User login
Study finds quality of topical steroid withdrawal videos on YouTube subpar
NEW ORLEANS –
“Video-sharing platforms such as YouTube are a great place for patients to connect and find community with others dealing with the same conditions,” senior author Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. “There is no doubt tremendous value in viewing the shared experience; however, it is important that medical advice be evidence based and validated. Seeking said advice from a medical professional such as a board-certified dermatologist will no doubt increase the likelihood that said guidance is supported by the literature and most importantly, will do no harm.”
Noting a trend of increased user-created content on social media and Internet sites about topical steroid withdrawal in recent years, Dr. Friedman, first author Erika McCormick, a fourth-year medical student at George Washington University, and colleagues used the keywords “topical steroid withdrawal” on YouTube to search for and analyze the top 10 most viewed videos on the subject.
Two independent reviewers used the modified DISCERN (mDISCERN) tool and the Global Quality Scale (GQS) to assess reliability and quality/scientific accuracy of videos, respectively. Average scores were generated for each video and the researchers used one way ANOVA, unpaired t-tests, and linear regression to analyze the ratings. For mDISCERN criteria, a point is given per each of five criteria for a possible score between 0 and 5. Examples of criteria included “Are the aims clear and achieved?” and “Is the information presented both balanced and unbiased”? For GQS, a score from 1 to 5 is designated based on criteria ranging from “poor quality, poor flow, most information missing” to “excellent quality and flow, very useful for patients.”
The researchers found that the mean combined mDISCERN score of the 10 videos was a 2, which indicates poor reliability and shortcomings. Similarly, the combined mean GQS score was 2.5, which suggests poor to moderate quality of videos, missing discussion of important topics, and limited use to patients. The researchers found no correlation between mDISCERN or GQS scores and length of video, duration on YouTube, or number of views, subscribers, or likes.
“We were disheartened that patient testimonial videos had the poorest quality and reliability of the information sources,” Ms. McCormick said in an interview. “Videos that included medical research and information from dermatologists had significantly higher quality and reliability scores than the remainder of videos.” Accurate information online is essential to help patients recognize topical steroid withdrawal and seek medical care, she continued.
Conversely, wide viewership of unreliable information “may contribute to fear of topical corticosteroids and dissuade use in patients with primary skin diseases that may benefit from this common treatment,” Dr. Friedman said. “Dermatologists must be aware of the content patients are consuming online, should guide patients in appraising quality and reliability of online resources, and must provide valid sources of additional information for their patients.” One such resource he recommended is the National Eczema Association, which has created online content for patients about topical steroid withdrawal.
Doris Day, MD, a New York–based dermatologist who was asked to comment on the study, said that many patients rely on YouTube as a go-to resource, with videos that can be watched at times of their choosing. “Oftentimes, the person on the video is relatable and has some general knowledge but is lacking the information that would be relevant and important for the individual patient,” said Dr. Day, who was not involved with the study. “The downside of this is that the person who takes that advice may not use the prescription properly or for the correct amount of time, which can lead to either undertreating or, even worse, overtreatment, which can have permanent consequences.”
One possible solution is for more doctors to create videos for YouTube, she added, “but that doesn’t guarantee that those would be the ones patients would choose to watch.” Another solution “is to have YouTube add qualifiers indicating that the information being discussed is not medical,” she suggested. “Ideally, patients will get all the information they need while they are in the office and also have clear written instructions and even a video they can review at a later time, made by the office, to help them feel they are getting personalized care and the attention they need.”
Ms. McCormick’s research is funded by a grant from Galderma. Dr. Friedman and Dr. Day had no relevant disclosures to report.
NEW ORLEANS –
“Video-sharing platforms such as YouTube are a great place for patients to connect and find community with others dealing with the same conditions,” senior author Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. “There is no doubt tremendous value in viewing the shared experience; however, it is important that medical advice be evidence based and validated. Seeking said advice from a medical professional such as a board-certified dermatologist will no doubt increase the likelihood that said guidance is supported by the literature and most importantly, will do no harm.”
Noting a trend of increased user-created content on social media and Internet sites about topical steroid withdrawal in recent years, Dr. Friedman, first author Erika McCormick, a fourth-year medical student at George Washington University, and colleagues used the keywords “topical steroid withdrawal” on YouTube to search for and analyze the top 10 most viewed videos on the subject.
Two independent reviewers used the modified DISCERN (mDISCERN) tool and the Global Quality Scale (GQS) to assess reliability and quality/scientific accuracy of videos, respectively. Average scores were generated for each video and the researchers used one way ANOVA, unpaired t-tests, and linear regression to analyze the ratings. For mDISCERN criteria, a point is given per each of five criteria for a possible score between 0 and 5. Examples of criteria included “Are the aims clear and achieved?” and “Is the information presented both balanced and unbiased”? For GQS, a score from 1 to 5 is designated based on criteria ranging from “poor quality, poor flow, most information missing” to “excellent quality and flow, very useful for patients.”
The researchers found that the mean combined mDISCERN score of the 10 videos was a 2, which indicates poor reliability and shortcomings. Similarly, the combined mean GQS score was 2.5, which suggests poor to moderate quality of videos, missing discussion of important topics, and limited use to patients. The researchers found no correlation between mDISCERN or GQS scores and length of video, duration on YouTube, or number of views, subscribers, or likes.
“We were disheartened that patient testimonial videos had the poorest quality and reliability of the information sources,” Ms. McCormick said in an interview. “Videos that included medical research and information from dermatologists had significantly higher quality and reliability scores than the remainder of videos.” Accurate information online is essential to help patients recognize topical steroid withdrawal and seek medical care, she continued.
Conversely, wide viewership of unreliable information “may contribute to fear of topical corticosteroids and dissuade use in patients with primary skin diseases that may benefit from this common treatment,” Dr. Friedman said. “Dermatologists must be aware of the content patients are consuming online, should guide patients in appraising quality and reliability of online resources, and must provide valid sources of additional information for their patients.” One such resource he recommended is the National Eczema Association, which has created online content for patients about topical steroid withdrawal.
Doris Day, MD, a New York–based dermatologist who was asked to comment on the study, said that many patients rely on YouTube as a go-to resource, with videos that can be watched at times of their choosing. “Oftentimes, the person on the video is relatable and has some general knowledge but is lacking the information that would be relevant and important for the individual patient,” said Dr. Day, who was not involved with the study. “The downside of this is that the person who takes that advice may not use the prescription properly or for the correct amount of time, which can lead to either undertreating or, even worse, overtreatment, which can have permanent consequences.”
One possible solution is for more doctors to create videos for YouTube, she added, “but that doesn’t guarantee that those would be the ones patients would choose to watch.” Another solution “is to have YouTube add qualifiers indicating that the information being discussed is not medical,” she suggested. “Ideally, patients will get all the information they need while they are in the office and also have clear written instructions and even a video they can review at a later time, made by the office, to help them feel they are getting personalized care and the attention they need.”
Ms. McCormick’s research is funded by a grant from Galderma. Dr. Friedman and Dr. Day had no relevant disclosures to report.
NEW ORLEANS –
“Video-sharing platforms such as YouTube are a great place for patients to connect and find community with others dealing with the same conditions,” senior author Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. “There is no doubt tremendous value in viewing the shared experience; however, it is important that medical advice be evidence based and validated. Seeking said advice from a medical professional such as a board-certified dermatologist will no doubt increase the likelihood that said guidance is supported by the literature and most importantly, will do no harm.”
Noting a trend of increased user-created content on social media and Internet sites about topical steroid withdrawal in recent years, Dr. Friedman, first author Erika McCormick, a fourth-year medical student at George Washington University, and colleagues used the keywords “topical steroid withdrawal” on YouTube to search for and analyze the top 10 most viewed videos on the subject.
Two independent reviewers used the modified DISCERN (mDISCERN) tool and the Global Quality Scale (GQS) to assess reliability and quality/scientific accuracy of videos, respectively. Average scores were generated for each video and the researchers used one way ANOVA, unpaired t-tests, and linear regression to analyze the ratings. For mDISCERN criteria, a point is given per each of five criteria for a possible score between 0 and 5. Examples of criteria included “Are the aims clear and achieved?” and “Is the information presented both balanced and unbiased”? For GQS, a score from 1 to 5 is designated based on criteria ranging from “poor quality, poor flow, most information missing” to “excellent quality and flow, very useful for patients.”
The researchers found that the mean combined mDISCERN score of the 10 videos was a 2, which indicates poor reliability and shortcomings. Similarly, the combined mean GQS score was 2.5, which suggests poor to moderate quality of videos, missing discussion of important topics, and limited use to patients. The researchers found no correlation between mDISCERN or GQS scores and length of video, duration on YouTube, or number of views, subscribers, or likes.
“We were disheartened that patient testimonial videos had the poorest quality and reliability of the information sources,” Ms. McCormick said in an interview. “Videos that included medical research and information from dermatologists had significantly higher quality and reliability scores than the remainder of videos.” Accurate information online is essential to help patients recognize topical steroid withdrawal and seek medical care, she continued.
Conversely, wide viewership of unreliable information “may contribute to fear of topical corticosteroids and dissuade use in patients with primary skin diseases that may benefit from this common treatment,” Dr. Friedman said. “Dermatologists must be aware of the content patients are consuming online, should guide patients in appraising quality and reliability of online resources, and must provide valid sources of additional information for their patients.” One such resource he recommended is the National Eczema Association, which has created online content for patients about topical steroid withdrawal.
Doris Day, MD, a New York–based dermatologist who was asked to comment on the study, said that many patients rely on YouTube as a go-to resource, with videos that can be watched at times of their choosing. “Oftentimes, the person on the video is relatable and has some general knowledge but is lacking the information that would be relevant and important for the individual patient,” said Dr. Day, who was not involved with the study. “The downside of this is that the person who takes that advice may not use the prescription properly or for the correct amount of time, which can lead to either undertreating or, even worse, overtreatment, which can have permanent consequences.”
One possible solution is for more doctors to create videos for YouTube, she added, “but that doesn’t guarantee that those would be the ones patients would choose to watch.” Another solution “is to have YouTube add qualifiers indicating that the information being discussed is not medical,” she suggested. “Ideally, patients will get all the information they need while they are in the office and also have clear written instructions and even a video they can review at a later time, made by the office, to help them feel they are getting personalized care and the attention they need.”
Ms. McCormick’s research is funded by a grant from Galderma. Dr. Friedman and Dr. Day had no relevant disclosures to report.
AT AAD 2023
Spironolactone: an ‘inexpensive, effective’ option for acne in women
HONOLULU – In the clinical experience of Julie C. Harper, MD, an increasing number of women with acne are turning to off-label, long-term treatment with spironolactone.
“Spironolactone is fairly accessible, inexpensive, and effective for our patients,” Dr. Harper, a dermatologist who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
An aldosterone receptor antagonist commonly used to treat high blood pressure and heart failure, spironolactone also has antiandrogenic properties with a proven track record for treating acne and hirsutism. It reduces androgen production, inhibits 5-alpha reductase, and increases sex hormone binding globulin. The dosing range for treating acne is 25 mg to 200 mg per day, but Dr. Harper prefers a maximum dose of 100 mg per day.
According to a systematic review of its use for acne in adult women, the most common side effect is menstrual irregularity, while other common side effects include breast tenderness/swelling, fatigue, and headaches.
“The higher the dose, the higher the rate of side effects,” she said. Concomitant use of an oral contraceptive lessens menstrual irregularities and prevents pregnancies, to avoid exposure during pregnancy and the hypothetical risk of feminization of the male fetus with exposure late in the first trimester. “Early in my career, I used to say if you’re going to be on spironolactone you’re also going to be on an oral contraceptive. But the longer I’ve practiced, I’ve learned that women who have a contraindication to birth control pills or who don’t want to take it can still benefit from an oral antiandrogen by being on spironolactone.”
A large retrospective analysis of 14-year data concluded that routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. “If you’re between the ages of 18 and 45, healthy, and not taking other medications where I’m worried about potassium levels, I’m not checking those levels at all,” Dr. Harper said.
Spironolactone labeling includes a boxed warning regarding the potential for tumorigenicity based on rat studies, but the dosages used in those studies were 25-250 times higher than the exposure dose in humans, Dr. Harper said.
Results from a systematic review and meta-analysis of seven studies in the medical literature found no evidence of an increased risk of breast cancer in women with exposure to spironolactone. “However, the certainty of the evidence was low and future studies are needed, including among diverse populations such as younger individuals and those with acne or hirsutism,” the study authors wrote.
In a separate study, researchers drew from patients in the Humana Insurance database from 2005 to 2017 to address whether spironolactone is associated with an increased risk of recurrence of breast cancer. Recurrent breast cancer was examined in 29,146 women with continuous health insurance for 2 years after a diagnosis of breast cancer. Of these, 746 were prescribed spironolactone, and the remainder were not. The researchers found that 123 women (16.5%) who were prescribed spironolactone had a breast cancer recurrence, compared with 3,649 women (12.8%) with a breast cancer recurrence who had not been prescribed spironolactone (P = .004). Adjusted Cox regression analysis following propensity matching showed no association between spironolactone and increased breast cancer recurrence (adjusted hazard ratio, 0.966; P = .953).
According to Dr. Harper, spironolactone may take about 3 months to kick in. “Likely this is a long-term treatment, and most of the time we’re going to be using it in combination with other acne treatments such as topical retinoids or topical benzoyl peroxide, oral antibiotics, or even isotretinoin.”
A study of long-term spironolactone use in 403 women found that the most common dose prescribed was 100 mg/day, and 68% of the women were concurrently prescribed a topical retinoid, 2.2% an oral antibiotic, and 40.7% an oral contraceptive.
The study population included 32 patients with a history of polycystic ovarian syndrome, 1 with a history of breast cancer, and 5 were hypercoagulable. Patients took the drug for a mean of 471 days. “As opposed to our antibiotics, where the course for patients is generally 3-4 months, when you start someone on spironolactone, they may end up staying on it,” Dr. Harper said.
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, Cassiopeia, Cutera, EPI, Galderma, L’Oreal, Ortho Dermatologics, Sol Gel, and Vyne. She also serves as a speaker or member of a speaker’s bureau for Almirall, Cassiopeia, Cutera, EPI, Galderma, Journey Almirall, L’Oreal, Ortho Dermatologics, Sun Pharmaceutical Industries, and Vyne.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the clinical experience of Julie C. Harper, MD, an increasing number of women with acne are turning to off-label, long-term treatment with spironolactone.
“Spironolactone is fairly accessible, inexpensive, and effective for our patients,” Dr. Harper, a dermatologist who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
An aldosterone receptor antagonist commonly used to treat high blood pressure and heart failure, spironolactone also has antiandrogenic properties with a proven track record for treating acne and hirsutism. It reduces androgen production, inhibits 5-alpha reductase, and increases sex hormone binding globulin. The dosing range for treating acne is 25 mg to 200 mg per day, but Dr. Harper prefers a maximum dose of 100 mg per day.
According to a systematic review of its use for acne in adult women, the most common side effect is menstrual irregularity, while other common side effects include breast tenderness/swelling, fatigue, and headaches.
“The higher the dose, the higher the rate of side effects,” she said. Concomitant use of an oral contraceptive lessens menstrual irregularities and prevents pregnancies, to avoid exposure during pregnancy and the hypothetical risk of feminization of the male fetus with exposure late in the first trimester. “Early in my career, I used to say if you’re going to be on spironolactone you’re also going to be on an oral contraceptive. But the longer I’ve practiced, I’ve learned that women who have a contraindication to birth control pills or who don’t want to take it can still benefit from an oral antiandrogen by being on spironolactone.”
A large retrospective analysis of 14-year data concluded that routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. “If you’re between the ages of 18 and 45, healthy, and not taking other medications where I’m worried about potassium levels, I’m not checking those levels at all,” Dr. Harper said.
Spironolactone labeling includes a boxed warning regarding the potential for tumorigenicity based on rat studies, but the dosages used in those studies were 25-250 times higher than the exposure dose in humans, Dr. Harper said.
Results from a systematic review and meta-analysis of seven studies in the medical literature found no evidence of an increased risk of breast cancer in women with exposure to spironolactone. “However, the certainty of the evidence was low and future studies are needed, including among diverse populations such as younger individuals and those with acne or hirsutism,” the study authors wrote.
In a separate study, researchers drew from patients in the Humana Insurance database from 2005 to 2017 to address whether spironolactone is associated with an increased risk of recurrence of breast cancer. Recurrent breast cancer was examined in 29,146 women with continuous health insurance for 2 years after a diagnosis of breast cancer. Of these, 746 were prescribed spironolactone, and the remainder were not. The researchers found that 123 women (16.5%) who were prescribed spironolactone had a breast cancer recurrence, compared with 3,649 women (12.8%) with a breast cancer recurrence who had not been prescribed spironolactone (P = .004). Adjusted Cox regression analysis following propensity matching showed no association between spironolactone and increased breast cancer recurrence (adjusted hazard ratio, 0.966; P = .953).
According to Dr. Harper, spironolactone may take about 3 months to kick in. “Likely this is a long-term treatment, and most of the time we’re going to be using it in combination with other acne treatments such as topical retinoids or topical benzoyl peroxide, oral antibiotics, or even isotretinoin.”
A study of long-term spironolactone use in 403 women found that the most common dose prescribed was 100 mg/day, and 68% of the women were concurrently prescribed a topical retinoid, 2.2% an oral antibiotic, and 40.7% an oral contraceptive.
The study population included 32 patients with a history of polycystic ovarian syndrome, 1 with a history of breast cancer, and 5 were hypercoagulable. Patients took the drug for a mean of 471 days. “As opposed to our antibiotics, where the course for patients is generally 3-4 months, when you start someone on spironolactone, they may end up staying on it,” Dr. Harper said.
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, Cassiopeia, Cutera, EPI, Galderma, L’Oreal, Ortho Dermatologics, Sol Gel, and Vyne. She also serves as a speaker or member of a speaker’s bureau for Almirall, Cassiopeia, Cutera, EPI, Galderma, Journey Almirall, L’Oreal, Ortho Dermatologics, Sun Pharmaceutical Industries, and Vyne.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the clinical experience of Julie C. Harper, MD, an increasing number of women with acne are turning to off-label, long-term treatment with spironolactone.
“Spironolactone is fairly accessible, inexpensive, and effective for our patients,” Dr. Harper, a dermatologist who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
An aldosterone receptor antagonist commonly used to treat high blood pressure and heart failure, spironolactone also has antiandrogenic properties with a proven track record for treating acne and hirsutism. It reduces androgen production, inhibits 5-alpha reductase, and increases sex hormone binding globulin. The dosing range for treating acne is 25 mg to 200 mg per day, but Dr. Harper prefers a maximum dose of 100 mg per day.
According to a systematic review of its use for acne in adult women, the most common side effect is menstrual irregularity, while other common side effects include breast tenderness/swelling, fatigue, and headaches.
“The higher the dose, the higher the rate of side effects,” she said. Concomitant use of an oral contraceptive lessens menstrual irregularities and prevents pregnancies, to avoid exposure during pregnancy and the hypothetical risk of feminization of the male fetus with exposure late in the first trimester. “Early in my career, I used to say if you’re going to be on spironolactone you’re also going to be on an oral contraceptive. But the longer I’ve practiced, I’ve learned that women who have a contraindication to birth control pills or who don’t want to take it can still benefit from an oral antiandrogen by being on spironolactone.”
A large retrospective analysis of 14-year data concluded that routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. “If you’re between the ages of 18 and 45, healthy, and not taking other medications where I’m worried about potassium levels, I’m not checking those levels at all,” Dr. Harper said.
Spironolactone labeling includes a boxed warning regarding the potential for tumorigenicity based on rat studies, but the dosages used in those studies were 25-250 times higher than the exposure dose in humans, Dr. Harper said.
Results from a systematic review and meta-analysis of seven studies in the medical literature found no evidence of an increased risk of breast cancer in women with exposure to spironolactone. “However, the certainty of the evidence was low and future studies are needed, including among diverse populations such as younger individuals and those with acne or hirsutism,” the study authors wrote.
In a separate study, researchers drew from patients in the Humana Insurance database from 2005 to 2017 to address whether spironolactone is associated with an increased risk of recurrence of breast cancer. Recurrent breast cancer was examined in 29,146 women with continuous health insurance for 2 years after a diagnosis of breast cancer. Of these, 746 were prescribed spironolactone, and the remainder were not. The researchers found that 123 women (16.5%) who were prescribed spironolactone had a breast cancer recurrence, compared with 3,649 women (12.8%) with a breast cancer recurrence who had not been prescribed spironolactone (P = .004). Adjusted Cox regression analysis following propensity matching showed no association between spironolactone and increased breast cancer recurrence (adjusted hazard ratio, 0.966; P = .953).
According to Dr. Harper, spironolactone may take about 3 months to kick in. “Likely this is a long-term treatment, and most of the time we’re going to be using it in combination with other acne treatments such as topical retinoids or topical benzoyl peroxide, oral antibiotics, or even isotretinoin.”
A study of long-term spironolactone use in 403 women found that the most common dose prescribed was 100 mg/day, and 68% of the women were concurrently prescribed a topical retinoid, 2.2% an oral antibiotic, and 40.7% an oral contraceptive.
The study population included 32 patients with a history of polycystic ovarian syndrome, 1 with a history of breast cancer, and 5 were hypercoagulable. Patients took the drug for a mean of 471 days. “As opposed to our antibiotics, where the course for patients is generally 3-4 months, when you start someone on spironolactone, they may end up staying on it,” Dr. Harper said.
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, Cassiopeia, Cutera, EPI, Galderma, L’Oreal, Ortho Dermatologics, Sol Gel, and Vyne. She also serves as a speaker or member of a speaker’s bureau for Almirall, Cassiopeia, Cutera, EPI, Galderma, Journey Almirall, L’Oreal, Ortho Dermatologics, Sun Pharmaceutical Industries, and Vyne.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Lebrikizumab monotherapy for AD found safe, effective during induction
, researchers reported in the New England Journal of Medicine.
The identically designed, 52-week, randomized, double-blind, placebo-controlled trials enrolled 851 adolescents and adults with moderate to severe AD and included a 16-week induction period followed by a 36-week maintenance period. At week 16, the results “show a rapid onset of action in multiple domains of the disease, such as skin clearance and itch,” wrote lead author Jonathan Silverberg, MD, PhD, director of clinical research and contact dermatitis, at George Washington University, Washington, and colleagues. “Although 16 weeks of treatment with lebrikizumab is not sufficient to assess its long-term safety, the results from the induction period of these two trials suggest a safety profile that is consistent with findings in previous trials,” they added.
Results presented at the European Academy of Dermatology and Venereology 2022 annual meeting, but not yet published, showed similar efficacy maintained through the end of the trial.
Eligible patients were randomly assigned to receive either lebrikizumab 250 mg (with a 500-mg loading dose given at baseline and at week 2) or placebo, administered subcutaneously every 2 weeks, with concomitant topical or systemic treatments prohibited through week 16 except when deemed appropriate as rescue therapy. In such cases, moderate-potency topical glucocorticoids were preferred as first-line rescue therapy, while the study drug was discontinued if systemic therapy was needed.
In both trials, the primary efficacy outcome – a score of 0 or 1 on the Investigator’s Global Assessment (IGA) – and a reduction of at least 2 points from baseline at week 16, was met by more patients treated with lebrikizumab than with placebo: 43.1% vs. 12.7% respectively in trial 1 (P < .001); and 33.2% vs. 10.8% in trial 2 (P < .001).
Similarly, in both trials, a higher percentage of the lebrikizumab than placebo patients had an EASI-75 response (75% improvement in the Eczema Area and Severity Index score): 58.8% vs. 16.2% (P < .001) in trial 1 and 52.1% vs. 18.1% (P < .001) in trial 2.
Improvement in itch was also significantly better in patients treated with lebrikizumab, compared with placebo. This was measured by a reduction of at least 4 points in the Pruritus NRS from baseline to week 16 and a reduction in the Sleep-Loss Scale score of at least 2 points from baseline to week 16 (P < .001 for both measures in both trials).
A higher percentage of placebo vs. lebrikizumab patients discontinued the trials during the induction phases (14.9% vs. 7.1% in trial 1 and 11.0% vs. 7.8% in trial 2), and the use of rescue medication was approximately three times and two times higher in both placebo groups respectively.
Conjunctivitis was the most common adverse event, occurring consistently more frequently in patients treated with lebrikizumab, compared with placebo (7.4% vs. 2.8% in trial 1 and 7.5% vs. 2.1% in trial 2).
“Although several theories have been proposed for the pathogenesis of conjunctivitis in patients with atopic dermatitis treated with this class of biologic agents, the mechanism remains unclear and warrants further study,” the investigators wrote.
Asked to comment on the new results, Zelma Chiesa Fuxench, MD, who was not involved in the research, said they “continue to demonstrate the superior efficacy and favorable safety profile” of lebrikizumab in adolescents and adults and support the results of earlier phase 2 studies. “The results of these studies thus far continue to offer more hope and the possibility of a better future for our patients with atopic dermatitis who are still struggling to achieve control of their disease.”
Dr. Chiesa Fuxench from the department of dermatology at the University of Pennsylvania, Philadelphia, said she looks forward to reviewing the full study results in which patients who achieved the primary outcomes of interest were then rerandomized to either placebo, or lebrikizumab every 2 weeks or every 4 weeks for the 36-week maintenance period “because we know that there is data for other biologics in atopic dermatitis (such as tralokinumab) that demonstrate that a decrease in the frequency of injections may be possible for patients who achieve disease control after an initial 16 weeks of therapy every 2 weeks.”
The research was supported by Dermira, a wholly owned subsidiary of Eli Lilly. Dr. Silverberg disclosed he is a consultant for Dermira and Eli Lilly, as are other coauthors on the paper who additionally disclosed grants from Dermira and other relationships with Eli Lilly such as advisory board membership and having received lecture fees. Three authors are Eli Lilly employees. Dr. Chiesa Fuxench disclosed that she is a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, Abbvie, and Incyte for which she has received honoraria for work related to AD. Dr. Chiesa Fuxench has also been a recipient of research grants from Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, researchers reported in the New England Journal of Medicine.
The identically designed, 52-week, randomized, double-blind, placebo-controlled trials enrolled 851 adolescents and adults with moderate to severe AD and included a 16-week induction period followed by a 36-week maintenance period. At week 16, the results “show a rapid onset of action in multiple domains of the disease, such as skin clearance and itch,” wrote lead author Jonathan Silverberg, MD, PhD, director of clinical research and contact dermatitis, at George Washington University, Washington, and colleagues. “Although 16 weeks of treatment with lebrikizumab is not sufficient to assess its long-term safety, the results from the induction period of these two trials suggest a safety profile that is consistent with findings in previous trials,” they added.
Results presented at the European Academy of Dermatology and Venereology 2022 annual meeting, but not yet published, showed similar efficacy maintained through the end of the trial.
Eligible patients were randomly assigned to receive either lebrikizumab 250 mg (with a 500-mg loading dose given at baseline and at week 2) or placebo, administered subcutaneously every 2 weeks, with concomitant topical or systemic treatments prohibited through week 16 except when deemed appropriate as rescue therapy. In such cases, moderate-potency topical glucocorticoids were preferred as first-line rescue therapy, while the study drug was discontinued if systemic therapy was needed.
In both trials, the primary efficacy outcome – a score of 0 or 1 on the Investigator’s Global Assessment (IGA) – and a reduction of at least 2 points from baseline at week 16, was met by more patients treated with lebrikizumab than with placebo: 43.1% vs. 12.7% respectively in trial 1 (P < .001); and 33.2% vs. 10.8% in trial 2 (P < .001).
Similarly, in both trials, a higher percentage of the lebrikizumab than placebo patients had an EASI-75 response (75% improvement in the Eczema Area and Severity Index score): 58.8% vs. 16.2% (P < .001) in trial 1 and 52.1% vs. 18.1% (P < .001) in trial 2.
Improvement in itch was also significantly better in patients treated with lebrikizumab, compared with placebo. This was measured by a reduction of at least 4 points in the Pruritus NRS from baseline to week 16 and a reduction in the Sleep-Loss Scale score of at least 2 points from baseline to week 16 (P < .001 for both measures in both trials).
A higher percentage of placebo vs. lebrikizumab patients discontinued the trials during the induction phases (14.9% vs. 7.1% in trial 1 and 11.0% vs. 7.8% in trial 2), and the use of rescue medication was approximately three times and two times higher in both placebo groups respectively.
Conjunctivitis was the most common adverse event, occurring consistently more frequently in patients treated with lebrikizumab, compared with placebo (7.4% vs. 2.8% in trial 1 and 7.5% vs. 2.1% in trial 2).
“Although several theories have been proposed for the pathogenesis of conjunctivitis in patients with atopic dermatitis treated with this class of biologic agents, the mechanism remains unclear and warrants further study,” the investigators wrote.
Asked to comment on the new results, Zelma Chiesa Fuxench, MD, who was not involved in the research, said they “continue to demonstrate the superior efficacy and favorable safety profile” of lebrikizumab in adolescents and adults and support the results of earlier phase 2 studies. “The results of these studies thus far continue to offer more hope and the possibility of a better future for our patients with atopic dermatitis who are still struggling to achieve control of their disease.”
Dr. Chiesa Fuxench from the department of dermatology at the University of Pennsylvania, Philadelphia, said she looks forward to reviewing the full study results in which patients who achieved the primary outcomes of interest were then rerandomized to either placebo, or lebrikizumab every 2 weeks or every 4 weeks for the 36-week maintenance period “because we know that there is data for other biologics in atopic dermatitis (such as tralokinumab) that demonstrate that a decrease in the frequency of injections may be possible for patients who achieve disease control after an initial 16 weeks of therapy every 2 weeks.”
The research was supported by Dermira, a wholly owned subsidiary of Eli Lilly. Dr. Silverberg disclosed he is a consultant for Dermira and Eli Lilly, as are other coauthors on the paper who additionally disclosed grants from Dermira and other relationships with Eli Lilly such as advisory board membership and having received lecture fees. Three authors are Eli Lilly employees. Dr. Chiesa Fuxench disclosed that she is a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, Abbvie, and Incyte for which she has received honoraria for work related to AD. Dr. Chiesa Fuxench has also been a recipient of research grants from Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, researchers reported in the New England Journal of Medicine.
The identically designed, 52-week, randomized, double-blind, placebo-controlled trials enrolled 851 adolescents and adults with moderate to severe AD and included a 16-week induction period followed by a 36-week maintenance period. At week 16, the results “show a rapid onset of action in multiple domains of the disease, such as skin clearance and itch,” wrote lead author Jonathan Silverberg, MD, PhD, director of clinical research and contact dermatitis, at George Washington University, Washington, and colleagues. “Although 16 weeks of treatment with lebrikizumab is not sufficient to assess its long-term safety, the results from the induction period of these two trials suggest a safety profile that is consistent with findings in previous trials,” they added.
Results presented at the European Academy of Dermatology and Venereology 2022 annual meeting, but not yet published, showed similar efficacy maintained through the end of the trial.
Eligible patients were randomly assigned to receive either lebrikizumab 250 mg (with a 500-mg loading dose given at baseline and at week 2) or placebo, administered subcutaneously every 2 weeks, with concomitant topical or systemic treatments prohibited through week 16 except when deemed appropriate as rescue therapy. In such cases, moderate-potency topical glucocorticoids were preferred as first-line rescue therapy, while the study drug was discontinued if systemic therapy was needed.
In both trials, the primary efficacy outcome – a score of 0 or 1 on the Investigator’s Global Assessment (IGA) – and a reduction of at least 2 points from baseline at week 16, was met by more patients treated with lebrikizumab than with placebo: 43.1% vs. 12.7% respectively in trial 1 (P < .001); and 33.2% vs. 10.8% in trial 2 (P < .001).
Similarly, in both trials, a higher percentage of the lebrikizumab than placebo patients had an EASI-75 response (75% improvement in the Eczema Area and Severity Index score): 58.8% vs. 16.2% (P < .001) in trial 1 and 52.1% vs. 18.1% (P < .001) in trial 2.
Improvement in itch was also significantly better in patients treated with lebrikizumab, compared with placebo. This was measured by a reduction of at least 4 points in the Pruritus NRS from baseline to week 16 and a reduction in the Sleep-Loss Scale score of at least 2 points from baseline to week 16 (P < .001 for both measures in both trials).
A higher percentage of placebo vs. lebrikizumab patients discontinued the trials during the induction phases (14.9% vs. 7.1% in trial 1 and 11.0% vs. 7.8% in trial 2), and the use of rescue medication was approximately three times and two times higher in both placebo groups respectively.
Conjunctivitis was the most common adverse event, occurring consistently more frequently in patients treated with lebrikizumab, compared with placebo (7.4% vs. 2.8% in trial 1 and 7.5% vs. 2.1% in trial 2).
“Although several theories have been proposed for the pathogenesis of conjunctivitis in patients with atopic dermatitis treated with this class of biologic agents, the mechanism remains unclear and warrants further study,” the investigators wrote.
Asked to comment on the new results, Zelma Chiesa Fuxench, MD, who was not involved in the research, said they “continue to demonstrate the superior efficacy and favorable safety profile” of lebrikizumab in adolescents and adults and support the results of earlier phase 2 studies. “The results of these studies thus far continue to offer more hope and the possibility of a better future for our patients with atopic dermatitis who are still struggling to achieve control of their disease.”
Dr. Chiesa Fuxench from the department of dermatology at the University of Pennsylvania, Philadelphia, said she looks forward to reviewing the full study results in which patients who achieved the primary outcomes of interest were then rerandomized to either placebo, or lebrikizumab every 2 weeks or every 4 weeks for the 36-week maintenance period “because we know that there is data for other biologics in atopic dermatitis (such as tralokinumab) that demonstrate that a decrease in the frequency of injections may be possible for patients who achieve disease control after an initial 16 weeks of therapy every 2 weeks.”
The research was supported by Dermira, a wholly owned subsidiary of Eli Lilly. Dr. Silverberg disclosed he is a consultant for Dermira and Eli Lilly, as are other coauthors on the paper who additionally disclosed grants from Dermira and other relationships with Eli Lilly such as advisory board membership and having received lecture fees. Three authors are Eli Lilly employees. Dr. Chiesa Fuxench disclosed that she is a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, Abbvie, and Incyte for which she has received honoraria for work related to AD. Dr. Chiesa Fuxench has also been a recipient of research grants from Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
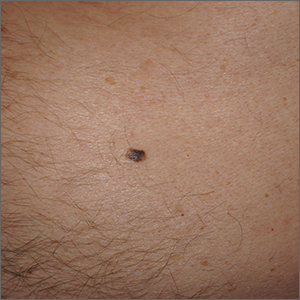
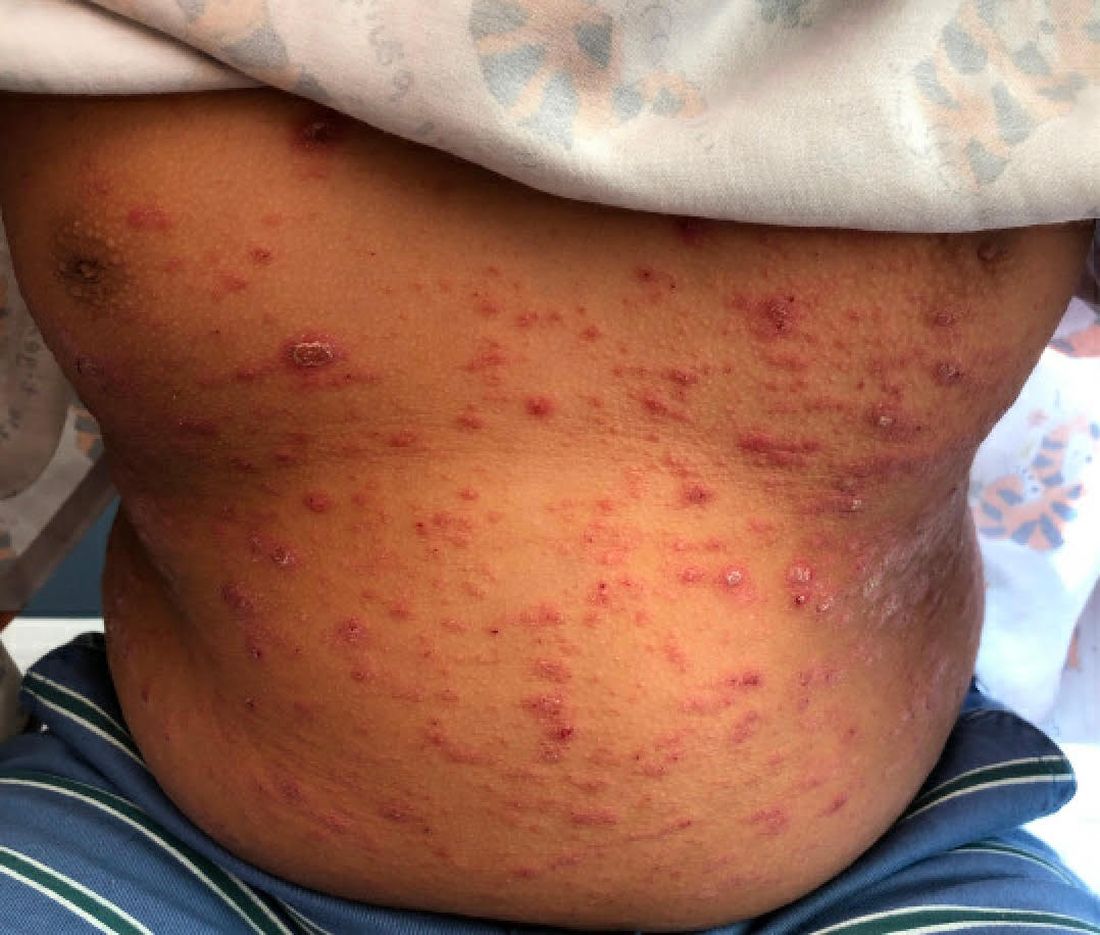
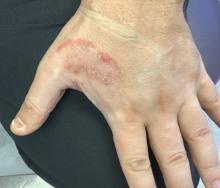
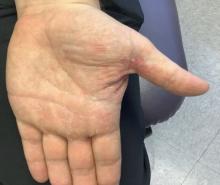
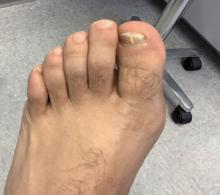
Solitary abdominal papule
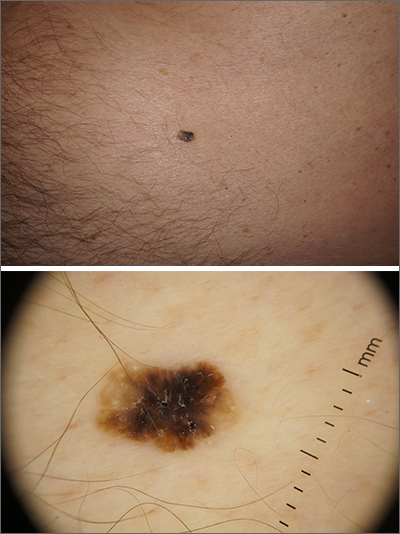
Dermoscopy revealed an 8-mm scaly brown-black papule that lacked melanocytic features (pigment network, globules, streaks, or homogeneous blue or brown color) but had milia-like cysts and so-called “fat fingers” (short, straight to curved radial projections1). These findings were consistent with a diagnosis of seborrheic keratosis (SK).
SKs go by many names and are often confused with nevi. Some patients might know them by such names as “age spots” or “liver spots.” Patients often have many SKs on their body; the back and skin folds are common locations. Patients may be unhappy about the way they look and may describe occasional discomfort when the SKs rub against clothes and inflammation that occurs spontaneously or with trauma.
Classic SKs have a well-demarcated border and waxy, stuck-on appearance. There are times when it is difficult to distinguish between an SK and a melanocytic lesion. Thus, a biopsy may be necessary. In addition, SKs are so common that collision lesions may occur. (Collision lesions result when 2 histologically distinct neoplasms occur adjacent to each other and cause an unusual clinical appearance with features of each lesion.) The atypical clinical features in a collision lesion may prompt a biopsy to exclude malignancy.
Dermoscopic features of SKs include well-demarcated borders, milia-like cysts (white circular inclusions), comedo-like openings (brown/black circular inclusions), fissures and ridges, hairpin vessels, and fat fingers.
Cryotherapy is a quick and efficient treatment when a patient would like the lesions removed. Curettage or light electrodessication may be less likely to cause post-inflammatory hypopigmentation in patients with darker skin types. These various destructive therapies are often considered cosmetic and are unlikely to be covered by insurance unless there is documentation of significant inflammation or discomfort. In this case, the lesion was not treated.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Wang S, Rabinovitz H, Oliviero M, et al. Solar lentigines, seborrheic keratoses, and lichen planus-like keratoses. In: Marghoob A, Malvehy J, Braun, R, eds. Atlas of Dermoscopy. 2nd ed. Informa Healthcare; 2012: 58-69.

Dermoscopy revealed an 8-mm scaly brown-black papule that lacked melanocytic features (pigment network, globules, streaks, or homogeneous blue or brown color) but had milia-like cysts and so-called “fat fingers” (short, straight to curved radial projections1). These findings were consistent with a diagnosis of seborrheic keratosis (SK).
SKs go by many names and are often confused with nevi. Some patients might know them by such names as “age spots” or “liver spots.” Patients often have many SKs on their body; the back and skin folds are common locations. Patients may be unhappy about the way they look and may describe occasional discomfort when the SKs rub against clothes and inflammation that occurs spontaneously or with trauma.
Classic SKs have a well-demarcated border and waxy, stuck-on appearance. There are times when it is difficult to distinguish between an SK and a melanocytic lesion. Thus, a biopsy may be necessary. In addition, SKs are so common that collision lesions may occur. (Collision lesions result when 2 histologically distinct neoplasms occur adjacent to each other and cause an unusual clinical appearance with features of each lesion.) The atypical clinical features in a collision lesion may prompt a biopsy to exclude malignancy.
Dermoscopic features of SKs include well-demarcated borders, milia-like cysts (white circular inclusions), comedo-like openings (brown/black circular inclusions), fissures and ridges, hairpin vessels, and fat fingers.
Cryotherapy is a quick and efficient treatment when a patient would like the lesions removed. Curettage or light electrodessication may be less likely to cause post-inflammatory hypopigmentation in patients with darker skin types. These various destructive therapies are often considered cosmetic and are unlikely to be covered by insurance unless there is documentation of significant inflammation or discomfort. In this case, the lesion was not treated.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Dermoscopy revealed an 8-mm scaly brown-black papule that lacked melanocytic features (pigment network, globules, streaks, or homogeneous blue or brown color) but had milia-like cysts and so-called “fat fingers” (short, straight to curved radial projections1). These findings were consistent with a diagnosis of seborrheic keratosis (SK).
SKs go by many names and are often confused with nevi. Some patients might know them by such names as “age spots” or “liver spots.” Patients often have many SKs on their body; the back and skin folds are common locations. Patients may be unhappy about the way they look and may describe occasional discomfort when the SKs rub against clothes and inflammation that occurs spontaneously or with trauma.
Classic SKs have a well-demarcated border and waxy, stuck-on appearance. There are times when it is difficult to distinguish between an SK and a melanocytic lesion. Thus, a biopsy may be necessary. In addition, SKs are so common that collision lesions may occur. (Collision lesions result when 2 histologically distinct neoplasms occur adjacent to each other and cause an unusual clinical appearance with features of each lesion.) The atypical clinical features in a collision lesion may prompt a biopsy to exclude malignancy.
Dermoscopic features of SKs include well-demarcated borders, milia-like cysts (white circular inclusions), comedo-like openings (brown/black circular inclusions), fissures and ridges, hairpin vessels, and fat fingers.
Cryotherapy is a quick and efficient treatment when a patient would like the lesions removed. Curettage or light electrodessication may be less likely to cause post-inflammatory hypopigmentation in patients with darker skin types. These various destructive therapies are often considered cosmetic and are unlikely to be covered by insurance unless there is documentation of significant inflammation or discomfort. In this case, the lesion was not treated.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Wang S, Rabinovitz H, Oliviero M, et al. Solar lentigines, seborrheic keratoses, and lichen planus-like keratoses. In: Marghoob A, Malvehy J, Braun, R, eds. Atlas of Dermoscopy. 2nd ed. Informa Healthcare; 2012: 58-69.
1. Wang S, Rabinovitz H, Oliviero M, et al. Solar lentigines, seborrheic keratoses, and lichen planus-like keratoses. In: Marghoob A, Malvehy J, Braun, R, eds. Atlas of Dermoscopy. 2nd ed. Informa Healthcare; 2012: 58-69.
Spinosad: New kid on the block for treating scabies
HONOLULU – , Anthony J. Mancini, MD, said during a presentation at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
In April 2021, spinosad topical suspension 0.9%, was approved by the Food and Drug Administration for treating scabies infestations in adult and pediatric patients 4 years of age and older – a first-in-class drug and the first new scabicide approved in 31 years. It was also approved for treating head lice in adults and children aged 6 months of age and older.
“Scabies has been described as the worst itch one can experience,” said Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago. “It’s a hallmark of the disease, it can persist for weeks, it’s most intense at night, and patients report various sensations. It’s believed to be a both type I and type IV hypersensitivity reaction.”
The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. Besides intense itching, the classic presentation consists of a skin rash composed of inflammatory papules, linear burrows and crusted papules (especially on the hands, feet, and groin), and at times, larger red nodules. “Scabies nodules can persist for many months,” he said.
The Global Burden of Disease Study 2015 cited scabies as having the greatest burden of disease in tropical regions, especially among children, adolescents, and the elderly. The greatest burden of disability-adjusted life years (DALYs) occurred in East and Southeast Asia, Oceana, and tropical South America, but in North America, there was a 24% increase in the DALY rate between 1990 and 2015.
In addition, the World Health Organization designated scabies as a neglected tropical disease in 2017 and included it in its 10-year road map for neglected tropical diseases 2021-2030 with goals of promoting disease awareness and encouraging research and achieving global control.
“In our country, we typically see scabies treated successfully without complications, but there can be complications, especially in underdeveloped areas, like Staph aureus and Group A beta-hemolytic streptococcal infections,” which can be fatal, said Dr. Mancini, who is also head of pediatric dermatology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Permethrin 5% cream is typically offered as first-line topical therapy in the United States for the treatment of scabies. However, in vitro studies and small investigator-initiated in vivo studies have reported that efficacy appears to be decreasing. In one of the trials, Italian researchers enrolled 155 patients who were treated with permethrin 5% for 8 hours for 2 consecutive days and repeated the treatment 5 days later . Following the course of permethrin, only 34 responded, 96 failed treatment, and 25 were lost to follow-up.
“The study authors concluded that mite resistance to permethrin 5% seems to be increasing, following a path like other ectoparasite resistance,” said Dr. Mancini, who was not involved with the study. “We may even be seeing more ivermectin resistance in some geographic locations, as well.”
According to new scabicide efficacy criteria established by the FDA in 2016, complete cure is now defined as meeting both clinical and confirmatory criteria. A clinical cure means that all signs and symptoms of scabies have completely resolved, including burrows, inflammatory/noninflammatory lesions, and pruritus. A confirmatory cure means there is an absence of mites, eggs, scybala (feces), and burrows via microscopy or dermoscopy.
Enter spinosad, which is derived from a naturally occurring soil microorganism known as Saccharopolyspora spinosa and is composed of two active molecules: spinosyn A and spinosyn D. According to Dr. Mancini, spinosad’s mechanism of action is unique from other medications used to treat ectoparasites. It activates nicotinic and GABA-gated sodium channels, leads to sodium influx in the insect nerves, hyperexcitation, then paralysis and death. Cross-resistance to other insecticides has not been reported, he added, and there is no known evidence of resistance to its active compound.
Approval of the drug was based on data from two phase 3 randomized clinical trials involving 551 index cases and household contacts. In the intent-to-treat population, with the two trials combined, complete cure was achieved in 78.1% of the spinosad-treated group, compared with 39.6% in the vehicle group (P < .0001), clinical cure was achieved in 79.6% of the spinosad group, compared with 41.2% in the vehicle group (P < .001), and microscopic cure occurred in 85.9% of the spinosad group, compared with 52.6% in the vehicle group (P < .001).
Of the 306 participants in the study, the only adverse events reported by more than one patient each included abdominal pain, back pain, cough, headache, neck pain, and decreased weight in two patients each (0.8%), which investigators believed were not attributable to the study drug. Adverse events that investigators considered to be potentially related to the study drug included burning sensation in two participants (0.7%) and dry skin in another (0.3%). In clinical trials reported in the prescribing information, adverse events occurring in greater than 1% of subjects included application-site irritation (3% spinosad vs. 0% vehicle) and dry skin (2% spinosad vs. 0% vehicle).
“Spinosad met the FDA’s new stringent criteria, with all signs and symptoms of scabies completely resolved and confirmed via microscopy or dermoscopy,” said Dr. Mancini, who was not involved in the trials. “The patented formulation drives the active compound to the stratum corneum, where mites live and breed. It’s a single full-body application, without any resistance observed to date. This is an exciting newer option for treating our scabies patients.”
In an interview at the meeting, John S. Barbieri, MD, MBA, of the department of dermatology, Brigham and Women’s Hospital, Boston, said that, while he has no clinical experience with spinosad for scabies, he welcomes a new option for the condition. “The fact that it has a different mechanism of action than permethrin is a good thing,” he said.
Dr. Mancini disclosed that he is a consultant or an adviser for ParaPRO, the manufacturer of spinosad, and Cassiopea, Castle Creek, Novan, Novartis, and Verrica. He was not involved in clinical trials of spinosad. Dr. Barbieri disclosed that he receives consulting fees from Dexcel.
Medscape and this news organization are owned by the same parent company.
HONOLULU – , Anthony J. Mancini, MD, said during a presentation at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
In April 2021, spinosad topical suspension 0.9%, was approved by the Food and Drug Administration for treating scabies infestations in adult and pediatric patients 4 years of age and older – a first-in-class drug and the first new scabicide approved in 31 years. It was also approved for treating head lice in adults and children aged 6 months of age and older.
“Scabies has been described as the worst itch one can experience,” said Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago. “It’s a hallmark of the disease, it can persist for weeks, it’s most intense at night, and patients report various sensations. It’s believed to be a both type I and type IV hypersensitivity reaction.”
The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. Besides intense itching, the classic presentation consists of a skin rash composed of inflammatory papules, linear burrows and crusted papules (especially on the hands, feet, and groin), and at times, larger red nodules. “Scabies nodules can persist for many months,” he said.
The Global Burden of Disease Study 2015 cited scabies as having the greatest burden of disease in tropical regions, especially among children, adolescents, and the elderly. The greatest burden of disability-adjusted life years (DALYs) occurred in East and Southeast Asia, Oceana, and tropical South America, but in North America, there was a 24% increase in the DALY rate between 1990 and 2015.
In addition, the World Health Organization designated scabies as a neglected tropical disease in 2017 and included it in its 10-year road map for neglected tropical diseases 2021-2030 with goals of promoting disease awareness and encouraging research and achieving global control.
“In our country, we typically see scabies treated successfully without complications, but there can be complications, especially in underdeveloped areas, like Staph aureus and Group A beta-hemolytic streptococcal infections,” which can be fatal, said Dr. Mancini, who is also head of pediatric dermatology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Permethrin 5% cream is typically offered as first-line topical therapy in the United States for the treatment of scabies. However, in vitro studies and small investigator-initiated in vivo studies have reported that efficacy appears to be decreasing. In one of the trials, Italian researchers enrolled 155 patients who were treated with permethrin 5% for 8 hours for 2 consecutive days and repeated the treatment 5 days later . Following the course of permethrin, only 34 responded, 96 failed treatment, and 25 were lost to follow-up.
“The study authors concluded that mite resistance to permethrin 5% seems to be increasing, following a path like other ectoparasite resistance,” said Dr. Mancini, who was not involved with the study. “We may even be seeing more ivermectin resistance in some geographic locations, as well.”
According to new scabicide efficacy criteria established by the FDA in 2016, complete cure is now defined as meeting both clinical and confirmatory criteria. A clinical cure means that all signs and symptoms of scabies have completely resolved, including burrows, inflammatory/noninflammatory lesions, and pruritus. A confirmatory cure means there is an absence of mites, eggs, scybala (feces), and burrows via microscopy or dermoscopy.
Enter spinosad, which is derived from a naturally occurring soil microorganism known as Saccharopolyspora spinosa and is composed of two active molecules: spinosyn A and spinosyn D. According to Dr. Mancini, spinosad’s mechanism of action is unique from other medications used to treat ectoparasites. It activates nicotinic and GABA-gated sodium channels, leads to sodium influx in the insect nerves, hyperexcitation, then paralysis and death. Cross-resistance to other insecticides has not been reported, he added, and there is no known evidence of resistance to its active compound.
Approval of the drug was based on data from two phase 3 randomized clinical trials involving 551 index cases and household contacts. In the intent-to-treat population, with the two trials combined, complete cure was achieved in 78.1% of the spinosad-treated group, compared with 39.6% in the vehicle group (P < .0001), clinical cure was achieved in 79.6% of the spinosad group, compared with 41.2% in the vehicle group (P < .001), and microscopic cure occurred in 85.9% of the spinosad group, compared with 52.6% in the vehicle group (P < .001).
Of the 306 participants in the study, the only adverse events reported by more than one patient each included abdominal pain, back pain, cough, headache, neck pain, and decreased weight in two patients each (0.8%), which investigators believed were not attributable to the study drug. Adverse events that investigators considered to be potentially related to the study drug included burning sensation in two participants (0.7%) and dry skin in another (0.3%). In clinical trials reported in the prescribing information, adverse events occurring in greater than 1% of subjects included application-site irritation (3% spinosad vs. 0% vehicle) and dry skin (2% spinosad vs. 0% vehicle).
“Spinosad met the FDA’s new stringent criteria, with all signs and symptoms of scabies completely resolved and confirmed via microscopy or dermoscopy,” said Dr. Mancini, who was not involved in the trials. “The patented formulation drives the active compound to the stratum corneum, where mites live and breed. It’s a single full-body application, without any resistance observed to date. This is an exciting newer option for treating our scabies patients.”
In an interview at the meeting, John S. Barbieri, MD, MBA, of the department of dermatology, Brigham and Women’s Hospital, Boston, said that, while he has no clinical experience with spinosad for scabies, he welcomes a new option for the condition. “The fact that it has a different mechanism of action than permethrin is a good thing,” he said.
Dr. Mancini disclosed that he is a consultant or an adviser for ParaPRO, the manufacturer of spinosad, and Cassiopea, Castle Creek, Novan, Novartis, and Verrica. He was not involved in clinical trials of spinosad. Dr. Barbieri disclosed that he receives consulting fees from Dexcel.
Medscape and this news organization are owned by the same parent company.
HONOLULU – , Anthony J. Mancini, MD, said during a presentation at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
In April 2021, spinosad topical suspension 0.9%, was approved by the Food and Drug Administration for treating scabies infestations in adult and pediatric patients 4 years of age and older – a first-in-class drug and the first new scabicide approved in 31 years. It was also approved for treating head lice in adults and children aged 6 months of age and older.
“Scabies has been described as the worst itch one can experience,” said Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago. “It’s a hallmark of the disease, it can persist for weeks, it’s most intense at night, and patients report various sensations. It’s believed to be a both type I and type IV hypersensitivity reaction.”
The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. Besides intense itching, the classic presentation consists of a skin rash composed of inflammatory papules, linear burrows and crusted papules (especially on the hands, feet, and groin), and at times, larger red nodules. “Scabies nodules can persist for many months,” he said.
The Global Burden of Disease Study 2015 cited scabies as having the greatest burden of disease in tropical regions, especially among children, adolescents, and the elderly. The greatest burden of disability-adjusted life years (DALYs) occurred in East and Southeast Asia, Oceana, and tropical South America, but in North America, there was a 24% increase in the DALY rate between 1990 and 2015.
In addition, the World Health Organization designated scabies as a neglected tropical disease in 2017 and included it in its 10-year road map for neglected tropical diseases 2021-2030 with goals of promoting disease awareness and encouraging research and achieving global control.
“In our country, we typically see scabies treated successfully without complications, but there can be complications, especially in underdeveloped areas, like Staph aureus and Group A beta-hemolytic streptococcal infections,” which can be fatal, said Dr. Mancini, who is also head of pediatric dermatology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Permethrin 5% cream is typically offered as first-line topical therapy in the United States for the treatment of scabies. However, in vitro studies and small investigator-initiated in vivo studies have reported that efficacy appears to be decreasing. In one of the trials, Italian researchers enrolled 155 patients who were treated with permethrin 5% for 8 hours for 2 consecutive days and repeated the treatment 5 days later . Following the course of permethrin, only 34 responded, 96 failed treatment, and 25 were lost to follow-up.
“The study authors concluded that mite resistance to permethrin 5% seems to be increasing, following a path like other ectoparasite resistance,” said Dr. Mancini, who was not involved with the study. “We may even be seeing more ivermectin resistance in some geographic locations, as well.”
According to new scabicide efficacy criteria established by the FDA in 2016, complete cure is now defined as meeting both clinical and confirmatory criteria. A clinical cure means that all signs and symptoms of scabies have completely resolved, including burrows, inflammatory/noninflammatory lesions, and pruritus. A confirmatory cure means there is an absence of mites, eggs, scybala (feces), and burrows via microscopy or dermoscopy.
Enter spinosad, which is derived from a naturally occurring soil microorganism known as Saccharopolyspora spinosa and is composed of two active molecules: spinosyn A and spinosyn D. According to Dr. Mancini, spinosad’s mechanism of action is unique from other medications used to treat ectoparasites. It activates nicotinic and GABA-gated sodium channels, leads to sodium influx in the insect nerves, hyperexcitation, then paralysis and death. Cross-resistance to other insecticides has not been reported, he added, and there is no known evidence of resistance to its active compound.
Approval of the drug was based on data from two phase 3 randomized clinical trials involving 551 index cases and household contacts. In the intent-to-treat population, with the two trials combined, complete cure was achieved in 78.1% of the spinosad-treated group, compared with 39.6% in the vehicle group (P < .0001), clinical cure was achieved in 79.6% of the spinosad group, compared with 41.2% in the vehicle group (P < .001), and microscopic cure occurred in 85.9% of the spinosad group, compared with 52.6% in the vehicle group (P < .001).
Of the 306 participants in the study, the only adverse events reported by more than one patient each included abdominal pain, back pain, cough, headache, neck pain, and decreased weight in two patients each (0.8%), which investigators believed were not attributable to the study drug. Adverse events that investigators considered to be potentially related to the study drug included burning sensation in two participants (0.7%) and dry skin in another (0.3%). In clinical trials reported in the prescribing information, adverse events occurring in greater than 1% of subjects included application-site irritation (3% spinosad vs. 0% vehicle) and dry skin (2% spinosad vs. 0% vehicle).
“Spinosad met the FDA’s new stringent criteria, with all signs and symptoms of scabies completely resolved and confirmed via microscopy or dermoscopy,” said Dr. Mancini, who was not involved in the trials. “The patented formulation drives the active compound to the stratum corneum, where mites live and breed. It’s a single full-body application, without any resistance observed to date. This is an exciting newer option for treating our scabies patients.”
In an interview at the meeting, John S. Barbieri, MD, MBA, of the department of dermatology, Brigham and Women’s Hospital, Boston, said that, while he has no clinical experience with spinosad for scabies, he welcomes a new option for the condition. “The fact that it has a different mechanism of action than permethrin is a good thing,” he said.
Dr. Mancini disclosed that he is a consultant or an adviser for ParaPRO, the manufacturer of spinosad, and Cassiopea, Castle Creek, Novan, Novartis, and Verrica. He was not involved in clinical trials of spinosad. Dr. Barbieri disclosed that he receives consulting fees from Dexcel.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Nearly one in three patients with IBD affected by skin lesions
People with inflammatory bowel disease (IBD) commonly develop skin lesions linked to their condition, but until now few researchers looked at how common they are.
, according to the prospective, single-center study.
“Skin lesions in IBD patients are much more prevalent than it is generally accepted. The lesions may be related to the pathogenesis of IBD, but it is very important to know that the modern biological therapies may also cause skin lesions,” said senior study author Laimas Jonaitis, MD, PhD, professor in the department of gastroenterology at Lithuanian University of Health Sciences in Kaunas.
“If the gastroenterologist is experienced and has enough competence, he or she may establish the diagnosis, but in all other cases it is wise and advisable to refer the patient to the dermatologist,” Dr. Jonaitis said. A referral should include the history and full treatment for IBD.
The results were presented as a poster at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
Dr. Jonaitis and colleagues conducted a literature analysis to determine the prevalence of extra-abdominal manifestations of IBD. The lack of published data prompted them to survey 152 consecutive patients with IBD receiving outpatient treatment at their institution. The patients completed questionnaires from January to October 2022 about any cutaneous lesions.
The mean age of patients was 42 years, and 58% were men. A majority, 72%, had ulcerative colitis, and 28% had Crohn’s disease.
Prevalence of skin lesions
A total of 43% of participants reported skin lesions, but only 30% of patients had lesions considered related to IBD or IBD therapy due to their emergence after the patient’s IBD diagnosis.
By IBD diagnosis, 29% of patients with ulcerative colitis and 33% of patients with Crohn’s disease had lesions related to their condition. The difference in skin lesion prevalence between the two groups was not significant (P > .05), the researchers noted.
The team further investigated the types of skin lesions deemed to be associated with IBD or IBD therapy.
Overall, they found psoriasis in nine patients, eczema in nine, erythema nodosum in six, pyoderma gangrenosum in five, allergic rash in four, and vitiligo in two. They found acne, epidermolysis bullosa acquisita, and hemorrhagic vasculitis in one patient each.
Specifically, among patients with ulcerative colitis, skin lesions were reported in 8 of 27 with left-sided colitis, 2 of 15 with ulcerative colitis proctitis, and 22 of 67 patients with pancolitis. The difference between the groups of proctitis and pancolitis was significant (P = .03).
Within the group with Crohn’s disease, skin lesions were reported in 3 of 15 patients with ileitis, 4 of 10 with colitis, and 7 of 17 with ileocolitis. The difference among these groups was not significant (P > .05).
The most common skin lesions observed in Crohn’s disease were erythema nodosum and eczema, and in ulcerative colitis, psoriasis and eczema, the researchers reported.
They also noted that the cutaneous lesions were significantly more prevalent in extensive ulcerative colitis compared with distal disease.
Skin lesions add to patient misery
“Skin lesions are considered a burden to patients with IBD and add to their suffering,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in the United Kingdom, who was not affiliated with the research.
The severity and location of the disease appears to play a role because researchers found extensive ulcerative colitis may carry a higher risk for the development of skin lesions, Dr. Mesilhy noted.
The first step when facing skin lesions is to control the disease activity via the best treatment option, Dr. Mesilhy suggested.
The study was independently supported. Dr. Jonaitis and Dr. Mesilhy have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with inflammatory bowel disease (IBD) commonly develop skin lesions linked to their condition, but until now few researchers looked at how common they are.
, according to the prospective, single-center study.
“Skin lesions in IBD patients are much more prevalent than it is generally accepted. The lesions may be related to the pathogenesis of IBD, but it is very important to know that the modern biological therapies may also cause skin lesions,” said senior study author Laimas Jonaitis, MD, PhD, professor in the department of gastroenterology at Lithuanian University of Health Sciences in Kaunas.
“If the gastroenterologist is experienced and has enough competence, he or she may establish the diagnosis, but in all other cases it is wise and advisable to refer the patient to the dermatologist,” Dr. Jonaitis said. A referral should include the history and full treatment for IBD.
The results were presented as a poster at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
Dr. Jonaitis and colleagues conducted a literature analysis to determine the prevalence of extra-abdominal manifestations of IBD. The lack of published data prompted them to survey 152 consecutive patients with IBD receiving outpatient treatment at their institution. The patients completed questionnaires from January to October 2022 about any cutaneous lesions.
The mean age of patients was 42 years, and 58% were men. A majority, 72%, had ulcerative colitis, and 28% had Crohn’s disease.
Prevalence of skin lesions
A total of 43% of participants reported skin lesions, but only 30% of patients had lesions considered related to IBD or IBD therapy due to their emergence after the patient’s IBD diagnosis.
By IBD diagnosis, 29% of patients with ulcerative colitis and 33% of patients with Crohn’s disease had lesions related to their condition. The difference in skin lesion prevalence between the two groups was not significant (P > .05), the researchers noted.
The team further investigated the types of skin lesions deemed to be associated with IBD or IBD therapy.
Overall, they found psoriasis in nine patients, eczema in nine, erythema nodosum in six, pyoderma gangrenosum in five, allergic rash in four, and vitiligo in two. They found acne, epidermolysis bullosa acquisita, and hemorrhagic vasculitis in one patient each.
Specifically, among patients with ulcerative colitis, skin lesions were reported in 8 of 27 with left-sided colitis, 2 of 15 with ulcerative colitis proctitis, and 22 of 67 patients with pancolitis. The difference between the groups of proctitis and pancolitis was significant (P = .03).
Within the group with Crohn’s disease, skin lesions were reported in 3 of 15 patients with ileitis, 4 of 10 with colitis, and 7 of 17 with ileocolitis. The difference among these groups was not significant (P > .05).
The most common skin lesions observed in Crohn’s disease were erythema nodosum and eczema, and in ulcerative colitis, psoriasis and eczema, the researchers reported.
They also noted that the cutaneous lesions were significantly more prevalent in extensive ulcerative colitis compared with distal disease.
Skin lesions add to patient misery
“Skin lesions are considered a burden to patients with IBD and add to their suffering,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in the United Kingdom, who was not affiliated with the research.
The severity and location of the disease appears to play a role because researchers found extensive ulcerative colitis may carry a higher risk for the development of skin lesions, Dr. Mesilhy noted.
The first step when facing skin lesions is to control the disease activity via the best treatment option, Dr. Mesilhy suggested.
The study was independently supported. Dr. Jonaitis and Dr. Mesilhy have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with inflammatory bowel disease (IBD) commonly develop skin lesions linked to their condition, but until now few researchers looked at how common they are.
, according to the prospective, single-center study.
“Skin lesions in IBD patients are much more prevalent than it is generally accepted. The lesions may be related to the pathogenesis of IBD, but it is very important to know that the modern biological therapies may also cause skin lesions,” said senior study author Laimas Jonaitis, MD, PhD, professor in the department of gastroenterology at Lithuanian University of Health Sciences in Kaunas.
“If the gastroenterologist is experienced and has enough competence, he or she may establish the diagnosis, but in all other cases it is wise and advisable to refer the patient to the dermatologist,” Dr. Jonaitis said. A referral should include the history and full treatment for IBD.
The results were presented as a poster at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
Dr. Jonaitis and colleagues conducted a literature analysis to determine the prevalence of extra-abdominal manifestations of IBD. The lack of published data prompted them to survey 152 consecutive patients with IBD receiving outpatient treatment at their institution. The patients completed questionnaires from January to October 2022 about any cutaneous lesions.
The mean age of patients was 42 years, and 58% were men. A majority, 72%, had ulcerative colitis, and 28% had Crohn’s disease.
Prevalence of skin lesions
A total of 43% of participants reported skin lesions, but only 30% of patients had lesions considered related to IBD or IBD therapy due to their emergence after the patient’s IBD diagnosis.
By IBD diagnosis, 29% of patients with ulcerative colitis and 33% of patients with Crohn’s disease had lesions related to their condition. The difference in skin lesion prevalence between the two groups was not significant (P > .05), the researchers noted.
The team further investigated the types of skin lesions deemed to be associated with IBD or IBD therapy.
Overall, they found psoriasis in nine patients, eczema in nine, erythema nodosum in six, pyoderma gangrenosum in five, allergic rash in four, and vitiligo in two. They found acne, epidermolysis bullosa acquisita, and hemorrhagic vasculitis in one patient each.
Specifically, among patients with ulcerative colitis, skin lesions were reported in 8 of 27 with left-sided colitis, 2 of 15 with ulcerative colitis proctitis, and 22 of 67 patients with pancolitis. The difference between the groups of proctitis and pancolitis was significant (P = .03).
Within the group with Crohn’s disease, skin lesions were reported in 3 of 15 patients with ileitis, 4 of 10 with colitis, and 7 of 17 with ileocolitis. The difference among these groups was not significant (P > .05).
The most common skin lesions observed in Crohn’s disease were erythema nodosum and eczema, and in ulcerative colitis, psoriasis and eczema, the researchers reported.
They also noted that the cutaneous lesions were significantly more prevalent in extensive ulcerative colitis compared with distal disease.
Skin lesions add to patient misery
“Skin lesions are considered a burden to patients with IBD and add to their suffering,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in the United Kingdom, who was not affiliated with the research.
The severity and location of the disease appears to play a role because researchers found extensive ulcerative colitis may carry a higher risk for the development of skin lesions, Dr. Mesilhy noted.
The first step when facing skin lesions is to control the disease activity via the best treatment option, Dr. Mesilhy suggested.
The study was independently supported. Dr. Jonaitis and Dr. Mesilhy have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ECCO 2023
A 9-year-old male presents with multiple thick scaly plaques on scalp, ears, and trunk
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
A 9-year-old male is seen in the clinic with a 1-year history of multiple thick scaly plaques on scalp, ears, and trunk. He has been treated with hydrocortisone 1% ointment with no change in the lesions. He had upper respiratory tract symptoms 3 weeks prior to the visit.
Examination reveals erythematous, well-demarcated plaques of the anterior scalp with thick overlying micaceous scale with some extension onto the forehead and temples. Additionally, erythematous scaly patches on the ear, axilla, and umbilicus were noted. There was no palmar or plantar involvement. He denied joint swelling, stiffness, or pain in the morning.
White male presents with pruritic, scaly, erythematous patches on his feet and left hand
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Biologics show signs of delaying arthritis in psoriasis patients
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
FROM LANCET RHEUMATOLOGY
FDA to review dupilumab for treating chronic spontaneous urticaria
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.