrew’s discharge that his doctors learned about MIS-C from colleagues in Europe. They recommended the whole family be tested for antibodies to the coronavirus. Although Andrew tested positive, the rest of the family – both parents, Andrew’s twin brother and two older siblings – all tested negative. Andrew’s mother is still not sure how he was exposed since the family had been observing a strict lockdown since mid-March. Both she and her husband were working remotely from home, and she says they all wore masks and were conscientious about hand-washing when they ventured out for groceries. She thinks Andrew must have been exposed at least a month before his illness began.
And she’s puzzled why the rest of her close-knit family wasn’t infected as well. “We are a Latino family,” Mrs. Lis said. “We are very used to being together, clustering in the same room.” Even when Andrew was sick, she says, all six of them huddled in his bedroom to comfort him.
Meanwhile, Andrew has made a quick recovery. Not long after his discharge in April, he turned 15 and resumed an exercise routine involving running, push-ups, and sit-ups. A few weeks later, an ECG showed Andrew’s heart was “perfect,” Mr. Lis said. Still, doctors have asked Andrew to follow up with a cardiologist every 3 months.
An eye on the long-term effects
The medical team at Montefiore is tracking the 40 children they have already treated and discharged. With kids showing few symptoms in the immediate aftermath, Dr. Choueiter hopes the long-term trajectory after MIS-C will be similar to what happens after Kawasaki disease.
“Usually children who have had coronary artery dilations [from Kawasaki disease] that have resolved within the first 6 weeks of the illness do well long-term,” said Dr. Choueiter, who runs the Kawasaki disease program at Montefiore.
The Montefiore team is asking patients affected by MIS-C to return for a checkup 1 week after discharge, then after 1 month, 3 months, 6 months, and a year. They will be evaluated by pediatric cardiologists, hematologists, rheumatologists and infectious disease specialists.
Montefiore and other children’s hospitals around the country are sharing information. Dr. Choueiter wants to establish an even longer-term monitoring program for MIS-C, comparable with registries that exist for other diseases.
Ms. Moholland is glad the hospital is being vigilant.
“The uncertainty of not knowing whether it could come back in his future is a little unsettling,” she said. “But I am hopeful.”
This story is part of a partnership that includes WNYC, NPR, and Kaiser Health News. A version of this article originally appeared on Kaiser Health News.
He’s a 5-year-old boy and would much rather talk about cartoons or the ideas for inventions that constantly pop into his head.
“Hold your horses, I think I know what I’m gonna make,” he said, holding up a finger in the middle of a conversation. “I’m gonna make something that lights up and attaches to things with glue, so if you don’t have a flashlight, you can just use it!”
In New York, at least 237 kids, including Israel, appear to have Multisystem Inflammatory Syndrome in Children (MIS-C). And state officials continue to track the syndrome, but the Centers for Disease Control and Prevention did not respond to repeated requests for information on how many children nationwide have been diagnosed so far with MIS-C.
A study published June 29 in the New England Journal of Medicine reported on 186 patients in 26 states who had been diagnosed with MIS-C. A researcher writing in the same issue added reports from other countries, finding that about 1,000 children worldwide have been diagnosed with MIS-C.
Tracking the long-term health effects of MIS-C
Israel is friendly and energetic, but he’s also really good at sitting still. During a recent checkup at the Children’s Hospital at Montefiore, New York, he had no complaints about all the stickers and wires a health aide attached to him for an EKG. And when Marc Foca, MD, an infectious disease specialist, came by to listen to his heart and lungs, and prod his abdomen, Israel barely seemed to notice.
There were still some tests pending, but overall, Dr. Foca said, “Israel looks like a totally healthy 5-year-old.”
“Stay safe!” Israel called out, as Dr. Foca left. It’s his new sign-off, instead of goodbye. His mother, Janelle Moholland, explained Israel came up with it himself. And she’s also hoping that, after a harrowing couple of weeks in early May, Israel himself will “stay safe.”
That’s why they’ve been returning to Montefiore for the periodic checkups, even though Israel seems to have recovered fully from both COVID-19 and MIS-C.
MIS-C is relatively rare, and it apparently responds well to treatment, but it is new enough – and mysterious enough – that doctors here want to make sure the children who recover don’t experience any related health complications in the future.
“We’ve seen these kids get really sick, and get better and recover and go home, yet we don’t know what the long-term outcomes are,” said Nadine Choueiter, MD, a pediatric cardiologist at Montefiore. “So that’s why we will be seeing them.”
When Israel first got sick at the end of April, his illness didn’t exactly look like COVID-19. He had persistent high fevers, with his temperature reaching 104° F – but no problems breathing. He wasn’t eating. He was barely drinking. He wasn’t using the bathroom. He had abdominal pains. His eyes were red.
They went to the ED a couple of times and visited an urgent care center, but the doctors sent them home without testing him for the coronavirus. Ms. Moholland, 29, said she felt powerless.
“There was nothing I could do but make him comfortable,” she said. “I literally had to just trust in a higher power and just hope that He would come through for us. It taught me a lot about patience and faith.”
As Israel grew sicker, and they still had no answers, Ms. Moholland grew frustrated. “I wish his pediatrician and [the ED and urgent care staff] had done what they were supposed to do and given him a test” when Israel first got sick, Ms. Moholland said. “What harm would it have done? He suffered for about 10 or 11 days that could have been avoided.”
In a later interview, she talked with NPR about how COVID-19 has disproportionately affected the African American community because of a combination of underlying health conditions and lack of access to good health care. She said she felt she, too, had fallen victim to those disparities.
“It affects me, personally, because I am African American, but you just never know,” she said. “It’s hard. We’re living in uncertain times – very uncertain times.”
Finally, the Children’s Hospital at Montefiore admitted Israel – and the test she’d been trying to get for days confirmed he had the virus.
“I was literally in tears, like begging them not to discharge me because I knew he was not fine,” she recalled.
Israel was in shock, and by the time he got to the hospital, doctors were on the lookout for MIS-C, so they recognized his symptoms – which were distinct from most people with COVID-19.
Doctors gave Israel fluids and intravenous immunoglobulin, a substance obtained from donated human plasma, which is used to treat deficiencies in the immune system.
Immunoglobulin has been effective in children like Israel because MIS-C appears to be caused by an immune overreaction to the initial coronavirus infection, according to Dr. Choueiter.
“The immune system starts attacking the body itself, including the arteries of the heart,” she said.
In some MIS-C cases – though not Israel’s – the attack occurs in the coronary arteries, inflaming and dilating them. That also happens in a different syndrome affecting children, Kawasaki disease. About 5% of Kawasaki patients experience aneurysms – which can fatally rupture blood vessels – after the initial condition subsides.
Dr. Choueiter and colleagues want to make sure MIS-C patients don’t face similar risks. So far, they’re cautiously optimistic.
“We have not seen any new decrease in heart function or any new coronary artery dilations,” she said. “When we check their blood, their inflammatory markers are back to normal. For the parents, the child is back to baseline, and it’s as if this illness is a nightmare that’s long gone.”
For a Pennsylvania teen, the MIS-C diagnosis came much later
Not every child who develops MIS-C tests positive for the coronavirus, though many will test positive for antibodies to the coronavirus, indicating they had been infected previously. That was the case with Andrew Lis, a boy from Pennsylvania who was the first MIS-C patient seen at the Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del.
Andrew had been a healthy 14-year-old boy before he got sick. He and his twin brother love sports and video games. He said the first symptom was a bad headache. He developed a fever the next day, then constipation and intense stomach pain.
“It was terrible,” Andrew said. “It was unbearable. I couldn’t really move a lot.”
His mother, Ingrid Lis, said they were thinking appendicitis, not coronavirus, at first. In fact, she hesitated to take Andrew to the hospital, for fear of exposing him to the virus. But after Andrew stopped eating because of his headache and stomach discomfort, “I knew I couldn’t keep him home anymore,” Mrs. Lis said.
Andrew was admitted to the hospital April 12, but that was before reports of the mysterious syndrome had started trickling out of Europe.
Over about 5 days in the pediatric ICU, Andrew’s condition deteriorated rapidly, as doctors struggled to figure out what was wrong. Puzzled, they tried treatments for scarlet fever, strep throat, and toxic shock syndrome. Andrew’s body broke out in rashes, then his heart began failing and he was put on a ventilator. Andrew’s father, Ed Lis, said doctors told the family to brace for the worst: “We’ve got a healthy kid who a few days ago was just having these sort of strange symptoms. And now they’re telling us that we could lose him.”
Though Andrew’s symptoms were atypical for Kawasaki disease, doctors decided to give him the standard treatment for that condition – administering intravenous immunoglobulin, the same treatment Israel Shippy received.
“Within the 24 hours of the infusion, he was a different person,” Mrs. Lis said. Andrew was removed from the ventilator, and his appetite eventually returned. “That’s when we knew that we had turned that corner.”
It wasn’t until after Andrew’s discharge that his doctors learned about MIS-C from colleagues in Europe. They recommended the whole family be tested for antibodies to the coronavirus. Although Andrew tested positive, the rest of the family – both parents, Andrew’s twin brother and two older siblings – all tested negative. Andrew’s mother is still not sure how he was exposed since the family had been observing a strict lockdown since mid-March. Both she and her husband were working remotely from home, and she says they all wore masks and were conscientious about hand-washing when they ventured out for groceries. She thinks Andrew must have been exposed at least a month before his illness began.
And she’s puzzled why the rest of her close-knit family wasn’t infected as well. “We are a Latino family,” Mrs. Lis said. “We are very used to being together, clustering in the same room.” Even when Andrew was sick, she says, all six of them huddled in his bedroom to comfort him.
Meanwhile, Andrew has made a quick recovery. Not long after his discharge in April, he turned 15 and resumed an exercise routine involving running, push-ups, and sit-ups. A few weeks later, an ECG showed Andrew’s heart was “perfect,” Mr. Lis said. Still, doctors have asked Andrew to follow up with a cardiologist every 3 months.
An eye on the long-term effects
The medical team at Montefiore is tracking the 40 children they have already treated and discharged. With kids showing few symptoms in the immediate aftermath, Dr. Choueiter hopes the long-term trajectory after MIS-C will be similar to what happens after Kawasaki disease.
“Usually children who have had coronary artery dilations [from Kawasaki disease] that have resolved within the first 6 weeks of the illness do well long-term,” said Dr. Choueiter, who runs the Kawasaki disease program at Montefiore.
The Montefiore team is asking patients affected by MIS-C to return for a checkup 1 week after discharge, then after 1 month, 3 months, 6 months, and a year. They will be evaluated by pediatric cardiologists, hematologists, rheumatologists and infectious disease specialists.
Montefiore and other children’s hospitals around the country are sharing information. Dr. Choueiter wants to establish an even longer-term monitoring program for MIS-C, comparable with registries that exist for other diseases.
Ms. Moholland is glad the hospital is being vigilant.
“The uncertainty of not knowing whether it could come back in his future is a little unsettling,” she said. “But I am hopeful.”
This story is part of a partnership that includes WNYC, NPR, and Kaiser Health News. A version of this article originally appeared on Kaiser Health News.
Prior beta-blockers predict extra burden of heart failure in women with ACS
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
PCI or not, mortality climbs with post-ACS bleeding complications
Patients with acute coronary syndromes (ACS) with later bleeding complications that were at least moderate in severity showed a 15-fold increased risk of dying within 30 days, compared with those without such bleeding, in a pooled analysis of four randomized antithrombotic-therapy trials.

Mortality 1 month to 1 year after a bleeding event was not as sharply increased, but there was still almost triple the risk seen in patients without bleeding complications.
In both cases, the risk increase was independent of whether percutaneous coronary intervention (PCI) had been part of the management of ACS, concludes the study, published in the July 14 issue of the Journal of the American College of Cardiology.
“We showed that postdischarge bleeding was associated with a pretty bad prognosis, in terms of all-cause mortality, regardless of the index treatment – PCI or medical therapy,” lead author Guillaume Marquis-Gravel, MD, MSc, Duke Clinical Research Institute, Durham, N.C., said in an interview.
“Our data suggest that we should care about bleeding prevention in patients who had a previous ACS, regardless of the treatment strategy, as much as we care for prevention of future ischemic events,” said Dr. Marquis-Gravel, who is also an interventional cardiologist at the Montreal Heart Institute.
“This large-scale analysis clearly demonstrates that bleeding events occurring among ACS patients with coronary stents carry the same prognostic significance in magnitude and time course as among patients who do not undergo PCI,” observed Derek Chew, MBBS, MPH, PhD, of Flinders University, Adelaide, Australia, and Jack Wei Chieh Tan, MBBS, MBA, of National Heart Centre, Singapore, in an accompanying editorial.
“Therefore, at least in the later phases of planning antithrombotic therapy, when weighting bleeding risk in these conditions, these estimates should not be ‘discounted’ for the absence or presence of PCI during the initial ACS management,” they wrote.
A “proven assumption”
“A great deal of research has previously been conducted to tailor DAPT [dual-antiplatelet therapy] and to minimize bleeding risk following PCI based on the proven assumption that bleeding is associated with adverse clinical outcomes,” Dr. Marques-Gravel explained.
“The prognostic impact of postdischarge bleeding has not been studied thoroughly in patients with ACS who were only treated medically with DAPT without PCI.” Yet this population makes up a large proportion of the ACS population, and patients are “generally older and sicker” and therefore at increased risk for both ischemic and bleeding events, he said.
The researchers explored those issues in a post hoc pooled analysis of four randomized comparisons of antithrombotic strategies in patients with ACS: APPRAISE-2, PLATO, TRACER, and TRILOGY ACS. The analyses tracked bleeding events that took place from a landmark time of 7 days after presentation with ACS over a median follow-up of 1 year in 45,011 patients (31.3% female), 48% of whom were managed with PCI.
Those treated with PCI, compared with those medically managed only, tended to be younger, more often male, more likely to have ST-segment elevation myocardial infarction (STEMI) as their ACS, and less likely to have cardiovascular comorbidities.
During the total follow-up of 48,717 person-years, the postdischarge rate of moderate, severe, or life-threatening bleeding defined by GUSTO criteria reached 2.6 events per 100 patient-years. A total of 2,149 patients died, and mortality was consistently higher in patients who had such bleeding complications. They showed an adjusted hazard ratio of 15.7 (95% confidence interval, 12.3-20.0) for mortality within 30 days, compared with patients without bleeds. Their HR for mortality at 30 days to 1 year was 2.7 (95% CI, 2.1-3.4).
The association between bleeding complications and mortality remained consistent, regardless of whether patients had undergone PCI for their ACS (interaction P = .240).
A pragmatic interpretation
Although an observational study can’t show causality between bleeding and mortality, Dr. Marquis-Gravel cautioned, “the fact that the majority of deaths occurred early after the bleeding event, within 30 days, is strongly suggestive of a causal relationship.”
He recommended a “pragmatic interpretation” of the study: “Bleeding avoidance strategies tested in PCI populations, including short-term DAPT or aspirin-free strategies, should also be considered in medically treated patients with ACS deemed at higher risk of bleeding.”
“It is clear that bleeding events after successful PCI for an ACS are independently associated with increased mortality and morbidity,” Debabrata Mukherjee, MD, of Texas Tech University, El Paso, said in an interview.
“Every effort should be made to minimize bleeding events with the use of appropriate access site for PCI, dosing, selection, and duration of antiplatelet and antithrombotic agents, and use of proton pump inhibitors when appropriate,” he said.
The clinical decision-making involved in this individualized approach “is often not easy,” said Dr. Mukherjee, who was not involved in the current study. “Integrating patients and clinical pharmacists in choosing optimal antithrombotic therapies post-MI is likely to be helpful” in the process.
Although “major bleeding following ACS increases the risk of mortality for both medically managed and PCI-managed patients with ACS, the vast majority of deaths, 90%, occur in those that have not had a bleed,” Mamas A. Mamas, DPhil, Keele University, Staffordshire, England, said in an interview.
“It is important to understand the causes of death in this population and think about how interventions may impact on this,” agreed Dr. Mamas, who was not involved in the study.
Dr. Marquis-Gravel reported receiving speaking fees and honoraria from Servier and Novartis; disclosures for the other authors are in the report. Dr. Chew reported receiving speaking fees and institutional grants in aid from Roche Diagnostics, AstraZeneca, and Edwards Lifesciences. Dr. Tan discloses receiving speaking fees and educational grants from Amgen, Roche Diagnostics, AstraZeneca, Bayer, and Abbott Vascular. Dr. Mukherjee and Dr. Mamas report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with acute coronary syndromes (ACS) with later bleeding complications that were at least moderate in severity showed a 15-fold increased risk of dying within 30 days, compared with those without such bleeding, in a pooled analysis of four randomized antithrombotic-therapy trials.

Mortality 1 month to 1 year after a bleeding event was not as sharply increased, but there was still almost triple the risk seen in patients without bleeding complications.
In both cases, the risk increase was independent of whether percutaneous coronary intervention (PCI) had been part of the management of ACS, concludes the study, published in the July 14 issue of the Journal of the American College of Cardiology.
“We showed that postdischarge bleeding was associated with a pretty bad prognosis, in terms of all-cause mortality, regardless of the index treatment – PCI or medical therapy,” lead author Guillaume Marquis-Gravel, MD, MSc, Duke Clinical Research Institute, Durham, N.C., said in an interview.
“Our data suggest that we should care about bleeding prevention in patients who had a previous ACS, regardless of the treatment strategy, as much as we care for prevention of future ischemic events,” said Dr. Marquis-Gravel, who is also an interventional cardiologist at the Montreal Heart Institute.
“This large-scale analysis clearly demonstrates that bleeding events occurring among ACS patients with coronary stents carry the same prognostic significance in magnitude and time course as among patients who do not undergo PCI,” observed Derek Chew, MBBS, MPH, PhD, of Flinders University, Adelaide, Australia, and Jack Wei Chieh Tan, MBBS, MBA, of National Heart Centre, Singapore, in an accompanying editorial.
“Therefore, at least in the later phases of planning antithrombotic therapy, when weighting bleeding risk in these conditions, these estimates should not be ‘discounted’ for the absence or presence of PCI during the initial ACS management,” they wrote.
A “proven assumption”
“A great deal of research has previously been conducted to tailor DAPT [dual-antiplatelet therapy] and to minimize bleeding risk following PCI based on the proven assumption that bleeding is associated with adverse clinical outcomes,” Dr. Marques-Gravel explained.
“The prognostic impact of postdischarge bleeding has not been studied thoroughly in patients with ACS who were only treated medically with DAPT without PCI.” Yet this population makes up a large proportion of the ACS population, and patients are “generally older and sicker” and therefore at increased risk for both ischemic and bleeding events, he said.
The researchers explored those issues in a post hoc pooled analysis of four randomized comparisons of antithrombotic strategies in patients with ACS: APPRAISE-2, PLATO, TRACER, and TRILOGY ACS. The analyses tracked bleeding events that took place from a landmark time of 7 days after presentation with ACS over a median follow-up of 1 year in 45,011 patients (31.3% female), 48% of whom were managed with PCI.
Those treated with PCI, compared with those medically managed only, tended to be younger, more often male, more likely to have ST-segment elevation myocardial infarction (STEMI) as their ACS, and less likely to have cardiovascular comorbidities.
During the total follow-up of 48,717 person-years, the postdischarge rate of moderate, severe, or life-threatening bleeding defined by GUSTO criteria reached 2.6 events per 100 patient-years. A total of 2,149 patients died, and mortality was consistently higher in patients who had such bleeding complications. They showed an adjusted hazard ratio of 15.7 (95% confidence interval, 12.3-20.0) for mortality within 30 days, compared with patients without bleeds. Their HR for mortality at 30 days to 1 year was 2.7 (95% CI, 2.1-3.4).
The association between bleeding complications and mortality remained consistent, regardless of whether patients had undergone PCI for their ACS (interaction P = .240).
A pragmatic interpretation
Although an observational study can’t show causality between bleeding and mortality, Dr. Marquis-Gravel cautioned, “the fact that the majority of deaths occurred early after the bleeding event, within 30 days, is strongly suggestive of a causal relationship.”
He recommended a “pragmatic interpretation” of the study: “Bleeding avoidance strategies tested in PCI populations, including short-term DAPT or aspirin-free strategies, should also be considered in medically treated patients with ACS deemed at higher risk of bleeding.”
“It is clear that bleeding events after successful PCI for an ACS are independently associated with increased mortality and morbidity,” Debabrata Mukherjee, MD, of Texas Tech University, El Paso, said in an interview.
“Every effort should be made to minimize bleeding events with the use of appropriate access site for PCI, dosing, selection, and duration of antiplatelet and antithrombotic agents, and use of proton pump inhibitors when appropriate,” he said.
The clinical decision-making involved in this individualized approach “is often not easy,” said Dr. Mukherjee, who was not involved in the current study. “Integrating patients and clinical pharmacists in choosing optimal antithrombotic therapies post-MI is likely to be helpful” in the process.
Although “major bleeding following ACS increases the risk of mortality for both medically managed and PCI-managed patients with ACS, the vast majority of deaths, 90%, occur in those that have not had a bleed,” Mamas A. Mamas, DPhil, Keele University, Staffordshire, England, said in an interview.
“It is important to understand the causes of death in this population and think about how interventions may impact on this,” agreed Dr. Mamas, who was not involved in the study.
Dr. Marquis-Gravel reported receiving speaking fees and honoraria from Servier and Novartis; disclosures for the other authors are in the report. Dr. Chew reported receiving speaking fees and institutional grants in aid from Roche Diagnostics, AstraZeneca, and Edwards Lifesciences. Dr. Tan discloses receiving speaking fees and educational grants from Amgen, Roche Diagnostics, AstraZeneca, Bayer, and Abbott Vascular. Dr. Mukherjee and Dr. Mamas report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with acute coronary syndromes (ACS) with later bleeding complications that were at least moderate in severity showed a 15-fold increased risk of dying within 30 days, compared with those without such bleeding, in a pooled analysis of four randomized antithrombotic-therapy trials.

Mortality 1 month to 1 year after a bleeding event was not as sharply increased, but there was still almost triple the risk seen in patients without bleeding complications.
In both cases, the risk increase was independent of whether percutaneous coronary intervention (PCI) had been part of the management of ACS, concludes the study, published in the July 14 issue of the Journal of the American College of Cardiology.
“We showed that postdischarge bleeding was associated with a pretty bad prognosis, in terms of all-cause mortality, regardless of the index treatment – PCI or medical therapy,” lead author Guillaume Marquis-Gravel, MD, MSc, Duke Clinical Research Institute, Durham, N.C., said in an interview.
“Our data suggest that we should care about bleeding prevention in patients who had a previous ACS, regardless of the treatment strategy, as much as we care for prevention of future ischemic events,” said Dr. Marquis-Gravel, who is also an interventional cardiologist at the Montreal Heart Institute.
“This large-scale analysis clearly demonstrates that bleeding events occurring among ACS patients with coronary stents carry the same prognostic significance in magnitude and time course as among patients who do not undergo PCI,” observed Derek Chew, MBBS, MPH, PhD, of Flinders University, Adelaide, Australia, and Jack Wei Chieh Tan, MBBS, MBA, of National Heart Centre, Singapore, in an accompanying editorial.
“Therefore, at least in the later phases of planning antithrombotic therapy, when weighting bleeding risk in these conditions, these estimates should not be ‘discounted’ for the absence or presence of PCI during the initial ACS management,” they wrote.
A “proven assumption”
“A great deal of research has previously been conducted to tailor DAPT [dual-antiplatelet therapy] and to minimize bleeding risk following PCI based on the proven assumption that bleeding is associated with adverse clinical outcomes,” Dr. Marques-Gravel explained.
“The prognostic impact of postdischarge bleeding has not been studied thoroughly in patients with ACS who were only treated medically with DAPT without PCI.” Yet this population makes up a large proportion of the ACS population, and patients are “generally older and sicker” and therefore at increased risk for both ischemic and bleeding events, he said.
The researchers explored those issues in a post hoc pooled analysis of four randomized comparisons of antithrombotic strategies in patients with ACS: APPRAISE-2, PLATO, TRACER, and TRILOGY ACS. The analyses tracked bleeding events that took place from a landmark time of 7 days after presentation with ACS over a median follow-up of 1 year in 45,011 patients (31.3% female), 48% of whom were managed with PCI.
Those treated with PCI, compared with those medically managed only, tended to be younger, more often male, more likely to have ST-segment elevation myocardial infarction (STEMI) as their ACS, and less likely to have cardiovascular comorbidities.
During the total follow-up of 48,717 person-years, the postdischarge rate of moderate, severe, or life-threatening bleeding defined by GUSTO criteria reached 2.6 events per 100 patient-years. A total of 2,149 patients died, and mortality was consistently higher in patients who had such bleeding complications. They showed an adjusted hazard ratio of 15.7 (95% confidence interval, 12.3-20.0) for mortality within 30 days, compared with patients without bleeds. Their HR for mortality at 30 days to 1 year was 2.7 (95% CI, 2.1-3.4).
The association between bleeding complications and mortality remained consistent, regardless of whether patients had undergone PCI for their ACS (interaction P = .240).
A pragmatic interpretation
Although an observational study can’t show causality between bleeding and mortality, Dr. Marquis-Gravel cautioned, “the fact that the majority of deaths occurred early after the bleeding event, within 30 days, is strongly suggestive of a causal relationship.”
He recommended a “pragmatic interpretation” of the study: “Bleeding avoidance strategies tested in PCI populations, including short-term DAPT or aspirin-free strategies, should also be considered in medically treated patients with ACS deemed at higher risk of bleeding.”
“It is clear that bleeding events after successful PCI for an ACS are independently associated with increased mortality and morbidity,” Debabrata Mukherjee, MD, of Texas Tech University, El Paso, said in an interview.
“Every effort should be made to minimize bleeding events with the use of appropriate access site for PCI, dosing, selection, and duration of antiplatelet and antithrombotic agents, and use of proton pump inhibitors when appropriate,” he said.
The clinical decision-making involved in this individualized approach “is often not easy,” said Dr. Mukherjee, who was not involved in the current study. “Integrating patients and clinical pharmacists in choosing optimal antithrombotic therapies post-MI is likely to be helpful” in the process.
Although “major bleeding following ACS increases the risk of mortality for both medically managed and PCI-managed patients with ACS, the vast majority of deaths, 90%, occur in those that have not had a bleed,” Mamas A. Mamas, DPhil, Keele University, Staffordshire, England, said in an interview.
“It is important to understand the causes of death in this population and think about how interventions may impact on this,” agreed Dr. Mamas, who was not involved in the study.
Dr. Marquis-Gravel reported receiving speaking fees and honoraria from Servier and Novartis; disclosures for the other authors are in the report. Dr. Chew reported receiving speaking fees and institutional grants in aid from Roche Diagnostics, AstraZeneca, and Edwards Lifesciences. Dr. Tan discloses receiving speaking fees and educational grants from Amgen, Roche Diagnostics, AstraZeneca, Bayer, and Abbott Vascular. Dr. Mukherjee and Dr. Mamas report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SGLT2 inhibitors, developed for T2D, now ‘belong to cardiologists and nephrologists’
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
FROM ADA 2020
Acetaminophen beats fentanyl in STEMI
Swapping out intravenous fentanyl in favor of IV acetaminophen in patients with ST-elevation MI (STEMI) provides comparable pain relief but with desirably higher blood levels of ticagrelor both immediately after primary percutaneous intervention and 1 hour post procedure.
That’s according to results of the Dutch ON-TIME 3 trial, presented by Anne H. Tavenier, MD, at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Our trial results have implications for the prehospital treatment of STEMI patients,” said Dr. Tavenier, a cardiologist at the Isala Clinic in Zwolle, the Netherlands.
The explanation for the success of this novel STEMI pain management strategy? The synthetic opioid fentanyl impairs gastrointestinal absorption of oral P2Y12 receptor antagonists such as ticagrelor. Opiates do so as well, whereas acetaminophen does not, she explained.
The potent platelet inhibition provided by oral P2Y12 inhibitors is crucial to successful primary PCI for STEMI. But these platelet inhibitory effects are inherently slowed in STEMI patients owing to hemodynamic changes and delayed GI absorption. And even though both American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend the use of opioids for pain control in STEMI patients, the fact is that these medications further delay the absorption of oral P2Y12 inhibitors. And this delay is further exacerbated by the nausea and vomiting which are common side effects of IV fentanyl, she continued.
The impetus for the ON-TIME 3 trial was straightforward, the cardiologist said: “For years, STEMI patients have been treated with morphine or morphinelike drugs like fentanyl because of pain or sympathetic stress. To date, trials investigating alternative analgesics to opioids have been scarce.”
ON-TIME 3 was a multicenter, open-label, phase 4 clinical trial in which 195 STEMI patients with a self-reported pain score of at least 4 on a 0-10 scale received crushed ticagrelor in the ambulance along with either 1,000 mg of IV acetaminophen or fentanyl at 1-2 mcg/kg.
Ticagrelor blood levels were significantly higher in the IV acetaminophen group when measured just prior to primary PCI (151 ng/mL versus 60 ng/mL in the IV fentanyl group; immediately after PCI (326 versus 115 ng/mL), and 1 hour post PCI (488 versus 372 ng/mL).
However, there was no significant between-group difference in levels of platelet reactivity units measured immediately after primary PCI, Dr. Tavenier added.
Discussant Christoph K. Naber, MD, PhD, confessed that prior to ON-TIME 3 he was unaware that administering opioids to STEMI patients results in delayed absorption of oral P2Y12 inhibitors. Upon delving into the literature, however, he found that this is indeed a well-documented problem.
“The open question I have about this very elegant trial is whether the increased P2Y12 levels will translate into a measurable difference in clinical outcomes,” said Dr. Naber, an interventional cardiologist at the Wilhemshaven (Germany) Clinic.
The answer to that question would require a larger, longer-term trial. And he’s disinclined to wait around for that to happen.
“I think when we look at the risk balance, the risk of switching from an opioid to acetaminophen, if it works for the patient, is rather low. So this might be something to introduce in my practice,” the cardiologist said.
Dr. Tavenier and Dr. Naber reported having no financial conflicts of interest.
SOURCE: Tavenier AH. EuroPCR 2020.
Swapping out intravenous fentanyl in favor of IV acetaminophen in patients with ST-elevation MI (STEMI) provides comparable pain relief but with desirably higher blood levels of ticagrelor both immediately after primary percutaneous intervention and 1 hour post procedure.
That’s according to results of the Dutch ON-TIME 3 trial, presented by Anne H. Tavenier, MD, at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Our trial results have implications for the prehospital treatment of STEMI patients,” said Dr. Tavenier, a cardiologist at the Isala Clinic in Zwolle, the Netherlands.
The explanation for the success of this novel STEMI pain management strategy? The synthetic opioid fentanyl impairs gastrointestinal absorption of oral P2Y12 receptor antagonists such as ticagrelor. Opiates do so as well, whereas acetaminophen does not, she explained.
The potent platelet inhibition provided by oral P2Y12 inhibitors is crucial to successful primary PCI for STEMI. But these platelet inhibitory effects are inherently slowed in STEMI patients owing to hemodynamic changes and delayed GI absorption. And even though both American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend the use of opioids for pain control in STEMI patients, the fact is that these medications further delay the absorption of oral P2Y12 inhibitors. And this delay is further exacerbated by the nausea and vomiting which are common side effects of IV fentanyl, she continued.
The impetus for the ON-TIME 3 trial was straightforward, the cardiologist said: “For years, STEMI patients have been treated with morphine or morphinelike drugs like fentanyl because of pain or sympathetic stress. To date, trials investigating alternative analgesics to opioids have been scarce.”
ON-TIME 3 was a multicenter, open-label, phase 4 clinical trial in which 195 STEMI patients with a self-reported pain score of at least 4 on a 0-10 scale received crushed ticagrelor in the ambulance along with either 1,000 mg of IV acetaminophen or fentanyl at 1-2 mcg/kg.
Ticagrelor blood levels were significantly higher in the IV acetaminophen group when measured just prior to primary PCI (151 ng/mL versus 60 ng/mL in the IV fentanyl group; immediately after PCI (326 versus 115 ng/mL), and 1 hour post PCI (488 versus 372 ng/mL).
However, there was no significant between-group difference in levels of platelet reactivity units measured immediately after primary PCI, Dr. Tavenier added.
Discussant Christoph K. Naber, MD, PhD, confessed that prior to ON-TIME 3 he was unaware that administering opioids to STEMI patients results in delayed absorption of oral P2Y12 inhibitors. Upon delving into the literature, however, he found that this is indeed a well-documented problem.
“The open question I have about this very elegant trial is whether the increased P2Y12 levels will translate into a measurable difference in clinical outcomes,” said Dr. Naber, an interventional cardiologist at the Wilhemshaven (Germany) Clinic.
The answer to that question would require a larger, longer-term trial. And he’s disinclined to wait around for that to happen.
“I think when we look at the risk balance, the risk of switching from an opioid to acetaminophen, if it works for the patient, is rather low. So this might be something to introduce in my practice,” the cardiologist said.
Dr. Tavenier and Dr. Naber reported having no financial conflicts of interest.
SOURCE: Tavenier AH. EuroPCR 2020.
Swapping out intravenous fentanyl in favor of IV acetaminophen in patients with ST-elevation MI (STEMI) provides comparable pain relief but with desirably higher blood levels of ticagrelor both immediately after primary percutaneous intervention and 1 hour post procedure.
That’s according to results of the Dutch ON-TIME 3 trial, presented by Anne H. Tavenier, MD, at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Our trial results have implications for the prehospital treatment of STEMI patients,” said Dr. Tavenier, a cardiologist at the Isala Clinic in Zwolle, the Netherlands.
The explanation for the success of this novel STEMI pain management strategy? The synthetic opioid fentanyl impairs gastrointestinal absorption of oral P2Y12 receptor antagonists such as ticagrelor. Opiates do so as well, whereas acetaminophen does not, she explained.
The potent platelet inhibition provided by oral P2Y12 inhibitors is crucial to successful primary PCI for STEMI. But these platelet inhibitory effects are inherently slowed in STEMI patients owing to hemodynamic changes and delayed GI absorption. And even though both American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend the use of opioids for pain control in STEMI patients, the fact is that these medications further delay the absorption of oral P2Y12 inhibitors. And this delay is further exacerbated by the nausea and vomiting which are common side effects of IV fentanyl, she continued.
The impetus for the ON-TIME 3 trial was straightforward, the cardiologist said: “For years, STEMI patients have been treated with morphine or morphinelike drugs like fentanyl because of pain or sympathetic stress. To date, trials investigating alternative analgesics to opioids have been scarce.”
ON-TIME 3 was a multicenter, open-label, phase 4 clinical trial in which 195 STEMI patients with a self-reported pain score of at least 4 on a 0-10 scale received crushed ticagrelor in the ambulance along with either 1,000 mg of IV acetaminophen or fentanyl at 1-2 mcg/kg.
Ticagrelor blood levels were significantly higher in the IV acetaminophen group when measured just prior to primary PCI (151 ng/mL versus 60 ng/mL in the IV fentanyl group; immediately after PCI (326 versus 115 ng/mL), and 1 hour post PCI (488 versus 372 ng/mL).
However, there was no significant between-group difference in levels of platelet reactivity units measured immediately after primary PCI, Dr. Tavenier added.
Discussant Christoph K. Naber, MD, PhD, confessed that prior to ON-TIME 3 he was unaware that administering opioids to STEMI patients results in delayed absorption of oral P2Y12 inhibitors. Upon delving into the literature, however, he found that this is indeed a well-documented problem.
“The open question I have about this very elegant trial is whether the increased P2Y12 levels will translate into a measurable difference in clinical outcomes,” said Dr. Naber, an interventional cardiologist at the Wilhemshaven (Germany) Clinic.
The answer to that question would require a larger, longer-term trial. And he’s disinclined to wait around for that to happen.
“I think when we look at the risk balance, the risk of switching from an opioid to acetaminophen, if it works for the patient, is rather low. So this might be something to introduce in my practice,” the cardiologist said.
Dr. Tavenier and Dr. Naber reported having no financial conflicts of interest.
SOURCE: Tavenier AH. EuroPCR 2020.
REPORTING FROM EUROPCR 2020