User login
Tease out genetic and structural causes of children’s hair loss
according to Maria Hordinsky, MD, of the University of Minnesota, Minneapolis.
The ectodermal dysplasias are a heterogeneous group of disorders in which a main feature is the absent, incomplete, or delayed development of one or more of the appendages derived from ectoderm, such as the hair follicle, Dr. Hordinsky said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Patients with pure hair and nail ectodermal dysplasia generally present with absent or sparse eyebrows and eyelashes, as well as follicular papules on the scalp and fragile, irregular hair, Dr. Hordinsky said. The condition is caused by a mutation in a gene associated with the production of keratin. In another rare form of hereditary hair loss – hypotrichosis simplex – patients are born with normal hair but lose it gradually from the scalp during the middle of the first decade of life.
The inability to grow long hair characterizes short anagen syndrome, a congenital disorder not to be confused with loose anagen syndrome, Dr. Hordinsky said. Patients with short anagen syndrome experience an idiopathic short anagen phase and as a result, an increased number of hairs in the telogen phase. Children with short anagen syndrome have unusually short hair in early childhood. “Parents typically complain that their children exhibit short hair even though they have never had a haircut,” she explained.
Trichothiodystrophy, a rare autosomal recessive disease, is distinguished by hair that is brittle and sulfur deficient, Dr. Hordinsky said. She cited a review of 112 patients with trichothiodystrophy in which additional distinguishing features included developmental delay/intellectual impairment (86%), short stature (73%), and ichthyosis (65%).
Some cases of hair loss in children have a structural basis, Dr. Hordinsky noted. Structural hair abnormalities include fractures of the hair shaft, extraneous matter on the hair shaft, and hair shaft irregularities such as coiling or twisting, she said.
In trichoptilosis, extensive cuticle loss results in fraying and splitting of the hair shaft, while in patients with trichoclasis, a fractured hair is splinted by a partially intact cuticle.
In trichorrhexis nodosa, the most common type of structural hair abnormality, “intact nodes [of hair] resemble two paintbrushes thrust together,” Dr. Hordinsky explained. Trichorrhexis nodosa may be congenital or acquired, and occurs in children with mental retardation and argininosuccinic aciduria, she said.
A hair shaft abnormality is the culprit behind uncombable hair syndrome, which can be inherited or can occur sporadically, Dr. Hordinsky said. The key feature of the condition is unruly hair caused by a distinctive hair shaft defect, “possibly related to an abnormality in the inner root sheath.” Abnormal hairs usually become apparent at about 3-4 years of age, but eyebrows and eyelashes appear normal. Many patients have a silvery blonde tint to their hair because of how the abnormal hairs reflect light, she said.
Dr. Hordinsky is a consultant for P&G, Concert, Cassiopea, and BioAZ; and receives grant/research support from Aclaris, Allergan, and the National Alopecia Areata Foundation. SDEF and this news organization are owned by Frontline Medical Communications.
according to Maria Hordinsky, MD, of the University of Minnesota, Minneapolis.
The ectodermal dysplasias are a heterogeneous group of disorders in which a main feature is the absent, incomplete, or delayed development of one or more of the appendages derived from ectoderm, such as the hair follicle, Dr. Hordinsky said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Patients with pure hair and nail ectodermal dysplasia generally present with absent or sparse eyebrows and eyelashes, as well as follicular papules on the scalp and fragile, irregular hair, Dr. Hordinsky said. The condition is caused by a mutation in a gene associated with the production of keratin. In another rare form of hereditary hair loss – hypotrichosis simplex – patients are born with normal hair but lose it gradually from the scalp during the middle of the first decade of life.
The inability to grow long hair characterizes short anagen syndrome, a congenital disorder not to be confused with loose anagen syndrome, Dr. Hordinsky said. Patients with short anagen syndrome experience an idiopathic short anagen phase and as a result, an increased number of hairs in the telogen phase. Children with short anagen syndrome have unusually short hair in early childhood. “Parents typically complain that their children exhibit short hair even though they have never had a haircut,” she explained.
Trichothiodystrophy, a rare autosomal recessive disease, is distinguished by hair that is brittle and sulfur deficient, Dr. Hordinsky said. She cited a review of 112 patients with trichothiodystrophy in which additional distinguishing features included developmental delay/intellectual impairment (86%), short stature (73%), and ichthyosis (65%).
Some cases of hair loss in children have a structural basis, Dr. Hordinsky noted. Structural hair abnormalities include fractures of the hair shaft, extraneous matter on the hair shaft, and hair shaft irregularities such as coiling or twisting, she said.
In trichoptilosis, extensive cuticle loss results in fraying and splitting of the hair shaft, while in patients with trichoclasis, a fractured hair is splinted by a partially intact cuticle.
In trichorrhexis nodosa, the most common type of structural hair abnormality, “intact nodes [of hair] resemble two paintbrushes thrust together,” Dr. Hordinsky explained. Trichorrhexis nodosa may be congenital or acquired, and occurs in children with mental retardation and argininosuccinic aciduria, she said.
A hair shaft abnormality is the culprit behind uncombable hair syndrome, which can be inherited or can occur sporadically, Dr. Hordinsky said. The key feature of the condition is unruly hair caused by a distinctive hair shaft defect, “possibly related to an abnormality in the inner root sheath.” Abnormal hairs usually become apparent at about 3-4 years of age, but eyebrows and eyelashes appear normal. Many patients have a silvery blonde tint to their hair because of how the abnormal hairs reflect light, she said.
Dr. Hordinsky is a consultant for P&G, Concert, Cassiopea, and BioAZ; and receives grant/research support from Aclaris, Allergan, and the National Alopecia Areata Foundation. SDEF and this news organization are owned by Frontline Medical Communications.
according to Maria Hordinsky, MD, of the University of Minnesota, Minneapolis.
The ectodermal dysplasias are a heterogeneous group of disorders in which a main feature is the absent, incomplete, or delayed development of one or more of the appendages derived from ectoderm, such as the hair follicle, Dr. Hordinsky said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Patients with pure hair and nail ectodermal dysplasia generally present with absent or sparse eyebrows and eyelashes, as well as follicular papules on the scalp and fragile, irregular hair, Dr. Hordinsky said. The condition is caused by a mutation in a gene associated with the production of keratin. In another rare form of hereditary hair loss – hypotrichosis simplex – patients are born with normal hair but lose it gradually from the scalp during the middle of the first decade of life.
The inability to grow long hair characterizes short anagen syndrome, a congenital disorder not to be confused with loose anagen syndrome, Dr. Hordinsky said. Patients with short anagen syndrome experience an idiopathic short anagen phase and as a result, an increased number of hairs in the telogen phase. Children with short anagen syndrome have unusually short hair in early childhood. “Parents typically complain that their children exhibit short hair even though they have never had a haircut,” she explained.
Trichothiodystrophy, a rare autosomal recessive disease, is distinguished by hair that is brittle and sulfur deficient, Dr. Hordinsky said. She cited a review of 112 patients with trichothiodystrophy in which additional distinguishing features included developmental delay/intellectual impairment (86%), short stature (73%), and ichthyosis (65%).
Some cases of hair loss in children have a structural basis, Dr. Hordinsky noted. Structural hair abnormalities include fractures of the hair shaft, extraneous matter on the hair shaft, and hair shaft irregularities such as coiling or twisting, she said.
In trichoptilosis, extensive cuticle loss results in fraying and splitting of the hair shaft, while in patients with trichoclasis, a fractured hair is splinted by a partially intact cuticle.
In trichorrhexis nodosa, the most common type of structural hair abnormality, “intact nodes [of hair] resemble two paintbrushes thrust together,” Dr. Hordinsky explained. Trichorrhexis nodosa may be congenital or acquired, and occurs in children with mental retardation and argininosuccinic aciduria, she said.
A hair shaft abnormality is the culprit behind uncombable hair syndrome, which can be inherited or can occur sporadically, Dr. Hordinsky said. The key feature of the condition is unruly hair caused by a distinctive hair shaft defect, “possibly related to an abnormality in the inner root sheath.” Abnormal hairs usually become apparent at about 3-4 years of age, but eyebrows and eyelashes appear normal. Many patients have a silvery blonde tint to their hair because of how the abnormal hairs reflect light, she said.
Dr. Hordinsky is a consultant for P&G, Concert, Cassiopea, and BioAZ; and receives grant/research support from Aclaris, Allergan, and the National Alopecia Areata Foundation. SDEF and this news organization are owned by Frontline Medical Communications.
FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Advise parents on validity of AD-associated conditions
including allergic rhinitis and asthma, according to Douglas W. Kress, MD, of the department of dermatology at the University of Pittsburgh.
Recent studies suggest that atopic dermatitis (AD) affects 10%-17% of the U.S. population, and 80%-90% of patients are diagnosed by the age of 5 years, Dr. Kress said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“There seem to be multiple pathways, which initiate and perpetuate the cutaneous inflammation of AD including exposure to allergens, irritants, and physical trauma, infection, stress, extremes in temperature and humidity,” Dr. Kress said. In addition, foods and airborne allergens may trigger AD.
Many parents may believe that certain factors are associated with AD, but most of these perceptions are not supported by evidence, said Dr. Kress, who is also chief of the division of pediatric dermatology at the Children’s Hospital of Pittsburgh. AD appears to be a disorder of T cell dysregulation dominated by Th2 lesions in acute cases and Th1 inflammation in patients with chronic lesions.
No associations have been proven between the development of AD in the first 18 months of life and any maternal dietary restrictions, according to a recent Cochrane review, nor is there evidence for an association between AD and the introduction of solid foods, exposure to fish oil, or exposure to animals at a young age, he said. In addition, a study published in 2016 showed a lack of evidence to support the use of specific allergen immunotherapy for AD.
However, evidence does support an association between the presence of AD in children and certain other conditions, Dr. Kress said. “Other associations include an increased incidence of alopecia areata, a threefold increase in autism spectrum disorders, and a twofold increase in ADHD in children with atopic dermatitis.”
The only known food allergy linked to AD severity is egg whites; reducing egg white exposure has been shown to improve AD in children with both conditions, he noted.
Although many patients with AD experience annoying but relatively mild symptoms, health care providers should be alert to the potential for infections, particularly with Staphylococcus aureus, and remember that an active egg white allergy has been associated with staphylococcal superantigen sensitization, said Dr. Kress. The increased risk for S. aureus in children with AD may stem from a tendency to underuse antibiotics in AD children, which results in a delayed treatment until the infection becomes overt. In addition, the increased pH in patients with AD might promote the development of pathogenic strains of staph. However, ceramide-based moisturizers could help inhibit these strains by increasing skin acidity.
For patients who have poor AD control with standard therapy, antibiotics may be used as adjunctive therapy. “Consider bleach baths and/or staph decolonization with mupirocin, both of which led to significant improvement in eczema severity compared to placebo,” Dr. Kress said. “Bleach may also have an anti-inflammatory effect.”
Dr. Kress disclosed relationships with Pfizer, Amgen, and Sanofi/Regeneron. SDEF and this news organization are owned by Frontline Medical Communications.
including allergic rhinitis and asthma, according to Douglas W. Kress, MD, of the department of dermatology at the University of Pittsburgh.
Recent studies suggest that atopic dermatitis (AD) affects 10%-17% of the U.S. population, and 80%-90% of patients are diagnosed by the age of 5 years, Dr. Kress said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“There seem to be multiple pathways, which initiate and perpetuate the cutaneous inflammation of AD including exposure to allergens, irritants, and physical trauma, infection, stress, extremes in temperature and humidity,” Dr. Kress said. In addition, foods and airborne allergens may trigger AD.
Many parents may believe that certain factors are associated with AD, but most of these perceptions are not supported by evidence, said Dr. Kress, who is also chief of the division of pediatric dermatology at the Children’s Hospital of Pittsburgh. AD appears to be a disorder of T cell dysregulation dominated by Th2 lesions in acute cases and Th1 inflammation in patients with chronic lesions.
No associations have been proven between the development of AD in the first 18 months of life and any maternal dietary restrictions, according to a recent Cochrane review, nor is there evidence for an association between AD and the introduction of solid foods, exposure to fish oil, or exposure to animals at a young age, he said. In addition, a study published in 2016 showed a lack of evidence to support the use of specific allergen immunotherapy for AD.
However, evidence does support an association between the presence of AD in children and certain other conditions, Dr. Kress said. “Other associations include an increased incidence of alopecia areata, a threefold increase in autism spectrum disorders, and a twofold increase in ADHD in children with atopic dermatitis.”
The only known food allergy linked to AD severity is egg whites; reducing egg white exposure has been shown to improve AD in children with both conditions, he noted.
Although many patients with AD experience annoying but relatively mild symptoms, health care providers should be alert to the potential for infections, particularly with Staphylococcus aureus, and remember that an active egg white allergy has been associated with staphylococcal superantigen sensitization, said Dr. Kress. The increased risk for S. aureus in children with AD may stem from a tendency to underuse antibiotics in AD children, which results in a delayed treatment until the infection becomes overt. In addition, the increased pH in patients with AD might promote the development of pathogenic strains of staph. However, ceramide-based moisturizers could help inhibit these strains by increasing skin acidity.
For patients who have poor AD control with standard therapy, antibiotics may be used as adjunctive therapy. “Consider bleach baths and/or staph decolonization with mupirocin, both of which led to significant improvement in eczema severity compared to placebo,” Dr. Kress said. “Bleach may also have an anti-inflammatory effect.”
Dr. Kress disclosed relationships with Pfizer, Amgen, and Sanofi/Regeneron. SDEF and this news organization are owned by Frontline Medical Communications.
including allergic rhinitis and asthma, according to Douglas W. Kress, MD, of the department of dermatology at the University of Pittsburgh.
Recent studies suggest that atopic dermatitis (AD) affects 10%-17% of the U.S. population, and 80%-90% of patients are diagnosed by the age of 5 years, Dr. Kress said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“There seem to be multiple pathways, which initiate and perpetuate the cutaneous inflammation of AD including exposure to allergens, irritants, and physical trauma, infection, stress, extremes in temperature and humidity,” Dr. Kress said. In addition, foods and airborne allergens may trigger AD.
Many parents may believe that certain factors are associated with AD, but most of these perceptions are not supported by evidence, said Dr. Kress, who is also chief of the division of pediatric dermatology at the Children’s Hospital of Pittsburgh. AD appears to be a disorder of T cell dysregulation dominated by Th2 lesions in acute cases and Th1 inflammation in patients with chronic lesions.
No associations have been proven between the development of AD in the first 18 months of life and any maternal dietary restrictions, according to a recent Cochrane review, nor is there evidence for an association between AD and the introduction of solid foods, exposure to fish oil, or exposure to animals at a young age, he said. In addition, a study published in 2016 showed a lack of evidence to support the use of specific allergen immunotherapy for AD.
However, evidence does support an association between the presence of AD in children and certain other conditions, Dr. Kress said. “Other associations include an increased incidence of alopecia areata, a threefold increase in autism spectrum disorders, and a twofold increase in ADHD in children with atopic dermatitis.”
