User login
New COVID vaccines force bivalents out
COVID vaccines will have a new formulation in 2023, according to a decision announced by the U.S. Food and Drug Administration, that will focus efforts on circulating variants. The move pushes last year’s bivalent vaccines out of circulation because they will no longer be authorized for use in the United States.
The updated mRNA vaccines for 2023-2024 are being revised to include a single component that corresponds to the Omicron variant XBB.1.5. Like the bivalents offered before, the new monovalents are being manufactured by Moderna and Pfizer.
The new vaccines are authorized for use in individuals age 6 months and older. And the new options are being developed using a similar process as previous formulations, according to the FDA.
Targeting circulating variants
In recent studies, regulators point out the extent of neutralization observed by the updated vaccines against currently circulating viral variants causing COVID-19, including EG.5, BA.2.86, appears to be of a similar magnitude to the extent of neutralization observed with previous versions of the vaccines against corresponding prior variants.
“This suggests that the vaccines are a good match for protecting against the currently circulating COVID-19 variants,” according to the report.
Hundreds of millions of people in the United States have already received previously approved mRNA COVID vaccines, according to regulators who say the benefit-to-risk profile is well understood as they move forward with new formulations.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
Timing the effort
On Sept. 12 the U.S. Centers for Disease Control and Prevention recommended that everyone 6 months and older get an updated COVID-19 vaccine. Updated vaccines from Pfizer-BioNTech and Moderna will be available later this week, according to the agency.
This article was updated 9/14/23.
A version of this article appeared on Medscape.com.
COVID vaccines will have a new formulation in 2023, according to a decision announced by the U.S. Food and Drug Administration, that will focus efforts on circulating variants. The move pushes last year’s bivalent vaccines out of circulation because they will no longer be authorized for use in the United States.
The updated mRNA vaccines for 2023-2024 are being revised to include a single component that corresponds to the Omicron variant XBB.1.5. Like the bivalents offered before, the new monovalents are being manufactured by Moderna and Pfizer.
The new vaccines are authorized for use in individuals age 6 months and older. And the new options are being developed using a similar process as previous formulations, according to the FDA.
Targeting circulating variants
In recent studies, regulators point out the extent of neutralization observed by the updated vaccines against currently circulating viral variants causing COVID-19, including EG.5, BA.2.86, appears to be of a similar magnitude to the extent of neutralization observed with previous versions of the vaccines against corresponding prior variants.
“This suggests that the vaccines are a good match for protecting against the currently circulating COVID-19 variants,” according to the report.
Hundreds of millions of people in the United States have already received previously approved mRNA COVID vaccines, according to regulators who say the benefit-to-risk profile is well understood as they move forward with new formulations.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
Timing the effort
On Sept. 12 the U.S. Centers for Disease Control and Prevention recommended that everyone 6 months and older get an updated COVID-19 vaccine. Updated vaccines from Pfizer-BioNTech and Moderna will be available later this week, according to the agency.
This article was updated 9/14/23.
A version of this article appeared on Medscape.com.
COVID vaccines will have a new formulation in 2023, according to a decision announced by the U.S. Food and Drug Administration, that will focus efforts on circulating variants. The move pushes last year’s bivalent vaccines out of circulation because they will no longer be authorized for use in the United States.
The updated mRNA vaccines for 2023-2024 are being revised to include a single component that corresponds to the Omicron variant XBB.1.5. Like the bivalents offered before, the new monovalents are being manufactured by Moderna and Pfizer.
The new vaccines are authorized for use in individuals age 6 months and older. And the new options are being developed using a similar process as previous formulations, according to the FDA.
Targeting circulating variants
In recent studies, regulators point out the extent of neutralization observed by the updated vaccines against currently circulating viral variants causing COVID-19, including EG.5, BA.2.86, appears to be of a similar magnitude to the extent of neutralization observed with previous versions of the vaccines against corresponding prior variants.
“This suggests that the vaccines are a good match for protecting against the currently circulating COVID-19 variants,” according to the report.
Hundreds of millions of people in the United States have already received previously approved mRNA COVID vaccines, according to regulators who say the benefit-to-risk profile is well understood as they move forward with new formulations.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
Timing the effort
On Sept. 12 the U.S. Centers for Disease Control and Prevention recommended that everyone 6 months and older get an updated COVID-19 vaccine. Updated vaccines from Pfizer-BioNTech and Moderna will be available later this week, according to the agency.
This article was updated 9/14/23.
A version of this article appeared on Medscape.com.
RSV season has started, and this year could be different
The Centers for Disease Control and Prevention issued a national alert to health officials Sept. 5, urging them to offer new medicines that can prevent severe cases of the respiratory virus in very young children and in older people. Those two groups are at the highest risk of potentially deadly complications from RSV.
Typically, the CDC considers the start of RSV season to occur when the rate of positive tests for the virus goes above 3% for 2 consecutive weeks. In Florida, the rate has been around 5% in recent weeks, and in Georgia, there has been an increase in RSV-related hospitalizations. Most of the hospitalizations in Georgia have been among infants less than a year old.
“Historically, such regional increases have predicted the beginning of RSV season nationally, with increased RSV activity spreading north and west over the following 2-3 months,” the CDC said.
Most children have been infected with RSV by the time they are 2 years old. Historically, up to 80,000 children under 5 years old are hospitalized annually because of the virus, and between 100 and 300 die from complications each year.
Those figures could be drastically different this year because new preventive treatments are available.
The CDC recommends that all children under 8 months old receive the newly approved monoclonal antibody treatment nirsevimab (Beyfortus). Children up to 19 months old at high risk of severe complications from RSV are also eligible for the single-dose shot. In clinical trials, the treatment was 80% effective at preventing RSV infections from becoming so severe that children had to be hospitalized. The protection lasted about 5 months.
Older people are also at a heightened risk of severe illness from RSV, and two new vaccines are available this season. The vaccines are called Arexvy and Abrysvo, and the single-dose shots are approved for people ages 60 years and older. They are more than 80% effective at making severe lower respiratory complications less likely.
Last year’s RSV season started during the summer and peaked in October and November, which was earlier than usual. There’s no indication yet of when RSV season may peak this year. Last year and throughout the pandemic, RSV held its historical pattern of starting in Florida.
A version of this article appeared on WebMD.com.
The Centers for Disease Control and Prevention issued a national alert to health officials Sept. 5, urging them to offer new medicines that can prevent severe cases of the respiratory virus in very young children and in older people. Those two groups are at the highest risk of potentially deadly complications from RSV.
Typically, the CDC considers the start of RSV season to occur when the rate of positive tests for the virus goes above 3% for 2 consecutive weeks. In Florida, the rate has been around 5% in recent weeks, and in Georgia, there has been an increase in RSV-related hospitalizations. Most of the hospitalizations in Georgia have been among infants less than a year old.
“Historically, such regional increases have predicted the beginning of RSV season nationally, with increased RSV activity spreading north and west over the following 2-3 months,” the CDC said.
Most children have been infected with RSV by the time they are 2 years old. Historically, up to 80,000 children under 5 years old are hospitalized annually because of the virus, and between 100 and 300 die from complications each year.
Those figures could be drastically different this year because new preventive treatments are available.
The CDC recommends that all children under 8 months old receive the newly approved monoclonal antibody treatment nirsevimab (Beyfortus). Children up to 19 months old at high risk of severe complications from RSV are also eligible for the single-dose shot. In clinical trials, the treatment was 80% effective at preventing RSV infections from becoming so severe that children had to be hospitalized. The protection lasted about 5 months.
Older people are also at a heightened risk of severe illness from RSV, and two new vaccines are available this season. The vaccines are called Arexvy and Abrysvo, and the single-dose shots are approved for people ages 60 years and older. They are more than 80% effective at making severe lower respiratory complications less likely.
Last year’s RSV season started during the summer and peaked in October and November, which was earlier than usual. There’s no indication yet of when RSV season may peak this year. Last year and throughout the pandemic, RSV held its historical pattern of starting in Florida.
A version of this article appeared on WebMD.com.
The Centers for Disease Control and Prevention issued a national alert to health officials Sept. 5, urging them to offer new medicines that can prevent severe cases of the respiratory virus in very young children and in older people. Those two groups are at the highest risk of potentially deadly complications from RSV.
Typically, the CDC considers the start of RSV season to occur when the rate of positive tests for the virus goes above 3% for 2 consecutive weeks. In Florida, the rate has been around 5% in recent weeks, and in Georgia, there has been an increase in RSV-related hospitalizations. Most of the hospitalizations in Georgia have been among infants less than a year old.
“Historically, such regional increases have predicted the beginning of RSV season nationally, with increased RSV activity spreading north and west over the following 2-3 months,” the CDC said.
Most children have been infected with RSV by the time they are 2 years old. Historically, up to 80,000 children under 5 years old are hospitalized annually because of the virus, and between 100 and 300 die from complications each year.
Those figures could be drastically different this year because new preventive treatments are available.
The CDC recommends that all children under 8 months old receive the newly approved monoclonal antibody treatment nirsevimab (Beyfortus). Children up to 19 months old at high risk of severe complications from RSV are also eligible for the single-dose shot. In clinical trials, the treatment was 80% effective at preventing RSV infections from becoming so severe that children had to be hospitalized. The protection lasted about 5 months.
Older people are also at a heightened risk of severe illness from RSV, and two new vaccines are available this season. The vaccines are called Arexvy and Abrysvo, and the single-dose shots are approved for people ages 60 years and older. They are more than 80% effective at making severe lower respiratory complications less likely.
Last year’s RSV season started during the summer and peaked in October and November, which was earlier than usual. There’s no indication yet of when RSV season may peak this year. Last year and throughout the pandemic, RSV held its historical pattern of starting in Florida.
A version of this article appeared on WebMD.com.
New Moderna vaccine to work against recent COVID variant
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
Long COVID and new migraines: What’s the link?
.
“I’ve also noticed visual disturbances, like flickering lights or blurred vision, which I later learned are called auras,” the 30-year-old medical billing specialist in Seattle told this news organization.
Mr. Solomon isn’t alone. It’s estimated that 1 out of 8 people with COVID develop long COVID. Of those persons, 44% also experience headaches. Research has found that many of those headaches are migraines – and many patients who are afflicted say they had never had a migraine before. These migraines tend to persist for at least 5 or 6 months, according to data from the American Headache Society.
What’s more, other patients may suddenly have more frequent or intense versions of headaches they’ve not noticed before.
The mechanism as to how long COVID could manifest migraines is not yet fully understood, but many doctors believe that inflammation caused by the virus plays a key role.
“To understand why some patients have migraine in long COVID, we have to go back to understand the role of inflammation in COVID-19 itself,” says Emad Estemalik, MD, clinical assistant professor of neurology at Cleveland Clinic Lerner College of Medicine and section head of headache medicine at Cleveland Clinic.
In COVID-19, inflammation occurs because of a cytokine storm. Cytokines, which are proteins essential for a strong immune system, can be overproduced in a patient with COVID, which causes too much inflammation in any organ in the body, including the brain. This can result in new daily headache for some patients.
A new study from Italian researchers found that many patients who develop migraines for the first time while ill with long COVID are middle-aged women (traditionally a late point in life for a first migraine) who have a family history of migraine. Potential causes could have to do with the immune system remaining persistently activated from inflammation during long COVID, as well as the activation of the trigeminovascular system in the brain, which contains neurons that can trigger a migraine.
What treatments can work for migraines related to long COVID?
Long COVID usually causes a constellation of other symptoms at the same time as migraine.
“It’s so important for patients to take an interdisciplinary approach,” Dr. Estemalik stresses. “Patients should make sure their doctors are addressing all of their symptoms.”
When it comes to specifically targeting migraines, standard treatments can be effective.
“In terms of treating migraine in long COVID patients, we don’t do anything different or special,” says Matthew E. Fink, MD, chair of neurology at Weill Cornell Medical College and chief of the Division of Stroke and Critical Care Neurology at New York–Presbyterian Hospital/Weill Cornell Medical Center. “We treat these patients with standard migraine medications.”
Mr. Solomon is following this course of action.
“My doctor prescribed triptans, which have been somewhat effective in reducing the severity and duration of the migraines,” he says. A daily supplement of magnesium and a daily dose of aspirin can also work for some patients, according to Dr. Fink.
Lifestyle modification is also a great idea.
“Patients should keep regular sleep hours, getting up and going to bed at the same time every day,” Dr. Fink continues. “Daily exercise is also recommended.”
Mr. Solomon suggests tracking migraine triggers and patterns in a journal.
“Try to identify lifestyle changes that help, like managing stress and staying hydrated,” Mr. Solomon advises. “Seeking support from health care professionals and support groups can make a significant difference.”
The best news of all: for patients that are diligent in following these strategies, they’ve been proven to work.
“We doctors are very optimistic when it comes to good outcomes for patients with long COVID and migraine,” Dr. Fink says. “I reassure my patients by telling them, ‘You will get better long-term.’ ”
A version of this article appeared on Medscape.com.
.
“I’ve also noticed visual disturbances, like flickering lights or blurred vision, which I later learned are called auras,” the 30-year-old medical billing specialist in Seattle told this news organization.
Mr. Solomon isn’t alone. It’s estimated that 1 out of 8 people with COVID develop long COVID. Of those persons, 44% also experience headaches. Research has found that many of those headaches are migraines – and many patients who are afflicted say they had never had a migraine before. These migraines tend to persist for at least 5 or 6 months, according to data from the American Headache Society.
What’s more, other patients may suddenly have more frequent or intense versions of headaches they’ve not noticed before.
The mechanism as to how long COVID could manifest migraines is not yet fully understood, but many doctors believe that inflammation caused by the virus plays a key role.
“To understand why some patients have migraine in long COVID, we have to go back to understand the role of inflammation in COVID-19 itself,” says Emad Estemalik, MD, clinical assistant professor of neurology at Cleveland Clinic Lerner College of Medicine and section head of headache medicine at Cleveland Clinic.
In COVID-19, inflammation occurs because of a cytokine storm. Cytokines, which are proteins essential for a strong immune system, can be overproduced in a patient with COVID, which causes too much inflammation in any organ in the body, including the brain. This can result in new daily headache for some patients.
A new study from Italian researchers found that many patients who develop migraines for the first time while ill with long COVID are middle-aged women (traditionally a late point in life for a first migraine) who have a family history of migraine. Potential causes could have to do with the immune system remaining persistently activated from inflammation during long COVID, as well as the activation of the trigeminovascular system in the brain, which contains neurons that can trigger a migraine.
What treatments can work for migraines related to long COVID?
Long COVID usually causes a constellation of other symptoms at the same time as migraine.
“It’s so important for patients to take an interdisciplinary approach,” Dr. Estemalik stresses. “Patients should make sure their doctors are addressing all of their symptoms.”
When it comes to specifically targeting migraines, standard treatments can be effective.
“In terms of treating migraine in long COVID patients, we don’t do anything different or special,” says Matthew E. Fink, MD, chair of neurology at Weill Cornell Medical College and chief of the Division of Stroke and Critical Care Neurology at New York–Presbyterian Hospital/Weill Cornell Medical Center. “We treat these patients with standard migraine medications.”
Mr. Solomon is following this course of action.
“My doctor prescribed triptans, which have been somewhat effective in reducing the severity and duration of the migraines,” he says. A daily supplement of magnesium and a daily dose of aspirin can also work for some patients, according to Dr. Fink.
Lifestyle modification is also a great idea.
“Patients should keep regular sleep hours, getting up and going to bed at the same time every day,” Dr. Fink continues. “Daily exercise is also recommended.”
Mr. Solomon suggests tracking migraine triggers and patterns in a journal.
“Try to identify lifestyle changes that help, like managing stress and staying hydrated,” Mr. Solomon advises. “Seeking support from health care professionals and support groups can make a significant difference.”
The best news of all: for patients that are diligent in following these strategies, they’ve been proven to work.
“We doctors are very optimistic when it comes to good outcomes for patients with long COVID and migraine,” Dr. Fink says. “I reassure my patients by telling them, ‘You will get better long-term.’ ”
A version of this article appeared on Medscape.com.
.
“I’ve also noticed visual disturbances, like flickering lights or blurred vision, which I later learned are called auras,” the 30-year-old medical billing specialist in Seattle told this news organization.
Mr. Solomon isn’t alone. It’s estimated that 1 out of 8 people with COVID develop long COVID. Of those persons, 44% also experience headaches. Research has found that many of those headaches are migraines – and many patients who are afflicted say they had never had a migraine before. These migraines tend to persist for at least 5 or 6 months, according to data from the American Headache Society.
What’s more, other patients may suddenly have more frequent or intense versions of headaches they’ve not noticed before.
The mechanism as to how long COVID could manifest migraines is not yet fully understood, but many doctors believe that inflammation caused by the virus plays a key role.
“To understand why some patients have migraine in long COVID, we have to go back to understand the role of inflammation in COVID-19 itself,” says Emad Estemalik, MD, clinical assistant professor of neurology at Cleveland Clinic Lerner College of Medicine and section head of headache medicine at Cleveland Clinic.
In COVID-19, inflammation occurs because of a cytokine storm. Cytokines, which are proteins essential for a strong immune system, can be overproduced in a patient with COVID, which causes too much inflammation in any organ in the body, including the brain. This can result in new daily headache for some patients.
A new study from Italian researchers found that many patients who develop migraines for the first time while ill with long COVID are middle-aged women (traditionally a late point in life for a first migraine) who have a family history of migraine. Potential causes could have to do with the immune system remaining persistently activated from inflammation during long COVID, as well as the activation of the trigeminovascular system in the brain, which contains neurons that can trigger a migraine.
What treatments can work for migraines related to long COVID?
Long COVID usually causes a constellation of other symptoms at the same time as migraine.
“It’s so important for patients to take an interdisciplinary approach,” Dr. Estemalik stresses. “Patients should make sure their doctors are addressing all of their symptoms.”
When it comes to specifically targeting migraines, standard treatments can be effective.
“In terms of treating migraine in long COVID patients, we don’t do anything different or special,” says Matthew E. Fink, MD, chair of neurology at Weill Cornell Medical College and chief of the Division of Stroke and Critical Care Neurology at New York–Presbyterian Hospital/Weill Cornell Medical Center. “We treat these patients with standard migraine medications.”
Mr. Solomon is following this course of action.
“My doctor prescribed triptans, which have been somewhat effective in reducing the severity and duration of the migraines,” he says. A daily supplement of magnesium and a daily dose of aspirin can also work for some patients, according to Dr. Fink.
Lifestyle modification is also a great idea.
“Patients should keep regular sleep hours, getting up and going to bed at the same time every day,” Dr. Fink continues. “Daily exercise is also recommended.”
Mr. Solomon suggests tracking migraine triggers and patterns in a journal.
“Try to identify lifestyle changes that help, like managing stress and staying hydrated,” Mr. Solomon advises. “Seeking support from health care professionals and support groups can make a significant difference.”
The best news of all: for patients that are diligent in following these strategies, they’ve been proven to work.
“We doctors are very optimistic when it comes to good outcomes for patients with long COVID and migraine,” Dr. Fink says. “I reassure my patients by telling them, ‘You will get better long-term.’ ”
A version of this article appeared on Medscape.com.
3D-printed meds customize the exact dose for sick children
Convincing kids to take their medicine could become much easier. Researchers at Texas A&M University are developing a new method of pharmaceutical 3D printing with pediatric patients in mind.
They hope to print precisely dosed tablets in child-friendly shapes and flavors. While the effort is focused on two drugs for pediatric AIDS, the process could be used to print other medicines, including for adults.
Researchers from Britain, Australia, and the University of Texas at Austin are also in the early stages of 3D-printed medication projects. It’s a promising venture in the broader pursuit of “personalized medicine,” tailoring treatments to each patient’s unique needs.
Drug mass production fails to address pediatric patients, who often need different dosages and combinations of medicines as they grow. As a result, adult tablets are often crushed and dissolved in liquid – known as compounding – and given to children. But this can harm drug quality and make doses less precise.
“Suppose the child needs 3.4 milligrams and only a 10-milligram tablet is available. Once you manipulate the dosage from solid to liquid, how do you ensure that it has the same amount of drug in it?” said co-principal investigator Mansoor Khan, PhD, a professor of pharmaceutical sciences at Texas A&M.
Most pharmacies lack the equipment to test compounded drug quality, he said. And liquified drugs taste bad because the pill coating has been ground away.
“Flavor is a big issue,” said Olive Eckstein, MD, an assistant professor of pediatric hematology-oncology at Texas Children’s Hospital and Baylor College of Medicine, who is not involved in the research. “Hospitals will sometimes delay discharging pediatric patients because they can’t take their meds orally and have to get an IV formulation.”
Updating pharmaceutical 3D printing
The FDA approved a 3D-printed drug in 2015, but since then, progress has stalled, largely because the method relied on solvents to bind drug particles together. Over time, solvents can compromise shelf life, according to co-principal investigator Mathew Kuttolamadom, PhD, an associate professor of engineering at Texas A&M.
The Texas A&M team is using a different method, without solvents. First, they create a powder mixture of the drug, a biocompatible polymer (such as lactose), and a sheen, a pigment that colors the tablet and allows heat to be absorbed. Flavoring can also be added. Next, the mixture is heated in the printer chamber.
“The polymer should melt just enough. That gives the tablet structural strength. But it should not melt too much, whereby the drug can start dissolving into the polymer,” Dr. Kuttolamadom said.
The tablets are finished with precise applications of laser heat. Using computer-aided design software, the researchers can create tablets in almost any shape, such as “stars or teddy bears,” he said.
After much trial and error, the researchers have printed tablets that won’t break apart or become soggy.
Now they are testing how different laser scan speeds affect the structure of the tablet, which in turn affects the rate at which drugs dissolve. Slowing down the laser imparts more energy, strengthening the tablet structure and making drugs dissolve slower, for a longer release inside the body.
The researchers hope to develop machine learning models to test different laser speed combinations. Eventually, they could create tablets that combine drugs with different dissolve rates.
“The outside could be a rapid release, and the inside could be an extended release or a sustained release, or even a completely different drug,” Dr. Kuttolamadom said.
Older patients who take many daily medications could benefit from the technology. “Personalized tablets could be printed at your local pharmacy,” he said, “even before you leave your doctor’s office.”
A version of this article first appeared on WebMD.com.
Convincing kids to take their medicine could become much easier. Researchers at Texas A&M University are developing a new method of pharmaceutical 3D printing with pediatric patients in mind.
They hope to print precisely dosed tablets in child-friendly shapes and flavors. While the effort is focused on two drugs for pediatric AIDS, the process could be used to print other medicines, including for adults.
Researchers from Britain, Australia, and the University of Texas at Austin are also in the early stages of 3D-printed medication projects. It’s a promising venture in the broader pursuit of “personalized medicine,” tailoring treatments to each patient’s unique needs.
Drug mass production fails to address pediatric patients, who often need different dosages and combinations of medicines as they grow. As a result, adult tablets are often crushed and dissolved in liquid – known as compounding – and given to children. But this can harm drug quality and make doses less precise.
“Suppose the child needs 3.4 milligrams and only a 10-milligram tablet is available. Once you manipulate the dosage from solid to liquid, how do you ensure that it has the same amount of drug in it?” said co-principal investigator Mansoor Khan, PhD, a professor of pharmaceutical sciences at Texas A&M.
Most pharmacies lack the equipment to test compounded drug quality, he said. And liquified drugs taste bad because the pill coating has been ground away.
“Flavor is a big issue,” said Olive Eckstein, MD, an assistant professor of pediatric hematology-oncology at Texas Children’s Hospital and Baylor College of Medicine, who is not involved in the research. “Hospitals will sometimes delay discharging pediatric patients because they can’t take their meds orally and have to get an IV formulation.”
Updating pharmaceutical 3D printing
The FDA approved a 3D-printed drug in 2015, but since then, progress has stalled, largely because the method relied on solvents to bind drug particles together. Over time, solvents can compromise shelf life, according to co-principal investigator Mathew Kuttolamadom, PhD, an associate professor of engineering at Texas A&M.
The Texas A&M team is using a different method, without solvents. First, they create a powder mixture of the drug, a biocompatible polymer (such as lactose), and a sheen, a pigment that colors the tablet and allows heat to be absorbed. Flavoring can also be added. Next, the mixture is heated in the printer chamber.
“The polymer should melt just enough. That gives the tablet structural strength. But it should not melt too much, whereby the drug can start dissolving into the polymer,” Dr. Kuttolamadom said.
The tablets are finished with precise applications of laser heat. Using computer-aided design software, the researchers can create tablets in almost any shape, such as “stars or teddy bears,” he said.
After much trial and error, the researchers have printed tablets that won’t break apart or become soggy.
Now they are testing how different laser scan speeds affect the structure of the tablet, which in turn affects the rate at which drugs dissolve. Slowing down the laser imparts more energy, strengthening the tablet structure and making drugs dissolve slower, for a longer release inside the body.
The researchers hope to develop machine learning models to test different laser speed combinations. Eventually, they could create tablets that combine drugs with different dissolve rates.
“The outside could be a rapid release, and the inside could be an extended release or a sustained release, or even a completely different drug,” Dr. Kuttolamadom said.
Older patients who take many daily medications could benefit from the technology. “Personalized tablets could be printed at your local pharmacy,” he said, “even before you leave your doctor’s office.”
A version of this article first appeared on WebMD.com.
Convincing kids to take their medicine could become much easier. Researchers at Texas A&M University are developing a new method of pharmaceutical 3D printing with pediatric patients in mind.
They hope to print precisely dosed tablets in child-friendly shapes and flavors. While the effort is focused on two drugs for pediatric AIDS, the process could be used to print other medicines, including for adults.
Researchers from Britain, Australia, and the University of Texas at Austin are also in the early stages of 3D-printed medication projects. It’s a promising venture in the broader pursuit of “personalized medicine,” tailoring treatments to each patient’s unique needs.
Drug mass production fails to address pediatric patients, who often need different dosages and combinations of medicines as they grow. As a result, adult tablets are often crushed and dissolved in liquid – known as compounding – and given to children. But this can harm drug quality and make doses less precise.
“Suppose the child needs 3.4 milligrams and only a 10-milligram tablet is available. Once you manipulate the dosage from solid to liquid, how do you ensure that it has the same amount of drug in it?” said co-principal investigator Mansoor Khan, PhD, a professor of pharmaceutical sciences at Texas A&M.
Most pharmacies lack the equipment to test compounded drug quality, he said. And liquified drugs taste bad because the pill coating has been ground away.
“Flavor is a big issue,” said Olive Eckstein, MD, an assistant professor of pediatric hematology-oncology at Texas Children’s Hospital and Baylor College of Medicine, who is not involved in the research. “Hospitals will sometimes delay discharging pediatric patients because they can’t take their meds orally and have to get an IV formulation.”
Updating pharmaceutical 3D printing
The FDA approved a 3D-printed drug in 2015, but since then, progress has stalled, largely because the method relied on solvents to bind drug particles together. Over time, solvents can compromise shelf life, according to co-principal investigator Mathew Kuttolamadom, PhD, an associate professor of engineering at Texas A&M.
The Texas A&M team is using a different method, without solvents. First, they create a powder mixture of the drug, a biocompatible polymer (such as lactose), and a sheen, a pigment that colors the tablet and allows heat to be absorbed. Flavoring can also be added. Next, the mixture is heated in the printer chamber.
“The polymer should melt just enough. That gives the tablet structural strength. But it should not melt too much, whereby the drug can start dissolving into the polymer,” Dr. Kuttolamadom said.
The tablets are finished with precise applications of laser heat. Using computer-aided design software, the researchers can create tablets in almost any shape, such as “stars or teddy bears,” he said.
After much trial and error, the researchers have printed tablets that won’t break apart or become soggy.
Now they are testing how different laser scan speeds affect the structure of the tablet, which in turn affects the rate at which drugs dissolve. Slowing down the laser imparts more energy, strengthening the tablet structure and making drugs dissolve slower, for a longer release inside the body.
The researchers hope to develop machine learning models to test different laser speed combinations. Eventually, they could create tablets that combine drugs with different dissolve rates.
“The outside could be a rapid release, and the inside could be an extended release or a sustained release, or even a completely different drug,” Dr. Kuttolamadom said.
Older patients who take many daily medications could benefit from the technology. “Personalized tablets could be printed at your local pharmacy,” he said, “even before you leave your doctor’s office.”
A version of this article first appeared on WebMD.com.
The new normal in body temperature
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.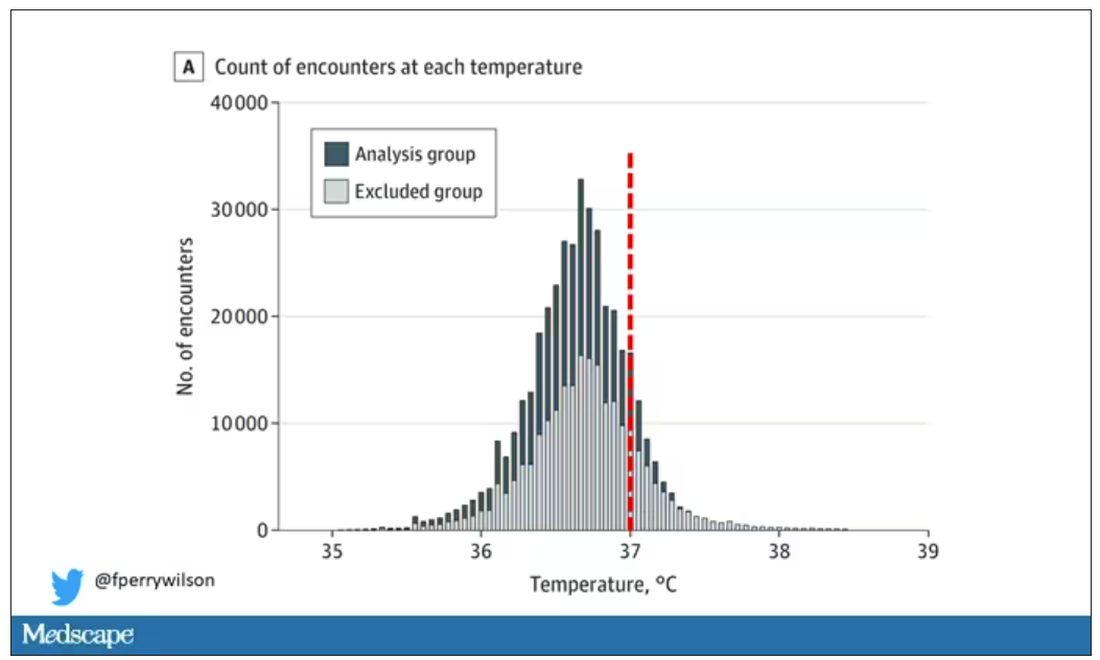
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.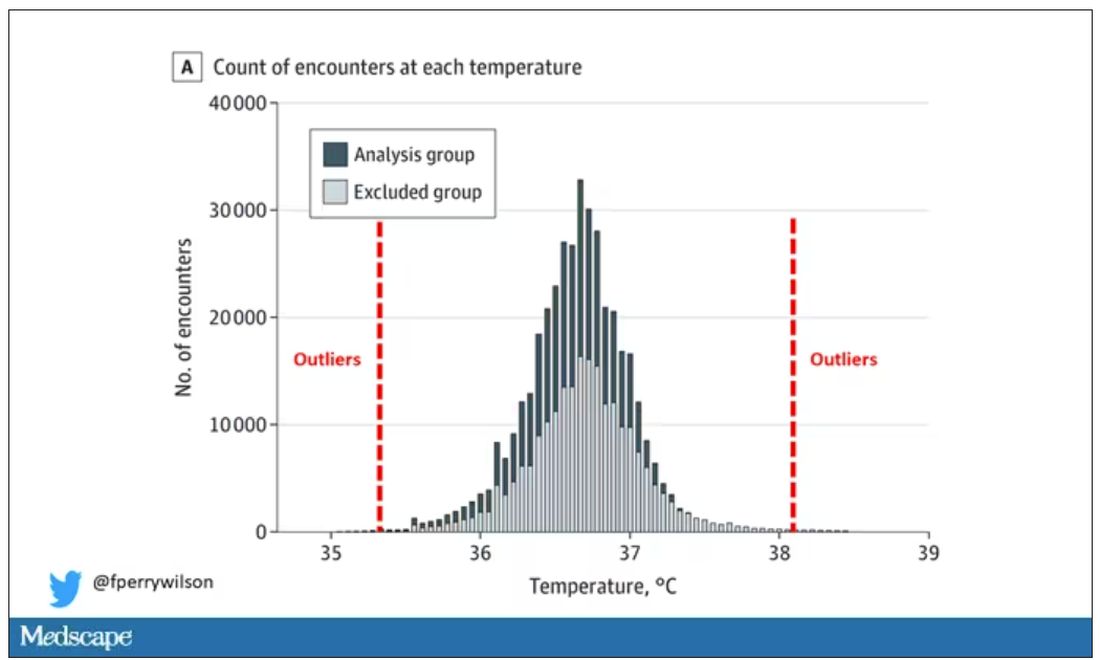
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.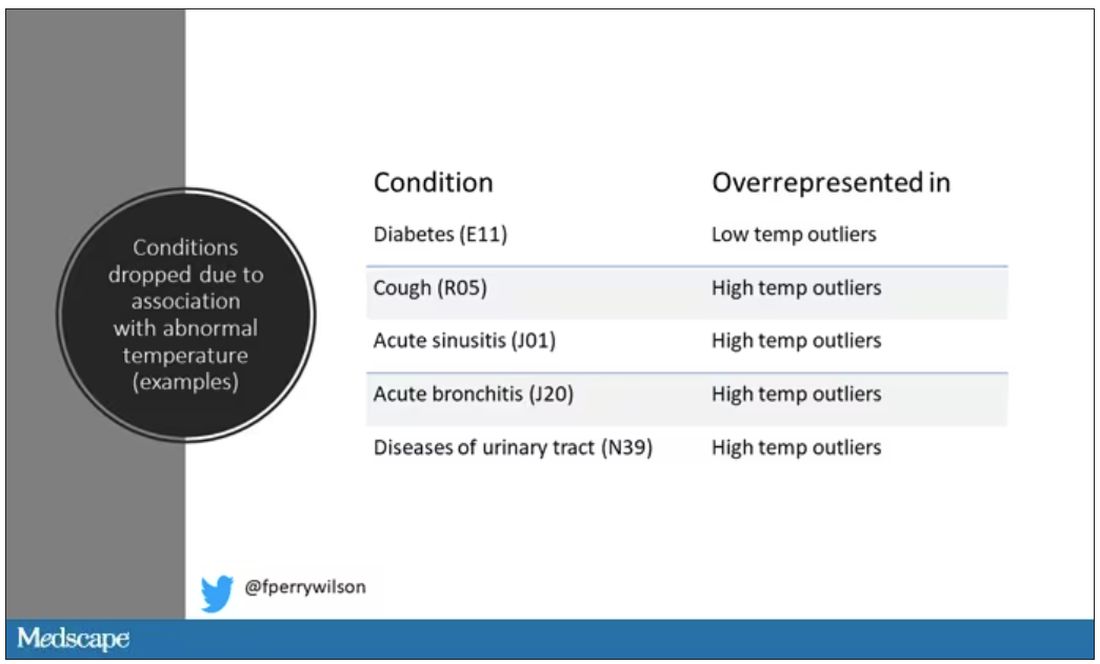
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.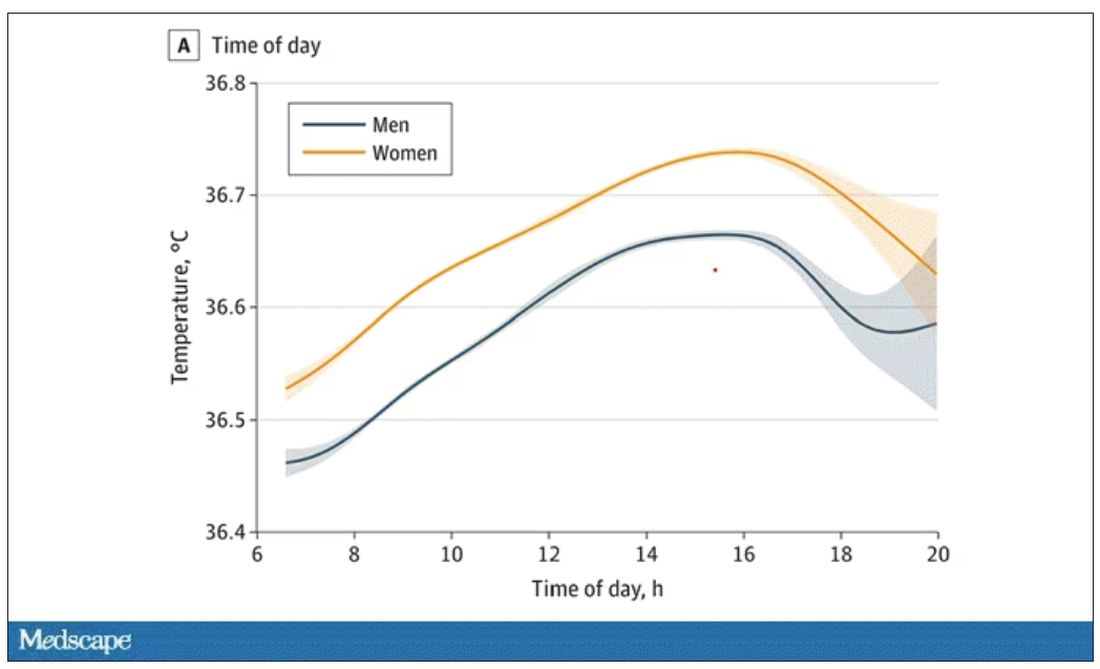
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.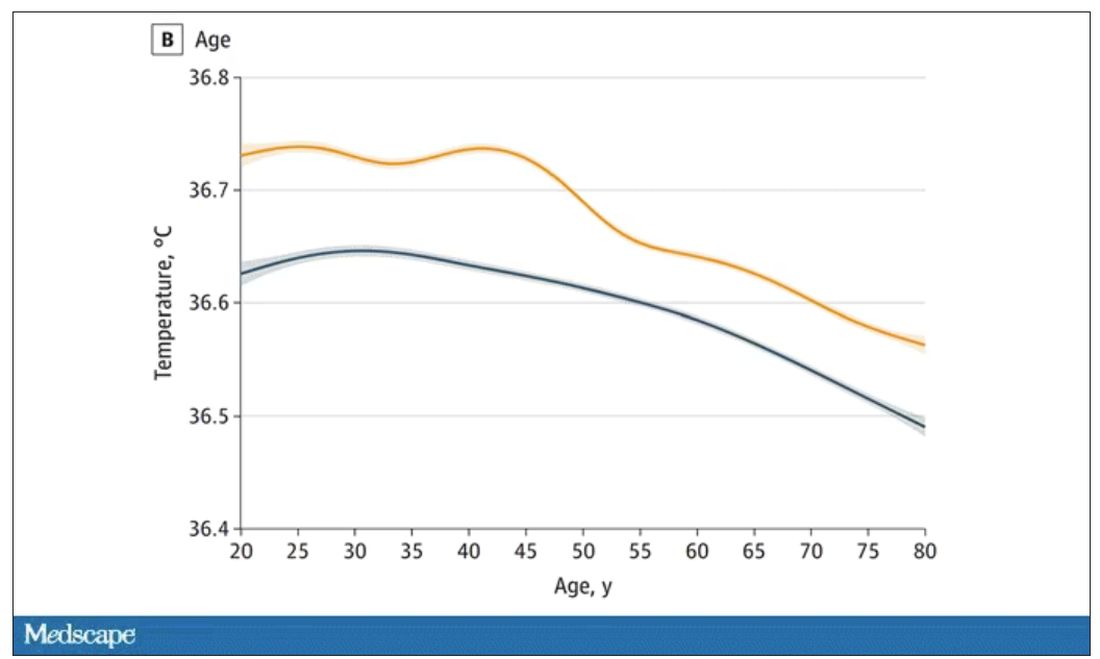
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.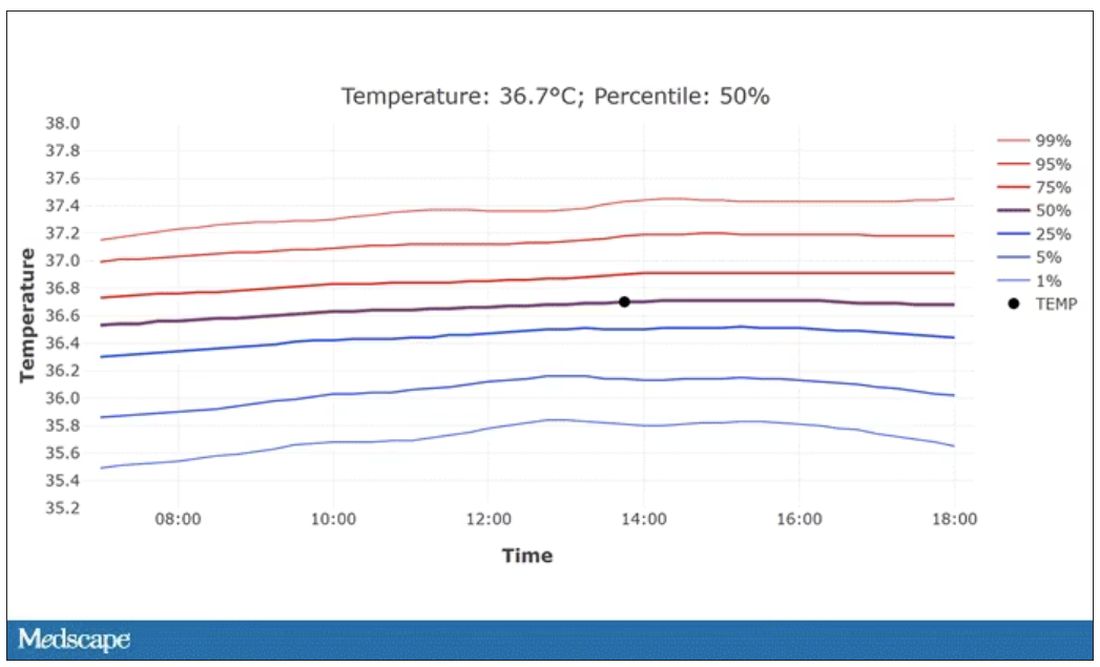
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
New AI-enhanced bandages poised to transform wound treatment
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
‘Missed opportunities’ for accurate diagnosing of women with vaginitis
Although the standard of care of diagnosing vaginitis is clinical evaluation, many practices do not perform accurate and comprehensive clinical examinations for a variety for reasons, and the Centers for Disease Control and Prevention currently recommends molecular testing, wrote Casey N. Pinto, PhD, of Penn State University, Hershey, and colleagues. The CDC also recommends testing women with vaginitis for Chlamydia trachomatis (CT) and Neissaria gonorrhoeae (NG) given the high rate of coinfections between vaginitis and these sexually transmitted infections, but data on cotesting in clinical practice are limited, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from a commercial administrative claims database for 1,359,289 women aged 18-50 years who were diagnosed with vaginitis between 2012 and 2017.
The women were categorized into groups based on type of vaginitis diagnosis: nucleic amplification test (NAAT), DNA probe test, traditional lab test, and those diagnosed clinically at an index visit but with no CPT code for further testing.
Overall, nearly half of the women (49.2%) had no CPT code for further vaginitis testing beyond clinical diagnosis. Of those with CPT codes for testing, 50.9% underwent traditional point-of-care testing, wet mount, or culture, 23.5% had a DNA probe, and 20.6% had NAAT testing.
Approximately one-third (34%) of women were cotested for CT/NG. Testing rates varied widely across the type of vaginitis test, from 70.8% of women who received NAAT to 22.8% of women with no CPT code. In multivariate analysis including age, region, and the Charlson Comorbidity Index (CCI), those tested with NAAT were eight times more likely to be cotested for CT/NG than those with no CPT code (odds ratio, 8.77; P < .0001).
Women who received a traditional test or DNA probe test for vaginitis also were more likely to have CT/NG testing than women with no CPT code, but only 1.8-2.5 times as likely.
“Our data suggest that most clinicians are not engaging the standard of care for testing and diagnosing vaginitis, or not engaging in comprehensive care by cotesting for vaginitis and CT/NG when patients may be at risk, resulting in missed opportunities for accurate diagnosis and potential associated coinfections,” the researchers wrote in their discussion. The higher rates for CT/NG testing among women receiving either NAAT or DNA probe vaginitis testing could be attributed to bundled testing, they noted, and the lower rate of CT/NG testing for patients with no CPT code could stem from limited access to microscopy or clinician preference for clinical diagnosis only, they said.
The findings were limited by several factors, including the lack of data on testing and diagnoses prior to the study period and not billed to insurance, and by the inability to account for variables including race, ethnicity, and socioeconomic status, the researchers noted.
However, the results highlight the need for more comprehensive care in vaginitis testing to take advantage of opportunities to identify CT or NG in women diagnosed with vaginitis, they concluded.
The study was supported by Becton, Dickinson and Company. Lead author Dr. Pinto disclosed consulting for Becton, Dickinson and Company, and receiving an honorarium from Roche.
Although the standard of care of diagnosing vaginitis is clinical evaluation, many practices do not perform accurate and comprehensive clinical examinations for a variety for reasons, and the Centers for Disease Control and Prevention currently recommends molecular testing, wrote Casey N. Pinto, PhD, of Penn State University, Hershey, and colleagues. The CDC also recommends testing women with vaginitis for Chlamydia trachomatis (CT) and Neissaria gonorrhoeae (NG) given the high rate of coinfections between vaginitis and these sexually transmitted infections, but data on cotesting in clinical practice are limited, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from a commercial administrative claims database for 1,359,289 women aged 18-50 years who were diagnosed with vaginitis between 2012 and 2017.
The women were categorized into groups based on type of vaginitis diagnosis: nucleic amplification test (NAAT), DNA probe test, traditional lab test, and those diagnosed clinically at an index visit but with no CPT code for further testing.
Overall, nearly half of the women (49.2%) had no CPT code for further vaginitis testing beyond clinical diagnosis. Of those with CPT codes for testing, 50.9% underwent traditional point-of-care testing, wet mount, or culture, 23.5% had a DNA probe, and 20.6% had NAAT testing.
Approximately one-third (34%) of women were cotested for CT/NG. Testing rates varied widely across the type of vaginitis test, from 70.8% of women who received NAAT to 22.8% of women with no CPT code. In multivariate analysis including age, region, and the Charlson Comorbidity Index (CCI), those tested with NAAT were eight times more likely to be cotested for CT/NG than those with no CPT code (odds ratio, 8.77; P < .0001).
Women who received a traditional test or DNA probe test for vaginitis also were more likely to have CT/NG testing than women with no CPT code, but only 1.8-2.5 times as likely.
“Our data suggest that most clinicians are not engaging the standard of care for testing and diagnosing vaginitis, or not engaging in comprehensive care by cotesting for vaginitis and CT/NG when patients may be at risk, resulting in missed opportunities for accurate diagnosis and potential associated coinfections,” the researchers wrote in their discussion. The higher rates for CT/NG testing among women receiving either NAAT or DNA probe vaginitis testing could be attributed to bundled testing, they noted, and the lower rate of CT/NG testing for patients with no CPT code could stem from limited access to microscopy or clinician preference for clinical diagnosis only, they said.
The findings were limited by several factors, including the lack of data on testing and diagnoses prior to the study period and not billed to insurance, and by the inability to account for variables including race, ethnicity, and socioeconomic status, the researchers noted.
However, the results highlight the need for more comprehensive care in vaginitis testing to take advantage of opportunities to identify CT or NG in women diagnosed with vaginitis, they concluded.
The study was supported by Becton, Dickinson and Company. Lead author Dr. Pinto disclosed consulting for Becton, Dickinson and Company, and receiving an honorarium from Roche.
Although the standard of care of diagnosing vaginitis is clinical evaluation, many practices do not perform accurate and comprehensive clinical examinations for a variety for reasons, and the Centers for Disease Control and Prevention currently recommends molecular testing, wrote Casey N. Pinto, PhD, of Penn State University, Hershey, and colleagues. The CDC also recommends testing women with vaginitis for Chlamydia trachomatis (CT) and Neissaria gonorrhoeae (NG) given the high rate of coinfections between vaginitis and these sexually transmitted infections, but data on cotesting in clinical practice are limited, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from a commercial administrative claims database for 1,359,289 women aged 18-50 years who were diagnosed with vaginitis between 2012 and 2017.
The women were categorized into groups based on type of vaginitis diagnosis: nucleic amplification test (NAAT), DNA probe test, traditional lab test, and those diagnosed clinically at an index visit but with no CPT code for further testing.
Overall, nearly half of the women (49.2%) had no CPT code for further vaginitis testing beyond clinical diagnosis. Of those with CPT codes for testing, 50.9% underwent traditional point-of-care testing, wet mount, or culture, 23.5% had a DNA probe, and 20.6% had NAAT testing.
Approximately one-third (34%) of women were cotested for CT/NG. Testing rates varied widely across the type of vaginitis test, from 70.8% of women who received NAAT to 22.8% of women with no CPT code. In multivariate analysis including age, region, and the Charlson Comorbidity Index (CCI), those tested with NAAT were eight times more likely to be cotested for CT/NG than those with no CPT code (odds ratio, 8.77; P < .0001).
Women who received a traditional test or DNA probe test for vaginitis also were more likely to have CT/NG testing than women with no CPT code, but only 1.8-2.5 times as likely.
“Our data suggest that most clinicians are not engaging the standard of care for testing and diagnosing vaginitis, or not engaging in comprehensive care by cotesting for vaginitis and CT/NG when patients may be at risk, resulting in missed opportunities for accurate diagnosis and potential associated coinfections,” the researchers wrote in their discussion. The higher rates for CT/NG testing among women receiving either NAAT or DNA probe vaginitis testing could be attributed to bundled testing, they noted, and the lower rate of CT/NG testing for patients with no CPT code could stem from limited access to microscopy or clinician preference for clinical diagnosis only, they said.
The findings were limited by several factors, including the lack of data on testing and diagnoses prior to the study period and not billed to insurance, and by the inability to account for variables including race, ethnicity, and socioeconomic status, the researchers noted.
However, the results highlight the need for more comprehensive care in vaginitis testing to take advantage of opportunities to identify CT or NG in women diagnosed with vaginitis, they concluded.
The study was supported by Becton, Dickinson and Company. Lead author Dr. Pinto disclosed consulting for Becton, Dickinson and Company, and receiving an honorarium from Roche.
FROM SEXUALLY TRANSMITTED DISEASES
Are you ready for RSV season? There’s a new preventive option
There is now an additional option for the prevention of respiratory syncytial virus (RSV), the most common cause of hospitalization among infants and children in the United States. In July, the US Food and Drug Administration (FDA) approved nirsevimab, an RSV preventive monoclonal antibody, for use in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to RSV during their second season.1 The Advisory Committee on Immunization Practices (ACIP) subsequently made 2 recommendations regarding use of nirsevimab, which I’ll detail in a moment.2
First, a word about RSV. The Centers for Disease Control and Prevention estimates that each year in children younger than 5 years, RSV is responsible for 1.5 million outpatient clinic visits, 520,000 emergency department visits, 58,000 to 80,000 hospitalizations, and 100 to 200 deaths.2 The risk for hospitalization from RSV is highest in the second and third months of life and decreases with increasing age.
There are some racial disparities in RSV severity, likely reflecting social drivers of health: ICU admission rates are 1.2 to 1.6 times higher among non-Hispanic Black infants younger than 6 months than among non-Hispanic White infants, and hospitalization rates are up to 5 times higher in American Indian and Alaska Native populations.2
What nirsevimab adds to the toolbox. Until recently, there was only 1 RSV preventive agent available: palivizumab, also a monoclonal antibody. The American Academy of Pediatrics has recommended palivizumab be used only for infants at high risk for RSV infection, due to its high cost and the need for monthly injections for the duration of an RSV season. In addition, the Academy has noted that palivizumab has limited effect on RSV hospitalizations on a population basis and does not appear to affect mortality.3
Nirsevimab has a longer half-life than palivizumab, and only 1 injection is needed for the RSV season. Early studies on nirsevimab demonstrate 79% effectiveness in preventing medical-attended lower respiratory tract infection, 80.6% effectiveness in preventing hospitalization, and 90% effectiveness in preventing ICU admission. The number needed to immunize with nirsevimab to prevent an outpatient visit is estimated to be 17; to prevent an ED visit, 48; and to prevent an inpatient admission, 128. Due to the low RSV death rate, the studies were not able to demonstrate reduced mortality.2
What the ACIP recommends. At a special meeting in July, the ACIP recommended 1 dose of nirsevimab for2:
- all infants younger than 8 months who are born during or entering their first RSV season
- children ages 8 to 19 months who are at increased risk for severe RSV disease and entering their second RSV season.
Those at risk include children with chronic lung disease of prematurity who required medical support any time during the 6-month period before the start of their second RSV season; those with severe immunocompromise; those with cystic fibrosis who have manifestations of severe lung disease or weight-for-length < 10th percentile; and American Indian and Alaska Native children.2
How to administer nirsevimab. The dose of nirsevimab is 50 mg IM for those weighing < 5 kg, 100 mg for those weighing ≥ 5 kg, and 200 mg for high-risk children entering their second RSV season.2 Nirsevimab can be co-administered with other recommended vaccines; however, both nirsevimab and palivizumab should not be used in the same child in the same RSV season.
Nirsevimab should be administered in the first week of life for infants born shortly before or during RSV season, and shortly before the season for infants younger than 8 months and those ages 8 to 19 months who are at high risk.4 The months of highest RSV transmission in most locations are December through February, but this can vary. Local epidemiology and advice from state and local health departments are the best source of information about when RSV season starts and ends in your area.
On the horizon. Nirsevimab will be included in the Vaccines for Children program and covered by commercial health plans with no cost sharing.5 A maternal vaccine to prevent RSV in newborns is likely to be approved by the FDA in the near future.
1. FDA. FDA approves new drug to prevent RSV in babies and toddlers [press release]. Published July 17, 2023. Accessed August 29, 2023. www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
2. Jones J. Evidence to recommendation framework: nirsevimab updates. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131586
3. American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–e638. doi: 10.1542/peds.2014-1666
4. Jones J. Proposed clinical consideration updates for nirsevimab. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-08-3/04-rsv-jones-508.pdf
5. Peacock G. Nirsevimab: implementation considerations. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131587
There is now an additional option for the prevention of respiratory syncytial virus (RSV), the most common cause of hospitalization among infants and children in the United States. In July, the US Food and Drug Administration (FDA) approved nirsevimab, an RSV preventive monoclonal antibody, for use in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to RSV during their second season.1 The Advisory Committee on Immunization Practices (ACIP) subsequently made 2 recommendations regarding use of nirsevimab, which I’ll detail in a moment.2
First, a word about RSV. The Centers for Disease Control and Prevention estimates that each year in children younger than 5 years, RSV is responsible for 1.5 million outpatient clinic visits, 520,000 emergency department visits, 58,000 to 80,000 hospitalizations, and 100 to 200 deaths.2 The risk for hospitalization from RSV is highest in the second and third months of life and decreases with increasing age.
There are some racial disparities in RSV severity, likely reflecting social drivers of health: ICU admission rates are 1.2 to 1.6 times higher among non-Hispanic Black infants younger than 6 months than among non-Hispanic White infants, and hospitalization rates are up to 5 times higher in American Indian and Alaska Native populations.2
What nirsevimab adds to the toolbox. Until recently, there was only 1 RSV preventive agent available: palivizumab, also a monoclonal antibody. The American Academy of Pediatrics has recommended palivizumab be used only for infants at high risk for RSV infection, due to its high cost and the need for monthly injections for the duration of an RSV season. In addition, the Academy has noted that palivizumab has limited effect on RSV hospitalizations on a population basis and does not appear to affect mortality.3
Nirsevimab has a longer half-life than palivizumab, and only 1 injection is needed for the RSV season. Early studies on nirsevimab demonstrate 79% effectiveness in preventing medical-attended lower respiratory tract infection, 80.6% effectiveness in preventing hospitalization, and 90% effectiveness in preventing ICU admission. The number needed to immunize with nirsevimab to prevent an outpatient visit is estimated to be 17; to prevent an ED visit, 48; and to prevent an inpatient admission, 128. Due to the low RSV death rate, the studies were not able to demonstrate reduced mortality.2
What the ACIP recommends. At a special meeting in July, the ACIP recommended 1 dose of nirsevimab for2:
- all infants younger than 8 months who are born during or entering their first RSV season
- children ages 8 to 19 months who are at increased risk for severe RSV disease and entering their second RSV season.
Those at risk include children with chronic lung disease of prematurity who required medical support any time during the 6-month period before the start of their second RSV season; those with severe immunocompromise; those with cystic fibrosis who have manifestations of severe lung disease or weight-for-length < 10th percentile; and American Indian and Alaska Native children.2
How to administer nirsevimab. The dose of nirsevimab is 50 mg IM for those weighing < 5 kg, 100 mg for those weighing ≥ 5 kg, and 200 mg for high-risk children entering their second RSV season.2 Nirsevimab can be co-administered with other recommended vaccines; however, both nirsevimab and palivizumab should not be used in the same child in the same RSV season.
Nirsevimab should be administered in the first week of life for infants born shortly before or during RSV season, and shortly before the season for infants younger than 8 months and those ages 8 to 19 months who are at high risk.4 The months of highest RSV transmission in most locations are December through February, but this can vary. Local epidemiology and advice from state and local health departments are the best source of information about when RSV season starts and ends in your area.
On the horizon. Nirsevimab will be included in the Vaccines for Children program and covered by commercial health plans with no cost sharing.5 A maternal vaccine to prevent RSV in newborns is likely to be approved by the FDA in the near future.
There is now an additional option for the prevention of respiratory syncytial virus (RSV), the most common cause of hospitalization among infants and children in the United States. In July, the US Food and Drug Administration (FDA) approved nirsevimab, an RSV preventive monoclonal antibody, for use in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to RSV during their second season.1 The Advisory Committee on Immunization Practices (ACIP) subsequently made 2 recommendations regarding use of nirsevimab, which I’ll detail in a moment.2
First, a word about RSV. The Centers for Disease Control and Prevention estimates that each year in children younger than 5 years, RSV is responsible for 1.5 million outpatient clinic visits, 520,000 emergency department visits, 58,000 to 80,000 hospitalizations, and 100 to 200 deaths.2 The risk for hospitalization from RSV is highest in the second and third months of life and decreases with increasing age.
There are some racial disparities in RSV severity, likely reflecting social drivers of health: ICU admission rates are 1.2 to 1.6 times higher among non-Hispanic Black infants younger than 6 months than among non-Hispanic White infants, and hospitalization rates are up to 5 times higher in American Indian and Alaska Native populations.2
What nirsevimab adds to the toolbox. Until recently, there was only 1 RSV preventive agent available: palivizumab, also a monoclonal antibody. The American Academy of Pediatrics has recommended palivizumab be used only for infants at high risk for RSV infection, due to its high cost and the need for monthly injections for the duration of an RSV season. In addition, the Academy has noted that palivizumab has limited effect on RSV hospitalizations on a population basis and does not appear to affect mortality.3
Nirsevimab has a longer half-life than palivizumab, and only 1 injection is needed for the RSV season. Early studies on nirsevimab demonstrate 79% effectiveness in preventing medical-attended lower respiratory tract infection, 80.6% effectiveness in preventing hospitalization, and 90% effectiveness in preventing ICU admission. The number needed to immunize with nirsevimab to prevent an outpatient visit is estimated to be 17; to prevent an ED visit, 48; and to prevent an inpatient admission, 128. Due to the low RSV death rate, the studies were not able to demonstrate reduced mortality.2
What the ACIP recommends. At a special meeting in July, the ACIP recommended 1 dose of nirsevimab for2:
- all infants younger than 8 months who are born during or entering their first RSV season
- children ages 8 to 19 months who are at increased risk for severe RSV disease and entering their second RSV season.
Those at risk include children with chronic lung disease of prematurity who required medical support any time during the 6-month period before the start of their second RSV season; those with severe immunocompromise; those with cystic fibrosis who have manifestations of severe lung disease or weight-for-length < 10th percentile; and American Indian and Alaska Native children.2
How to administer nirsevimab. The dose of nirsevimab is 50 mg IM for those weighing < 5 kg, 100 mg for those weighing ≥ 5 kg, and 200 mg for high-risk children entering their second RSV season.2 Nirsevimab can be co-administered with other recommended vaccines; however, both nirsevimab and palivizumab should not be used in the same child in the same RSV season.
Nirsevimab should be administered in the first week of life for infants born shortly before or during RSV season, and shortly before the season for infants younger than 8 months and those ages 8 to 19 months who are at high risk.4 The months of highest RSV transmission in most locations are December through February, but this can vary. Local epidemiology and advice from state and local health departments are the best source of information about when RSV season starts and ends in your area.
On the horizon. Nirsevimab will be included in the Vaccines for Children program and covered by commercial health plans with no cost sharing.5 A maternal vaccine to prevent RSV in newborns is likely to be approved by the FDA in the near future.
1. FDA. FDA approves new drug to prevent RSV in babies and toddlers [press release]. Published July 17, 2023. Accessed August 29, 2023. www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
2. Jones J. Evidence to recommendation framework: nirsevimab updates. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131586
3. American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–e638. doi: 10.1542/peds.2014-1666
4. Jones J. Proposed clinical consideration updates for nirsevimab. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-08-3/04-rsv-jones-508.pdf
5. Peacock G. Nirsevimab: implementation considerations. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131587
1. FDA. FDA approves new drug to prevent RSV in babies and toddlers [press release]. Published July 17, 2023. Accessed August 29, 2023. www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
2. Jones J. Evidence to recommendation framework: nirsevimab updates. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131586
3. American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–e638. doi: 10.1542/peds.2014-1666
4. Jones J. Proposed clinical consideration updates for nirsevimab. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-08-3/04-rsv-jones-508.pdf
5. Peacock G. Nirsevimab: implementation considerations. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131587
Sepsis too often neglected in hospitals
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.


