User login
The ED Sailed Smoothly in the Early COVID-19 Days
TOPLINE:
There were few cases of SARS-CoV-2 infections among emergency department (ED) healthcare personnel and no substantial changes in the delivery of emergency medical care during the initial phase of the COVID-19 pandemic.
METHODOLOGY:
- This multicenter prospective cohort study of US ED healthcare personnel called Project COVERED was conducted from May to December 2020 to evaluate the following outcomes:
- The possibility of infected ED personnel reporting to work
- The burden of COVID-19 symptoms on an ED personnel’s work status
- The association between SARS-CoV-2 infection levels and ED staffing
- Project COVERED enrolled 1673 ED healthcare personnel with 29,825 person weeks of observational data from 25 geographically diverse EDs.
- The presence of any SARS-CoV-2 infection was determined using reverse transcription polymerase chain reaction or IgG antibody testing at baseline, week 2, week 4, and every four subsequent weeks through week 20.
- Investigators also collected weekly data on ED staffing and the incidence of SARS-CoV-2 infections in healthcare facilities.
TAKEAWAY:
- Despite the absence of widespread natural immunity or COVID-19 vaccine availability during the time of this study, only 4.5% of ED healthcare personnel tested positive for SARS-CoV-2 infections, with more than half (57.3%) not experiencing any symptoms.
- Most personnel (83%) who experienced symptoms associated with COVID-19 reported working at least one shift in the ED and nearly all of them continued to work until they received laboratory confirmation of their infection.
- The working time lost as a result of COVID-19 and related concerns was minimal, as 89 healthcare personnel reported 90 person weeks of missed work (0.3% of all weeks).
- During this study, physician-staffing levels ranged from 98.7% to 102.0% of normal staffing, with similar values noted for nursing and nonclinical staffs. Reduced staffing was rare, even during COVID-19 surges.
IN PRACTICE:
“Our findings suggest that the cumulative interaction between infected healthcare personnel and others resulted in a negligible risk of transmission on the scale of public health emergencies,” the authors wrote.
SOURCE:
This study was led by Kurt D. Weber, MD, Department of Emergency Medicine, Orlando Health, Orlando, Florida, and published online in Annals of Emergency Medicine.
LIMITATIONS:
Data regarding the Delta variant surges that occurred toward the end of December and the ED status after the advent of the COVID-19 vaccine were not recorded. There may also have been a selection bias risk in this study because the volunteer participants may have exhibited behaviors like social distancing and use of protective equipment, which may have decreased their risk for infections.
DISCLOSURES:
This study was funded by a cooperative agreement from the Centers for Disease Control and Prevention and the Institute for Clinical and Translational Science at the University of Iowa through a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
There were few cases of SARS-CoV-2 infections among emergency department (ED) healthcare personnel and no substantial changes in the delivery of emergency medical care during the initial phase of the COVID-19 pandemic.
METHODOLOGY:
- This multicenter prospective cohort study of US ED healthcare personnel called Project COVERED was conducted from May to December 2020 to evaluate the following outcomes:
- The possibility of infected ED personnel reporting to work
- The burden of COVID-19 symptoms on an ED personnel’s work status
- The association between SARS-CoV-2 infection levels and ED staffing
- Project COVERED enrolled 1673 ED healthcare personnel with 29,825 person weeks of observational data from 25 geographically diverse EDs.
- The presence of any SARS-CoV-2 infection was determined using reverse transcription polymerase chain reaction or IgG antibody testing at baseline, week 2, week 4, and every four subsequent weeks through week 20.
- Investigators also collected weekly data on ED staffing and the incidence of SARS-CoV-2 infections in healthcare facilities.
TAKEAWAY:
- Despite the absence of widespread natural immunity or COVID-19 vaccine availability during the time of this study, only 4.5% of ED healthcare personnel tested positive for SARS-CoV-2 infections, with more than half (57.3%) not experiencing any symptoms.
- Most personnel (83%) who experienced symptoms associated with COVID-19 reported working at least one shift in the ED and nearly all of them continued to work until they received laboratory confirmation of their infection.
- The working time lost as a result of COVID-19 and related concerns was minimal, as 89 healthcare personnel reported 90 person weeks of missed work (0.3% of all weeks).
- During this study, physician-staffing levels ranged from 98.7% to 102.0% of normal staffing, with similar values noted for nursing and nonclinical staffs. Reduced staffing was rare, even during COVID-19 surges.
IN PRACTICE:
“Our findings suggest that the cumulative interaction between infected healthcare personnel and others resulted in a negligible risk of transmission on the scale of public health emergencies,” the authors wrote.
SOURCE:
This study was led by Kurt D. Weber, MD, Department of Emergency Medicine, Orlando Health, Orlando, Florida, and published online in Annals of Emergency Medicine.
LIMITATIONS:
Data regarding the Delta variant surges that occurred toward the end of December and the ED status after the advent of the COVID-19 vaccine were not recorded. There may also have been a selection bias risk in this study because the volunteer participants may have exhibited behaviors like social distancing and use of protective equipment, which may have decreased their risk for infections.
DISCLOSURES:
This study was funded by a cooperative agreement from the Centers for Disease Control and Prevention and the Institute for Clinical and Translational Science at the University of Iowa through a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
There were few cases of SARS-CoV-2 infections among emergency department (ED) healthcare personnel and no substantial changes in the delivery of emergency medical care during the initial phase of the COVID-19 pandemic.
METHODOLOGY:
- This multicenter prospective cohort study of US ED healthcare personnel called Project COVERED was conducted from May to December 2020 to evaluate the following outcomes:
- The possibility of infected ED personnel reporting to work
- The burden of COVID-19 symptoms on an ED personnel’s work status
- The association between SARS-CoV-2 infection levels and ED staffing
- Project COVERED enrolled 1673 ED healthcare personnel with 29,825 person weeks of observational data from 25 geographically diverse EDs.
- The presence of any SARS-CoV-2 infection was determined using reverse transcription polymerase chain reaction or IgG antibody testing at baseline, week 2, week 4, and every four subsequent weeks through week 20.
- Investigators also collected weekly data on ED staffing and the incidence of SARS-CoV-2 infections in healthcare facilities.
TAKEAWAY:
- Despite the absence of widespread natural immunity or COVID-19 vaccine availability during the time of this study, only 4.5% of ED healthcare personnel tested positive for SARS-CoV-2 infections, with more than half (57.3%) not experiencing any symptoms.
- Most personnel (83%) who experienced symptoms associated with COVID-19 reported working at least one shift in the ED and nearly all of them continued to work until they received laboratory confirmation of their infection.
- The working time lost as a result of COVID-19 and related concerns was minimal, as 89 healthcare personnel reported 90 person weeks of missed work (0.3% of all weeks).
- During this study, physician-staffing levels ranged from 98.7% to 102.0% of normal staffing, with similar values noted for nursing and nonclinical staffs. Reduced staffing was rare, even during COVID-19 surges.
IN PRACTICE:
“Our findings suggest that the cumulative interaction between infected healthcare personnel and others resulted in a negligible risk of transmission on the scale of public health emergencies,” the authors wrote.
SOURCE:
This study was led by Kurt D. Weber, MD, Department of Emergency Medicine, Orlando Health, Orlando, Florida, and published online in Annals of Emergency Medicine.
LIMITATIONS:
Data regarding the Delta variant surges that occurred toward the end of December and the ED status after the advent of the COVID-19 vaccine were not recorded. There may also have been a selection bias risk in this study because the volunteer participants may have exhibited behaviors like social distancing and use of protective equipment, which may have decreased their risk for infections.
DISCLOSURES:
This study was funded by a cooperative agreement from the Centers for Disease Control and Prevention and the Institute for Clinical and Translational Science at the University of Iowa through a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
What We’ve Learned About Remote Learning
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Underlying Mental Illness and Risk of Severe Outcomes Associated With COVID-19
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
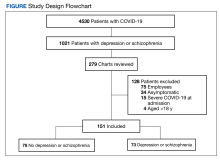
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
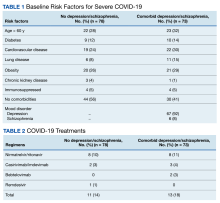
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
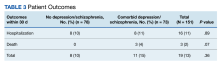
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
No Increased Stroke Risk After COVID-19 Bivalent Vaccine
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
How COVID-19 Treatments Affect Patients With IBD
TOPLINE:
Inflammatory bowel disease (IBD) therapies for patients may need to be briefly halted during treatment for COVID-19, but it does not escalate IBD flares, with prior vaccination for COVID-19 helping reduce complications from the virus.
METHODOLOGY:
- Patients with IBD who receive immunosuppressive agents are at an increased risk of developing severe SARS-CoV-2 infection; however, the effects of COVID-19 vaccination and treatment on the outcomes in patients with IBD are less known.
- Researchers assessed the effect of COVID-19 medications in 127 patients with IBD (age ≥ 18 years; 54% women) who were diagnosed with COVID-19 after the advent of vaccines and release of antiviral therapies.
- Patients were stratified into those who received treatment for COVID-19 (n = 44), defined as the use of antivirals and/or intravenous antibodies, and those who did not receive treatment for COVID-19 (n = 83).
- The primary outcome was the development of a severe SARS-CoV-2 infection (defined by the need for oxygen supplements, corticosteroids and/or antibiotic treatment, or hospitalization).
- The secondary outcomes were the percentage of patients who had their IBD therapy withheld and rates of IBD flare post COVID-19.
TAKEAWAY:
- The likelihood of being treated for COVID-19 was higher in patients on corticosteroids (odds ratio [OR], 4.61; P = .002) or in those undergoing advanced IBD therapies (OR, 2.78; P = .041) prior to infection.
- Advanced age at the time of infection (adjusted OR [aOR], 1.06; P = .018) and corticosteroid treatment prior to contracting COVID-19 (aOR, 9.86; P = .001) were associated with an increased risk for severe infection.
- After adjustment for multiple factors, the likelihood of withholding IBD treatment was higher in patients being treated for COVID-19 (aOR, 6.95; P = .007).
IN PRACTICE:
“Patients with IBD on advanced therapies were frequently treated for acute COVID-19. Although COVID-19 treatment was associated with temporary withholding of IBD therapy, it did not result in increased IBD flares,” the authors wrote.
SOURCE:
The investigation, led by Laura C. Sahyoun, MD, Section of Digestive Diseases, Yale School of Medicine, New Haven, Connecticut, was published online in Digestive Diseases and Sciences.
LIMITATIONS:
Owing to the small sample size, the outcomes comparing antivirals to intravenous antibodies and SARS-CoV-2 strain prevalence could not be assessed. This single-center study also may not reflect the different clinical practices pertaining to IBD and COVID-19 treatments.
DISCLOSURES:
The study did not receive any specific funding. One author reported receiving speaker fees and being part of advisory boards, and another author received research support and reported being a part of advisory boards.
A version of this article appeared on Medscape.com.
TOPLINE:
Inflammatory bowel disease (IBD) therapies for patients may need to be briefly halted during treatment for COVID-19, but it does not escalate IBD flares, with prior vaccination for COVID-19 helping reduce complications from the virus.
METHODOLOGY:
- Patients with IBD who receive immunosuppressive agents are at an increased risk of developing severe SARS-CoV-2 infection; however, the effects of COVID-19 vaccination and treatment on the outcomes in patients with IBD are less known.
- Researchers assessed the effect of COVID-19 medications in 127 patients with IBD (age ≥ 18 years; 54% women) who were diagnosed with COVID-19 after the advent of vaccines and release of antiviral therapies.
- Patients were stratified into those who received treatment for COVID-19 (n = 44), defined as the use of antivirals and/or intravenous antibodies, and those who did not receive treatment for COVID-19 (n = 83).
- The primary outcome was the development of a severe SARS-CoV-2 infection (defined by the need for oxygen supplements, corticosteroids and/or antibiotic treatment, or hospitalization).
- The secondary outcomes were the percentage of patients who had their IBD therapy withheld and rates of IBD flare post COVID-19.
TAKEAWAY:
- The likelihood of being treated for COVID-19 was higher in patients on corticosteroids (odds ratio [OR], 4.61; P = .002) or in those undergoing advanced IBD therapies (OR, 2.78; P = .041) prior to infection.
- Advanced age at the time of infection (adjusted OR [aOR], 1.06; P = .018) and corticosteroid treatment prior to contracting COVID-19 (aOR, 9.86; P = .001) were associated with an increased risk for severe infection.
- After adjustment for multiple factors, the likelihood of withholding IBD treatment was higher in patients being treated for COVID-19 (aOR, 6.95; P = .007).
