User login
This Rash Really Stinks!
ANSWER
The correct diagnosis is Darier disease (choice “d”).
DISCUSSION
Darier disease, also known as Darier-White disease or keratosis follicularis, is an inherited defect transmitted by autosomal dominant mode. The pathophysiologic process is a breakdown of cell adhesion that normally binds keratin filaments to tiny connecting fibers called desmosomes.
Darier disease manifests with a “branny” papulosquamous rash, typically arising in the third decade of life and affecting the chest, scalp, back, and intertriginous areas. The nail and intraoral findings noted in this patient are typical. In the author’s experience, the former is more commonly seen and is essentially pathognomic for the disease.
Darier disease is relatively rare, occurring in 1:30,000 to 1:100,000 population, depending on the geographic area studied. Men and women are equally affected, although it is more common in those with darker skin.
The differential outlined in the answer choices is reasonable, considering the condition’s rarity and how unlikely it is to manifest solely in the inframammary area. One could conclude that, just as with psoriasis (choice “b”) and seborrhea, intertrigo (choice “c”) is not always a primary process. And although yeast infection (choice “a”) can complicate any florid rash in this area, topical and oral anti-yeast treatment had utterly failed to help.
TREATMENT
Isotretinoin is used in cases such as this one, but it only offers temporary relief. For less severe cases, oral antibiotics (eg minocycline) or topical steroids (used with caution given the risk for atrophy in the inframammary area) often suffice. This patient’s prognosis is guarded at best, although control of the worst is certainly possible.
ANSWER
The correct diagnosis is Darier disease (choice “d”).
DISCUSSION
Darier disease, also known as Darier-White disease or keratosis follicularis, is an inherited defect transmitted by autosomal dominant mode. The pathophysiologic process is a breakdown of cell adhesion that normally binds keratin filaments to tiny connecting fibers called desmosomes.
Darier disease manifests with a “branny” papulosquamous rash, typically arising in the third decade of life and affecting the chest, scalp, back, and intertriginous areas. The nail and intraoral findings noted in this patient are typical. In the author’s experience, the former is more commonly seen and is essentially pathognomic for the disease.
Darier disease is relatively rare, occurring in 1:30,000 to 1:100,000 population, depending on the geographic area studied. Men and women are equally affected, although it is more common in those with darker skin.
The differential outlined in the answer choices is reasonable, considering the condition’s rarity and how unlikely it is to manifest solely in the inframammary area. One could conclude that, just as with psoriasis (choice “b”) and seborrhea, intertrigo (choice “c”) is not always a primary process. And although yeast infection (choice “a”) can complicate any florid rash in this area, topical and oral anti-yeast treatment had utterly failed to help.
TREATMENT
Isotretinoin is used in cases such as this one, but it only offers temporary relief. For less severe cases, oral antibiotics (eg minocycline) or topical steroids (used with caution given the risk for atrophy in the inframammary area) often suffice. This patient’s prognosis is guarded at best, although control of the worst is certainly possible.
ANSWER
The correct diagnosis is Darier disease (choice “d”).
DISCUSSION
Darier disease, also known as Darier-White disease or keratosis follicularis, is an inherited defect transmitted by autosomal dominant mode. The pathophysiologic process is a breakdown of cell adhesion that normally binds keratin filaments to tiny connecting fibers called desmosomes.
Darier disease manifests with a “branny” papulosquamous rash, typically arising in the third decade of life and affecting the chest, scalp, back, and intertriginous areas. The nail and intraoral findings noted in this patient are typical. In the author’s experience, the former is more commonly seen and is essentially pathognomic for the disease.
Darier disease is relatively rare, occurring in 1:30,000 to 1:100,000 population, depending on the geographic area studied. Men and women are equally affected, although it is more common in those with darker skin.
The differential outlined in the answer choices is reasonable, considering the condition’s rarity and how unlikely it is to manifest solely in the inframammary area. One could conclude that, just as with psoriasis (choice “b”) and seborrhea, intertrigo (choice “c”) is not always a primary process. And although yeast infection (choice “a”) can complicate any florid rash in this area, topical and oral anti-yeast treatment had utterly failed to help.
TREATMENT
Isotretinoin is used in cases such as this one, but it only offers temporary relief. For less severe cases, oral antibiotics (eg minocycline) or topical steroids (used with caution given the risk for atrophy in the inframammary area) often suffice. This patient’s prognosis is guarded at best, although control of the worst is certainly possible.

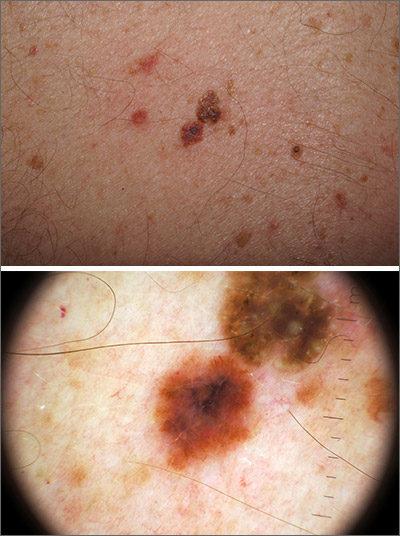
A 50-year-old woman is referred to dermatology with a “yeast” infection of several years’ duration. The condition causes considerable discomfort, especially during hot weather when the rash emits a very objectionable odor.
The florid, white, scaly rash under her breasts is a stark contrast to the patient’s type V skin. On both sides, the affected skin perfectly matches the inframammary fold. There are sharp margins and uniform moist scaling.
Looking elsewhere, 7 of 10 fingernails exhibit longitudinal white and red streaks, along with triangular nicks in the edges of several nails. The roof of the patient’s mouth is studded with fleshy nodules measuring 0.6 to 1.0 cm. Several pits are seen on her palms.
The patient is in no distress but is quite agitated by the lack of effective treatment. She reports trying a number of prescription and OTC anti-yeast creams, lotions, and oral medications, none of which resolved the problem.
History-taking reveals a family history of skin problems, although neither the patient nor anyone else in the family has ever been seen by a dermatologist. No one has ever suggested that a biopsy be done.
A punch biopsy is performed on the affected inframammary skin. The pathology report shows acantholysis with focal dyskeratotic keratinocytes. Intraepidermal separation is seen throughout the specimen.
Vasodilatory medications found protective against rosacea
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Managing hyperhidrosis, HS: Ask questions first
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Outlier lesion on the back
In addition to the patient’s SK, the second finding was diagnosed as a thin melanoma. The clinical appearance of SKs and nevi or melanoma can overlap. Dermoscopy is a helpful tool in distinguishing between them, even when juxtaposed in a collision lesion such as this.1
Dermoscopy of the superior portion of the lesion demonstrated a well-demarcated brown, waxy papule with milia-like cysts, consistent with an SK. Inferiorly, the dermoscopic features included atypical pigment network, asymmetrical streaks, and blue-white veil, suggestive of melanoma or an atypical melanocytic neoplasm. A deep-shave biopsy was performed of the lower section, aiming for a narrow margin (1-3 mm) of normal skin. The biopsy confirmed a superficial spreading melanoma with a Breslow depth of 0.5 mm with 0 mitoses per high-power field.
A deep-shave biopsy was chosen over a punch biopsy because the latter would be unlikely to sample the entire lesion.
One month after the initial biopsy, a wide local excision with a 1-cm margin was performed. The planned follow-up for the patient was skin exams every 3 months for the first year, every 6 months for the next 4 years, and then annually for life.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Blum A, Siggs G, Marghoob AA, et al. Collision skin lesions-results of a multicenter study of the International Dermoscopy Society (IDS). Dermatol Pract Concept. 2017;7:51-62. doi:10.5826/dpc.0704a12
In addition to the patient’s SK, the second finding was diagnosed as a thin melanoma. The clinical appearance of SKs and nevi or melanoma can overlap. Dermoscopy is a helpful tool in distinguishing between them, even when juxtaposed in a collision lesion such as this.1
Dermoscopy of the superior portion of the lesion demonstrated a well-demarcated brown, waxy papule with milia-like cysts, consistent with an SK. Inferiorly, the dermoscopic features included atypical pigment network, asymmetrical streaks, and blue-white veil, suggestive of melanoma or an atypical melanocytic neoplasm. A deep-shave biopsy was performed of the lower section, aiming for a narrow margin (1-3 mm) of normal skin. The biopsy confirmed a superficial spreading melanoma with a Breslow depth of 0.5 mm with 0 mitoses per high-power field.
A deep-shave biopsy was chosen over a punch biopsy because the latter would be unlikely to sample the entire lesion.
One month after the initial biopsy, a wide local excision with a 1-cm margin was performed. The planned follow-up for the patient was skin exams every 3 months for the first year, every 6 months for the next 4 years, and then annually for life.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
In addition to the patient’s SK, the second finding was diagnosed as a thin melanoma. The clinical appearance of SKs and nevi or melanoma can overlap. Dermoscopy is a helpful tool in distinguishing between them, even when juxtaposed in a collision lesion such as this.1
Dermoscopy of the superior portion of the lesion demonstrated a well-demarcated brown, waxy papule with milia-like cysts, consistent with an SK. Inferiorly, the dermoscopic features included atypical pigment network, asymmetrical streaks, and blue-white veil, suggestive of melanoma or an atypical melanocytic neoplasm. A deep-shave biopsy was performed of the lower section, aiming for a narrow margin (1-3 mm) of normal skin. The biopsy confirmed a superficial spreading melanoma with a Breslow depth of 0.5 mm with 0 mitoses per high-power field.
A deep-shave biopsy was chosen over a punch biopsy because the latter would be unlikely to sample the entire lesion.
One month after the initial biopsy, a wide local excision with a 1-cm margin was performed. The planned follow-up for the patient was skin exams every 3 months for the first year, every 6 months for the next 4 years, and then annually for life.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Blum A, Siggs G, Marghoob AA, et al. Collision skin lesions-results of a multicenter study of the International Dermoscopy Society (IDS). Dermatol Pract Concept. 2017;7:51-62. doi:10.5826/dpc.0704a12
1. Blum A, Siggs G, Marghoob AA, et al. Collision skin lesions-results of a multicenter study of the International Dermoscopy Society (IDS). Dermatol Pract Concept. 2017;7:51-62. doi:10.5826/dpc.0704a12
Is it possible to classify dermatologists and internists into different patterns of prescribing behavior?
An exploratory analysis recently published in the Journal of the American Academy of Dermatology examines whether it is possible to classify dermatologists and internists into different patterns of prescribing behavior for patients with acne.
“Prior research has highlighted that prescribing for acne may not be aligned with guideline recommendations, including the overuse of oral antibiotics and lack of use of concomitant topical medications such as topical retinoids,” the study’s corresponding author, John S. Barbieri, MD, MBA, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
“In addition, there is substantial variation in prescribing practices among clinicians. . By identifying such groups, it would facilitate future qualitative interviews to understand factors that might contribute to clinicians having certain prescribing patterns, which could help guide implementation science work to better align practices with evidence and guidelines.”
For the study, which appeared online on March 1, Dr. Barbieri and colleague David J. Margolis, MD, PhD, professor of dermatology and epidemiology at the University of Pennsylvania, evaluated all clinical encounters associated with an ICD-9 or ICD-10 code for acne that occurred in the university’s departments of dermatology and internal medicine between Jan. 1, 2011, and Dec. 31, 2019. They used a machine-learning method known as k-means clustering to cluster clinicians based on their relative use of acne medications, as well as the ratio of spironolactone versus tetracycline use among female patients and stratified their analyses by specialty.
Of the 116 dermatologists included in the analysis, the researchers identified three clusters. The first cluster included 17 dermatologists (14.7%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and low use of spironolactone, compared with oral antibiotics, among women with acne. Physicians in this cluster were more likely to be male and to have more years in practice.
The second cluster included 46 dermatologists (39.6%) and was marked by high use of spironolactone and low use of isotretinoin. The third cluster included 53 dermatologists (45.7%) and was characterized by high use of topical retinoids and frequent use of systemic medications.
Of the 86 internists included in the study, the researchers identified three clusters. The first cluster included 39 internists (45.4%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and limited use of spironolactone. The second cluster included 34 internists (39.5%) and was marked by low use of topical retinoids and systemic medications. The third cluster included 13 clinicians (15.1%), most of whom were nurse practitioners, physician assistants, and other advanced practice providers. This cluster was characterized by high use of topical retinoids and relatively high use of spironolactone.
“There are likely opportunities to improve the use of topical retinoids by internists caring for patients with acne, since these are a first-line treatment option that may be underutilized by internists,” Dr. Barbieri said in the interview. “Future work is needed to identify underlying factors associated with different prescribing phenotypes among both dermatologists and internists. By understanding these factors, we can develop implementation science efforts to align prescribing behavior with best practices based on the guidelines and available evidence.”
He acknowledged certain limitations of the analysis, including its single-center design and the lack of data on patient characteristics. “Future studies are needed to examine whether our results generalize to other settings,” he said.
Dr. Barbieri disclosed that he receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. The authors had no other disclosures.
An exploratory analysis recently published in the Journal of the American Academy of Dermatology examines whether it is possible to classify dermatologists and internists into different patterns of prescribing behavior for patients with acne.
“Prior research has highlighted that prescribing for acne may not be aligned with guideline recommendations, including the overuse of oral antibiotics and lack of use of concomitant topical medications such as topical retinoids,” the study’s corresponding author, John S. Barbieri, MD, MBA, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
“In addition, there is substantial variation in prescribing practices among clinicians. . By identifying such groups, it would facilitate future qualitative interviews to understand factors that might contribute to clinicians having certain prescribing patterns, which could help guide implementation science work to better align practices with evidence and guidelines.”
For the study, which appeared online on March 1, Dr. Barbieri and colleague David J. Margolis, MD, PhD, professor of dermatology and epidemiology at the University of Pennsylvania, evaluated all clinical encounters associated with an ICD-9 or ICD-10 code for acne that occurred in the university’s departments of dermatology and internal medicine between Jan. 1, 2011, and Dec. 31, 2019. They used a machine-learning method known as k-means clustering to cluster clinicians based on their relative use of acne medications, as well as the ratio of spironolactone versus tetracycline use among female patients and stratified their analyses by specialty.
Of the 116 dermatologists included in the analysis, the researchers identified three clusters. The first cluster included 17 dermatologists (14.7%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and low use of spironolactone, compared with oral antibiotics, among women with acne. Physicians in this cluster were more likely to be male and to have more years in practice.
The second cluster included 46 dermatologists (39.6%) and was marked by high use of spironolactone and low use of isotretinoin. The third cluster included 53 dermatologists (45.7%) and was characterized by high use of topical retinoids and frequent use of systemic medications.
Of the 86 internists included in the study, the researchers identified three clusters. The first cluster included 39 internists (45.4%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and limited use of spironolactone. The second cluster included 34 internists (39.5%) and was marked by low use of topical retinoids and systemic medications. The third cluster included 13 clinicians (15.1%), most of whom were nurse practitioners, physician assistants, and other advanced practice providers. This cluster was characterized by high use of topical retinoids and relatively high use of spironolactone.
“There are likely opportunities to improve the use of topical retinoids by internists caring for patients with acne, since these are a first-line treatment option that may be underutilized by internists,” Dr. Barbieri said in the interview. “Future work is needed to identify underlying factors associated with different prescribing phenotypes among both dermatologists and internists. By understanding these factors, we can develop implementation science efforts to align prescribing behavior with best practices based on the guidelines and available evidence.”
He acknowledged certain limitations of the analysis, including its single-center design and the lack of data on patient characteristics. “Future studies are needed to examine whether our results generalize to other settings,” he said.
Dr. Barbieri disclosed that he receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. The authors had no other disclosures.
An exploratory analysis recently published in the Journal of the American Academy of Dermatology examines whether it is possible to classify dermatologists and internists into different patterns of prescribing behavior for patients with acne.
“Prior research has highlighted that prescribing for acne may not be aligned with guideline recommendations, including the overuse of oral antibiotics and lack of use of concomitant topical medications such as topical retinoids,” the study’s corresponding author, John S. Barbieri, MD, MBA, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
“In addition, there is substantial variation in prescribing practices among clinicians. . By identifying such groups, it would facilitate future qualitative interviews to understand factors that might contribute to clinicians having certain prescribing patterns, which could help guide implementation science work to better align practices with evidence and guidelines.”
For the study, which appeared online on March 1, Dr. Barbieri and colleague David J. Margolis, MD, PhD, professor of dermatology and epidemiology at the University of Pennsylvania, evaluated all clinical encounters associated with an ICD-9 or ICD-10 code for acne that occurred in the university’s departments of dermatology and internal medicine between Jan. 1, 2011, and Dec. 31, 2019. They used a machine-learning method known as k-means clustering to cluster clinicians based on their relative use of acne medications, as well as the ratio of spironolactone versus tetracycline use among female patients and stratified their analyses by specialty.
Of the 116 dermatologists included in the analysis, the researchers identified three clusters. The first cluster included 17 dermatologists (14.7%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and low use of spironolactone, compared with oral antibiotics, among women with acne. Physicians in this cluster were more likely to be male and to have more years in practice.
The second cluster included 46 dermatologists (39.6%) and was marked by high use of spironolactone and low use of isotretinoin. The third cluster included 53 dermatologists (45.7%) and was characterized by high use of topical retinoids and frequent use of systemic medications.
Of the 86 internists included in the study, the researchers identified three clusters. The first cluster included 39 internists (45.4%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and limited use of spironolactone. The second cluster included 34 internists (39.5%) and was marked by low use of topical retinoids and systemic medications. The third cluster included 13 clinicians (15.1%), most of whom were nurse practitioners, physician assistants, and other advanced practice providers. This cluster was characterized by high use of topical retinoids and relatively high use of spironolactone.
“There are likely opportunities to improve the use of topical retinoids by internists caring for patients with acne, since these are a first-line treatment option that may be underutilized by internists,” Dr. Barbieri said in the interview. “Future work is needed to identify underlying factors associated with different prescribing phenotypes among both dermatologists and internists. By understanding these factors, we can develop implementation science efforts to align prescribing behavior with best practices based on the guidelines and available evidence.”
He acknowledged certain limitations of the analysis, including its single-center design and the lack of data on patient characteristics. “Future studies are needed to examine whether our results generalize to other settings,” he said.
Dr. Barbieri disclosed that he receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. The authors had no other disclosures.
Bacteriotherapy passes early test in phase 1 atopic dermatitis study
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
FROM NATURE MEDICINE
Atopic dermatitis in children linked to elevated risk of chronic school absenteeism
.
In addition, parents of children with AD have significantly increased absenteeism from work compared with parents of children without AD.
Those are among key findings from a cross-sectional analysis of data from the Medical Expenditure Panel Surveys (MEPS), reported by Brian T. Cheng and Jonathan I. Silverberg, MD, PhD, MPH. The results were published online March 1 in the Journal of the American Academy of Dermatology.
“Atopic dermatitis is a debilitating disease that profoundly impacts children and their ability to attend school,” the study’s senior author, Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, said in an interview. “This is clinically relevant because school absenteeism is a sign of poorly controlled disease and should prompt clinicians to step up their game and aim for tighter control of the child’s atopic dermatitis.”
In an effort to determine the burden and predictors of chronic school absenteeism in children with AD, Mr. Cheng, a medical student at Northwestern University, Chicago, and Dr. Silverberg conducted a cross-sectional retrospective analysis of 124,267 children, adolescents, and young adults between the ages of 3 and 22 years from the 2000-2015 MEPS, which are representative surveys of the U.S. noninstitutionalized population conducted by the Agency for Healthcare Research and Quality. They used ICD-9 codes to determine a diagnosis of AD, psoriasis, and comorbidities; the primary outcome was chronic school absenteeism, defined as missing 15 or more days per year in the United States. MEPS also recorded the number of workdays that parents missed to care for their children or a relative.
The 124,267 individuals evaluated ranged in age between 3 and 22 years. Of these, 3,132 had AD and 200 had psoriasis. In the full cohort, chronic school absenteeism was higher among females, younger children, and those with lower household incomes, and public insurance.
Among children with AD, and those with psoriasis, 68% and 63% missed one or more day of school due to illness, respectively, while 4% in each group missed 15 days or more. Logistic regression analysis revealed that AD was associated with chronic absenteeism overall (adjusted odds ratio, 1.42), and with more severe disease (aOR, 1.33 for mild to moderate disease; aOR, 2.00 for severe disease).
On the other hand, the researchers did not observe any statistical difference in chronic absenteeism among children with versus those without psoriasis (aOR, 1.26).
The researchers also found that parents of children with versus parents of children without AD had a higher prevalence of absenteeism from work (an aOR of 1.28 among fathers, P = .009; and an aOR of 1.24 among mothers, P = .003).
In other findings, chronic absenteeism among children with AD was associated with poor/near poor/low income (aOR, 4.61) and comorbid disease (aOR, 3.35 for depression and aOR, 3.83 for asthma).
The investigators recommend that clinicians screen for and aim to reduce school absenteeism and parental work absenteeism in children with AD.
“I typically ask ‘Has (child’s name) missed any school because of their eczema?’ and follow-up with ‘What about from asthma or allergies?’ ” Dr. Silverberg said. “If the parent’s answer is yes to the first question, then I follow-up with more open-ended probing questions to understand why. Is it from all the doctor visits? Not sleeping well? Severe itch or pain? Poor sleep? Feeling sad or depressed? An answer of yes to each of these would prompt a potentially different treatment decision.”
The study received financial support from the Dermatology Foundation. The authors reported having no financial disclosures.
.
In addition, parents of children with AD have significantly increased absenteeism from work compared with parents of children without AD.
Those are among key findings from a cross-sectional analysis of data from the Medical Expenditure Panel Surveys (MEPS), reported by Brian T. Cheng and Jonathan I. Silverberg, MD, PhD, MPH. The results were published online March 1 in the Journal of the American Academy of Dermatology.
“Atopic dermatitis is a debilitating disease that profoundly impacts children and their ability to attend school,” the study’s senior author, Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, said in an interview. “This is clinically relevant because school absenteeism is a sign of poorly controlled disease and should prompt clinicians to step up their game and aim for tighter control of the child’s atopic dermatitis.”
In an effort to determine the burden and predictors of chronic school absenteeism in children with AD, Mr. Cheng, a medical student at Northwestern University, Chicago, and Dr. Silverberg conducted a cross-sectional retrospective analysis of 124,267 children, adolescents, and young adults between the ages of 3 and 22 years from the 2000-2015 MEPS, which are representative surveys of the U.S. noninstitutionalized population conducted by the Agency for Healthcare Research and Quality. They used ICD-9 codes to determine a diagnosis of AD, psoriasis, and comorbidities; the primary outcome was chronic school absenteeism, defined as missing 15 or more days per year in the United States. MEPS also recorded the number of workdays that parents missed to care for their children or a relative.
The 124,267 individuals evaluated ranged in age between 3 and 22 years. Of these, 3,132 had AD and 200 had psoriasis. In the full cohort, chronic school absenteeism was higher among females, younger children, and those with lower household incomes, and public insurance.
Among children with AD, and those with psoriasis, 68% and 63% missed one or more day of school due to illness, respectively, while 4% in each group missed 15 days or more. Logistic regression analysis revealed that AD was associated with chronic absenteeism overall (adjusted odds ratio, 1.42), and with more severe disease (aOR, 1.33 for mild to moderate disease; aOR, 2.00 for severe disease).
On the other hand, the researchers did not observe any statistical difference in chronic absenteeism among children with versus those without psoriasis (aOR, 1.26).
The researchers also found that parents of children with versus parents of children without AD had a higher prevalence of absenteeism from work (an aOR of 1.28 among fathers, P = .009; and an aOR of 1.24 among mothers, P = .003).
In other findings, chronic absenteeism among children with AD was associated with poor/near poor/low income (aOR, 4.61) and comorbid disease (aOR, 3.35 for depression and aOR, 3.83 for asthma).
The investigators recommend that clinicians screen for and aim to reduce school absenteeism and parental work absenteeism in children with AD.
“I typically ask ‘Has (child’s name) missed any school because of their eczema?’ and follow-up with ‘What about from asthma or allergies?’ ” Dr. Silverberg said. “If the parent’s answer is yes to the first question, then I follow-up with more open-ended probing questions to understand why. Is it from all the doctor visits? Not sleeping well? Severe itch or pain? Poor sleep? Feeling sad or depressed? An answer of yes to each of these would prompt a potentially different treatment decision.”
The study received financial support from the Dermatology Foundation. The authors reported having no financial disclosures.
.
In addition, parents of children with AD have significantly increased absenteeism from work compared with parents of children without AD.
Those are among key findings from a cross-sectional analysis of data from the Medical Expenditure Panel Surveys (MEPS), reported by Brian T. Cheng and Jonathan I. Silverberg, MD, PhD, MPH. The results were published online March 1 in the Journal of the American Academy of Dermatology.
“Atopic dermatitis is a debilitating disease that profoundly impacts children and their ability to attend school,” the study’s senior author, Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, said in an interview. “This is clinically relevant because school absenteeism is a sign of poorly controlled disease and should prompt clinicians to step up their game and aim for tighter control of the child’s atopic dermatitis.”
In an effort to determine the burden and predictors of chronic school absenteeism in children with AD, Mr. Cheng, a medical student at Northwestern University, Chicago, and Dr. Silverberg conducted a cross-sectional retrospective analysis of 124,267 children, adolescents, and young adults between the ages of 3 and 22 years from the 2000-2015 MEPS, which are representative surveys of the U.S. noninstitutionalized population conducted by the Agency for Healthcare Research and Quality. They used ICD-9 codes to determine a diagnosis of AD, psoriasis, and comorbidities; the primary outcome was chronic school absenteeism, defined as missing 15 or more days per year in the United States. MEPS also recorded the number of workdays that parents missed to care for their children or a relative.
The 124,267 individuals evaluated ranged in age between 3 and 22 years. Of these, 3,132 had AD and 200 had psoriasis. In the full cohort, chronic school absenteeism was higher among females, younger children, and those with lower household incomes, and public insurance.
Among children with AD, and those with psoriasis, 68% and 63% missed one or more day of school due to illness, respectively, while 4% in each group missed 15 days or more. Logistic regression analysis revealed that AD was associated with chronic absenteeism overall (adjusted odds ratio, 1.42), and with more severe disease (aOR, 1.33 for mild to moderate disease; aOR, 2.00 for severe disease).
On the other hand, the researchers did not observe any statistical difference in chronic absenteeism among children with versus those without psoriasis (aOR, 1.26).
The researchers also found that parents of children with versus parents of children without AD had a higher prevalence of absenteeism from work (an aOR of 1.28 among fathers, P = .009; and an aOR of 1.24 among mothers, P = .003).
In other findings, chronic absenteeism among children with AD was associated with poor/near poor/low income (aOR, 4.61) and comorbid disease (aOR, 3.35 for depression and aOR, 3.83 for asthma).
The investigators recommend that clinicians screen for and aim to reduce school absenteeism and parental work absenteeism in children with AD.
“I typically ask ‘Has (child’s name) missed any school because of their eczema?’ and follow-up with ‘What about from asthma or allergies?’ ” Dr. Silverberg said. “If the parent’s answer is yes to the first question, then I follow-up with more open-ended probing questions to understand why. Is it from all the doctor visits? Not sleeping well? Severe itch or pain? Poor sleep? Feeling sad or depressed? An answer of yes to each of these would prompt a potentially different treatment decision.”
The study received financial support from the Dermatology Foundation. The authors reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Atopic dermatitis, sleep difficulties often intertwined
According to Phyllis C. Zee, MD, PhD, proinflammatory cytokines influence neural processes that affect sleep and circadian rhythm. “It’s almost like when you’re most vulnerable, when you’re sleeping, the immune system is kind of poised for attack,” Dr. Zee, chief of the division of sleep medicine at Northwestern University, Chicago, said at the Revolutionizing Atopic Dermatitis symposium. “This is normal, and perhaps in some of these inflammatory disorders, it’s gone a little haywire.”
Circulation of interleukins and cytokines are high in the morning, become lower in the afternoon, and then get higher again in the evening hours and into the night during sleep, she continued. “Whereas if you look at something like blood flow, it increases on a diurnal basis,” she said. “It’s higher during the day and a little bit lower during the mid-day, and a little bit higher during the evening. That parallels changes in the sebum production of the skin and the transepidermal water loss, which has been implicated in some of the symptoms of AD. What’s curious about this is that the transdermal/epidermal water loss is really highest during the sleep period. Some of this is sleep gated, but some of this is circadian gated as well. There’s a bidirectional relationship between sleep and immunity.”
Disturbance of sleep can have multiple consequences. It can activate the hypothalamic-pituitary-adrenal axis through autonomic activation, increase brain metabolic activity, trigger mood disturbances and cognitive impairment, and cause daytime sleepiness and health consequences that affect cardiometabolic and immunologic health.
One study conducted by Anna B. Fishbein, MD, Dr. Zee, and colleagues at Northwestern examined the effects of sleep duration and sleep disruption and movements in 38 children with and without moderate to severe AD. It found that children with AD get about 1 hour less of sleep per night overall, compared with age-matched healthy controls. “It’s not so much difficulty falling asleep, but more difficulty staying asleep as determined by wake after sleep onset,” said Dr. Zee, who is also a professor of neurology at Northwestern.
A study of 34,613 adults who participated in the 2012 National Health and Nutrition Examination Survey found that eczema increased the odds of fatigue (odds ratio, 2.97), daytime sleepiness (OR, 2.66), and regular insomnia (OR, 2.36).
“Very importantly, it predicted poor health,” said Dr. Zee, who was one of the study’s coauthors. “This gives us an opportunity to think about how we can improve sleep to improve outcomes.”
Dr. Zee advises dermatologists and primary care clinicians to ask patients with AD about their sleep health by using a screening tool such as the self-reported STOP questionnaire, which consists of the following questions: “Do you snore loudly?” “Do you often feel tired, fatigued, or sleepy during daytime?” “Has anyone observed you stop breathing during your sleep?” “Do you have or are you being treated for high blood pressure?”
Other clinical indicators of a sleep disorder, such as obstructive sleep apnea (OSA), include having a neck circumference of 17 inches or greater in men and 16 inches or greater in women. “You want to also do a brief upper-airway examination, the Mallampati classification where you say to the patient, ‘open your mouth, don’t stick your mouth out too much,’ and you look at how crowded the upper airway is,” Dr. Zee said . “Someone with a Mallampati score of 3 has a very high risk of having sleep apnea.”
She also recommends asking patients with AD if they have difficulty falling asleep or staying asleep 3 or more nights per week, and about the frequency and duration of awakenings. “Maybe they have insomnia as a disorder,” she said. “If they have trouble falling asleep, maybe they have a circadian rhythm disorder. You want to ask about snoring, choking, and stop breathing episodes, because those are symptoms of sleep apnea. You want to ask about itch, uncomfortable sensations in the limbs during sleep or while trying to get to sleep, because that may be something like restless legs syndrome. Sleep disorder assessment is important because it impair daytime function, cognition, attention, and disruptive behavior, especially in children.”
For the management of insomnia, try behavioral approaches first. “You don’t want to try medications from the get-go,” Dr. Zee advised. Techniques include sleep hygiene and stimulus control therapy, “to make the bedroom a safe place to sleep. Lower the temperature a little bit and get rid of the allergens as much as possible. Relaxation and cognitive-behavioral therapy can also help. If you get a lot of light during the day, structure your physical activity, and watch what and when you eat.”
An OSA diagnosis requires evaluation of objective information from a sleep study. Common treatments of mild to moderate OSA include nasal continuous positive airway pressure and oral appliances.
Dr. Zee disclosed that she had received research funding from the National Institutes of Health, Jazz Pharmaceuticals, Harmony and Apnimed. She also serves on the scientific advisory board of Eisai, Jazz, CVS-Caremark, Takeda, and Sanofi-Aventis, and holds stock in Teva.
According to Phyllis C. Zee, MD, PhD, proinflammatory cytokines influence neural processes that affect sleep and circadian rhythm. “It’s almost like when you’re most vulnerable, when you’re sleeping, the immune system is kind of poised for attack,” Dr. Zee, chief of the division of sleep medicine at Northwestern University, Chicago, said at the Revolutionizing Atopic Dermatitis symposium. “This is normal, and perhaps in some of these inflammatory disorders, it’s gone a little haywire.”
Circulation of interleukins and cytokines are high in the morning, become lower in the afternoon, and then get higher again in the evening hours and into the night during sleep, she continued. “Whereas if you look at something like blood flow, it increases on a diurnal basis,” she said. “It’s higher during the day and a little bit lower during the mid-day, and a little bit higher during the evening. That parallels changes in the sebum production of the skin and the transepidermal water loss, which has been implicated in some of the symptoms of AD. What’s curious about this is that the transdermal/epidermal water loss is really highest during the sleep period. Some of this is sleep gated, but some of this is circadian gated as well. There’s a bidirectional relationship between sleep and immunity.”
Disturbance of sleep can have multiple consequences. It can activate the hypothalamic-pituitary-adrenal axis through autonomic activation, increase brain metabolic activity, trigger mood disturbances and cognitive impairment, and cause daytime sleepiness and health consequences that affect cardiometabolic and immunologic health.
One study conducted by Anna B. Fishbein, MD, Dr. Zee, and colleagues at Northwestern examined the effects of sleep duration and sleep disruption and movements in 38 children with and without moderate to severe AD. It found that children with AD get about 1 hour less of sleep per night overall, compared with age-matched healthy controls. “It’s not so much difficulty falling asleep, but more difficulty staying asleep as determined by wake after sleep onset,” said Dr. Zee, who is also a professor of neurology at Northwestern.
A study of 34,613 adults who participated in the 2012 National Health and Nutrition Examination Survey found that eczema increased the odds of fatigue (odds ratio, 2.97), daytime sleepiness (OR, 2.66), and regular insomnia (OR, 2.36).
“Very importantly, it predicted poor health,” said Dr. Zee, who was one of the study’s coauthors. “This gives us an opportunity to think about how we can improve sleep to improve outcomes.”
Dr. Zee advises dermatologists and primary care clinicians to ask patients with AD about their sleep health by using a screening tool such as the self-reported STOP questionnaire, which consists of the following questions: “Do you snore loudly?” “Do you often feel tired, fatigued, or sleepy during daytime?” “Has anyone observed you stop breathing during your sleep?” “Do you have or are you being treated for high blood pressure?”
Other clinical indicators of a sleep disorder, such as obstructive sleep apnea (OSA), include having a neck circumference of 17 inches or greater in men and 16 inches or greater in women. “You want to also do a brief upper-airway examination, the Mallampati classification where you say to the patient, ‘open your mouth, don’t stick your mouth out too much,’ and you look at how crowded the upper airway is,” Dr. Zee said . “Someone with a Mallampati score of 3 has a very high risk of having sleep apnea.”
She also recommends asking patients with AD if they have difficulty falling asleep or staying asleep 3 or more nights per week, and about the frequency and duration of awakenings. “Maybe they have insomnia as a disorder,” she said. “If they have trouble falling asleep, maybe they have a circadian rhythm disorder. You want to ask about snoring, choking, and stop breathing episodes, because those are symptoms of sleep apnea. You want to ask about itch, uncomfortable sensations in the limbs during sleep or while trying to get to sleep, because that may be something like restless legs syndrome. Sleep disorder assessment is important because it impair daytime function, cognition, attention, and disruptive behavior, especially in children.”
For the management of insomnia, try behavioral approaches first. “You don’t want to try medications from the get-go,” Dr. Zee advised. Techniques include sleep hygiene and stimulus control therapy, “to make the bedroom a safe place to sleep. Lower the temperature a little bit and get rid of the allergens as much as possible. Relaxation and cognitive-behavioral therapy can also help. If you get a lot of light during the day, structure your physical activity, and watch what and when you eat.”
An OSA diagnosis requires evaluation of objective information from a sleep study. Common treatments of mild to moderate OSA include nasal continuous positive airway pressure and oral appliances.
Dr. Zee disclosed that she had received research funding from the National Institutes of Health, Jazz Pharmaceuticals, Harmony and Apnimed. She also serves on the scientific advisory board of Eisai, Jazz, CVS-Caremark, Takeda, and Sanofi-Aventis, and holds stock in Teva.
According to Phyllis C. Zee, MD, PhD, proinflammatory cytokines influence neural processes that affect sleep and circadian rhythm. “It’s almost like when you’re most vulnerable, when you’re sleeping, the immune system is kind of poised for attack,” Dr. Zee, chief of the division of sleep medicine at Northwestern University, Chicago, said at the Revolutionizing Atopic Dermatitis symposium. “This is normal, and perhaps in some of these inflammatory disorders, it’s gone a little haywire.”
Circulation of interleukins and cytokines are high in the morning, become lower in the afternoon, and then get higher again in the evening hours and into the night during sleep, she continued. “Whereas if you look at something like blood flow, it increases on a diurnal basis,” she said. “It’s higher during the day and a little bit lower during the mid-day, and a little bit higher during the evening. That parallels changes in the sebum production of the skin and the transepidermal water loss, which has been implicated in some of the symptoms of AD. What’s curious about this is that the transdermal/epidermal water loss is really highest during the sleep period. Some of this is sleep gated, but some of this is circadian gated as well. There’s a bidirectional relationship between sleep and immunity.”
Disturbance of sleep can have multiple consequences. It can activate the hypothalamic-pituitary-adrenal axis through autonomic activation, increase brain metabolic activity, trigger mood disturbances and cognitive impairment, and cause daytime sleepiness and health consequences that affect cardiometabolic and immunologic health.
One study conducted by Anna B. Fishbein, MD, Dr. Zee, and colleagues at Northwestern examined the effects of sleep duration and sleep disruption and movements in 38 children with and without moderate to severe AD. It found that children with AD get about 1 hour less of sleep per night overall, compared with age-matched healthy controls. “It’s not so much difficulty falling asleep, but more difficulty staying asleep as determined by wake after sleep onset,” said Dr. Zee, who is also a professor of neurology at Northwestern.
A study of 34,613 adults who participated in the 2012 National Health and Nutrition Examination Survey found that eczema increased the odds of fatigue (odds ratio, 2.97), daytime sleepiness (OR, 2.66), and regular insomnia (OR, 2.36).
“Very importantly, it predicted poor health,” said Dr. Zee, who was one of the study’s coauthors. “This gives us an opportunity to think about how we can improve sleep to improve outcomes.”
Dr. Zee advises dermatologists and primary care clinicians to ask patients with AD about their sleep health by using a screening tool such as the self-reported STOP questionnaire, which consists of the following questions: “Do you snore loudly?” “Do you often feel tired, fatigued, or sleepy during daytime?” “Has anyone observed you stop breathing during your sleep?” “Do you have or are you being treated for high blood pressure?”
Other clinical indicators of a sleep disorder, such as obstructive sleep apnea (OSA), include having a neck circumference of 17 inches or greater in men and 16 inches or greater in women. “You want to also do a brief upper-airway examination, the Mallampati classification where you say to the patient, ‘open your mouth, don’t stick your mouth out too much,’ and you look at how crowded the upper airway is,” Dr. Zee said . “Someone with a Mallampati score of 3 has a very high risk of having sleep apnea.”
She also recommends asking patients with AD if they have difficulty falling asleep or staying asleep 3 or more nights per week, and about the frequency and duration of awakenings. “Maybe they have insomnia as a disorder,” she said. “If they have trouble falling asleep, maybe they have a circadian rhythm disorder. You want to ask about snoring, choking, and stop breathing episodes, because those are symptoms of sleep apnea. You want to ask about itch, uncomfortable sensations in the limbs during sleep or while trying to get to sleep, because that may be something like restless legs syndrome. Sleep disorder assessment is important because it impair daytime function, cognition, attention, and disruptive behavior, especially in children.”
For the management of insomnia, try behavioral approaches first. “You don’t want to try medications from the get-go,” Dr. Zee advised. Techniques include sleep hygiene and stimulus control therapy, “to make the bedroom a safe place to sleep. Lower the temperature a little bit and get rid of the allergens as much as possible. Relaxation and cognitive-behavioral therapy can also help. If you get a lot of light during the day, structure your physical activity, and watch what and when you eat.”
An OSA diagnosis requires evaluation of objective information from a sleep study. Common treatments of mild to moderate OSA include nasal continuous positive airway pressure and oral appliances.
Dr. Zee disclosed that she had received research funding from the National Institutes of Health, Jazz Pharmaceuticals, Harmony and Apnimed. She also serves on the scientific advisory board of Eisai, Jazz, CVS-Caremark, Takeda, and Sanofi-Aventis, and holds stock in Teva.
FROM REVOLUTIONIZING AD 2020
What drives treatment satisfaction among adults with atopic dermatitis?
.
Satisfaction scores were higher when specialists prescribed systemic therapy, but were lower when nonspecialists prescribed systemic therapy and when specialists prescribed only topical therapy.
Those are among key findings from an analysis of the Medical Expenditure Panel Surveys reported by Brian T. Cheng during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“AD management is complex,” said Mr. Cheng, a medical student at Northwestern University, Chicago. “It includes patient education about trigger avoidance, over-the-counter and prescription topical therapies, as well as systemic therapies. Previous studies have shown major decrements to quality of life as well as atopic and non-atopic comorbidities in these patients. The burden of AD and their comorbidities, as well as their management, may impact patient satisfaction.”
Prior studies have demonstrated that patient satisfaction is associated with improvements in clinical outcomes, increased patient retention, and reduced malpractice claims (Br J Dermatol. 2001 Oct;145[4]:617-23, Arch Dermatol 2008 Feb;144[2]:263-5). However, since data on patient satisfaction in AD are limited, Mr. Cheng and the study’s senior author, Jonathan I. Silverberg, MD, PhD, MPH, set out to examine overall patient satisfaction among adults with AD, to determine associations of patient satisfaction with patterns of health care utilization, and to identify predictors of higher satisfaction among these adults.
The researchers conducted a cross-sectional retrospective analysis of 3,810 patients from the 2000-2015 Medical Expenditure Panel Surveys, representative surveys of the U.S. noninstitutionalized population conducted annually by the Agency for Healthcare Research and Quality. They used ICD-9 codes 691 and 692 to determine AD diagnosis and five Consumer Assessment of Health Plans Survey (CAHPS) questions to assess patients’ satisfaction with their clinicians. “These questions have been extensively validated to correlate with global satisfaction,” Mr. Cheng said. “These are not disease-specific and allow for comparison across multiple diseases.”
Next, the researchers created a composite satisfaction score based on the methods of Anthony Jerant, MD, of the University of California, Davis, and colleagues. They adjusted each question in the CAHPS survey to have an equal weight and then summed these into a composite satisfaction score. “We examined patient satisfaction comparing across diseases, and based on the guidelines from the AHRQ to isolate that impact of patient-physician interaction, we adjusted for sociodemographics, mental and physical health status, self-reported health rating, as well as multimorbidity and comorbid diseases.”
Compared with adults who are healthy, adults with AD had lower patient satisfaction overall. “Moreover, people with AD had lower satisfaction compared to those with psoriasis, which may reflect more substantial itch burden as well as the greater comorbid disease challenges in management,” Mr. Cheng said. “It may also reflect the renaissance in psoriasis treatment over the last 10-20 years, giving a wider spectrum of treatment and thus a higher patient satisfaction.”
Among adults with AD, lower satisfaction was consistent across all domains of CAHPS. For the question of “How often health providers listen carefully to you” the adjusted OR (aOR) was 0.87 (P = .008). For the question of “How often health providers explain things in a way that was easy to understand” the aOR was 0.89 (P = .003). For the question of “How often health providers spent enough time with you” the aOR was 0.86 (P = .0001). For “How often providers showed respect for what you had to say” the aOR was 0.91 (P = .02).
Recognizing that treatment regimens are complex and used differently by provider type, the researchers examined interactions between specialists (dermatologists and allergists) and treatment type. “Previous studies found dermatologists treat more severe, chronic AD,” Mr. Cheng said. “We found here that there was lower satisfaction among those treated with topical therapy and by specialists, which may reflect inadequate disease control. We also found lower satisfaction among those treated with systemic therapy by primary care physicians. This may reflect that these patients are not achieving optimal therapy. We found that satisfaction was highest among those treated with systemic therapy and by dermatologists and allergists.”
Socioeconomic, racial/ethnic, and health care disparities were observed in terms of satisfaction among this cohort. The following characteristics were significantly associated with lower patient satisfaction, compared with the general cohort of adults with AD: poor to low income (aOR, –1.82; P less than .0001), multiracial/other race (aOR, –2.34; P = .0001), Hispanic ethnicity (aOR, –1.40; P = .007), and having no insurance coverage (aOR, –4.53; P less than .0001).
“Moreover, those with multimorbidity had even lower satisfaction,” Mr. Cheng said. “In previous studies, AD has been linked with many other comorbidities. This may reflect that these patients are not being adequately managed overall. So, there’s a need here for multidisciplinary care to ensure that all of these comorbidities and the full spectrum of symptoms are being managed adequately.”
He concluded that future research is needed to determine strategies to optimize patient satisfaction in adults with AD.
“I’m not sure how much more provocative you can get in terms of data,” added Dr. Silverberg, director of clinical research and contact dermatitis at George Washington University, Washington. “It’s really eye-opening. I think many clinicians may feel like they’re doing a perfect job in managing this disease. These data suggest that at least at the national level that may not be the case.”
Mr. Cheng reported having no financial disclosures. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
.
Satisfaction scores were higher when specialists prescribed systemic therapy, but were lower when nonspecialists prescribed systemic therapy and when specialists prescribed only topical therapy.
Those are among key findings from an analysis of the Medical Expenditure Panel Surveys reported by Brian T. Cheng during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“AD management is complex,” said Mr. Cheng, a medical student at Northwestern University, Chicago. “It includes patient education about trigger avoidance, over-the-counter and prescription topical therapies, as well as systemic therapies. Previous studies have shown major decrements to quality of life as well as atopic and non-atopic comorbidities in these patients. The burden of AD and their comorbidities, as well as their management, may impact patient satisfaction.”
Prior studies have demonstrated that patient satisfaction is associated with improvements in clinical outcomes, increased patient retention, and reduced malpractice claims (Br J Dermatol. 2001 Oct;145[4]:617-23, Arch Dermatol 2008 Feb;144[2]:263-5). However, since data on patient satisfaction in AD are limited, Mr. Cheng and the study’s senior author, Jonathan I. Silverberg, MD, PhD, MPH, set out to examine overall patient satisfaction among adults with AD, to determine associations of patient satisfaction with patterns of health care utilization, and to identify predictors of higher satisfaction among these adults.
The researchers conducted a cross-sectional retrospective analysis of 3,810 patients from the 2000-2015 Medical Expenditure Panel Surveys, representative surveys of the U.S. noninstitutionalized population conducted annually by the Agency for Healthcare Research and Quality. They used ICD-9 codes 691 and 692 to determine AD diagnosis and five Consumer Assessment of Health Plans Survey (CAHPS) questions to assess patients’ satisfaction with their clinicians. “These questions have been extensively validated to correlate with global satisfaction,” Mr. Cheng said. “These are not disease-specific and allow for comparison across multiple diseases.”
Next, the researchers created a composite satisfaction score based on the methods of Anthony Jerant, MD, of the University of California, Davis, and colleagues. They adjusted each question in the CAHPS survey to have an equal weight and then summed these into a composite satisfaction score. “We examined patient satisfaction comparing across diseases, and based on the guidelines from the AHRQ to isolate that impact of patient-physician interaction, we adjusted for sociodemographics, mental and physical health status, self-reported health rating, as well as multimorbidity and comorbid diseases.”
Compared with adults who are healthy, adults with AD had lower patient satisfaction overall. “Moreover, people with AD had lower satisfaction compared to those with psoriasis, which may reflect more substantial itch burden as well as the greater comorbid disease challenges in management,” Mr. Cheng said. “It may also reflect the renaissance in psoriasis treatment over the last 10-20 years, giving a wider spectrum of treatment and thus a higher patient satisfaction.”
Among adults with AD, lower satisfaction was consistent across all domains of CAHPS. For the question of “How often health providers listen carefully to you” the adjusted OR (aOR) was 0.87 (P = .008). For the question of “How often health providers explain things in a way that was easy to understand” the aOR was 0.89 (P = .003). For the question of “How often health providers spent enough time with you” the aOR was 0.86 (P = .0001). For “How often providers showed respect for what you had to say” the aOR was 0.91 (P = .02).
Recognizing that treatment regimens are complex and used differently by provider type, the researchers examined interactions between specialists (dermatologists and allergists) and treatment type. “Previous studies found dermatologists treat more severe, chronic AD,” Mr. Cheng said. “We found here that there was lower satisfaction among those treated with topical therapy and by specialists, which may reflect inadequate disease control. We also found lower satisfaction among those treated with systemic therapy by primary care physicians. This may reflect that these patients are not achieving optimal therapy. We found that satisfaction was highest among those treated with systemic therapy and by dermatologists and allergists.”
Socioeconomic, racial/ethnic, and health care disparities were observed in terms of satisfaction among this cohort. The following characteristics were significantly associated with lower patient satisfaction, compared with the general cohort of adults with AD: poor to low income (aOR, –1.82; P less than .0001), multiracial/other race (aOR, –2.34; P = .0001), Hispanic ethnicity (aOR, –1.40; P = .007), and having no insurance coverage (aOR, –4.53; P less than .0001).
“Moreover, those with multimorbidity had even lower satisfaction,” Mr. Cheng said. “In previous studies, AD has been linked with many other comorbidities. This may reflect that these patients are not being adequately managed overall. So, there’s a need here for multidisciplinary care to ensure that all of these comorbidities and the full spectrum of symptoms are being managed adequately.”
He concluded that future research is needed to determine strategies to optimize patient satisfaction in adults with AD.
“I’m not sure how much more provocative you can get in terms of data,” added Dr. Silverberg, director of clinical research and contact dermatitis at George Washington University, Washington. “It’s really eye-opening. I think many clinicians may feel like they’re doing a perfect job in managing this disease. These data suggest that at least at the national level that may not be the case.”
Mr. Cheng reported having no financial disclosures. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
.
Satisfaction scores were higher when specialists prescribed systemic therapy, but were lower when nonspecialists prescribed systemic therapy and when specialists prescribed only topical therapy.
Those are among key findings from an analysis of the Medical Expenditure Panel Surveys reported by Brian T. Cheng during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“AD management is complex,” said Mr. Cheng, a medical student at Northwestern University, Chicago. “It includes patient education about trigger avoidance, over-the-counter and prescription topical therapies, as well as systemic therapies. Previous studies have shown major decrements to quality of life as well as atopic and non-atopic comorbidities in these patients. The burden of AD and their comorbidities, as well as their management, may impact patient satisfaction.”
Prior studies have demonstrated that patient satisfaction is associated with improvements in clinical outcomes, increased patient retention, and reduced malpractice claims (Br J Dermatol. 2001 Oct;145[4]:617-23, Arch Dermatol 2008 Feb;144[2]:263-5). However, since data on patient satisfaction in AD are limited, Mr. Cheng and the study’s senior author, Jonathan I. Silverberg, MD, PhD, MPH, set out to examine overall patient satisfaction among adults with AD, to determine associations of patient satisfaction with patterns of health care utilization, and to identify predictors of higher satisfaction among these adults.
The researchers conducted a cross-sectional retrospective analysis of 3,810 patients from the 2000-2015 Medical Expenditure Panel Surveys, representative surveys of the U.S. noninstitutionalized population conducted annually by the Agency for Healthcare Research and Quality. They used ICD-9 codes 691 and 692 to determine AD diagnosis and five Consumer Assessment of Health Plans Survey (CAHPS) questions to assess patients’ satisfaction with their clinicians. “These questions have been extensively validated to correlate with global satisfaction,” Mr. Cheng said. “These are not disease-specific and allow for comparison across multiple diseases.”
Next, the researchers created a composite satisfaction score based on the methods of Anthony Jerant, MD, of the University of California, Davis, and colleagues. They adjusted each question in the CAHPS survey to have an equal weight and then summed these into a composite satisfaction score. “We examined patient satisfaction comparing across diseases, and based on the guidelines from the AHRQ to isolate that impact of patient-physician interaction, we adjusted for sociodemographics, mental and physical health status, self-reported health rating, as well as multimorbidity and comorbid diseases.”
Compared with adults who are healthy, adults with AD had lower patient satisfaction overall. “Moreover, people with AD had lower satisfaction compared to those with psoriasis, which may reflect more substantial itch burden as well as the greater comorbid disease challenges in management,” Mr. Cheng said. “It may also reflect the renaissance in psoriasis treatment over the last 10-20 years, giving a wider spectrum of treatment and thus a higher patient satisfaction.”
Among adults with AD, lower satisfaction was consistent across all domains of CAHPS. For the question of “How often health providers listen carefully to you” the adjusted OR (aOR) was 0.87 (P = .008). For the question of “How often health providers explain things in a way that was easy to understand” the aOR was 0.89 (P = .003). For the question of “How often health providers spent enough time with you” the aOR was 0.86 (P = .0001). For “How often providers showed respect for what you had to say” the aOR was 0.91 (P = .02).
Recognizing that treatment regimens are complex and used differently by provider type, the researchers examined interactions between specialists (dermatologists and allergists) and treatment type. “Previous studies found dermatologists treat more severe, chronic AD,” Mr. Cheng said. “We found here that there was lower satisfaction among those treated with topical therapy and by specialists, which may reflect inadequate disease control. We also found lower satisfaction among those treated with systemic therapy by primary care physicians. This may reflect that these patients are not achieving optimal therapy. We found that satisfaction was highest among those treated with systemic therapy and by dermatologists and allergists.”
Socioeconomic, racial/ethnic, and health care disparities were observed in terms of satisfaction among this cohort. The following characteristics were significantly associated with lower patient satisfaction, compared with the general cohort of adults with AD: poor to low income (aOR, –1.82; P less than .0001), multiracial/other race (aOR, –2.34; P = .0001), Hispanic ethnicity (aOR, –1.40; P = .007), and having no insurance coverage (aOR, –4.53; P less than .0001).
“Moreover, those with multimorbidity had even lower satisfaction,” Mr. Cheng said. “In previous studies, AD has been linked with many other comorbidities. This may reflect that these patients are not being adequately managed overall. So, there’s a need here for multidisciplinary care to ensure that all of these comorbidities and the full spectrum of symptoms are being managed adequately.”
He concluded that future research is needed to determine strategies to optimize patient satisfaction in adults with AD.
“I’m not sure how much more provocative you can get in terms of data,” added Dr. Silverberg, director of clinical research and contact dermatitis at George Washington University, Washington. “It’s really eye-opening. I think many clinicians may feel like they’re doing a perfect job in managing this disease. These data suggest that at least at the national level that may not be the case.”
Mr. Cheng reported having no financial disclosures. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020
Novel oral agent effective in teens with atopic dermatitis
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.