User login
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
The Comparison
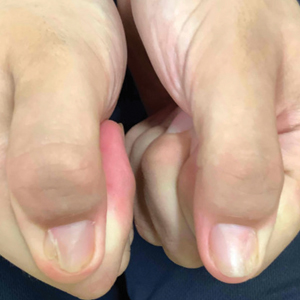
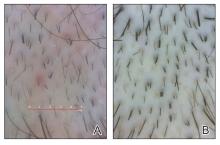
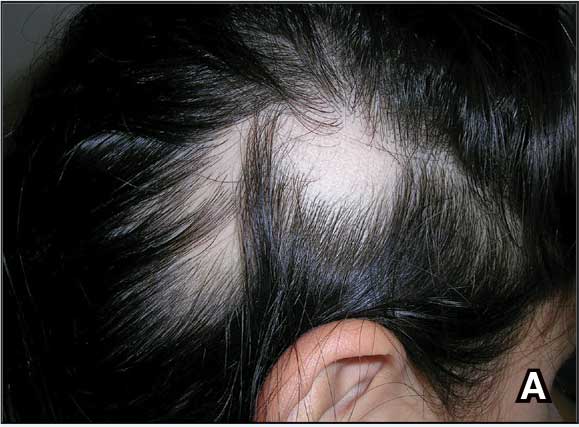
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.
young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.
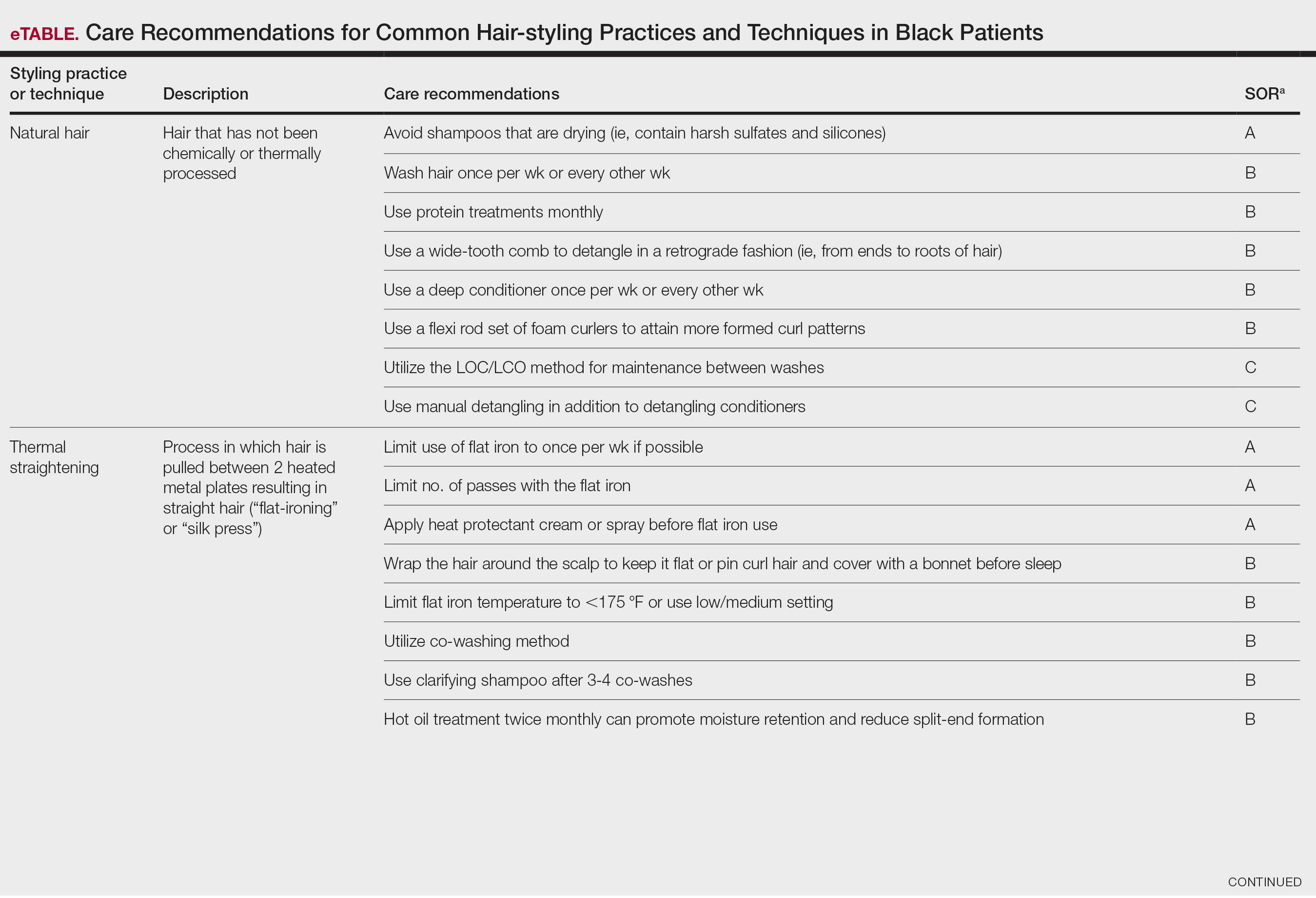
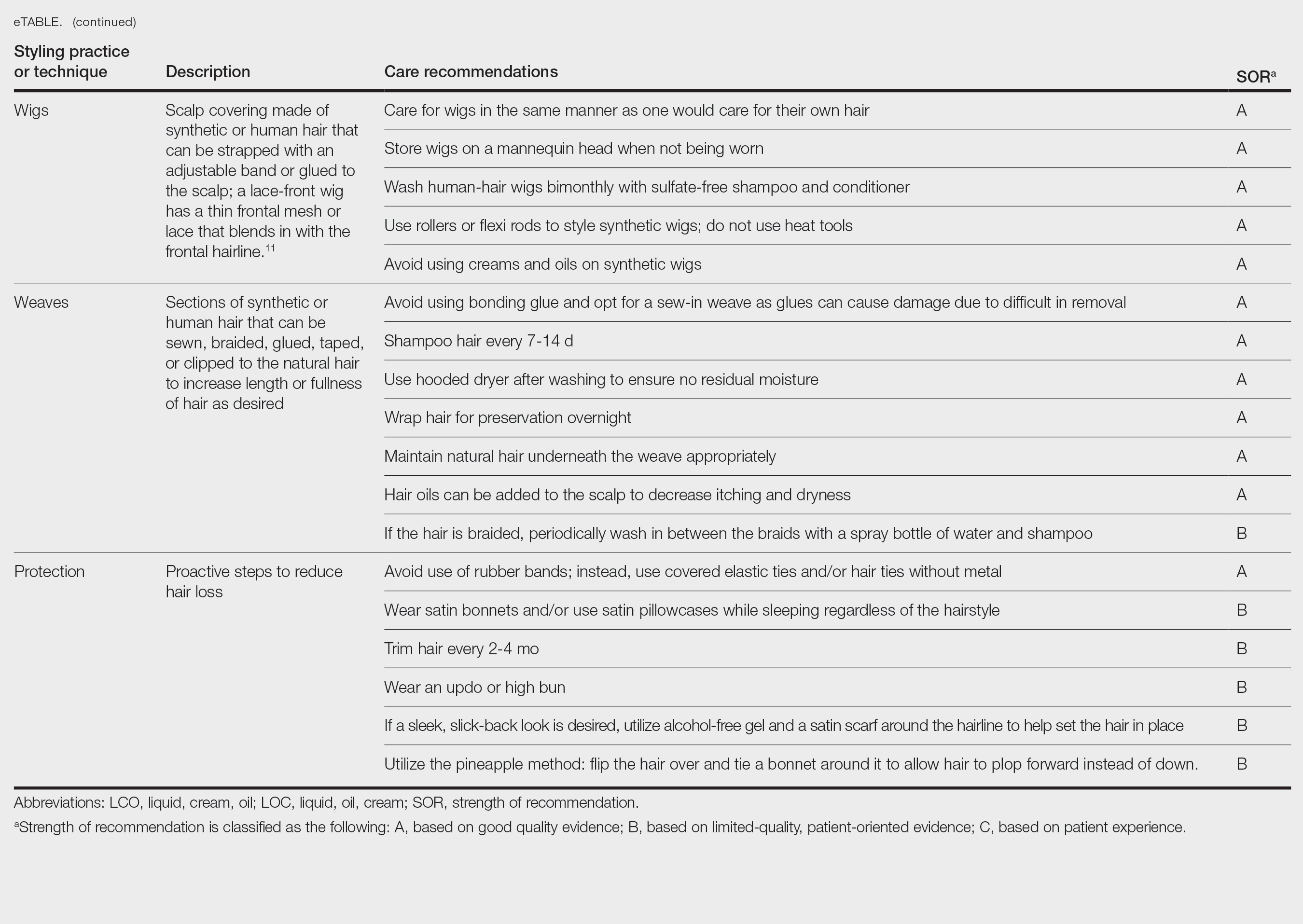
Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
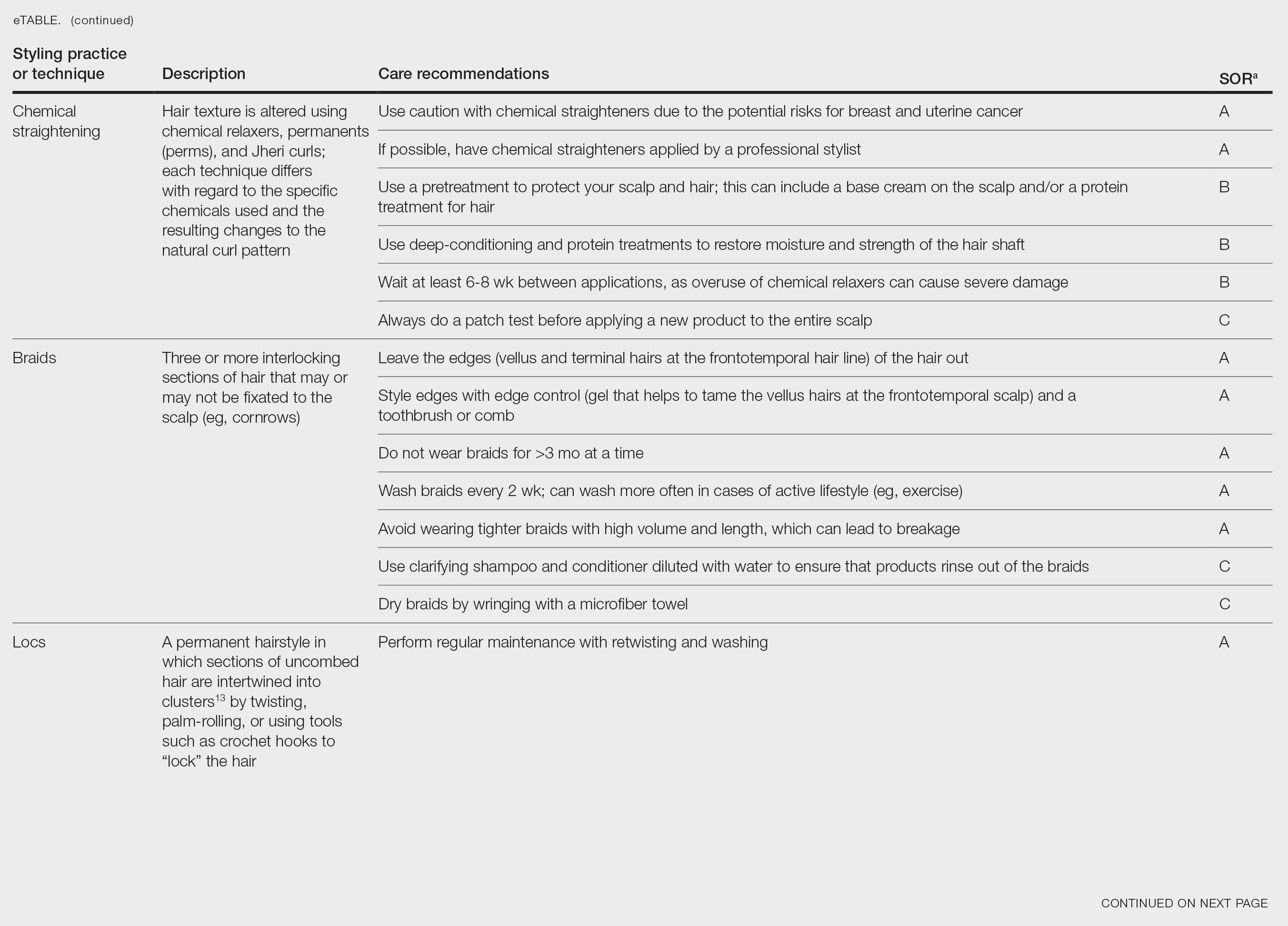
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.



Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.



Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
PRACTICE POINTS
- There is a dearth in understanding of hair care practices in Black women among health care professionals.
- Increased knowledge and cultural understanding of past and present hair care practices in Black women enhances patient care.
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8
Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
A 14-year-old boy with a history of toe walking, attention-deficit/hyperactivity disorder, and mixed receptive expressive language disorder presented to our pediatric dermatology clinic with fingernail abnormalities that had been present since birth. Physical examination revealed narrowing and longitudinal splitting of the nail plates with triangular lacunae and progressive improvement appreciated toward the fifth digits. The nail changes were most prominent on the first digits. A review of the patient’s medical record revealed incidental bilateral iliac horns of the pelvis on radiographs taken at age 18 months. The patient reported waxing and waning knee pain that worsened with prolonged activity and when climbing stairs. Urinalysis demonstrated mild hematuria without proteinuria. The patient was normotensive. There was no evidence of glaucoma, cataracts, or hyperpigmentation of the pupillary margin (Lester iris) on ophthalmologic examination. Genetic testing was performed.
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products

Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
PRACTICE POINTS
- Early identification of androgenetic alopecia (AGA) is key in pediatric patients, especially in those with a family history of AGA and comorbidities such as seborrheic dermatitis, acne, or sleep disturbances.
- It is important to evaluate pediatric patients with AGA for hormonal imbalances and deficiencies in vitamin D and iron to guide treatment.
- Use targeted therapies such as topical minoxidil to treat pediatric AGA while also monitoring for adverse effects. For optimal outcomes, encourage consistent medication use and regular follow-up.
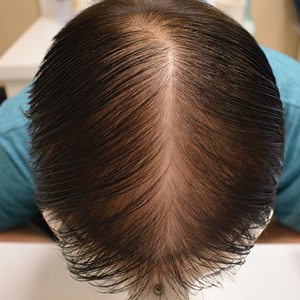
Treating Onychomycosis: Pearls from a Podiatrist
LAS VEGAS —
According to Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, most cases of onychomycosis are caused by the dermatophytes Trichophyton rubrum and T mentagrophytes, although the cause can also be a mixed infection. “Dermatophytes are going to impact the nails first, and molds may come in and join the party later,” she said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference.
