User login
Fluoroquinolones linked to sudden death risk for those on hemodialysis
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at victoria.persampiere@jefferson.edu or via fpnews@mdedge.com.
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at victoria.persampiere@jefferson.edu or via fpnews@mdedge.com.
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at victoria.persampiere@jefferson.edu or via fpnews@mdedge.com.
Molluscum Contagiosum Superimposed on Lymphangioma Circumscriptum
To the Editor:
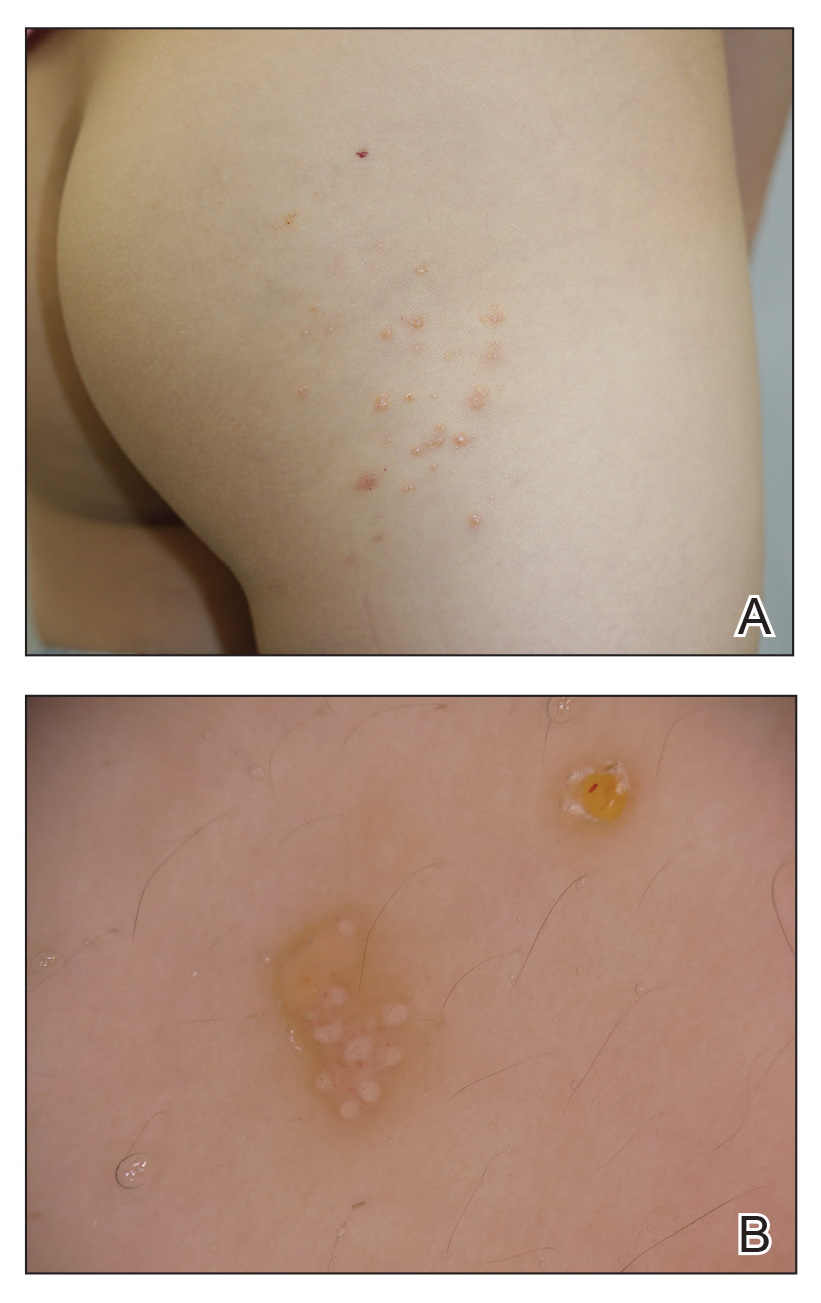
Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system.1 It is postulated to arise from abnormal lymphatic cisterns, and it grows separately from the normal lymphatic system. These cisterns are connected to malformed dermal lymphatic channels, and the contraction of smooth muscles lining cisterns will cause dilatation of connected lymphatic channels in the papillary dermis due to back pressure,1,2 which causes a classic LC manifestation characterized by multiple translucent, sometimes red-brown, small vesicles grouped together. Lymphangioma circumscriptum can be difficult to differentiate from molluscum contagiosum (MC) due to the similar morphology.1 We present a notable case of MC superimposed on LC.
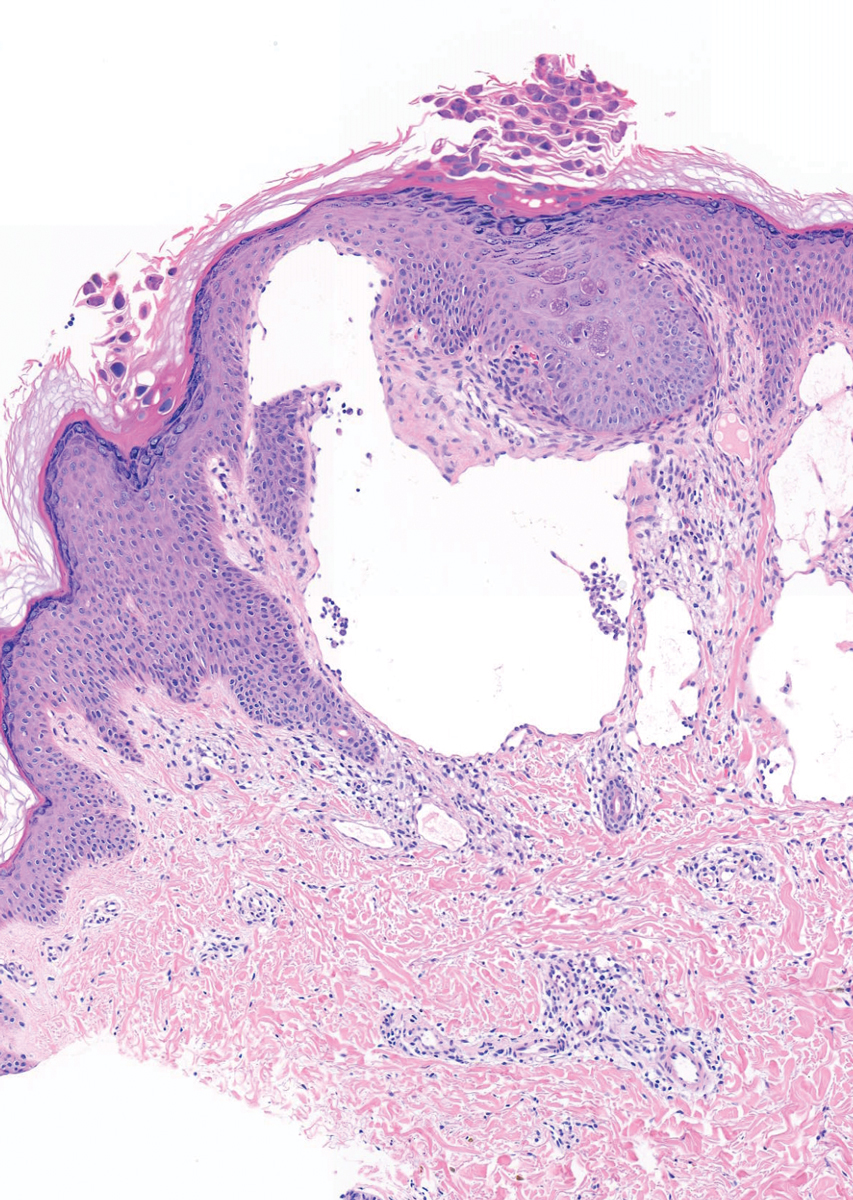
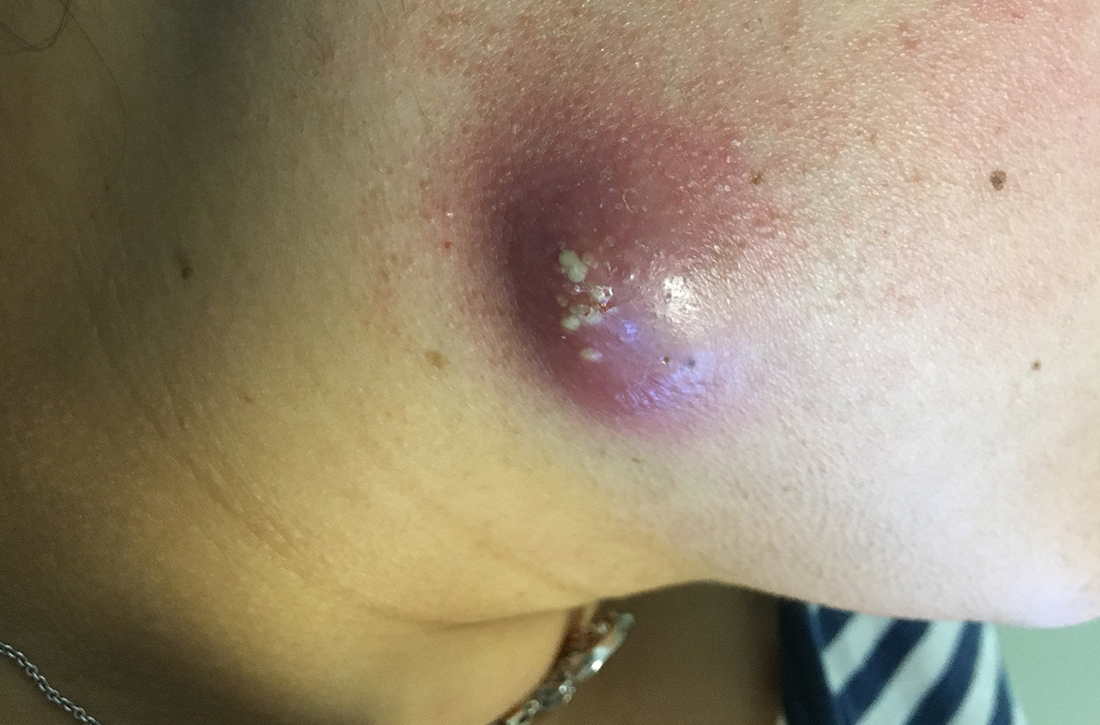
A 6-year-old girl presented with multiple grouped, clear, vesicular papules on the right buttock of 18 months’ duration. Some of the papules showed tiny whitish pearl-like particles on the top (Figure 1). Similar lesions were not present elsewhere on the body. She had no underlying disease and did not have a history of procedure, edema, or malformation of the lower extremities. Histopathology from one of the lesions showed dilated cystic lymphatic spaces in the papillary dermis lined with flattened endothelium and cup-shaped downward proliferation of the epidermis with presence of large intracytoplasmic inclusion bodies—features of both LC and MC (Figure 2). We waited 4 additional months for the MC lesions to self-resolve, but they persisted. The patient’s mother strongly requested for their removal, and the residual MC lesions were carefully removed by CO2 laser. To prevent unnecessary physical damage to underlying LC lesions and minimize scarring, we opted to use the CO2 laser and not simple curettage. She currently is under periodic observation with no signs of clinical recurrence of MC, but the LC lesions naturally persisted.
Due to its vesicular and sometimes warty appearance, LC can sometimes be hard to differentiate from MC. In one report, a vesicular plaquelike lesion on the trunk initially was misdiagnosed and treated as MC but was histologically confirmed as LC several years later.3 Our case demonstrates the coexistence of MC and LC. Although this phenomenon may be coincidental, we have not noticed any additional MC lesions on the body and MC only existed over the LC lesions, implying a possible pathophysiologic relationship. It is unlikely that MC might have preceded the development of LC. Although acquired LC exists, it has mostly been reported in the genital region of patients with conditions leading to lymphatic obstruction such as surgery, radiation therapy, malignancy, or serious infections.4 Because our patient developed lesions at an early age without any remarkable medical history, it is likely that she had congenital LC that was secondarily infected by the MC virus. Vesicular lesions in LC are known to rupture easily and may serve as a vulnerable entry site for pathogens. Subsequent secondary bacterial infections are common, with Staphylococcus aureus being the most prominent entity.1 However, secondary viral infection rarely is reported. It is possible that the abnormally dilated lymphatic channels of LC that lack communication with the normal lymphatic system have contributed to an LC site-specific vulnerability to MC virus. Further studies and subsequent reports are required to confirm this hypothesis.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111/j.1365-4632.2009.04226.x
- Fatima S, Uddin N, Idrees R, et al. Lymphangioma circumscriptum: clinicopathological spectrum of 29 cases. J Coll Physicians Surg Pak. 2015;25:658-661. doi:09.2015/JCPSP.658661
- Patel GA, Siperstein RD, Ragi G, Schwartz RA. Zosteriform lymphangioma circumscriptum. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:179-182.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487. doi:10.1111/ijd.13264
To the Editor:
Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system.1 It is postulated to arise from abnormal lymphatic cisterns, and it grows separately from the normal lymphatic system. These cisterns are connected to malformed dermal lymphatic channels, and the contraction of smooth muscles lining cisterns will cause dilatation of connected lymphatic channels in the papillary dermis due to back pressure,1,2 which causes a classic LC manifestation characterized by multiple translucent, sometimes red-brown, small vesicles grouped together. Lymphangioma circumscriptum can be difficult to differentiate from molluscum contagiosum (MC) due to the similar morphology.1 We present a notable case of MC superimposed on LC.
A 6-year-old girl presented with multiple grouped, clear, vesicular papules on the right buttock of 18 months’ duration. Some of the papules showed tiny whitish pearl-like particles on the top (Figure 1). Similar lesions were not present elsewhere on the body. She had no underlying disease and did not have a history of procedure, edema, or malformation of the lower extremities. Histopathology from one of the lesions showed dilated cystic lymphatic spaces in the papillary dermis lined with flattened endothelium and cup-shaped downward proliferation of the epidermis with presence of large intracytoplasmic inclusion bodies—features of both LC and MC (Figure 2). We waited 4 additional months for the MC lesions to self-resolve, but they persisted. The patient’s mother strongly requested for their removal, and the residual MC lesions were carefully removed by CO2 laser. To prevent unnecessary physical damage to underlying LC lesions and minimize scarring, we opted to use the CO2 laser and not simple curettage. She currently is under periodic observation with no signs of clinical recurrence of MC, but the LC lesions naturally persisted.
Due to its vesicular and sometimes warty appearance, LC can sometimes be hard to differentiate from MC. In one report, a vesicular plaquelike lesion on the trunk initially was misdiagnosed and treated as MC but was histologically confirmed as LC several years later.3 Our case demonstrates the coexistence of MC and LC. Although this phenomenon may be coincidental, we have not noticed any additional MC lesions on the body and MC only existed over the LC lesions, implying a possible pathophysiologic relationship. It is unlikely that MC might have preceded the development of LC. Although acquired LC exists, it has mostly been reported in the genital region of patients with conditions leading to lymphatic obstruction such as surgery, radiation therapy, malignancy, or serious infections.4 Because our patient developed lesions at an early age without any remarkable medical history, it is likely that she had congenital LC that was secondarily infected by the MC virus. Vesicular lesions in LC are known to rupture easily and may serve as a vulnerable entry site for pathogens. Subsequent secondary bacterial infections are common, with Staphylococcus aureus being the most prominent entity.1 However, secondary viral infection rarely is reported. It is possible that the abnormally dilated lymphatic channels of LC that lack communication with the normal lymphatic system have contributed to an LC site-specific vulnerability to MC virus. Further studies and subsequent reports are required to confirm this hypothesis.
To the Editor:
Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system.1 It is postulated to arise from abnormal lymphatic cisterns, and it grows separately from the normal lymphatic system. These cisterns are connected to malformed dermal lymphatic channels, and the contraction of smooth muscles lining cisterns will cause dilatation of connected lymphatic channels in the papillary dermis due to back pressure,1,2 which causes a classic LC manifestation characterized by multiple translucent, sometimes red-brown, small vesicles grouped together. Lymphangioma circumscriptum can be difficult to differentiate from molluscum contagiosum (MC) due to the similar morphology.1 We present a notable case of MC superimposed on LC.
A 6-year-old girl presented with multiple grouped, clear, vesicular papules on the right buttock of 18 months’ duration. Some of the papules showed tiny whitish pearl-like particles on the top (Figure 1). Similar lesions were not present elsewhere on the body. She had no underlying disease and did not have a history of procedure, edema, or malformation of the lower extremities. Histopathology from one of the lesions showed dilated cystic lymphatic spaces in the papillary dermis lined with flattened endothelium and cup-shaped downward proliferation of the epidermis with presence of large intracytoplasmic inclusion bodies—features of both LC and MC (Figure 2). We waited 4 additional months for the MC lesions to self-resolve, but they persisted. The patient’s mother strongly requested for their removal, and the residual MC lesions were carefully removed by CO2 laser. To prevent unnecessary physical damage to underlying LC lesions and minimize scarring, we opted to use the CO2 laser and not simple curettage. She currently is under periodic observation with no signs of clinical recurrence of MC, but the LC lesions naturally persisted.
Due to its vesicular and sometimes warty appearance, LC can sometimes be hard to differentiate from MC. In one report, a vesicular plaquelike lesion on the trunk initially was misdiagnosed and treated as MC but was histologically confirmed as LC several years later.3 Our case demonstrates the coexistence of MC and LC. Although this phenomenon may be coincidental, we have not noticed any additional MC lesions on the body and MC only existed over the LC lesions, implying a possible pathophysiologic relationship. It is unlikely that MC might have preceded the development of LC. Although acquired LC exists, it has mostly been reported in the genital region of patients with conditions leading to lymphatic obstruction such as surgery, radiation therapy, malignancy, or serious infections.4 Because our patient developed lesions at an early age without any remarkable medical history, it is likely that she had congenital LC that was secondarily infected by the MC virus. Vesicular lesions in LC are known to rupture easily and may serve as a vulnerable entry site for pathogens. Subsequent secondary bacterial infections are common, with Staphylococcus aureus being the most prominent entity.1 However, secondary viral infection rarely is reported. It is possible that the abnormally dilated lymphatic channels of LC that lack communication with the normal lymphatic system have contributed to an LC site-specific vulnerability to MC virus. Further studies and subsequent reports are required to confirm this hypothesis.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111/j.1365-4632.2009.04226.x
- Fatima S, Uddin N, Idrees R, et al. Lymphangioma circumscriptum: clinicopathological spectrum of 29 cases. J Coll Physicians Surg Pak. 2015;25:658-661. doi:09.2015/JCPSP.658661
- Patel GA, Siperstein RD, Ragi G, Schwartz RA. Zosteriform lymphangioma circumscriptum. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:179-182.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487. doi:10.1111/ijd.13264
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111/j.1365-4632.2009.04226.x
- Fatima S, Uddin N, Idrees R, et al. Lymphangioma circumscriptum: clinicopathological spectrum of 29 cases. J Coll Physicians Surg Pak. 2015;25:658-661. doi:09.2015/JCPSP.658661
- Patel GA, Siperstein RD, Ragi G, Schwartz RA. Zosteriform lymphangioma circumscriptum. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:179-182.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487. doi:10.1111/ijd.13264
Practice Points
- Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system that can be misdiagnosed as molluscum contagiosum (MC).
- Secondary infection of LC is common, with Staphylococcus aureus being the most common entity, but MC virus also can be secondarily infected.
ACIP recommends Shingrix for younger immunocompromised adults; updates pneumococcal vaccine guidance
The U.S. Centers for Disease Control and Prevention Advisory Committee of Immunization Practices has voted to recommend Shingrix (zoster vaccine recombinant, adjuvanted) for the prevention of shingles in immunodeficient or immunosuppressed adults aged 19 or older. The recommendation was approved Oct. 20 by a unanimous vote.
Shingles is a reactivation of varicella zoster virus (VZV), the virus that causes chickenpox. There are about 1 million cases of shingles in the United States every year, according to CDC estimates, and one in three Americans will develop shingles over their lifetime. While adults older than 50 are one of the most vulnerable groups to reinfection – with about 99% having been infected with VZV – a weakened immune system is another common risk factor.
The Food and Drug Administration originally approved Shingrix in 2017 for the prevention of shingles in adults over 50; in July of this year, the vaccine was approved for immunodeficient adults aged 18 or older. The approval and subsequent recommendation by ACIP were based on clinical studies of Shingrix in adults being treated for hematologic malignancies or those who had undergone an autologous hematopoietic stem cell transplant.
According to a press statement from the FDA, “Further safety and immunogenicity data were generated in adults who were, or were anticipated to be, immunodeficient or immunosuppressed due to known disease or therapy, including patients with HIV, solid tumors, and renal transplants.”
For adults with functional immune systems, Shingrix is administered in two doses, 2-6 months apart. For immunocompromised individuals, the second dose can be given 1-2 months after the first dose.
During the same meeting, ACIP also voted to recommend pneumococcal vaccines for routine use in adults older than 65 and in adults aged 19-64 with chronic conditions such as diabetes, chronic heart disease, chronic liver disease, and HIV, and disease risk factors like smoking and alcoholism. The recommendation only applies to those who have not received a pneumococcal conjugate vaccine or whose vaccination history is unknown. The recommendation states that qualifying adults should be vaccinated with the 15-valent pneumococcal conjugate vaccine Vaxneuvance followed by Pneumovax23, or a single dose of the 20-valent pneumococcal conjugate vaccine Prevnar 20.
These ACIP recommendations will now be sent to the directors of the CDC and the U.S. Department of Health & Human Services for review and approval. If approved, the recommendations are considered finalized and will be published in a future Morbidity and Mortality Weekly Report.
A version of this article first appeared on Medscape.com.
The U.S. Centers for Disease Control and Prevention Advisory Committee of Immunization Practices has voted to recommend Shingrix (zoster vaccine recombinant, adjuvanted) for the prevention of shingles in immunodeficient or immunosuppressed adults aged 19 or older. The recommendation was approved Oct. 20 by a unanimous vote.
Shingles is a reactivation of varicella zoster virus (VZV), the virus that causes chickenpox. There are about 1 million cases of shingles in the United States every year, according to CDC estimates, and one in three Americans will develop shingles over their lifetime. While adults older than 50 are one of the most vulnerable groups to reinfection – with about 99% having been infected with VZV – a weakened immune system is another common risk factor.
The Food and Drug Administration originally approved Shingrix in 2017 for the prevention of shingles in adults over 50; in July of this year, the vaccine was approved for immunodeficient adults aged 18 or older. The approval and subsequent recommendation by ACIP were based on clinical studies of Shingrix in adults being treated for hematologic malignancies or those who had undergone an autologous hematopoietic stem cell transplant.
According to a press statement from the FDA, “Further safety and immunogenicity data were generated in adults who were, or were anticipated to be, immunodeficient or immunosuppressed due to known disease or therapy, including patients with HIV, solid tumors, and renal transplants.”
For adults with functional immune systems, Shingrix is administered in two doses, 2-6 months apart. For immunocompromised individuals, the second dose can be given 1-2 months after the first dose.
During the same meeting, ACIP also voted to recommend pneumococcal vaccines for routine use in adults older than 65 and in adults aged 19-64 with chronic conditions such as diabetes, chronic heart disease, chronic liver disease, and HIV, and disease risk factors like smoking and alcoholism. The recommendation only applies to those who have not received a pneumococcal conjugate vaccine or whose vaccination history is unknown. The recommendation states that qualifying adults should be vaccinated with the 15-valent pneumococcal conjugate vaccine Vaxneuvance followed by Pneumovax23, or a single dose of the 20-valent pneumococcal conjugate vaccine Prevnar 20.
These ACIP recommendations will now be sent to the directors of the CDC and the U.S. Department of Health & Human Services for review and approval. If approved, the recommendations are considered finalized and will be published in a future Morbidity and Mortality Weekly Report.
A version of this article first appeared on Medscape.com.
The U.S. Centers for Disease Control and Prevention Advisory Committee of Immunization Practices has voted to recommend Shingrix (zoster vaccine recombinant, adjuvanted) for the prevention of shingles in immunodeficient or immunosuppressed adults aged 19 or older. The recommendation was approved Oct. 20 by a unanimous vote.
Shingles is a reactivation of varicella zoster virus (VZV), the virus that causes chickenpox. There are about 1 million cases of shingles in the United States every year, according to CDC estimates, and one in three Americans will develop shingles over their lifetime. While adults older than 50 are one of the most vulnerable groups to reinfection – with about 99% having been infected with VZV – a weakened immune system is another common risk factor.
The Food and Drug Administration originally approved Shingrix in 2017 for the prevention of shingles in adults over 50; in July of this year, the vaccine was approved for immunodeficient adults aged 18 or older. The approval and subsequent recommendation by ACIP were based on clinical studies of Shingrix in adults being treated for hematologic malignancies or those who had undergone an autologous hematopoietic stem cell transplant.
According to a press statement from the FDA, “Further safety and immunogenicity data were generated in adults who were, or were anticipated to be, immunodeficient or immunosuppressed due to known disease or therapy, including patients with HIV, solid tumors, and renal transplants.”
For adults with functional immune systems, Shingrix is administered in two doses, 2-6 months apart. For immunocompromised individuals, the second dose can be given 1-2 months after the first dose.
During the same meeting, ACIP also voted to recommend pneumococcal vaccines for routine use in adults older than 65 and in adults aged 19-64 with chronic conditions such as diabetes, chronic heart disease, chronic liver disease, and HIV, and disease risk factors like smoking and alcoholism. The recommendation only applies to those who have not received a pneumococcal conjugate vaccine or whose vaccination history is unknown. The recommendation states that qualifying adults should be vaccinated with the 15-valent pneumococcal conjugate vaccine Vaxneuvance followed by Pneumovax23, or a single dose of the 20-valent pneumococcal conjugate vaccine Prevnar 20.
These ACIP recommendations will now be sent to the directors of the CDC and the U.S. Department of Health & Human Services for review and approval. If approved, the recommendations are considered finalized and will be published in a future Morbidity and Mortality Weekly Report.
A version of this article first appeared on Medscape.com.
Sepsis multiplies in-hospital mortality risk in COPD
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
White House announces vaccination plans for younger children
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
Fungal infection can mimic lung cancer metastases
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
REPORTING FROM CHEST 2021
Painful facial abscess
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.
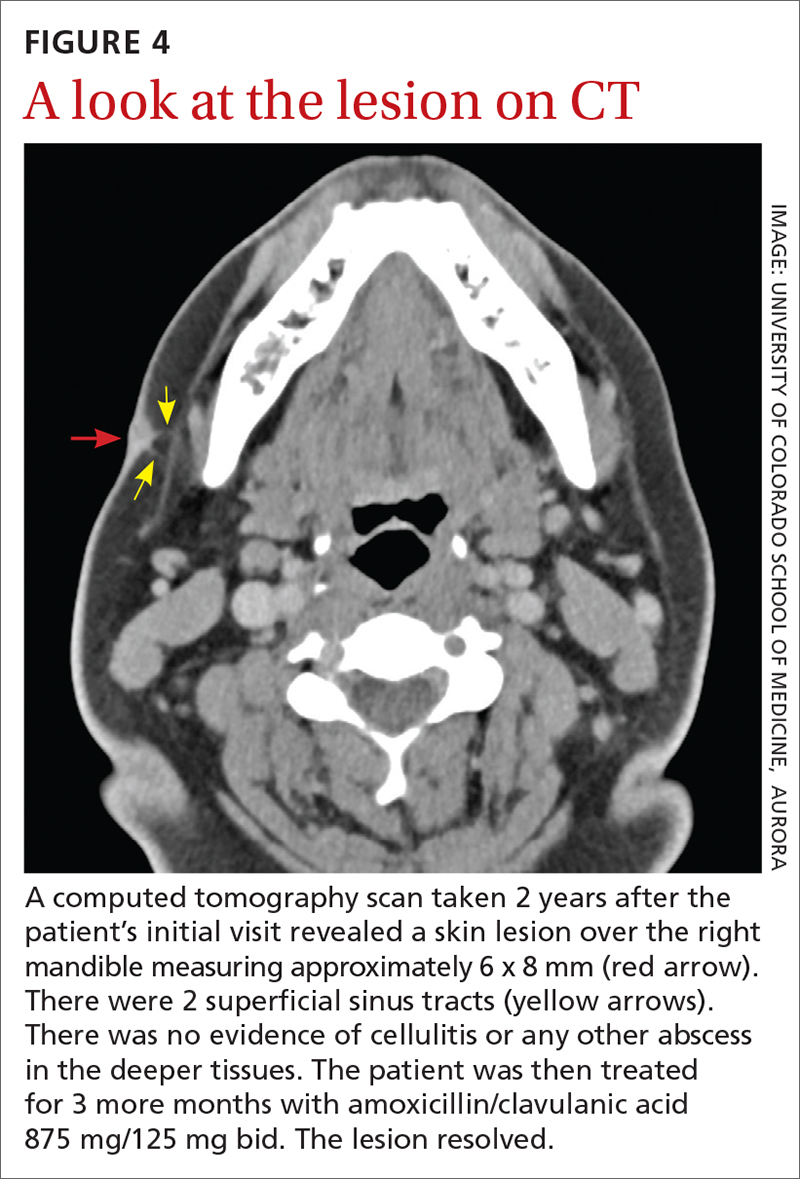
We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
Influenza vaccine update, 2021-22
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.
Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1
In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.
The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4
Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.
Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1
In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.
The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4
Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.
Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1
In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.
The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4
Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
Children and COVID: Vaccinations lower than ever as cases continue to drop
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.