User login
Blastomycosislike Pyoderma: Verrucous Hyperpigmented Plaques on the Pretibial Shins
To the Editor:
Blastomycosislike pyoderma (BLP), also commonly referred to as pyoderma vegetans, is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 It is characterized by a collection of neutrophilic abscesses with pseudoepitheliomatous hyperplasia that coalesce into crusted plaques.
A 15-year-old adolescent girl with a history of type 1 diabetes mellitus was admitted for diabetic ketoacidosis. The patient presented with bilateral pretibial lesions of 6 years’ duration that developed after swimming in a pool following reported trauma to the site. These pruritic plaques had grown slowly and were occasionally tender. Of note, with episodes of hyperglycemia, the lesions developed purulent drainage.
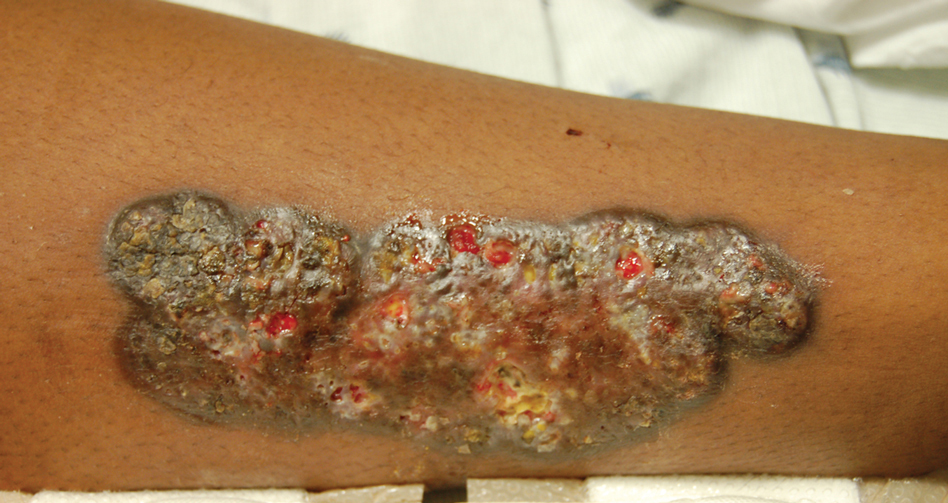
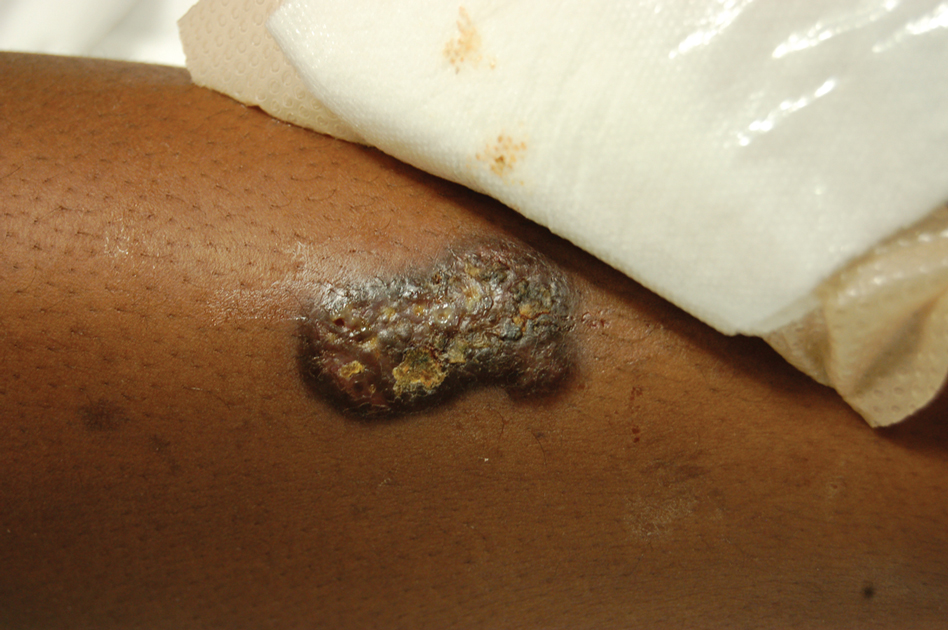
Upon admission to the hospital and subsequent dermatology consultation, physical examination revealed the right pretibial shin had a 15×5-cm, gray-brown, hyperpigmented, verrucous, tender plaque with purulent drainage and overlying crust (Figure 1). The left pretibial shin had a similar smaller lesion (Figure 2). Laboratory test results were notable for a white blood cell count of 41.84 cells/µL (reference range, 3.8–10.5 cells/µL), blood glucose level of 586 mg/dL (reference range, 70–99 mg/dL), and hemoglobin A1c of 11.7% (reference range, 4.0%–5.6%). A biopsy specimen from the right pretibial shin was stained with hematoxylin and eosin for dermatopathologic evaluation as well as sent for tissue culture. Tissue and wound cultures grew Staphylococcus aureus and group B Streptococcus with no fungal or acid-fast bacilli growth.
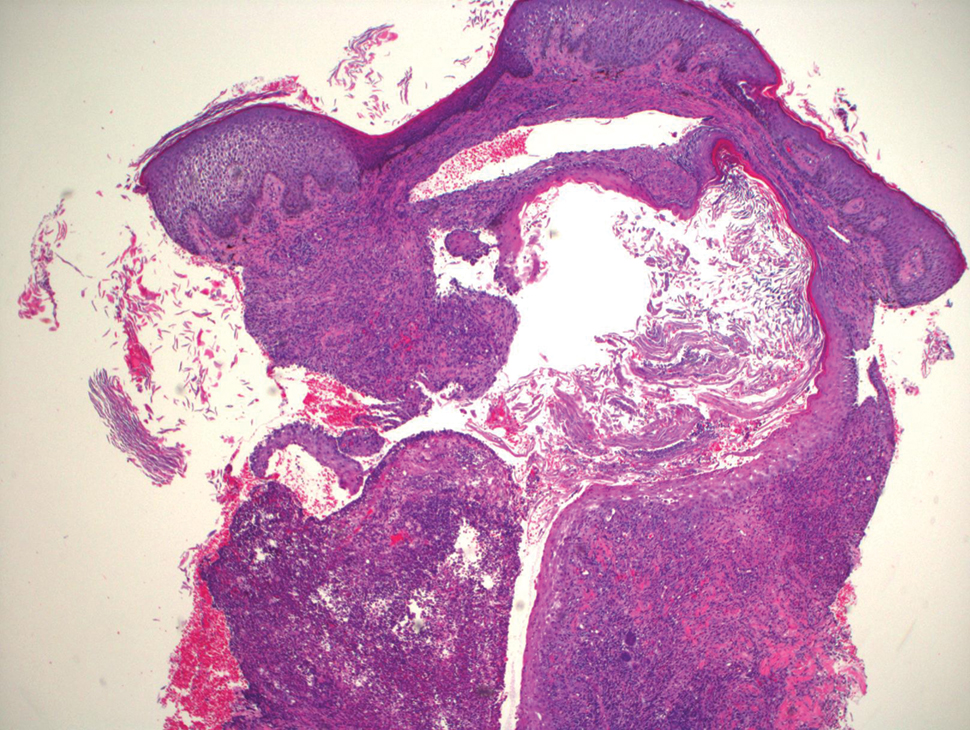
Blood cultures were negative for bacteria. Results of radiographic imaging were negative for osteomyelitis. Biopsy specimens from the right pretibial plaque showed a markedly inflamed, ruptured follicular unit with a dense dermal lympho-neutrophilic infiltrate and overlying pseudoepitheliomatous hyperplasia (Figure 3). Periodic acid–Schiff, Gomori methenamine-silver, acid-fast bacilli, and Giemsa stains were negative for organisms. No granules consistent with a Splendore-Hoeppli phenomenon were observed. These observations were consistent with a diagnosis of BLP.