The only known food allergy linked to AD severity is egg whites; reducing egg white exposure has been shown to improve AD in children with both conditions, he noted.
Although many patients with AD experience annoying but relatively mild symptoms, health care providers should be alert to the potential for infections, particularly with Staphylococcus aureus, and remember that an active egg white allergy has been associated with staphylococcal superantigen sensitization, said Dr. Kress. The increased risk for S. aureus in children with AD may stem from a tendency to underuse antibiotics in AD children, which results in a delayed treatment until the infection becomes overt. In addition, the increased pH in patients with AD might promote the development of pathogenic strains of staph. However, ceramide-based moisturizers could help inhibit these strains by increasing skin acidity.
For patients who have poor AD control with standard therapy, antibiotics may be used as adjunctive therapy. “Consider bleach baths and/or staph decolonization with mupirocin, both of which led to significant improvement in eczema severity compared to placebo,” Dr. Kress said. “Bleach may also have an anti-inflammatory effect.”
Dr. Kress disclosed relationships with Pfizer, Amgen, and Sanofi/Regeneron. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Study offers snapshot of esophageal strictures in EB patients
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
REPORTING FROM SPD 2018
Key clinical point: Esophageal strictures are common complications of patients with severe types of epidermolysis bullosa.
Major finding: Most epidermolysis bullosa patients (85.5%) presented with dysphagia, while the preferred method of evaluation was video fluoroscopy (57.7%).
Study details: A multicenter study of 497 stricture events in 125 patients with epidermolysis bullosa.
Disclosures: The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. Dr. Pope reported having no financial disclosures.
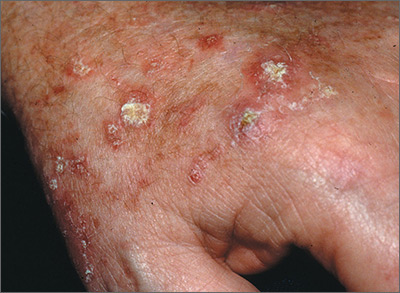
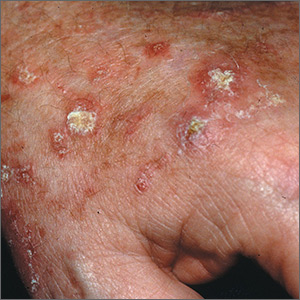
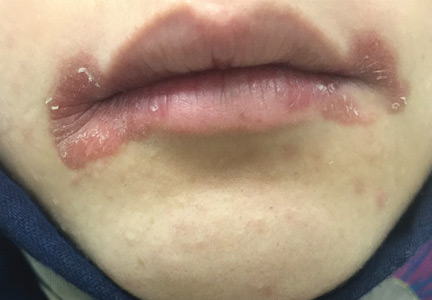
Rough skin on hands
The FP recognized that the lesions on the back of the hands were due to sun damage and included actinic keratosis. However, he was concerned that some of the thicker lesions could be squamous cell carcinoma or SCC in situ.
He discussed options with the patient, which included biopsy of the thickest lesions, cryotherapy, and/or field treatment with a topical agent. The decision was made to biopsy the 2 thickest and whitest lesions and do cryotherapy on some of the other lesions. Shave biopsies were performed and the patient was encouraged to wear sunscreen and minimize his sun exposure. (See the Watch & Learn video on “Shave biopsy.”) On the follow-up visit 2 weeks later, the FP noted that the biopsies and frozen areas were healing. Both biopsies were hypertrophic actinic keratoses only.
The FP then prescribed 5-fluorouracil to be used for 3 to 4 weeks on the remaining lesions on the backs of his hands and forearms. The patient was directed to treat the right arm first to allow more time for the left hand to heal. He was told to stop the 5-fluorouracil if the area became too painful. The patient was also told to treat his left arm the same way after allowing the right arm to heal. Any areas of skin ulceration could be treated with plain petrolatum, but no topical steroids are needed.
Six months later, the patient’s arms and hands looked much better. The patient required regular follow-up.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP recognized that the lesions on the back of the hands were due to sun damage and included actinic keratosis. However, he was concerned that some of the thicker lesions could be squamous cell carcinoma or SCC in situ.
He discussed options with the patient, which included biopsy of the thickest lesions, cryotherapy, and/or field treatment with a topical agent. The decision was made to biopsy the 2 thickest and whitest lesions and do cryotherapy on some of the other lesions. Shave biopsies were performed and the patient was encouraged to wear sunscreen and minimize his sun exposure. (See the Watch & Learn video on “Shave biopsy.”) On the follow-up visit 2 weeks later, the FP noted that the biopsies and frozen areas were healing. Both biopsies were hypertrophic actinic keratoses only.
The FP then prescribed 5-fluorouracil to be used for 3 to 4 weeks on the remaining lesions on the backs of his hands and forearms. The patient was directed to treat the right arm first to allow more time for the left hand to heal. He was told to stop the 5-fluorouracil if the area became too painful. The patient was also told to treat his left arm the same way after allowing the right arm to heal. Any areas of skin ulceration could be treated with plain petrolatum, but no topical steroids are needed.
Six months later, the patient’s arms and hands looked much better. The patient required regular follow-up.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP recognized that the lesions on the back of the hands were due to sun damage and included actinic keratosis. However, he was concerned that some of the thicker lesions could be squamous cell carcinoma or SCC in situ.
He discussed options with the patient, which included biopsy of the thickest lesions, cryotherapy, and/or field treatment with a topical agent. The decision was made to biopsy the 2 thickest and whitest lesions and do cryotherapy on some of the other lesions. Shave biopsies were performed and the patient was encouraged to wear sunscreen and minimize his sun exposure. (See the Watch & Learn video on “Shave biopsy.”) On the follow-up visit 2 weeks later, the FP noted that the biopsies and frozen areas were healing. Both biopsies were hypertrophic actinic keratoses only.
The FP then prescribed 5-fluorouracil to be used for 3 to 4 weeks on the remaining lesions on the backs of his hands and forearms. The patient was directed to treat the right arm first to allow more time for the left hand to heal. He was told to stop the 5-fluorouracil if the area became too painful. The patient was also told to treat his left arm the same way after allowing the right arm to heal. Any areas of skin ulceration could be treated with plain petrolatum, but no topical steroids are needed.
Six months later, the patient’s arms and hands looked much better. The patient required regular follow-up.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
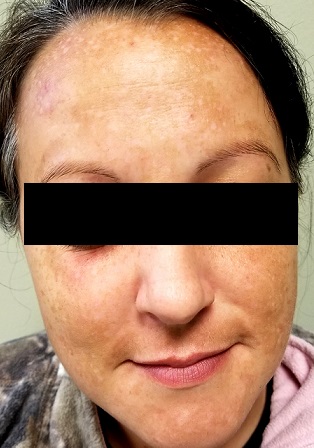
The Mother of All Skin Problems
Each time this 32-year-old woman has a baby—she’s had four to date—she notices that sections of her face darken. Early on, she observed a pattern in which the coming of winter coincided with a lightening of these affected areas—but now the effect lasts year-round, with progressive darkening. She has not tried any products (OTC or prescription) for this problem.
Growing up in the South, the patient and her family spent most summers boating, swimming, and fishing. Her use of sunscreen was sporadic, but she would tan easily regardless.
Her health is good, aside from a 15-year history of smoking.
EXAMINATION
There is excessive hyperpigmentation (brown) on the patient’s face. It follows a mask-like pattern, including her maxilla and the periphery of her face.
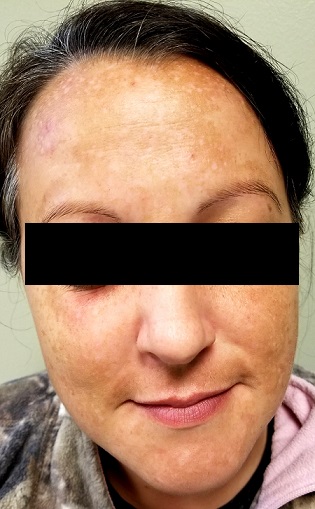
Elsewhere, there is abundant evidence of excessive sun exposure, with focal hyperpigmentation and telangiectasias on her arms. She has type IV skin, consistent with her Native American ancestry.
What is the diagnosis?
DISCUSSION
Melasma, also known as chloasma and dubbed the “mask of pregnancy,” is an extremely common problem that results from a combination of naturally dark skin, lots of sun exposure, and increased levels of estrogenic hormones. The latter can result from pregnancy or from oral contraceptive or estrogen replacement therapy use. Another precipitating factor is thyroid disease, certain types of which lead to an increase in melanocytic stimulating hormone.
Melasma, as one might expect, is seen almost exclusively in women, though a rare male is affected. It is especially common among Latina, Native American, and African-American women, whose melanocytes are especially able to produce pigment.
There are several treatments for melasma, none of them perfect, including tretinoin, azelaic acid, chemical peels, dermabrasion, and lasers. The most common treatment is hydroquinone cream, available in the US in both OTC (2%) and prescription (4%) strengths. However, hydroquinone is available OTC in stronger formulations (15% to 20%) in many Central and South American countries; unfortunately, many women who obtain and use these products experience exogenous ochronosis—a worsening or even precipitation of melasma, resulting from excessive production of tyrosinase.
Any treatment must be used in conjunction with rigorous sunscreen application. A full-spectrum product, with titanium dioxide and zinc oxide as the only active ingredients, must be used, because chemical-laden sunscreens don’t do as good a job covering UVA, UVB, and visible light. Convincing women who are unaccustomed to needing sunscreen to use it religiously is part of what makes treating melasma difficult.
The differential for melasma includes postinflammatory hyperpigmentation (eg, following an episode of contact dermatitis) and simple solar lentigines.
This patient was treated with hydroquinone 4% cream bid, plus sunscreen. She was also given information about other treatment options, such as laser and dermabrasion.
TAKE-HOME LEARNING POINTS
- Melasma, also known at chloasma, is quite common, especially among women with darker skin who live in sunny parts of the world.
- It results from a combination of dark skin, an increased level of estrogenic hormones (eg, with pregnancy, birth control pills, or estrogen replacement therapy), and excessive exposure to UV light.
- While hydroquinone cream can be an effective treatment, the maximum strength should be 4%; overuse of stronger concentrations (available in other countries, such as Mexico), can actually cause melasma to worsen.
Each time this 32-year-old woman has a baby—she’s had four to date—she notices that sections of her face darken. Early on, she observed a pattern in which the coming of winter coincided with a lightening of these affected areas—but now the effect lasts year-round, with progressive darkening. She has not tried any products (OTC or prescription) for this problem.
Growing up in the South, the patient and her family spent most summers boating, swimming, and fishing. Her use of sunscreen was sporadic, but she would tan easily regardless.
Her health is good, aside from a 15-year history of smoking.
EXAMINATION
There is excessive hyperpigmentation (brown) on the patient’s face. It follows a mask-like pattern, including her maxilla and the periphery of her face.

Elsewhere, there is abundant evidence of excessive sun exposure, with focal hyperpigmentation and telangiectasias on her arms. She has type IV skin, consistent with her Native American ancestry.
What is the diagnosis?
DISCUSSION
Melasma, also known as chloasma and dubbed the “mask of pregnancy,” is an extremely common problem that results from a combination of naturally dark skin, lots of sun exposure, and increased levels of estrogenic hormones. The latter can result from pregnancy or from oral contraceptive or estrogen replacement therapy use. Another precipitating factor is thyroid disease, certain types of which lead to an increase in melanocytic stimulating hormone.
Melasma, as one might expect, is seen almost exclusively in women, though a rare male is affected. It is especially common among Latina, Native American, and African-American women, whose melanocytes are especially able to produce pigment.
There are several treatments for melasma, none of them perfect, including tretinoin, azelaic acid, chemical peels, dermabrasion, and lasers. The most common treatment is hydroquinone cream, available in the US in both OTC (2%) and prescription (4%) strengths. However, hydroquinone is available OTC in stronger formulations (15% to 20%) in many Central and South American countries; unfortunately, many women who obtain and use these products experience exogenous ochronosis—a worsening or even precipitation of melasma, resulting from excessive production of tyrosinase.
Any treatment must be used in conjunction with rigorous sunscreen application. A full-spectrum product, with titanium dioxide and zinc oxide as the only active ingredients, must be used, because chemical-laden sunscreens don’t do as good a job covering UVA, UVB, and visible light. Convincing women who are unaccustomed to needing sunscreen to use it religiously is part of what makes treating melasma difficult.
The differential for melasma includes postinflammatory hyperpigmentation (eg, following an episode of contact dermatitis) and simple solar lentigines.
This patient was treated with hydroquinone 4% cream bid, plus sunscreen. She was also given information about other treatment options, such as laser and dermabrasion.
TAKE-HOME LEARNING POINTS
- Melasma, also known at chloasma, is quite common, especially among women with darker skin who live in sunny parts of the world.
- It results from a combination of dark skin, an increased level of estrogenic hormones (eg, with pregnancy, birth control pills, or estrogen replacement therapy), and excessive exposure to UV light.
- While hydroquinone cream can be an effective treatment, the maximum strength should be 4%; overuse of stronger concentrations (available in other countries, such as Mexico), can actually cause melasma to worsen.
Each time this 32-year-old woman has a baby—she’s had four to date—she notices that sections of her face darken. Early on, she observed a pattern in which the coming of winter coincided with a lightening of these affected areas—but now the effect lasts year-round, with progressive darkening. She has not tried any products (OTC or prescription) for this problem.
Growing up in the South, the patient and her family spent most summers boating, swimming, and fishing. Her use of sunscreen was sporadic, but she would tan easily regardless.
Her health is good, aside from a 15-year history of smoking.
EXAMINATION
There is excessive hyperpigmentation (brown) on the patient’s face. It follows a mask-like pattern, including her maxilla and the periphery of her face.

Elsewhere, there is abundant evidence of excessive sun exposure, with focal hyperpigmentation and telangiectasias on her arms. She has type IV skin, consistent with her Native American ancestry.
What is the diagnosis?
DISCUSSION
Melasma, also known as chloasma and dubbed the “mask of pregnancy,” is an extremely common problem that results from a combination of naturally dark skin, lots of sun exposure, and increased levels of estrogenic hormones. The latter can result from pregnancy or from oral contraceptive or estrogen replacement therapy use. Another precipitating factor is thyroid disease, certain types of which lead to an increase in melanocytic stimulating hormone.
Melasma, as one might expect, is seen almost exclusively in women, though a rare male is affected. It is especially common among Latina, Native American, and African-American women, whose melanocytes are especially able to produce pigment.
There are several treatments for melasma, none of them perfect, including tretinoin, azelaic acid, chemical peels, dermabrasion, and lasers. The most common treatment is hydroquinone cream, available in the US in both OTC (2%) and prescription (4%) strengths. However, hydroquinone is available OTC in stronger formulations (15% to 20%) in many Central and South American countries; unfortunately, many women who obtain and use these products experience exogenous ochronosis—a worsening or even precipitation of melasma, resulting from excessive production of tyrosinase.
Any treatment must be used in conjunction with rigorous sunscreen application. A full-spectrum product, with titanium dioxide and zinc oxide as the only active ingredients, must be used, because chemical-laden sunscreens don’t do as good a job covering UVA, UVB, and visible light. Convincing women who are unaccustomed to needing sunscreen to use it religiously is part of what makes treating melasma difficult.
The differential for melasma includes postinflammatory hyperpigmentation (eg, following an episode of contact dermatitis) and simple solar lentigines.
This patient was treated with hydroquinone 4% cream bid, plus sunscreen. She was also given information about other treatment options, such as laser and dermabrasion.
TAKE-HOME LEARNING POINTS
- Melasma, also known at chloasma, is quite common, especially among women with darker skin who live in sunny parts of the world.
- It results from a combination of dark skin, an increased level of estrogenic hormones (eg, with pregnancy, birth control pills, or estrogen replacement therapy), and excessive exposure to UV light.
- While hydroquinone cream can be an effective treatment, the maximum strength should be 4%; overuse of stronger concentrations (available in other countries, such as Mexico), can actually cause melasma to worsen.
Foster cultural competence when examining hair, scalp of ethnic patients
LAKE TAHOE, CALIF. – The way Susan C. Taylor, MD, sees it,
At the annual meeting of the Society for Pediatric Dermatology, Dr. Taylor, a dermatologist at the University of Pennsylvania, Philadelphia, defined culturally competent care as a patient-centered approach in which clinicians establish a rapport with the patient and the caregiver. “It’s important that we ask the right questions,” she said. “In doing so, we have to be familiar with common hair care practices. It’s important that we respect our patients’ values, their goals, their health needs, and, of course, their cultural background. Finally, we have to engage in shared decision making. That’s where we can improve compliance and lead to an overall very satisfactory patient visit.”
To illustrate this point, she discussed the case of a four-year-old black female with a 9-month history of bad dandruff. The child’s mother reports thick flakes that never go away. She takes pride in caring for her daughter’s hair, and shampoos it every two weeks. “She tells me that during the 2.5 hours that it takes her on Saturdays to shampoo, detangle, and comb her daughter’s hair, which she then braids or cornrows and adorns with barrettes or balls, it is a great bonding experience for the two of them,” Dr. Taylor said. “The flakes are temporarily better after she ‘greases’ her daughter’s scalp, but after 2-3 days, they are back.”
In a case like this, Dr. Taylor recommends asking the parent or caregiver to join you while you examine the scalp. This way, the focus becomes the child’s scalp, and the parent is not just staring at your expressions. “The child also observes the pediatric dermatologist and parent/caregiver working together as a team,” she said. “You also want to ask the parent to remove the hair adornments. This makes the child feel more comfortable. It also allows you to observe how the hair is being managed. Are the adornments being removed gently? Is there aggressive pulling of the hair when they take out the braids? Is the child visibly wincing in pain? If the latter two happen this is a teachable moment. You can point out, ‘It looks like Susie is in pain. Let’s do it a little more gently. That might prevent further hair breakage.’ ”
The differential diagnosis of a scaly pediatric scalp includes infrequent shampooing, seborrheic dermatitis, tinea capitis, atopic dermatitis, psoriasis, and sebopsoriasis. The type of hairstyle also factors in. For example, cornrows are a popular hair styling option among ethnic patients. “These are very popular and very time consuming and may lead to infrequent shampooing,” she said. “If they’re put in very tightly or if they have beads or other adornments, it can lead to traction alopecia.” Twists, meanwhile, can create tension on the hair, while puffs can cause traction alopecia if they’re pulled too tightly. “Although dreadlocks are more common among adolescents, we’re seeing them more commonly in young children,” she said. “They can be very long and wavy and lead to traction alopecia.”
The time required to shampoo, detangle, and style tightly coiled African American hair can be significant. For example, in children with cornrows or braids with extensions, Dr. Taylor said that it might require 30 minutes to as long as 2 or 3 hours to remove their current hairstyle, followed by shampooing and conditioning. “Detangling can take at least 15 minutes. In tightly coiled African American hair, studies have demonstrated that detangling while the hair is wet is best, because you have fewer forces on that comb and the hair is less likely to break, as opposed to detangling when the hair is dry. After the wet hair is detangled and the conditioner is rinsed out, a leave-in conditioner is often applied. Then the hair is detangled again, which can take up to an hour, followed by styling, which can take 1-3 hours. That gives you some insight as to why there can often be infrequent shampooing.”
The recommended frequency of shampooing depends on the hairstyle selected. Many children with braids and extensions will have those braids and extensions taken out every six to 12 weeks, “but that doesn’t mean that the scalp can’t be shampooed,” Dr. Taylor said. “The scalp should be shampooed more often. Economics and socioeconomic status play into the frequency of shampooing. For example, if a parent or a caregiver sends a child to a hair stylist, that can range in price from $45 to $65 or more. Time also factors in. In the black community in particular, it’s a ritual on a Saturday to get your hair done. If the parent or caregiver works on weekends, that’s going to impact the frequency of shampooing.”
Dr. Taylor underscored the importance of framing the history-taking process to avoid common pitfall questions like “Do you wash the child’s hair every day or every other day?” or “Do you use dandruff shampoo every day?” It is important to remember that “the parent’s inherent perception is of a doctor who does not have my hair probably does not understand my hair or my child’s hair,” she said. “It’s unlikely that you’re going to find a parent who shampoos their child’s hair every day or every other day. Maybe once a week, probably biweekly. It’s important to ask culturally competent questions.”
She also advises against asking about shampooing when you’re examining the child’s hair, “because there’s going to be the perception that you may think the scalp is dirty,” Dr. Taylor explained. “You probably want to ask that when gathering the history of present illness. The culturally competent question is going to be, ‘Do you wash her/his hair weekly, every other week, monthly, or does it depend on the hair style?’ ”
Body language is also important. “Don’t lean in from afar when examining the patient,” she said. “Get up close and touch the child’s hair.” If you choose to wear surgical gloves for the exam “don’t hold your hands in the surgical scrub position,” she recommended. “Hold your hands in a more neutral position. I think it’s important to touch the hair.”
Referring back to the 4-year-old black child with bad dandruff, she said that a diagnosis of seborrheic dermatitis is unlikely since that condition usually occurs during puberty. “You should have a high index of suspicion for tinea capitis,” she said. “If the patient has occipital lymphadenopathy plus scaling of the scalp or alopecia, that’s enough to presumptively treat for tinea capitis. There are studies that support that.”
For established tinea capitis, Dr. Taylor advises parents to wash barrettes and other hair adornments in hot soapy water or in the dishwasher. She also recommends disposal of hair oil, pomade, and grease and shampooing the child’s hair with ketoconazole and use a conditioner to decrease household and patient spread, which decreases transmissible fungal spores. “There’s a misperception that the application of hair oils and grease can increase the rate of tinea capitis,” she noted. “That’s not true. However, if hair grease and hair oil is applied to the scalp within one week of culture, it could produce a false negative culture.”
For established seborrheic dermatitis, antifungal shampoos including ketoconazole, ciclopirox, and selenium sulfide may be too drying for ethnic hair, “which already has a propensity to break,” she said. “Instead, we recommend a 5-10 minute scalp contact time with the shampoo and avoid contact with strands of hair. Shampoo hair strands with a conditioning shampoo followed by a conditioner to limit hair breakage. We suggest once weekly or biweekly shampooing.”
Dr. Taylor disclosed that she has advisory board and/or investigator relationships with Aclaris Therapeutics, Allergan, Beiersdorf, Croma Pharmaceuticals, Galderma, Isdin, Johnson & Johnson, and Unilever. She also acknowledged Candrice R. Heath, MD, a dermatologist based in Newark, Delaware, for her assistance with the presentation content.
dbrunk@mdedge.com
LAKE TAHOE, CALIF. – The way Susan C. Taylor, MD, sees it,
At the annual meeting of the Society for Pediatric Dermatology, Dr. Taylor, a dermatologist at the University of Pennsylvania, Philadelphia, defined culturally competent care as a patient-centered approach in which clinicians establish a rapport with the patient and the caregiver. “It’s important that we ask the right questions,” she said. “In doing so, we have to be familiar with common hair care practices. It’s important that we respect our patients’ values, their goals, their health needs, and, of course, their cultural background. Finally, we have to engage in shared decision making. That’s where we can improve compliance and lead to an overall very satisfactory patient visit.”
To illustrate this point, she discussed the case of a four-year-old black female with a 9-month history of bad dandruff. The child’s mother reports thick flakes that never go away. She takes pride in caring for her daughter’s hair, and shampoos it every two weeks. “She tells me that during the 2.5 hours that it takes her on Saturdays to shampoo, detangle, and comb her daughter’s hair, which she then braids or cornrows and adorns with barrettes or balls, it is a great bonding experience for the two of them,” Dr. Taylor said. “The flakes are temporarily better after she ‘greases’ her daughter’s scalp, but after 2-3 days, they are back.”
In a case like this, Dr. Taylor recommends asking the parent or caregiver to join you while you examine the scalp. This way, the focus becomes the child’s scalp, and the parent is not just staring at your expressions. “The child also observes the pediatric dermatologist and parent/caregiver working together as a team,” she said. “You also want to ask the parent to remove the hair adornments. This makes the child feel more comfortable. It also allows you to observe how the hair is being managed. Are the adornments being removed gently? Is there aggressive pulling of the hair when they take out the braids? Is the child visibly wincing in pain? If the latter two happen this is a teachable moment. You can point out, ‘It looks like Susie is in pain. Let’s do it a little more gently. That might prevent further hair breakage.’ ”
The differential diagnosis of a scaly pediatric scalp includes infrequent shampooing, seborrheic dermatitis, tinea capitis, atopic dermatitis, psoriasis, and sebopsoriasis. The type of hairstyle also factors in. For example, cornrows are a popular hair styling option among ethnic patients. “These are very popular and very time consuming and may lead to infrequent shampooing,” she said. “If they’re put in very tightly or if they have beads or other adornments, it can lead to traction alopecia.” Twists, meanwhile, can create tension on the hair, while puffs can cause traction alopecia if they’re pulled too tightly. “Although dreadlocks are more common among adolescents, we’re seeing them more commonly in young children,” she said. “They can be very long and wavy and lead to traction alopecia.”
The time required to shampoo, detangle, and style tightly coiled African American hair can be significant. For example, in children with cornrows or braids with extensions, Dr. Taylor said that it might require 30 minutes to as long as 2 or 3 hours to remove their current hairstyle, followed by shampooing and conditioning. “Detangling can take at least 15 minutes. In tightly coiled African American hair, studies have demonstrated that detangling while the hair is wet is best, because you have fewer forces on that comb and the hair is less likely to break, as opposed to detangling when the hair is dry. After the wet hair is detangled and the conditioner is rinsed out, a leave-in conditioner is often applied. Then the hair is detangled again, which can take up to an hour, followed by styling, which can take 1-3 hours. That gives you some insight as to why there can often be infrequent shampooing.”
The recommended frequency of shampooing depends on the hairstyle selected. Many children with braids and extensions will have those braids and extensions taken out every six to 12 weeks, “but that doesn’t mean that the scalp can’t be shampooed,” Dr. Taylor said. “The scalp should be shampooed more often. Economics and socioeconomic status play into the frequency of shampooing. For example, if a parent or a caregiver sends a child to a hair stylist, that can range in price from $45 to $65 or more. Time also factors in. In the black community in particular, it’s a ritual on a Saturday to get your hair done. If the parent or caregiver works on weekends, that’s going to impact the frequency of shampooing.”
Dr. Taylor underscored the importance of framing the history-taking process to avoid common pitfall questions like “Do you wash the child’s hair every day or every other day?” or “Do you use dandruff shampoo every day?” It is important to remember that “the parent’s inherent perception is of a doctor who does not have my hair probably does not understand my hair or my child’s hair,” she said. “It’s unlikely that you’re going to find a parent who shampoos their child’s hair every day or every other day. Maybe once a week, probably biweekly. It’s important to ask culturally competent questions.”
She also advises against asking about shampooing when you’re examining the child’s hair, “because there’s going to be the perception that you may think the scalp is dirty,” Dr. Taylor explained. “You probably want to ask that when gathering the history of present illness. The culturally competent question is going to be, ‘Do you wash her/his hair weekly, every other week, monthly, or does it depend on the hair style?’ ”
Body language is also important. “Don’t lean in from afar when examining the patient,” she said. “Get up close and touch the child’s hair.” If you choose to wear surgical gloves for the exam “don’t hold your hands in the surgical scrub position,” she recommended. “Hold your hands in a more neutral position. I think it’s important to touch the hair.”
Referring back to the 4-year-old black child with bad dandruff, she said that a diagnosis of seborrheic dermatitis is unlikely since that condition usually occurs during puberty. “You should have a high index of suspicion for tinea capitis,” she said. “If the patient has occipital lymphadenopathy plus scaling of the scalp or alopecia, that’s enough to presumptively treat for tinea capitis. There are studies that support that.”
For established tinea capitis, Dr. Taylor advises parents to wash barrettes and other hair adornments in hot soapy water or in the dishwasher. She also recommends disposal of hair oil, pomade, and grease and shampooing the child’s hair with ketoconazole and use a conditioner to decrease household and patient spread, which decreases transmissible fungal spores. “There’s a misperception that the application of hair oils and grease can increase the rate of tinea capitis,” she noted. “That’s not true. However, if hair grease and hair oil is applied to the scalp within one week of culture, it could produce a false negative culture.”
For established seborrheic dermatitis, antifungal shampoos including ketoconazole, ciclopirox, and selenium sulfide may be too drying for ethnic hair, “which already has a propensity to break,” she said. “Instead, we recommend a 5-10 minute scalp contact time with the shampoo and avoid contact with strands of hair. Shampoo hair strands with a conditioning shampoo followed by a conditioner to limit hair breakage. We suggest once weekly or biweekly shampooing.”
Dr. Taylor disclosed that she has advisory board and/or investigator relationships with Aclaris Therapeutics, Allergan, Beiersdorf, Croma Pharmaceuticals, Galderma, Isdin, Johnson & Johnson, and Unilever. She also acknowledged Candrice R. Heath, MD, a dermatologist based in Newark, Delaware, for her assistance with the presentation content.
dbrunk@mdedge.com
LAKE TAHOE, CALIF. – The way Susan C. Taylor, MD, sees it,
At the annual meeting of the Society for Pediatric Dermatology, Dr. Taylor, a dermatologist at the University of Pennsylvania, Philadelphia, defined culturally competent care as a patient-centered approach in which clinicians establish a rapport with the patient and the caregiver. “It’s important that we ask the right questions,” she said. “In doing so, we have to be familiar with common hair care practices. It’s important that we respect our patients’ values, their goals, their health needs, and, of course, their cultural background. Finally, we have to engage in shared decision making. That’s where we can improve compliance and lead to an overall very satisfactory patient visit.”
To illustrate this point, she discussed the case of a four-year-old black female with a 9-month history of bad dandruff. The child’s mother reports thick flakes that never go away. She takes pride in caring for her daughter’s hair, and shampoos it every two weeks. “She tells me that during the 2.5 hours that it takes her on Saturdays to shampoo, detangle, and comb her daughter’s hair, which she then braids or cornrows and adorns with barrettes or balls, it is a great bonding experience for the two of them,” Dr. Taylor said. “The flakes are temporarily better after she ‘greases’ her daughter’s scalp, but after 2-3 days, they are back.”
In a case like this, Dr. Taylor recommends asking the parent or caregiver to join you while you examine the scalp. This way, the focus becomes the child’s scalp, and the parent is not just staring at your expressions. “The child also observes the pediatric dermatologist and parent/caregiver working together as a team,” she said. “You also want to ask the parent to remove the hair adornments. This makes the child feel more comfortable. It also allows you to observe how the hair is being managed. Are the adornments being removed gently? Is there aggressive pulling of the hair when they take out the braids? Is the child visibly wincing in pain? If the latter two happen this is a teachable moment. You can point out, ‘It looks like Susie is in pain. Let’s do it a little more gently. That might prevent further hair breakage.’ ”
The differential diagnosis of a scaly pediatric scalp includes infrequent shampooing, seborrheic dermatitis, tinea capitis, atopic dermatitis, psoriasis, and sebopsoriasis. The type of hairstyle also factors in. For example, cornrows are a popular hair styling option among ethnic patients. “These are very popular and very time consuming and may lead to infrequent shampooing,” she said. “If they’re put in very tightly or if they have beads or other adornments, it can lead to traction alopecia.” Twists, meanwhile, can create tension on the hair, while puffs can cause traction alopecia if they’re pulled too tightly. “Although dreadlocks are more common among adolescents, we’re seeing them more commonly in young children,” she said. “They can be very long and wavy and lead to traction alopecia.”
The time required to shampoo, detangle, and style tightly coiled African American hair can be significant. For example, in children with cornrows or braids with extensions, Dr. Taylor said that it might require 30 minutes to as long as 2 or 3 hours to remove their current hairstyle, followed by shampooing and conditioning. “Detangling can take at least 15 minutes. In tightly coiled African American hair, studies have demonstrated that detangling while the hair is wet is best, because you have fewer forces on that comb and the hair is less likely to break, as opposed to detangling when the hair is dry. After the wet hair is detangled and the conditioner is rinsed out, a leave-in conditioner is often applied. Then the hair is detangled again, which can take up to an hour, followed by styling, which can take 1-3 hours. That gives you some insight as to why there can often be infrequent shampooing.”
The recommended frequency of shampooing depends on the hairstyle selected. Many children with braids and extensions will have those braids and extensions taken out every six to 12 weeks, “but that doesn’t mean that the scalp can’t be shampooed,” Dr. Taylor said. “The scalp should be shampooed more often. Economics and socioeconomic status play into the frequency of shampooing. For example, if a parent or a caregiver sends a child to a hair stylist, that can range in price from $45 to $65 or more. Time also factors in. In the black community in particular, it’s a ritual on a Saturday to get your hair done. If the parent or caregiver works on weekends, that’s going to impact the frequency of shampooing.”
Dr. Taylor underscored the importance of framing the history-taking process to avoid common pitfall questions like “Do you wash the child’s hair every day or every other day?” or “Do you use dandruff shampoo every day?” It is important to remember that “the parent’s inherent perception is of a doctor who does not have my hair probably does not understand my hair or my child’s hair,” she said. “It’s unlikely that you’re going to find a parent who shampoos their child’s hair every day or every other day. Maybe once a week, probably biweekly. It’s important to ask culturally competent questions.”
She also advises against asking about shampooing when you’re examining the child’s hair, “because there’s going to be the perception that you may think the scalp is dirty,” Dr. Taylor explained. “You probably want to ask that when gathering the history of present illness. The culturally competent question is going to be, ‘Do you wash her/his hair weekly, every other week, monthly, or does it depend on the hair style?’ ”
Body language is also important. “Don’t lean in from afar when examining the patient,” she said. “Get up close and touch the child’s hair.” If you choose to wear surgical gloves for the exam “don’t hold your hands in the surgical scrub position,” she recommended. “Hold your hands in a more neutral position. I think it’s important to touch the hair.”
Referring back to the 4-year-old black child with bad dandruff, she said that a diagnosis of seborrheic dermatitis is unlikely since that condition usually occurs during puberty. “You should have a high index of suspicion for tinea capitis,” she said. “If the patient has occipital lymphadenopathy plus scaling of the scalp or alopecia, that’s enough to presumptively treat for tinea capitis. There are studies that support that.”
For established tinea capitis, Dr. Taylor advises parents to wash barrettes and other hair adornments in hot soapy water or in the dishwasher. She also recommends disposal of hair oil, pomade, and grease and shampooing the child’s hair with ketoconazole and use a conditioner to decrease household and patient spread, which decreases transmissible fungal spores. “There’s a misperception that the application of hair oils and grease can increase the rate of tinea capitis,” she noted. “That’s not true. However, if hair grease and hair oil is applied to the scalp within one week of culture, it could produce a false negative culture.”
For established seborrheic dermatitis, antifungal shampoos including ketoconazole, ciclopirox, and selenium sulfide may be too drying for ethnic hair, “which already has a propensity to break,” she said. “Instead, we recommend a 5-10 minute scalp contact time with the shampoo and avoid contact with strands of hair. Shampoo hair strands with a conditioning shampoo followed by a conditioner to limit hair breakage. We suggest once weekly or biweekly shampooing.”
Dr. Taylor disclosed that she has advisory board and/or investigator relationships with Aclaris Therapeutics, Allergan, Beiersdorf, Croma Pharmaceuticals, Galderma, Isdin, Johnson & Johnson, and Unilever. She also acknowledged Candrice R. Heath, MD, a dermatologist based in Newark, Delaware, for her assistance with the presentation content.
dbrunk@mdedge.com
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
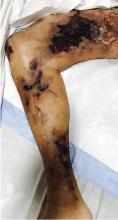
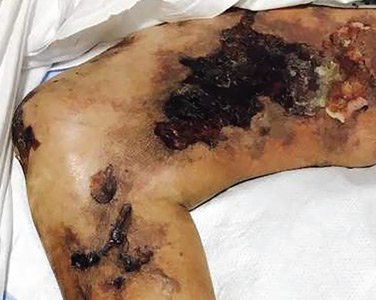
Calcific uremic arteriolopathy
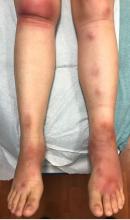
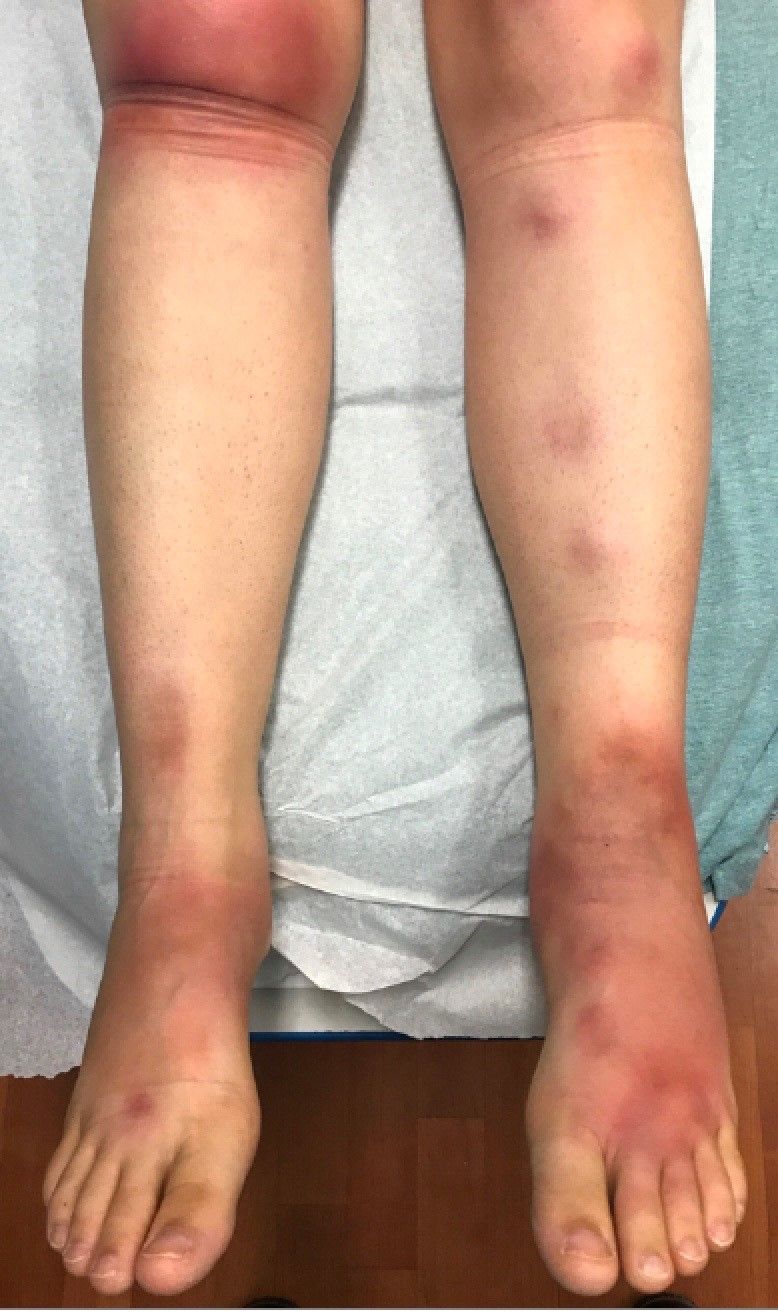
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
A 51-year-old man with end-stage renal disease, on peritoneal dialysis for the past 4 years, presented to the emergency department with severe pain in both legs. The pain had started 2 months previously and had progressively worsened. After multiple admissions in the past for hyperkalemia and volume overload due to noncompliance, he had been advised to switch to hemodialysis.
See related article and editorial
Laboratory analysis revealed the following values:
- Serum creatinine 12.62 mg/dL (reference range 0.73–1.22)
- Blood urea nitrogen 159 mg/dL (9–24)
- Serum calcium corrected for serum albumin 8.1 mg/dL (8.4–10.0)
- Serum phosphorus 10.6 mg/dL (2.7–4.8).
His history of end-stage renal disease, failure of peritoneal dialysis, high calcium-phosphorus product (8.1 mg/dL × 10.6 mg/dL = 85.9 mg2/dL2, reference range ≤ 55), and characteristic physical findings led to the diagnosis of calcific uremic arteriolopathy.
CALCIFIC UREMIC ARTERIOLOPATHY
Calcific uremic arteriolopathy or “calciphylaxis,” seen most often in patients with end-stage renal disease, is caused by calcium deposition in the media of the dermo-hypodermic arterioles, leading to infarction of adjacent tissue.1–3 A high calcium-phosphorus product (> 55) has been implicated in its development; however, the calcium-phosphorus product can be normal despite hyperphosphatemia, which itself may promote ectopic calcification.
Early ischemic manifestations include livedo reticularis and painful retiform purpura on the thighs and other areas of high adiposity. Lesions evolve into violaceous plaquelike subcutaneous nodules that can infarct, become necrotic, ulcerate, and become infected. Punch biopsy demonstrating arteriolar calcification, subintimal fibrosis, and thrombosis confirms the diagnosis.
Differential diagnosis
Warfarin necrosis can cause large, irregular, bloody bullae that ulcerate and turn into eschar that may resemble lesions of calcific uremic arteriolopathy. Our patient, however, had no exposure to warfarin.
Pemphigus foliaceus, an immunoglobulin G4-mediated autoimmune disorder targeted against desmoglein-1, leads to the formation of fragile blisters that easily rupture when rubbed (Nikolsky sign). Lesions evolve into scaling, crusty erosions on an erythematous base. With tender blisters and lack of mucous membrane involvement, pemphigus foliaceus shares similarities with calcific uremic arteriolopathy, but the presence of necrotic eschar surrounded by violaceous plaques in our patient made it an unlikely diagnosis.
Cryofibrinogenemia. In the right clinical scenario, ie, in a patient with vasculitis, malignancy, infection, cryoglobulinemia, or collagen diseases, cryofibrinogen-mediated cold-induced occlusive lesions may mimic calcific uremic arteriolopathy, with painful or pruritic erythema, purpura, livedo reticularis, necrosis, and ulceration.4 Our patient had no color changes with exposure to cold, nor any history of Raynaud phenomenon or joint pain, making the diagnosis of cryofibrinogenemia less likely.
Nephrogenic systemic fibrosis. Gadolinium contrast medium in magnetic resonance imaging can cause nephrogenic systemic fibrosis, characterized by erythematous papules that coalesce into brawny plaques with surrounding woody induration, which may resemble lesions of calcific uremic arteriolopathy.5 However, our patient had not been exposed to gadolinium.
Management
Management is multidisciplinary and includes the following1:
- Hemodialysis, modified to optimize calcium balance2
- Intravenous sodium thiosulfate: the exact mechanism of action remains unclear, but it is thought to play a role in chelating calcium from tissue deposits, thus decreasing pain and promoting regression of skin lesions3
- Wound care, including chemical debridement agents, negative-pressure wound therapy, and surgical debridement for infected wounds6
- Pain management with opioid analgesics.
The patient was treated with all these measures. However, he died of sudden cardiac arrest during the same admission.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56(4):569–579. doi:10.1016/j.jaad.2006.08.065
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66(1):133–146. doi:10.1053/j.ajkd.2015.01.034
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis 2000; 35(4):588–597. pmid:10739777
- Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol 2013; 19(3):142–148. doi:10.1097/RHU.0b013e318289e06e
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006; 18(6):614–617. doi:10.1097/01.bor.0000245725.94887.8d
- Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill Professional; 2003:558–562.
Palmoplantar exanthema and liver dysfunction
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
Angular cheilitis induced by iron deficiency anemia
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
What is your diagnosis?
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
A 16-year-old female came to the dermatology clinic for acne follow-up. She reported some improvement on her acne since she started taking OCs. She also had been using benzoyl peroxide and tretinoin on her face. In addition to the acne, she also wanted us to check some tender bumps she had been getting on her shins after she came back from a camping trip. Initially she thought they were bug bites, but the lesions were getting larger, more tender, and not improving with diphenhydramine.
The physical exam did not reveal acute distress. She was afebrile. On skin examination, she had comedones, papules and scars on her face, chest, and back. On her shins there were several erythematous tender nodules and plaques. There was no edema on her legs and pulses were present.