User login
Slow-growing lesion on eyebrow
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
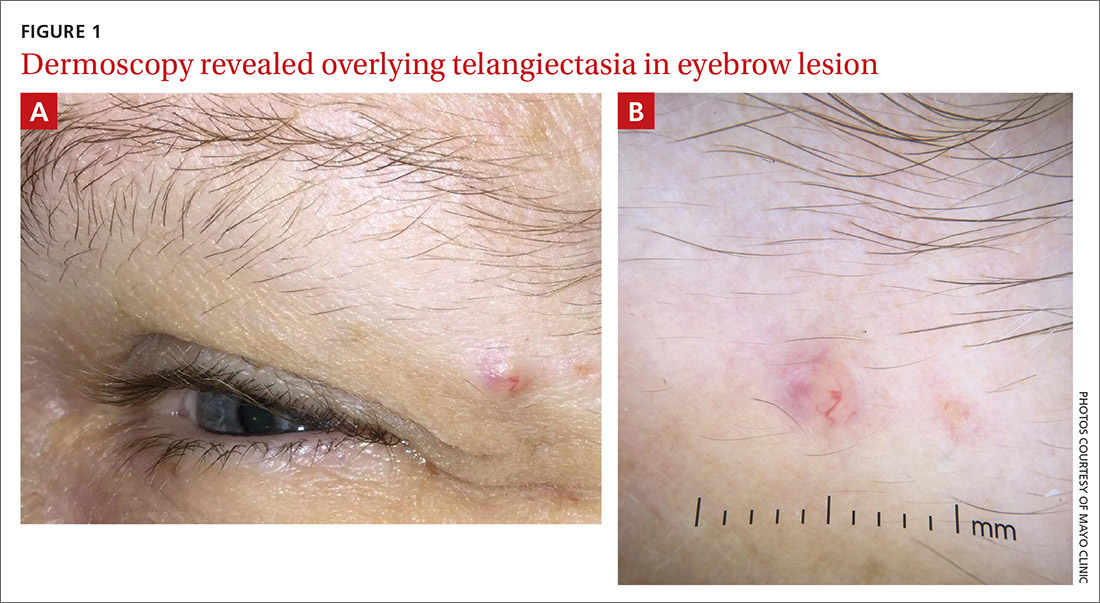
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
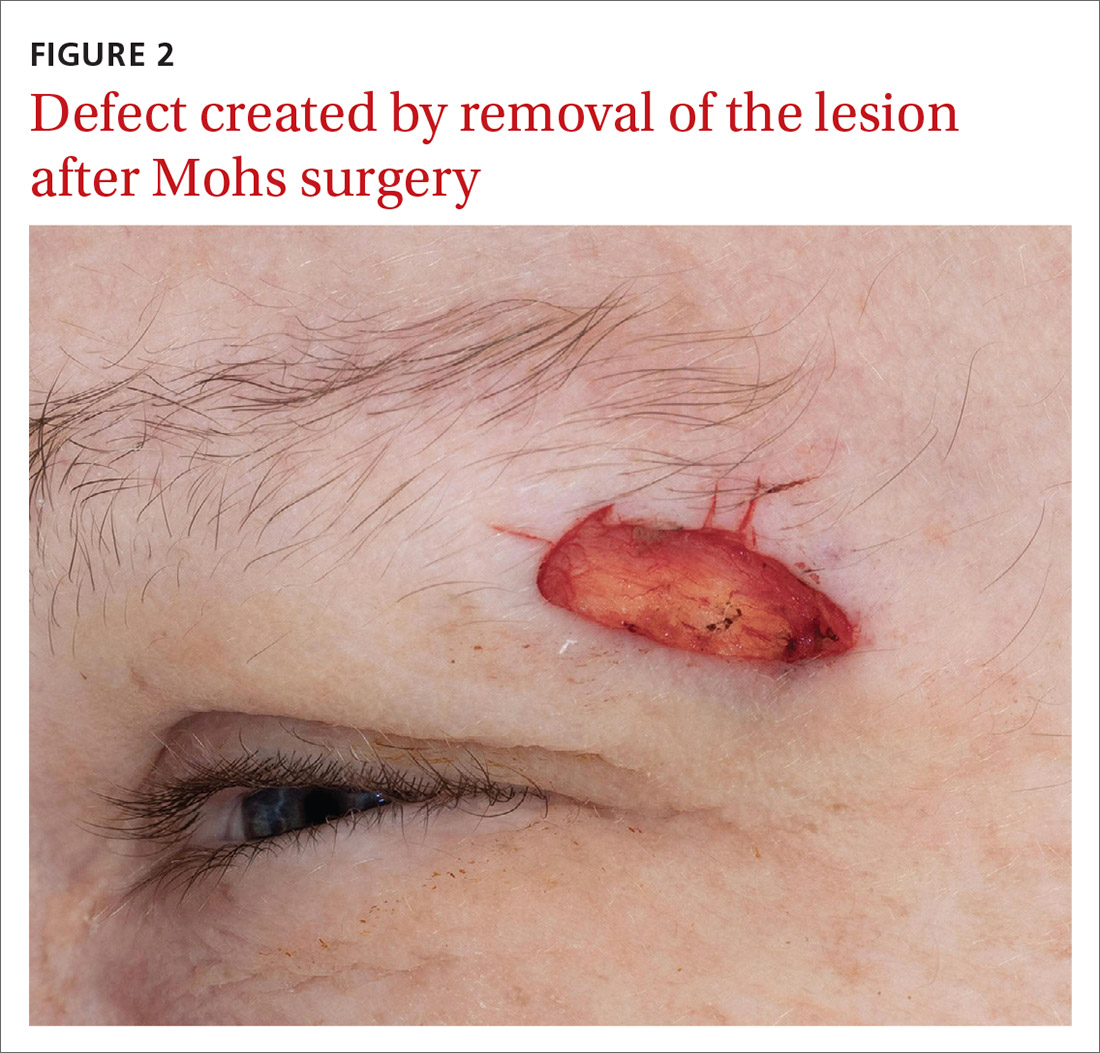
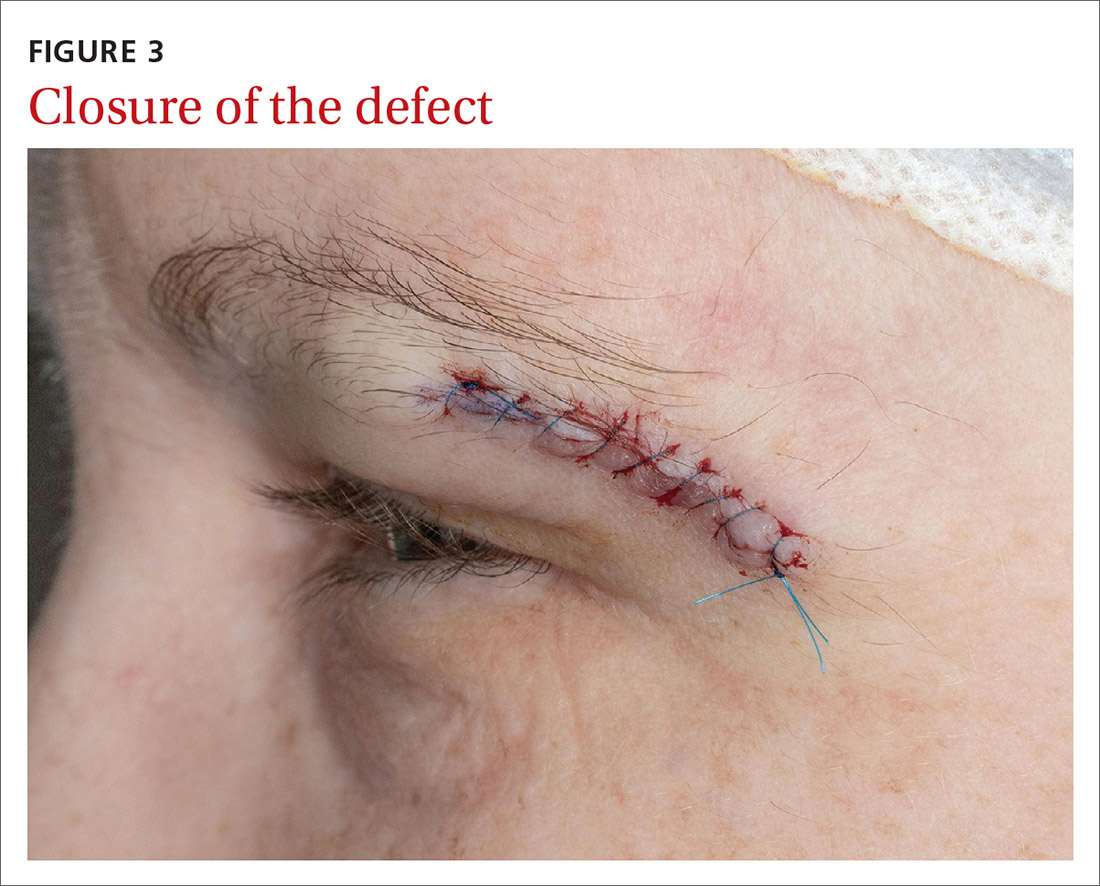
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.
The dermatologist recommended yearly skin examination for 5 years for the patient.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.
The dermatologist recommended yearly skin examination for 5 years for the patient.
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.
The dermatologist recommended yearly skin examination for 5 years for the patient.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
New skin papules
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
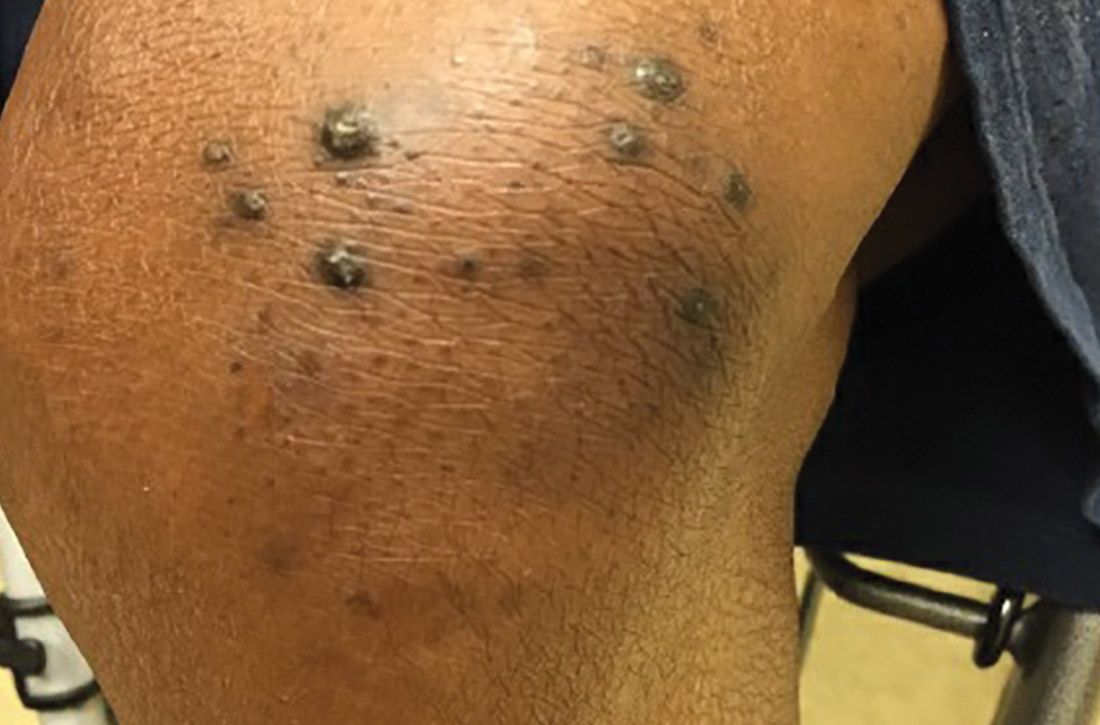
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
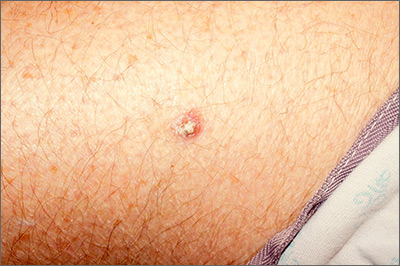
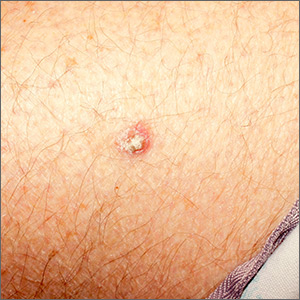
Rough lesion on the thigh
A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037
A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037
Severe atopic dermatitis often puts a dent in quality of life
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
FROM REVOLUTIONIZING AD 2020
ACR, AAD, AAO, RDS issue joint statement on safe use of hydroxychloroquine
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
FROM ARTHRITIS & rHEUMATOLOGY
Peanut sublingual immunotherapy feasible and effective in toddlers
Sublingual immunotherapy for the treatment of peanut allergy is safe and effective, even in children as young as age 1 year.
In a double-blind, placebo-controlled, food challenge (DBPCFC) of some 36 peanut-allergic children (mean age 2.2 years, range 1-4 years), those who were randomly assigned to receive peanut sublingual immunotherapy (PNSLIT) showed significant desensitization compared with those who received placebo.
In addition, there was a “strong potential” for sustained unresponsiveness at 3 months for the toddlers who received the active treatment.
The findings were presented in a late breaking oral abstract session at the 2021 American Academy of Allergy, Asthma & Immunology virtual annual meeting (Abstract L2).
“A year ago, the Food and Drug Administration approved the oral agent Palforzia (peanut allergen powder) for the treatment of peanut allergy in children 4 and older, and it is a great option, but I think what we have learned over time is that this approach is not for everybody,” Edwin H. Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, said in an interview.
Palforzia is a powder that is mixed in food like yogurt or pudding which the child then eats daily, according to a rigorous schedule. But Palforzia treatment presents some difficulties.
“Palforzia requires getting the powder dose, mixing it with food, like pudding or apple sauce, then eating it, which can take up to 30 minutes depending on age and kids’ cooperation. It tastes and smells like peanut which can cause aversion. Kids have to refrain from exercise or strenuous activity for at least 30 minutes before and after dosing and have to be observed for up to 2 hours post dose for symptoms,” Dr. Kim said.
“It’s a great drug, but the treatment could be overly difficult for certain families to be able to do, and in some cases the side effects may be more than certain patients are able or willing to handle, so there is a real urgent need for alternative approaches,” Dr. Kim said. “SLIT is several drops under the tongue, held for 2 minutes, swallowed and done.”
In the current placebo-controlled study, he and his group tested the feasibility, efficacy, and safety of the sublingual approach to peanut allergy in children age 4 years and younger.
Both groups were similar with regard to gender, race, ethnicity, atopic history, peanut skin prick test, and qualifying DBPCFC, and all children were previously allergic with positive blood and skin tests, with a positive reaction during baseline food challenge, thus proving the allergy and establishing the baseline threshold.
“We have learned from some studies, for instance the DEVIL and LEAP studies, that strongly suggest that the immune systems in younger patients may be more amenable to change, and there may be some justification for early intervention,” he said.
