User login
Widespread Necrotizing Purpura and Lucio Phenomenon as the First Diagnostic Presentation of Diffuse Nonnodular Lepromatous Leprosy
Case Report
A 70-year-old man living in Esna, Luxor, Egypt presented to the Department of Rheumatology and Rehabilitation with widespread gangrenous skin lesions associated with ulcers of 2 weeks’ duration. One year prior, the patient had an insidious onset of nocturnal fever, bilateral leg edema, and numbness and a tingling sensation in both hands. He presented some laboratory and radiologic investigations that were performed at another hospital prior to the current presentation, which revealed thrombocytopenia, mild splenomegaly, and generalized lymphadenopathy. An excisional left axillary lymph node biopsy was performed at another hospital prior to the current presentation, and the pathology report provided by the patient described a reactive, foamy, histiocyte-rich lesion, suggesting a diagnosis of hemophagocytic lymphohistiocytosis. The patient had no diabetes or hypertension and no history of deep vein thrombosis, stroke, or unintentional weight loss. No medications were taken prior to the onset of the skin lesions, and his family history was irrelevant.
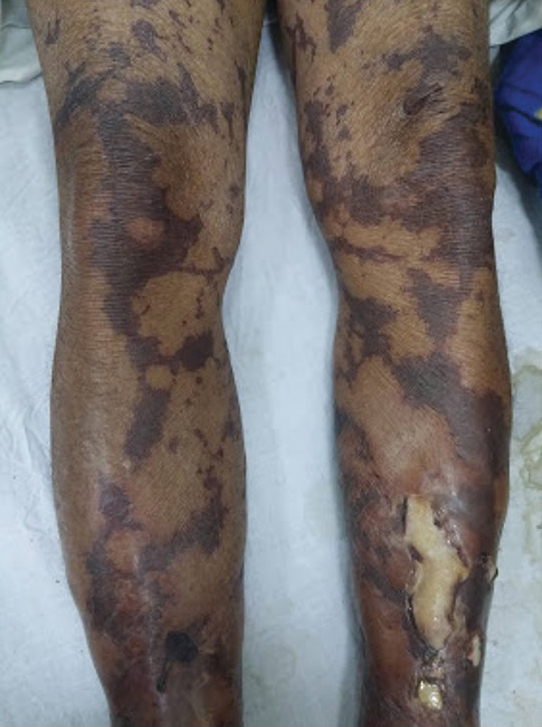
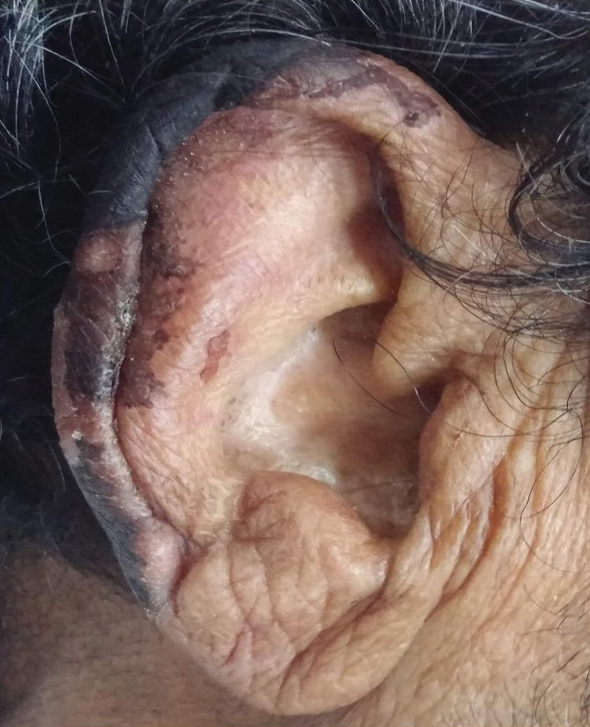
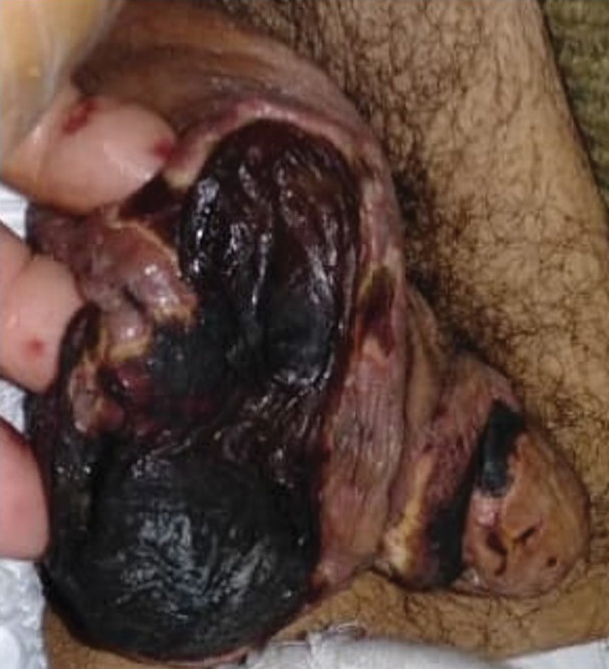
General examination at the current presentation revealed a fever (temperature, 101.3 °F [38.5 °C]), a normal heart rate (90 beats per minute), normal blood pressure (120/80 mmHg), normal respiratory rate (14 breaths per minute), accentuated heart sounds, and normal vesicular breathing without adventitious sounds. He had saddle nose, loss of the outer third of the eyebrows, and marked reduction in the density of the eyelashes (madarosis). Bilateral pitting edema of the legs also was present. Neurologic examination revealed hypoesthesia in a glove-and-stocking pattern, thickened peripheral nerves, and trophic changes over both hands; however, he had normal muscle power and deep reflexes. Joint examination revealed no abnormalities. Skin examination revealed widespread, reticulated, necrotizing, purpuric lesions on the arms, legs, abdomen, and ears, some associated with gangrenous ulcerations and hemorrhagic blisters. Scattered vasculitic ulcers and gangrenous patches were seen on the fingers. A gangrenous ulcer mimicking Fournier gangrene was seen involving the scrotal skin in addition to a gangrenous lesion on the glans penis (Figure 1–3). Unaffected skin appeared smooth, shiny, and edematous and showed no nodular lesions. Peripheral pulsations were intact.
Positive findings from a wide panel of laboratory investigations included an elevated erythrocyte sedimentation rate (103 mm for the first hour [reference range, 0–22 mm]), high C-reactive protein (50.7 mg/L [reference range, up to 6 mg/L]), anemia (hemoglobin count, 7.3 g/dL [reference range, 13.5–17.5 g/dL]), thrombocytopenia (45×103/mm3 [reference range, 150×103/mm3), low serum albumin (2.3 g/dL [reference range, 3.4–5.4 g/dL]), elevated IgG and IgM anticardiolipin antibodies (IgG, 21.4 IgG phospholipid [GPL] units [reference range, <10 IgG phospholipid (GPL) units]; IgM, 59.4 IgM phospholipid (MPL) units [reference range, <7 IgM phospholipid (MPL) units]), positive lupus anticoagulant panel test, elevated anti-β2 glycoprotein antibodies (IgG, 17.5
Nerve conduction velocity showed axonal sensory polyneuropathy. Motor nerve conduction studies for median and ulnar nerves were within normal range. Lower-limb nerves assessment was limited by the ulcerated areas and marked edema. Echocardiography was unremarkable. Arterial Doppler studies were only available for the upper limbs and were unremarkable.