Blastomycosislike pyoderma is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 Pediatric cases also are uncommon. Blastomycosislike pyoderma most commonly is caused by infection with S aureus or group A streptococci, but several other organisms have been implicated.2 Clinically, BLP is similar to cutaneous botryomycosis, as both are caused by similar organisms.3 However, while BLP is limited to the skin, botryomycosis may involve visceral organs.
Blastomycosislike pyoderma typically presents as verrucous, hyperkeratotic, purulent plaques with raised borders. It most commonly occurs on the face, scalp, axillae, trunk, and distal extremities. Predisposing factors include immunosuppressed states such as poor nutrition, HIV, malignancy, alcoholism, and diabetes mellitus.3,4 Hyperglycemia is thought to suppress helper T cell (TH1)–dependent immunity, which may explain why our patient’s lesions worsened with hyperglycemic episodes.5Histopathology revealed pseudoepitheliomatous hyperplasia with neutrophilic abscesses.1 The distinguishing feature between botryomycosis and BLP is the development of grains known as the Splendore-Hoeppli phenomenon in botryomycosis.6 The grains are eosinophilic and contain the causative infectious agent. The presence of these grains is consistent with botryomycosis but is not pathognomonic, as it also can be found in several bacterial, fungal, and parasitic infections.3,6
The differential diagnosis of BLP includes atypical mycobacterial infection, pyoderma gangrenosum, fungal infection, and tuberculosis verrucosa cutis.7
Although BLP is caused by bacteria, response to systemic antibiotics is variable. Other treatment modalities include dapsone, systemic and intralesional corticosteroids, retinoids, debridement, CO2 laser, and excision.6,8 Lesions typically start out localized, but it is not uncommon for them to spread to distal or vulnerable tissue, such as sites of trauma or inflammation. Our patient was started on oral trimethoprim-sulfamethoxazole and showed improvement, but she worsened with subsequent hyperglycemic episodes when antibiotics were discontinued.
1. Adis¸en E, Tezel F, Gürer MA. Pyoderma vegetans: a case for discussion. Acta Derm Venereol. 2009;89:186-188.
2. Scuderi S, O’Brien B, Robertson I, et al. Heterogeneity of blastomycosis-like pyoderma: a selection of cases from the last 35 years. Australas J Dermatol. 2017;58:139-141.
3. Marschalko, M. Pyoderma vegetans: report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol Alp Pannonica Adriat. 1995;4:55-59.
4. Cerullo L, Zussman J, Young L. An unusual presentation of blastomycosislike pyoderma (pyoderma vegetans) and a review of the literature. Cutis. 2009;84:201-204.
5. Tanaka Y. Immunosuppressive mechanisms in diabetes mellitus [in Japanese]. Nihon Rinsho. 2008;66:2233-2237.
6. Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol. 2008;35:979-988.
7. Lee YS, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
8. Kobraei KB, Wesson SK. Blastomycosis-like pyoderma: response to systemic retinoid therapy. Int J Dermatol. 2010;49:1336-1338.
To the Editor:
Blastomycosislike pyoderma (BLP), also commonly referred to as pyoderma vegetans, is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 It is characterized by a collection of neutrophilic abscesses with pseudoepitheliomatous hyperplasia that coalesce into crusted plaques.
A 15-year-old adolescent girl with a history of type 1 diabetes mellitus was admitted for diabetic ketoacidosis. The patient presented with bilateral pretibial lesions of 6 years’ duration that developed after swimming in a pool following reported trauma to the site. These pruritic plaques had grown slowly and were occasionally tender. Of note, with episodes of hyperglycemia, the lesions developed purulent drainage.
Upon admission to the hospital and subsequent dermatology consultation, physical examination revealed the right pretibial shin had a 15×5-cm, gray-brown, hyperpigmented, verrucous, tender plaque with purulent drainage and overlying crust (Figure 1). The left pretibial shin had a similar smaller lesion (Figure 2). Laboratory test results were notable for a white blood cell count of 41.84 cells/µL (reference range, 3.8–10.5 cells/µL), blood glucose level of 586 mg/dL (reference range, 70–99 mg/dL), and hemoglobin A1c of 11.7% (reference range, 4.0%–5.6%). A biopsy specimen from the right pretibial shin was stained with hematoxylin and eosin for dermatopathologic evaluation as well as sent for tissue culture. Tissue and wound cultures grew Staphylococcus aureus and group B Streptococcus with no fungal or acid-fast bacilli growth.


Blood cultures were negative for bacteria. Results of radiographic imaging were negative for osteomyelitis. Biopsy specimens from the right pretibial plaque showed a markedly inflamed, ruptured follicular unit with a dense dermal lympho-neutrophilic infiltrate and overlying pseudoepitheliomatous hyperplasia (Figure 3). Periodic acid–Schiff, Gomori methenamine-silver, acid-fast bacilli, and Giemsa stains were negative for organisms. No granules consistent with a Splendore-Hoeppli phenomenon were observed. These observations were consistent with a diagnosis of BLP.

Blastomycosislike pyoderma is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 Pediatric cases also are uncommon. Blastomycosislike pyoderma most commonly is caused by infection with S aureus or group A streptococci, but several other organisms have been implicated.2 Clinically, BLP is similar to cutaneous botryomycosis, as both are caused by similar organisms.3 However, while BLP is limited to the skin, botryomycosis may involve visceral organs.
Blastomycosislike pyoderma typically presents as verrucous, hyperkeratotic, purulent plaques with raised borders. It most commonly occurs on the face, scalp, axillae, trunk, and distal extremities. Predisposing factors include immunosuppressed states such as poor nutrition, HIV, malignancy, alcoholism, and diabetes mellitus.3,4 Hyperglycemia is thought to suppress helper T cell (TH1)–dependent immunity, which may explain why our patient’s lesions worsened with hyperglycemic episodes.5Histopathology revealed pseudoepitheliomatous hyperplasia with neutrophilic abscesses.1 The distinguishing feature between botryomycosis and BLP is the development of grains known as the Splendore-Hoeppli phenomenon in botryomycosis.6 The grains are eosinophilic and contain the causative infectious agent. The presence of these grains is consistent with botryomycosis but is not pathognomonic, as it also can be found in several bacterial, fungal, and parasitic infections.3,6
The differential diagnosis of BLP includes atypical mycobacterial infection, pyoderma gangrenosum, fungal infection, and tuberculosis verrucosa cutis.7
Although BLP is caused by bacteria, response to systemic antibiotics is variable. Other treatment modalities include dapsone, systemic and intralesional corticosteroids, retinoids, debridement, CO2 laser, and excision.6,8 Lesions typically start out localized, but it is not uncommon for them to spread to distal or vulnerable tissue, such as sites of trauma or inflammation. Our patient was started on oral trimethoprim-sulfamethoxazole and showed improvement, but she worsened with subsequent hyperglycemic episodes when antibiotics were discontinued.
To the Editor:
Blastomycosislike pyoderma (BLP), also commonly referred to as pyoderma vegetans, is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 It is characterized by a collection of neutrophilic abscesses with pseudoepitheliomatous hyperplasia that coalesce into crusted plaques.
A 15-year-old adolescent girl with a history of type 1 diabetes mellitus was admitted for diabetic ketoacidosis. The patient presented with bilateral pretibial lesions of 6 years’ duration that developed after swimming in a pool following reported trauma to the site. These pruritic plaques had grown slowly and were occasionally tender. Of note, with episodes of hyperglycemia, the lesions developed purulent drainage.
Upon admission to the hospital and subsequent dermatology consultation, physical examination revealed the right pretibial shin had a 15×5-cm, gray-brown, hyperpigmented, verrucous, tender plaque with purulent drainage and overlying crust (Figure 1). The left pretibial shin had a similar smaller lesion (Figure 2). Laboratory test results were notable for a white blood cell count of 41.84 cells/µL (reference range, 3.8–10.5 cells/µL), blood glucose level of 586 mg/dL (reference range, 70–99 mg/dL), and hemoglobin A1c of 11.7% (reference range, 4.0%–5.6%). A biopsy specimen from the right pretibial shin was stained with hematoxylin and eosin for dermatopathologic evaluation as well as sent for tissue culture. Tissue and wound cultures grew Staphylococcus aureus and group B Streptococcus with no fungal or acid-fast bacilli growth.


Blood cultures were negative for bacteria. Results of radiographic imaging were negative for osteomyelitis. Biopsy specimens from the right pretibial plaque showed a markedly inflamed, ruptured follicular unit with a dense dermal lympho-neutrophilic infiltrate and overlying pseudoepitheliomatous hyperplasia (Figure 3). Periodic acid–Schiff, Gomori methenamine-silver, acid-fast bacilli, and Giemsa stains were negative for organisms. No granules consistent with a Splendore-Hoeppli phenomenon were observed. These observations were consistent with a diagnosis of BLP.

Blastomycosislike pyoderma is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 Pediatric cases also are uncommon. Blastomycosislike pyoderma most commonly is caused by infection with S aureus or group A streptococci, but several other organisms have been implicated.2 Clinically, BLP is similar to cutaneous botryomycosis, as both are caused by similar organisms.3 However, while BLP is limited to the skin, botryomycosis may involve visceral organs.
Blastomycosislike pyoderma typically presents as verrucous, hyperkeratotic, purulent plaques with raised borders. It most commonly occurs on the face, scalp, axillae, trunk, and distal extremities. Predisposing factors include immunosuppressed states such as poor nutrition, HIV, malignancy, alcoholism, and diabetes mellitus.3,4 Hyperglycemia is thought to suppress helper T cell (TH1)–dependent immunity, which may explain why our patient’s lesions worsened with hyperglycemic episodes.5Histopathology revealed pseudoepitheliomatous hyperplasia with neutrophilic abscesses.1 The distinguishing feature between botryomycosis and BLP is the development of grains known as the Splendore-Hoeppli phenomenon in botryomycosis.6 The grains are eosinophilic and contain the causative infectious agent. The presence of these grains is consistent with botryomycosis but is not pathognomonic, as it also can be found in several bacterial, fungal, and parasitic infections.3,6
The differential diagnosis of BLP includes atypical mycobacterial infection, pyoderma gangrenosum, fungal infection, and tuberculosis verrucosa cutis.7
Although BLP is caused by bacteria, response to systemic antibiotics is variable. Other treatment modalities include dapsone, systemic and intralesional corticosteroids, retinoids, debridement, CO2 laser, and excision.6,8 Lesions typically start out localized, but it is not uncommon for them to spread to distal or vulnerable tissue, such as sites of trauma or inflammation. Our patient was started on oral trimethoprim-sulfamethoxazole and showed improvement, but she worsened with subsequent hyperglycemic episodes when antibiotics were discontinued.
1. Adis¸en E, Tezel F, Gürer MA. Pyoderma vegetans: a case for discussion. Acta Derm Venereol. 2009;89:186-188.
2. Scuderi S, O’Brien B, Robertson I, et al. Heterogeneity of blastomycosis-like pyoderma: a selection of cases from the last 35 years. Australas J Dermatol. 2017;58:139-141.
3. Marschalko, M. Pyoderma vegetans: report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol Alp Pannonica Adriat. 1995;4:55-59.
4. Cerullo L, Zussman J, Young L. An unusual presentation of blastomycosislike pyoderma (pyoderma vegetans) and a review of the literature. Cutis. 2009;84:201-204.
5. Tanaka Y. Immunosuppressive mechanisms in diabetes mellitus [in Japanese]. Nihon Rinsho. 2008;66:2233-2237.
6. Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol. 2008;35:979-988.
7. Lee YS, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
8. Kobraei KB, Wesson SK. Blastomycosis-like pyoderma: response to systemic retinoid therapy. Int J Dermatol. 2010;49:1336-1338.
1. Adis¸en E, Tezel F, Gürer MA. Pyoderma vegetans: a case for discussion. Acta Derm Venereol. 2009;89:186-188.
2. Scuderi S, O’Brien B, Robertson I, et al. Heterogeneity of blastomycosis-like pyoderma: a selection of cases from the last 35 years. Australas J Dermatol. 2017;58:139-141.
3. Marschalko, M. Pyoderma vegetans: report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol Alp Pannonica Adriat. 1995;4:55-59.
4. Cerullo L, Zussman J, Young L. An unusual presentation of blastomycosislike pyoderma (pyoderma vegetans) and a review of the literature. Cutis. 2009;84:201-204.
5. Tanaka Y. Immunosuppressive mechanisms in diabetes mellitus [in Japanese]. Nihon Rinsho. 2008;66:2233-2237.
6. Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol. 2008;35:979-988.
7. Lee YS, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
8. Kobraei KB, Wesson SK. Blastomycosis-like pyoderma: response to systemic retinoid therapy. Int J Dermatol. 2010;49:1336-1338.
Practice Points
- Blastomycosislike pyoderma is a rare condition secondary to bacterial infection, but as the name suggests, it also can resemble cutaneous blastomycosis.
- Blastomycosislike pyoderma most commonly occurs in immunocompromised patients.
- The most common histologic findings include suppurative and neutrophilic inflammation with pseudoepitheliomatous hyperplasia.
COVID cases spike as questions remain about Omicron’s threat
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
HIV: FDA stops all islatravir oral and implant trials
, the investigational drug’s developer, Merck, announced in a press release.
Investigational new drug applications were halted for the oral and implant formulations of islatravir, a nucleoside reverse transcriptase translocation inhibitor, for pre-exposure prophylaxis (PrEP); the injectable formulation of islatravir for treatment and prophylaxis; and the oral doravirine/islatravir (DOR/ISL) once-daily treatment, the company announced.
The FDA’s hold followed observations that total lymphocyte and T-cell counts had dropped in some participants receiving islatravir in clinical studies.
The trials have dealt a major setback to Merck’s HIV program momentum: Thirteen trials are now on hold (six on partial hold and seven on full hold). Seven of the trials were in phase 3. But primarily the news is disappointing for patients looking for options with the confounding disease.
Tristan Barber, MD, an HIV consultant with Royal Free London National Health Service Foundation Trust, told this news organization that “the hold on these studies is a blow for those hoping for longer-acting therapies for HIV treatment and prevention. Islatravir and [investigational drug] MK-8507 were being explored in oral and other formulations and potentially would offer a non-integrase, two-drug option, increasing options for people with HIV. Whilst we don’t know the clinical significance of these CD4 drops, [Merck] made the correct decision in pausing these studies until the data is clearer.”
Merck announced in November that it had stopped dosing in the phase 2 IMAGINE-DR clinical trial of islatravir in combination with MK-8507. MK-8507 and islatravir, alone and combined, are investigational and not approved for use.
In that trial as well, decreases were observed in total lymphocyte and T-cell counts in study participants randomly assigned to receive the combination. A review by the external Data Monitoring Committee determined that the drop was related to treatment with the combination.
“We are grateful to the participants and the study investigators for their ongoing contributions to this research,” Joan Butterton, MD, vice president of infectious diseases in Global Clinical Development at Merck Research Laboratories, said in a statement. “Merck continues to investigate the potential of islatravir and nucleoside reverse transcriptase translocation inhibitors and remains committed to helping to address unmet needs in HIV treatment and prevention.”
In light of the hold, no new studies using islatravir may be initiated. People currently receiving islatravir as part of the studies for PrEP, as well as injectable islatravir for treatment and prophylaxis, will no longer receive the study drug, and T-cell and lymphocyte counts will be monitored for recovery.
Those participating in the PrEP studies will be offered approved, once-daily, oral PrEP and those in studies of DOR/ISL who already started treatment will continue to receive study medication under a partial clinical hold.
A full list of the trials that have been placed on full or partial clinical holds can be found in the press release.
In an interview with this news organization, Monica Gandhi, MD, MPH, director of University of California, San Francisco’s Gladstone Center for AIDS Research, described the news of the islatravir trial holds as “very disappointing.”
“There were high hopes for this drug,” she said, adding that the hope was it would be paired with Gilead’s lenacapavir (another long-acting agent) for treatment and be able to give a once-weekly option for HIV treatment.
Lenacapavir is Gilead’s potential first-in-class, long-acting HIV-1 capsid inhibitor in development for treatment and prevention of HIV.
“Moreover,” she said, “additional hope was that, because of [islatravir’s] long half-life, it could be used as a monthly medication for pre-exposure prophylaxis.”
Gilead and Merck have decided to stop all dosing of participants in the phase 2 clinical trial evaluating an oral, weekly combination treatment of islatravir and lenacapavir in people living with HIV who are virologically suppressed on antiretroviral therapy, according to Merck’s press release.
Participants in that trial will stop taking the study drug and restart their previous antiretroviral regimen. According to the press release, both companies are considering whether a different dosing of islatravir combined with lenacapavir may become a once-weekly oral therapy option for people living with HIV.
Neither Merck nor Gilead representatives responded to request for comment by publication time.
Dr. Barber reported conference support, speaker fees, and advisory board honoraria from Gilead, Janssen, Merck, Roche, Thera, and ViiV and research/educational grants from Gilead, Roche, and ViiV. Dr. Gandhi has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the investigational drug’s developer, Merck, announced in a press release.
Investigational new drug applications were halted for the oral and implant formulations of islatravir, a nucleoside reverse transcriptase translocation inhibitor, for pre-exposure prophylaxis (PrEP); the injectable formulation of islatravir for treatment and prophylaxis; and the oral doravirine/islatravir (DOR/ISL) once-daily treatment, the company announced.
The FDA’s hold followed observations that total lymphocyte and T-cell counts had dropped in some participants receiving islatravir in clinical studies.
The trials have dealt a major setback to Merck’s HIV program momentum: Thirteen trials are now on hold (six on partial hold and seven on full hold). Seven of the trials were in phase 3. But primarily the news is disappointing for patients looking for options with the confounding disease.
Tristan Barber, MD, an HIV consultant with Royal Free London National Health Service Foundation Trust, told this news organization that “the hold on these studies is a blow for those hoping for longer-acting therapies for HIV treatment and prevention. Islatravir and [investigational drug] MK-8507 were being explored in oral and other formulations and potentially would offer a non-integrase, two-drug option, increasing options for people with HIV. Whilst we don’t know the clinical significance of these CD4 drops, [Merck] made the correct decision in pausing these studies until the data is clearer.”
Merck announced in November that it had stopped dosing in the phase 2 IMAGINE-DR clinical trial of islatravir in combination with MK-8507. MK-8507 and islatravir, alone and combined, are investigational and not approved for use.
In that trial as well, decreases were observed in total lymphocyte and T-cell counts in study participants randomly assigned to receive the combination. A review by the external Data Monitoring Committee determined that the drop was related to treatment with the combination.
“We are grateful to the participants and the study investigators for their ongoing contributions to this research,” Joan Butterton, MD, vice president of infectious diseases in Global Clinical Development at Merck Research Laboratories, said in a statement. “Merck continues to investigate the potential of islatravir and nucleoside reverse transcriptase translocation inhibitors and remains committed to helping to address unmet needs in HIV treatment and prevention.”
In light of the hold, no new studies using islatravir may be initiated. People currently receiving islatravir as part of the studies for PrEP, as well as injectable islatravir for treatment and prophylaxis, will no longer receive the study drug, and T-cell and lymphocyte counts will be monitored for recovery.
Those participating in the PrEP studies will be offered approved, once-daily, oral PrEP and those in studies of DOR/ISL who already started treatment will continue to receive study medication under a partial clinical hold.
A full list of the trials that have been placed on full or partial clinical holds can be found in the press release.
In an interview with this news organization, Monica Gandhi, MD, MPH, director of University of California, San Francisco’s Gladstone Center for AIDS Research, described the news of the islatravir trial holds as “very disappointing.”
“There were high hopes for this drug,” she said, adding that the hope was it would be paired with Gilead’s lenacapavir (another long-acting agent) for treatment and be able to give a once-weekly option for HIV treatment.
Lenacapavir is Gilead’s potential first-in-class, long-acting HIV-1 capsid inhibitor in development for treatment and prevention of HIV.
“Moreover,” she said, “additional hope was that, because of [islatravir’s] long half-life, it could be used as a monthly medication for pre-exposure prophylaxis.”
Gilead and Merck have decided to stop all dosing of participants in the phase 2 clinical trial evaluating an oral, weekly combination treatment of islatravir and lenacapavir in people living with HIV who are virologically suppressed on antiretroviral therapy, according to Merck’s press release.
Participants in that trial will stop taking the study drug and restart their previous antiretroviral regimen. According to the press release, both companies are considering whether a different dosing of islatravir combined with lenacapavir may become a once-weekly oral therapy option for people living with HIV.
Neither Merck nor Gilead representatives responded to request for comment by publication time.
Dr. Barber reported conference support, speaker fees, and advisory board honoraria from Gilead, Janssen, Merck, Roche, Thera, and ViiV and research/educational grants from Gilead, Roche, and ViiV. Dr. Gandhi has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the investigational drug’s developer, Merck, announced in a press release.
Investigational new drug applications were halted for the oral and implant formulations of islatravir, a nucleoside reverse transcriptase translocation inhibitor, for pre-exposure prophylaxis (PrEP); the injectable formulation of islatravir for treatment and prophylaxis; and the oral doravirine/islatravir (DOR/ISL) once-daily treatment, the company announced.
The FDA’s hold followed observations that total lymphocyte and T-cell counts had dropped in some participants receiving islatravir in clinical studies.
The trials have dealt a major setback to Merck’s HIV program momentum: Thirteen trials are now on hold (six on partial hold and seven on full hold). Seven of the trials were in phase 3. But primarily the news is disappointing for patients looking for options with the confounding disease.
Tristan Barber, MD, an HIV consultant with Royal Free London National Health Service Foundation Trust, told this news organization that “the hold on these studies is a blow for those hoping for longer-acting therapies for HIV treatment and prevention. Islatravir and [investigational drug] MK-8507 were being explored in oral and other formulations and potentially would offer a non-integrase, two-drug option, increasing options for people with HIV. Whilst we don’t know the clinical significance of these CD4 drops, [Merck] made the correct decision in pausing these studies until the data is clearer.”
Merck announced in November that it had stopped dosing in the phase 2 IMAGINE-DR clinical trial of islatravir in combination with MK-8507. MK-8507 and islatravir, alone and combined, are investigational and not approved for use.
In that trial as well, decreases were observed in total lymphocyte and T-cell counts in study participants randomly assigned to receive the combination. A review by the external Data Monitoring Committee determined that the drop was related to treatment with the combination.
“We are grateful to the participants and the study investigators for their ongoing contributions to this research,” Joan Butterton, MD, vice president of infectious diseases in Global Clinical Development at Merck Research Laboratories, said in a statement. “Merck continues to investigate the potential of islatravir and nucleoside reverse transcriptase translocation inhibitors and remains committed to helping to address unmet needs in HIV treatment and prevention.”
In light of the hold, no new studies using islatravir may be initiated. People currently receiving islatravir as part of the studies for PrEP, as well as injectable islatravir for treatment and prophylaxis, will no longer receive the study drug, and T-cell and lymphocyte counts will be monitored for recovery.
Those participating in the PrEP studies will be offered approved, once-daily, oral PrEP and those in studies of DOR/ISL who already started treatment will continue to receive study medication under a partial clinical hold.
A full list of the trials that have been placed on full or partial clinical holds can be found in the press release.
In an interview with this news organization, Monica Gandhi, MD, MPH, director of University of California, San Francisco’s Gladstone Center for AIDS Research, described the news of the islatravir trial holds as “very disappointing.”
“There were high hopes for this drug,” she said, adding that the hope was it would be paired with Gilead’s lenacapavir (another long-acting agent) for treatment and be able to give a once-weekly option for HIV treatment.
Lenacapavir is Gilead’s potential first-in-class, long-acting HIV-1 capsid inhibitor in development for treatment and prevention of HIV.
“Moreover,” she said, “additional hope was that, because of [islatravir’s] long half-life, it could be used as a monthly medication for pre-exposure prophylaxis.”
Gilead and Merck have decided to stop all dosing of participants in the phase 2 clinical trial evaluating an oral, weekly combination treatment of islatravir and lenacapavir in people living with HIV who are virologically suppressed on antiretroviral therapy, according to Merck’s press release.
Participants in that trial will stop taking the study drug and restart their previous antiretroviral regimen. According to the press release, both companies are considering whether a different dosing of islatravir combined with lenacapavir may become a once-weekly oral therapy option for people living with HIV.
Neither Merck nor Gilead representatives responded to request for comment by publication time.
Dr. Barber reported conference support, speaker fees, and advisory board honoraria from Gilead, Janssen, Merck, Roche, Thera, and ViiV and research/educational grants from Gilead, Roche, and ViiV. Dr. Gandhi has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New hepatitis B vaccination recommendations praised amid low awareness
An updated recommendation from the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) calling for universal hepatitis B vaccination of all adults aged 59 and younger has boosted the call to improve clinicians’ awareness of the increasing infection and low vaccination rates – and raise the issue with patients.
“This new recommendation from [the] ACIP will be instrumental [in] raising adult hepatitis B vaccination rates in the U.S. to levels that will allow us to finally eliminate hepatitis B in this country,” said Rita K. Kuwahara, MD, a primary care internal medicine physician and health policy fellow at Georgetown University, in Washington, D.C., in addressing the issue at the U.S. Conference on HIV/AIDS (USCHA) this month.
“We have the tools to prevent hepatitis B, and since we have such safe and highly effective vaccines to protect against community [spread], we should not have a single new infection in our nation,” she asserted.
The unanimously approved updated ACIP recommendation was issued in November and still requires adoption by the CDC director. The ACIP specifically recommends that adults aged 19 to 59 and those 60 years and older with risk factors for infection “should” receive the hepatitis B vaccine, and it further stipulates that those 60 years and older without known risk factors for hepatitis B “may” receive the vaccine.
The recommendation was previously only for adults at risk for hepatitis B infection due to a variety of factors, including sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, and HIV infection.
“The number of risk factors was long, and for a busy primary care provider to have to go through a lengthy risk-based protocol like that, it may not happen,” Dr. Kuwahara told this news organization.
“Now we have a really helpful new recommendation that is simply age based, and clinicians can just tell patients that if they were born before this certain period, a hepatitis B vaccination is recommended.”
The change comes amid a troubling trajectory of hepatitis B, with up to 2.4 million individuals currently having chronic hepatitis B in the U.S. and infection rates soaring by 100% to more than 400% in states with high opioid use, such as West Virginia, Kentucky, Tennessee, and Maine, Dr. Kuwahara said.
Notably, hepatitis B is the leading cause of liver disease, and one in four individuals with unmanaged chronic hepatitis B goes on to develop liver failure and/or cirrhosis or liver cancer, which has a 5-year survival rate of only 18%.
Despite the rising infection rates, only 25%-30% of adults in the U.S. are reported to be currently vaccinated for hepatitis B, even though safe and highly effective vaccines are available, notably including a new two-dose vaccine (Heplisav-B) that can be provided over just a month (vs. other hepatitis B vaccines requiring 3 doses over 6 months).
Clinician awareness of low vaccination rates lacking
Dr. Kuwahara noted that awareness among clinicians of the issues surrounding hepatitis B appears low, with one small survey that she and her colleagues conducted of 30 primary care physicians showing that not one of the respondents was aware of the low vaccination rate.
Dr. Kuwahara says a key reason for the low awareness to discuss the hepatitis B vaccination with adults is the common impression that the responsibility for the vaccination lies in the hands of pediatricians.
But that’s only half correct – universal vaccination for hepatitis B in all infants and children is indeed currently the policy in the U.S. – but that was not implemented in all states until the mid-to-late ‘90s, meaning the millions of adults over the age of about 25 to 30, born before that period, are likely not fully vaccinated against hepatitis B.
“When I was in medical school, there wasn’t a lot of discussion of how low the hepatitis B vaccination rate was because everyone knew there was universal childhood vaccination, and I think there was an assumption that it had been going on for a long time,” Dr. Kuwahara said. “So I think it’s clearly a misconception, and it’s really important to improve clinician awareness around the issue.”
Opioid use a key factor in rising infection rates
Importantly, a large proportion of opioid users are among the population of patients born before the mid-’90s – and those adults have a particularly high risk of transmission, with data indicating that 36% of new hepatitis B infections are the result of the opioid epidemic, Dr. Kuwahara noted.
“In the opioid epidemic, we have seen some of the greatest increases in acute hepatitis B presenting in adults aged 30 to 49 years old, as most adults in this age range would not have been vaccinated as children in the U.S.,” she said.
Approximately two-thirds of individuals with chronic hepatitis B are reportedly not even aware of their infection status due to ineffective prevention and vaccination programs, adding to the spread of infection, Dr. Kuwahara said.
Meanwhile, COVID-19 has only exacerbated the problem, with record-high instances of overdoses and overdose-related deaths during the pandemic, she explained.
However, the pandemic, and specifically the sweeping innovations that have been implemented in desperate efforts to bring COVID-19 vaccines to the public, could in fact represent a critical opportunity for hepatitis B prevention, Dr. Kuwahara said.
“Significant resources and federal funding have already been invested to develop a robust infrastructure for multi-dose COVID-19 vaccine administration during the pandemic, which has resulted in millions of people across the U.S. receiving the COVID-19 vaccine in easily accessible settings within their communities,” she said.
“It is essential that we expand the infrastructure development ... so that we may use this infrastructure to administer other vaccines such as the hepatitis B vaccine to adults throughout the nation and prevent additional outbreaks.”
Implementation of vaccine recommendations key
Dr. Kuwahara outlined key measures that will be important in implementing the hepatitis B vaccine recommendations:
- Awareness of the hepatitis B vaccination recommendations at the primary care level: “The first step in implementing universal [guidelines] will be to ensure that health care providers, particularly in primary care, are aware of the new ACIP guidelines so that they can speak with their patients about this and appropriately order hepatitis B testing and vaccination,” she said.
- Availability of vaccines: In addition to making sure primary care clinics are well stocked with hepatitis B vaccines, the vaccines should also be available in pharmacies and other convenient nonclinical settings through community outreach, similar to COVID-19 vaccines.
- Follow-up: Systems should be established to remind patients to receive follow-up doses.
- Public funding for vaccines: Policy changes will need to occur to allocate appropriate Section 317 funding to provide hepatitis B vaccines to adults without health insurance coverage, Dr. Kuwahara said, underscoring concerns about health equity in vaccination.
- Track vaccinations: Communication should be established between places administering vaccines and primary care providers to make sure that vaccination status can be documented in a reliable setting.
Dr. Kuwahara also noted that a federal immunization information system will be essential to track vaccines across a lifespan, providing one integrated vaccine record that can be accessed even when patients travel or move to different states.
Commenting on the issue, Frank Hood, manager of hepatitis advocacy for The AIDS Institute in Washington, D.C., added that, in addition to simplifying the process, the new age-based recommendation removes the issue of perceived judgement from the advice.
“The previous recommendations were more risk based, and patients may tend to say ‘oh, I don’t have any of those behaviors,’ and there can be some stigma,” he said. “But having something that says everyone in these age groups should be or may be vaccinated just makes it much easier and covers a greater number of individuals.”
Mr. Hood further underscored the need for continued diligence in improving measures to prevent and eradicate HBV as well as other infectious diseases.
“It is imperative that the systems being built now to respond to future infectious disease outbreaks are done so in a way to equitably support the efforts and end goal of eliminating current infectious disease epidemics like viral hepatitis and HIV,” he emphasized.“Elimination can’t be achieved if we leave people behind.”
Dr. Kuwahara and Mr. Hood had no disclosures to report.
A version of this article first appeared on Medscape.com.
An updated recommendation from the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) calling for universal hepatitis B vaccination of all adults aged 59 and younger has boosted the call to improve clinicians’ awareness of the increasing infection and low vaccination rates – and raise the issue with patients.
“This new recommendation from [the] ACIP will be instrumental [in] raising adult hepatitis B vaccination rates in the U.S. to levels that will allow us to finally eliminate hepatitis B in this country,” said Rita K. Kuwahara, MD, a primary care internal medicine physician and health policy fellow at Georgetown University, in Washington, D.C., in addressing the issue at the U.S. Conference on HIV/AIDS (USCHA) this month.
“We have the tools to prevent hepatitis B, and since we have such safe and highly effective vaccines to protect against community [spread], we should not have a single new infection in our nation,” she asserted.
The unanimously approved updated ACIP recommendation was issued in November and still requires adoption by the CDC director. The ACIP specifically recommends that adults aged 19 to 59 and those 60 years and older with risk factors for infection “should” receive the hepatitis B vaccine, and it further stipulates that those 60 years and older without known risk factors for hepatitis B “may” receive the vaccine.
The recommendation was previously only for adults at risk for hepatitis B infection due to a variety of factors, including sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, and HIV infection.
“The number of risk factors was long, and for a busy primary care provider to have to go through a lengthy risk-based protocol like that, it may not happen,” Dr. Kuwahara told this news organization.
“Now we have a really helpful new recommendation that is simply age based, and clinicians can just tell patients that if they were born before this certain period, a hepatitis B vaccination is recommended.”
The change comes amid a troubling trajectory of hepatitis B, with up to 2.4 million individuals currently having chronic hepatitis B in the U.S. and infection rates soaring by 100% to more than 400% in states with high opioid use, such as West Virginia, Kentucky, Tennessee, and Maine, Dr. Kuwahara said.
Notably, hepatitis B is the leading cause of liver disease, and one in four individuals with unmanaged chronic hepatitis B goes on to develop liver failure and/or cirrhosis or liver cancer, which has a 5-year survival rate of only 18%.
Despite the rising infection rates, only 25%-30% of adults in the U.S. are reported to be currently vaccinated for hepatitis B, even though safe and highly effective vaccines are available, notably including a new two-dose vaccine (Heplisav-B) that can be provided over just a month (vs. other hepatitis B vaccines requiring 3 doses over 6 months).
Clinician awareness of low vaccination rates lacking
Dr. Kuwahara noted that awareness among clinicians of the issues surrounding hepatitis B appears low, with one small survey that she and her colleagues conducted of 30 primary care physicians showing that not one of the respondents was aware of the low vaccination rate.
Dr. Kuwahara says a key reason for the low awareness to discuss the hepatitis B vaccination with adults is the common impression that the responsibility for the vaccination lies in the hands of pediatricians.
But that’s only half correct – universal vaccination for hepatitis B in all infants and children is indeed currently the policy in the U.S. – but that was not implemented in all states until the mid-to-late ‘90s, meaning the millions of adults over the age of about 25 to 30, born before that period, are likely not fully vaccinated against hepatitis B.
“When I was in medical school, there wasn’t a lot of discussion of how low the hepatitis B vaccination rate was because everyone knew there was universal childhood vaccination, and I think there was an assumption that it had been going on for a long time,” Dr. Kuwahara said. “So I think it’s clearly a misconception, and it’s really important to improve clinician awareness around the issue.”
Opioid use a key factor in rising infection rates
Importantly, a large proportion of opioid users are among the population of patients born before the mid-’90s – and those adults have a particularly high risk of transmission, with data indicating that 36% of new hepatitis B infections are the result of the opioid epidemic, Dr. Kuwahara noted.
“In the opioid epidemic, we have seen some of the greatest increases in acute hepatitis B presenting in adults aged 30 to 49 years old, as most adults in this age range would not have been vaccinated as children in the U.S.,” she said.
Approximately two-thirds of individuals with chronic hepatitis B are reportedly not even aware of their infection status due to ineffective prevention and vaccination programs, adding to the spread of infection, Dr. Kuwahara said.
Meanwhile, COVID-19 has only exacerbated the problem, with record-high instances of overdoses and overdose-related deaths during the pandemic, she explained.
However, the pandemic, and specifically the sweeping innovations that have been implemented in desperate efforts to bring COVID-19 vaccines to the public, could in fact represent a critical opportunity for hepatitis B prevention, Dr. Kuwahara said.
“Significant resources and federal funding have already been invested to develop a robust infrastructure for multi-dose COVID-19 vaccine administration during the pandemic, which has resulted in millions of people across the U.S. receiving the COVID-19 vaccine in easily accessible settings within their communities,” she said.
“It is essential that we expand the infrastructure development ... so that we may use this infrastructure to administer other vaccines such as the hepatitis B vaccine to adults throughout the nation and prevent additional outbreaks.”
Implementation of vaccine recommendations key
Dr. Kuwahara outlined key measures that will be important in implementing the hepatitis B vaccine recommendations:
- Awareness of the hepatitis B vaccination recommendations at the primary care level: “The first step in implementing universal [guidelines] will be to ensure that health care providers, particularly in primary care, are aware of the new ACIP guidelines so that they can speak with their patients about this and appropriately order hepatitis B testing and vaccination,” she said.
- Availability of vaccines: In addition to making sure primary care clinics are well stocked with hepatitis B vaccines, the vaccines should also be available in pharmacies and other convenient nonclinical settings through community outreach, similar to COVID-19 vaccines.
- Follow-up: Systems should be established to remind patients to receive follow-up doses.
- Public funding for vaccines: Policy changes will need to occur to allocate appropriate Section 317 funding to provide hepatitis B vaccines to adults without health insurance coverage, Dr. Kuwahara said, underscoring concerns about health equity in vaccination.
- Track vaccinations: Communication should be established between places administering vaccines and primary care providers to make sure that vaccination status can be documented in a reliable setting.
Dr. Kuwahara also noted that a federal immunization information system will be essential to track vaccines across a lifespan, providing one integrated vaccine record that can be accessed even when patients travel or move to different states.
Commenting on the issue, Frank Hood, manager of hepatitis advocacy for The AIDS Institute in Washington, D.C., added that, in addition to simplifying the process, the new age-based recommendation removes the issue of perceived judgement from the advice.
“The previous recommendations were more risk based, and patients may tend to say ‘oh, I don’t have any of those behaviors,’ and there can be some stigma,” he said. “But having something that says everyone in these age groups should be or may be vaccinated just makes it much easier and covers a greater number of individuals.”
Mr. Hood further underscored the need for continued diligence in improving measures to prevent and eradicate HBV as well as other infectious diseases.
“It is imperative that the systems being built now to respond to future infectious disease outbreaks are done so in a way to equitably support the efforts and end goal of eliminating current infectious disease epidemics like viral hepatitis and HIV,” he emphasized.“Elimination can’t be achieved if we leave people behind.”
Dr. Kuwahara and Mr. Hood had no disclosures to report.
A version of this article first appeared on Medscape.com.
An updated recommendation from the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) calling for universal hepatitis B vaccination of all adults aged 59 and younger has boosted the call to improve clinicians’ awareness of the increasing infection and low vaccination rates – and raise the issue with patients.
“This new recommendation from [the] ACIP will be instrumental [in] raising adult hepatitis B vaccination rates in the U.S. to levels that will allow us to finally eliminate hepatitis B in this country,” said Rita K. Kuwahara, MD, a primary care internal medicine physician and health policy fellow at Georgetown University, in Washington, D.C., in addressing the issue at the U.S. Conference on HIV/AIDS (USCHA) this month.
“We have the tools to prevent hepatitis B, and since we have such safe and highly effective vaccines to protect against community [spread], we should not have a single new infection in our nation,” she asserted.
The unanimously approved updated ACIP recommendation was issued in November and still requires adoption by the CDC director. The ACIP specifically recommends that adults aged 19 to 59 and those 60 years and older with risk factors for infection “should” receive the hepatitis B vaccine, and it further stipulates that those 60 years and older without known risk factors for hepatitis B “may” receive the vaccine.
The recommendation was previously only for adults at risk for hepatitis B infection due to a variety of factors, including sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, and HIV infection.
“The number of risk factors was long, and for a busy primary care provider to have to go through a lengthy risk-based protocol like that, it may not happen,” Dr. Kuwahara told this news organization.
“Now we have a really helpful new recommendation that is simply age based, and clinicians can just tell patients that if they were born before this certain period, a hepatitis B vaccination is recommended.”
The change comes amid a troubling trajectory of hepatitis B, with up to 2.4 million individuals currently having chronic hepatitis B in the U.S. and infection rates soaring by 100% to more than 400% in states with high opioid use, such as West Virginia, Kentucky, Tennessee, and Maine, Dr. Kuwahara said.
Notably, hepatitis B is the leading cause of liver disease, and one in four individuals with unmanaged chronic hepatitis B goes on to develop liver failure and/or cirrhosis or liver cancer, which has a 5-year survival rate of only 18%.
Despite the rising infection rates, only 25%-30% of adults in the U.S. are reported to be currently vaccinated for hepatitis B, even though safe and highly effective vaccines are available, notably including a new two-dose vaccine (Heplisav-B) that can be provided over just a month (vs. other hepatitis B vaccines requiring 3 doses over 6 months).
Clinician awareness of low vaccination rates lacking
Dr. Kuwahara noted that awareness among clinicians of the issues surrounding hepatitis B appears low, with one small survey that she and her colleagues conducted of 30 primary care physicians showing that not one of the respondents was aware of the low vaccination rate.
Dr. Kuwahara says a key reason for the low awareness to discuss the hepatitis B vaccination with adults is the common impression that the responsibility for the vaccination lies in the hands of pediatricians.
But that’s only half correct – universal vaccination for hepatitis B in all infants and children is indeed currently the policy in the U.S. – but that was not implemented in all states until the mid-to-late ‘90s, meaning the millions of adults over the age of about 25 to 30, born before that period, are likely not fully vaccinated against hepatitis B.
“When I was in medical school, there wasn’t a lot of discussion of how low the hepatitis B vaccination rate was because everyone knew there was universal childhood vaccination, and I think there was an assumption that it had been going on for a long time,” Dr. Kuwahara said. “So I think it’s clearly a misconception, and it’s really important to improve clinician awareness around the issue.”
Opioid use a key factor in rising infection rates
Importantly, a large proportion of opioid users are among the population of patients born before the mid-’90s – and those adults have a particularly high risk of transmission, with data indicating that 36% of new hepatitis B infections are the result of the opioid epidemic, Dr. Kuwahara noted.
“In the opioid epidemic, we have seen some of the greatest increases in acute hepatitis B presenting in adults aged 30 to 49 years old, as most adults in this age range would not have been vaccinated as children in the U.S.,” she said.
Approximately two-thirds of individuals with chronic hepatitis B are reportedly not even aware of their infection status due to ineffective prevention and vaccination programs, adding to the spread of infection, Dr. Kuwahara said.
Meanwhile, COVID-19 has only exacerbated the problem, with record-high instances of overdoses and overdose-related deaths during the pandemic, she explained.
However, the pandemic, and specifically the sweeping innovations that have been implemented in desperate efforts to bring COVID-19 vaccines to the public, could in fact represent a critical opportunity for hepatitis B prevention, Dr. Kuwahara said.
“Significant resources and federal funding have already been invested to develop a robust infrastructure for multi-dose COVID-19 vaccine administration during the pandemic, which has resulted in millions of people across the U.S. receiving the COVID-19 vaccine in easily accessible settings within their communities,” she said.
“It is essential that we expand the infrastructure development ... so that we may use this infrastructure to administer other vaccines such as the hepatitis B vaccine to adults throughout the nation and prevent additional outbreaks.”
Implementation of vaccine recommendations key
Dr. Kuwahara outlined key measures that will be important in implementing the hepatitis B vaccine recommendations:
- Awareness of the hepatitis B vaccination recommendations at the primary care level: “The first step in implementing universal [guidelines] will be to ensure that health care providers, particularly in primary care, are aware of the new ACIP guidelines so that they can speak with their patients about this and appropriately order hepatitis B testing and vaccination,” she said.
- Availability of vaccines: In addition to making sure primary care clinics are well stocked with hepatitis B vaccines, the vaccines should also be available in pharmacies and other convenient nonclinical settings through community outreach, similar to COVID-19 vaccines.
- Follow-up: Systems should be established to remind patients to receive follow-up doses.
- Public funding for vaccines: Policy changes will need to occur to allocate appropriate Section 317 funding to provide hepatitis B vaccines to adults without health insurance coverage, Dr. Kuwahara said, underscoring concerns about health equity in vaccination.
- Track vaccinations: Communication should be established between places administering vaccines and primary care providers to make sure that vaccination status can be documented in a reliable setting.
Dr. Kuwahara also noted that a federal immunization information system will be essential to track vaccines across a lifespan, providing one integrated vaccine record that can be accessed even when patients travel or move to different states.
Commenting on the issue, Frank Hood, manager of hepatitis advocacy for The AIDS Institute in Washington, D.C., added that, in addition to simplifying the process, the new age-based recommendation removes the issue of perceived judgement from the advice.
“The previous recommendations were more risk based, and patients may tend to say ‘oh, I don’t have any of those behaviors,’ and there can be some stigma,” he said. “But having something that says everyone in these age groups should be or may be vaccinated just makes it much easier and covers a greater number of individuals.”
Mr. Hood further underscored the need for continued diligence in improving measures to prevent and eradicate HBV as well as other infectious diseases.
“It is imperative that the systems being built now to respond to future infectious disease outbreaks are done so in a way to equitably support the efforts and end goal of eliminating current infectious disease epidemics like viral hepatitis and HIV,” he emphasized.“Elimination can’t be achieved if we leave people behind.”
Dr. Kuwahara and Mr. Hood had no disclosures to report.
A version of this article first appeared on Medscape.com.
COVID-19 interrupted global poliovirus surveillance and immunization
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HIV testing dips during pandemic raise transmission concerns
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
Inadequate routine diabetes screening common in HIV
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 asymptomatic infection rate remains high
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Appendicitis: Up-front antibiotics OK in select patients
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Children and COVID: Weekly cases resume their climb
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
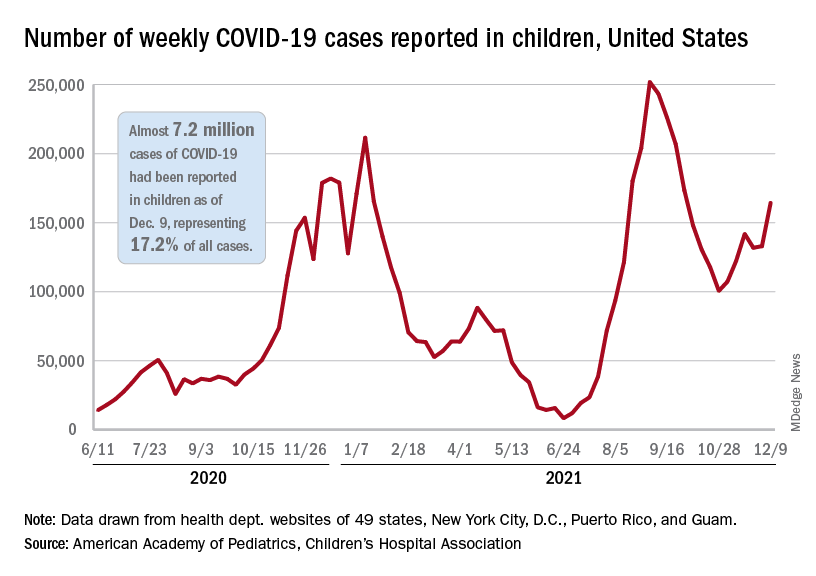
according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.