“Based on both of those ideas, we wanted to take our sublingual approach, which we have shown to have a pretty good efficacy in older children, and bring it down to this younger group and see if it still could have the same efficacy and also maintain what seems to be a very good safety signal.”
The researchers randomly assigned the children to receive PNSLIT at a daily maintenance dose of 4 mg peanut protein (n = 19) or to receive placebo (n = 17) for 36 months.
“There was a 5- to 6-month buildup period where the SLIT dose was increased every 1-2 weeks up to the target dose of 4 mg, and then the final dose of 4 mg was continued through to the end of the study,” Dr. Kim noted.
Over a total of 20,593 potential dosing days, the children took 91.2% of SLIT doses and 93.5% of placebo doses.
At the end of the 3-year study period, the children were challenged by DBPCFC with up to 4,333 mg of peanut protein.
Sustained unresponsiveness was assessed by an identical DBPCFC after discontinuation of the immunotherapy for 3 months.
Cumulative tolerated dose increased from a median of 143 mg to 4,443 mg in the PNSLIT group, compared with a median of 43 mg to 143 mg in the placebo group (P < .0001).
Fourteen of the children receiving PNSLIT, and none of the children receiving placebo, passed the desensitization food challenge. Twelve of the children receiving PNSLIT and two of the children receiving placebo passed the sustained unresponsiveness challenge.
Children who underwent the immunotherapy saw a decrease in their peanut skin prick test from 10 mm to 3.25 mm, compared to an increase from 11.5 mm to 12 mm with placebo (P < .0001).
The most common side effect reported was itching or irritation in the mouth. Most side effects resolved on their own, although some patients used an antihistamine. Getting children as young as 1 to hold the dose under their tongue was a challenge in some instances, but it eventually worked out, Dr. Kim said.
“It took a lot of work from the parents as well as from our research coordinators in trying to train these young kids to, first of all, allow us to put the peanut medication in the mouth and then to try as best as possible to keep it in their mouth for up to 2 minutes, but the families involved in our study were very dedicated and so we were able to get through that,” he said.
Study merits larger numbers
“Among the 36 who completed the 3 years of therapy, the authors report significant rates of desensitization among treated children compared with those receiving placebo. Furthermore, this effect was persistent for at least 3 months after stopping therapy in a subgroup of the children,” said Leonard B. Bacharier, MD, director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
“Overall, these findings suggest the promise of peanut SLIT, which should be studied in larger numbers of preschool children,” Dr. Bacharier, who was not part of the study, said in an interview.
Jonathan A. Bernstein, MD, professor of medicine, University of Cincinnati, agreed.
“It’s a well-designed study, it’s small, but it’s promising,” Dr. Bernstein, who was not involved with the study, said in an interview.
“They did show that most of the patients who got the sublingual therapy were able to get to the target dose and develop tolerance, so I think it’s promising. We know that this stuff works. This is just more data from a well-controlled study in a younger population,” he said.
“We do OIT [oral immunotherapy] and sublingual but we don’t do it in such young children in our practice. The youngest is 3 years old, because they have to understand what is going on and cooperate. If they don’t cooperate it’s not possible.”
Dr. Kim reported financial relationships with DBV Technologies, Kenota Health, Ukko, Aimmune Therapeutics, ALK, AllerGenis, Belhaven Pharma, Duke Clinical Research Institute, Nutricia, NIH/NIAID, NIH/NCCIH, NIH/Immune Tolerance Network, FARE, and the Wallace Foundation. Dr. Bacharier and Dr. Bernstein have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy for the treatment of peanut allergy is safe and effective, even in children as young as age 1 year.
In a double-blind, placebo-controlled, food challenge (DBPCFC) of some 36 peanut-allergic children (mean age 2.2 years, range 1-4 years), those who were randomly assigned to receive peanut sublingual immunotherapy (PNSLIT) showed significant desensitization compared with those who received placebo.
In addition, there was a “strong potential” for sustained unresponsiveness at 3 months for the toddlers who received the active treatment.
The findings were presented in a late breaking oral abstract session at the 2021 American Academy of Allergy, Asthma & Immunology virtual annual meeting (Abstract L2).
“A year ago, the Food and Drug Administration approved the oral agent Palforzia (peanut allergen powder) for the treatment of peanut allergy in children 4 and older, and it is a great option, but I think what we have learned over time is that this approach is not for everybody,” Edwin H. Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, said in an interview.
Palforzia is a powder that is mixed in food like yogurt or pudding which the child then eats daily, according to a rigorous schedule. But Palforzia treatment presents some difficulties.
“Palforzia requires getting the powder dose, mixing it with food, like pudding or apple sauce, then eating it, which can take up to 30 minutes depending on age and kids’ cooperation. It tastes and smells like peanut which can cause aversion. Kids have to refrain from exercise or strenuous activity for at least 30 minutes before and after dosing and have to be observed for up to 2 hours post dose for symptoms,” Dr. Kim said.
“It’s a great drug, but the treatment could be overly difficult for certain families to be able to do, and in some cases the side effects may be more than certain patients are able or willing to handle, so there is a real urgent need for alternative approaches,” Dr. Kim said. “SLIT is several drops under the tongue, held for 2 minutes, swallowed and done.”
In the current placebo-controlled study, he and his group tested the feasibility, efficacy, and safety of the sublingual approach to peanut allergy in children age 4 years and younger.
Both groups were similar with regard to gender, race, ethnicity, atopic history, peanut skin prick test, and qualifying DBPCFC, and all children were previously allergic with positive blood and skin tests, with a positive reaction during baseline food challenge, thus proving the allergy and establishing the baseline threshold.
“We have learned from some studies, for instance the DEVIL and LEAP studies, that strongly suggest that the immune systems in younger patients may be more amenable to change, and there may be some justification for early intervention,” he said.
“Based on both of those ideas, we wanted to take our sublingual approach, which we have shown to have a pretty good efficacy in older children, and bring it down to this younger group and see if it still could have the same efficacy and also maintain what seems to be a very good safety signal.”
The researchers randomly assigned the children to receive PNSLIT at a daily maintenance dose of 4 mg peanut protein (n = 19) or to receive placebo (n = 17) for 36 months.
“There was a 5- to 6-month buildup period where the SLIT dose was increased every 1-2 weeks up to the target dose of 4 mg, and then the final dose of 4 mg was continued through to the end of the study,” Dr. Kim noted.
Over a total of 20,593 potential dosing days, the children took 91.2% of SLIT doses and 93.5% of placebo doses.
At the end of the 3-year study period, the children were challenged by DBPCFC with up to 4,333 mg of peanut protein.
Sustained unresponsiveness was assessed by an identical DBPCFC after discontinuation of the immunotherapy for 3 months.
Cumulative tolerated dose increased from a median of 143 mg to 4,443 mg in the PNSLIT group, compared with a median of 43 mg to 143 mg in the placebo group (P < .0001).
Fourteen of the children receiving PNSLIT, and none of the children receiving placebo, passed the desensitization food challenge. Twelve of the children receiving PNSLIT and two of the children receiving placebo passed the sustained unresponsiveness challenge.
Children who underwent the immunotherapy saw a decrease in their peanut skin prick test from 10 mm to 3.25 mm, compared to an increase from 11.5 mm to 12 mm with placebo (P < .0001).
The most common side effect reported was itching or irritation in the mouth. Most side effects resolved on their own, although some patients used an antihistamine. Getting children as young as 1 to hold the dose under their tongue was a challenge in some instances, but it eventually worked out, Dr. Kim said.
“It took a lot of work from the parents as well as from our research coordinators in trying to train these young kids to, first of all, allow us to put the peanut medication in the mouth and then to try as best as possible to keep it in their mouth for up to 2 minutes, but the families involved in our study were very dedicated and so we were able to get through that,” he said.
Study merits larger numbers
“Among the 36 who completed the 3 years of therapy, the authors report significant rates of desensitization among treated children compared with those receiving placebo. Furthermore, this effect was persistent for at least 3 months after stopping therapy in a subgroup of the children,” said Leonard B. Bacharier, MD, director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
“Overall, these findings suggest the promise of peanut SLIT, which should be studied in larger numbers of preschool children,” Dr. Bacharier, who was not part of the study, said in an interview.
Jonathan A. Bernstein, MD, professor of medicine, University of Cincinnati, agreed.
“It’s a well-designed study, it’s small, but it’s promising,” Dr. Bernstein, who was not involved with the study, said in an interview.
“They did show that most of the patients who got the sublingual therapy were able to get to the target dose and develop tolerance, so I think it’s promising. We know that this stuff works. This is just more data from a well-controlled study in a younger population,” he said.
“We do OIT [oral immunotherapy] and sublingual but we don’t do it in such young children in our practice. The youngest is 3 years old, because they have to understand what is going on and cooperate. If they don’t cooperate it’s not possible.”
Dr. Kim reported financial relationships with DBV Technologies, Kenota Health, Ukko, Aimmune Therapeutics, ALK, AllerGenis, Belhaven Pharma, Duke Clinical Research Institute, Nutricia, NIH/NIAID, NIH/NCCIH, NIH/Immune Tolerance Network, FARE, and the Wallace Foundation. Dr. Bacharier and Dr. Bernstein have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy for the treatment of peanut allergy is safe and effective, even in children as young as age 1 year.
In a double-blind, placebo-controlled, food challenge (DBPCFC) of some 36 peanut-allergic children (mean age 2.2 years, range 1-4 years), those who were randomly assigned to receive peanut sublingual immunotherapy (PNSLIT) showed significant desensitization compared with those who received placebo.
In addition, there was a “strong potential” for sustained unresponsiveness at 3 months for the toddlers who received the active treatment.
The findings were presented in a late breaking oral abstract session at the 2021 American Academy of Allergy, Asthma & Immunology virtual annual meeting (Abstract L2).
“A year ago, the Food and Drug Administration approved the oral agent Palforzia (peanut allergen powder) for the treatment of peanut allergy in children 4 and older, and it is a great option, but I think what we have learned over time is that this approach is not for everybody,” Edwin H. Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, said in an interview.
Palforzia is a powder that is mixed in food like yogurt or pudding which the child then eats daily, according to a rigorous schedule. But Palforzia treatment presents some difficulties.
“Palforzia requires getting the powder dose, mixing it with food, like pudding or apple sauce, then eating it, which can take up to 30 minutes depending on age and kids’ cooperation. It tastes and smells like peanut which can cause aversion. Kids have to refrain from exercise or strenuous activity for at least 30 minutes before and after dosing and have to be observed for up to 2 hours post dose for symptoms,” Dr. Kim said.
“It’s a great drug, but the treatment could be overly difficult for certain families to be able to do, and in some cases the side effects may be more than certain patients are able or willing to handle, so there is a real urgent need for alternative approaches,” Dr. Kim said. “SLIT is several drops under the tongue, held for 2 minutes, swallowed and done.”
In the current placebo-controlled study, he and his group tested the feasibility, efficacy, and safety of the sublingual approach to peanut allergy in children age 4 years and younger.
Both groups were similar with regard to gender, race, ethnicity, atopic history, peanut skin prick test, and qualifying DBPCFC, and all children were previously allergic with positive blood and skin tests, with a positive reaction during baseline food challenge, thus proving the allergy and establishing the baseline threshold.
“We have learned from some studies, for instance the DEVIL and LEAP studies, that strongly suggest that the immune systems in younger patients may be more amenable to change, and there may be some justification for early intervention,” he said.
“Based on both of those ideas, we wanted to take our sublingual approach, which we have shown to have a pretty good efficacy in older children, and bring it down to this younger group and see if it still could have the same efficacy and also maintain what seems to be a very good safety signal.”
The researchers randomly assigned the children to receive PNSLIT at a daily maintenance dose of 4 mg peanut protein (n = 19) or to receive placebo (n = 17) for 36 months.
“There was a 5- to 6-month buildup period where the SLIT dose was increased every 1-2 weeks up to the target dose of 4 mg, and then the final dose of 4 mg was continued through to the end of the study,” Dr. Kim noted.
Over a total of 20,593 potential dosing days, the children took 91.2% of SLIT doses and 93.5% of placebo doses.
At the end of the 3-year study period, the children were challenged by DBPCFC with up to 4,333 mg of peanut protein.
Sustained unresponsiveness was assessed by an identical DBPCFC after discontinuation of the immunotherapy for 3 months.
Cumulative tolerated dose increased from a median of 143 mg to 4,443 mg in the PNSLIT group, compared with a median of 43 mg to 143 mg in the placebo group (P < .0001).
Fourteen of the children receiving PNSLIT, and none of the children receiving placebo, passed the desensitization food challenge. Twelve of the children receiving PNSLIT and two of the children receiving placebo passed the sustained unresponsiveness challenge.
Children who underwent the immunotherapy saw a decrease in their peanut skin prick test from 10 mm to 3.25 mm, compared to an increase from 11.5 mm to 12 mm with placebo (P < .0001).
The most common side effect reported was itching or irritation in the mouth. Most side effects resolved on their own, although some patients used an antihistamine. Getting children as young as 1 to hold the dose under their tongue was a challenge in some instances, but it eventually worked out, Dr. Kim said.
“It took a lot of work from the parents as well as from our research coordinators in trying to train these young kids to, first of all, allow us to put the peanut medication in the mouth and then to try as best as possible to keep it in their mouth for up to 2 minutes, but the families involved in our study were very dedicated and so we were able to get through that,” he said.
Study merits larger numbers
“Among the 36 who completed the 3 years of therapy, the authors report significant rates of desensitization among treated children compared with those receiving placebo. Furthermore, this effect was persistent for at least 3 months after stopping therapy in a subgroup of the children,” said Leonard B. Bacharier, MD, director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
“Overall, these findings suggest the promise of peanut SLIT, which should be studied in larger numbers of preschool children,” Dr. Bacharier, who was not part of the study, said in an interview.
Jonathan A. Bernstein, MD, professor of medicine, University of Cincinnati, agreed.
“It’s a well-designed study, it’s small, but it’s promising,” Dr. Bernstein, who was not involved with the study, said in an interview.
“They did show that most of the patients who got the sublingual therapy were able to get to the target dose and develop tolerance, so I think it’s promising. We know that this stuff works. This is just more data from a well-controlled study in a younger population,” he said.
“We do OIT [oral immunotherapy] and sublingual but we don’t do it in such young children in our practice. The youngest is 3 years old, because they have to understand what is going on and cooperate. If they don’t cooperate it’s not possible.”
Dr. Kim reported financial relationships with DBV Technologies, Kenota Health, Ukko, Aimmune Therapeutics, ALK, AllerGenis, Belhaven Pharma, Duke Clinical Research Institute, Nutricia, NIH/NIAID, NIH/NCCIH, NIH/Immune Tolerance Network, FARE, and the Wallace Foundation. Dr. Bacharier and Dr. Bernstein have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAAAI
Data on atopic dermatitis risk factors are accumulating
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
FROM REVOLUTIONIZING AD 2020
AI detects ugly-duckling skin lesions for melanoma follow-up
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
Methotrexate-associated hepatotoxicity risk differs between psoriasis, PsA, and RA patients
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Painful hand and foot plaques
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.