User login
Cases of potentially deadly fungus jump 200%: CDC
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
Phase 3 prurigo nodularis trial shows positive results for nemolizumab
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
AT AAD 2023
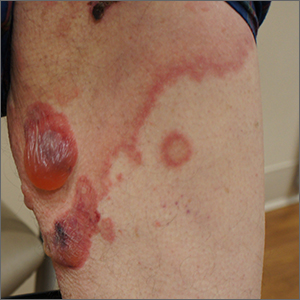
Blisters on arms and legs
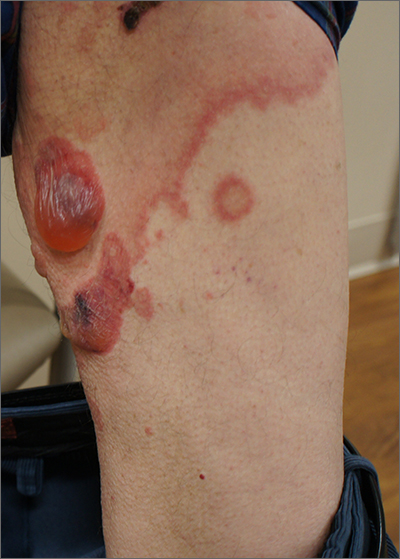
This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573

This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573
Novel therapy shows promise for treating skin-predominant dermatomyositis
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2023
Studies validate IL-17 as hidradenitis suppurativa drug target
NEW ORLEANS – In two phase 3 trials, bimekizumab, a monoclonal antibody targeting two types of interleukin-17 — IL-17A and IL-17F — reduced the abscess and inflammatory nodule count better than placebo in the chronic inflammatory skin condition hidradenitis suppurativa (HS), according to results presented together during a late-breaker session at the annual meeting of the American Academy of Dermatology.
“We are very excited to add this data to what we already have around IL-17 inhibition. This clearly validates this target for the control of HS,” reported lead investigator Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston.
The trials, called BE HEARD I and BE HEARD II, enrolled 505 and 509 patients with HS, respectively. About 50% of patients in BE HEARD I and 60% of patients in BE HEARD II had Hurley stage 3 disease, which is the most severe of the three stratifications. The remainder were in Hurley stage 2. The mean duration of HS was 8.3 and 7.1 years, respectively.
Patients in both studies were randomized to one of four groups – either to a dosing regimen of 320 mg of bimekizumab administered by subcutaneous injection or to a placebo group. Both trials comprised double-blind 16-week initial and 32-week maintenance treatment periods.
In one experimental group, bimekizumab was given once every 2 weeks for the full course of the 48-week study (Q2W/Q2W). In another, patients started on the every-2-week schedule for 16 weeks and then were switched to every-4-week dosing (Q2W/Q4W). In the third group, patients started and remained on the every-4-week schedule (Q4W/Q4W). Patients in a fourth group started on placebo and switched at 16 weeks to the every-2-week bimekizumab schedule (placebo/Q2W).
Results at primary endpoint
The primary endpoint was HiSCR50, signifying a 50% reduction from baseline in abscess and inflammatory nodule count on the Hidradenitis Suppurativa Clinical Response (HiSCR) assessment tool. At 16 weeks, the initial Q2W dose in two of the groups outperformed the placebo in both BE HEARD I (47.8% vs. 28.7%) and BE HEARD II (52.0% vs. 32.2%). The response rates in the Q4W arm in BE HEARD I (45.3%) and BE HEARD II (53.8%) were also higher than the placebo, but the difference was only significant in BE HEARD II.
At 48 weeks, the proportion of patients with an HiSCR50 response climbed in all groups in both trials. The patterns were generally the same with slightly higher numerical responses among the groups that received the every-2-week dosing schedule relative to the every-4-week schedule.
In BE HEARD I at 48 weeks, the HiSCR50 response rate was about 60% for those who started and remained on every-2-week bimekizumab (Q2W/Q2W) or were switched at 16 weeks to every-4-week bimekizumab (Q2W/Q4W). For those who started and remained on every-4-week bimekizumab and the group started on placebo and switched to every-2-week bimekizumab, the response rates were 52.7% and 45.3%, respectively.
In BE HEARD II, the HiSCR50 response rates were higher in all groups, including the placebo, and the patterns of response were similar at 48 weeks. Most patients reached the HiSCR50 response – 79.8% (Q2W/Q2W), 78.4% (Q2W/Q4W), 76.7% (Q4W/Q4W), and 65.9 % (placebo/Q2W) of patients.
It is notable that, although there was rapid increase in the proportion of placebo patients reaching HiSCR50 after the switch at 16 weeks, there appeared to be an advantage at 48 weeks for starting on full-dose bimekizumab over starting on placebo.
In this trial, patients were listed as nonresponders if they received antibiotics at any time and for any reason after randomization. This might have concealed an even greater benefit of bimekizumab, Dr. Kimball said, but the study design element was considered necessary to isolate the activity of the study drug.
“In future HS trials, it will be helpful to address the difficulty of handling the impact of antibiotics and pain medications [in assessing results],” Dr. Kimball said.
Clinically meaningful secondary endpoint
For HS patients, the secondary endpoint of HiSCR75 might be considered the most meaningful, according to Dr. Kimball. She said that this higher bar not only documents a higher level of efficacy but correlates with meaningful improvement in quality of life. In the two trials combined, more than 55% of patients on continuous bimekizumab achieved HiSCR75 at week 48 in the observed case analysis, according to a news release from biopharmaceutical company UCB, developer of bimekizumab.
In BE HEARD I, the HiSCR75 rates were 33.4% and 24.7% for the every-2-week and every-4-week bimekizumab doses, respectively. The 33.4% response was statistically superior to placebo (18.4%). In BE HEARD II, both the every-2-week dose (35.7%) and the every-4-week dose (33.7%) were superior to the 15.6% response in placebo patients.
The improvements in quality of life as measured with the Dermatology Life Quality Index (DLQI), reflected the changes in disease activity. Relative to about a 3-point reduction from baseline in the placebo groups of the two trials, the 5-point reduction for either the 2-week or 4-week bimekizumab groups in each clinical trial were highly significant, Dr. Kimball said.
Bimekizumab was relatively well tolerated, although it shares the increased risk for candidiasis observed with this agent when used in psoriasis and with other IL-17 inhibitors, such as secukinumab (Cosentyx), in general. The risk of candidiasis appeared to be dose related, but cases were generally mild and easily managed, according to Dr. Kimball. She noted that only three patients discontinued treatment for this reason. Discontinuations for a treatment-related adverse event overall was less than 4% at 16 weeks.
This is only the third phase 3 trial ever completed in patients with HS. In fact, Dr. Kimball has led all of the phase 3 trials so far, including clinical studies of adalimumab (Humira), published in 2016, and of secukinumab, published earlier this year. All were positive studies.
“This is amazing news for our patients,” Dr. Kimball said. HS remains a challenging disease, even with a growing number of options showing benefit in large studies, she said, and the high rate of response, particularly at the level of HiSCR75, “is a huge milestone for what we can achieve.”
Multiple treatment options important
Her assessment was echoed by other experts, including Christopher J. Sayed, MD, an associate professor of dermatology at the University of North Carolina at Chapel Hill, who publishes frequently about this disease.
“It is incredibly exciting to see the strong phase 3 data on bimekizumab, particularly the deep responses at the HiSCR75 in a majority of patients after the first year,” he said.
Importantly, he does not see the growing array of treatment options as necessarily competitive for a disease with heterogeneous manifestations and variable responses to any one agent.
“While this may be a major step forward, it will still be critical to see more drugs come along for those who do not respond fully enough or have comorbidities that prevent the use of IL-17 and TNF [tumor necrosis factor] antagonists,” he said.
Bimekizumab is not approved for any indication in the United States; it is approved for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy in the EU/EEA, where it is marketed as Bimzelx, according to UCB. Dr. Kimball reports financial relationships with AbbVie, Janssen, Kymera, Lilly, Novartis, Pfizer, and UCB. Dr. Sayed reports financial relationships with AbbVie, InflaRx, and UCB.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – In two phase 3 trials, bimekizumab, a monoclonal antibody targeting two types of interleukin-17 — IL-17A and IL-17F — reduced the abscess and inflammatory nodule count better than placebo in the chronic inflammatory skin condition hidradenitis suppurativa (HS), according to results presented together during a late-breaker session at the annual meeting of the American Academy of Dermatology.
“We are very excited to add this data to what we already have around IL-17 inhibition. This clearly validates this target for the control of HS,” reported lead investigator Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston.
The trials, called BE HEARD I and BE HEARD II, enrolled 505 and 509 patients with HS, respectively. About 50% of patients in BE HEARD I and 60% of patients in BE HEARD II had Hurley stage 3 disease, which is the most severe of the three stratifications. The remainder were in Hurley stage 2. The mean duration of HS was 8.3 and 7.1 years, respectively.
Patients in both studies were randomized to one of four groups – either to a dosing regimen of 320 mg of bimekizumab administered by subcutaneous injection or to a placebo group. Both trials comprised double-blind 16-week initial and 32-week maintenance treatment periods.
In one experimental group, bimekizumab was given once every 2 weeks for the full course of the 48-week study (Q2W/Q2W). In another, patients started on the every-2-week schedule for 16 weeks and then were switched to every-4-week dosing (Q2W/Q4W). In the third group, patients started and remained on the every-4-week schedule (Q4W/Q4W). Patients in a fourth group started on placebo and switched at 16 weeks to the every-2-week bimekizumab schedule (placebo/Q2W).
Results at primary endpoint
The primary endpoint was HiSCR50, signifying a 50% reduction from baseline in abscess and inflammatory nodule count on the Hidradenitis Suppurativa Clinical Response (HiSCR) assessment tool. At 16 weeks, the initial Q2W dose in two of the groups outperformed the placebo in both BE HEARD I (47.8% vs. 28.7%) and BE HEARD II (52.0% vs. 32.2%). The response rates in the Q4W arm in BE HEARD I (45.3%) and BE HEARD II (53.8%) were also higher than the placebo, but the difference was only significant in BE HEARD II.
At 48 weeks, the proportion of patients with an HiSCR50 response climbed in all groups in both trials. The patterns were generally the same with slightly higher numerical responses among the groups that received the every-2-week dosing schedule relative to the every-4-week schedule.
In BE HEARD I at 48 weeks, the HiSCR50 response rate was about 60% for those who started and remained on every-2-week bimekizumab (Q2W/Q2W) or were switched at 16 weeks to every-4-week bimekizumab (Q2W/Q4W). For those who started and remained on every-4-week bimekizumab and the group started on placebo and switched to every-2-week bimekizumab, the response rates were 52.7% and 45.3%, respectively.
In BE HEARD II, the HiSCR50 response rates were higher in all groups, including the placebo, and the patterns of response were similar at 48 weeks. Most patients reached the HiSCR50 response – 79.8% (Q2W/Q2W), 78.4% (Q2W/Q4W), 76.7% (Q4W/Q4W), and 65.9 % (placebo/Q2W) of patients.
It is notable that, although there was rapid increase in the proportion of placebo patients reaching HiSCR50 after the switch at 16 weeks, there appeared to be an advantage at 48 weeks for starting on full-dose bimekizumab over starting on placebo.
In this trial, patients were listed as nonresponders if they received antibiotics at any time and for any reason after randomization. This might have concealed an even greater benefit of bimekizumab, Dr. Kimball said, but the study design element was considered necessary to isolate the activity of the study drug.
“In future HS trials, it will be helpful to address the difficulty of handling the impact of antibiotics and pain medications [in assessing results],” Dr. Kimball said.
Clinically meaningful secondary endpoint
For HS patients, the secondary endpoint of HiSCR75 might be considered the most meaningful, according to Dr. Kimball. She said that this higher bar not only documents a higher level of efficacy but correlates with meaningful improvement in quality of life. In the two trials combined, more than 55% of patients on continuous bimekizumab achieved HiSCR75 at week 48 in the observed case analysis, according to a news release from biopharmaceutical company UCB, developer of bimekizumab.
In BE HEARD I, the HiSCR75 rates were 33.4% and 24.7% for the every-2-week and every-4-week bimekizumab doses, respectively. The 33.4% response was statistically superior to placebo (18.4%). In BE HEARD II, both the every-2-week dose (35.7%) and the every-4-week dose (33.7%) were superior to the 15.6% response in placebo patients.
The improvements in quality of life as measured with the Dermatology Life Quality Index (DLQI), reflected the changes in disease activity. Relative to about a 3-point reduction from baseline in the placebo groups of the two trials, the 5-point reduction for either the 2-week or 4-week bimekizumab groups in each clinical trial were highly significant, Dr. Kimball said.
Bimekizumab was relatively well tolerated, although it shares the increased risk for candidiasis observed with this agent when used in psoriasis and with other IL-17 inhibitors, such as secukinumab (Cosentyx), in general. The risk of candidiasis appeared to be dose related, but cases were generally mild and easily managed, according to Dr. Kimball. She noted that only three patients discontinued treatment for this reason. Discontinuations for a treatment-related adverse event overall was less than 4% at 16 weeks.
This is only the third phase 3 trial ever completed in patients with HS. In fact, Dr. Kimball has led all of the phase 3 trials so far, including clinical studies of adalimumab (Humira), published in 2016, and of secukinumab, published earlier this year. All were positive studies.
“This is amazing news for our patients,” Dr. Kimball said. HS remains a challenging disease, even with a growing number of options showing benefit in large studies, she said, and the high rate of response, particularly at the level of HiSCR75, “is a huge milestone for what we can achieve.”
Multiple treatment options important
Her assessment was echoed by other experts, including Christopher J. Sayed, MD, an associate professor of dermatology at the University of North Carolina at Chapel Hill, who publishes frequently about this disease.
“It is incredibly exciting to see the strong phase 3 data on bimekizumab, particularly the deep responses at the HiSCR75 in a majority of patients after the first year,” he said.
Importantly, he does not see the growing array of treatment options as necessarily competitive for a disease with heterogeneous manifestations and variable responses to any one agent.
“While this may be a major step forward, it will still be critical to see more drugs come along for those who do not respond fully enough or have comorbidities that prevent the use of IL-17 and TNF [tumor necrosis factor] antagonists,” he said.
Bimekizumab is not approved for any indication in the United States; it is approved for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy in the EU/EEA, where it is marketed as Bimzelx, according to UCB. Dr. Kimball reports financial relationships with AbbVie, Janssen, Kymera, Lilly, Novartis, Pfizer, and UCB. Dr. Sayed reports financial relationships with AbbVie, InflaRx, and UCB.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – In two phase 3 trials, bimekizumab, a monoclonal antibody targeting two types of interleukin-17 — IL-17A and IL-17F — reduced the abscess and inflammatory nodule count better than placebo in the chronic inflammatory skin condition hidradenitis suppurativa (HS), according to results presented together during a late-breaker session at the annual meeting of the American Academy of Dermatology.
“We are very excited to add this data to what we already have around IL-17 inhibition. This clearly validates this target for the control of HS,” reported lead investigator Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston.
The trials, called BE HEARD I and BE HEARD II, enrolled 505 and 509 patients with HS, respectively. About 50% of patients in BE HEARD I and 60% of patients in BE HEARD II had Hurley stage 3 disease, which is the most severe of the three stratifications. The remainder were in Hurley stage 2. The mean duration of HS was 8.3 and 7.1 years, respectively.
Patients in both studies were randomized to one of four groups – either to a dosing regimen of 320 mg of bimekizumab administered by subcutaneous injection or to a placebo group. Both trials comprised double-blind 16-week initial and 32-week maintenance treatment periods.
In one experimental group, bimekizumab was given once every 2 weeks for the full course of the 48-week study (Q2W/Q2W). In another, patients started on the every-2-week schedule for 16 weeks and then were switched to every-4-week dosing (Q2W/Q4W). In the third group, patients started and remained on the every-4-week schedule (Q4W/Q4W). Patients in a fourth group started on placebo and switched at 16 weeks to the every-2-week bimekizumab schedule (placebo/Q2W).
Results at primary endpoint
The primary endpoint was HiSCR50, signifying a 50% reduction from baseline in abscess and inflammatory nodule count on the Hidradenitis Suppurativa Clinical Response (HiSCR) assessment tool. At 16 weeks, the initial Q2W dose in two of the groups outperformed the placebo in both BE HEARD I (47.8% vs. 28.7%) and BE HEARD II (52.0% vs. 32.2%). The response rates in the Q4W arm in BE HEARD I (45.3%) and BE HEARD II (53.8%) were also higher than the placebo, but the difference was only significant in BE HEARD II.
At 48 weeks, the proportion of patients with an HiSCR50 response climbed in all groups in both trials. The patterns were generally the same with slightly higher numerical responses among the groups that received the every-2-week dosing schedule relative to the every-4-week schedule.
In BE HEARD I at 48 weeks, the HiSCR50 response rate was about 60% for those who started and remained on every-2-week bimekizumab (Q2W/Q2W) or were switched at 16 weeks to every-4-week bimekizumab (Q2W/Q4W). For those who started and remained on every-4-week bimekizumab and the group started on placebo and switched to every-2-week bimekizumab, the response rates were 52.7% and 45.3%, respectively.
In BE HEARD II, the HiSCR50 response rates were higher in all groups, including the placebo, and the patterns of response were similar at 48 weeks. Most patients reached the HiSCR50 response – 79.8% (Q2W/Q2W), 78.4% (Q2W/Q4W), 76.7% (Q4W/Q4W), and 65.9 % (placebo/Q2W) of patients.
It is notable that, although there was rapid increase in the proportion of placebo patients reaching HiSCR50 after the switch at 16 weeks, there appeared to be an advantage at 48 weeks for starting on full-dose bimekizumab over starting on placebo.
In this trial, patients were listed as nonresponders if they received antibiotics at any time and for any reason after randomization. This might have concealed an even greater benefit of bimekizumab, Dr. Kimball said, but the study design element was considered necessary to isolate the activity of the study drug.
“In future HS trials, it will be helpful to address the difficulty of handling the impact of antibiotics and pain medications [in assessing results],” Dr. Kimball said.
Clinically meaningful secondary endpoint
For HS patients, the secondary endpoint of HiSCR75 might be considered the most meaningful, according to Dr. Kimball. She said that this higher bar not only documents a higher level of efficacy but correlates with meaningful improvement in quality of life. In the two trials combined, more than 55% of patients on continuous bimekizumab achieved HiSCR75 at week 48 in the observed case analysis, according to a news release from biopharmaceutical company UCB, developer of bimekizumab.
In BE HEARD I, the HiSCR75 rates were 33.4% and 24.7% for the every-2-week and every-4-week bimekizumab doses, respectively. The 33.4% response was statistically superior to placebo (18.4%). In BE HEARD II, both the every-2-week dose (35.7%) and the every-4-week dose (33.7%) were superior to the 15.6% response in placebo patients.
The improvements in quality of life as measured with the Dermatology Life Quality Index (DLQI), reflected the changes in disease activity. Relative to about a 3-point reduction from baseline in the placebo groups of the two trials, the 5-point reduction for either the 2-week or 4-week bimekizumab groups in each clinical trial were highly significant, Dr. Kimball said.
Bimekizumab was relatively well tolerated, although it shares the increased risk for candidiasis observed with this agent when used in psoriasis and with other IL-17 inhibitors, such as secukinumab (Cosentyx), in general. The risk of candidiasis appeared to be dose related, but cases were generally mild and easily managed, according to Dr. Kimball. She noted that only three patients discontinued treatment for this reason. Discontinuations for a treatment-related adverse event overall was less than 4% at 16 weeks.
This is only the third phase 3 trial ever completed in patients with HS. In fact, Dr. Kimball has led all of the phase 3 trials so far, including clinical studies of adalimumab (Humira), published in 2016, and of secukinumab, published earlier this year. All were positive studies.
“This is amazing news for our patients,” Dr. Kimball said. HS remains a challenging disease, even with a growing number of options showing benefit in large studies, she said, and the high rate of response, particularly at the level of HiSCR75, “is a huge milestone for what we can achieve.”
Multiple treatment options important
Her assessment was echoed by other experts, including Christopher J. Sayed, MD, an associate professor of dermatology at the University of North Carolina at Chapel Hill, who publishes frequently about this disease.
“It is incredibly exciting to see the strong phase 3 data on bimekizumab, particularly the deep responses at the HiSCR75 in a majority of patients after the first year,” he said.
Importantly, he does not see the growing array of treatment options as necessarily competitive for a disease with heterogeneous manifestations and variable responses to any one agent.
“While this may be a major step forward, it will still be critical to see more drugs come along for those who do not respond fully enough or have comorbidities that prevent the use of IL-17 and TNF [tumor necrosis factor] antagonists,” he said.
Bimekizumab is not approved for any indication in the United States; it is approved for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy in the EU/EEA, where it is marketed as Bimzelx, according to UCB. Dr. Kimball reports financial relationships with AbbVie, Janssen, Kymera, Lilly, Novartis, Pfizer, and UCB. Dr. Sayed reports financial relationships with AbbVie, InflaRx, and UCB.
A version of this article first appeared on Medscape.com.
AT AAD 2023
Melanoma screening: Consensus statement offers greater clarity
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Novel single-use patch shows promise for primary axillary hyperhidrosis
NEW ORLEANS – , results from a pivotal randomized trial showed.
“This is a new kind of device that is going to be a nice tool to have for treating patients who have hyperhidrosis of the axilla,” the study’s lead investigator, David M. Pariser, MD, who practices dermatology in Norfolk, Va., said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
In a study known as SAHARA, investigators at 11 sites evaluated the efficacy of the targeted alkali thermolysis (TAT) patch, a single-use disposable device. The patch consists of a thin sodium layer on an adhesive overlay. It’s applied to the dry axilla, and as the patient sweats during treatment, the sweat reacts with the sodium. According to Dr. Pariser, this interaction generates precisely targeted thermal energy that targets sweat glands, leading to a reduction in excessive sweat production for up to three months.
The researchers enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 and randomized them to either an active TAT or a sham patch for up to 3 minutes. Their mean age was about 33 years, and slightly more than half were women. “If significant discomfort or pain was noted, [the patch] treatment was halted; otherwise, it was left on for 3 minutes,” said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. “The treated area was thoroughly cleaned after treatment, and the TAT patch was deactivated. This process was repeated on the other axilla.”
The HDSS, Gravimetric Sweat Production (GSP), and quality of life assessments for bother and impact were measured through 12 weeks. The quality of life assessments were an exploratory endpoint and scored from 0 to 4, with 4 being extremely bothered or impacted and 0 not being bothered or impacted at all. The primary efficacy endpoint was the proportion of treated patients achieving a 1 or 2 on the HDSS at week 4, compared with sham treatment.
Secondary endpoints included the proportion of patients with an improvement of at least 2 grades from baseline to 4 weeks in HDSS by treatment group; mean improvement in the quality of life scale bother by treatment group; mean improvement in the quality of life scale impact by treatment group; and the proportion of subjects with at least 50% improvement in GSP from baseline to 4 weeks in the active patch group only.
Adverse events (AEs) were divided into 3 categories: AEs at the treatment site (or skin reactions within the treated part of the axilla); procedure-related AEs (those that are the result of treatment, but not in the treated part of the axilla), and non-axillary AEs.
Dr. Pariser reported that at 4 weeks, 63.6% of patients in the active patch group versus 44.2% of those in the sham group improved to an HDSS score of 1 or 2 (P = .0332) and that 43.2% of those in the active patch group versus 16.3% of those in the sham group (P = .0107) achieved a 2-point or greater HDSS improvement. In addition, 9.1% of those in the active patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” he commented.
In other findings, 60.5% of patients in the active patch group showed at least a 50% reduction in GSP, compared with 32.6% of those in the sham group (P = .0102), with mean reductions of 57.3 mg/5min and 18.2 mg/5min, respectively (P = .0036). As for quality-of-life outcome scores, bother associated with hyperhidrosis was reduced by 1.52 points in active versus 0.61 in sham subjects (P = .0005), while impact was reduced by 1.44 in active versus 0.57 in sham subjects (P = .0004).
Adverse events
A total of 13 patients in the active patch group experienced AEs at the treatment site, including six with erythema; four with erosion; two with burning, itching or stinging; and one with underarm odor. “The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” said Dr. Pariser said.
Most adverse events resolved in fewer than 2 weeks, and all were mild to moderate. No serious adverse events occurred. Only five adverse events occurred in the sham group.
The TAT patch is currently undergoing review by the Food and Drug Administration, and according to Dr. Pariser, no other body sites have been treated with the device.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized hyperhidrosis as “an exceedingly common medical condition that is commonly overlooked even though it has a tremendous burden on quality of life. I should know, as both someone who manages a large cohort of these patients but also as someone who suffers from it.”
Treatment options “have historically been limited, many of which are off-label and some which are difficult to access due to cost and/or duration/frequency of treatment,” added Dr. Friedman, who was not involved with the study. “The TAT patch offers a new, targeted, in-office, practical procedure-based approach to treat primary axillary hyperhidrosis. Innovation is certainly welcomed and needed, and I am curious to see how this technology is employed in practice once approved.”
The device is being developed by Candesant Biomedical. Dr. Pariser disclosed that he is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol Myers Squibb, Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – , results from a pivotal randomized trial showed.
“This is a new kind of device that is going to be a nice tool to have for treating patients who have hyperhidrosis of the axilla,” the study’s lead investigator, David M. Pariser, MD, who practices dermatology in Norfolk, Va., said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
In a study known as SAHARA, investigators at 11 sites evaluated the efficacy of the targeted alkali thermolysis (TAT) patch, a single-use disposable device. The patch consists of a thin sodium layer on an adhesive overlay. It’s applied to the dry axilla, and as the patient sweats during treatment, the sweat reacts with the sodium. According to Dr. Pariser, this interaction generates precisely targeted thermal energy that targets sweat glands, leading to a reduction in excessive sweat production for up to three months.
The researchers enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 and randomized them to either an active TAT or a sham patch for up to 3 minutes. Their mean age was about 33 years, and slightly more than half were women. “If significant discomfort or pain was noted, [the patch] treatment was halted; otherwise, it was left on for 3 minutes,” said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. “The treated area was thoroughly cleaned after treatment, and the TAT patch was deactivated. This process was repeated on the other axilla.”
The HDSS, Gravimetric Sweat Production (GSP), and quality of life assessments for bother and impact were measured through 12 weeks. The quality of life assessments were an exploratory endpoint and scored from 0 to 4, with 4 being extremely bothered or impacted and 0 not being bothered or impacted at all. The primary efficacy endpoint was the proportion of treated patients achieving a 1 or 2 on the HDSS at week 4, compared with sham treatment.
Secondary endpoints included the proportion of patients with an improvement of at least 2 grades from baseline to 4 weeks in HDSS by treatment group; mean improvement in the quality of life scale bother by treatment group; mean improvement in the quality of life scale impact by treatment group; and the proportion of subjects with at least 50% improvement in GSP from baseline to 4 weeks in the active patch group only.
Adverse events (AEs) were divided into 3 categories: AEs at the treatment site (or skin reactions within the treated part of the axilla); procedure-related AEs (those that are the result of treatment, but not in the treated part of the axilla), and non-axillary AEs.
Dr. Pariser reported that at 4 weeks, 63.6% of patients in the active patch group versus 44.2% of those in the sham group improved to an HDSS score of 1 or 2 (P = .0332) and that 43.2% of those in the active patch group versus 16.3% of those in the sham group (P = .0107) achieved a 2-point or greater HDSS improvement. In addition, 9.1% of those in the active patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” he commented.
In other findings, 60.5% of patients in the active patch group showed at least a 50% reduction in GSP, compared with 32.6% of those in the sham group (P = .0102), with mean reductions of 57.3 mg/5min and 18.2 mg/5min, respectively (P = .0036). As for quality-of-life outcome scores, bother associated with hyperhidrosis was reduced by 1.52 points in active versus 0.61 in sham subjects (P = .0005), while impact was reduced by 1.44 in active versus 0.57 in sham subjects (P = .0004).
Adverse events
A total of 13 patients in the active patch group experienced AEs at the treatment site, including six with erythema; four with erosion; two with burning, itching or stinging; and one with underarm odor. “The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” said Dr. Pariser said.
Most adverse events resolved in fewer than 2 weeks, and all were mild to moderate. No serious adverse events occurred. Only five adverse events occurred in the sham group.
The TAT patch is currently undergoing review by the Food and Drug Administration, and according to Dr. Pariser, no other body sites have been treated with the device.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized hyperhidrosis as “an exceedingly common medical condition that is commonly overlooked even though it has a tremendous burden on quality of life. I should know, as both someone who manages a large cohort of these patients but also as someone who suffers from it.”
Treatment options “have historically been limited, many of which are off-label and some which are difficult to access due to cost and/or duration/frequency of treatment,” added Dr. Friedman, who was not involved with the study. “The TAT patch offers a new, targeted, in-office, practical procedure-based approach to treat primary axillary hyperhidrosis. Innovation is certainly welcomed and needed, and I am curious to see how this technology is employed in practice once approved.”
The device is being developed by Candesant Biomedical. Dr. Pariser disclosed that he is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol Myers Squibb, Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – , results from a pivotal randomized trial showed.
“This is a new kind of device that is going to be a nice tool to have for treating patients who have hyperhidrosis of the axilla,” the study’s lead investigator, David M. Pariser, MD, who practices dermatology in Norfolk, Va., said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
In a study known as SAHARA, investigators at 11 sites evaluated the efficacy of the targeted alkali thermolysis (TAT) patch, a single-use disposable device. The patch consists of a thin sodium layer on an adhesive overlay. It’s applied to the dry axilla, and as the patient sweats during treatment, the sweat reacts with the sodium. According to Dr. Pariser, this interaction generates precisely targeted thermal energy that targets sweat glands, leading to a reduction in excessive sweat production for up to three months.
The researchers enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 and randomized them to either an active TAT or a sham patch for up to 3 minutes. Their mean age was about 33 years, and slightly more than half were women. “If significant discomfort or pain was noted, [the patch] treatment was halted; otherwise, it was left on for 3 minutes,” said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. “The treated area was thoroughly cleaned after treatment, and the TAT patch was deactivated. This process was repeated on the other axilla.”
The HDSS, Gravimetric Sweat Production (GSP), and quality of life assessments for bother and impact were measured through 12 weeks. The quality of life assessments were an exploratory endpoint and scored from 0 to 4, with 4 being extremely bothered or impacted and 0 not being bothered or impacted at all. The primary efficacy endpoint was the proportion of treated patients achieving a 1 or 2 on the HDSS at week 4, compared with sham treatment.
Secondary endpoints included the proportion of patients with an improvement of at least 2 grades from baseline to 4 weeks in HDSS by treatment group; mean improvement in the quality of life scale bother by treatment group; mean improvement in the quality of life scale impact by treatment group; and the proportion of subjects with at least 50% improvement in GSP from baseline to 4 weeks in the active patch group only.
Adverse events (AEs) were divided into 3 categories: AEs at the treatment site (or skin reactions within the treated part of the axilla); procedure-related AEs (those that are the result of treatment, but not in the treated part of the axilla), and non-axillary AEs.
Dr. Pariser reported that at 4 weeks, 63.6% of patients in the active patch group versus 44.2% of those in the sham group improved to an HDSS score of 1 or 2 (P = .0332) and that 43.2% of those in the active patch group versus 16.3% of those in the sham group (P = .0107) achieved a 2-point or greater HDSS improvement. In addition, 9.1% of those in the active patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” he commented.
In other findings, 60.5% of patients in the active patch group showed at least a 50% reduction in GSP, compared with 32.6% of those in the sham group (P = .0102), with mean reductions of 57.3 mg/5min and 18.2 mg/5min, respectively (P = .0036). As for quality-of-life outcome scores, bother associated with hyperhidrosis was reduced by 1.52 points in active versus 0.61 in sham subjects (P = .0005), while impact was reduced by 1.44 in active versus 0.57 in sham subjects (P = .0004).
Adverse events
A total of 13 patients in the active patch group experienced AEs at the treatment site, including six with erythema; four with erosion; two with burning, itching or stinging; and one with underarm odor. “The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” said Dr. Pariser said.
Most adverse events resolved in fewer than 2 weeks, and all were mild to moderate. No serious adverse events occurred. Only five adverse events occurred in the sham group.
The TAT patch is currently undergoing review by the Food and Drug Administration, and according to Dr. Pariser, no other body sites have been treated with the device.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized hyperhidrosis as “an exceedingly common medical condition that is commonly overlooked even though it has a tremendous burden on quality of life. I should know, as both someone who manages a large cohort of these patients but also as someone who suffers from it.”
Treatment options “have historically been limited, many of which are off-label and some which are difficult to access due to cost and/or duration/frequency of treatment,” added Dr. Friedman, who was not involved with the study. “The TAT patch offers a new, targeted, in-office, practical procedure-based approach to treat primary axillary hyperhidrosis. Innovation is certainly welcomed and needed, and I am curious to see how this technology is employed in practice once approved.”
The device is being developed by Candesant Biomedical. Dr. Pariser disclosed that he is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol Myers Squibb, Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
Dr. Friedman reported having no relevant disclosures.
AT AAD 2023
CSU in children: Study identifies biomarkers associated with responses to different treatments
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
AT AAD 2023
JAK inhibitor safety warnings drawn from rheumatologic data may be misleading in dermatology
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
AT AAD 2023
Lanolin gets nod for Allergen of the Year
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
FROM ACDS 2023