User login
Food allergy test breakthrough: Less risk, more useful results
What would you do if you believed you had a serious health issue, but the best way to find out for sure might kill you?
That’s the reality for patients who wish to confirm or rule out a food allergy, says Sindy Tang, PhD, an associate professor of mechanical engineering at Stanford (Calif.) University.
And it’s the reason Dr. Tang and her colleagues are developing a food allergy test that’s not only safer, but also more reliable than today’s tests. In a paper in the journal Lab on a Chip, Dr. Tang and her colleagues outline the basis for this future test, which isolates a food allergy marker from the blood using a magnetic field.
How today’s food allergy tests fall short
The gold standard for food allergy diagnosis is something called the oral food challenge. That’s when the patient eats gradually increasing amounts of a problem food – say, peanuts – every 15 to 30 minutes to see if symptoms occur. This means highly allergic patients may risk anaphylaxis, an allergic reaction that causes inflammation so severe that breathing becomes restricted and blood pressure drops. Because of that, a clinical team must be at the ready with treatments like oxygen, epinephrine, or albuterol.
“The test is very accurate, but it’s also potentially unsafe and even fatal in rare cases,” Dr. Tang says. “That’s led to many sham tests advertised online that claim to use hair samples for food tests, but those are inaccurate and potentially dangerous, since they may give someone a false sense of confidence about a food they should avoid.”
Less risky tests are available, such as skin-prick tests – those involve scratching a small amount of the food into a patient’s arm – as well as blood tests that measure allergen-specific antibodies.
“Unfortunately, both of those are not that accurate and have high false-positive rates,” Dr. Tang says. “The best method is the oral food challenge, which many patients are afraid to do, not surprisingly.”
The future of food allergy testing: faster, safer, more reliable
In their study, the Stanford researchers focused on a type of white blood cell known as basophils, which release histamine when triggered by allergens. By using magnetic nanoparticles that bind to some blood cells but not basophils, they were able to separate basophils from the blood with a magnetic field in just 10 minutes.
Once isolated, the basophils are exposed to potential allergens. If they react, that’s a sign of an allergy.
Basophils have been isolated in labs before but not nearly this quickly and efficiently, Dr. Tang says.
“For true basophil activation, you need the blood to be fresh, which is challenging when you have to send it to a lab,” Dr. Tang says. “Being able to do this kind of test within a clinic or an in-house lab would be a big step forward.”
Next steps
While this represents a breakthrough in basophil activation testing, more research is needed to fully develop the system for clinical use. It must be standardized, automated, and miniaturized, the researchers say.
That said, the results give hope to those with food allergies that tomorrow’s gold-standard test will require only a blood sample without an emergency team standing by.
A version of this article first appeared on WebMD.com.
What would you do if you believed you had a serious health issue, but the best way to find out for sure might kill you?
That’s the reality for patients who wish to confirm or rule out a food allergy, says Sindy Tang, PhD, an associate professor of mechanical engineering at Stanford (Calif.) University.
And it’s the reason Dr. Tang and her colleagues are developing a food allergy test that’s not only safer, but also more reliable than today’s tests. In a paper in the journal Lab on a Chip, Dr. Tang and her colleagues outline the basis for this future test, which isolates a food allergy marker from the blood using a magnetic field.
How today’s food allergy tests fall short
The gold standard for food allergy diagnosis is something called the oral food challenge. That’s when the patient eats gradually increasing amounts of a problem food – say, peanuts – every 15 to 30 minutes to see if symptoms occur. This means highly allergic patients may risk anaphylaxis, an allergic reaction that causes inflammation so severe that breathing becomes restricted and blood pressure drops. Because of that, a clinical team must be at the ready with treatments like oxygen, epinephrine, or albuterol.
“The test is very accurate, but it’s also potentially unsafe and even fatal in rare cases,” Dr. Tang says. “That’s led to many sham tests advertised online that claim to use hair samples for food tests, but those are inaccurate and potentially dangerous, since they may give someone a false sense of confidence about a food they should avoid.”
Less risky tests are available, such as skin-prick tests – those involve scratching a small amount of the food into a patient’s arm – as well as blood tests that measure allergen-specific antibodies.
“Unfortunately, both of those are not that accurate and have high false-positive rates,” Dr. Tang says. “The best method is the oral food challenge, which many patients are afraid to do, not surprisingly.”
The future of food allergy testing: faster, safer, more reliable
In their study, the Stanford researchers focused on a type of white blood cell known as basophils, which release histamine when triggered by allergens. By using magnetic nanoparticles that bind to some blood cells but not basophils, they were able to separate basophils from the blood with a magnetic field in just 10 minutes.
Once isolated, the basophils are exposed to potential allergens. If they react, that’s a sign of an allergy.
Basophils have been isolated in labs before but not nearly this quickly and efficiently, Dr. Tang says.
“For true basophil activation, you need the blood to be fresh, which is challenging when you have to send it to a lab,” Dr. Tang says. “Being able to do this kind of test within a clinic or an in-house lab would be a big step forward.”
Next steps
While this represents a breakthrough in basophil activation testing, more research is needed to fully develop the system for clinical use. It must be standardized, automated, and miniaturized, the researchers say.
That said, the results give hope to those with food allergies that tomorrow’s gold-standard test will require only a blood sample without an emergency team standing by.
A version of this article first appeared on WebMD.com.
What would you do if you believed you had a serious health issue, but the best way to find out for sure might kill you?
That’s the reality for patients who wish to confirm or rule out a food allergy, says Sindy Tang, PhD, an associate professor of mechanical engineering at Stanford (Calif.) University.
And it’s the reason Dr. Tang and her colleagues are developing a food allergy test that’s not only safer, but also more reliable than today’s tests. In a paper in the journal Lab on a Chip, Dr. Tang and her colleagues outline the basis for this future test, which isolates a food allergy marker from the blood using a magnetic field.
How today’s food allergy tests fall short
The gold standard for food allergy diagnosis is something called the oral food challenge. That’s when the patient eats gradually increasing amounts of a problem food – say, peanuts – every 15 to 30 minutes to see if symptoms occur. This means highly allergic patients may risk anaphylaxis, an allergic reaction that causes inflammation so severe that breathing becomes restricted and blood pressure drops. Because of that, a clinical team must be at the ready with treatments like oxygen, epinephrine, or albuterol.
“The test is very accurate, but it’s also potentially unsafe and even fatal in rare cases,” Dr. Tang says. “That’s led to many sham tests advertised online that claim to use hair samples for food tests, but those are inaccurate and potentially dangerous, since they may give someone a false sense of confidence about a food they should avoid.”
Less risky tests are available, such as skin-prick tests – those involve scratching a small amount of the food into a patient’s arm – as well as blood tests that measure allergen-specific antibodies.
“Unfortunately, both of those are not that accurate and have high false-positive rates,” Dr. Tang says. “The best method is the oral food challenge, which many patients are afraid to do, not surprisingly.”
The future of food allergy testing: faster, safer, more reliable
In their study, the Stanford researchers focused on a type of white blood cell known as basophils, which release histamine when triggered by allergens. By using magnetic nanoparticles that bind to some blood cells but not basophils, they were able to separate basophils from the blood with a magnetic field in just 10 minutes.
Once isolated, the basophils are exposed to potential allergens. If they react, that’s a sign of an allergy.
Basophils have been isolated in labs before but not nearly this quickly and efficiently, Dr. Tang says.
“For true basophil activation, you need the blood to be fresh, which is challenging when you have to send it to a lab,” Dr. Tang says. “Being able to do this kind of test within a clinic or an in-house lab would be a big step forward.”
Next steps
While this represents a breakthrough in basophil activation testing, more research is needed to fully develop the system for clinical use. It must be standardized, automated, and miniaturized, the researchers say.
That said, the results give hope to those with food allergies that tomorrow’s gold-standard test will require only a blood sample without an emergency team standing by.
A version of this article first appeared on WebMD.com.
Intravenous Immunoglobulin in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia: A Pharmacoeconomic Analysis
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board (research@sharp.com), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
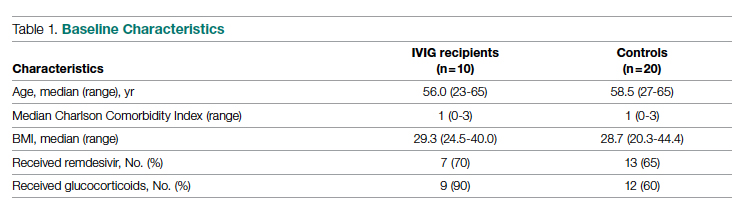
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
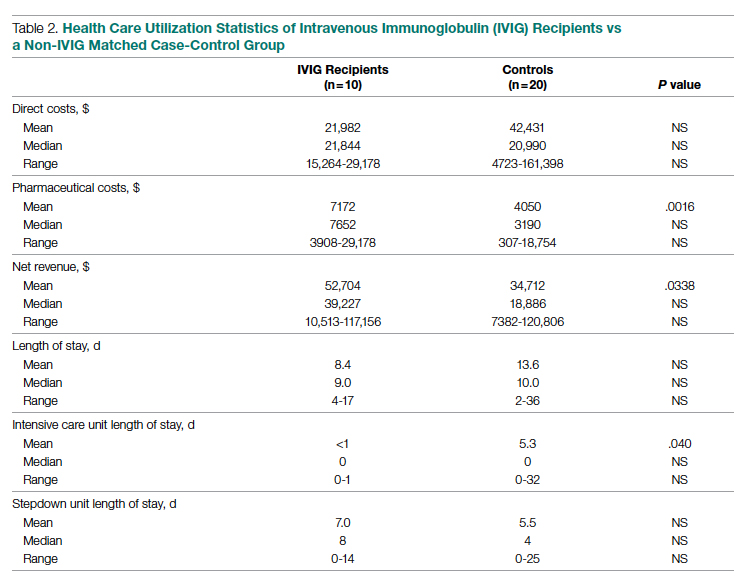
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; gsakoulas@health.ucsd.edu
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board (research@sharp.com), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; gsakoulas@health.ucsd.edu
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board (research@sharp.com), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; gsakoulas@health.ucsd.edu
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
Food allergy risk not greater in C-section infants
Cesarean births are not likely linked to an elevated risk of food allergy during the first year of life, an Australian study found.
Published online in the Journal of Allergy and Clinical Immunology, the findings may help assess the risks and benefits of cesarean delivery and reassure women who require it that their babies are not more likely to develop food allergy, according to Rachel L. Peters, PhD, an epidemiologist at the Murdoch Child Research Institute (MCRI) in Melbourne, and colleagues.
Dr. Peters’ group undertook the analysis to clarify a possible association between mode of delivery and food allergy risk, which has remained unclear owing to the absence of studies with both challenge-proven food allergy outcomes and detailed information on the type and timing of cesarean delivery.
“The infant immune system undergoes rapid development during the neonatal period,” Dr. Peters said in an MCRI press release, and the mode of delivery may interfere with the normal development of the immune system. “Babies born by cesarean have less exposure to the bacteria from the mother’s gut and vagina, which influence the composition of the baby’s microbiome and immune system development. However, this doesn’t appear to play a major role in the development of food allergy,” she said.
The HealthNuts study
In the period 2007-2011, the longitudinal population-based HealthNuts cohort study enrolled 5,276 12-month-olds who underwent skin prick testing and oral food challenge for sensitization to egg, peanut, sesame, and either shellfish or cow’s milk. It linked the resulting data to additional birth statistics from the Victorian Perinatal Data Collection when children turned 6.
Birth data were obtained on 2,045 babies, and in this subgroup with linked data, 30% were born by cesarean – similar to the 31.7% of U.S. cesarean births in 2019 – and 12.7% of these had food allergy versus 13.2% of those delivered vaginally.
Compared with vaginal birth, C-section was not associated with the risk of food allergy (adjusted odds ratio [aOR] 0.95, 95% confidence interval [CI], 0.70-0.30).
Nor did the timing of the C-section have an effect. Cesarean delivery either before labor or after onset of labor was not associated with the risk of food allergy (aOR, 0.83, 95% CI, 0.55-1.23) and aOR, 1.13, 95% CI, 0.75-1.72), respectively.
Compared with vaginal delivery, elective or emergency cesarean was not associated with food allergy risk (aOR, 1.05, 95% CI, 0.71-1.55, and aOR, 0.86, 95% CI, 0.56-1.31).
Similarly, no evidence emerged of an effect modification by breastfeeding, older siblings, pet dog ownership, or maternal allergy.
“This study is helpful because in addition to blood and skin tests, it also used food challenge, which is the gold standard,” Terri Brown-Whitehorn, MD, an attending physician in the division of allergy and immunology at Children’s Hospital of Philadelphia, said in an interview. “If no actual food is given, the other tests could lead to false positives.”
Dr. Brown-Whitehorn, who was not involved in the MCRI research, said the findings are not likely to affect most decisions about C-sections because most are not voluntary. “But if a mother had a first baby by emergency cesarean section, she might be given the option of having the next one by the same method.”
She said the current advice is to introduce even high-risk foods to a child’s diet early on to ward off the development of food allergies.
According to the microbial exposure hypothesis, it was previously thought that a potential link between cesarean birth and allergy might reflect differences in early exposure to maternal flora beneficial to the immune system in the vagina during delivery. A C-section might bypass the opportunity for neonatal gut colonization with maternal gut and vaginal flora, thereby raising allergy risk. A 2018 meta-analysis, for example, suggested cesarean birth could raise the risk for food allergies by 21%.
In other research from HealthNuts, 30% of child peanut allergy and 90% of egg allergy appear to resolve naturally by age 6. These numbers are somewhat higher than what Dr. Brown-Whitehorn sees. “We find that about 20% of peanut allergies and about 70% or 80% – maybe a bit less – of egg allergies resolve by age 6.”
This research was supported by the National Health & Medical Research Council of Australia, the Ilhan Food Allergy Foundation, AnaphylaxiStop, the Charles and Sylvia Viertel Medical Research Foundation, the Victorian Government’s Operational Infrastructure Support Program, and the Melbourne Children’s Clinician-Scientist Fellowship.
Dr. Peters disclosed no competing interests. Several coauthors reported research support or employment with private companies and one is the inventor of an MCRI-held patent. Dr. Brown-Whitehorn had no competing interests to disclose.
Cesarean births are not likely linked to an elevated risk of food allergy during the first year of life, an Australian study found.
Published online in the Journal of Allergy and Clinical Immunology, the findings may help assess the risks and benefits of cesarean delivery and reassure women who require it that their babies are not more likely to develop food allergy, according to Rachel L. Peters, PhD, an epidemiologist at the Murdoch Child Research Institute (MCRI) in Melbourne, and colleagues.
Dr. Peters’ group undertook the analysis to clarify a possible association between mode of delivery and food allergy risk, which has remained unclear owing to the absence of studies with both challenge-proven food allergy outcomes and detailed information on the type and timing of cesarean delivery.
“The infant immune system undergoes rapid development during the neonatal period,” Dr. Peters said in an MCRI press release, and the mode of delivery may interfere with the normal development of the immune system. “Babies born by cesarean have less exposure to the bacteria from the mother’s gut and vagina, which influence the composition of the baby’s microbiome and immune system development. However, this doesn’t appear to play a major role in the development of food allergy,” she said.
The HealthNuts study
In the period 2007-2011, the longitudinal population-based HealthNuts cohort study enrolled 5,276 12-month-olds who underwent skin prick testing and oral food challenge for sensitization to egg, peanut, sesame, and either shellfish or cow’s milk. It linked the resulting data to additional birth statistics from the Victorian Perinatal Data Collection when children turned 6.
Birth data were obtained on 2,045 babies, and in this subgroup with linked data, 30% were born by cesarean – similar to the 31.7% of U.S. cesarean births in 2019 – and 12.7% of these had food allergy versus 13.2% of those delivered vaginally.
Compared with vaginal birth, C-section was not associated with the risk of food allergy (adjusted odds ratio [aOR] 0.95, 95% confidence interval [CI], 0.70-0.30).
Nor did the timing of the C-section have an effect. Cesarean delivery either before labor or after onset of labor was not associated with the risk of food allergy (aOR, 0.83, 95% CI, 0.55-1.23) and aOR, 1.13, 95% CI, 0.75-1.72), respectively.
Compared with vaginal delivery, elective or emergency cesarean was not associated with food allergy risk (aOR, 1.05, 95% CI, 0.71-1.55, and aOR, 0.86, 95% CI, 0.56-1.31).
Similarly, no evidence emerged of an effect modification by breastfeeding, older siblings, pet dog ownership, or maternal allergy.
“This study is helpful because in addition to blood and skin tests, it also used food challenge, which is the gold standard,” Terri Brown-Whitehorn, MD, an attending physician in the division of allergy and immunology at Children’s Hospital of Philadelphia, said in an interview. “If no actual food is given, the other tests could lead to false positives.”
Dr. Brown-Whitehorn, who was not involved in the MCRI research, said the findings are not likely to affect most decisions about C-sections because most are not voluntary. “But if a mother had a first baby by emergency cesarean section, she might be given the option of having the next one by the same method.”
She said the current advice is to introduce even high-risk foods to a child’s diet early on to ward off the development of food allergies.
According to the microbial exposure hypothesis, it was previously thought that a potential link between cesarean birth and allergy might reflect differences in early exposure to maternal flora beneficial to the immune system in the vagina during delivery. A C-section might bypass the opportunity for neonatal gut colonization with maternal gut and vaginal flora, thereby raising allergy risk. A 2018 meta-analysis, for example, suggested cesarean birth could raise the risk for food allergies by 21%.
In other research from HealthNuts, 30% of child peanut allergy and 90% of egg allergy appear to resolve naturally by age 6. These numbers are somewhat higher than what Dr. Brown-Whitehorn sees. “We find that about 20% of peanut allergies and about 70% or 80% – maybe a bit less – of egg allergies resolve by age 6.”
This research was supported by the National Health & Medical Research Council of Australia, the Ilhan Food Allergy Foundation, AnaphylaxiStop, the Charles and Sylvia Viertel Medical Research Foundation, the Victorian Government’s Operational Infrastructure Support Program, and the Melbourne Children’s Clinician-Scientist Fellowship.
Dr. Peters disclosed no competing interests. Several coauthors reported research support or employment with private companies and one is the inventor of an MCRI-held patent. Dr. Brown-Whitehorn had no competing interests to disclose.
Cesarean births are not likely linked to an elevated risk of food allergy during the first year of life, an Australian study found.
Published online in the Journal of Allergy and Clinical Immunology, the findings may help assess the risks and benefits of cesarean delivery and reassure women who require it that their babies are not more likely to develop food allergy, according to Rachel L. Peters, PhD, an epidemiologist at the Murdoch Child Research Institute (MCRI) in Melbourne, and colleagues.
Dr. Peters’ group undertook the analysis to clarify a possible association between mode of delivery and food allergy risk, which has remained unclear owing to the absence of studies with both challenge-proven food allergy outcomes and detailed information on the type and timing of cesarean delivery.
“The infant immune system undergoes rapid development during the neonatal period,” Dr. Peters said in an MCRI press release, and the mode of delivery may interfere with the normal development of the immune system. “Babies born by cesarean have less exposure to the bacteria from the mother’s gut and vagina, which influence the composition of the baby’s microbiome and immune system development. However, this doesn’t appear to play a major role in the development of food allergy,” she said.
The HealthNuts study
In the period 2007-2011, the longitudinal population-based HealthNuts cohort study enrolled 5,276 12-month-olds who underwent skin prick testing and oral food challenge for sensitization to egg, peanut, sesame, and either shellfish or cow’s milk. It linked the resulting data to additional birth statistics from the Victorian Perinatal Data Collection when children turned 6.
Birth data were obtained on 2,045 babies, and in this subgroup with linked data, 30% were born by cesarean – similar to the 31.7% of U.S. cesarean births in 2019 – and 12.7% of these had food allergy versus 13.2% of those delivered vaginally.
Compared with vaginal birth, C-section was not associated with the risk of food allergy (adjusted odds ratio [aOR] 0.95, 95% confidence interval [CI], 0.70-0.30).
Nor did the timing of the C-section have an effect. Cesarean delivery either before labor or after onset of labor was not associated with the risk of food allergy (aOR, 0.83, 95% CI, 0.55-1.23) and aOR, 1.13, 95% CI, 0.75-1.72), respectively.
Compared with vaginal delivery, elective or emergency cesarean was not associated with food allergy risk (aOR, 1.05, 95% CI, 0.71-1.55, and aOR, 0.86, 95% CI, 0.56-1.31).
Similarly, no evidence emerged of an effect modification by breastfeeding, older siblings, pet dog ownership, or maternal allergy.
“This study is helpful because in addition to blood and skin tests, it also used food challenge, which is the gold standard,” Terri Brown-Whitehorn, MD, an attending physician in the division of allergy and immunology at Children’s Hospital of Philadelphia, said in an interview. “If no actual food is given, the other tests could lead to false positives.”
Dr. Brown-Whitehorn, who was not involved in the MCRI research, said the findings are not likely to affect most decisions about C-sections because most are not voluntary. “But if a mother had a first baby by emergency cesarean section, she might be given the option of having the next one by the same method.”
She said the current advice is to introduce even high-risk foods to a child’s diet early on to ward off the development of food allergies.
According to the microbial exposure hypothesis, it was previously thought that a potential link between cesarean birth and allergy might reflect differences in early exposure to maternal flora beneficial to the immune system in the vagina during delivery. A C-section might bypass the opportunity for neonatal gut colonization with maternal gut and vaginal flora, thereby raising allergy risk. A 2018 meta-analysis, for example, suggested cesarean birth could raise the risk for food allergies by 21%.
In other research from HealthNuts, 30% of child peanut allergy and 90% of egg allergy appear to resolve naturally by age 6. These numbers are somewhat higher than what Dr. Brown-Whitehorn sees. “We find that about 20% of peanut allergies and about 70% or 80% – maybe a bit less – of egg allergies resolve by age 6.”
This research was supported by the National Health & Medical Research Council of Australia, the Ilhan Food Allergy Foundation, AnaphylaxiStop, the Charles and Sylvia Viertel Medical Research Foundation, the Victorian Government’s Operational Infrastructure Support Program, and the Melbourne Children’s Clinician-Scientist Fellowship.
Dr. Peters disclosed no competing interests. Several coauthors reported research support or employment with private companies and one is the inventor of an MCRI-held patent. Dr. Brown-Whitehorn had no competing interests to disclose.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Low-calorie ketogenic diet improves immune function
According to the latest evidence, This development was revealed during the 8th International Scientific Symposium New Frontiers in Scientific Research, organized by PronoKal Group and held in Barcelona. During this conference, international multidisciplinary experts in the study and management of obesity presented the latest data on the benefits of treatment based on a very-low-calorie ketogenic diet.
“Nutritional ketosis has gained great interest in recent years because it is shown to have beneficial properties for health and promotes healthy aging, increasing longevity,” said Ana Belén Crujeiras, BSc, PhD, principal investigator of the Health Research Institute of Santiago de Compostela-Galician Health Service (IDIS-SERGAS) Group of Epigenomics in Endocrinology and Nutrition and the Biomedical Research Networking Center for Obesity and Nutrition Physiopathology (CIBEROBN). “Furthermore, in the case of obesity, we have more and more evidence that it is an effective treatment, mainly because to achieve this metabolic state (ketosis), routes that require the combustion of fats are activated, and this induces body weight loss.”
The specialist stressed that several strategies are used to induce nutritional ketosis. They are characterized by low carbohydrate consumption (low-carbohydrate and high-fat diet; low-carbohydrate, low-fat diet; and intermittent fasting). But Dr. Crujeiras warned that to use it as a treatment for a disease such as obesity, it must be backed by strong and solid scientific evidence, moving away from the concept of fad diets.
In this sense, since 2010, Dr. Crujeiras’ team has developed several studies focused on analyzing the efficacy and safety of treatment with a very-low-calorie ketogenic diet, the results of which have been published in high-impact journals.
Dr. Crujeiras commented on the main conclusions drawn from these investigations. “Our work has shown that the very-low-calorie ketogenic diet is effective for rapid weight loss and maintenance of lost weight, as well as reducing fat mass, primarily visceral fat mass.
“In this sense, a very interesting result is that despite the strong weight loss it induces, it preserves muscle mass and function and improves resting metabolic rate. These two variables are important, because all therapeutic strategies that exist to lose weight lead to a significant reduction in fat-free mass and also a reduction in energy expenditure at rest. This factor is associated with the risk of regaining lost weight, which is currently the great challenge in the treatment of obesity,” she added.
Specific methylation pattern
Dr. Crujeiras indicated that other notable evidence is the favorable impact on an emotional and psychological level. “To determine whether the caloric restriction of this diet and the strong weight loss that it involves were associated with an increased desire to eat, we also carried out an analysis with psychobiological tests. These results led us to conclude that this guideline is accompanied by a reduction of anxiety about food and an improvement in psychobiological parameters, thus increasing the quality of life of these patients.”
The specialist also mentioned that studies currently in progress show that the beneficial effect of this diet could be mediated by epigenetic mechanisms. “In our group, we have identified a specific DNA methylation pattern in people with obesity and we wondered if the very-low-calorie ketogenic diet would be able to reverse that methylome.
“We conducted a study in which we collected blood samples from patients on the very-low-calorie ketogenic diet (600 to 800 kcal/day) drawn before treatment, at peak ketosis, and at the end of treatment. After determining the global pattern of DNA in all patients with obesity targeted with this strategy and through bioinformatic analysis, we were able to obtain a methylation pattern. The results showed that after weight loss on the very-low-calorie ketogenic diet, the methylome that obese people initially had [was] reversed and matched that of normal-weight people.
“Continuing with this bioinformatic analysis more comprehensively, we wanted to see what kind of genes were differentially methylated, especially by the action of the ketosis itself. We found that most of the genes that exhibited differential methylation (in total, 292 identified) belonged to pathways that were involved in the regulation of metabolism, adipose tissue function, CNS function, and also carcinogenesis,” she continued.
Immunomodulatory effect
Dr. Crujeiras said that her research group also observed the modulatory role of the very-low-calorie ketogenic diet in the functioning of the immune system, “something that was not seen after similar weight loss induced by bariatric surgery. We analyzed this data in the context of the situation created by COVID-19, taking into account the evidence that people with obesity, compared to those with normal weight, have a higher risk of becoming infected and of having a poor evolution of the infection.”
In this regard, Dr. Crujeiras’ team launched an investigation to study the ACE2 gene methylation pattern, comparing obesity with normal weight and the situation after following a very-low-calorie ketogenic diet or undergoing bariatric surgery. “We observed that the methylation pattern of this gene in obese people was increased, compared to normal-weight people,” she explained, “and this increase was observed mainly in visceral adipose tissue. However, we did not see this in subcutaneous adipose tissue, which is in agreement with the hypothesis that visceral adipose tissue is that mostly associated with obesity-related comorbidities.
“Likewise, the very-low-calorie ketogenic diet was associated with decreased ACE2 methylation, along with increased exposure of this gene. However, after bariatric surgery, no significant changes were observed, so we deduce that we are protecting the patient in some way from inflammation and, therefore, from the potential of serious illness if they become infected.
“In light of these results, we wanted to dig deeper into what was happening with the immune system of obese patients and that inflammation after a very-low-calorie ketogenic diet. We conducted a new study, currently under review in the journal Clinical Nutrition, with the same approach, comparing this diet with a standard hypocaloric balanced diet and bariatric surgery, in which we analyzed a wide battery of cytokines (32). We have observed a differential pattern between the very-low-calorie ketogenic diet and bariatric surgery.
“The results confirm our hypothesis that the very-low-calorie ketogenic diet remodels the inflammatory status of obese patients, and we were also able to verify that the increase in ketone bodies has immunomodulatory properties that were previously demonstrated in preclinical and animal models, which is associated with increased immune function in these patients,” added Dr. Crujeiras.
Personalization and weight regain
In regard to the next steps to take in the knowledge and clinical application of the benefits of this dietary strategy, Dr. Crujeiras said that despite the fact that this diet is known to be effective, it is currently prescribed in a standard manner to all patients, “but there is some variability in the response and also a high risk of regaining weight, as is the case with any nutritional intervention strategy, with that ‘regain’ of lost weight being the main challenge in the treatment of obesity. In this sense, the epigenomic and epigenetic markers that we have identified could help us optimize treatment.”
She added that the future lies in establishing an algorithm that encompasses the patient’s exposome data, along with their genetic and epigenetic profile, to properly classify patients and prescribe a personalized precision therapeutic strategy.
Luca Busetto, MD, cochair of the Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO), also insisted on the challenge posed by the individualized application of the very-low-calorie ketogenic diet, emphasizing that this diet should always be prescribed by a doctor after an appropriate assessment of the patient. “Obesity is not a matter of willpower or motivation, and most people with obesity have struggled their entire lives and failed because their biology tends to cause weight regain. Therefore, we should try to offer them the options that we currently have, including the very-low-calorie ketogenic diet, adapting them as much as possible to the profile of each patient.”
During his speech, Dr. Busetto presented the recent European guidelines for the management of obesity in adults with a very-low-calorie ketogenic diet, endorsed by EASO, and analyzed the main strengths of these recommendations.
Dr. Busetto remarked that three important points clearly justify the use of the very-low-calorie ketogenic diet. The first is the speed with which the initial weight loss occurs. Recent studies have looked at the benefits of a significant loss of excess weight early in a weight-loss diet, and although this is an association rather than a cause, the results strongly suggest that rapid initial weight loss increases the chance of the result being maintained in the long term. This clashes with the traditional recommendation of losing weight little by little as a strategy to achieve long-term results, but it must be taken into account that there are many myths in the treatment of obesity that current evidence is dismantling with new data – and this is one of them.
Secondly, the effect of the very-low-calorie ketogenic diet can be added to other treatments. This has been demonstrated by studies carried out with liraglutide that showed that this dietary strategy optimizes results, compared with patients who had been treated only with this drug. The third point that justifies the use of the very-low-calorie ketogenic diet is the management of obesity comorbidities. Several investigations demonstrate the effectiveness of this diet in this regard, especially in the case of type 2 diabetes. Data suggest that the substantial weight reductions achieved with it also favor the remission of these comorbidities in many patients.
EASO ‘approval’
Dr. Busetto pointed out that, based on this evidence, the OMTF proposed the development of standards to be included in the EASO guidelines, since there had been no specific recommendation on the very-low-calorie ketogenic diet.
“The main objective of these European guidelines was to provide data referenced by scientific evidence and to suggest a common protocol for the use of this dietary strategy,” he added.
For this, a very exhaustive meta-analysis was carried out, researching all the publications that compared the very-low-calorie ketogenic diet with other diets. The results showed the superiority of the former method for the reduction of body mass index and weight and fat mass, with no difference in lean (muscle) mass, despite significant weight loss in these patients.
This evidence also demonstrates a reduction and an improvement in metabolic markers, specifically glucose metabolism and lipid metabolism.
“The final conclusions of the study corroborate that the very-low-calorie ketogenic diet can be recommended as an effective and safe tool for people with obesity, especially those with severe obesity or comorbidities (joint disease, preoperative period to bariatric surgery, metabolic and cardiovascular diseases) who need immediate, substantial weight loss. In addition, it can be prescribed to specific groups of patients with obesity after considering potential contraindications and under medical follow-up,” said Dr. Busetto.
In Dr. Busetto’s opinion, it would be convenient to refer to this approach as a method, instead of as a diet, “because, in reality, the state of ketosis is limited in time, and if the ketogenic phase is stopped without a continuity plan, obviously weight is regained. In addition, the method approach may increase adherence by patients.”
Finally, Dr. Busetto emphasized the importance of integrating this type of treatment into a long-term lifestyle strategy (including habits, exercise, and nutritional advice). “We must start from the basis that obesity is a chronic and relapsing disease, whose management should also be chronic and probably maintained throughout life.”
Dr. Crujeiras and Dr. Busetto have disclosed no relevant financial relationships. PronoKal Group has recently become part of Nestlé Health Science.
A version of this article appeared on Medscape.com. It was translated from Medscape Spanish Edition.
According to the latest evidence, This development was revealed during the 8th International Scientific Symposium New Frontiers in Scientific Research, organized by PronoKal Group and held in Barcelona. During this conference, international multidisciplinary experts in the study and management of obesity presented the latest data on the benefits of treatment based on a very-low-calorie ketogenic diet.
“Nutritional ketosis has gained great interest in recent years because it is shown to have beneficial properties for health and promotes healthy aging, increasing longevity,” said Ana Belén Crujeiras, BSc, PhD, principal investigator of the Health Research Institute of Santiago de Compostela-Galician Health Service (IDIS-SERGAS) Group of Epigenomics in Endocrinology and Nutrition and the Biomedical Research Networking Center for Obesity and Nutrition Physiopathology (CIBEROBN). “Furthermore, in the case of obesity, we have more and more evidence that it is an effective treatment, mainly because to achieve this metabolic state (ketosis), routes that require the combustion of fats are activated, and this induces body weight loss.”
The specialist stressed that several strategies are used to induce nutritional ketosis. They are characterized by low carbohydrate consumption (low-carbohydrate and high-fat diet; low-carbohydrate, low-fat diet; and intermittent fasting). But Dr. Crujeiras warned that to use it as a treatment for a disease such as obesity, it must be backed by strong and solid scientific evidence, moving away from the concept of fad diets.
In this sense, since 2010, Dr. Crujeiras’ team has developed several studies focused on analyzing the efficacy and safety of treatment with a very-low-calorie ketogenic diet, the results of which have been published in high-impact journals.
Dr. Crujeiras commented on the main conclusions drawn from these investigations. “Our work has shown that the very-low-calorie ketogenic diet is effective for rapid weight loss and maintenance of lost weight, as well as reducing fat mass, primarily visceral fat mass.
“In this sense, a very interesting result is that despite the strong weight loss it induces, it preserves muscle mass and function and improves resting metabolic rate. These two variables are important, because all therapeutic strategies that exist to lose weight lead to a significant reduction in fat-free mass and also a reduction in energy expenditure at rest. This factor is associated with the risk of regaining lost weight, which is currently the great challenge in the treatment of obesity,” she added.
Specific methylation pattern
Dr. Crujeiras indicated that other notable evidence is the favorable impact on an emotional and psychological level. “To determine whether the caloric restriction of this diet and the strong weight loss that it involves were associated with an increased desire to eat, we also carried out an analysis with psychobiological tests. These results led us to conclude that this guideline is accompanied by a reduction of anxiety about food and an improvement in psychobiological parameters, thus increasing the quality of life of these patients.”
The specialist also mentioned that studies currently in progress show that the beneficial effect of this diet could be mediated by epigenetic mechanisms. “In our group, we have identified a specific DNA methylation pattern in people with obesity and we wondered if the very-low-calorie ketogenic diet would be able to reverse that methylome.
“We conducted a study in which we collected blood samples from patients on the very-low-calorie ketogenic diet (600 to 800 kcal/day) drawn before treatment, at peak ketosis, and at the end of treatment. After determining the global pattern of DNA in all patients with obesity targeted with this strategy and through bioinformatic analysis, we were able to obtain a methylation pattern. The results showed that after weight loss on the very-low-calorie ketogenic diet, the methylome that obese people initially had [was] reversed and matched that of normal-weight people.
“Continuing with this bioinformatic analysis more comprehensively, we wanted to see what kind of genes were differentially methylated, especially by the action of the ketosis itself. We found that most of the genes that exhibited differential methylation (in total, 292 identified) belonged to pathways that were involved in the regulation of metabolism, adipose tissue function, CNS function, and also carcinogenesis,” she continued.
Immunomodulatory effect
Dr. Crujeiras said that her research group also observed the modulatory role of the very-low-calorie ketogenic diet in the functioning of the immune system, “something that was not seen after similar weight loss induced by bariatric surgery. We analyzed this data in the context of the situation created by COVID-19, taking into account the evidence that people with obesity, compared to those with normal weight, have a higher risk of becoming infected and of having a poor evolution of the infection.”
In this regard, Dr. Crujeiras’ team launched an investigation to study the ACE2 gene methylation pattern, comparing obesity with normal weight and the situation after following a very-low-calorie ketogenic diet or undergoing bariatric surgery. “We observed that the methylation pattern of this gene in obese people was increased, compared to normal-weight people,” she explained, “and this increase was observed mainly in visceral adipose tissue. However, we did not see this in subcutaneous adipose tissue, which is in agreement with the hypothesis that visceral adipose tissue is that mostly associated with obesity-related comorbidities.
“Likewise, the very-low-calorie ketogenic diet was associated with decreased ACE2 methylation, along with increased exposure of this gene. However, after bariatric surgery, no significant changes were observed, so we deduce that we are protecting the patient in some way from inflammation and, therefore, from the potential of serious illness if they become infected.
“In light of these results, we wanted to dig deeper into what was happening with the immune system of obese patients and that inflammation after a very-low-calorie ketogenic diet. We conducted a new study, currently under review in the journal Clinical Nutrition, with the same approach, comparing this diet with a standard hypocaloric balanced diet and bariatric surgery, in which we analyzed a wide battery of cytokines (32). We have observed a differential pattern between the very-low-calorie ketogenic diet and bariatric surgery.
“The results confirm our hypothesis that the very-low-calorie ketogenic diet remodels the inflammatory status of obese patients, and we were also able to verify that the increase in ketone bodies has immunomodulatory properties that were previously demonstrated in preclinical and animal models, which is associated with increased immune function in these patients,” added Dr. Crujeiras.
Personalization and weight regain
In regard to the next steps to take in the knowledge and clinical application of the benefits of this dietary strategy, Dr. Crujeiras said that despite the fact that this diet is known to be effective, it is currently prescribed in a standard manner to all patients, “but there is some variability in the response and also a high risk of regaining weight, as is the case with any nutritional intervention strategy, with that ‘regain’ of lost weight being the main challenge in the treatment of obesity. In this sense, the epigenomic and epigenetic markers that we have identified could help us optimize treatment.”
She added that the future lies in establishing an algorithm that encompasses the patient’s exposome data, along with their genetic and epigenetic profile, to properly classify patients and prescribe a personalized precision therapeutic strategy.
Luca Busetto, MD, cochair of the Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO), also insisted on the challenge posed by the individualized application of the very-low-calorie ketogenic diet, emphasizing that this diet should always be prescribed by a doctor after an appropriate assessment of the patient. “Obesity is not a matter of willpower or motivation, and most people with obesity have struggled their entire lives and failed because their biology tends to cause weight regain. Therefore, we should try to offer them the options that we currently have, including the very-low-calorie ketogenic diet, adapting them as much as possible to the profile of each patient.”
During his speech, Dr. Busetto presented the recent European guidelines for the management of obesity in adults with a very-low-calorie ketogenic diet, endorsed by EASO, and analyzed the main strengths of these recommendations.
Dr. Busetto remarked that three important points clearly justify the use of the very-low-calorie ketogenic diet. The first is the speed with which the initial weight loss occurs. Recent studies have looked at the benefits of a significant loss of excess weight early in a weight-loss diet, and although this is an association rather than a cause, the results strongly suggest that rapid initial weight loss increases the chance of the result being maintained in the long term. This clashes with the traditional recommendation of losing weight little by little as a strategy to achieve long-term results, but it must be taken into account that there are many myths in the treatment of obesity that current evidence is dismantling with new data – and this is one of them.
Secondly, the effect of the very-low-calorie ketogenic diet can be added to other treatments. This has been demonstrated by studies carried out with liraglutide that showed that this dietary strategy optimizes results, compared with patients who had been treated only with this drug. The third point that justifies the use of the very-low-calorie ketogenic diet is the management of obesity comorbidities. Several investigations demonstrate the effectiveness of this diet in this regard, especially in the case of type 2 diabetes. Data suggest that the substantial weight reductions achieved with it also favor the remission of these comorbidities in many patients.
EASO ‘approval’
Dr. Busetto pointed out that, based on this evidence, the OMTF proposed the development of standards to be included in the EASO guidelines, since there had been no specific recommendation on the very-low-calorie ketogenic diet.
“The main objective of these European guidelines was to provide data referenced by scientific evidence and to suggest a common protocol for the use of this dietary strategy,” he added.
For this, a very exhaustive meta-analysis was carried out, researching all the publications that compared the very-low-calorie ketogenic diet with other diets. The results showed the superiority of the former method for the reduction of body mass index and weight and fat mass, with no difference in lean (muscle) mass, despite significant weight loss in these patients.
This evidence also demonstrates a reduction and an improvement in metabolic markers, specifically glucose metabolism and lipid metabolism.
“The final conclusions of the study corroborate that the very-low-calorie ketogenic diet can be recommended as an effective and safe tool for people with obesity, especially those with severe obesity or comorbidities (joint disease, preoperative period to bariatric surgery, metabolic and cardiovascular diseases) who need immediate, substantial weight loss. In addition, it can be prescribed to specific groups of patients with obesity after considering potential contraindications and under medical follow-up,” said Dr. Busetto.
In Dr. Busetto’s opinion, it would be convenient to refer to this approach as a method, instead of as a diet, “because, in reality, the state of ketosis is limited in time, and if the ketogenic phase is stopped without a continuity plan, obviously weight is regained. In addition, the method approach may increase adherence by patients.”
Finally, Dr. Busetto emphasized the importance of integrating this type of treatment into a long-term lifestyle strategy (including habits, exercise, and nutritional advice). “We must start from the basis that obesity is a chronic and relapsing disease, whose management should also be chronic and probably maintained throughout life.”
Dr. Crujeiras and Dr. Busetto have disclosed no relevant financial relationships. PronoKal Group has recently become part of Nestlé Health Science.
A version of this article appeared on Medscape.com. It was translated from Medscape Spanish Edition.
According to the latest evidence, This development was revealed during the 8th International Scientific Symposium New Frontiers in Scientific Research, organized by PronoKal Group and held in Barcelona. During this conference, international multidisciplinary experts in the study and management of obesity presented the latest data on the benefits of treatment based on a very-low-calorie ketogenic diet.
“Nutritional ketosis has gained great interest in recent years because it is shown to have beneficial properties for health and promotes healthy aging, increasing longevity,” said Ana Belén Crujeiras, BSc, PhD, principal investigator of the Health Research Institute of Santiago de Compostela-Galician Health Service (IDIS-SERGAS) Group of Epigenomics in Endocrinology and Nutrition and the Biomedical Research Networking Center for Obesity and Nutrition Physiopathology (CIBEROBN). “Furthermore, in the case of obesity, we have more and more evidence that it is an effective treatment, mainly because to achieve this metabolic state (ketosis), routes that require the combustion of fats are activated, and this induces body weight loss.”
The specialist stressed that several strategies are used to induce nutritional ketosis. They are characterized by low carbohydrate consumption (low-carbohydrate and high-fat diet; low-carbohydrate, low-fat diet; and intermittent fasting). But Dr. Crujeiras warned that to use it as a treatment for a disease such as obesity, it must be backed by strong and solid scientific evidence, moving away from the concept of fad diets.
In this sense, since 2010, Dr. Crujeiras’ team has developed several studies focused on analyzing the efficacy and safety of treatment with a very-low-calorie ketogenic diet, the results of which have been published in high-impact journals.
Dr. Crujeiras commented on the main conclusions drawn from these investigations. “Our work has shown that the very-low-calorie ketogenic diet is effective for rapid weight loss and maintenance of lost weight, as well as reducing fat mass, primarily visceral fat mass.
“In this sense, a very interesting result is that despite the strong weight loss it induces, it preserves muscle mass and function and improves resting metabolic rate. These two variables are important, because all therapeutic strategies that exist to lose weight lead to a significant reduction in fat-free mass and also a reduction in energy expenditure at rest. This factor is associated with the risk of regaining lost weight, which is currently the great challenge in the treatment of obesity,” she added.
Specific methylation pattern
Dr. Crujeiras indicated that other notable evidence is the favorable impact on an emotional and psychological level. “To determine whether the caloric restriction of this diet and the strong weight loss that it involves were associated with an increased desire to eat, we also carried out an analysis with psychobiological tests. These results led us to conclude that this guideline is accompanied by a reduction of anxiety about food and an improvement in psychobiological parameters, thus increasing the quality of life of these patients.”
The specialist also mentioned that studies currently in progress show that the beneficial effect of this diet could be mediated by epigenetic mechanisms. “In our group, we have identified a specific DNA methylation pattern in people with obesity and we wondered if the very-low-calorie ketogenic diet would be able to reverse that methylome.
“We conducted a study in which we collected blood samples from patients on the very-low-calorie ketogenic diet (600 to 800 kcal/day) drawn before treatment, at peak ketosis, and at the end of treatment. After determining the global pattern of DNA in all patients with obesity targeted with this strategy and through bioinformatic analysis, we were able to obtain a methylation pattern. The results showed that after weight loss on the very-low-calorie ketogenic diet, the methylome that obese people initially had [was] reversed and matched that of normal-weight people.
“Continuing with this bioinformatic analysis more comprehensively, we wanted to see what kind of genes were differentially methylated, especially by the action of the ketosis itself. We found that most of the genes that exhibited differential methylation (in total, 292 identified) belonged to pathways that were involved in the regulation of metabolism, adipose tissue function, CNS function, and also carcinogenesis,” she continued.
Immunomodulatory effect
Dr. Crujeiras said that her research group also observed the modulatory role of the very-low-calorie ketogenic diet in the functioning of the immune system, “something that was not seen after similar weight loss induced by bariatric surgery. We analyzed this data in the context of the situation created by COVID-19, taking into account the evidence that people with obesity, compared to those with normal weight, have a higher risk of becoming infected and of having a poor evolution of the infection.”
In this regard, Dr. Crujeiras’ team launched an investigation to study the ACE2 gene methylation pattern, comparing obesity with normal weight and the situation after following a very-low-calorie ketogenic diet or undergoing bariatric surgery. “We observed that the methylation pattern of this gene in obese people was increased, compared to normal-weight people,” she explained, “and this increase was observed mainly in visceral adipose tissue. However, we did not see this in subcutaneous adipose tissue, which is in agreement with the hypothesis that visceral adipose tissue is that mostly associated with obesity-related comorbidities.
“Likewise, the very-low-calorie ketogenic diet was associated with decreased ACE2 methylation, along with increased exposure of this gene. However, after bariatric surgery, no significant changes were observed, so we deduce that we are protecting the patient in some way from inflammation and, therefore, from the potential of serious illness if they become infected.
“In light of these results, we wanted to dig deeper into what was happening with the immune system of obese patients and that inflammation after a very-low-calorie ketogenic diet. We conducted a new study, currently under review in the journal Clinical Nutrition, with the same approach, comparing this diet with a standard hypocaloric balanced diet and bariatric surgery, in which we analyzed a wide battery of cytokines (32). We have observed a differential pattern between the very-low-calorie ketogenic diet and bariatric surgery.
“The results confirm our hypothesis that the very-low-calorie ketogenic diet remodels the inflammatory status of obese patients, and we were also able to verify that the increase in ketone bodies has immunomodulatory properties that were previously demonstrated in preclinical and animal models, which is associated with increased immune function in these patients,” added Dr. Crujeiras.
Personalization and weight regain
In regard to the next steps to take in the knowledge and clinical application of the benefits of this dietary strategy, Dr. Crujeiras said that despite the fact that this diet is known to be effective, it is currently prescribed in a standard manner to all patients, “but there is some variability in the response and also a high risk of regaining weight, as is the case with any nutritional intervention strategy, with that ‘regain’ of lost weight being the main challenge in the treatment of obesity. In this sense, the epigenomic and epigenetic markers that we have identified could help us optimize treatment.”
She added that the future lies in establishing an algorithm that encompasses the patient’s exposome data, along with their genetic and epigenetic profile, to properly classify patients and prescribe a personalized precision therapeutic strategy.
Luca Busetto, MD, cochair of the Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO), also insisted on the challenge posed by the individualized application of the very-low-calorie ketogenic diet, emphasizing that this diet should always be prescribed by a doctor after an appropriate assessment of the patient. “Obesity is not a matter of willpower or motivation, and most people with obesity have struggled their entire lives and failed because their biology tends to cause weight regain. Therefore, we should try to offer them the options that we currently have, including the very-low-calorie ketogenic diet, adapting them as much as possible to the profile of each patient.”
During his speech, Dr. Busetto presented the recent European guidelines for the management of obesity in adults with a very-low-calorie ketogenic diet, endorsed by EASO, and analyzed the main strengths of these recommendations.
Dr. Busetto remarked that three important points clearly justify the use of the very-low-calorie ketogenic diet. The first is the speed with which the initial weight loss occurs. Recent studies have looked at the benefits of a significant loss of excess weight early in a weight-loss diet, and although this is an association rather than a cause, the results strongly suggest that rapid initial weight loss increases the chance of the result being maintained in the long term. This clashes with the traditional recommendation of losing weight little by little as a strategy to achieve long-term results, but it must be taken into account that there are many myths in the treatment of obesity that current evidence is dismantling with new data – and this is one of them.
Secondly, the effect of the very-low-calorie ketogenic diet can be added to other treatments. This has been demonstrated by studies carried out with liraglutide that showed that this dietary strategy optimizes results, compared with patients who had been treated only with this drug. The third point that justifies the use of the very-low-calorie ketogenic diet is the management of obesity comorbidities. Several investigations demonstrate the effectiveness of this diet in this regard, especially in the case of type 2 diabetes. Data suggest that the substantial weight reductions achieved with it also favor the remission of these comorbidities in many patients.
EASO ‘approval’
Dr. Busetto pointed out that, based on this evidence, the OMTF proposed the development of standards to be included in the EASO guidelines, since there had been no specific recommendation on the very-low-calorie ketogenic diet.
“The main objective of these European guidelines was to provide data referenced by scientific evidence and to suggest a common protocol for the use of this dietary strategy,” he added.
For this, a very exhaustive meta-analysis was carried out, researching all the publications that compared the very-low-calorie ketogenic diet with other diets. The results showed the superiority of the former method for the reduction of body mass index and weight and fat mass, with no difference in lean (muscle) mass, despite significant weight loss in these patients.
This evidence also demonstrates a reduction and an improvement in metabolic markers, specifically glucose metabolism and lipid metabolism.
“The final conclusions of the study corroborate that the very-low-calorie ketogenic diet can be recommended as an effective and safe tool for people with obesity, especially those with severe obesity or comorbidities (joint disease, preoperative period to bariatric surgery, metabolic and cardiovascular diseases) who need immediate, substantial weight loss. In addition, it can be prescribed to specific groups of patients with obesity after considering potential contraindications and under medical follow-up,” said Dr. Busetto.
In Dr. Busetto’s opinion, it would be convenient to refer to this approach as a method, instead of as a diet, “because, in reality, the state of ketosis is limited in time, and if the ketogenic phase is stopped without a continuity plan, obviously weight is regained. In addition, the method approach may increase adherence by patients.”
Finally, Dr. Busetto emphasized the importance of integrating this type of treatment into a long-term lifestyle strategy (including habits, exercise, and nutritional advice). “We must start from the basis that obesity is a chronic and relapsing disease, whose management should also be chronic and probably maintained throughout life.”
Dr. Crujeiras and Dr. Busetto have disclosed no relevant financial relationships. PronoKal Group has recently become part of Nestlé Health Science.
A version of this article appeared on Medscape.com. It was translated from Medscape Spanish Edition.
43-year-old male • fatigue • unintentional weight loss • pancytopenia • Dx?
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; isabella.brout@bmc.org
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; isabella.brout@bmc.org
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; isabella.brout@bmc.org
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
Medical ‘myths’ persist despite evidence, says professor of medicine
These long-held pieces of dogma – or “medical myths” – were engraved during training or early in the careers of many physicians, and are difficult to overcome, noted Douglas Paauw, MD, professor of medicine at the University of Washington, Seattle.
“I think that myths persist because medical professionals get taught one way in training, given a ‘truth’ or ‘This is the way we do it,’ and then do not ever rethink, ‘Is it true?’ ” he said in an interview. “Studies pop up to question conventional wisdom, but unless the studies get highly publicized, they aren’t noticed.”
During his presentation, Dr. Paauw discussed three of what he considers to be some of the some of the medical myths that are in greatest need of being dispelled.
Shellfish allergy and radiocontrast
A myth persists that people with a shellfish allergy could have an allergic reaction when a contrast agent is used for a scan, he said.
This belief arose, because fish and shellfish contain iodine, and allergic reactions to seafood are fairly common, and contrast agents contain iodine, too, Dr. Paauw said.
The belief is widespread, with 65% of radiologists and 88.9% of interventional cardiologists saying they ask about seafood or shellfish allergies before administering contrast. And a third of radiologists and 50% of cardiologists said they would withhold contrast media or recommend a premedication for patients with such an allergy.
But the belief makes no sense, Dr. Pauuw said. Iodine is present in many other foods, including milk and bread, and allergies to shellfish are because of parvalbumin protein and tropomyosins, not iodine.
Colonoscopy dogma
It’s been long believed that people need to be on a clear, liquid diet for 1 or 2 days and need to drink a bowel-prep liquid before a colonoscopy, noted Dr. Paauw.
But the evidence shows this isn’t necessary, he said.
A 2020 study found that a low-residual diet, allowing foods such as meat, eggs, dairy, and bread, were comparable to the clear liquid diet in terms of bowel prep and detection of polyps during the exam. The patients on the low-residual diet had less nausea, less vomiting, and less hunger, and expressed more willingness to have a repeat colonoscopy.
“Let them eat,” Dr. Paauw said in his presentation.
Metronidazole and alcohol
There is a belief that patients shouldn’t drink alcohol if they are taking metronidazole, because of concerns about nausea, vomiting, flushing and other symptoms – also known as a disulfiramlike reaction, Dr. Paauw explained.
Case reports have been published, but the cases were presented as though a metronidazole-ethanol reaction was an established fact, and the authors didn’t provide evidence to justify this, Dr. Paauw said.
But it’s been shown in rat models that metronidazole can increase levels of acetaldehyde, the trigger of symptoms, in the colon, but not in the blood. And in a small placebo-controlled, randomized trial, six people were given metronidazole and ethanol and, after regular blood testing, no difference was seen in acetaldehyde blood levels, vital signs, or symptoms.
The Centers for Disease Control and Prevention has said that avoiding alcohol while taking metronidazole is unnecessary, said Dr. Paauw.
Sinus headaches
Contrary to common belief, headaches thought to be “sinus headaches” are usually migraine headaches, Dr. Paauw said.
In one study, 2,991 patients with six headaches in the previous 6 months were self-diagnosed or were physician-diagnosed with sinus headaches. But 88% of these headaches met the International Headache Society criteria for migraine headache.
Dr. Paauw said he hopes that clinicians reconsider the evidence regularly when deciding how to treat their patients, and not rely on bits of dogma.
“They stay with us,” he said, “and sometimes there are other ways to do it.”
Shien Tze, MD, an internist in Fargo, N,D,, said that patients sometimes also hold misconceptions, based on outdated dogma, that he needs to dispel.
“I try to convince them that this is a myth that is not based on evidence, not based on science,” he said. “I think it depends on the way you say it. If you say it in a calm, firm, not wishy-washy way, the patients believe you.”
Dr. Paauw reported no relevant financial disclosures. He serves on the editorial advisory board of Internal Medicine News, and he contributes “Myth of the Month” and “Pearl of the Month” columns to this publication.
These long-held pieces of dogma – or “medical myths” – were engraved during training or early in the careers of many physicians, and are difficult to overcome, noted Douglas Paauw, MD, professor of medicine at the University of Washington, Seattle.
“I think that myths persist because medical professionals get taught one way in training, given a ‘truth’ or ‘This is the way we do it,’ and then do not ever rethink, ‘Is it true?’ ” he said in an interview. “Studies pop up to question conventional wisdom, but unless the studies get highly publicized, they aren’t noticed.”
During his presentation, Dr. Paauw discussed three of what he considers to be some of the some of the medical myths that are in greatest need of being dispelled.
Shellfish allergy and radiocontrast
A myth persists that people with a shellfish allergy could have an allergic reaction when a contrast agent is used for a scan, he said.
This belief arose, because fish and shellfish contain iodine, and allergic reactions to seafood are fairly common, and contrast agents contain iodine, too, Dr. Paauw said.
The belief is widespread, with 65% of radiologists and 88.9% of interventional cardiologists saying they ask about seafood or shellfish allergies before administering contrast. And a third of radiologists and 50% of cardiologists said they would withhold contrast media or recommend a premedication for patients with such an allergy.
But the belief makes no sense, Dr. Pauuw said. Iodine is present in many other foods, including milk and bread, and allergies to shellfish are because of parvalbumin protein and tropomyosins, not iodine.
Colonoscopy dogma
It’s been long believed that people need to be on a clear, liquid diet for 1 or 2 days and need to drink a bowel-prep liquid before a colonoscopy, noted Dr. Paauw.
But the evidence shows this isn’t necessary, he said.
A 2020 study found that a low-residual diet, allowing foods such as meat, eggs, dairy, and bread, were comparable to the clear liquid diet in terms of bowel prep and detection of polyps during the exam. The patients on the low-residual diet had less nausea, less vomiting, and less hunger, and expressed more willingness to have a repeat colonoscopy.
“Let them eat,” Dr. Paauw said in his presentation.
Metronidazole and alcohol
There is a belief that patients shouldn’t drink alcohol if they are taking metronidazole, because of concerns about nausea, vomiting, flushing and other symptoms – also known as a disulfiramlike reaction, Dr. Paauw explained.
Case reports have been published, but the cases were presented as though a metronidazole-ethanol reaction was an established fact, and the authors didn’t provide evidence to justify this, Dr. Paauw said.
But it’s been shown in rat models that metronidazole can increase levels of acetaldehyde, the trigger of symptoms, in the colon, but not in the blood. And in a small placebo-controlled, randomized trial, six people were given metronidazole and ethanol and, after regular blood testing, no difference was seen in acetaldehyde blood levels, vital signs, or symptoms.
The Centers for Disease Control and Prevention has said that avoiding alcohol while taking metronidazole is unnecessary, said Dr. Paauw.
Sinus headaches
Contrary to common belief, headaches thought to be “sinus headaches” are usually migraine headaches, Dr. Paauw said.
In one study, 2,991 patients with six headaches in the previous 6 months were self-diagnosed or were physician-diagnosed with sinus headaches. But 88% of these headaches met the International Headache Society criteria for migraine headache.
Dr. Paauw said he hopes that clinicians reconsider the evidence regularly when deciding how to treat their patients, and not rely on bits of dogma.
“They stay with us,” he said, “and sometimes there are other ways to do it.”
Shien Tze, MD, an internist in Fargo, N,D,, said that patients sometimes also hold misconceptions, based on outdated dogma, that he needs to dispel.
“I try to convince them that this is a myth that is not based on evidence, not based on science,” he said. “I think it depends on the way you say it. If you say it in a calm, firm, not wishy-washy way, the patients believe you.”
Dr. Paauw reported no relevant financial disclosures. He serves on the editorial advisory board of Internal Medicine News, and he contributes “Myth of the Month” and “Pearl of the Month” columns to this publication.
These long-held pieces of dogma – or “medical myths” – were engraved during training or early in the careers of many physicians, and are difficult to overcome, noted Douglas Paauw, MD, professor of medicine at the University of Washington, Seattle.
“I think that myths persist because medical professionals get taught one way in training, given a ‘truth’ or ‘This is the way we do it,’ and then do not ever rethink, ‘Is it true?’ ” he said in an interview. “Studies pop up to question conventional wisdom, but unless the studies get highly publicized, they aren’t noticed.”
During his presentation, Dr. Paauw discussed three of what he considers to be some of the some of the medical myths that are in greatest need of being dispelled.
Shellfish allergy and radiocontrast
A myth persists that people with a shellfish allergy could have an allergic reaction when a contrast agent is used for a scan, he said.
This belief arose, because fish and shellfish contain iodine, and allergic reactions to seafood are fairly common, and contrast agents contain iodine, too, Dr. Paauw said.
The belief is widespread, with 65% of radiologists and 88.9% of interventional cardiologists saying they ask about seafood or shellfish allergies before administering contrast. And a third of radiologists and 50% of cardiologists said they would withhold contrast media or recommend a premedication for patients with such an allergy.
But the belief makes no sense, Dr. Pauuw said. Iodine is present in many other foods, including milk and bread, and allergies to shellfish are because of parvalbumin protein and tropomyosins, not iodine.
Colonoscopy dogma
It’s been long believed that people need to be on a clear, liquid diet for 1 or 2 days and need to drink a bowel-prep liquid before a colonoscopy, noted Dr. Paauw.
But the evidence shows this isn’t necessary, he said.
A 2020 study found that a low-residual diet, allowing foods such as meat, eggs, dairy, and bread, were comparable to the clear liquid diet in terms of bowel prep and detection of polyps during the exam. The patients on the low-residual diet had less nausea, less vomiting, and less hunger, and expressed more willingness to have a repeat colonoscopy.
“Let them eat,” Dr. Paauw said in his presentation.
Metronidazole and alcohol
There is a belief that patients shouldn’t drink alcohol if they are taking metronidazole, because of concerns about nausea, vomiting, flushing and other symptoms – also known as a disulfiramlike reaction, Dr. Paauw explained.
Case reports have been published, but the cases were presented as though a metronidazole-ethanol reaction was an established fact, and the authors didn’t provide evidence to justify this, Dr. Paauw said.
But it’s been shown in rat models that metronidazole can increase levels of acetaldehyde, the trigger of symptoms, in the colon, but not in the blood. And in a small placebo-controlled, randomized trial, six people were given metronidazole and ethanol and, after regular blood testing, no difference was seen in acetaldehyde blood levels, vital signs, or symptoms.
The Centers for Disease Control and Prevention has said that avoiding alcohol while taking metronidazole is unnecessary, said Dr. Paauw.
Sinus headaches
Contrary to common belief, headaches thought to be “sinus headaches” are usually migraine headaches, Dr. Paauw said.
In one study, 2,991 patients with six headaches in the previous 6 months were self-diagnosed or were physician-diagnosed with sinus headaches. But 88% of these headaches met the International Headache Society criteria for migraine headache.
Dr. Paauw said he hopes that clinicians reconsider the evidence regularly when deciding how to treat their patients, and not rely on bits of dogma.
“They stay with us,” he said, “and sometimes there are other ways to do it.”
Shien Tze, MD, an internist in Fargo, N,D,, said that patients sometimes also hold misconceptions, based on outdated dogma, that he needs to dispel.
“I try to convince them that this is a myth that is not based on evidence, not based on science,” he said. “I think it depends on the way you say it. If you say it in a calm, firm, not wishy-washy way, the patients believe you.”
Dr. Paauw reported no relevant financial disclosures. He serves on the editorial advisory board of Internal Medicine News, and he contributes “Myth of the Month” and “Pearl of the Month” columns to this publication.
AT INTERNAL MEDICINE 2022
Maternal autoimmune diseases up risk of mental illness in children
Mental disorders were significantly more likely in children whose mothers had one of five common autoimmune diseases, a new study found.
Previous research has linked both maternal and paternal autoimmune diseases and specific mental disorders, such as attention-deficit/hyperactivity disorder (ADHD), but most of these studies focused on specific conditions in relatively small populations. The new study included data on more than 2 million births, making it one of the largest efforts to date to examine the association, according to the researchers, whose findings were published in JAMA Network Open.
Previous evidence of the possible association between certain maternal autoimmune diseases and mental disorders in offspring has been “scattered and limited,” which “hampered an overall understanding” of the link, Fei Li, MD, the corresponding author of the study, told this news organization.
Dr. Li, of Shanghai Jiao Tong University China, and colleagues reviewed data from a Danish registry cohort of singleton births with up to 38 years of follow-up. They explored associations between a range of maternal autoimmune diseases diagnosed before childbirth and the risks of mental disorders in children in early childhood through young adulthood.
The study population included 2,254,234 births and 38,916,359 person-years. Data on mental health were collected from the Psychiatric Central Research Register and the country’s National Patient Register. The median age of the children at the time of assessment was 16.7 years; approximately half were male.
A total of 50,863 children (2.26%) were born to mothers who had been diagnosed with autoimmune diseases before childbirth. During the follow-up period, 5,460 children of mothers with autoimmune diseases and 303,092 children of mothers without autoimmune diseases were diagnosed with a mental disorder (10.73% vs. 13.76%), according to the researchers.
The risk of being diagnosed with a mental disorder was significantly higher among children of mothers with any autoimmune disease (hazard ratio [HR,], 1.16), with an incidence of 9.38 vs. 7.91 per 1,000 person-years, the researchers reported.
The increased risk persisted when the results were classified by organ system, including connective tissue (HR, 1.11), endocrine (HR, 1.19), gastrointestinal (HR, 1.11), blood (HR, 1.10), nervous (HR, 1.17), and skin (HR, 1.19).
The five autoimmune diseases in mothers that were most commonly associated mental health disorders in children were type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and psoriasis vulgaris.
The greatest risk for children of mothers with any autoimmune disease was observed for organic conditions such as delirium, (HR, 1.54), followed by obsessive-compulsive disorder (HR, 1.42), schizophrenia (HR, 1.54), and mood problems (HR, 1.12).
Children of mothers with any autoimmune disorder also had a significantly increased risk of autism (HR, 1.21), intellectual disability (HR, 1.19), and ADHD (HR, 1.19).
The results add to evidence that activation of the maternal immune system may drive changes in the brain and behavioral problems, which has been observed in animal studies, the researchers wrote.
Potential underlying mechanisms in need of more exploration include genetic risk factors, maternal transmission of autoantibodies to the fetus during pregnancy, and the increased risk of obstetric complications, such as preterm birth, for women with autoimmune disorders that could affect mental development in children, they added.
The study findings were limited by several factors, including the lack of data on potential exacerbation of autoimmune disease activity during pregnancy and its effect on the fetus, the researchers noted. Other limitations included potential detection bias, lack of data on mental disorders in adulthood, and potential changes in diagnostic criteria over the long study period.
The results were strengthened by the use of a population-based registry, the large sample size, and ability to consider a range of confounders, the researchers said.
“This study could help acquire a comprehensive compilation of the associations between maternal autoimmune disorders diagnosed before childbirth and offspring’s mental disorders from childhood through early adulthood,” Dr. Li said in an interview.
For clinicians, Dr. Li said, the findings suggest that the offspring of mothers with autoimmune diseases may benefit from long-term surveillance for mental health disorders.
“Further studies should provide more evidence on the detailed associations of specific maternal autoimmune diseases with a full spectrum of mental disorders in offspring, and more research on underlying mechanisms is needed as well,” she said.
Pay early attention
M. Susan Jay, MD, an adjunct professor of pediatrics at the Medical College of Wisconsin, Milwaukee, said previous efforts to examine the association between maternal autoimmunity were hampered by study design, small samples, and self-report of disease history – problems the new research avoids.
The large patient population allowed for detailed subgroup analysis of different conditions and outcomes. Another advantage was the availability of sociodemographic and clinical information, which allowed for the elimination of confounding factors, said Dr. Jay, who was not involved in the research.
“It would be prudent to follow children of mothers with autoimmune disorders before or during pregnancy for mental health issues, and if identified clinically, to offer psychological and developmental behavioral support options,” Dr. Jay added.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mental disorders were significantly more likely in children whose mothers had one of five common autoimmune diseases, a new study found.
Previous research has linked both maternal and paternal autoimmune diseases and specific mental disorders, such as attention-deficit/hyperactivity disorder (ADHD), but most of these studies focused on specific conditions in relatively small populations. The new study included data on more than 2 million births, making it one of the largest efforts to date to examine the association, according to the researchers, whose findings were published in JAMA Network Open.
Previous evidence of the possible association between certain maternal autoimmune diseases and mental disorders in offspring has been “scattered and limited,” which “hampered an overall understanding” of the link, Fei Li, MD, the corresponding author of the study, told this news organization.
Dr. Li, of Shanghai Jiao Tong University China, and colleagues reviewed data from a Danish registry cohort of singleton births with up to 38 years of follow-up. They explored associations between a range of maternal autoimmune diseases diagnosed before childbirth and the risks of mental disorders in children in early childhood through young adulthood.
The study population included 2,254,234 births and 38,916,359 person-years. Data on mental health were collected from the Psychiatric Central Research Register and the country’s National Patient Register. The median age of the children at the time of assessment was 16.7 years; approximately half were male.
A total of 50,863 children (2.26%) were born to mothers who had been diagnosed with autoimmune diseases before childbirth. During the follow-up period, 5,460 children of mothers with autoimmune diseases and 303,092 children of mothers without autoimmune diseases were diagnosed with a mental disorder (10.73% vs. 13.76%), according to the researchers.
The risk of being diagnosed with a mental disorder was significantly higher among children of mothers with any autoimmune disease (hazard ratio [HR,], 1.16), with an incidence of 9.38 vs. 7.91 per 1,000 person-years, the researchers reported.
The increased risk persisted when the results were classified by organ system, including connective tissue (HR, 1.11), endocrine (HR, 1.19), gastrointestinal (HR, 1.11), blood (HR, 1.10), nervous (HR, 1.17), and skin (HR, 1.19).
The five autoimmune diseases in mothers that were most commonly associated mental health disorders in children were type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and psoriasis vulgaris.
The greatest risk for children of mothers with any autoimmune disease was observed for organic conditions such as delirium, (HR, 1.54), followed by obsessive-compulsive disorder (HR, 1.42), schizophrenia (HR, 1.54), and mood problems (HR, 1.12).
Children of mothers with any autoimmune disorder also had a significantly increased risk of autism (HR, 1.21), intellectual disability (HR, 1.19), and ADHD (HR, 1.19).
The results add to evidence that activation of the maternal immune system may drive changes in the brain and behavioral problems, which has been observed in animal studies, the researchers wrote.
Potential underlying mechanisms in need of more exploration include genetic risk factors, maternal transmission of autoantibodies to the fetus during pregnancy, and the increased risk of obstetric complications, such as preterm birth, for women with autoimmune disorders that could affect mental development in children, they added.
The study findings were limited by several factors, including the lack of data on potential exacerbation of autoimmune disease activity during pregnancy and its effect on the fetus, the researchers noted. Other limitations included potential detection bias, lack of data on mental disorders in adulthood, and potential changes in diagnostic criteria over the long study period.
The results were strengthened by the use of a population-based registry, the large sample size, and ability to consider a range of confounders, the researchers said.
“This study could help acquire a comprehensive compilation of the associations between maternal autoimmune disorders diagnosed before childbirth and offspring’s mental disorders from childhood through early adulthood,” Dr. Li said in an interview.
For clinicians, Dr. Li said, the findings suggest that the offspring of mothers with autoimmune diseases may benefit from long-term surveillance for mental health disorders.
“Further studies should provide more evidence on the detailed associations of specific maternal autoimmune diseases with a full spectrum of mental disorders in offspring, and more research on underlying mechanisms is needed as well,” she said.
Pay early attention
M. Susan Jay, MD, an adjunct professor of pediatrics at the Medical College of Wisconsin, Milwaukee, said previous efforts to examine the association between maternal autoimmunity were hampered by study design, small samples, and self-report of disease history – problems the new research avoids.
The large patient population allowed for detailed subgroup analysis of different conditions and outcomes. Another advantage was the availability of sociodemographic and clinical information, which allowed for the elimination of confounding factors, said Dr. Jay, who was not involved in the research.
“It would be prudent to follow children of mothers with autoimmune disorders before or during pregnancy for mental health issues, and if identified clinically, to offer psychological and developmental behavioral support options,” Dr. Jay added.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mental disorders were significantly more likely in children whose mothers had one of five common autoimmune diseases, a new study found.
Previous research has linked both maternal and paternal autoimmune diseases and specific mental disorders, such as attention-deficit/hyperactivity disorder (ADHD), but most of these studies focused on specific conditions in relatively small populations. The new study included data on more than 2 million births, making it one of the largest efforts to date to examine the association, according to the researchers, whose findings were published in JAMA Network Open.
Previous evidence of the possible association between certain maternal autoimmune diseases and mental disorders in offspring has been “scattered and limited,” which “hampered an overall understanding” of the link, Fei Li, MD, the corresponding author of the study, told this news organization.
Dr. Li, of Shanghai Jiao Tong University China, and colleagues reviewed data from a Danish registry cohort of singleton births with up to 38 years of follow-up. They explored associations between a range of maternal autoimmune diseases diagnosed before childbirth and the risks of mental disorders in children in early childhood through young adulthood.
The study population included 2,254,234 births and 38,916,359 person-years. Data on mental health were collected from the Psychiatric Central Research Register and the country’s National Patient Register. The median age of the children at the time of assessment was 16.7 years; approximately half were male.
A total of 50,863 children (2.26%) were born to mothers who had been diagnosed with autoimmune diseases before childbirth. During the follow-up period, 5,460 children of mothers with autoimmune diseases and 303,092 children of mothers without autoimmune diseases were diagnosed with a mental disorder (10.73% vs. 13.76%), according to the researchers.
The risk of being diagnosed with a mental disorder was significantly higher among children of mothers with any autoimmune disease (hazard ratio [HR,], 1.16), with an incidence of 9.38 vs. 7.91 per 1,000 person-years, the researchers reported.
The increased risk persisted when the results were classified by organ system, including connective tissue (HR, 1.11), endocrine (HR, 1.19), gastrointestinal (HR, 1.11), blood (HR, 1.10), nervous (HR, 1.17), and skin (HR, 1.19).
The five autoimmune diseases in mothers that were most commonly associated mental health disorders in children were type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and psoriasis vulgaris.
The greatest risk for children of mothers with any autoimmune disease was observed for organic conditions such as delirium, (HR, 1.54), followed by obsessive-compulsive disorder (HR, 1.42), schizophrenia (HR, 1.54), and mood problems (HR, 1.12).
Children of mothers with any autoimmune disorder also had a significantly increased risk of autism (HR, 1.21), intellectual disability (HR, 1.19), and ADHD (HR, 1.19).
The results add to evidence that activation of the maternal immune system may drive changes in the brain and behavioral problems, which has been observed in animal studies, the researchers wrote.
Potential underlying mechanisms in need of more exploration include genetic risk factors, maternal transmission of autoantibodies to the fetus during pregnancy, and the increased risk of obstetric complications, such as preterm birth, for women with autoimmune disorders that could affect mental development in children, they added.
The study findings were limited by several factors, including the lack of data on potential exacerbation of autoimmune disease activity during pregnancy and its effect on the fetus, the researchers noted. Other limitations included potential detection bias, lack of data on mental disorders in adulthood, and potential changes in diagnostic criteria over the long study period.
The results were strengthened by the use of a population-based registry, the large sample size, and ability to consider a range of confounders, the researchers said.
“This study could help acquire a comprehensive compilation of the associations between maternal autoimmune disorders diagnosed before childbirth and offspring’s mental disorders from childhood through early adulthood,” Dr. Li said in an interview.
For clinicians, Dr. Li said, the findings suggest that the offspring of mothers with autoimmune diseases may benefit from long-term surveillance for mental health disorders.
“Further studies should provide more evidence on the detailed associations of specific maternal autoimmune diseases with a full spectrum of mental disorders in offspring, and more research on underlying mechanisms is needed as well,” she said.
Pay early attention
M. Susan Jay, MD, an adjunct professor of pediatrics at the Medical College of Wisconsin, Milwaukee, said previous efforts to examine the association between maternal autoimmunity were hampered by study design, small samples, and self-report of disease history – problems the new research avoids.
The large patient population allowed for detailed subgroup analysis of different conditions and outcomes. Another advantage was the availability of sociodemographic and clinical information, which allowed for the elimination of confounding factors, said Dr. Jay, who was not involved in the research.
“It would be prudent to follow children of mothers with autoimmune disorders before or during pregnancy for mental health issues, and if identified clinically, to offer psychological and developmental behavioral support options,” Dr. Jay added.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antibiotics use and vaccine antibody levels
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).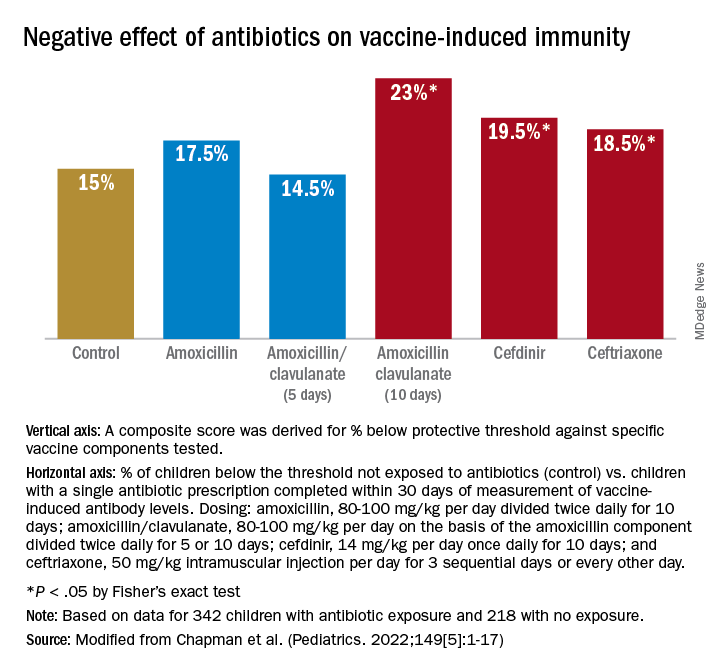
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
Probiotic LGG doesn’t lessen eczema, asthma, or rhinitis risk by age 7
Giving the probiotic supplement Lactobacillus rhamnosus GG (LGG) to high-risk infants in the first 6 months of life is not effective in lessening incidence of eczema, asthma, or rhinitis in later childhood, researchers have found.
The researchers, led by Michael D. Cabana, MD, MPH, with the Children’s Hospital of Montefiore, New York, said they cannot support its use in this population of children at high risk for allergic disease. Findings were published in Pediatrics.
Jonathan Spergel, MD, PhD, chief of the allergy program at Children’s Hospital of Philadelphia, who was not part of the study, said the “small, but very interesting study adds to the literature indicating that allergy prevention needs to be a multifactorial approach and simply adding LGG in a select population makes no difference.”
He noted that the study of probiotics for allergic conditions is complex as it depends on many factors, such as the child’s environment, including exposure to pets and pollution, and whether the child was delivered vaginally or by cesarean section.
Study builds on previous work
The new study builds on the same researchers’ randomized, double-masked, parallel-arm, controlled Trial of Infant Probiotic Supplementation (TIPS). That study investigated whether daily administration of LGG in the first 6 months to children at high risk for allergic disease because of asthma in a parent, could decrease their cumulative incidence of eczema. Investigators found LGG had no effect.
These additional results included participants at least 7 years old and also included physician-diagnosed asthma and physician-diagnosed rhinitis as secondary outcomes.
Retention rate over the 7-year follow-up was 56%; 49 (53%) of 92 in the intervention group and 54 (59%) of 92 in the control group.
The researchers performed modified intention-to-treat analyses with all children who received treatment in the study arm to which they had been randomized.
Eczema was diagnosed in 78 participants, asthma in 32, and rhinitis in 15. Incidence of eczema was high in infancy, but low thereafter. Incidence rates for asthma and rhinitis were constant throughout childhood.
The researchers used modeling to compare the incidence of each outcome between the intervention and control groups, adjusting for mode of delivery and how long a child was breastfed.
Cesarean delivery was linked to a greater incidence of rhinitis, with a hazard ratio of 3.33 (95% confidence interval, 1.21-9.21).
Finding the right strain
Heather Cassell, MD, a pediatric allergist and immunologist at University of Arizona, Tucson, who was not part of the study, said in an interview that many researchers, including those at her institution, are trying to find which strain of probiotic might be beneficial in lowering risk for allergic disease.
Though it appears LGG doesn’t have an effect, she said, another strain might be successful and this helps zero in on the right one.
The TIPS trial showed that there were no significant side effects from giving LGG early, which is good information to have as the search resumes for the right strain, she said.
“We know that there’s probably some immune dysregulation in kids with asthma, eczema, other allergies, but we don’t fully know the extent of it,” she said, adding that it may be that skin flora or respiratory flora and microbiomes in other parts of the body play a role.
“We don’t have bacteria just in our guts,” she noted. “It may be a combination of strains or a combination of bacteria.”
The authors, Dr. Spergel, and Dr. Cassell reported no relevant financial relationships.
Giving the probiotic supplement Lactobacillus rhamnosus GG (LGG) to high-risk infants in the first 6 months of life is not effective in lessening incidence of eczema, asthma, or rhinitis in later childhood, researchers have found.
The researchers, led by Michael D. Cabana, MD, MPH, with the Children’s Hospital of Montefiore, New York, said they cannot support its use in this population of children at high risk for allergic disease. Findings were published in Pediatrics.
Jonathan Spergel, MD, PhD, chief of the allergy program at Children’s Hospital of Philadelphia, who was not part of the study, said the “small, but very interesting study adds to the literature indicating that allergy prevention needs to be a multifactorial approach and simply adding LGG in a select population makes no difference.”
He noted that the study of probiotics for allergic conditions is complex as it depends on many factors, such as the child’s environment, including exposure to pets and pollution, and whether the child was delivered vaginally or by cesarean section.
Study builds on previous work
The new study builds on the same researchers’ randomized, double-masked, parallel-arm, controlled Trial of Infant Probiotic Supplementation (TIPS). That study investigated whether daily administration of LGG in the first 6 months to children at high risk for allergic disease because of asthma in a parent, could decrease their cumulative incidence of eczema. Investigators found LGG had no effect.
These additional results included participants at least 7 years old and also included physician-diagnosed asthma and physician-diagnosed rhinitis as secondary outcomes.
Retention rate over the 7-year follow-up was 56%; 49 (53%) of 92 in the intervention group and 54 (59%) of 92 in the control group.
The researchers performed modified intention-to-treat analyses with all children who received treatment in the study arm to which they had been randomized.
Eczema was diagnosed in 78 participants, asthma in 32, and rhinitis in 15. Incidence of eczema was high in infancy, but low thereafter. Incidence rates for asthma and rhinitis were constant throughout childhood.
The researchers used modeling to compare the incidence of each outcome between the intervention and control groups, adjusting for mode of delivery and how long a child was breastfed.
Cesarean delivery was linked to a greater incidence of rhinitis, with a hazard ratio of 3.33 (95% confidence interval, 1.21-9.21).
Finding the right strain
Heather Cassell, MD, a pediatric allergist and immunologist at University of Arizona, Tucson, who was not part of the study, said in an interview that many researchers, including those at her institution, are trying to find which strain of probiotic might be beneficial in lowering risk for allergic disease.
Though it appears LGG doesn’t have an effect, she said, another strain might be successful and this helps zero in on the right one.
The TIPS trial showed that there were no significant side effects from giving LGG early, which is good information to have as the search resumes for the right strain, she said.
“We know that there’s probably some immune dysregulation in kids with asthma, eczema, other allergies, but we don’t fully know the extent of it,” she said, adding that it may be that skin flora or respiratory flora and microbiomes in other parts of the body play a role.
“We don’t have bacteria just in our guts,” she noted. “It may be a combination of strains or a combination of bacteria.”
The authors, Dr. Spergel, and Dr. Cassell reported no relevant financial relationships.
Giving the probiotic supplement Lactobacillus rhamnosus GG (LGG) to high-risk infants in the first 6 months of life is not effective in lessening incidence of eczema, asthma, or rhinitis in later childhood, researchers have found.
The researchers, led by Michael D. Cabana, MD, MPH, with the Children’s Hospital of Montefiore, New York, said they cannot support its use in this population of children at high risk for allergic disease. Findings were published in Pediatrics.
Jonathan Spergel, MD, PhD, chief of the allergy program at Children’s Hospital of Philadelphia, who was not part of the study, said the “small, but very interesting study adds to the literature indicating that allergy prevention needs to be a multifactorial approach and simply adding LGG in a select population makes no difference.”
He noted that the study of probiotics for allergic conditions is complex as it depends on many factors, such as the child’s environment, including exposure to pets and pollution, and whether the child was delivered vaginally or by cesarean section.
Study builds on previous work
The new study builds on the same researchers’ randomized, double-masked, parallel-arm, controlled Trial of Infant Probiotic Supplementation (TIPS). That study investigated whether daily administration of LGG in the first 6 months to children at high risk for allergic disease because of asthma in a parent, could decrease their cumulative incidence of eczema. Investigators found LGG had no effect.
These additional results included participants at least 7 years old and also included physician-diagnosed asthma and physician-diagnosed rhinitis as secondary outcomes.
Retention rate over the 7-year follow-up was 56%; 49 (53%) of 92 in the intervention group and 54 (59%) of 92 in the control group.
The researchers performed modified intention-to-treat analyses with all children who received treatment in the study arm to which they had been randomized.
Eczema was diagnosed in 78 participants, asthma in 32, and rhinitis in 15. Incidence of eczema was high in infancy, but low thereafter. Incidence rates for asthma and rhinitis were constant throughout childhood.
The researchers used modeling to compare the incidence of each outcome between the intervention and control groups, adjusting for mode of delivery and how long a child was breastfed.
Cesarean delivery was linked to a greater incidence of rhinitis, with a hazard ratio of 3.33 (95% confidence interval, 1.21-9.21).
Finding the right strain
Heather Cassell, MD, a pediatric allergist and immunologist at University of Arizona, Tucson, who was not part of the study, said in an interview that many researchers, including those at her institution, are trying to find which strain of probiotic might be beneficial in lowering risk for allergic disease.
Though it appears LGG doesn’t have an effect, she said, another strain might be successful and this helps zero in on the right one.
The TIPS trial showed that there were no significant side effects from giving LGG early, which is good information to have as the search resumes for the right strain, she said.
“We know that there’s probably some immune dysregulation in kids with asthma, eczema, other allergies, but we don’t fully know the extent of it,” she said, adding that it may be that skin flora or respiratory flora and microbiomes in other parts of the body play a role.
“We don’t have bacteria just in our guts,” she noted. “It may be a combination of strains or a combination of bacteria.”
The authors, Dr. Spergel, and Dr. Cassell reported no relevant financial relationships.
FROM PEDIATRICS
Treat or refer? New primary care flow diagrams for allergy patients
Most patients with allergy problems first see PCPs, not allergists, the authors write in Allergy. The new flow diagrams help PCPs treat anaphylaxis, asthma, drug allergy, food allergy, and urticaria.
“The European Academy of Allergy and Clinical Immunology established the Logogram Task Force to create a set of simple flow diagrams to assist allergy nonspecialist, generalist, and primary care teams in the diagnosis of five common allergic diseases encountered in primary care,” lead author Dermot Ryan, MB BCh, BAO, FRGCP, of the University of Edinburgh told this news organization.
“The source documents were mainstream guidelines coupled with ancillary literature,” he added in an email. “A multi-disciplinary taskforce ... distilled these guidelines into accessible, comprehensible, usable, and context-specific flow diagrams.”
The flow diagrams developed in Europe can be used by providers in the United States and elsewhere
“These diagrams are consistent with practices in the U.S.,” Christina E. Ciaccio, MD, an associate professor of pediatrics and the section chief of pediatric allergy and immunology at the University of Chicago Medicine, said in an email. “They will prove helpful to PCPs in the U.S. and elsewhere, particularly to young physicians new to practice.
“Treating allergies is part of the ‘bread-and-butter’ practice of primary care physicians in the U.S.,” Dr. Ciaccio, who was not involved in developing the flow diagrams, explained. “Up to 30% of Americans are atopic, and the vast majority seek treatment advice from their PCP first.”
The flow diagrams can help providers in developing countries, where allergic diseases are common, provide the best patient care possible, she said.
At some point, a PCP may need to think beyond flow diagrams and refer the patient to an allergist
“If the treatment plan for a patient falls outside first- or second-line medications, or if a diagnosis is unclear with preliminary testing, a PCP may reach out to an allergy/immunology specialist to assist in providing care,” Dr. Ciaccio advised. “Allergists may provide treatment options, such as immunotherapy, that the PCP does not offer. PCPs also often reach out to allergy team members for help with patients whose allergies are not ‘run-of-the-mill.’
“The flow diagrams are complex and may not be practical in the middle of a busy clinic,” she cautioned. “However, when a patient comes into a primary care clinic with an atypical presentation of an allergic disease, the diagrams are likely to help a physician feel confident that an allergist is the right physician for consultation.”
Patricia Lynne Lugar, MD, an associate professor of medicine in pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C., noted that providers in the U.S. can use the flow diagrams because the definitions, differential diagnosis, and treatments for the conditions they cover are similar.
“The flow diagrams are comprehensive, and they attempt to condense a great deal of information into summary points. They are very useful in the U.S., and not just for generalists,” Dr. Lugar, who also was not involved in the project, said. “Even emergency rooms would benefit from these flow diagrams, especially regarding the recognition of symptoms and differential diagnosis.”
Asthma and seasonal and environmental allergies are often managed by PCPs, and the flow diagrams would help them decide when to refer their patients to an allergist, she added in an email.
Dr. Lugar advises PCPs to “recognize the symptoms of an allergic condition, offer treatment based on confidence the diagnosis is correct, and offer a referral for testing to confirm the allergy.
“Because 50% or more of asthmatics are allergic, all asthmatics should be offered an allergy evaluation to determine their allergies and avoid exacerbating the asthma,” she added. “I do not see the flow diagrams as comprehensive enough to manage chronic urticaria, asthma, venom allergy, and drug allergy.”
With food allergy, environmental allergy, venom allergy, or anaphylaxis, “allergists are experts at considering the differential diagnosis and providing the next steps in the diagnostic workup,” Dr. Lugar said. “Allergists can also provide special treatments, such as allergen-specific immunotherapy or desensitization.”
The flow diagrams guide nonspecialists in diagnosis and treatment of their patients with allergy, with supplementary information as needed. The diagrams recommend referral to a specialist when appropriate, as in cases of anaphylaxis, or chronic urticaria.
The task force was funded by EAACI. Dr. Ryan and several other authors report financial relationships with pharmaceutical companies. Dr. Ciaccio and Dr. Lugar report no such relationships.
A version of this article first appeared on Medscape.com.
Most patients with allergy problems first see PCPs, not allergists, the authors write in Allergy. The new flow diagrams help PCPs treat anaphylaxis, asthma, drug allergy, food allergy, and urticaria.
“The European Academy of Allergy and Clinical Immunology established the Logogram Task Force to create a set of simple flow diagrams to assist allergy nonspecialist, generalist, and primary care teams in the diagnosis of five common allergic diseases encountered in primary care,” lead author Dermot Ryan, MB BCh, BAO, FRGCP, of the University of Edinburgh told this news organization.
“The source documents were mainstream guidelines coupled with ancillary literature,” he added in an email. “A multi-disciplinary taskforce ... distilled these guidelines into accessible, comprehensible, usable, and context-specific flow diagrams.”
The flow diagrams developed in Europe can be used by providers in the United States and elsewhere
“These diagrams are consistent with practices in the U.S.,” Christina E. Ciaccio, MD, an associate professor of pediatrics and the section chief of pediatric allergy and immunology at the University of Chicago Medicine, said in an email. “They will prove helpful to PCPs in the U.S. and elsewhere, particularly to young physicians new to practice.
“Treating allergies is part of the ‘bread-and-butter’ practice of primary care physicians in the U.S.,” Dr. Ciaccio, who was not involved in developing the flow diagrams, explained. “Up to 30% of Americans are atopic, and the vast majority seek treatment advice from their PCP first.”
The flow diagrams can help providers in developing countries, where allergic diseases are common, provide the best patient care possible, she said.
At some point, a PCP may need to think beyond flow diagrams and refer the patient to an allergist
“If the treatment plan for a patient falls outside first- or second-line medications, or if a diagnosis is unclear with preliminary testing, a PCP may reach out to an allergy/immunology specialist to assist in providing care,” Dr. Ciaccio advised. “Allergists may provide treatment options, such as immunotherapy, that the PCP does not offer. PCPs also often reach out to allergy team members for help with patients whose allergies are not ‘run-of-the-mill.’
“The flow diagrams are complex and may not be practical in the middle of a busy clinic,” she cautioned. “However, when a patient comes into a primary care clinic with an atypical presentation of an allergic disease, the diagrams are likely to help a physician feel confident that an allergist is the right physician for consultation.”
Patricia Lynne Lugar, MD, an associate professor of medicine in pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C., noted that providers in the U.S. can use the flow diagrams because the definitions, differential diagnosis, and treatments for the conditions they cover are similar.
“The flow diagrams are comprehensive, and they attempt to condense a great deal of information into summary points. They are very useful in the U.S., and not just for generalists,” Dr. Lugar, who also was not involved in the project, said. “Even emergency rooms would benefit from these flow diagrams, especially regarding the recognition of symptoms and differential diagnosis.”
Asthma and seasonal and environmental allergies are often managed by PCPs, and the flow diagrams would help them decide when to refer their patients to an allergist, she added in an email.
Dr. Lugar advises PCPs to “recognize the symptoms of an allergic condition, offer treatment based on confidence the diagnosis is correct, and offer a referral for testing to confirm the allergy.
“Because 50% or more of asthmatics are allergic, all asthmatics should be offered an allergy evaluation to determine their allergies and avoid exacerbating the asthma,” she added. “I do not see the flow diagrams as comprehensive enough to manage chronic urticaria, asthma, venom allergy, and drug allergy.”
With food allergy, environmental allergy, venom allergy, or anaphylaxis, “allergists are experts at considering the differential diagnosis and providing the next steps in the diagnostic workup,” Dr. Lugar said. “Allergists can also provide special treatments, such as allergen-specific immunotherapy or desensitization.”
The flow diagrams guide nonspecialists in diagnosis and treatment of their patients with allergy, with supplementary information as needed. The diagrams recommend referral to a specialist when appropriate, as in cases of anaphylaxis, or chronic urticaria.
The task force was funded by EAACI. Dr. Ryan and several other authors report financial relationships with pharmaceutical companies. Dr. Ciaccio and Dr. Lugar report no such relationships.
A version of this article first appeared on Medscape.com.
Most patients with allergy problems first see PCPs, not allergists, the authors write in Allergy. The new flow diagrams help PCPs treat anaphylaxis, asthma, drug allergy, food allergy, and urticaria.
“The European Academy of Allergy and Clinical Immunology established the Logogram Task Force to create a set of simple flow diagrams to assist allergy nonspecialist, generalist, and primary care teams in the diagnosis of five common allergic diseases encountered in primary care,” lead author Dermot Ryan, MB BCh, BAO, FRGCP, of the University of Edinburgh told this news organization.
“The source documents were mainstream guidelines coupled with ancillary literature,” he added in an email. “A multi-disciplinary taskforce ... distilled these guidelines into accessible, comprehensible, usable, and context-specific flow diagrams.”
The flow diagrams developed in Europe can be used by providers in the United States and elsewhere
“These diagrams are consistent with practices in the U.S.,” Christina E. Ciaccio, MD, an associate professor of pediatrics and the section chief of pediatric allergy and immunology at the University of Chicago Medicine, said in an email. “They will prove helpful to PCPs in the U.S. and elsewhere, particularly to young physicians new to practice.
“Treating allergies is part of the ‘bread-and-butter’ practice of primary care physicians in the U.S.,” Dr. Ciaccio, who was not involved in developing the flow diagrams, explained. “Up to 30% of Americans are atopic, and the vast majority seek treatment advice from their PCP first.”
The flow diagrams can help providers in developing countries, where allergic diseases are common, provide the best patient care possible, she said.
At some point, a PCP may need to think beyond flow diagrams and refer the patient to an allergist
“If the treatment plan for a patient falls outside first- or second-line medications, or if a diagnosis is unclear with preliminary testing, a PCP may reach out to an allergy/immunology specialist to assist in providing care,” Dr. Ciaccio advised. “Allergists may provide treatment options, such as immunotherapy, that the PCP does not offer. PCPs also often reach out to allergy team members for help with patients whose allergies are not ‘run-of-the-mill.’
“The flow diagrams are complex and may not be practical in the middle of a busy clinic,” she cautioned. “However, when a patient comes into a primary care clinic with an atypical presentation of an allergic disease, the diagrams are likely to help a physician feel confident that an allergist is the right physician for consultation.”
Patricia Lynne Lugar, MD, an associate professor of medicine in pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C., noted that providers in the U.S. can use the flow diagrams because the definitions, differential diagnosis, and treatments for the conditions they cover are similar.
“The flow diagrams are comprehensive, and they attempt to condense a great deal of information into summary points. They are very useful in the U.S., and not just for generalists,” Dr. Lugar, who also was not involved in the project, said. “Even emergency rooms would benefit from these flow diagrams, especially regarding the recognition of symptoms and differential diagnosis.”
Asthma and seasonal and environmental allergies are often managed by PCPs, and the flow diagrams would help them decide when to refer their patients to an allergist, she added in an email.
Dr. Lugar advises PCPs to “recognize the symptoms of an allergic condition, offer treatment based on confidence the diagnosis is correct, and offer a referral for testing to confirm the allergy.
“Because 50% or more of asthmatics are allergic, all asthmatics should be offered an allergy evaluation to determine their allergies and avoid exacerbating the asthma,” she added. “I do not see the flow diagrams as comprehensive enough to manage chronic urticaria, asthma, venom allergy, and drug allergy.”
With food allergy, environmental allergy, venom allergy, or anaphylaxis, “allergists are experts at considering the differential diagnosis and providing the next steps in the diagnostic workup,” Dr. Lugar said. “Allergists can also provide special treatments, such as allergen-specific immunotherapy or desensitization.”
The flow diagrams guide nonspecialists in diagnosis and treatment of their patients with allergy, with supplementary information as needed. The diagrams recommend referral to a specialist when appropriate, as in cases of anaphylaxis, or chronic urticaria.
The task force was funded by EAACI. Dr. Ryan and several other authors report financial relationships with pharmaceutical companies. Dr. Ciaccio and Dr. Lugar report no such relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY