User login
Recent Evidence for Home Phototherapy Benefits May Improve Access for Patients with Psoriasis
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Merkel Cell: Immunotherapy Not Used for Many Patients With Metastatic Disease
PHOENIX — Immunotherapy has revolutionized outcomes for patients are better at high-volume centers.
The study has important implications, said study author Shayan Cheraghlou, MD, an incoming fellow in Mohs surgery at New York University, New York City. “We can see that in a real-world setting, these agents have an impact on survival,” he said. “We also found high-volume centers were significantly more likely to use the agents than low-volume centers.” He presented the findings at the annual meeting of the American College of Mohs Surgery.
MCC is a neuroendocrine skin cancer with a high rate of mortality, and even though it remains relatively rare, its incidence has been rising rapidly since the late 1990s and continues to increase. There were no approved treatments available until 2017, when the US Food and Drug Administration (FDA) approved the immunotherapy drug avelumab (Bavencio) to treat advanced MCC. Two years later, pembrolizumab (Keytruda) also received regulatory approval for MCC, and these two agents have revolutionized outcomes.
“In clinical trial settings, these agents led to significant and durable responses, and they are now the recommended treatments in guidelines for metastatic Merkel cell carcinoma,” said Dr. Cheraghlou. “However, we don’t have data as to how they are being used in the real-world setting and if survival outcomes are similar.”
Real World vs Clinical Trials
Real-world outcomes can differ from clinical trial data, and the adoption of novel therapeutics can be gradual. The goal of this study was to see if clinical trial data matched what was being observed in actual clinical use and if the agents were being used uniformly in centers across the United States.
The authors used data from the National Cancer Database that included patients diagnosed with cancer from 2004 to 2019 and identified 1017 adult cases of metastatic MCC. They then looked at the association of a variety of patient characteristics, tumors, and system factors with the likelihood of receiving systemic treatment for their disease.
“Our first finding was maybe the least surprising,” he said. “Patients who received these therapeutic agents had significantly improved survival compared to those who have not.”
Those who received immunotherapy had a 35% decrease in the risk for death per year compared with those who did not. The 1-, 3-, and 5-year survival rates were 47.2%, 21.8%, and 16.5%, respectively, for patients who did not receive immunotherapy compared with 62.7%, 34.4%, and 23.6%, respectively, for those who were treated with these agents.
Dr. Cheraghlou noted that they started to get some “surprising” findings when they looked at utilization data. “While it has been increasing over time, it is not as high as it should be,” he emphasized.
From 2017 to 2019, 54.2% of patients with metastatic MCC received immunotherapy. The data also showed an increase in use from 45.1% in 2017 to 63.0% in 2019. “This is an effective treatment for aggressive malignancy, so we have to ask why more patients aren’t getting them,” said Dr. Cheraghlou.
Their findings did suggest one possible reason, and that was that high-volume centers were significantly more likely to use the agents than low-volume centers. Centers that were in the top percentile for MCC case volume were three times as likely to use immunotherapy for MCC compared with other institutions. “So, if you have metastatic Merkel cell carcinoma and go to a low volume center, you may be less likely to get potential lifesaving treatment,” he noted.
Implications Going Forward
Dr. Cheraghlou concluded his presentation by pointing out that this study has important implications. The data showed that in a real-world setting, these agents have an impact on survival, but all eligible patients do not have access. “In other countries, there are established referral patterns for all patients with aggressive rare malignancies and really all cancers,” he added. “But in the US, cancer care is more decentralized. Studies like this and others show that high-volume centers have much better outcomes for aggressive rare malignancies, and we should be looking at why this is the case and mitigating these disparities and outcomes.”
Commenting on the study results, Jeffrey M. Farma, MD, co-director of the Melanoma and Skin Cancer Program and professor of surgical oncology at Fox Chase Cancer Center, Philadelphia, referred to the two immunotherapies that have been approved for MCC since 2017, which have demonstrated a survival benefit and improved outcomes in patients with metastatic MCC.
“In their study, immunotherapy was associated with improved outcomes,” said Dr. Farma. “This study highlights the continued lag of implementation of guidelines when new therapies are approved, and that for rare cancers like Merkel cell carcinoma, being treated at high-volume centers and the regionalization of care can lead to improved outcomes for patients.”
Dr. Cheraghlou and Dr. Farma had no disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — Immunotherapy has revolutionized outcomes for patients are better at high-volume centers.
The study has important implications, said study author Shayan Cheraghlou, MD, an incoming fellow in Mohs surgery at New York University, New York City. “We can see that in a real-world setting, these agents have an impact on survival,” he said. “We also found high-volume centers were significantly more likely to use the agents than low-volume centers.” He presented the findings at the annual meeting of the American College of Mohs Surgery.
MCC is a neuroendocrine skin cancer with a high rate of mortality, and even though it remains relatively rare, its incidence has been rising rapidly since the late 1990s and continues to increase. There were no approved treatments available until 2017, when the US Food and Drug Administration (FDA) approved the immunotherapy drug avelumab (Bavencio) to treat advanced MCC. Two years later, pembrolizumab (Keytruda) also received regulatory approval for MCC, and these two agents have revolutionized outcomes.
“In clinical trial settings, these agents led to significant and durable responses, and they are now the recommended treatments in guidelines for metastatic Merkel cell carcinoma,” said Dr. Cheraghlou. “However, we don’t have data as to how they are being used in the real-world setting and if survival outcomes are similar.”
Real World vs Clinical Trials
Real-world outcomes can differ from clinical trial data, and the adoption of novel therapeutics can be gradual. The goal of this study was to see if clinical trial data matched what was being observed in actual clinical use and if the agents were being used uniformly in centers across the United States.
The authors used data from the National Cancer Database that included patients diagnosed with cancer from 2004 to 2019 and identified 1017 adult cases of metastatic MCC. They then looked at the association of a variety of patient characteristics, tumors, and system factors with the likelihood of receiving systemic treatment for their disease.
“Our first finding was maybe the least surprising,” he said. “Patients who received these therapeutic agents had significantly improved survival compared to those who have not.”
Those who received immunotherapy had a 35% decrease in the risk for death per year compared with those who did not. The 1-, 3-, and 5-year survival rates were 47.2%, 21.8%, and 16.5%, respectively, for patients who did not receive immunotherapy compared with 62.7%, 34.4%, and 23.6%, respectively, for those who were treated with these agents.
Dr. Cheraghlou noted that they started to get some “surprising” findings when they looked at utilization data. “While it has been increasing over time, it is not as high as it should be,” he emphasized.
From 2017 to 2019, 54.2% of patients with metastatic MCC received immunotherapy. The data also showed an increase in use from 45.1% in 2017 to 63.0% in 2019. “This is an effective treatment for aggressive malignancy, so we have to ask why more patients aren’t getting them,” said Dr. Cheraghlou.
Their findings did suggest one possible reason, and that was that high-volume centers were significantly more likely to use the agents than low-volume centers. Centers that were in the top percentile for MCC case volume were three times as likely to use immunotherapy for MCC compared with other institutions. “So, if you have metastatic Merkel cell carcinoma and go to a low volume center, you may be less likely to get potential lifesaving treatment,” he noted.
Implications Going Forward
Dr. Cheraghlou concluded his presentation by pointing out that this study has important implications. The data showed that in a real-world setting, these agents have an impact on survival, but all eligible patients do not have access. “In other countries, there are established referral patterns for all patients with aggressive rare malignancies and really all cancers,” he added. “But in the US, cancer care is more decentralized. Studies like this and others show that high-volume centers have much better outcomes for aggressive rare malignancies, and we should be looking at why this is the case and mitigating these disparities and outcomes.”
Commenting on the study results, Jeffrey M. Farma, MD, co-director of the Melanoma and Skin Cancer Program and professor of surgical oncology at Fox Chase Cancer Center, Philadelphia, referred to the two immunotherapies that have been approved for MCC since 2017, which have demonstrated a survival benefit and improved outcomes in patients with metastatic MCC.
“In their study, immunotherapy was associated with improved outcomes,” said Dr. Farma. “This study highlights the continued lag of implementation of guidelines when new therapies are approved, and that for rare cancers like Merkel cell carcinoma, being treated at high-volume centers and the regionalization of care can lead to improved outcomes for patients.”
Dr. Cheraghlou and Dr. Farma had no disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — Immunotherapy has revolutionized outcomes for patients are better at high-volume centers.
The study has important implications, said study author Shayan Cheraghlou, MD, an incoming fellow in Mohs surgery at New York University, New York City. “We can see that in a real-world setting, these agents have an impact on survival,” he said. “We also found high-volume centers were significantly more likely to use the agents than low-volume centers.” He presented the findings at the annual meeting of the American College of Mohs Surgery.
MCC is a neuroendocrine skin cancer with a high rate of mortality, and even though it remains relatively rare, its incidence has been rising rapidly since the late 1990s and continues to increase. There were no approved treatments available until 2017, when the US Food and Drug Administration (FDA) approved the immunotherapy drug avelumab (Bavencio) to treat advanced MCC. Two years later, pembrolizumab (Keytruda) also received regulatory approval for MCC, and these two agents have revolutionized outcomes.
“In clinical trial settings, these agents led to significant and durable responses, and they are now the recommended treatments in guidelines for metastatic Merkel cell carcinoma,” said Dr. Cheraghlou. “However, we don’t have data as to how they are being used in the real-world setting and if survival outcomes are similar.”
Real World vs Clinical Trials
Real-world outcomes can differ from clinical trial data, and the adoption of novel therapeutics can be gradual. The goal of this study was to see if clinical trial data matched what was being observed in actual clinical use and if the agents were being used uniformly in centers across the United States.
The authors used data from the National Cancer Database that included patients diagnosed with cancer from 2004 to 2019 and identified 1017 adult cases of metastatic MCC. They then looked at the association of a variety of patient characteristics, tumors, and system factors with the likelihood of receiving systemic treatment for their disease.
“Our first finding was maybe the least surprising,” he said. “Patients who received these therapeutic agents had significantly improved survival compared to those who have not.”
Those who received immunotherapy had a 35% decrease in the risk for death per year compared with those who did not. The 1-, 3-, and 5-year survival rates were 47.2%, 21.8%, and 16.5%, respectively, for patients who did not receive immunotherapy compared with 62.7%, 34.4%, and 23.6%, respectively, for those who were treated with these agents.
Dr. Cheraghlou noted that they started to get some “surprising” findings when they looked at utilization data. “While it has been increasing over time, it is not as high as it should be,” he emphasized.
From 2017 to 2019, 54.2% of patients with metastatic MCC received immunotherapy. The data also showed an increase in use from 45.1% in 2017 to 63.0% in 2019. “This is an effective treatment for aggressive malignancy, so we have to ask why more patients aren’t getting them,” said Dr. Cheraghlou.
Their findings did suggest one possible reason, and that was that high-volume centers were significantly more likely to use the agents than low-volume centers. Centers that were in the top percentile for MCC case volume were three times as likely to use immunotherapy for MCC compared with other institutions. “So, if you have metastatic Merkel cell carcinoma and go to a low volume center, you may be less likely to get potential lifesaving treatment,” he noted.
Implications Going Forward
Dr. Cheraghlou concluded his presentation by pointing out that this study has important implications. The data showed that in a real-world setting, these agents have an impact on survival, but all eligible patients do not have access. “In other countries, there are established referral patterns for all patients with aggressive rare malignancies and really all cancers,” he added. “But in the US, cancer care is more decentralized. Studies like this and others show that high-volume centers have much better outcomes for aggressive rare malignancies, and we should be looking at why this is the case and mitigating these disparities and outcomes.”
Commenting on the study results, Jeffrey M. Farma, MD, co-director of the Melanoma and Skin Cancer Program and professor of surgical oncology at Fox Chase Cancer Center, Philadelphia, referred to the two immunotherapies that have been approved for MCC since 2017, which have demonstrated a survival benefit and improved outcomes in patients with metastatic MCC.
“In their study, immunotherapy was associated with improved outcomes,” said Dr. Farma. “This study highlights the continued lag of implementation of guidelines when new therapies are approved, and that for rare cancers like Merkel cell carcinoma, being treated at high-volume centers and the regionalization of care can lead to improved outcomes for patients.”
Dr. Cheraghlou and Dr. Farma had no disclosures.
A version of this article appeared on Medscape.com.
FROM ACMS 2024
Use of Radiotherapy for Nonmelanoma Skin Cancer Increasing, Study Finds
PHOENIX — More specifically, the persistent growth in the use of superficial radiotherapy (SRT) devices and electronic brachytherapy (eBT) to treat nonmelanoma skin cancer (NMSC) has exceeded that of traditional procedures among dermatologists using these modalities, according to Christian Gronbeck, MD, a resident in dermatology at the University of Connecticut Health Center, Farmington.
“These services increased substantially over the study period,” Dr. Gronbeck said at the annual meeting of the American College of Mohs Surgery, where he presented the results of the study. “Our findings suggest that those using eBT/SRT were frequently general dermatologists and non-fellowship–trained Mohs surgeons who have less formalized surgical training.”
He also noted that billing for these services also rose substantially, which is being driven by growing utilization and an increased SRT payment rate.
Surgical approaches are standard for most NMSC cases, but some patients are not good surgical candidates because of medical comorbidities and/or other factors, and radiotherapy is emerging as a potential treatment option for those patients. Traditionally, radiotherapy was administered by radiation oncologists, but with the growing availability of SRT devices and the introduction of eBT, dermatologists are now treating patients with these modalities.
“It is a potential treatment option for nonmelanoma skin cancer and keloids, and these lower energy devices can be used in the outpatient setting,” said Dr. Gronbeck. “Treatment typically involves a series of fractions over a period of several weeks. There has been recent growth in the use of radiotherapy despite this being a secondary option in skin cancer, primarily when surgery is contraindicated.”
Steady Expansion of Use
Dr. Gronbeck and colleagues sought to gain a better understanding of the use of SRT and eBT for NMSC among dermatologists, as well as trends in cost. Data were obtained from the 2016-2021 Medicare Public Use Files to evaluate the trend in the volume of Medicare Part B claims for eBT (CPT 0394T) and SRT (CPT 77401) by dermatologists, and they also looked at related billable services for radiotherapy.