“The distal subungual onychomycosis (DSO) type is still the most common, but don’t forget that onychomycosis and nail psoriasis can happen at the same time. What we can’t lose sight of is that onychomycosis is a disease of the nail bed, which ultimately affects the nail plate; it’s not a disease of the nail plate first.”
Her diagnostic approach combines periodic acid-Schiff (PAS) staining with fungal culture “because I like to know the speciation,” she said. “PAS doesn’t give me the speciation; fungal cultures should. PCR can be expensive, but that can give me speciation.”
How Does This Happen?
Fungal DSO occurs because of exposure to a dermatophyte, which can be as simple as tinea pedis. “Perhaps it’s the environment in the shoe,” said Vlahovic, one of the authors of a textbook on onychomycosis. “That’s something I’m always concentrating on with the patient. What is your foot hygiene like? What’s your shoe and sock wear? What’s your level of physical activity? You can have trauma to the hyponychium, where the skin and the nail meet. Maybe they trim their nails too close to the skin, or maybe there’s another skin condition like psoriasis.”
The dermatophyte, she continued, enters and invades the nail at the hyponychium and uses the keratinase enzyme to digest keratin in the nail bed. Mild inflammation develops, and pH changes cause focal parakeratosis and subungual hyperkeratosis in the form of onycholysis and subungual debris. “Hyphae then invade the lamina of the nail plate, which causes brittle nails,” she said. “The compromised hyponychium creates a reservoir for molds and bacteria.”
Therapies approved by the Food and Drug Administration (FDA) for onychomycosis include the topical agents efinaconazole, tavaborole, and ciclopirox; the oral agents terbinafine and itraconazole; and laser therapy. Off-label, Vlahovic said that she sometimes uses oral fluconazole, pulsed dosing for terbinafine, and booster doses of terbinafine or any approved oral antifungal agent. Pulse dosing for itraconazole is FDA-approved for fingernails but not for toenails.
“We don’t have any oral antifungals that are approved for children, but we do have weight-based dosing,” she noted. Other off-label treatments for onychomycosis that patients may come across while browsing the internet but do not penetrate the nail plate, include products containing tolnaftate, tree oil, and undecylenic acid, “which is a very long-chain antifungal,” Vlahovic said. “It’s so huge that it can’t get through the nail plate. These products must get through the nail plate into the nail bed where the infection is.”
According to therapeutic recommendations for the treatment of toenail onychomycosis in the United States, published in 2021, terbinafine is the primary choice for oral treatment and efinaconazole 10% for topical treatment. There are no current treatment recommendations for pregnant or lactating patients. “I always defer to the obstetrician,” said Vlahovic, a coauthor of the recommendations. For pediatric patients, there are approved topical medications: Efinaconazole and tavaborole for ages 6 and up and ciclopirox for ages 12 years or older.
Treatment recommendations for adults vary based on clinical presentation and patient characteristics. Questions to consider: Are they older? Do they have diabetes? Are they able to reach their feet to apply medication? What other medications are they taking? Are there any kidney or liver issues that are cause for concern?
Another question to consider is whether they have concurrent nail psoriasis. “When I have those patients, I often treat the onychomycosis first and the nail psoriasis second,” she said.
Evidence for Lasers Weak
Though laser therapy is FDA approved for the temporary increase of clear nails in onychomycosis, Vlahovic is underwhelmed by the evidence of its use for onychomycosis. According to a systematic review of 261 studies, only 1 reported treatment success as 16.7%, and clinical cures ranged from 13% to 16%. “Many of the existing studies were so poorly done in terms of protocols; it was frustrating,” she said. “No study has reported complete cure. There’s a lack of standardization across laser companies and a lack of standardization across protocols.”
Before starting oral antifungal therapy, Vlahovic uses the Onychomycosis Severity Index to determine the number of nails involved and the proportion of nails that are affected. She also wants to know if the patient is taking any medication that might interfere with an oral antifungal and gets baseline liver function tests (LFTs) to document results in the chart. “You want to discuss the pros and cons of oral antifungal therapy, and you want to set realistic expectations,” she added. “These medications are not cosmetic products; they are meant to kill fungus. Sometimes patients lose sight of that.”
Vlahovic routinely offers pulse dosing of terbinafine, which is FDA approved at a dose of 250 mg/d for 90 days. Pulse dosing involves taking terbinafine 250 mg twice a day for 1 week, followed by a 3-week break. This cycle is repeated three or four times. A clinical trial found no significant difference in outcome between patients who received pulsed vs continuous terbinafine dosing for the treatment of dermatophyte onychomycosis.
What About Oral Antifungal Safety?
For patients who ask about the safety of oral antifungals, Vlahovic characterized them as “well tolerated and safe in an immunocompetent population.” In a meta-analysis of 122 studies of about 22,000 patients, the pooled risk for treatment discontinuation because of adverse events was 3.4% for terbinafine 250 mg/d and 4.21% for itraconazole 200 mg/d. The risk for liver injury requiring termination of treatment and the risk of having symptomatic elevation of LFTs were less than 2% for all regimens.
According to the best available published evidence, Vlahovic said, the onychomycosis recurrence rate ranges from 6% to 40%. “That’s a wild number. We really have no idea what the true recurrence rate is, and that’s a problem.”
Vlahovic disclosed having been a consultant to and an investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
LAS VEGAS —
According to Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, most cases of onychomycosis are caused by the dermatophytes Trichophyton rubrum and T mentagrophytes, although the cause can also be a mixed infection. “Dermatophytes are going to impact the nails first, and molds may come in and join the party later,” she said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference.
“The distal subungual onychomycosis (DSO) type is still the most common, but don’t forget that onychomycosis and nail psoriasis can happen at the same time. What we can’t lose sight of is that onychomycosis is a disease of the nail bed, which ultimately affects the nail plate; it’s not a disease of the nail plate first.”
Her diagnostic approach combines periodic acid-Schiff (PAS) staining with fungal culture “because I like to know the speciation,” she said. “PAS doesn’t give me the speciation; fungal cultures should. PCR can be expensive, but that can give me speciation.”
How Does This Happen?
Fungal DSO occurs because of exposure to a dermatophyte, which can be as simple as tinea pedis. “Perhaps it’s the environment in the shoe,” said Vlahovic, one of the authors of a textbook on onychomycosis. “That’s something I’m always concentrating on with the patient. What is your foot hygiene like? What’s your shoe and sock wear? What’s your level of physical activity? You can have trauma to the hyponychium, where the skin and the nail meet. Maybe they trim their nails too close to the skin, or maybe there’s another skin condition like psoriasis.”
The dermatophyte, she continued, enters and invades the nail at the hyponychium and uses the keratinase enzyme to digest keratin in the nail bed. Mild inflammation develops, and pH changes cause focal parakeratosis and subungual hyperkeratosis in the form of onycholysis and subungual debris. “Hyphae then invade the lamina of the nail plate, which causes brittle nails,” she said. “The compromised hyponychium creates a reservoir for molds and bacteria.”
Therapies approved by the Food and Drug Administration (FDA) for onychomycosis include the topical agents efinaconazole, tavaborole, and ciclopirox; the oral agents terbinafine and itraconazole; and laser therapy. Off-label, Vlahovic said that she sometimes uses oral fluconazole, pulsed dosing for terbinafine, and booster doses of terbinafine or any approved oral antifungal agent. Pulse dosing for itraconazole is FDA-approved for fingernails but not for toenails.
“We don’t have any oral antifungals that are approved for children, but we do have weight-based dosing,” she noted. Other off-label treatments for onychomycosis that patients may come across while browsing the internet but do not penetrate the nail plate, include products containing tolnaftate, tree oil, and undecylenic acid, “which is a very long-chain antifungal,” Vlahovic said. “It’s so huge that it can’t get through the nail plate. These products must get through the nail plate into the nail bed where the infection is.”
According to therapeutic recommendations for the treatment of toenail onychomycosis in the United States, published in 2021, terbinafine is the primary choice for oral treatment and efinaconazole 10% for topical treatment. There are no current treatment recommendations for pregnant or lactating patients. “I always defer to the obstetrician,” said Vlahovic, a coauthor of the recommendations. For pediatric patients, there are approved topical medications: Efinaconazole and tavaborole for ages 6 and up and ciclopirox for ages 12 years or older.
Treatment recommendations for adults vary based on clinical presentation and patient characteristics. Questions to consider: Are they older? Do they have diabetes? Are they able to reach their feet to apply medication? What other medications are they taking? Are there any kidney or liver issues that are cause for concern?
Another question to consider is whether they have concurrent nail psoriasis. “When I have those patients, I often treat the onychomycosis first and the nail psoriasis second,” she said.
Evidence for Lasers Weak
Though laser therapy is FDA approved for the temporary increase of clear nails in onychomycosis, Vlahovic is underwhelmed by the evidence of its use for onychomycosis. According to a systematic review of 261 studies, only 1 reported treatment success as 16.7%, and clinical cures ranged from 13% to 16%. “Many of the existing studies were so poorly done in terms of protocols; it was frustrating,” she said. “No study has reported complete cure. There’s a lack of standardization across laser companies and a lack of standardization across protocols.”
Before starting oral antifungal therapy, Vlahovic uses the Onychomycosis Severity Index to determine the number of nails involved and the proportion of nails that are affected. She also wants to know if the patient is taking any medication that might interfere with an oral antifungal and gets baseline liver function tests (LFTs) to document results in the chart. “You want to discuss the pros and cons of oral antifungal therapy, and you want to set realistic expectations,” she added. “These medications are not cosmetic products; they are meant to kill fungus. Sometimes patients lose sight of that.”
Vlahovic routinely offers pulse dosing of terbinafine, which is FDA approved at a dose of 250 mg/d for 90 days. Pulse dosing involves taking terbinafine 250 mg twice a day for 1 week, followed by a 3-week break. This cycle is repeated three or four times. A clinical trial found no significant difference in outcome between patients who received pulsed vs continuous terbinafine dosing for the treatment of dermatophyte onychomycosis.
What About Oral Antifungal Safety?
For patients who ask about the safety of oral antifungals, Vlahovic characterized them as “well tolerated and safe in an immunocompetent population.” In a meta-analysis of 122 studies of about 22,000 patients, the pooled risk for treatment discontinuation because of adverse events was 3.4% for terbinafine 250 mg/d and 4.21% for itraconazole 200 mg/d. The risk for liver injury requiring termination of treatment and the risk of having symptomatic elevation of LFTs were less than 2% for all regimens.
According to the best available published evidence, Vlahovic said, the onychomycosis recurrence rate ranges from 6% to 40%. “That’s a wild number. We really have no idea what the true recurrence rate is, and that’s a problem.”
Vlahovic disclosed having been a consultant to and an investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
LAS VEGAS —
According to Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, most cases of onychomycosis are caused by the dermatophytes Trichophyton rubrum and T mentagrophytes, although the cause can also be a mixed infection. “Dermatophytes are going to impact the nails first, and molds may come in and join the party later,” she said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference.
“The distal subungual onychomycosis (DSO) type is still the most common, but don’t forget that onychomycosis and nail psoriasis can happen at the same time. What we can’t lose sight of is that onychomycosis is a disease of the nail bed, which ultimately affects the nail plate; it’s not a disease of the nail plate first.”
Her diagnostic approach combines periodic acid-Schiff (PAS) staining with fungal culture “because I like to know the speciation,” she said. “PAS doesn’t give me the speciation; fungal cultures should. PCR can be expensive, but that can give me speciation.”
How Does This Happen?
Fungal DSO occurs because of exposure to a dermatophyte, which can be as simple as tinea pedis. “Perhaps it’s the environment in the shoe,” said Vlahovic, one of the authors of a textbook on onychomycosis. “That’s something I’m always concentrating on with the patient. What is your foot hygiene like? What’s your shoe and sock wear? What’s your level of physical activity? You can have trauma to the hyponychium, where the skin and the nail meet. Maybe they trim their nails too close to the skin, or maybe there’s another skin condition like psoriasis.”
The dermatophyte, she continued, enters and invades the nail at the hyponychium and uses the keratinase enzyme to digest keratin in the nail bed. Mild inflammation develops, and pH changes cause focal parakeratosis and subungual hyperkeratosis in the form of onycholysis and subungual debris. “Hyphae then invade the lamina of the nail plate, which causes brittle nails,” she said. “The compromised hyponychium creates a reservoir for molds and bacteria.”
Therapies approved by the Food and Drug Administration (FDA) for onychomycosis include the topical agents efinaconazole, tavaborole, and ciclopirox; the oral agents terbinafine and itraconazole; and laser therapy. Off-label, Vlahovic said that she sometimes uses oral fluconazole, pulsed dosing for terbinafine, and booster doses of terbinafine or any approved oral antifungal agent. Pulse dosing for itraconazole is FDA-approved for fingernails but not for toenails.
“We don’t have any oral antifungals that are approved for children, but we do have weight-based dosing,” she noted. Other off-label treatments for onychomycosis that patients may come across while browsing the internet but do not penetrate the nail plate, include products containing tolnaftate, tree oil, and undecylenic acid, “which is a very long-chain antifungal,” Vlahovic said. “It’s so huge that it can’t get through the nail plate. These products must get through the nail plate into the nail bed where the infection is.”
According to therapeutic recommendations for the treatment of toenail onychomycosis in the United States, published in 2021, terbinafine is the primary choice for oral treatment and efinaconazole 10% for topical treatment. There are no current treatment recommendations for pregnant or lactating patients. “I always defer to the obstetrician,” said Vlahovic, a coauthor of the recommendations. For pediatric patients, there are approved topical medications: Efinaconazole and tavaborole for ages 6 and up and ciclopirox for ages 12 years or older.
Treatment recommendations for adults vary based on clinical presentation and patient characteristics. Questions to consider: Are they older? Do they have diabetes? Are they able to reach their feet to apply medication? What other medications are they taking? Are there any kidney or liver issues that are cause for concern?
Another question to consider is whether they have concurrent nail psoriasis. “When I have those patients, I often treat the onychomycosis first and the nail psoriasis second,” she said.
Evidence for Lasers Weak
Though laser therapy is FDA approved for the temporary increase of clear nails in onychomycosis, Vlahovic is underwhelmed by the evidence of its use for onychomycosis. According to a systematic review of 261 studies, only 1 reported treatment success as 16.7%, and clinical cures ranged from 13% to 16%. “Many of the existing studies were so poorly done in terms of protocols; it was frustrating,” she said. “No study has reported complete cure. There’s a lack of standardization across laser companies and a lack of standardization across protocols.”
Before starting oral antifungal therapy, Vlahovic uses the Onychomycosis Severity Index to determine the number of nails involved and the proportion of nails that are affected. She also wants to know if the patient is taking any medication that might interfere with an oral antifungal and gets baseline liver function tests (LFTs) to document results in the chart. “You want to discuss the pros and cons of oral antifungal therapy, and you want to set realistic expectations,” she added. “These medications are not cosmetic products; they are meant to kill fungus. Sometimes patients lose sight of that.”
Vlahovic routinely offers pulse dosing of terbinafine, which is FDA approved at a dose of 250 mg/d for 90 days. Pulse dosing involves taking terbinafine 250 mg twice a day for 1 week, followed by a 3-week break. This cycle is repeated three or four times. A clinical trial found no significant difference in outcome between patients who received pulsed vs continuous terbinafine dosing for the treatment of dermatophyte onychomycosis.
What About Oral Antifungal Safety?
For patients who ask about the safety of oral antifungals, Vlahovic characterized them as “well tolerated and safe in an immunocompetent population.” In a meta-analysis of 122 studies of about 22,000 patients, the pooled risk for treatment discontinuation because of adverse events was 3.4% for terbinafine 250 mg/d and 4.21% for itraconazole 200 mg/d. The risk for liver injury requiring termination of treatment and the risk of having symptomatic elevation of LFTs were less than 2% for all regimens.
According to the best available published evidence, Vlahovic said, the onychomycosis recurrence rate ranges from 6% to 40%. “That’s a wild number. We really have no idea what the true recurrence rate is, and that’s a problem.”
Vlahovic disclosed having been a consultant to and an investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
FROM SDPA 2024
Low-Dose Oral Minoxidil: Expert Consensus Provide Guidance for Treating Hair Loss
. With large randomized, controlled trials lacking, the guidelines authors and other dermatologists said the paper provides practical pointers that should increase clinicians’ confidence in prescribing LDOM for hair loss.
Comfort and Confidence
Benjamin N. Ungar, MD, director of the Alopecia Center of Excellence at Mount Sinai Icahn School of Medicine, New York City, said he hopes that the guidelines will “make dermatologists in practice more comfortable with the use of low-dose oral minoxidil to treat different kinds of hair loss, and therefore, more patients will benefit.” He was not an author of the paper, which was published online in JAMA Dermatology on November 20, but was asked to comment.
Members of the multidisciplinary Low-Dose Oral Minoxidil Initiation steering committee recruited dermatologists with hair loss expertise from 12 countries. Using a modified four-round Delphi process that required at least 70% agreement, the group of 43 dermatologists crafted 76 consensus statements. “Notably,” said Co-senior author Jennifer Fu, MD, director of the Hair Disorders Clinic at the University of California, San Francisco, “27 items achieved at least 90% consensus after the first two rounds, indicating broad agreement in expert practice.”
Indications for LDOM
At least 90% of experts concurred regarding the appropriateness of LDOM use for androgenetic alopecia (AGA) and age-related thinning and in cases where topical minoxidil proves ineffective or problematic. Additional situations in which LDOM might provide direct benefit involve follicular miniaturization, such as alopecia areata, or hair cycle disruption, such as chemotherapy. The authors also recommended considering LDOM over topical minoxidil when the latter is more expensive and when patients desire enhanced hypertrichosis.
Contraindications and Precautions
Before prescribing LDOM, the authors wrote, clinicians may consult with primary care or cardiology when contraindications (cardiovascular issues, pregnancy/nursing, and potential drug interactions) or precautions (history of tachycardia or arrhythmia, hypotension, or impaired kidney function) exist. Patients with precautions may require blood pressure monitoring, as well as monitoring for adverse effects of treatment. The panel also suggested the latter for all patients at the time of LDOM initiation and dose escalation. The authors advised against routine baseline laboratory and EKG testing in cases without relevant precautions.
Dosing Considerations
Along with systemic adverse event risk and baseline hair loss severity, key dosing considerations include patient age, sex, and whether patients desire hypertrichosis. Consensus on daily doses for adolescent females and males begins at 0.625 mg and 1.25 mg, respectively, and ranges up to 2.5 mg for adolescent females vs 5 mg for adult females and adolescent and adult males.
Presently, said Ungar, many dermatologists — including some who prescribe LDOM — remain uncomfortable even with very low doses, perhaps because of an invalid perception of cardiovascular safety issues including potential hypotension and pericardial effusions. However, recently published data include a review published November 7 in the Journal of the American Academy of Dermatology, which showed no significant effect of LDOM on blood pressure. And in a September Journal of Drugs in Dermatology article the authors found no impact on pericardial effusions in a 100-patient cohort.
Some dermatologists worry about the impact hypertrichosis may have on patients, Ungar added. Although incidence estimates range from 15% to 30%, he said, more than half of his patients experience hypertrichosis. “However, most continue treatment because the beneficial effects outweigh the effect of hypertrichosis.”
Practical Roadmap
Adam Friedman, MD, who was not involved with the publication, applauds its inclusion of pragmatic clinical guidance, which he said consensus papers often lack. “This paper sets a great roadmap for working low-dose oral minoxidil into your clinical practice, Friedman, professor and chair of dermatology at George Washington University, Washington, DC, said in an interview.
Rather than limiting LDOM use to AGA, he said, the paper is most helpful in showing the spectrum of disease states for which the expert panel prescribes LDOM. “We use it as adjunctive therapy for many other things, both scarring and nonscarring hair loss,” he added.
In appropriate clinical contexts, the authors wrote, clinicians may consider combining LDOM with spironolactone or beta-blockers. Friedman said that in his hands, combining LDOM with a 5-alpha reductase inhibitor (5ARI) is “absolutely outstanding.” Minoxidil increases blood flow to the scalp, he explained, while 5ARIs prevent production of dihydrotestosterone, which miniaturizes hair.
Fu said, “We hope these consensus outcomes will be helpful to dermatology colleagues as they consider using LDOM to treat hair loss in their adult and adolescent patient populations. We anticipate that these guidelines will be updated as additional evidence-based data emerges and are encouraged that we are already seeing new publications on this topic.”
Important areas for future research, she noted, include pediatric use of LDOM, the comparative efficacy of topical vs oral minoxidil, the safety of oral minoxidil for patients with a history of allergic contact dermatitis to topical minoxidil, and the use of other off-label forms of minoxidil, such as compounded oral minoxidil and sublingual minoxidil.
The study was funded by the University of California, San Francisco, Department of Dermatology Medical Student Summer Research Fellowship Program. Fu reported personal fees from Pfizer, Eli Lilly and Company, and Sun Pharma outside of the study. The full list of author disclosures can be found in the paper. Ungar and Friedman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
. With large randomized, controlled trials lacking, the guidelines authors and other dermatologists said the paper provides practical pointers that should increase clinicians’ confidence in prescribing LDOM for hair loss.
Comfort and Confidence
Benjamin N. Ungar, MD, director of the Alopecia Center of Excellence at Mount Sinai Icahn School of Medicine, New York City, said he hopes that the guidelines will “make dermatologists in practice more comfortable with the use of low-dose oral minoxidil to treat different kinds of hair loss, and therefore, more patients will benefit.” He was not an author of the paper, which was published online in JAMA Dermatology on November 20, but was asked to comment.
Members of the multidisciplinary Low-Dose Oral Minoxidil Initiation steering committee recruited dermatologists with hair loss expertise from 12 countries. Using a modified four-round Delphi process that required at least 70% agreement, the group of 43 dermatologists crafted 76 consensus statements. “Notably,” said Co-senior author Jennifer Fu, MD, director of the Hair Disorders Clinic at the University of California, San Francisco, “27 items achieved at least 90% consensus after the first two rounds, indicating broad agreement in expert practice.”
Indications for LDOM
At least 90% of experts concurred regarding the appropriateness of LDOM use for androgenetic alopecia (AGA) and age-related thinning and in cases where topical minoxidil proves ineffective or problematic. Additional situations in which LDOM might provide direct benefit involve follicular miniaturization, such as alopecia areata, or hair cycle disruption, such as chemotherapy. The authors also recommended considering LDOM over topical minoxidil when the latter is more expensive and when patients desire enhanced hypertrichosis.
Contraindications and Precautions
Before prescribing LDOM, the authors wrote, clinicians may consult with primary care or cardiology when contraindications (cardiovascular issues, pregnancy/nursing, and potential drug interactions) or precautions (history of tachycardia or arrhythmia, hypotension, or impaired kidney function) exist. Patients with precautions may require blood pressure monitoring, as well as monitoring for adverse effects of treatment. The panel also suggested the latter for all patients at the time of LDOM initiation and dose escalation. The authors advised against routine baseline laboratory and EKG testing in cases without relevant precautions.
Dosing Considerations
Along with systemic adverse event risk and baseline hair loss severity, key dosing considerations include patient age, sex, and whether patients desire hypertrichosis. Consensus on daily doses for adolescent females and males begins at 0.625 mg and 1.25 mg, respectively, and ranges up to 2.5 mg for adolescent females vs 5 mg for adult females and adolescent and adult males.
Presently, said Ungar, many dermatologists — including some who prescribe LDOM — remain uncomfortable even with very low doses, perhaps because of an invalid perception of cardiovascular safety issues including potential hypotension and pericardial effusions. However, recently published data include a review published November 7 in the Journal of the American Academy of Dermatology, which showed no significant effect of LDOM on blood pressure. And in a September Journal of Drugs in Dermatology article the authors found no impact on pericardial effusions in a 100-patient cohort.
Some dermatologists worry about the impact hypertrichosis may have on patients, Ungar added. Although incidence estimates range from 15% to 30%, he said, more than half of his patients experience hypertrichosis. “However, most continue treatment because the beneficial effects outweigh the effect of hypertrichosis.”
Practical Roadmap
Adam Friedman, MD, who was not involved with the publication, applauds its inclusion of pragmatic clinical guidance, which he said consensus papers often lack. “This paper sets a great roadmap for working low-dose oral minoxidil into your clinical practice, Friedman, professor and chair of dermatology at George Washington University, Washington, DC, said in an interview.
Rather than limiting LDOM use to AGA, he said, the paper is most helpful in showing the spectrum of disease states for which the expert panel prescribes LDOM. “We use it as adjunctive therapy for many other things, both scarring and nonscarring hair loss,” he added.
In appropriate clinical contexts, the authors wrote, clinicians may consider combining LDOM with spironolactone or beta-blockers. Friedman said that in his hands, combining LDOM with a 5-alpha reductase inhibitor (5ARI) is “absolutely outstanding.” Minoxidil increases blood flow to the scalp, he explained, while 5ARIs prevent production of dihydrotestosterone, which miniaturizes hair.
Fu said, “We hope these consensus outcomes will be helpful to dermatology colleagues as they consider using LDOM to treat hair loss in their adult and adolescent patient populations. We anticipate that these guidelines will be updated as additional evidence-based data emerges and are encouraged that we are already seeing new publications on this topic.”
Important areas for future research, she noted, include pediatric use of LDOM, the comparative efficacy of topical vs oral minoxidil, the safety of oral minoxidil for patients with a history of allergic contact dermatitis to topical minoxidil, and the use of other off-label forms of minoxidil, such as compounded oral minoxidil and sublingual minoxidil.
The study was funded by the University of California, San Francisco, Department of Dermatology Medical Student Summer Research Fellowship Program. Fu reported personal fees from Pfizer, Eli Lilly and Company, and Sun Pharma outside of the study. The full list of author disclosures can be found in the paper. Ungar and Friedman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
. With large randomized, controlled trials lacking, the guidelines authors and other dermatologists said the paper provides practical pointers that should increase clinicians’ confidence in prescribing LDOM for hair loss.
Comfort and Confidence
Benjamin N. Ungar, MD, director of the Alopecia Center of Excellence at Mount Sinai Icahn School of Medicine, New York City, said he hopes that the guidelines will “make dermatologists in practice more comfortable with the use of low-dose oral minoxidil to treat different kinds of hair loss, and therefore, more patients will benefit.” He was not an author of the paper, which was published online in JAMA Dermatology on November 20, but was asked to comment.
Members of the multidisciplinary Low-Dose Oral Minoxidil Initiation steering committee recruited dermatologists with hair loss expertise from 12 countries. Using a modified four-round Delphi process that required at least 70% agreement, the group of 43 dermatologists crafted 76 consensus statements. “Notably,” said Co-senior author Jennifer Fu, MD, director of the Hair Disorders Clinic at the University of California, San Francisco, “27 items achieved at least 90% consensus after the first two rounds, indicating broad agreement in expert practice.”
Indications for LDOM
At least 90% of experts concurred regarding the appropriateness of LDOM use for androgenetic alopecia (AGA) and age-related thinning and in cases where topical minoxidil proves ineffective or problematic. Additional situations in which LDOM might provide direct benefit involve follicular miniaturization, such as alopecia areata, or hair cycle disruption, such as chemotherapy. The authors also recommended considering LDOM over topical minoxidil when the latter is more expensive and when patients desire enhanced hypertrichosis.
Contraindications and Precautions
Before prescribing LDOM, the authors wrote, clinicians may consult with primary care or cardiology when contraindications (cardiovascular issues, pregnancy/nursing, and potential drug interactions) or precautions (history of tachycardia or arrhythmia, hypotension, or impaired kidney function) exist. Patients with precautions may require blood pressure monitoring, as well as monitoring for adverse effects of treatment. The panel also suggested the latter for all patients at the time of LDOM initiation and dose escalation. The authors advised against routine baseline laboratory and EKG testing in cases without relevant precautions.
Dosing Considerations
Along with systemic adverse event risk and baseline hair loss severity, key dosing considerations include patient age, sex, and whether patients desire hypertrichosis. Consensus on daily doses for adolescent females and males begins at 0.625 mg and 1.25 mg, respectively, and ranges up to 2.5 mg for adolescent females vs 5 mg for adult females and adolescent and adult males.
Presently, said Ungar, many dermatologists — including some who prescribe LDOM — remain uncomfortable even with very low doses, perhaps because of an invalid perception of cardiovascular safety issues including potential hypotension and pericardial effusions. However, recently published data include a review published November 7 in the Journal of the American Academy of Dermatology, which showed no significant effect of LDOM on blood pressure. And in a September Journal of Drugs in Dermatology article the authors found no impact on pericardial effusions in a 100-patient cohort.
Some dermatologists worry about the impact hypertrichosis may have on patients, Ungar added. Although incidence estimates range from 15% to 30%, he said, more than half of his patients experience hypertrichosis. “However, most continue treatment because the beneficial effects outweigh the effect of hypertrichosis.”
Practical Roadmap
Adam Friedman, MD, who was not involved with the publication, applauds its inclusion of pragmatic clinical guidance, which he said consensus papers often lack. “This paper sets a great roadmap for working low-dose oral minoxidil into your clinical practice, Friedman, professor and chair of dermatology at George Washington University, Washington, DC, said in an interview.
Rather than limiting LDOM use to AGA, he said, the paper is most helpful in showing the spectrum of disease states for which the expert panel prescribes LDOM. “We use it as adjunctive therapy for many other things, both scarring and nonscarring hair loss,” he added.
In appropriate clinical contexts, the authors wrote, clinicians may consider combining LDOM with spironolactone or beta-blockers. Friedman said that in his hands, combining LDOM with a 5-alpha reductase inhibitor (5ARI) is “absolutely outstanding.” Minoxidil increases blood flow to the scalp, he explained, while 5ARIs prevent production of dihydrotestosterone, which miniaturizes hair.
Fu said, “We hope these consensus outcomes will be helpful to dermatology colleagues as they consider using LDOM to treat hair loss in their adult and adolescent patient populations. We anticipate that these guidelines will be updated as additional evidence-based data emerges and are encouraged that we are already seeing new publications on this topic.”
Important areas for future research, she noted, include pediatric use of LDOM, the comparative efficacy of topical vs oral minoxidil, the safety of oral minoxidil for patients with a history of allergic contact dermatitis to topical minoxidil, and the use of other off-label forms of minoxidil, such as compounded oral minoxidil and sublingual minoxidil.
The study was funded by the University of California, San Francisco, Department of Dermatology Medical Student Summer Research Fellowship Program. Fu reported personal fees from Pfizer, Eli Lilly and Company, and Sun Pharma outside of the study. The full list of author disclosures can be found in the paper. Ungar and Friedman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Levonorgestrel IUDs Linked to Higher Skin Side Effects
TOPLINE:
, with some differences between the available levonorgestrel IUDs.
METHODOLOGY:
- Researchers reviewed the US Food and Drug Administration (FDA) Adverse Events Reporting System (FAERS) through December 2023 for adverse events associated with levonorgestrel IUDs where IUDs were the only suspected cause, focusing on acne, alopecia, and hirsutism.
- They included 139,348 reports for the levonorgestrel IUDs (Mirena, Liletta, Kyleena, Skyla) and 50,450 reports for the copper IUD (Paragard).
TAKEAWAY:
- Levonorgestrel IUD users showed higher odds of reporting acne (odds ratio [OR], 3.21), alopecia (OR, 5.96), and hirsutism (OR, 15.48; all P < .0001) than copper IUD users.
- The Kyleena 19.5 mg levonorgestrel IUD was associated with the highest odds of acne reports (OR, 3.42), followed by the Mirena 52 mg (OR, 3.40) and Skyla 13.5 mg (OR, 2.30) levonorgestrel IUDs (all P < .0001).
- The Mirena IUD was associated with the highest odds of alopecia and hirsutism reports (OR, 6.62 and 17.43, respectively), followed by the Kyleena (ORs, 2.90 and 8.17, respectively) and Skyla (ORs, 2.69 and 1.48, respectively) IUDs (all P < .0001).
- Reports of acne, alopecia, and hirsutism were not significantly different between the Liletta 52 mg levonorgestrel IUD and the copper IUD.
IN PRACTICE:
“Overall, we identified significant associations between levonorgestrel IUDs and androgenic cutaneous adverse events,” the authors wrote. “Counseling prior to initiation of levonorgestrel IUDs should include information on possible cutaneous AEs including acne, alopecia, and hirsutism to guide contraceptive shared decision making,” they added.
SOURCE:
The study was led by Lydia Cassard, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio, and was published online November 3 in Journal of the American Academy of Dermatology.
LIMITATIONS:
FAERS database reports could not be verified, and differences in FDA approval dates for IUDs could have influenced reporting rates. Moreover, a lack of data on prior medication use limits the ability to determine if these AEs are a result of changes in androgenic or antiandrogenic medication use. Cutaneous adverse events associated with copper IUDs may have been underreported because of assumptions that a nonhormonal device would not cause these adverse events.
DISCLOSURES:
The authors did not report any funding source or conflict of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, with some differences between the available levonorgestrel IUDs.
METHODOLOGY:
- Researchers reviewed the US Food and Drug Administration (FDA) Adverse Events Reporting System (FAERS) through December 2023 for adverse events associated with levonorgestrel IUDs where IUDs were the only suspected cause, focusing on acne, alopecia, and hirsutism.
- They included 139,348 reports for the levonorgestrel IUDs (Mirena, Liletta, Kyleena, Skyla) and 50,450 reports for the copper IUD (Paragard).
TAKEAWAY:
- Levonorgestrel IUD users showed higher odds of reporting acne (odds ratio [OR], 3.21), alopecia (OR, 5.96), and hirsutism (OR, 15.48; all P < .0001) than copper IUD users.
- The Kyleena 19.5 mg levonorgestrel IUD was associated with the highest odds of acne reports (OR, 3.42), followed by the Mirena 52 mg (OR, 3.40) and Skyla 13.5 mg (OR, 2.30) levonorgestrel IUDs (all P < .0001).
- The Mirena IUD was associated with the highest odds of alopecia and hirsutism reports (OR, 6.62 and 17.43, respectively), followed by the Kyleena (ORs, 2.90 and 8.17, respectively) and Skyla (ORs, 2.69 and 1.48, respectively) IUDs (all P < .0001).
- Reports of acne, alopecia, and hirsutism were not significantly different between the Liletta 52 mg levonorgestrel IUD and the copper IUD.
IN PRACTICE:
“Overall, we identified significant associations between levonorgestrel IUDs and androgenic cutaneous adverse events,” the authors wrote. “Counseling prior to initiation of levonorgestrel IUDs should include information on possible cutaneous AEs including acne, alopecia, and hirsutism to guide contraceptive shared decision making,” they added.
SOURCE:
The study was led by Lydia Cassard, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio, and was published online November 3 in Journal of the American Academy of Dermatology.
LIMITATIONS:
FAERS database reports could not be verified, and differences in FDA approval dates for IUDs could have influenced reporting rates. Moreover, a lack of data on prior medication use limits the ability to determine if these AEs are a result of changes in androgenic or antiandrogenic medication use. Cutaneous adverse events associated with copper IUDs may have been underreported because of assumptions that a nonhormonal device would not cause these adverse events.
DISCLOSURES:
The authors did not report any funding source or conflict of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, with some differences between the available levonorgestrel IUDs.
METHODOLOGY:
- Researchers reviewed the US Food and Drug Administration (FDA) Adverse Events Reporting System (FAERS) through December 2023 for adverse events associated with levonorgestrel IUDs where IUDs were the only suspected cause, focusing on acne, alopecia, and hirsutism.
- They included 139,348 reports for the levonorgestrel IUDs (Mirena, Liletta, Kyleena, Skyla) and 50,450 reports for the copper IUD (Paragard).
TAKEAWAY:
- Levonorgestrel IUD users showed higher odds of reporting acne (odds ratio [OR], 3.21), alopecia (OR, 5.96), and hirsutism (OR, 15.48; all P < .0001) than copper IUD users.
- The Kyleena 19.5 mg levonorgestrel IUD was associated with the highest odds of acne reports (OR, 3.42), followed by the Mirena 52 mg (OR, 3.40) and Skyla 13.5 mg (OR, 2.30) levonorgestrel IUDs (all P < .0001).
- The Mirena IUD was associated with the highest odds of alopecia and hirsutism reports (OR, 6.62 and 17.43, respectively), followed by the Kyleena (ORs, 2.90 and 8.17, respectively) and Skyla (ORs, 2.69 and 1.48, respectively) IUDs (all P < .0001).
- Reports of acne, alopecia, and hirsutism were not significantly different between the Liletta 52 mg levonorgestrel IUD and the copper IUD.
IN PRACTICE:
“Overall, we identified significant associations between levonorgestrel IUDs and androgenic cutaneous adverse events,” the authors wrote. “Counseling prior to initiation of levonorgestrel IUDs should include information on possible cutaneous AEs including acne, alopecia, and hirsutism to guide contraceptive shared decision making,” they added.
SOURCE:
The study was led by Lydia Cassard, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio, and was published online November 3 in Journal of the American Academy of Dermatology.
LIMITATIONS:
FAERS database reports could not be verified, and differences in FDA approval dates for IUDs could have influenced reporting rates. Moreover, a lack of data on prior medication use limits the ability to determine if these AEs are a result of changes in androgenic or antiandrogenic medication use. Cutaneous adverse events associated with copper IUDs may have been underreported because of assumptions that a nonhormonal device would not cause these adverse events.
DISCLOSURES:
The authors did not report any funding source or conflict of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Post COVID-19, Long-term Risk for Autoimmune, Autoinflammatory Skin Disorders Increased, Study Finds
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Expert Reviews Options for Revitalizing Dystrophic Nails
LAS VEGAS —
“With the fingernails, we don’t often see onychomycosis, but with toenails, we certainly do,” Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. “But toenails are subject to a lot of forces beyond just fungal [infections]. We have the wear and tear of wearing shoes, gait, and other physical activity.”
For example, she continued, some runners develop second-toenail dystrophy “because there’s constant repetitive trauma to the toenail, and [poorly fitting] shoes can contribute to that. Biomechanical issues are a unique consideration when you’re dealing with toenail issues.”
Vlahovic highlighted several options that can help improve the appearance of dystrophic nails as they recover or grow back:
Urea nail preparations: To temporarily soften the nail.
Genadur (hydroxypropyl chitosan): This product “is used mainly for psoriatic nails, but I use it for all different kinds of nail dystrophy,” she said.
DermaNail (acetyl mandelic acid solution): This can be used for brittle nails and fingernails. Vlahovic said she recommends it be used on toenails “in addition to the onychomycosis and other nail dystrophy treatments that I’m doing because it really helps to hydrate the nail unit.”
Kerasal Fungal Nail Renewal (ingredients include propylene glycol, urea, glycerin, and lactic acid): This product is used “for smoothing out the appearance of the nail,” she said.
KeryFlex: Applied in an office setting, this resin-based product restores the appearance of an individual’s natural nails. “It comes in two colors [and] absorbs the shock of what is going on mechanically with the feet,” Vlahovic said. “So, if I’m treating a ballet dancer performing en pointe, or a soccer player, it’s something I can use to protect the nail, but also to make it cosmetically more acceptable.”
NECPro: A nail reconstruction method that involves the use of a composite used mainly by podiatrists, it “helps you not only create a barrier, but to create a natural-looking color that matches your own nail color,” she said.
In Vlahovic’s experience, KeryFlex and NECPro last 6-8 weeks. “You can use nail polish on top of them if you’d like, but they’re basically cosmetic barriers to protect the nail unit,” she said.
Vlahovic has disclosed being a consultant and investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
LAS VEGAS —
“With the fingernails, we don’t often see onychomycosis, but with toenails, we certainly do,” Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. “But toenails are subject to a lot of forces beyond just fungal [infections]. We have the wear and tear of wearing shoes, gait, and other physical activity.”
For example, she continued, some runners develop second-toenail dystrophy “because there’s constant repetitive trauma to the toenail, and [poorly fitting] shoes can contribute to that. Biomechanical issues are a unique consideration when you’re dealing with toenail issues.”
Vlahovic highlighted several options that can help improve the appearance of dystrophic nails as they recover or grow back:
Urea nail preparations: To temporarily soften the nail.
Genadur (hydroxypropyl chitosan): This product “is used mainly for psoriatic nails, but I use it for all different kinds of nail dystrophy,” she said.
DermaNail (acetyl mandelic acid solution): This can be used for brittle nails and fingernails. Vlahovic said she recommends it be used on toenails “in addition to the onychomycosis and other nail dystrophy treatments that I’m doing because it really helps to hydrate the nail unit.”
Kerasal Fungal Nail Renewal (ingredients include propylene glycol, urea, glycerin, and lactic acid): This product is used “for smoothing out the appearance of the nail,” she said.
KeryFlex: Applied in an office setting, this resin-based product restores the appearance of an individual’s natural nails. “It comes in two colors [and] absorbs the shock of what is going on mechanically with the feet,” Vlahovic said. “So, if I’m treating a ballet dancer performing en pointe, or a soccer player, it’s something I can use to protect the nail, but also to make it cosmetically more acceptable.”
NECPro: A nail reconstruction method that involves the use of a composite used mainly by podiatrists, it “helps you not only create a barrier, but to create a natural-looking color that matches your own nail color,” she said.
In Vlahovic’s experience, KeryFlex and NECPro last 6-8 weeks. “You can use nail polish on top of them if you’d like, but they’re basically cosmetic barriers to protect the nail unit,” she said.
Vlahovic has disclosed being a consultant and investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
LAS VEGAS —
“With the fingernails, we don’t often see onychomycosis, but with toenails, we certainly do,” Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. “But toenails are subject to a lot of forces beyond just fungal [infections]. We have the wear and tear of wearing shoes, gait, and other physical activity.”
For example, she continued, some runners develop second-toenail dystrophy “because there’s constant repetitive trauma to the toenail, and [poorly fitting] shoes can contribute to that. Biomechanical issues are a unique consideration when you’re dealing with toenail issues.”
Vlahovic highlighted several options that can help improve the appearance of dystrophic nails as they recover or grow back:
Urea nail preparations: To temporarily soften the nail.
Genadur (hydroxypropyl chitosan): This product “is used mainly for psoriatic nails, but I use it for all different kinds of nail dystrophy,” she said.
DermaNail (acetyl mandelic acid solution): This can be used for brittle nails and fingernails. Vlahovic said she recommends it be used on toenails “in addition to the onychomycosis and other nail dystrophy treatments that I’m doing because it really helps to hydrate the nail unit.”
Kerasal Fungal Nail Renewal (ingredients include propylene glycol, urea, glycerin, and lactic acid): This product is used “for smoothing out the appearance of the nail,” she said.
KeryFlex: Applied in an office setting, this resin-based product restores the appearance of an individual’s natural nails. “It comes in two colors [and] absorbs the shock of what is going on mechanically with the feet,” Vlahovic said. “So, if I’m treating a ballet dancer performing en pointe, or a soccer player, it’s something I can use to protect the nail, but also to make it cosmetically more acceptable.”
NECPro: A nail reconstruction method that involves the use of a composite used mainly by podiatrists, it “helps you not only create a barrier, but to create a natural-looking color that matches your own nail color,” she said.
In Vlahovic’s experience, KeryFlex and NECPro last 6-8 weeks. “You can use nail polish on top of them if you’d like, but they’re basically cosmetic barriers to protect the nail unit,” she said.
Vlahovic has disclosed being a consultant and investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
FROM SDPA 24