User login
FDA accepts application for topical molluscum treatment
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
Pembrolizumab before and after melanoma surgery boosts outcomes
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Be vigilant about suspected cases of measles, expert advises
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Widespread flaky red skin
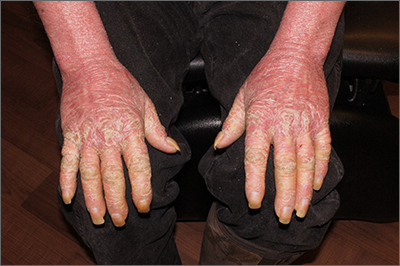
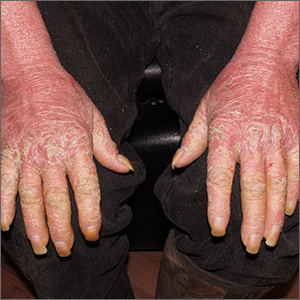
This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
Nicotinamide does not prevent skin cancer after organ transplant
published in the New England Journal of Medicine.
“No signal of efficacy was observed,” said investigators led by Nicholas Allen, MPH, of the University of Sydney department of dermatology.
These results fill an “important gap in our understanding” and “will probably change the practice of many skin-cancer physicians,” two experts on the topic commented in a related editorial.
The editorialists are David Miller, MD, PhD, a dermatologist and medical oncologist at Massachusetts General Hospital, and Kevin Emerick, MD, a head and neck surgeon as Massachusetts Eye and Ear, both in Boston.
Transplant patients have 50 times the risk of nonmelanoma skin cancers – also known as keratinocyte cancers – than the general public, owing to immunosuppression, and their lesions are more aggressive and are more likely to metastasize, they explain.
Nicotinamide (vitamin B3) has been shown to prevent nonmelanoma skin cancers in healthy, immunocompetent people, so physicians routinely prescribe it to transplant patients on the assumption that it will do the same for them, they comment.
The Australian investigators decided to put the assumption to the test.
The team randomly assigned 79 patients who had undergone solid-organ transplant to receive nicotinamide 500 mg twice a day and 79 other patients to receive twice-daily placebo for a year. Participants underwent dermatology exams every 3 months to check for new lesions.
The participants were at high risk for new lesions; some had had more than 40 in the previous 5 years. The two groups were well balanced; kidney transplants were the most common.
At 12 months, there was virtually no difference in the incidence of new nonmelanoma skin cancers: 207 in the nicotinamide group and 210 in the placebo group (P = .96).
There was also no significant difference in squamous cell and basal cell carcinoma counts or actinic keratosis counts.
“The interpretation of the results is straightforward: nicotinamide lacks clinical usefulness in preventing the development of keratinocyte carcinomas in solid-organ transplant recipients,” the team concludes.
As for why nicotinamide didn’t work in the trial, the investigators say it could be because it is not potent enough to overcome the stifling of antitumor immunity and DNA-repair enzymes with immunosuppression.
Fewer than half of participants in the trial reported using sunscreen at any point during the study, which is in line with past reports that transplant patients don’t routinely use sunscreen.
Two other strategies for preventing squamous cell carcinoma after transplant – use of oral retinoids and mTOR inhibitors – are problematic for various reasons, and use was low in both study arms.
Editorialists Dr. Miller and Dr. Emerick suggest a possible new approach: immune checkpoint inhibitors before transplant to reduce the risk of nonmelanoma skin cancer afterward. They say the strategy should be explored and that ongoing efforts to minimize or eliminate the need for immunosuppression after transplant are promising.
The investigators originally planned to enroll 254 persons, but the trial was stopped early because of poor recruitment. Potential participants may already have been taking nicotinamide, which is commonly used, and that may have affected recruitment, the investigators say.
The work was funded by Australia’s National Health and Medical Research Council. Dr. Allen has disclosed no relevant financial relationships. One investigator has received speaker’s fees from BMS. Another is a consultant for many companies, including Amgen, BMS, GlaxoSmithKline, and Merck. Dr. Emerick is an advisor for Regeneron, Sanofi, and Castle Biosciences. Dr. Miller is a researcher or consultant for those companies as well as Pfizer and others and has stock options in Avstera.
A version of this article first appeared on Medscape.com.
published in the New England Journal of Medicine.
“No signal of efficacy was observed,” said investigators led by Nicholas Allen, MPH, of the University of Sydney department of dermatology.
These results fill an “important gap in our understanding” and “will probably change the practice of many skin-cancer physicians,” two experts on the topic commented in a related editorial.
The editorialists are David Miller, MD, PhD, a dermatologist and medical oncologist at Massachusetts General Hospital, and Kevin Emerick, MD, a head and neck surgeon as Massachusetts Eye and Ear, both in Boston.
Transplant patients have 50 times the risk of nonmelanoma skin cancers – also known as keratinocyte cancers – than the general public, owing to immunosuppression, and their lesions are more aggressive and are more likely to metastasize, they explain.
Nicotinamide (vitamin B3) has been shown to prevent nonmelanoma skin cancers in healthy, immunocompetent people, so physicians routinely prescribe it to transplant patients on the assumption that it will do the same for them, they comment.
The Australian investigators decided to put the assumption to the test.
The team randomly assigned 79 patients who had undergone solid-organ transplant to receive nicotinamide 500 mg twice a day and 79 other patients to receive twice-daily placebo for a year. Participants underwent dermatology exams every 3 months to check for new lesions.
The participants were at high risk for new lesions; some had had more than 40 in the previous 5 years. The two groups were well balanced; kidney transplants were the most common.
At 12 months, there was virtually no difference in the incidence of new nonmelanoma skin cancers: 207 in the nicotinamide group and 210 in the placebo group (P = .96).
There was also no significant difference in squamous cell and basal cell carcinoma counts or actinic keratosis counts.
“The interpretation of the results is straightforward: nicotinamide lacks clinical usefulness in preventing the development of keratinocyte carcinomas in solid-organ transplant recipients,” the team concludes.
As for why nicotinamide didn’t work in the trial, the investigators say it could be because it is not potent enough to overcome the stifling of antitumor immunity and DNA-repair enzymes with immunosuppression.
Fewer than half of participants in the trial reported using sunscreen at any point during the study, which is in line with past reports that transplant patients don’t routinely use sunscreen.
Two other strategies for preventing squamous cell carcinoma after transplant – use of oral retinoids and mTOR inhibitors – are problematic for various reasons, and use was low in both study arms.
Editorialists Dr. Miller and Dr. Emerick suggest a possible new approach: immune checkpoint inhibitors before transplant to reduce the risk of nonmelanoma skin cancer afterward. They say the strategy should be explored and that ongoing efforts to minimize or eliminate the need for immunosuppression after transplant are promising.
The investigators originally planned to enroll 254 persons, but the trial was stopped early because of poor recruitment. Potential participants may already have been taking nicotinamide, which is commonly used, and that may have affected recruitment, the investigators say.
The work was funded by Australia’s National Health and Medical Research Council. Dr. Allen has disclosed no relevant financial relationships. One investigator has received speaker’s fees from BMS. Another is a consultant for many companies, including Amgen, BMS, GlaxoSmithKline, and Merck. Dr. Emerick is an advisor for Regeneron, Sanofi, and Castle Biosciences. Dr. Miller is a researcher or consultant for those companies as well as Pfizer and others and has stock options in Avstera.
A version of this article first appeared on Medscape.com.
published in the New England Journal of Medicine.
“No signal of efficacy was observed,” said investigators led by Nicholas Allen, MPH, of the University of Sydney department of dermatology.
These results fill an “important gap in our understanding” and “will probably change the practice of many skin-cancer physicians,” two experts on the topic commented in a related editorial.
The editorialists are David Miller, MD, PhD, a dermatologist and medical oncologist at Massachusetts General Hospital, and Kevin Emerick, MD, a head and neck surgeon as Massachusetts Eye and Ear, both in Boston.
Transplant patients have 50 times the risk of nonmelanoma skin cancers – also known as keratinocyte cancers – than the general public, owing to immunosuppression, and their lesions are more aggressive and are more likely to metastasize, they explain.
Nicotinamide (vitamin B3) has been shown to prevent nonmelanoma skin cancers in healthy, immunocompetent people, so physicians routinely prescribe it to transplant patients on the assumption that it will do the same for them, they comment.
The Australian investigators decided to put the assumption to the test.
The team randomly assigned 79 patients who had undergone solid-organ transplant to receive nicotinamide 500 mg twice a day and 79 other patients to receive twice-daily placebo for a year. Participants underwent dermatology exams every 3 months to check for new lesions.
The participants were at high risk for new lesions; some had had more than 40 in the previous 5 years. The two groups were well balanced; kidney transplants were the most common.
At 12 months, there was virtually no difference in the incidence of new nonmelanoma skin cancers: 207 in the nicotinamide group and 210 in the placebo group (P = .96).
There was also no significant difference in squamous cell and basal cell carcinoma counts or actinic keratosis counts.
“The interpretation of the results is straightforward: nicotinamide lacks clinical usefulness in preventing the development of keratinocyte carcinomas in solid-organ transplant recipients,” the team concludes.
As for why nicotinamide didn’t work in the trial, the investigators say it could be because it is not potent enough to overcome the stifling of antitumor immunity and DNA-repair enzymes with immunosuppression.
Fewer than half of participants in the trial reported using sunscreen at any point during the study, which is in line with past reports that transplant patients don’t routinely use sunscreen.
Two other strategies for preventing squamous cell carcinoma after transplant – use of oral retinoids and mTOR inhibitors – are problematic for various reasons, and use was low in both study arms.
Editorialists Dr. Miller and Dr. Emerick suggest a possible new approach: immune checkpoint inhibitors before transplant to reduce the risk of nonmelanoma skin cancer afterward. They say the strategy should be explored and that ongoing efforts to minimize or eliminate the need for immunosuppression after transplant are promising.
The investigators originally planned to enroll 254 persons, but the trial was stopped early because of poor recruitment. Potential participants may already have been taking nicotinamide, which is commonly used, and that may have affected recruitment, the investigators say.
The work was funded by Australia’s National Health and Medical Research Council. Dr. Allen has disclosed no relevant financial relationships. One investigator has received speaker’s fees from BMS. Another is a consultant for many companies, including Amgen, BMS, GlaxoSmithKline, and Merck. Dr. Emerick is an advisor for Regeneron, Sanofi, and Castle Biosciences. Dr. Miller is a researcher or consultant for those companies as well as Pfizer and others and has stock options in Avstera.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
How to help pediatricians apply peanut allergy guidelines
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
Skin reactions from melanoma targeted and immune therapies range from pruritus to SJS
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
AT MELANOMA 2023
Can skin care aid use of diabetes devices?
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Expert dispels myths about hair care in patients with skin of color
HONOLULU –
“This is false,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! With little manipulation, length may be retained, since tightly coiled hair has a higher likelihood of breakage, she said. “But washing the scalp and hair is recommended for tightly coiled hair weekly or every other week. Exclusively co-washing – a technique where hair conditioner is used instead of shampooing – is also not advised due to scalp build-up.”
Other myths she addressed include the following:
“I have a weak spot (or stress spot) on the top of my scalp.” These terms may be used to describe hair on a spot that goes through cycles of breaking off and re-growing. This is false. “If someone were to say that, and we see short hairs on the top of a patient’s scalp, with or without tenderness, pruritus, or pain, we want to recognize that as possibly an early sign of central centrifugal cicatricial alopecia [CCCA],” she said. “We want to pick up cases of CCCA forme fruste [central hair breakage] early.”
Medicated shampoos are helpful for all patients with seborrheic dermatitis. This notion is more complicated. “In theory, medicated shampoos like ketoconazole should be helpful, but if the shampoos are too drying for the hair and they cause further hair breakage, that’s going to be a problem as well,” explained Dr. Heath, who was the senior author of an article on how to address common conditions affecting pediatric and adolescent patients with skin of color. For patients with tightly coiled hair, she recommends applying antifungal shampoos to the scalp only, waiting 5-10 minutes, rinsing, and shampooing the scalp and hair with a moisturizing shampoo and rinsing. They can then condition with a moisturizing conditioner and style their hair as desired.
Don’t touch a Black woman’s hair. That unwritten rule may apply to a woman you pass on the street, she said, but not during clinical exams in cases where clinicians and patients seeking hair loss treatment have different hair types. “Touch the hair; don’t do a lean-in exam,” emphasized Dr. Heath, who is the inaugural faculty scholar at Temple University Lewis Katz School of Medicine’s Office of Health Equity Diversity, and Inclusion. “You want to perform the scalp and hair exam with cultural humility.” Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to their concerns, especially in race-discordant patient-physician interactions.
Going natural (chemical-free) will solve scarring hair loss problems. This is false. “Genetic defects in the hair shaft have been described as the cause of some CCCA cases, so we need to stop solely blaming the patient for that condition,” she said. Dr. Heath noted that the transition point between natural hair and relaxed hair is highly prone to breakage. She suggests low or lower tension options such as knotless braids, and crochet hairstyles, and when patients have locs, they should be shoulder length or higher to reduce tension.
Dr. Heath disclosed that she has served as a consultant or adviser for Arcutis, CeraVe, Janssen Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, Leo, Lilly, Pfizer, and Regeneron Pharmaceuticals.
Medscape and this news organization are owned by the same parent company.
HONOLULU –
“This is false,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! With little manipulation, length may be retained, since tightly coiled hair has a higher likelihood of breakage, she said. “But washing the scalp and hair is recommended for tightly coiled hair weekly or every other week. Exclusively co-washing – a technique where hair conditioner is used instead of shampooing – is also not advised due to scalp build-up.”
Other myths she addressed include the following:
“I have a weak spot (or stress spot) on the top of my scalp.” These terms may be used to describe hair on a spot that goes through cycles of breaking off and re-growing. This is false. “If someone were to say that, and we see short hairs on the top of a patient’s scalp, with or without tenderness, pruritus, or pain, we want to recognize that as possibly an early sign of central centrifugal cicatricial alopecia [CCCA],” she said. “We want to pick up cases of CCCA forme fruste [central hair breakage] early.”
Medicated shampoos are helpful for all patients with seborrheic dermatitis. This notion is more complicated. “In theory, medicated shampoos like ketoconazole should be helpful, but if the shampoos are too drying for the hair and they cause further hair breakage, that’s going to be a problem as well,” explained Dr. Heath, who was the senior author of an article on how to address common conditions affecting pediatric and adolescent patients with skin of color. For patients with tightly coiled hair, she recommends applying antifungal shampoos to the scalp only, waiting 5-10 minutes, rinsing, and shampooing the scalp and hair with a moisturizing shampoo and rinsing. They can then condition with a moisturizing conditioner and style their hair as desired.
Don’t touch a Black woman’s hair. That unwritten rule may apply to a woman you pass on the street, she said, but not during clinical exams in cases where clinicians and patients seeking hair loss treatment have different hair types. “Touch the hair; don’t do a lean-in exam,” emphasized Dr. Heath, who is the inaugural faculty scholar at Temple University Lewis Katz School of Medicine’s Office of Health Equity Diversity, and Inclusion. “You want to perform the scalp and hair exam with cultural humility.” Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to their concerns, especially in race-discordant patient-physician interactions.
Going natural (chemical-free) will solve scarring hair loss problems. This is false. “Genetic defects in the hair shaft have been described as the cause of some CCCA cases, so we need to stop solely blaming the patient for that condition,” she said. Dr. Heath noted that the transition point between natural hair and relaxed hair is highly prone to breakage. She suggests low or lower tension options such as knotless braids, and crochet hairstyles, and when patients have locs, they should be shoulder length or higher to reduce tension.
Dr. Heath disclosed that she has served as a consultant or adviser for Arcutis, CeraVe, Janssen Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, Leo, Lilly, Pfizer, and Regeneron Pharmaceuticals.
Medscape and this news organization are owned by the same parent company.
HONOLULU –
“This is false,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! With little manipulation, length may be retained, since tightly coiled hair has a higher likelihood of breakage, she said. “But washing the scalp and hair is recommended for tightly coiled hair weekly or every other week. Exclusively co-washing – a technique where hair conditioner is used instead of shampooing – is also not advised due to scalp build-up.”
Other myths she addressed include the following:
“I have a weak spot (or stress spot) on the top of my scalp.” These terms may be used to describe hair on a spot that goes through cycles of breaking off and re-growing. This is false. “If someone were to say that, and we see short hairs on the top of a patient’s scalp, with or without tenderness, pruritus, or pain, we want to recognize that as possibly an early sign of central centrifugal cicatricial alopecia [CCCA],” she said. “We want to pick up cases of CCCA forme fruste [central hair breakage] early.”
Medicated shampoos are helpful for all patients with seborrheic dermatitis. This notion is more complicated. “In theory, medicated shampoos like ketoconazole should be helpful, but if the shampoos are too drying for the hair and they cause further hair breakage, that’s going to be a problem as well,” explained Dr. Heath, who was the senior author of an article on how to address common conditions affecting pediatric and adolescent patients with skin of color. For patients with tightly coiled hair, she recommends applying antifungal shampoos to the scalp only, waiting 5-10 minutes, rinsing, and shampooing the scalp and hair with a moisturizing shampoo and rinsing. They can then condition with a moisturizing conditioner and style their hair as desired.
Don’t touch a Black woman’s hair. That unwritten rule may apply to a woman you pass on the street, she said, but not during clinical exams in cases where clinicians and patients seeking hair loss treatment have different hair types. “Touch the hair; don’t do a lean-in exam,” emphasized Dr. Heath, who is the inaugural faculty scholar at Temple University Lewis Katz School of Medicine’s Office of Health Equity Diversity, and Inclusion. “You want to perform the scalp and hair exam with cultural humility.” Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to their concerns, especially in race-discordant patient-physician interactions.
Going natural (chemical-free) will solve scarring hair loss problems. This is false. “Genetic defects in the hair shaft have been described as the cause of some CCCA cases, so we need to stop solely blaming the patient for that condition,” she said. Dr. Heath noted that the transition point between natural hair and relaxed hair is highly prone to breakage. She suggests low or lower tension options such as knotless braids, and crochet hairstyles, and when patients have locs, they should be shoulder length or higher to reduce tension.
Dr. Heath disclosed that she has served as a consultant or adviser for Arcutis, CeraVe, Janssen Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, Leo, Lilly, Pfizer, and Regeneron Pharmaceuticals.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPE LIVE! HAWAII DERMATOLOGY SEMINAR
Isolated nail psoriasis may bring arthritis into play
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.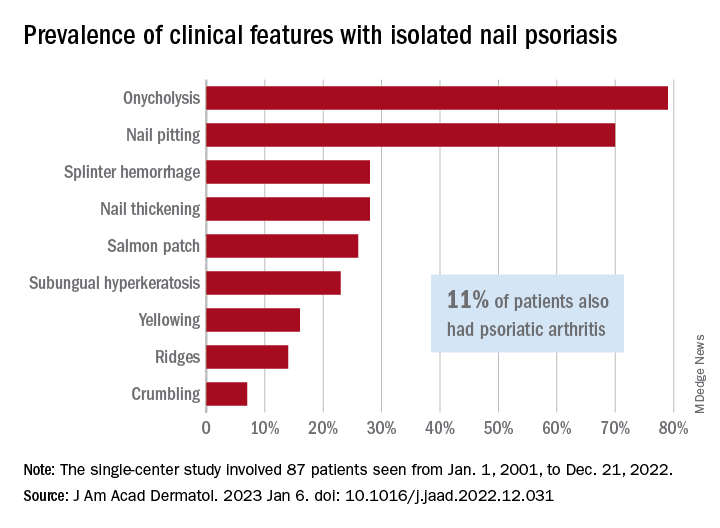
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY