User login
Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

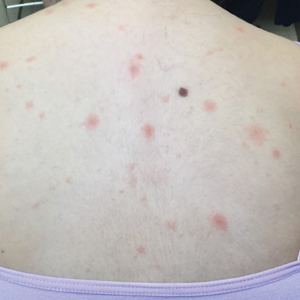
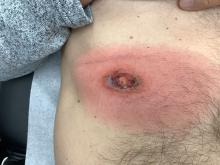
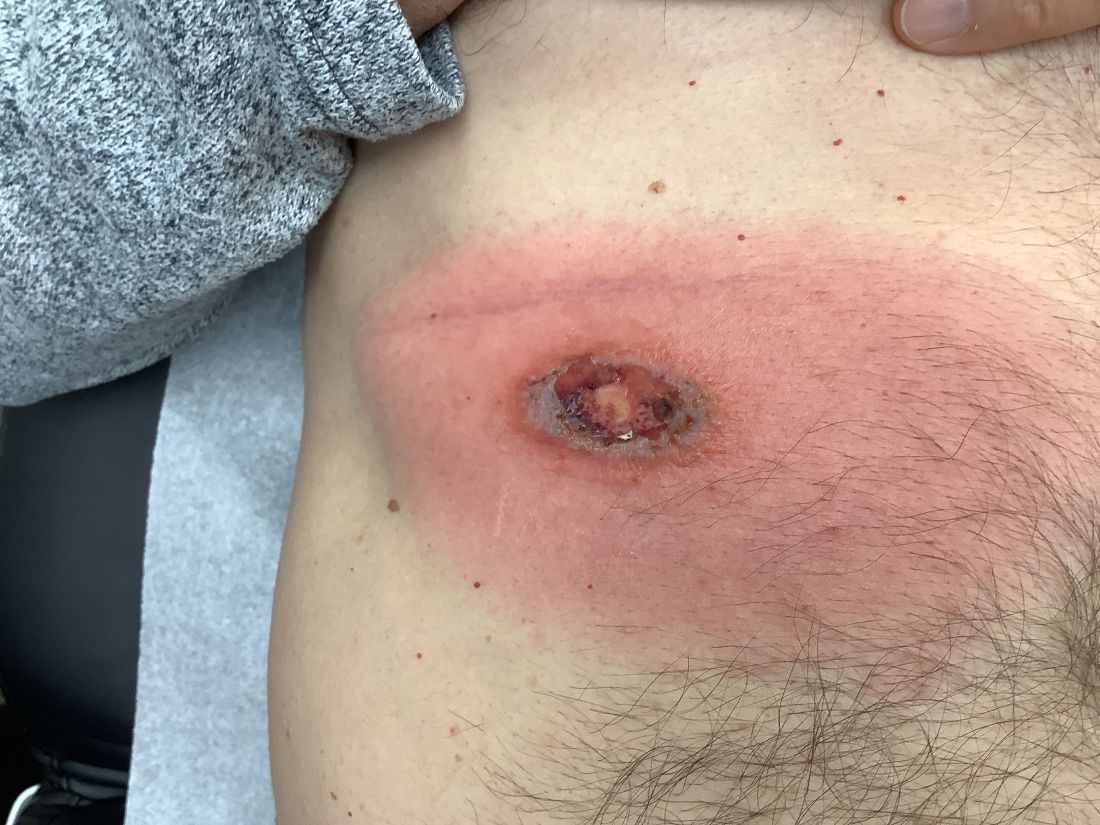
A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
Practice Points
- Dermatologists should familiarize themselves with the physical examination findings of cat scratch disease.
- There are numerous clinical variants and triggers of pityriasis rosea (PR).
- There may be a new infectious trigger for PR, and exposure to cats prior to a classic PR eruption should raise one’s suspicion as a possible cause.
Primary care clinicians should spearhead HIV prevention
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
Diffuse Pruritic Eruption in an Immunocompromised Patient
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.
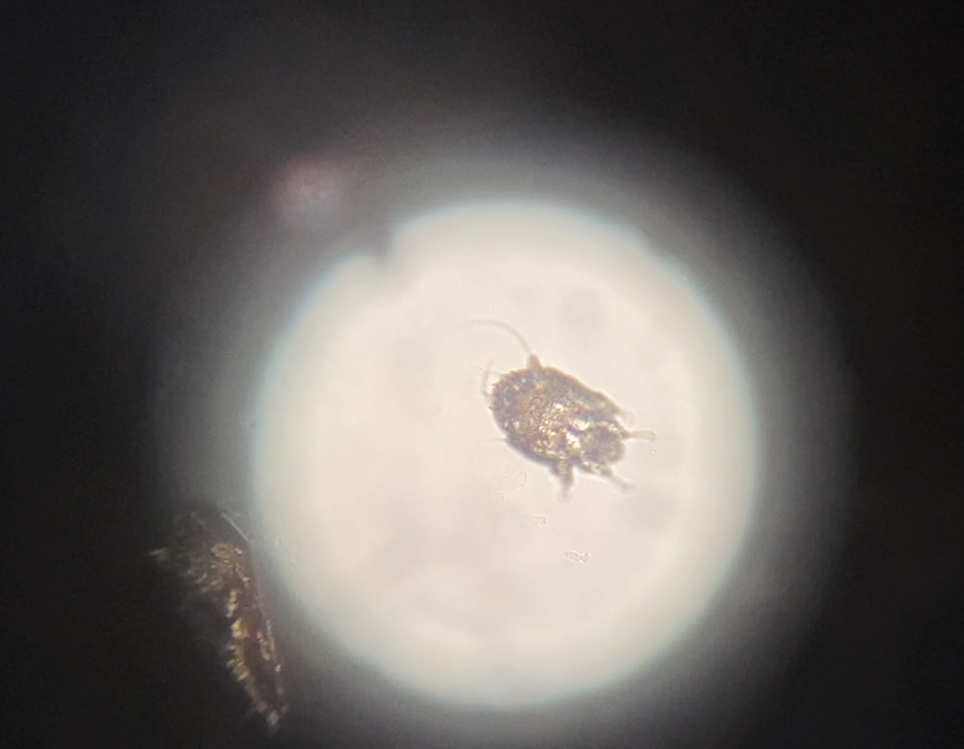
Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.

Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.

Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
A 54-year-old man presented to our dermatology clinic for evaluation of a widespread intensely pruritic rash of 4 weeks’ duration. Calamine lotion and oral hydroxyzine provided minimal relief. He was being treated for a myeloproliferative disorder with immunosuppressive therapy consisting of a combination of cladribine, low-dose cytarabine, and fedratinib. Physical examination revealed multiple excoriated papules and indurated nodules on the extensor and flexor surfaces of the arms and legs (top), chest, midline of the back (bottom), and groin. No lesions were noted on the volar aspect of the patient’s wrists or interdigital spaces, and no central puncta or scales were present. He denied any preceding arthropod bites, trauma, new environmental exposures, or changes to his medications. Scrapings from several representative lesions were obtained for mineral oil preparation and microscopic evaluation.
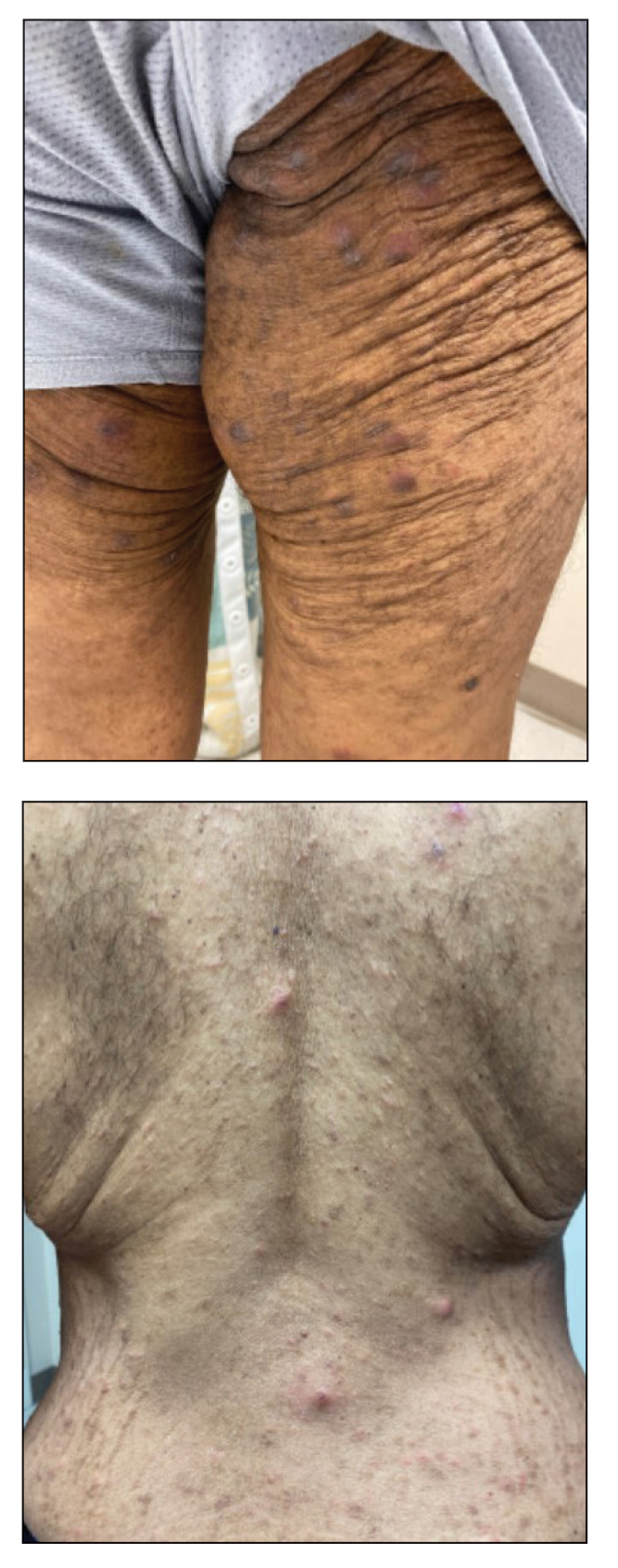
Paxlovid and Lagevrio benefit COVID outpatients in Omicron era
The American College of Physicians has issued an updated version of its living, rapid practice point guideline on the best treatment options for outpatients with confirmed COVID-19 in the era of the dominant Omicron variant of SARS-CoV-2. The recommendations in version 2 apply to persons presenting with mild to moderate infection and symptom onset in the past 5 days who are at high risk for progression to severe disease and potential hospitalization or death.
Version 1 appeared in late 2022.
While outpatient management is appropriate for most patients, treatment should be personalized and based on careful risk stratification and informed decision-making, said the guideline authors, led by Amir Qaseem, MD, PhD, MHA, vice president of clinical policy and the Center for Evidence Reviews at the ACP in Philadelphia.
Practice points
- Consider the oral antivirals nirmatrelvir-ritonavir (Paxlovid) or molnupiravir (Lagevrio) for symptomatic outpatients with confirmed mild to moderate COVID-19 who are within 5 days of the onset of symptoms and at high risk for progressing to severe disease.
New evidence for the Omicron variant suggests a possible net benefit of the antiviral molnupiravir versus standard or no treatment in terms of reducing recovery time if treatment is initiated within 5 days of symptom onset. Nirmatrelvir-ritonavir was associated with reductions in COVID-19 hospitalization and all-cause mortality.
“The practice points only address [whether] treatments work compared to placebo, no treatment, or usual care,” cautioned Linda L. Humphrey, MD, MPH, MACP, chair of the ACP’s Population Health and Medical Science Committee and a professor of medicine at Oregon Health and Science University VA Portland Health Care System. The ACP continues to monitor the evidence. “Once enough evidence has emerged, it will be possible to compare treatments to each other. Until that time we are unable to determine if there is an advantage to using one treatment over another.”
- Do not use the antiparasitic ivermectin (Stromectol) or the monoclonal antibody sotrovimab (Xevudy) to treat this patient population. “It is not expected to be effective against the Omicron variant,” Dr. Humphrey said.
There was no evidence to support the use of medications such as corticosteroids, antibiotics, antihistamines, SSRIs, and multiple other agents.
“The guideline is not a departure from previous knowledge and reflects what appears in other guidelines and is already being done generally in practice,” said Mirella Salvatore, MD, an associate professor of medicine and population health sciences at Weill Cornell Medicine, New York, who was not involved in the ACP statement. It is therefore unlikely the recommendations will trigger controversy or negative feedback, added Dr. Salvatore, who is also a spokesperson for the Infectious Diseases Society of America. “We believe that our evidence-based approach, which considers the balance of benefits and harms of various treatments, will be embraced by the physician community,” Dr. Humphrey said.
The updated recommendations are based on new data from the evidence review of multiple treatments, which concluded that both nirmatrelvir-ritonavir and molnupiravir likely improve outcomes for outpatients with mild to moderate COVID-19. The review was conducted after the emergence of the Omicron variant by the ACP Center for Evidence Reviews at Cochrane Austria/University for Continuing Education Krems (Austria).
Review details
Inclusion criteria were modified to focus on the Omicron variant by limiting eligible studies to only those enrolling patients on or after Nov. 26, 2021. The investigators included two randomized controlled trials and six retrospective cohort studies and ranked quality of evidence for the effectiveness of the following treatments, compared with usual care or no treatment: azithromycin, camostat mesylate, chloroquine-hydroxychloroquine, chlorpheniramine, colchicine, convalescent plasma, corticosteroids, ensitrelvir, favipiravir, fluvoxamine, ivermectin, lopinavir-ritonavir, molnupiravir, neutralizing monoclonal antibodies, metformin, niclosamide, nitazoxanide, nirmatrelvir-ritonavir, and remdesivir.
It compared results for all-cause and COVID-specific mortality, recovery, time to recovery, COVID hospitalization, and adverse and serious adverse events.
Nirmatrelvir-ritonavir was associated with a reduction in hospitalization caused by COVID-19 of 0.7% versus 1.2% (moderate certainty of evidence [COE]) and a reduction in all-cause mortality of less than 0.1% versus 0.2% (moderate COE).
Molnupiravir led to a higher recovery rate of 31.8% versus 22.6% (moderate COE) and a reduced time to recovery of 9 versus 15 median days (moderate COE). It had no effect, however, on all-cause mortality: 0.02% versus 0.04% (moderate COE). Nor did it affect the incidence of serious adverse events: 0.4% versus 0.3% (moderate COE).
“There have been no head-to-head comparative studies of these two treatments, but nirmatrelvir-ritonavir appears to be the preferred treatment,” Dr. Salvatore said. She noted that molnupiravir cannot be used in pregnant women or young persons under age 18, while nirmatrelvir-ritonavir carries the risk of drug interactions. Viral rebound and recurrence of symptoms have been reported in some patients receiving nirmatrelvir-ritonavir.
In other review findings, ivermectin had no effect on time to recovery (moderate COE) and adverse events versus placebo (low COE). Sotrovimab resulted in no difference in all-cause mortality, compared with no treatment (low COE). There were no eligible studies for all of the other treatments of interest nor were there any that specifically evaluated the benefits and harms of treatments for the Omicron variant.
The panel pointed to the need for more evaluation of the efficacy, effectiveness, and comparative effectiveness, as well as harms of pharmacologic and biologic treatments of COVID-19 in the outpatient setting, particularly in the context of changing dominant SARS-CoV-2 variants and subvariants.
Another area requiring further research is the effectiveness of retreatment in patients with previous COVID-19 infection. Subgroup analyses are also needed to assess whether the efficacy and effectiveness of outpatient treatments vary by age, sex, socioeconomic status, and comorbid conditions – or by SARS-CoV-2 variant, immunity status (prior SARS-CoV-2 infection, vaccination status, or time since infection or vaccination), symptom duration, or disease severity.
Dr. Salvatore agreed that more research is needed in special convalescent groups. “For instance, those with cancer who are immunocompromised may need longer treatment and adjunctive treatment with convalescent plasma. But is difficult to find a large enough study with 5,000 immunocompromised patients.”
Financial support for the development of the practice points came exclusively from the ACP operating budget. The evidence review was funded by the ACP. The authors disclosed no relevant high-level competing interests with regard to this guidance, although several authors reported intellectual interests in various areas of research. Dr. Salvatore disclosed no conflicts of interest relevant to her comments but is engaged in influenza research for Genentech.
The American College of Physicians has issued an updated version of its living, rapid practice point guideline on the best treatment options for outpatients with confirmed COVID-19 in the era of the dominant Omicron variant of SARS-CoV-2. The recommendations in version 2 apply to persons presenting with mild to moderate infection and symptom onset in the past 5 days who are at high risk for progression to severe disease and potential hospitalization or death.
Version 1 appeared in late 2022.
While outpatient management is appropriate for most patients, treatment should be personalized and based on careful risk stratification and informed decision-making, said the guideline authors, led by Amir Qaseem, MD, PhD, MHA, vice president of clinical policy and the Center for Evidence Reviews at the ACP in Philadelphia.
Practice points
- Consider the oral antivirals nirmatrelvir-ritonavir (Paxlovid) or molnupiravir (Lagevrio) for symptomatic outpatients with confirmed mild to moderate COVID-19 who are within 5 days of the onset of symptoms and at high risk for progressing to severe disease.
New evidence for the Omicron variant suggests a possible net benefit of the antiviral molnupiravir versus standard or no treatment in terms of reducing recovery time if treatment is initiated within 5 days of symptom onset. Nirmatrelvir-ritonavir was associated with reductions in COVID-19 hospitalization and all-cause mortality.
“The practice points only address [whether] treatments work compared to placebo, no treatment, or usual care,” cautioned Linda L. Humphrey, MD, MPH, MACP, chair of the ACP’s Population Health and Medical Science Committee and a professor of medicine at Oregon Health and Science University VA Portland Health Care System. The ACP continues to monitor the evidence. “Once enough evidence has emerged, it will be possible to compare treatments to each other. Until that time we are unable to determine if there is an advantage to using one treatment over another.”
- Do not use the antiparasitic ivermectin (Stromectol) or the monoclonal antibody sotrovimab (Xevudy) to treat this patient population. “It is not expected to be effective against the Omicron variant,” Dr. Humphrey said.
There was no evidence to support the use of medications such as corticosteroids, antibiotics, antihistamines, SSRIs, and multiple other agents.
“The guideline is not a departure from previous knowledge and reflects what appears in other guidelines and is already being done generally in practice,” said Mirella Salvatore, MD, an associate professor of medicine and population health sciences at Weill Cornell Medicine, New York, who was not involved in the ACP statement. It is therefore unlikely the recommendations will trigger controversy or negative feedback, added Dr. Salvatore, who is also a spokesperson for the Infectious Diseases Society of America. “We believe that our evidence-based approach, which considers the balance of benefits and harms of various treatments, will be embraced by the physician community,” Dr. Humphrey said.
The updated recommendations are based on new data from the evidence review of multiple treatments, which concluded that both nirmatrelvir-ritonavir and molnupiravir likely improve outcomes for outpatients with mild to moderate COVID-19. The review was conducted after the emergence of the Omicron variant by the ACP Center for Evidence Reviews at Cochrane Austria/University for Continuing Education Krems (Austria).
Review details
Inclusion criteria were modified to focus on the Omicron variant by limiting eligible studies to only those enrolling patients on or after Nov. 26, 2021. The investigators included two randomized controlled trials and six retrospective cohort studies and ranked quality of evidence for the effectiveness of the following treatments, compared with usual care or no treatment: azithromycin, camostat mesylate, chloroquine-hydroxychloroquine, chlorpheniramine, colchicine, convalescent plasma, corticosteroids, ensitrelvir, favipiravir, fluvoxamine, ivermectin, lopinavir-ritonavir, molnupiravir, neutralizing monoclonal antibodies, metformin, niclosamide, nitazoxanide, nirmatrelvir-ritonavir, and remdesivir.
It compared results for all-cause and COVID-specific mortality, recovery, time to recovery, COVID hospitalization, and adverse and serious adverse events.
Nirmatrelvir-ritonavir was associated with a reduction in hospitalization caused by COVID-19 of 0.7% versus 1.2% (moderate certainty of evidence [COE]) and a reduction in all-cause mortality of less than 0.1% versus 0.2% (moderate COE).
Molnupiravir led to a higher recovery rate of 31.8% versus 22.6% (moderate COE) and a reduced time to recovery of 9 versus 15 median days (moderate COE). It had no effect, however, on all-cause mortality: 0.02% versus 0.04% (moderate COE). Nor did it affect the incidence of serious adverse events: 0.4% versus 0.3% (moderate COE).
“There have been no head-to-head comparative studies of these two treatments, but nirmatrelvir-ritonavir appears to be the preferred treatment,” Dr. Salvatore said. She noted that molnupiravir cannot be used in pregnant women or young persons under age 18, while nirmatrelvir-ritonavir carries the risk of drug interactions. Viral rebound and recurrence of symptoms have been reported in some patients receiving nirmatrelvir-ritonavir.
In other review findings, ivermectin had no effect on time to recovery (moderate COE) and adverse events versus placebo (low COE). Sotrovimab resulted in no difference in all-cause mortality, compared with no treatment (low COE). There were no eligible studies for all of the other treatments of interest nor were there any that specifically evaluated the benefits and harms of treatments for the Omicron variant.
The panel pointed to the need for more evaluation of the efficacy, effectiveness, and comparative effectiveness, as well as harms of pharmacologic and biologic treatments of COVID-19 in the outpatient setting, particularly in the context of changing dominant SARS-CoV-2 variants and subvariants.
Another area requiring further research is the effectiveness of retreatment in patients with previous COVID-19 infection. Subgroup analyses are also needed to assess whether the efficacy and effectiveness of outpatient treatments vary by age, sex, socioeconomic status, and comorbid conditions – or by SARS-CoV-2 variant, immunity status (prior SARS-CoV-2 infection, vaccination status, or time since infection or vaccination), symptom duration, or disease severity.
Dr. Salvatore agreed that more research is needed in special convalescent groups. “For instance, those with cancer who are immunocompromised may need longer treatment and adjunctive treatment with convalescent plasma. But is difficult to find a large enough study with 5,000 immunocompromised patients.”
Financial support for the development of the practice points came exclusively from the ACP operating budget. The evidence review was funded by the ACP. The authors disclosed no relevant high-level competing interests with regard to this guidance, although several authors reported intellectual interests in various areas of research. Dr. Salvatore disclosed no conflicts of interest relevant to her comments but is engaged in influenza research for Genentech.
The American College of Physicians has issued an updated version of its living, rapid practice point guideline on the best treatment options for outpatients with confirmed COVID-19 in the era of the dominant Omicron variant of SARS-CoV-2. The recommendations in version 2 apply to persons presenting with mild to moderate infection and symptom onset in the past 5 days who are at high risk for progression to severe disease and potential hospitalization or death.
Version 1 appeared in late 2022.
While outpatient management is appropriate for most patients, treatment should be personalized and based on careful risk stratification and informed decision-making, said the guideline authors, led by Amir Qaseem, MD, PhD, MHA, vice president of clinical policy and the Center for Evidence Reviews at the ACP in Philadelphia.
Practice points
- Consider the oral antivirals nirmatrelvir-ritonavir (Paxlovid) or molnupiravir (Lagevrio) for symptomatic outpatients with confirmed mild to moderate COVID-19 who are within 5 days of the onset of symptoms and at high risk for progressing to severe disease.
New evidence for the Omicron variant suggests a possible net benefit of the antiviral molnupiravir versus standard or no treatment in terms of reducing recovery time if treatment is initiated within 5 days of symptom onset. Nirmatrelvir-ritonavir was associated with reductions in COVID-19 hospitalization and all-cause mortality.
“The practice points only address [whether] treatments work compared to placebo, no treatment, or usual care,” cautioned Linda L. Humphrey, MD, MPH, MACP, chair of the ACP’s Population Health and Medical Science Committee and a professor of medicine at Oregon Health and Science University VA Portland Health Care System. The ACP continues to monitor the evidence. “Once enough evidence has emerged, it will be possible to compare treatments to each other. Until that time we are unable to determine if there is an advantage to using one treatment over another.”
- Do not use the antiparasitic ivermectin (Stromectol) or the monoclonal antibody sotrovimab (Xevudy) to treat this patient population. “It is not expected to be effective against the Omicron variant,” Dr. Humphrey said.
There was no evidence to support the use of medications such as corticosteroids, antibiotics, antihistamines, SSRIs, and multiple other agents.
“The guideline is not a departure from previous knowledge and reflects what appears in other guidelines and is already being done generally in practice,” said Mirella Salvatore, MD, an associate professor of medicine and population health sciences at Weill Cornell Medicine, New York, who was not involved in the ACP statement. It is therefore unlikely the recommendations will trigger controversy or negative feedback, added Dr. Salvatore, who is also a spokesperson for the Infectious Diseases Society of America. “We believe that our evidence-based approach, which considers the balance of benefits and harms of various treatments, will be embraced by the physician community,” Dr. Humphrey said.
The updated recommendations are based on new data from the evidence review of multiple treatments, which concluded that both nirmatrelvir-ritonavir and molnupiravir likely improve outcomes for outpatients with mild to moderate COVID-19. The review was conducted after the emergence of the Omicron variant by the ACP Center for Evidence Reviews at Cochrane Austria/University for Continuing Education Krems (Austria).
Review details
Inclusion criteria were modified to focus on the Omicron variant by limiting eligible studies to only those enrolling patients on or after Nov. 26, 2021. The investigators included two randomized controlled trials and six retrospective cohort studies and ranked quality of evidence for the effectiveness of the following treatments, compared with usual care or no treatment: azithromycin, camostat mesylate, chloroquine-hydroxychloroquine, chlorpheniramine, colchicine, convalescent plasma, corticosteroids, ensitrelvir, favipiravir, fluvoxamine, ivermectin, lopinavir-ritonavir, molnupiravir, neutralizing monoclonal antibodies, metformin, niclosamide, nitazoxanide, nirmatrelvir-ritonavir, and remdesivir.
It compared results for all-cause and COVID-specific mortality, recovery, time to recovery, COVID hospitalization, and adverse and serious adverse events.
Nirmatrelvir-ritonavir was associated with a reduction in hospitalization caused by COVID-19 of 0.7% versus 1.2% (moderate certainty of evidence [COE]) and a reduction in all-cause mortality of less than 0.1% versus 0.2% (moderate COE).
Molnupiravir led to a higher recovery rate of 31.8% versus 22.6% (moderate COE) and a reduced time to recovery of 9 versus 15 median days (moderate COE). It had no effect, however, on all-cause mortality: 0.02% versus 0.04% (moderate COE). Nor did it affect the incidence of serious adverse events: 0.4% versus 0.3% (moderate COE).
“There have been no head-to-head comparative studies of these two treatments, but nirmatrelvir-ritonavir appears to be the preferred treatment,” Dr. Salvatore said. She noted that molnupiravir cannot be used in pregnant women or young persons under age 18, while nirmatrelvir-ritonavir carries the risk of drug interactions. Viral rebound and recurrence of symptoms have been reported in some patients receiving nirmatrelvir-ritonavir.
In other review findings, ivermectin had no effect on time to recovery (moderate COE) and adverse events versus placebo (low COE). Sotrovimab resulted in no difference in all-cause mortality, compared with no treatment (low COE). There were no eligible studies for all of the other treatments of interest nor were there any that specifically evaluated the benefits and harms of treatments for the Omicron variant.
The panel pointed to the need for more evaluation of the efficacy, effectiveness, and comparative effectiveness, as well as harms of pharmacologic and biologic treatments of COVID-19 in the outpatient setting, particularly in the context of changing dominant SARS-CoV-2 variants and subvariants.
Another area requiring further research is the effectiveness of retreatment in patients with previous COVID-19 infection. Subgroup analyses are also needed to assess whether the efficacy and effectiveness of outpatient treatments vary by age, sex, socioeconomic status, and comorbid conditions – or by SARS-CoV-2 variant, immunity status (prior SARS-CoV-2 infection, vaccination status, or time since infection or vaccination), symptom duration, or disease severity.
Dr. Salvatore agreed that more research is needed in special convalescent groups. “For instance, those with cancer who are immunocompromised may need longer treatment and adjunctive treatment with convalescent plasma. But is difficult to find a large enough study with 5,000 immunocompromised patients.”
Financial support for the development of the practice points came exclusively from the ACP operating budget. The evidence review was funded by the ACP. The authors disclosed no relevant high-level competing interests with regard to this guidance, although several authors reported intellectual interests in various areas of research. Dr. Salvatore disclosed no conflicts of interest relevant to her comments but is engaged in influenza research for Genentech.
FROM ANNALS OF INTERNAL MEDICINE
New antibiotic could combat multidrug-resistant superbugs
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
FROM CELL
Antigen tests: After pandemic success, time for bigger role?
Before the pandemic, most of the public probably had a fleeting and limited familiarity with lateral flow tests (LFTs), also known as rapid antigen tests. Perhaps they used, or awaited the results of, a lateral flow home pregnancy test, which detects human chorionic gonadotropin in urine.
Then came COVID-19, and the need for large-scale testing. By late 2022, more than 3 billion tests for SARS-CoV-2 had been done worldwide. Although testing with reverse-transcription polymerase chain reaction (PCR) is the gold standard for diagnosing COVID, LFTs made possible large-scale testing at low cost with rapid results.
As of Sept. 12, the Food and Drug Administration lists 32 rapid antigen tests with emergency use authorizations (EUAs) for home use.
Now, many experts conclude, it’s time to expand the role of LFTs so the technology can help detect a host of other diseases. In a Nature Reviews bioengineering report, global experts from the United States, the United Kingdom, and other countries pointed out that commercial LFTs are currently not available for four of the eight known priority diseases of epidemic potential: Crimean-Congo hemorrhagic fever, Middle East respiratory syndrome coronavirus, Nipah and other henipaviruses, and Rift Valley fever.
Expansion should not only include more tests for more diseases, some experts say, but also make use of existing technology to provide “full-circle” care. After a rapid test, for instance, users could download a mobile phone app, transmit the results to their health care provider, and then set up an appointment if needed or get a prescribed medication at the pharmacy.
Medical community on board
Clinicians support increased availability of LFTs, said Eric J. Topol, MD, professor and executive vice president of Scripps Research, La Jolla, Calif.“Rapid antigen tests are critical, made a big difference in the pandemic, and will be used increasingly for many other applications in the years ahead,” Dr. Topol said in an email.
Physicians welcome their potential, agreed William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. At the start of the pandemic, when he was briefed about a lateral flow device in development, he said, “I was blown away by the technology, ease of use, rapidity of getting a result, its reasonable accuracy and its anticipated relatively low price.”
Clinicians would probably see many advantages to having more LFTs for more diseases, Dr. Schaffner said, because they are of use not only at home but also in doctors’ offices and in emergency departments. Their increased use “would help [people] make quick decisions about treatment, especially for flu and COVID.”
How LFTs work
LFTs are capable of targeting antigens, such as for the COVID tests, and antibodies such as IgG or IgM. The tests are also capable of detecting nucleic acids, although the availability of these tests is currently rare.
First, a sample from blood, urine, saliva or other bodily sources is placed onto a sample pad. It travels to a conjugate pad containing antibodies. If the target being looked for is present, the target and antibodies bind and, as the sample moves along to the test line, produces a positive result line along with the control line (to show that the test worked).
Global market outlook
By 2030, the lateral flow assays market is predicted to rise to $14.1 billion, according to a report issued in September by the firm Research and Markets. In 2022, the market was estimated at $9.4 billion, with $3.6 billion of that in the United States.
The report details the performances of 55 major competitors, such as Abbott Laboratories, Siemens, and QuidelOrtho, but smaller companies and start-ups are also involved in LFT development.
LFTs: Pros and cons
Although LFTs give rapid results, their accuracy is lower than that of PCR, especially the sensitivity. For COVID antigen LFTs, the sensitivity ranges from 34.1% to 88.1%, with an overall specificity of 99.6%, according to a Cochrane Review report. The analytical sensitivity performance of PCR testing for COVID is near 100%.
Everyone acknowledges the accuracy challenge of LFTs. The technologies “are generally thought to have limitations of detection that for some applications may present a challenge,” said Douglas C. Bryant, president and CEO of QuidelOrtho, San Diego, which counts the QuickVue rapid test for COVID detection among its products.
However, Mr. Bryant added, “as we saw during the pandemic, there was a place for more sensitive PCR-based technologies that are often run in a lab and there was a place for the use of rapid tests: The key is knowing the strengths and best use cases when applying the different technologies.”
One strength, he said, was that the tests “were shown to be highly effective at detecting active, infectious cases of SARS-CoV-2 and the rapid turnaround time allowed patients to isolate themselves from others quickly to help curb the spread of infection to others.” Another advantage was the ability to screen high-risk populations such as nursing homes to detect positive cases and help prevent outbreaks.
The pandemic familiarized people with the tests, said Jeremy Stackawitz, CEO of Senzo, a start-up in vitro diagnostics company developing an amplified LFT platform for rapid tests for flu, tuberculosis, COVID, and Clostridioides difficile. People liked using them. Physicians generally accepted them. It works great with tele-doc. It works great with personalized medicine.
Now, he said, people used to the COVID self-tests are asking: “Where is my strep test? Where is my sexual health test?”
FDA’s perspective on LFTs
The FDA has no one-size-fits-all standard for evaluating LFTs.
“LFTs are evaluated with respect to their individual indications and the pathway under which they are being reviewed,” said James McKinney, an FDA spokesperson. “A performance recommendation for one type of lateral flow test may not be appropriate for another.”
EUAs, such as those given for the COVID at-home tests, require different levels of evidence than traditional premarket review, he said, whether de novo marketing authorization, 510(k) premarket notification, or premarket approval. The EUAs are evaluated with a risk-benefit analysis to speed up the time it takes to make the devices available.
And, Mr. McKinney said, for some devices, the FDA provides recommendations on the expected performance through guidance documents. For instance, for rapid devices developed to detect influenza A virus antigen, the FDA recommends including enough sample to generate sensitivity of greater than 60% and testing at least 50 samples.
LFTs: The potential, the challenges
Mr. Stackawitz predicted that, as more LFT self-tests become available, more people will seek care, just as they did with the COVID rapid tests. A 22-year-old who thinks he has chlamydia may balk at going to a doctor right away. However, “if he can go buy a soda and a test at CVS, it’s different, it really is. With a little anonymity, people will seek care.”
He has a vision shared by other experts: That testing technology will evolve so that after getting the results at home, people would follow through by sending those results to their health care provider and obtaining needed care or medication. In his opinion, this is superior to the traditional way, which often involves visiting a doctor with symptoms, going for tests, waiting for results, and then beginning treatment.
“It would make more sense if you came in knowing your results,” Mr. Stackawitz said. “It’s a much smarter pathway, gives better outcomes for the patient, is much quicker and at much less cost. And it frees up time for doctors. I think most physicians would embrace that.”
Although rapid testing is gaining well-deserved recognition, funding is an issue, according to the Nature Reviews report. Those experts warned that “a reduction in funding for LFT research post COVID-19 may hamper efforts to capitalize on gains in decentralized testing, especially self-testing, which may be critical to address future pandemic threats.”
A version of this article first appeared on Medscape.com.
Before the pandemic, most of the public probably had a fleeting and limited familiarity with lateral flow tests (LFTs), also known as rapid antigen tests. Perhaps they used, or awaited the results of, a lateral flow home pregnancy test, which detects human chorionic gonadotropin in urine.
Then came COVID-19, and the need for large-scale testing. By late 2022, more than 3 billion tests for SARS-CoV-2 had been done worldwide. Although testing with reverse-transcription polymerase chain reaction (PCR) is the gold standard for diagnosing COVID, LFTs made possible large-scale testing at low cost with rapid results.
As of Sept. 12, the Food and Drug Administration lists 32 rapid antigen tests with emergency use authorizations (EUAs) for home use.
Now, many experts conclude, it’s time to expand the role of LFTs so the technology can help detect a host of other diseases. In a Nature Reviews bioengineering report, global experts from the United States, the United Kingdom, and other countries pointed out that commercial LFTs are currently not available for four of the eight known priority diseases of epidemic potential: Crimean-Congo hemorrhagic fever, Middle East respiratory syndrome coronavirus, Nipah and other henipaviruses, and Rift Valley fever.
Expansion should not only include more tests for more diseases, some experts say, but also make use of existing technology to provide “full-circle” care. After a rapid test, for instance, users could download a mobile phone app, transmit the results to their health care provider, and then set up an appointment if needed or get a prescribed medication at the pharmacy.
Medical community on board
Clinicians support increased availability of LFTs, said Eric J. Topol, MD, professor and executive vice president of Scripps Research, La Jolla, Calif.“Rapid antigen tests are critical, made a big difference in the pandemic, and will be used increasingly for many other applications in the years ahead,” Dr. Topol said in an email.
Physicians welcome their potential, agreed William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. At the start of the pandemic, when he was briefed about a lateral flow device in development, he said, “I was blown away by the technology, ease of use, rapidity of getting a result, its reasonable accuracy and its anticipated relatively low price.”
Clinicians would probably see many advantages to having more LFTs for more diseases, Dr. Schaffner said, because they are of use not only at home but also in doctors’ offices and in emergency departments. Their increased use “would help [people] make quick decisions about treatment, especially for flu and COVID.”
How LFTs work
LFTs are capable of targeting antigens, such as for the COVID tests, and antibodies such as IgG or IgM. The tests are also capable of detecting nucleic acids, although the availability of these tests is currently rare.
First, a sample from blood, urine, saliva or other bodily sources is placed onto a sample pad. It travels to a conjugate pad containing antibodies. If the target being looked for is present, the target and antibodies bind and, as the sample moves along to the test line, produces a positive result line along with the control line (to show that the test worked).
Global market outlook
By 2030, the lateral flow assays market is predicted to rise to $14.1 billion, according to a report issued in September by the firm Research and Markets. In 2022, the market was estimated at $9.4 billion, with $3.6 billion of that in the United States.
The report details the performances of 55 major competitors, such as Abbott Laboratories, Siemens, and QuidelOrtho, but smaller companies and start-ups are also involved in LFT development.
LFTs: Pros and cons
Although LFTs give rapid results, their accuracy is lower than that of PCR, especially the sensitivity. For COVID antigen LFTs, the sensitivity ranges from 34.1% to 88.1%, with an overall specificity of 99.6%, according to a Cochrane Review report. The analytical sensitivity performance of PCR testing for COVID is near 100%.
Everyone acknowledges the accuracy challenge of LFTs. The technologies “are generally thought to have limitations of detection that for some applications may present a challenge,” said Douglas C. Bryant, president and CEO of QuidelOrtho, San Diego, which counts the QuickVue rapid test for COVID detection among its products.
However, Mr. Bryant added, “as we saw during the pandemic, there was a place for more sensitive PCR-based technologies that are often run in a lab and there was a place for the use of rapid tests: The key is knowing the strengths and best use cases when applying the different technologies.”
One strength, he said, was that the tests “were shown to be highly effective at detecting active, infectious cases of SARS-CoV-2 and the rapid turnaround time allowed patients to isolate themselves from others quickly to help curb the spread of infection to others.” Another advantage was the ability to screen high-risk populations such as nursing homes to detect positive cases and help prevent outbreaks.
The pandemic familiarized people with the tests, said Jeremy Stackawitz, CEO of Senzo, a start-up in vitro diagnostics company developing an amplified LFT platform for rapid tests for flu, tuberculosis, COVID, and Clostridioides difficile. People liked using them. Physicians generally accepted them. It works great with tele-doc. It works great with personalized medicine.
Now, he said, people used to the COVID self-tests are asking: “Where is my strep test? Where is my sexual health test?”
FDA’s perspective on LFTs
The FDA has no one-size-fits-all standard for evaluating LFTs.
“LFTs are evaluated with respect to their individual indications and the pathway under which they are being reviewed,” said James McKinney, an FDA spokesperson. “A performance recommendation for one type of lateral flow test may not be appropriate for another.”
EUAs, such as those given for the COVID at-home tests, require different levels of evidence than traditional premarket review, he said, whether de novo marketing authorization, 510(k) premarket notification, or premarket approval. The EUAs are evaluated with a risk-benefit analysis to speed up the time it takes to make the devices available.
And, Mr. McKinney said, for some devices, the FDA provides recommendations on the expected performance through guidance documents. For instance, for rapid devices developed to detect influenza A virus antigen, the FDA recommends including enough sample to generate sensitivity of greater than 60% and testing at least 50 samples.
LFTs: The potential, the challenges
Mr. Stackawitz predicted that, as more LFT self-tests become available, more people will seek care, just as they did with the COVID rapid tests. A 22-year-old who thinks he has chlamydia may balk at going to a doctor right away. However, “if he can go buy a soda and a test at CVS, it’s different, it really is. With a little anonymity, people will seek care.”
He has a vision shared by other experts: That testing technology will evolve so that after getting the results at home, people would follow through by sending those results to their health care provider and obtaining needed care or medication. In his opinion, this is superior to the traditional way, which often involves visiting a doctor with symptoms, going for tests, waiting for results, and then beginning treatment.
“It would make more sense if you came in knowing your results,” Mr. Stackawitz said. “It’s a much smarter pathway, gives better outcomes for the patient, is much quicker and at much less cost. And it frees up time for doctors. I think most physicians would embrace that.”
Although rapid testing is gaining well-deserved recognition, funding is an issue, according to the Nature Reviews report. Those experts warned that “a reduction in funding for LFT research post COVID-19 may hamper efforts to capitalize on gains in decentralized testing, especially self-testing, which may be critical to address future pandemic threats.”
A version of this article first appeared on Medscape.com.
Before the pandemic, most of the public probably had a fleeting and limited familiarity with lateral flow tests (LFTs), also known as rapid antigen tests. Perhaps they used, or awaited the results of, a lateral flow home pregnancy test, which detects human chorionic gonadotropin in urine.
Then came COVID-19, and the need for large-scale testing. By late 2022, more than 3 billion tests for SARS-CoV-2 had been done worldwide. Although testing with reverse-transcription polymerase chain reaction (PCR) is the gold standard for diagnosing COVID, LFTs made possible large-scale testing at low cost with rapid results.
As of Sept. 12, the Food and Drug Administration lists 32 rapid antigen tests with emergency use authorizations (EUAs) for home use.
Now, many experts conclude, it’s time to expand the role of LFTs so the technology can help detect a host of other diseases. In a Nature Reviews bioengineering report, global experts from the United States, the United Kingdom, and other countries pointed out that commercial LFTs are currently not available for four of the eight known priority diseases of epidemic potential: Crimean-Congo hemorrhagic fever, Middle East respiratory syndrome coronavirus, Nipah and other henipaviruses, and Rift Valley fever.
Expansion should not only include more tests for more diseases, some experts say, but also make use of existing technology to provide “full-circle” care. After a rapid test, for instance, users could download a mobile phone app, transmit the results to their health care provider, and then set up an appointment if needed or get a prescribed medication at the pharmacy.
Medical community on board
Clinicians support increased availability of LFTs, said Eric J. Topol, MD, professor and executive vice president of Scripps Research, La Jolla, Calif.“Rapid antigen tests are critical, made a big difference in the pandemic, and will be used increasingly for many other applications in the years ahead,” Dr. Topol said in an email.
Physicians welcome their potential, agreed William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. At the start of the pandemic, when he was briefed about a lateral flow device in development, he said, “I was blown away by the technology, ease of use, rapidity of getting a result, its reasonable accuracy and its anticipated relatively low price.”
Clinicians would probably see many advantages to having more LFTs for more diseases, Dr. Schaffner said, because they are of use not only at home but also in doctors’ offices and in emergency departments. Their increased use “would help [people] make quick decisions about treatment, especially for flu and COVID.”
How LFTs work
LFTs are capable of targeting antigens, such as for the COVID tests, and antibodies such as IgG or IgM. The tests are also capable of detecting nucleic acids, although the availability of these tests is currently rare.
First, a sample from blood, urine, saliva or other bodily sources is placed onto a sample pad. It travels to a conjugate pad containing antibodies. If the target being looked for is present, the target and antibodies bind and, as the sample moves along to the test line, produces a positive result line along with the control line (to show that the test worked).
Global market outlook
By 2030, the lateral flow assays market is predicted to rise to $14.1 billion, according to a report issued in September by the firm Research and Markets. In 2022, the market was estimated at $9.4 billion, with $3.6 billion of that in the United States.
The report details the performances of 55 major competitors, such as Abbott Laboratories, Siemens, and QuidelOrtho, but smaller companies and start-ups are also involved in LFT development.
LFTs: Pros and cons
Although LFTs give rapid results, their accuracy is lower than that of PCR, especially the sensitivity. For COVID antigen LFTs, the sensitivity ranges from 34.1% to 88.1%, with an overall specificity of 99.6%, according to a Cochrane Review report. The analytical sensitivity performance of PCR testing for COVID is near 100%.
Everyone acknowledges the accuracy challenge of LFTs. The technologies “are generally thought to have limitations of detection that for some applications may present a challenge,” said Douglas C. Bryant, president and CEO of QuidelOrtho, San Diego, which counts the QuickVue rapid test for COVID detection among its products.
However, Mr. Bryant added, “as we saw during the pandemic, there was a place for more sensitive PCR-based technologies that are often run in a lab and there was a place for the use of rapid tests: The key is knowing the strengths and best use cases when applying the different technologies.”
One strength, he said, was that the tests “were shown to be highly effective at detecting active, infectious cases of SARS-CoV-2 and the rapid turnaround time allowed patients to isolate themselves from others quickly to help curb the spread of infection to others.” Another advantage was the ability to screen high-risk populations such as nursing homes to detect positive cases and help prevent outbreaks.
The pandemic familiarized people with the tests, said Jeremy Stackawitz, CEO of Senzo, a start-up in vitro diagnostics company developing an amplified LFT platform for rapid tests for flu, tuberculosis, COVID, and Clostridioides difficile. People liked using them. Physicians generally accepted them. It works great with tele-doc. It works great with personalized medicine.
Now, he said, people used to the COVID self-tests are asking: “Where is my strep test? Where is my sexual health test?”
FDA’s perspective on LFTs
The FDA has no one-size-fits-all standard for evaluating LFTs.
“LFTs are evaluated with respect to their individual indications and the pathway under which they are being reviewed,” said James McKinney, an FDA spokesperson. “A performance recommendation for one type of lateral flow test may not be appropriate for another.”
EUAs, such as those given for the COVID at-home tests, require different levels of evidence than traditional premarket review, he said, whether de novo marketing authorization, 510(k) premarket notification, or premarket approval. The EUAs are evaluated with a risk-benefit analysis to speed up the time it takes to make the devices available.
And, Mr. McKinney said, for some devices, the FDA provides recommendations on the expected performance through guidance documents. For instance, for rapid devices developed to detect influenza A virus antigen, the FDA recommends including enough sample to generate sensitivity of greater than 60% and testing at least 50 samples.
LFTs: The potential, the challenges
Mr. Stackawitz predicted that, as more LFT self-tests become available, more people will seek care, just as they did with the COVID rapid tests. A 22-year-old who thinks he has chlamydia may balk at going to a doctor right away. However, “if he can go buy a soda and a test at CVS, it’s different, it really is. With a little anonymity, people will seek care.”
He has a vision shared by other experts: That testing technology will evolve so that after getting the results at home, people would follow through by sending those results to their health care provider and obtaining needed care or medication. In his opinion, this is superior to the traditional way, which often involves visiting a doctor with symptoms, going for tests, waiting for results, and then beginning treatment.
“It would make more sense if you came in knowing your results,” Mr. Stackawitz said. “It’s a much smarter pathway, gives better outcomes for the patient, is much quicker and at much less cost. And it frees up time for doctors. I think most physicians would embrace that.”
Although rapid testing is gaining well-deserved recognition, funding is an issue, according to the Nature Reviews report. Those experts warned that “a reduction in funding for LFT research post COVID-19 may hamper efforts to capitalize on gains in decentralized testing, especially self-testing, which may be critical to address future pandemic threats.”
A version of this article first appeared on Medscape.com.
PCPs prep for ‘less predictable’ respiratory virus season
Hospitalizations for COVID-19 in the United States have increased for 8 weeks in a row.
Data from Florida and Georgia signal that respiratory syncytial virus (RSV) season has begun.
As for flu shots, experts say patients with long COVID should get them in 2023, although federal health agencies have not addressed that specific question.
Paul G. Auwaerter, MD, MBA, an infectious disease consultant, said many patients in his primary care practice worry about “the big three” – COVID, influenza, and RSV.
They discussed how to handle COVID boosters, the use of Paxlovid, vaccine hesitancy, and the correct order of operations for patients getting vaccinated against all three diseases.
Paul G. Auwaerter, MD, MBA, clinical director of the division of infectious diseases and the Sherrilyn and Ken Fisher Professor of Medicine at Johns Hopkins University, BaltimoreQuestion: How should primary care physicians be preparing to handle what everyone is predicting will be a major surge in cases of respiratory infections?
Auwaerter: Although I’m an infectious disease consultant, I still have a small primary care practice. So, I field questions for my patients all the time, and many patients, especially those with health problems, are worried about the big three: RSV, COVID, and influenza – at least, my more motivated patients are.
People frequently ask if they need the COVID booster. I think that’s been something many people think maybe they can avoid. The good news is that the early in vitro data suggest that the XBB1.5x-based vaccine seems to offer sufficient neutralizing activity against the circulating newer variants since the vaccine was approved earlier this year. I am suggesting that everyone get a booster, especially those at high risk, because we know that the risk for hospitalization decreases based on earlier studies for 4-6 months after a COVID booster. We can simultaneously administer the revised COVID booster vaccine and the annual influenza vaccine. The timing is good, as influenza immunization should be accomplished by October or early November at the latest. Like many parts of the country, we in Maryland are in the middle of a COVID boomlet. I have issued more Paxlovid prescriptions since mid-August than I did all spring and early summer.
Q: Are you seeing a lot of rebound COVID in your patients taking Paxlovid [nirmatrelvir/ritonavir]?
Dr. Auwaerter: I think the frequency is probably around 10%. It has been quoted much higher – at 20% – but careful studies have put it down at just single digits. I think it just depends on symptomatology and how you ask the question. But I think it’s important that I try to persuade people to take a direct-acting antiviral if they’re in a high-risk category rather than tough it out. Increasing data suggest taking an antiviral also reduces the risk for long COVID. Also, we know that rebound symptoms are not always infectious virus. Sometimes, they’re just inflammatory. Unless a person is immune suppressed, they rarely have a culturable virus 7-8 days after onset of symptoms. So, for most people, I don’t administer second courses of Paxlovid, although I know some physicians do. One has to realize the risk for hospitalization from a rebound is tiny, and many people don’t even have infectious virus when they take the second course of a drug such as Paxlovid.
Q: You mentioned motivated patients, which seems to be an important factor to consider, particularly for new vaccines.
Dr. Auwaerter: There are always early adopters who are less afraid. And then some people say: This is a brand-new vaccine; I’m going to wait for a year to let this shake out, and make sure it seems safe. People more engaged in their health have asked me about the RSV vaccine. For anyone who has cardiopulmonary problems and other major health problems, I’ve advised it. But if someone’s in good health and 65 or 70, the RSV illness is probably pretty mild if they get it. For them, I would say the vaccine is optional.
For people over 75, I have been advising the RSV vaccine because that is a group we tend to see hospitalized with RSV; they’re the highest-risk group, similar to COVID. The older you are, the more likely this infection will land you in the hospital. You can acquire RSV even if you don’t have young grandchildren around.
Q: You have called respiratory virus seasons unstable? What does it mean, and what is the significance for clinicians?
Dr. Auwaerter: It’s less predictable than in the past. If you had a cough and fever, you could think it was influenza if you knew you had influenza circulating in your community. Maybe you thought about RSV for your immunocompromised or older patients, but we didn’t have any therapy for it anyway. I sometimes refer to the respiratory virus season as a cage match between the major infections. Last year, RSV came out first, and we got some influenza and COVID. What does the situation look like this year? I don’t know at this point, but we are seeing more COVID earlier. What’s different is we continue to have the emergence of viral variants of SARS-CoV-2. Also, with both influenza and COVID, it’s harder to make a clinical judgment about what people have.
I think we have to rely more on tests to treat these patients. Options include having point of care testing in the office for rapid results (molecular assays preferred) for both influenza and SARS-CoV-2 or home antigen testing. There are home kits that do test for both if influenza is known to be circulating significantly in the community. But there are still barriers. For one, COVID and COVID/influenza antigen kits are no longer free, although some health insurance companies do provide COVID kits free of charge. In offices, you don’t want to have ill people with respiratory infections in your waiting room unless you can isolate or have negative pressure rooms. Do you ask for masking in your offices? Telemedicine has been a big help since the pandemic in managing nonsevere respiratory infections at home; however, you must be licensed in the state to practice, which limits helping your out-of-state patients.
Q: How has the advent of in-home antigen tests changed practice?
Dr. Auwaerter: Home antigen tests have been groundbreaking in facilitating care. When I see patients via telemedicine, I don’t want to prescribe medications for influenza and COVID to people simultaneously. I want to pick one or the other – and now I’m able to ask for a COVID test or a COVID/influenza test if the patient or family is able to get a kit. Some offices do have real-time molecular testing, which is the ideal and the CDC-recommended approach, but they’re expensive, and not everyone has access to them.
Q: People talk about the “tripledemic,” but does doing so ignore the fourth horseman of the respiratory apocalypse: pneumococcal pneumonia?
Dr. Auwaerter: Pneumonia remains a leading cause of hospitalization, except we’ve seen much more viral than bacterial pneumonia in recent years of the pandemic. We’ve lost sight, and pneumococcal pneumonia is important, especially in older patients. What we have seen pretty clearly is a rise in group A streptococcal infections. This is another consequence of the pandemic, where people did not socialize for a year or 2. There was much less group A strep infection in younger children, and even in adults, the amount of invasive group A streptococcal infections has clearly taken a jump, according to the NHS in Great Britain. Our pediatric practices here at Johns Hopkins are seeing far more cases of acute rheumatic fever than they’ve seen in decades. And I think, again, this is a consequence of the frequency of group A strep infections definitely taking an uptick. And that was no doubt probably from social mitigation measures and just an interruption in normal circumstances that bacterial and respiratory pathogens tend to circulate and colonize.
Q: Do you have any concerns about immunogenicity or side effects associated with receiving several vaccines at once?
Dr. Auwaerter: I think three injections at once is only for the heroic, and there is actually no guidance for getting all three at the moment. COVID, RSV, and influenza are not live vaccines. I’ve been recommending the new COVID booster and flu together, and then wait 2 weeks and then get RSV or vice-versa. A part of the reason is RSV is new. People have gotten COVID and flu vaccines before; they’re no different than in the past in terms of anticipating adverse effects. But RSV is new, so I’ve usually been recommending that as a standalone to gauge if there are issues as an RSV booster may be recommended at some point down the road.
Q: Unfortunately, some people are going to see or hear misinformation that the COVID boosters have not been properly tested or proven safe. What’s your response to the patient who says something to that effect?
Dr. Auwaerter: My response is, the basic components of the vaccine are the same, right? If you have the mRNA vaccine, you’re getting the vaccine components, the lipids, and the mRNA coding for spike proteins, which has just been modified slightly to adjust to the Omicron subvariant composition. We do the same thing with the influenza vaccine every year, and we don’t see much change in the side effect profile. I think it’s important for my staff in the office and myself to be very comfortable to field questions such as these.
We try to inform all of our staff about a vaccine, especially a new one like RSV, just so they have some comfort level with it, whether they’re getting it or not. Vaccine-hesitant patients need very little to dissuade and to take a pass – to the probable detriment of their health and their family’s health. We know the influenza vaccine helps reduce absenteeism and transmission in addition to reducing serious illness in high-risk patients. Even COVID vaccine efficacy is not as robust as initially reported, falling from 95% to under 70% depending on the study – you are provided with protection against serious illness and hospitalization. The same goes for influenza, and that’s how we try to pitch it to people. Are they going to get the flu? Maybe, but you didn’t land in the hospital. That’s why it’s these vaccines are so important.
Spencer H. Durham, PharmD, associate clinical professor in the department of pharmacy practice at Auburn (Ala.) University, and clinical pharmacist, Internal Medicine & Infectious Diseases, at the UAB Heersink School of Medicine in Huntsville.Q: What is known, if anything, about the risks/desirability of giving three vaccinations at once to patients (particularly older patients) – flu, COVID-19 and RSV? Any potential vaccine interactions physicians should know about?
Dr. Durham: There are currently no data about giving all three of these vaccines together at the same time. However, there is both data and practical experience of giving both the flu and COVID vaccines at the same time. The best approach right now for these three vaccines would be to get the flu and COVID vaccines at the same time, then give the RSV vaccine at a different date. In general, they should be separated by about 2 weeks, although it does not matter in what order they are given (that is, patients could get RSV first, then flu/COVID, or they could get flu/COVID first, followed by RSV).
Having said this, there is no theoretical reason why patients couldn’t get all three at once, so if there is only one opportunity to vaccinate a patient, then it would be okay to give all three. But, if the patient can come for two separate visits, the recommendation would currently be to separate these. In the future, there likely will be data on giving all three vaccines at once, so it may not be an issue to administer all three at the same time.
Lastly, I would point out that the RSV vaccine is not necessarily recommended for everyone age 60 and above. The Advisory Committee on Immunization Practices recommends using shared clinical decision-making to determine if that vaccine is right for the patient. In general, the flu and COVID vaccines are recommended for everyone, although the specific COVID recommendations for fall 2023 have not yet been released. There are no particular vaccine interactions that are concerning with these vaccines.
Q: What if any special considerations are there regarding the storage, handling, and ordering of these vaccines? Should primary care practices take any special steps they might not already be taking?
Dr. Durham: I don’t think there are any special considerations that providers might not already be doing. All of the vaccines do require refrigeration, but each individual product may vary some on beyond-use dates or how long they are good after being reconstituted. All providers administering these vaccines should carefully examine the labeling of each individual product to ensure correct storage and handling. In addition, the Centers for Disease Control and Prevention has an online toolkit for vaccine storage and handling and can be found at https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/index.html.
Santina J. G. Wheat, MD, MPH, vice chair of diversity, equity, and inclusion, department of family and community medicine, and associate professor of family and community medicine, Northwestern University, ChicagoQ: What can primary care doctors/family physicians and their staff do to increase patient access to the vaccines? Any lessons learned from the earlier phases of the pandemic that might pertain not only to COVID-19 but also to RSV and/or influenza?
Dr. Wheat: I think the most important thing family physicians can do is speak with their patients about the importance of vaccines and specific recommendations they have for the situations of individuals and families. When vaccines started becoming available, I had many patients who wanted to hear from me – as their primary physician – what I truly thought and what I was planning to do for my own family.
I also think if our teams can know where vaccines are easily accessible, that makes it much easier for our patients. I have heard great stories and seen my own clinical support staff look at websites with patients to help them find the best location to get vaccines. In particular, about the RSV vaccine, I have had a handful of patients already come to ask me about my recommendations. When vaccines are available at my location, I find it much easier for my patients to be willing to get vaccinated. Similarly, if I am sending patients to pick up a prescription and they can get it at the same time, I have found success in them being willing to be vaccinated while picking up their prescription. In both instances, they do not need to make an additional stop; they are just able to be vaccinated while already at the clinic or pharmacy.
Q: Do you see any extra difficulties involved in trying to get groups of patients – in this case, older people – to be receptive to three vaccines, especially in this climate where it appears a growing number of people are hostile to immunization?
Dr. Wheat: Recently, I have found myself negotiating vaccines with patients not just with these, but as recommendations have changed for vaccines such as the pneumococcal vaccines and the hepatitis B vaccines. I think primary care providers can recommend all of them, but still help patients prioritize what is most important for that patient and family. For example, if welcoming a new baby soon, they might prioritize the vaccines for pertussis or influenza over the hepatitis vaccine with a plan to revisit the conversations later.
I have had some patients tell me they have gotten enough vaccines – and we know that even before the pandemic there was resistance to the influenza vaccine for some. I think we need to be prepared to address the concerns and, at times, the apathy. We also need to ask every time, because we never know which visit will be the one when a patient agrees.
Dr. Auwaerter reported financial relationships with Pfizer, Shionogi, Gilead, and Wellstat. Dr. Durham and Dr. Wheat disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Hospitalizations for COVID-19 in the United States have increased for 8 weeks in a row.
Data from Florida and Georgia signal that respiratory syncytial virus (RSV) season has begun.
As for flu shots, experts say patients with long COVID should get them in 2023, although federal health agencies have not addressed that specific question.
Paul G. Auwaerter, MD, MBA, an infectious disease consultant, said many patients in his primary care practice worry about “the big three” – COVID, influenza, and RSV.
They discussed how to handle COVID boosters, the use of Paxlovid, vaccine hesitancy, and the correct order of operations for patients getting vaccinated against all three diseases.
Paul G. Auwaerter, MD, MBA, clinical director of the division of infectious diseases and the Sherrilyn and Ken Fisher Professor of Medicine at Johns Hopkins University, BaltimoreQuestion: How should primary care physicians be preparing to handle what everyone is predicting will be a major surge in cases of respiratory infections?
Auwaerter: Although I’m an infectious disease consultant, I still have a small primary care practice. So, I field questions for my patients all the time, and many patients, especially those with health problems, are worried about the big three: RSV, COVID, and influenza – at least, my more motivated patients are.
People frequently ask if they need the COVID booster. I think that’s been something many people think maybe they can avoid. The good news is that the early in vitro data suggest that the XBB1.5x-based vaccine seems to offer sufficient neutralizing activity against the circulating newer variants since the vaccine was approved earlier this year. I am suggesting that everyone get a booster, especially those at high risk, because we know that the risk for hospitalization decreases based on earlier studies for 4-6 months after a COVID booster. We can simultaneously administer the revised COVID booster vaccine and the annual influenza vaccine. The timing is good, as influenza immunization should be accomplished by October or early November at the latest. Like many parts of the country, we in Maryland are in the middle of a COVID boomlet. I have issued more Paxlovid prescriptions since mid-August than I did all spring and early summer.
Q: Are you seeing a lot of rebound COVID in your patients taking Paxlovid [nirmatrelvir/ritonavir]?
Dr. Auwaerter: I think the frequency is probably around 10%. It has been quoted much higher – at 20% – but careful studies have put it down at just single digits. I think it just depends on symptomatology and how you ask the question. But I think it’s important that I try to persuade people to take a direct-acting antiviral if they’re in a high-risk category rather than tough it out. Increasing data suggest taking an antiviral also reduces the risk for long COVID. Also, we know that rebound symptoms are not always infectious virus. Sometimes, they’re just inflammatory. Unless a person is immune suppressed, they rarely have a culturable virus 7-8 days after onset of symptoms. So, for most people, I don’t administer second courses of Paxlovid, although I know some physicians do. One has to realize the risk for hospitalization from a rebound is tiny, and many people don’t even have infectious virus when they take the second course of a drug such as Paxlovid.
Q: You mentioned motivated patients, which seems to be an important factor to consider, particularly for new vaccines.
Dr. Auwaerter: There are always early adopters who are less afraid. And then some people say: This is a brand-new vaccine; I’m going to wait for a year to let this shake out, and make sure it seems safe. People more engaged in their health have asked me about the RSV vaccine. For anyone who has cardiopulmonary problems and other major health problems, I’ve advised it. But if someone’s in good health and 65 or 70, the RSV illness is probably pretty mild if they get it. For them, I would say the vaccine is optional.
For people over 75, I have been advising the RSV vaccine because that is a group we tend to see hospitalized with RSV; they’re the highest-risk group, similar to COVID. The older you are, the more likely this infection will land you in the hospital. You can acquire RSV even if you don’t have young grandchildren around.
Q: You have called respiratory virus seasons unstable? What does it mean, and what is the significance for clinicians?
Dr. Auwaerter: It’s less predictable than in the past. If you had a cough and fever, you could think it was influenza if you knew you had influenza circulating in your community. Maybe you thought about RSV for your immunocompromised or older patients, but we didn’t have any therapy for it anyway. I sometimes refer to the respiratory virus season as a cage match between the major infections. Last year, RSV came out first, and we got some influenza and COVID. What does the situation look like this year? I don’t know at this point, but we are seeing more COVID earlier. What’s different is we continue to have the emergence of viral variants of SARS-CoV-2. Also, with both influenza and COVID, it’s harder to make a clinical judgment about what people have.
I think we have to rely more on tests to treat these patients. Options include having point of care testing in the office for rapid results (molecular assays preferred) for both influenza and SARS-CoV-2 or home antigen testing. There are home kits that do test for both if influenza is known to be circulating significantly in the community. But there are still barriers. For one, COVID and COVID/influenza antigen kits are no longer free, although some health insurance companies do provide COVID kits free of charge. In offices, you don’t want to have ill people with respiratory infections in your waiting room unless you can isolate or have negative pressure rooms. Do you ask for masking in your offices? Telemedicine has been a big help since the pandemic in managing nonsevere respiratory infections at home; however, you must be licensed in the state to practice, which limits helping your out-of-state patients.
Q: How has the advent of in-home antigen tests changed practice?
Dr. Auwaerter: Home antigen tests have been groundbreaking in facilitating care. When I see patients via telemedicine, I don’t want to prescribe medications for influenza and COVID to people simultaneously. I want to pick one or the other – and now I’m able to ask for a COVID test or a COVID/influenza test if the patient or family is able to get a kit. Some offices do have real-time molecular testing, which is the ideal and the CDC-recommended approach, but they’re expensive, and not everyone has access to them.
Q: People talk about the “tripledemic,” but does doing so ignore the fourth horseman of the respiratory apocalypse: pneumococcal pneumonia?
Dr. Auwaerter: Pneumonia remains a leading cause of hospitalization, except we’ve seen much more viral than bacterial pneumonia in recent years of the pandemic. We’ve lost sight, and pneumococcal pneumonia is important, especially in older patients. What we have seen pretty clearly is a rise in group A streptococcal infections. This is another consequence of the pandemic, where people did not socialize for a year or 2. There was much less group A strep infection in younger children, and even in adults, the amount of invasive group A streptococcal infections has clearly taken a jump, according to the NHS in Great Britain. Our pediatric practices here at Johns Hopkins are seeing far more cases of acute rheumatic fever than they’ve seen in decades. And I think, again, this is a consequence of the frequency of group A strep infections definitely taking an uptick. And that was no doubt probably from social mitigation measures and just an interruption in normal circumstances that bacterial and respiratory pathogens tend to circulate and colonize.
Q: Do you have any concerns about immunogenicity or side effects associated with receiving several vaccines at once?
Dr. Auwaerter: I think three injections at once is only for the heroic, and there is actually no guidance for getting all three at the moment. COVID, RSV, and influenza are not live vaccines. I’ve been recommending the new COVID booster and flu together, and then wait 2 weeks and then get RSV or vice-versa. A part of the reason is RSV is new. People have gotten COVID and flu vaccines before; they’re no different than in the past in terms of anticipating adverse effects. But RSV is new, so I’ve usually been recommending that as a standalone to gauge if there are issues as an RSV booster may be recommended at some point down the road.
Q: Unfortunately, some people are going to see or hear misinformation that the COVID boosters have not been properly tested or proven safe. What’s your response to the patient who says something to that effect?
Dr. Auwaerter: My response is, the basic components of the vaccine are the same, right? If you have the mRNA vaccine, you’re getting the vaccine components, the lipids, and the mRNA coding for spike proteins, which has just been modified slightly to adjust to the Omicron subvariant composition. We do the same thing with the influenza vaccine every year, and we don’t see much change in the side effect profile. I think it’s important for my staff in the office and myself to be very comfortable to field questions such as these.
We try to inform all of our staff about a vaccine, especially a new one like RSV, just so they have some comfort level with it, whether they’re getting it or not. Vaccine-hesitant patients need very little to dissuade and to take a pass – to the probable detriment of their health and their family’s health. We know the influenza vaccine helps reduce absenteeism and transmission in addition to reducing serious illness in high-risk patients. Even COVID vaccine efficacy is not as robust as initially reported, falling from 95% to under 70% depending on the study – you are provided with protection against serious illness and hospitalization. The same goes for influenza, and that’s how we try to pitch it to people. Are they going to get the flu? Maybe, but you didn’t land in the hospital. That’s why it’s these vaccines are so important.
Spencer H. Durham, PharmD, associate clinical professor in the department of pharmacy practice at Auburn (Ala.) University, and clinical pharmacist, Internal Medicine & Infectious Diseases, at the UAB Heersink School of Medicine in Huntsville.Q: What is known, if anything, about the risks/desirability of giving three vaccinations at once to patients (particularly older patients) – flu, COVID-19 and RSV? Any potential vaccine interactions physicians should know about?
Dr. Durham: There are currently no data about giving all three of these vaccines together at the same time. However, there is both data and practical experience of giving both the flu and COVID vaccines at the same time. The best approach right now for these three vaccines would be to get the flu and COVID vaccines at the same time, then give the RSV vaccine at a different date. In general, they should be separated by about 2 weeks, although it does not matter in what order they are given (that is, patients could get RSV first, then flu/COVID, or they could get flu/COVID first, followed by RSV).
Having said this, there is no theoretical reason why patients couldn’t get all three at once, so if there is only one opportunity to vaccinate a patient, then it would be okay to give all three. But, if the patient can come for two separate visits, the recommendation would currently be to separate these. In the future, there likely will be data on giving all three vaccines at once, so it may not be an issue to administer all three at the same time.
Lastly, I would point out that the RSV vaccine is not necessarily recommended for everyone age 60 and above. The Advisory Committee on Immunization Practices recommends using shared clinical decision-making to determine if that vaccine is right for the patient. In general, the flu and COVID vaccines are recommended for everyone, although the specific COVID recommendations for fall 2023 have not yet been released. There are no particular vaccine interactions that are concerning with these vaccines.
Q: What if any special considerations are there regarding the storage, handling, and ordering of these vaccines? Should primary care practices take any special steps they might not already be taking?
Dr. Durham: I don’t think there are any special considerations that providers might not already be doing. All of the vaccines do require refrigeration, but each individual product may vary some on beyond-use dates or how long they are good after being reconstituted. All providers administering these vaccines should carefully examine the labeling of each individual product to ensure correct storage and handling. In addition, the Centers for Disease Control and Prevention has an online toolkit for vaccine storage and handling and can be found at https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/index.html.
Santina J. G. Wheat, MD, MPH, vice chair of diversity, equity, and inclusion, department of family and community medicine, and associate professor of family and community medicine, Northwestern University, ChicagoQ: What can primary care doctors/family physicians and their staff do to increase patient access to the vaccines? Any lessons learned from the earlier phases of the pandemic that might pertain not only to COVID-19 but also to RSV and/or influenza?
Dr. Wheat: I think the most important thing family physicians can do is speak with their patients about the importance of vaccines and specific recommendations they have for the situations of individuals and families. When vaccines started becoming available, I had many patients who wanted to hear from me – as their primary physician – what I truly thought and what I was planning to do for my own family.
I also think if our teams can know where vaccines are easily accessible, that makes it much easier for our patients. I have heard great stories and seen my own clinical support staff look at websites with patients to help them find the best location to get vaccines. In particular, about the RSV vaccine, I have had a handful of patients already come to ask me about my recommendations. When vaccines are available at my location, I find it much easier for my patients to be willing to get vaccinated. Similarly, if I am sending patients to pick up a prescription and they can get it at the same time, I have found success in them being willing to be vaccinated while picking up their prescription. In both instances, they do not need to make an additional stop; they are just able to be vaccinated while already at the clinic or pharmacy.
Q: Do you see any extra difficulties involved in trying to get groups of patients – in this case, older people – to be receptive to three vaccines, especially in this climate where it appears a growing number of people are hostile to immunization?
Dr. Wheat: Recently, I have found myself negotiating vaccines with patients not just with these, but as recommendations have changed for vaccines such as the pneumococcal vaccines and the hepatitis B vaccines. I think primary care providers can recommend all of them, but still help patients prioritize what is most important for that patient and family. For example, if welcoming a new baby soon, they might prioritize the vaccines for pertussis or influenza over the hepatitis vaccine with a plan to revisit the conversations later.
I have had some patients tell me they have gotten enough vaccines – and we know that even before the pandemic there was resistance to the influenza vaccine for some. I think we need to be prepared to address the concerns and, at times, the apathy. We also need to ask every time, because we never know which visit will be the one when a patient agrees.
Dr. Auwaerter reported financial relationships with Pfizer, Shionogi, Gilead, and Wellstat. Dr. Durham and Dr. Wheat disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Hospitalizations for COVID-19 in the United States have increased for 8 weeks in a row.
Data from Florida and Georgia signal that respiratory syncytial virus (RSV) season has begun.
As for flu shots, experts say patients with long COVID should get them in 2023, although federal health agencies have not addressed that specific question.
Paul G. Auwaerter, MD, MBA, an infectious disease consultant, said many patients in his primary care practice worry about “the big three” – COVID, influenza, and RSV.
They discussed how to handle COVID boosters, the use of Paxlovid, vaccine hesitancy, and the correct order of operations for patients getting vaccinated against all three diseases.
Paul G. Auwaerter, MD, MBA, clinical director of the division of infectious diseases and the Sherrilyn and Ken Fisher Professor of Medicine at Johns Hopkins University, BaltimoreQuestion: How should primary care physicians be preparing to handle what everyone is predicting will be a major surge in cases of respiratory infections?
Auwaerter: Although I’m an infectious disease consultant, I still have a small primary care practice. So, I field questions for my patients all the time, and many patients, especially those with health problems, are worried about the big three: RSV, COVID, and influenza – at least, my more motivated patients are.
People frequently ask if they need the COVID booster. I think that’s been something many people think maybe they can avoid. The good news is that the early in vitro data suggest that the XBB1.5x-based vaccine seems to offer sufficient neutralizing activity against the circulating newer variants since the vaccine was approved earlier this year. I am suggesting that everyone get a booster, especially those at high risk, because we know that the risk for hospitalization decreases based on earlier studies for 4-6 months after a COVID booster. We can simultaneously administer the revised COVID booster vaccine and the annual influenza vaccine. The timing is good, as influenza immunization should be accomplished by October or early November at the latest. Like many parts of the country, we in Maryland are in the middle of a COVID boomlet. I have issued more Paxlovid prescriptions since mid-August than I did all spring and early summer.
Q: Are you seeing a lot of rebound COVID in your patients taking Paxlovid [nirmatrelvir/ritonavir]?
Dr. Auwaerter: I think the frequency is probably around 10%. It has been quoted much higher – at 20% – but careful studies have put it down at just single digits. I think it just depends on symptomatology and how you ask the question. But I think it’s important that I try to persuade people to take a direct-acting antiviral if they’re in a high-risk category rather than tough it out. Increasing data suggest taking an antiviral also reduces the risk for long COVID. Also, we know that rebound symptoms are not always infectious virus. Sometimes, they’re just inflammatory. Unless a person is immune suppressed, they rarely have a culturable virus 7-8 days after onset of symptoms. So, for most people, I don’t administer second courses of Paxlovid, although I know some physicians do. One has to realize the risk for hospitalization from a rebound is tiny, and many people don’t even have infectious virus when they take the second course of a drug such as Paxlovid.
Q: You mentioned motivated patients, which seems to be an important factor to consider, particularly for new vaccines.
Dr. Auwaerter: There are always early adopters who are less afraid. And then some people say: This is a brand-new vaccine; I’m going to wait for a year to let this shake out, and make sure it seems safe. People more engaged in their health have asked me about the RSV vaccine. For anyone who has cardiopulmonary problems and other major health problems, I’ve advised it. But if someone’s in good health and 65 or 70, the RSV illness is probably pretty mild if they get it. For them, I would say the vaccine is optional.
For people over 75, I have been advising the RSV vaccine because that is a group we tend to see hospitalized with RSV; they’re the highest-risk group, similar to COVID. The older you are, the more likely this infection will land you in the hospital. You can acquire RSV even if you don’t have young grandchildren around.
Q: You have called respiratory virus seasons unstable? What does it mean, and what is the significance for clinicians?
Dr. Auwaerter: It’s less predictable than in the past. If you had a cough and fever, you could think it was influenza if you knew you had influenza circulating in your community. Maybe you thought about RSV for your immunocompromised or older patients, but we didn’t have any therapy for it anyway. I sometimes refer to the respiratory virus season as a cage match between the major infections. Last year, RSV came out first, and we got some influenza and COVID. What does the situation look like this year? I don’t know at this point, but we are seeing more COVID earlier. What’s different is we continue to have the emergence of viral variants of SARS-CoV-2. Also, with both influenza and COVID, it’s harder to make a clinical judgment about what people have.
I think we have to rely more on tests to treat these patients. Options include having point of care testing in the office for rapid results (molecular assays preferred) for both influenza and SARS-CoV-2 or home antigen testing. There are home kits that do test for both if influenza is known to be circulating significantly in the community. But there are still barriers. For one, COVID and COVID/influenza antigen kits are no longer free, although some health insurance companies do provide COVID kits free of charge. In offices, you don’t want to have ill people with respiratory infections in your waiting room unless you can isolate or have negative pressure rooms. Do you ask for masking in your offices? Telemedicine has been a big help since the pandemic in managing nonsevere respiratory infections at home; however, you must be licensed in the state to practice, which limits helping your out-of-state patients.
Q: How has the advent of in-home antigen tests changed practice?
Dr. Auwaerter: Home antigen tests have been groundbreaking in facilitating care. When I see patients via telemedicine, I don’t want to prescribe medications for influenza and COVID to people simultaneously. I want to pick one or the other – and now I’m able to ask for a COVID test or a COVID/influenza test if the patient or family is able to get a kit. Some offices do have real-time molecular testing, which is the ideal and the CDC-recommended approach, but they’re expensive, and not everyone has access to them.
Q: People talk about the “tripledemic,” but does doing so ignore the fourth horseman of the respiratory apocalypse: pneumococcal pneumonia?
Dr. Auwaerter: Pneumonia remains a leading cause of hospitalization, except we’ve seen much more viral than bacterial pneumonia in recent years of the pandemic. We’ve lost sight, and pneumococcal pneumonia is important, especially in older patients. What we have seen pretty clearly is a rise in group A streptococcal infections. This is another consequence of the pandemic, where people did not socialize for a year or 2. There was much less group A strep infection in younger children, and even in adults, the amount of invasive group A streptococcal infections has clearly taken a jump, according to the NHS in Great Britain. Our pediatric practices here at Johns Hopkins are seeing far more cases of acute rheumatic fever than they’ve seen in decades. And I think, again, this is a consequence of the frequency of group A strep infections definitely taking an uptick. And that was no doubt probably from social mitigation measures and just an interruption in normal circumstances that bacterial and respiratory pathogens tend to circulate and colonize.
Q: Do you have any concerns about immunogenicity or side effects associated with receiving several vaccines at once?
Dr. Auwaerter: I think three injections at once is only for the heroic, and there is actually no guidance for getting all three at the moment. COVID, RSV, and influenza are not live vaccines. I’ve been recommending the new COVID booster and flu together, and then wait 2 weeks and then get RSV or vice-versa. A part of the reason is RSV is new. People have gotten COVID and flu vaccines before; they’re no different than in the past in terms of anticipating adverse effects. But RSV is new, so I’ve usually been recommending that as a standalone to gauge if there are issues as an RSV booster may be recommended at some point down the road.
Q: Unfortunately, some people are going to see or hear misinformation that the COVID boosters have not been properly tested or proven safe. What’s your response to the patient who says something to that effect?
Dr. Auwaerter: My response is, the basic components of the vaccine are the same, right? If you have the mRNA vaccine, you’re getting the vaccine components, the lipids, and the mRNA coding for spike proteins, which has just been modified slightly to adjust to the Omicron subvariant composition. We do the same thing with the influenza vaccine every year, and we don’t see much change in the side effect profile. I think it’s important for my staff in the office and myself to be very comfortable to field questions such as these.
We try to inform all of our staff about a vaccine, especially a new one like RSV, just so they have some comfort level with it, whether they’re getting it or not. Vaccine-hesitant patients need very little to dissuade and to take a pass – to the probable detriment of their health and their family’s health. We know the influenza vaccine helps reduce absenteeism and transmission in addition to reducing serious illness in high-risk patients. Even COVID vaccine efficacy is not as robust as initially reported, falling from 95% to under 70% depending on the study – you are provided with protection against serious illness and hospitalization. The same goes for influenza, and that’s how we try to pitch it to people. Are they going to get the flu? Maybe, but you didn’t land in the hospital. That’s why it’s these vaccines are so important.
Spencer H. Durham, PharmD, associate clinical professor in the department of pharmacy practice at Auburn (Ala.) University, and clinical pharmacist, Internal Medicine & Infectious Diseases, at the UAB Heersink School of Medicine in Huntsville.Q: What is known, if anything, about the risks/desirability of giving three vaccinations at once to patients (particularly older patients) – flu, COVID-19 and RSV? Any potential vaccine interactions physicians should know about?
Dr. Durham: There are currently no data about giving all three of these vaccines together at the same time. However, there is both data and practical experience of giving both the flu and COVID vaccines at the same time. The best approach right now for these three vaccines would be to get the flu and COVID vaccines at the same time, then give the RSV vaccine at a different date. In general, they should be separated by about 2 weeks, although it does not matter in what order they are given (that is, patients could get RSV first, then flu/COVID, or they could get flu/COVID first, followed by RSV).
Having said this, there is no theoretical reason why patients couldn’t get all three at once, so if there is only one opportunity to vaccinate a patient, then it would be okay to give all three. But, if the patient can come for two separate visits, the recommendation would currently be to separate these. In the future, there likely will be data on giving all three vaccines at once, so it may not be an issue to administer all three at the same time.
Lastly, I would point out that the RSV vaccine is not necessarily recommended for everyone age 60 and above. The Advisory Committee on Immunization Practices recommends using shared clinical decision-making to determine if that vaccine is right for the patient. In general, the flu and COVID vaccines are recommended for everyone, although the specific COVID recommendations for fall 2023 have not yet been released. There are no particular vaccine interactions that are concerning with these vaccines.
Q: What if any special considerations are there regarding the storage, handling, and ordering of these vaccines? Should primary care practices take any special steps they might not already be taking?
Dr. Durham: I don’t think there are any special considerations that providers might not already be doing. All of the vaccines do require refrigeration, but each individual product may vary some on beyond-use dates or how long they are good after being reconstituted. All providers administering these vaccines should carefully examine the labeling of each individual product to ensure correct storage and handling. In addition, the Centers for Disease Control and Prevention has an online toolkit for vaccine storage and handling and can be found at https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/index.html.
Santina J. G. Wheat, MD, MPH, vice chair of diversity, equity, and inclusion, department of family and community medicine, and associate professor of family and community medicine, Northwestern University, ChicagoQ: What can primary care doctors/family physicians and their staff do to increase patient access to the vaccines? Any lessons learned from the earlier phases of the pandemic that might pertain not only to COVID-19 but also to RSV and/or influenza?
Dr. Wheat: I think the most important thing family physicians can do is speak with their patients about the importance of vaccines and specific recommendations they have for the situations of individuals and families. When vaccines started becoming available, I had many patients who wanted to hear from me – as their primary physician – what I truly thought and what I was planning to do for my own family.
I also think if our teams can know where vaccines are easily accessible, that makes it much easier for our patients. I have heard great stories and seen my own clinical support staff look at websites with patients to help them find the best location to get vaccines. In particular, about the RSV vaccine, I have had a handful of patients already come to ask me about my recommendations. When vaccines are available at my location, I find it much easier for my patients to be willing to get vaccinated. Similarly, if I am sending patients to pick up a prescription and they can get it at the same time, I have found success in them being willing to be vaccinated while picking up their prescription. In both instances, they do not need to make an additional stop; they are just able to be vaccinated while already at the clinic or pharmacy.
Q: Do you see any extra difficulties involved in trying to get groups of patients – in this case, older people – to be receptive to three vaccines, especially in this climate where it appears a growing number of people are hostile to immunization?
Dr. Wheat: Recently, I have found myself negotiating vaccines with patients not just with these, but as recommendations have changed for vaccines such as the pneumococcal vaccines and the hepatitis B vaccines. I think primary care providers can recommend all of them, but still help patients prioritize what is most important for that patient and family. For example, if welcoming a new baby soon, they might prioritize the vaccines for pertussis or influenza over the hepatitis vaccine with a plan to revisit the conversations later.
I have had some patients tell me they have gotten enough vaccines – and we know that even before the pandemic there was resistance to the influenza vaccine for some. I think we need to be prepared to address the concerns and, at times, the apathy. We also need to ask every time, because we never know which visit will be the one when a patient agrees.
Dr. Auwaerter reported financial relationships with Pfizer, Shionogi, Gilead, and Wellstat. Dr. Durham and Dr. Wheat disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
SGLT2 inhibitors: No benefit or harm in hospitalized COVID-19
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Bad blood: Could brain bleeds be contagious?
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A White male presented with a purulent erythematous edematous plaque with central necrosis and ulceration on his right flank
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.