User login
Epilepsy Linked to Higher COVID Hospitalization, Death Rates
, data from two linked studies showed.
Results showed that individuals with epilepsy had a 60% higher risk for hospitalization and a 33% higher risk of dying from COVID-19 than those without the disorder. However, during the pandemic, the number of hospitalizations and ER visits by people with epilepsy dropped by as much as 30%.
“The neurotropic effects of Sars-CoV-2 might explain some of this increased risk for people with epilepsy, or epilepsy might be associated with alterations in the immune system, predisposing to more severe COVID-19,” wrote the investigators, led by Owen Pickrell, MBBChirm, PhD, Swansea University, United Kingdom.
The findings were published online March 5 in Epilepsia.
Skill Shifting
Epilepsy is one of the most common neurological conditions and affects approximately 50 million people worldwide, with significant comorbidity and an increased risk for early death.
During the pandemic, clinicians treating people with epilepsy and other conditions shifted their skills to treat an ever-increasing number of patients with COVID-19, which may have hindered epilepsy-specific services for a time.
To further explore how the COVID-19 pandemic may have affected the health of this patient population, researchers analyzed health records from a large database with information about hospital admissions, primary care visits, COVID-19 vaccination status, and demographics of 90% of Welsh residents.
Those living with epilepsy before or during the study period (March 1, 2020, to June 31, 2021) were identified and compared with controls without epilepsy.
The analysis included approximately 27,280 people with epilepsy and 136,400 matched controls. Among those with epilepsy, there were 158 deaths (0.58%) and 933 hospitalizations (3.4%). In comparison, there were 370 deaths (0.27%) and 1871 hospitalizations (1.4%) in the control group.
Unadjusted analyses showed the risk of dying from COVID-19 for those with epilepsy vs controls was more than twofold higher (hazard ratio [HR], 2.15; 95% CI; 1.78-2.59) and the increase in the risk for hospitalization was similar (HR, 2.15; 95% CI; 1.94-2.37).
After adjusting for 40 comorbidities, including serious mental illness, asthma, and diabetes, those with epilepsy had a 60% increased risk for hospitalization (adjusted HR [aHR], 1.60) and a 33% increased risk for death (aHR, 1.33) than those without epilepsy (all P < .0001).
The findings “may have implications for prioritizing future COVID-19 treatments and vaccinations for people with epilepsy,” the investigators wrote.
Study limitations included the inability to account for the effect of vaccinations or prior infections with SARS-CoV-2. Moreover, the study did not account for geographical or temporal variations in prevalence and COVID-19 variants.
Consultations Canceled
In the related study, researchers analyzed healthcare utilization by people with epilepsy before and after the pandemic using the same database. Results showed hospital admissions, ER visits, and outpatient visits significantly decreased during the pandemic.
In the year before the pandemic, people with epilepsy had double the rate of ER visits (rate ratio [RR], 2.36), hospital admissions (RR, 2.08), and outpatient appointments (RR, 1.92) compared with matched controls.
However, during the pandemic there was a greater reduction in hospital admissions (RR, 0.70; 95% CI, 0.69-0.72) and ER visits (RR, 0.78; 95% CI, 0.77-0.70) in those with epilepsy versus matched controls (RR, 0.82; 95% CI, 0.81-0.83) as well as hospital visits and ER visits (RR, 0.87; 95% CI, 0.86-0.88; all P < .0001). New epilepsy diagnoses also decreased during the pandemic (RR, 0.73; P < .0001)
The redeployment of epileptologists during the pandemic also meant that epilepsy consultations and investigations were canceled, making it harder for people with epilepsy to access specialty care, the researchers noted.
“Our research also showed that there were fewer new diagnoses of epilepsy and fewer contacts with health services by people with epilepsy, during the period we examined,” Huw Strafford, lead data analyst for the studies, said in a release.
Both studies were funded by Health and Care Research Wales. Dr. Pickrell reported receiving speaker fees from UCB Pharma and Angelini Pharma, travel grants from Angelini Pharma, and an unrestricted grant from UCB Pharma.
A version of this article appeared on Medscape.com .
, data from two linked studies showed.
Results showed that individuals with epilepsy had a 60% higher risk for hospitalization and a 33% higher risk of dying from COVID-19 than those without the disorder. However, during the pandemic, the number of hospitalizations and ER visits by people with epilepsy dropped by as much as 30%.
“The neurotropic effects of Sars-CoV-2 might explain some of this increased risk for people with epilepsy, or epilepsy might be associated with alterations in the immune system, predisposing to more severe COVID-19,” wrote the investigators, led by Owen Pickrell, MBBChirm, PhD, Swansea University, United Kingdom.
The findings were published online March 5 in Epilepsia.
Skill Shifting
Epilepsy is one of the most common neurological conditions and affects approximately 50 million people worldwide, with significant comorbidity and an increased risk for early death.
During the pandemic, clinicians treating people with epilepsy and other conditions shifted their skills to treat an ever-increasing number of patients with COVID-19, which may have hindered epilepsy-specific services for a time.
To further explore how the COVID-19 pandemic may have affected the health of this patient population, researchers analyzed health records from a large database with information about hospital admissions, primary care visits, COVID-19 vaccination status, and demographics of 90% of Welsh residents.
Those living with epilepsy before or during the study period (March 1, 2020, to June 31, 2021) were identified and compared with controls without epilepsy.
The analysis included approximately 27,280 people with epilepsy and 136,400 matched controls. Among those with epilepsy, there were 158 deaths (0.58%) and 933 hospitalizations (3.4%). In comparison, there were 370 deaths (0.27%) and 1871 hospitalizations (1.4%) in the control group.
Unadjusted analyses showed the risk of dying from COVID-19 for those with epilepsy vs controls was more than twofold higher (hazard ratio [HR], 2.15; 95% CI; 1.78-2.59) and the increase in the risk for hospitalization was similar (HR, 2.15; 95% CI; 1.94-2.37).
After adjusting for 40 comorbidities, including serious mental illness, asthma, and diabetes, those with epilepsy had a 60% increased risk for hospitalization (adjusted HR [aHR], 1.60) and a 33% increased risk for death (aHR, 1.33) than those without epilepsy (all P < .0001).
The findings “may have implications for prioritizing future COVID-19 treatments and vaccinations for people with epilepsy,” the investigators wrote.
Study limitations included the inability to account for the effect of vaccinations or prior infections with SARS-CoV-2. Moreover, the study did not account for geographical or temporal variations in prevalence and COVID-19 variants.
Consultations Canceled
In the related study, researchers analyzed healthcare utilization by people with epilepsy before and after the pandemic using the same database. Results showed hospital admissions, ER visits, and outpatient visits significantly decreased during the pandemic.
In the year before the pandemic, people with epilepsy had double the rate of ER visits (rate ratio [RR], 2.36), hospital admissions (RR, 2.08), and outpatient appointments (RR, 1.92) compared with matched controls.
However, during the pandemic there was a greater reduction in hospital admissions (RR, 0.70; 95% CI, 0.69-0.72) and ER visits (RR, 0.78; 95% CI, 0.77-0.70) in those with epilepsy versus matched controls (RR, 0.82; 95% CI, 0.81-0.83) as well as hospital visits and ER visits (RR, 0.87; 95% CI, 0.86-0.88; all P < .0001). New epilepsy diagnoses also decreased during the pandemic (RR, 0.73; P < .0001)
The redeployment of epileptologists during the pandemic also meant that epilepsy consultations and investigations were canceled, making it harder for people with epilepsy to access specialty care, the researchers noted.
“Our research also showed that there were fewer new diagnoses of epilepsy and fewer contacts with health services by people with epilepsy, during the period we examined,” Huw Strafford, lead data analyst for the studies, said in a release.
Both studies were funded by Health and Care Research Wales. Dr. Pickrell reported receiving speaker fees from UCB Pharma and Angelini Pharma, travel grants from Angelini Pharma, and an unrestricted grant from UCB Pharma.
A version of this article appeared on Medscape.com .
, data from two linked studies showed.
Results showed that individuals with epilepsy had a 60% higher risk for hospitalization and a 33% higher risk of dying from COVID-19 than those without the disorder. However, during the pandemic, the number of hospitalizations and ER visits by people with epilepsy dropped by as much as 30%.
“The neurotropic effects of Sars-CoV-2 might explain some of this increased risk for people with epilepsy, or epilepsy might be associated with alterations in the immune system, predisposing to more severe COVID-19,” wrote the investigators, led by Owen Pickrell, MBBChirm, PhD, Swansea University, United Kingdom.
The findings were published online March 5 in Epilepsia.
Skill Shifting
Epilepsy is one of the most common neurological conditions and affects approximately 50 million people worldwide, with significant comorbidity and an increased risk for early death.
During the pandemic, clinicians treating people with epilepsy and other conditions shifted their skills to treat an ever-increasing number of patients with COVID-19, which may have hindered epilepsy-specific services for a time.
To further explore how the COVID-19 pandemic may have affected the health of this patient population, researchers analyzed health records from a large database with information about hospital admissions, primary care visits, COVID-19 vaccination status, and demographics of 90% of Welsh residents.
Those living with epilepsy before or during the study period (March 1, 2020, to June 31, 2021) were identified and compared with controls without epilepsy.
The analysis included approximately 27,280 people with epilepsy and 136,400 matched controls. Among those with epilepsy, there were 158 deaths (0.58%) and 933 hospitalizations (3.4%). In comparison, there were 370 deaths (0.27%) and 1871 hospitalizations (1.4%) in the control group.
Unadjusted analyses showed the risk of dying from COVID-19 for those with epilepsy vs controls was more than twofold higher (hazard ratio [HR], 2.15; 95% CI; 1.78-2.59) and the increase in the risk for hospitalization was similar (HR, 2.15; 95% CI; 1.94-2.37).
After adjusting for 40 comorbidities, including serious mental illness, asthma, and diabetes, those with epilepsy had a 60% increased risk for hospitalization (adjusted HR [aHR], 1.60) and a 33% increased risk for death (aHR, 1.33) than those without epilepsy (all P < .0001).
The findings “may have implications for prioritizing future COVID-19 treatments and vaccinations for people with epilepsy,” the investigators wrote.
Study limitations included the inability to account for the effect of vaccinations or prior infections with SARS-CoV-2. Moreover, the study did not account for geographical or temporal variations in prevalence and COVID-19 variants.
Consultations Canceled
In the related study, researchers analyzed healthcare utilization by people with epilepsy before and after the pandemic using the same database. Results showed hospital admissions, ER visits, and outpatient visits significantly decreased during the pandemic.
In the year before the pandemic, people with epilepsy had double the rate of ER visits (rate ratio [RR], 2.36), hospital admissions (RR, 2.08), and outpatient appointments (RR, 1.92) compared with matched controls.
However, during the pandemic there was a greater reduction in hospital admissions (RR, 0.70; 95% CI, 0.69-0.72) and ER visits (RR, 0.78; 95% CI, 0.77-0.70) in those with epilepsy versus matched controls (RR, 0.82; 95% CI, 0.81-0.83) as well as hospital visits and ER visits (RR, 0.87; 95% CI, 0.86-0.88; all P < .0001). New epilepsy diagnoses also decreased during the pandemic (RR, 0.73; P < .0001)
The redeployment of epileptologists during the pandemic also meant that epilepsy consultations and investigations were canceled, making it harder for people with epilepsy to access specialty care, the researchers noted.
“Our research also showed that there were fewer new diagnoses of epilepsy and fewer contacts with health services by people with epilepsy, during the period we examined,” Huw Strafford, lead data analyst for the studies, said in a release.
Both studies were funded by Health and Care Research Wales. Dr. Pickrell reported receiving speaker fees from UCB Pharma and Angelini Pharma, travel grants from Angelini Pharma, and an unrestricted grant from UCB Pharma.
A version of this article appeared on Medscape.com .
FROM EPILEPSIA
Cognitive Deficits After Most Severe COVID Cases Associated With 9-Point IQ Drop
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
COVID Virus Can Remain in the Body Over a Year
Scientists at the University of California, San Francisco, have discovered that remnants of the COVID-19 virus can linger in blood and tissue for more than a year after a person is first infected.
In their research on long COVID, the scientists found COVID antigens in the blood for up to 14 months after infection, and in tissue samples for more than 2 years after infection.
“These two studies provide some of the strongest evidence so far that COVID antigens can persist in some people, even though we think they have normal immune responses,” Michael Peluso, MD, an infectious disease researcher in the UCSF School of Medicine, who led both studies, said in a statement.
Scientists don’t know what causes long COVID, in which symptoms of the illness persist months or years after recovery. The most common symptoms are extreme fatigue, shortness of breath, loss of smell, and muscle aches.
The UCSF research team examined blood samples from 171 infected people and found the COVID “spike” protein was still present up to 14 months after infection in some people. The antigens were found more often in people who were hospitalized with COVID or who reported being very sick but were not hospitalized.
Researchers next looked at the UCSF Long COVID Tissue Bank, which contains samples donated by patients with and without long COVID.
They found portions of viral RNA in the tissue up to 2 years after people were infected, though there was no evidence of reinfection. Those viral fragments were found in connective tissue where immune cells are, suggesting that the fragments caused the immune system to attack, according to the researchers.
The UCSF team is running clinical trials to find out if monoclonal antibodies or antiviral drugs can remove the virus.
The findings were presented in Denver this week at the Conference on Retroviruses and Opportunistic Infections.
A version of this article appeared on WebMD.com.
Scientists at the University of California, San Francisco, have discovered that remnants of the COVID-19 virus can linger in blood and tissue for more than a year after a person is first infected.
In their research on long COVID, the scientists found COVID antigens in the blood for up to 14 months after infection, and in tissue samples for more than 2 years after infection.
“These two studies provide some of the strongest evidence so far that COVID antigens can persist in some people, even though we think they have normal immune responses,” Michael Peluso, MD, an infectious disease researcher in the UCSF School of Medicine, who led both studies, said in a statement.
Scientists don’t know what causes long COVID, in which symptoms of the illness persist months or years after recovery. The most common symptoms are extreme fatigue, shortness of breath, loss of smell, and muscle aches.
The UCSF research team examined blood samples from 171 infected people and found the COVID “spike” protein was still present up to 14 months after infection in some people. The antigens were found more often in people who were hospitalized with COVID or who reported being very sick but were not hospitalized.
Researchers next looked at the UCSF Long COVID Tissue Bank, which contains samples donated by patients with and without long COVID.
They found portions of viral RNA in the tissue up to 2 years after people were infected, though there was no evidence of reinfection. Those viral fragments were found in connective tissue where immune cells are, suggesting that the fragments caused the immune system to attack, according to the researchers.
The UCSF team is running clinical trials to find out if monoclonal antibodies or antiviral drugs can remove the virus.
The findings were presented in Denver this week at the Conference on Retroviruses and Opportunistic Infections.
A version of this article appeared on WebMD.com.
Scientists at the University of California, San Francisco, have discovered that remnants of the COVID-19 virus can linger in blood and tissue for more than a year after a person is first infected.
In their research on long COVID, the scientists found COVID antigens in the blood for up to 14 months after infection, and in tissue samples for more than 2 years after infection.
“These two studies provide some of the strongest evidence so far that COVID antigens can persist in some people, even though we think they have normal immune responses,” Michael Peluso, MD, an infectious disease researcher in the UCSF School of Medicine, who led both studies, said in a statement.
Scientists don’t know what causes long COVID, in which symptoms of the illness persist months or years after recovery. The most common symptoms are extreme fatigue, shortness of breath, loss of smell, and muscle aches.
The UCSF research team examined blood samples from 171 infected people and found the COVID “spike” protein was still present up to 14 months after infection in some people. The antigens were found more often in people who were hospitalized with COVID or who reported being very sick but were not hospitalized.
Researchers next looked at the UCSF Long COVID Tissue Bank, which contains samples donated by patients with and without long COVID.
They found portions of viral RNA in the tissue up to 2 years after people were infected, though there was no evidence of reinfection. Those viral fragments were found in connective tissue where immune cells are, suggesting that the fragments caused the immune system to attack, according to the researchers.
The UCSF team is running clinical trials to find out if monoclonal antibodies or antiviral drugs can remove the virus.
The findings were presented in Denver this week at the Conference on Retroviruses and Opportunistic Infections.
A version of this article appeared on WebMD.com.
New Data on Mild COVID’s Risk for Neurologic, Psychiatric Disorders
While severe COVID-19 is associated with a significantly higher risk for psychiatric and neurologic disorders a year after infection, mild does not carry the same risk, a new study shows.
However, less severe COVID-19 was not linked to a higher incidence of psychiatric diagnoses and was associated with only a slightly higher risk for neurologic disorders.
The new research challenges previous findings of long-term risk for psychiatric and neurologic disorders associated with SARS-CoV-2 in patients who had not been hospitalized for the condition.
“Our study does not support previous findings of substantial post-acute neurologic and psychiatric morbidities among the general population of SARS-CoV-2-infected individuals but does corroborate an elevated risk among the most severe cases with COVID-19,” the authors wrote.
The study was published online on February 21 in Neurology.
‘Alarming’ Findings
Previous studies have reported nervous system symptoms in patients who have experienced COVID-19, which may persist for several weeks or months after the acute phase, even in milder cases.
But these findings haven’t been consistent across all studies, and few studies have addressed the potential effect of different viral variants and vaccination status on post-acute psychiatric and neurologic morbidities.
“Our study was partly motivated by our strong research interest in the associations between infectious disease and later chronic disease and partly by international studies, such as those conducted in the US Veterans Health databases, that have suggested substantial risks of psychiatric and neurological conditions associated with infection,” senior author Anders Hviid, MSc, DrMedSci, head of the department and professor of pharmacoepidemiology, Statens Serum Institut, Copenhagen, Denmark, told this news organization.
Investigators drew on data from the Danish National Patient Registry to compare the risk for neurologic and psychiatric disorders during the 12 months after acute COVID-19 infection to risk among people who never tested positive.
They examined data on all recorded hospital contacts between January 2005 and January 2023 for a discharge diagnosis of at least one of 11 psychiatric illnesses or at least one of 30 neurologic disorders.
The researchers compared the incidence of each disorder within 1-12 months after infection with those of COVID-naive individuals and stratified analyses according to time since infection, vaccination status, variant period, age, sex, and infection severity.
The final study cohort included 1.8 million individuals who tested positive during the study period and 1.5 who didn’t. Three quarters of those who tested positive were infected primarily with the Omicron variant.
Hospitalized vs Nonhospitalized
Overall, individuals who tested positive had a 24% lower risk for psychiatric disorders during the post-acute period (incident rate ratio [IRR], 0.76; 95% CI, 0.74-0.78) compared with the control group, but a 5% higher risk for any neurologic disorder (IRR, 1.05; 95% CI, 1.04-1.07).
Age, sex, and variant had less influence on risk than infection severity, where the differences between hospitalized and nonhospitalized patients were significant.
Compared with COVID-negative individuals, the risk for any psychiatric disorder was double for hospitalized patients (IRR, 2.05; 95% CI, 1.78-2.37) but was 25% lower among nonhospitalized patients (IRR, 0.75; 95% CI, 0.73-0.77).
For neurologic disorders, the IRR for hospitalized patients was 2.44 (95% CI, 2.29-2.60) compared with COVID-negative individuals vs an IRR of only 1.02 (95% CI, 1.01-1.04) among nonhospitalized patients.
“In a general population, there was little support for clinically relevant post-acute risk increases of psychiatric and neurologic disorders associated with SARS-CoV-2 infection without hospitalization. This was particularly true for vaccinated individuals and for the more recent variants,” the authors wrote, adding that the only exception was for change in sense and smell.
‘Flaws’ in Previous Studies?
The findings in hospitalized patients were in line with previous findings, but those in nonhospitalized patients stand out, they added.
Previous studies were done predominantly in older males with comorbidities and those who were more socioeconomically disadvantaged, which could lead to a bias, Dr. Hviid said.
Those other studies “had a number of fundamental flaws that we do not believe our study has,” Dr. Hviid said. “Our study was conducted in the general population, with free and universal testing and healthcare.”
Researchers stress that sequelae after infection are predominantly associated with severe illness.
“Today, a healthy vaccinated adult having an asymptomatic or mild bout of COVID-19 with the current variants shouldn’t fear developing serious psychiatric or neurologic disorders in the months or years after infection.”
One limitation is that only hospital contacts were included, omitting possible diagnoses given outside hospital settings.
‘Extreme Caution’ Required
The link between COVID-19 and brain health is “complex,” and the new findings should be viewed cautiously, said Maxime Taquet, MRCPsych, PhD, National Institute for Health and Care Research clinical lecturer and specialty registrar in Psychiatry, Oxford Health NHS Foundation Trust, England, who commented on the findings.
Previous research by Dr. Taquet, who was not involved in the current study, found an increased risk for neurologic and psychiatric diagnoses during the first 6 months after COVID-19 diagnosis.
The current study “contributes to better understanding this link by providing data from another country with a different organization of healthcare provision than the US, where most of the existing data come from,” Dr. Taquet said.
However, “some observations — for example, that COVID-19 is associated with a 50% reduction in the risk of autism, a condition present from very early in life — call for extreme caution in the interpretation of the findings, as they suggest that residual bias has not been accounted for,” Dr. Taquet continued.
Authors of an accompanying editorial, Eric Chow, MD, MS, MPH, of the Division of Allergy and Infectious Diseases, University of Washington, School of Public Health, and Anita Chopra, MD, of the post-COVID Clinic, University of Washington, Seattle, called the study a “critical contribution to the published literature.”
The association of neurologic and psychiatric diagnoses with severe disease “is a reminder of the importance of risk reduction by combining vaccinations with improved indoor ventilation and masking,” they concluded.
The study was supported by a grant from the Independent Research Fund Denmark. Dr. Hviid and coauthors, Dr. Chopra, and Dr. Taquet reported no relevant financial relationships. Dr. Chow received a travel award from the Infectious Diseases Society of America to attend ID Week 2022.
A version of this article appeared on Medscape.com.
While severe COVID-19 is associated with a significantly higher risk for psychiatric and neurologic disorders a year after infection, mild does not carry the same risk, a new study shows.
However, less severe COVID-19 was not linked to a higher incidence of psychiatric diagnoses and was associated with only a slightly higher risk for neurologic disorders.
The new research challenges previous findings of long-term risk for psychiatric and neurologic disorders associated with SARS-CoV-2 in patients who had not been hospitalized for the condition.
“Our study does not support previous findings of substantial post-acute neurologic and psychiatric morbidities among the general population of SARS-CoV-2-infected individuals but does corroborate an elevated risk among the most severe cases with COVID-19,” the authors wrote.
The study was published online on February 21 in Neurology.
‘Alarming’ Findings
Previous studies have reported nervous system symptoms in patients who have experienced COVID-19, which may persist for several weeks or months after the acute phase, even in milder cases.
But these findings haven’t been consistent across all studies, and few studies have addressed the potential effect of different viral variants and vaccination status on post-acute psychiatric and neurologic morbidities.
“Our study was partly motivated by our strong research interest in the associations between infectious disease and later chronic disease and partly by international studies, such as those conducted in the US Veterans Health databases, that have suggested substantial risks of psychiatric and neurological conditions associated with infection,” senior author Anders Hviid, MSc, DrMedSci, head of the department and professor of pharmacoepidemiology, Statens Serum Institut, Copenhagen, Denmark, told this news organization.
Investigators drew on data from the Danish National Patient Registry to compare the risk for neurologic and psychiatric disorders during the 12 months after acute COVID-19 infection to risk among people who never tested positive.
They examined data on all recorded hospital contacts between January 2005 and January 2023 for a discharge diagnosis of at least one of 11 psychiatric illnesses or at least one of 30 neurologic disorders.
The researchers compared the incidence of each disorder within 1-12 months after infection with those of COVID-naive individuals and stratified analyses according to time since infection, vaccination status, variant period, age, sex, and infection severity.
The final study cohort included 1.8 million individuals who tested positive during the study period and 1.5 who didn’t. Three quarters of those who tested positive were infected primarily with the Omicron variant.
Hospitalized vs Nonhospitalized
Overall, individuals who tested positive had a 24% lower risk for psychiatric disorders during the post-acute period (incident rate ratio [IRR], 0.76; 95% CI, 0.74-0.78) compared with the control group, but a 5% higher risk for any neurologic disorder (IRR, 1.05; 95% CI, 1.04-1.07).
Age, sex, and variant had less influence on risk than infection severity, where the differences between hospitalized and nonhospitalized patients were significant.
Compared with COVID-negative individuals, the risk for any psychiatric disorder was double for hospitalized patients (IRR, 2.05; 95% CI, 1.78-2.37) but was 25% lower among nonhospitalized patients (IRR, 0.75; 95% CI, 0.73-0.77).
For neurologic disorders, the IRR for hospitalized patients was 2.44 (95% CI, 2.29-2.60) compared with COVID-negative individuals vs an IRR of only 1.02 (95% CI, 1.01-1.04) among nonhospitalized patients.
“In a general population, there was little support for clinically relevant post-acute risk increases of psychiatric and neurologic disorders associated with SARS-CoV-2 infection without hospitalization. This was particularly true for vaccinated individuals and for the more recent variants,” the authors wrote, adding that the only exception was for change in sense and smell.
‘Flaws’ in Previous Studies?
The findings in hospitalized patients were in line with previous findings, but those in nonhospitalized patients stand out, they added.
Previous studies were done predominantly in older males with comorbidities and those who were more socioeconomically disadvantaged, which could lead to a bias, Dr. Hviid said.
Those other studies “had a number of fundamental flaws that we do not believe our study has,” Dr. Hviid said. “Our study was conducted in the general population, with free and universal testing and healthcare.”
Researchers stress that sequelae after infection are predominantly associated with severe illness.
“Today, a healthy vaccinated adult having an asymptomatic or mild bout of COVID-19 with the current variants shouldn’t fear developing serious psychiatric or neurologic disorders in the months or years after infection.”
One limitation is that only hospital contacts were included, omitting possible diagnoses given outside hospital settings.
‘Extreme Caution’ Required
The link between COVID-19 and brain health is “complex,” and the new findings should be viewed cautiously, said Maxime Taquet, MRCPsych, PhD, National Institute for Health and Care Research clinical lecturer and specialty registrar in Psychiatry, Oxford Health NHS Foundation Trust, England, who commented on the findings.
Previous research by Dr. Taquet, who was not involved in the current study, found an increased risk for neurologic and psychiatric diagnoses during the first 6 months after COVID-19 diagnosis.
The current study “contributes to better understanding this link by providing data from another country with a different organization of healthcare provision than the US, where most of the existing data come from,” Dr. Taquet said.
However, “some observations — for example, that COVID-19 is associated with a 50% reduction in the risk of autism, a condition present from very early in life — call for extreme caution in the interpretation of the findings, as they suggest that residual bias has not been accounted for,” Dr. Taquet continued.
Authors of an accompanying editorial, Eric Chow, MD, MS, MPH, of the Division of Allergy and Infectious Diseases, University of Washington, School of Public Health, and Anita Chopra, MD, of the post-COVID Clinic, University of Washington, Seattle, called the study a “critical contribution to the published literature.”
The association of neurologic and psychiatric diagnoses with severe disease “is a reminder of the importance of risk reduction by combining vaccinations with improved indoor ventilation and masking,” they concluded.
The study was supported by a grant from the Independent Research Fund Denmark. Dr. Hviid and coauthors, Dr. Chopra, and Dr. Taquet reported no relevant financial relationships. Dr. Chow received a travel award from the Infectious Diseases Society of America to attend ID Week 2022.
A version of this article appeared on Medscape.com.
While severe COVID-19 is associated with a significantly higher risk for psychiatric and neurologic disorders a year after infection, mild does not carry the same risk, a new study shows.
However, less severe COVID-19 was not linked to a higher incidence of psychiatric diagnoses and was associated with only a slightly higher risk for neurologic disorders.
The new research challenges previous findings of long-term risk for psychiatric and neurologic disorders associated with SARS-CoV-2 in patients who had not been hospitalized for the condition.
“Our study does not support previous findings of substantial post-acute neurologic and psychiatric morbidities among the general population of SARS-CoV-2-infected individuals but does corroborate an elevated risk among the most severe cases with COVID-19,” the authors wrote.
The study was published online on February 21 in Neurology.
‘Alarming’ Findings
Previous studies have reported nervous system symptoms in patients who have experienced COVID-19, which may persist for several weeks or months after the acute phase, even in milder cases.
But these findings haven’t been consistent across all studies, and few studies have addressed the potential effect of different viral variants and vaccination status on post-acute psychiatric and neurologic morbidities.
“Our study was partly motivated by our strong research interest in the associations between infectious disease and later chronic disease and partly by international studies, such as those conducted in the US Veterans Health databases, that have suggested substantial risks of psychiatric and neurological conditions associated with infection,” senior author Anders Hviid, MSc, DrMedSci, head of the department and professor of pharmacoepidemiology, Statens Serum Institut, Copenhagen, Denmark, told this news organization.
Investigators drew on data from the Danish National Patient Registry to compare the risk for neurologic and psychiatric disorders during the 12 months after acute COVID-19 infection to risk among people who never tested positive.
They examined data on all recorded hospital contacts between January 2005 and January 2023 for a discharge diagnosis of at least one of 11 psychiatric illnesses or at least one of 30 neurologic disorders.
The researchers compared the incidence of each disorder within 1-12 months after infection with those of COVID-naive individuals and stratified analyses according to time since infection, vaccination status, variant period, age, sex, and infection severity.
The final study cohort included 1.8 million individuals who tested positive during the study period and 1.5 who didn’t. Three quarters of those who tested positive were infected primarily with the Omicron variant.
Hospitalized vs Nonhospitalized
Overall, individuals who tested positive had a 24% lower risk for psychiatric disorders during the post-acute period (incident rate ratio [IRR], 0.76; 95% CI, 0.74-0.78) compared with the control group, but a 5% higher risk for any neurologic disorder (IRR, 1.05; 95% CI, 1.04-1.07).
Age, sex, and variant had less influence on risk than infection severity, where the differences between hospitalized and nonhospitalized patients were significant.
Compared with COVID-negative individuals, the risk for any psychiatric disorder was double for hospitalized patients (IRR, 2.05; 95% CI, 1.78-2.37) but was 25% lower among nonhospitalized patients (IRR, 0.75; 95% CI, 0.73-0.77).
For neurologic disorders, the IRR for hospitalized patients was 2.44 (95% CI, 2.29-2.60) compared with COVID-negative individuals vs an IRR of only 1.02 (95% CI, 1.01-1.04) among nonhospitalized patients.
“In a general population, there was little support for clinically relevant post-acute risk increases of psychiatric and neurologic disorders associated with SARS-CoV-2 infection without hospitalization. This was particularly true for vaccinated individuals and for the more recent variants,” the authors wrote, adding that the only exception was for change in sense and smell.
‘Flaws’ in Previous Studies?
The findings in hospitalized patients were in line with previous findings, but those in nonhospitalized patients stand out, they added.
Previous studies were done predominantly in older males with comorbidities and those who were more socioeconomically disadvantaged, which could lead to a bias, Dr. Hviid said.
Those other studies “had a number of fundamental flaws that we do not believe our study has,” Dr. Hviid said. “Our study was conducted in the general population, with free and universal testing and healthcare.”
Researchers stress that sequelae after infection are predominantly associated with severe illness.
“Today, a healthy vaccinated adult having an asymptomatic or mild bout of COVID-19 with the current variants shouldn’t fear developing serious psychiatric or neurologic disorders in the months or years after infection.”
One limitation is that only hospital contacts were included, omitting possible diagnoses given outside hospital settings.
‘Extreme Caution’ Required
The link between COVID-19 and brain health is “complex,” and the new findings should be viewed cautiously, said Maxime Taquet, MRCPsych, PhD, National Institute for Health and Care Research clinical lecturer and specialty registrar in Psychiatry, Oxford Health NHS Foundation Trust, England, who commented on the findings.
Previous research by Dr. Taquet, who was not involved in the current study, found an increased risk for neurologic and psychiatric diagnoses during the first 6 months after COVID-19 diagnosis.
The current study “contributes to better understanding this link by providing data from another country with a different organization of healthcare provision than the US, where most of the existing data come from,” Dr. Taquet said.
However, “some observations — for example, that COVID-19 is associated with a 50% reduction in the risk of autism, a condition present from very early in life — call for extreme caution in the interpretation of the findings, as they suggest that residual bias has not been accounted for,” Dr. Taquet continued.
Authors of an accompanying editorial, Eric Chow, MD, MS, MPH, of the Division of Allergy and Infectious Diseases, University of Washington, School of Public Health, and Anita Chopra, MD, of the post-COVID Clinic, University of Washington, Seattle, called the study a “critical contribution to the published literature.”
The association of neurologic and psychiatric diagnoses with severe disease “is a reminder of the importance of risk reduction by combining vaccinations with improved indoor ventilation and masking,” they concluded.
The study was supported by a grant from the Independent Research Fund Denmark. Dr. Hviid and coauthors, Dr. Chopra, and Dr. Taquet reported no relevant financial relationships. Dr. Chow received a travel award from the Infectious Diseases Society of America to attend ID Week 2022.
A version of this article appeared on Medscape.com.
COVID-19 Is a Very Weird Virus
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Increased Risk of New Rheumatic Disease Follows COVID-19 Infection
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Cognitive Deficits After Most Severe COVID Cases Associated With 9-Point IQ Drop
and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg, in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post-COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg, in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post-COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg, in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post-COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Paxlovid Lowers Risk of COVID-19 Hospitalization, Study Finds
This medicine has been approved for use in the United States for people over 12 years old who are at risk of having a severe COVID-19 infection.
The study was published in the Journal of Antimicrobial Chemotherapy.
Study authors examined the health records of almost 45,000 outpatients who tested positive for COVID-19 from January to August 2022. This sample period was when the Omicron strain was dominant.
The average patient age was 47. Sixty-two percent were White, 24% were Black, 6% were Hispanic, and 8% had an unknown ethnicity. A slight majority, 51%, had received two or more vaccine doses before the study period.
From the study group, 201 people were hospitalized within 28 days of their positive COVID test.
Almost 5,000 people in the study group received Paxlovid. The use of Paxlovid was the best indicator of avoiding hospitalization, with three of those people being hospitalized.
“Patients who were treated with Paxlovid were twice as likely to have received at least two doses of COVID-19 vaccine,” the University of Minnesota’s CIDRAP reported. “They were also more likely to be 70 years or older.”
People taking Paxlovid were more likely to be White and to live in middle- or upper-income areas.
“COVID-19 hospitalization risk was reduced by 84% among [Paxlovid] recipients in a large, diverse healthcare system during the Omicron wave,” the study’s authors wrote. “These results suggest that [Paxlovid] remained highly effective in a setting substantially different than the original clinical trials.”
A version of this article appeared on WebMD.com.
This medicine has been approved for use in the United States for people over 12 years old who are at risk of having a severe COVID-19 infection.
The study was published in the Journal of Antimicrobial Chemotherapy.
Study authors examined the health records of almost 45,000 outpatients who tested positive for COVID-19 from January to August 2022. This sample period was when the Omicron strain was dominant.
The average patient age was 47. Sixty-two percent were White, 24% were Black, 6% were Hispanic, and 8% had an unknown ethnicity. A slight majority, 51%, had received two or more vaccine doses before the study period.
From the study group, 201 people were hospitalized within 28 days of their positive COVID test.
Almost 5,000 people in the study group received Paxlovid. The use of Paxlovid was the best indicator of avoiding hospitalization, with three of those people being hospitalized.
“Patients who were treated with Paxlovid were twice as likely to have received at least two doses of COVID-19 vaccine,” the University of Minnesota’s CIDRAP reported. “They were also more likely to be 70 years or older.”
People taking Paxlovid were more likely to be White and to live in middle- or upper-income areas.
“COVID-19 hospitalization risk was reduced by 84% among [Paxlovid] recipients in a large, diverse healthcare system during the Omicron wave,” the study’s authors wrote. “These results suggest that [Paxlovid] remained highly effective in a setting substantially different than the original clinical trials.”
A version of this article appeared on WebMD.com.
This medicine has been approved for use in the United States for people over 12 years old who are at risk of having a severe COVID-19 infection.
The study was published in the Journal of Antimicrobial Chemotherapy.
Study authors examined the health records of almost 45,000 outpatients who tested positive for COVID-19 from January to August 2022. This sample period was when the Omicron strain was dominant.
The average patient age was 47. Sixty-two percent were White, 24% were Black, 6% were Hispanic, and 8% had an unknown ethnicity. A slight majority, 51%, had received two or more vaccine doses before the study period.
From the study group, 201 people were hospitalized within 28 days of their positive COVID test.
Almost 5,000 people in the study group received Paxlovid. The use of Paxlovid was the best indicator of avoiding hospitalization, with three of those people being hospitalized.
“Patients who were treated with Paxlovid were twice as likely to have received at least two doses of COVID-19 vaccine,” the University of Minnesota’s CIDRAP reported. “They were also more likely to be 70 years or older.”
People taking Paxlovid were more likely to be White and to live in middle- or upper-income areas.
“COVID-19 hospitalization risk was reduced by 84% among [Paxlovid] recipients in a large, diverse healthcare system during the Omicron wave,” the study’s authors wrote. “These results suggest that [Paxlovid] remained highly effective in a setting substantially different than the original clinical trials.”
A version of this article appeared on WebMD.com.
Dermatologic Reactions Following COVID-19 Vaccination: A Case Series
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).
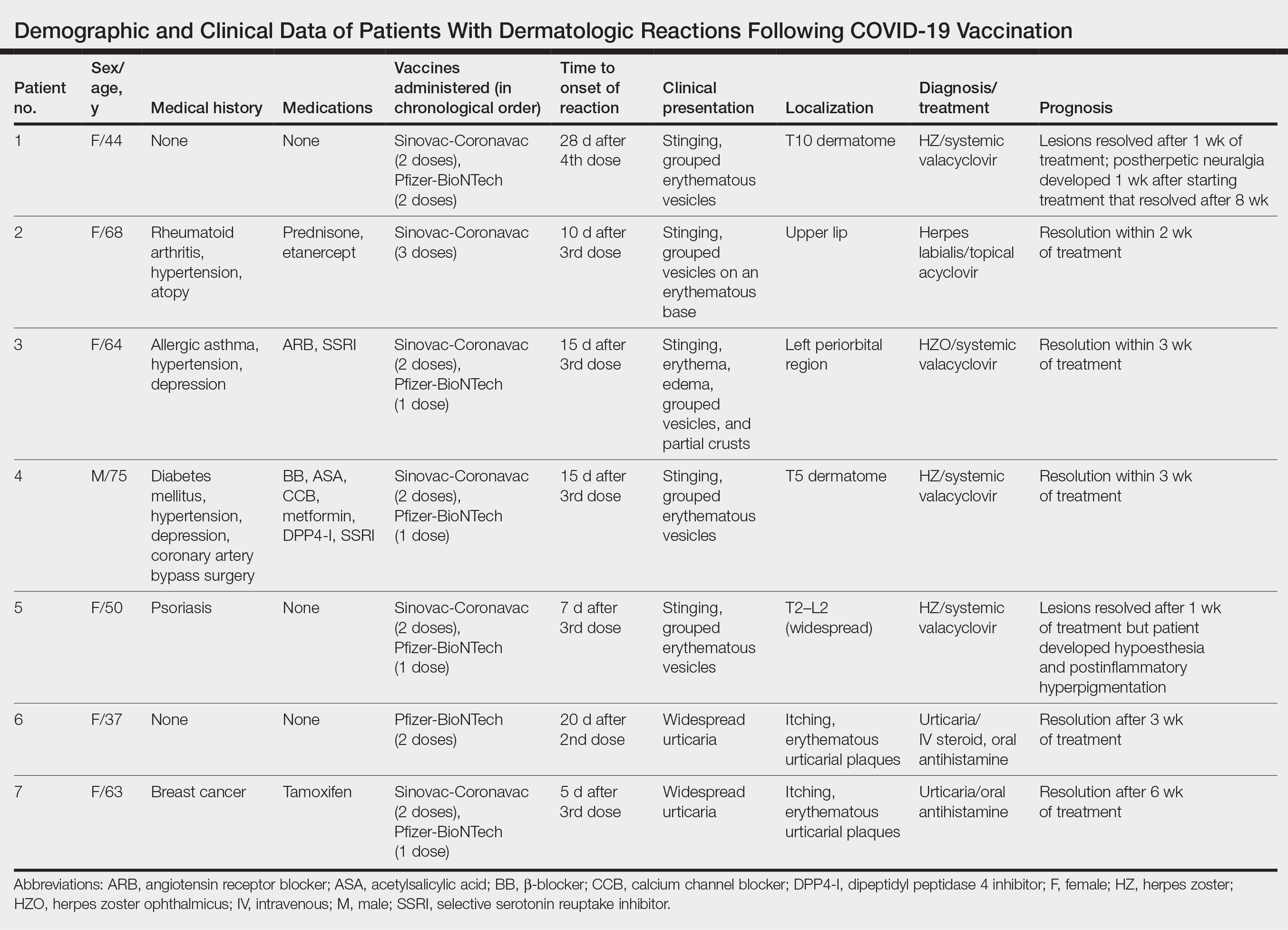
Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Practice Points
- Cutaneous reactions have been reported following COVID-19 vaccination.
- Herpes infections and urticarial reactions can be associated with COVID-19 vaccination, regardless of the delay in onset between the injection and symptom development.
Mixing Paxlovid With Specific Immunosuppressants Risks Serious Adverse Reactions
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.



