User login
The right indoor relative humidity could ward off COVID
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF THE ROYAL SOCIETY INTERFACE
Children and COVID: Weekly cases maintain a low-level plateau
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
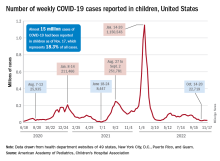
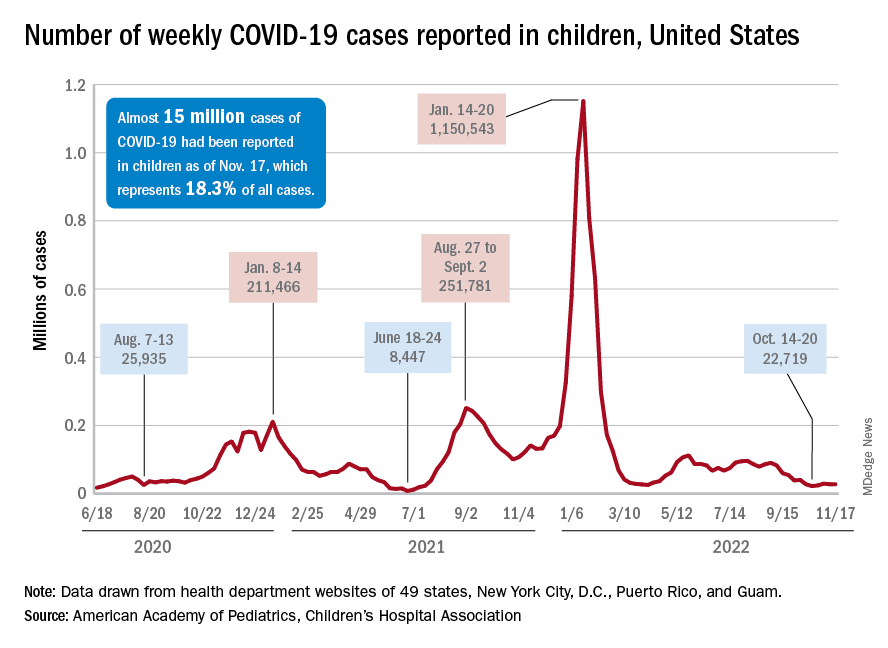
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
AAP issues guidelines to combat rise in respiratory illness
Updated guidance from the group outlines measures to optimize resources to manage a surge of patients filling hospital beds, emergency departments, and physicians’ practices.
A separate document from the AAP endorses giving extra doses of palivizumab, a monoclonal antibody used to prevent severe infection in infants at high risk of respiratory syncytial virus (RSV), as long as the illness is prevalent in the community.
Upticks in rates of RSV and influenza, along with a crisis in children’s mental health, prompted the AAP and the Children’s Hospital Association to petition the Biden administration on Nov. 14 to declare an emergency. Such a move would free up extra funding and waivers to allow physicians and hospitals to pool resources, the organizations said.
Despite those challenges, the AAP stressed in its new guidance that routine care, such as immunizations and chronic disease management, “cannot be neglected.”
Shifting resources
Officials at some children’s hospitals said that they have already implemented many of the AAP’s recommended measures for providing care during a surge, such as cross-training staff who usually treat adults, expanding telehealth and urgent care, and optimizing the use of ancillary care spaces.
“A lot of this is just reinforcing the things that I think children’s hospitals have been doing,” Lindsay Ragsdale, MD, chief medical officer for Kentucky Children’s Hospital, Lexington, said. “Can we shift adults around? Can we use an adult unit? Can we use an occupied space creatively? We’re really thinking outside the box.”
Andrew Pavia, MD, chief of the division of pediatric infectious diseases at University of Utah Health, Salt Lake City, said large children’s hospitals have been actively sharing practices for handling a surge through various channels, but the new guidance could be a useful “checklist” for small hospitals and physician practices that lack well-developed plans.
The AAP’s suggestions for pediatricians in outpatient settings include stocking up on personal protective equipment, using social media and office staff to increase communication with families, and keeping abreast of wait times at local emergency departments.
Addressing a subset of kids
In updated guidance for palivizumab, the AAP noted that earlier-than-usual circulation of RSV prompted pediatricians in some areas to begin administering the drug in the summer and early fall.
Palivizumab is typically given in five consecutive monthly intramuscular injections during RSV season, starting in November. Eligible infants and young children include those born prematurely or who have conditions such as chronic lung disease, hemodynamically significant congenital heart disease, or a suppressed immune system.
The AAP said it supports giving extra doses if RSV activity “persists at high levels in a given region through the fall and winter.” Published studies are sparse but contain “no evidence of increased frequency or severity of adverse events with later doses in a five-dose series nor with doses beyond five doses,” the group added.
The guidance may encourage payers to pick up the tab for extra doses, which are priced at more than $1,800 for cash customers, Dr. Pavia said. However, that recommendation addresses “a pretty small part of the problem overall because the injections are used for a very small subset of kids who are at the highest risk, and more than 80% of hospitalizations for RSV are among healthy kids,” he added.
Dr. Ragsdale and Dr. Pavia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Updated guidance from the group outlines measures to optimize resources to manage a surge of patients filling hospital beds, emergency departments, and physicians’ practices.
A separate document from the AAP endorses giving extra doses of palivizumab, a monoclonal antibody used to prevent severe infection in infants at high risk of respiratory syncytial virus (RSV), as long as the illness is prevalent in the community.