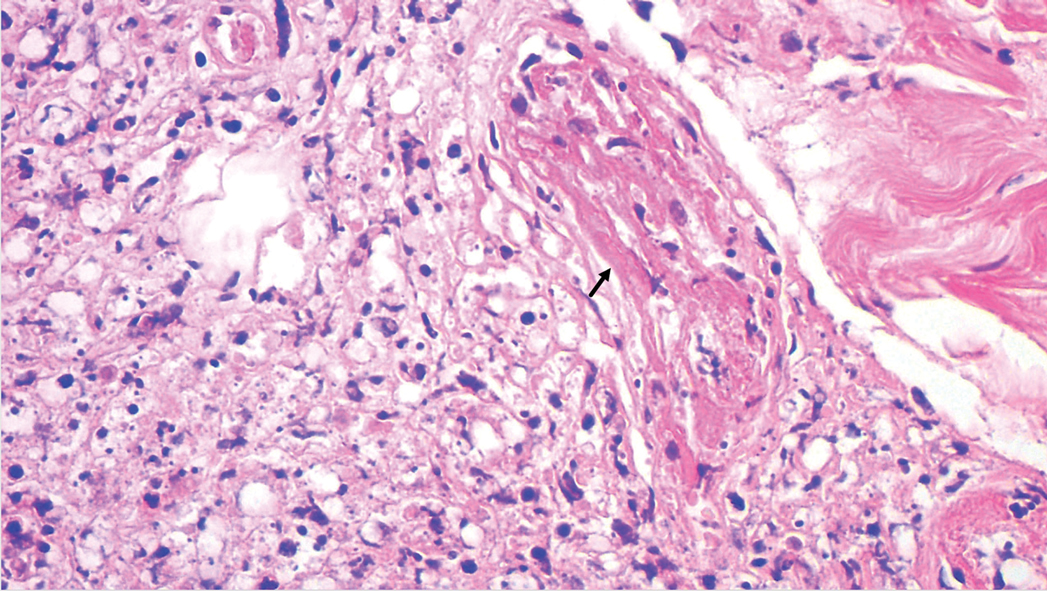
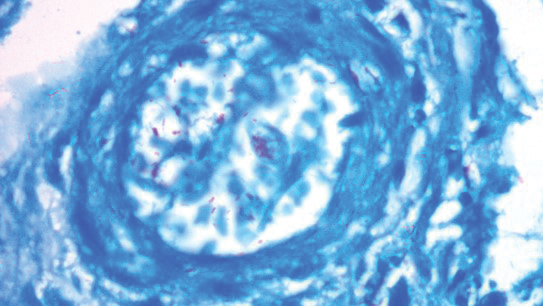
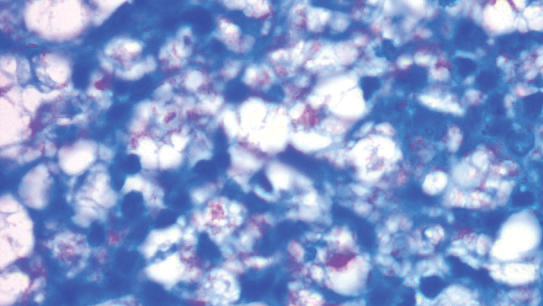
A punch biopsy was taken from one of the necrotizing purpuric lesions on the legs, and histopathologic examination revealed foci of epidermal necrosis and subepidermal separation and superficial and deep perivascular and periadnexal infiltrates extending into the fat lobules. The infiltrates were mainly made up of foamy macrophages, and some contained globi (lepra cells), in addition to lymphocytes and many neutrophils with nuclear dust. Blood vessels in the superficial and deep dermis and in the subcutaneous fat showed fibrinoid necrosis in their walls with neutrophils infiltrating the walls and thrombi in the lumens (Figure 4). Modified Ziehl-Neelsen staining revealed clumps of acid-fast lepra bacilli inside vascular lumina and endothelial cell lining and within the foamy macrophages (Figure 5). Slit-skin smear examination was performed twice and yielded negative results. The slide and paraffin block of the already performed lymph node biopsy were retrieved. Examination revealed aggregates of foamy histiocytes surrounded by lymphocytes and plasma cells replacing normal lymphoid follicles. Modified Ziehl-Neelsen stain was performed, and clusters of acid-fast bacilli were detected within the foamy histiocytic infiltrate (Figure 6).
According to the results of the skin biopsy, the revised result of the lymph node biopsy, and the pattern of neurologic deficit together with clinical and laboratory correlation, the patient was diagnosed with diffuse nonnodular lepromatous leprosy presenting with Lucio phenomenon (Lucio leprosy) and associated with lepromatous lymphadenitis.
The patient received the following treatment: methylprednisolone 500 mg (intravenous pulse therapy) followed by daily oral administration of prednisolone 10 mg, rifampicin 300 mg, dapsone 100 mg, clofazimine 100 mg, acetylsalicylic acid 150 mg, and enoxaparin sodium 80 mg. In addition, the scrotal Fournier gangrene–like lesion was treated by surgical debridement followed by vacuum therapy. By the second week after treatment, the gangrenous lesions of the fingers developed a line of demarcation, and the skin infarctions started to recede.
Comment
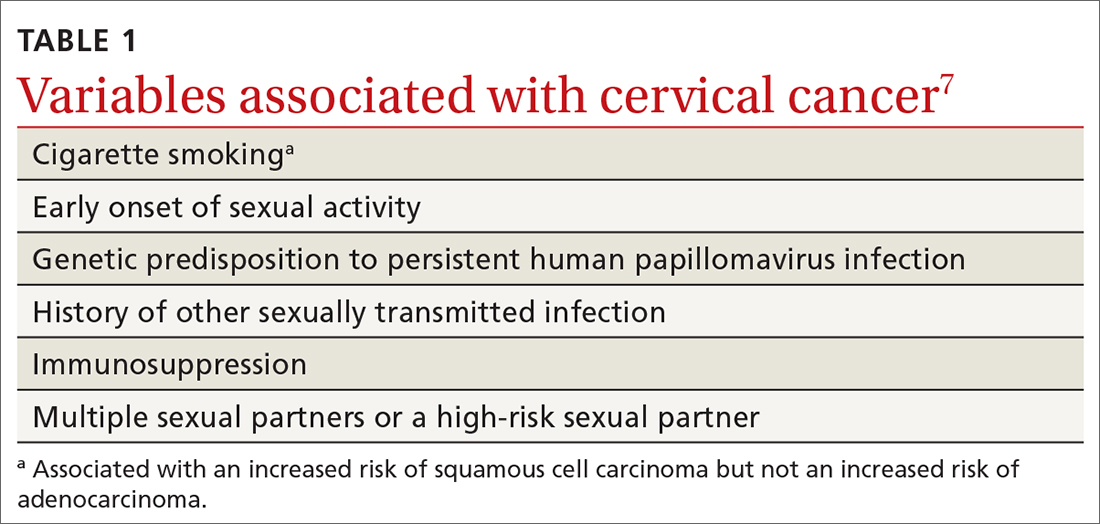
Despite a decrease in its prevalence through a World Health Organization (WHO)–empowered eradication program, leprosy still represents a health problem in endemic areas.1,2 It is characterized by a wide range of immune responses to Mycobacterium leprae, displaying a spectrum of clinical and histopathologic manifestations that vary from the tuberculoid or paucibacillary pole with a strong cell-mediated immune response and fewer organisms to the lepromatous or multibacillary pole with weaker cell-mediated immune response and higher loads of organisms.3 In addition to its well-known cutaneous and neurologic manifestations, leprosy can present with a variety of manifestations, including constitutional symptoms, musculoskeletal manifestations, and serologic abnormalities; thus, leprosy can mimic rheumatoid arthritis, spondyloarthritis, and vasculitis—a pitfall that may result in misdiagnosis as a rheumatologic disorder.3-7
The chronic course of leprosy can be disrupted by acute, immunologically mediated reactions known as lepra reactions, of which there are 3 types.8 Type I lepra reactions are cell mediated and occur mainly in patients with borderline disease, often representing an upgrade toward the tuberculoid pole; less often they represent a downgrade reaction. Nerves become painful and swollen with possible loss of function, and skin lesions become edematous and tender; sometimes arthritis develops.9 Type II lepra reactions, also known as erythema nodosum leprosum (ENL), occur in borderline lepromatous and lepromatous patients with a high bacillary load. They are characterized by fever, body aches, tender cutaneous/subcutaneous nodules that may ulcerate, possible bullous lesions, painful nerve swellings, swollen joints, iritis, lymphadenitis, glomerulonephritis, epididymo-orchitis, and hepatic affection. Both immune-complex and delayed hypersensitivity reactions play a role in ENL.8,10 The third reaction is a rare aggressive type known as Lucio phenomenon or Lucio leprosy, which presents with irregular-shaped, angulated, or stellar necrotizing purpuric lesions (hemorrhagic infacrtions) developing mainly on the extremities. The lesions evolve into ulcers that heal with atrophic scarring.2,11 Lucio phenomenon develops as a result of thrombotic vascular occlusion secondary to massive invasion of vascular endothelial cells by lepra bacilli.2,11-14 Involvement of the scrotal skin, such as in our patient, is rare.
Lucio phenomenon mainly is seen in Mexico and Central America, and few cases have been documented in Cuba, South America, the United States, India, Polynesia, South Africa, and Southeast Asia.15-17 It specifically occurs in patients with untreated, diffuse, nonnodular lepromatous leprosy (pure and primitive diffuse lepromatous leprosy (DLL)/diffuse leprosy of Lucio and Latapí). This type of leprosy was first described by Lucio and Alvarado18 in 1852 as a distinct form of lepromatous leprosy characterized by widespread and dense infiltration of the whole skin by lepra bacilli without the typical nodular lesions of leprosy, rendering its diagnosis challenging, especially in sporadic cases. Other manifestations of DLL include complete alopecia of the eyebrows and eyelashes, destructive rhinitis, and areas of anhidrosis and dyesthesia.2
Latapí and Chévez-Zomora19 defined Lucio phenomenon in 1948 as a form of histopathologic vasculitis restricted to patients with DLL. Histopathologically, in addition to the infiltration of the skin with acid-fast bacilli–laden foamy histiocytes, lesions of Lucio phenomenon show features of necrotizing (leukocytoclastic) vasculitis with fibrinoid necrosis20 or vascular thrombi with minimal perivascular lymphocytic infiltrate and no evidence of vasculitis.11 Medium to large vessels in the deep dermis and subcutaneous tissue show infiltration of their walls with a large number of macrophages laden with acid-fast bacilli.11 Cases with histopathologic features mimicking antiphospholipid syndrome with endothelial cell proliferation, thrombosis, and mild mononuclear cell infiltrate also may be seen.20 In all cases, ischemic epidermal necrosis is seen, as well as acid-fast bacilli, both singly and in clusters (globi) within endothelial cells and inside blood vessel lumina.
Although Lucio phenomenon initially was thought to be immune-complex mediated like ENL, it has been suggested that the main trigger is thrombotic vascular occlusion secondary to massive invasion of the vascular endothelial cells by the lepra bacilli, resulting in necrosis.14 Bacterial lipopolysaccharides promote the release of IL-1 and tumor necrosis factor α, which in turn stimulate the production of prostaglandins, IL-6, and coagulation factor III, leading to vascular thrombosis and tissue necrosis.21,22 Moreover, antiphospholipid antibodies, which have been found to be induced in response to certain infectious agents in genetically predisposed individuals,23 have been reported in patients with leprosy, mainly in association with lepromatous leprosy. The reported prevalence of anticardiolipin antibodies ranged from 37% to 98%, whereas anti-β2-glycoprotein I antibodies ranged from 3% to 19%, and antiprothrombin antibodies ranged from 6% to 45%.24,25 Antiphospholipid antibodies have been reported to play a role in the pathogenesis of Lucio phenomenon.11,13,15,26 Our case supports this hypothesis with positive anticardiolipin antibodies, anti-β2 glycoprotein antibodies, and positive lupus anticoagulant.
In accordance with Curi et al,2 who reported 5 cases of DLL with Lucio phenomenon, our patient showed a similar presentation with positive inflammatory markers in association with a negative autoimmune profile (ANA, ANCA-C&P) and negative venereal disease research laboratory test. It is important to mention that a positive autoimmune profile (ANA, ANCA-C&P) can be present in leprotic patients, causing possible diagnostic confusion with collagen diseases.27,28
An interesting finding in our case was the negative slit-skin smear results. Although the specificity of slit-skin smear is 100%, as it directly demonstrates the presence of acid-fast bacilli,29 its sensitivity is low and varies from 10% to 50%.30 The detection of acid-fast bacilli in tissue sections is reported to be a better method for confirming the diagnosis of leprosy.31
The provisional impression of hemophagocytic lymphohistiocytosis in the lymph node biopsy in our patient was excluded upon detection of acid-fast bacilli in the foamy histiocytes infiltrating the lymph node; moreover, the normal serum lipids and serum ferritin argued against this diagnosis.32 Leprosy tends to involve the lymph nodes, particularly in borderline, borderline lepromatous, and lepromatous forms.33 The incidence of lymph node involvement accompanied by skin lesions with the presence of acid-fast bacilli in the lymph nodes is 92.2%.34
Our patient showed an excellent response to antileprotic treatment, which was administered according to the WHO multidrug therapy guidelines for multibacillary leprosy,35 combined with low-dose prednisolone, acetylsalicylic acid, and anticoagulant treatment. Thalidomide and high-dose prednisolone (60 mg/d) combined with antileprotic treatment also have been reported to be successful in managing recurrent infarctions in leprosy.36 The Fournier-like gangrenous ulcer of the scrotum was managed by surgical debridement and vacuum therapy.
It is noteworthy that the WHO elimination goal for leprosy was to reduce the prevalence to less than 1 case per 10,000 population. Egypt is among the first countries in North Africa and the Middle East regions to achieve this target supervised by the National Leprosy Control Program as early as 1994; this was further reduced to 0.33 cases per 10,000 population in 2004, and reduced again in 2009; however, certain foci showed a prevalence rate more than the elimination target, particularly in the cities of Qena (1.12) and Sohag (2.47).37 Esna, where our patient is from, is an endemic area in Egypt.38
Conclusion
1. World Health Organization. World Health Statistics: 2011. World Health Organization; 2011. https://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_Full.pdf
2. Curi PF, Villaroel JS, Migliore N, et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016;35:1397-1401.
3. Shrestha B, Li YQ, Fu P. Leprosy mimics adult onset Still’s disease in a Chinese patient. Egypt Rheumatol. 2018;40:217-220.
4. Prasad S, Misra R, Aggarwal A, et al. Leprosy revealed in a rheumatology clinic: a case series. Int J Rheum Dis. 2013;16:129-133.
5. Chao G, Fang L, Lu C. Leprosy with ANA positive mistaken for connective tissue disease. Clin Rheumatol. 2013;32:645-648.
6. Chauhan S, Wakhlu A, Agarwal V. Arthritis in leprosy. Rheumatology. 2010;49:2237-2242.
7. Rath D, Bhargava S, Kundu BK. Leprosy mimicking common rheumatologic entities: a trial for the clinician in the era of biologics. Case Rep Rheumatol. 2014;2014:429698.
8. Cuevas J, Rodríguez-Peralto JL, Carrillo R, et al. Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg. 2007;26:126-130.
9. Henriques CC, Lopéz B, Mestre T, et al. Leprosy and rheumatoid arthritis: consequence or association? BMJ Case Rep. 2012;13:1-4.
10. Vázquez-Botet M, Sánchez JL. Erythema nodosum leprosum. Int J Dermatol. 1987;26:436-437.
11. Nunzie E, Ortega Cabrera LV, Macanchi Moncayo FM, et al. Lucio leprosy with Lucio’s phenomenon, digital gangrene and anticardiolipin antibodies. Lepr Rev. 2014;85:194-200.
12. Salvi S, Chopra A. Leprosy in a rheumatology setting: a challenging mimic to expose. Clin Rheumatol. 2013;32:1557-1563.
13. Azulay-Abulafia L, Pereira SL, Hardmann D, et al. Lucio phenomenon. vasculitis or occlusive vasculopathy? Hautarzt. 2006;57:1101-1105.
14. Benard G, Sakai-Valente NY, Bianconcini Trindade MA. Concomittant Lucio phenomenon and erythema nodosum in a leprosy patient: clues for their distinct pathogenesis. Am J Dermatopathol. 2009;31:288-292.
15. Rocha RH, Emerich PS, Diniz LM, et al. Lucio’s phenomenon: exuberant case report and review of Brazilian cases. An Bras Dermatol. 2016;91(suppl 5):S60-S63.
16. Costa IM, Kawano LB, Pereira CP, et al. Lucio’s phenomenon: a case report and review of the literature. Int J Dermatol. 2005;44:566-571.
17. Kumari R, Thappa DM, Basu D. A fatal case of Lucio phenomenon from India. Dermatol Online J. 2008;14:10.
18. Lucio R, Alvarado I. Opúsculo Sobre el Mal de San Lázaro o Elefantiasis de los Griegos. M. Murguía; 1852.
19. Latapí F, Chévez-Zamora A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948;16:421-437.
20. Vargas OF. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78:248-260.
21. Latapí FR, Chevez-Zamora A. La lepra manchada de Lucio. Rev Dermatol Mex. 1978;22:102-107.
22. Monteiro R, Abreu MA, Tiezzi MG, et al. Fenômeno de Lúcio: mais um caso relatado no Brasil. An Bras Dermatol. 2012;87:296-300.
23. Gharavi EE, Chaimovich H, Cucucrull E, et al. Induction of antiphospholipid antibodies by immunization with synthetic bacterial & viral peptides. Lupus. 1999;8:449-455.
24. de Larrañaga GF, Forastiero RR, Martinuzzo ME, et al. High prevalence of antiphospholipid antibodies in leprosy: evaluation of antigen reactivity. Lupus. 2000;9:594-600.
25. Loizou S, Singh S, Wypkema E, et al. Anticardiolipin, anti-beta(2)-glycoprotein I and antiprothrombin antibodies in black South African patients with infectious disease. Ann Rheum Dis. 2003;62:1106-1111.
26. Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association; role of antiphospholipid antibodies. BMC Infect Dis. 2005,5:74.
27. Horta-Baas G, Hernández-Cabrera MF, Barile-Fabris LA, et al. Multibacillary leprosy mimicking systemic lupus erythematosus: case report and literature review. Lupus. 2015;24:1095-1102.
28. Pradhan V, Badakere SS, Shankar KU. Increased incidence of cytoplasmic ANCA (cANCA) and other auto antibodies in leprosy patients from western India. Lepr Rev. 2004;75:50-56.
29. Oskam L. Diagnosis and classification of leprosy. Lepr Rev. 2002;73:17-26.
30. Rao PN. Recent advances in the control programs and therapy of leprosy. Indian J Dermatol Venereol Leprol. 2004;70:269-276.
31. Rao PN, Pratap D, Ramana Reddy AV, et al. Evaluation of leprosy patients with 1 to 5 skin lesions with relevance to their grouping into paucibacillary or multibacillary disease. Indian J Dermatol Venereol Leprol. 2006;72:207-210.
32. Rosado FGN, Kim AS. Hemophagocytic lymphohistiocytosis. an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727.
33. Kar HK, Mohanty HC, Mohanty GN, et al. Clinicopathological study of lymph node involvement in leprosy. Lepr India. 1983;55:725-738.
34. Gupta JC, Panda PK, Shrivastava KK, et al. A histopathologic study of lymph nodes in 43 cases of leprosy. Lepr India. 1978;50:196-203.
35. WHO Expert Committee on Leprosy. Seventh Report. World Health Organization; 1998. https://apps.who.int/iris/bitstream/handle/10665/42060/WHO_TRS_874.pdf?sequence=1&isAllowed=y
36. Misra DP, Parida JR, Chowdhury AC, et al. Lepra reaction with Lucio phenomenon mimicking cutaneous vasculitis. Case Rep Immunol. 2014;2014:641989.
37. Amer A, Mansour A. Epidemiological study of leprosy in Egypt: 2005-2009. Egypt J Dermatol Venereol. 2014;34:70-73.
38. World Health Organization. Screening campaign aims to eliminate leprosy in Egypt. Published May 9, 2018. Accessed September 8, 2021. http://www.emro.who.int/egy/egypt-events/last-miless-activities-on-eliminating-leprosy-from-egypt.html
Case Report
A 70-year-old man living in Esna, Luxor, Egypt presented to the Department of Rheumatology and Rehabilitation with widespread gangrenous skin lesions associated with ulcers of 2 weeks’ duration. One year prior, the patient had an insidious onset of nocturnal fever, bilateral leg edema, and numbness and a tingling sensation in both hands. He presented some laboratory and radiologic investigations that were performed at another hospital prior to the current presentation, which revealed thrombocytopenia, mild splenomegaly, and generalized lymphadenopathy. An excisional left axillary lymph node biopsy was performed at another hospital prior to the current presentation, and the pathology report provided by the patient described a reactive, foamy, histiocyte-rich lesion, suggesting a diagnosis of hemophagocytic lymphohistiocytosis. The patient had no diabetes or hypertension and no history of deep vein thrombosis, stroke, or unintentional weight loss. No medications were taken prior to the onset of the skin lesions, and his family history was irrelevant.
General examination at the current presentation revealed a fever (temperature, 101.3 °F [38.5 °C]), a normal heart rate (90 beats per minute), normal blood pressure (120/80 mmHg), normal respiratory rate (14 breaths per minute), accentuated heart sounds, and normal vesicular breathing without adventitious sounds. He had saddle nose, loss of the outer third of the eyebrows, and marked reduction in the density of the eyelashes (madarosis). Bilateral pitting edema of the legs also was present. Neurologic examination revealed hypoesthesia in a glove-and-stocking pattern, thickened peripheral nerves, and trophic changes over both hands; however, he had normal muscle power and deep reflexes. Joint examination revealed no abnormalities. Skin examination revealed widespread, reticulated, necrotizing, purpuric lesions on the arms, legs, abdomen, and ears, some associated with gangrenous ulcerations and hemorrhagic blisters. Scattered vasculitic ulcers and gangrenous patches were seen on the fingers. A gangrenous ulcer mimicking Fournier gangrene was seen involving the scrotal skin in addition to a gangrenous lesion on the glans penis (Figure 1–3). Unaffected skin appeared smooth, shiny, and edematous and showed no nodular lesions. Peripheral pulsations were intact.



Positive findings from a wide panel of laboratory investigations included an elevated erythrocyte sedimentation rate (103 mm for the first hour [reference range, 0–22 mm]), high C-reactive protein (50.7 mg/L [reference range, up to 6 mg/L]), anemia (hemoglobin count, 7.3 g/dL [reference range, 13.5–17.5 g/dL]), thrombocytopenia (45×103/mm3 [reference range, 150×103/mm3), low serum albumin (2.3 g/dL [reference range, 3.4–5.4 g/dL]), elevated IgG and IgM anticardiolipin antibodies (IgG, 21.4 IgG phospholipid [GPL] units [reference range, <10 IgG phospholipid (GPL) units]; IgM, 59.4 IgM phospholipid (MPL) units [reference range, <7 IgM phospholipid (MPL) units]), positive lupus anticoagulant panel test, elevated anti-β2 glycoprotein antibodies (IgG, 17.5
Nerve conduction velocity showed axonal sensory polyneuropathy. Motor nerve conduction studies for median and ulnar nerves were within normal range. Lower-limb nerves assessment was limited by the ulcerated areas and marked edema. Echocardiography was unremarkable. Arterial Doppler studies were only available for the upper limbs and were unremarkable.
A punch biopsy was taken from one of the necrotizing purpuric lesions on the legs, and histopathologic examination revealed foci of epidermal necrosis and subepidermal separation and superficial and deep perivascular and periadnexal infiltrates extending into the fat lobules. The infiltrates were mainly made up of foamy macrophages, and some contained globi (lepra cells), in addition to lymphocytes and many neutrophils with nuclear dust. Blood vessels in the superficial and deep dermis and in the subcutaneous fat showed fibrinoid necrosis in their walls with neutrophils infiltrating the walls and thrombi in the lumens (Figure 4). Modified Ziehl-Neelsen staining revealed clumps of acid-fast lepra bacilli inside vascular lumina and endothelial cell lining and within the foamy macrophages (Figure 5). Slit-skin smear examination was performed twice and yielded negative results. The slide and paraffin block of the already performed lymph node biopsy were retrieved. Examination revealed aggregates of foamy histiocytes surrounded by lymphocytes and plasma cells replacing normal lymphoid follicles. Modified Ziehl-Neelsen stain was performed, and clusters of acid-fast bacilli were detected within the foamy histiocytic infiltrate (Figure 6).



According to the results of the skin biopsy, the revised result of the lymph node biopsy, and the pattern of neurologic deficit together with clinical and laboratory correlation, the patient was diagnosed with diffuse nonnodular lepromatous leprosy presenting with Lucio phenomenon (Lucio leprosy) and associated with lepromatous lymphadenitis.
The patient received the following treatment: methylprednisolone 500 mg (intravenous pulse therapy) followed by daily oral administration of prednisolone 10 mg, rifampicin 300 mg, dapsone 100 mg, clofazimine 100 mg, acetylsalicylic acid 150 mg, and enoxaparin sodium 80 mg. In addition, the scrotal Fournier gangrene–like lesion was treated by surgical debridement followed by vacuum therapy. By the second week after treatment, the gangrenous lesions of the fingers developed a line of demarcation, and the skin infarctions started to recede.
Comment
Despite a decrease in its prevalence through a World Health Organization (WHO)–empowered eradication program, leprosy still represents a health problem in endemic areas.1,2 It is characterized by a wide range of immune responses to Mycobacterium leprae, displaying a spectrum of clinical and histopathologic manifestations that vary from the tuberculoid or paucibacillary pole with a strong cell-mediated immune response and fewer organisms to the lepromatous or multibacillary pole with weaker cell-mediated immune response and higher loads of organisms.3 In addition to its well-known cutaneous and neurologic manifestations, leprosy can present with a variety of manifestations, including constitutional symptoms, musculoskeletal manifestations, and serologic abnormalities; thus, leprosy can mimic rheumatoid arthritis, spondyloarthritis, and vasculitis—a pitfall that may result in misdiagnosis as a rheumatologic disorder.3-7
The chronic course of leprosy can be disrupted by acute, immunologically mediated reactions known as lepra reactions, of which there are 3 types.8 Type I lepra reactions are cell mediated and occur mainly in patients with borderline disease, often representing an upgrade toward the tuberculoid pole; less often they represent a downgrade reaction. Nerves become painful and swollen with possible loss of function, and skin lesions become edematous and tender; sometimes arthritis develops.9 Type II lepra reactions, also known as erythema nodosum leprosum (ENL), occur in borderline lepromatous and lepromatous patients with a high bacillary load. They are characterized by fever, body aches, tender cutaneous/subcutaneous nodules that may ulcerate, possible bullous lesions, painful nerve swellings, swollen joints, iritis, lymphadenitis, glomerulonephritis, epididymo-orchitis, and hepatic affection. Both immune-complex and delayed hypersensitivity reactions play a role in ENL.8,10 The third reaction is a rare aggressive type known as Lucio phenomenon or Lucio leprosy, which presents with irregular-shaped, angulated, or stellar necrotizing purpuric lesions (hemorrhagic infacrtions) developing mainly on the extremities. The lesions evolve into ulcers that heal with atrophic scarring.2,11 Lucio phenomenon develops as a result of thrombotic vascular occlusion secondary to massive invasion of vascular endothelial cells by lepra bacilli.2,11-14 Involvement of the scrotal skin, such as in our patient, is rare.
Lucio phenomenon mainly is seen in Mexico and Central America, and few cases have been documented in Cuba, South America, the United States, India, Polynesia, South Africa, and Southeast Asia.15-17 It specifically occurs in patients with untreated, diffuse, nonnodular lepromatous leprosy (pure and primitive diffuse lepromatous leprosy (DLL)/diffuse leprosy of Lucio and Latapí). This type of leprosy was first described by Lucio and Alvarado18 in 1852 as a distinct form of lepromatous leprosy characterized by widespread and dense infiltration of the whole skin by lepra bacilli without the typical nodular lesions of leprosy, rendering its diagnosis challenging, especially in sporadic cases. Other manifestations of DLL include complete alopecia of the eyebrows and eyelashes, destructive rhinitis, and areas of anhidrosis and dyesthesia.2
Latapí and Chévez-Zomora19 defined Lucio phenomenon in 1948 as a form of histopathologic vasculitis restricted to patients with DLL. Histopathologically, in addition to the infiltration of the skin with acid-fast bacilli–laden foamy histiocytes, lesions of Lucio phenomenon show features of necrotizing (leukocytoclastic) vasculitis with fibrinoid necrosis20 or vascular thrombi with minimal perivascular lymphocytic infiltrate and no evidence of vasculitis.11 Medium to large vessels in the deep dermis and subcutaneous tissue show infiltration of their walls with a large number of macrophages laden with acid-fast bacilli.11 Cases with histopathologic features mimicking antiphospholipid syndrome with endothelial cell proliferation, thrombosis, and mild mononuclear cell infiltrate also may be seen.20 In all cases, ischemic epidermal necrosis is seen, as well as acid-fast bacilli, both singly and in clusters (globi) within endothelial cells and inside blood vessel lumina.
Although Lucio phenomenon initially was thought to be immune-complex mediated like ENL, it has been suggested that the main trigger is thrombotic vascular occlusion secondary to massive invasion of the vascular endothelial cells by the lepra bacilli, resulting in necrosis.14 Bacterial lipopolysaccharides promote the release of IL-1 and tumor necrosis factor α, which in turn stimulate the production of prostaglandins, IL-6, and coagulation factor III, leading to vascular thrombosis and tissue necrosis.21,22 Moreover, antiphospholipid antibodies, which have been found to be induced in response to certain infectious agents in genetically predisposed individuals,23 have been reported in patients with leprosy, mainly in association with lepromatous leprosy. The reported prevalence of anticardiolipin antibodies ranged from 37% to 98%, whereas anti-β2-glycoprotein I antibodies ranged from 3% to 19%, and antiprothrombin antibodies ranged from 6% to 45%.24,25 Antiphospholipid antibodies have been reported to play a role in the pathogenesis of Lucio phenomenon.11,13,15,26 Our case supports this hypothesis with positive anticardiolipin antibodies, anti-β2 glycoprotein antibodies, and positive lupus anticoagulant.
In accordance with Curi et al,2 who reported 5 cases of DLL with Lucio phenomenon, our patient showed a similar presentation with positive inflammatory markers in association with a negative autoimmune profile (ANA, ANCA-C&P) and negative venereal disease research laboratory test. It is important to mention that a positive autoimmune profile (ANA, ANCA-C&P) can be present in leprotic patients, causing possible diagnostic confusion with collagen diseases.27,28
An interesting finding in our case was the negative slit-skin smear results. Although the specificity of slit-skin smear is 100%, as it directly demonstrates the presence of acid-fast bacilli,29 its sensitivity is low and varies from 10% to 50%.30 The detection of acid-fast bacilli in tissue sections is reported to be a better method for confirming the diagnosis of leprosy.31
The provisional impression of hemophagocytic lymphohistiocytosis in the lymph node biopsy in our patient was excluded upon detection of acid-fast bacilli in the foamy histiocytes infiltrating the lymph node; moreover, the normal serum lipids and serum ferritin argued against this diagnosis.32 Leprosy tends to involve the lymph nodes, particularly in borderline, borderline lepromatous, and lepromatous forms.33 The incidence of lymph node involvement accompanied by skin lesions with the presence of acid-fast bacilli in the lymph nodes is 92.2%.34
Our patient showed an excellent response to antileprotic treatment, which was administered according to the WHO multidrug therapy guidelines for multibacillary leprosy,35 combined with low-dose prednisolone, acetylsalicylic acid, and anticoagulant treatment. Thalidomide and high-dose prednisolone (60 mg/d) combined with antileprotic treatment also have been reported to be successful in managing recurrent infarctions in leprosy.36 The Fournier-like gangrenous ulcer of the scrotum was managed by surgical debridement and vacuum therapy.
It is noteworthy that the WHO elimination goal for leprosy was to reduce the prevalence to less than 1 case per 10,000 population. Egypt is among the first countries in North Africa and the Middle East regions to achieve this target supervised by the National Leprosy Control Program as early as 1994; this was further reduced to 0.33 cases per 10,000 population in 2004, and reduced again in 2009; however, certain foci showed a prevalence rate more than the elimination target, particularly in the cities of Qena (1.12) and Sohag (2.47).37 Esna, where our patient is from, is an endemic area in Egypt.38
Conclusion
Case Report
A 70-year-old man living in Esna, Luxor, Egypt presented to the Department of Rheumatology and Rehabilitation with widespread gangrenous skin lesions associated with ulcers of 2 weeks’ duration. One year prior, the patient had an insidious onset of nocturnal fever, bilateral leg edema, and numbness and a tingling sensation in both hands. He presented some laboratory and radiologic investigations that were performed at another hospital prior to the current presentation, which revealed thrombocytopenia, mild splenomegaly, and generalized lymphadenopathy. An excisional left axillary lymph node biopsy was performed at another hospital prior to the current presentation, and the pathology report provided by the patient described a reactive, foamy, histiocyte-rich lesion, suggesting a diagnosis of hemophagocytic lymphohistiocytosis. The patient had no diabetes or hypertension and no history of deep vein thrombosis, stroke, or unintentional weight loss. No medications were taken prior to the onset of the skin lesions, and his family history was irrelevant.
General examination at the current presentation revealed a fever (temperature, 101.3 °F [38.5 °C]), a normal heart rate (90 beats per minute), normal blood pressure (120/80 mmHg), normal respiratory rate (14 breaths per minute), accentuated heart sounds, and normal vesicular breathing without adventitious sounds. He had saddle nose, loss of the outer third of the eyebrows, and marked reduction in the density of the eyelashes (madarosis). Bilateral pitting edema of the legs also was present. Neurologic examination revealed hypoesthesia in a glove-and-stocking pattern, thickened peripheral nerves, and trophic changes over both hands; however, he had normal muscle power and deep reflexes. Joint examination revealed no abnormalities. Skin examination revealed widespread, reticulated, necrotizing, purpuric lesions on the arms, legs, abdomen, and ears, some associated with gangrenous ulcerations and hemorrhagic blisters. Scattered vasculitic ulcers and gangrenous patches were seen on the fingers. A gangrenous ulcer mimicking Fournier gangrene was seen involving the scrotal skin in addition to a gangrenous lesion on the glans penis (Figure 1–3). Unaffected skin appeared smooth, shiny, and edematous and showed no nodular lesions. Peripheral pulsations were intact.



Positive findings from a wide panel of laboratory investigations included an elevated erythrocyte sedimentation rate (103 mm for the first hour [reference range, 0–22 mm]), high C-reactive protein (50.7 mg/L [reference range, up to 6 mg/L]), anemia (hemoglobin count, 7.3 g/dL [reference range, 13.5–17.5 g/dL]), thrombocytopenia (45×103/mm3 [reference range, 150×103/mm3), low serum albumin (2.3 g/dL [reference range, 3.4–5.4 g/dL]), elevated IgG and IgM anticardiolipin antibodies (IgG, 21.4 IgG phospholipid [GPL] units [reference range, <10 IgG phospholipid (GPL) units]; IgM, 59.4 IgM phospholipid (MPL) units [reference range, <7 IgM phospholipid (MPL) units]), positive lupus anticoagulant panel test, elevated anti-β2 glycoprotein antibodies (IgG, 17.5
Nerve conduction velocity showed axonal sensory polyneuropathy. Motor nerve conduction studies for median and ulnar nerves were within normal range. Lower-limb nerves assessment was limited by the ulcerated areas and marked edema. Echocardiography was unremarkable. Arterial Doppler studies were only available for the upper limbs and were unremarkable.
A punch biopsy was taken from one of the necrotizing purpuric lesions on the legs, and histopathologic examination revealed foci of epidermal necrosis and subepidermal separation and superficial and deep perivascular and periadnexal infiltrates extending into the fat lobules. The infiltrates were mainly made up of foamy macrophages, and some contained globi (lepra cells), in addition to lymphocytes and many neutrophils with nuclear dust. Blood vessels in the superficial and deep dermis and in the subcutaneous fat showed fibrinoid necrosis in their walls with neutrophils infiltrating the walls and thrombi in the lumens (Figure 4). Modified Ziehl-Neelsen staining revealed clumps of acid-fast lepra bacilli inside vascular lumina and endothelial cell lining and within the foamy macrophages (Figure 5). Slit-skin smear examination was performed twice and yielded negative results. The slide and paraffin block of the already performed lymph node biopsy were retrieved. Examination revealed aggregates of foamy histiocytes surrounded by lymphocytes and plasma cells replacing normal lymphoid follicles. Modified Ziehl-Neelsen stain was performed, and clusters of acid-fast bacilli were detected within the foamy histiocytic infiltrate (Figure 6).



According to the results of the skin biopsy, the revised result of the lymph node biopsy, and the pattern of neurologic deficit together with clinical and laboratory correlation, the patient was diagnosed with diffuse nonnodular lepromatous leprosy presenting with Lucio phenomenon (Lucio leprosy) and associated with lepromatous lymphadenitis.
The patient received the following treatment: methylprednisolone 500 mg (intravenous pulse therapy) followed by daily oral administration of prednisolone 10 mg, rifampicin 300 mg, dapsone 100 mg, clofazimine 100 mg, acetylsalicylic acid 150 mg, and enoxaparin sodium 80 mg. In addition, the scrotal Fournier gangrene–like lesion was treated by surgical debridement followed by vacuum therapy. By the second week after treatment, the gangrenous lesions of the fingers developed a line of demarcation, and the skin infarctions started to recede.
Comment
Despite a decrease in its prevalence through a World Health Organization (WHO)–empowered eradication program, leprosy still represents a health problem in endemic areas.1,2 It is characterized by a wide range of immune responses to Mycobacterium leprae, displaying a spectrum of clinical and histopathologic manifestations that vary from the tuberculoid or paucibacillary pole with a strong cell-mediated immune response and fewer organisms to the lepromatous or multibacillary pole with weaker cell-mediated immune response and higher loads of organisms.3 In addition to its well-known cutaneous and neurologic manifestations, leprosy can present with a variety of manifestations, including constitutional symptoms, musculoskeletal manifestations, and serologic abnormalities; thus, leprosy can mimic rheumatoid arthritis, spondyloarthritis, and vasculitis—a pitfall that may result in misdiagnosis as a rheumatologic disorder.3-7
The chronic course of leprosy can be disrupted by acute, immunologically mediated reactions known as lepra reactions, of which there are 3 types.8 Type I lepra reactions are cell mediated and occur mainly in patients with borderline disease, often representing an upgrade toward the tuberculoid pole; less often they represent a downgrade reaction. Nerves become painful and swollen with possible loss of function, and skin lesions become edematous and tender; sometimes arthritis develops.9 Type II lepra reactions, also known as erythema nodosum leprosum (ENL), occur in borderline lepromatous and lepromatous patients with a high bacillary load. They are characterized by fever, body aches, tender cutaneous/subcutaneous nodules that may ulcerate, possible bullous lesions, painful nerve swellings, swollen joints, iritis, lymphadenitis, glomerulonephritis, epididymo-orchitis, and hepatic affection. Both immune-complex and delayed hypersensitivity reactions play a role in ENL.8,10 The third reaction is a rare aggressive type known as Lucio phenomenon or Lucio leprosy, which presents with irregular-shaped, angulated, or stellar necrotizing purpuric lesions (hemorrhagic infacrtions) developing mainly on the extremities. The lesions evolve into ulcers that heal with atrophic scarring.2,11 Lucio phenomenon develops as a result of thrombotic vascular occlusion secondary to massive invasion of vascular endothelial cells by lepra bacilli.2,11-14 Involvement of the scrotal skin, such as in our patient, is rare.
Lucio phenomenon mainly is seen in Mexico and Central America, and few cases have been documented in Cuba, South America, the United States, India, Polynesia, South Africa, and Southeast Asia.15-17 It specifically occurs in patients with untreated, diffuse, nonnodular lepromatous leprosy (pure and primitive diffuse lepromatous leprosy (DLL)/diffuse leprosy of Lucio and Latapí). This type of leprosy was first described by Lucio and Alvarado18 in 1852 as a distinct form of lepromatous leprosy characterized by widespread and dense infiltration of the whole skin by lepra bacilli without the typical nodular lesions of leprosy, rendering its diagnosis challenging, especially in sporadic cases. Other manifestations of DLL include complete alopecia of the eyebrows and eyelashes, destructive rhinitis, and areas of anhidrosis and dyesthesia.2
Latapí and Chévez-Zomora19 defined Lucio phenomenon in 1948 as a form of histopathologic vasculitis restricted to patients with DLL. Histopathologically, in addition to the infiltration of the skin with acid-fast bacilli–laden foamy histiocytes, lesions of Lucio phenomenon show features of necrotizing (leukocytoclastic) vasculitis with fibrinoid necrosis20 or vascular thrombi with minimal perivascular lymphocytic infiltrate and no evidence of vasculitis.11 Medium to large vessels in the deep dermis and subcutaneous tissue show infiltration of their walls with a large number of macrophages laden with acid-fast bacilli.11 Cases with histopathologic features mimicking antiphospholipid syndrome with endothelial cell proliferation, thrombosis, and mild mononuclear cell infiltrate also may be seen.20 In all cases, ischemic epidermal necrosis is seen, as well as acid-fast bacilli, both singly and in clusters (globi) within endothelial cells and inside blood vessel lumina.
Although Lucio phenomenon initially was thought to be immune-complex mediated like ENL, it has been suggested that the main trigger is thrombotic vascular occlusion secondary to massive invasion of the vascular endothelial cells by the lepra bacilli, resulting in necrosis.14 Bacterial lipopolysaccharides promote the release of IL-1 and tumor necrosis factor α, which in turn stimulate the production of prostaglandins, IL-6, and coagulation factor III, leading to vascular thrombosis and tissue necrosis.21,22 Moreover, antiphospholipid antibodies, which have been found to be induced in response to certain infectious agents in genetically predisposed individuals,23 have been reported in patients with leprosy, mainly in association with lepromatous leprosy. The reported prevalence of anticardiolipin antibodies ranged from 37% to 98%, whereas anti-β2-glycoprotein I antibodies ranged from 3% to 19%, and antiprothrombin antibodies ranged from 6% to 45%.24,25 Antiphospholipid antibodies have been reported to play a role in the pathogenesis of Lucio phenomenon.11,13,15,26 Our case supports this hypothesis with positive anticardiolipin antibodies, anti-β2 glycoprotein antibodies, and positive lupus anticoagulant.
In accordance with Curi et al,2 who reported 5 cases of DLL with Lucio phenomenon, our patient showed a similar presentation with positive inflammatory markers in association with a negative autoimmune profile (ANA, ANCA-C&P) and negative venereal disease research laboratory test. It is important to mention that a positive autoimmune profile (ANA, ANCA-C&P) can be present in leprotic patients, causing possible diagnostic confusion with collagen diseases.27,28
An interesting finding in our case was the negative slit-skin smear results. Although the specificity of slit-skin smear is 100%, as it directly demonstrates the presence of acid-fast bacilli,29 its sensitivity is low and varies from 10% to 50%.30 The detection of acid-fast bacilli in tissue sections is reported to be a better method for confirming the diagnosis of leprosy.31
The provisional impression of hemophagocytic lymphohistiocytosis in the lymph node biopsy in our patient was excluded upon detection of acid-fast bacilli in the foamy histiocytes infiltrating the lymph node; moreover, the normal serum lipids and serum ferritin argued against this diagnosis.32 Leprosy tends to involve the lymph nodes, particularly in borderline, borderline lepromatous, and lepromatous forms.33 The incidence of lymph node involvement accompanied by skin lesions with the presence of acid-fast bacilli in the lymph nodes is 92.2%.34
Our patient showed an excellent response to antileprotic treatment, which was administered according to the WHO multidrug therapy guidelines for multibacillary leprosy,35 combined with low-dose prednisolone, acetylsalicylic acid, and anticoagulant treatment. Thalidomide and high-dose prednisolone (60 mg/d) combined with antileprotic treatment also have been reported to be successful in managing recurrent infarctions in leprosy.36 The Fournier-like gangrenous ulcer of the scrotum was managed by surgical debridement and vacuum therapy.
It is noteworthy that the WHO elimination goal for leprosy was to reduce the prevalence to less than 1 case per 10,000 population. Egypt is among the first countries in North Africa and the Middle East regions to achieve this target supervised by the National Leprosy Control Program as early as 1994; this was further reduced to 0.33 cases per 10,000 population in 2004, and reduced again in 2009; however, certain foci showed a prevalence rate more than the elimination target, particularly in the cities of Qena (1.12) and Sohag (2.47).37 Esna, where our patient is from, is an endemic area in Egypt.38
Conclusion
1. World Health Organization. World Health Statistics: 2011. World Health Organization; 2011. https://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_Full.pdf
2. Curi PF, Villaroel JS, Migliore N, et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016;35:1397-1401.
3. Shrestha B, Li YQ, Fu P. Leprosy mimics adult onset Still’s disease in a Chinese patient. Egypt Rheumatol. 2018;40:217-220.
4. Prasad S, Misra R, Aggarwal A, et al. Leprosy revealed in a rheumatology clinic: a case series. Int J Rheum Dis. 2013;16:129-133.
5. Chao G, Fang L, Lu C. Leprosy with ANA positive mistaken for connective tissue disease. Clin Rheumatol. 2013;32:645-648.
6. Chauhan S, Wakhlu A, Agarwal V. Arthritis in leprosy. Rheumatology. 2010;49:2237-2242.
7. Rath D, Bhargava S, Kundu BK. Leprosy mimicking common rheumatologic entities: a trial for the clinician in the era of biologics. Case Rep Rheumatol. 2014;2014:429698.
8. Cuevas J, Rodríguez-Peralto JL, Carrillo R, et al. Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg. 2007;26:126-130.
9. Henriques CC, Lopéz B, Mestre T, et al. Leprosy and rheumatoid arthritis: consequence or association? BMJ Case Rep. 2012;13:1-4.
10. Vázquez-Botet M, Sánchez JL. Erythema nodosum leprosum. Int J Dermatol. 1987;26:436-437.
11. Nunzie E, Ortega Cabrera LV, Macanchi Moncayo FM, et al. Lucio leprosy with Lucio’s phenomenon, digital gangrene and anticardiolipin antibodies. Lepr Rev. 2014;85:194-200.
12. Salvi S, Chopra A. Leprosy in a rheumatology setting: a challenging mimic to expose. Clin Rheumatol. 2013;32:1557-1563.
13. Azulay-Abulafia L, Pereira SL, Hardmann D, et al. Lucio phenomenon. vasculitis or occlusive vasculopathy? Hautarzt. 2006;57:1101-1105.
14. Benard G, Sakai-Valente NY, Bianconcini Trindade MA. Concomittant Lucio phenomenon and erythema nodosum in a leprosy patient: clues for their distinct pathogenesis. Am J Dermatopathol. 2009;31:288-292.
15. Rocha RH, Emerich PS, Diniz LM, et al. Lucio’s phenomenon: exuberant case report and review of Brazilian cases. An Bras Dermatol. 2016;91(suppl 5):S60-S63.
16. Costa IM, Kawano LB, Pereira CP, et al. Lucio’s phenomenon: a case report and review of the literature. Int J Dermatol. 2005;44:566-571.
17. Kumari R, Thappa DM, Basu D. A fatal case of Lucio phenomenon from India. Dermatol Online J. 2008;14:10.
18. Lucio R, Alvarado I. Opúsculo Sobre el Mal de San Lázaro o Elefantiasis de los Griegos. M. Murguía; 1852.
19. Latapí F, Chévez-Zamora A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948;16:421-437.
20. Vargas OF. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78:248-260.
21. Latapí FR, Chevez-Zamora A. La lepra manchada de Lucio. Rev Dermatol Mex. 1978;22:102-107.
22. Monteiro R, Abreu MA, Tiezzi MG, et al. Fenômeno de Lúcio: mais um caso relatado no Brasil. An Bras Dermatol. 2012;87:296-300.
23. Gharavi EE, Chaimovich H, Cucucrull E, et al. Induction of antiphospholipid antibodies by immunization with synthetic bacterial & viral peptides. Lupus. 1999;8:449-455.
24. de Larrañaga GF, Forastiero RR, Martinuzzo ME, et al. High prevalence of antiphospholipid antibodies in leprosy: evaluation of antigen reactivity. Lupus. 2000;9:594-600.
25. Loizou S, Singh S, Wypkema E, et al. Anticardiolipin, anti-beta(2)-glycoprotein I and antiprothrombin antibodies in black South African patients with infectious disease. Ann Rheum Dis. 2003;62:1106-1111.
26. Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association; role of antiphospholipid antibodies. BMC Infect Dis. 2005,5:74.
27. Horta-Baas G, Hernández-Cabrera MF, Barile-Fabris LA, et al. Multibacillary leprosy mimicking systemic lupus erythematosus: case report and literature review. Lupus. 2015;24:1095-1102.
28. Pradhan V, Badakere SS, Shankar KU. Increased incidence of cytoplasmic ANCA (cANCA) and other auto antibodies in leprosy patients from western India. Lepr Rev. 2004;75:50-56.
29. Oskam L. Diagnosis and classification of leprosy. Lepr Rev. 2002;73:17-26.
30. Rao PN. Recent advances in the control programs and therapy of leprosy. Indian J Dermatol Venereol Leprol. 2004;70:269-276.
31. Rao PN, Pratap D, Ramana Reddy AV, et al. Evaluation of leprosy patients with 1 to 5 skin lesions with relevance to their grouping into paucibacillary or multibacillary disease. Indian J Dermatol Venereol Leprol. 2006;72:207-210.
32. Rosado FGN, Kim AS. Hemophagocytic lymphohistiocytosis. an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727.
33. Kar HK, Mohanty HC, Mohanty GN, et al. Clinicopathological study of lymph node involvement in leprosy. Lepr India. 1983;55:725-738.
34. Gupta JC, Panda PK, Shrivastava KK, et al. A histopathologic study of lymph nodes in 43 cases of leprosy. Lepr India. 1978;50:196-203.
35. WHO Expert Committee on Leprosy. Seventh Report. World Health Organization; 1998. https://apps.who.int/iris/bitstream/handle/10665/42060/WHO_TRS_874.pdf?sequence=1&isAllowed=y
36. Misra DP, Parida JR, Chowdhury AC, et al. Lepra reaction with Lucio phenomenon mimicking cutaneous vasculitis. Case Rep Immunol. 2014;2014:641989.
37. Amer A, Mansour A. Epidemiological study of leprosy in Egypt: 2005-2009. Egypt J Dermatol Venereol. 2014;34:70-73.
38. World Health Organization. Screening campaign aims to eliminate leprosy in Egypt. Published May 9, 2018. Accessed September 8, 2021. http://www.emro.who.int/egy/egypt-events/last-miless-activities-on-eliminating-leprosy-from-egypt.html
1. World Health Organization. World Health Statistics: 2011. World Health Organization; 2011. https://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_Full.pdf
2. Curi PF, Villaroel JS, Migliore N, et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016;35:1397-1401.
3. Shrestha B, Li YQ, Fu P. Leprosy mimics adult onset Still’s disease in a Chinese patient. Egypt Rheumatol. 2018;40:217-220.
4. Prasad S, Misra R, Aggarwal A, et al. Leprosy revealed in a rheumatology clinic: a case series. Int J Rheum Dis. 2013;16:129-133.
5. Chao G, Fang L, Lu C. Leprosy with ANA positive mistaken for connective tissue disease. Clin Rheumatol. 2013;32:645-648.
6. Chauhan S, Wakhlu A, Agarwal V. Arthritis in leprosy. Rheumatology. 2010;49:2237-2242.
7. Rath D, Bhargava S, Kundu BK. Leprosy mimicking common rheumatologic entities: a trial for the clinician in the era of biologics. Case Rep Rheumatol. 2014;2014:429698.
8. Cuevas J, Rodríguez-Peralto JL, Carrillo R, et al. Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg. 2007;26:126-130.
9. Henriques CC, Lopéz B, Mestre T, et al. Leprosy and rheumatoid arthritis: consequence or association? BMJ Case Rep. 2012;13:1-4.
10. Vázquez-Botet M, Sánchez JL. Erythema nodosum leprosum. Int J Dermatol. 1987;26:436-437.
11. Nunzie E, Ortega Cabrera LV, Macanchi Moncayo FM, et al. Lucio leprosy with Lucio’s phenomenon, digital gangrene and anticardiolipin antibodies. Lepr Rev. 2014;85:194-200.
12. Salvi S, Chopra A. Leprosy in a rheumatology setting: a challenging mimic to expose. Clin Rheumatol. 2013;32:1557-1563.
13. Azulay-Abulafia L, Pereira SL, Hardmann D, et al. Lucio phenomenon. vasculitis or occlusive vasculopathy? Hautarzt. 2006;57:1101-1105.
14. Benard G, Sakai-Valente NY, Bianconcini Trindade MA. Concomittant Lucio phenomenon and erythema nodosum in a leprosy patient: clues for their distinct pathogenesis. Am J Dermatopathol. 2009;31:288-292.
15. Rocha RH, Emerich PS, Diniz LM, et al. Lucio’s phenomenon: exuberant case report and review of Brazilian cases. An Bras Dermatol. 2016;91(suppl 5):S60-S63.
16. Costa IM, Kawano LB, Pereira CP, et al. Lucio’s phenomenon: a case report and review of the literature. Int J Dermatol. 2005;44:566-571.
17. Kumari R, Thappa DM, Basu D. A fatal case of Lucio phenomenon from India. Dermatol Online J. 2008;14:10.
18. Lucio R, Alvarado I. Opúsculo Sobre el Mal de San Lázaro o Elefantiasis de los Griegos. M. Murguía; 1852.
19. Latapí F, Chévez-Zamora A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948;16:421-437.
20. Vargas OF. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78:248-260.
21. Latapí FR, Chevez-Zamora A. La lepra manchada de Lucio. Rev Dermatol Mex. 1978;22:102-107.
22. Monteiro R, Abreu MA, Tiezzi MG, et al. Fenômeno de Lúcio: mais um caso relatado no Brasil. An Bras Dermatol. 2012;87:296-300.
23. Gharavi EE, Chaimovich H, Cucucrull E, et al. Induction of antiphospholipid antibodies by immunization with synthetic bacterial & viral peptides. Lupus. 1999;8:449-455.
24. de Larrañaga GF, Forastiero RR, Martinuzzo ME, et al. High prevalence of antiphospholipid antibodies in leprosy: evaluation of antigen reactivity. Lupus. 2000;9:594-600.
25. Loizou S, Singh S, Wypkema E, et al. Anticardiolipin, anti-beta(2)-glycoprotein I and antiprothrombin antibodies in black South African patients with infectious disease. Ann Rheum Dis. 2003;62:1106-1111.
26. Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association; role of antiphospholipid antibodies. BMC Infect Dis. 2005,5:74.
27. Horta-Baas G, Hernández-Cabrera MF, Barile-Fabris LA, et al. Multibacillary leprosy mimicking systemic lupus erythematosus: case report and literature review. Lupus. 2015;24:1095-1102.
28. Pradhan V, Badakere SS, Shankar KU. Increased incidence of cytoplasmic ANCA (cANCA) and other auto antibodies in leprosy patients from western India. Lepr Rev. 2004;75:50-56.
29. Oskam L. Diagnosis and classification of leprosy. Lepr Rev. 2002;73:17-26.
30. Rao PN. Recent advances in the control programs and therapy of leprosy. Indian J Dermatol Venereol Leprol. 2004;70:269-276.
31. Rao PN, Pratap D, Ramana Reddy AV, et al. Evaluation of leprosy patients with 1 to 5 skin lesions with relevance to their grouping into paucibacillary or multibacillary disease. Indian J Dermatol Venereol Leprol. 2006;72:207-210.
32. Rosado FGN, Kim AS. Hemophagocytic lymphohistiocytosis. an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727.
33. Kar HK, Mohanty HC, Mohanty GN, et al. Clinicopathological study of lymph node involvement in leprosy. Lepr India. 1983;55:725-738.
34. Gupta JC, Panda PK, Shrivastava KK, et al. A histopathologic study of lymph nodes in 43 cases of leprosy. Lepr India. 1978;50:196-203.
35. WHO Expert Committee on Leprosy. Seventh Report. World Health Organization; 1998. https://apps.who.int/iris/bitstream/handle/10665/42060/WHO_TRS_874.pdf?sequence=1&isAllowed=y
36. Misra DP, Parida JR, Chowdhury AC, et al. Lepra reaction with Lucio phenomenon mimicking cutaneous vasculitis. Case Rep Immunol. 2014;2014:641989.
37. Amer A, Mansour A. Epidemiological study of leprosy in Egypt: 2005-2009. Egypt J Dermatol Venereol. 2014;34:70-73.
38. World Health Organization. Screening campaign aims to eliminate leprosy in Egypt. Published May 9, 2018. Accessed September 8, 2021. http://www.emro.who.int/egy/egypt-events/last-miless-activities-on-eliminating-leprosy-from-egypt.html
Practice Points
- Leprosy is a great mimicker of many connective tissue diseases, including vasculitis.
- Antiphospholipid antibodies are involved in Lucio phenomenon.
- Prompt treatment is important in Lucio phenomenon to avoid morbidity and mortality.
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
Oral step-down therapy for infective endocarditis
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Risk for severe COVID-19 and death plummets with Pfizer booster
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Mumps: Sometimes forgotten but not gone
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
New HIV PrEP guidelines call for clinicians to talk to patients about HIV prevention meds
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
Despite ‘getting it wrong’ we must continue to do what’s right
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
Cervical cancer update: The latest on screening & management
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7
Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access
ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22
Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.
Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; Linda.speer@utoledo.edu
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7
Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access
ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22
Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.
Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; Linda.speer@utoledo.edu
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7
Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access
ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22
Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.
Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; Linda.speer@utoledo.edu
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
PRACTICE RECOMMENDATIONS
› Encourage eligible patients to be vaccinated against human papillomavirus (HPV) because the vaccine is highly effective for preventing cervical dysplasia, especially when given to patients previously unexposed to the virus. A
› Screen for cervical disease with either cytology plus HPV testing or primary HPV testing with secondary triage for cytology; both protocols are more accurate than screening with cervical cytology alone, and allow you to widen the screening interval. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
2021 CDC guidelines on sexually transmitted infections
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.
Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1
Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.
Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1
Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.
Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1
Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html