User login
What’s the future of telehealth? It’s ‘complicated’
pre-AAD meeting.
“We have seen large numbers of children struggle with access to school and access to health care because of lack of access to devices, challenges of broadband Internet access, culture, language, and educational barriers – just having trouble being comfortable with this technology,” said Natalie Pageler, MD, a pediatric intensivist and chief medical information officer at Stanford Children’s Health, Palo Alto, Calif.
“There are also privacy concerns, especially in situations where there are multiple families within a household. Finally, it’s important to remember that policy and reimbursement issues may have a significant effect on some of the socioeconomic barriers,” she added. “For example, many of our families who don’t have access to audio and video may be able to do a telephone call, but it’s important that telephone calls be considered a form of telehealth and be reimbursed to help increase the access to health care by these families. It also makes it easier to facilitate coordination of care. All of this leads to decreased time and costs for patients, families, and providers.”
Within the first few weeks of the pandemic, Dr. Pageler and colleagues at Stanford Children’s Health observed an increase from about 20 telehealth visits per day to more than 700 per day, which has held stable. While the benefits of telehealth are clear, many perceived barriers exist. In a study conducted prior to the COVID-19 pandemic, researchers identified a wide variety of barriers to implementation of telehealth, led by reimbursement, followed by poor business model sustainability, lack of provider time, and provider interest.
“Some of the barriers, like patient preferences for inpatient care, lack of provider interest in telehealth, and lack of provider time were easily overcome during the COVID pandemic,” Dr. Pageler said. “We dedicated the time to train immediately, because the need was so great.”
In 2018, Patrick McMahon, MD, and colleagues at Children’s Hospital of Philadelphia, launched a teledermatology program that provided direct-to-patient “E-visits” and recently pivoted to using this service only for acne patients through a program called “Acne Express.” The out-of-pocket cost to patients is $50 per consult and nearly 1,500 cases have been completed since 2018, which has saved patients and their parents an estimated 65,000 miles driving to the clinic.
“In the last year we have piloted something called “E-Consults,” which is a provider-to-provider, store-and-forward service,” said Dr. McMahon, a pediatric dermatologist and director of teledermatology at CHOP. “That service is not currently reimbursable, but it’s funded through our hospital. We also have live video visits between provider and patient. That is reimbursable. We have done about 7,500 of those.”
In a 2020 unpublished membership survey of SPD members, Dr. McMahon and colleagues posed the question, “How has teledermatology positively impacted your practice over the past year?” The top three responses were that teledermatology was safe during COVID-19, it provided easy access for follow-up, and it was convenient. In response to the question, “What is the most fundamental change needed for successful delivery of pediatric teledermatology?” the top three responses were reimbursement, improved technology, and regulatory changes.
“When we asked about struggles and difficulties, a lot of responses surrounded the lack of connectivity, both from a technological standpoint and also that lack of connectivity we would feel in person – a lack of rapport,” Dr. McMahon said. “There’s also the inability for us to touch and feel when we examine, and we worry about misdiagnosing. There are also concerns about disparities and for us being sedentary – sitting in one place staring at a screen.”
To optimize the teledermatology experience, he suggested four pillars: educate, optimize, reach out, and tailor. “I think we need to draw upon some of the digital education we already have, including a handout for patients [on the SPD website] that offers tips on taking a clear photograph on their smartphones,” he said. “We’re also trying to use some of the cases and learnings from our teledermatology experiences to teach the providers. We are setting up CME modules that are sort of a flashcard-based teaching mechanism.”
To optimize teledermatology experiences, he continued, tracking demographics, diagnoses, number of cases, and turnaround time is helpful. “We can then track who’s coming in to see us at follow-up after a new visit through telehealth,” Dr. McMahon said. “This helps us repurpose things, pivot as needed, and find any glitches. Surveying the families is also critical. Finally, we need clinical support to tee-up visits and to ensure photos are submitted and efficient, and to match diagnoses and family preference with the right modality.”
Another panelist, Justin M. Ko, MD, MBA, who chairs the American Academy of Dermatology’s Task Force on Augmented Intelligence, said that digitally enabled and artificial intelligence (AI)-augmented care delivery offers a “unique opportunity” for increasing access and increasing the value of care delivered to patients.
“The role that we play as clinicians is central, and I think we can make significant strides by doing two things,” said Dr. Ko, chief of medical dermatology for Stanford (Calif.) Health Care. “One: extending the reach of our expertise, and the second: scaling the impact of the care we deliver by clinician-driven, patient-centered, digitally-enabled, AI-augmented care delivery innovation. This opportunity for digital care transformation is more than just a transition from in-person visits to video visits. We have to look at this as an opportunity to leverage the unique aspects of digital capabilities and fundamentally reimagine how we deliver care.”
The AAD’s Position Statement on Augmented Intelligence was published in 2019.
Between March and June of 2021, Neil S. Prose, MD, conducted about 300 televisits with patients. “I had a few spectacular visits where, for example, a teenage patient who had been challenging showed me all of her artwork and we became instantly more connected,” said Dr. Prose, professor of dermatology, pediatrics, and global health at Duke University, Durham, N.C. “Then there’s the potential for a long-term improvement in health care for some patients.”
But there were also downsides to the process, he said, including dropped connections, poor picture and sound quality, patient no-shows, and patients reporting they were unable to schedule a telemedicine visit. “The problems I was experiencing were not just between me and my patients; the problems are systemic, and they have to do with various factors: the portal, the equipment, Internet access, and inadequate or no health insurance,” said Dr. Prose, past president of the SPD.
Portal-related challenges include a lack of focus on culture, literacy, and numeracy, “and these worsen inequities,” he said. “Another issue related to portal design has to do with language. Very few of the portals allow patients to participate in Spanish. This has been particularly difficult for those of us who use Epic. The next issue has to deal with the devices the patients are using. Cell phone visits can be very problematic. Unfortunately, lower-income Americans have a lower level of technology adoption, and many are relying on smartphones for their Internet access. That’s the root of some of our problems.”
To achieve digital health equity, Dr. Prose emphasized the need for federal mandates for tools for digital health access usable by underserved populations and federal policies that increase broadband access and view it as a human right. He also underscored the importance of federal policies that ensure continuation of adequate telemedicine reimbursement beyond the pandemic and urged health institutions to invest in portals that address the needs of the underserved.
“What is the future of telemedicine? The answer is complicated,” said Dr. Prose, who recommended a recently published article in JAMA on digital health equity. “There have been several rumblings of large insurers who plan to pull the rug on telemedicine as soon as the pandemic is more or less over. So, all of our projections about this being a wonderful trend for the future may be for naught if the insurers don’t step up to the table.”
None of the presenters reported having financial disclosures.
pre-AAD meeting.
“We have seen large numbers of children struggle with access to school and access to health care because of lack of access to devices, challenges of broadband Internet access, culture, language, and educational barriers – just having trouble being comfortable with this technology,” said Natalie Pageler, MD, a pediatric intensivist and chief medical information officer at Stanford Children’s Health, Palo Alto, Calif.
“There are also privacy concerns, especially in situations where there are multiple families within a household. Finally, it’s important to remember that policy and reimbursement issues may have a significant effect on some of the socioeconomic barriers,” she added. “For example, many of our families who don’t have access to audio and video may be able to do a telephone call, but it’s important that telephone calls be considered a form of telehealth and be reimbursed to help increase the access to health care by these families. It also makes it easier to facilitate coordination of care. All of this leads to decreased time and costs for patients, families, and providers.”
Within the first few weeks of the pandemic, Dr. Pageler and colleagues at Stanford Children’s Health observed an increase from about 20 telehealth visits per day to more than 700 per day, which has held stable. While the benefits of telehealth are clear, many perceived barriers exist. In a study conducted prior to the COVID-19 pandemic, researchers identified a wide variety of barriers to implementation of telehealth, led by reimbursement, followed by poor business model sustainability, lack of provider time, and provider interest.
“Some of the barriers, like patient preferences for inpatient care, lack of provider interest in telehealth, and lack of provider time were easily overcome during the COVID pandemic,” Dr. Pageler said. “We dedicated the time to train immediately, because the need was so great.”
In 2018, Patrick McMahon, MD, and colleagues at Children’s Hospital of Philadelphia, launched a teledermatology program that provided direct-to-patient “E-visits” and recently pivoted to using this service only for acne patients through a program called “Acne Express.” The out-of-pocket cost to patients is $50 per consult and nearly 1,500 cases have been completed since 2018, which has saved patients and their parents an estimated 65,000 miles driving to the clinic.
“In the last year we have piloted something called “E-Consults,” which is a provider-to-provider, store-and-forward service,” said Dr. McMahon, a pediatric dermatologist and director of teledermatology at CHOP. “That service is not currently reimbursable, but it’s funded through our hospital. We also have live video visits between provider and patient. That is reimbursable. We have done about 7,500 of those.”
In a 2020 unpublished membership survey of SPD members, Dr. McMahon and colleagues posed the question, “How has teledermatology positively impacted your practice over the past year?” The top three responses were that teledermatology was safe during COVID-19, it provided easy access for follow-up, and it was convenient. In response to the question, “What is the most fundamental change needed for successful delivery of pediatric teledermatology?” the top three responses were reimbursement, improved technology, and regulatory changes.
“When we asked about struggles and difficulties, a lot of responses surrounded the lack of connectivity, both from a technological standpoint and also that lack of connectivity we would feel in person – a lack of rapport,” Dr. McMahon said. “There’s also the inability for us to touch and feel when we examine, and we worry about misdiagnosing. There are also concerns about disparities and for us being sedentary – sitting in one place staring at a screen.”
To optimize the teledermatology experience, he suggested four pillars: educate, optimize, reach out, and tailor. “I think we need to draw upon some of the digital education we already have, including a handout for patients [on the SPD website] that offers tips on taking a clear photograph on their smartphones,” he said. “We’re also trying to use some of the cases and learnings from our teledermatology experiences to teach the providers. We are setting up CME modules that are sort of a flashcard-based teaching mechanism.”
To optimize teledermatology experiences, he continued, tracking demographics, diagnoses, number of cases, and turnaround time is helpful. “We can then track who’s coming in to see us at follow-up after a new visit through telehealth,” Dr. McMahon said. “This helps us repurpose things, pivot as needed, and find any glitches. Surveying the families is also critical. Finally, we need clinical support to tee-up visits and to ensure photos are submitted and efficient, and to match diagnoses and family preference with the right modality.”
Another panelist, Justin M. Ko, MD, MBA, who chairs the American Academy of Dermatology’s Task Force on Augmented Intelligence, said that digitally enabled and artificial intelligence (AI)-augmented care delivery offers a “unique opportunity” for increasing access and increasing the value of care delivered to patients.
“The role that we play as clinicians is central, and I think we can make significant strides by doing two things,” said Dr. Ko, chief of medical dermatology for Stanford (Calif.) Health Care. “One: extending the reach of our expertise, and the second: scaling the impact of the care we deliver by clinician-driven, patient-centered, digitally-enabled, AI-augmented care delivery innovation. This opportunity for digital care transformation is more than just a transition from in-person visits to video visits. We have to look at this as an opportunity to leverage the unique aspects of digital capabilities and fundamentally reimagine how we deliver care.”
The AAD’s Position Statement on Augmented Intelligence was published in 2019.
Between March and June of 2021, Neil S. Prose, MD, conducted about 300 televisits with patients. “I had a few spectacular visits where, for example, a teenage patient who had been challenging showed me all of her artwork and we became instantly more connected,” said Dr. Prose, professor of dermatology, pediatrics, and global health at Duke University, Durham, N.C. “Then there’s the potential for a long-term improvement in health care for some patients.”
But there were also downsides to the process, he said, including dropped connections, poor picture and sound quality, patient no-shows, and patients reporting they were unable to schedule a telemedicine visit. “The problems I was experiencing were not just between me and my patients; the problems are systemic, and they have to do with various factors: the portal, the equipment, Internet access, and inadequate or no health insurance,” said Dr. Prose, past president of the SPD.
Portal-related challenges include a lack of focus on culture, literacy, and numeracy, “and these worsen inequities,” he said. “Another issue related to portal design has to do with language. Very few of the portals allow patients to participate in Spanish. This has been particularly difficult for those of us who use Epic. The next issue has to deal with the devices the patients are using. Cell phone visits can be very problematic. Unfortunately, lower-income Americans have a lower level of technology adoption, and many are relying on smartphones for their Internet access. That’s the root of some of our problems.”
To achieve digital health equity, Dr. Prose emphasized the need for federal mandates for tools for digital health access usable by underserved populations and federal policies that increase broadband access and view it as a human right. He also underscored the importance of federal policies that ensure continuation of adequate telemedicine reimbursement beyond the pandemic and urged health institutions to invest in portals that address the needs of the underserved.
“What is the future of telemedicine? The answer is complicated,” said Dr. Prose, who recommended a recently published article in JAMA on digital health equity. “There have been several rumblings of large insurers who plan to pull the rug on telemedicine as soon as the pandemic is more or less over. So, all of our projections about this being a wonderful trend for the future may be for naught if the insurers don’t step up to the table.”
None of the presenters reported having financial disclosures.
pre-AAD meeting.
“We have seen large numbers of children struggle with access to school and access to health care because of lack of access to devices, challenges of broadband Internet access, culture, language, and educational barriers – just having trouble being comfortable with this technology,” said Natalie Pageler, MD, a pediatric intensivist and chief medical information officer at Stanford Children’s Health, Palo Alto, Calif.
“There are also privacy concerns, especially in situations where there are multiple families within a household. Finally, it’s important to remember that policy and reimbursement issues may have a significant effect on some of the socioeconomic barriers,” she added. “For example, many of our families who don’t have access to audio and video may be able to do a telephone call, but it’s important that telephone calls be considered a form of telehealth and be reimbursed to help increase the access to health care by these families. It also makes it easier to facilitate coordination of care. All of this leads to decreased time and costs for patients, families, and providers.”
Within the first few weeks of the pandemic, Dr. Pageler and colleagues at Stanford Children’s Health observed an increase from about 20 telehealth visits per day to more than 700 per day, which has held stable. While the benefits of telehealth are clear, many perceived barriers exist. In a study conducted prior to the COVID-19 pandemic, researchers identified a wide variety of barriers to implementation of telehealth, led by reimbursement, followed by poor business model sustainability, lack of provider time, and provider interest.
“Some of the barriers, like patient preferences for inpatient care, lack of provider interest in telehealth, and lack of provider time were easily overcome during the COVID pandemic,” Dr. Pageler said. “We dedicated the time to train immediately, because the need was so great.”
In 2018, Patrick McMahon, MD, and colleagues at Children’s Hospital of Philadelphia, launched a teledermatology program that provided direct-to-patient “E-visits” and recently pivoted to using this service only for acne patients through a program called “Acne Express.” The out-of-pocket cost to patients is $50 per consult and nearly 1,500 cases have been completed since 2018, which has saved patients and their parents an estimated 65,000 miles driving to the clinic.
“In the last year we have piloted something called “E-Consults,” which is a provider-to-provider, store-and-forward service,” said Dr. McMahon, a pediatric dermatologist and director of teledermatology at CHOP. “That service is not currently reimbursable, but it’s funded through our hospital. We also have live video visits between provider and patient. That is reimbursable. We have done about 7,500 of those.”
In a 2020 unpublished membership survey of SPD members, Dr. McMahon and colleagues posed the question, “How has teledermatology positively impacted your practice over the past year?” The top three responses were that teledermatology was safe during COVID-19, it provided easy access for follow-up, and it was convenient. In response to the question, “What is the most fundamental change needed for successful delivery of pediatric teledermatology?” the top three responses were reimbursement, improved technology, and regulatory changes.
“When we asked about struggles and difficulties, a lot of responses surrounded the lack of connectivity, both from a technological standpoint and also that lack of connectivity we would feel in person – a lack of rapport,” Dr. McMahon said. “There’s also the inability for us to touch and feel when we examine, and we worry about misdiagnosing. There are also concerns about disparities and for us being sedentary – sitting in one place staring at a screen.”
To optimize the teledermatology experience, he suggested four pillars: educate, optimize, reach out, and tailor. “I think we need to draw upon some of the digital education we already have, including a handout for patients [on the SPD website] that offers tips on taking a clear photograph on their smartphones,” he said. “We’re also trying to use some of the cases and learnings from our teledermatology experiences to teach the providers. We are setting up CME modules that are sort of a flashcard-based teaching mechanism.”
To optimize teledermatology experiences, he continued, tracking demographics, diagnoses, number of cases, and turnaround time is helpful. “We can then track who’s coming in to see us at follow-up after a new visit through telehealth,” Dr. McMahon said. “This helps us repurpose things, pivot as needed, and find any glitches. Surveying the families is also critical. Finally, we need clinical support to tee-up visits and to ensure photos are submitted and efficient, and to match diagnoses and family preference with the right modality.”
Another panelist, Justin M. Ko, MD, MBA, who chairs the American Academy of Dermatology’s Task Force on Augmented Intelligence, said that digitally enabled and artificial intelligence (AI)-augmented care delivery offers a “unique opportunity” for increasing access and increasing the value of care delivered to patients.
“The role that we play as clinicians is central, and I think we can make significant strides by doing two things,” said Dr. Ko, chief of medical dermatology for Stanford (Calif.) Health Care. “One: extending the reach of our expertise, and the second: scaling the impact of the care we deliver by clinician-driven, patient-centered, digitally-enabled, AI-augmented care delivery innovation. This opportunity for digital care transformation is more than just a transition from in-person visits to video visits. We have to look at this as an opportunity to leverage the unique aspects of digital capabilities and fundamentally reimagine how we deliver care.”
The AAD’s Position Statement on Augmented Intelligence was published in 2019.
Between March and June of 2021, Neil S. Prose, MD, conducted about 300 televisits with patients. “I had a few spectacular visits where, for example, a teenage patient who had been challenging showed me all of her artwork and we became instantly more connected,” said Dr. Prose, professor of dermatology, pediatrics, and global health at Duke University, Durham, N.C. “Then there’s the potential for a long-term improvement in health care for some patients.”
But there were also downsides to the process, he said, including dropped connections, poor picture and sound quality, patient no-shows, and patients reporting they were unable to schedule a telemedicine visit. “The problems I was experiencing were not just between me and my patients; the problems are systemic, and they have to do with various factors: the portal, the equipment, Internet access, and inadequate or no health insurance,” said Dr. Prose, past president of the SPD.
Portal-related challenges include a lack of focus on culture, literacy, and numeracy, “and these worsen inequities,” he said. “Another issue related to portal design has to do with language. Very few of the portals allow patients to participate in Spanish. This has been particularly difficult for those of us who use Epic. The next issue has to deal with the devices the patients are using. Cell phone visits can be very problematic. Unfortunately, lower-income Americans have a lower level of technology adoption, and many are relying on smartphones for their Internet access. That’s the root of some of our problems.”
To achieve digital health equity, Dr. Prose emphasized the need for federal mandates for tools for digital health access usable by underserved populations and federal policies that increase broadband access and view it as a human right. He also underscored the importance of federal policies that ensure continuation of adequate telemedicine reimbursement beyond the pandemic and urged health institutions to invest in portals that address the needs of the underserved.
“What is the future of telemedicine? The answer is complicated,” said Dr. Prose, who recommended a recently published article in JAMA on digital health equity. “There have been several rumblings of large insurers who plan to pull the rug on telemedicine as soon as the pandemic is more or less over. So, all of our projections about this being a wonderful trend for the future may be for naught if the insurers don’t step up to the table.”
None of the presenters reported having financial disclosures.
FROM THE SPD PRE-AAD MEETING
Guarding against nonmelanoma skin cancer in solid organ transplant recipients
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
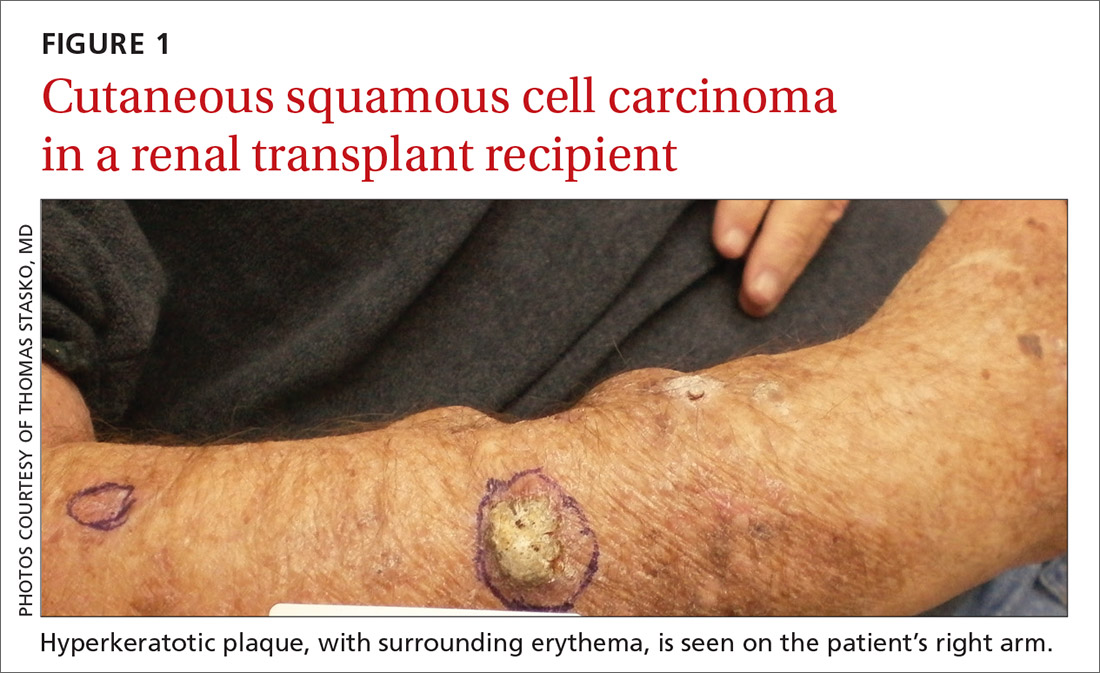
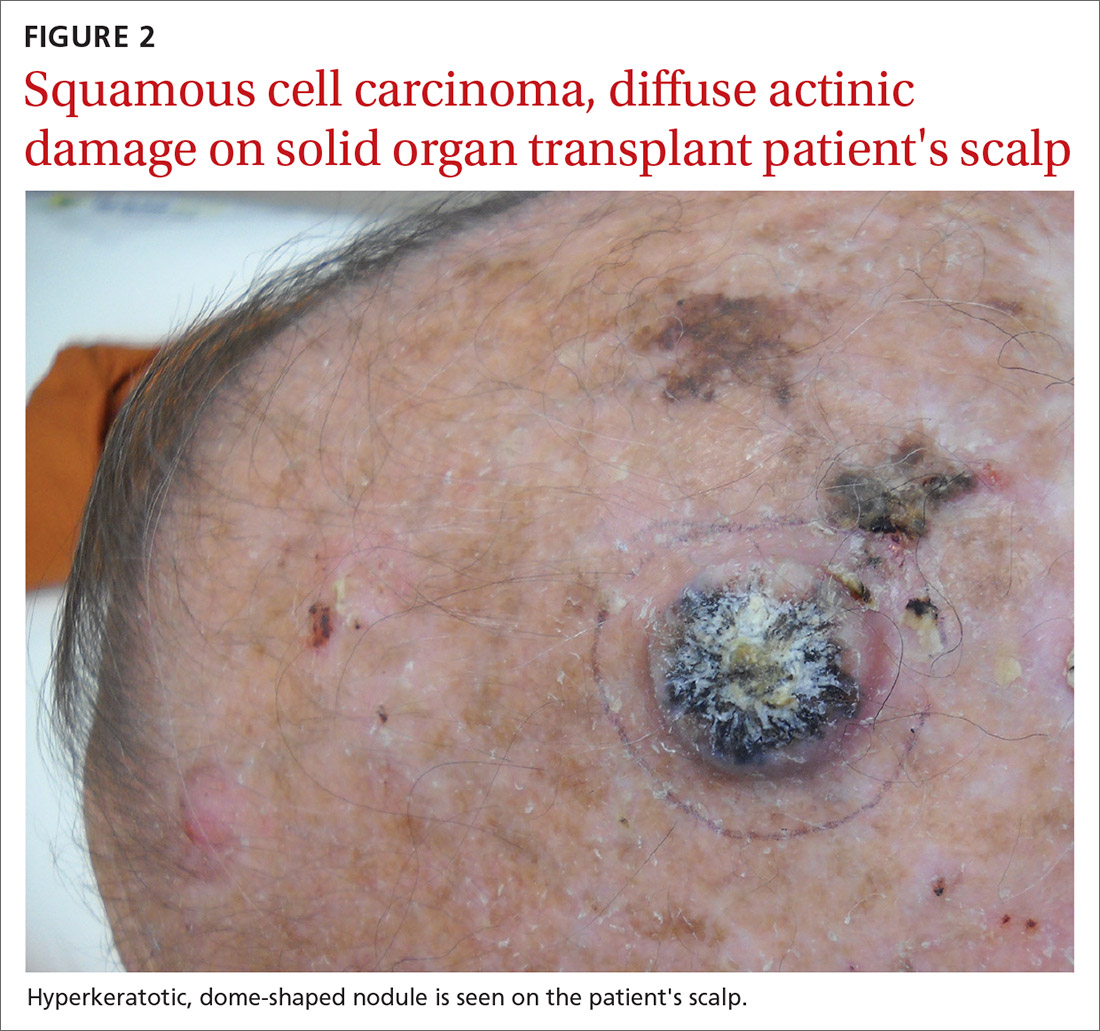
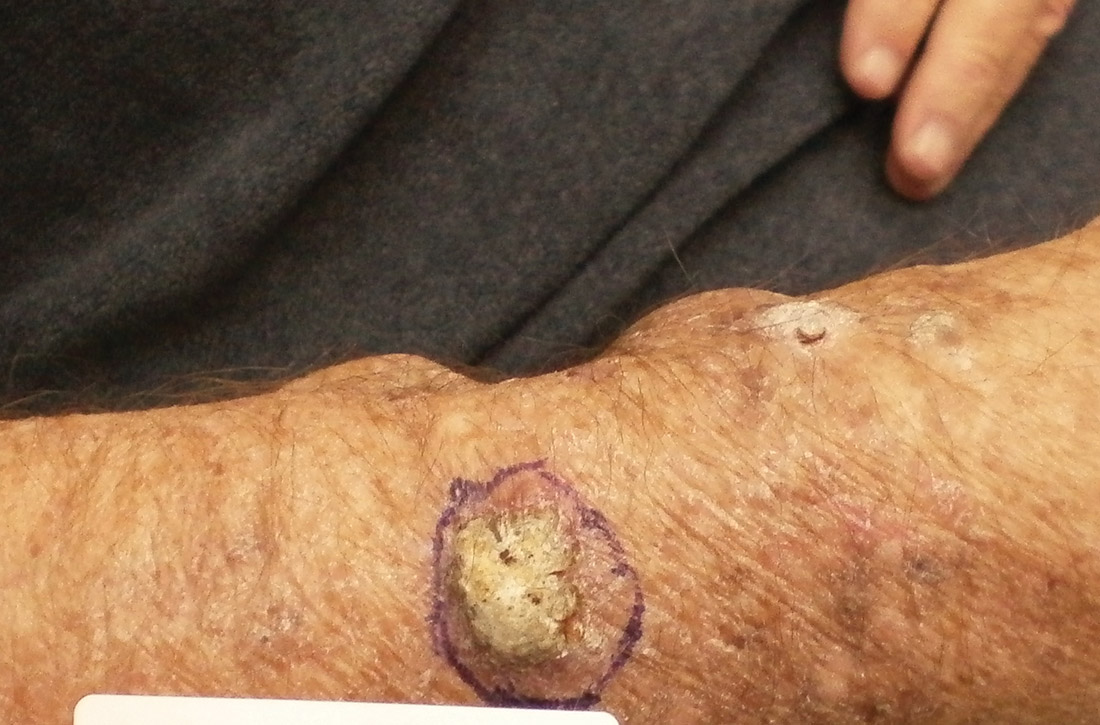
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
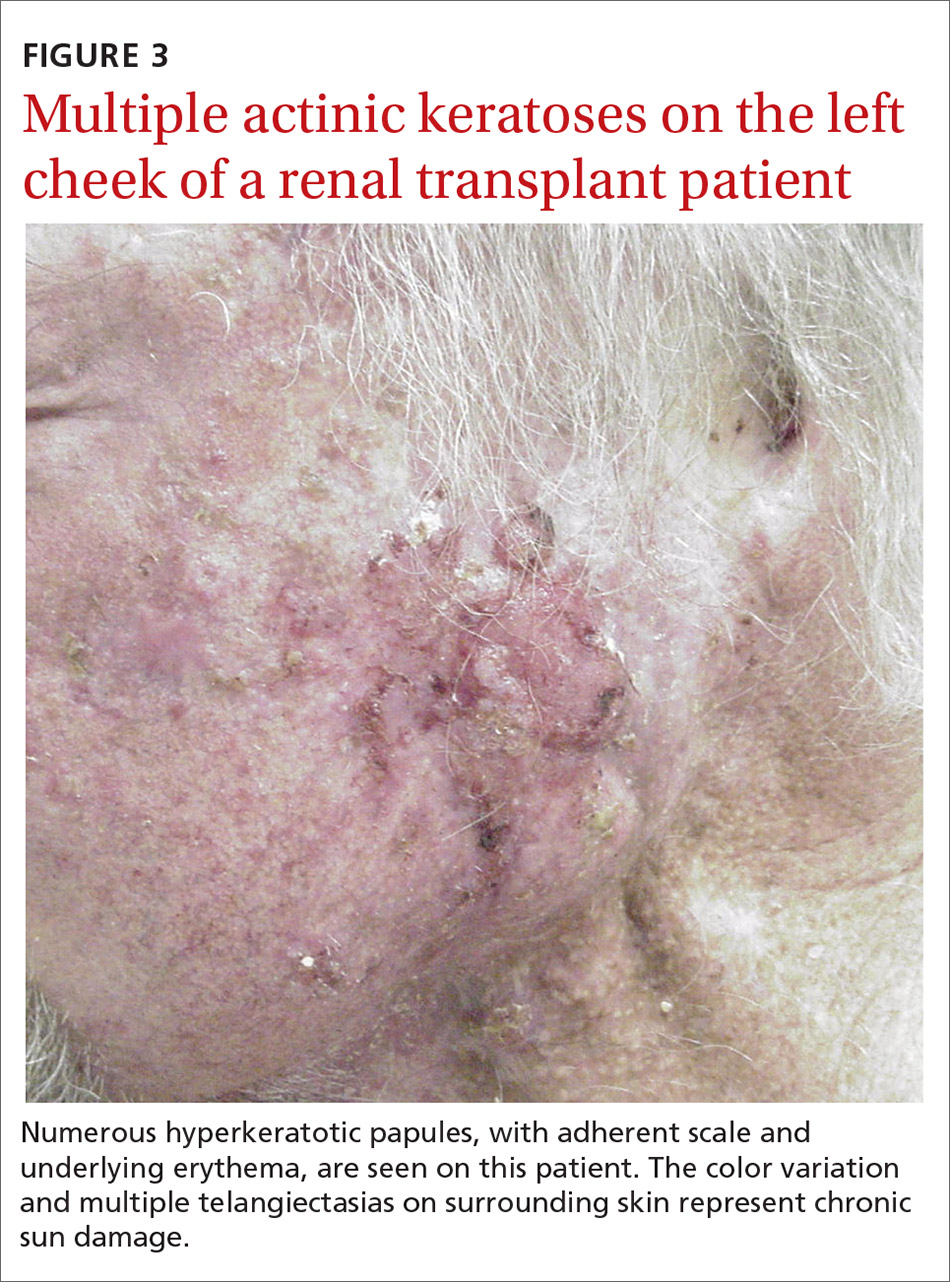
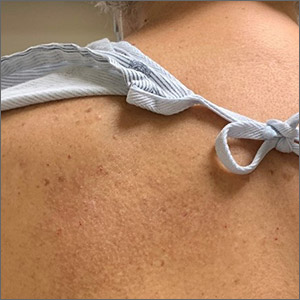
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
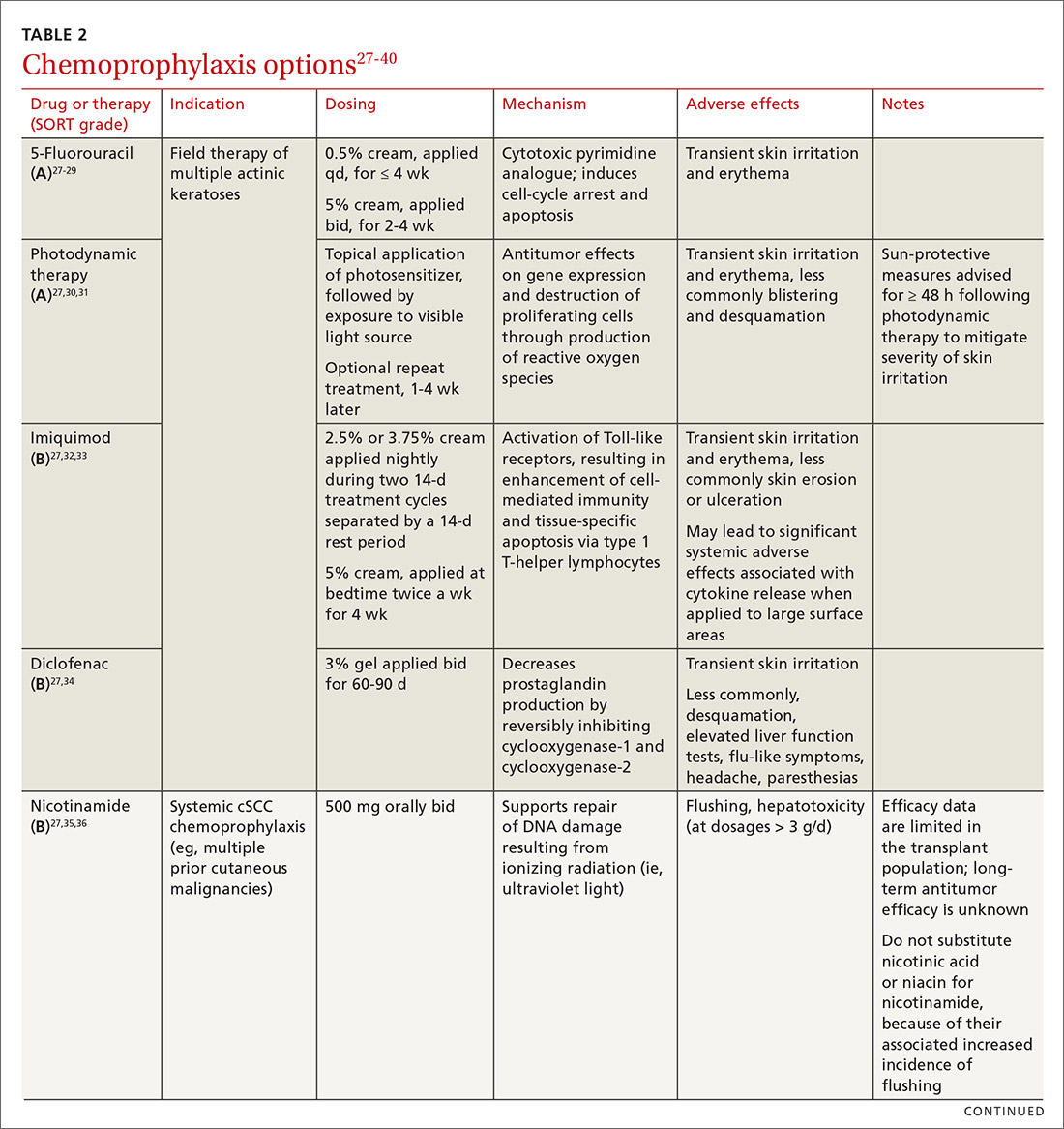
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
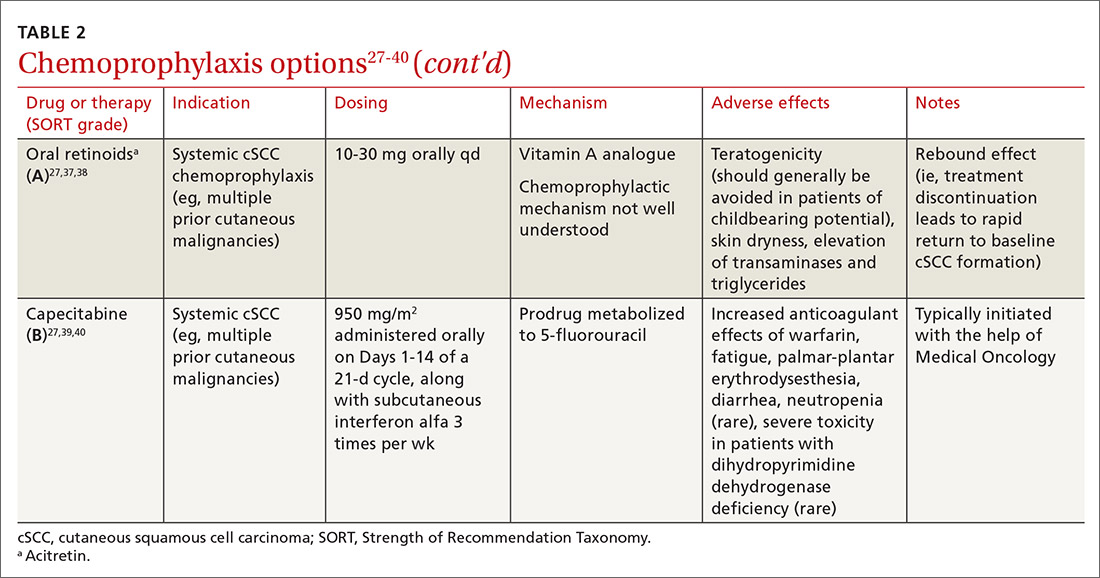
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
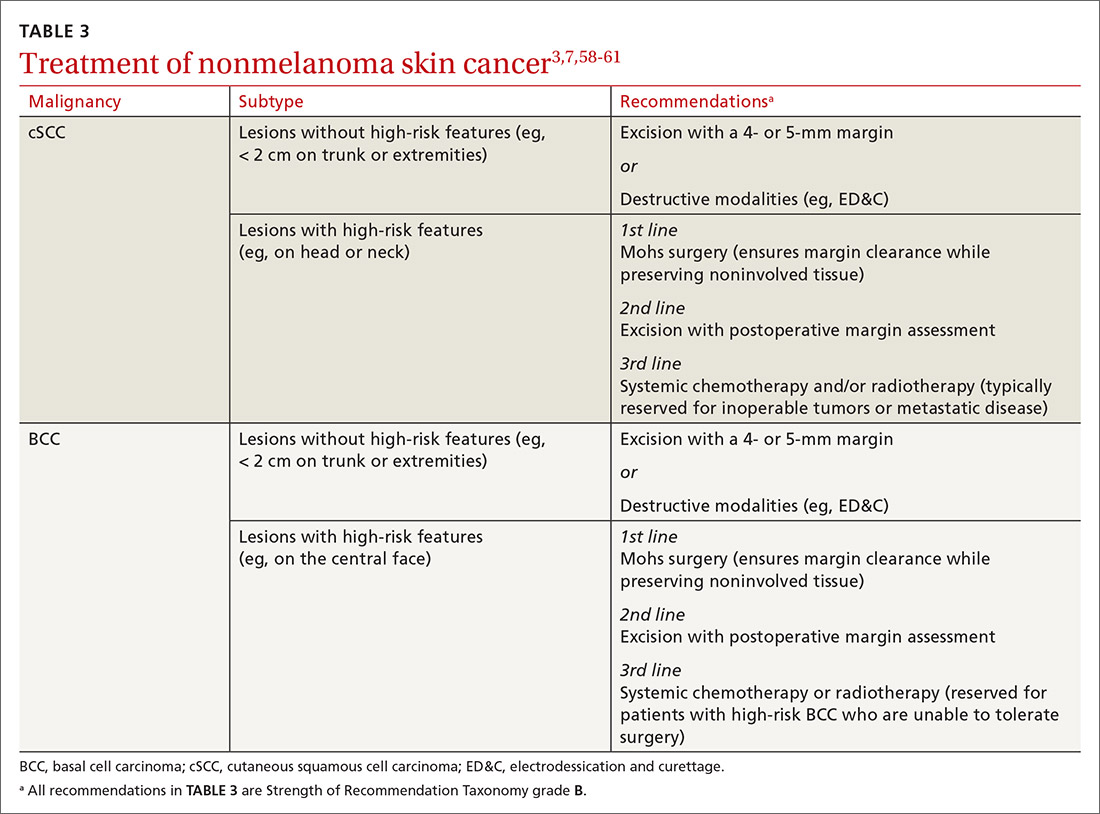
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
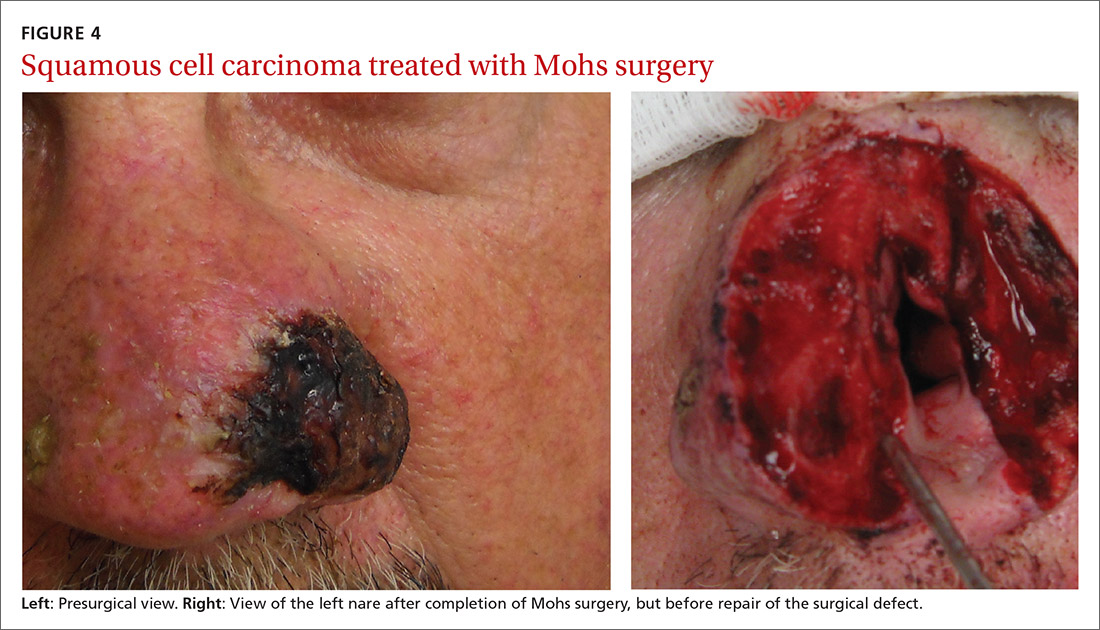
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; Lindsey-Collins@ouhsc.edu
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
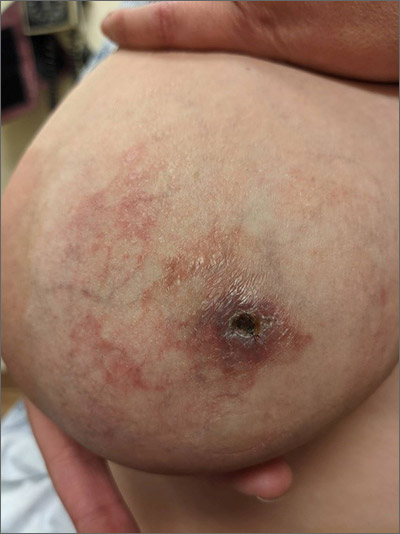
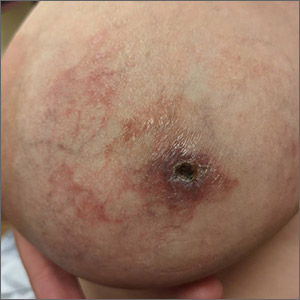
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; Lindsey-Collins@ouhsc.edu
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; Lindsey-Collins@ouhsc.edu
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
PRACTICE RECOMMENDATIONS
› Conduct a full-body skin examination at least once annually for solid organ transplant recipients. C
› Encourage daily use of broad-spectrum SPF ≥ 30 sunscreen and sun-protective clothing (long sleeves, pants, wide-brimmed hats) for these patients. A
› Consider chemoprophylactic agents for patients at especially high risk of nonmelanoma skin cancer. A
› Treat nonmelanoma skin cancer in a solid organ transplant recipient aggressively because of their increased risk of recurrence, local invasion, and metastasis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Itchy rash on back
A unilateral, neuropathic itch accompanied by postinflammatory pigmentation changes or lichenification at the medial inferior tip of the scapula are the hallmarks of notalgia paresthetica (NP).
NP is thought to result from nerve impingement or chronic nerve trauma to the posterior rami of the upper thoracic spinal nerves. The hyperpigmentation and lichenification arise from repeated scratching or rubbing of the skin.
NP is a clinical diagnosis and does not require biopsy or imaging. The differential diagnosis includes brachioradial pruritus, postherpetic neuralgia, multiple sclerosis, and other small fiber neuropathies.
The standard treatment is topical capsaicin 0.025% tid for 5 weeks, with repeat treatments (for a few days or weeks) if there are relapses. Higher doses (0.075%) may work more quickly but may also lead to more burning. A lidocaine 5% patch bid can also be considered. Second-line treatment options include cutaneous electrical field stimulation or transcutaneous electrical nerve stimulation, gabapentin, or oxcarbazepine. Topical steroids are considered ineffective for this condition.1
The patient in this case was started on capsaicin 0.025%. She was encouraged to keep her skin moisturized and well hydrated because dyshidrosis can exacerbate itching. A prescription for gabapentin was offered in case topical treatments were unsuccessful, but she declined after she weighed the risks of adverse effects against her current symptoms.
Photo courtesy of Daniel Stulberg, MD, and text courtesy of Nathan Birnbaum, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 5th ed. Elsevier; 2017.

A unilateral, neuropathic itch accompanied by postinflammatory pigmentation changes or lichenification at the medial inferior tip of the scapula are the hallmarks of notalgia paresthetica (NP).
NP is thought to result from nerve impingement or chronic nerve trauma to the posterior rami of the upper thoracic spinal nerves. The hyperpigmentation and lichenification arise from repeated scratching or rubbing of the skin.
NP is a clinical diagnosis and does not require biopsy or imaging. The differential diagnosis includes brachioradial pruritus, postherpetic neuralgia, multiple sclerosis, and other small fiber neuropathies.
The standard treatment is topical capsaicin 0.025% tid for 5 weeks, with repeat treatments (for a few days or weeks) if there are relapses. Higher doses (0.075%) may work more quickly but may also lead to more burning. A lidocaine 5% patch bid can also be considered. Second-line treatment options include cutaneous electrical field stimulation or transcutaneous electrical nerve stimulation, gabapentin, or oxcarbazepine. Topical steroids are considered ineffective for this condition.1
The patient in this case was started on capsaicin 0.025%. She was encouraged to keep her skin moisturized and well hydrated because dyshidrosis can exacerbate itching. A prescription for gabapentin was offered in case topical treatments were unsuccessful, but she declined after she weighed the risks of adverse effects against her current symptoms.
Photo courtesy of Daniel Stulberg, MD, and text courtesy of Nathan Birnbaum, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque

A unilateral, neuropathic itch accompanied by postinflammatory pigmentation changes or lichenification at the medial inferior tip of the scapula are the hallmarks of notalgia paresthetica (NP).
NP is thought to result from nerve impingement or chronic nerve trauma to the posterior rami of the upper thoracic spinal nerves. The hyperpigmentation and lichenification arise from repeated scratching or rubbing of the skin.
NP is a clinical diagnosis and does not require biopsy or imaging. The differential diagnosis includes brachioradial pruritus, postherpetic neuralgia, multiple sclerosis, and other small fiber neuropathies.
The standard treatment is topical capsaicin 0.025% tid for 5 weeks, with repeat treatments (for a few days or weeks) if there are relapses. Higher doses (0.075%) may work more quickly but may also lead to more burning. A lidocaine 5% patch bid can also be considered. Second-line treatment options include cutaneous electrical field stimulation or transcutaneous electrical nerve stimulation, gabapentin, or oxcarbazepine. Topical steroids are considered ineffective for this condition.1
The patient in this case was started on capsaicin 0.025%. She was encouraged to keep her skin moisturized and well hydrated because dyshidrosis can exacerbate itching. A prescription for gabapentin was offered in case topical treatments were unsuccessful, but she declined after she weighed the risks of adverse effects against her current symptoms.
Photo courtesy of Daniel Stulberg, MD, and text courtesy of Nathan Birnbaum, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 5th ed. Elsevier; 2017.
1. Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 5th ed. Elsevier; 2017.
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers (david.beck@nih.gov).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity.
“As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers (david.beck@nih.gov).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity.
“As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers (david.beck@nih.gov).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity.
“As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV
Novel analysis quantifies the benefit of melanoma screening
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS, AND PREVENTION
Painful thickened breast lesion
Treatment was attempted for both a suspected spider bite (2 weeks of topical triamcinolone 0.1%) and presumed cellulitis (oral doxycycline 100 mg bid/5 d), but neither improved her condition. Concerned for the possibility of cutaneous breast cancer, a punch biopsy was ordered and revealed diffuse dermal angiomatosis (DDA).
DDA is an uncommon proliferation of cutaneous blood vessels causing a reticular blood vessel pattern, as seen in this image. Typically, DDA is associated with tissue hypoxia due to arterial insufficiency from peripheral artery disease. In recent years, there have been numerous case reports of painful ulcerated lesions and reticular blood vessels occurring in women with large, pendulous breasts, increased body mass index, and a history of smoking. One theory suggests that the weight of the breasts causes tissue to stretch, compressing the blood vessels. This, combined with smoking, leads to localized hypoxia and DDA.
Treatments have included oral isotretinoin, calcium channel blockers, aspirin, or pentoxifylline to help circulation. Smoking cessation is recommended, as well as reduction mammoplasty to decrease the stretch on the tissues and relieve the local hypoxia. Although invasive, breast reduction surgery has moved to the forefront of therapy, with reports having shown resolution of the ulcers and pain.1
Two important aspects of clinical medicine are highlighted by this case. First, nonhealing lesions that are not responding to prescribed therapies may require biopsy to rule out malignancy. Second, when there is difficulty making a diagnosis, especially with uncommon diseases, biopsy and input from a pathologist can be extremely helpful.
In this case, the patient was referred to Plastic Surgery and scheduled for reduction mammoplasty. The patient was advised to stop smoking for at least 4 weeks prior to the surgery to possibly improve her condition and reduce the likelihood of postoperative complications.
Photo courtesy of Michael Louie, MD, and text courtesy of Michael Louie, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Galambos J, Meuli-Simmen C, Schmid R, et al. Diffuse dermal angiomatosis of the breast: a distinct entity in the spectrum of cutaneous reactive angiomatoses—clinicopathologic study of two cases and comprehensive review of the literature. Case Rep Dermatol 2017;9:194-205. https://doi.org/10.1159/000480721
Treatment was attempted for both a suspected spider bite (2 weeks of topical triamcinolone 0.1%) and presumed cellulitis (oral doxycycline 100 mg bid/5 d), but neither improved her condition. Concerned for the possibility of cutaneous breast cancer, a punch biopsy was ordered and revealed diffuse dermal angiomatosis (DDA).
DDA is an uncommon proliferation of cutaneous blood vessels causing a reticular blood vessel pattern, as seen in this image. Typically, DDA is associated with tissue hypoxia due to arterial insufficiency from peripheral artery disease. In recent years, there have been numerous case reports of painful ulcerated lesions and reticular blood vessels occurring in women with large, pendulous breasts, increased body mass index, and a history of smoking. One theory suggests that the weight of the breasts causes tissue to stretch, compressing the blood vessels. This, combined with smoking, leads to localized hypoxia and DDA.
Treatments have included oral isotretinoin, calcium channel blockers, aspirin, or pentoxifylline to help circulation. Smoking cessation is recommended, as well as reduction mammoplasty to decrease the stretch on the tissues and relieve the local hypoxia. Although invasive, breast reduction surgery has moved to the forefront of therapy, with reports having shown resolution of the ulcers and pain.1
Two important aspects of clinical medicine are highlighted by this case. First, nonhealing lesions that are not responding to prescribed therapies may require biopsy to rule out malignancy. Second, when there is difficulty making a diagnosis, especially with uncommon diseases, biopsy and input from a pathologist can be extremely helpful.
In this case, the patient was referred to Plastic Surgery and scheduled for reduction mammoplasty. The patient was advised to stop smoking for at least 4 weeks prior to the surgery to possibly improve her condition and reduce the likelihood of postoperative complications.
Photo courtesy of Michael Louie, MD, and text courtesy of Michael Louie, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Treatment was attempted for both a suspected spider bite (2 weeks of topical triamcinolone 0.1%) and presumed cellulitis (oral doxycycline 100 mg bid/5 d), but neither improved her condition. Concerned for the possibility of cutaneous breast cancer, a punch biopsy was ordered and revealed diffuse dermal angiomatosis (DDA).
DDA is an uncommon proliferation of cutaneous blood vessels causing a reticular blood vessel pattern, as seen in this image. Typically, DDA is associated with tissue hypoxia due to arterial insufficiency from peripheral artery disease. In recent years, there have been numerous case reports of painful ulcerated lesions and reticular blood vessels occurring in women with large, pendulous breasts, increased body mass index, and a history of smoking. One theory suggests that the weight of the breasts causes tissue to stretch, compressing the blood vessels. This, combined with smoking, leads to localized hypoxia and DDA.
Treatments have included oral isotretinoin, calcium channel blockers, aspirin, or pentoxifylline to help circulation. Smoking cessation is recommended, as well as reduction mammoplasty to decrease the stretch on the tissues and relieve the local hypoxia. Although invasive, breast reduction surgery has moved to the forefront of therapy, with reports having shown resolution of the ulcers and pain.1
Two important aspects of clinical medicine are highlighted by this case. First, nonhealing lesions that are not responding to prescribed therapies may require biopsy to rule out malignancy. Second, when there is difficulty making a diagnosis, especially with uncommon diseases, biopsy and input from a pathologist can be extremely helpful.
In this case, the patient was referred to Plastic Surgery and scheduled for reduction mammoplasty. The patient was advised to stop smoking for at least 4 weeks prior to the surgery to possibly improve her condition and reduce the likelihood of postoperative complications.
Photo courtesy of Michael Louie, MD, and text courtesy of Michael Louie, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Galambos J, Meuli-Simmen C, Schmid R, et al. Diffuse dermal angiomatosis of the breast: a distinct entity in the spectrum of cutaneous reactive angiomatoses—clinicopathologic study of two cases and comprehensive review of the literature. Case Rep Dermatol 2017;9:194-205. https://doi.org/10.1159/000480721
Galambos J, Meuli-Simmen C, Schmid R, et al. Diffuse dermal angiomatosis of the breast: a distinct entity in the spectrum of cutaneous reactive angiomatoses—clinicopathologic study of two cases and comprehensive review of the literature. Case Rep Dermatol 2017;9:194-205. https://doi.org/10.1159/000480721
Oral sarecycline promising for papulopustular rosacea
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
National Psoriasis Foundation recommends some stop methotrexate for 2 weeks after J&J vaccine
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Contact dermatitis content varies among social media sites
on YouTube, Facebook, Instagram, Google, Twitter, and Reddit.
Data on social media use suggest that approximately 65% of U.S. adults regularly use social media, and 40% of individuals use it in making medical decisions, Morgan Nguyen, a medical student at Northwestern University, Chicago, said at the annual meeting of the American Contact Dermatitis Society, held virtually this year.
“Dermatologists’ awareness of social media discussions can further their understanding of where patients go for information and what they might encounter,” she said. In particular, “contact dermatitis practitioners can tailor their counseling by knowing what their patients are seeing online.”
To characterize the social media landscape for content related to allergic contact dermatitis (ACD), Ms. Nguyen and colleagues assessed metrics on content and authorship on six different platforms.
For YouTube, the authors reviewed 15 videos related to ACD with views ranging from 24,262 to 232,300. Of these videos, two were produced as medical education, four were produced by patients, and nine were produced by physicians. The content of many videos was poor quality, with an average QUEST score of 7.4/28 overall and 8.7 for physician videos. Video quality was not associated with increased views. Video titles included “What to do if you have a rash on your face,” and “Contact dermatitis on lips!”
Overall, Instagram was more popular than Twitter, particularly among patients. The investigators searched using the hashtags #ContactDermatitis, #AllergicContactDermatitis, and #ContactDerm and reviewed the 100 most recent posts for authorship. The most recent 100 posts occurred over 16 days; physicians, patients, and companies each contributed approximately one-third of the content, but patient content was more focused on symptoms, treatment progress, and advice.
For Instagram, the hashtag search phrase made a notable difference in authorship, Ms. Nguyen said. Physicians were disproportionately more likely to use #AllergicContactDermatitis (43%) compared with patients (22%).
On Twitter, the most recent 100 posts were spread over 152 days, and professional organizations and companies generated approximately two-thirds of the posts. The #ContactDermatitis hashtag was the most common, and accounted for 94% of tweets.
Although patient support groups specific to ACD exist on Facebook, the researchers found none on Reddit. These two venues are designed for creating online communities, rather than simply providing information, and the researchers searched for support groups related to contact dermatitis. One of the main differences between the two is that Facebook allows for the creation of private groups, while Reddit is an open forum.
The largest contact dermatitis Facebook group, the “Eczema, Contact Dermatitis and Patch Testing Alliance,” had 4,665 members at the time of the study, and most groups were private. Although no support groups existed on Reddit, titles of Reddit forums discussing ACD included allergies, askdoctors, fragrance, haircarescience, legaladvice, skincareaddicts, beauty, dermatologyquestions, medical_advice, skincare_addiction, tretinoin, and vulvodynia.
For Google, the researchers used terms similar to “contact dermatitis” as generated by the Google Keyword Planner tool, and used Google Adwords data to estimate monthly searches. The top estimated term was “contact dermatitis,” with 8,322 searches, followed by “contact dermatitis pictures,” with 1,666 searches, and “contact dermatitis treatment” with 595 searches. By contrast, “allergic dermatitis” had an estimated 346 monthly searches, and “allergic contact dermatitis” had 194.
Overall, approximately 9,000 searches each month involve “contact dermatitis,” “allergic contact dermatitis,” or “allergic dermatitis,” said Ms. Nguyen. However, these estimated searches seemed comparatively low, given the high burden of ACD, she said. Although ACD ranks eighth among skin diseases based on health care costs, psoriasis (fourteenth based on health care costs) shows an estimated monthly Google search volume of 600,462, she pointed out.
The study findings were limited by several factors including the potential impact of the COVID-19 pandemic on social media use, and by the lack of specificity associated with the search term “contact dermatitis,” which is not unique to ACD, Ms. Nguyen said.
Although more research on quality assessment is needed, the results suggest that social media is a popular venue for ACD patients to seek and share information, Ms. Nguyen emphasized. There is an opportunity for patch testing physicians to create and disperse educational content for patients using these sites, she concluded.
Study highlights education opportunities
“Due to the pandemic, patients have been increasingly interacting with online resources in lieu of coming to a physician’s office,” corresponding author Walter J. Liszewski, MD, of the department of dermatology, Northwestern University, Chicago, said in an interview. “As social media is increasingly used by patients and physicians, it is important to get a sense of its footprint,” he said.
He and Ms. Nguyen were surprised by several of their findings: First, searches for ACD on Google were not particularly common given its relatively high prevalence and economic cost to society. In addition, they found that physicians often used different language than that of patients to describe ACD on Twitter and Instagram. They were also surprised at how often ACD appeared in Reddit posts, which they noted highlights that ACD impacts multiple sections of society.
The greatest challenge in studying social media and medicine is the quality of material available, Dr. Liszewski and Ms. Nguyen observed, emphasizing that while there are numerous videos on ACD on YouTube, the quality is highly variable, and there is a need for more patient-centered, educational materials. However, the results of their study highlight the opportunity for physicians and industry to create medically-accurate educational materials, they added.
Ms. Nguyen and Dr. Liszewski had no financial conflicts to disclose.
on YouTube, Facebook, Instagram, Google, Twitter, and Reddit.
Data on social media use suggest that approximately 65% of U.S. adults regularly use social media, and 40% of individuals use it in making medical decisions, Morgan Nguyen, a medical student at Northwestern University, Chicago, said at the annual meeting of the American Contact Dermatitis Society, held virtually this year.
“Dermatologists’ awareness of social media discussions can further their understanding of where patients go for information and what they might encounter,” she said. In particular, “contact dermatitis practitioners can tailor their counseling by knowing what their patients are seeing online.”
To characterize the social media landscape for content related to allergic contact dermatitis (ACD), Ms. Nguyen and colleagues assessed metrics on content and authorship on six different platforms.
For YouTube, the authors reviewed 15 videos related to ACD with views ranging from 24,262 to 232,300. Of these videos, two were produced as medical education, four were produced by patients, and nine were produced by physicians. The content of many videos was poor quality, with an average QUEST score of 7.4/28 overall and 8.7 for physician videos. Video quality was not associated with increased views. Video titles included “What to do if you have a rash on your face,” and “Contact dermatitis on lips!”
Overall, Instagram was more popular than Twitter, particularly among patients. The investigators searched using the hashtags #ContactDermatitis, #AllergicContactDermatitis, and #ContactDerm and reviewed the 100 most recent posts for authorship. The most recent 100 posts occurred over 16 days; physicians, patients, and companies each contributed approximately one-third of the content, but patient content was more focused on symptoms, treatment progress, and advice.
For Instagram, the hashtag search phrase made a notable difference in authorship, Ms. Nguyen said. Physicians were disproportionately more likely to use #AllergicContactDermatitis (43%) compared with patients (22%).
On Twitter, the most recent 100 posts were spread over 152 days, and professional organizations and companies generated approximately two-thirds of the posts. The #ContactDermatitis hashtag was the most common, and accounted for 94% of tweets.
Although patient support groups specific to ACD exist on Facebook, the researchers found none on Reddit. These two venues are designed for creating online communities, rather than simply providing information, and the researchers searched for support groups related to contact dermatitis. One of the main differences between the two is that Facebook allows for the creation of private groups, while Reddit is an open forum.
The largest contact dermatitis Facebook group, the “Eczema, Contact Dermatitis and Patch Testing Alliance,” had 4,665 members at the time of the study, and most groups were private. Although no support groups existed on Reddit, titles of Reddit forums discussing ACD included allergies, askdoctors, fragrance, haircarescience, legaladvice, skincareaddicts, beauty, dermatologyquestions, medical_advice, skincare_addiction, tretinoin, and vulvodynia.
For Google, the researchers used terms similar to “contact dermatitis” as generated by the Google Keyword Planner tool, and used Google Adwords data to estimate monthly searches. The top estimated term was “contact dermatitis,” with 8,322 searches, followed by “contact dermatitis pictures,” with 1,666 searches, and “contact dermatitis treatment” with 595 searches. By contrast, “allergic dermatitis” had an estimated 346 monthly searches, and “allergic contact dermatitis” had 194.
Overall, approximately 9,000 searches each month involve “contact dermatitis,” “allergic contact dermatitis,” or “allergic dermatitis,” said Ms. Nguyen. However, these estimated searches seemed comparatively low, given the high burden of ACD, she said. Although ACD ranks eighth among skin diseases based on health care costs, psoriasis (fourteenth based on health care costs) shows an estimated monthly Google search volume of 600,462, she pointed out.
The study findings were limited by several factors including the potential impact of the COVID-19 pandemic on social media use, and by the lack of specificity associated with the search term “contact dermatitis,” which is not unique to ACD, Ms. Nguyen said.
Although more research on quality assessment is needed, the results suggest that social media is a popular venue for ACD patients to seek and share information, Ms. Nguyen emphasized. There is an opportunity for patch testing physicians to create and disperse educational content for patients using these sites, she concluded.
Study highlights education opportunities
“Due to the pandemic, patients have been increasingly interacting with online resources in lieu of coming to a physician’s office,” corresponding author Walter J. Liszewski, MD, of the department of dermatology, Northwestern University, Chicago, said in an interview. “As social media is increasingly used by patients and physicians, it is important to get a sense of its footprint,” he said.
He and Ms. Nguyen were surprised by several of their findings: First, searches for ACD on Google were not particularly common given its relatively high prevalence and economic cost to society. In addition, they found that physicians often used different language than that of patients to describe ACD on Twitter and Instagram. They were also surprised at how often ACD appeared in Reddit posts, which they noted highlights that ACD impacts multiple sections of society.
The greatest challenge in studying social media and medicine is the quality of material available, Dr. Liszewski and Ms. Nguyen observed, emphasizing that while there are numerous videos on ACD on YouTube, the quality is highly variable, and there is a need for more patient-centered, educational materials. However, the results of their study highlight the opportunity for physicians and industry to create medically-accurate educational materials, they added.
Ms. Nguyen and Dr. Liszewski had no financial conflicts to disclose.
on YouTube, Facebook, Instagram, Google, Twitter, and Reddit.
Data on social media use suggest that approximately 65% of U.S. adults regularly use social media, and 40% of individuals use it in making medical decisions, Morgan Nguyen, a medical student at Northwestern University, Chicago, said at the annual meeting of the American Contact Dermatitis Society, held virtually this year.
“Dermatologists’ awareness of social media discussions can further their understanding of where patients go for information and what they might encounter,” she said. In particular, “contact dermatitis practitioners can tailor their counseling by knowing what their patients are seeing online.”
To characterize the social media landscape for content related to allergic contact dermatitis (ACD), Ms. Nguyen and colleagues assessed metrics on content and authorship on six different platforms.
For YouTube, the authors reviewed 15 videos related to ACD with views ranging from 24,262 to 232,300. Of these videos, two were produced as medical education, four were produced by patients, and nine were produced by physicians. The content of many videos was poor quality, with an average QUEST score of 7.4/28 overall and 8.7 for physician videos. Video quality was not associated with increased views. Video titles included “What to do if you have a rash on your face,” and “Contact dermatitis on lips!”
Overall, Instagram was more popular than Twitter, particularly among patients. The investigators searched using the hashtags #ContactDermatitis, #AllergicContactDermatitis, and #ContactDerm and reviewed the 100 most recent posts for authorship. The most recent 100 posts occurred over 16 days; physicians, patients, and companies each contributed approximately one-third of the content, but patient content was more focused on symptoms, treatment progress, and advice.
For Instagram, the hashtag search phrase made a notable difference in authorship, Ms. Nguyen said. Physicians were disproportionately more likely to use #AllergicContactDermatitis (43%) compared with patients (22%).
On Twitter, the most recent 100 posts were spread over 152 days, and professional organizations and companies generated approximately two-thirds of the posts. The #ContactDermatitis hashtag was the most common, and accounted for 94% of tweets.
Although patient support groups specific to ACD exist on Facebook, the researchers found none on Reddit. These two venues are designed for creating online communities, rather than simply providing information, and the researchers searched for support groups related to contact dermatitis. One of the main differences between the two is that Facebook allows for the creation of private groups, while Reddit is an open forum.
The largest contact dermatitis Facebook group, the “Eczema, Contact Dermatitis and Patch Testing Alliance,” had 4,665 members at the time of the study, and most groups were private. Although no support groups existed on Reddit, titles of Reddit forums discussing ACD included allergies, askdoctors, fragrance, haircarescience, legaladvice, skincareaddicts, beauty, dermatologyquestions, medical_advice, skincare_addiction, tretinoin, and vulvodynia.
For Google, the researchers used terms similar to “contact dermatitis” as generated by the Google Keyword Planner tool, and used Google Adwords data to estimate monthly searches. The top estimated term was “contact dermatitis,” with 8,322 searches, followed by “contact dermatitis pictures,” with 1,666 searches, and “contact dermatitis treatment” with 595 searches. By contrast, “allergic dermatitis” had an estimated 346 monthly searches, and “allergic contact dermatitis” had 194.
Overall, approximately 9,000 searches each month involve “contact dermatitis,” “allergic contact dermatitis,” or “allergic dermatitis,” said Ms. Nguyen. However, these estimated searches seemed comparatively low, given the high burden of ACD, she said. Although ACD ranks eighth among skin diseases based on health care costs, psoriasis (fourteenth based on health care costs) shows an estimated monthly Google search volume of 600,462, she pointed out.
The study findings were limited by several factors including the potential impact of the COVID-19 pandemic on social media use, and by the lack of specificity associated with the search term “contact dermatitis,” which is not unique to ACD, Ms. Nguyen said.
Although more research on quality assessment is needed, the results suggest that social media is a popular venue for ACD patients to seek and share information, Ms. Nguyen emphasized. There is an opportunity for patch testing physicians to create and disperse educational content for patients using these sites, she concluded.
Study highlights education opportunities
“Due to the pandemic, patients have been increasingly interacting with online resources in lieu of coming to a physician’s office,” corresponding author Walter J. Liszewski, MD, of the department of dermatology, Northwestern University, Chicago, said in an interview. “As social media is increasingly used by patients and physicians, it is important to get a sense of its footprint,” he said.
He and Ms. Nguyen were surprised by several of their findings: First, searches for ACD on Google were not particularly common given its relatively high prevalence and economic cost to society. In addition, they found that physicians often used different language than that of patients to describe ACD on Twitter and Instagram. They were also surprised at how often ACD appeared in Reddit posts, which they noted highlights that ACD impacts multiple sections of society.
The greatest challenge in studying social media and medicine is the quality of material available, Dr. Liszewski and Ms. Nguyen observed, emphasizing that while there are numerous videos on ACD on YouTube, the quality is highly variable, and there is a need for more patient-centered, educational materials. However, the results of their study highlight the opportunity for physicians and industry to create medically-accurate educational materials, they added.
Ms. Nguyen and Dr. Liszewski had no financial conflicts to disclose.
FROM ACDS 2021