Of 12,050 dermatologists, 293 (2.4%) were identified as utilizing eBT or SRT in 2021, representing a 75.4% increase from 2016. The usage of both eBT and SRT increased by 59.6% and 148.4%, respectively, from 2016 to 2021.
There were notable geographic differences in the utilization of radiotherapy. “Florida, California, Texas, and Arizona had the highest utilization,” Dr. Gronbeck said, although during the study period, utilization increased in other states, including North Carolina and Alabama.
When looking at geographic regions as a whole, the highest number of dermatologists using radiotherapy were located in the South (n = 143, 50.9%), followed by the West (n = 69, 23.6%). Utilization was more common in metro areas than in nonmetro/rural areas (86% vs 14%).
Differences were also noted among dermatologists. Those who performed eBT/SRT than those who did not were significantly more likely to have had 15 or more years of independent practice (70.1% vs 48.6%), be in a small private dermatology practice (62.7% vs 47.5%), and be non–fellowship-trained Mohs surgeons (33.5% vs 10.2%). Dermatologists utilizing radiotherapy were also more likely to treat Medicare beneficiaries who were older, with a mean age over 75 years (39.3% vs 31.1%) and a mean hierarchical condition category (HCC) score, above the national average (55.2% vs 44.6%).
Dr. Gronbeck and colleagues also looked at cost. The number of direct payments for eBT/SRT payments increased throughout the study period, from 3,678,224 in 2016 to 11,680,925 in 2021, nearly a 218% increase. The change in payments for services related to eBT/SRT, such as radiotherapy simulation, radiotherapy dosing, and ultrasound guidance, increased by 621.4% during this same timeframe.
Radiotherapy in dermatology has primarily been assessed through retrospective studies. “Our findings suggest that eBT and SRT are more frequently utilized by dermatologists managing older and sicker patients, but further studies are needed to identify whether these interventions are truly addressing poor surgical candidates,” Dr. Gronbeck said.
The Centers for Medicare & Medicaid Services (CMS) has recently proposed changes in Medicare coverage in seven states for Image-Guided Superficial Radiation Therapy (image-guided SRT or IGSRT) for the treatment of NMSC. The proposed local coverage determination, or LCD, if finalized in its current form, would affect residents in North Carolina, South Carolina, Virginia, West Virginia, Alabama, Georgia, and Tennessee.
“These changes would mean more restrictive coverage,” said Dr. Gronbeck, and further support the need for “improved clinical data and development of guidelines to support evidence-based utilization.”
Surgical Management Standard, but SRT Has a Role
Asked to comment on the findings, Seemal R. Desai, MD, president of the American Academy of Dermatology (AAD), who was not involved with the study, reiterated that according to this abstract, efficacy has mainly been assessed through retrospective studies, and results are likely inferior to Mohs surgery, require multiple treatment visits, and are associated with significant costs. More study is needed for the use of radiation therapy in dermatology, he told this news organization.
“The Academy supports continued research and studies for therapies that can help improve patient outcomes and offer treatment options, as well as further studies on long-term outcomes for treatments like superficial radiation therapy,” he said.
“Well-designed studies can certainly be helpful to better assess efficacy and outcomes,” Dr. Desai continued. “This is why the Academy supports the idea of scientific studies that continue to expand the body of literature and data, which can help dermatologists tailor therapeutic options for their patients.”
As for general dermatologists using radiation therapy, he pointed out that SRT was developed within the dermatology specialty with dermatologists being the experts in delivering SRT for patients with NMSCs when indicated. “SRT has been used for over 100 years to treat skin cancer,” said Dr. Desai, of the department of dermatology, UT Southwestern Medical Center, Dallas. “While certain radiation devices have historically been used by dermatologists, dermatologists engaged in providing superficial radiation therapy must have adequate education and training to administer this therapy safely and effectively.”
The AAD Association (AADA) has a position statement that supports the use of SRT as an option for the treatment of basal cell carcinoma and squamous cell carcinoma in certain circumstances. “This could be when surgical intervention is contraindicated or refused and after the benefits and risk of treatment alternatives have been discussed with the patient,” he said. “Based on current evidence, surgical management remains the most effective treatment for NMSC.”
Dr. Desai added that the AADA is also concerned that if the Proposed LCD is finalized by CMS, it “could restrict dermatologists from performing SRT and impede patient access to SRT as a potential treatment when indicated.”
The study was independently supported. Dr. Gronbeck and Dr. Desai reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
PHOENIX — More specifically, the persistent growth in the use of superficial radiotherapy (SRT) devices and electronic brachytherapy (eBT) to treat nonmelanoma skin cancer (NMSC) has exceeded that of traditional procedures among dermatologists using these modalities, according to Christian Gronbeck, MD, a resident in dermatology at the University of Connecticut Health Center, Farmington.
“These services increased substantially over the study period,” Dr. Gronbeck said at the annual meeting of the American College of Mohs Surgery, where he presented the results of the study. “Our findings suggest that those using eBT/SRT were frequently general dermatologists and non-fellowship–trained Mohs surgeons who have less formalized surgical training.”
He also noted that billing for these services also rose substantially, which is being driven by growing utilization and an increased SRT payment rate.
Surgical approaches are standard for most NMSC cases, but some patients are not good surgical candidates because of medical comorbidities and/or other factors, and radiotherapy is emerging as a potential treatment option for those patients. Traditionally, radiotherapy was administered by radiation oncologists, but with the growing availability of SRT devices and the introduction of eBT, dermatologists are now treating patients with these modalities.
“It is a potential treatment option for nonmelanoma skin cancer and keloids, and these lower energy devices can be used in the outpatient setting,” said Dr. Gronbeck. “Treatment typically involves a series of fractions over a period of several weeks. There has been recent growth in the use of radiotherapy despite this being a secondary option in skin cancer, primarily when surgery is contraindicated.”
Steady Expansion of Use
Dr. Gronbeck and colleagues sought to gain a better understanding of the use of SRT and eBT for NMSC among dermatologists, as well as trends in cost. Data were obtained from the 2016-2021 Medicare Public Use Files to evaluate the trend in the volume of Medicare Part B claims for eBT (CPT 0394T) and SRT (CPT 77401) by dermatologists, and they also looked at related billable services for radiotherapy.
Of 12,050 dermatologists, 293 (2.4%) were identified as utilizing eBT or SRT in 2021, representing a 75.4% increase from 2016. The usage of both eBT and SRT increased by 59.6% and 148.4%, respectively, from 2016 to 2021.
There were notable geographic differences in the utilization of radiotherapy. “Florida, California, Texas, and Arizona had the highest utilization,” Dr. Gronbeck said, although during the study period, utilization increased in other states, including North Carolina and Alabama.
When looking at geographic regions as a whole, the highest number of dermatologists using radiotherapy were located in the South (n = 143, 50.9%), followed by the West (n = 69, 23.6%). Utilization was more common in metro areas than in nonmetro/rural areas (86% vs 14%).
Differences were also noted among dermatologists. Those who performed eBT/SRT than those who did not were significantly more likely to have had 15 or more years of independent practice (70.1% vs 48.6%), be in a small private dermatology practice (62.7% vs 47.5%), and be non–fellowship-trained Mohs surgeons (33.5% vs 10.2%). Dermatologists utilizing radiotherapy were also more likely to treat Medicare beneficiaries who were older, with a mean age over 75 years (39.3% vs 31.1%) and a mean hierarchical condition category (HCC) score, above the national average (55.2% vs 44.6%).
Dr. Gronbeck and colleagues also looked at cost. The number of direct payments for eBT/SRT payments increased throughout the study period, from 3,678,224 in 2016 to 11,680,925 in 2021, nearly a 218% increase. The change in payments for services related to eBT/SRT, such as radiotherapy simulation, radiotherapy dosing, and ultrasound guidance, increased by 621.4% during this same timeframe.
Radiotherapy in dermatology has primarily been assessed through retrospective studies. “Our findings suggest that eBT and SRT are more frequently utilized by dermatologists managing older and sicker patients, but further studies are needed to identify whether these interventions are truly addressing poor surgical candidates,” Dr. Gronbeck said.
The Centers for Medicare & Medicaid Services (CMS) has recently proposed changes in Medicare coverage in seven states for Image-Guided Superficial Radiation Therapy (image-guided SRT or IGSRT) for the treatment of NMSC. The proposed local coverage determination, or LCD, if finalized in its current form, would affect residents in North Carolina, South Carolina, Virginia, West Virginia, Alabama, Georgia, and Tennessee.
“These changes would mean more restrictive coverage,” said Dr. Gronbeck, and further support the need for “improved clinical data and development of guidelines to support evidence-based utilization.”
Surgical Management Standard, but SRT Has a Role
Asked to comment on the findings, Seemal R. Desai, MD, president of the American Academy of Dermatology (AAD), who was not involved with the study, reiterated that according to this abstract, efficacy has mainly been assessed through retrospective studies, and results are likely inferior to Mohs surgery, require multiple treatment visits, and are associated with significant costs. More study is needed for the use of radiation therapy in dermatology, he told this news organization.
“The Academy supports continued research and studies for therapies that can help improve patient outcomes and offer treatment options, as well as further studies on long-term outcomes for treatments like superficial radiation therapy,” he said.
“Well-designed studies can certainly be helpful to better assess efficacy and outcomes,” Dr. Desai continued. “This is why the Academy supports the idea of scientific studies that continue to expand the body of literature and data, which can help dermatologists tailor therapeutic options for their patients.”
As for general dermatologists using radiation therapy, he pointed out that SRT was developed within the dermatology specialty with dermatologists being the experts in delivering SRT for patients with NMSCs when indicated. “SRT has been used for over 100 years to treat skin cancer,” said Dr. Desai, of the department of dermatology, UT Southwestern Medical Center, Dallas. “While certain radiation devices have historically been used by dermatologists, dermatologists engaged in providing superficial radiation therapy must have adequate education and training to administer this therapy safely and effectively.”
The AAD Association (AADA) has a position statement that supports the use of SRT as an option for the treatment of basal cell carcinoma and squamous cell carcinoma in certain circumstances. “This could be when surgical intervention is contraindicated or refused and after the benefits and risk of treatment alternatives have been discussed with the patient,” he said. “Based on current evidence, surgical management remains the most effective treatment for NMSC.”
Dr. Desai added that the AADA is also concerned that if the Proposed LCD is finalized by CMS, it “could restrict dermatologists from performing SRT and impede patient access to SRT as a potential treatment when indicated.”
The study was independently supported. Dr. Gronbeck and Dr. Desai reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
PHOENIX — More specifically, the persistent growth in the use of superficial radiotherapy (SRT) devices and electronic brachytherapy (eBT) to treat nonmelanoma skin cancer (NMSC) has exceeded that of traditional procedures among dermatologists using these modalities, according to Christian Gronbeck, MD, a resident in dermatology at the University of Connecticut Health Center, Farmington.
“These services increased substantially over the study period,” Dr. Gronbeck said at the annual meeting of the American College of Mohs Surgery, where he presented the results of the study. “Our findings suggest that those using eBT/SRT were frequently general dermatologists and non-fellowship–trained Mohs surgeons who have less formalized surgical training.”
He also noted that billing for these services also rose substantially, which is being driven by growing utilization and an increased SRT payment rate.
Surgical approaches are standard for most NMSC cases, but some patients are not good surgical candidates because of medical comorbidities and/or other factors, and radiotherapy is emerging as a potential treatment option for those patients. Traditionally, radiotherapy was administered by radiation oncologists, but with the growing availability of SRT devices and the introduction of eBT, dermatologists are now treating patients with these modalities.
“It is a potential treatment option for nonmelanoma skin cancer and keloids, and these lower energy devices can be used in the outpatient setting,” said Dr. Gronbeck. “Treatment typically involves a series of fractions over a period of several weeks. There has been recent growth in the use of radiotherapy despite this being a secondary option in skin cancer, primarily when surgery is contraindicated.”
Steady Expansion of Use
Dr. Gronbeck and colleagues sought to gain a better understanding of the use of SRT and eBT for NMSC among dermatologists, as well as trends in cost. Data were obtained from the 2016-2021 Medicare Public Use Files to evaluate the trend in the volume of Medicare Part B claims for eBT (CPT 0394T) and SRT (CPT 77401) by dermatologists, and they also looked at related billable services for radiotherapy.
Of 12,050 dermatologists, 293 (2.4%) were identified as utilizing eBT or SRT in 2021, representing a 75.4% increase from 2016. The usage of both eBT and SRT increased by 59.6% and 148.4%, respectively, from 2016 to 2021.
There were notable geographic differences in the utilization of radiotherapy. “Florida, California, Texas, and Arizona had the highest utilization,” Dr. Gronbeck said, although during the study period, utilization increased in other states, including North Carolina and Alabama.
When looking at geographic regions as a whole, the highest number of dermatologists using radiotherapy were located in the South (n = 143, 50.9%), followed by the West (n = 69, 23.6%). Utilization was more common in metro areas than in nonmetro/rural areas (86% vs 14%).
Differences were also noted among dermatologists. Those who performed eBT/SRT than those who did not were significantly more likely to have had 15 or more years of independent practice (70.1% vs 48.6%), be in a small private dermatology practice (62.7% vs 47.5%), and be non–fellowship-trained Mohs surgeons (33.5% vs 10.2%). Dermatologists utilizing radiotherapy were also more likely to treat Medicare beneficiaries who were older, with a mean age over 75 years (39.3% vs 31.1%) and a mean hierarchical condition category (HCC) score, above the national average (55.2% vs 44.6%).
Dr. Gronbeck and colleagues also looked at cost. The number of direct payments for eBT/SRT payments increased throughout the study period, from 3,678,224 in 2016 to 11,680,925 in 2021, nearly a 218% increase. The change in payments for services related to eBT/SRT, such as radiotherapy simulation, radiotherapy dosing, and ultrasound guidance, increased by 621.4% during this same timeframe.
Radiotherapy in dermatology has primarily been assessed through retrospective studies. “Our findings suggest that eBT and SRT are more frequently utilized by dermatologists managing older and sicker patients, but further studies are needed to identify whether these interventions are truly addressing poor surgical candidates,” Dr. Gronbeck said.
The Centers for Medicare & Medicaid Services (CMS) has recently proposed changes in Medicare coverage in seven states for Image-Guided Superficial Radiation Therapy (image-guided SRT or IGSRT) for the treatment of NMSC. The proposed local coverage determination, or LCD, if finalized in its current form, would affect residents in North Carolina, South Carolina, Virginia, West Virginia, Alabama, Georgia, and Tennessee.
“These changes would mean more restrictive coverage,” said Dr. Gronbeck, and further support the need for “improved clinical data and development of guidelines to support evidence-based utilization.”
Surgical Management Standard, but SRT Has a Role
Asked to comment on the findings, Seemal R. Desai, MD, president of the American Academy of Dermatology (AAD), who was not involved with the study, reiterated that according to this abstract, efficacy has mainly been assessed through retrospective studies, and results are likely inferior to Mohs surgery, require multiple treatment visits, and are associated with significant costs. More study is needed for the use of radiation therapy in dermatology, he told this news organization.
“The Academy supports continued research and studies for therapies that can help improve patient outcomes and offer treatment options, as well as further studies on long-term outcomes for treatments like superficial radiation therapy,” he said.
“Well-designed studies can certainly be helpful to better assess efficacy and outcomes,” Dr. Desai continued. “This is why the Academy supports the idea of scientific studies that continue to expand the body of literature and data, which can help dermatologists tailor therapeutic options for their patients.”
As for general dermatologists using radiation therapy, he pointed out that SRT was developed within the dermatology specialty with dermatologists being the experts in delivering SRT for patients with NMSCs when indicated. “SRT has been used for over 100 years to treat skin cancer,” said Dr. Desai, of the department of dermatology, UT Southwestern Medical Center, Dallas. “While certain radiation devices have historically been used by dermatologists, dermatologists engaged in providing superficial radiation therapy must have adequate education and training to administer this therapy safely and effectively.”
The AAD Association (AADA) has a position statement that supports the use of SRT as an option for the treatment of basal cell carcinoma and squamous cell carcinoma in certain circumstances. “This could be when surgical intervention is contraindicated or refused and after the benefits and risk of treatment alternatives have been discussed with the patient,” he said. “Based on current evidence, surgical management remains the most effective treatment for NMSC.”
Dr. Desai added that the AADA is also concerned that if the Proposed LCD is finalized by CMS, it “could restrict dermatologists from performing SRT and impede patient access to SRT as a potential treatment when indicated.”
The study was independently supported. Dr. Gronbeck and Dr. Desai reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ACMS 2024
Study Highlights Atopic Dermatitis Features, Treatments Among Older Patients
.
The researchers reviewed charts of patients aged 60 years and older who were seen at either a private or county dermatology clinic in Houston between 2009 and 2020 and had been diagnosed with AD by a dermatologist. The findings of their cross-sectional study further supports that AD in this age group “presents as a unique phenotype compared to AD in younger ages, which may inform dermatologists’ diagnosis of AD in these patients” they wrote.
The 791 patients in the study had an average age of 69.3 years, were predominantly women (60.1%), and were racially diverse, with almost 40% being non-Hispanic White individuals. Others were non-Hispanic Black individuals (21.8%), Hispanics (20.4%), and non-Hispanic Asian/Pacific Islanders (11.7%).
Use of topicals, mainly topical corticosteroids (92.2%), was the most frequent treatment prescribed. Oral corticosteroids and antihistamines were “frequent systemic treatments” in this population, prescribed to 10.4% and 12.1%, respectively, “likely due to management prior to a diagnosis of AD by a dermatologist,” wrote first author Hannah Y. Wang, Baylor College of Medicine, Houston, and her coauthors, including Soo Jung Kim, MD, PhD, of the department of dermatology at Baylor.
Other treatments included dupilumab in 5.4%, systemic immunosuppressants (including methotrexate, cyclosporine, and mycophenolate) in 5.4%, and UVB-phototherapy in 2.7%.
Approximately 40% of the patients had a history of allergic rhinitis, while 20% had a history of asthma. Lichenification was noted in 14.5% of patients and nummular lesions in almost 13%. Other rash characteristics — ichthyosis and hyperpigmented patches — were less frequent, seen in 9.7% and 9.1%, respectively.
AD in this older population was most commonly documented on the extensors (49.9%) and the trunk (46%) and less commonly on the hands (19.8%) and feet (9%) — a distribution that is similar to past reports, the authors wrote.
Asked to comment on the findings, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization that the data relating to clinical morphology are consistent with past reports and with his own experiences. Lichenification is a “tell-tale sign of chronic disease” and may indicate undertreatment, and the frequency of nummular plaques is unsurprising because “nummular dermatitis as an independent eczema tends to occur more so in the elderly.”
More important, he said, was the finding regarding the use of oral corticosteroid and antihistamine, “both of which are advocated against in the management of AD.”
More research is “needed to elucidate the unique features of elderly AD in pathophysiology and optimal treatments,” the authors wrote, noting that age-related factors potentially affecting AD in this population include reduced skin barrier function, immune dysregulation, and environmental exposures.
The study, Dr. Friedman said, “shines a spotlight on this demographic — they exist, they suffer, and they are at times being managed with less-than-optimal options.” Clinical trials of “the welcome additions to our historically limited armament often lack a substantial elderly study population,” he said, and Medicare makes it “painful to get these game-changing drugs for this large patient population.”
The study authors and Dr. Friedman, who was not involved with the study, reported no conflicts of interest.
A version of this article appeared on Medscape.com.
.
The researchers reviewed charts of patients aged 60 years and older who were seen at either a private or county dermatology clinic in Houston between 2009 and 2020 and had been diagnosed with AD by a dermatologist. The findings of their cross-sectional study further supports that AD in this age group “presents as a unique phenotype compared to AD in younger ages, which may inform dermatologists’ diagnosis of AD in these patients” they wrote.
The 791 patients in the study had an average age of 69.3 years, were predominantly women (60.1%), and were racially diverse, with almost 40% being non-Hispanic White individuals. Others were non-Hispanic Black individuals (21.8%), Hispanics (20.4%), and non-Hispanic Asian/Pacific Islanders (11.7%).
Use of topicals, mainly topical corticosteroids (92.2%), was the most frequent treatment prescribed. Oral corticosteroids and antihistamines were “frequent systemic treatments” in this population, prescribed to 10.4% and 12.1%, respectively, “likely due to management prior to a diagnosis of AD by a dermatologist,” wrote first author Hannah Y. Wang, Baylor College of Medicine, Houston, and her coauthors, including Soo Jung Kim, MD, PhD, of the department of dermatology at Baylor.
Other treatments included dupilumab in 5.4%, systemic immunosuppressants (including methotrexate, cyclosporine, and mycophenolate) in 5.4%, and UVB-phototherapy in 2.7%.
Approximately 40% of the patients had a history of allergic rhinitis, while 20% had a history of asthma. Lichenification was noted in 14.5% of patients and nummular lesions in almost 13%. Other rash characteristics — ichthyosis and hyperpigmented patches — were less frequent, seen in 9.7% and 9.1%, respectively.
AD in this older population was most commonly documented on the extensors (49.9%) and the trunk (46%) and less commonly on the hands (19.8%) and feet (9%) — a distribution that is similar to past reports, the authors wrote.
Asked to comment on the findings, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization that the data relating to clinical morphology are consistent with past reports and with his own experiences. Lichenification is a “tell-tale sign of chronic disease” and may indicate undertreatment, and the frequency of nummular plaques is unsurprising because “nummular dermatitis as an independent eczema tends to occur more so in the elderly.”
More important, he said, was the finding regarding the use of oral corticosteroid and antihistamine, “both of which are advocated against in the management of AD.”
More research is “needed to elucidate the unique features of elderly AD in pathophysiology and optimal treatments,” the authors wrote, noting that age-related factors potentially affecting AD in this population include reduced skin barrier function, immune dysregulation, and environmental exposures.
The study, Dr. Friedman said, “shines a spotlight on this demographic — they exist, they suffer, and they are at times being managed with less-than-optimal options.” Clinical trials of “the welcome additions to our historically limited armament often lack a substantial elderly study population,” he said, and Medicare makes it “painful to get these game-changing drugs for this large patient population.”
The study authors and Dr. Friedman, who was not involved with the study, reported no conflicts of interest.
A version of this article appeared on Medscape.com.
.
The researchers reviewed charts of patients aged 60 years and older who were seen at either a private or county dermatology clinic in Houston between 2009 and 2020 and had been diagnosed with AD by a dermatologist. The findings of their cross-sectional study further supports that AD in this age group “presents as a unique phenotype compared to AD in younger ages, which may inform dermatologists’ diagnosis of AD in these patients” they wrote.
The 791 patients in the study had an average age of 69.3 years, were predominantly women (60.1%), and were racially diverse, with almost 40% being non-Hispanic White individuals. Others were non-Hispanic Black individuals (21.8%), Hispanics (20.4%), and non-Hispanic Asian/Pacific Islanders (11.7%).
Use of topicals, mainly topical corticosteroids (92.2%), was the most frequent treatment prescribed. Oral corticosteroids and antihistamines were “frequent systemic treatments” in this population, prescribed to 10.4% and 12.1%, respectively, “likely due to management prior to a diagnosis of AD by a dermatologist,” wrote first author Hannah Y. Wang, Baylor College of Medicine, Houston, and her coauthors, including Soo Jung Kim, MD, PhD, of the department of dermatology at Baylor.
Other treatments included dupilumab in 5.4%, systemic immunosuppressants (including methotrexate, cyclosporine, and mycophenolate) in 5.4%, and UVB-phototherapy in 2.7%.
Approximately 40% of the patients had a history of allergic rhinitis, while 20% had a history of asthma. Lichenification was noted in 14.5% of patients and nummular lesions in almost 13%. Other rash characteristics — ichthyosis and hyperpigmented patches — were less frequent, seen in 9.7% and 9.1%, respectively.
AD in this older population was most commonly documented on the extensors (49.9%) and the trunk (46%) and less commonly on the hands (19.8%) and feet (9%) — a distribution that is similar to past reports, the authors wrote.
Asked to comment on the findings, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization that the data relating to clinical morphology are consistent with past reports and with his own experiences. Lichenification is a “tell-tale sign of chronic disease” and may indicate undertreatment, and the frequency of nummular plaques is unsurprising because “nummular dermatitis as an independent eczema tends to occur more so in the elderly.”
More important, he said, was the finding regarding the use of oral corticosteroid and antihistamine, “both of which are advocated against in the management of AD.”
More research is “needed to elucidate the unique features of elderly AD in pathophysiology and optimal treatments,” the authors wrote, noting that age-related factors potentially affecting AD in this population include reduced skin barrier function, immune dysregulation, and environmental exposures.
The study, Dr. Friedman said, “shines a spotlight on this demographic — they exist, they suffer, and they are at times being managed with less-than-optimal options.” Clinical trials of “the welcome additions to our historically limited armament often lack a substantial elderly study population,” he said, and Medicare makes it “painful to get these game-changing drugs for this large patient population.”
The study authors and Dr. Friedman, who was not involved with the study, reported no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAAD INTERNATIONAL
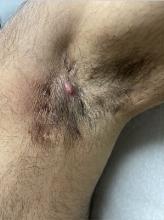
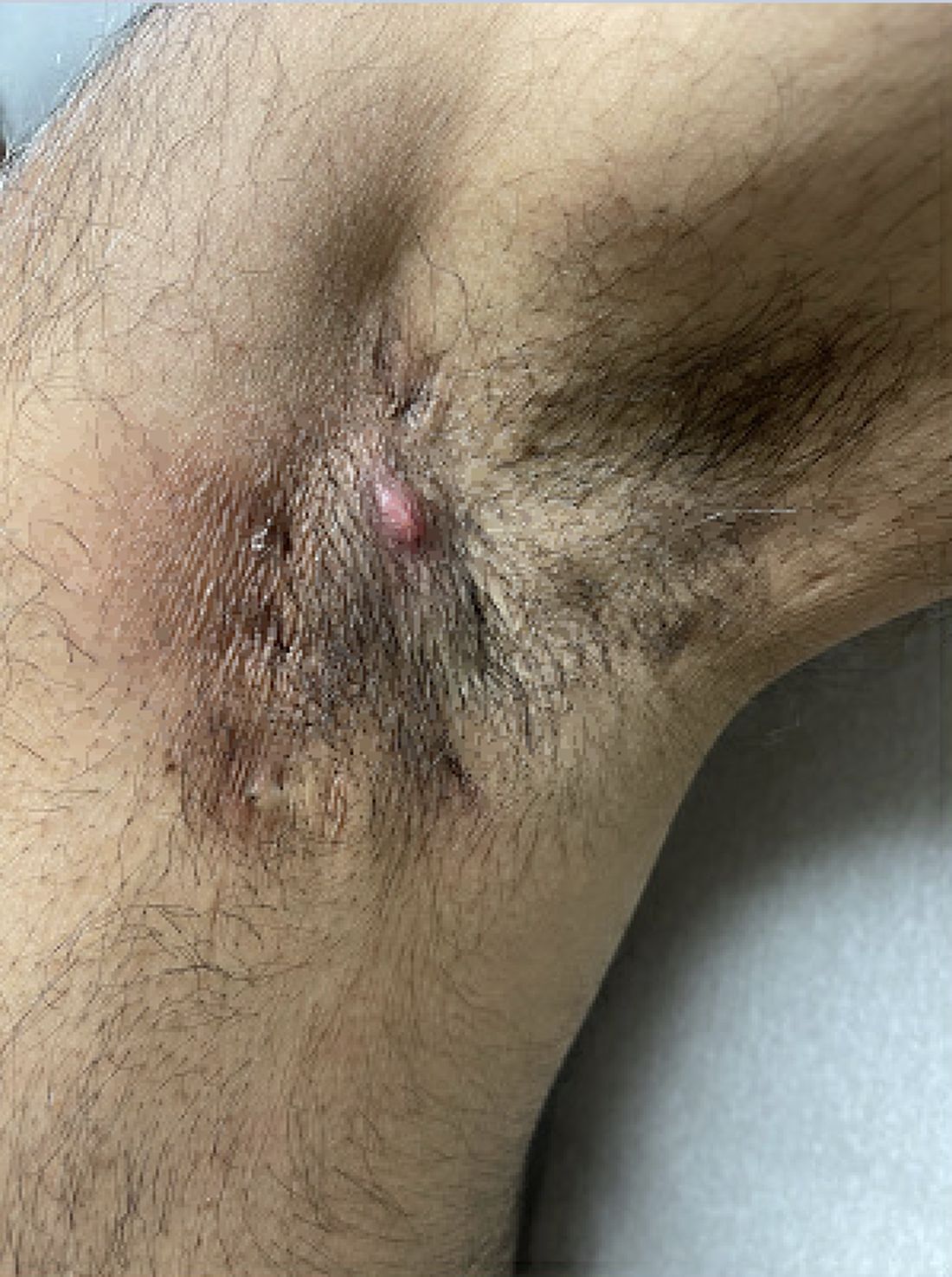
Treatments for Early HS Range From Topical Therapies to Laser Hair Removal
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
Diagnosing Mild Hidradenitis Suppurativa: Early Stage Can Mimic Other Diseases
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
An 8-year-old girl presented with papules on her bilateral eyelid margins
, with an equal distribution across genders and ethnicities.1 It is caused by mutations in the ECM1 gene2 on chromosome 1q21. This leads to the abnormal deposition of hyaline material in various tissues across different organ systems, with the classic manifestations known as the “string of pearls” sign and a hoarse cry or voice.
The rarity of lipoid proteinosis often leads to challenges in diagnosis. Particularly when deviating from the common association with consanguinity, the potential for de novo mutations or a broader genetic variability in disease expression is highlighted. Our patient presents with symptoms that are pathognomonic to LP with moniliform blepharosis and hoarseness of the voice, in addition to scarring of the extremities.
Other common clinical manifestations in patients with LP include cobblestoning of the mucosa; hyperkeratosis of the elbows, knees, and hands; and calcification of the amygdala with neuroimaging.3
Genetic testing that identifies a loss-of-function mutation in ECM1 offers diagnostic confirmation. Patients often need multidisciplinary care involving dermatology; ear, nose, throat; neurology; and genetics. Treatment of LP is mostly symptomatic with unsatisfactory resolution of cutaneous changes, with retinoids such as acitretin used as the first-line option and surgery as a consideration for laryngeal hyaline deposits.2 Although LP can affect different organ systems, patients tend to have a normal lifespan.
LP is a rare disorder that dermatologists often learn about during textbook sessions or didactics in residency but do not see in practice for decades, or if ever. This case highlights the need to review the classic presentations of rare conditions.
This case and the photos were submitted by Ms. Chang, BS, Western University of Health Sciences, College of Osteopathic Medicine, Pomona, California; Dr. Connie Chang, Verdugo Dermatology, Glendale, California; and Dr. Yuchieh Kathryn Chang, MD Anderson Cancer Center, Houston, Texas. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Mcgrath JA. Handb Clin Neurol. 2015:132:317-22. doi: 10.1016/B978-0-444-62702-5.00023-8.
2. Hamada Tet al. Hum Mol Genet. 2002 Apr 1;11(7):833-40. doi: 10.1093/hmg/11.7.833.
3. Frenkel B et al. Clin Oral Investig. 2017 Sep;21(7):2245-51 doi: 10.1007/s00784-016-2017-7.
, with an equal distribution across genders and ethnicities.1 It is caused by mutations in the ECM1 gene2 on chromosome 1q21. This leads to the abnormal deposition of hyaline material in various tissues across different organ systems, with the classic manifestations known as the “string of pearls” sign and a hoarse cry or voice.
The rarity of lipoid proteinosis often leads to challenges in diagnosis. Particularly when deviating from the common association with consanguinity, the potential for de novo mutations or a broader genetic variability in disease expression is highlighted. Our patient presents with symptoms that are pathognomonic to LP with moniliform blepharosis and hoarseness of the voice, in addition to scarring of the extremities.
Other common clinical manifestations in patients with LP include cobblestoning of the mucosa; hyperkeratosis of the elbows, knees, and hands; and calcification of the amygdala with neuroimaging.3
Genetic testing that identifies a loss-of-function mutation in ECM1 offers diagnostic confirmation. Patients often need multidisciplinary care involving dermatology; ear, nose, throat; neurology; and genetics. Treatment of LP is mostly symptomatic with unsatisfactory resolution of cutaneous changes, with retinoids such as acitretin used as the first-line option and surgery as a consideration for laryngeal hyaline deposits.2 Although LP can affect different organ systems, patients tend to have a normal lifespan.
LP is a rare disorder that dermatologists often learn about during textbook sessions or didactics in residency but do not see in practice for decades, or if ever. This case highlights the need to review the classic presentations of rare conditions.
This case and the photos were submitted by Ms. Chang, BS, Western University of Health Sciences, College of Osteopathic Medicine, Pomona, California; Dr. Connie Chang, Verdugo Dermatology, Glendale, California; and Dr. Yuchieh Kathryn Chang, MD Anderson Cancer Center, Houston, Texas. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Mcgrath JA. Handb Clin Neurol. 2015:132:317-22. doi: 10.1016/B978-0-444-62702-5.00023-8.
2. Hamada Tet al. Hum Mol Genet. 2002 Apr 1;11(7):833-40. doi: 10.1093/hmg/11.7.833.
3. Frenkel B et al. Clin Oral Investig. 2017 Sep;21(7):2245-51 doi: 10.1007/s00784-016-2017-7.
, with an equal distribution across genders and ethnicities.1 It is caused by mutations in the ECM1 gene2 on chromosome 1q21. This leads to the abnormal deposition of hyaline material in various tissues across different organ systems, with the classic manifestations known as the “string of pearls” sign and a hoarse cry or voice.
The rarity of lipoid proteinosis often leads to challenges in diagnosis. Particularly when deviating from the common association with consanguinity, the potential for de novo mutations or a broader genetic variability in disease expression is highlighted. Our patient presents with symptoms that are pathognomonic to LP with moniliform blepharosis and hoarseness of the voice, in addition to scarring of the extremities.
Other common clinical manifestations in patients with LP include cobblestoning of the mucosa; hyperkeratosis of the elbows, knees, and hands; and calcification of the amygdala with neuroimaging.3
Genetic testing that identifies a loss-of-function mutation in ECM1 offers diagnostic confirmation. Patients often need multidisciplinary care involving dermatology; ear, nose, throat; neurology; and genetics. Treatment of LP is mostly symptomatic with unsatisfactory resolution of cutaneous changes, with retinoids such as acitretin used as the first-line option and surgery as a consideration for laryngeal hyaline deposits.2 Although LP can affect different organ systems, patients tend to have a normal lifespan.
LP is a rare disorder that dermatologists often learn about during textbook sessions or didactics in residency but do not see in practice for decades, or if ever. This case highlights the need to review the classic presentations of rare conditions.
This case and the photos were submitted by Ms. Chang, BS, Western University of Health Sciences, College of Osteopathic Medicine, Pomona, California; Dr. Connie Chang, Verdugo Dermatology, Glendale, California; and Dr. Yuchieh Kathryn Chang, MD Anderson Cancer Center, Houston, Texas. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Mcgrath JA. Handb Clin Neurol. 2015:132:317-22. doi: 10.1016/B978-0-444-62702-5.00023-8.
2. Hamada Tet al. Hum Mol Genet. 2002 Apr 1;11(7):833-40. doi: 10.1093/hmg/11.7.833.
3. Frenkel B et al. Clin Oral Investig. 2017 Sep;21(7):2245-51 doi: 10.1007/s00784-016-2017-7.
Specialists Are ‘Underwater’ With Some Insurance-Preferred Biosimilars
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Pediatric Dermatologists Beat ChatGPT on Board Questions
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
Global Analysis Identifies Drugs Associated With SJS-TEN in Children
TOPLINE:
METHODOLOGY:
- SJS and TEN are rare, life-threatening mucocutaneous reactions mainly associated with medications, but large pharmacovigilance studies of drugs associated with SJS-TEN in the pediatric population are still lacking.
- Using the WHO’s pharmacovigilance database (VigiBase) containing individual case safety reports from January 1967 to July 2022, researchers identified 7342 adverse drug reaction reports of SJS-TEN in children (younger than 18 years; median age, 9 years) in all six continents. Median onset was 5 days, and 3.2% were fatal.
- They analyzed drugs reported as suspected treatments, and for each molecule, they performed a case–non-case study to assess a potential pharmacovigilance signal by computing the information component (IC).
- A positive IC value suggested more frequent reporting of a specific drug-adverse reaction pair. A positive IC025, a traditional threshold for statistical signal detection, is suggestive of a potential pharmacovigilance signal.
TAKEAWAY:
- Overall, 165 drugs were associated with a diagnosis of SJS-TEN; antiepileptic and anti-infectious drugs were the most common drug classes represented.
- The five most frequently reported drugs were carbamazepine (11.7%), lamotrigine (10.6%), sulfamethoxazole-trimethoprim (9%), acetaminophen (8.4%), and phenytoin (6.6%). The five drugs with the highest IC025 were lamotrigine, carbamazepine, phenobarbital, phenytoin, and nimesulide.
- All antiepileptics, many antibiotic families, dapsone, antiretroviral drugs, some antifungal drugs, and nonsteroidal anti-inflammatory drugs were identified in reports, with penicillins the most frequently reported antibiotic family and sulfonamides having the strongest pharmacovigilance signal.
- Vaccines were not associated with significant signals.
IN PRACTICE:
The study provides an update on “the spectrum of drugs potentially associated with SJS-TEN in the pediatric population,” the authors concluded, and “underlines the importance of reporting to pharmacovigilance the suspicion of this severe side effect of drugs with the most precise and detailed clinical description possible.”
SOURCE:
The study, led by Pauline Bataille, MD, of the Department of Pediatric Dermatology, Hôpital Necker-Enfants Malades, Paris City University, France, was published online in the Journal of the European Academy of Dermatology and Venereology.
LIMITATIONS:
Limitations include the possibility that some cases could have had an infectious or idiopathic cause not related to a drug and the lack of detailed clinical data in the database.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflict of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- SJS and TEN are rare, life-threatening mucocutaneous reactions mainly associated with medications, but large pharmacovigilance studies of drugs associated with SJS-TEN in the pediatric population are still lacking.
- Using the WHO’s pharmacovigilance database (VigiBase) containing individual case safety reports from January 1967 to July 2022, researchers identified 7342 adverse drug reaction reports of SJS-TEN in children (younger than 18 years; median age, 9 years) in all six continents. Median onset was 5 days, and 3.2% were fatal.
- They analyzed drugs reported as suspected treatments, and for each molecule, they performed a case–non-case study to assess a potential pharmacovigilance signal by computing the information component (IC).
- A positive IC value suggested more frequent reporting of a specific drug-adverse reaction pair. A positive IC025, a traditional threshold for statistical signal detection, is suggestive of a potential pharmacovigilance signal.
TAKEAWAY:
- Overall, 165 drugs were associated with a diagnosis of SJS-TEN; antiepileptic and anti-infectious drugs were the most common drug classes represented.
- The five most frequently reported drugs were carbamazepine (11.7%), lamotrigine (10.6%), sulfamethoxazole-trimethoprim (9%), acetaminophen (8.4%), and phenytoin (6.6%). The five drugs with the highest IC025 were lamotrigine, carbamazepine, phenobarbital, phenytoin, and nimesulide.
- All antiepileptics, many antibiotic families, dapsone, antiretroviral drugs, some antifungal drugs, and nonsteroidal anti-inflammatory drugs were identified in reports, with penicillins the most frequently reported antibiotic family and sulfonamides having the strongest pharmacovigilance signal.
- Vaccines were not associated with significant signals.
IN PRACTICE:
The study provides an update on “the spectrum of drugs potentially associated with SJS-TEN in the pediatric population,” the authors concluded, and “underlines the importance of reporting to pharmacovigilance the suspicion of this severe side effect of drugs with the most precise and detailed clinical description possible.”
SOURCE:
The study, led by Pauline Bataille, MD, of the Department of Pediatric Dermatology, Hôpital Necker-Enfants Malades, Paris City University, France, was published online in the Journal of the European Academy of Dermatology and Venereology.
LIMITATIONS:
Limitations include the possibility that some cases could have had an infectious or idiopathic cause not related to a drug and the lack of detailed clinical data in the database.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflict of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- SJS and TEN are rare, life-threatening mucocutaneous reactions mainly associated with medications, but large pharmacovigilance studies of drugs associated with SJS-TEN in the pediatric population are still lacking.
- Using the WHO’s pharmacovigilance database (VigiBase) containing individual case safety reports from January 1967 to July 2022, researchers identified 7342 adverse drug reaction reports of SJS-TEN in children (younger than 18 years; median age, 9 years) in all six continents. Median onset was 5 days, and 3.2% were fatal.
- They analyzed drugs reported as suspected treatments, and for each molecule, they performed a case–non-case study to assess a potential pharmacovigilance signal by computing the information component (IC).
- A positive IC value suggested more frequent reporting of a specific drug-adverse reaction pair. A positive IC025, a traditional threshold for statistical signal detection, is suggestive of a potential pharmacovigilance signal.
TAKEAWAY:
- Overall, 165 drugs were associated with a diagnosis of SJS-TEN; antiepileptic and anti-infectious drugs were the most common drug classes represented.
- The five most frequently reported drugs were carbamazepine (11.7%), lamotrigine (10.6%), sulfamethoxazole-trimethoprim (9%), acetaminophen (8.4%), and phenytoin (6.6%). The five drugs with the highest IC025 were lamotrigine, carbamazepine, phenobarbital, phenytoin, and nimesulide.
- All antiepileptics, many antibiotic families, dapsone, antiretroviral drugs, some antifungal drugs, and nonsteroidal anti-inflammatory drugs were identified in reports, with penicillins the most frequently reported antibiotic family and sulfonamides having the strongest pharmacovigilance signal.
- Vaccines were not associated with significant signals.
IN PRACTICE:
The study provides an update on “the spectrum of drugs potentially associated with SJS-TEN in the pediatric population,” the authors concluded, and “underlines the importance of reporting to pharmacovigilance the suspicion of this severe side effect of drugs with the most precise and detailed clinical description possible.”
SOURCE:
The study, led by Pauline Bataille, MD, of the Department of Pediatric Dermatology, Hôpital Necker-Enfants Malades, Paris City University, France, was published online in the Journal of the European Academy of Dermatology and Venereology.
LIMITATIONS:
Limitations include the possibility that some cases could have had an infectious or idiopathic cause not related to a drug and the lack of detailed clinical data in the database.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflict of interest.
A version of this article first appeared on Medscape.com.