User login
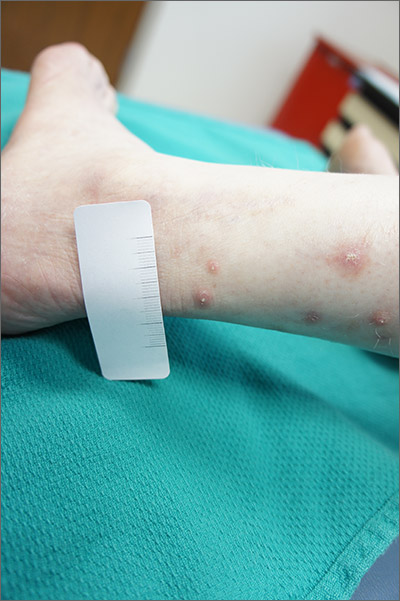
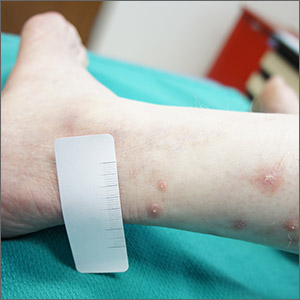
Itchy leg papules
Punch biopsy revealed collagen extravasation consistent with acquired reactive perforating collagenosis, an uncommon acquired disease that is seen in patients with longstanding renal disease or diabetes mellitus. The patient’s history of end-stage renal disease, paired with the eruptive nature of the pink papules with central firm plugs, pointed to the diagnosis.
The differential diagnosis for lesions like these includes prurigo nodularis and eruptive keratoacanthomas. Prurigo nodules would itch but likely lack a central plug. Eruptive keratoacanthomas would have a central keratinaceous plug but would be less likely to itch. A biopsy can help distinguish these entities.
Reactive perforating collagenosis can affect up to 10% of hemodialysis patients. It also can be associated with human immunodeficiency virus, hyperparathyroidism, hypothyroidism, liver disease, and sclerosing cholangitis. One theory of the etiology is that underlying disease causes skin itching and the subsequent trauma from scratching causes reactivity.
The work-up consists of a punch biopsy of the entire lesion or central plug. A biopsy limited to the edge of the lesion, or one that is shallow, may fail to connect altered dermal collagen with its follicular elimination and be misread as dermatitis.
Lesions often resolve spontaneously, but disease can be widespread. Topical steroids and antihistamines may reduce itching. Narrowband UVB, topical or systemic retinoids, tetracyclines, and cryotherapy all have had reported success. Narrowband UVB is especially helpful for uremic pruritus and, if available, may be the treatment of choice.
This patient was treated with topical steroids, oral cetirizine 10 mg/d, and cryotherapy to the most stubborn lesions. Over 3 months, the number of lesions and severity of symptoms improved. She continued hemodialysis and awaits a renal transplant.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660.
Punch biopsy revealed collagen extravasation consistent with acquired reactive perforating collagenosis, an uncommon acquired disease that is seen in patients with longstanding renal disease or diabetes mellitus. The patient’s history of end-stage renal disease, paired with the eruptive nature of the pink papules with central firm plugs, pointed to the diagnosis.
The differential diagnosis for lesions like these includes prurigo nodularis and eruptive keratoacanthomas. Prurigo nodules would itch but likely lack a central plug. Eruptive keratoacanthomas would have a central keratinaceous plug but would be less likely to itch. A biopsy can help distinguish these entities.
Reactive perforating collagenosis can affect up to 10% of hemodialysis patients. It also can be associated with human immunodeficiency virus, hyperparathyroidism, hypothyroidism, liver disease, and sclerosing cholangitis. One theory of the etiology is that underlying disease causes skin itching and the subsequent trauma from scratching causes reactivity.
The work-up consists of a punch biopsy of the entire lesion or central plug. A biopsy limited to the edge of the lesion, or one that is shallow, may fail to connect altered dermal collagen with its follicular elimination and be misread as dermatitis.
Lesions often resolve spontaneously, but disease can be widespread. Topical steroids and antihistamines may reduce itching. Narrowband UVB, topical or systemic retinoids, tetracyclines, and cryotherapy all have had reported success. Narrowband UVB is especially helpful for uremic pruritus and, if available, may be the treatment of choice.
This patient was treated with topical steroids, oral cetirizine 10 mg/d, and cryotherapy to the most stubborn lesions. Over 3 months, the number of lesions and severity of symptoms improved. She continued hemodialysis and awaits a renal transplant.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Punch biopsy revealed collagen extravasation consistent with acquired reactive perforating collagenosis, an uncommon acquired disease that is seen in patients with longstanding renal disease or diabetes mellitus. The patient’s history of end-stage renal disease, paired with the eruptive nature of the pink papules with central firm plugs, pointed to the diagnosis.
The differential diagnosis for lesions like these includes prurigo nodularis and eruptive keratoacanthomas. Prurigo nodules would itch but likely lack a central plug. Eruptive keratoacanthomas would have a central keratinaceous plug but would be less likely to itch. A biopsy can help distinguish these entities.
Reactive perforating collagenosis can affect up to 10% of hemodialysis patients. It also can be associated with human immunodeficiency virus, hyperparathyroidism, hypothyroidism, liver disease, and sclerosing cholangitis. One theory of the etiology is that underlying disease causes skin itching and the subsequent trauma from scratching causes reactivity.
The work-up consists of a punch biopsy of the entire lesion or central plug. A biopsy limited to the edge of the lesion, or one that is shallow, may fail to connect altered dermal collagen with its follicular elimination and be misread as dermatitis.
Lesions often resolve spontaneously, but disease can be widespread. Topical steroids and antihistamines may reduce itching. Narrowband UVB, topical or systemic retinoids, tetracyclines, and cryotherapy all have had reported success. Narrowband UVB is especially helpful for uremic pruritus and, if available, may be the treatment of choice.
This patient was treated with topical steroids, oral cetirizine 10 mg/d, and cryotherapy to the most stubborn lesions. Over 3 months, the number of lesions and severity of symptoms improved. She continued hemodialysis and awaits a renal transplant.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660.
Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660.
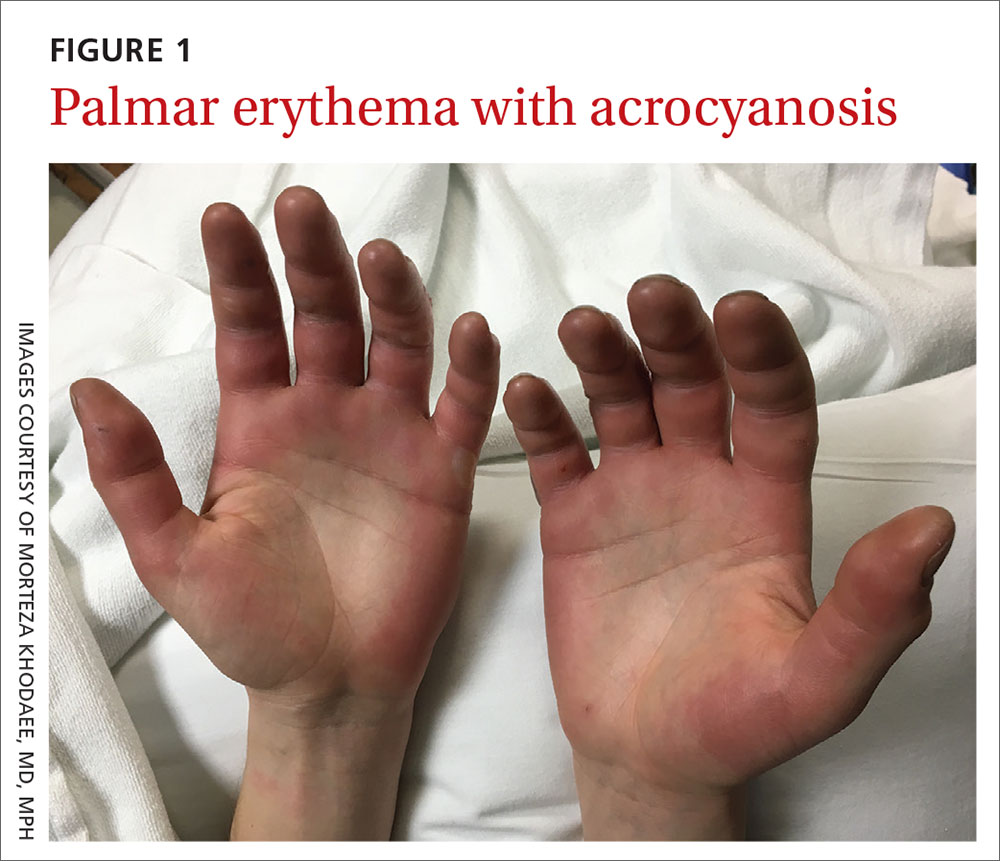
Acute bilateral hand edema and vesiculation
A 27-year-old man presented to the urgent care clinic with acute bilateral hand swelling, blisters, numbness, and pain. History taking revealed that these symptoms developed after he was locked outside of his apartment for 45 minutes in –22°C (–8°F) weather following a night of heavy drinking.
On physical examination, the patient had a temperature of 36.2°C (97.2°F) and a heart rate of 116 beats/min. He had
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Second-degree frostbite
Frostbite is the result of tissue freezing, which generally occurs after prolonged exposure to freezing temperatures (typically –4°C or below).1,2 The majority (~90%) of frostbite injuries occur in the hands and feet; however, frostbite has also been observed in the face, perineum, buttocks, and male genitalia.3
Frostbite is a clinical diagnosis based on a history of sustained exposure to freezing temperatures, paresthesia of affected areas, and typical skin changes. Evidence is lacking regarding the epidemiology of frostbite within the general population.2
Pathophysiology. Intra- and extracellular ice crystal formation causes fluid and electrolyte disturbances, cell dehydration, lipid denaturation, and subsequent cell death.1 After thawing, progressive tissue ischemia can occur as a result of endothelial damage and dysfunction, intravascular sludging, increased inflammatory markers, an influx of free radicals, and microvascular thrombosis.1
Classification. Traditionally, frostbite has been classified according to a 4-tiered system based on tissue appearance after rewarming.2 First-degree frostbite is characterized by white plaques with surrounding erythema; second degree by edema and clear or cloudy vesicles; third degree by hemorrhagic bullae; and fourth degree by cold and hard tissue that eventually progresses to gangrene.2
A simpler scheme designates frostbite as either superficial (corresponding to first- or second-degree frostbite) or deep (corresponding to third- or fourth-degree frostbite) with presumed muscle and bone involvement.2
Continue to: Risk factors
Risk factors. Frostbite is often associated with risk factors such as alcohol or drug intoxication, vehicular failure or trauma, immobilizing trauma, psychiatric illness, homelessness, Raynaud phenomenon, peripheral vascular disease, diabetes, inadequate clothing, previous cold-weather injury, outdoor winter recreation, and the use of certain medications (eg, beta-blockers).1-3 Apart from environmental exposure, frostbite can also occur by direct contact with freezing materials, such as ice packs or industrial refrigerants.3
Differential includes nonfreezing injuries
Frostnip, pernio, and trench foot are other cold-weather injuries distinguished by the absence of tissue freezing.4 Raynaud phenomenon is a condition that is triggered by either cold temperatures or emotional stress.5
Frostnip is characterized by pallor and paresthesia of exposed areas. It may precede frostbite, but it quickly resolves after rewarming.2
Pernio occurs when skin is exposed to damp, cold, nonfreezing environments.6 It results in edematous and inflammatory skin lesions that may be painful, pruritic, violaceous, or erythematous.6 These lesions are typically found over the fingers, toes, nose, ears, buttocks, or thighs.4,6 Pernio may be classified as either primary or secondary disease.5 Primary pernio is considered idiopathic.6 Secondary pernio is thought to be either drug induced or due to underlying autoimmune diseases, such as hepatitis or cryopathy.6
Trench foot develops under similar conditions to pernio but requires exposure to a wet environment for at least 10 to 14 hours.7 It is characterized by foot pain, paresthesia, pruritus, edema, erythema, cyanosis, blisters, and even gangrene if left untreated.7
Continue to: Raynaud phenomenon
Raynaud phenomenon results from transient, acral vasocontraction and manifests as well-demarcated pallor, cyanosis, and then erythema as the affected body part reperfuses.5 Similar to pernio, it can be categorized as either primary or secondary.5 Primary phenomenon is idiopathic. Secondary phenomenon is thought to be a result of autoimmune disease, use of certain medications, occupational vibratory exposure, obstructive vascular disease, or infection
In the absence of a history of exposure to subfreezing temperatures, frostbite can be excluded from the differential diagnosis.
Treatment entails rewarming
The aim of frostbite treatment is to save injured cells and minimize tissue loss.1 This is accomplished through rapid rewarming and—in severe cases—reperfusion techniques.
Tissue should be rewarmed in a 37°C to 39°C water bath with povidone iodine or chlorhexidine added for antiseptic effect.1 All efforts should be made to avoid refreezing or trauma, as this could worsen the initial injury.2 Oral or intravenous hydration may be offered to optimize fluid status.1 Supplemental oxygen may be administered to maintain saturations above 90%.1 Nonsteroidal anti-inflammatory drugs are helpful for analgesia and anti-inflammatory effect, and opioids can be used for breakthrough pain.1 It is recommended that blisters be drained in a sterile fashion and that all affected tissue be covered with topical aloe vera and a loose dressing.1,2,4
Treatment of severe frostbite. Angiography should be performed on all patients with third- or fourth-degree frostbite.3 If imaging shows evidence of vascular occlusion, tissue plasminogen activator (tPA) and heparin can be initiated within 24 hours to reduce the risk for amputation.8-10
Continue to: Iloprost is another...
Iloprost is another proposed treatment for severe frostbite. It is a prostacyclin analog that may lower the amputation rate in patients with at least third-degree frostbite.11 Unlike tPA, iloprost may be given to trauma patients, and it can be used more than 24 hours after injury.2
In cases of fourth-degree frostbite that is not successfully reperfused, amputation is delayed until dry gangrene develops. This often takes weeks to months.12
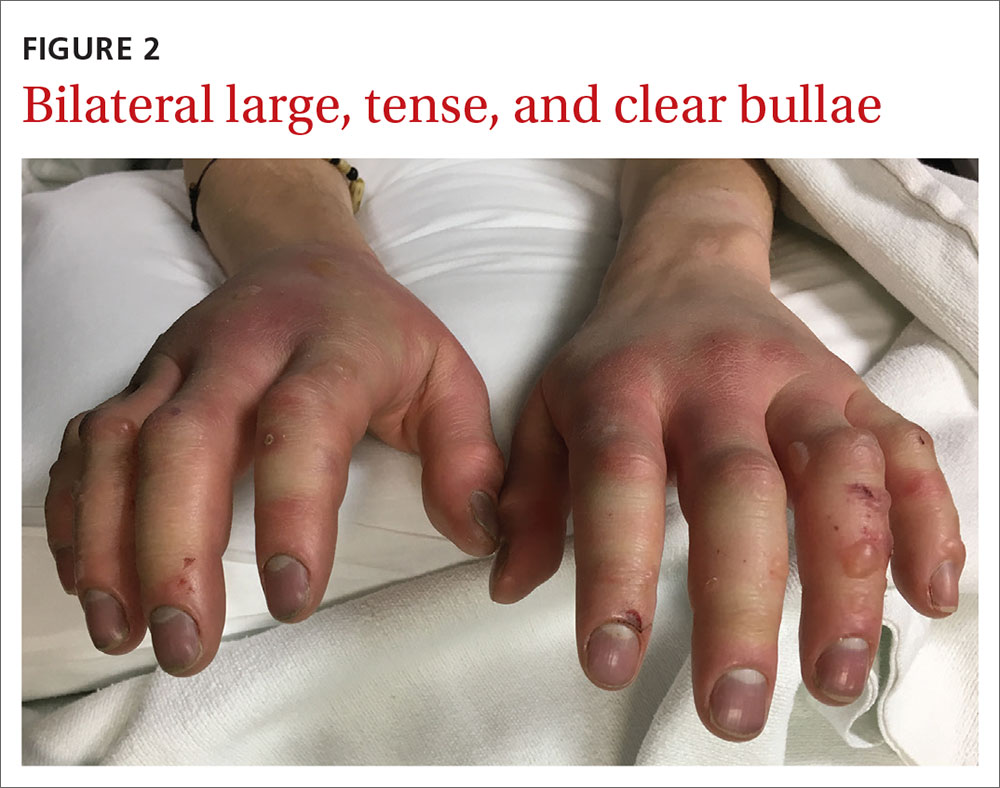
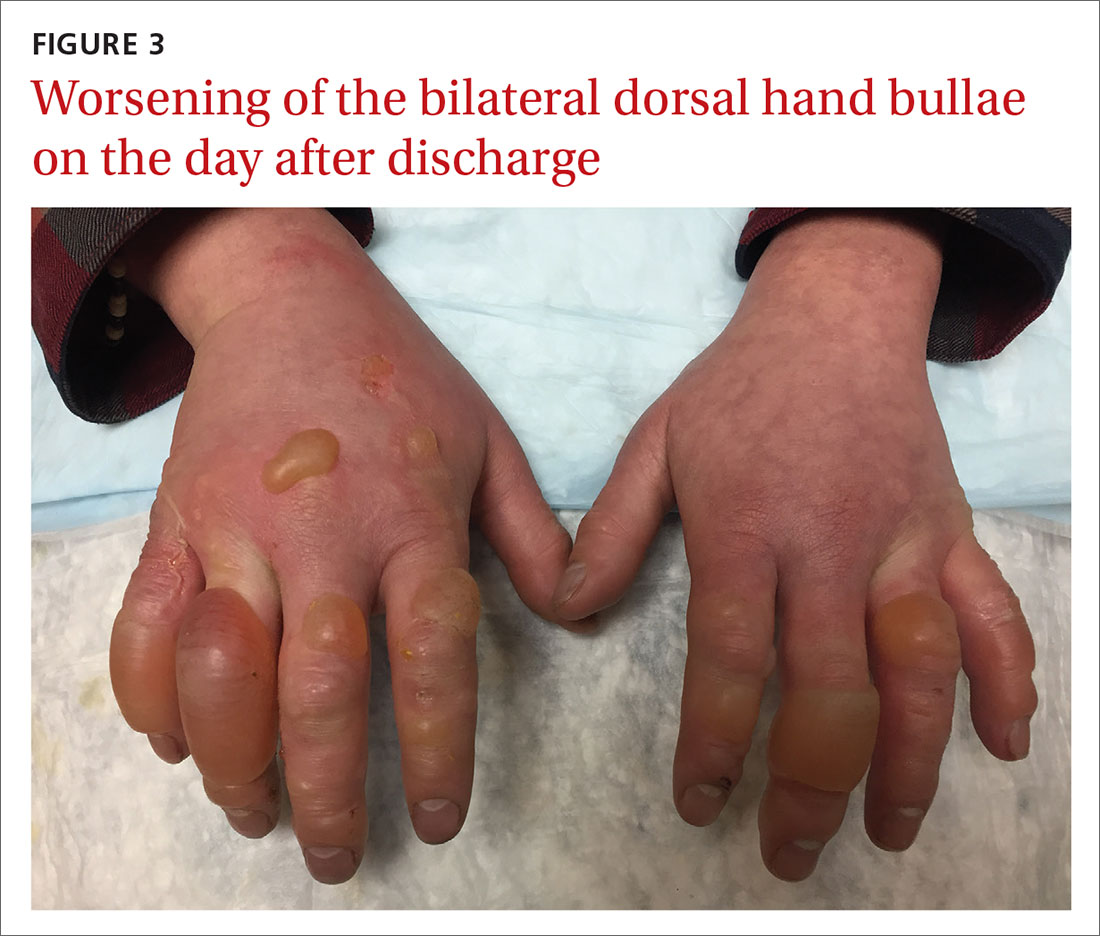
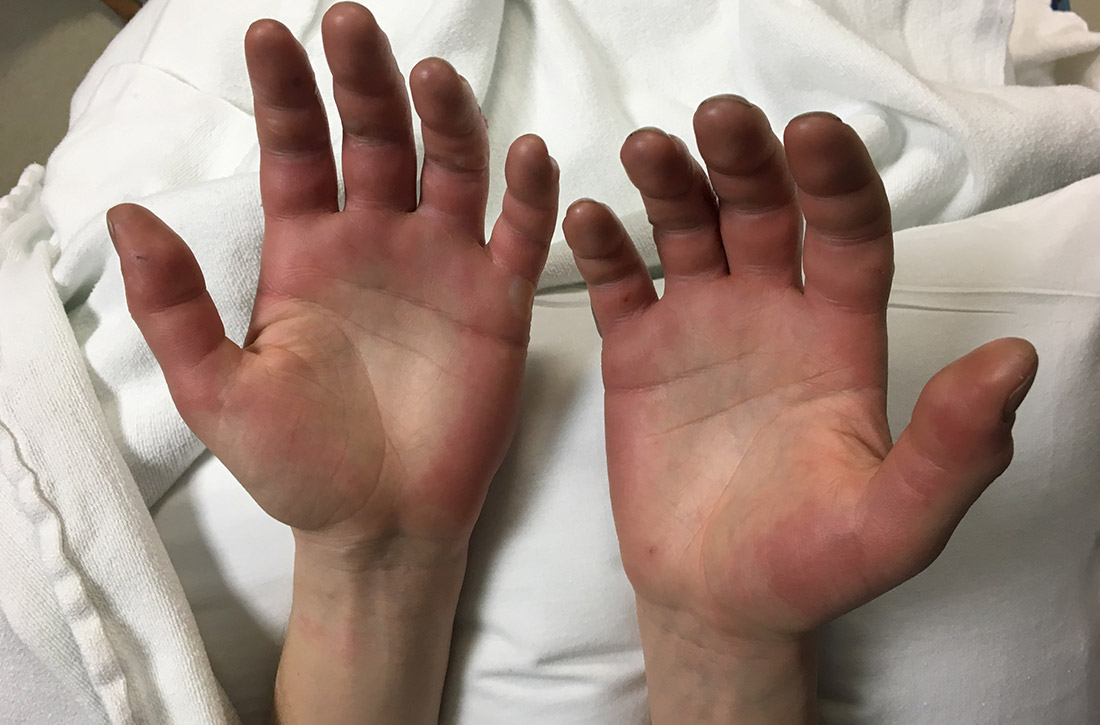
Our patient underwent rewarming and was orally rehydrated. He was discharged home with ibuprofen, oxycodone-acetaminophen, topical aloe vera, and loose dressings. His bullae enlarged the next day (FIGURE 3). One week later, his blisters were debrided and dressed with silver sulfadiazine at his plastic surgery follow-up. He experienced sensory deficits for a few months, but eventually made a full recovery after 6 months with no remaining sequelae.
ACKNOWLEDGEMENT
The authors thank Lisa Kim, MD, for her clinical care of this patient.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ cuanschutz.edu
1. Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
2. Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016;117:79-93.
3. Millet JD, Brown RK, Levi B, et al. Frostbite: spectrum of imaging findings and guidelines for management. Radiographics. 2016;36:2154-2169.
4. Long WB 3rd, Edlich RF, Winters KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67-78.
5. Baker JS, Miranpuri S. Perniosis: a case report with literature review. J Am Podiatr Med Assoc. 2016;106:138-140.
6. Bush JS, Watson S. Trench foot. Updated February 3, 2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK482364/. Accessed April 22, 2020.
7. Musa R, Qurie A. Raynaud disease (Raynaud phenomenon, Raynaud syndrome). Updated February 14, 2019. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK499833/. Accessed April 22, 2020.
8. Bruen KJ, Ballard JR, Morris SE, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546-551; discussion 551-553.
9. Gonzaga T, Jenabzadeh K, Anderson CP, et al. Use of intra-arterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care Res. 2016;37:e323-e334.
10. Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350-1354; discussion 1354-1355.
11. Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189-190.
12. McIntosh SE, Opacic M, Freer L, et al; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environ Med. 2014;25(4 suppl):S43-S54.
A 27-year-old man presented to the urgent care clinic with acute bilateral hand swelling, blisters, numbness, and pain. History taking revealed that these symptoms developed after he was locked outside of his apartment for 45 minutes in –22°C (–8°F) weather following a night of heavy drinking.
On physical examination, the patient had a temperature of 36.2°C (97.2°F) and a heart rate of 116 beats/min. He had
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Second-degree frostbite
Frostbite is the result of tissue freezing, which generally occurs after prolonged exposure to freezing temperatures (typically –4°C or below).1,2 The majority (~90%) of frostbite injuries occur in the hands and feet; however, frostbite has also been observed in the face, perineum, buttocks, and male genitalia.3
Frostbite is a clinical diagnosis based on a history of sustained exposure to freezing temperatures, paresthesia of affected areas, and typical skin changes. Evidence is lacking regarding the epidemiology of frostbite within the general population.2
Pathophysiology. Intra- and extracellular ice crystal formation causes fluid and electrolyte disturbances, cell dehydration, lipid denaturation, and subsequent cell death.1 After thawing, progressive tissue ischemia can occur as a result of endothelial damage and dysfunction, intravascular sludging, increased inflammatory markers, an influx of free radicals, and microvascular thrombosis.1
Classification. Traditionally, frostbite has been classified according to a 4-tiered system based on tissue appearance after rewarming.2 First-degree frostbite is characterized by white plaques with surrounding erythema; second degree by edema and clear or cloudy vesicles; third degree by hemorrhagic bullae; and fourth degree by cold and hard tissue that eventually progresses to gangrene.2
A simpler scheme designates frostbite as either superficial (corresponding to first- or second-degree frostbite) or deep (corresponding to third- or fourth-degree frostbite) with presumed muscle and bone involvement.2
Continue to: Risk factors
Risk factors. Frostbite is often associated with risk factors such as alcohol or drug intoxication, vehicular failure or trauma, immobilizing trauma, psychiatric illness, homelessness, Raynaud phenomenon, peripheral vascular disease, diabetes, inadequate clothing, previous cold-weather injury, outdoor winter recreation, and the use of certain medications (eg, beta-blockers).1-3 Apart from environmental exposure, frostbite can also occur by direct contact with freezing materials, such as ice packs or industrial refrigerants.3
Differential includes nonfreezing injuries
Frostnip, pernio, and trench foot are other cold-weather injuries distinguished by the absence of tissue freezing.4 Raynaud phenomenon is a condition that is triggered by either cold temperatures or emotional stress.5
Frostnip is characterized by pallor and paresthesia of exposed areas. It may precede frostbite, but it quickly resolves after rewarming.2
Pernio occurs when skin is exposed to damp, cold, nonfreezing environments.6 It results in edematous and inflammatory skin lesions that may be painful, pruritic, violaceous, or erythematous.6 These lesions are typically found over the fingers, toes, nose, ears, buttocks, or thighs.4,6 Pernio may be classified as either primary or secondary disease.5 Primary pernio is considered idiopathic.6 Secondary pernio is thought to be either drug induced or due to underlying autoimmune diseases, such as hepatitis or cryopathy.6
Trench foot develops under similar conditions to pernio but requires exposure to a wet environment for at least 10 to 14 hours.7 It is characterized by foot pain, paresthesia, pruritus, edema, erythema, cyanosis, blisters, and even gangrene if left untreated.7
Continue to: Raynaud phenomenon
Raynaud phenomenon results from transient, acral vasocontraction and manifests as well-demarcated pallor, cyanosis, and then erythema as the affected body part reperfuses.5 Similar to pernio, it can be categorized as either primary or secondary.5 Primary phenomenon is idiopathic. Secondary phenomenon is thought to be a result of autoimmune disease, use of certain medications, occupational vibratory exposure, obstructive vascular disease, or infection
In the absence of a history of exposure to subfreezing temperatures, frostbite can be excluded from the differential diagnosis.
Treatment entails rewarming
The aim of frostbite treatment is to save injured cells and minimize tissue loss.1 This is accomplished through rapid rewarming and—in severe cases—reperfusion techniques.
Tissue should be rewarmed in a 37°C to 39°C water bath with povidone iodine or chlorhexidine added for antiseptic effect.1 All efforts should be made to avoid refreezing or trauma, as this could worsen the initial injury.2 Oral or intravenous hydration may be offered to optimize fluid status.1 Supplemental oxygen may be administered to maintain saturations above 90%.1 Nonsteroidal anti-inflammatory drugs are helpful for analgesia and anti-inflammatory effect, and opioids can be used for breakthrough pain.1 It is recommended that blisters be drained in a sterile fashion and that all affected tissue be covered with topical aloe vera and a loose dressing.1,2,4
Treatment of severe frostbite. Angiography should be performed on all patients with third- or fourth-degree frostbite.3 If imaging shows evidence of vascular occlusion, tissue plasminogen activator (tPA) and heparin can be initiated within 24 hours to reduce the risk for amputation.8-10
Continue to: Iloprost is another...
Iloprost is another proposed treatment for severe frostbite. It is a prostacyclin analog that may lower the amputation rate in patients with at least third-degree frostbite.11 Unlike tPA, iloprost may be given to trauma patients, and it can be used more than 24 hours after injury.2
In cases of fourth-degree frostbite that is not successfully reperfused, amputation is delayed until dry gangrene develops. This often takes weeks to months.12
Our patient underwent rewarming and was orally rehydrated. He was discharged home with ibuprofen, oxycodone-acetaminophen, topical aloe vera, and loose dressings. His bullae enlarged the next day (FIGURE 3). One week later, his blisters were debrided and dressed with silver sulfadiazine at his plastic surgery follow-up. He experienced sensory deficits for a few months, but eventually made a full recovery after 6 months with no remaining sequelae.
ACKNOWLEDGEMENT
The authors thank Lisa Kim, MD, for her clinical care of this patient.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ cuanschutz.edu
A 27-year-old man presented to the urgent care clinic with acute bilateral hand swelling, blisters, numbness, and pain. History taking revealed that these symptoms developed after he was locked outside of his apartment for 45 minutes in –22°C (–8°F) weather following a night of heavy drinking.
On physical examination, the patient had a temperature of 36.2°C (97.2°F) and a heart rate of 116 beats/min. He had
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Second-degree frostbite
Frostbite is the result of tissue freezing, which generally occurs after prolonged exposure to freezing temperatures (typically –4°C or below).1,2 The majority (~90%) of frostbite injuries occur in the hands and feet; however, frostbite has also been observed in the face, perineum, buttocks, and male genitalia.3
Frostbite is a clinical diagnosis based on a history of sustained exposure to freezing temperatures, paresthesia of affected areas, and typical skin changes. Evidence is lacking regarding the epidemiology of frostbite within the general population.2
Pathophysiology. Intra- and extracellular ice crystal formation causes fluid and electrolyte disturbances, cell dehydration, lipid denaturation, and subsequent cell death.1 After thawing, progressive tissue ischemia can occur as a result of endothelial damage and dysfunction, intravascular sludging, increased inflammatory markers, an influx of free radicals, and microvascular thrombosis.1
Classification. Traditionally, frostbite has been classified according to a 4-tiered system based on tissue appearance after rewarming.2 First-degree frostbite is characterized by white plaques with surrounding erythema; second degree by edema and clear or cloudy vesicles; third degree by hemorrhagic bullae; and fourth degree by cold and hard tissue that eventually progresses to gangrene.2
A simpler scheme designates frostbite as either superficial (corresponding to first- or second-degree frostbite) or deep (corresponding to third- or fourth-degree frostbite) with presumed muscle and bone involvement.2
Continue to: Risk factors
Risk factors. Frostbite is often associated with risk factors such as alcohol or drug intoxication, vehicular failure or trauma, immobilizing trauma, psychiatric illness, homelessness, Raynaud phenomenon, peripheral vascular disease, diabetes, inadequate clothing, previous cold-weather injury, outdoor winter recreation, and the use of certain medications (eg, beta-blockers).1-3 Apart from environmental exposure, frostbite can also occur by direct contact with freezing materials, such as ice packs or industrial refrigerants.3
Differential includes nonfreezing injuries
Frostnip, pernio, and trench foot are other cold-weather injuries distinguished by the absence of tissue freezing.4 Raynaud phenomenon is a condition that is triggered by either cold temperatures or emotional stress.5
Frostnip is characterized by pallor and paresthesia of exposed areas. It may precede frostbite, but it quickly resolves after rewarming.2
Pernio occurs when skin is exposed to damp, cold, nonfreezing environments.6 It results in edematous and inflammatory skin lesions that may be painful, pruritic, violaceous, or erythematous.6 These lesions are typically found over the fingers, toes, nose, ears, buttocks, or thighs.4,6 Pernio may be classified as either primary or secondary disease.5 Primary pernio is considered idiopathic.6 Secondary pernio is thought to be either drug induced or due to underlying autoimmune diseases, such as hepatitis or cryopathy.6
Trench foot develops under similar conditions to pernio but requires exposure to a wet environment for at least 10 to 14 hours.7 It is characterized by foot pain, paresthesia, pruritus, edema, erythema, cyanosis, blisters, and even gangrene if left untreated.7
Continue to: Raynaud phenomenon
Raynaud phenomenon results from transient, acral vasocontraction and manifests as well-demarcated pallor, cyanosis, and then erythema as the affected body part reperfuses.5 Similar to pernio, it can be categorized as either primary or secondary.5 Primary phenomenon is idiopathic. Secondary phenomenon is thought to be a result of autoimmune disease, use of certain medications, occupational vibratory exposure, obstructive vascular disease, or infection
In the absence of a history of exposure to subfreezing temperatures, frostbite can be excluded from the differential diagnosis.
Treatment entails rewarming
The aim of frostbite treatment is to save injured cells and minimize tissue loss.1 This is accomplished through rapid rewarming and—in severe cases—reperfusion techniques.
Tissue should be rewarmed in a 37°C to 39°C water bath with povidone iodine or chlorhexidine added for antiseptic effect.1 All efforts should be made to avoid refreezing or trauma, as this could worsen the initial injury.2 Oral or intravenous hydration may be offered to optimize fluid status.1 Supplemental oxygen may be administered to maintain saturations above 90%.1 Nonsteroidal anti-inflammatory drugs are helpful for analgesia and anti-inflammatory effect, and opioids can be used for breakthrough pain.1 It is recommended that blisters be drained in a sterile fashion and that all affected tissue be covered with topical aloe vera and a loose dressing.1,2,4
Treatment of severe frostbite. Angiography should be performed on all patients with third- or fourth-degree frostbite.3 If imaging shows evidence of vascular occlusion, tissue plasminogen activator (tPA) and heparin can be initiated within 24 hours to reduce the risk for amputation.8-10
Continue to: Iloprost is another...
Iloprost is another proposed treatment for severe frostbite. It is a prostacyclin analog that may lower the amputation rate in patients with at least third-degree frostbite.11 Unlike tPA, iloprost may be given to trauma patients, and it can be used more than 24 hours after injury.2
In cases of fourth-degree frostbite that is not successfully reperfused, amputation is delayed until dry gangrene develops. This often takes weeks to months.12
Our patient underwent rewarming and was orally rehydrated. He was discharged home with ibuprofen, oxycodone-acetaminophen, topical aloe vera, and loose dressings. His bullae enlarged the next day (FIGURE 3). One week later, his blisters were debrided and dressed with silver sulfadiazine at his plastic surgery follow-up. He experienced sensory deficits for a few months, but eventually made a full recovery after 6 months with no remaining sequelae.
ACKNOWLEDGEMENT
The authors thank Lisa Kim, MD, for her clinical care of this patient.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ cuanschutz.edu
1. Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
2. Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016;117:79-93.
3. Millet JD, Brown RK, Levi B, et al. Frostbite: spectrum of imaging findings and guidelines for management. Radiographics. 2016;36:2154-2169.
4. Long WB 3rd, Edlich RF, Winters KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67-78.
5. Baker JS, Miranpuri S. Perniosis: a case report with literature review. J Am Podiatr Med Assoc. 2016;106:138-140.
6. Bush JS, Watson S. Trench foot. Updated February 3, 2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK482364/. Accessed April 22, 2020.
7. Musa R, Qurie A. Raynaud disease (Raynaud phenomenon, Raynaud syndrome). Updated February 14, 2019. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK499833/. Accessed April 22, 2020.
8. Bruen KJ, Ballard JR, Morris SE, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546-551; discussion 551-553.
9. Gonzaga T, Jenabzadeh K, Anderson CP, et al. Use of intra-arterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care Res. 2016;37:e323-e334.
10. Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350-1354; discussion 1354-1355.
11. Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189-190.
12. McIntosh SE, Opacic M, Freer L, et al; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environ Med. 2014;25(4 suppl):S43-S54.
1. Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
2. Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016;117:79-93.
3. Millet JD, Brown RK, Levi B, et al. Frostbite: spectrum of imaging findings and guidelines for management. Radiographics. 2016;36:2154-2169.
4. Long WB 3rd, Edlich RF, Winters KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67-78.
5. Baker JS, Miranpuri S. Perniosis: a case report with literature review. J Am Podiatr Med Assoc. 2016;106:138-140.
6. Bush JS, Watson S. Trench foot. Updated February 3, 2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK482364/. Accessed April 22, 2020.
7. Musa R, Qurie A. Raynaud disease (Raynaud phenomenon, Raynaud syndrome). Updated February 14, 2019. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK499833/. Accessed April 22, 2020.
8. Bruen KJ, Ballard JR, Morris SE, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546-551; discussion 551-553.
9. Gonzaga T, Jenabzadeh K, Anderson CP, et al. Use of intra-arterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care Res. 2016;37:e323-e334.
10. Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350-1354; discussion 1354-1355.
11. Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189-190.
12. McIntosh SE, Opacic M, Freer L, et al; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environ Med. 2014;25(4 suppl):S43-S54.
Sun-damage selfies give kids motivation to protect skin
Photo-manipulated selfies can provide adolescents an influential window into the wrinkled, sun-damaged future that may be theirs if they’re not careful, a new study suggests.
In the study, researchers found that Brazilian teenagers, especially girls, were more likely to protect themselves from the sun if they got glimpses of how sun exposure could damage their faces. “The intervention used in this study was effective in convincing a substantial part of the students to take up regular sunscreen use and to examine their own skin regularly,” they wrote. “Moreover, these effects were maintained for at least half a year.”
The study, led by Titus J. Brinker, MD, of the department of dermatology, in the National Center for Tumor Diseases, German Cancer Research Center in Heidelberg, Germany, appeared online on May 6 in JAMA Dermatology (2020 May 6. doi: 10.1001/jamadermatol.2020.0511.
Dr. Brinker and colleagues launched the study in 2018 at eight public schools that serve grades 9-12 in Itaúna, a city in southeast Brazil, randomly assigning 1,573 students (52% girls, 48% boys; mean age, 16 years) to the intervention or control group.
Those in the intervention group attended seminars in which medical students showed them selfies of their classmates altered with a mobile phone app called Sunface, developed by Dr. Brinker.
The app, which takes the skin types of the subjects into account, was described by the Vice news site as “terrifying” in a 2018 article. It “could very well scare you into using sunscreen and wearing hats,” the author of that article wrote.
The app appeared to do just that – but not universally, according to the new study.
At 6 months, there was no change in sun protection habits in the control group. But among those remaining in the intervention group, the use of daily sunscreen significantly increased from 15% (110 of 734 students) during the 30 days prior to the survey, to 23% (139 of 607 students) at the 6-month follow-up (P less than .001), as did the percentage of those who performed at least one skin self-examination within the 6 months (25% to 49%; P less than .001). The students were slightly less likely to use tanning beds within the previous month (19% to 15%; P = .04); the researchers speculate that it’s easier to gain a new healthy habit than get rid of an old unhealthy one.
Girls were much more likely to change their habits than boys. The number needed to treat to reach the primary endpoint, daily sunscreen use, was 8 for girls and 31 for boys.
The researchers noted that the dropout rate was higher in the intervention group (17%) vs. the control group (6%). “The intervention may have led to strong adverse reactions in some students, leading to the observed higher dropout rate in the intervention group,” they wrote. Changes to the way the app is used could improve the dropout rate, but potentially hurt the intervention’s impact, they added.
In an accompanying editorial in JAMA Dermatology, two health intervention researchers wrote that “this work represents a needed shift toward scalable interventions that bring messaging to target populations using their preferred technology” (2020 May 6. doi: 10.1001/jamadermatol.2020.0510).
Referring to the finding that sunscreen use did not change much among the boys in the study, the authors, Sherry L. Pagoto, PhD, of the Institute for Collaborations on Health, Interventions, and Policy at the University of Connecticut, Storrs, and Alan C. Geller, MPH, RN, of the Harvard TH Chan School of Public Health, Boston, also noted that “teen boys have been largely resistant to traditional and nontraditional forms of sun safety education.”
“Teasing out sex differences is important,” they added, “because sun protection interventions woven into existing programs at pools, beaches, and sporting events might be more appealing and enduring for boys, particularly if the technology they regularly use is leveraged.”
Dr. Brinker disclosed receiving an award from La Fondation la Roche-Posay, which also provided support for the study which partially funded the study, for his research on the Sunface app. The University of Itaúna provided other study funding. Several other study authors had various disclosures. Dr. Pagoto disclosed consulting work and personal fees from Johnson & Johnson, unrelated to the topic of the commentary; Dr. Geller had no disclosures.
SOURCES: Brinker TJ et al. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0511; Pagoto SL and Geller AC. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0510.
Photo-manipulated selfies can provide adolescents an influential window into the wrinkled, sun-damaged future that may be theirs if they’re not careful, a new study suggests.
In the study, researchers found that Brazilian teenagers, especially girls, were more likely to protect themselves from the sun if they got glimpses of how sun exposure could damage their faces. “The intervention used in this study was effective in convincing a substantial part of the students to take up regular sunscreen use and to examine their own skin regularly,” they wrote. “Moreover, these effects were maintained for at least half a year.”
The study, led by Titus J. Brinker, MD, of the department of dermatology, in the National Center for Tumor Diseases, German Cancer Research Center in Heidelberg, Germany, appeared online on May 6 in JAMA Dermatology (2020 May 6. doi: 10.1001/jamadermatol.2020.0511.
Dr. Brinker and colleagues launched the study in 2018 at eight public schools that serve grades 9-12 in Itaúna, a city in southeast Brazil, randomly assigning 1,573 students (52% girls, 48% boys; mean age, 16 years) to the intervention or control group.
Those in the intervention group attended seminars in which medical students showed them selfies of their classmates altered with a mobile phone app called Sunface, developed by Dr. Brinker.
The app, which takes the skin types of the subjects into account, was described by the Vice news site as “terrifying” in a 2018 article. It “could very well scare you into using sunscreen and wearing hats,” the author of that article wrote.
The app appeared to do just that – but not universally, according to the new study.
At 6 months, there was no change in sun protection habits in the control group. But among those remaining in the intervention group, the use of daily sunscreen significantly increased from 15% (110 of 734 students) during the 30 days prior to the survey, to 23% (139 of 607 students) at the 6-month follow-up (P less than .001), as did the percentage of those who performed at least one skin self-examination within the 6 months (25% to 49%; P less than .001). The students were slightly less likely to use tanning beds within the previous month (19% to 15%; P = .04); the researchers speculate that it’s easier to gain a new healthy habit than get rid of an old unhealthy one.
Girls were much more likely to change their habits than boys. The number needed to treat to reach the primary endpoint, daily sunscreen use, was 8 for girls and 31 for boys.
The researchers noted that the dropout rate was higher in the intervention group (17%) vs. the control group (6%). “The intervention may have led to strong adverse reactions in some students, leading to the observed higher dropout rate in the intervention group,” they wrote. Changes to the way the app is used could improve the dropout rate, but potentially hurt the intervention’s impact, they added.
In an accompanying editorial in JAMA Dermatology, two health intervention researchers wrote that “this work represents a needed shift toward scalable interventions that bring messaging to target populations using their preferred technology” (2020 May 6. doi: 10.1001/jamadermatol.2020.0510).
Referring to the finding that sunscreen use did not change much among the boys in the study, the authors, Sherry L. Pagoto, PhD, of the Institute for Collaborations on Health, Interventions, and Policy at the University of Connecticut, Storrs, and Alan C. Geller, MPH, RN, of the Harvard TH Chan School of Public Health, Boston, also noted that “teen boys have been largely resistant to traditional and nontraditional forms of sun safety education.”
“Teasing out sex differences is important,” they added, “because sun protection interventions woven into existing programs at pools, beaches, and sporting events might be more appealing and enduring for boys, particularly if the technology they regularly use is leveraged.”
Dr. Brinker disclosed receiving an award from La Fondation la Roche-Posay, which also provided support for the study which partially funded the study, for his research on the Sunface app. The University of Itaúna provided other study funding. Several other study authors had various disclosures. Dr. Pagoto disclosed consulting work and personal fees from Johnson & Johnson, unrelated to the topic of the commentary; Dr. Geller had no disclosures.
SOURCES: Brinker TJ et al. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0511; Pagoto SL and Geller AC. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0510.
Photo-manipulated selfies can provide adolescents an influential window into the wrinkled, sun-damaged future that may be theirs if they’re not careful, a new study suggests.
In the study, researchers found that Brazilian teenagers, especially girls, were more likely to protect themselves from the sun if they got glimpses of how sun exposure could damage their faces. “The intervention used in this study was effective in convincing a substantial part of the students to take up regular sunscreen use and to examine their own skin regularly,” they wrote. “Moreover, these effects were maintained for at least half a year.”
The study, led by Titus J. Brinker, MD, of the department of dermatology, in the National Center for Tumor Diseases, German Cancer Research Center in Heidelberg, Germany, appeared online on May 6 in JAMA Dermatology (2020 May 6. doi: 10.1001/jamadermatol.2020.0511.
Dr. Brinker and colleagues launched the study in 2018 at eight public schools that serve grades 9-12 in Itaúna, a city in southeast Brazil, randomly assigning 1,573 students (52% girls, 48% boys; mean age, 16 years) to the intervention or control group.
Those in the intervention group attended seminars in which medical students showed them selfies of their classmates altered with a mobile phone app called Sunface, developed by Dr. Brinker.
The app, which takes the skin types of the subjects into account, was described by the Vice news site as “terrifying” in a 2018 article. It “could very well scare you into using sunscreen and wearing hats,” the author of that article wrote.
The app appeared to do just that – but not universally, according to the new study.
At 6 months, there was no change in sun protection habits in the control group. But among those remaining in the intervention group, the use of daily sunscreen significantly increased from 15% (110 of 734 students) during the 30 days prior to the survey, to 23% (139 of 607 students) at the 6-month follow-up (P less than .001), as did the percentage of those who performed at least one skin self-examination within the 6 months (25% to 49%; P less than .001). The students were slightly less likely to use tanning beds within the previous month (19% to 15%; P = .04); the researchers speculate that it’s easier to gain a new healthy habit than get rid of an old unhealthy one.
Girls were much more likely to change their habits than boys. The number needed to treat to reach the primary endpoint, daily sunscreen use, was 8 for girls and 31 for boys.
The researchers noted that the dropout rate was higher in the intervention group (17%) vs. the control group (6%). “The intervention may have led to strong adverse reactions in some students, leading to the observed higher dropout rate in the intervention group,” they wrote. Changes to the way the app is used could improve the dropout rate, but potentially hurt the intervention’s impact, they added.
In an accompanying editorial in JAMA Dermatology, two health intervention researchers wrote that “this work represents a needed shift toward scalable interventions that bring messaging to target populations using their preferred technology” (2020 May 6. doi: 10.1001/jamadermatol.2020.0510).
Referring to the finding that sunscreen use did not change much among the boys in the study, the authors, Sherry L. Pagoto, PhD, of the Institute for Collaborations on Health, Interventions, and Policy at the University of Connecticut, Storrs, and Alan C. Geller, MPH, RN, of the Harvard TH Chan School of Public Health, Boston, also noted that “teen boys have been largely resistant to traditional and nontraditional forms of sun safety education.”
“Teasing out sex differences is important,” they added, “because sun protection interventions woven into existing programs at pools, beaches, and sporting events might be more appealing and enduring for boys, particularly if the technology they regularly use is leveraged.”
Dr. Brinker disclosed receiving an award from La Fondation la Roche-Posay, which also provided support for the study which partially funded the study, for his research on the Sunface app. The University of Itaúna provided other study funding. Several other study authors had various disclosures. Dr. Pagoto disclosed consulting work and personal fees from Johnson & Johnson, unrelated to the topic of the commentary; Dr. Geller had no disclosures.
SOURCES: Brinker TJ et al. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0511; Pagoto SL and Geller AC. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0510.
FROM JAMA DERMATOLOGY
Recent-onset bloody nodule
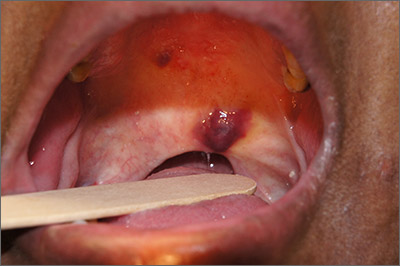
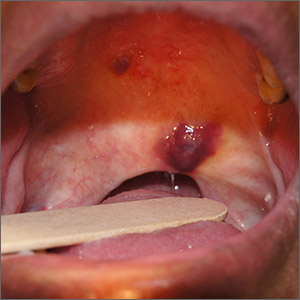
A 45-year-old man presented to the Dermatology Clinic with a 4-month history of a bump on his left upper back. The lesion was tender and had been draining clear fluid and intermittent blood; he denied any preceding trauma. He had been seen both by his primary care physician and by a physician at an urgent care clinic, where he was told to use an antibiotic ointment and benzoyl peroxide daily on the area and advised to seek a dermatology consult should it not resolve. He did not see any improvement from these measures.
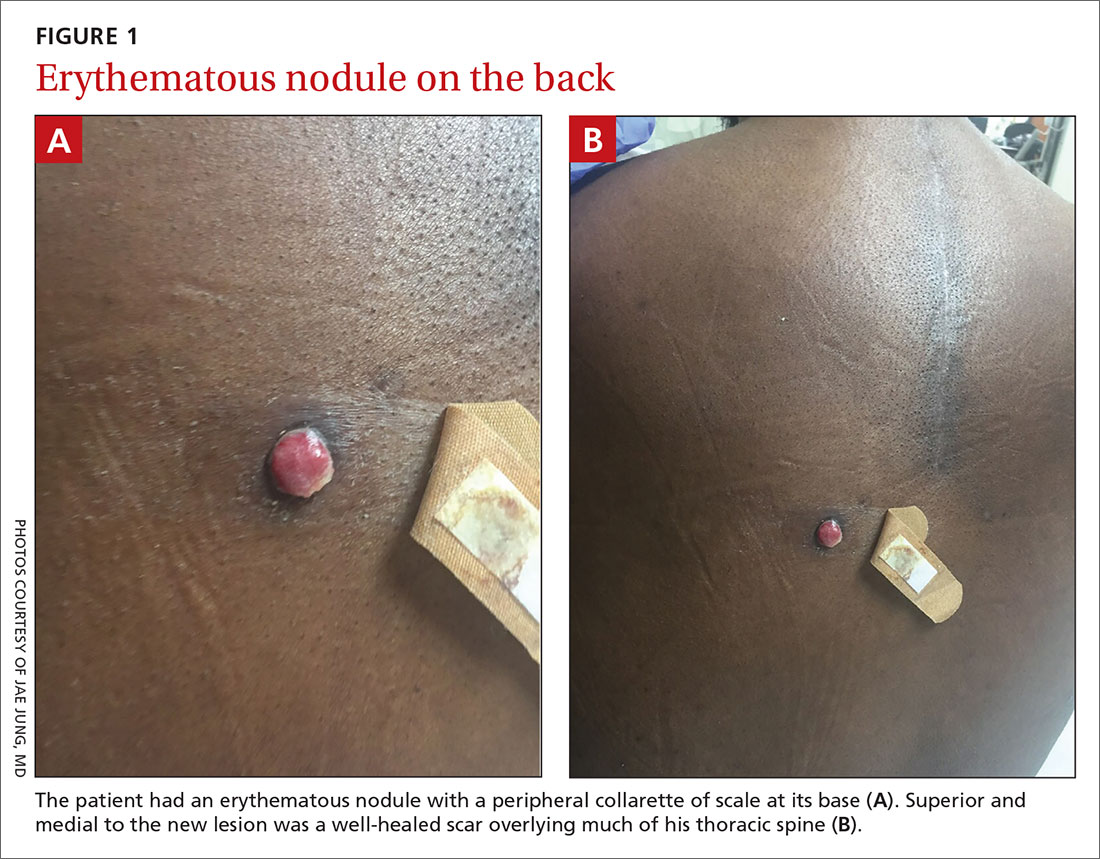
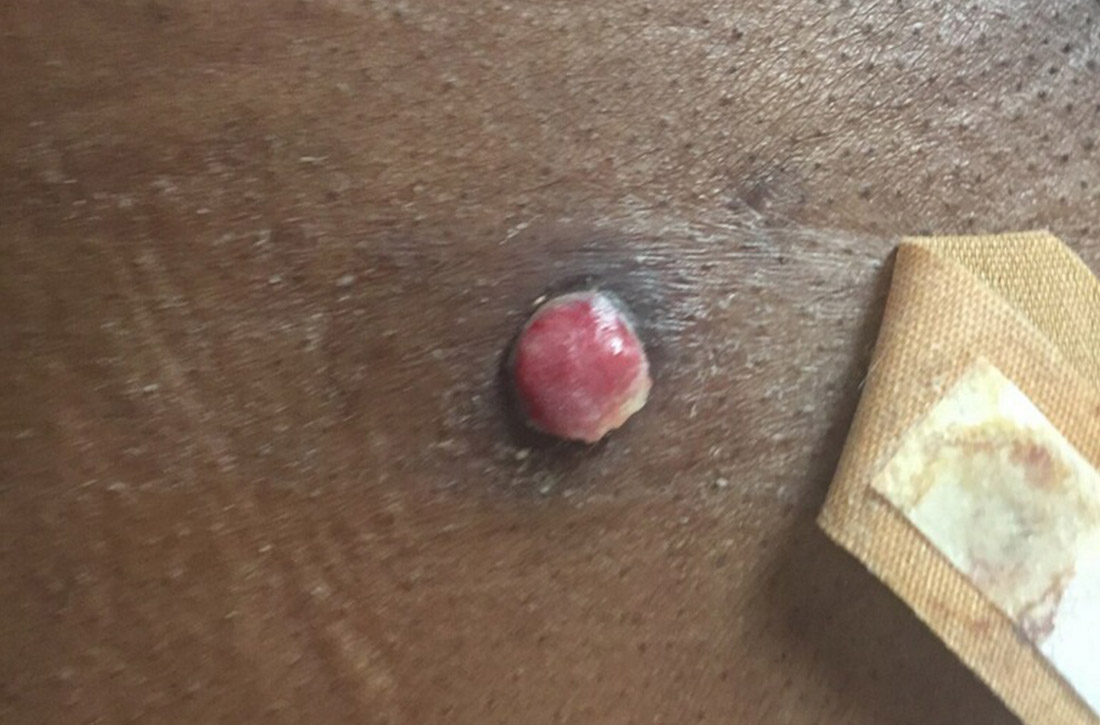
Physical exam revealed a 0.8-cm erythematous nodule with a peripheral collarette of scale at its base. The bandage used to cover the nodule was stained with hemorrhagic crust (FIGURE 1A). Superior and medial to the new lesion was a well-healed scar overlying much of the patient’s thoracic spine (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic renal cell carcinoma
The nodule initially appeared to be a benign pyogenic granuloma. In fact, a biopsy of the nodule showed a profile similar to that of a pyogenic granuloma and it exhibited granulation tissue. However, further questioning revealed that the patient had a history of metastatic clear cell renal carcinoma. (The scar was from a prior unrelated orthopedic surgery.) Immunohistochemical stains showed positive staining in the cells of interest for PAX8 and CK8, 2 markers for renal cell lineage.
Cutaneous metastasis to the skin is a rare event, representing roughly 2% of all skin tumors.1 Anatomically, lesions tend to appear on the head and neck in men and anterior chest and abdomen in women.2 Eruptions on the back, as seen in our patient, are relatively rare. The primary source of the metastasis also is gender dependent. Melanoma is the most common source overall; but in women, breast cancer represents the large majority of cutaneous metastases3 while in men lung, large intestine, and oral cavity tumors are the most common origin.3 Renal metastases are the fourth most common cause in men.3
The clinical morphology of cutaneous metastases is protean; the most common manifestations are nodules, papules, plaques, tumors, and ulcers.2 Rare manifestations include alopecia plaques, erysipelas, herpes zoster–like eruptions,4 and pyogenic granuloma–like manifestations, as in our case. Pyogenic granuloma–like manifestations have been described in renal cell carcinoma, breast carcinoma, acute myelogenous leukemia,5 and hepatocellular carcinoma.6
Differential includes an array of erythematous nodules
The differential diagnosis of a lesion with the appearance of a pyogenic granuloma is variable.
Pyogenic granulomas tend to arise over a short period of time. They are more common in children and pregnant women. Pyogenic granulomas can manifest anywhere but often are reported on the digits and extremities. Clinical history is important to ensure no history of internal malignancy.
Continue to: Bartonella henselae
Bartonella henselae, known as “cat scratch disease,” also can present as a friable, erythematous nodule reminiscent of a pyogenic granuloma. Patients with bartonella henselae usually are immunocompromised and/or have had close contact with a cat.7
Kaposi sarcoma is a vascular tumor that may manifest as erythematous papules or nodules. Erythematous or violaceous patches or plaques may be present before a nodule arises. Kaposi sarcoma may manifest on the legs of elderly patients or anywhere on immunocompromised patients. Immunohistochemical stains for human herpesvirus-8 can clinch the diagnosis.8
Amelanotic melanoma may be impossible to discern clinically from a pyogenic granuloma. It appears as erythematous, violaceous, or flesh-colored nodules. Histologic evaluation is paramount in the diagnosis.9
Clinical suspicion should prompt a biopsy
The diagnosis of metastatic renal cell carcinoma is made on clinical suspicion and skin biopsy. Dermoscopy is an important tool in the evaluation of primary cutaneous tumors. Due to the rarity of cutaneous metastases, studies on dermoscopic findings in cutaneous metastases are limited to case series. One series showed a vascular dermoscopy pattern in 15 of 17 cases (88%).10
In light of this nonspecific pattern, it’s wise to consider biopsy of a pyogenic granuloma–like lesion or one with a vascular pattern on dermoscopy in any patient with a history of malignancy. Any lesion suspected of being a pyogenic granuloma that does not respond to conservative measures also would warrant a biopsy. Definitive diagnosis is made based upon histologic evaluation.
Continue to: Surgery is the cornerstone of treatment
Surgery is the cornerstone of treatment
Upon diagnosis, immediate referral for further local and systemic control is recommended. Treatment may consist of any combination of surgery, chemotherapy, immunotherapy, or radiation.11
In this case, our patient was referred to Oncology for further treatment. Unfortunately, cutaneous metastases portend a very poor prognosis, with approximate survival times of 7.5 months.12
CORRESPONDENCE
M. Tye Haeberle, MD, 3810 Springhurst Boulevard, Ste 200, Louisville, KY 40241; tye.haeberle@gmail.com
1. Nashan D, Meiss F, Braun-Flaco M, et al. Cutaneous metastases from internal malignancies. Dermatol Ther. 2010;23:567-580.
2. Alcaraz IM, Cerroni LM, Rütten AM, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
3. Lookingbill D, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
4. Hussein MR. Skin metastases: a pathologist’s perspective. J Cutan Pathol. 2010;37:E1-E20.
5. Hager C, Cohen P. Cutaneous lesions of metastatic visceral malignancy mimicking pyogenic granuloma. Cancer Invest. 1999;17:385-390.
6. Kubota Y, Koga T, Nakayama J. Cutaneous metastasis from hepatocellular carcinoma resembling pyogenic granuloma. Clin Exp Dermatol. 1999;24:78-80.
7. Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203-219.
8. Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460.
9. Wee E, Wolfe R, Mclean C, et al. Clinically amelanotic or hypomelanotic melanoma: anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79:645-651.
10. Chernoff K, Marghoob A, Lacouture M, et al. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;4:429-433.
11. Adibi M, Thomas AZ, Borregales LD, et al. Surgical considerations for patients with metastatic renal cell carcinoma. Urol Oncol. 2015;33:528-537.
12. Saeed S, Keehm C, Morgan M. Cutaneous metastases: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol. 1994;31:419-430.
A 45-year-old man presented to the Dermatology Clinic with a 4-month history of a bump on his left upper back. The lesion was tender and had been draining clear fluid and intermittent blood; he denied any preceding trauma. He had been seen both by his primary care physician and by a physician at an urgent care clinic, where he was told to use an antibiotic ointment and benzoyl peroxide daily on the area and advised to seek a dermatology consult should it not resolve. He did not see any improvement from these measures.
Physical exam revealed a 0.8-cm erythematous nodule with a peripheral collarette of scale at its base. The bandage used to cover the nodule was stained with hemorrhagic crust (FIGURE 1A). Superior and medial to the new lesion was a well-healed scar overlying much of the patient’s thoracic spine (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic renal cell carcinoma
The nodule initially appeared to be a benign pyogenic granuloma. In fact, a biopsy of the nodule showed a profile similar to that of a pyogenic granuloma and it exhibited granulation tissue. However, further questioning revealed that the patient had a history of metastatic clear cell renal carcinoma. (The scar was from a prior unrelated orthopedic surgery.) Immunohistochemical stains showed positive staining in the cells of interest for PAX8 and CK8, 2 markers for renal cell lineage.
Cutaneous metastasis to the skin is a rare event, representing roughly 2% of all skin tumors.1 Anatomically, lesions tend to appear on the head and neck in men and anterior chest and abdomen in women.2 Eruptions on the back, as seen in our patient, are relatively rare. The primary source of the metastasis also is gender dependent. Melanoma is the most common source overall; but in women, breast cancer represents the large majority of cutaneous metastases3 while in men lung, large intestine, and oral cavity tumors are the most common origin.3 Renal metastases are the fourth most common cause in men.3
The clinical morphology of cutaneous metastases is protean; the most common manifestations are nodules, papules, plaques, tumors, and ulcers.2 Rare manifestations include alopecia plaques, erysipelas, herpes zoster–like eruptions,4 and pyogenic granuloma–like manifestations, as in our case. Pyogenic granuloma–like manifestations have been described in renal cell carcinoma, breast carcinoma, acute myelogenous leukemia,5 and hepatocellular carcinoma.6
Differential includes an array of erythematous nodules
The differential diagnosis of a lesion with the appearance of a pyogenic granuloma is variable.
Pyogenic granulomas tend to arise over a short period of time. They are more common in children and pregnant women. Pyogenic granulomas can manifest anywhere but often are reported on the digits and extremities. Clinical history is important to ensure no history of internal malignancy.
Continue to: Bartonella henselae
Bartonella henselae, known as “cat scratch disease,” also can present as a friable, erythematous nodule reminiscent of a pyogenic granuloma. Patients with bartonella henselae usually are immunocompromised and/or have had close contact with a cat.7
Kaposi sarcoma is a vascular tumor that may manifest as erythematous papules or nodules. Erythematous or violaceous patches or plaques may be present before a nodule arises. Kaposi sarcoma may manifest on the legs of elderly patients or anywhere on immunocompromised patients. Immunohistochemical stains for human herpesvirus-8 can clinch the diagnosis.8
Amelanotic melanoma may be impossible to discern clinically from a pyogenic granuloma. It appears as erythematous, violaceous, or flesh-colored nodules. Histologic evaluation is paramount in the diagnosis.9
Clinical suspicion should prompt a biopsy
The diagnosis of metastatic renal cell carcinoma is made on clinical suspicion and skin biopsy. Dermoscopy is an important tool in the evaluation of primary cutaneous tumors. Due to the rarity of cutaneous metastases, studies on dermoscopic findings in cutaneous metastases are limited to case series. One series showed a vascular dermoscopy pattern in 15 of 17 cases (88%).10
In light of this nonspecific pattern, it’s wise to consider biopsy of a pyogenic granuloma–like lesion or one with a vascular pattern on dermoscopy in any patient with a history of malignancy. Any lesion suspected of being a pyogenic granuloma that does not respond to conservative measures also would warrant a biopsy. Definitive diagnosis is made based upon histologic evaluation.
Continue to: Surgery is the cornerstone of treatment
Surgery is the cornerstone of treatment
Upon diagnosis, immediate referral for further local and systemic control is recommended. Treatment may consist of any combination of surgery, chemotherapy, immunotherapy, or radiation.11
In this case, our patient was referred to Oncology for further treatment. Unfortunately, cutaneous metastases portend a very poor prognosis, with approximate survival times of 7.5 months.12
CORRESPONDENCE
M. Tye Haeberle, MD, 3810 Springhurst Boulevard, Ste 200, Louisville, KY 40241; tye.haeberle@gmail.com
A 45-year-old man presented to the Dermatology Clinic with a 4-month history of a bump on his left upper back. The lesion was tender and had been draining clear fluid and intermittent blood; he denied any preceding trauma. He had been seen both by his primary care physician and by a physician at an urgent care clinic, where he was told to use an antibiotic ointment and benzoyl peroxide daily on the area and advised to seek a dermatology consult should it not resolve. He did not see any improvement from these measures.
Physical exam revealed a 0.8-cm erythematous nodule with a peripheral collarette of scale at its base. The bandage used to cover the nodule was stained with hemorrhagic crust (FIGURE 1A). Superior and medial to the new lesion was a well-healed scar overlying much of the patient’s thoracic spine (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic renal cell carcinoma
The nodule initially appeared to be a benign pyogenic granuloma. In fact, a biopsy of the nodule showed a profile similar to that of a pyogenic granuloma and it exhibited granulation tissue. However, further questioning revealed that the patient had a history of metastatic clear cell renal carcinoma. (The scar was from a prior unrelated orthopedic surgery.) Immunohistochemical stains showed positive staining in the cells of interest for PAX8 and CK8, 2 markers for renal cell lineage.
Cutaneous metastasis to the skin is a rare event, representing roughly 2% of all skin tumors.1 Anatomically, lesions tend to appear on the head and neck in men and anterior chest and abdomen in women.2 Eruptions on the back, as seen in our patient, are relatively rare. The primary source of the metastasis also is gender dependent. Melanoma is the most common source overall; but in women, breast cancer represents the large majority of cutaneous metastases3 while in men lung, large intestine, and oral cavity tumors are the most common origin.3 Renal metastases are the fourth most common cause in men.3
The clinical morphology of cutaneous metastases is protean; the most common manifestations are nodules, papules, plaques, tumors, and ulcers.2 Rare manifestations include alopecia plaques, erysipelas, herpes zoster–like eruptions,4 and pyogenic granuloma–like manifestations, as in our case. Pyogenic granuloma–like manifestations have been described in renal cell carcinoma, breast carcinoma, acute myelogenous leukemia,5 and hepatocellular carcinoma.6
Differential includes an array of erythematous nodules
The differential diagnosis of a lesion with the appearance of a pyogenic granuloma is variable.
Pyogenic granulomas tend to arise over a short period of time. They are more common in children and pregnant women. Pyogenic granulomas can manifest anywhere but often are reported on the digits and extremities. Clinical history is important to ensure no history of internal malignancy.
Continue to: Bartonella henselae
Bartonella henselae, known as “cat scratch disease,” also can present as a friable, erythematous nodule reminiscent of a pyogenic granuloma. Patients with bartonella henselae usually are immunocompromised and/or have had close contact with a cat.7
Kaposi sarcoma is a vascular tumor that may manifest as erythematous papules or nodules. Erythematous or violaceous patches or plaques may be present before a nodule arises. Kaposi sarcoma may manifest on the legs of elderly patients or anywhere on immunocompromised patients. Immunohistochemical stains for human herpesvirus-8 can clinch the diagnosis.8
Amelanotic melanoma may be impossible to discern clinically from a pyogenic granuloma. It appears as erythematous, violaceous, or flesh-colored nodules. Histologic evaluation is paramount in the diagnosis.9
Clinical suspicion should prompt a biopsy
The diagnosis of metastatic renal cell carcinoma is made on clinical suspicion and skin biopsy. Dermoscopy is an important tool in the evaluation of primary cutaneous tumors. Due to the rarity of cutaneous metastases, studies on dermoscopic findings in cutaneous metastases are limited to case series. One series showed a vascular dermoscopy pattern in 15 of 17 cases (88%).10
In light of this nonspecific pattern, it’s wise to consider biopsy of a pyogenic granuloma–like lesion or one with a vascular pattern on dermoscopy in any patient with a history of malignancy. Any lesion suspected of being a pyogenic granuloma that does not respond to conservative measures also would warrant a biopsy. Definitive diagnosis is made based upon histologic evaluation.
Continue to: Surgery is the cornerstone of treatment
Surgery is the cornerstone of treatment
Upon diagnosis, immediate referral for further local and systemic control is recommended. Treatment may consist of any combination of surgery, chemotherapy, immunotherapy, or radiation.11
In this case, our patient was referred to Oncology for further treatment. Unfortunately, cutaneous metastases portend a very poor prognosis, with approximate survival times of 7.5 months.12
CORRESPONDENCE
M. Tye Haeberle, MD, 3810 Springhurst Boulevard, Ste 200, Louisville, KY 40241; tye.haeberle@gmail.com
1. Nashan D, Meiss F, Braun-Flaco M, et al. Cutaneous metastases from internal malignancies. Dermatol Ther. 2010;23:567-580.
2. Alcaraz IM, Cerroni LM, Rütten AM, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
3. Lookingbill D, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
4. Hussein MR. Skin metastases: a pathologist’s perspective. J Cutan Pathol. 2010;37:E1-E20.
5. Hager C, Cohen P. Cutaneous lesions of metastatic visceral malignancy mimicking pyogenic granuloma. Cancer Invest. 1999;17:385-390.
6. Kubota Y, Koga T, Nakayama J. Cutaneous metastasis from hepatocellular carcinoma resembling pyogenic granuloma. Clin Exp Dermatol. 1999;24:78-80.
7. Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203-219.
8. Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460.
9. Wee E, Wolfe R, Mclean C, et al. Clinically amelanotic or hypomelanotic melanoma: anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79:645-651.
10. Chernoff K, Marghoob A, Lacouture M, et al. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;4:429-433.
11. Adibi M, Thomas AZ, Borregales LD, et al. Surgical considerations for patients with metastatic renal cell carcinoma. Urol Oncol. 2015;33:528-537.
12. Saeed S, Keehm C, Morgan M. Cutaneous metastases: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol. 1994;31:419-430.
1. Nashan D, Meiss F, Braun-Flaco M, et al. Cutaneous metastases from internal malignancies. Dermatol Ther. 2010;23:567-580.
2. Alcaraz IM, Cerroni LM, Rütten AM, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
3. Lookingbill D, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
4. Hussein MR. Skin metastases: a pathologist’s perspective. J Cutan Pathol. 2010;37:E1-E20.
5. Hager C, Cohen P. Cutaneous lesions of metastatic visceral malignancy mimicking pyogenic granuloma. Cancer Invest. 1999;17:385-390.
6. Kubota Y, Koga T, Nakayama J. Cutaneous metastasis from hepatocellular carcinoma resembling pyogenic granuloma. Clin Exp Dermatol. 1999;24:78-80.
7. Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203-219.
8. Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460.
9. Wee E, Wolfe R, Mclean C, et al. Clinically amelanotic or hypomelanotic melanoma: anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79:645-651.
10. Chernoff K, Marghoob A, Lacouture M, et al. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;4:429-433.
11. Adibi M, Thomas AZ, Borregales LD, et al. Surgical considerations for patients with metastatic renal cell carcinoma. Urol Oncol. 2015;33:528-537.
12. Saeed S, Keehm C, Morgan M. Cutaneous metastases: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol. 1994;31:419-430.
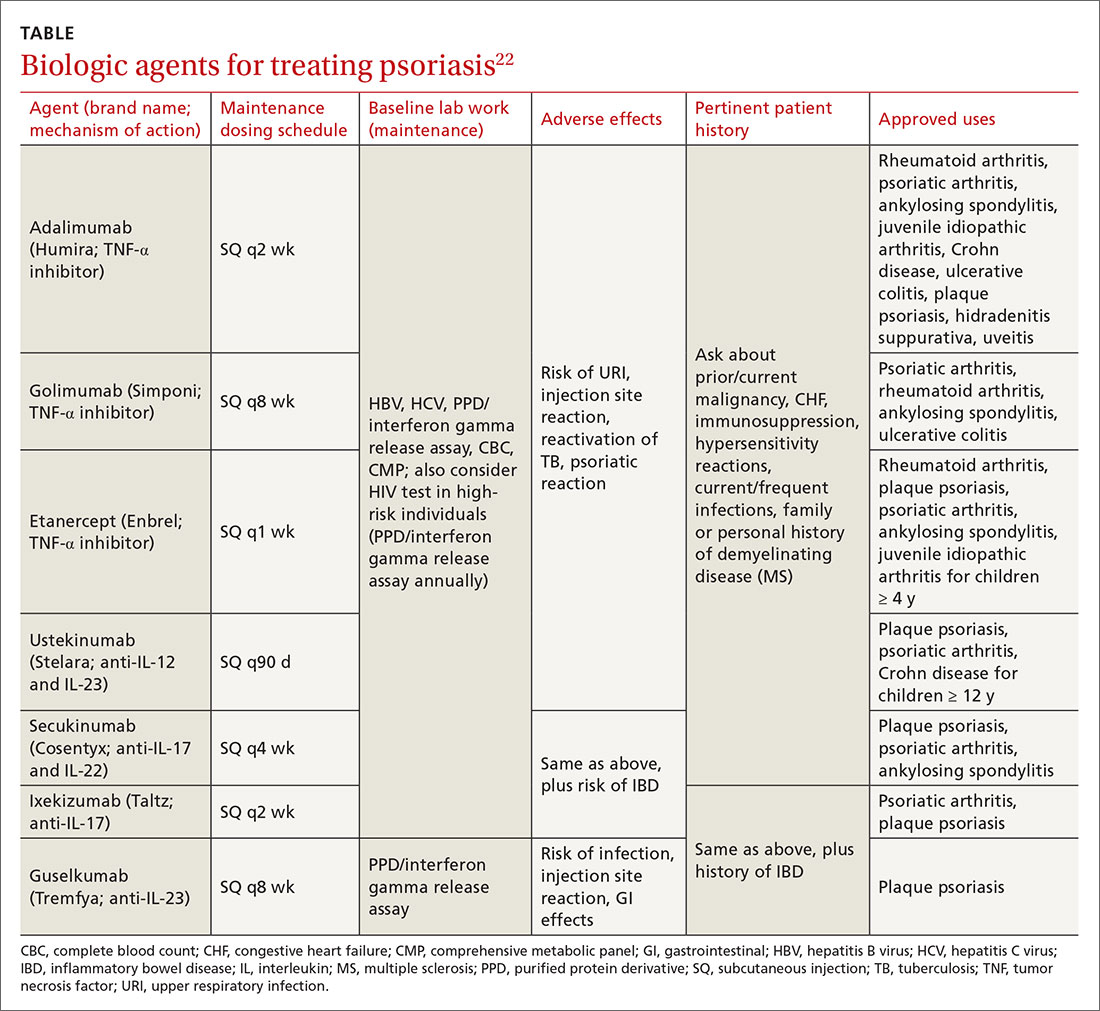
The many variants of psoriasis
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
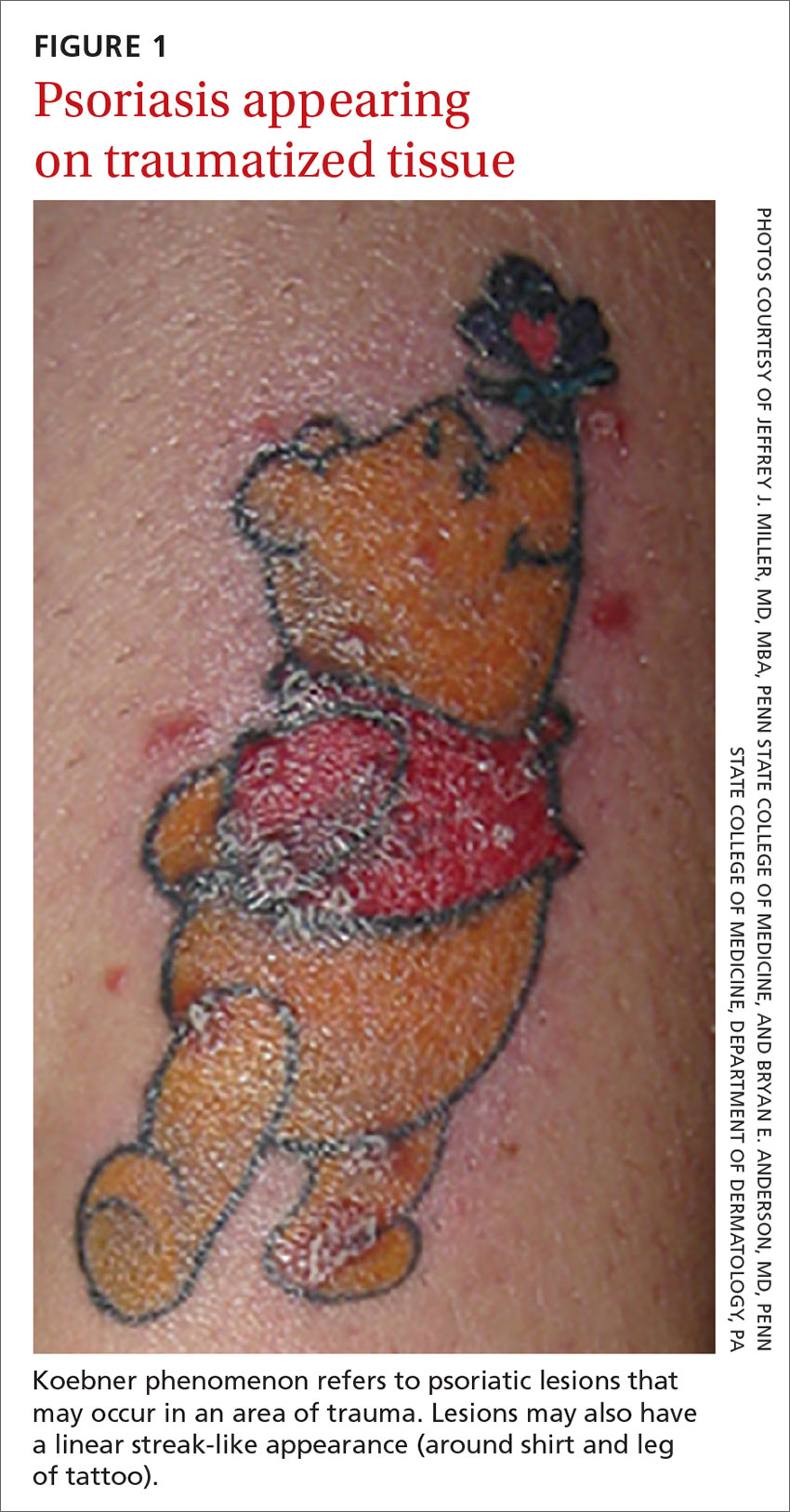
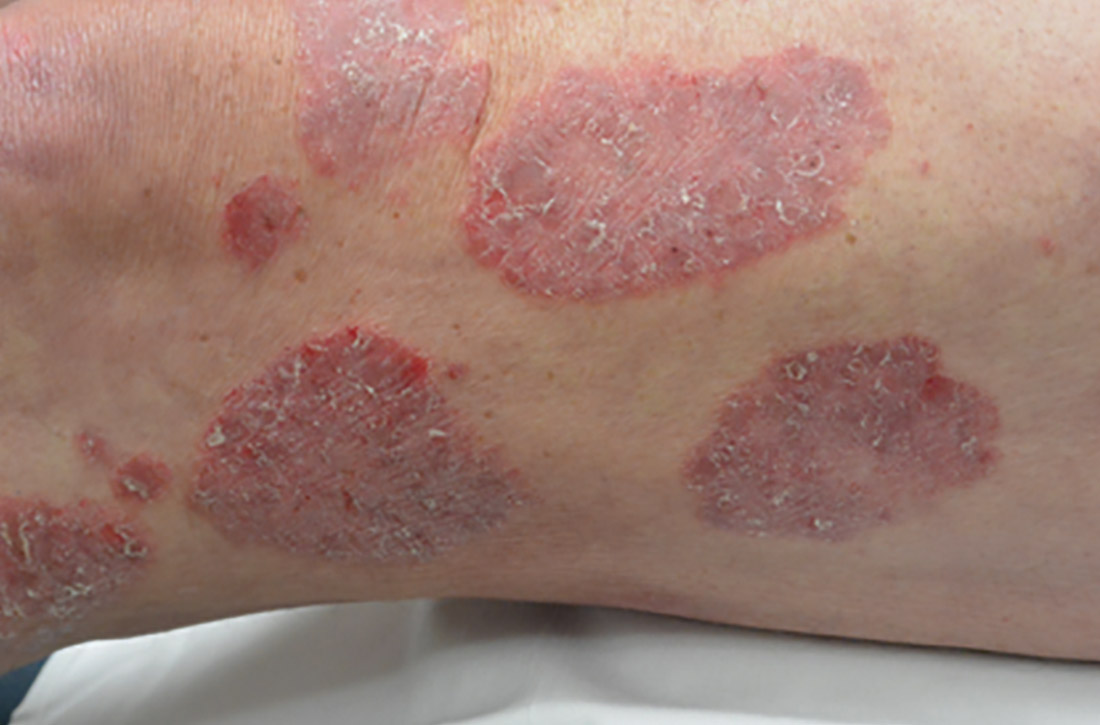
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.
Continue to: Chronic plaque-type psoriasis
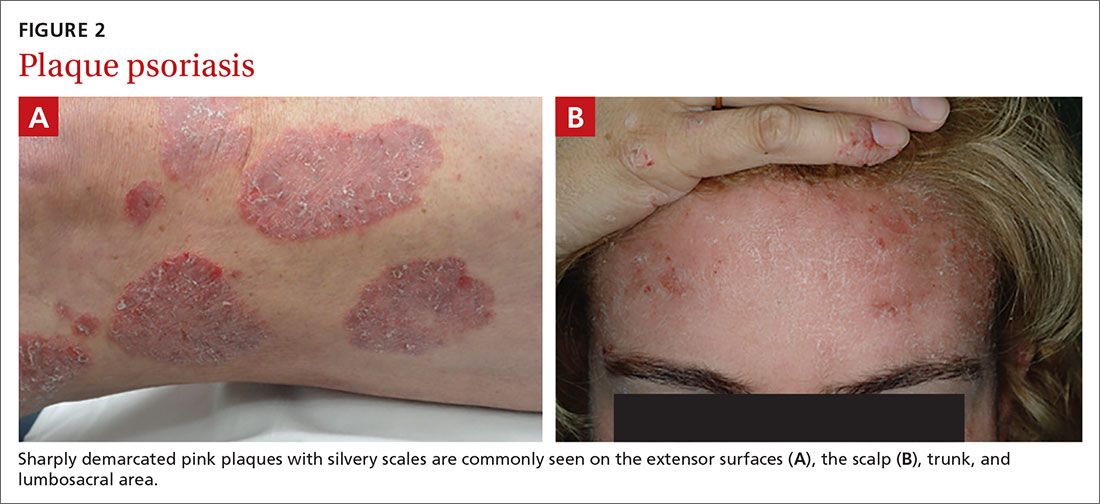
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.
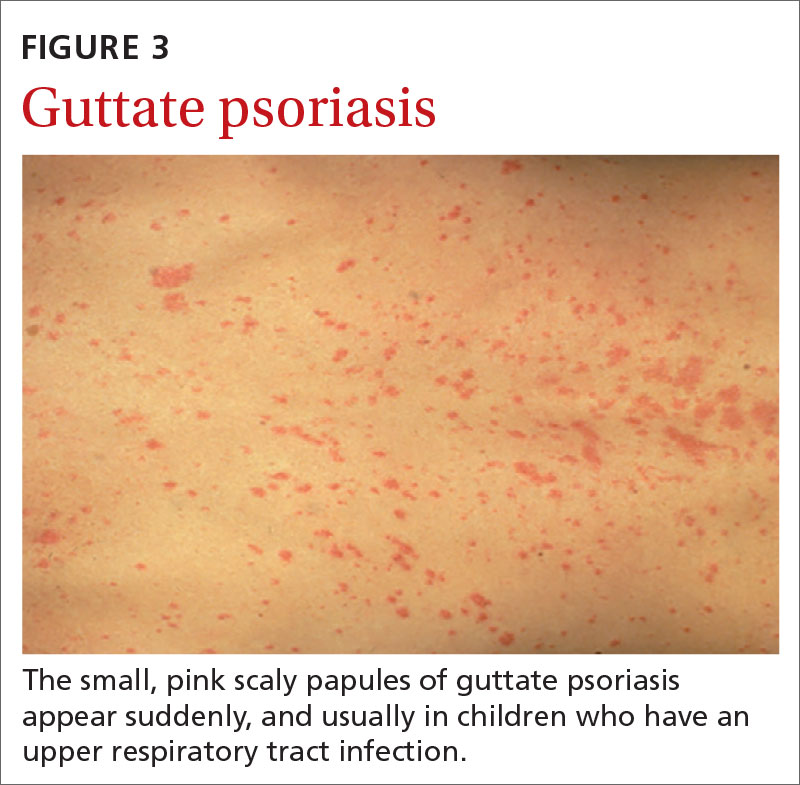
Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.
Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.
Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
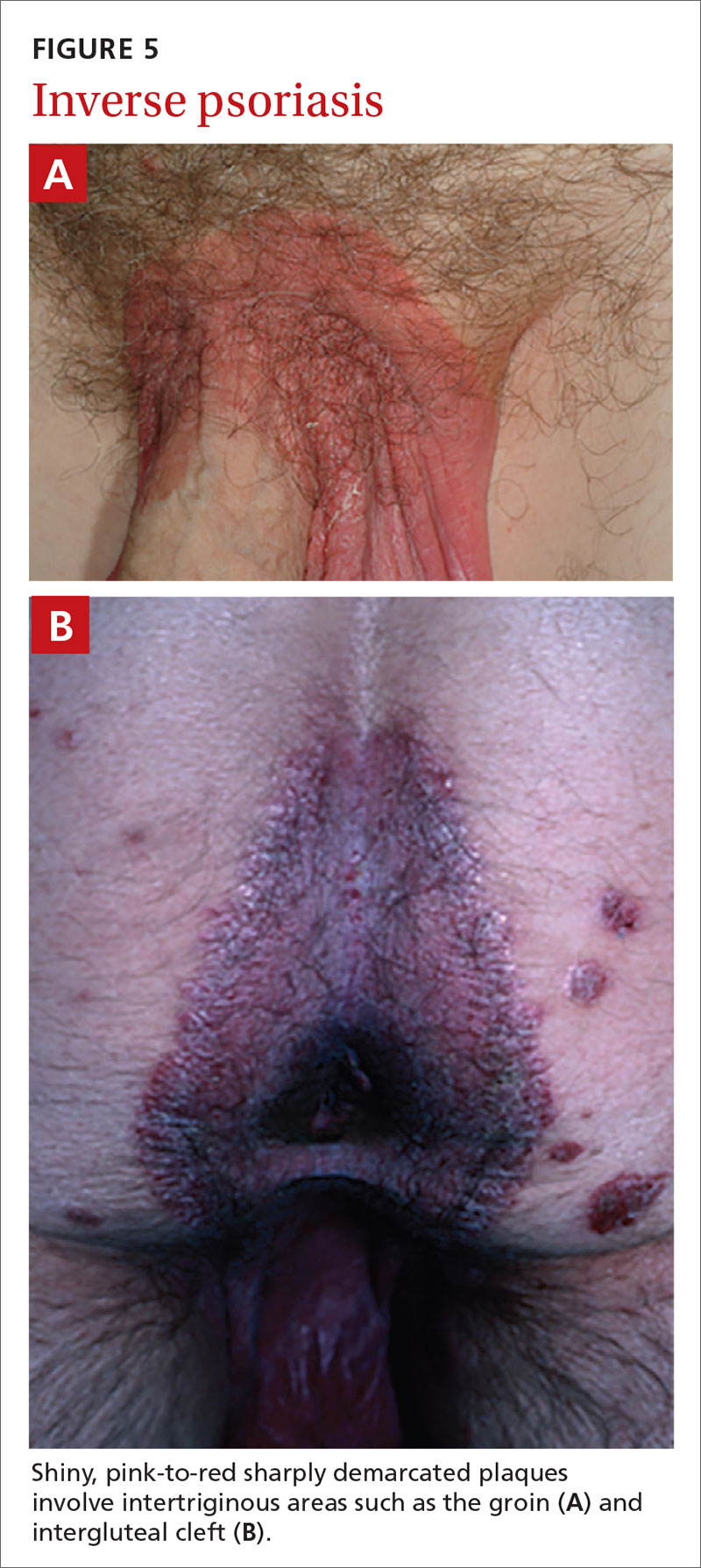
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.
Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
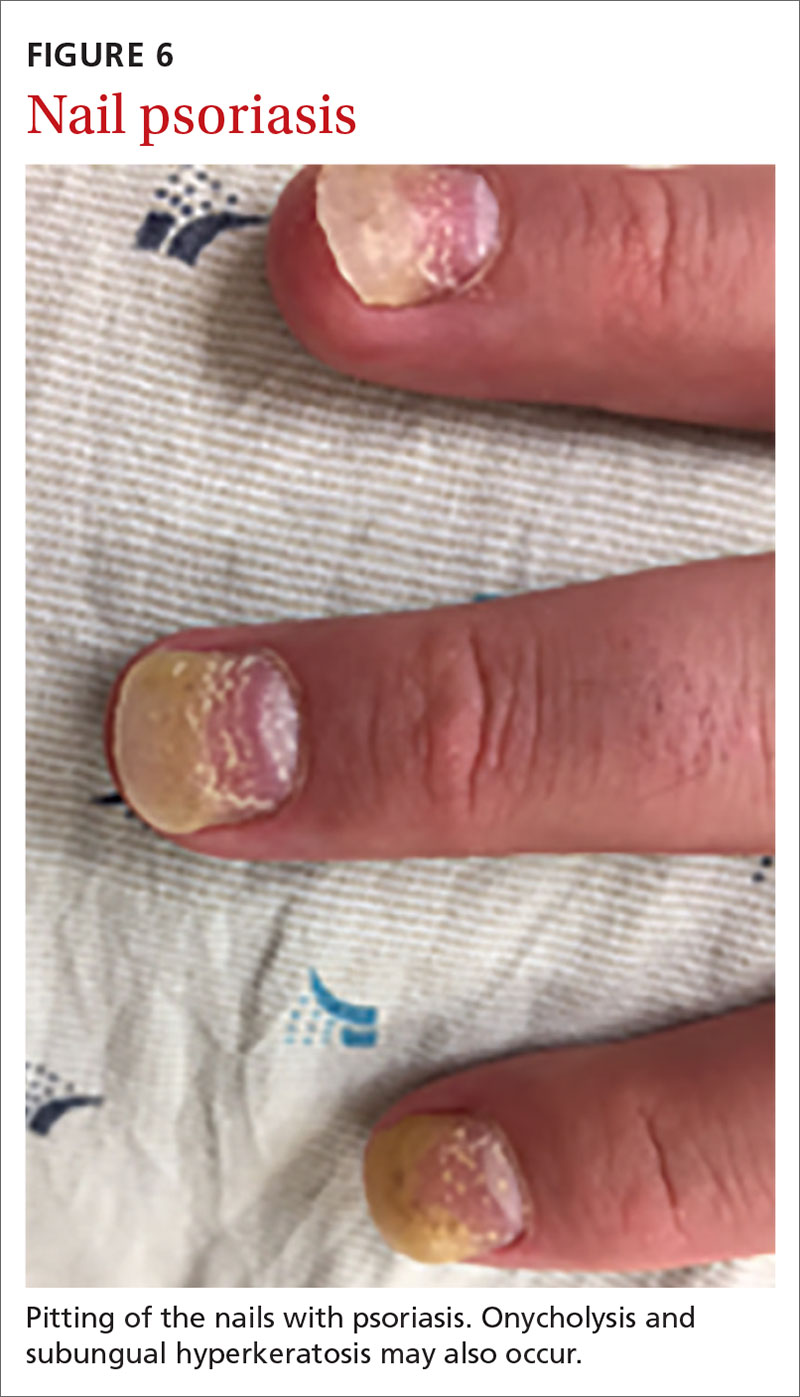
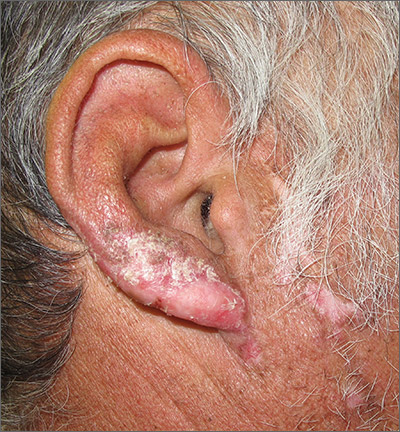
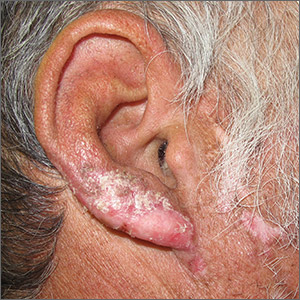
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.
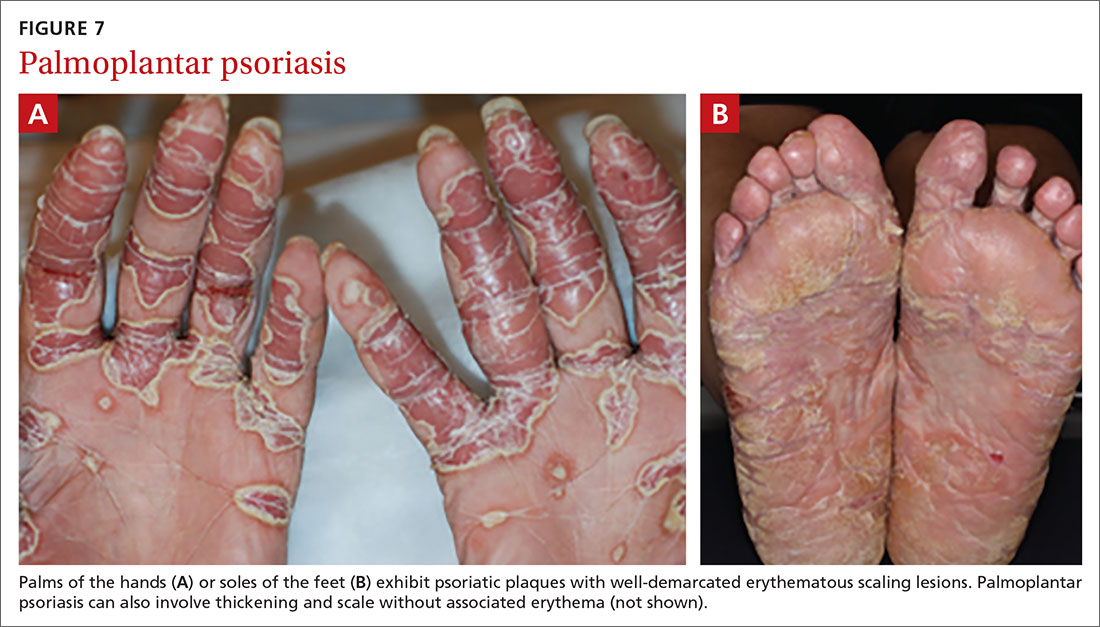
Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.
Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
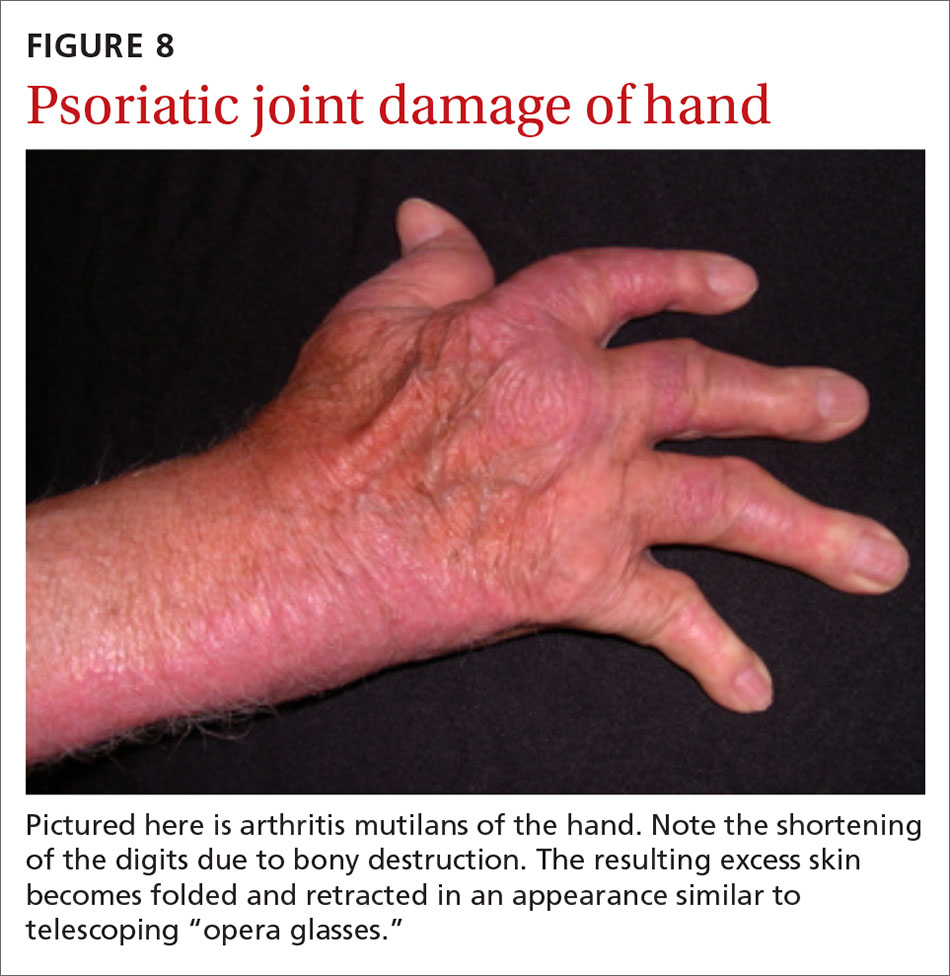
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).
Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
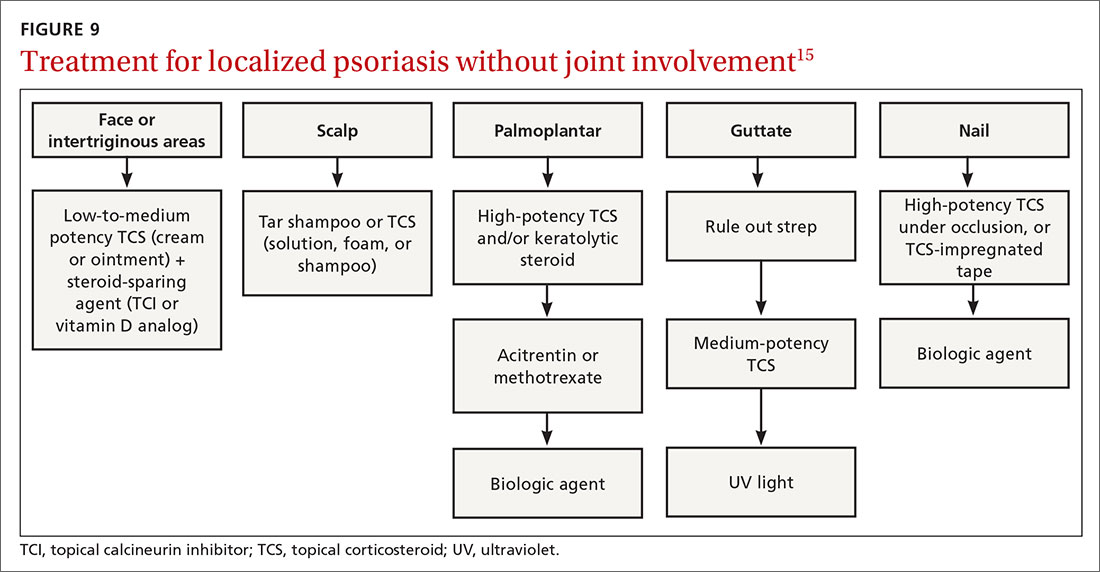
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15
If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22
Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; kclebak@pennstatehealth.psu.edu.
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.
Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.
Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.
Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.
Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.
Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.
Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.
Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).
Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15
If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22
Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; kclebak@pennstatehealth.psu.edu.
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.
Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.
Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.
Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.
Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.
Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.
Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.
Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).
Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15
If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22
Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; kclebak@pennstatehealth.psu.edu.
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
PRACTICE RECOMMENDATIONS
› Consider guttate psoriasis if small (often < 1 cm) pink scaly papules appear suddenly, particularly in a child who has an upper respiratory tract infection. C
› Document extent of disease using a tool such as the Psoriasis Area and Severity Index, which calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions. C
› Consider prescribing UV light treatment or a combination of alcitretin and topical corticosteroid if > 10% of the body surface area is involved but joints are not affected. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Ear and nose plaques
Based on the clinical appearance of hyperpigmented and hypopigmented scaly plaques in sun-exposed facial areas (with associated scarring and follicular plugging in affected lesions), the patient was given a diagnosis of discoid lupus erythematosus. The diagnosis was confirmed with a punch biopsy that showed characteristic interface dermatitis with superficial and deep perivascular and peri-adnexal lymphocytes. Direct immunofluorescence showed a band of C3 at the dermo-epidermal junction, consistent with various forms of cutaneous lupus.
A review of systems was negative for signs of systemic disease, including a lack of weakness, joint pain/swelling, fever, or known blood clots. An antinuclear antibody panel was also negative, largely excluding systemic disease.
Discoid lupus erythematosus is a form of cutaneous lupus. Patients often are genetically predisposed to autoimmune disease, and disease may be triggered by sun exposure. Up to 80% of patients develop lesions that are limited to the head and neck. Up to 25% of patients develop systemic lupus erythematosus that may occur months—or even decades—after the initial diagnosis. Lesions may lead to longstanding scars; this cannot always be prevented.
Two- to 3-week trials of topical potent or superpotent steroids applied to affected plaques may lead to clearance. Additionally, lesions may be treated with intralesional triamcinolone 3 mg/mL to 10 mg/mL and repeated every 3 to 4 weeks. Systemic therapies include hydroxychloroquine, chloroquine, retinoids, steroid sparing immunomodulators, and rarely prednisone.
This patient tolerated topical therapy well and though some scars remained, he was able to control flares with 2- to 3-week courses of clobetasol 0.05% twice daily. He is followed 2 to 3 times annually and has not developed signs or serologic findings of systemic disease over several years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Jessop S, Whitelaw DA, Grainge MJ, et al. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2017;5:CD002954.
Based on the clinical appearance of hyperpigmented and hypopigmented scaly plaques in sun-exposed facial areas (with associated scarring and follicular plugging in affected lesions), the patient was given a diagnosis of discoid lupus erythematosus. The diagnosis was confirmed with a punch biopsy that showed characteristic interface dermatitis with superficial and deep perivascular and peri-adnexal lymphocytes. Direct immunofluorescence showed a band of C3 at the dermo-epidermal junction, consistent with various forms of cutaneous lupus.
A review of systems was negative for signs of systemic disease, including a lack of weakness, joint pain/swelling, fever, or known blood clots. An antinuclear antibody panel was also negative, largely excluding systemic disease.
Discoid lupus erythematosus is a form of cutaneous lupus. Patients often are genetically predisposed to autoimmune disease, and disease may be triggered by sun exposure. Up to 80% of patients develop lesions that are limited to the head and neck. Up to 25% of patients develop systemic lupus erythematosus that may occur months—or even decades—after the initial diagnosis. Lesions may lead to longstanding scars; this cannot always be prevented.
Two- to 3-week trials of topical potent or superpotent steroids applied to affected plaques may lead to clearance. Additionally, lesions may be treated with intralesional triamcinolone 3 mg/mL to 10 mg/mL and repeated every 3 to 4 weeks. Systemic therapies include hydroxychloroquine, chloroquine, retinoids, steroid sparing immunomodulators, and rarely prednisone.
This patient tolerated topical therapy well and though some scars remained, he was able to control flares with 2- to 3-week courses of clobetasol 0.05% twice daily. He is followed 2 to 3 times annually and has not developed signs or serologic findings of systemic disease over several years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Based on the clinical appearance of hyperpigmented and hypopigmented scaly plaques in sun-exposed facial areas (with associated scarring and follicular plugging in affected lesions), the patient was given a diagnosis of discoid lupus erythematosus. The diagnosis was confirmed with a punch biopsy that showed characteristic interface dermatitis with superficial and deep perivascular and peri-adnexal lymphocytes. Direct immunofluorescence showed a band of C3 at the dermo-epidermal junction, consistent with various forms of cutaneous lupus.
A review of systems was negative for signs of systemic disease, including a lack of weakness, joint pain/swelling, fever, or known blood clots. An antinuclear antibody panel was also negative, largely excluding systemic disease.
Discoid lupus erythematosus is a form of cutaneous lupus. Patients often are genetically predisposed to autoimmune disease, and disease may be triggered by sun exposure. Up to 80% of patients develop lesions that are limited to the head and neck. Up to 25% of patients develop systemic lupus erythematosus that may occur months—or even decades—after the initial diagnosis. Lesions may lead to longstanding scars; this cannot always be prevented.
Two- to 3-week trials of topical potent or superpotent steroids applied to affected plaques may lead to clearance. Additionally, lesions may be treated with intralesional triamcinolone 3 mg/mL to 10 mg/mL and repeated every 3 to 4 weeks. Systemic therapies include hydroxychloroquine, chloroquine, retinoids, steroid sparing immunomodulators, and rarely prednisone.
This patient tolerated topical therapy well and though some scars remained, he was able to control flares with 2- to 3-week courses of clobetasol 0.05% twice daily. He is followed 2 to 3 times annually and has not developed signs or serologic findings of systemic disease over several years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Jessop S, Whitelaw DA, Grainge MJ, et al. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2017;5:CD002954.
Jessop S, Whitelaw DA, Grainge MJ, et al. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2017;5:CD002954.
Sensitizer prevalent in many hypoallergenic products for children
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
cpalmer@mdedge.com
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
cpalmer@mdedge.com
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
cpalmer@mdedge.com
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
Multiple atopic dermatitis therapies completed or close to completing phase 3 studies
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Major , Jonathan I. Silverberg, MD, PhD, said during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
“In the next 2-3 years, we may have nine new treatments approved for atopic dermatitis,” said Dr. Silverberg, director of clinical research and contact dermatitis at the University.
All nine medications he discussed are either in ongoing pivotal phase 3 randomized controlled trials or have completed their phase 3 developmental programs. “This is not theoretical; these are things you’re going to be using in your toolbox imminently,” he stressed.
Oral JAK inhibitors
The Janus kinase (JAK) pathway is the intracellular signaling mediator that interacts with extracellular inflammatory cytokines, including interleukin-4, -13, and -31, which are familiar to dermatologists because they’re targeted by potent biologic monoclonal antibody therapies. For example, IL-4 goes through JAK1 and 3, while IL-31 signals through JAK1 and 2.
“You really need to know the key JAK and STAT pathways involved in atopic dermatitis because it will help you determine the selectivity of the agents you’re going to be using,” the dermatologist advised.
Three oral, once-daily JAK inhibitors – abrocitinib, upadacitinib, and baricitinib – are in an advanced stage of development.
“Upadacitinib and abrocitinib may be the two most potent options coming to market soon for us to be thinking about,” Dr. Silverberg said.
Abrocitinib: Three positive phase 3 studies featuring this selective JAK1 inhibitor have been completed in adults with moderate to severe atopic dermatitis (AD). The most recent, JADE COMPARE, featured a head-to-head randomized comparison of abrocitinib and the injectable IL-4/IL-13 inhibitor dupilumab. The results of this 837-patient study haven’t yet been formally presented at a conference because of the COVID-19 pandemic. However, Pfizer recently announced that abrocitinib at 200 mg/day achieved significantly greater improvements than dupilumab (Dupixent) in the coprimary endpoints of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (that is, clear or almost clear) and disease extent based upon 75% reduction from baseline in Eczema Area and Severity Index (EASI 75) at 12 weeks. The same was true at 16 weeks.
Also, a significantly larger proportion of abrocitinib-treated patients achieved at least a 4-point reduction in itch severity as measured using the Peak Pruritus Numerical Rating Scale at week 2. The company plans to file for regulatory approval later this year.
The JADE COMPARE data are exciting because of a pressing unmet need for treatment options that are even more powerful than dupilumab, Dr. Silverberg said.
Upadacitinib: This is selective JAK1 inhibitor is not as far along in the developmental pipeline as abrocitinib, but the efficacy appears to be comparable. In a phase 2 study of 126 adults with moderate to severe AD, upadacitinib at the top dose of 30 mg/day achieved efficacy results Dr. Silverberg deemed “quite extraordinary,” with a rate of IGA score of 0 or 1 of 50% at 16 weeks and an EASI 75 response rate of 69%. Those findings numerically eclipsed results seen in an earlier phase 3 pivotal trial for dupilumab, in which the IGA 0/1 rate was 37% and EASI 75 was 48%, albeit with the caveat that cross-trial comparisons must be taken with a large grain of salt.
Baricitinib: Multiple phase 3 studies of this JAK 1/2 inhibitor have reported positive results. At the top dose of 4 mg/day, baricitinib appears to be less effective than dupilumab in its earlier pivotal trials.
“This may be a good oral option for our patients. It could be similar to the Otezla [apremilast] story in psoriasis: It’s perhaps not as effective as a lot of the biologics, but patients often prefer an oral option,” Dr. Silverberg said.
Of note, in one large, placebo-controlled, phase 3 study of baricitinib on top of background low- or medium-potency topical steroids, the IGA 0/1 rate at 16 weeks with placebo plus topical steroids was a modest 14.7%, which underscores that this long-time workhorse topical therapy is objectively less effective than most physicians think. In contrast, the IGA 0/1 rate with baricitinib at 4 mg/day plus topical steroids was a more respectable 30.6%.
All three oral JAK inhibitors have rapid onset of efficacy, a key advantage over the biologic agents.
“The issue you have to keep in mind is safety. The safety in the atopic dermatitis population was overall quite good for all three drugs. However, safety concerns have come up with JAK inhibitors in rheumatoid arthritis. I think that’s the part we watch the most in this. The efficacy has become clear. Now the question is where does the safety take us,” he said.
Novel injectable biologics
Nemolizumab: This humanized monoclonal antibody inhibits IL-31 receptor alpha. Mounting evidence implicates IL-31 as both a proinflammatory and immunomodulatory cytokine linking the immune and neural systems.
Early on, most researchers pigeonholed IL-31 as being a key player only in the itch factor in AD. Not so. Indeed, Dr. Silverberg was the lead investigator in a recent phase 2b study of nemolizumab that demonstrated the biologic is also effective at rapidly clearing AD lesions. The study, which evaluated three different doses in 226 adults with moderate to severe AD and severe pruritus who were on background topical corticosteroids, showed that nemolizumab at 30 mg every 4 weeks trounced placebo in terms of itch reduction: The 69% drop from baseline in Peak Pruritus Numeric Rating Scale at week 16 was twice that in controls, with a significant difference apparent even at week 1.
But in addition, the 33% IGA 0/1 rate at the same time point bested the 12% rate in controls. The EASI 75 response rate was significantly higher as well – 49% versus 19% – as was the EASI 90 response of 33%, compared with 9% in controls. Moreover, nemolizumab-treated patients used close to 40% less topical steroids during the study (J Allergy Clin Immunol. 2020 Jan;145[1]:173-82).
“This is something that’s fascinating. The study gets into the idea that a subset of atopic dermatitis patients have the itch that rashes, and perhaps if you break the itch/scratch cycle you can modify the lesions. Or the effect may even be due to the direct anti-inflammatory action of IL-31 blockade,” Dr. Silverberg observed.
It appeared that a plateau hadn’t been reached for some endpoints out at week 24, when the study ended. Japanese phase 3 studies have been completed, with what he called “great results,” and others are ongoing in the United States.
Tralokinumab: This fully human monoclonal antibody binds to IL-13, but unlike dupilumab, it doesn’t also inhibit IL-4. Tralokinumab met all primary and secondary endpoints in three pivotal phase 3 clinical trials, known as ECZTRA 1-3, that assessed it as treatment for moderate to severe AD in adults and showed an overall adverse event rate comparable with placebo. Leo Pharma, the Danish company developing the biologic, has announced it will file for marketing approval before the end of 2020. Phase 3 data would have been presented at the annual meeting of the American Academy of Dermatology in Denver, had it not been canceled. Dr. Silverberg said that, based upon phase 2 results, it appears tralokinumab may not be quite as effective as dupilumab in the overall AD population, but he predicted the newcomer will still play a useful role.
“The complexities of the immune system are such that some patients will respond better to one drug than another. I think we still have a lot to learn about who the patients are for these novel assets,” he said.
Lebrikizumab: This is another selective IL-13 inhibitor, but this one binds to IL-13 in a slightly different way than tralokinumab. The Food and Drug Administration granted it Fast Track status in December 2019. Twin placebo-controlled phase 3 studies of lebrikizumab as monotherapy for moderate to severe AD are ongoing, and another phase 3 trial of the biologic in combination with topical steroids is planned. Based upon the results of a phase 2b study, the highest dose studied – 250 mg every 2 weeks – appears to be at least as effective as dupilumab.
Nonsteroidal topical agents
These three late-stage topical creams – ruxolitinib, delgocitinib, and tapinarof – have previously received considerable coverage in Dermatology News. Ruxolitinib, a selective JAK1/2 inhibitor, has completed a positive phase 3 trial in adolescents and adults with mild to moderate AD. Delgocitinib, a pan-JAK1/2/3 and Tyrosine kinase 2 inhibitor, is already approved in an ointment formulation in Japan, and the cream formulation is in phase 2 studies in the United States and Europe. Tapinarof has a unique mechanism of action – it’s an aryl hydrocarbon receptor modulator – and is now in phase 3 in adolescents and adults with moderate to severe AD.
These three drugs appear to offer efficacy that’s comparable to or even better than medium-potency topical steroids, and without the notorious steroidal side effects that have caused widespread parental steroid-phobia. Potential applications for other inflammatory diseases, including vitiligo and psoriasis, are under study.
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to those pharmaceutical companies and more than a dozen others.
Case series suggests biologics, JAK inhibitors safe during pandemic
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
aotto@mdedge.com
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
aotto@mdedge.com
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
aotto@mdedge.com
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Purple oral plaques
The papules were clinically consistent with Kaposi sarcoma (KS) and confirmed with a biopsy from the buccal mucosa. The patient had not been given a diagnosis of human immunodeficiency virus (HIV) prior to this presentation. However, the physician confirmed the diagnosis with RNA titers. A CD4 count < 40 cells/mm3 (reference range, 500-1200 cells/mm3) pointed to her progression to AIDS. A chest X-ray revealed multiple nodules; a subsequent biopsy indicated that they were consistent with KS.
KS is a low-grade tumor of vascular origin associated with human herpesvirus-8 (HHV-8). It most often presents on the skin as flat to raised pink to deep purple lesions. It can manifest in the oral mucosa, viscera, and other organs, which can portend a worse prognosis because of the risks associated with bleeding and organ perforation.
There are 4 types of KS.
- Classic KS occurs most often in elderly men of Mediterranean descent.
- HIV-associated KS can occur at any time during HIV infection but is more common as CD4 counts fall. HIV-associated KS increased in frequency dramatically in the United States during the early years of the HIV pandemic prior to effective antiretroviral therapy (ART).
- Endemic KS occurs in equatorial Africa, where there is a natural increased transmission rate of HHV-8.
- Iatrogenic KS can occur following treatment with immunosuppressive therapies.
Our patient was admitted to the Infectious Disease Service and given ART. Chemotherapy was discussed (and sometimes is warranted in extensive visceral disease) but the patient and her specialists opted for ART alone. In addition to ART, she was started on daily trimethoprim-sulfamethoxazole for pneumocystis prophylaxis.
At 6 months’ follow-up with Infectious Disease, the patient’s oral lesions resolved, CD4 count increased above 200 cells/mm3, and HIV RNA titers fell.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Thariat J, Kirova Y, Sio T, et al. Mucosal Kaposi sarcoma, a Rare Cancer Network study. Rare Tumors. 2012;4:E49.
The papules were clinically consistent with Kaposi sarcoma (KS) and confirmed with a biopsy from the buccal mucosa. The patient had not been given a diagnosis of human immunodeficiency virus (HIV) prior to this presentation. However, the physician confirmed the diagnosis with RNA titers. A CD4 count < 40 cells/mm3 (reference range, 500-1200 cells/mm3) pointed to her progression to AIDS. A chest X-ray revealed multiple nodules; a subsequent biopsy indicated that they were consistent with KS.
KS is a low-grade tumor of vascular origin associated with human herpesvirus-8 (HHV-8). It most often presents on the skin as flat to raised pink to deep purple lesions. It can manifest in the oral mucosa, viscera, and other organs, which can portend a worse prognosis because of the risks associated with bleeding and organ perforation.
There are 4 types of KS.
- Classic KS occurs most often in elderly men of Mediterranean descent.
- HIV-associated KS can occur at any time during HIV infection but is more common as CD4 counts fall. HIV-associated KS increased in frequency dramatically in the United States during the early years of the HIV pandemic prior to effective antiretroviral therapy (ART).
- Endemic KS occurs in equatorial Africa, where there is a natural increased transmission rate of HHV-8.
- Iatrogenic KS can occur following treatment with immunosuppressive therapies.
Our patient was admitted to the Infectious Disease Service and given ART. Chemotherapy was discussed (and sometimes is warranted in extensive visceral disease) but the patient and her specialists opted for ART alone. In addition to ART, she was started on daily trimethoprim-sulfamethoxazole for pneumocystis prophylaxis.
At 6 months’ follow-up with Infectious Disease, the patient’s oral lesions resolved, CD4 count increased above 200 cells/mm3, and HIV RNA titers fell.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
The papules were clinically consistent with Kaposi sarcoma (KS) and confirmed with a biopsy from the buccal mucosa. The patient had not been given a diagnosis of human immunodeficiency virus (HIV) prior to this presentation. However, the physician confirmed the diagnosis with RNA titers. A CD4 count < 40 cells/mm3 (reference range, 500-1200 cells/mm3) pointed to her progression to AIDS. A chest X-ray revealed multiple nodules; a subsequent biopsy indicated that they were consistent with KS.
KS is a low-grade tumor of vascular origin associated with human herpesvirus-8 (HHV-8). It most often presents on the skin as flat to raised pink to deep purple lesions. It can manifest in the oral mucosa, viscera, and other organs, which can portend a worse prognosis because of the risks associated with bleeding and organ perforation.
There are 4 types of KS.
- Classic KS occurs most often in elderly men of Mediterranean descent.
- HIV-associated KS can occur at any time during HIV infection but is more common as CD4 counts fall. HIV-associated KS increased in frequency dramatically in the United States during the early years of the HIV pandemic prior to effective antiretroviral therapy (ART).
- Endemic KS occurs in equatorial Africa, where there is a natural increased transmission rate of HHV-8.
- Iatrogenic KS can occur following treatment with immunosuppressive therapies.
Our patient was admitted to the Infectious Disease Service and given ART. Chemotherapy was discussed (and sometimes is warranted in extensive visceral disease) but the patient and her specialists opted for ART alone. In addition to ART, she was started on daily trimethoprim-sulfamethoxazole for pneumocystis prophylaxis.
At 6 months’ follow-up with Infectious Disease, the patient’s oral lesions resolved, CD4 count increased above 200 cells/mm3, and HIV RNA titers fell.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Thariat J, Kirova Y, Sio T, et al. Mucosal Kaposi sarcoma, a Rare Cancer Network study. Rare Tumors. 2012;4:E49.
Thariat J, Kirova Y, Sio T, et al. Mucosal Kaposi sarcoma, a Rare Cancer Network study. Rare Tumors. 2012;4:E49.