IN PRACTICE:
“Patients with IBD on advanced therapies were frequently treated for acute COVID-19. Although COVID-19 treatment was associated with temporary withholding of IBD therapy, it did not result in increased IBD flares,” the authors wrote.
SOURCE:
The investigation, led by Laura C. Sahyoun, MD, Section of Digestive Diseases, Yale School of Medicine, New Haven, Connecticut, was published online in Digestive Diseases and Sciences.
LIMITATIONS:
Owing to the small sample size, the outcomes comparing antivirals to intravenous antibodies and SARS-CoV-2 strain prevalence could not be assessed. This single-center study also may not reflect the different clinical practices pertaining to IBD and COVID-19 treatments.
DISCLOSURES:
The study did not receive any specific funding. One author reported receiving speaker fees and being part of advisory boards, and another author received research support and reported being a part of advisory boards.
A version of this article appeared on Medscape.com.
TOPLINE:
Inflammatory bowel disease (IBD) therapies for patients may need to be briefly halted during treatment for COVID-19, but it does not escalate IBD flares, with prior vaccination for COVID-19 helping reduce complications from the virus.
METHODOLOGY:
- Patients with IBD who receive immunosuppressive agents are at an increased risk of developing severe SARS-CoV-2 infection; however, the effects of COVID-19 vaccination and treatment on the outcomes in patients with IBD are less known.
- Researchers assessed the effect of COVID-19 medications in 127 patients with IBD (age ≥ 18 years; 54% women) who were diagnosed with COVID-19 after the advent of vaccines and release of antiviral therapies.
- Patients were stratified into those who received treatment for COVID-19 (n = 44), defined as the use of antivirals and/or intravenous antibodies, and those who did not receive treatment for COVID-19 (n = 83).
- The primary outcome was the development of a severe SARS-CoV-2 infection (defined by the need for oxygen supplements, corticosteroids and/or antibiotic treatment, or hospitalization).
- The secondary outcomes were the percentage of patients who had their IBD therapy withheld and rates of IBD flare post COVID-19.
TAKEAWAY:
- The likelihood of being treated for COVID-19 was higher in patients on corticosteroids (odds ratio [OR], 4.61; P = .002) or in those undergoing advanced IBD therapies (OR, 2.78; P = .041) prior to infection.
- Advanced age at the time of infection (adjusted OR [aOR], 1.06; P = .018) and corticosteroid treatment prior to contracting COVID-19 (aOR, 9.86; P = .001) were associated with an increased risk for severe infection.
- After adjustment for multiple factors, the likelihood of withholding IBD treatment was higher in patients being treated for COVID-19 (aOR, 6.95; P = .007).
IN PRACTICE:
“Patients with IBD on advanced therapies were frequently treated for acute COVID-19. Although COVID-19 treatment was associated with temporary withholding of IBD therapy, it did not result in increased IBD flares,” the authors wrote.
SOURCE:
The investigation, led by Laura C. Sahyoun, MD, Section of Digestive Diseases, Yale School of Medicine, New Haven, Connecticut, was published online in Digestive Diseases and Sciences.
LIMITATIONS:
Owing to the small sample size, the outcomes comparing antivirals to intravenous antibodies and SARS-CoV-2 strain prevalence could not be assessed. This single-center study also may not reflect the different clinical practices pertaining to IBD and COVID-19 treatments.
DISCLOSURES:
The study did not receive any specific funding. One author reported receiving speaker fees and being part of advisory boards, and another author received research support and reported being a part of advisory boards.
A version of this article appeared on Medscape.com.
Severe Flu Confers Higher Risk for Neurologic Disorders Versus COVID
TOPLINE:
, results of a large study show.
METHODOLOGY:
- Researchers used healthcare claims data to compare 77,300 people hospitalized with COVID-19 with 77,300 hospitalized with influenza. The study did not include individuals with long COVID.
- In the final sample of 154,500 participants, the mean age was 51 years, and more than half (58%) were female.
- Investigators followed participants from both cohorts for a year to find out how many of them had medical care for six of the most common neurologic disorders: migraine, epilepsy, stroke, neuropathy, movement disorders, and dementia.
- If participants had one of these neurologic disorders prior to the original hospitalization, the primary outcome involved subsequent healthcare encounters for the neurologic diagnosis.
TAKEAWAY:
- Participants hospitalized with COVID-19 versus influenza were significantly less likely to require care in the following year for migraine (2% vs 3.2%), epilepsy (1.6% vs 2.1%), neuropathy (1.9% vs 3.6%), movement disorders (1.5% vs 2.5%), stroke (2% vs 2.4%), and dementia (2% vs 2.3%) (all P < .001).
- After adjusting for age, sex, and other health conditions, researchers found that people hospitalized with COVID-19 had a 35% lower risk of receiving care for migraine, a 22% lower risk of receiving care for epilepsy, and a 44% lower risk of receiving care for neuropathy than those with influenza. They also had a 36% lower risk of receiving care for movement disorders, a 10% lower risk for stroke (all P < .001), as well as a 7% lower risk for dementia (P = .0007).
- In participants who did not have a preexisting neurologic condition at the time of hospitalization for either COVID-19 or influenza, 2.8% hospitalized with COVID-19 developed one in the next year compared with 5% of those hospitalized with influenza.
IN PRACTICE:
“While the results were not what we expected to find, they are reassuring in that we found being hospitalized with COVID did not lead to more care for common neurologic conditions when compared to being hospitalized with influenza,” study investigator Brian C. Callaghan, MD, of University of Michigan, Ann Arbor, said in a press release.
SOURCE:
Adam de Havenon, MD, of Yale University in New Haven, Connecticut, led the study, which was published online on March 20 in Neurology.
LIMITATIONS:
The study relied on ICD codes in health claims databases, which could introduce misclassification bias. Also, by selecting only individuals who had associated hospital-based care, there may have been a selection bias based on disease severity.
DISCLOSURES:
The study was funded by the American Academy of Neurology. Dr. De Havenon reported receiving consultant fees from Integra and Novo Nordisk and royalty fees from UpToDate and has equity in Titin KM and Certus. Dr. Callaghan has consulted for DynaMed and the Vaccine Injury Compensation Program. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
, results of a large study show.
METHODOLOGY:
- Researchers used healthcare claims data to compare 77,300 people hospitalized with COVID-19 with 77,300 hospitalized with influenza. The study did not include individuals with long COVID.
- In the final sample of 154,500 participants, the mean age was 51 years, and more than half (58%) were female.
- Investigators followed participants from both cohorts for a year to find out how many of them had medical care for six of the most common neurologic disorders: migraine, epilepsy, stroke, neuropathy, movement disorders, and dementia.
- If participants had one of these neurologic disorders prior to the original hospitalization, the primary outcome involved subsequent healthcare encounters for the neurologic diagnosis.
TAKEAWAY:
- Participants hospitalized with COVID-19 versus influenza were significantly less likely to require care in the following year for migraine (2% vs 3.2%), epilepsy (1.6% vs 2.1%), neuropathy (1.9% vs 3.6%), movement disorders (1.5% vs 2.5%), stroke (2% vs 2.4%), and dementia (2% vs 2.3%) (all P < .001).
- After adjusting for age, sex, and other health conditions, researchers found that people hospitalized with COVID-19 had a 35% lower risk of receiving care for migraine, a 22% lower risk of receiving care for epilepsy, and a 44% lower risk of receiving care for neuropathy than those with influenza. They also had a 36% lower risk of receiving care for movement disorders, a 10% lower risk for stroke (all P < .001), as well as a 7% lower risk for dementia (P = .0007).
- In participants who did not have a preexisting neurologic condition at the time of hospitalization for either COVID-19 or influenza, 2.8% hospitalized with COVID-19 developed one in the next year compared with 5% of those hospitalized with influenza.
IN PRACTICE:
“While the results were not what we expected to find, they are reassuring in that we found being hospitalized with COVID did not lead to more care for common neurologic conditions when compared to being hospitalized with influenza,” study investigator Brian C. Callaghan, MD, of University of Michigan, Ann Arbor, said in a press release.
SOURCE:
Adam de Havenon, MD, of Yale University in New Haven, Connecticut, led the study, which was published online on March 20 in Neurology.
LIMITATIONS:
The study relied on ICD codes in health claims databases, which could introduce misclassification bias. Also, by selecting only individuals who had associated hospital-based care, there may have been a selection bias based on disease severity.
DISCLOSURES:
The study was funded by the American Academy of Neurology. Dr. De Havenon reported receiving consultant fees from Integra and Novo Nordisk and royalty fees from UpToDate and has equity in Titin KM and Certus. Dr. Callaghan has consulted for DynaMed and the Vaccine Injury Compensation Program. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
, results of a large study show.
METHODOLOGY:
- Researchers used healthcare claims data to compare 77,300 people hospitalized with COVID-19 with 77,300 hospitalized with influenza. The study did not include individuals with long COVID.
- In the final sample of 154,500 participants, the mean age was 51 years, and more than half (58%) were female.
- Investigators followed participants from both cohorts for a year to find out how many of them had medical care for six of the most common neurologic disorders: migraine, epilepsy, stroke, neuropathy, movement disorders, and dementia.
- If participants had one of these neurologic disorders prior to the original hospitalization, the primary outcome involved subsequent healthcare encounters for the neurologic diagnosis.
TAKEAWAY:
- Participants hospitalized with COVID-19 versus influenza were significantly less likely to require care in the following year for migraine (2% vs 3.2%), epilepsy (1.6% vs 2.1%), neuropathy (1.9% vs 3.6%), movement disorders (1.5% vs 2.5%), stroke (2% vs 2.4%), and dementia (2% vs 2.3%) (all P < .001).
- After adjusting for age, sex, and other health conditions, researchers found that people hospitalized with COVID-19 had a 35% lower risk of receiving care for migraine, a 22% lower risk of receiving care for epilepsy, and a 44% lower risk of receiving care for neuropathy than those with influenza. They also had a 36% lower risk of receiving care for movement disorders, a 10% lower risk for stroke (all P < .001), as well as a 7% lower risk for dementia (P = .0007).
- In participants who did not have a preexisting neurologic condition at the time of hospitalization for either COVID-19 or influenza, 2.8% hospitalized with COVID-19 developed one in the next year compared with 5% of those hospitalized with influenza.
IN PRACTICE:
“While the results were not what we expected to find, they are reassuring in that we found being hospitalized with COVID did not lead to more care for common neurologic conditions when compared to being hospitalized with influenza,” study investigator Brian C. Callaghan, MD, of University of Michigan, Ann Arbor, said in a press release.
SOURCE:
Adam de Havenon, MD, of Yale University in New Haven, Connecticut, led the study, which was published online on March 20 in Neurology.
LIMITATIONS:
The study relied on ICD codes in health claims databases, which could introduce misclassification bias. Also, by selecting only individuals who had associated hospital-based care, there may have been a selection bias based on disease severity.
DISCLOSURES:
The study was funded by the American Academy of Neurology. Dr. De Havenon reported receiving consultant fees from Integra and Novo Nordisk and royalty fees from UpToDate and has equity in Titin KM and Certus. Dr. Callaghan has consulted for DynaMed and the Vaccine Injury Compensation Program. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
Getting Reluctant Patients to ‘Yes’ on COVID Vaccination
No matter how much we’d like to leave it in the dust, COVID-19 remains prevalent and potent. Tens of thousands of people still contract COVID per week in the United States. Hundreds die. And those who don’t may still develop long COVID.
Pleas from public health officials for people to get a COVID vaccine or booster shot have been ignored by many people. About 80% of eligible Americans haven’t taken any kind of COVID booster. Meantime, the virus continues to mutate, eroding the efficacy of the vaccine’s past versions.
How to get more people to get the jab? Vaccine hesitancy, said infectious disease specialist William Schaffner, MD, is likely rooted in a lack of trust in authority, whether it’s public health officials or politicians.
Dr. Schaffner, professor of infectious diseases at the Vanderbilt University School of Medicine, Nashville, Tennessee, and former medical director of the National Foundation for Infectious Diseases, recommended five strategies physicians can try when discussing the importance of staying up to date on COVID vaccines with patients.
#1: Be Patient With Your Patient
First and foremost, if doctors are feeling reluctance from their patients, they need to know “what they shouldn’t do,” Dr. Schaffner said.
When a patient initially doesn’t want the vaccine, doctors shouldn’t express surprise. “Do not scold or berate or belittle. Do not give the impression the patient is somehow wrong or has failed a test of some sort,” Dr. Schaffner said.
Step back and affirm that they understand what the patient is saying so they feel reassured, even if they don’t agree or it’s based on falsehoods about the vaccine.
He said patients need to feel “the doctor heard them; it’s okay to tell the doctor this.” When you affirm what the patient says, it puts them at ease and provides a smoother road to eventually getting them to say “yes.”
But if there’s still a roadblock, don’t bulldoze them. “You don’t want to punish the patient ... let them know you’ll continue to hear them,” Dr. Schaffner said.
#2: Always Acknowledge a Concern
Fear of side effects is great among some patients, even if the risks are low, Dr. Schaffner said. Patients may be hesitant because they’re afraid they’ll become one of the “two or three in a million” who suffer extremely rare side effects from the vaccine, Dr. Schaffner said.
In that case, doctors should acknowledge their concern is valid, he said. Never be dismissive. Ask the patients how they feel about the vaccine, listen to their responses, and let them know “I hear you. This is a new mRNA vaccine…you have concern about that,” Dr. Schaffner said.
Doctors can segue into how there’s little reason to wait for some elusive perfectly risk-free vaccine when they can help themselves right now.
“The adverse events that occur with vaccines occur within 2 months [and are typically mild]. I don’t know of a single vaccine that has genuinely long-term implications,” Dr. Schaffner said. “We should remember that old French philosopher Voltaire. He admonished us: Waiting for perfection is the great enemy of the current good.”
#3: Make a Strong Recommendation
Here’s something that may seem obvious: Don’t treat the vaccine as an afterthought. “Survey after survey tells us this ... it has everything to do with the strength of the recommendation,” Dr. Schaffner said.
Doctors typically make strong treatment recommendations such conditions as diabetes or high blood pressure, but “when it comes to vaccines, they’re often rather nonchalant,” he said.
If a patient is eligible for a vaccine, doctors should tell the patient they need to get it — not that you think they should get it. “Doctors have to make a firm recommendation: ‘You’re eligible for a vaccine ... and you need to get it ... you’ll receive it on your way out.’ It then becomes a distinct and strong recommendation,” he said.
#4: Appeal to Patients’ Hearts, Not Their Minds
In the opening of Charles Dickens’s novel “Hard Times,” the stern school superintendent, Mr. Gradgrind, scolds his students by beating their brow with the notion that, “Facts alone are wanted in life. Plant nothing else and root out everything else.”
The idea that facts alone can sway a vaccine-resistant patient is wrong. “It often doesn’t happen that way,” Dr. Schaffner said. “I don’t think facts do that. Psychologists tell us, yes, information is important, but it’s rarely sufficient to change behavior.”
Data and studies are foundational to medicine, but the key is to change how a patient feels about the data they’re presented with, not how they think about it. “Don’t attack their brain so much but their heart,” Dr. Schaffner said.
Dr. Schaffner has stressed with his patients that the COVID vaccine has become “the social norm,” suggesting virtually everyone he knows has received it and had no problem.
Once questions have been answered about whether the vaccine works and its various side effects, doctors could remind the patient, “You know, everyone in my office is getting the vaccine, and we’re trying to provide this protection to every patient,” he said.
You’re then delving deeper into their emotions and crossing a barrier that facts alone can’t breach.
#5: Make it Personal
Lead by example and personalize the fight against the virus. This allows doctors to act as if they’re building an alliance with their patients by framing the vaccine not as something that only affects them but can also confer benefits to a broader social circle.
Even after using these methods, patients may remain resistant, apprehensive, or even indifferent. In cases like these, Dr. Schaffner said it’s a good idea to let it go for the time being.
Let the patient know they “have access to you and can keep speaking with you about it” in the future, he said. “It takes more time, and you have to be cognizant of the nature of the conversation.”
Everybody is unique, but with trust, patience, and awareness of the patient’s feelings, doctors have a better shot at finding common ground with their patients and convincing them the vaccine is in their best interest, he said.
A version of this article first appeared on Medscape.com.
No matter how much we’d like to leave it in the dust, COVID-19 remains prevalent and potent. Tens of thousands of people still contract COVID per week in the United States. Hundreds die. And those who don’t may still develop long COVID.
Pleas from public health officials for people to get a COVID vaccine or booster shot have been ignored by many people. About 80% of eligible Americans haven’t taken any kind of COVID booster. Meantime, the virus continues to mutate, eroding the efficacy of the vaccine’s past versions.
How to get more people to get the jab? Vaccine hesitancy, said infectious disease specialist William Schaffner, MD, is likely rooted in a lack of trust in authority, whether it’s public health officials or politicians.
Dr. Schaffner, professor of infectious diseases at the Vanderbilt University School of Medicine, Nashville, Tennessee, and former medical director of the National Foundation for Infectious Diseases, recommended five strategies physicians can try when discussing the importance of staying up to date on COVID vaccines with patients.
#1: Be Patient With Your Patient
First and foremost, if doctors are feeling reluctance from their patients, they need to know “what they shouldn’t do,” Dr. Schaffner said.
When a patient initially doesn’t want the vaccine, doctors shouldn’t express surprise. “Do not scold or berate or belittle. Do not give the impression the patient is somehow wrong or has failed a test of some sort,” Dr. Schaffner said.
Step back and affirm that they understand what the patient is saying so they feel reassured, even if they don’t agree or it’s based on falsehoods about the vaccine.
He said patients need to feel “the doctor heard them; it’s okay to tell the doctor this.” When you affirm what the patient says, it puts them at ease and provides a smoother road to eventually getting them to say “yes.”
But if there’s still a roadblock, don’t bulldoze them. “You don’t want to punish the patient ... let them know you’ll continue to hear them,” Dr. Schaffner said.
#2: Always Acknowledge a Concern
Fear of side effects is great among some patients, even if the risks are low, Dr. Schaffner said. Patients may be hesitant because they’re afraid they’ll become one of the “two or three in a million” who suffer extremely rare side effects from the vaccine, Dr. Schaffner said.
In that case, doctors should acknowledge their concern is valid, he said. Never be dismissive. Ask the patients how they feel about the vaccine, listen to their responses, and let them know “I hear you. This is a new mRNA vaccine…you have concern about that,” Dr. Schaffner said.
Doctors can segue into how there’s little reason to wait for some elusive perfectly risk-free vaccine when they can help themselves right now.
“The adverse events that occur with vaccines occur within 2 months [and are typically mild]. I don’t know of a single vaccine that has genuinely long-term implications,” Dr. Schaffner said. “We should remember that old French philosopher Voltaire. He admonished us: Waiting for perfection is the great enemy of the current good.”
#3: Make a Strong Recommendation
Here’s something that may seem obvious: Don’t treat the vaccine as an afterthought. “Survey after survey tells us this ... it has everything to do with the strength of the recommendation,” Dr. Schaffner said.
Doctors typically make strong treatment recommendations such conditions as diabetes or high blood pressure, but “when it comes to vaccines, they’re often rather nonchalant,” he said.
If a patient is eligible for a vaccine, doctors should tell the patient they need to get it — not that you think they should get it. “Doctors have to make a firm recommendation: ‘You’re eligible for a vaccine ... and you need to get it ... you’ll receive it on your way out.’ It then becomes a distinct and strong recommendation,” he said.
#4: Appeal to Patients’ Hearts, Not Their Minds
In the opening of Charles Dickens’s novel “Hard Times,” the stern school superintendent, Mr. Gradgrind, scolds his students by beating their brow with the notion that, “Facts alone are wanted in life. Plant nothing else and root out everything else.”
The idea that facts alone can sway a vaccine-resistant patient is wrong. “It often doesn’t happen that way,” Dr. Schaffner said. “I don’t think facts do that. Psychologists tell us, yes, information is important, but it’s rarely sufficient to change behavior.”
Data and studies are foundational to medicine, but the key is to change how a patient feels about the data they’re presented with, not how they think about it. “Don’t attack their brain so much but their heart,” Dr. Schaffner said.
Dr. Schaffner has stressed with his patients that the COVID vaccine has become “the social norm,” suggesting virtually everyone he knows has received it and had no problem.
Once questions have been answered about whether the vaccine works and its various side effects, doctors could remind the patient, “You know, everyone in my office is getting the vaccine, and we’re trying to provide this protection to every patient,” he said.
You’re then delving deeper into their emotions and crossing a barrier that facts alone can’t breach.
#5: Make it Personal
Lead by example and personalize the fight against the virus. This allows doctors to act as if they’re building an alliance with their patients by framing the vaccine not as something that only affects them but can also confer benefits to a broader social circle.
Even after using these methods, patients may remain resistant, apprehensive, or even indifferent. In cases like these, Dr. Schaffner said it’s a good idea to let it go for the time being.
Let the patient know they “have access to you and can keep speaking with you about it” in the future, he said. “It takes more time, and you have to be cognizant of the nature of the conversation.”
Everybody is unique, but with trust, patience, and awareness of the patient’s feelings, doctors have a better shot at finding common ground with their patients and convincing them the vaccine is in their best interest, he said.
A version of this article first appeared on Medscape.com.
No matter how much we’d like to leave it in the dust, COVID-19 remains prevalent and potent. Tens of thousands of people still contract COVID per week in the United States. Hundreds die. And those who don’t may still develop long COVID.
Pleas from public health officials for people to get a COVID vaccine or booster shot have been ignored by many people. About 80% of eligible Americans haven’t taken any kind of COVID booster. Meantime, the virus continues to mutate, eroding the efficacy of the vaccine’s past versions.
How to get more people to get the jab? Vaccine hesitancy, said infectious disease specialist William Schaffner, MD, is likely rooted in a lack of trust in authority, whether it’s public health officials or politicians.
Dr. Schaffner, professor of infectious diseases at the Vanderbilt University School of Medicine, Nashville, Tennessee, and former medical director of the National Foundation for Infectious Diseases, recommended five strategies physicians can try when discussing the importance of staying up to date on COVID vaccines with patients.
#1: Be Patient With Your Patient
First and foremost, if doctors are feeling reluctance from their patients, they need to know “what they shouldn’t do,” Dr. Schaffner said.
When a patient initially doesn’t want the vaccine, doctors shouldn’t express surprise. “Do not scold or berate or belittle. Do not give the impression the patient is somehow wrong or has failed a test of some sort,” Dr. Schaffner said.
Step back and affirm that they understand what the patient is saying so they feel reassured, even if they don’t agree or it’s based on falsehoods about the vaccine.
He said patients need to feel “the doctor heard them; it’s okay to tell the doctor this.” When you affirm what the patient says, it puts them at ease and provides a smoother road to eventually getting them to say “yes.”
But if there’s still a roadblock, don’t bulldoze them. “You don’t want to punish the patient ... let them know you’ll continue to hear them,” Dr. Schaffner said.
#2: Always Acknowledge a Concern
Fear of side effects is great among some patients, even if the risks are low, Dr. Schaffner said. Patients may be hesitant because they’re afraid they’ll become one of the “two or three in a million” who suffer extremely rare side effects from the vaccine, Dr. Schaffner said.
In that case, doctors should acknowledge their concern is valid, he said. Never be dismissive. Ask the patients how they feel about the vaccine, listen to their responses, and let them know “I hear you. This is a new mRNA vaccine…you have concern about that,” Dr. Schaffner said.
Doctors can segue into how there’s little reason to wait for some elusive perfectly risk-free vaccine when they can help themselves right now.
“The adverse events that occur with vaccines occur within 2 months [and are typically mild]. I don’t know of a single vaccine that has genuinely long-term implications,” Dr. Schaffner said. “We should remember that old French philosopher Voltaire. He admonished us: Waiting for perfection is the great enemy of the current good.”
#3: Make a Strong Recommendation
Here’s something that may seem obvious: Don’t treat the vaccine as an afterthought. “Survey after survey tells us this ... it has everything to do with the strength of the recommendation,” Dr. Schaffner said.
Doctors typically make strong treatment recommendations such conditions as diabetes or high blood pressure, but “when it comes to vaccines, they’re often rather nonchalant,” he said.
If a patient is eligible for a vaccine, doctors should tell the patient they need to get it — not that you think they should get it. “Doctors have to make a firm recommendation: ‘You’re eligible for a vaccine ... and you need to get it ... you’ll receive it on your way out.’ It then becomes a distinct and strong recommendation,” he said.
#4: Appeal to Patients’ Hearts, Not Their Minds
In the opening of Charles Dickens’s novel “Hard Times,” the stern school superintendent, Mr. Gradgrind, scolds his students by beating their brow with the notion that, “Facts alone are wanted in life. Plant nothing else and root out everything else.”
The idea that facts alone can sway a vaccine-resistant patient is wrong. “It often doesn’t happen that way,” Dr. Schaffner said. “I don’t think facts do that. Psychologists tell us, yes, information is important, but it’s rarely sufficient to change behavior.”
Data and studies are foundational to medicine, but the key is to change how a patient feels about the data they’re presented with, not how they think about it. “Don’t attack their brain so much but their heart,” Dr. Schaffner said.
Dr. Schaffner has stressed with his patients that the COVID vaccine has become “the social norm,” suggesting virtually everyone he knows has received it and had no problem.
Once questions have been answered about whether the vaccine works and its various side effects, doctors could remind the patient, “You know, everyone in my office is getting the vaccine, and we’re trying to provide this protection to every patient,” he said.
You’re then delving deeper into their emotions and crossing a barrier that facts alone can’t breach.
#5: Make it Personal
Lead by example and personalize the fight against the virus. This allows doctors to act as if they’re building an alliance with their patients by framing the vaccine not as something that only affects them but can also confer benefits to a broader social circle.
Even after using these methods, patients may remain resistant, apprehensive, or even indifferent. In cases like these, Dr. Schaffner said it’s a good idea to let it go for the time being.
Let the patient know they “have access to you and can keep speaking with you about it” in the future, he said. “It takes more time, and you have to be cognizant of the nature of the conversation.”
Everybody is unique, but with trust, patience, and awareness of the patient’s feelings, doctors have a better shot at finding common ground with their patients and convincing them the vaccine is in their best interest, he said.
A version of this article first appeared on Medscape.com.
COVID Levels Decline, but Other Viruses Remain High
COVID-19 may be headed toward a springtime retreat.
The indication comes from declining levels of SARS-CoV-2 being detected in wastewater over the past 3 weeks. Virus levels are already considered “low” throughout western U.S. states. Detections are at medium levels in the Midwest and South, while high levels persist in the Northeast, according to WastewaterSCAN.
But it’s not time to let your guard down because high levels of other viruses that cause stomach and respiratory illnesses continue to circulate widely nationwide. Wastewater data currently shows threats from flu, RSV, norovirus, and rotavirus.
The rate of positive flu tests reported to the CDC had been a downward trend since peaking around a rate of 16% in mid-January, but positive test rates are now climbing again, with the most recent weekly rate back around 15%. So far this flu season, 116 children and an estimated 20,000 adults have died from the flu, according to the CDC’s weekly flu publication, FluView.
RSV wastewater detection remains high, especially in the Midwest and Northeast, WastewaterSCAN data shows. But positive RSV test results reported to the CDC are at the lowest point of the 2023 to 2024 season, with less than 2,000 positive results listed for the week of March 9, down from a peak of more than 14,000 cases around Christmas.
About 12% of norovirus tests reported to the CDC in the last 3 weeks of February were positive, mirroring an upward trend observed during the same time period last year. In 2023, norovirus peaked in the U.S. in March with a positive test rate around 16%, CDC data show.
Last year, COVID also followed a downward springtime trend. Around this time last year, there were about 20,000 weekly hospital admissions due to COVID-19, compared to just over 13,000 in early March this year. All COVID metrics, including the positive test rate, hospitalizations, and ER visits, are currently trending downward, the CDC’s COVID Data Tracker indicates. The positive COVID test rate is 5%, and just 1% of ER visits in the U.S. involve a COVID-19 diagnosis.
“We’re seeing a downward trend, which is fantastic,” Marlene Wolfe, PhD, WastewaterSCAN’s program director, told USA Today. “Hopefully, that pattern continues as we enjoy some warmer weather and longer daylight.”
A version of this article appeared on WebMD.com.
COVID-19 may be headed toward a springtime retreat.
The indication comes from declining levels of SARS-CoV-2 being detected in wastewater over the past 3 weeks. Virus levels are already considered “low” throughout western U.S. states. Detections are at medium levels in the Midwest and South, while high levels persist in the Northeast, according to WastewaterSCAN.
But it’s not time to let your guard down because high levels of other viruses that cause stomach and respiratory illnesses continue to circulate widely nationwide. Wastewater data currently shows threats from flu, RSV, norovirus, and rotavirus.
The rate of positive flu tests reported to the CDC had been a downward trend since peaking around a rate of 16% in mid-January, but positive test rates are now climbing again, with the most recent weekly rate back around 15%. So far this flu season, 116 children and an estimated 20,000 adults have died from the flu, according to the CDC’s weekly flu publication, FluView.
RSV wastewater detection remains high, especially in the Midwest and Northeast, WastewaterSCAN data shows. But positive RSV test results reported to the CDC are at the lowest point of the 2023 to 2024 season, with less than 2,000 positive results listed for the week of March 9, down from a peak of more than 14,000 cases around Christmas.
About 12% of norovirus tests reported to the CDC in the last 3 weeks of February were positive, mirroring an upward trend observed during the same time period last year. In 2023, norovirus peaked in the U.S. in March with a positive test rate around 16%, CDC data show.
Last year, COVID also followed a downward springtime trend. Around this time last year, there were about 20,000 weekly hospital admissions due to COVID-19, compared to just over 13,000 in early March this year. All COVID metrics, including the positive test rate, hospitalizations, and ER visits, are currently trending downward, the CDC’s COVID Data Tracker indicates. The positive COVID test rate is 5%, and just 1% of ER visits in the U.S. involve a COVID-19 diagnosis.
“We’re seeing a downward trend, which is fantastic,” Marlene Wolfe, PhD, WastewaterSCAN’s program director, told USA Today. “Hopefully, that pattern continues as we enjoy some warmer weather and longer daylight.”
A version of this article appeared on WebMD.com.
COVID-19 may be headed toward a springtime retreat.
The indication comes from declining levels of SARS-CoV-2 being detected in wastewater over the past 3 weeks. Virus levels are already considered “low” throughout western U.S. states. Detections are at medium levels in the Midwest and South, while high levels persist in the Northeast, according to WastewaterSCAN.
But it’s not time to let your guard down because high levels of other viruses that cause stomach and respiratory illnesses continue to circulate widely nationwide. Wastewater data currently shows threats from flu, RSV, norovirus, and rotavirus.
The rate of positive flu tests reported to the CDC had been a downward trend since peaking around a rate of 16% in mid-January, but positive test rates are now climbing again, with the most recent weekly rate back around 15%. So far this flu season, 116 children and an estimated 20,000 adults have died from the flu, according to the CDC’s weekly flu publication, FluView.
RSV wastewater detection remains high, especially in the Midwest and Northeast, WastewaterSCAN data shows. But positive RSV test results reported to the CDC are at the lowest point of the 2023 to 2024 season, with less than 2,000 positive results listed for the week of March 9, down from a peak of more than 14,000 cases around Christmas.
About 12% of norovirus tests reported to the CDC in the last 3 weeks of February were positive, mirroring an upward trend observed during the same time period last year. In 2023, norovirus peaked in the U.S. in March with a positive test rate around 16%, CDC data show.
Last year, COVID also followed a downward springtime trend. Around this time last year, there were about 20,000 weekly hospital admissions due to COVID-19, compared to just over 13,000 in early March this year. All COVID metrics, including the positive test rate, hospitalizations, and ER visits, are currently trending downward, the CDC’s COVID Data Tracker indicates. The positive COVID test rate is 5%, and just 1% of ER visits in the U.S. involve a COVID-19 diagnosis.
“We’re seeing a downward trend, which is fantastic,” Marlene Wolfe, PhD, WastewaterSCAN’s program director, told USA Today. “Hopefully, that pattern continues as we enjoy some warmer weather and longer daylight.”
A version of this article appeared on WebMD.com.
Immunomodulators Do Not Affect COVID-19 Vaccine Efficacy
TOPLINE:
The results of a recent study suggest that biologics and small molecule inhibitors (SMIs) do not impair the protective effect of COVID-19 vaccine against hospitalization in patients with psoriasis and hidradenitis suppurativa (HS).
METHODOLOGY:
- It remains unknown whether immunomodulatory therapies impair COVID-19 vaccine efficacy and increase hospitalization rates linked to COVID-19 in patients with inflammatory skin conditions such as psoriasis or HS.
- Researchers conducted a cross-sectional study using data from the Epic Cosmos database from January 2020 to October 2023, identifying 30,845 patients with psoriasis or HS.
- Overall, 22,293 patients with documented completion of their primary COVID-19 vaccine series were included in the analysis.
- Of the vaccinated patients, they compared 7046 patients with psoriasis on SMIs and 2033 with psoriasis or HS on biologics with 13,214 patients who did not receive biologics or SMIs.
- The primary outcome was the COVID-19 hospitalization rate.
- Treatment with biologics did not increase COVID-19-related hospitalization rates in vaccinated patients with psoriasis or HS (hospitalization rate, 6.0% for both those taking and those not taking a biologic; P > .99).
- Similarly, hospitalization rates did not significantly differ between vaccinated patients who received SMIs vs those who did not (7.1% vs 6.0%; P = .0596).
IN PRACTICE:
These findings “encourage dermatologists to continue treating [psoriasis]/HS confidently despite the ongoing COVID-19 pandemic,” the authors concluded.
SOURCE:
The study led by Bella R. Lee from Ohio State University Wexner Medical Center, Columbus, was published online on March 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Multivariable adjustments could not be performed in this study due to unavailability of individual-level data, and hospital admissions that occurred outside the Epic system were not captured.
DISCLOSURES:
The study did not receive any funding. All authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
The results of a recent study suggest that biologics and small molecule inhibitors (SMIs) do not impair the protective effect of COVID-19 vaccine against hospitalization in patients with psoriasis and hidradenitis suppurativa (HS).
METHODOLOGY:
- It remains unknown whether immunomodulatory therapies impair COVID-19 vaccine efficacy and increase hospitalization rates linked to COVID-19 in patients with inflammatory skin conditions such as psoriasis or HS.
- Researchers conducted a cross-sectional study using data from the Epic Cosmos database from January 2020 to October 2023, identifying 30,845 patients with psoriasis or HS.
- Overall, 22,293 patients with documented completion of their primary COVID-19 vaccine series were included in the analysis.
- Of the vaccinated patients, they compared 7046 patients with psoriasis on SMIs and 2033 with psoriasis or HS on biologics with 13,214 patients who did not receive biologics or SMIs.
- The primary outcome was the COVID-19 hospitalization rate.
- Treatment with biologics did not increase COVID-19-related hospitalization rates in vaccinated patients with psoriasis or HS (hospitalization rate, 6.0% for both those taking and those not taking a biologic; P > .99).
- Similarly, hospitalization rates did not significantly differ between vaccinated patients who received SMIs vs those who did not (7.1% vs 6.0%; P = .0596).
IN PRACTICE:
These findings “encourage dermatologists to continue treating [psoriasis]/HS confidently despite the ongoing COVID-19 pandemic,” the authors concluded.
SOURCE:
The study led by Bella R. Lee from Ohio State University Wexner Medical Center, Columbus, was published online on March 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Multivariable adjustments could not be performed in this study due to unavailability of individual-level data, and hospital admissions that occurred outside the Epic system were not captured.
DISCLOSURES:
The study did not receive any funding. All authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
The results of a recent study suggest that biologics and small molecule inhibitors (SMIs) do not impair the protective effect of COVID-19 vaccine against hospitalization in patients with psoriasis and hidradenitis suppurativa (HS).
METHODOLOGY:
- It remains unknown whether immunomodulatory therapies impair COVID-19 vaccine efficacy and increase hospitalization rates linked to COVID-19 in patients with inflammatory skin conditions such as psoriasis or HS.
- Researchers conducted a cross-sectional study using data from the Epic Cosmos database from January 2020 to October 2023, identifying 30,845 patients with psoriasis or HS.
- Overall, 22,293 patients with documented completion of their primary COVID-19 vaccine series were included in the analysis.
- Of the vaccinated patients, they compared 7046 patients with psoriasis on SMIs and 2033 with psoriasis or HS on biologics with 13,214 patients who did not receive biologics or SMIs.
- The primary outcome was the COVID-19 hospitalization rate.
- Treatment with biologics did not increase COVID-19-related hospitalization rates in vaccinated patients with psoriasis or HS (hospitalization rate, 6.0% for both those taking and those not taking a biologic; P > .99).
- Similarly, hospitalization rates did not significantly differ between vaccinated patients who received SMIs vs those who did not (7.1% vs 6.0%; P = .0596).
IN PRACTICE:
These findings “encourage dermatologists to continue treating [psoriasis]/HS confidently despite the ongoing COVID-19 pandemic,” the authors concluded.
SOURCE:
The study led by Bella R. Lee from Ohio State University Wexner Medical Center, Columbus, was published online on March 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Multivariable adjustments could not be performed in this study due to unavailability of individual-level data, and hospital admissions that occurred outside the Epic system were not captured.
DISCLOSURES:
The study did not receive any funding. All authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Hormones and Viruses Influence Each Other: Consider These Connections in Your Patients
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.