User login
S-ICD ‘noninferior’ to transvenous-lead ICD in head-to-head PRAETORIAN trial
by turning in a “noninferior” performance when it was compared with transvenous-lead devices in a first-of-its-kind head-to-head study.
Patients implanted with the subcutaneous-lead S-ICD (Boston Scientific) defibrillator showed a 4-year risk for inappropriate shocks or device-related complications similar to that seen with standard transvenous-lead implantable cardioverter defibrillators (ICD) in a randomized comparison.
At the same time, the S-ICD did its job by showing a highly significant three-fourths reduction in risk for lead-related complications, compared with ICDs with standard leads, in the trial with more than 800 patients, called PRAETORIAN.
The study population represented a mix of patients seen in “real-world” practice who have an ICD indication, of whom about two-thirds had ischemic cardiomyopathy, said Reinoud Knops, MD, PhD, Academic Medical Center, Hilversum, the Netherlands. About 80% received the devices for primary prevention.
Knops, the trial’s principal investigator, presented the results online May 8 as one of the Heart Rhythm Society 2020 Scientific Sessions virtual presentations.
“I think the PRAETORIAN trial has really shown now, in a conventional ICD population – the real-world patients that we treat with ICD therapy, the single-chamber ICD cohort – that the S-ICD is a really good alternative option,” he said to reporters during a media briefing.
“The main conclusion is that the S-ICD should be considered in all patients who need an ICD who do not have a pacing indication,” Knops said.
This latter part is critical, because the S-ICD does not provide pacing therapy, including antitachycardia pacing (ATP) and cardiac resynchronization therapy (CRT), and the trial did not enter patients considered likely to benefit from it. For example, it excluded anyone with bradycardia or treatment-refractory monomorphic ventricular tachycardia (VT) and patients considered appropriate for CRT.
In fact, there are a lot reasons clinicians might prefer a transvenous-lead ICD over the S-ICD, observed Anne B. Curtis, MD, University at Buffalo, State University of New York, who is not associated with PRAETORIAN.
A transvenous-lead system might be preferred in older patients, those with heart failure, and those with a lot of comorbidities. “A lot of these patients already have cardiomyopathies, so they’re more likely to develop atrial fibrillation or a need for CRT,” conditions that might make a transvenous-lead system the better choice, Curtis told theheart.org | Medscape Cardiology.
“For a lot of patients, you’re always thinking that you may have a need for that kind of therapy.”
In contrast, younger patients who perhaps have survived cardiac arrest and probably don’t have heart failure, and so may be less likely to benefit from pacing therapy, Curtis said, “are the kind of patient who you would probably lean very strongly toward for an S-ICD rather than a transvenous ICD.”
Remaining patients, those who might be considered candidates for either kind of device, are actually “a fairly limited subset,” she said.
The trial randomized 849 patients in Europe and the United States, from March 2011 to January 2017, who had a class I or IIa indication for an ICD but no bradycardia or need for CRT or ATP, to be implanted with an S-ICD or a transvenous-lead ICD.
The rates of the primary end point, a composite of device-related complications and inappropriate shocks at a median follow-up of 4 years, were comparable, at 15.1% in the S-ICD group and 15.7% for those with transvenous-lead ICDs.
The incidence of device-related complications numerically favored the S-ICD group, and the incidence of inappropriate shocks numerically favored the transvenous-lead group, but neither difference reached significance.
Knops said the PRAETORIAN researchers are seeking addition funding to extend the follow-up to 8 years. “We will get more insight into the durability of the S-ICD when we follow these patients longer,” he told theheart.org | Medscape Cardiology.
The investigator-initiated trial received support from Boston Scientific. Knops discloses receiving consultancy fees and research grants from Abbott, Boston Scientific, Medtronic, and Cairdac, and holding stock options from AtaCor Medical.
This article first appeared on Medscape.com.
by turning in a “noninferior” performance when it was compared with transvenous-lead devices in a first-of-its-kind head-to-head study.
Patients implanted with the subcutaneous-lead S-ICD (Boston Scientific) defibrillator showed a 4-year risk for inappropriate shocks or device-related complications similar to that seen with standard transvenous-lead implantable cardioverter defibrillators (ICD) in a randomized comparison.
At the same time, the S-ICD did its job by showing a highly significant three-fourths reduction in risk for lead-related complications, compared with ICDs with standard leads, in the trial with more than 800 patients, called PRAETORIAN.
The study population represented a mix of patients seen in “real-world” practice who have an ICD indication, of whom about two-thirds had ischemic cardiomyopathy, said Reinoud Knops, MD, PhD, Academic Medical Center, Hilversum, the Netherlands. About 80% received the devices for primary prevention.
Knops, the trial’s principal investigator, presented the results online May 8 as one of the Heart Rhythm Society 2020 Scientific Sessions virtual presentations.
“I think the PRAETORIAN trial has really shown now, in a conventional ICD population – the real-world patients that we treat with ICD therapy, the single-chamber ICD cohort – that the S-ICD is a really good alternative option,” he said to reporters during a media briefing.
“The main conclusion is that the S-ICD should be considered in all patients who need an ICD who do not have a pacing indication,” Knops said.
This latter part is critical, because the S-ICD does not provide pacing therapy, including antitachycardia pacing (ATP) and cardiac resynchronization therapy (CRT), and the trial did not enter patients considered likely to benefit from it. For example, it excluded anyone with bradycardia or treatment-refractory monomorphic ventricular tachycardia (VT) and patients considered appropriate for CRT.
In fact, there are a lot reasons clinicians might prefer a transvenous-lead ICD over the S-ICD, observed Anne B. Curtis, MD, University at Buffalo, State University of New York, who is not associated with PRAETORIAN.
A transvenous-lead system might be preferred in older patients, those with heart failure, and those with a lot of comorbidities. “A lot of these patients already have cardiomyopathies, so they’re more likely to develop atrial fibrillation or a need for CRT,” conditions that might make a transvenous-lead system the better choice, Curtis told theheart.org | Medscape Cardiology.
“For a lot of patients, you’re always thinking that you may have a need for that kind of therapy.”
In contrast, younger patients who perhaps have survived cardiac arrest and probably don’t have heart failure, and so may be less likely to benefit from pacing therapy, Curtis said, “are the kind of patient who you would probably lean very strongly toward for an S-ICD rather than a transvenous ICD.”
Remaining patients, those who might be considered candidates for either kind of device, are actually “a fairly limited subset,” she said.
The trial randomized 849 patients in Europe and the United States, from March 2011 to January 2017, who had a class I or IIa indication for an ICD but no bradycardia or need for CRT or ATP, to be implanted with an S-ICD or a transvenous-lead ICD.
The rates of the primary end point, a composite of device-related complications and inappropriate shocks at a median follow-up of 4 years, were comparable, at 15.1% in the S-ICD group and 15.7% for those with transvenous-lead ICDs.
The incidence of device-related complications numerically favored the S-ICD group, and the incidence of inappropriate shocks numerically favored the transvenous-lead group, but neither difference reached significance.
Knops said the PRAETORIAN researchers are seeking addition funding to extend the follow-up to 8 years. “We will get more insight into the durability of the S-ICD when we follow these patients longer,” he told theheart.org | Medscape Cardiology.
The investigator-initiated trial received support from Boston Scientific. Knops discloses receiving consultancy fees and research grants from Abbott, Boston Scientific, Medtronic, and Cairdac, and holding stock options from AtaCor Medical.
This article first appeared on Medscape.com.
by turning in a “noninferior” performance when it was compared with transvenous-lead devices in a first-of-its-kind head-to-head study.
Patients implanted with the subcutaneous-lead S-ICD (Boston Scientific) defibrillator showed a 4-year risk for inappropriate shocks or device-related complications similar to that seen with standard transvenous-lead implantable cardioverter defibrillators (ICD) in a randomized comparison.
At the same time, the S-ICD did its job by showing a highly significant three-fourths reduction in risk for lead-related complications, compared with ICDs with standard leads, in the trial with more than 800 patients, called PRAETORIAN.
The study population represented a mix of patients seen in “real-world” practice who have an ICD indication, of whom about two-thirds had ischemic cardiomyopathy, said Reinoud Knops, MD, PhD, Academic Medical Center, Hilversum, the Netherlands. About 80% received the devices for primary prevention.
Knops, the trial’s principal investigator, presented the results online May 8 as one of the Heart Rhythm Society 2020 Scientific Sessions virtual presentations.
“I think the PRAETORIAN trial has really shown now, in a conventional ICD population – the real-world patients that we treat with ICD therapy, the single-chamber ICD cohort – that the S-ICD is a really good alternative option,” he said to reporters during a media briefing.
“The main conclusion is that the S-ICD should be considered in all patients who need an ICD who do not have a pacing indication,” Knops said.
This latter part is critical, because the S-ICD does not provide pacing therapy, including antitachycardia pacing (ATP) and cardiac resynchronization therapy (CRT), and the trial did not enter patients considered likely to benefit from it. For example, it excluded anyone with bradycardia or treatment-refractory monomorphic ventricular tachycardia (VT) and patients considered appropriate for CRT.
In fact, there are a lot reasons clinicians might prefer a transvenous-lead ICD over the S-ICD, observed Anne B. Curtis, MD, University at Buffalo, State University of New York, who is not associated with PRAETORIAN.
A transvenous-lead system might be preferred in older patients, those with heart failure, and those with a lot of comorbidities. “A lot of these patients already have cardiomyopathies, so they’re more likely to develop atrial fibrillation or a need for CRT,” conditions that might make a transvenous-lead system the better choice, Curtis told theheart.org | Medscape Cardiology.
“For a lot of patients, you’re always thinking that you may have a need for that kind of therapy.”
In contrast, younger patients who perhaps have survived cardiac arrest and probably don’t have heart failure, and so may be less likely to benefit from pacing therapy, Curtis said, “are the kind of patient who you would probably lean very strongly toward for an S-ICD rather than a transvenous ICD.”
Remaining patients, those who might be considered candidates for either kind of device, are actually “a fairly limited subset,” she said.
The trial randomized 849 patients in Europe and the United States, from March 2011 to January 2017, who had a class I or IIa indication for an ICD but no bradycardia or need for CRT or ATP, to be implanted with an S-ICD or a transvenous-lead ICD.
The rates of the primary end point, a composite of device-related complications and inappropriate shocks at a median follow-up of 4 years, were comparable, at 15.1% in the S-ICD group and 15.7% for those with transvenous-lead ICDs.
The incidence of device-related complications numerically favored the S-ICD group, and the incidence of inappropriate shocks numerically favored the transvenous-lead group, but neither difference reached significance.
Knops said the PRAETORIAN researchers are seeking addition funding to extend the follow-up to 8 years. “We will get more insight into the durability of the S-ICD when we follow these patients longer,” he told theheart.org | Medscape Cardiology.
The investigator-initiated trial received support from Boston Scientific. Knops discloses receiving consultancy fees and research grants from Abbott, Boston Scientific, Medtronic, and Cairdac, and holding stock options from AtaCor Medical.
This article first appeared on Medscape.com.
Silent brain infarcts found in 3% of AFib patients, tied to cognitive decline
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
FROM HEART RHYTHM 2020
Hypertriglyceridemia: A strategic approach
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?
CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
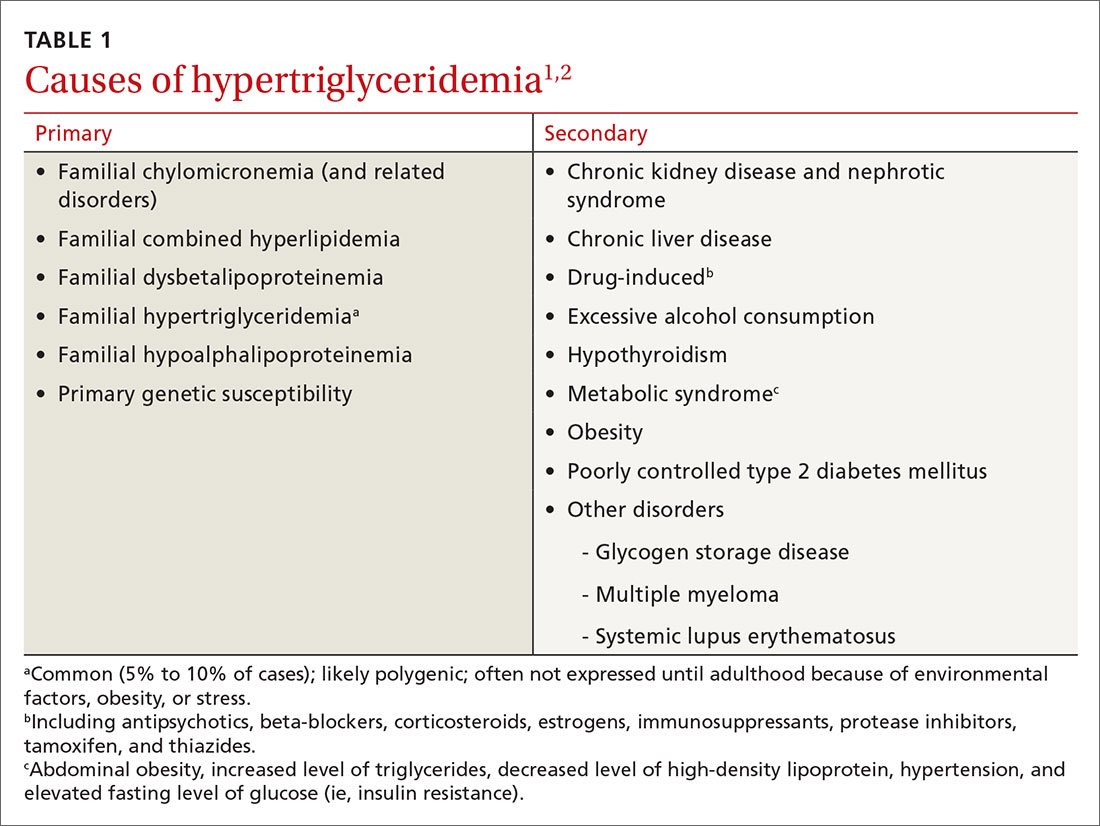
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.
Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
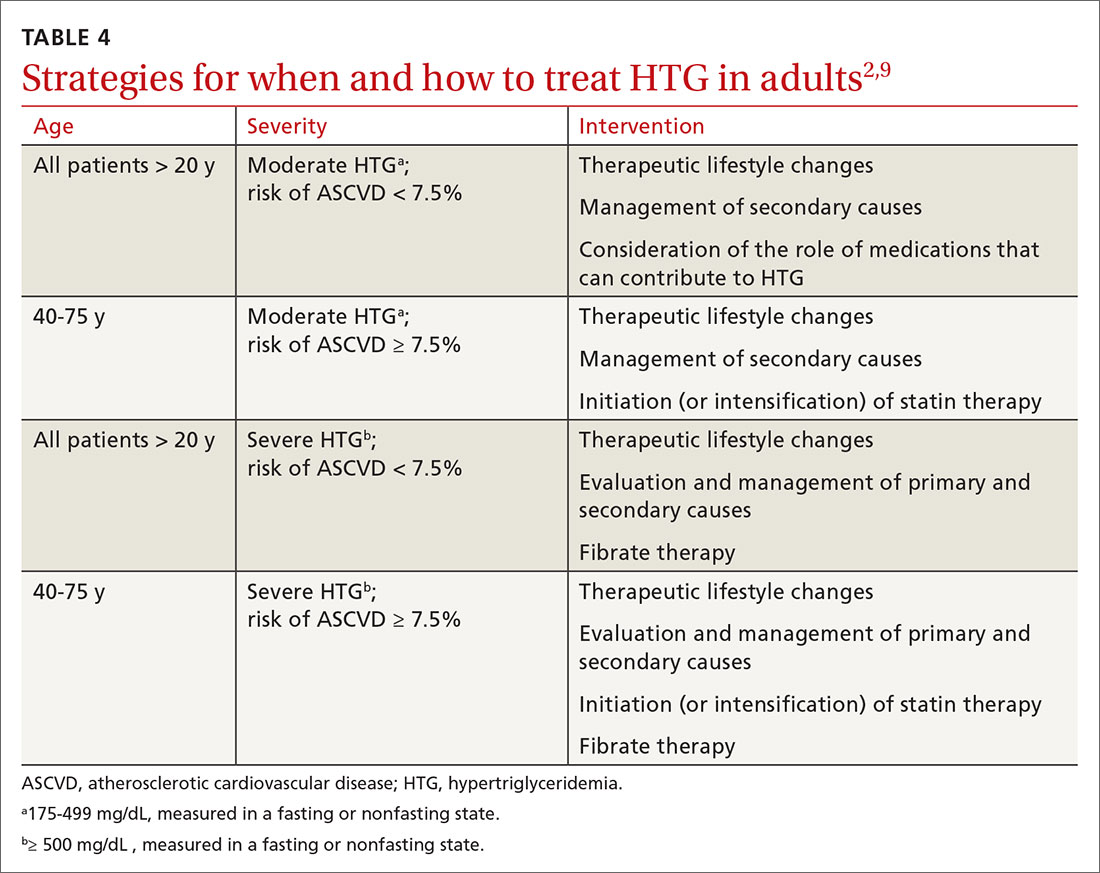
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
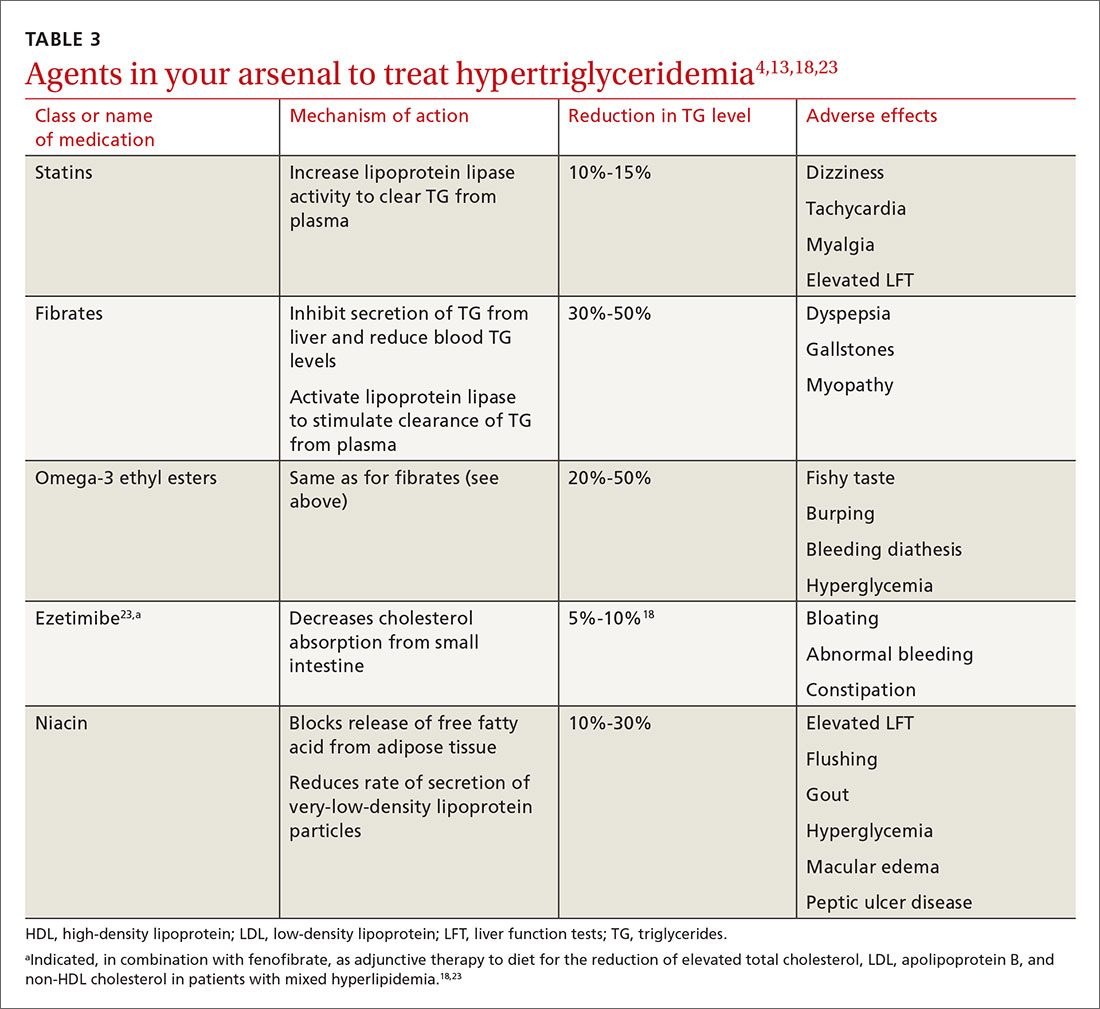
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2
Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).
Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; Ashwini.KamathMulki@lvhn.org.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?
CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.
Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2
Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).
Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; Ashwini.KamathMulki@lvhn.org.
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?
CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.
Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2
Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).
Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; Ashwini.KamathMulki@lvhn.org.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
PRACTICE RECOMMENDATIONS
› Evaluate patients for hypertriglyceridemia when they have a comorbid condition (eg, type 2 diabetes, obesity, hypothyroidism, metabolic syndrome, alcoholism). B
› Do not require fasting status when evaluating triglycerides in a lipid panel. B
› Make therapeutic lifestyle changes first-line treatment for hypertriglyceridemia. C
› Prescribe fibrates for severe hypertriglyceridemia to reduce the risk and recurrence of pancreatitis. A
› Prescribe a statin and an omega-3 fatty acid (fish oil) to lower the triglyceride level and thus reduce resulting atherogenicity when the risk of atherosclerotic cardiovascular disease is > 7.5%. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Evidence builds linking anticoagulation to COVID-19 survival
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
Coronary CT angiography gives superior MI risk prediction
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
FROM ACC 2020
UNTOUCHED: Inappropriate shocks cut by subcutaneous ICD improvements
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
FROM HEART RHYTHM 2020
Coffee drinking linked with fewer arrhythmias
Moderate, daily coffee consumption had no apparent adverse effect for triggering incident heart arrhythmias, and even linked with a small but statistically significant drop in arrhythmias in an analysis of prospectively collected data from nearly 300,000 U.K. residents.
“In this large, population-based, prospective study, moderate habitual coffee drinking was associated with a lower risk of arrhythmia,” EunJeong Kim, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
Her analysis found that on average each additional daily cup of coffee that people said they drank reduced the incidence of arrhythmic episodes by a statistically significant 3%, compared with those who drank fewer daily cups. The relationship held for people who reported drinking as many as five or six cups of coffee daily.
“The main message of our study is that it does not appear to be deleterious to continue with moderate amounts of habitual coffee intake regarding a risk of overall arrhythmia,” said Dr. Kim, a cardiac electrophysiologist at the University of California, San Francisco.
Evidence builds for coffee’s safety
The finding adds to a substantial existing evidence base documenting the safety of moderate, habitual coffee drinking when it comes to heart rhythms. For example, a recent report from the Physicians Health Study of nearly 19,000 American men showed a statistically significant decrease in the incidence of atrial fibrillation during an average follow-up of 9 years among men who reported drinking one to three cups of coffee daily (J Am Heart Assoc. 2019 Aug 6;8[15]:e011346). In addition, a recent review of several reports found that “mild-to-moderate habitual consumption of caffeinated beverages, particularly a daily intake of 2-3 cups of coffee or tea, appears to be safe across a broad range of cardiovascular conditions, and may even be beneficial with respect to diabetes mellitus, atherosclerosis, heart failure, arrhythmia and total mortality,” but also concluded that “acute consumption of high doses of caffeine, particularly in the form of energy drinks, is best avoided”(Trends Cardiovasc Med. 2019 Aug;29[6]:345-50). Specifically about cardiac arrhythmias, the review said “while caffeine is commonly considered a trigger for arrhythmias by physicians and patients alike there is minimal evidence to support this misconception. Rather caffeine is associated with a mild reduction in the incidence of atrial fibrillation in observational studies.”
“There has been a lot of public interest about a possible association of caffeine and arrhythmias,” but an adverse effect from daily consumption of a moderate amount of coffee “is more legend and anecdote than fact based,” commented Andrew D. Krahn, MD, an electrophysiologist, professor of medicine, and head of cardiology at the University of British Columbia and St. Paul’s Hospital in Vancouver. “Increasingly we’re finding that there really is nothing here” when the proarrhythmic effects of moderate coffee undergo detailed assessment, he said in an interview.
What the study did
The study run by Dr. Kim and her associates used prospectively collected data from 296,227 participants in the UK Biobank during 2006-2016 who had complete data on their coffee intake and for the other covariables used in the analysis. During an average 5.25 years of follow-up, these people had more than 13,000 incident arrhythmic events, including 4,748 episodes of atrial fibrillation or flutter and 798 supraventricular tachycardia events, as well as fewer numbers of ventricular arrhythmias and many episodes of less clinically relevant events like skipped beats.
The multivariate analysis the researchers ran controlled for more than 20 demographic, lifestyle, and clinical variables, including adjustment for tea intake but not for consumption of other caffeine-containing drinks.
The adjusted analysis showed an average, statistically significant 3% incremental drop in both all incident arrhythmias and in incident atrial fibrillation episodes for each additional cup of coffee drunk a day, for up to 6 daily cups.
A strength of this study is that it included a large number of people, Dr. Krahn noted, and “the UK Biobank includes a very diverse, community-based sample” of people, said Dr. Kim. The analysis excluded people with prevalent arrhythmia at baseline, so the study couldn’t address the impact of coffee consumption in this setting. A limitation of the study is that participants in the UK Biobank are all volunteers, which could result in a selection bias, Dr. Krahn said.
What it tells us
While the main message from the results is that moderate daily coffee drinking is not arrhythmogenic, “it is also possible that coffee is beneficial” based on the small but statistically significant decline in new-onset events, Dr. Kim added. “Multiple studies revealed that caffeine and potentially other constituents in coffee have antioxidant and anti-inflammatory properties. Multiple studies have reported the potential benefit of coffee in multiple chronic medical conditions such as cardiovascular disease, diabetes, and certain types of cancers, as well as for all-cause mortality.”
“It’s plausible that a moderate amount of coffee intake a day will not cause big physiologic changes, and moderate coffee intake may link with other characteristics” of moderate behavior that result in average or better than average outcomes, Dr. Krahn commented. “These results add to the existing data in a different and large population,” which strengthens the case that moderate coffee intake isn’t harmful, he said.
The study received no commercial funding. Dr. Kim and Dr. Krahn had no disclosures. The senior author on Dr. Kim’s study, Gregory M. Marcus, MD, has been a consultant to Johnson & Johnson and Incardia, has an equity interest in Incardia, and has received research funding from Baylis, Eight Sleep, and Medtronic.
SOURCE: Kim EJ et al. Heart Rhythm 2020, abstract D-PO01-032.
Moderate, daily coffee consumption had no apparent adverse effect for triggering incident heart arrhythmias, and even linked with a small but statistically significant drop in arrhythmias in an analysis of prospectively collected data from nearly 300,000 U.K. residents.
“In this large, population-based, prospective study, moderate habitual coffee drinking was associated with a lower risk of arrhythmia,” EunJeong Kim, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
Her analysis found that on average each additional daily cup of coffee that people said they drank reduced the incidence of arrhythmic episodes by a statistically significant 3%, compared with those who drank fewer daily cups. The relationship held for people who reported drinking as many as five or six cups of coffee daily.
“The main message of our study is that it does not appear to be deleterious to continue with moderate amounts of habitual coffee intake regarding a risk of overall arrhythmia,” said Dr. Kim, a cardiac electrophysiologist at the University of California, San Francisco.
Evidence builds for coffee’s safety
The finding adds to a substantial existing evidence base documenting the safety of moderate, habitual coffee drinking when it comes to heart rhythms. For example, a recent report from the Physicians Health Study of nearly 19,000 American men showed a statistically significant decrease in the incidence of atrial fibrillation during an average follow-up of 9 years among men who reported drinking one to three cups of coffee daily (J Am Heart Assoc. 2019 Aug 6;8[15]:e011346). In addition, a recent review of several reports found that “mild-to-moderate habitual consumption of caffeinated beverages, particularly a daily intake of 2-3 cups of coffee or tea, appears to be safe across a broad range of cardiovascular conditions, and may even be beneficial with respect to diabetes mellitus, atherosclerosis, heart failure, arrhythmia and total mortality,” but also concluded that “acute consumption of high doses of caffeine, particularly in the form of energy drinks, is best avoided”(Trends Cardiovasc Med. 2019 Aug;29[6]:345-50). Specifically about cardiac arrhythmias, the review said “while caffeine is commonly considered a trigger for arrhythmias by physicians and patients alike there is minimal evidence to support this misconception. Rather caffeine is associated with a mild reduction in the incidence of atrial fibrillation in observational studies.”
“There has been a lot of public interest about a possible association of caffeine and arrhythmias,” but an adverse effect from daily consumption of a moderate amount of coffee “is more legend and anecdote than fact based,” commented Andrew D. Krahn, MD, an electrophysiologist, professor of medicine, and head of cardiology at the University of British Columbia and St. Paul’s Hospital in Vancouver. “Increasingly we’re finding that there really is nothing here” when the proarrhythmic effects of moderate coffee undergo detailed assessment, he said in an interview.
What the study did
The study run by Dr. Kim and her associates used prospectively collected data from 296,227 participants in the UK Biobank during 2006-2016 who had complete data on their coffee intake and for the other covariables used in the analysis. During an average 5.25 years of follow-up, these people had more than 13,000 incident arrhythmic events, including 4,748 episodes of atrial fibrillation or flutter and 798 supraventricular tachycardia events, as well as fewer numbers of ventricular arrhythmias and many episodes of less clinically relevant events like skipped beats.
The multivariate analysis the researchers ran controlled for more than 20 demographic, lifestyle, and clinical variables, including adjustment for tea intake but not for consumption of other caffeine-containing drinks.
The adjusted analysis showed an average, statistically significant 3% incremental drop in both all incident arrhythmias and in incident atrial fibrillation episodes for each additional cup of coffee drunk a day, for up to 6 daily cups.
A strength of this study is that it included a large number of people, Dr. Krahn noted, and “the UK Biobank includes a very diverse, community-based sample” of people, said Dr. Kim. The analysis excluded people with prevalent arrhythmia at baseline, so the study couldn’t address the impact of coffee consumption in this setting. A limitation of the study is that participants in the UK Biobank are all volunteers, which could result in a selection bias, Dr. Krahn said.
What it tells us
While the main message from the results is that moderate daily coffee drinking is not arrhythmogenic, “it is also possible that coffee is beneficial” based on the small but statistically significant decline in new-onset events, Dr. Kim added. “Multiple studies revealed that caffeine and potentially other constituents in coffee have antioxidant and anti-inflammatory properties. Multiple studies have reported the potential benefit of coffee in multiple chronic medical conditions such as cardiovascular disease, diabetes, and certain types of cancers, as well as for all-cause mortality.”
“It’s plausible that a moderate amount of coffee intake a day will not cause big physiologic changes, and moderate coffee intake may link with other characteristics” of moderate behavior that result in average or better than average outcomes, Dr. Krahn commented. “These results add to the existing data in a different and large population,” which strengthens the case that moderate coffee intake isn’t harmful, he said.
The study received no commercial funding. Dr. Kim and Dr. Krahn had no disclosures. The senior author on Dr. Kim’s study, Gregory M. Marcus, MD, has been a consultant to Johnson & Johnson and Incardia, has an equity interest in Incardia, and has received research funding from Baylis, Eight Sleep, and Medtronic.
SOURCE: Kim EJ et al. Heart Rhythm 2020, abstract D-PO01-032.
Moderate, daily coffee consumption had no apparent adverse effect for triggering incident heart arrhythmias, and even linked with a small but statistically significant drop in arrhythmias in an analysis of prospectively collected data from nearly 300,000 U.K. residents.
“In this large, population-based, prospective study, moderate habitual coffee drinking was associated with a lower risk of arrhythmia,” EunJeong Kim, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
Her analysis found that on average each additional daily cup of coffee that people said they drank reduced the incidence of arrhythmic episodes by a statistically significant 3%, compared with those who drank fewer daily cups. The relationship held for people who reported drinking as many as five or six cups of coffee daily.
“The main message of our study is that it does not appear to be deleterious to continue with moderate amounts of habitual coffee intake regarding a risk of overall arrhythmia,” said Dr. Kim, a cardiac electrophysiologist at the University of California, San Francisco.
Evidence builds for coffee’s safety
The finding adds to a substantial existing evidence base documenting the safety of moderate, habitual coffee drinking when it comes to heart rhythms. For example, a recent report from the Physicians Health Study of nearly 19,000 American men showed a statistically significant decrease in the incidence of atrial fibrillation during an average follow-up of 9 years among men who reported drinking one to three cups of coffee daily (J Am Heart Assoc. 2019 Aug 6;8[15]:e011346). In addition, a recent review of several reports found that “mild-to-moderate habitual consumption of caffeinated beverages, particularly a daily intake of 2-3 cups of coffee or tea, appears to be safe across a broad range of cardiovascular conditions, and may even be beneficial with respect to diabetes mellitus, atherosclerosis, heart failure, arrhythmia and total mortality,” but also concluded that “acute consumption of high doses of caffeine, particularly in the form of energy drinks, is best avoided”(Trends Cardiovasc Med. 2019 Aug;29[6]:345-50). Specifically about cardiac arrhythmias, the review said “while caffeine is commonly considered a trigger for arrhythmias by physicians and patients alike there is minimal evidence to support this misconception. Rather caffeine is associated with a mild reduction in the incidence of atrial fibrillation in observational studies.”
“There has been a lot of public interest about a possible association of caffeine and arrhythmias,” but an adverse effect from daily consumption of a moderate amount of coffee “is more legend and anecdote than fact based,” commented Andrew D. Krahn, MD, an electrophysiologist, professor of medicine, and head of cardiology at the University of British Columbia and St. Paul’s Hospital in Vancouver. “Increasingly we’re finding that there really is nothing here” when the proarrhythmic effects of moderate coffee undergo detailed assessment, he said in an interview.
What the study did
The study run by Dr. Kim and her associates used prospectively collected data from 296,227 participants in the UK Biobank during 2006-2016 who had complete data on their coffee intake and for the other covariables used in the analysis. During an average 5.25 years of follow-up, these people had more than 13,000 incident arrhythmic events, including 4,748 episodes of atrial fibrillation or flutter and 798 supraventricular tachycardia events, as well as fewer numbers of ventricular arrhythmias and many episodes of less clinically relevant events like skipped beats.
The multivariate analysis the researchers ran controlled for more than 20 demographic, lifestyle, and clinical variables, including adjustment for tea intake but not for consumption of other caffeine-containing drinks.
The adjusted analysis showed an average, statistically significant 3% incremental drop in both all incident arrhythmias and in incident atrial fibrillation episodes for each additional cup of coffee drunk a day, for up to 6 daily cups.
A strength of this study is that it included a large number of people, Dr. Krahn noted, and “the UK Biobank includes a very diverse, community-based sample” of people, said Dr. Kim. The analysis excluded people with prevalent arrhythmia at baseline, so the study couldn’t address the impact of coffee consumption in this setting. A limitation of the study is that participants in the UK Biobank are all volunteers, which could result in a selection bias, Dr. Krahn said.
What it tells us
While the main message from the results is that moderate daily coffee drinking is not arrhythmogenic, “it is also possible that coffee is beneficial” based on the small but statistically significant decline in new-onset events, Dr. Kim added. “Multiple studies revealed that caffeine and potentially other constituents in coffee have antioxidant and anti-inflammatory properties. Multiple studies have reported the potential benefit of coffee in multiple chronic medical conditions such as cardiovascular disease, diabetes, and certain types of cancers, as well as for all-cause mortality.”
“It’s plausible that a moderate amount of coffee intake a day will not cause big physiologic changes, and moderate coffee intake may link with other characteristics” of moderate behavior that result in average or better than average outcomes, Dr. Krahn commented. “These results add to the existing data in a different and large population,” which strengthens the case that moderate coffee intake isn’t harmful, he said.
The study received no commercial funding. Dr. Kim and Dr. Krahn had no disclosures. The senior author on Dr. Kim’s study, Gregory M. Marcus, MD, has been a consultant to Johnson & Johnson and Incardia, has an equity interest in Incardia, and has received research funding from Baylis, Eight Sleep, and Medtronic.
SOURCE: Kim EJ et al. Heart Rhythm 2020, abstract D-PO01-032.
FROM HEART RHYTHM 2020
Adolescent obesity, diabetes linked to atherosclerotic signs
significantly greater than their normal-weight peers, according to a longitudinal study published online in the Journal of the American Heart Association.
The study evaluated 448 adolescents over 5 years for changes in a variety of metrics to determine changes in arterial structure, including carotid intima media thickness (cIMT), carotid-femoral pulse-wave velocity (PWV), and augmentation index (Aix). The average age of the study group was 17.6 years. The three study groups broke down accordingly: 141 with normal weight, 156 with obesity, and 151 with type 2 diabetes. Patients were evaluated at baseline and 5 years later.
“The presence of obesity and especially type 2 diabetes in adolescents accelerates the early vascular aging process associated with several key risk factors,” wrote Justin R. Ryder, PhD, an assistant professor of pediatrics at the University of Minnesota, Minneapolis, and colleagues.
The researchers also noted that systolic hypertension was associated with changes in cIMT and arterial stiffness comparable to obesity and diabetes. “These data add further evidence underscoring the importance of efforts targeting prevention and treatment of obesity, type 2 diabetes, and elevated blood pressure among youth, with a goal of delaying and/or preventing the progression of early vascular aging,” Dr. Ryder and colleagues wrote.
Obese patients, when compared with normal-weight participants, had the following average increases: common cIMT by 0.05 mm, bulb cIMT by 0.02 mm, internal cIMT by 0.03 mm, and PWV carotid-femoral by 0.38 m/sec, all statistically significant differences. Patients with diabetes, compared with normal-weight participants, registered the following average increases: common cIMT by 0.05 mm, bulb cIMT by 0.06 mm, internal cIMT by 0.04 mm, Aix by 4.67%, and PWV carotid-femoral by 0.74 m/sec. All differences were highly significant at P less than .001.
The results also showed that higher baseline systolic blood pressure was associated with significantly greater average increases in the following factors: common cIMT by 0.007 mm, bulb cIMT by 0.009 mm, internal cIMT by 0.008 mm, and PWV carotid-femoral by 0.66 m/sec.
Drilling down into the data, the study reported that males had greater increases in bulb cIMT and incremental elastic modulus as well as reduced Aix, compared with females. Nonwhites also had greater increases in bulb cIMT than did whites. Age was associated with greater increases in bulb and internal cIMT and Aix.
“Our data support the concept that male sex is an independent and primary risk factor for accelerated early vascular aging,” Dr. Ryder and colleagues wrote. The study also determined that type 2 diabetes is a more prominent risk factor than obesity for early vascular aging.
The size of the study population, specifically adolescents with diabetes, is a study strength, Dr. Ryder and colleagues noted. Other strengths they pointed to are the 5-year duration and the robust panel of noninvasive measures, although not using hard cardiovascular outcomes is an acknowledged limitation.
“It should also be noted that many of the youth with type 2 diabetes were on medications for glycemic control, lipids, and/or blood pressure regulation,” Dr. Ryder and colleagues wrote. “Despite this, the vascular profiles worsened over time.”
The study showed “a really significant change” in the carotid anatomy in adolescents with obesity and type 2 diabetes over 5 years, Robert Eckel, MD, professor at the University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Notably, the PWV is not just anatomy; now we’re talking about function. In other words, the augmentation index and PWV will assess the compliance of the artery.”
The findings suggest that atherosclerosis begins with thickening of the arterial walls. “The question is, is thickness reversible?” Dr. Eckel said. “It’s probably not very reversible, so these are early changes that ultimately in the middle years or latter years are associated with major cardiovascular disease.”
They key lesson from the study, Dr. Eckel noted, is to “prevent obesity. If you prevent obesity in the teenage years, you basically prevent diabetes.”
Dr. Ryder disclosed receiving support from Boehringer Ingelheim in the form of drug/placebo. The National Institutes of Health provided funding. Dr. Eckel has no relevant relationships to disclose.
SOURCE: Ryder JR et al. J Am Heart Assoc. 2020 May 6:e014891. doi: 10.1161/JAHA.119.014891.
significantly greater than their normal-weight peers, according to a longitudinal study published online in the Journal of the American Heart Association.
The study evaluated 448 adolescents over 5 years for changes in a variety of metrics to determine changes in arterial structure, including carotid intima media thickness (cIMT), carotid-femoral pulse-wave velocity (PWV), and augmentation index (Aix). The average age of the study group was 17.6 years. The three study groups broke down accordingly: 141 with normal weight, 156 with obesity, and 151 with type 2 diabetes. Patients were evaluated at baseline and 5 years later.
“The presence of obesity and especially type 2 diabetes in adolescents accelerates the early vascular aging process associated with several key risk factors,” wrote Justin R. Ryder, PhD, an assistant professor of pediatrics at the University of Minnesota, Minneapolis, and colleagues.
The researchers also noted that systolic hypertension was associated with changes in cIMT and arterial stiffness comparable to obesity and diabetes. “These data add further evidence underscoring the importance of efforts targeting prevention and treatment of obesity, type 2 diabetes, and elevated blood pressure among youth, with a goal of delaying and/or preventing the progression of early vascular aging,” Dr. Ryder and colleagues wrote.
Obese patients, when compared with normal-weight participants, had the following average increases: common cIMT by 0.05 mm, bulb cIMT by 0.02 mm, internal cIMT by 0.03 mm, and PWV carotid-femoral by 0.38 m/sec, all statistically significant differences. Patients with diabetes, compared with normal-weight participants, registered the following average increases: common cIMT by 0.05 mm, bulb cIMT by 0.06 mm, internal cIMT by 0.04 mm, Aix by 4.67%, and PWV carotid-femoral by 0.74 m/sec. All differences were highly significant at P less than .001.
The results also showed that higher baseline systolic blood pressure was associated with significantly greater average increases in the following factors: common cIMT by 0.007 mm, bulb cIMT by 0.009 mm, internal cIMT by 0.008 mm, and PWV carotid-femoral by 0.66 m/sec.
Drilling down into the data, the study reported that males had greater increases in bulb cIMT and incremental elastic modulus as well as reduced Aix, compared with females. Nonwhites also had greater increases in bulb cIMT than did whites. Age was associated with greater increases in bulb and internal cIMT and Aix.
“Our data support the concept that male sex is an independent and primary risk factor for accelerated early vascular aging,” Dr. Ryder and colleagues wrote. The study also determined that type 2 diabetes is a more prominent risk factor than obesity for early vascular aging.
The size of the study population, specifically adolescents with diabetes, is a study strength, Dr. Ryder and colleagues noted. Other strengths they pointed to are the 5-year duration and the robust panel of noninvasive measures, although not using hard cardiovascular outcomes is an acknowledged limitation.
“It should also be noted that many of the youth with type 2 diabetes were on medications for glycemic control, lipids, and/or blood pressure regulation,” Dr. Ryder and colleagues wrote. “Despite this, the vascular profiles worsened over time.”
The study showed “a really significant change” in the carotid anatomy in adolescents with obesity and type 2 diabetes over 5 years, Robert Eckel, MD, professor at the University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Notably, the PWV is not just anatomy; now we’re talking about function. In other words, the augmentation index and PWV will assess the compliance of the artery.”
The findings suggest that atherosclerosis begins with thickening of the arterial walls. “The question is, is thickness reversible?” Dr. Eckel said. “It’s probably not very reversible, so these are early changes that ultimately in the middle years or latter years are associated with major cardiovascular disease.”
They key lesson from the study, Dr. Eckel noted, is to “prevent obesity. If you prevent obesity in the teenage years, you basically prevent diabetes.”
Dr. Ryder disclosed receiving support from Boehringer Ingelheim in the form of drug/placebo. The National Institutes of Health provided funding. Dr. Eckel has no relevant relationships to disclose.
SOURCE: Ryder JR et al. J Am Heart Assoc. 2020 May 6:e014891. doi: 10.1161/JAHA.119.014891.
significantly greater than their normal-weight peers, according to a longitudinal study published online in the Journal of the American Heart Association.
The study evaluated 448 adolescents over 5 years for changes in a variety of metrics to determine changes in arterial structure, including carotid intima media thickness (cIMT), carotid-femoral pulse-wave velocity (PWV), and augmentation index (Aix). The average age of the study group was 17.6 years. The three study groups broke down accordingly: 141 with normal weight, 156 with obesity, and 151 with type 2 diabetes. Patients were evaluated at baseline and 5 years later.
“The presence of obesity and especially type 2 diabetes in adolescents accelerates the early vascular aging process associated with several key risk factors,” wrote Justin R. Ryder, PhD, an assistant professor of pediatrics at the University of Minnesota, Minneapolis, and colleagues.
The researchers also noted that systolic hypertension was associated with changes in cIMT and arterial stiffness comparable to obesity and diabetes. “These data add further evidence underscoring the importance of efforts targeting prevention and treatment of obesity, type 2 diabetes, and elevated blood pressure among youth, with a goal of delaying and/or preventing the progression of early vascular aging,” Dr. Ryder and colleagues wrote.
Obese patients, when compared with normal-weight participants, had the following average increases: common cIMT by 0.05 mm, bulb cIMT by 0.02 mm, internal cIMT by 0.03 mm, and PWV carotid-femoral by 0.38 m/sec, all statistically significant differences. Patients with diabetes, compared with normal-weight participants, registered the following average increases: common cIMT by 0.05 mm, bulb cIMT by 0.06 mm, internal cIMT by 0.04 mm, Aix by 4.67%, and PWV carotid-femoral by 0.74 m/sec. All differences were highly significant at P less than .001.
The results also showed that higher baseline systolic blood pressure was associated with significantly greater average increases in the following factors: common cIMT by 0.007 mm, bulb cIMT by 0.009 mm, internal cIMT by 0.008 mm, and PWV carotid-femoral by 0.66 m/sec.
Drilling down into the data, the study reported that males had greater increases in bulb cIMT and incremental elastic modulus as well as reduced Aix, compared with females. Nonwhites also had greater increases in bulb cIMT than did whites. Age was associated with greater increases in bulb and internal cIMT and Aix.
“Our data support the concept that male sex is an independent and primary risk factor for accelerated early vascular aging,” Dr. Ryder and colleagues wrote. The study also determined that type 2 diabetes is a more prominent risk factor than obesity for early vascular aging.
The size of the study population, specifically adolescents with diabetes, is a study strength, Dr. Ryder and colleagues noted. Other strengths they pointed to are the 5-year duration and the robust panel of noninvasive measures, although not using hard cardiovascular outcomes is an acknowledged limitation.
“It should also be noted that many of the youth with type 2 diabetes were on medications for glycemic control, lipids, and/or blood pressure regulation,” Dr. Ryder and colleagues wrote. “Despite this, the vascular profiles worsened over time.”
The study showed “a really significant change” in the carotid anatomy in adolescents with obesity and type 2 diabetes over 5 years, Robert Eckel, MD, professor at the University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Notably, the PWV is not just anatomy; now we’re talking about function. In other words, the augmentation index and PWV will assess the compliance of the artery.”
The findings suggest that atherosclerosis begins with thickening of the arterial walls. “The question is, is thickness reversible?” Dr. Eckel said. “It’s probably not very reversible, so these are early changes that ultimately in the middle years or latter years are associated with major cardiovascular disease.”
They key lesson from the study, Dr. Eckel noted, is to “prevent obesity. If you prevent obesity in the teenage years, you basically prevent diabetes.”
Dr. Ryder disclosed receiving support from Boehringer Ingelheim in the form of drug/placebo. The National Institutes of Health provided funding. Dr. Eckel has no relevant relationships to disclose.
SOURCE: Ryder JR et al. J Am Heart Assoc. 2020 May 6:e014891. doi: 10.1161/JAHA.119.014891.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Results from 11 AHA-funded COVID-19 studies expected within months
Work is set to start in June, with findings reported in as few as 6 months. The Cleveland Clinic will coordinate the efforts, collecting and disseminating the findings.
There were more than 750 research proposals in less than a month after the association announced its COVID-19 and Its Cardiovascular Impact Rapid Response Grant initiative.
“We were just blown away and so impressed to see this level of interest and commitment from the teams submitting such thorough proposals so quickly,” AHA President Robert Harrington, MD, chair of the department of medicine at Stanford (Calif.) University, said in a press statement. “There’s so much we don’t know about this unique coronavirus, and we continue to see emerging complications affecting both heart and brain health for which we desperately need answers and we need them quickly.”
The projects include the following:
- A Comprehensive Assessment of Arterial and Venous Thrombotic Complications in Patients with COVID-19, led by Columbia University, New York City.
- Repurposing Drugs for Treatment of Cardiomyopathy Caused by Coronavirus-2 (SARS-CoV-2), led by Brigham and Women’s Hospital and Harvard Medical School, Boston.
- Risk of Severe Morbidity and Mortality of Coronavirus Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications, led by Kaiser Permanente Southern California.
- Deep Learning Using Chest Radiographs to Predict COVID-19 Cardiopulmonary Risk, led by Massachusetts General Hospital, Boston.
- Cardiovascular Outcomes and Biomarker Titrated Corticosteroid Dosing for SARS COV-2 (COVID-19): A Randomized Controlled Trial, led by the Mayo Clinic, Rochester Minn.
- Outcomes for Patients With Hypertension, Diabetes, and Heart Disease in the Coronavirus Pandemic: Impact of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Treatment, led by Stanford University.
- Rapid COVID-19-on-A-Chip to Screen Competitive Targets for SARS-CoV-2 Spike Binding Sites, led by University of California, Los Angeles.
- COVID-19 Infection, African American Women and Cardiovascular Health, led by University of California, San Francisco.
- Myocardial Virus and Gene Expression in SARS CoV-2 Positive Patients with Clinically Important Myocardial Dysfunction, led by the University of Colorado, Aurora.
- The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection, led by the University of Massachusetts, Worcester.
- Harnessing Glycomics to Understand Myocardial Injury in COVID-19, led by the University of Nebraska Medical Center, Omaha.
The AHA also awarded $800,000 for short-term projects to members of its new Health Technologies & Innovation Strategically Focused Research Network.
Cincinnati Children’s Hospital will assess the use of ejection fraction to triage COVID-19 patients; Johns Hopkins University, Baltimore, will assess smartphones for “virtual check-in” for stroke symptoms; Stanford will assess digital tracking of COVID-19 patients with cardiovascular complications; and the University of Michigan, Ann Arbor, will assess a system to track physiological and cardiovascular consequences of the infection.
Work is set to start in June, with findings reported in as few as 6 months. The Cleveland Clinic will coordinate the efforts, collecting and disseminating the findings.
There were more than 750 research proposals in less than a month after the association announced its COVID-19 and Its Cardiovascular Impact Rapid Response Grant initiative.
“We were just blown away and so impressed to see this level of interest and commitment from the teams submitting such thorough proposals so quickly,” AHA President Robert Harrington, MD, chair of the department of medicine at Stanford (Calif.) University, said in a press statement. “There’s so much we don’t know about this unique coronavirus, and we continue to see emerging complications affecting both heart and brain health for which we desperately need answers and we need them quickly.”
The projects include the following:
- A Comprehensive Assessment of Arterial and Venous Thrombotic Complications in Patients with COVID-19, led by Columbia University, New York City.
- Repurposing Drugs for Treatment of Cardiomyopathy Caused by Coronavirus-2 (SARS-CoV-2), led by Brigham and Women’s Hospital and Harvard Medical School, Boston.
- Risk of Severe Morbidity and Mortality of Coronavirus Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications, led by Kaiser Permanente Southern California.
- Deep Learning Using Chest Radiographs to Predict COVID-19 Cardiopulmonary Risk, led by Massachusetts General Hospital, Boston.
- Cardiovascular Outcomes and Biomarker Titrated Corticosteroid Dosing for SARS COV-2 (COVID-19): A Randomized Controlled Trial, led by the Mayo Clinic, Rochester Minn.
- Outcomes for Patients With Hypertension, Diabetes, and Heart Disease in the Coronavirus Pandemic: Impact of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Treatment, led by Stanford University.
- Rapid COVID-19-on-A-Chip to Screen Competitive Targets for SARS-CoV-2 Spike Binding Sites, led by University of California, Los Angeles.
- COVID-19 Infection, African American Women and Cardiovascular Health, led by University of California, San Francisco.
- Myocardial Virus and Gene Expression in SARS CoV-2 Positive Patients with Clinically Important Myocardial Dysfunction, led by the University of Colorado, Aurora.
- The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection, led by the University of Massachusetts, Worcester.
- Harnessing Glycomics to Understand Myocardial Injury in COVID-19, led by the University of Nebraska Medical Center, Omaha.
The AHA also awarded $800,000 for short-term projects to members of its new Health Technologies & Innovation Strategically Focused Research Network.
Cincinnati Children’s Hospital will assess the use of ejection fraction to triage COVID-19 patients; Johns Hopkins University, Baltimore, will assess smartphones for “virtual check-in” for stroke symptoms; Stanford will assess digital tracking of COVID-19 patients with cardiovascular complications; and the University of Michigan, Ann Arbor, will assess a system to track physiological and cardiovascular consequences of the infection.
Work is set to start in June, with findings reported in as few as 6 months. The Cleveland Clinic will coordinate the efforts, collecting and disseminating the findings.
There were more than 750 research proposals in less than a month after the association announced its COVID-19 and Its Cardiovascular Impact Rapid Response Grant initiative.
“We were just blown away and so impressed to see this level of interest and commitment from the teams submitting such thorough proposals so quickly,” AHA President Robert Harrington, MD, chair of the department of medicine at Stanford (Calif.) University, said in a press statement. “There’s so much we don’t know about this unique coronavirus, and we continue to see emerging complications affecting both heart and brain health for which we desperately need answers and we need them quickly.”
The projects include the following:
- A Comprehensive Assessment of Arterial and Venous Thrombotic Complications in Patients with COVID-19, led by Columbia University, New York City.
- Repurposing Drugs for Treatment of Cardiomyopathy Caused by Coronavirus-2 (SARS-CoV-2), led by Brigham and Women’s Hospital and Harvard Medical School, Boston.
- Risk of Severe Morbidity and Mortality of Coronavirus Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications, led by Kaiser Permanente Southern California.
- Deep Learning Using Chest Radiographs to Predict COVID-19 Cardiopulmonary Risk, led by Massachusetts General Hospital, Boston.
- Cardiovascular Outcomes and Biomarker Titrated Corticosteroid Dosing for SARS COV-2 (COVID-19): A Randomized Controlled Trial, led by the Mayo Clinic, Rochester Minn.
- Outcomes for Patients With Hypertension, Diabetes, and Heart Disease in the Coronavirus Pandemic: Impact of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Treatment, led by Stanford University.
- Rapid COVID-19-on-A-Chip to Screen Competitive Targets for SARS-CoV-2 Spike Binding Sites, led by University of California, Los Angeles.
- COVID-19 Infection, African American Women and Cardiovascular Health, led by University of California, San Francisco.
- Myocardial Virus and Gene Expression in SARS CoV-2 Positive Patients with Clinically Important Myocardial Dysfunction, led by the University of Colorado, Aurora.
- The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection, led by the University of Massachusetts, Worcester.
- Harnessing Glycomics to Understand Myocardial Injury in COVID-19, led by the University of Nebraska Medical Center, Omaha.
The AHA also awarded $800,000 for short-term projects to members of its new Health Technologies & Innovation Strategically Focused Research Network.
Cincinnati Children’s Hospital will assess the use of ejection fraction to triage COVID-19 patients; Johns Hopkins University, Baltimore, will assess smartphones for “virtual check-in” for stroke symptoms; Stanford will assess digital tracking of COVID-19 patients with cardiovascular complications; and the University of Michigan, Ann Arbor, will assess a system to track physiological and cardiovascular consequences of the infection.
FDA approves dapagliflozin for low-EF heart failure
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.