Upticks in rates of RSV and influenza, along with a crisis in children’s mental health, prompted the AAP and the Children’s Hospital Association to petition the Biden administration on Nov. 14 to declare an emergency. Such a move would free up extra funding and waivers to allow physicians and hospitals to pool resources, the organizations said.
Despite those challenges, the AAP stressed in its new guidance that routine care, such as immunizations and chronic disease management, “cannot be neglected.”
Shifting resources
Officials at some children’s hospitals said that they have already implemented many of the AAP’s recommended measures for providing care during a surge, such as cross-training staff who usually treat adults, expanding telehealth and urgent care, and optimizing the use of ancillary care spaces.
“A lot of this is just reinforcing the things that I think children’s hospitals have been doing,” Lindsay Ragsdale, MD, chief medical officer for Kentucky Children’s Hospital, Lexington, said. “Can we shift adults around? Can we use an adult unit? Can we use an occupied space creatively? We’re really thinking outside the box.”
Andrew Pavia, MD, chief of the division of pediatric infectious diseases at University of Utah Health, Salt Lake City, said large children’s hospitals have been actively sharing practices for handling a surge through various channels, but the new guidance could be a useful “checklist” for small hospitals and physician practices that lack well-developed plans.
The AAP’s suggestions for pediatricians in outpatient settings include stocking up on personal protective equipment, using social media and office staff to increase communication with families, and keeping abreast of wait times at local emergency departments.
Addressing a subset of kids
In updated guidance for palivizumab, the AAP noted that earlier-than-usual circulation of RSV prompted pediatricians in some areas to begin administering the drug in the summer and early fall.
Palivizumab is typically given in five consecutive monthly intramuscular injections during RSV season, starting in November. Eligible infants and young children include those born prematurely or who have conditions such as chronic lung disease, hemodynamically significant congenital heart disease, or a suppressed immune system.
The AAP said it supports giving extra doses if RSV activity “persists at high levels in a given region through the fall and winter.” Published studies are sparse but contain “no evidence of increased frequency or severity of adverse events with later doses in a five-dose series nor with doses beyond five doses,” the group added.
The guidance may encourage payers to pick up the tab for extra doses, which are priced at more than $1,800 for cash customers, Dr. Pavia said. However, that recommendation addresses “a pretty small part of the problem overall because the injections are used for a very small subset of kids who are at the highest risk, and more than 80% of hospitalizations for RSV are among healthy kids,” he added.
Dr. Ragsdale and Dr. Pavia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Updated guidance from the group outlines measures to optimize resources to manage a surge of patients filling hospital beds, emergency departments, and physicians’ practices.
A separate document from the AAP endorses giving extra doses of palivizumab, a monoclonal antibody used to prevent severe infection in infants at high risk of respiratory syncytial virus (RSV), as long as the illness is prevalent in the community.
Upticks in rates of RSV and influenza, along with a crisis in children’s mental health, prompted the AAP and the Children’s Hospital Association to petition the Biden administration on Nov. 14 to declare an emergency. Such a move would free up extra funding and waivers to allow physicians and hospitals to pool resources, the organizations said.
Despite those challenges, the AAP stressed in its new guidance that routine care, such as immunizations and chronic disease management, “cannot be neglected.”
Shifting resources
Officials at some children’s hospitals said that they have already implemented many of the AAP’s recommended measures for providing care during a surge, such as cross-training staff who usually treat adults, expanding telehealth and urgent care, and optimizing the use of ancillary care spaces.
“A lot of this is just reinforcing the things that I think children’s hospitals have been doing,” Lindsay Ragsdale, MD, chief medical officer for Kentucky Children’s Hospital, Lexington, said. “Can we shift adults around? Can we use an adult unit? Can we use an occupied space creatively? We’re really thinking outside the box.”
Andrew Pavia, MD, chief of the division of pediatric infectious diseases at University of Utah Health, Salt Lake City, said large children’s hospitals have been actively sharing practices for handling a surge through various channels, but the new guidance could be a useful “checklist” for small hospitals and physician practices that lack well-developed plans.
The AAP’s suggestions for pediatricians in outpatient settings include stocking up on personal protective equipment, using social media and office staff to increase communication with families, and keeping abreast of wait times at local emergency departments.
Addressing a subset of kids
In updated guidance for palivizumab, the AAP noted that earlier-than-usual circulation of RSV prompted pediatricians in some areas to begin administering the drug in the summer and early fall.
Palivizumab is typically given in five consecutive monthly intramuscular injections during RSV season, starting in November. Eligible infants and young children include those born prematurely or who have conditions such as chronic lung disease, hemodynamically significant congenital heart disease, or a suppressed immune system.
The AAP said it supports giving extra doses if RSV activity “persists at high levels in a given region through the fall and winter.” Published studies are sparse but contain “no evidence of increased frequency or severity of adverse events with later doses in a five-dose series nor with doses beyond five doses,” the group added.
The guidance may encourage payers to pick up the tab for extra doses, which are priced at more than $1,800 for cash customers, Dr. Pavia said. However, that recommendation addresses “a pretty small part of the problem overall because the injections are used for a very small subset of kids who are at the highest risk, and more than 80% of hospitalizations for RSV are among healthy kids,” he added.
Dr. Ragsdale and Dr. Pavia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HIV: Treating ‘symptom clusters’ could help improve QOL
TAMPA – People living with HIV experience many symptoms that can be grouped into “clusters” to help guide therapy and ideally treat more than one symptom at a time in an effort to improve quality of life, according to a study presented at the annual meeting of the Association of Nurses in AIDS Care.
Interestingly, these symptoms were more common among people living with HIV who are older than 45 years versus those who are younger, with one exception.
“In HIV, with exception of anxiety, we saw older people had more symptoms than younger ones,” said Natalie Wilson, PhD, assistant professor of community health systems at the University of California, San Francisco.
Dr. Wilson and colleagues performed a study that also suggests the older group experienced more distress from their symptoms than the younger cohort, again with the exception of anxiety.
Symptom clusters are two or more related symptoms that occur together with or without the same etiology. “Imagine you can’t sleep and the next day you’re tired and have no energy, you have trouble remembering someone’s name ... and then the next night you get so anxious about not getting sleep that it keeps you from sleeping.” That’s an example of a symptom cluster, Dr. Wilson said.
A high burden
“Why should we even evaluate symptom clusters?” Dr. Wilson asked. “The symptom burden is still high in people living with HIV. The medications got better but the symptoms remain.”
A high symptom burden also is linked to lower adherence to antiretrovirals. Also, considering groups of symptoms together could lead to targeted interventions that treat multiple symptoms, she said, “instead of treating one symptom at a time and increasing the pill burden for people living with HIV.”
Accelerated aging concerns
In addition, people living with HIV can experience accelerated aging, which is one reason Dr. Wilson and colleagues chose the 45-year-old cutoff in the study. Living with inflammation from HIV and the toxicity of earlier treatments likely contribute.
“Those over age 45 have higher rates of age-associated noncommunicable comorbidities developing at an earlier age than uninfected people with comparable lifestyles and demographics,” Dr. Wilson said.
In the full study, published in the Journal of Pain and Symptom Management, a total 2,000 people living with HIV completed the 20-item HIV Symptom Index. The participants reported their symptoms on their first visit to one of six national HIV Centers of Excellence. People were asked to report presence or absence of a particular symptom, and if they had it, how distressing it was on a scale of 1 “doesn’t bother me” to 4 “bothers me a lot.”
Younger people not only reported more anxiety but were also more distressed by it, Dr. Wilson said. The older group was more likely to be distressed by muscle aches and joint pain, trouble remembering things, and more.
The mean age in the younger group was 35 years, and it was 55 years in the older group. A total of 86% in the younger group and 87% in the older were men, and there were some differences by race, Dr. Wilson said.
More research needed
“These findings warrant further confirmation,” Dr. Wilson added. Future work could evaluate whether symptom clusters share etiology and how symptom clusters change over time. “We need to look at outcomes over time. Can we predict poorer outcomes, such as cardiac events, over time based on symptom clusters?”
Also, as part of HIV treatment success in recent years, “Our guidelines are moving people out further – if you’re undetectable sometimes you can come back at 6 months or 1 year.” The question, she said, is then: “Do we need to watch people with certain symptom clusters more closely?”
Limitations of the study include a lack of information on symptom causes and severity and its cross-sectional design.
‘Absolutely useful’
The study is “absolutely useful,” said session moderator Cheryl Netherly, an HIV nurse and clinical educator for CAN Community Health headquartered in Sarasota, Fla.
“One of the things that she mentioned was people with HIV, especially long-term HIV, they’re aging faster than the population without HIV. So, that is really important to look at.”
People living with HIV and dying from age-related comorbidities is something “we never thought would happen,” Ms. Netherly said. “Unfortunately, we’re now losing them to the different things like kidney issues, heart disease, and diabetes.”
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Wilson and Ms. Netherly disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV experience many symptoms that can be grouped into “clusters” to help guide therapy and ideally treat more than one symptom at a time in an effort to improve quality of life, according to a study presented at the annual meeting of the Association of Nurses in AIDS Care.
Interestingly, these symptoms were more common among people living with HIV who are older than 45 years versus those who are younger, with one exception.
“In HIV, with exception of anxiety, we saw older people had more symptoms than younger ones,” said Natalie Wilson, PhD, assistant professor of community health systems at the University of California, San Francisco.
Dr. Wilson and colleagues performed a study that also suggests the older group experienced more distress from their symptoms than the younger cohort, again with the exception of anxiety.
Symptom clusters are two or more related symptoms that occur together with or without the same etiology. “Imagine you can’t sleep and the next day you’re tired and have no energy, you have trouble remembering someone’s name ... and then the next night you get so anxious about not getting sleep that it keeps you from sleeping.” That’s an example of a symptom cluster, Dr. Wilson said.
A high burden
“Why should we even evaluate symptom clusters?” Dr. Wilson asked. “The symptom burden is still high in people living with HIV. The medications got better but the symptoms remain.”
A high symptom burden also is linked to lower adherence to antiretrovirals. Also, considering groups of symptoms together could lead to targeted interventions that treat multiple symptoms, she said, “instead of treating one symptom at a time and increasing the pill burden for people living with HIV.”
Accelerated aging concerns
In addition, people living with HIV can experience accelerated aging, which is one reason Dr. Wilson and colleagues chose the 45-year-old cutoff in the study. Living with inflammation from HIV and the toxicity of earlier treatments likely contribute.
“Those over age 45 have higher rates of age-associated noncommunicable comorbidities developing at an earlier age than uninfected people with comparable lifestyles and demographics,” Dr. Wilson said.
In the full study, published in the Journal of Pain and Symptom Management, a total 2,000 people living with HIV completed the 20-item HIV Symptom Index. The participants reported their symptoms on their first visit to one of six national HIV Centers of Excellence. People were asked to report presence or absence of a particular symptom, and if they had it, how distressing it was on a scale of 1 “doesn’t bother me” to 4 “bothers me a lot.”
Younger people not only reported more anxiety but were also more distressed by it, Dr. Wilson said. The older group was more likely to be distressed by muscle aches and joint pain, trouble remembering things, and more.
The mean age in the younger group was 35 years, and it was 55 years in the older group. A total of 86% in the younger group and 87% in the older were men, and there were some differences by race, Dr. Wilson said.
More research needed
“These findings warrant further confirmation,” Dr. Wilson added. Future work could evaluate whether symptom clusters share etiology and how symptom clusters change over time. “We need to look at outcomes over time. Can we predict poorer outcomes, such as cardiac events, over time based on symptom clusters?”
Also, as part of HIV treatment success in recent years, “Our guidelines are moving people out further – if you’re undetectable sometimes you can come back at 6 months or 1 year.” The question, she said, is then: “Do we need to watch people with certain symptom clusters more closely?”
Limitations of the study include a lack of information on symptom causes and severity and its cross-sectional design.
‘Absolutely useful’
The study is “absolutely useful,” said session moderator Cheryl Netherly, an HIV nurse and clinical educator for CAN Community Health headquartered in Sarasota, Fla.
“One of the things that she mentioned was people with HIV, especially long-term HIV, they’re aging faster than the population without HIV. So, that is really important to look at.”
People living with HIV and dying from age-related comorbidities is something “we never thought would happen,” Ms. Netherly said. “Unfortunately, we’re now losing them to the different things like kidney issues, heart disease, and diabetes.”
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Wilson and Ms. Netherly disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV experience many symptoms that can be grouped into “clusters” to help guide therapy and ideally treat more than one symptom at a time in an effort to improve quality of life, according to a study presented at the annual meeting of the Association of Nurses in AIDS Care.
Interestingly, these symptoms were more common among people living with HIV who are older than 45 years versus those who are younger, with one exception.
“In HIV, with exception of anxiety, we saw older people had more symptoms than younger ones,” said Natalie Wilson, PhD, assistant professor of community health systems at the University of California, San Francisco.
Dr. Wilson and colleagues performed a study that also suggests the older group experienced more distress from their symptoms than the younger cohort, again with the exception of anxiety.
Symptom clusters are two or more related symptoms that occur together with or without the same etiology. “Imagine you can’t sleep and the next day you’re tired and have no energy, you have trouble remembering someone’s name ... and then the next night you get so anxious about not getting sleep that it keeps you from sleeping.” That’s an example of a symptom cluster, Dr. Wilson said.
A high burden
“Why should we even evaluate symptom clusters?” Dr. Wilson asked. “The symptom burden is still high in people living with HIV. The medications got better but the symptoms remain.”
A high symptom burden also is linked to lower adherence to antiretrovirals. Also, considering groups of symptoms together could lead to targeted interventions that treat multiple symptoms, she said, “instead of treating one symptom at a time and increasing the pill burden for people living with HIV.”
Accelerated aging concerns
In addition, people living with HIV can experience accelerated aging, which is one reason Dr. Wilson and colleagues chose the 45-year-old cutoff in the study. Living with inflammation from HIV and the toxicity of earlier treatments likely contribute.
“Those over age 45 have higher rates of age-associated noncommunicable comorbidities developing at an earlier age than uninfected people with comparable lifestyles and demographics,” Dr. Wilson said.
In the full study, published in the Journal of Pain and Symptom Management, a total 2,000 people living with HIV completed the 20-item HIV Symptom Index. The participants reported their symptoms on their first visit to one of six national HIV Centers of Excellence. People were asked to report presence or absence of a particular symptom, and if they had it, how distressing it was on a scale of 1 “doesn’t bother me” to 4 “bothers me a lot.”
Younger people not only reported more anxiety but were also more distressed by it, Dr. Wilson said. The older group was more likely to be distressed by muscle aches and joint pain, trouble remembering things, and more.
The mean age in the younger group was 35 years, and it was 55 years in the older group. A total of 86% in the younger group and 87% in the older were men, and there were some differences by race, Dr. Wilson said.
More research needed
“These findings warrant further confirmation,” Dr. Wilson added. Future work could evaluate whether symptom clusters share etiology and how symptom clusters change over time. “We need to look at outcomes over time. Can we predict poorer outcomes, such as cardiac events, over time based on symptom clusters?”
Also, as part of HIV treatment success in recent years, “Our guidelines are moving people out further – if you’re undetectable sometimes you can come back at 6 months or 1 year.” The question, she said, is then: “Do we need to watch people with certain symptom clusters more closely?”
Limitations of the study include a lack of information on symptom causes and severity and its cross-sectional design.
‘Absolutely useful’
The study is “absolutely useful,” said session moderator Cheryl Netherly, an HIV nurse and clinical educator for CAN Community Health headquartered in Sarasota, Fla.
“One of the things that she mentioned was people with HIV, especially long-term HIV, they’re aging faster than the population without HIV. So, that is really important to look at.”
People living with HIV and dying from age-related comorbidities is something “we never thought would happen,” Ms. Netherly said. “Unfortunately, we’re now losing them to the different things like kidney issues, heart disease, and diabetes.”
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Wilson and Ms. Netherly disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As STDs proliferate, companies rush to market at-home test kits. But are they reliable?
Among the more remarkable legacies of the COVID-19 pandemic is how quickly federal regulators, the health care industry, and consumers moved to make at-home testing a reliable tool for managing a public health crisis.
But that fast-track focus is missing from another, less publicized epidemic: an explosion in sexually transmitted diseases that can cause chronic pain and infertility among infected adults and disable or kill infected newborns. The disparity has amplified calls from researchers, public health advocates, and health care companies urging the federal government to greenlight at-home testing kits that could vastly multiply the number of Americans testing for STDs.
Online shoppers can already choose from more than a dozen self-testing kits, typically ranging in price from $69 to $500, depending on the brand and the variety of infections they can detect.
But, except for HIV tests, the Food and Drug Administration hasn’t approved STD test kits for use outside a medical setting. That leaves consumers unsure about their reliability even as at-home use grows dramatically.
The STD epidemic is “out of control,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins University Center for Health Security. “We know we are missing diagnoses. We know that contact tracing is happening late or not at all. If we’re really serious about tackling the STD crisis, we have to get more people diagnosed.”
Preliminary data for 2021 showed nearly 2.5 million reported cases of chlamydia, gonorrhea, and syphilis in the United States, according to the Centers for Disease Control and Prevention. Reported cases of syphilis and gonorrhea have been climbing for about a decade. In its most recent prevalence estimate, the agency said that on any given day, one in five Americans are infected with any of eight common STDs.
The push to make at-home testing for STDs as easy and commonplace as at-home COVID and pregnancy testing is coming from several sectors. Public health officials say their overextended staffers can’t handle the staggering need for testing and surveillance. Diagnostic and pharmaceutical companies see a business opportunity in the unmet demand.
The medical science underpinning STD testing is not particularly new or mysterious. Depending on the test, it may involve collecting a urine sample, pricking a finger for blood, or swabbing the mouth, genitals, or anus for discharge or cell samples. Medical centers and community health clinics have performed such testing for decades.
The issue for regulators is whether sampling kits can be reliably adapted for in-home use. Unlike rapid antigen tests for COVID, which produce results in 15-20 minutes, the home STD kits on the market require patients to collect their own samples, and then package and mail them to a lab for analysis.
In the past 3 years, as the pandemic prompted clinics that provide low-cost care to drastically curtail in-person services, a number of public health departments – among them state agencies in Alabama, Alaska, and Maryland – have started mailing free STD test kits to residents. Universities and nonprofits are also spearheading at-home testing efforts.
And dozens of commercial enterprises are jumping into or ramping up direct-to-consumer sales. Everly Health, a digital health company that sells a variety of lab tests online, reported sales for its suite of STD kits grew 120% in the first half of this year compared with the first half of 2021.
CVS Health began selling its own bundled STD kit in October, priced at $99.99. Unlike most home kits, CVS’ version is available in stores.
Hologic, Abbott, and Molecular Testing Labs are among the companies urgently developing tests. And Cue Health, which sells COVID tests, is poised to launch a clinical trial for a rapid home test for chlamydia and gonorrhea that would set a new bar, providing results in about 20 minutes.
Alberto Gutierrez, who formerly led the FDA office that oversees diagnostic tests, said agency officials have been concerned about the reliability of home tests for years. The FDA wants companies to prove that home collection kits are as accurate as those used in clinics, and that samples don’t degrade during shipping.
“The agency doesn’t believe these tests are legally marketed at this point,” said Mr. Gutierrez, a partner at NDA Partners, a consulting firm that advises companies seeking to bring health care products to market.
“CVS should not be selling that test,” he added.
In response to KHN questions, the FDA said it considers home collection kits, which can include swabs, lancets, transport tubes, and chemicals to stabilize the samples, to be devices that require agency review. The FDA “generally does not comment” on whether it plans to take action on any specific case, the statement said.
CVS spokesperson Mary Gattuso said the pharmacy chain is following the law. “We are committed to ensuring the products we offer are safe, work as intended, comply with regulations, and satisfy customers,” Ms. Gattuso said.
Everly Health and other companies described their kits as laboratory-developed tests, akin to the diagnostics some hospitals create for in-house use. And they contend their tests can be legally marketed because their labs have been certified by a different agency, the Centers for Medicare & Medicaid Services.
“The instruments and assays used by the laboratories we use are comparable to – and often the same as – those used by the labs a doctor’s office uses,” said Liz Kwo, MD, chief medical officer at Everly Health. “Our at-home sample collection methods, like dried blood spots and saliva, have been widely used for decades.”
Home collection kits appeal to Uxmal Caldera, 27, of Miami Beach, who prefers to test in the privacy of his home. Mr. Caldera, who doesn’t have a car, said home testing saves him the time and expense of getting to a clinic.
Mr. Caldera has been testing himself for HIV and other STDs every 3 months for more than a year, part of routine monitoring for people taking PrEP, a regimen of daily pills to prevent HIV infection.
“Doing it by yourself is not hard at all,” said Mr. Caldera, who is uninsured but receives the tests free through a community foundation. “The instructions are really clear. I get the results in maybe 4 days. For sure, I would recommend it to other people.”
Leandro Mena, MD, director of the CDC’s division of STD prevention, said he would like to see at-home STD testing become as routine as home pregnancy tests. An estimated 16 million–20 million tests for gonorrhea and chlamydia are performed in the United States each year, Dr. Mena said. Widespread use of at-home STD testing could double or triple that number.
He noted that doctors have years of experience using home collection kits.
The Johns Hopkins Center for Point-of-Care Technologies Research for Sexually Transmitted Diseases has distributed roughly 23,000 at-home STD kits since 2004, said Charlotte Gaydos, DrPH, a principal investigator with the center. The FDA generally allows such use if it’s part of research overseen by medical professionals. The center’s tests are now used by the Alaska health department, as well as Native American tribes in Arizona and Oklahoma.
Dr. Gaydos has published dozens of studies establishing that home collection kits for diseases such as chlamydia and gonorrhea are accurate and easy to use.
“There’s a huge amount of data showing that home testing works,” said Dr. Gaydos.
But Dr. Gaydos noted that her studies have been limited to small sample sizes. She said she doesn’t have the millions of dollars in funding it would take to run the sort of comprehensive trial the FDA typically requires for approval.
Jenny Mahn, director of clinical and sexual health at the National Coalition of STD Directors, said many public health labs are reluctant to handle home kits. “The public health labs won’t touch it without FDA’s blessing.”
Public health clinics often provide STD testing at little to no cost, while health insurance typically covers in-person testing at a private practice. But most consumers pay out-of-pocket for direct-to-consumer kits. Commercial pricing puts them out of reach for many people, particularly teens and young adults, who account for nearly half of STDs.
Adalja said the FDA has a history of moving slowly on home testing. The agency spent 7 years evaluating the first home HIV test it approved, which hit the market in 2012.
“Home testing is the way of the future,” said Laura Lindberg, PhD, a professor of public health at Rutgers University, Piscataway, N.J. “The pandemic opened the door to testing and treatment at home without traveling to a health care provider, and we aren’t going to be able to put the genie back in the bottle.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Among the more remarkable legacies of the COVID-19 pandemic is how quickly federal regulators, the health care industry, and consumers moved to make at-home testing a reliable tool for managing a public health crisis.
But that fast-track focus is missing from another, less publicized epidemic: an explosion in sexually transmitted diseases that can cause chronic pain and infertility among infected adults and disable or kill infected newborns. The disparity has amplified calls from researchers, public health advocates, and health care companies urging the federal government to greenlight at-home testing kits that could vastly multiply the number of Americans testing for STDs.
Online shoppers can already choose from more than a dozen self-testing kits, typically ranging in price from $69 to $500, depending on the brand and the variety of infections they can detect.
But, except for HIV tests, the Food and Drug Administration hasn’t approved STD test kits for use outside a medical setting. That leaves consumers unsure about their reliability even as at-home use grows dramatically.
The STD epidemic is “out of control,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins University Center for Health Security. “We know we are missing diagnoses. We know that contact tracing is happening late or not at all. If we’re really serious about tackling the STD crisis, we have to get more people diagnosed.”
Preliminary data for 2021 showed nearly 2.5 million reported cases of chlamydia, gonorrhea, and syphilis in the United States, according to the Centers for Disease Control and Prevention. Reported cases of syphilis and gonorrhea have been climbing for about a decade. In its most recent prevalence estimate, the agency said that on any given day, one in five Americans are infected with any of eight common STDs.
The push to make at-home testing for STDs as easy and commonplace as at-home COVID and pregnancy testing is coming from several sectors. Public health officials say their overextended staffers can’t handle the staggering need for testing and surveillance. Diagnostic and pharmaceutical companies see a business opportunity in the unmet demand.
The medical science underpinning STD testing is not particularly new or mysterious. Depending on the test, it may involve collecting a urine sample, pricking a finger for blood, or swabbing the mouth, genitals, or anus for discharge or cell samples. Medical centers and community health clinics have performed such testing for decades.
The issue for regulators is whether sampling kits can be reliably adapted for in-home use. Unlike rapid antigen tests for COVID, which produce results in 15-20 minutes, the home STD kits on the market require patients to collect their own samples, and then package and mail them to a lab for analysis.
In the past 3 years, as the pandemic prompted clinics that provide low-cost care to drastically curtail in-person services, a number of public health departments – among them state agencies in Alabama, Alaska, and Maryland – have started mailing free STD test kits to residents. Universities and nonprofits are also spearheading at-home testing efforts.
And dozens of commercial enterprises are jumping into or ramping up direct-to-consumer sales. Everly Health, a digital health company that sells a variety of lab tests online, reported sales for its suite of STD kits grew 120% in the first half of this year compared with the first half of 2021.
CVS Health began selling its own bundled STD kit in October, priced at $99.99. Unlike most home kits, CVS’ version is available in stores.
Hologic, Abbott, and Molecular Testing Labs are among the companies urgently developing tests. And Cue Health, which sells COVID tests, is poised to launch a clinical trial for a rapid home test for chlamydia and gonorrhea that would set a new bar, providing results in about 20 minutes.
Alberto Gutierrez, who formerly led the FDA office that oversees diagnostic tests, said agency officials have been concerned about the reliability of home tests for years. The FDA wants companies to prove that home collection kits are as accurate as those used in clinics, and that samples don’t degrade during shipping.
“The agency doesn’t believe these tests are legally marketed at this point,” said Mr. Gutierrez, a partner at NDA Partners, a consulting firm that advises companies seeking to bring health care products to market.
“CVS should not be selling that test,” he added.
In response to KHN questions, the FDA said it considers home collection kits, which can include swabs, lancets, transport tubes, and chemicals to stabilize the samples, to be devices that require agency review. The FDA “generally does not comment” on whether it plans to take action on any specific case, the statement said.
CVS spokesperson Mary Gattuso said the pharmacy chain is following the law. “We are committed to ensuring the products we offer are safe, work as intended, comply with regulations, and satisfy customers,” Ms. Gattuso said.
Everly Health and other companies described their kits as laboratory-developed tests, akin to the diagnostics some hospitals create for in-house use. And they contend their tests can be legally marketed because their labs have been certified by a different agency, the Centers for Medicare & Medicaid Services.
“The instruments and assays used by the laboratories we use are comparable to – and often the same as – those used by the labs a doctor’s office uses,” said Liz Kwo, MD, chief medical officer at Everly Health. “Our at-home sample collection methods, like dried blood spots and saliva, have been widely used for decades.”
Home collection kits appeal to Uxmal Caldera, 27, of Miami Beach, who prefers to test in the privacy of his home. Mr. Caldera, who doesn’t have a car, said home testing saves him the time and expense of getting to a clinic.
Mr. Caldera has been testing himself for HIV and other STDs every 3 months for more than a year, part of routine monitoring for people taking PrEP, a regimen of daily pills to prevent HIV infection.
“Doing it by yourself is not hard at all,” said Mr. Caldera, who is uninsured but receives the tests free through a community foundation. “The instructions are really clear. I get the results in maybe 4 days. For sure, I would recommend it to other people.”
Leandro Mena, MD, director of the CDC’s division of STD prevention, said he would like to see at-home STD testing become as routine as home pregnancy tests. An estimated 16 million–20 million tests for gonorrhea and chlamydia are performed in the United States each year, Dr. Mena said. Widespread use of at-home STD testing could double or triple that number.
He noted that doctors have years of experience using home collection kits.
The Johns Hopkins Center for Point-of-Care Technologies Research for Sexually Transmitted Diseases has distributed roughly 23,000 at-home STD kits since 2004, said Charlotte Gaydos, DrPH, a principal investigator with the center. The FDA generally allows such use if it’s part of research overseen by medical professionals. The center’s tests are now used by the Alaska health department, as well as Native American tribes in Arizona and Oklahoma.
Dr. Gaydos has published dozens of studies establishing that home collection kits for diseases such as chlamydia and gonorrhea are accurate and easy to use.
“There’s a huge amount of data showing that home testing works,” said Dr. Gaydos.
But Dr. Gaydos noted that her studies have been limited to small sample sizes. She said she doesn’t have the millions of dollars in funding it would take to run the sort of comprehensive trial the FDA typically requires for approval.
Jenny Mahn, director of clinical and sexual health at the National Coalition of STD Directors, said many public health labs are reluctant to handle home kits. “The public health labs won’t touch it without FDA’s blessing.”
Public health clinics often provide STD testing at little to no cost, while health insurance typically covers in-person testing at a private practice. But most consumers pay out-of-pocket for direct-to-consumer kits. Commercial pricing puts them out of reach for many people, particularly teens and young adults, who account for nearly half of STDs.
Adalja said the FDA has a history of moving slowly on home testing. The agency spent 7 years evaluating the first home HIV test it approved, which hit the market in 2012.
“Home testing is the way of the future,” said Laura Lindberg, PhD, a professor of public health at Rutgers University, Piscataway, N.J. “The pandemic opened the door to testing and treatment at home without traveling to a health care provider, and we aren’t going to be able to put the genie back in the bottle.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Among the more remarkable legacies of the COVID-19 pandemic is how quickly federal regulators, the health care industry, and consumers moved to make at-home testing a reliable tool for managing a public health crisis.
But that fast-track focus is missing from another, less publicized epidemic: an explosion in sexually transmitted diseases that can cause chronic pain and infertility among infected adults and disable or kill infected newborns. The disparity has amplified calls from researchers, public health advocates, and health care companies urging the federal government to greenlight at-home testing kits that could vastly multiply the number of Americans testing for STDs.
Online shoppers can already choose from more than a dozen self-testing kits, typically ranging in price from $69 to $500, depending on the brand and the variety of infections they can detect.
But, except for HIV tests, the Food and Drug Administration hasn’t approved STD test kits for use outside a medical setting. That leaves consumers unsure about their reliability even as at-home use grows dramatically.
The STD epidemic is “out of control,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins University Center for Health Security. “We know we are missing diagnoses. We know that contact tracing is happening late or not at all. If we’re really serious about tackling the STD crisis, we have to get more people diagnosed.”
Preliminary data for 2021 showed nearly 2.5 million reported cases of chlamydia, gonorrhea, and syphilis in the United States, according to the Centers for Disease Control and Prevention. Reported cases of syphilis and gonorrhea have been climbing for about a decade. In its most recent prevalence estimate, the agency said that on any given day, one in five Americans are infected with any of eight common STDs.
The push to make at-home testing for STDs as easy and commonplace as at-home COVID and pregnancy testing is coming from several sectors. Public health officials say their overextended staffers can’t handle the staggering need for testing and surveillance. Diagnostic and pharmaceutical companies see a business opportunity in the unmet demand.
The medical science underpinning STD testing is not particularly new or mysterious. Depending on the test, it may involve collecting a urine sample, pricking a finger for blood, or swabbing the mouth, genitals, or anus for discharge or cell samples. Medical centers and community health clinics have performed such testing for decades.
The issue for regulators is whether sampling kits can be reliably adapted for in-home use. Unlike rapid antigen tests for COVID, which produce results in 15-20 minutes, the home STD kits on the market require patients to collect their own samples, and then package and mail them to a lab for analysis.
In the past 3 years, as the pandemic prompted clinics that provide low-cost care to drastically curtail in-person services, a number of public health departments – among them state agencies in Alabama, Alaska, and Maryland – have started mailing free STD test kits to residents. Universities and nonprofits are also spearheading at-home testing efforts.
And dozens of commercial enterprises are jumping into or ramping up direct-to-consumer sales. Everly Health, a digital health company that sells a variety of lab tests online, reported sales for its suite of STD kits grew 120% in the first half of this year compared with the first half of 2021.
CVS Health began selling its own bundled STD kit in October, priced at $99.99. Unlike most home kits, CVS’ version is available in stores.
Hologic, Abbott, and Molecular Testing Labs are among the companies urgently developing tests. And Cue Health, which sells COVID tests, is poised to launch a clinical trial for a rapid home test for chlamydia and gonorrhea that would set a new bar, providing results in about 20 minutes.
Alberto Gutierrez, who formerly led the FDA office that oversees diagnostic tests, said agency officials have been concerned about the reliability of home tests for years. The FDA wants companies to prove that home collection kits are as accurate as those used in clinics, and that samples don’t degrade during shipping.
“The agency doesn’t believe these tests are legally marketed at this point,” said Mr. Gutierrez, a partner at NDA Partners, a consulting firm that advises companies seeking to bring health care products to market.
“CVS should not be selling that test,” he added.
In response to KHN questions, the FDA said it considers home collection kits, which can include swabs, lancets, transport tubes, and chemicals to stabilize the samples, to be devices that require agency review. The FDA “generally does not comment” on whether it plans to take action on any specific case, the statement said.
CVS spokesperson Mary Gattuso said the pharmacy chain is following the law. “We are committed to ensuring the products we offer are safe, work as intended, comply with regulations, and satisfy customers,” Ms. Gattuso said.
Everly Health and other companies described their kits as laboratory-developed tests, akin to the diagnostics some hospitals create for in-house use. And they contend their tests can be legally marketed because their labs have been certified by a different agency, the Centers for Medicare & Medicaid Services.
“The instruments and assays used by the laboratories we use are comparable to – and often the same as – those used by the labs a doctor’s office uses,” said Liz Kwo, MD, chief medical officer at Everly Health. “Our at-home sample collection methods, like dried blood spots and saliva, have been widely used for decades.”
Home collection kits appeal to Uxmal Caldera, 27, of Miami Beach, who prefers to test in the privacy of his home. Mr. Caldera, who doesn’t have a car, said home testing saves him the time and expense of getting to a clinic.
Mr. Caldera has been testing himself for HIV and other STDs every 3 months for more than a year, part of routine monitoring for people taking PrEP, a regimen of daily pills to prevent HIV infection.
“Doing it by yourself is not hard at all,” said Mr. Caldera, who is uninsured but receives the tests free through a community foundation. “The instructions are really clear. I get the results in maybe 4 days. For sure, I would recommend it to other people.”
Leandro Mena, MD, director of the CDC’s division of STD prevention, said he would like to see at-home STD testing become as routine as home pregnancy tests. An estimated 16 million–20 million tests for gonorrhea and chlamydia are performed in the United States each year, Dr. Mena said. Widespread use of at-home STD testing could double or triple that number.
He noted that doctors have years of experience using home collection kits.
The Johns Hopkins Center for Point-of-Care Technologies Research for Sexually Transmitted Diseases has distributed roughly 23,000 at-home STD kits since 2004, said Charlotte Gaydos, DrPH, a principal investigator with the center. The FDA generally allows such use if it’s part of research overseen by medical professionals. The center’s tests are now used by the Alaska health department, as well as Native American tribes in Arizona and Oklahoma.
Dr. Gaydos has published dozens of studies establishing that home collection kits for diseases such as chlamydia and gonorrhea are accurate and easy to use.
“There’s a huge amount of data showing that home testing works,” said Dr. Gaydos.
But Dr. Gaydos noted that her studies have been limited to small sample sizes. She said she doesn’t have the millions of dollars in funding it would take to run the sort of comprehensive trial the FDA typically requires for approval.
Jenny Mahn, director of clinical and sexual health at the National Coalition of STD Directors, said many public health labs are reluctant to handle home kits. “The public health labs won’t touch it without FDA’s blessing.”
Public health clinics often provide STD testing at little to no cost, while health insurance typically covers in-person testing at a private practice. But most consumers pay out-of-pocket for direct-to-consumer kits. Commercial pricing puts them out of reach for many people, particularly teens and young adults, who account for nearly half of STDs.
Adalja said the FDA has a history of moving slowly on home testing. The agency spent 7 years evaluating the first home HIV test it approved, which hit the market in 2012.
“Home testing is the way of the future,” said Laura Lindberg, PhD, a professor of public health at Rutgers University, Piscataway, N.J. “The pandemic opened the door to testing and treatment at home without traveling to a health care provider, and we aren’t going to be able to put the genie back in the bottle.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Experts explain the ‘perfect storm’ of rampant RSV and flu
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Hypertension linked to risk of severe COVID
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
FROM PLOS ONE
HIV: Greater parental involvement needed with young men who have sex with men
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AIDS AND BEHAVIOR
Bepirovirsen: Is a ‘functional cure’ for HBV on the horizon?
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
Flu vaccination associated with reduced stroke risk
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM LANCET PUBLIC HEALTH