User login
Continuous cuffless monitoring may fuel lifestyle change to lower BP
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
FROM AHA HYPERTENSION 2022
Ultrasonic renal denervation passes 2-month test in uncontrolled HTN: RADIANCE II
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.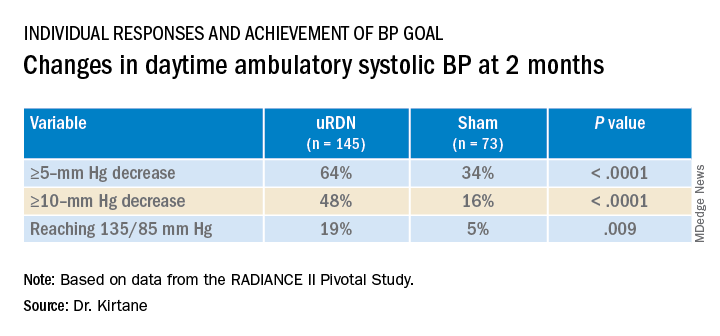
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).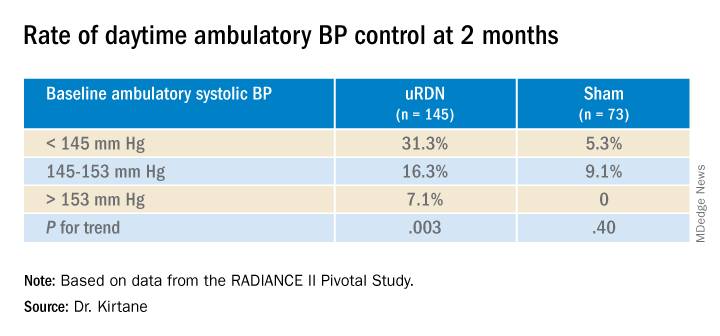
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
TAVR now used in almost 50% of younger severe aortic stenosis patients
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
New science reveals the best way to take a pill
I want to tell you a story about forgetfulness and haste, and how the combination of the two can lead to frightening consequences. A few years ago, I was lying in bed about to turn out the light when I realized I’d forgotten to take “my pill.”
Like some 161 million other American adults, I was then a consumer of a prescription medication. Being conscientious, I got up, retrieved said pill, and tossed it back. Being lazy, I didn’t bother to grab a glass of water to help the thing go down. Instead, I promptly returned to bed, threw a pillow over my head, and prepared for sleep.
Within seconds, I began to feel a burning sensation in my chest. After about a minute, that burn became a crippling pain. Not wanting to alarm my wife, I went into the living room, where I spent the next 30 minutes doubled over in agony. Was I having a heart attack? I phoned my sister, a hospitalist in Texas. She advised me to take myself to the ED to get checked out.
If only I’d known then about “Duke.” He could have told me how critical body posture is when people swallow pills.
Who’s Duke?
Duke is a computer representation of a 34-year-old, anatomically normal human male created by computer scientists at the IT’IS Foundation, a nonprofit group based in Switzerland that works on a variety of projects in health care technology. Using Duke, Rajat Mittal, PhD, a professor of medicine at the Johns Hopkins University, Baltimore, created a computer model called “StomachSim” to explore the process of digestion.
Their research, published in the journal Physics of Fluids, turned up several surprising findings about the dynamics of swallowing pills – the most common way medication is used worldwide.
Dr. Mittal said he chose to study the stomach because the functions of most other organ systems, from the heart to the brain, have already attracted plenty of attention from scientists.
“As I was looking to initiate research in some new directions, the implications of stomach biomechanics on important conditions such as diabetes, obesity, and gastroparesis became apparent to me,” he said. “It was clear that bioengineering research in this arena lags other more ‘sexy’ areas such as cardiovascular flows by at least 20 years, and there seemed to be a great opportunity to do impactful work.”
Your posture may help a pill work better
Several well-known things affect a pill’s ability to disperse its contents into the gut and be used by the body, such as the stomach’s contents (a heavy breakfast, a mix of liquids like juice, milk, and coffee) and the motion of the organ’s walls. But Dr. Mittal’s group learned that Duke’s posture also played a major role.
The researchers ran Duke through computer simulations in varying postures: upright, leaning right, leaning left, and leaning back, while keeping all the other parts of their analyses (like the things mentioned above) the same.
They found that posture determined as much as 83% of how quickly a pill disperses into the intestines. The most efficient position was leaning right. The least was leaning left, which prevented the pill from reaching the antrum, or bottom section of the stomach, and thus kept all but traces of the dissolved drug from entering the duodenum, where the stomach joins the small intestine. (Interestingly, Jews who observe Passover are advised to recline to the left during the meal as a symbol of freedom and leisure.)
That makes sense if you think about the stomach’s shape, which looks kind of like a bean, curving from the left to the right side of the body. Because of gravity, your position will change where the pill lands.
a condition in which the stomach loses the ability to empty properly.
How this could help people
Among the groups most likely to benefit from such studies, Dr. Mittal said, are the elderly – who both take a lot of pills and are more prone to trouble swallowing because of age-related changes in their esophagus – and the bedridden, who can’t easily shift their posture. The findings may also lead to improvements in the ability to treat people with gastroparesis, a particular problem for people with diabetes.
Future studies with Duke and similar simulations will look at how the GI system digests proteins, carbohydrates, and fatty meals, Dr. Mittal said.
In the meantime, Dr. Mittal offered the following advice: “Standing or sitting upright after taking a pill is fine. If you have to take a pill lying down, stay on your back or on your right side. Avoid lying on your left side after taking a pill.”
As for what happened to me, any gastroenterologist reading this has figured out that my condition was not heart-related. Instead, I likely was having a bout of pill esophagitis, irritation that can result from medications that aggravate the mucosa of the food tube. Although painful, esophagitis isn’t life-threatening. After about an hour, the pain began to subside, and by the next morning I was fine, with only a faint ache in my chest to remind me of my earlier torment. (Researchers noted an increase in the condition early in the COVID-19 pandemic, linked to the antibiotic doxycycline.)
And, in the interest of accuracy, my pill problem began above the stomach. Nothing in the Hopkins research suggests that the alignment of the esophagus plays a role in how drugs disperse in the gut – unless, of course, it prevents those pills from reaching the stomach in the first place.
A version of this article first appeared on WebMD.com.
I want to tell you a story about forgetfulness and haste, and how the combination of the two can lead to frightening consequences. A few years ago, I was lying in bed about to turn out the light when I realized I’d forgotten to take “my pill.”
Like some 161 million other American adults, I was then a consumer of a prescription medication. Being conscientious, I got up, retrieved said pill, and tossed it back. Being lazy, I didn’t bother to grab a glass of water to help the thing go down. Instead, I promptly returned to bed, threw a pillow over my head, and prepared for sleep.
Within seconds, I began to feel a burning sensation in my chest. After about a minute, that burn became a crippling pain. Not wanting to alarm my wife, I went into the living room, where I spent the next 30 minutes doubled over in agony. Was I having a heart attack? I phoned my sister, a hospitalist in Texas. She advised me to take myself to the ED to get checked out.
If only I’d known then about “Duke.” He could have told me how critical body posture is when people swallow pills.
Who’s Duke?
Duke is a computer representation of a 34-year-old, anatomically normal human male created by computer scientists at the IT’IS Foundation, a nonprofit group based in Switzerland that works on a variety of projects in health care technology. Using Duke, Rajat Mittal, PhD, a professor of medicine at the Johns Hopkins University, Baltimore, created a computer model called “StomachSim” to explore the process of digestion.
Their research, published in the journal Physics of Fluids, turned up several surprising findings about the dynamics of swallowing pills – the most common way medication is used worldwide.
