User login
Don’t let amoxicillin shortage go to waste, antibiotic stewards say
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
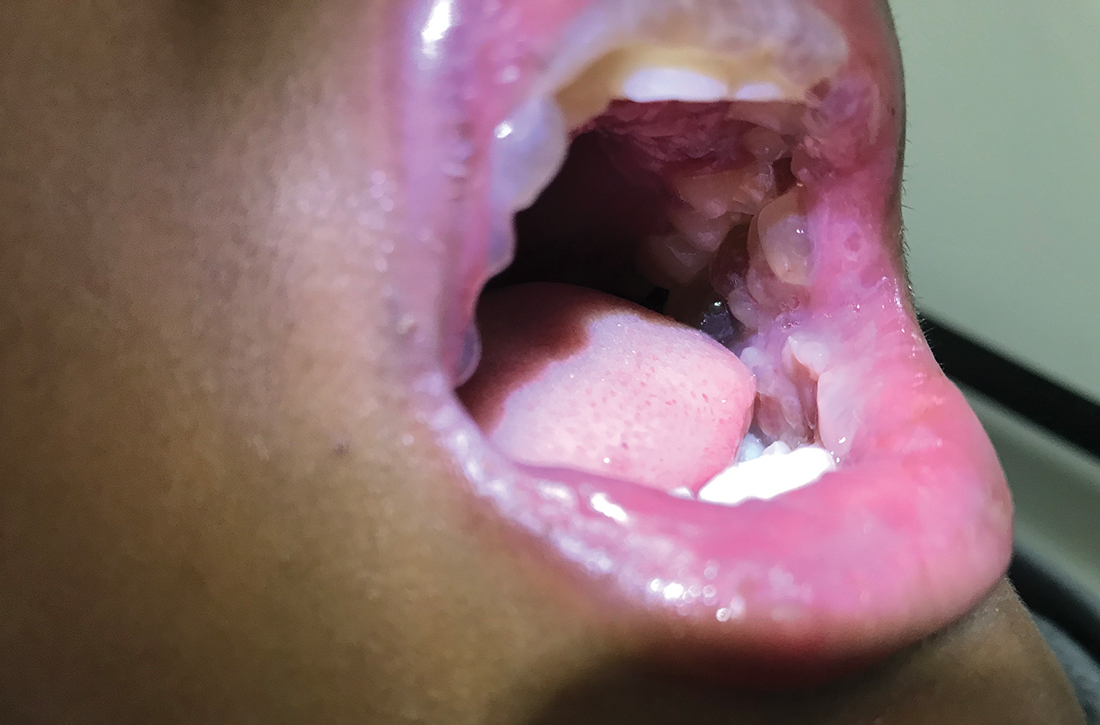
Severe pediatric oral mucositis
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).
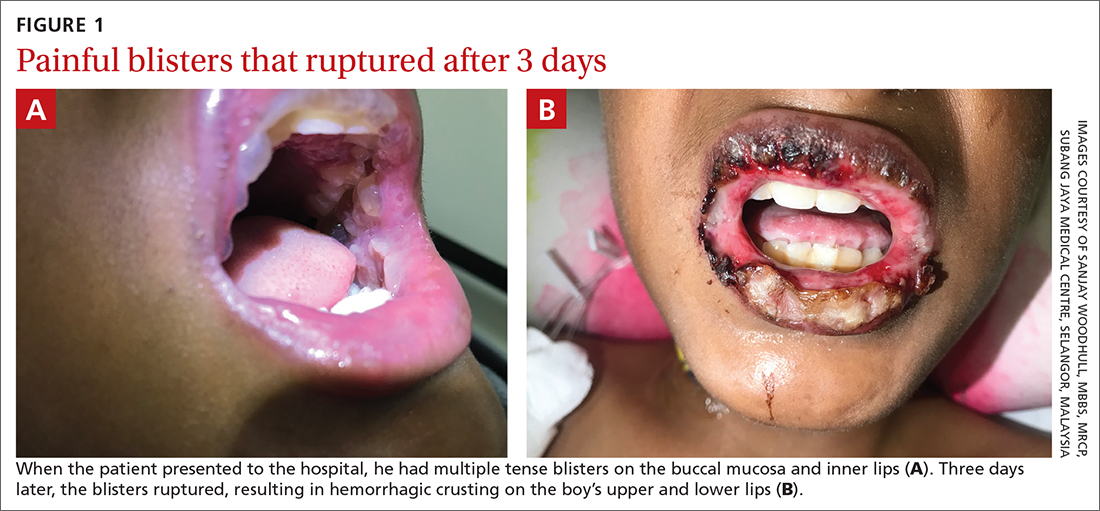
The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
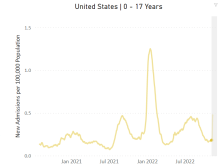
RSV causes 1 in 50 deaths in children under age 5: European study
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
FROM LANCET RESPIRATORY MEDICINE
Children and COVID: Weekly cases continue to hold fairly steady
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.
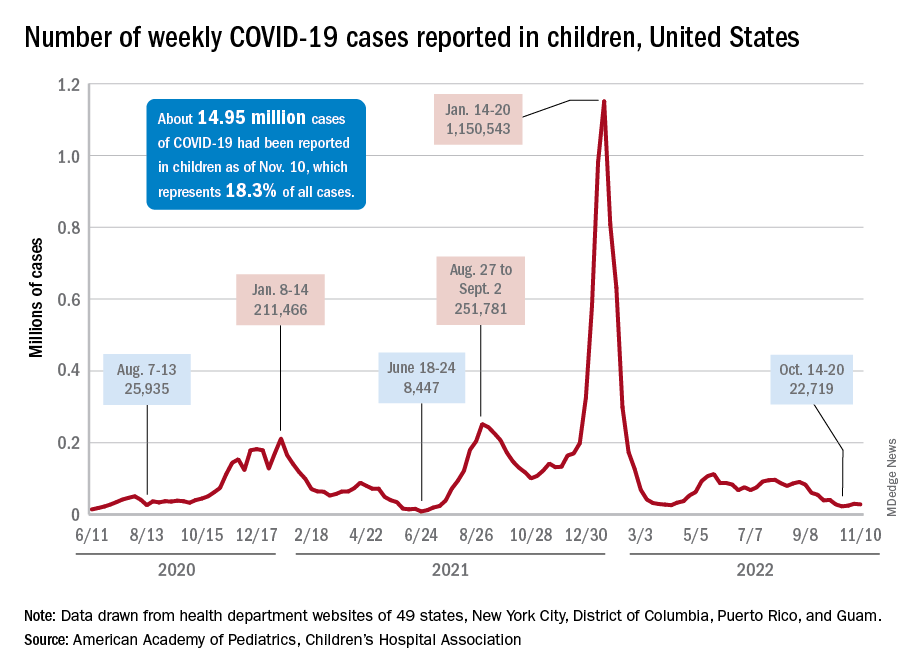
The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
Love them or hate them, masks in schools work
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
No benefit of rivaroxaban in COVID outpatients: PREVENT-HD
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Children from poorer ZIP codes often untreated for ear infections
Children from socially disadvantaged backgrounds are less likely to be treated for middle ear infections and are likely to experience serious complications from the condition – potentially with lifelong economic consequences – researchers have found.
Problems such as hearing loss and chronic ear infections were more common for children who lived in areas marked by difficult socioeconomic circumstances, according to the researchers, who linked the complications to a lack of adequate treatment in this population.
“We are treating socially disadvantaged kids differently than we are treating more advantaged kids,” said Jason Qian, MD, a resident in otolaryngology and head and neck surgery at Stanford (Calif.) University, who helped conduct the new study. “We have to think about social inequalities so we can ensure all kids are receiving the same level and type of care.”
In the United States, 80% of children will experience otitis media during their lifetime. Untreated ear infections can lead to symptoms ranging from mild discharge from the ear to life-threatening conditions like mastoiditis and intracranial abscesses.
For the new study, published online in JAMA Otolaryngology–Head & Neck Surgery, Dr. Qian and colleagues looked at 4.8 million children with private health insurance across the United States using a database with information on inpatient and outpatient visits and medication use. The researchers identified patients between January 2003 and March 2021 who received treatment for recurrent and suppurative otitis media, those who received tympanostomy tubes, and children who experienced severe complications from undertreated ear infections.
Social disadvantage was assessed using the Social Deprivation Index (SDI), a tool used to measure indicators of poverty throughout the United States based on seven demographic factors including level of educational attainment, the number of single-parent households, the share of people living in overcrowded homes, and other factors.
Every point increase in the SDI score was associated with a 14% lower likelihood of being treated for recurrent ear infections despite having them and a 28% greater chance of being hospitalized for severe ear infections, according to the researchers.
Previous research established that children with government health insurance or no coverage have more difficulty receiving proper treatment for ear infections. Although people with commercial insurance are generally wealthier than those without private coverage, Dr. Qian said, the new data indicate that significant social disparities in care exist even within this group.
Although some studies have found that wealthier children are more likely to develop otitis media, Dr. Qian’s group said that association likely reflects the better access to health care money affords.
“We found that socially disadvantaged children not only have a higher burden of otitis media but are also undertreated both medically and surgically for [ear infections]. Because chronic and complicated forms of otitis media can cause childhood hearing loss, which in turn limits academic and economic potential, undertreatment of [otitis media] in socially disadvantaged populations can contribute to generational cycles of poverty, unemployment, and low pay,” they write.
“The biggest take home is that we are not treating children equitably when it comes to ear infections,” Dr. Qian added. “In order to give children equal access to care, we as health care providers need to find strategies to do better.”
The study was supported by the Stanford Center for Population Health Science Data Core, which is supported by a grant from the National Institutes of Health and internal funding. Dr. Qian has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
Children from socially disadvantaged backgrounds are less likely to be treated for middle ear infections and are likely to experience serious complications from the condition – potentially with lifelong economic consequences – researchers have found.
Problems such as hearing loss and chronic ear infections were more common for children who lived in areas marked by difficult socioeconomic circumstances, according to the researchers, who linked the complications to a lack of adequate treatment in this population.
“We are treating socially disadvantaged kids differently than we are treating more advantaged kids,” said Jason Qian, MD, a resident in otolaryngology and head and neck surgery at Stanford (Calif.) University, who helped conduct the new study. “We have to think about social inequalities so we can ensure all kids are receiving the same level and type of care.”
In the United States, 80% of children will experience otitis media during their lifetime. Untreated ear infections can lead to symptoms ranging from mild discharge from the ear to life-threatening conditions like mastoiditis and intracranial abscesses.
For the new study, published online in JAMA Otolaryngology–Head & Neck Surgery, Dr. Qian and colleagues looked at 4.8 million children with private health insurance across the United States using a database with information on inpatient and outpatient visits and medication use. The researchers identified patients between January 2003 and March 2021 who received treatment for recurrent and suppurative otitis media, those who received tympanostomy tubes, and children who experienced severe complications from undertreated ear infections.
Social disadvantage was assessed using the Social Deprivation Index (SDI), a tool used to measure indicators of poverty throughout the United States based on seven demographic factors including level of educational attainment, the number of single-parent households, the share of people living in overcrowded homes, and other factors.
Every point increase in the SDI score was associated with a 14% lower likelihood of being treated for recurrent ear infections despite having them and a 28% greater chance of being hospitalized for severe ear infections, according to the researchers.
Previous research established that children with government health insurance or no coverage have more difficulty receiving proper treatment for ear infections. Although people with commercial insurance are generally wealthier than those without private coverage, Dr. Qian said, the new data indicate that significant social disparities in care exist even within this group.
Although some studies have found that wealthier children are more likely to develop otitis media, Dr. Qian’s group said that association likely reflects the better access to health care money affords.
“We found that socially disadvantaged children not only have a higher burden of otitis media but are also undertreated both medically and surgically for [ear infections]. Because chronic and complicated forms of otitis media can cause childhood hearing loss, which in turn limits academic and economic potential, undertreatment of [otitis media] in socially disadvantaged populations can contribute to generational cycles of poverty, unemployment, and low pay,” they write.
“The biggest take home is that we are not treating children equitably when it comes to ear infections,” Dr. Qian added. “In order to give children equal access to care, we as health care providers need to find strategies to do better.”
The study was supported by the Stanford Center for Population Health Science Data Core, which is supported by a grant from the National Institutes of Health and internal funding. Dr. Qian has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
Children from socially disadvantaged backgrounds are less likely to be treated for middle ear infections and are likely to experience serious complications from the condition – potentially with lifelong economic consequences – researchers have found.
Problems such as hearing loss and chronic ear infections were more common for children who lived in areas marked by difficult socioeconomic circumstances, according to the researchers, who linked the complications to a lack of adequate treatment in this population.
“We are treating socially disadvantaged kids differently than we are treating more advantaged kids,” said Jason Qian, MD, a resident in otolaryngology and head and neck surgery at Stanford (Calif.) University, who helped conduct the new study. “We have to think about social inequalities so we can ensure all kids are receiving the same level and type of care.”
In the United States, 80% of children will experience otitis media during their lifetime. Untreated ear infections can lead to symptoms ranging from mild discharge from the ear to life-threatening conditions like mastoiditis and intracranial abscesses.
For the new study, published online in JAMA Otolaryngology–Head & Neck Surgery, Dr. Qian and colleagues looked at 4.8 million children with private health insurance across the United States using a database with information on inpatient and outpatient visits and medication use. The researchers identified patients between January 2003 and March 2021 who received treatment for recurrent and suppurative otitis media, those who received tympanostomy tubes, and children who experienced severe complications from undertreated ear infections.
Social disadvantage was assessed using the Social Deprivation Index (SDI), a tool used to measure indicators of poverty throughout the United States based on seven demographic factors including level of educational attainment, the number of single-parent households, the share of people living in overcrowded homes, and other factors.
Every point increase in the SDI score was associated with a 14% lower likelihood of being treated for recurrent ear infections despite having them and a 28% greater chance of being hospitalized for severe ear infections, according to the researchers.
Previous research established that children with government health insurance or no coverage have more difficulty receiving proper treatment for ear infections. Although people with commercial insurance are generally wealthier than those without private coverage, Dr. Qian said, the new data indicate that significant social disparities in care exist even within this group.
Although some studies have found that wealthier children are more likely to develop otitis media, Dr. Qian’s group said that association likely reflects the better access to health care money affords.
“We found that socially disadvantaged children not only have a higher burden of otitis media but are also undertreated both medically and surgically for [ear infections]. Because chronic and complicated forms of otitis media can cause childhood hearing loss, which in turn limits academic and economic potential, undertreatment of [otitis media] in socially disadvantaged populations can contribute to generational cycles of poverty, unemployment, and low pay,” they write.
“The biggest take home is that we are not treating children equitably when it comes to ear infections,” Dr. Qian added. “In order to give children equal access to care, we as health care providers need to find strategies to do better.”
The study was supported by the Stanford Center for Population Health Science Data Core, which is supported by a grant from the National Institutes of Health and internal funding. Dr. Qian has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
Monkeypox in children appears rare and relatively mild
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An adolescent male presents with an eroded bump on the temple
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.