User login
Physician sues AMA for defamation over 2022 election controversy
If Willarda Edwards, MD, MBA, had won her 2022 campaign for president-elect of the American Medical Association (AMA), she would have been the second Black woman to head the group.
The lawsuit sheds light on the power dynamics of a politically potent organization that has more than 271,000 members and holds assets of $1.2 billion. The AMA president is one of the most visible figures in American medicine.
“The AMA impugned Dr. Edwards with these false charges, which destroyed her candidacy and irreparably damaged her reputation,” according to the complaint, which was filed Nov. 9, 2022, in Baltimore County Circuit Court. The case was later moved to federal court.
The AMA “previously rejected our attempt to resolve this matter without litigation,” Dr. Edwards’ attorney, Timothy Maloney, told this news organization. An AMA spokesman said the organization had no comment on Dr. Edwards’ suit.
Dr. Edwards is a past president of the National Medical Association, MedChi, the Baltimore City Medical Society, the Monumental City Medical Society, and the Sickle Cell Disease Association of America. She joined the AMA in 1994 and has served as a trustee since 2016.
As chair of the AMA Task Force on Health Equity, “she helped lead the way in consensus building and driving action that in 2019 resulted in the AMA House of Delegates establishing the AMA Center on Health Equity,” according to her AMA bio page.
‘Quid pro quo’ alleged
In June 2022, Dr. Edwards was one of three individuals running to be AMA president-elect.
According to Dr. Edwards’ complaint, she was “incorrectly advised by colleagues” that Virginia urologist William Reha, MD, had decided not to seek the AMA vice-speakership in 2023. This was important because both Dr. Edwards and Dr. Reha were in the Southeastern delegation. It could be in Dr. Edwards’ favor if Dr. Reha was not running, as it would mean one less leadership candidate from the same region.
Dr. Edwards called Dr. Reha on June 6 to discuss the matter. When they talked, Dr. Reha allegedly recorded the call without Dr. Edwards’ knowledge or permission – a felony in Maryland – and also steered her toward discussions about how his decision could benefit her campaign, according to the complaint.
The suit alleges that Dr. Reha’s questions were “clearly calculated to draw some statements by Dr. Edwards that he could use later to thwart her candidacy and to benefit her opponent.”
On June 10, at the AMA’s House of Delegates meeting in Chicago, Dr. Edwards was taken aside and questioned by members of the AMA’s Election Campaign Committee, according to the complaint. They accused her of “vote trading” but did not provide any evidence or a copy of a complaint they said had been filed against her, the suit said.
Dr. Edwards was given no opportunity to produce her own evidence or rebut the accusations, the suit alleges.
Just before the delegates started formal business on June 13, House Speaker Bruce Scott, MD, read a statement to the assembly saying that a complaint of a possible campaign violation had been filed against Dr. Edwards.
Dr. Scott told the delegates that “committee members interviewed the complainant and multiple other individuals said to have knowledge of the circumstances. In addition to conducting multiple interviews, the committee reviewed evidence that was deemed credible and corroborated that a campaign violation did in fact occur,” according to the complaint.
The supposed violation: A “quid pro quo” in which an unnamed delegation would support Dr. Edwards’ current candidacy, and the Southeastern delegation would support a future candidate from that other unnamed delegation.
Dr. Edwards was given a short opportunity to speak, in which she denied any violations.
According to a news report, Dr. Edwards said, “I’ve been in the House of Delegates for 30 years, and you know me as a process person – a person who truly believes in the process and trying to follow the complexities of our election campaign.”
The lawsuit alleges that “this defamatory conduct was repeated the next day to more than 600 delegates just minutes prior to the casting of votes, when Dr Scott repeated these allegations.”
Dr. Edwards lost the election.
AMA: Nothing more to add
The suit alleges that neither the Election Campaign Committee nor the AMA itself has made any accusers or complaints available to Dr. Edwards and that it has not provided any audio or written evidence of her alleged violation.
In July, the AMA’s Southeastern delegation told its membership, “We continue to maintain that Willarda was ‘set up’ ... The whole affair lacked any reasonable semblance of due process.”
The delegation has filed a counter claim against the AMA seeking “to address this lack of due process as well as the reputational harm” to the delegation.
The AMA said that it has nothing it can produce. “The Speaker of the House presented a verbal report to the attending delegates,” said a spokesman. “The Speaker’s report remains the only remarks from an AMA officer, and no additional remarks can be expected at this time.”
He added that there “is no official transcript of the Speaker’s report.”
A version of this article first appeared on Medscape.com.
If Willarda Edwards, MD, MBA, had won her 2022 campaign for president-elect of the American Medical Association (AMA), she would have been the second Black woman to head the group.
The lawsuit sheds light on the power dynamics of a politically potent organization that has more than 271,000 members and holds assets of $1.2 billion. The AMA president is one of the most visible figures in American medicine.
“The AMA impugned Dr. Edwards with these false charges, which destroyed her candidacy and irreparably damaged her reputation,” according to the complaint, which was filed Nov. 9, 2022, in Baltimore County Circuit Court. The case was later moved to federal court.
The AMA “previously rejected our attempt to resolve this matter without litigation,” Dr. Edwards’ attorney, Timothy Maloney, told this news organization. An AMA spokesman said the organization had no comment on Dr. Edwards’ suit.
Dr. Edwards is a past president of the National Medical Association, MedChi, the Baltimore City Medical Society, the Monumental City Medical Society, and the Sickle Cell Disease Association of America. She joined the AMA in 1994 and has served as a trustee since 2016.
As chair of the AMA Task Force on Health Equity, “she helped lead the way in consensus building and driving action that in 2019 resulted in the AMA House of Delegates establishing the AMA Center on Health Equity,” according to her AMA bio page.
‘Quid pro quo’ alleged
In June 2022, Dr. Edwards was one of three individuals running to be AMA president-elect.
According to Dr. Edwards’ complaint, she was “incorrectly advised by colleagues” that Virginia urologist William Reha, MD, had decided not to seek the AMA vice-speakership in 2023. This was important because both Dr. Edwards and Dr. Reha were in the Southeastern delegation. It could be in Dr. Edwards’ favor if Dr. Reha was not running, as it would mean one less leadership candidate from the same region.
Dr. Edwards called Dr. Reha on June 6 to discuss the matter. When they talked, Dr. Reha allegedly recorded the call without Dr. Edwards’ knowledge or permission – a felony in Maryland – and also steered her toward discussions about how his decision could benefit her campaign, according to the complaint.
The suit alleges that Dr. Reha’s questions were “clearly calculated to draw some statements by Dr. Edwards that he could use later to thwart her candidacy and to benefit her opponent.”
On June 10, at the AMA’s House of Delegates meeting in Chicago, Dr. Edwards was taken aside and questioned by members of the AMA’s Election Campaign Committee, according to the complaint. They accused her of “vote trading” but did not provide any evidence or a copy of a complaint they said had been filed against her, the suit said.
Dr. Edwards was given no opportunity to produce her own evidence or rebut the accusations, the suit alleges.
Just before the delegates started formal business on June 13, House Speaker Bruce Scott, MD, read a statement to the assembly saying that a complaint of a possible campaign violation had been filed against Dr. Edwards.
Dr. Scott told the delegates that “committee members interviewed the complainant and multiple other individuals said to have knowledge of the circumstances. In addition to conducting multiple interviews, the committee reviewed evidence that was deemed credible and corroborated that a campaign violation did in fact occur,” according to the complaint.
The supposed violation: A “quid pro quo” in which an unnamed delegation would support Dr. Edwards’ current candidacy, and the Southeastern delegation would support a future candidate from that other unnamed delegation.
Dr. Edwards was given a short opportunity to speak, in which she denied any violations.
According to a news report, Dr. Edwards said, “I’ve been in the House of Delegates for 30 years, and you know me as a process person – a person who truly believes in the process and trying to follow the complexities of our election campaign.”
The lawsuit alleges that “this defamatory conduct was repeated the next day to more than 600 delegates just minutes prior to the casting of votes, when Dr Scott repeated these allegations.”
Dr. Edwards lost the election.
AMA: Nothing more to add
The suit alleges that neither the Election Campaign Committee nor the AMA itself has made any accusers or complaints available to Dr. Edwards and that it has not provided any audio or written evidence of her alleged violation.
In July, the AMA’s Southeastern delegation told its membership, “We continue to maintain that Willarda was ‘set up’ ... The whole affair lacked any reasonable semblance of due process.”
The delegation has filed a counter claim against the AMA seeking “to address this lack of due process as well as the reputational harm” to the delegation.
The AMA said that it has nothing it can produce. “The Speaker of the House presented a verbal report to the attending delegates,” said a spokesman. “The Speaker’s report remains the only remarks from an AMA officer, and no additional remarks can be expected at this time.”
He added that there “is no official transcript of the Speaker’s report.”
A version of this article first appeared on Medscape.com.
If Willarda Edwards, MD, MBA, had won her 2022 campaign for president-elect of the American Medical Association (AMA), she would have been the second Black woman to head the group.
The lawsuit sheds light on the power dynamics of a politically potent organization that has more than 271,000 members and holds assets of $1.2 billion. The AMA president is one of the most visible figures in American medicine.
“The AMA impugned Dr. Edwards with these false charges, which destroyed her candidacy and irreparably damaged her reputation,” according to the complaint, which was filed Nov. 9, 2022, in Baltimore County Circuit Court. The case was later moved to federal court.
The AMA “previously rejected our attempt to resolve this matter without litigation,” Dr. Edwards’ attorney, Timothy Maloney, told this news organization. An AMA spokesman said the organization had no comment on Dr. Edwards’ suit.
Dr. Edwards is a past president of the National Medical Association, MedChi, the Baltimore City Medical Society, the Monumental City Medical Society, and the Sickle Cell Disease Association of America. She joined the AMA in 1994 and has served as a trustee since 2016.
As chair of the AMA Task Force on Health Equity, “she helped lead the way in consensus building and driving action that in 2019 resulted in the AMA House of Delegates establishing the AMA Center on Health Equity,” according to her AMA bio page.
‘Quid pro quo’ alleged
In June 2022, Dr. Edwards was one of three individuals running to be AMA president-elect.
According to Dr. Edwards’ complaint, she was “incorrectly advised by colleagues” that Virginia urologist William Reha, MD, had decided not to seek the AMA vice-speakership in 2023. This was important because both Dr. Edwards and Dr. Reha were in the Southeastern delegation. It could be in Dr. Edwards’ favor if Dr. Reha was not running, as it would mean one less leadership candidate from the same region.
Dr. Edwards called Dr. Reha on June 6 to discuss the matter. When they talked, Dr. Reha allegedly recorded the call without Dr. Edwards’ knowledge or permission – a felony in Maryland – and also steered her toward discussions about how his decision could benefit her campaign, according to the complaint.
The suit alleges that Dr. Reha’s questions were “clearly calculated to draw some statements by Dr. Edwards that he could use later to thwart her candidacy and to benefit her opponent.”
On June 10, at the AMA’s House of Delegates meeting in Chicago, Dr. Edwards was taken aside and questioned by members of the AMA’s Election Campaign Committee, according to the complaint. They accused her of “vote trading” but did not provide any evidence or a copy of a complaint they said had been filed against her, the suit said.
Dr. Edwards was given no opportunity to produce her own evidence or rebut the accusations, the suit alleges.
Just before the delegates started formal business on June 13, House Speaker Bruce Scott, MD, read a statement to the assembly saying that a complaint of a possible campaign violation had been filed against Dr. Edwards.
Dr. Scott told the delegates that “committee members interviewed the complainant and multiple other individuals said to have knowledge of the circumstances. In addition to conducting multiple interviews, the committee reviewed evidence that was deemed credible and corroborated that a campaign violation did in fact occur,” according to the complaint.
The supposed violation: A “quid pro quo” in which an unnamed delegation would support Dr. Edwards’ current candidacy, and the Southeastern delegation would support a future candidate from that other unnamed delegation.
Dr. Edwards was given a short opportunity to speak, in which she denied any violations.
According to a news report, Dr. Edwards said, “I’ve been in the House of Delegates for 30 years, and you know me as a process person – a person who truly believes in the process and trying to follow the complexities of our election campaign.”
The lawsuit alleges that “this defamatory conduct was repeated the next day to more than 600 delegates just minutes prior to the casting of votes, when Dr Scott repeated these allegations.”
Dr. Edwards lost the election.
AMA: Nothing more to add
The suit alleges that neither the Election Campaign Committee nor the AMA itself has made any accusers or complaints available to Dr. Edwards and that it has not provided any audio or written evidence of her alleged violation.
In July, the AMA’s Southeastern delegation told its membership, “We continue to maintain that Willarda was ‘set up’ ... The whole affair lacked any reasonable semblance of due process.”
The delegation has filed a counter claim against the AMA seeking “to address this lack of due process as well as the reputational harm” to the delegation.
The AMA said that it has nothing it can produce. “The Speaker of the House presented a verbal report to the attending delegates,” said a spokesman. “The Speaker’s report remains the only remarks from an AMA officer, and no additional remarks can be expected at this time.”
He added that there “is no official transcript of the Speaker’s report.”
A version of this article first appeared on Medscape.com.
Mothers with disabilities less likely to start breastfeeding
Mothers with intellectual or developmental disabilities are less likely to initiate breastfeeding and to receive in-hospital breastfeeding support than are those without a disability, new data suggest.
In a population-based cohort study of more than 600,000 mothers, patients with an intellectual or developmental disability were about 18% less likely to have a chance to initiate breastfeeding during their hospital stay.
“Overall, we did see lower rates of breastfeeding practices and supports in people with intellectual and developmental disabilities, as well as those with multiple disabilities, compared to people without disabilities,” study author Hilary K. Brown, PhD, assistant professor of health and society at University of Toronto Scarborough in Ontario, told this news organization.
The study was published in The Lancet Public Health.
Disparities in breastfeeding
“There hasn’t been a lot of research on breastfeeding outcomes in people with disabilities,” said Dr. Brown, who noted that the study outcomes were based on the WHO-UNICEF Baby Friendly Hospital Initiative guidelines. “There have been a number of qualitative studies that have suggested that they do experience barriers accessing care related to breastfeeding and different challenges related to breastfeeding. But as far as quantitative outcomes, there has only been a handful of studies.”
To examine these outcomes, the investigators analyzed health administrative data from Ontario. They included in their analysis all birthing parents aged 15-49 years who had a single live birth between April 1, 2012, and March 31, 2018. Patients with a physical disability, sensory disability, intellectual or developmental disability, or two or more disabilities were identified via diagnostic algorithms and were compared with individuals without disabilities with respect to the opportunity to initiate breastfeeding, to engage in in-hospital breastfeeding, to engage in exclusive breastfeeding at hospital discharge, to have skin-to-skin contact, and to be provided with breastfeeding assistance.
The investigators considered a physical disability to encompass conditions such as congenital anomalies, musculoskeletal disorders, neurologic disorders, or permanent injuries. They defined sensory disability as hearing loss or vision loss. Intellectual or developmental disability was defined as having autism spectrum disorder, chromosomal anomaly, fetal alcohol spectrum disorder, or other intellectual disability. Patients with multiple disabilities had two or more of these conditions.
The study population included 634,111 birthing parents, of whom 54,476 (8.6%) had a physical disability, 19,227 (3.0%) had a sensory disability, 1,048 (0.2%) had an intellectual or developmental disability, 4,050 (0.6%) had multiple disabilities, and 555,310 (87.6%) had no disability.
The investigators found that patients with intellectual or developmental disabilities were less likely than were those without a disability to have an opportunity to initiate breastfeeding (adjusted relative risk [aRR], 0.82), to engage in any in-hospital breastfeeding (aRR, 0.85), to be breastfeeding exclusively at hospital discharge (aRR, 0.73), to have skin-to-skin contact (aRR, 0.90), and to receive breastfeeding assistance (aRR, 0.85) compared with patients without a disability.
They also found that individuals with multiple disabilities were less likely to have an opportunity to initiate breastfeeding (aRR, 0.93), to engage in any in-hospital breastfeeding (aRR, 0.93), to be exclusively breastfeeding at hospital discharge (aRR, 0.90), to have skin-to-skin contact (aRR, 0.93), and to receive breastfeeding assistance (aRR, 0.95) compared with patients without a disability.
An understudied population
Commenting on the study, Lori Feldman-Winter, MD, MPH, professor of pediatrics at Rowan University in Camden, N.J., said that one of its strengths is that it included patients who may be excluded from studies of breastfeeding practices. The finding of few differences in breastfeeding practices and supports for people with physical and sensory disabilities, compared with those without disabilities, was positive, she added.
“This is an understudied population, and it is important to call out that there may be practices related to breastfeeding care that suffer, due to implicit bias regarding persons with intellectual and multiple disabilities,” said Dr. Feldman-Winter. “The good news is that other disabilities did not show the same disparities. This study also shows how important it is to measure potential gaps in care across multiple sociodemographic and other variables, such as disabilities, to ensure equitable and inclusive care.”
Health care professionals need to be aware of disparities in breastfeeding care, she added. They need to be open to exploring potential biases when it comes to providing equitable care.
R. Douglas Wilson, MD, president of the Society of Obstetricians and Gynaecologists of Canada and professor emeritus of obstetrics and gynecology at the University of Calgary in Alberta, noted that the size of the cohort represents a strength of the study and that the findings suggest the possible need for closer follow-up of a new mother who is breastfeeding and who has an intellectual disability or multiple disabilities.
“You might keep that patient in hospital for an extra day, and then the home care nurse may look in on them more frequently than they would for someone who does not need that extra oversight,” said Dr. Wilson. When their patients are pregnant, obstetricians and gynecologists can find out whether their patients intend to breastfeed and put them in touch with nurses or lactation consultants to assist them, he added.
The study was funded by the National Institutes of Health and the Canada Research Chairs Program. Dr. Brown, Dr. Feldman-Winter, and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mothers with intellectual or developmental disabilities are less likely to initiate breastfeeding and to receive in-hospital breastfeeding support than are those without a disability, new data suggest.
In a population-based cohort study of more than 600,000 mothers, patients with an intellectual or developmental disability were about 18% less likely to have a chance to initiate breastfeeding during their hospital stay.
“Overall, we did see lower rates of breastfeeding practices and supports in people with intellectual and developmental disabilities, as well as those with multiple disabilities, compared to people without disabilities,” study author Hilary K. Brown, PhD, assistant professor of health and society at University of Toronto Scarborough in Ontario, told this news organization.
The study was published in The Lancet Public Health.
Disparities in breastfeeding
“There hasn’t been a lot of research on breastfeeding outcomes in people with disabilities,” said Dr. Brown, who noted that the study outcomes were based on the WHO-UNICEF Baby Friendly Hospital Initiative guidelines. “There have been a number of qualitative studies that have suggested that they do experience barriers accessing care related to breastfeeding and different challenges related to breastfeeding. But as far as quantitative outcomes, there has only been a handful of studies.”
To examine these outcomes, the investigators analyzed health administrative data from Ontario. They included in their analysis all birthing parents aged 15-49 years who had a single live birth between April 1, 2012, and March 31, 2018. Patients with a physical disability, sensory disability, intellectual or developmental disability, or two or more disabilities were identified via diagnostic algorithms and were compared with individuals without disabilities with respect to the opportunity to initiate breastfeeding, to engage in in-hospital breastfeeding, to engage in exclusive breastfeeding at hospital discharge, to have skin-to-skin contact, and to be provided with breastfeeding assistance.
The investigators considered a physical disability to encompass conditions such as congenital anomalies, musculoskeletal disorders, neurologic disorders, or permanent injuries. They defined sensory disability as hearing loss or vision loss. Intellectual or developmental disability was defined as having autism spectrum disorder, chromosomal anomaly, fetal alcohol spectrum disorder, or other intellectual disability. Patients with multiple disabilities had two or more of these conditions.
The study population included 634,111 birthing parents, of whom 54,476 (8.6%) had a physical disability, 19,227 (3.0%) had a sensory disability, 1,048 (0.2%) had an intellectual or developmental disability, 4,050 (0.6%) had multiple disabilities, and 555,310 (87.6%) had no disability.
The investigators found that patients with intellectual or developmental disabilities were less likely than were those without a disability to have an opportunity to initiate breastfeeding (adjusted relative risk [aRR], 0.82), to engage in any in-hospital breastfeeding (aRR, 0.85), to be breastfeeding exclusively at hospital discharge (aRR, 0.73), to have skin-to-skin contact (aRR, 0.90), and to receive breastfeeding assistance (aRR, 0.85) compared with patients without a disability.
They also found that individuals with multiple disabilities were less likely to have an opportunity to initiate breastfeeding (aRR, 0.93), to engage in any in-hospital breastfeeding (aRR, 0.93), to be exclusively breastfeeding at hospital discharge (aRR, 0.90), to have skin-to-skin contact (aRR, 0.93), and to receive breastfeeding assistance (aRR, 0.95) compared with patients without a disability.
An understudied population
Commenting on the study, Lori Feldman-Winter, MD, MPH, professor of pediatrics at Rowan University in Camden, N.J., said that one of its strengths is that it included patients who may be excluded from studies of breastfeeding practices. The finding of few differences in breastfeeding practices and supports for people with physical and sensory disabilities, compared with those without disabilities, was positive, she added.
“This is an understudied population, and it is important to call out that there may be practices related to breastfeeding care that suffer, due to implicit bias regarding persons with intellectual and multiple disabilities,” said Dr. Feldman-Winter. “The good news is that other disabilities did not show the same disparities. This study also shows how important it is to measure potential gaps in care across multiple sociodemographic and other variables, such as disabilities, to ensure equitable and inclusive care.”
Health care professionals need to be aware of disparities in breastfeeding care, she added. They need to be open to exploring potential biases when it comes to providing equitable care.
R. Douglas Wilson, MD, president of the Society of Obstetricians and Gynaecologists of Canada and professor emeritus of obstetrics and gynecology at the University of Calgary in Alberta, noted that the size of the cohort represents a strength of the study and that the findings suggest the possible need for closer follow-up of a new mother who is breastfeeding and who has an intellectual disability or multiple disabilities.
“You might keep that patient in hospital for an extra day, and then the home care nurse may look in on them more frequently than they would for someone who does not need that extra oversight,” said Dr. Wilson. When their patients are pregnant, obstetricians and gynecologists can find out whether their patients intend to breastfeed and put them in touch with nurses or lactation consultants to assist them, he added.
The study was funded by the National Institutes of Health and the Canada Research Chairs Program. Dr. Brown, Dr. Feldman-Winter, and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mothers with intellectual or developmental disabilities are less likely to initiate breastfeeding and to receive in-hospital breastfeeding support than are those without a disability, new data suggest.
In a population-based cohort study of more than 600,000 mothers, patients with an intellectual or developmental disability were about 18% less likely to have a chance to initiate breastfeeding during their hospital stay.
“Overall, we did see lower rates of breastfeeding practices and supports in people with intellectual and developmental disabilities, as well as those with multiple disabilities, compared to people without disabilities,” study author Hilary K. Brown, PhD, assistant professor of health and society at University of Toronto Scarborough in Ontario, told this news organization.
The study was published in The Lancet Public Health.
Disparities in breastfeeding
“There hasn’t been a lot of research on breastfeeding outcomes in people with disabilities,” said Dr. Brown, who noted that the study outcomes were based on the WHO-UNICEF Baby Friendly Hospital Initiative guidelines. “There have been a number of qualitative studies that have suggested that they do experience barriers accessing care related to breastfeeding and different challenges related to breastfeeding. But as far as quantitative outcomes, there has only been a handful of studies.”
To examine these outcomes, the investigators analyzed health administrative data from Ontario. They included in their analysis all birthing parents aged 15-49 years who had a single live birth between April 1, 2012, and March 31, 2018. Patients with a physical disability, sensory disability, intellectual or developmental disability, or two or more disabilities were identified via diagnostic algorithms and were compared with individuals without disabilities with respect to the opportunity to initiate breastfeeding, to engage in in-hospital breastfeeding, to engage in exclusive breastfeeding at hospital discharge, to have skin-to-skin contact, and to be provided with breastfeeding assistance.
The investigators considered a physical disability to encompass conditions such as congenital anomalies, musculoskeletal disorders, neurologic disorders, or permanent injuries. They defined sensory disability as hearing loss or vision loss. Intellectual or developmental disability was defined as having autism spectrum disorder, chromosomal anomaly, fetal alcohol spectrum disorder, or other intellectual disability. Patients with multiple disabilities had two or more of these conditions.
The study population included 634,111 birthing parents, of whom 54,476 (8.6%) had a physical disability, 19,227 (3.0%) had a sensory disability, 1,048 (0.2%) had an intellectual or developmental disability, 4,050 (0.6%) had multiple disabilities, and 555,310 (87.6%) had no disability.
The investigators found that patients with intellectual or developmental disabilities were less likely than were those without a disability to have an opportunity to initiate breastfeeding (adjusted relative risk [aRR], 0.82), to engage in any in-hospital breastfeeding (aRR, 0.85), to be breastfeeding exclusively at hospital discharge (aRR, 0.73), to have skin-to-skin contact (aRR, 0.90), and to receive breastfeeding assistance (aRR, 0.85) compared with patients without a disability.
They also found that individuals with multiple disabilities were less likely to have an opportunity to initiate breastfeeding (aRR, 0.93), to engage in any in-hospital breastfeeding (aRR, 0.93), to be exclusively breastfeeding at hospital discharge (aRR, 0.90), to have skin-to-skin contact (aRR, 0.93), and to receive breastfeeding assistance (aRR, 0.95) compared with patients without a disability.
An understudied population
Commenting on the study, Lori Feldman-Winter, MD, MPH, professor of pediatrics at Rowan University in Camden, N.J., said that one of its strengths is that it included patients who may be excluded from studies of breastfeeding practices. The finding of few differences in breastfeeding practices and supports for people with physical and sensory disabilities, compared with those without disabilities, was positive, she added.
“This is an understudied population, and it is important to call out that there may be practices related to breastfeeding care that suffer, due to implicit bias regarding persons with intellectual and multiple disabilities,” said Dr. Feldman-Winter. “The good news is that other disabilities did not show the same disparities. This study also shows how important it is to measure potential gaps in care across multiple sociodemographic and other variables, such as disabilities, to ensure equitable and inclusive care.”
Health care professionals need to be aware of disparities in breastfeeding care, she added. They need to be open to exploring potential biases when it comes to providing equitable care.
R. Douglas Wilson, MD, president of the Society of Obstetricians and Gynaecologists of Canada and professor emeritus of obstetrics and gynecology at the University of Calgary in Alberta, noted that the size of the cohort represents a strength of the study and that the findings suggest the possible need for closer follow-up of a new mother who is breastfeeding and who has an intellectual disability or multiple disabilities.
“You might keep that patient in hospital for an extra day, and then the home care nurse may look in on them more frequently than they would for someone who does not need that extra oversight,” said Dr. Wilson. When their patients are pregnant, obstetricians and gynecologists can find out whether their patients intend to breastfeed and put them in touch with nurses or lactation consultants to assist them, he added.
The study was funded by the National Institutes of Health and the Canada Research Chairs Program. Dr. Brown, Dr. Feldman-Winter, and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PUBLIC HEALTH
Black Veterans Disproportionately Denied VA Benefits
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
By the numbers: Cardiology slow to add women, IMGs join more quickly
Despite Mark Twain’s assertion that “there are three kinds of lies: lies, damned lies, and statistics,” we’re going to dive into 20 years’ worth of data and, hopefully, come up with a few statistics that shed some light on the specialty’s workforce since Cardiology News published its first issue in February 2003.
We start with a major issue over these last 20 years: The participation of women in the specialty.
Back in July of 2002, just a few months before the first issue of Cardiology News was published, W. Bruce Fye, MD, then-president of the American College of Cardiology, wrote, “We need to do more to attract female medical graduates to our specialty because they represent almost one-half of the new doctors trained in this country. Cardiology needs to take full advantage of this large talent pool”
Data from the American Medical Association confirm that assertion: Of the nearly 20,000 postgraduate cardiologists in practice that year, only 7.8% were women. And that was at a time when more than 42% of medical school graduates were women, Dr. Fye noted, while also pointing out that “only 10% of cardiology trainees are female, and just 6% of ACC fellows are women.”
The gap between men and women has closed somewhat in the last 20 years, but the specialty continues to lag behind the profession as a whole. Women represented 16.7% of cardiologists in 2022, versus 37% of physicians overall, AMA data show. In 2019, for the first time, the majority of U.S. medical school students (50.5%) were women, according to the Association of American Medical Colleges.
A look at residency numbers from the Accreditation Council for Graduate Medical Education shows that continued slow improvement in the number of women can be expected, as 25.5% of cardiovascular disease residents were women during the 2021-2022 academic year. Only 2 of the 19 other internal medicine subspecialties were lower, and they happened to be interventional cardiology (20.1%) and clinical cardiac electrophysiology (14.5%).
When men are added to the mix, cardiovascular disease had a total of 3,320 active residents training in 268 programs in 2021-2022, making it the largest of the IM subspecialties in both respects. The resident total is up 57% since 2003, when it came in at 2,117, while programs have increased 55% from the 173 that were operating 2 decades ago. During the year in the middle (2011-2012), there were 2,521 residents in 187 programs, so a larger share of the growth has occurred in the last 10 years, the ACGME data indicate.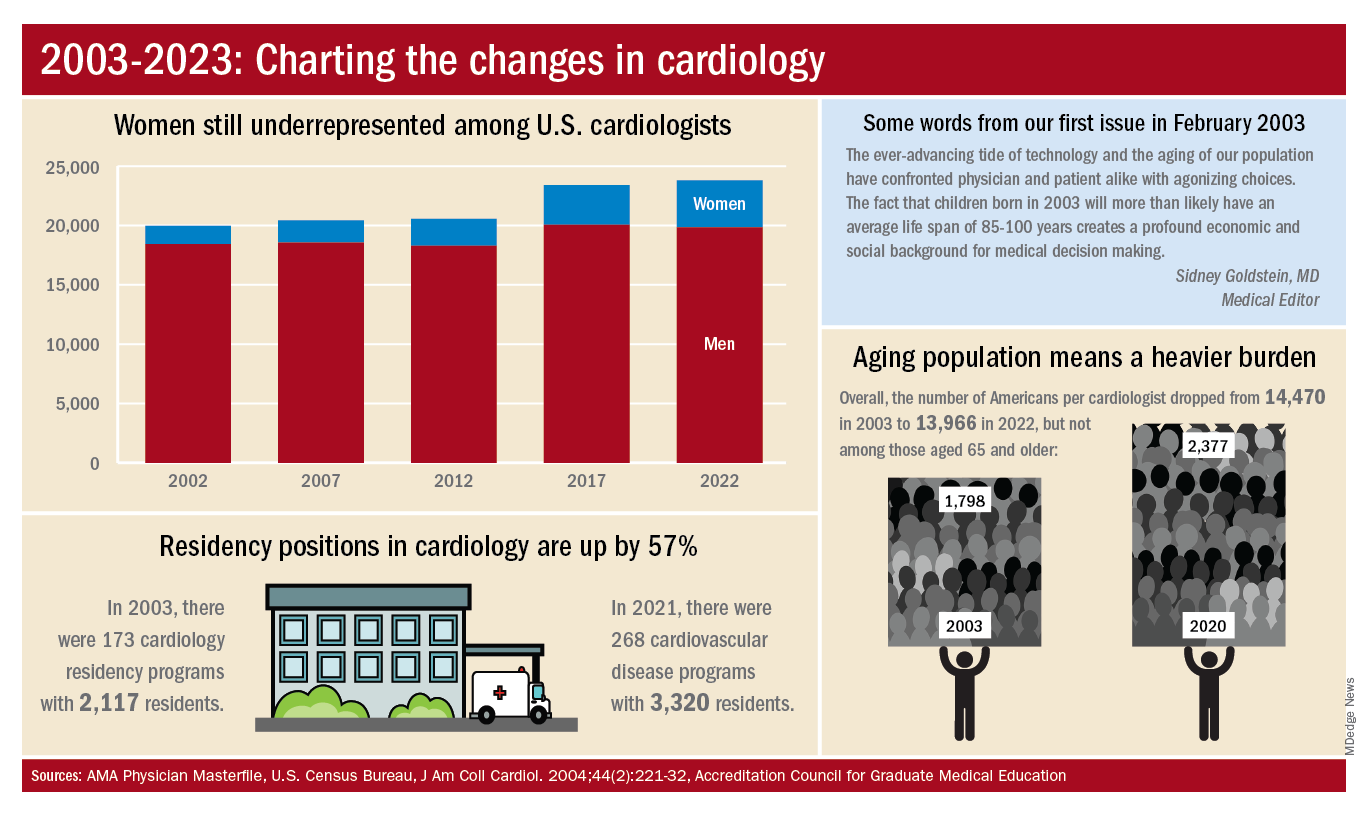
The shortage of cardiologists that Dr. Fye and others wrote about 20 years ago has not gone away. A 2018 report from health consulting firm PYA noted the increase in obesity and the low number of medical school graduates choosing the specialty. “Older and fewer physicians specializing in cardiology, coupled with the aging of baby boomers and gravitation toward practice in urban areas, will continue to exacerbate shortages in physician services in the specialty of cardiology, especially in rural areas, over the next decade,” PYA principal Lyle Oelrich wrote.
A little math appears to back up the claims of a cardiologist shortage. Based on census figures for the U.S. population in 2003, there were 14,470 Americans for each of the cardiologists reported by the AMA. That figure dropped to 13,966 by 2022, which seems like an improvement, but it comes with a caveat. The number of Americans aged 65 years and older increased from 1,798 to 2,377 per cardiologist as of 2020, the latest year for which population data were available by age.
One source of growth in the cardiology workforce has been perhaps its most significant minority: international medical graduates. Even by 2004, IMGs represented a much larger segment of all cardiologists (30.0%) than did women (9.3%), based on AMA data. To put it another way, there were more IMGs specializing in cardiovascular disease (6,615) in 2004 than there were women (3,963) in 2022.
The latest data on cardiology training programs – overall numbers were not available – put IMGs at 39.2% for the 2019-2020 academic year. The 2022 fellowship match provides a slightly smaller proportion of IMGs (37.4%) filling cardiovascular disease positions, according to the National Resident Matching Program.
Despite Mark Twain’s assertion that “there are three kinds of lies: lies, damned lies, and statistics,” we’re going to dive into 20 years’ worth of data and, hopefully, come up with a few statistics that shed some light on the specialty’s workforce since Cardiology News published its first issue in February 2003.
We start with a major issue over these last 20 years: The participation of women in the specialty.
Back in July of 2002, just a few months before the first issue of Cardiology News was published, W. Bruce Fye, MD, then-president of the American College of Cardiology, wrote, “We need to do more to attract female medical graduates to our specialty because they represent almost one-half of the new doctors trained in this country. Cardiology needs to take full advantage of this large talent pool”
Data from the American Medical Association confirm that assertion: Of the nearly 20,000 postgraduate cardiologists in practice that year, only 7.8% were women. And that was at a time when more than 42% of medical school graduates were women, Dr. Fye noted, while also pointing out that “only 10% of cardiology trainees are female, and just 6% of ACC fellows are women.”
The gap between men and women has closed somewhat in the last 20 years, but the specialty continues to lag behind the profession as a whole. Women represented 16.7% of cardiologists in 2022, versus 37% of physicians overall, AMA data show. In 2019, for the first time, the majority of U.S. medical school students (50.5%) were women, according to the Association of American Medical Colleges.
A look at residency numbers from the Accreditation Council for Graduate Medical Education shows that continued slow improvement in the number of women can be expected, as 25.5% of cardiovascular disease residents were women during the 2021-2022 academic year. Only 2 of the 19 other internal medicine subspecialties were lower, and they happened to be interventional cardiology (20.1%) and clinical cardiac electrophysiology (14.5%).
When men are added to the mix, cardiovascular disease had a total of 3,320 active residents training in 268 programs in 2021-2022, making it the largest of the IM subspecialties in both respects. The resident total is up 57% since 2003, when it came in at 2,117, while programs have increased 55% from the 173 that were operating 2 decades ago. During the year in the middle (2011-2012), there were 2,521 residents in 187 programs, so a larger share of the growth has occurred in the last 10 years, the ACGME data indicate.
The shortage of cardiologists that Dr. Fye and others wrote about 20 years ago has not gone away. A 2018 report from health consulting firm PYA noted the increase in obesity and the low number of medical school graduates choosing the specialty. “Older and fewer physicians specializing in cardiology, coupled with the aging of baby boomers and gravitation toward practice in urban areas, will continue to exacerbate shortages in physician services in the specialty of cardiology, especially in rural areas, over the next decade,” PYA principal Lyle Oelrich wrote.
A little math appears to back up the claims of a cardiologist shortage. Based on census figures for the U.S. population in 2003, there were 14,470 Americans for each of the cardiologists reported by the AMA. That figure dropped to 13,966 by 2022, which seems like an improvement, but it comes with a caveat. The number of Americans aged 65 years and older increased from 1,798 to 2,377 per cardiologist as of 2020, the latest year for which population data were available by age.
One source of growth in the cardiology workforce has been perhaps its most significant minority: international medical graduates. Even by 2004, IMGs represented a much larger segment of all cardiologists (30.0%) than did women (9.3%), based on AMA data. To put it another way, there were more IMGs specializing in cardiovascular disease (6,615) in 2004 than there were women (3,963) in 2022.
The latest data on cardiology training programs – overall numbers were not available – put IMGs at 39.2% for the 2019-2020 academic year. The 2022 fellowship match provides a slightly smaller proportion of IMGs (37.4%) filling cardiovascular disease positions, according to the National Resident Matching Program.
Despite Mark Twain’s assertion that “there are three kinds of lies: lies, damned lies, and statistics,” we’re going to dive into 20 years’ worth of data and, hopefully, come up with a few statistics that shed some light on the specialty’s workforce since Cardiology News published its first issue in February 2003.
We start with a major issue over these last 20 years: The participation of women in the specialty.
Back in July of 2002, just a few months before the first issue of Cardiology News was published, W. Bruce Fye, MD, then-president of the American College of Cardiology, wrote, “We need to do more to attract female medical graduates to our specialty because they represent almost one-half of the new doctors trained in this country. Cardiology needs to take full advantage of this large talent pool”
Data from the American Medical Association confirm that assertion: Of the nearly 20,000 postgraduate cardiologists in practice that year, only 7.8% were women. And that was at a time when more than 42% of medical school graduates were women, Dr. Fye noted, while also pointing out that “only 10% of cardiology trainees are female, and just 6% of ACC fellows are women.”
The gap between men and women has closed somewhat in the last 20 years, but the specialty continues to lag behind the profession as a whole. Women represented 16.7% of cardiologists in 2022, versus 37% of physicians overall, AMA data show. In 2019, for the first time, the majority of U.S. medical school students (50.5%) were women, according to the Association of American Medical Colleges.
A look at residency numbers from the Accreditation Council for Graduate Medical Education shows that continued slow improvement in the number of women can be expected, as 25.5% of cardiovascular disease residents were women during the 2021-2022 academic year. Only 2 of the 19 other internal medicine subspecialties were lower, and they happened to be interventional cardiology (20.1%) and clinical cardiac electrophysiology (14.5%).
When men are added to the mix, cardiovascular disease had a total of 3,320 active residents training in 268 programs in 2021-2022, making it the largest of the IM subspecialties in both respects. The resident total is up 57% since 2003, when it came in at 2,117, while programs have increased 55% from the 173 that were operating 2 decades ago. During the year in the middle (2011-2012), there were 2,521 residents in 187 programs, so a larger share of the growth has occurred in the last 10 years, the ACGME data indicate.
The shortage of cardiologists that Dr. Fye and others wrote about 20 years ago has not gone away. A 2018 report from health consulting firm PYA noted the increase in obesity and the low number of medical school graduates choosing the specialty. “Older and fewer physicians specializing in cardiology, coupled with the aging of baby boomers and gravitation toward practice in urban areas, will continue to exacerbate shortages in physician services in the specialty of cardiology, especially in rural areas, over the next decade,” PYA principal Lyle Oelrich wrote.
A little math appears to back up the claims of a cardiologist shortage. Based on census figures for the U.S. population in 2003, there were 14,470 Americans for each of the cardiologists reported by the AMA. That figure dropped to 13,966 by 2022, which seems like an improvement, but it comes with a caveat. The number of Americans aged 65 years and older increased from 1,798 to 2,377 per cardiologist as of 2020, the latest year for which population data were available by age.
One source of growth in the cardiology workforce has been perhaps its most significant minority: international medical graduates. Even by 2004, IMGs represented a much larger segment of all cardiologists (30.0%) than did women (9.3%), based on AMA data. To put it another way, there were more IMGs specializing in cardiovascular disease (6,615) in 2004 than there were women (3,963) in 2022.
The latest data on cardiology training programs – overall numbers were not available – put IMGs at 39.2% for the 2019-2020 academic year. The 2022 fellowship match provides a slightly smaller proportion of IMGs (37.4%) filling cardiovascular disease positions, according to the National Resident Matching Program.
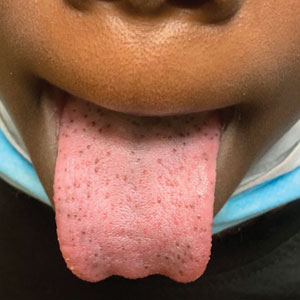
Hyperpigmented Papules on the Tongue of a Child
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
A 9-year-old Black boy presented to the dermatology clinic for evaluation of dark spots on the tongue. The family first noted these spots 5 months prior and reported that they remained stable during that time. The patient’s medical history was notable for autism spectrum disorder and multiple food allergies. His family history was negative for similar oral pigmentation or other pigmentary anomalies. A review of systems was positive only for selective eating and rare nosebleeds. Physical examination revealed numerous dark brown, pinpoint papules across the dorsal aspect of the tongue. No hyperpigmentation of the buccal mucosae, lips, palms, or soles was identified. Several light brown streaks were present on the fingernails and toenails, consistent with longitudinal melanonychia. A prior complete blood cell count was within reference range.
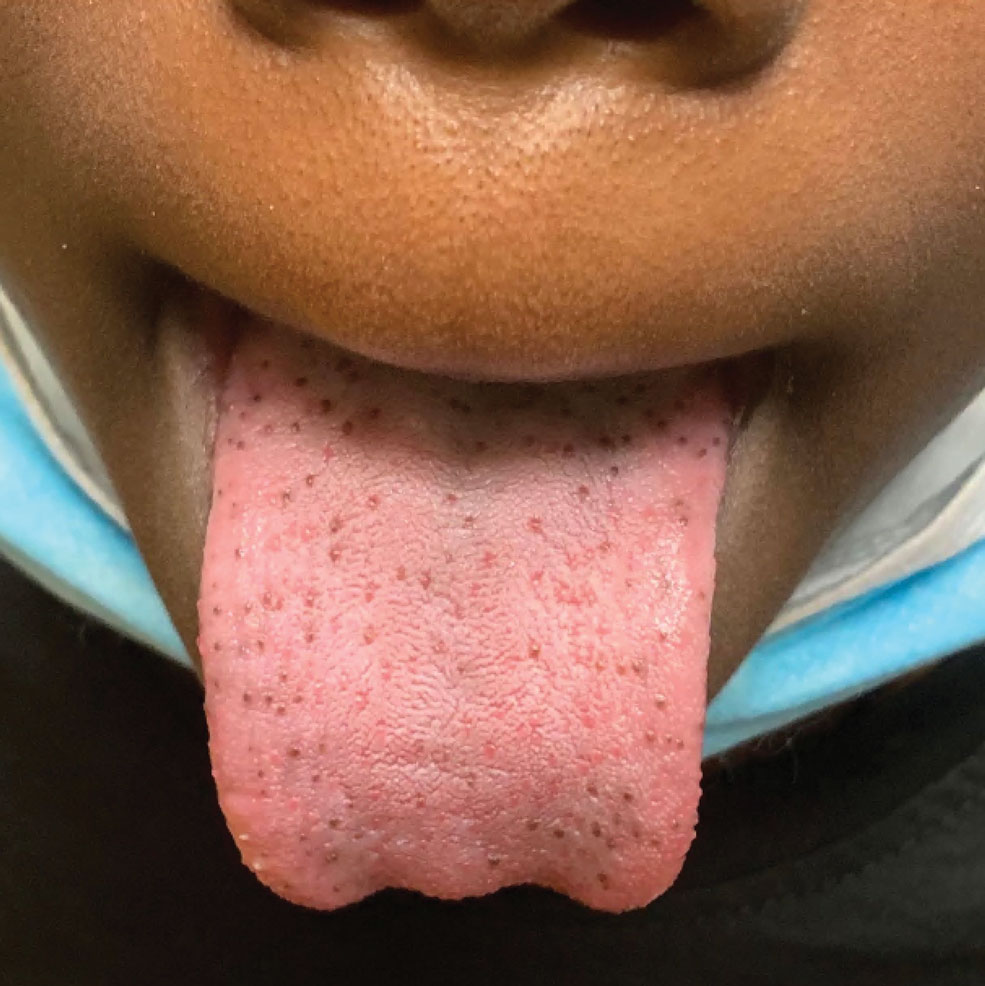
Alopecia Areata in Skin of Color Patients: New Considerations Sparked by the Approval of Baricitinib
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
Study evaluates features of alopecia areata in Hispanic/Latinx patients
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Black HFrEF patients get more empagliflozin benefit in EMPEROR analyses
CHICAGO – Black patients with heart failure with reduced ejection fraction (HFrEF) may receive more benefit from treatment with a sodium-glucose cotransporter-2 (SGLT2) inhibitor than do White patients, according to a new report.
A secondary analysis of data collected from the pivotal trials that assessed the SGLT2 inhibitor empagliflozin in patients with HFrEF, EMPEROR-Reduced, and in patients with heart failure with preserved ejection fraction (HFpEF), EMPEROR-Preserved, was presented by Subodh Verma, MD, PhD, at the American Heart Association scientific sessions.
The “hypothesis-generating” analysis of data from EMPEROR-Reduced showed “a suggestion of a greater benefit of empagliflozin” in Black, compared with White patients, for the study’s primary endpoint (cardiovascular death or hospitalization for heart failure) as well as for first and total hospitalizations for heart failure, he reported.
However, a similar but separate analysis that compared Black and White patients with heart failure who received treatment with a second agent, dapagliflozin, from the same SGLT2-inhibitor class did not show any suggestion of heterogeneity in the drug’s effect based on race.
Race-linked heterogeneity in empagliflozin’s effect
In EMPEROR-Reduced, which randomized 3,730 patients with heart failure and a left ventricular ejection fraction of 40% or less, treatment of White patients with empagliflozin (Jardiance) produced a nonsignificant 16% relative reduction in the rate of the primary endpoint, compared with placebo, during a median 16-month follow-up.
By contrast, among Black patients, treatment with empagliflozin produced a significant 56% reduction in the primary endpoint, compared with placebo-treated patients, a significant heterogeneity (P = .02) in effect between the two race subgroups, said Dr. Verma, a cardiac surgeon and professor at the University of Toronto.
The analysis he reported used combined data from EMPEROR-Reduced and the companion trial EMPEROR-Preserved, which randomized 5,988 patients with heart failure and a left ventricular ejection fraction greater than 40% to treatment with either empagliflozin or placebo and followed them for a median of 26 months.
To assess the effects of the randomized treatments in the two racial subgroups, Dr. Verma and associates used pooled data from both trials, but only from the 3,502 patients enrolled in the Americas, which included 3,024 White patients and 478 Black patients. Analysis of the patients in this subgroup who were randomized to placebo showed a significantly excess rate of the primary outcome among Blacks, who tallied 49% more of the primary outcome events during follow-up than did White patients, Dr. Verma reported. The absolute rate of the primary outcome without empagliflozin treatment was 13.15 events/100 patient-years of follow-up in White patients and 20.83 events/100 patient-years in Black patients.
The impact of empagliflozin was not statistically heterogeneous in the total pool of patients that included both those with HFrEF and those with HFpEF. The drug reduced the primary outcome incidence by a significant 20% in White patients, and by a significant 44% among Black patients.
But this point-estimate difference in efficacy, when coupled with the underlying difference in risk for an event between the two racial groups, meant that the number-needed-to-treat to prevent one primary outcome event was 42 among White patients and 12 among Black patients.
Race-linked treatment responses only in HFrEF
This suggestion of an imbalance in treatment efficacy was especially apparent among patients with HFrEF. In addition to the heterogeneity for the primary outcome, the Black and White subgroups also showed significantly divergent results for the outcomes of first hospitalization for heart failure, with a nonsignificant 21% relative reduction with empagliflozin treatment in Whites but a significant 65% relative cut in this endpoint with empagliflozin in Blacks, and for total hospitalizations for heart failure, which showed a similar level of significant heterogeneity between the two race subgroups.
In contrast, the patients with HFpEF showed no signal at all for heterogeneous outcome rates between Black and White subgroups.
One other study outcome, change in symptom burden measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), also showed suggestion of a race-based imbalance. The adjusted mean difference from baseline in the KCCQ clinical summary score was 1.50 points higher with empagliflozin treatment, compared with placebo among all White patients (those with HFrEF and those with HFpEF), and compared with a 5.25-point increase with empagliflozin over placebo among all Black patients with heart failure in the pooled American EMPEROR dataset, a difference between White and Black patients that just missed significance (P = .06). Again, this difference was especially notable and significant among the patients with HFrEF, where the adjusted mean difference in KCCQ was a 0.77-point increase in White patients and a 6.71-point increase among Black patients (P = .043),
These results also appeared in a report published simultaneously with Dr. Verma’s talk.
But two other analyses that assessed a possible race-based difference in empagliflozin’s effect on renal protection and on functional status showed no suggestion of heterogeneity.
Dr. Verma stressed caution about the limitations of these analyses because they involved a relatively small number of Black patients, and were possibly subject to unadjusted confounding from differences in baseline characteristics between the Black and White patients.
Black patients also had a number-needed-to-treat advantage with dapagliflozin
The finding that Black patients with heart failure potentially get more bang for the buck from treatment with an SGLT2 inhibitor by having a lower number needed to treat also showed up in a separate report at the meeting that assessed the treatment effect from dapagliflozin (Farxiga) in Black and White patients in a pooled analysis of the DAPA-HF pivotal trial of patients with HFrEF and the DELIVER pivotal trial of patients with HFpEF. The pooled cohort included a total of 11,007, but for the analysis by race the investigators also limited their focus to patients from the Americas with 2,626 White patients and 381 Black patients.
Assessment of the effect of dapagliflozin on the primary outcome of cardiovascular death or hospitalization for heart failure among all patients, both those with HFrEF and those with HFpEF, again showed that event rates among patients treated with placebo were significantly higher in Black, compared with White patients, and this led to a difference in the number needed to treat to prevent one primary outcome event of 12 in Blacks and 17 in Whites, Jawad H. Butt, MD said in a talk at the meeting.
Although treatment with dapagliflozin reduced the rate of the primary outcome in this subgroup of patients from the DAPA-HF trial and the DELIVER trial by similar rates in Black and White patients, event rates were higher in the Black patients resulting in “greater benefit in absolute terms” for Black patients, explained Dr. Butt, a cardiologist at Rigshospitalet in Copenhagen.
But in contrast to the empagliflozin findings reported by Dr. Verma, the combined data from the dapagliflozin trials showed no suggestion of heterogeneity in the beneficial effect of dapagliflozin based on left ventricular ejection fraction. In the Black patients, for example, the relative benefit from dapagliflozin on the primary outcome was consistent across the full spectrum of patients with HFrEF and HFpEF.
EMPEROR-Reduced and EMPEROR-Preserved were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). The DAPA-HF and DELIVER trials were sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Verma has received honoraria, research support, or both from AstraZeneca, Boehringer Ingelheim, and Lilly, and from numerous other companies. Dr. Butt has been a consultant to and received travel grants from AstraZeneca, honoraria from Novartis, and has been an adviser to Bayer.
CHICAGO – Black patients with heart failure with reduced ejection fraction (HFrEF) may receive more benefit from treatment with a sodium-glucose cotransporter-2 (SGLT2) inhibitor than do White patients, according to a new report.
A secondary analysis of data collected from the pivotal trials that assessed the SGLT2 inhibitor empagliflozin in patients with HFrEF, EMPEROR-Reduced, and in patients with heart failure with preserved ejection fraction (HFpEF), EMPEROR-Preserved, was presented by Subodh Verma, MD, PhD, at the American Heart Association scientific sessions.
The “hypothesis-generating” analysis of data from EMPEROR-Reduced showed “a suggestion of a greater benefit of empagliflozin” in Black, compared with White patients, for the study’s primary endpoint (cardiovascular death or hospitalization for heart failure) as well as for first and total hospitalizations for heart failure, he reported.
However, a similar but separate analysis that compared Black and White patients with heart failure who received treatment with a second agent, dapagliflozin, from the same SGLT2-inhibitor class did not show any suggestion of heterogeneity in the drug’s effect based on race.
Race-linked heterogeneity in empagliflozin’s effect
In EMPEROR-Reduced, which randomized 3,730 patients with heart failure and a left ventricular ejection fraction of 40% or less, treatment of White patients with empagliflozin (Jardiance) produced a nonsignificant 16% relative reduction in the rate of the primary endpoint, compared with placebo, during a median 16-month follow-up.
By contrast, among Black patients, treatment with empagliflozin produced a significant 56% reduction in the primary endpoint, compared with placebo-treated patients, a significant heterogeneity (P = .02) in effect between the two race subgroups, said Dr. Verma, a cardiac surgeon and professor at the University of Toronto.
The analysis he reported used combined data from EMPEROR-Reduced and the companion trial EMPEROR-Preserved, which randomized 5,988 patients with heart failure and a left ventricular ejection fraction greater than 40% to treatment with either empagliflozin or placebo and followed them for a median of 26 months.
To assess the effects of the randomized treatments in the two racial subgroups, Dr. Verma and associates used pooled data from both trials, but only from the 3,502 patients enrolled in the Americas, which included 3,024 White patients and 478 Black patients. Analysis of the patients in this subgroup who were randomized to placebo showed a significantly excess rate of the primary outcome among Blacks, who tallied 49% more of the primary outcome events during follow-up than did White patients, Dr. Verma reported. The absolute rate of the primary outcome without empagliflozin treatment was 13.15 events/100 patient-years of follow-up in White patients and 20.83 events/100 patient-years in Black patients.
The impact of empagliflozin was not statistically heterogeneous in the total pool of patients that included both those with HFrEF and those with HFpEF. The drug reduced the primary outcome incidence by a significant 20% in White patients, and by a significant 44% among Black patients.
But this point-estimate difference in efficacy, when coupled with the underlying difference in risk for an event between the two racial groups, meant that the number-needed-to-treat to prevent one primary outcome event was 42 among White patients and 12 among Black patients.
Race-linked treatment responses only in HFrEF
This suggestion of an imbalance in treatment efficacy was especially apparent among patients with HFrEF. In addition to the heterogeneity for the primary outcome, the Black and White subgroups also showed significantly divergent results for the outcomes of first hospitalization for heart failure, with a nonsignificant 21% relative reduction with empagliflozin treatment in Whites but a significant 65% relative cut in this endpoint with empagliflozin in Blacks, and for total hospitalizations for heart failure, which showed a similar level of significant heterogeneity between the two race subgroups.
In contrast, the patients with HFpEF showed no signal at all for heterogeneous outcome rates between Black and White subgroups.
One other study outcome, change in symptom burden measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), also showed suggestion of a race-based imbalance. The adjusted mean difference from baseline in the KCCQ clinical summary score was 1.50 points higher with empagliflozin treatment, compared with placebo among all White patients (those with HFrEF and those with HFpEF), and compared with a 5.25-point increase with empagliflozin over placebo among all Black patients with heart failure in the pooled American EMPEROR dataset, a difference between White and Black patients that just missed significance (P = .06). Again, this difference was especially notable and significant among the patients with HFrEF, where the adjusted mean difference in KCCQ was a 0.77-point increase in White patients and a 6.71-point increase among Black patients (P = .043),
These results also appeared in a report published simultaneously with Dr. Verma’s talk.
But two other analyses that assessed a possible race-based difference in empagliflozin’s effect on renal protection and on functional status showed no suggestion of heterogeneity.
Dr. Verma stressed caution about the limitations of these analyses because they involved a relatively small number of Black patients, and were possibly subject to unadjusted confounding from differences in baseline characteristics between the Black and White patients.
Black patients also had a number-needed-to-treat advantage with dapagliflozin
The finding that Black patients with heart failure potentially get more bang for the buck from treatment with an SGLT2 inhibitor by having a lower number needed to treat also showed up in a separate report at the meeting that assessed the treatment effect from dapagliflozin (Farxiga) in Black and White patients in a pooled analysis of the DAPA-HF pivotal trial of patients with HFrEF and the DELIVER pivotal trial of patients with HFpEF. The pooled cohort included a total of 11,007, but for the analysis by race the investigators also limited their focus to patients from the Americas with 2,626 White patients and 381 Black patients.
Assessment of the effect of dapagliflozin on the primary outcome of cardiovascular death or hospitalization for heart failure among all patients, both those with HFrEF and those with HFpEF, again showed that event rates among patients treated with placebo were significantly higher in Black, compared with White patients, and this led to a difference in the number needed to treat to prevent one primary outcome event of 12 in Blacks and 17 in Whites, Jawad H. Butt, MD said in a talk at the meeting.
Although treatment with dapagliflozin reduced the rate of the primary outcome in this subgroup of patients from the DAPA-HF trial and the DELIVER trial by similar rates in Black and White patients, event rates were higher in the Black patients resulting in “greater benefit in absolute terms” for Black patients, explained Dr. Butt, a cardiologist at Rigshospitalet in Copenhagen.
But in contrast to the empagliflozin findings reported by Dr. Verma, the combined data from the dapagliflozin trials showed no suggestion of heterogeneity in the beneficial effect of dapagliflozin based on left ventricular ejection fraction. In the Black patients, for example, the relative benefit from dapagliflozin on the primary outcome was consistent across the full spectrum of patients with HFrEF and HFpEF.
EMPEROR-Reduced and EMPEROR-Preserved were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). The DAPA-HF and DELIVER trials were sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Verma has received honoraria, research support, or both from AstraZeneca, Boehringer Ingelheim, and Lilly, and from numerous other companies. Dr. Butt has been a consultant to and received travel grants from AstraZeneca, honoraria from Novartis, and has been an adviser to Bayer.
CHICAGO – Black patients with heart failure with reduced ejection fraction (HFrEF) may receive more benefit from treatment with a sodium-glucose cotransporter-2 (SGLT2) inhibitor than do White patients, according to a new report.
A secondary analysis of data collected from the pivotal trials that assessed the SGLT2 inhibitor empagliflozin in patients with HFrEF, EMPEROR-Reduced, and in patients with heart failure with preserved ejection fraction (HFpEF), EMPEROR-Preserved, was presented by Subodh Verma, MD, PhD, at the American Heart Association scientific sessions.
The “hypothesis-generating” analysis of data from EMPEROR-Reduced showed “a suggestion of a greater benefit of empagliflozin” in Black, compared with White patients, for the study’s primary endpoint (cardiovascular death or hospitalization for heart failure) as well as for first and total hospitalizations for heart failure, he reported.
However, a similar but separate analysis that compared Black and White patients with heart failure who received treatment with a second agent, dapagliflozin, from the same SGLT2-inhibitor class did not show any suggestion of heterogeneity in the drug’s effect based on race.
Race-linked heterogeneity in empagliflozin’s effect
In EMPEROR-Reduced, which randomized 3,730 patients with heart failure and a left ventricular ejection fraction of 40% or less, treatment of White patients with empagliflozin (Jardiance) produced a nonsignificant 16% relative reduction in the rate of the primary endpoint, compared with placebo, during a median 16-month follow-up.
By contrast, among Black patients, treatment with empagliflozin produced a significant 56% reduction in the primary endpoint, compared with placebo-treated patients, a significant heterogeneity (P = .02) in effect between the two race subgroups, said Dr. Verma, a cardiac surgeon and professor at the University of Toronto.
The analysis he reported used combined data from EMPEROR-Reduced and the companion trial EMPEROR-Preserved, which randomized 5,988 patients with heart failure and a left ventricular ejection fraction greater than 40% to treatment with either empagliflozin or placebo and followed them for a median of 26 months.
To assess the effects of the randomized treatments in the two racial subgroups, Dr. Verma and associates used pooled data from both trials, but only from the 3,502 patients enrolled in the Americas, which included 3,024 White patients and 478 Black patients. Analysis of the patients in this subgroup who were randomized to placebo showed a significantly excess rate of the primary outcome among Blacks, who tallied 49% more of the primary outcome events during follow-up than did White patients, Dr. Verma reported. The absolute rate of the primary outcome without empagliflozin treatment was 13.15 events/100 patient-years of follow-up in White patients and 20.83 events/100 patient-years in Black patients.
The impact of empagliflozin was not statistically heterogeneous in the total pool of patients that included both those with HFrEF and those with HFpEF. The drug reduced the primary outcome incidence by a significant 20% in White patients, and by a significant 44% among Black patients.
But this point-estimate difference in efficacy, when coupled with the underlying difference in risk for an event between the two racial groups, meant that the number-needed-to-treat to prevent one primary outcome event was 42 among White patients and 12 among Black patients.
Race-linked treatment responses only in HFrEF
This suggestion of an imbalance in treatment efficacy was especially apparent among patients with HFrEF. In addition to the heterogeneity for the primary outcome, the Black and White subgroups also showed significantly divergent results for the outcomes of first hospitalization for heart failure, with a nonsignificant 21% relative reduction with empagliflozin treatment in Whites but a significant 65% relative cut in this endpoint with empagliflozin in Blacks, and for total hospitalizations for heart failure, which showed a similar level of significant heterogeneity between the two race subgroups.
In contrast, the patients with HFpEF showed no signal at all for heterogeneous outcome rates between Black and White subgroups.
One other study outcome, change in symptom burden measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), also showed suggestion of a race-based imbalance. The adjusted mean difference from baseline in the KCCQ clinical summary score was 1.50 points higher with empagliflozin treatment, compared with placebo among all White patients (those with HFrEF and those with HFpEF), and compared with a 5.25-point increase with empagliflozin over placebo among all Black patients with heart failure in the pooled American EMPEROR dataset, a difference between White and Black patients that just missed significance (P = .06). Again, this difference was especially notable and significant among the patients with HFrEF, where the adjusted mean difference in KCCQ was a 0.77-point increase in White patients and a 6.71-point increase among Black patients (P = .043),
These results also appeared in a report published simultaneously with Dr. Verma’s talk.
But two other analyses that assessed a possible race-based difference in empagliflozin’s effect on renal protection and on functional status showed no suggestion of heterogeneity.
Dr. Verma stressed caution about the limitations of these analyses because they involved a relatively small number of Black patients, and were possibly subject to unadjusted confounding from differences in baseline characteristics between the Black and White patients.
Black patients also had a number-needed-to-treat advantage with dapagliflozin
The finding that Black patients with heart failure potentially get more bang for the buck from treatment with an SGLT2 inhibitor by having a lower number needed to treat also showed up in a separate report at the meeting that assessed the treatment effect from dapagliflozin (Farxiga) in Black and White patients in a pooled analysis of the DAPA-HF pivotal trial of patients with HFrEF and the DELIVER pivotal trial of patients with HFpEF. The pooled cohort included a total of 11,007, but for the analysis by race the investigators also limited their focus to patients from the Americas with 2,626 White patients and 381 Black patients.
Assessment of the effect of dapagliflozin on the primary outcome of cardiovascular death or hospitalization for heart failure among all patients, both those with HFrEF and those with HFpEF, again showed that event rates among patients treated with placebo were significantly higher in Black, compared with White patients, and this led to a difference in the number needed to treat to prevent one primary outcome event of 12 in Blacks and 17 in Whites, Jawad H. Butt, MD said in a talk at the meeting.
Although treatment with dapagliflozin reduced the rate of the primary outcome in this subgroup of patients from the DAPA-HF trial and the DELIVER trial by similar rates in Black and White patients, event rates were higher in the Black patients resulting in “greater benefit in absolute terms” for Black patients, explained Dr. Butt, a cardiologist at Rigshospitalet in Copenhagen.
But in contrast to the empagliflozin findings reported by Dr. Verma, the combined data from the dapagliflozin trials showed no suggestion of heterogeneity in the beneficial effect of dapagliflozin based on left ventricular ejection fraction. In the Black patients, for example, the relative benefit from dapagliflozin on the primary outcome was consistent across the full spectrum of patients with HFrEF and HFpEF.
EMPEROR-Reduced and EMPEROR-Preserved were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). The DAPA-HF and DELIVER trials were sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Verma has received honoraria, research support, or both from AstraZeneca, Boehringer Ingelheim, and Lilly, and from numerous other companies. Dr. Butt has been a consultant to and received travel grants from AstraZeneca, honoraria from Novartis, and has been an adviser to Bayer.
AT AHA 2022
Hospitals with more diverse and uninsured patients more likely to provide delayed fracture care
Regardless of individual patient-level characteristics such as race, ethnicity, or insurance status, these patients were more likely to miss the recommended 24-hour benchmark for surgery.
“Institutions that treat a less diverse patient population appeared to be more resilient to the mix of insurance status in their patient population and were more likely to meet time-to-surgery benchmarks, regardless of patient insurance status or population-based insurance mix,” write study author Ida Leah Gitajn, MD, an orthopedic trauma surgeon at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues.
“While it is unsurprising that increased delays were associated with underfunded institutions, the association between institutional-level racial disparity and surgical delays implies structural health systems bias,” the authors wrote.
The study was published online in JAMA Network Open.
Site performance varied
Racial inequalities in health care utilization and outcomes have been documented in many medical specialties, including orthopedic trauma, the study authors write. However, previous studies evaluating racial disparities in fracture care have been limited to patient-level associations rather than hospital-level factors.
The investigators conducted a secondary analysis of prospectively collected multicenter data for 2,565 patients with hip and femur fractures enrolled in two randomized trials at 23 sites in the United States and Canada. The researchers assessed whether disparities in meeting 24-hour time-to-surgery benchmarks exist at the patient level or at the institutional level, evaluating the association of race, ethnicity, and insurance status.
The cohort study used data from the Program of Randomized Trials to Evaluate Preoperative Antiseptic Skin Solutions in Orthopaedic Trauma (PREP-IT), which enrolled patients from 2018-2021 and followed them for 1 year. All patients with hip and femur fractures enrolled in the PREP-IT program were included in the analysis, which was conducted from April to September of this year.
The cohort included 2,565 patients with an average age of about 65 years. About 82% of patients were White, 13.4% were Black, 3.2% were Asian, and 1.1% were classified as another race or ethnicity. Among the study population, 32.5% of participants were employed, and 92.2% had health insurance. Nearly 40% had a femur fracture with an average injury severity score of 10.4.
Overall, 596 patients (23.2%) didn’t meet the 24-hour time-to-operating-room benchmark. Patients who didn’t meet the 24-hour surgical window were more likely to be older, women, and have a femur fracture. They were less likely to be employed.
The 23 sites had variability in meeting the 24-hour benchmark, race and ethnicity distribution, and population-based health insurance. Institutions met benchmarks at frequencies ranging from 45.2% (for 196 of 433 procedures) to 97.4% (37 of 38 procedures). Minority race and ethnicity distribution ranged from 0% (in 99 procedures) to 58.2% (in 53 of 91 procedures). The proportion of uninsured patients ranged from 0% (in 64 procedures) to 34.2% (in 13 of 38 procedures).
At the patient level, there was no association between missing the 24-hour benchmark and race or ethnicity, and there was no independent association between hospital population racial composition and surgical delay. In an analysis that controlled for patient-level characteristics, there was no association between missing the 24-hour benchmark and patient-level insurance status.
There was an independent association, however, between the hospital population insurance coverage and hospital population racial composition as an interaction term, suggesting a moderating effect (P = .03), the study authors write.
At low rates of uninsured patients, the probability of missing the 24-hour benchmark was 12.5%-14.6% when racial composition varied from 0%-50% minority patients. In contrast, at higher rates of uninsured patients, the risk of missing the 24-hour window was higher among more diverse populations. For instance, at 30% uninsured, the risk of missing the benchmark was 0.5% when the racial composition was low and 17.6% at 50% minority patients.
Additional studies are needed to understand the findings and how health system programs or structures play a role, the authors write. For instance, well-funded health systems that care for a higher proportion of insured patients likely have quality improvement programs and other support structures, such as operating room access, that ensure appropriate time-to-surgery benchmarks for time-sensitive fractures, they say.
Addressing inequalities
Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery, New York, said, “Despite these disparities being reported and well documented in recent years, unfortunately, not enough has been done to address them or understand their fundamental root causes.”
Dr. Amen, who wasn’t involved with this study, has researched racial and ethnic disparities in hip fracture surgery care across the United States. He and his colleagues found disparities in delayed time-to-surgery, particularly for Black patients.
“We live in a country and society where we want and strive for equality of care for patients regardless of race, ethnicity, gender, sexual orientation, or background,” he said. “We have a moral imperative to address these disparities as health care providers, not only among ourselves, but also in conjunction with lawmakers, hospital administrators, and health policy specialists.”
Uma Srikumaran, MD, an associate professor of orthopedic surgery at Johns Hopkins University, Baltimore, wasn’t involved with this study but has researched racial disparities in the timing of radiographic assessment and surgical treatment of hip fractures.
“Though we understand that racial disparities are pervasive in health care, we have a great deal left to understand about the extent of those disparities and all the various factors that contribute to them,” Dr. Srikumaran told this news organization.
Dr. Srikumaran and colleagues have found that Black patients had longer wait times for evaluation and surgery than White patients.
“We all want to get to the solutions, but those can be difficult to execute without an intricate understanding of the problem,” he said. “We should encourage this type of research all throughout health care in general but also very locally, as solutions are not likely to be one-size-fits-all.”
Dr. Srikumaran pointed to the need to measure the problem in specific pathologies, populations, geographies, hospital types, and other factors.
“Studying the trends of this issue will help us determine whether our national or local initiatives are making a difference and which interventions are most effective for a particular hospital, geographic location, or particular pathology,” he said. “Accordingly, if a particular hospital or health system isn’t looking at differences in the delivery of care by race, they are missing an opportunity to ensure equity and raise overall quality.”
The study was supported by funding from the Patient Centered Outcomes Research Institute. Dr. Gitajn reported receiving personal fees for consulting and teaching work from Stryker outside the submitted work. Dr. Amen and Dr. Srikumaran reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regardless of individual patient-level characteristics such as race, ethnicity, or insurance status, these patients were more likely to miss the recommended 24-hour benchmark for surgery.
“Institutions that treat a less diverse patient population appeared to be more resilient to the mix of insurance status in their patient population and were more likely to meet time-to-surgery benchmarks, regardless of patient insurance status or population-based insurance mix,” write study author Ida Leah Gitajn, MD, an orthopedic trauma surgeon at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues.
“While it is unsurprising that increased delays were associated with underfunded institutions, the association between institutional-level racial disparity and surgical delays implies structural health systems bias,” the authors wrote.
The study was published online in JAMA Network Open.
Site performance varied
Racial inequalities in health care utilization and outcomes have been documented in many medical specialties, including orthopedic trauma, the study authors write. However, previous studies evaluating racial disparities in fracture care have been limited to patient-level associations rather than hospital-level factors.
The investigators conducted a secondary analysis of prospectively collected multicenter data for 2,565 patients with hip and femur fractures enrolled in two randomized trials at 23 sites in the United States and Canada. The researchers assessed whether disparities in meeting 24-hour time-to-surgery benchmarks exist at the patient level or at the institutional level, evaluating the association of race, ethnicity, and insurance status.
The cohort study used data from the Program of Randomized Trials to Evaluate Preoperative Antiseptic Skin Solutions in Orthopaedic Trauma (PREP-IT), which enrolled patients from 2018-2021 and followed them for 1 year. All patients with hip and femur fractures enrolled in the PREP-IT program were included in the analysis, which was conducted from April to September of this year.
The cohort included 2,565 patients with an average age of about 65 years. About 82% of patients were White, 13.4% were Black, 3.2% were Asian, and 1.1% were classified as another race or ethnicity. Among the study population, 32.5% of participants were employed, and 92.2% had health insurance. Nearly 40% had a femur fracture with an average injury severity score of 10.4.
Overall, 596 patients (23.2%) didn’t meet the 24-hour time-to-operating-room benchmark. Patients who didn’t meet the 24-hour surgical window were more likely to be older, women, and have a femur fracture. They were less likely to be employed.
The 23 sites had variability in meeting the 24-hour benchmark, race and ethnicity distribution, and population-based health insurance. Institutions met benchmarks at frequencies ranging from 45.2% (for 196 of 433 procedures) to 97.4% (37 of 38 procedures). Minority race and ethnicity distribution ranged from 0% (in 99 procedures) to 58.2% (in 53 of 91 procedures). The proportion of uninsured patients ranged from 0% (in 64 procedures) to 34.2% (in 13 of 38 procedures).
At the patient level, there was no association between missing the 24-hour benchmark and race or ethnicity, and there was no independent association between hospital population racial composition and surgical delay. In an analysis that controlled for patient-level characteristics, there was no association between missing the 24-hour benchmark and patient-level insurance status.
There was an independent association, however, between the hospital population insurance coverage and hospital population racial composition as an interaction term, suggesting a moderating effect (P = .03), the study authors write.
At low rates of uninsured patients, the probability of missing the 24-hour benchmark was 12.5%-14.6% when racial composition varied from 0%-50% minority patients. In contrast, at higher rates of uninsured patients, the risk of missing the 24-hour window was higher among more diverse populations. For instance, at 30% uninsured, the risk of missing the benchmark was 0.5% when the racial composition was low and 17.6% at 50% minority patients.
Additional studies are needed to understand the findings and how health system programs or structures play a role, the authors write. For instance, well-funded health systems that care for a higher proportion of insured patients likely have quality improvement programs and other support structures, such as operating room access, that ensure appropriate time-to-surgery benchmarks for time-sensitive fractures, they say.
Addressing inequalities
Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery, New York, said, “Despite these disparities being reported and well documented in recent years, unfortunately, not enough has been done to address them or understand their fundamental root causes.”
Dr. Amen, who wasn’t involved with this study, has researched racial and ethnic disparities in hip fracture surgery care across the United States. He and his colleagues found disparities in delayed time-to-surgery, particularly for Black patients.
“We live in a country and society where we want and strive for equality of care for patients regardless of race, ethnicity, gender, sexual orientation, or background,” he said. “We have a moral imperative to address these disparities as health care providers, not only among ourselves, but also in conjunction with lawmakers, hospital administrators, and health policy specialists.”
Uma Srikumaran, MD, an associate professor of orthopedic surgery at Johns Hopkins University, Baltimore, wasn’t involved with this study but has researched racial disparities in the timing of radiographic assessment and surgical treatment of hip fractures.
“Though we understand that racial disparities are pervasive in health care, we have a great deal left to understand about the extent of those disparities and all the various factors that contribute to them,” Dr. Srikumaran told this news organization.
Dr. Srikumaran and colleagues have found that Black patients had longer wait times for evaluation and surgery than White patients.
“We all want to get to the solutions, but those can be difficult to execute without an intricate understanding of the problem,” he said. “We should encourage this type of research all throughout health care in general but also very locally, as solutions are not likely to be one-size-fits-all.”
Dr. Srikumaran pointed to the need to measure the problem in specific pathologies, populations, geographies, hospital types, and other factors.
“Studying the trends of this issue will help us determine whether our national or local initiatives are making a difference and which interventions are most effective for a particular hospital, geographic location, or particular pathology,” he said. “Accordingly, if a particular hospital or health system isn’t looking at differences in the delivery of care by race, they are missing an opportunity to ensure equity and raise overall quality.”
The study was supported by funding from the Patient Centered Outcomes Research Institute. Dr. Gitajn reported receiving personal fees for consulting and teaching work from Stryker outside the submitted work. Dr. Amen and Dr. Srikumaran reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regardless of individual patient-level characteristics such as race, ethnicity, or insurance status, these patients were more likely to miss the recommended 24-hour benchmark for surgery.
“Institutions that treat a less diverse patient population appeared to be more resilient to the mix of insurance status in their patient population and were more likely to meet time-to-surgery benchmarks, regardless of patient insurance status or population-based insurance mix,” write study author Ida Leah Gitajn, MD, an orthopedic trauma surgeon at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues.
“While it is unsurprising that increased delays were associated with underfunded institutions, the association between institutional-level racial disparity and surgical delays implies structural health systems bias,” the authors wrote.
The study was published online in JAMA Network Open.
Site performance varied
Racial inequalities in health care utilization and outcomes have been documented in many medical specialties, including orthopedic trauma, the study authors write. However, previous studies evaluating racial disparities in fracture care have been limited to patient-level associations rather than hospital-level factors.
The investigators conducted a secondary analysis of prospectively collected multicenter data for 2,565 patients with hip and femur fractures enrolled in two randomized trials at 23 sites in the United States and Canada. The researchers assessed whether disparities in meeting 24-hour time-to-surgery benchmarks exist at the patient level or at the institutional level, evaluating the association of race, ethnicity, and insurance status.
The cohort study used data from the Program of Randomized Trials to Evaluate Preoperative Antiseptic Skin Solutions in Orthopaedic Trauma (PREP-IT), which enrolled patients from 2018-2021 and followed them for 1 year. All patients with hip and femur fractures enrolled in the PREP-IT program were included in the analysis, which was conducted from April to September of this year.
The cohort included 2,565 patients with an average age of about 65 years. About 82% of patients were White, 13.4% were Black, 3.2% were Asian, and 1.1% were classified as another race or ethnicity. Among the study population, 32.5% of participants were employed, and 92.2% had health insurance. Nearly 40% had a femur fracture with an average injury severity score of 10.4.
Overall, 596 patients (23.2%) didn’t meet the 24-hour time-to-operating-room benchmark. Patients who didn’t meet the 24-hour surgical window were more likely to be older, women, and have a femur fracture. They were less likely to be employed.
The 23 sites had variability in meeting the 24-hour benchmark, race and ethnicity distribution, and population-based health insurance. Institutions met benchmarks at frequencies ranging from 45.2% (for 196 of 433 procedures) to 97.4% (37 of 38 procedures). Minority race and ethnicity distribution ranged from 0% (in 99 procedures) to 58.2% (in 53 of 91 procedures). The proportion of uninsured patients ranged from 0% (in 64 procedures) to 34.2% (in 13 of 38 procedures).
At the patient level, there was no association between missing the 24-hour benchmark and race or ethnicity, and there was no independent association between hospital population racial composition and surgical delay. In an analysis that controlled for patient-level characteristics, there was no association between missing the 24-hour benchmark and patient-level insurance status.
There was an independent association, however, between the hospital population insurance coverage and hospital population racial composition as an interaction term, suggesting a moderating effect (P = .03), the study authors write.
At low rates of uninsured patients, the probability of missing the 24-hour benchmark was 12.5%-14.6% when racial composition varied from 0%-50% minority patients. In contrast, at higher rates of uninsured patients, the risk of missing the 24-hour window was higher among more diverse populations. For instance, at 30% uninsured, the risk of missing the benchmark was 0.5% when the racial composition was low and 17.6% at 50% minority patients.
Additional studies are needed to understand the findings and how health system programs or structures play a role, the authors write. For instance, well-funded health systems that care for a higher proportion of insured patients likely have quality improvement programs and other support structures, such as operating room access, that ensure appropriate time-to-surgery benchmarks for time-sensitive fractures, they say.
Addressing inequalities
Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery, New York, said, “Despite these disparities being reported and well documented in recent years, unfortunately, not enough has been done to address them or understand their fundamental root causes.”
Dr. Amen, who wasn’t involved with this study, has researched racial and ethnic disparities in hip fracture surgery care across the United States. He and his colleagues found disparities in delayed time-to-surgery, particularly for Black patients.
“We live in a country and society where we want and strive for equality of care for patients regardless of race, ethnicity, gender, sexual orientation, or background,” he said. “We have a moral imperative to address these disparities as health care providers, not only among ourselves, but also in conjunction with lawmakers, hospital administrators, and health policy specialists.”
Uma Srikumaran, MD, an associate professor of orthopedic surgery at Johns Hopkins University, Baltimore, wasn’t involved with this study but has researched racial disparities in the timing of radiographic assessment and surgical treatment of hip fractures.
“Though we understand that racial disparities are pervasive in health care, we have a great deal left to understand about the extent of those disparities and all the various factors that contribute to them,” Dr. Srikumaran told this news organization.
Dr. Srikumaran and colleagues have found that Black patients had longer wait times for evaluation and surgery than White patients.
“We all want to get to the solutions, but those can be difficult to execute without an intricate understanding of the problem,” he said. “We should encourage this type of research all throughout health care in general but also very locally, as solutions are not likely to be one-size-fits-all.”
Dr. Srikumaran pointed to the need to measure the problem in specific pathologies, populations, geographies, hospital types, and other factors.
“Studying the trends of this issue will help us determine whether our national or local initiatives are making a difference and which interventions are most effective for a particular hospital, geographic location, or particular pathology,” he said. “Accordingly, if a particular hospital or health system isn’t looking at differences in the delivery of care by race, they are missing an opportunity to ensure equity and raise overall quality.”
The study was supported by funding from the Patient Centered Outcomes Research Institute. Dr. Gitajn reported receiving personal fees for consulting and teaching work from Stryker outside the submitted work. Dr. Amen and Dr. Srikumaran reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Medical degree program put on probation for ‘infrastructure’ issues
after a national accrediting agency’s onsite survey uncovered “infrastructure” problems earlier this year. Those include faculty shortages and inadequate student access to financial aid as well as to career and wellness counseling.
The inspection was conducted by the Liaison Committee on Medical Education (LCME), an accrediting body sponsored by the Association of American Medical Colleges and the American Medical Association.
While participation is voluntary, institutions must comply with 12 standards to maintain their standing. These include hiring qualified faculty and providing students with financial aid and debt management counseling.
Jeannette South-Paul, MD, Meharry’s senior vice president and chief academic officer, said in an interview that the degree program remains fully accredited despite the fact that LCME representatives found “notable areas of concern,” including the “need for some infrastructure updates and additional educational and financial resources for students.”
Specifically, students did not have sufficient access to advising services, broadband internet, and study spaces. In addition, faculty shortages caused delays in student evaluations, she said.
The new status does not affect the ability of students to complete their medical degrees or residency programs, she said. Dr. South-Paul added that school officials have begun addressing several of the issues and anticipate a swift resolution “guided by an aggressive action plan over the next 18-24 months.”
The university, located in Nashville, Tenn., has had accreditation problems before. In January, following a site visit and low scores on annual resident surveys, the Accreditation Council for Graduate Medical Education (ACGME) placed several of the schools’ residency and fellowship programs on probationary status.
At the time, school officials said that all programs would remain accredited, and they committed to expanding available resources, such as hiring additional staff and an independent expert to make program recommendations. A follow-up site visit was scheduled for August.
Regarding the most recent accreditation challenges, Veronica M. Catanese, MD, MBA, co-secretary of LCME, said the organization could only disclose the accreditation status of a medical school.
“LCME is not able to discuss any details concerning the accreditation of individual medical education programs, including the review process, resulting decisions, or survey results,” she said.
Established medical education programs typically undergo a self-study process and a full survey visit every 8 years. According to LCME’s website, a full survey visit may be conducted sooner if concerns arise about the program’s quality or sustainability.
The LCME program directory lists Meharry Medical College’s accreditation status as “full, on probation.” The next survey visit is scheduled for the 2023-2024 school year.
LCME accreditation is a prerequisite for having access to federal grants and programs, such as Title VII funding, which helps increase minority participation in health care careers. In addition, most state licensure boards and ACGME-affiliated residency programs require applicants to graduate from an LCME-accredited school.
Last year, when Meharry Medical College received pandemic aid money as part of the CARES Act, the school distributed nearly $10 million in scholarships to students – many of whom come from modest-income families and struggle to afford college tuition.
But in general, endowments to historically Black colleges and universities (HBCUs) are often at least 70% smaller than those made to non-HBCUs, which raises the question: Does the lack of funding make it more difficult for schools such as Meharry to maintain accreditation standards?
“Many different factors played into this finding by LCME,” said Dr. South-Paul. “It is a well-known fact that HBCUs have historically not been as well funded or possess the same size endowments as their mainstream academic peers. That is true of Meharry, but it would not be accurate to say this probation is because we are an HBCU.”
Similarly, Dr. Catanese said there is no evidence that HBCUs and non-HBCUs differ in their ability to meet LCME accreditation standards.
About half of the school’s residency and fellowship programs continue to have accreditation problems. According to ACGME’s database, the internal medicine program is currently on “continued accreditation with warning” status. The psychiatry and ob.gyn. programs are on “probationary accreditation” after receiving warnings in previous years.
Meharry was chartered in 1915 but was founded in 1876 as one of the first medical schools in the South for Black Americans.
A version of this article first appeared on Medscape.com.
after a national accrediting agency’s onsite survey uncovered “infrastructure” problems earlier this year. Those include faculty shortages and inadequate student access to financial aid as well as to career and wellness counseling.
The inspection was conducted by the Liaison Committee on Medical Education (LCME), an accrediting body sponsored by the Association of American Medical Colleges and the American Medical Association.
While participation is voluntary, institutions must comply with 12 standards to maintain their standing. These include hiring qualified faculty and providing students with financial aid and debt management counseling.
Jeannette South-Paul, MD, Meharry’s senior vice president and chief academic officer, said in an interview that the degree program remains fully accredited despite the fact that LCME representatives found “notable areas of concern,” including the “need for some infrastructure updates and additional educational and financial resources for students.”
Specifically, students did not have sufficient access to advising services, broadband internet, and study spaces. In addition, faculty shortages caused delays in student evaluations, she said.
The new status does not affect the ability of students to complete their medical degrees or residency programs, she said. Dr. South-Paul added that school officials have begun addressing several of the issues and anticipate a swift resolution “guided by an aggressive action plan over the next 18-24 months.”
The university, located in Nashville, Tenn., has had accreditation problems before. In January, following a site visit and low scores on annual resident surveys, the Accreditation Council for Graduate Medical Education (ACGME) placed several of the schools’ residency and fellowship programs on probationary status.
At the time, school officials said that all programs would remain accredited, and they committed to expanding available resources, such as hiring additional staff and an independent expert to make program recommendations. A follow-up site visit was scheduled for August.
Regarding the most recent accreditation challenges, Veronica M. Catanese, MD, MBA, co-secretary of LCME, said the organization could only disclose the accreditation status of a medical school.
“LCME is not able to discuss any details concerning the accreditation of individual medical education programs, including the review process, resulting decisions, or survey results,” she said.
Established medical education programs typically undergo a self-study process and a full survey visit every 8 years. According to LCME’s website, a full survey visit may be conducted sooner if concerns arise about the program’s quality or sustainability.
The LCME program directory lists Meharry Medical College’s accreditation status as “full, on probation.” The next survey visit is scheduled for the 2023-2024 school year.
LCME accreditation is a prerequisite for having access to federal grants and programs, such as Title VII funding, which helps increase minority participation in health care careers. In addition, most state licensure boards and ACGME-affiliated residency programs require applicants to graduate from an LCME-accredited school.
Last year, when Meharry Medical College received pandemic aid money as part of the CARES Act, the school distributed nearly $10 million in scholarships to students – many of whom come from modest-income families and struggle to afford college tuition.
But in general, endowments to historically Black colleges and universities (HBCUs) are often at least 70% smaller than those made to non-HBCUs, which raises the question: Does the lack of funding make it more difficult for schools such as Meharry to maintain accreditation standards?
“Many different factors played into this finding by LCME,” said Dr. South-Paul. “It is a well-known fact that HBCUs have historically not been as well funded or possess the same size endowments as their mainstream academic peers. That is true of Meharry, but it would not be accurate to say this probation is because we are an HBCU.”
Similarly, Dr. Catanese said there is no evidence that HBCUs and non-HBCUs differ in their ability to meet LCME accreditation standards.
About half of the school’s residency and fellowship programs continue to have accreditation problems. According to ACGME’s database, the internal medicine program is currently on “continued accreditation with warning” status. The psychiatry and ob.gyn. programs are on “probationary accreditation” after receiving warnings in previous years.
Meharry was chartered in 1915 but was founded in 1876 as one of the first medical schools in the South for Black Americans.
A version of this article first appeared on Medscape.com.
after a national accrediting agency’s onsite survey uncovered “infrastructure” problems earlier this year. Those include faculty shortages and inadequate student access to financial aid as well as to career and wellness counseling.
The inspection was conducted by the Liaison Committee on Medical Education (LCME), an accrediting body sponsored by the Association of American Medical Colleges and the American Medical Association.
While participation is voluntary, institutions must comply with 12 standards to maintain their standing. These include hiring qualified faculty and providing students with financial aid and debt management counseling.
Jeannette South-Paul, MD, Meharry’s senior vice president and chief academic officer, said in an interview that the degree program remains fully accredited despite the fact that LCME representatives found “notable areas of concern,” including the “need for some infrastructure updates and additional educational and financial resources for students.”
Specifically, students did not have sufficient access to advising services, broadband internet, and study spaces. In addition, faculty shortages caused delays in student evaluations, she said.
The new status does not affect the ability of students to complete their medical degrees or residency programs, she said. Dr. South-Paul added that school officials have begun addressing several of the issues and anticipate a swift resolution “guided by an aggressive action plan over the next 18-24 months.”
The university, located in Nashville, Tenn., has had accreditation problems before. In January, following a site visit and low scores on annual resident surveys, the Accreditation Council for Graduate Medical Education (ACGME) placed several of the schools’ residency and fellowship programs on probationary status.
At the time, school officials said that all programs would remain accredited, and they committed to expanding available resources, such as hiring additional staff and an independent expert to make program recommendations. A follow-up site visit was scheduled for August.
Regarding the most recent accreditation challenges, Veronica M. Catanese, MD, MBA, co-secretary of LCME, said the organization could only disclose the accreditation status of a medical school.
“LCME is not able to discuss any details concerning the accreditation of individual medical education programs, including the review process, resulting decisions, or survey results,” she said.
Established medical education programs typically undergo a self-study process and a full survey visit every 8 years. According to LCME’s website, a full survey visit may be conducted sooner if concerns arise about the program’s quality or sustainability.
The LCME program directory lists Meharry Medical College’s accreditation status as “full, on probation.” The next survey visit is scheduled for the 2023-2024 school year.
LCME accreditation is a prerequisite for having access to federal grants and programs, such as Title VII funding, which helps increase minority participation in health care careers. In addition, most state licensure boards and ACGME-affiliated residency programs require applicants to graduate from an LCME-accredited school.
Last year, when Meharry Medical College received pandemic aid money as part of the CARES Act, the school distributed nearly $10 million in scholarships to students – many of whom come from modest-income families and struggle to afford college tuition.
But in general, endowments to historically Black colleges and universities (HBCUs) are often at least 70% smaller than those made to non-HBCUs, which raises the question: Does the lack of funding make it more difficult for schools such as Meharry to maintain accreditation standards?
“Many different factors played into this finding by LCME,” said Dr. South-Paul. “It is a well-known fact that HBCUs have historically not been as well funded or possess the same size endowments as their mainstream academic peers. That is true of Meharry, but it would not be accurate to say this probation is because we are an HBCU.”
Similarly, Dr. Catanese said there is no evidence that HBCUs and non-HBCUs differ in their ability to meet LCME accreditation standards.
About half of the school’s residency and fellowship programs continue to have accreditation problems. According to ACGME’s database, the internal medicine program is currently on “continued accreditation with warning” status. The psychiatry and ob.gyn. programs are on “probationary accreditation” after receiving warnings in previous years.
Meharry was chartered in 1915 but was founded in 1876 as one of the first medical schools in the South for Black Americans.
A version of this article first appeared on Medscape.com.