Dr. Mittal said he chose to study the stomach because the functions of most other organ systems, from the heart to the brain, have already attracted plenty of attention from scientists.
“As I was looking to initiate research in some new directions, the implications of stomach biomechanics on important conditions such as diabetes, obesity, and gastroparesis became apparent to me,” he said. “It was clear that bioengineering research in this arena lags other more ‘sexy’ areas such as cardiovascular flows by at least 20 years, and there seemed to be a great opportunity to do impactful work.”
Your posture may help a pill work better
Several well-known things affect a pill’s ability to disperse its contents into the gut and be used by the body, such as the stomach’s contents (a heavy breakfast, a mix of liquids like juice, milk, and coffee) and the motion of the organ’s walls. But Dr. Mittal’s group learned that Duke’s posture also played a major role.
The researchers ran Duke through computer simulations in varying postures: upright, leaning right, leaning left, and leaning back, while keeping all the other parts of their analyses (like the things mentioned above) the same.
They found that posture determined as much as 83% of how quickly a pill disperses into the intestines. The most efficient position was leaning right. The least was leaning left, which prevented the pill from reaching the antrum, or bottom section of the stomach, and thus kept all but traces of the dissolved drug from entering the duodenum, where the stomach joins the small intestine. (Interestingly, Jews who observe Passover are advised to recline to the left during the meal as a symbol of freedom and leisure.)
That makes sense if you think about the stomach’s shape, which looks kind of like a bean, curving from the left to the right side of the body. Because of gravity, your position will change where the pill lands.
a condition in which the stomach loses the ability to empty properly.
How this could help people
Among the groups most likely to benefit from such studies, Dr. Mittal said, are the elderly – who both take a lot of pills and are more prone to trouble swallowing because of age-related changes in their esophagus – and the bedridden, who can’t easily shift their posture. The findings may also lead to improvements in the ability to treat people with gastroparesis, a particular problem for people with diabetes.
Future studies with Duke and similar simulations will look at how the GI system digests proteins, carbohydrates, and fatty meals, Dr. Mittal said.
In the meantime, Dr. Mittal offered the following advice: “Standing or sitting upright after taking a pill is fine. If you have to take a pill lying down, stay on your back or on your right side. Avoid lying on your left side after taking a pill.”
As for what happened to me, any gastroenterologist reading this has figured out that my condition was not heart-related. Instead, I likely was having a bout of pill esophagitis, irritation that can result from medications that aggravate the mucosa of the food tube. Although painful, esophagitis isn’t life-threatening. After about an hour, the pain began to subside, and by the next morning I was fine, with only a faint ache in my chest to remind me of my earlier torment. (Researchers noted an increase in the condition early in the COVID-19 pandemic, linked to the antibiotic doxycycline.)
And, in the interest of accuracy, my pill problem began above the stomach. Nothing in the Hopkins research suggests that the alignment of the esophagus plays a role in how drugs disperse in the gut – unless, of course, it prevents those pills from reaching the stomach in the first place.
A version of this article first appeared on WebMD.com.
I want to tell you a story about forgetfulness and haste, and how the combination of the two can lead to frightening consequences. A few years ago, I was lying in bed about to turn out the light when I realized I’d forgotten to take “my pill.”
Like some 161 million other American adults, I was then a consumer of a prescription medication. Being conscientious, I got up, retrieved said pill, and tossed it back. Being lazy, I didn’t bother to grab a glass of water to help the thing go down. Instead, I promptly returned to bed, threw a pillow over my head, and prepared for sleep.
Within seconds, I began to feel a burning sensation in my chest. After about a minute, that burn became a crippling pain. Not wanting to alarm my wife, I went into the living room, where I spent the next 30 minutes doubled over in agony. Was I having a heart attack? I phoned my sister, a hospitalist in Texas. She advised me to take myself to the ED to get checked out.
If only I’d known then about “Duke.” He could have told me how critical body posture is when people swallow pills.
Who’s Duke?
Duke is a computer representation of a 34-year-old, anatomically normal human male created by computer scientists at the IT’IS Foundation, a nonprofit group based in Switzerland that works on a variety of projects in health care technology. Using Duke, Rajat Mittal, PhD, a professor of medicine at the Johns Hopkins University, Baltimore, created a computer model called “StomachSim” to explore the process of digestion.
Their research, published in the journal Physics of Fluids, turned up several surprising findings about the dynamics of swallowing pills – the most common way medication is used worldwide.
Dr. Mittal said he chose to study the stomach because the functions of most other organ systems, from the heart to the brain, have already attracted plenty of attention from scientists.
“As I was looking to initiate research in some new directions, the implications of stomach biomechanics on important conditions such as diabetes, obesity, and gastroparesis became apparent to me,” he said. “It was clear that bioengineering research in this arena lags other more ‘sexy’ areas such as cardiovascular flows by at least 20 years, and there seemed to be a great opportunity to do impactful work.”
Your posture may help a pill work better
Several well-known things affect a pill’s ability to disperse its contents into the gut and be used by the body, such as the stomach’s contents (a heavy breakfast, a mix of liquids like juice, milk, and coffee) and the motion of the organ’s walls. But Dr. Mittal’s group learned that Duke’s posture also played a major role.
The researchers ran Duke through computer simulations in varying postures: upright, leaning right, leaning left, and leaning back, while keeping all the other parts of their analyses (like the things mentioned above) the same.
They found that posture determined as much as 83% of how quickly a pill disperses into the intestines. The most efficient position was leaning right. The least was leaning left, which prevented the pill from reaching the antrum, or bottom section of the stomach, and thus kept all but traces of the dissolved drug from entering the duodenum, where the stomach joins the small intestine. (Interestingly, Jews who observe Passover are advised to recline to the left during the meal as a symbol of freedom and leisure.)
That makes sense if you think about the stomach’s shape, which looks kind of like a bean, curving from the left to the right side of the body. Because of gravity, your position will change where the pill lands.
a condition in which the stomach loses the ability to empty properly.
How this could help people
Among the groups most likely to benefit from such studies, Dr. Mittal said, are the elderly – who both take a lot of pills and are more prone to trouble swallowing because of age-related changes in their esophagus – and the bedridden, who can’t easily shift their posture. The findings may also lead to improvements in the ability to treat people with gastroparesis, a particular problem for people with diabetes.
Future studies with Duke and similar simulations will look at how the GI system digests proteins, carbohydrates, and fatty meals, Dr. Mittal said.
In the meantime, Dr. Mittal offered the following advice: “Standing or sitting upright after taking a pill is fine. If you have to take a pill lying down, stay on your back or on your right side. Avoid lying on your left side after taking a pill.”
As for what happened to me, any gastroenterologist reading this has figured out that my condition was not heart-related. Instead, I likely was having a bout of pill esophagitis, irritation that can result from medications that aggravate the mucosa of the food tube. Although painful, esophagitis isn’t life-threatening. After about an hour, the pain began to subside, and by the next morning I was fine, with only a faint ache in my chest to remind me of my earlier torment. (Researchers noted an increase in the condition early in the COVID-19 pandemic, linked to the antibiotic doxycycline.)
And, in the interest of accuracy, my pill problem began above the stomach. Nothing in the Hopkins research suggests that the alignment of the esophagus plays a role in how drugs disperse in the gut – unless, of course, it prevents those pills from reaching the stomach in the first place.
A version of this article first appeared on WebMD.com.
Walking intensity and step count are linked to health benefits
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
FROM JAMA INTERNAL MEDICINE
Two states aim to curb diet pill sales to minors
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Cumulative blood pressure load: A better predictor of CV events?
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Should patients stand for office BP readings?
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
Autoimmune diseases linked to spike in post-MI events
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION














