User login
Check all components in cases of suspected shoe allergy
according to a retrospective study of more than 30,000 patients.
Contact allergy to shoes remains a common but difficult problem for many reasons, including the limited information from shoe manufacturers, differences in shoe manufacturing processes, and changes in shoe trends, said Raina Bembry, MD, a dermatitis research fellow at Duke University, Durham, N.C., in a presentation at the annual meeting of the American Contact Dermatitis Society.
The North American Contact Dermatitis Group (NACDG) published data on shoe allergens from 2001-2004 in a 2007 review. To update this information to reflect changes in shoe manufacturing and trends, she and her coinvestigators characterized demographics, clinical characteristics, patch test results, and occupational data for NACDG patients with shoe contact allergy. They identified 33,661 patients who were patch tested with the standard series with or without a supplemental allergen between 2005 and 2018; over half were over aged 40.
The primary focus was individuals with a confirmed shoe (defined as shoes, boots, sandals, or slippers) as the source of a screening allergen or supplemental allergen, a positive patch test, and the foot as one of three sites of involvement. A total of 352 individuals met these criteria and had a confirmed final diagnosis of allergic contact dermatitis, Dr. Bembry said. Compared with individuals who had positive patch tests without a confirmed diagnosis, those with confirmed allergic dermatitis were significantly more likely to be male (odds ratio, 3.36) and less likely to be over aged 40 years (OR, 0.49).
The most common NACDG screening allergen, potassium dichromate, was found in 29.8% of the study population, followed by p-tert-butylphenol formaldehyde resin in 20.1%, thiuram mix (13.3%), mixed dialkyl thioureas (12.6%) and carba mix (12%).
Notably, 29.8% of the patients showed positive patch test reactions to supplemental allergens, and 12.2% only reacted to supplemental allergens, Dr. Bembry said.
The results were limited by several factors, including referral selection bias, reliance on clinical judgment for patch test interpretations, and lack of data on the specifics of the supplemental allergens other than the source code, she said. In addition, the study does not identify nonshoe sources of foot contact allergy, and six screening allergens were not testing during this study period.
Overall, the findings were similar to those from previous studies in that patients affected with contact dermatitis from shoe allergens tended to be younger and male, with no occupational relevance to the reaction, said Dr. Bembry.
The finding that almost 20% of allergens were not found with the screening series emphasizes the value of testing not only relevant supplemental allergens, but also patient products and shoe components, she concluded.
Dr. Bembry had no financial conflicts to disclose. Coauthor Amber Atwater, MD, the immediate past president of the ACDS, and associate professor of dermatology at Duke University, disclosed receiving the Pfizer Independent Grant for Learning & Change and consulting for Henkel.
according to a retrospective study of more than 30,000 patients.
Contact allergy to shoes remains a common but difficult problem for many reasons, including the limited information from shoe manufacturers, differences in shoe manufacturing processes, and changes in shoe trends, said Raina Bembry, MD, a dermatitis research fellow at Duke University, Durham, N.C., in a presentation at the annual meeting of the American Contact Dermatitis Society.
The North American Contact Dermatitis Group (NACDG) published data on shoe allergens from 2001-2004 in a 2007 review. To update this information to reflect changes in shoe manufacturing and trends, she and her coinvestigators characterized demographics, clinical characteristics, patch test results, and occupational data for NACDG patients with shoe contact allergy. They identified 33,661 patients who were patch tested with the standard series with or without a supplemental allergen between 2005 and 2018; over half were over aged 40.
The primary focus was individuals with a confirmed shoe (defined as shoes, boots, sandals, or slippers) as the source of a screening allergen or supplemental allergen, a positive patch test, and the foot as one of three sites of involvement. A total of 352 individuals met these criteria and had a confirmed final diagnosis of allergic contact dermatitis, Dr. Bembry said. Compared with individuals who had positive patch tests without a confirmed diagnosis, those with confirmed allergic dermatitis were significantly more likely to be male (odds ratio, 3.36) and less likely to be over aged 40 years (OR, 0.49).
The most common NACDG screening allergen, potassium dichromate, was found in 29.8% of the study population, followed by p-tert-butylphenol formaldehyde resin in 20.1%, thiuram mix (13.3%), mixed dialkyl thioureas (12.6%) and carba mix (12%).
Notably, 29.8% of the patients showed positive patch test reactions to supplemental allergens, and 12.2% only reacted to supplemental allergens, Dr. Bembry said.
The results were limited by several factors, including referral selection bias, reliance on clinical judgment for patch test interpretations, and lack of data on the specifics of the supplemental allergens other than the source code, she said. In addition, the study does not identify nonshoe sources of foot contact allergy, and six screening allergens were not testing during this study period.
Overall, the findings were similar to those from previous studies in that patients affected with contact dermatitis from shoe allergens tended to be younger and male, with no occupational relevance to the reaction, said Dr. Bembry.
The finding that almost 20% of allergens were not found with the screening series emphasizes the value of testing not only relevant supplemental allergens, but also patient products and shoe components, she concluded.
Dr. Bembry had no financial conflicts to disclose. Coauthor Amber Atwater, MD, the immediate past president of the ACDS, and associate professor of dermatology at Duke University, disclosed receiving the Pfizer Independent Grant for Learning & Change and consulting for Henkel.
according to a retrospective study of more than 30,000 patients.
Contact allergy to shoes remains a common but difficult problem for many reasons, including the limited information from shoe manufacturers, differences in shoe manufacturing processes, and changes in shoe trends, said Raina Bembry, MD, a dermatitis research fellow at Duke University, Durham, N.C., in a presentation at the annual meeting of the American Contact Dermatitis Society.
The North American Contact Dermatitis Group (NACDG) published data on shoe allergens from 2001-2004 in a 2007 review. To update this information to reflect changes in shoe manufacturing and trends, she and her coinvestigators characterized demographics, clinical characteristics, patch test results, and occupational data for NACDG patients with shoe contact allergy. They identified 33,661 patients who were patch tested with the standard series with or without a supplemental allergen between 2005 and 2018; over half were over aged 40.
The primary focus was individuals with a confirmed shoe (defined as shoes, boots, sandals, or slippers) as the source of a screening allergen or supplemental allergen, a positive patch test, and the foot as one of three sites of involvement. A total of 352 individuals met these criteria and had a confirmed final diagnosis of allergic contact dermatitis, Dr. Bembry said. Compared with individuals who had positive patch tests without a confirmed diagnosis, those with confirmed allergic dermatitis were significantly more likely to be male (odds ratio, 3.36) and less likely to be over aged 40 years (OR, 0.49).
The most common NACDG screening allergen, potassium dichromate, was found in 29.8% of the study population, followed by p-tert-butylphenol formaldehyde resin in 20.1%, thiuram mix (13.3%), mixed dialkyl thioureas (12.6%) and carba mix (12%).
Notably, 29.8% of the patients showed positive patch test reactions to supplemental allergens, and 12.2% only reacted to supplemental allergens, Dr. Bembry said.
The results were limited by several factors, including referral selection bias, reliance on clinical judgment for patch test interpretations, and lack of data on the specifics of the supplemental allergens other than the source code, she said. In addition, the study does not identify nonshoe sources of foot contact allergy, and six screening allergens were not testing during this study period.
Overall, the findings were similar to those from previous studies in that patients affected with contact dermatitis from shoe allergens tended to be younger and male, with no occupational relevance to the reaction, said Dr. Bembry.
The finding that almost 20% of allergens were not found with the screening series emphasizes the value of testing not only relevant supplemental allergens, but also patient products and shoe components, she concluded.
Dr. Bembry had no financial conflicts to disclose. Coauthor Amber Atwater, MD, the immediate past president of the ACDS, and associate professor of dermatology at Duke University, disclosed receiving the Pfizer Independent Grant for Learning & Change and consulting for Henkel.
FROM ACDS 2021
Will psoriasis patients embrace proactive topical therapy?
Long-term proactive topical management of plaque psoriasis with twice-weekly calcipotriene/betamethasone dipropionate foam has been shown in a high-quality randomized trial to be more effective than conventional reactive management – but will patients go for it?
Bruce E. Strober, MD, PhD, has his doubts, and he shared them with Linda Stein Gold, MD, after she presented updated results from the 52-week PSO-LONG trial at Innovations in Dermatology: Virtual Spring Conference 2021.
. And while they did so in this study with an assist in the form of monthly office visits and nudging from investigators, in real-world clinical practice that’s unlikely to happen, according to Dr. Strober, of Yale University, New Haven, Conn.
“It makes sense to do what’s being done in this study, there’s no doubt, but I’m concerned about adherence and whether patients are really going to do it,” he said.
“Adherence is going to be everything here, and you know patients don’t like to apply topicals to their body. Once they’re clear they’re just going to walk away from the topical,” Dr. Strober predicted.
Dr. Stein Gold countered: “When a study goes on for a full year, it starts to reflect real life.”
Moreover, the PSO-LONG trial provides the first high-quality evidence physicians can share with patients demonstrating that proactive management pays off in terms of fewer relapses and more time in remission over the long haul, added Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
PSO-LONG was a double-blind, international, phase 3 study including 545 adults with plaque psoriasis who had clear or almost-clear skin after 4 weeks of once-daily calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam (Enstilar), and were then randomized to twice-weekly proactive management or to a reactive approach involving application of vehicle on the same twice-weekly schedule. Relapses resulted in rescue therapy with 4 weeks of once-daily Cal/BD foam.
The primary endpoint was the median time to first relapse: 56 days with the proactive approach, a significant improvement over the 30 days with the reactive approach. Over the course of 52 weeks, the proactive group spent an additional 41 days in remission, compared with the reactive group. Patients randomized to twice-weekly Cal/BD foam averaged 3.1 relapses per year, compared with 4.8 with reactive management. The side-effect profiles in the two study arms were similar.
Mean Physician Global Assessment scores and Psoriasis Area and Activity Index scores for the proactive group clearly separated from the reactive group by week 4, with those differences maintained throughout the year. The area under the curve for distribution for the Physician Global Assessment score was 15% lower in the proactive group, and 20% lower for the modified PASI score.
“These results suggest that proactive management – a concept that’s been used for atopic dermatitis – could be applied to patients with psoriasis to prolong remission,” Dr. Stein Gold concluded at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Asked how confident she is that patients in the real world truly will do this, Dr. Stein Gold replied: “You know, I don’t know. We hope so. Now we can tell them we actually have some data that supports treating the cleared areas. And it’s only twice a week, separated on Mondays and Thursdays.”
“I take a much more reactive approach,” Dr. Strober said. “I advise patients to get back in there with their topical steroid as soon as they see any signs of recurrence.
He added that he’s eager to see if a proactive management approach such as the one that was successful in PSO-LONG is also beneficial using some of the promising topical agents with nonsteroidal mechanisms of action, which are advancing through the developmental pipeline.
Late in 2020, the Food and Drug Administration approved an expanded indication for Cal/BD foam, which includes the PSO-LONG data on the efficacy and safety of long-term twice-weekly therapy in adults in product labeling. The combination spray/foam was previously approved by the FDA as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid as daily therapy.
The PSO-LONG trial was funded by LEO Pharma. Dr. Stein Gold reported serving as a paid investigator and/or consultant to LEO and numerous other pharmaceutical companies. Dr. Strober, reported serving as a consultant to more than two dozen pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Long-term proactive topical management of plaque psoriasis with twice-weekly calcipotriene/betamethasone dipropionate foam has been shown in a high-quality randomized trial to be more effective than conventional reactive management – but will patients go for it?
Bruce E. Strober, MD, PhD, has his doubts, and he shared them with Linda Stein Gold, MD, after she presented updated results from the 52-week PSO-LONG trial at Innovations in Dermatology: Virtual Spring Conference 2021.
. And while they did so in this study with an assist in the form of monthly office visits and nudging from investigators, in real-world clinical practice that’s unlikely to happen, according to Dr. Strober, of Yale University, New Haven, Conn.
“It makes sense to do what’s being done in this study, there’s no doubt, but I’m concerned about adherence and whether patients are really going to do it,” he said.
“Adherence is going to be everything here, and you know patients don’t like to apply topicals to their body. Once they’re clear they’re just going to walk away from the topical,” Dr. Strober predicted.
Dr. Stein Gold countered: “When a study goes on for a full year, it starts to reflect real life.”
Moreover, the PSO-LONG trial provides the first high-quality evidence physicians can share with patients demonstrating that proactive management pays off in terms of fewer relapses and more time in remission over the long haul, added Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
PSO-LONG was a double-blind, international, phase 3 study including 545 adults with plaque psoriasis who had clear or almost-clear skin after 4 weeks of once-daily calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam (Enstilar), and were then randomized to twice-weekly proactive management or to a reactive approach involving application of vehicle on the same twice-weekly schedule. Relapses resulted in rescue therapy with 4 weeks of once-daily Cal/BD foam.
The primary endpoint was the median time to first relapse: 56 days with the proactive approach, a significant improvement over the 30 days with the reactive approach. Over the course of 52 weeks, the proactive group spent an additional 41 days in remission, compared with the reactive group. Patients randomized to twice-weekly Cal/BD foam averaged 3.1 relapses per year, compared with 4.8 with reactive management. The side-effect profiles in the two study arms were similar.
Mean Physician Global Assessment scores and Psoriasis Area and Activity Index scores for the proactive group clearly separated from the reactive group by week 4, with those differences maintained throughout the year. The area under the curve for distribution for the Physician Global Assessment score was 15% lower in the proactive group, and 20% lower for the modified PASI score.
“These results suggest that proactive management – a concept that’s been used for atopic dermatitis – could be applied to patients with psoriasis to prolong remission,” Dr. Stein Gold concluded at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Asked how confident she is that patients in the real world truly will do this, Dr. Stein Gold replied: “You know, I don’t know. We hope so. Now we can tell them we actually have some data that supports treating the cleared areas. And it’s only twice a week, separated on Mondays and Thursdays.”
“I take a much more reactive approach,” Dr. Strober said. “I advise patients to get back in there with their topical steroid as soon as they see any signs of recurrence.
He added that he’s eager to see if a proactive management approach such as the one that was successful in PSO-LONG is also beneficial using some of the promising topical agents with nonsteroidal mechanisms of action, which are advancing through the developmental pipeline.
Late in 2020, the Food and Drug Administration approved an expanded indication for Cal/BD foam, which includes the PSO-LONG data on the efficacy and safety of long-term twice-weekly therapy in adults in product labeling. The combination spray/foam was previously approved by the FDA as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid as daily therapy.
The PSO-LONG trial was funded by LEO Pharma. Dr. Stein Gold reported serving as a paid investigator and/or consultant to LEO and numerous other pharmaceutical companies. Dr. Strober, reported serving as a consultant to more than two dozen pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Long-term proactive topical management of plaque psoriasis with twice-weekly calcipotriene/betamethasone dipropionate foam has been shown in a high-quality randomized trial to be more effective than conventional reactive management – but will patients go for it?
Bruce E. Strober, MD, PhD, has his doubts, and he shared them with Linda Stein Gold, MD, after she presented updated results from the 52-week PSO-LONG trial at Innovations in Dermatology: Virtual Spring Conference 2021.
. And while they did so in this study with an assist in the form of monthly office visits and nudging from investigators, in real-world clinical practice that’s unlikely to happen, according to Dr. Strober, of Yale University, New Haven, Conn.
“It makes sense to do what’s being done in this study, there’s no doubt, but I’m concerned about adherence and whether patients are really going to do it,” he said.
“Adherence is going to be everything here, and you know patients don’t like to apply topicals to their body. Once they’re clear they’re just going to walk away from the topical,” Dr. Strober predicted.
Dr. Stein Gold countered: “When a study goes on for a full year, it starts to reflect real life.”
Moreover, the PSO-LONG trial provides the first high-quality evidence physicians can share with patients demonstrating that proactive management pays off in terms of fewer relapses and more time in remission over the long haul, added Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
PSO-LONG was a double-blind, international, phase 3 study including 545 adults with plaque psoriasis who had clear or almost-clear skin after 4 weeks of once-daily calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam (Enstilar), and were then randomized to twice-weekly proactive management or to a reactive approach involving application of vehicle on the same twice-weekly schedule. Relapses resulted in rescue therapy with 4 weeks of once-daily Cal/BD foam.
The primary endpoint was the median time to first relapse: 56 days with the proactive approach, a significant improvement over the 30 days with the reactive approach. Over the course of 52 weeks, the proactive group spent an additional 41 days in remission, compared with the reactive group. Patients randomized to twice-weekly Cal/BD foam averaged 3.1 relapses per year, compared with 4.8 with reactive management. The side-effect profiles in the two study arms were similar.
Mean Physician Global Assessment scores and Psoriasis Area and Activity Index scores for the proactive group clearly separated from the reactive group by week 4, with those differences maintained throughout the year. The area under the curve for distribution for the Physician Global Assessment score was 15% lower in the proactive group, and 20% lower for the modified PASI score.
“These results suggest that proactive management – a concept that’s been used for atopic dermatitis – could be applied to patients with psoriasis to prolong remission,” Dr. Stein Gold concluded at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Asked how confident she is that patients in the real world truly will do this, Dr. Stein Gold replied: “You know, I don’t know. We hope so. Now we can tell them we actually have some data that supports treating the cleared areas. And it’s only twice a week, separated on Mondays and Thursdays.”
“I take a much more reactive approach,” Dr. Strober said. “I advise patients to get back in there with their topical steroid as soon as they see any signs of recurrence.
He added that he’s eager to see if a proactive management approach such as the one that was successful in PSO-LONG is also beneficial using some of the promising topical agents with nonsteroidal mechanisms of action, which are advancing through the developmental pipeline.
Late in 2020, the Food and Drug Administration approved an expanded indication for Cal/BD foam, which includes the PSO-LONG data on the efficacy and safety of long-term twice-weekly therapy in adults in product labeling. The combination spray/foam was previously approved by the FDA as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid as daily therapy.
The PSO-LONG trial was funded by LEO Pharma. Dr. Stein Gold reported serving as a paid investigator and/or consultant to LEO and numerous other pharmaceutical companies. Dr. Strober, reported serving as a consultant to more than two dozen pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Contact allergen of the year found in foam in shin guards, footwear
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
FROM ACDS 2021
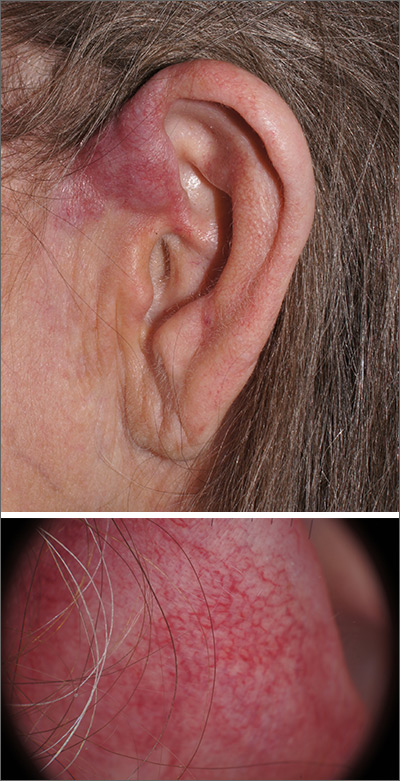
Pink plaque on the ear
A 4-mm punch biopsy was performed and revealed B-cell lymphoma, consistent with extranodal marginal zone lymphoma. The plaque was palpated carefully and the location of branches of the superficial temporal artery, which usually course anterior to the helix, were mapped, and avoided as a biopsy site.
Marginal zone lymphoma is a relatively indolent B-cell lymphoma that occurs in adults in mucosal associated lymphoid tissue, most often in the gastrointestinal (GI) tract. Neoplasms also occur in the lungs, eyes, and skin. Initial symptoms vary according to the site of manifestation. Patients with GI tumors may present with GI bleeding, abdominal pain, and weight loss. Pulmonary lesions are often asymptomatic and picked up on chest imaging for other indications. Chronic gastritis associated with Helicobacter pylori contributes to cases and occasionally eradication of H. pylori may clear patients of disease. Autoimmune diseases, particularly Sjögren disease and Hashimoto thyroiditis, also have a causative association. Rarely, transformation into high-grade disease occurs.
On the skin, marginal zone lymphoma may be exhibited as soft salmon-colored patches with serpentine vascular markings, as seen here on dermoscopy.1 The differential diagnosis includes keloid scar, basal cell carcinoma, Merkel cell carcinoma, arthropod bites, and amelanotic melanoma.
This patient had been under the care of Medical Oncology for surveillance and was offered observation as a potential treatment strategy because of the relatively indolent nature of the tumor. However, because the tumor was painful and growing, she opted for focal palliative radiation therapy.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Marghoob AA, Scope A, et al. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J Eur Acad Dermatol Venereol. 2018;32:53-56. doi:10.1111/jdv.14549
A 4-mm punch biopsy was performed and revealed B-cell lymphoma, consistent with extranodal marginal zone lymphoma. The plaque was palpated carefully and the location of branches of the superficial temporal artery, which usually course anterior to the helix, were mapped, and avoided as a biopsy site.
Marginal zone lymphoma is a relatively indolent B-cell lymphoma that occurs in adults in mucosal associated lymphoid tissue, most often in the gastrointestinal (GI) tract. Neoplasms also occur in the lungs, eyes, and skin. Initial symptoms vary according to the site of manifestation. Patients with GI tumors may present with GI bleeding, abdominal pain, and weight loss. Pulmonary lesions are often asymptomatic and picked up on chest imaging for other indications. Chronic gastritis associated with Helicobacter pylori contributes to cases and occasionally eradication of H. pylori may clear patients of disease. Autoimmune diseases, particularly Sjögren disease and Hashimoto thyroiditis, also have a causative association. Rarely, transformation into high-grade disease occurs.
On the skin, marginal zone lymphoma may be exhibited as soft salmon-colored patches with serpentine vascular markings, as seen here on dermoscopy.1 The differential diagnosis includes keloid scar, basal cell carcinoma, Merkel cell carcinoma, arthropod bites, and amelanotic melanoma.
This patient had been under the care of Medical Oncology for surveillance and was offered observation as a potential treatment strategy because of the relatively indolent nature of the tumor. However, because the tumor was painful and growing, she opted for focal palliative radiation therapy.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
A 4-mm punch biopsy was performed and revealed B-cell lymphoma, consistent with extranodal marginal zone lymphoma. The plaque was palpated carefully and the location of branches of the superficial temporal artery, which usually course anterior to the helix, were mapped, and avoided as a biopsy site.
Marginal zone lymphoma is a relatively indolent B-cell lymphoma that occurs in adults in mucosal associated lymphoid tissue, most often in the gastrointestinal (GI) tract. Neoplasms also occur in the lungs, eyes, and skin. Initial symptoms vary according to the site of manifestation. Patients with GI tumors may present with GI bleeding, abdominal pain, and weight loss. Pulmonary lesions are often asymptomatic and picked up on chest imaging for other indications. Chronic gastritis associated with Helicobacter pylori contributes to cases and occasionally eradication of H. pylori may clear patients of disease. Autoimmune diseases, particularly Sjögren disease and Hashimoto thyroiditis, also have a causative association. Rarely, transformation into high-grade disease occurs.
On the skin, marginal zone lymphoma may be exhibited as soft salmon-colored patches with serpentine vascular markings, as seen here on dermoscopy.1 The differential diagnosis includes keloid scar, basal cell carcinoma, Merkel cell carcinoma, arthropod bites, and amelanotic melanoma.
This patient had been under the care of Medical Oncology for surveillance and was offered observation as a potential treatment strategy because of the relatively indolent nature of the tumor. However, because the tumor was painful and growing, she opted for focal palliative radiation therapy.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Marghoob AA, Scope A, et al. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J Eur Acad Dermatol Venereol. 2018;32:53-56. doi:10.1111/jdv.14549
1. Geller S, Marghoob AA, Scope A, et al. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J Eur Acad Dermatol Venereol. 2018;32:53-56. doi:10.1111/jdv.14549
Itch response faster with abrocitinib in trial comparing JAK inhibitor to dupilumab
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.
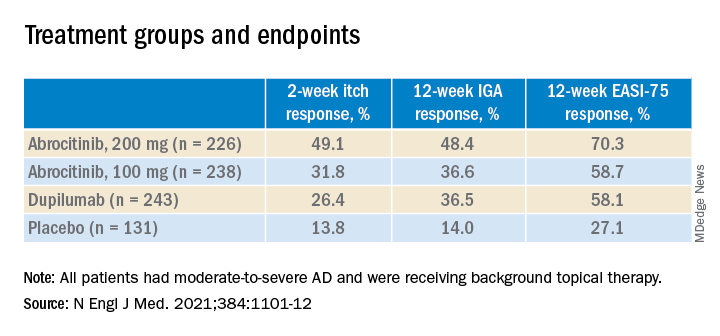
The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.

The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.

The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
Ruxolitinib cream for atopic dermatitis is in regulatory home stretch
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
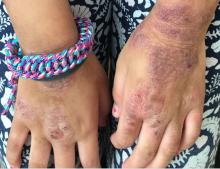
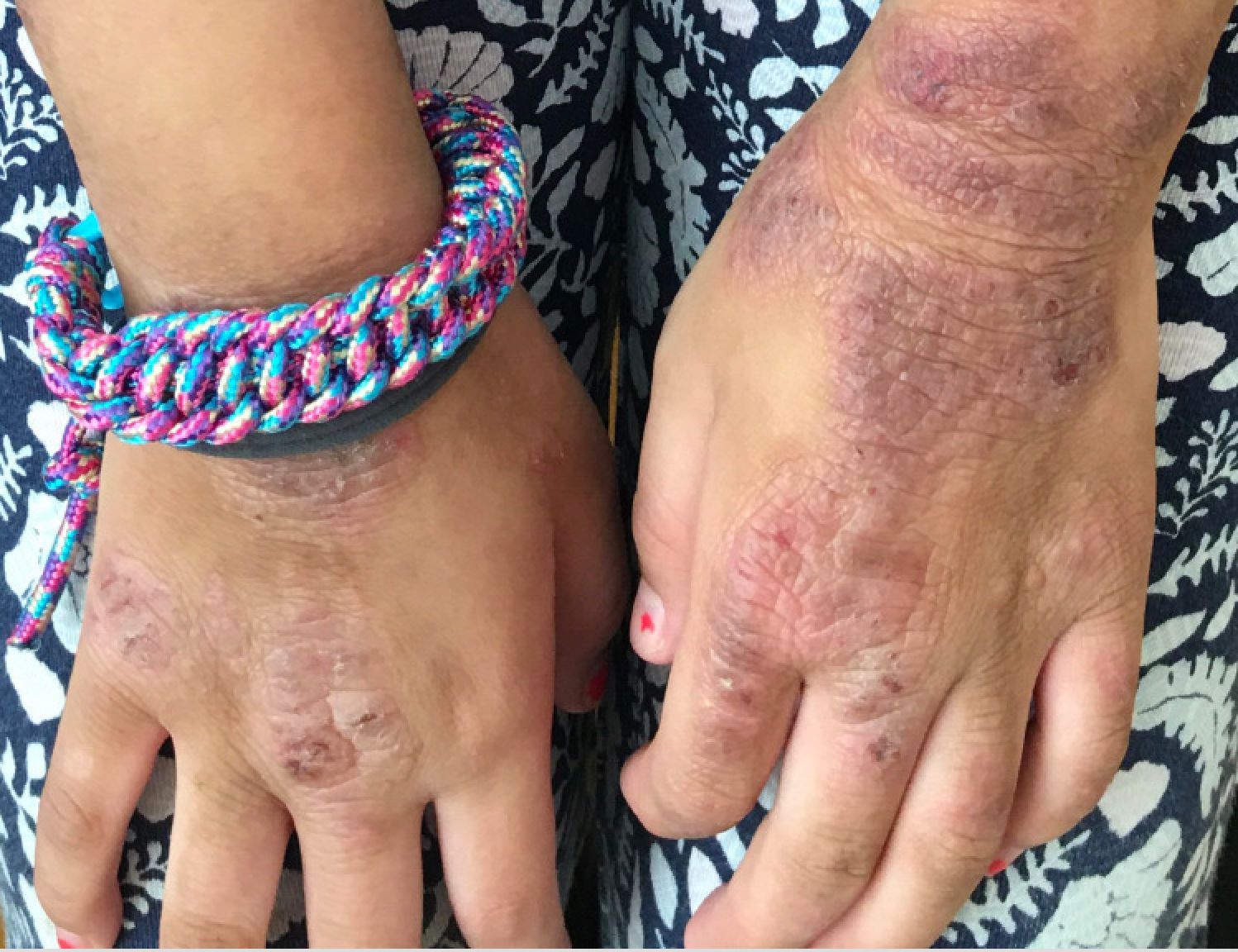
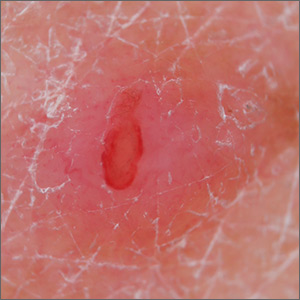
A young girl presents with ‘itchy, rashy’ hands
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Examination findings of the bilateral hands and wrists demonstrate plaques of erythema, lichenification, and scale of the dorsal surfaces of the hands and digits. Closer inspection reveals fissuring and erythematous crust of the affected skin but normal nails. The rest of the skin exam is unremarkable.
Pink papule on thigh

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10
An 80-year-old patient presents with an asymptomatic firm pink plaque on his shoulder
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
AAP issues five recommendations for common dermatologic problems
The American Academy of Pediatrics recently issued five recommendations for the most common dermatologic problems in primary care pediatrics.
Topics include diagnostic and management strategies for a variety of conditions, including atopic dermatitis, fungal infections, and autoimmune conditions.
The AAP Section on Dermatology created the recommendations, which were then reviewed and approved by “more than a dozen relevant AAP committees, councils, and sections,” before final approval by the AAP executive committee and board of directors.
The final list represents a collaborative effort with the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, which aims “to promote conversations between clinicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm, [and] truly necessary.”
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said that the recommendations are “a fine set of suggestions to help health care providers with some of their pediatric dermatology issues.”
• To begin, the AAP recommended against use of combination topical steroid antifungals for candida skin infections, diaper dermatitis, and tinea corporis, despite approvals for these indications.
“Many providers are unaware that the combination products contain a relatively high-potency topical steroid,” the AAP wrote, noting that “combination products are also often expensive and not covered by pharmacy plans.”
Diaper dermatitis responds best to barrier creams and ointments alone, according to the AAP. If needed, a topical, low-potency steroid may be used no more than twice a day, and tapered with improvement. Similarly, the AAP recommended a separate, low-potency steroid for tinea corporis if pruritus is severe.
• In contrast with this call for minimal treatment intensity, the AAP recommended a more intensive approach to tinea capitis, advising against topical medications alone.
“Topical treatments cannot penetrate the hair shaft itself, which is where the infection lies; thus, monotherapy with topical medications is insufficient to effectively treat the infection,” the AAP wrote. “This insufficient treatment can lead to increased health care costs resulting from multiple visits and the prescribing of ineffective medications.”
While medicated shampoos may still be used as adjunctive treatments for tinea capitis, the AAP recommended primary therapy with either griseofulvin or terbinafine, slightly favoring terbinafine because of adequate efficacy, lesser expense, and shorter regimen.
According to Dr. Eichenfield, a more thorough workup should also be considered.
“Consider culturing possible tinea capitis, so that oral antifungals can be used judiciously and not used for other scaling scalp diagnoses,” he said.
• For most cases of atopic dermatitis, the AAP advised against oral or injected corticosteroids, despite rapid efficacy, because of potential for adverse events, such as adrenal suppression, growth retardation, and disease worsening upon discontinuation. Instead, they recommended topical therapies, “good skin care practices,” and if necessary, “phototherapy and/or steroid-sparing systemic agents.”
“Systemic corticosteroids should only be prescribed for severe flares once all other treatment options have been exhausted and should be limited to a short course for the purpose of bridging to a steroid-sparing agent,” the AAP wrote.
Dr. Eichenfield emphasized this point, noting that new therapies have expanded treatment options.
“Be aware of the advances in atopic dermatitis,” he said, “with newer topical medications and with a new systemic biologic agent approved for moderate to severe refractory atopic dermatitis for ages 6 and older.”
• Turning to diagnostic strategies, the AAP recommended against routine laboratory testing for associated autoimmune diseases among patients with vitiligo, unless clinical signs and/or symptoms of such diseases are present.
“There is no convincing evidence that extensive workups in the absence of specific clinical suspicion improves outcomes for patients and may in fact beget additional costs and harms,” the AAP wrote. “Although many studies suggest ordering these tests, it is based largely on the increased cosegregation of vitiligo and thyroid disease and not on improved outcomes from having identified an abnormal laboratory test result.”
• Similarly, the AAP advised practitioners to avoid routinely testing patients with alopecia areata for other diseases if relevant symptoms and signs aren’t present.
“As in the case of vitiligo, it is more common to find thyroid autoantibodies or subclinical hypothyroidism than overt thyroid disease, unless there are clinically suspicious findings,” the AAP wrote. “Patients identified as having subclinical hypothyroidism are not currently treated and may even have resolution of the abnormal TSH.”
Before drawing blood, Dr. Eichenfield suggested that clinicians first ask the right questions.
“Be comfortable with screening questions about growth, weight, or activity changes to assist with decisions for thyroid screening in a patient with vitiligo or alopecia areata,” he said.
Choosing Wisely is an initiative of the American Board of Internal Medicine. The AAP and Dr. Eichenfield reported no conflicts of interest.
The American Academy of Pediatrics recently issued five recommendations for the most common dermatologic problems in primary care pediatrics.
Topics include diagnostic and management strategies for a variety of conditions, including atopic dermatitis, fungal infections, and autoimmune conditions.
The AAP Section on Dermatology created the recommendations, which were then reviewed and approved by “more than a dozen relevant AAP committees, councils, and sections,” before final approval by the AAP executive committee and board of directors.
The final list represents a collaborative effort with the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, which aims “to promote conversations between clinicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm, [and] truly necessary.”
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said that the recommendations are “a fine set of suggestions to help health care providers with some of their pediatric dermatology issues.”
• To begin, the AAP recommended against use of combination topical steroid antifungals for candida skin infections, diaper dermatitis, and tinea corporis, despite approvals for these indications.
“Many providers are unaware that the combination products contain a relatively high-potency topical steroid,” the AAP wrote, noting that “combination products are also often expensive and not covered by pharmacy plans.”
Diaper dermatitis responds best to barrier creams and ointments alone, according to the AAP. If needed, a topical, low-potency steroid may be used no more than twice a day, and tapered with improvement. Similarly, the AAP recommended a separate, low-potency steroid for tinea corporis if pruritus is severe.
• In contrast with this call for minimal treatment intensity, the AAP recommended a more intensive approach to tinea capitis, advising against topical medications alone.
“Topical treatments cannot penetrate the hair shaft itself, which is where the infection lies; thus, monotherapy with topical medications is insufficient to effectively treat the infection,” the AAP wrote. “This insufficient treatment can lead to increased health care costs resulting from multiple visits and the prescribing of ineffective medications.”
While medicated shampoos may still be used as adjunctive treatments for tinea capitis, the AAP recommended primary therapy with either griseofulvin or terbinafine, slightly favoring terbinafine because of adequate efficacy, lesser expense, and shorter regimen.
According to Dr. Eichenfield, a more thorough workup should also be considered.
“Consider culturing possible tinea capitis, so that oral antifungals can be used judiciously and not used for other scaling scalp diagnoses,” he said.
• For most cases of atopic dermatitis, the AAP advised against oral or injected corticosteroids, despite rapid efficacy, because of potential for adverse events, such as adrenal suppression, growth retardation, and disease worsening upon discontinuation. Instead, they recommended topical therapies, “good skin care practices,” and if necessary, “phototherapy and/or steroid-sparing systemic agents.”
“Systemic corticosteroids should only be prescribed for severe flares once all other treatment options have been exhausted and should be limited to a short course for the purpose of bridging to a steroid-sparing agent,” the AAP wrote.
Dr. Eichenfield emphasized this point, noting that new therapies have expanded treatment options.
“Be aware of the advances in atopic dermatitis,” he said, “with newer topical medications and with a new systemic biologic agent approved for moderate to severe refractory atopic dermatitis for ages 6 and older.”
• Turning to diagnostic strategies, the AAP recommended against routine laboratory testing for associated autoimmune diseases among patients with vitiligo, unless clinical signs and/or symptoms of such diseases are present.
“There is no convincing evidence that extensive workups in the absence of specific clinical suspicion improves outcomes for patients and may in fact beget additional costs and harms,” the AAP wrote. “Although many studies suggest ordering these tests, it is based largely on the increased cosegregation of vitiligo and thyroid disease and not on improved outcomes from having identified an abnormal laboratory test result.”
• Similarly, the AAP advised practitioners to avoid routinely testing patients with alopecia areata for other diseases if relevant symptoms and signs aren’t present.
“As in the case of vitiligo, it is more common to find thyroid autoantibodies or subclinical hypothyroidism than overt thyroid disease, unless there are clinically suspicious findings,” the AAP wrote. “Patients identified as having subclinical hypothyroidism are not currently treated and may even have resolution of the abnormal TSH.”
Before drawing blood, Dr. Eichenfield suggested that clinicians first ask the right questions.
“Be comfortable with screening questions about growth, weight, or activity changes to assist with decisions for thyroid screening in a patient with vitiligo or alopecia areata,” he said.
Choosing Wisely is an initiative of the American Board of Internal Medicine. The AAP and Dr. Eichenfield reported no conflicts of interest.
The American Academy of Pediatrics recently issued five recommendations for the most common dermatologic problems in primary care pediatrics.
Topics include diagnostic and management strategies for a variety of conditions, including atopic dermatitis, fungal infections, and autoimmune conditions.
The AAP Section on Dermatology created the recommendations, which were then reviewed and approved by “more than a dozen relevant AAP committees, councils, and sections,” before final approval by the AAP executive committee and board of directors.
The final list represents a collaborative effort with the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, which aims “to promote conversations between clinicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm, [and] truly necessary.”
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said that the recommendations are “a fine set of suggestions to help health care providers with some of their pediatric dermatology issues.”
• To begin, the AAP recommended against use of combination topical steroid antifungals for candida skin infections, diaper dermatitis, and tinea corporis, despite approvals for these indications.
“Many providers are unaware that the combination products contain a relatively high-potency topical steroid,” the AAP wrote, noting that “combination products are also often expensive and not covered by pharmacy plans.”
Diaper dermatitis responds best to barrier creams and ointments alone, according to the AAP. If needed, a topical, low-potency steroid may be used no more than twice a day, and tapered with improvement. Similarly, the AAP recommended a separate, low-potency steroid for tinea corporis if pruritus is severe.
• In contrast with this call for minimal treatment intensity, the AAP recommended a more intensive approach to tinea capitis, advising against topical medications alone.
“Topical treatments cannot penetrate the hair shaft itself, which is where the infection lies; thus, monotherapy with topical medications is insufficient to effectively treat the infection,” the AAP wrote. “This insufficient treatment can lead to increased health care costs resulting from multiple visits and the prescribing of ineffective medications.”
While medicated shampoos may still be used as adjunctive treatments for tinea capitis, the AAP recommended primary therapy with either griseofulvin or terbinafine, slightly favoring terbinafine because of adequate efficacy, lesser expense, and shorter regimen.
According to Dr. Eichenfield, a more thorough workup should also be considered.
“Consider culturing possible tinea capitis, so that oral antifungals can be used judiciously and not used for other scaling scalp diagnoses,” he said.
• For most cases of atopic dermatitis, the AAP advised against oral or injected corticosteroids, despite rapid efficacy, because of potential for adverse events, such as adrenal suppression, growth retardation, and disease worsening upon discontinuation. Instead, they recommended topical therapies, “good skin care practices,” and if necessary, “phototherapy and/or steroid-sparing systemic agents.”
“Systemic corticosteroids should only be prescribed for severe flares once all other treatment options have been exhausted and should be limited to a short course for the purpose of bridging to a steroid-sparing agent,” the AAP wrote.
Dr. Eichenfield emphasized this point, noting that new therapies have expanded treatment options.
“Be aware of the advances in atopic dermatitis,” he said, “with newer topical medications and with a new systemic biologic agent approved for moderate to severe refractory atopic dermatitis for ages 6 and older.”
• Turning to diagnostic strategies, the AAP recommended against routine laboratory testing for associated autoimmune diseases among patients with vitiligo, unless clinical signs and/or symptoms of such diseases are present.
“There is no convincing evidence that extensive workups in the absence of specific clinical suspicion improves outcomes for patients and may in fact beget additional costs and harms,” the AAP wrote. “Although many studies suggest ordering these tests, it is based largely on the increased cosegregation of vitiligo and thyroid disease and not on improved outcomes from having identified an abnormal laboratory test result.”
• Similarly, the AAP advised practitioners to avoid routinely testing patients with alopecia areata for other diseases if relevant symptoms and signs aren’t present.
“As in the case of vitiligo, it is more common to find thyroid autoantibodies or subclinical hypothyroidism than overt thyroid disease, unless there are clinically suspicious findings,” the AAP wrote. “Patients identified as having subclinical hypothyroidism are not currently treated and may even have resolution of the abnormal TSH.”
Before drawing blood, Dr. Eichenfield suggested that clinicians first ask the right questions.
“Be comfortable with screening questions about growth, weight, or activity changes to assist with decisions for thyroid screening in a patient with vitiligo or alopecia areata,” he said.
Choosing Wisely is an initiative of the American Board of Internal Medicine. The AAP and Dr. Eichenfield reported no conflicts of interest.
FROM CHOOSING WISELY AND THE AAP