User login
Prostate Cancer Treatment Associated With More Complications
TOPLINE:
Radiotherapy increases the risk for bladder cancer and radiation-specific complications, according to the new cohort study.
METHODOLOGY:
- Researchers conducted a cohort study to try to characterize long-term treatment-related adverse effects and complications in patients treated for prostate cancer, compared with a general population of older males.
- They used data from the Prostate Cancer Prevention Trial and the Selenium and Vitamin E Cancer Prevention Trial, linked with Medicare claims. A total of 29,196 participants were included in the study’s control group. Of 3946 patients diagnosed with prostate cancer, 655 were treated with prostatectomy, and 1056 were treated with radiotherapy.
- Participants were followed for a median of 10.2 years, with specific follow-up durations being 10.5 years and 8.5 years for the prostatectomy and radiotherapy groups, respectively.
- The study analyzed ten potential treatment-related complications using Medicare claims data, including urinary incontinence, erectile dysfunction, and secondary cancers.
- Multivariable Cox regression was used to adjust for age, race, and year of time-at-risk initiation, with stratification by study and intervention arm.
TAKEAWAY:
- At 12 years, there was a 7.23 increase in hazard risk for urinary or sexual complications for patients who had prostatectomy, compared with controls (P < .001).
- Radiotherapy-treated patients had a nearly three times greater hazard risk for bladder cancer and a 100-fold increased hazard risk for radiation-specific complications, such as radiation cystitis and radiation proctitis (P < .001).
- The incidence of any treatment-related complication per 1000 person-years was 124.26 for prostatectomy, 62.15 for radiotherapy, and 23.61 for untreated participants.
- The authors stated that these findings highlight the importance of patient counseling before prostate cancer screening and treatment.
IN PRACTICE:
“We found that, after accounting for baseline population rates, most patients with PCA undergoing treatment experience complications associated with worse quality of life and/or new health risks. The magnitude of these risks, compared with the relatively small benefit found by randomized clinical trials of PCA screening and treatment, should be explicitly reflected in national cancer screening and treatment guidelines and be integral to shared decision-making with patients before initiation of PSA screening, biopsy, or PCA treatment,” wrote the authors of the study.
SOURCE:
The study was led by Joseph M. Unger, PhD, SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Center in Seattle, Washington. It was published online on November 7, 2024, in JAMA Oncology.
LIMITATIONS:
The study did not account for multiple comparisons, which may affect the statistical significance of some findings. Claims data are subject to misclassification and may underreport complications that are not reported to a physician. The study did not differentiate among strategies of prostatectomy or radiotherapy, which may result in different patterns of complications. The cohort comprised men enrolled in large, randomized prevention trials, which may limit the generalizability of the incidence estimates. Confounding by unknown factors cannot be ruled out, affecting the attribution of risks to prostate cancer treatment alone.
DISCLOSURES:
Unger disclosed consulting fees from AstraZeneca and Loxo/Lilly outside the submitted work. One coauthor reported grants from the US National Cancer Institute during the conduct of the study. Another coauthor reported employment with Flatiron Health at the time of manuscript submission and review. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Radiotherapy increases the risk for bladder cancer and radiation-specific complications, according to the new cohort study.
METHODOLOGY:
- Researchers conducted a cohort study to try to characterize long-term treatment-related adverse effects and complications in patients treated for prostate cancer, compared with a general population of older males.
- They used data from the Prostate Cancer Prevention Trial and the Selenium and Vitamin E Cancer Prevention Trial, linked with Medicare claims. A total of 29,196 participants were included in the study’s control group. Of 3946 patients diagnosed with prostate cancer, 655 were treated with prostatectomy, and 1056 were treated with radiotherapy.
- Participants were followed for a median of 10.2 years, with specific follow-up durations being 10.5 years and 8.5 years for the prostatectomy and radiotherapy groups, respectively.
- The study analyzed ten potential treatment-related complications using Medicare claims data, including urinary incontinence, erectile dysfunction, and secondary cancers.
- Multivariable Cox regression was used to adjust for age, race, and year of time-at-risk initiation, with stratification by study and intervention arm.
TAKEAWAY:
- At 12 years, there was a 7.23 increase in hazard risk for urinary or sexual complications for patients who had prostatectomy, compared with controls (P < .001).
- Radiotherapy-treated patients had a nearly three times greater hazard risk for bladder cancer and a 100-fold increased hazard risk for radiation-specific complications, such as radiation cystitis and radiation proctitis (P < .001).
- The incidence of any treatment-related complication per 1000 person-years was 124.26 for prostatectomy, 62.15 for radiotherapy, and 23.61 for untreated participants.
- The authors stated that these findings highlight the importance of patient counseling before prostate cancer screening and treatment.
IN PRACTICE:
“We found that, after accounting for baseline population rates, most patients with PCA undergoing treatment experience complications associated with worse quality of life and/or new health risks. The magnitude of these risks, compared with the relatively small benefit found by randomized clinical trials of PCA screening and treatment, should be explicitly reflected in national cancer screening and treatment guidelines and be integral to shared decision-making with patients before initiation of PSA screening, biopsy, or PCA treatment,” wrote the authors of the study.
SOURCE:
The study was led by Joseph M. Unger, PhD, SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Center in Seattle, Washington. It was published online on November 7, 2024, in JAMA Oncology.
LIMITATIONS:
The study did not account for multiple comparisons, which may affect the statistical significance of some findings. Claims data are subject to misclassification and may underreport complications that are not reported to a physician. The study did not differentiate among strategies of prostatectomy or radiotherapy, which may result in different patterns of complications. The cohort comprised men enrolled in large, randomized prevention trials, which may limit the generalizability of the incidence estimates. Confounding by unknown factors cannot be ruled out, affecting the attribution of risks to prostate cancer treatment alone.
DISCLOSURES:
Unger disclosed consulting fees from AstraZeneca and Loxo/Lilly outside the submitted work. One coauthor reported grants from the US National Cancer Institute during the conduct of the study. Another coauthor reported employment with Flatiron Health at the time of manuscript submission and review. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Radiotherapy increases the risk for bladder cancer and radiation-specific complications, according to the new cohort study.
METHODOLOGY:
- Researchers conducted a cohort study to try to characterize long-term treatment-related adverse effects and complications in patients treated for prostate cancer, compared with a general population of older males.
- They used data from the Prostate Cancer Prevention Trial and the Selenium and Vitamin E Cancer Prevention Trial, linked with Medicare claims. A total of 29,196 participants were included in the study’s control group. Of 3946 patients diagnosed with prostate cancer, 655 were treated with prostatectomy, and 1056 were treated with radiotherapy.
- Participants were followed for a median of 10.2 years, with specific follow-up durations being 10.5 years and 8.5 years for the prostatectomy and radiotherapy groups, respectively.
- The study analyzed ten potential treatment-related complications using Medicare claims data, including urinary incontinence, erectile dysfunction, and secondary cancers.
- Multivariable Cox regression was used to adjust for age, race, and year of time-at-risk initiation, with stratification by study and intervention arm.
TAKEAWAY:
- At 12 years, there was a 7.23 increase in hazard risk for urinary or sexual complications for patients who had prostatectomy, compared with controls (P < .001).
- Radiotherapy-treated patients had a nearly three times greater hazard risk for bladder cancer and a 100-fold increased hazard risk for radiation-specific complications, such as radiation cystitis and radiation proctitis (P < .001).
- The incidence of any treatment-related complication per 1000 person-years was 124.26 for prostatectomy, 62.15 for radiotherapy, and 23.61 for untreated participants.
- The authors stated that these findings highlight the importance of patient counseling before prostate cancer screening and treatment.
IN PRACTICE:
“We found that, after accounting for baseline population rates, most patients with PCA undergoing treatment experience complications associated with worse quality of life and/or new health risks. The magnitude of these risks, compared with the relatively small benefit found by randomized clinical trials of PCA screening and treatment, should be explicitly reflected in national cancer screening and treatment guidelines and be integral to shared decision-making with patients before initiation of PSA screening, biopsy, or PCA treatment,” wrote the authors of the study.
SOURCE:
The study was led by Joseph M. Unger, PhD, SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Center in Seattle, Washington. It was published online on November 7, 2024, in JAMA Oncology.
LIMITATIONS:
The study did not account for multiple comparisons, which may affect the statistical significance of some findings. Claims data are subject to misclassification and may underreport complications that are not reported to a physician. The study did not differentiate among strategies of prostatectomy or radiotherapy, which may result in different patterns of complications. The cohort comprised men enrolled in large, randomized prevention trials, which may limit the generalizability of the incidence estimates. Confounding by unknown factors cannot be ruled out, affecting the attribution of risks to prostate cancer treatment alone.
DISCLOSURES:
Unger disclosed consulting fees from AstraZeneca and Loxo/Lilly outside the submitted work. One coauthor reported grants from the US National Cancer Institute during the conduct of the study. Another coauthor reported employment with Flatiron Health at the time of manuscript submission and review. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Gardasil 9 at 10 Years: Vaccine Protects Against Multiple Cancers
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Is Being ‘Manly’ a Threat to a Man’s Health?
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here.
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
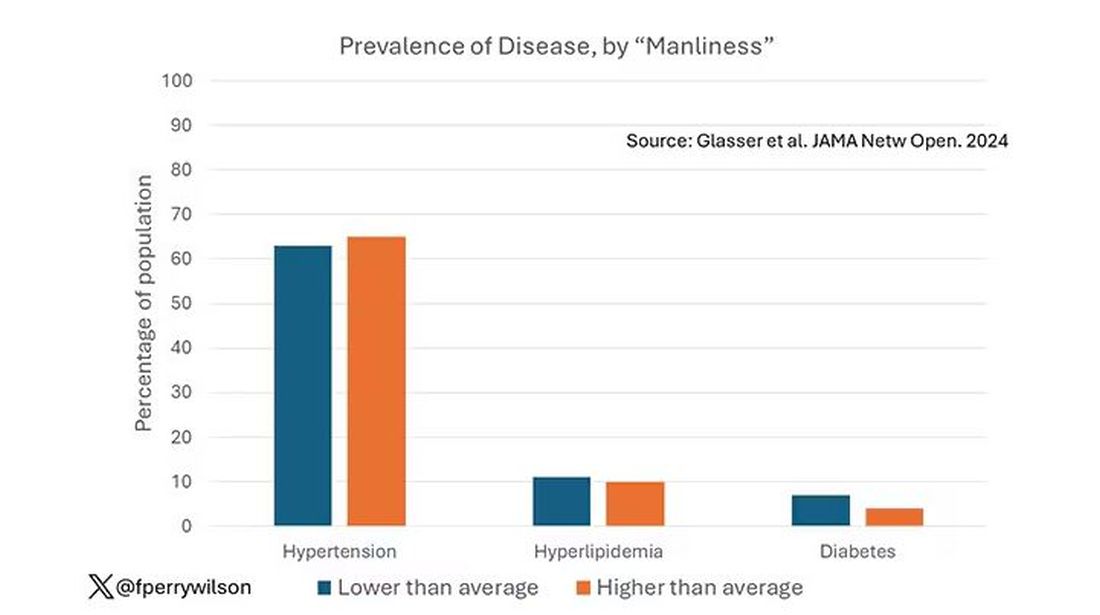
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
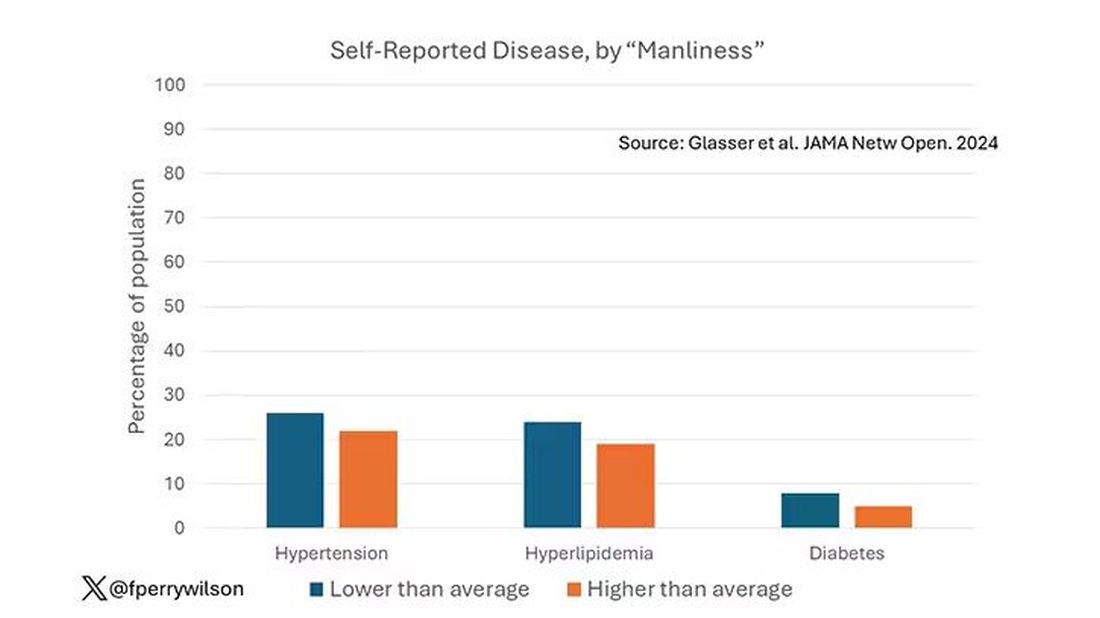
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
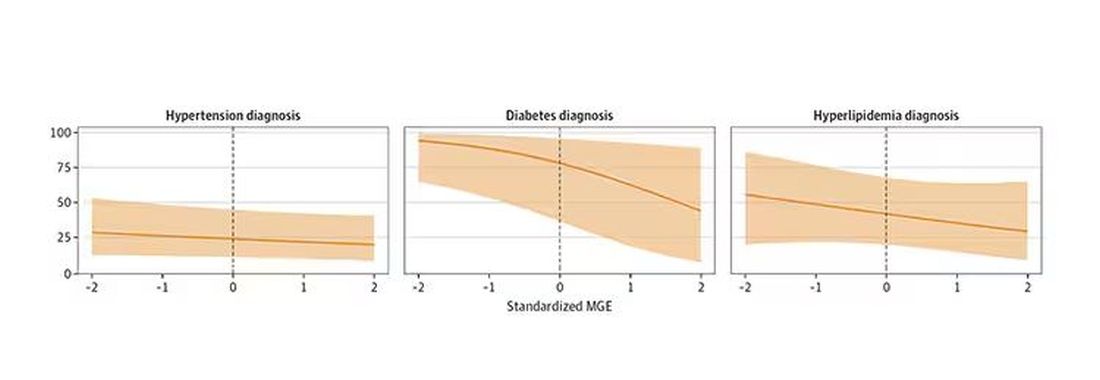
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here.
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here.
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The Genitals Are a Window Into Health: Sex as a Vital Sign
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, a urologist and sexual medicine specialist in the Washington, DC, area. And I am so thrilled because my co-fellow, the brilliant and famous Dr. Ashley Winter, a board-certified urologist and a certified menopause practitioner, who sees patients in our practice from Los Angeles, is joining us today to talk about sex as a vital sign.
Ashley Winter, MD: To have the best sexual function, you need many different systems to work. You need your hormones to be in the right place. You need your blood vessels to dilate when you want them to. You need your nerves to connect to your genitalia to make them responsive. The way people say, “The eyes are the window into the soul” — well, the genitals are the window into the cardiovascular system, the peripheral nervous system, and the hormonal system. It’s so dynamic. Patients can understand how this reflects their health. We just need healthcare providers to hammer home how those things connect.
Rubin: If you’re a primary care doctor seeing a patient and you want to educate them on diabetes or high blood pressure, how can you “ ‘sell it with ‘sex”? How can you use sex to educate them about these important medical conditions?
Winter: I hate using it as a fear tactic, but sometimes you have to. Time and again, I’ve seen men with severe profound erectile dysfunction at a young age, with chronically uncontrolled diabetes.
Diabetes can impair the peripheral nerves, resulting in peripheral neuropathy. The same way that it can affect the fingers and toes, diabetes can affect the penis, even before those other areas. Diabetes can also lead to other conditions such as low testosterone, which also affects the function of the penis.
I’m being brutally honest when I tell patients that diabetes control is critical to having a wonderful sexspan — the duration of your life where you’re able to be sexually active and have great sex and do it in the way that you want.
Chronic conditions such as high cholesterol or hypertension can affect your ability to become erect or aroused whether you have a penis or a vulva, and even your ability to have an orgasm.
Rubin: None of my doctors has ever asked me about these issues. But we have to bring them up with patients because they›re not going to bring them up to us. I always say in the review of systems, we shouldn›t just ask, “Do you have any sexual problems?” (which nobody ever does) and move past the question about men, women or both. We should be asking, “Do you have any issues with libido? Do you want to talk about it? Any issues with erection, arousal, orgasm, or sexual pain?”
When you can talk about those things, you can treat the patient from a whole physiologic perspective. For example, how does their sciatica affect their sexual pain? How does their antidepressant cause a delayed orgasm? How does their low testosterone level affect their energy level, their libido, and their desire?
We see so much shame and guilt in sexual health, to the extent that patients feel broken. We can help them understand the anatomy and physiology and explain that they aren’t broken. Instead, it’s “You need this medicine for your crippling anxiety, and that’s why your orgasm is delayed, and so can we augment it or add or subtract something to help you with it.”
Winter: In a primary care setting, where we are considering the patient›s overall health, we strive for medication compliance, but a huge part of medication noncompliance is sexual side effects, whether it›s antidepressants, beta-blockers, birth control, or this new world of GLP-1 agonists.
Rubin: I would add breast cancer treatments. Many patients go off their anastrozole or their tamoxifen because of the sexual side effects.
Winter: This is where we get to the crux of this discussion about sex being a vital sign — something you need to check routinely. We need to become comfortable with it, because then we are unlocking the ability to treat every patient like a whole person, give them better outcomes, improve their compliance, and have a really powerful tool for education.
Rubin: We have a growing toolbox for all genders when it comes to sexual health. We have FDA- approved medications for low libido in women. We use testosterone in men in an evidence-based way to safely improve libido. We use medications to help with the genitourinary syndrome of menopause. Orgasm is a challenging one, but we have devices that can help with those reflexes. And working with people who specialize in sexual pain can be extremely helpful for patients.
Dr. Winter, having practiced in different settings, what would you tell the primary care doctors who don’t want to talk about libido or who minimize sexual complaints because they don’t know how to navigate them?
Winter: I do not envy the challenge of being a primary care provider in the healthcare world we are living in. I think it is the hardest job. The ultimate takeaway is to just normalize the conversation and be able to validate what is happening. Have a few basic tools, and then have referrals. It›s not that you have to have all the time in the world or you have to treat every condition, but you have to start the conversation, be comfortable with it, and then get patients hooked up with the right resources.
Rubin: Every doctor of every kind can connect with patients and try to understand what they care about. What are their goals? What do they want for their families, for their relationships, for their quality of life? And how can we work collaboratively as a team to help them with those things?
Sex is a huge part of people’s lives. If we don’t ask about it; if we don’t look into it; and if we don’t admit that our physiology, our medications, and our surgeries can affect sexual health and functioning, how can we improve people’s lives? We can do so much as a team when we consider sex as a true vital sign.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC, has disclosed ties with Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, a urologist and sexual medicine specialist in the Washington, DC, area. And I am so thrilled because my co-fellow, the brilliant and famous Dr. Ashley Winter, a board-certified urologist and a certified menopause practitioner, who sees patients in our practice from Los Angeles, is joining us today to talk about sex as a vital sign.
Ashley Winter, MD: To have the best sexual function, you need many different systems to work. You need your hormones to be in the right place. You need your blood vessels to dilate when you want them to. You need your nerves to connect to your genitalia to make them responsive. The way people say, “The eyes are the window into the soul” — well, the genitals are the window into the cardiovascular system, the peripheral nervous system, and the hormonal system. It’s so dynamic. Patients can understand how this reflects their health. We just need healthcare providers to hammer home how those things connect.
Rubin: If you’re a primary care doctor seeing a patient and you want to educate them on diabetes or high blood pressure, how can you “ ‘sell it with ‘sex”? How can you use sex to educate them about these important medical conditions?
Winter: I hate using it as a fear tactic, but sometimes you have to. Time and again, I’ve seen men with severe profound erectile dysfunction at a young age, with chronically uncontrolled diabetes.
Diabetes can impair the peripheral nerves, resulting in peripheral neuropathy. The same way that it can affect the fingers and toes, diabetes can affect the penis, even before those other areas. Diabetes can also lead to other conditions such as low testosterone, which also affects the function of the penis.
I’m being brutally honest when I tell patients that diabetes control is critical to having a wonderful sexspan — the duration of your life where you’re able to be sexually active and have great sex and do it in the way that you want.
Chronic conditions such as high cholesterol or hypertension can affect your ability to become erect or aroused whether you have a penis or a vulva, and even your ability to have an orgasm.
Rubin: None of my doctors has ever asked me about these issues. But we have to bring them up with patients because they›re not going to bring them up to us. I always say in the review of systems, we shouldn›t just ask, “Do you have any sexual problems?” (which nobody ever does) and move past the question about men, women or both. We should be asking, “Do you have any issues with libido? Do you want to talk about it? Any issues with erection, arousal, orgasm, or sexual pain?”
When you can talk about those things, you can treat the patient from a whole physiologic perspective. For example, how does their sciatica affect their sexual pain? How does their antidepressant cause a delayed orgasm? How does their low testosterone level affect their energy level, their libido, and their desire?
We see so much shame and guilt in sexual health, to the extent that patients feel broken. We can help them understand the anatomy and physiology and explain that they aren’t broken. Instead, it’s “You need this medicine for your crippling anxiety, and that’s why your orgasm is delayed, and so can we augment it or add or subtract something to help you with it.”
Winter: In a primary care setting, where we are considering the patient›s overall health, we strive for medication compliance, but a huge part of medication noncompliance is sexual side effects, whether it›s antidepressants, beta-blockers, birth control, or this new world of GLP-1 agonists.
Rubin: I would add breast cancer treatments. Many patients go off their anastrozole or their tamoxifen because of the sexual side effects.
Winter: This is where we get to the crux of this discussion about sex being a vital sign — something you need to check routinely. We need to become comfortable with it, because then we are unlocking the ability to treat every patient like a whole person, give them better outcomes, improve their compliance, and have a really powerful tool for education.
Rubin: We have a growing toolbox for all genders when it comes to sexual health. We have FDA- approved medications for low libido in women. We use testosterone in men in an evidence-based way to safely improve libido. We use medications to help with the genitourinary syndrome of menopause. Orgasm is a challenging one, but we have devices that can help with those reflexes. And working with people who specialize in sexual pain can be extremely helpful for patients.
Dr. Winter, having practiced in different settings, what would you tell the primary care doctors who don’t want to talk about libido or who minimize sexual complaints because they don’t know how to navigate them?
Winter: I do not envy the challenge of being a primary care provider in the healthcare world we are living in. I think it is the hardest job. The ultimate takeaway is to just normalize the conversation and be able to validate what is happening. Have a few basic tools, and then have referrals. It›s not that you have to have all the time in the world or you have to treat every condition, but you have to start the conversation, be comfortable with it, and then get patients hooked up with the right resources.
Rubin: Every doctor of every kind can connect with patients and try to understand what they care about. What are their goals? What do they want for their families, for their relationships, for their quality of life? And how can we work collaboratively as a team to help them with those things?
Sex is a huge part of people’s lives. If we don’t ask about it; if we don’t look into it; and if we don’t admit that our physiology, our medications, and our surgeries can affect sexual health and functioning, how can we improve people’s lives? We can do so much as a team when we consider sex as a true vital sign.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC, has disclosed ties with Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, a urologist and sexual medicine specialist in the Washington, DC, area. And I am so thrilled because my co-fellow, the brilliant and famous Dr. Ashley Winter, a board-certified urologist and a certified menopause practitioner, who sees patients in our practice from Los Angeles, is joining us today to talk about sex as a vital sign.
Ashley Winter, MD: To have the best sexual function, you need many different systems to work. You need your hormones to be in the right place. You need your blood vessels to dilate when you want them to. You need your nerves to connect to your genitalia to make them responsive. The way people say, “The eyes are the window into the soul” — well, the genitals are the window into the cardiovascular system, the peripheral nervous system, and the hormonal system. It’s so dynamic. Patients can understand how this reflects their health. We just need healthcare providers to hammer home how those things connect.
Rubin: If you’re a primary care doctor seeing a patient and you want to educate them on diabetes or high blood pressure, how can you “ ‘sell it with ‘sex”? How can you use sex to educate them about these important medical conditions?
Winter: I hate using it as a fear tactic, but sometimes you have to. Time and again, I’ve seen men with severe profound erectile dysfunction at a young age, with chronically uncontrolled diabetes.
Diabetes can impair the peripheral nerves, resulting in peripheral neuropathy. The same way that it can affect the fingers and toes, diabetes can affect the penis, even before those other areas. Diabetes can also lead to other conditions such as low testosterone, which also affects the function of the penis.
I’m being brutally honest when I tell patients that diabetes control is critical to having a wonderful sexspan — the duration of your life where you’re able to be sexually active and have great sex and do it in the way that you want.
Chronic conditions such as high cholesterol or hypertension can affect your ability to become erect or aroused whether you have a penis or a vulva, and even your ability to have an orgasm.
Rubin: None of my doctors has ever asked me about these issues. But we have to bring them up with patients because they›re not going to bring them up to us. I always say in the review of systems, we shouldn›t just ask, “Do you have any sexual problems?” (which nobody ever does) and move past the question about men, women or both. We should be asking, “Do you have any issues with libido? Do you want to talk about it? Any issues with erection, arousal, orgasm, or sexual pain?”
When you can talk about those things, you can treat the patient from a whole physiologic perspective. For example, how does their sciatica affect their sexual pain? How does their antidepressant cause a delayed orgasm? How does their low testosterone level affect their energy level, their libido, and their desire?
We see so much shame and guilt in sexual health, to the extent that patients feel broken. We can help them understand the anatomy and physiology and explain that they aren’t broken. Instead, it’s “You need this medicine for your crippling anxiety, and that’s why your orgasm is delayed, and so can we augment it or add or subtract something to help you with it.”
Winter: In a primary care setting, where we are considering the patient›s overall health, we strive for medication compliance, but a huge part of medication noncompliance is sexual side effects, whether it›s antidepressants, beta-blockers, birth control, or this new world of GLP-1 agonists.
Rubin: I would add breast cancer treatments. Many patients go off their anastrozole or their tamoxifen because of the sexual side effects.
Winter: This is where we get to the crux of this discussion about sex being a vital sign — something you need to check routinely. We need to become comfortable with it, because then we are unlocking the ability to treat every patient like a whole person, give them better outcomes, improve their compliance, and have a really powerful tool for education.
Rubin: We have a growing toolbox for all genders when it comes to sexual health. We have FDA- approved medications for low libido in women. We use testosterone in men in an evidence-based way to safely improve libido. We use medications to help with the genitourinary syndrome of menopause. Orgasm is a challenging one, but we have devices that can help with those reflexes. And working with people who specialize in sexual pain can be extremely helpful for patients.
Dr. Winter, having practiced in different settings, what would you tell the primary care doctors who don’t want to talk about libido or who minimize sexual complaints because they don’t know how to navigate them?
Winter: I do not envy the challenge of being a primary care provider in the healthcare world we are living in. I think it is the hardest job. The ultimate takeaway is to just normalize the conversation and be able to validate what is happening. Have a few basic tools, and then have referrals. It›s not that you have to have all the time in the world or you have to treat every condition, but you have to start the conversation, be comfortable with it, and then get patients hooked up with the right resources.
Rubin: Every doctor of every kind can connect with patients and try to understand what they care about. What are their goals? What do they want for their families, for their relationships, for their quality of life? And how can we work collaboratively as a team to help them with those things?
Sex is a huge part of people’s lives. If we don’t ask about it; if we don’t look into it; and if we don’t admit that our physiology, our medications, and our surgeries can affect sexual health and functioning, how can we improve people’s lives? We can do so much as a team when we consider sex as a true vital sign.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC, has disclosed ties with Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
Groups With Highest Unmet Need for PrEP Highlighted in Analysis
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2024
SBRT or Prostatectomy for Localized Prostate Cancer: Is One Better?
TOPLINE:
according to a phase 3, open-label, randomized trial evaluating quality-of-life outcomes.
METHODOLOGY:
- Compared with prostatectomy, radiotherapy may offer better urinary and sexual outcomes but a higher risk for bowel toxicity in patients with localized prostate cancer. However, a comparison has not been performed in a randomized trial using more modern treatment options, such as SBRT.
- Researchers conducted the multicenter PACE-A trial to compare and evaluate quality-of-life outcomes among 123 patients (median age, 65.5 years) with low- to intermediate-risk localized prostate cancer who were randomly assigned to undergo either SBRT (n = 63) or radical prostatectomy (n = 60).
- Of the 123 patients, 97 (79%) had a Gleason score of 3+4 and 116 (94%) had National Comprehensive Cancer Network intermediate risk. The median follow-up was 60.7 months.
- The co–primary endpoints were urinary continence, measured by the number of absorbent urinary pads required per day, and bowel function, assessed using the Expanded Prostate Cancer Index Composite Short Form (EPIC-26).
- Secondary endpoints included erectile function (measured using the International Index of Erectile Function 5 questionnaire) , clinician-reported genitourinary and gastrointestinal toxicity, and International Prostate Symptom Score. Other patient-reported outcomes included EPIC-26 domain scores for urinary irritative/obstructive symptoms, and overall urinary, bowel, and sexual issues.
TAKEAWAY:
- At 2 years, only 6.5% (three of 46) of patients who ultimately received SBRT used one or more urinary pads daily compared with 50% (16 of 32) of patients who underwent prostatectomy (P < .001). Patients in the prostatectomy group reported worse EPIC-26 urinary incontinence domain scores (median, 77.3 vs 100; P = .003).
- Patients who underwent prostatectomy also had significantly worse sexual function scores (median, 18 vs 62.5 with SBRT; P < .001). Erectile dysfunction events of grade 2 or higher were significantly more common in patients who underwent prostatectomy (63% vs 18%).
- However, at 2 years, the bowel domain scores in the prostatectomy group were significantly higher than in the SBRT group (median, 100 vs 87.5), with a mean difference of 8.9.
- Overall, clinician-reported genitourinary and gastrointestinal toxicities were low in both treatment groups.
IN PRACTICE:
“PACE-A provides level 1 evidence of better outcomes of urinary continence and sexual function with worse bowel bother for SBRT, compared with prostatectomy,” the authors wrote, adding that the trial “provides contemporary toxicity estimates to optimize treatment decisions and maximize individual quality of life.”
SOURCE:
The study, led by Nicholas van As, of The Royal Marsden Hospital and The Institute of Cancer Research in London, was published online in European Urology.
LIMITATIONS:
The small sample size and differential dropout from allocated treatment could have introduced bias. Data completeness was another limitation.
DISCLOSURES:
The study was supported by grants from the Royal Marsden NHS Foundation Trust. Several authors reported having various ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
according to a phase 3, open-label, randomized trial evaluating quality-of-life outcomes.
METHODOLOGY:
- Compared with prostatectomy, radiotherapy may offer better urinary and sexual outcomes but a higher risk for bowel toxicity in patients with localized prostate cancer. However, a comparison has not been performed in a randomized trial using more modern treatment options, such as SBRT.
- Researchers conducted the multicenter PACE-A trial to compare and evaluate quality-of-life outcomes among 123 patients (median age, 65.5 years) with low- to intermediate-risk localized prostate cancer who were randomly assigned to undergo either SBRT (n = 63) or radical prostatectomy (n = 60).
- Of the 123 patients, 97 (79%) had a Gleason score of 3+4 and 116 (94%) had National Comprehensive Cancer Network intermediate risk. The median follow-up was 60.7 months.
- The co–primary endpoints were urinary continence, measured by the number of absorbent urinary pads required per day, and bowel function, assessed using the Expanded Prostate Cancer Index Composite Short Form (EPIC-26).
- Secondary endpoints included erectile function (measured using the International Index of Erectile Function 5 questionnaire) , clinician-reported genitourinary and gastrointestinal toxicity, and International Prostate Symptom Score. Other patient-reported outcomes included EPIC-26 domain scores for urinary irritative/obstructive symptoms, and overall urinary, bowel, and sexual issues.
TAKEAWAY:
- At 2 years, only 6.5% (three of 46) of patients who ultimately received SBRT used one or more urinary pads daily compared with 50% (16 of 32) of patients who underwent prostatectomy (P < .001). Patients in the prostatectomy group reported worse EPIC-26 urinary incontinence domain scores (median, 77.3 vs 100; P = .003).
- Patients who underwent prostatectomy also had significantly worse sexual function scores (median, 18 vs 62.5 with SBRT; P < .001). Erectile dysfunction events of grade 2 or higher were significantly more common in patients who underwent prostatectomy (63% vs 18%).
- However, at 2 years, the bowel domain scores in the prostatectomy group were significantly higher than in the SBRT group (median, 100 vs 87.5), with a mean difference of 8.9.
- Overall, clinician-reported genitourinary and gastrointestinal toxicities were low in both treatment groups.
IN PRACTICE:
“PACE-A provides level 1 evidence of better outcomes of urinary continence and sexual function with worse bowel bother for SBRT, compared with prostatectomy,” the authors wrote, adding that the trial “provides contemporary toxicity estimates to optimize treatment decisions and maximize individual quality of life.”
SOURCE:
The study, led by Nicholas van As, of The Royal Marsden Hospital and The Institute of Cancer Research in London, was published online in European Urology.
LIMITATIONS:
The small sample size and differential dropout from allocated treatment could have introduced bias. Data completeness was another limitation.
DISCLOSURES:
The study was supported by grants from the Royal Marsden NHS Foundation Trust. Several authors reported having various ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
according to a phase 3, open-label, randomized trial evaluating quality-of-life outcomes.
METHODOLOGY:
- Compared with prostatectomy, radiotherapy may offer better urinary and sexual outcomes but a higher risk for bowel toxicity in patients with localized prostate cancer. However, a comparison has not been performed in a randomized trial using more modern treatment options, such as SBRT.
- Researchers conducted the multicenter PACE-A trial to compare and evaluate quality-of-life outcomes among 123 patients (median age, 65.5 years) with low- to intermediate-risk localized prostate cancer who were randomly assigned to undergo either SBRT (n = 63) or radical prostatectomy (n = 60).
- Of the 123 patients, 97 (79%) had a Gleason score of 3+4 and 116 (94%) had National Comprehensive Cancer Network intermediate risk. The median follow-up was 60.7 months.
- The co–primary endpoints were urinary continence, measured by the number of absorbent urinary pads required per day, and bowel function, assessed using the Expanded Prostate Cancer Index Composite Short Form (EPIC-26).
- Secondary endpoints included erectile function (measured using the International Index of Erectile Function 5 questionnaire) , clinician-reported genitourinary and gastrointestinal toxicity, and International Prostate Symptom Score. Other patient-reported outcomes included EPIC-26 domain scores for urinary irritative/obstructive symptoms, and overall urinary, bowel, and sexual issues.
TAKEAWAY:
- At 2 years, only 6.5% (three of 46) of patients who ultimately received SBRT used one or more urinary pads daily compared with 50% (16 of 32) of patients who underwent prostatectomy (P < .001). Patients in the prostatectomy group reported worse EPIC-26 urinary incontinence domain scores (median, 77.3 vs 100; P = .003).
- Patients who underwent prostatectomy also had significantly worse sexual function scores (median, 18 vs 62.5 with SBRT; P < .001). Erectile dysfunction events of grade 2 or higher were significantly more common in patients who underwent prostatectomy (63% vs 18%).
- However, at 2 years, the bowel domain scores in the prostatectomy group were significantly higher than in the SBRT group (median, 100 vs 87.5), with a mean difference of 8.9.
- Overall, clinician-reported genitourinary and gastrointestinal toxicities were low in both treatment groups.
IN PRACTICE:
“PACE-A provides level 1 evidence of better outcomes of urinary continence and sexual function with worse bowel bother for SBRT, compared with prostatectomy,” the authors wrote, adding that the trial “provides contemporary toxicity estimates to optimize treatment decisions and maximize individual quality of life.”
SOURCE:
The study, led by Nicholas van As, of The Royal Marsden Hospital and The Institute of Cancer Research in London, was published online in European Urology.
LIMITATIONS:
The small sample size and differential dropout from allocated treatment could have introduced bias. Data completeness was another limitation.
DISCLOSURES:
The study was supported by grants from the Royal Marsden NHS Foundation Trust. Several authors reported having various ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Sperm Appear to Have a Nonreproductive Function
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM ANDROLOGY
What Should You Do When a Patient Asks for a PSA Test?
Many patients ask us to request a prostate-specific antigen (PSA) test. According to the Brazilian Ministry of Health, prostate cancer is the second most common type of cancer in the male population in all regions of our country. It is the second-leading cause of cancer death in the male population, reaffirming its epidemiologic importance in Brazil. On the other hand, a Ministry of Health technical paper recommends against population-based screening for prostate cancer. So, what should we do?
First, it is important to distinguish early diagnosis from screening. Early diagnosis is the identification of cancer in early stages in people with signs and symptoms. Screening is characterized by the systematic application of exams — digital rectal exam and PSA test — in asymptomatic people, with the aim of identifying cancer in an early stage.
A recent European epidemiologic study reinforced this thesis and helps guide us.
The study included men aged 35-84 years from 26 European countries. Data on cancer incidence and mortality were collected between 1980 and 2017. The data suggested overdiagnosis of prostate cancer, which varied over time and among populations. The findings supported previous recommendations that any implementation of prostate cancer screening should be carefully designed, with an emphasis on minimizing the harms of overdiagnosis.
The clinical evolution of prostate cancer is still not well understood. Increasing age is associated with increased mortality. Many men with less aggressive disease tend to die with cancer rather than die of cancer. However, it is not always possible at the time of diagnosis to determine which tumors will be aggressive and which will grow slowly.
On the other hand, with screening, many of these indolent cancers are unnecessarily detected, generating excessive exams and treatments with negative repercussions (eg, pain, bleeding, infections, stress, and urinary and sexual dysfunction).
So, how should we as clinicians proceed regarding screening?
We should request the PSA test and emphasize the importance of digital rectal exam by a urologist for those at high risk for prostatic neoplasia (ie, those with family history) or those with urinary symptoms that may be associated with prostate cancer.
In general, we should draw attention to the possible risks and benefits of testing and adopt a shared decision-making approach with asymptomatic men or those at low risk who wish to have the screening exam. But achieving a shared decision is not a simple task.
I always have a thorough conversation with patients, but I confess that I request the exam in most cases.
Dr. Wajngarten is a professor of cardiology, Faculty of Medicine, at the University of São Paulo in Brazil. Dr. Wajngarten reported no conflicts of interest.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Many patients ask us to request a prostate-specific antigen (PSA) test. According to the Brazilian Ministry of Health, prostate cancer is the second most common type of cancer in the male population in all regions of our country. It is the second-leading cause of cancer death in the male population, reaffirming its epidemiologic importance in Brazil. On the other hand, a Ministry of Health technical paper recommends against population-based screening for prostate cancer. So, what should we do?
First, it is important to distinguish early diagnosis from screening. Early diagnosis is the identification of cancer in early stages in people with signs and symptoms. Screening is characterized by the systematic application of exams — digital rectal exam and PSA test — in asymptomatic people, with the aim of identifying cancer in an early stage.
A recent European epidemiologic study reinforced this thesis and helps guide us.
The study included men aged 35-84 years from 26 European countries. Data on cancer incidence and mortality were collected between 1980 and 2017. The data suggested overdiagnosis of prostate cancer, which varied over time and among populations. The findings supported previous recommendations that any implementation of prostate cancer screening should be carefully designed, with an emphasis on minimizing the harms of overdiagnosis.
The clinical evolution of prostate cancer is still not well understood. Increasing age is associated with increased mortality. Many men with less aggressive disease tend to die with cancer rather than die of cancer. However, it is not always possible at the time of diagnosis to determine which tumors will be aggressive and which will grow slowly.
On the other hand, with screening, many of these indolent cancers are unnecessarily detected, generating excessive exams and treatments with negative repercussions (eg, pain, bleeding, infections, stress, and urinary and sexual dysfunction).
So, how should we as clinicians proceed regarding screening?
We should request the PSA test and emphasize the importance of digital rectal exam by a urologist for those at high risk for prostatic neoplasia (ie, those with family history) or those with urinary symptoms that may be associated with prostate cancer.
In general, we should draw attention to the possible risks and benefits of testing and adopt a shared decision-making approach with asymptomatic men or those at low risk who wish to have the screening exam. But achieving a shared decision is not a simple task.
I always have a thorough conversation with patients, but I confess that I request the exam in most cases.
Dr. Wajngarten is a professor of cardiology, Faculty of Medicine, at the University of São Paulo in Brazil. Dr. Wajngarten reported no conflicts of interest.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Many patients ask us to request a prostate-specific antigen (PSA) test. According to the Brazilian Ministry of Health, prostate cancer is the second most common type of cancer in the male population in all regions of our country. It is the second-leading cause of cancer death in the male population, reaffirming its epidemiologic importance in Brazil. On the other hand, a Ministry of Health technical paper recommends against population-based screening for prostate cancer. So, what should we do?
First, it is important to distinguish early diagnosis from screening. Early diagnosis is the identification of cancer in early stages in people with signs and symptoms. Screening is characterized by the systematic application of exams — digital rectal exam and PSA test — in asymptomatic people, with the aim of identifying cancer in an early stage.
A recent European epidemiologic study reinforced this thesis and helps guide us.
The study included men aged 35-84 years from 26 European countries. Data on cancer incidence and mortality were collected between 1980 and 2017. The data suggested overdiagnosis of prostate cancer, which varied over time and among populations. The findings supported previous recommendations that any implementation of prostate cancer screening should be carefully designed, with an emphasis on minimizing the harms of overdiagnosis.
The clinical evolution of prostate cancer is still not well understood. Increasing age is associated with increased mortality. Many men with less aggressive disease tend to die with cancer rather than die of cancer. However, it is not always possible at the time of diagnosis to determine which tumors will be aggressive and which will grow slowly.
On the other hand, with screening, many of these indolent cancers are unnecessarily detected, generating excessive exams and treatments with negative repercussions (eg, pain, bleeding, infections, stress, and urinary and sexual dysfunction).
So, how should we as clinicians proceed regarding screening?
We should request the PSA test and emphasize the importance of digital rectal exam by a urologist for those at high risk for prostatic neoplasia (ie, those with family history) or those with urinary symptoms that may be associated with prostate cancer.
In general, we should draw attention to the possible risks and benefits of testing and adopt a shared decision-making approach with asymptomatic men or those at low risk who wish to have the screening exam. But achieving a shared decision is not a simple task.
I always have a thorough conversation with patients, but I confess that I request the exam in most cases.
Dr. Wajngarten is a professor of cardiology, Faculty of Medicine, at the University of São Paulo in Brazil. Dr. Wajngarten reported no conflicts of interest.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New Treatment Effective for Male Postpartum Depression
A psychosocial intervention designed to improve depressive symptoms and promote good parenting skills can be an effective way of treating male postpartum depression, according to new research.
In a study conducted in Pakistan, about 70% fathers with postpartum depression who received the intervention showed complete remission of their depressive symptoms and experienced enhanced relationships with their children and domestic partners.
Called Learning Through Play Plus Dads (LTP + Dads), the intervention, which can be delivered by community health workers, could improve paternal mental health and child development not only in Pakistan but also in other populations, the authors stated.
The results of the study were published on October 2, 2024, in JAMA Psychiatry.
Stigmatized and Understudied
“Pakistan is a patriarchal society with strict gender roles, and male mental health, particularly postpartum depression in new fathers, is stigmatized and understudied,” lead investigator Ishrat Husain, MD, a senior scientist at the Centre for Addiction and Mental Health and associate professor of psychiatry at the University of Toronto in Ontario, Canada, said in an interview.
“Historically, and rightly so, the focus has always been on the mother, but men also experience significant emotional challenges as they adapt to being a parent. Fathers are also in need of support,” said Husain.
Male postpartum depression is prevalent in all populations. Globally, about 10% fathers have postpartum depression. But in societies like Pakistan, rates of male postpartum depression have been reported to be as high as 23.5%.
The study included 357 fathers aged 18 years or older (mean age, 31.44 years) with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, diagnosis of major depressive episode and a child younger than 30 months.
They were randomly assigned either to receive treatment as usual (n = 186) or to participate in the LTP + Dads program (n = 171). LTP + Dads is a parenting and mental health initiative adapted from a similar program for Pakistani mothers. It combines parenting skills training, play therapy, and cognitive behavioral therapy. In this study, the initiative was delivered by community health workers in 12 group sessions over 4 months. Sessions took place weekly for the first 2 months and biweekly thereafter.
The researchers assessed changes in the 17-item Hamilton Depression Rating Scale (HDRS-17) score at 4 months and at 6 months. They also looked at anxiety symptoms; parenting stress; intimate partner violence; functioning; quality of life; and child social, emotional, and physical health outcomes.
Improved Child Development
There were significantly greater reductions in HDRS-17 scores in the LTP + Dads group than in the treatment as usual group at 4 months (group difference ratio [GDR], 0.66; P < .001) and at 6 months (GDR, 0.67; P < .001).
Similar results were seen for anxiety (GDR, 0.62; P < .001), parenting stress (GDR, −12.5; P < .001), intimate partner violence (GDR, 0.89; P = .05), disability (GDR, 0.77; P = .03), and health-related quality of life (GDR, 12.7; P < .001) at 4 months. The differences in depression and parenting stress were sustained at 6 months.
In addition, children of fathers who received the parenting intervention showed significantly greater improvements in social-emotional development scores (mean difference, −20.8; P < .001) at 6 months than children of those who received the treatment as usual.
“We believe that this program could also be successful in other countries, including Canada,” said Husain. “Canada is multicultural, and similar patterns of male postpartum depression probably exist here. We know that cultural and social pressures create barriers to seeking mental health support for men. Stigma and cultural beliefs often prevent new fathers from seeking the help they need. Programs like LTP + Dads can help men transition to their new role as fathers by giving them support to process their emotions,” he said.
Husain added that the program will be expanded throughout Pakistan to include about 4000 fathers and their partners.
‘Remarkable’ Success Rate
“Postpartum depression in men is still something that people are trying to understand,” John Ogrodniczuk, MD, professor of psychiatry and director of the psychotherapy program at The University of British Columbia, Vancouver, Canada, said in an interview. He did not participate in the study.
“Obviously, men aren’t going through the same endocrine changes that women are, but nonetheless, a lot of men do actually struggle with it,” said Ogrodniczuk, who is also the founder of HeadsUpGuys, a mental health resource for men.
“Understandably, most of the literature is around postpartum depression in women, not so much around men. The positive results seen here are interesting, especially in a country that is patriarchal and where there is not a lot of uptake of mental health interventions and services by men,” he said.
“The success rate of this psychosocial intervention is remarkable, so I am excited to see that the researchers have secured funding to expand the study and validate their results with a larger group of participants,” Simon B. Sherry, PhD, professor of psychology and neuroscience at Dalhousie University, Halifax, Nova Scotia, Canada, said in an interview.
“I am also encouraged by the inclusion of play-based activities in addition to cognitive behavioral therapy. Perhaps more than any other role we hold through life, the role of parent comes with copious societal and personal expectations, plus with all that pressure, transitioning into that role is hard for everyone, but especially for those with postpartum depression. Supporting parents and improving their mental well-being goes a long way toward raising mentally healthy kids,” said Sherry, who was not part of the study.
The study was funded by a grant from Grand Challenges Canada, an Academic Scholars Award from the Department of Psychiatry at the University of Toronto, and a Tier 2 Canada Research Chair from the Canadian Institutes of Health Research. Husain reported receiving grants from COMPASS Pathfinder, stock options from Mindset Pharma, and personal fees from Wake Network, outside the submitted work. He previously served as a trustee for the Pakistan Institute of Living and Learning. Ogrodniczuk and Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A psychosocial intervention designed to improve depressive symptoms and promote good parenting skills can be an effective way of treating male postpartum depression, according to new research.
In a study conducted in Pakistan, about 70% fathers with postpartum depression who received the intervention showed complete remission of their depressive symptoms and experienced enhanced relationships with their children and domestic partners.
Called Learning Through Play Plus Dads (LTP + Dads), the intervention, which can be delivered by community health workers, could improve paternal mental health and child development not only in Pakistan but also in other populations, the authors stated.
The results of the study were published on October 2, 2024, in JAMA Psychiatry.
Stigmatized and Understudied
“Pakistan is a patriarchal society with strict gender roles, and male mental health, particularly postpartum depression in new fathers, is stigmatized and understudied,” lead investigator Ishrat Husain, MD, a senior scientist at the Centre for Addiction and Mental Health and associate professor of psychiatry at the University of Toronto in Ontario, Canada, said in an interview.
“Historically, and rightly so, the focus has always been on the mother, but men also experience significant emotional challenges as they adapt to being a parent. Fathers are also in need of support,” said Husain.
Male postpartum depression is prevalent in all populations. Globally, about 10% fathers have postpartum depression. But in societies like Pakistan, rates of male postpartum depression have been reported to be as high as 23.5%.
The study included 357 fathers aged 18 years or older (mean age, 31.44 years) with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, diagnosis of major depressive episode and a child younger than 30 months.
They were randomly assigned either to receive treatment as usual (n = 186) or to participate in the LTP + Dads program (n = 171). LTP + Dads is a parenting and mental health initiative adapted from a similar program for Pakistani mothers. It combines parenting skills training, play therapy, and cognitive behavioral therapy. In this study, the initiative was delivered by community health workers in 12 group sessions over 4 months. Sessions took place weekly for the first 2 months and biweekly thereafter.
The researchers assessed changes in the 17-item Hamilton Depression Rating Scale (HDRS-17) score at 4 months and at 6 months. They also looked at anxiety symptoms; parenting stress; intimate partner violence; functioning; quality of life; and child social, emotional, and physical health outcomes.
Improved Child Development
There were significantly greater reductions in HDRS-17 scores in the LTP + Dads group than in the treatment as usual group at 4 months (group difference ratio [GDR], 0.66; P < .001) and at 6 months (GDR, 0.67; P < .001).
Similar results were seen for anxiety (GDR, 0.62; P < .001), parenting stress (GDR, −12.5; P < .001), intimate partner violence (GDR, 0.89; P = .05), disability (GDR, 0.77; P = .03), and health-related quality of life (GDR, 12.7; P < .001) at 4 months. The differences in depression and parenting stress were sustained at 6 months.
In addition, children of fathers who received the parenting intervention showed significantly greater improvements in social-emotional development scores (mean difference, −20.8; P < .001) at 6 months than children of those who received the treatment as usual.
“We believe that this program could also be successful in other countries, including Canada,” said Husain. “Canada is multicultural, and similar patterns of male postpartum depression probably exist here. We know that cultural and social pressures create barriers to seeking mental health support for men. Stigma and cultural beliefs often prevent new fathers from seeking the help they need. Programs like LTP + Dads can help men transition to their new role as fathers by giving them support to process their emotions,” he said.
Husain added that the program will be expanded throughout Pakistan to include about 4000 fathers and their partners.
‘Remarkable’ Success Rate
“Postpartum depression in men is still something that people are trying to understand,” John Ogrodniczuk, MD, professor of psychiatry and director of the psychotherapy program at The University of British Columbia, Vancouver, Canada, said in an interview. He did not participate in the study.
“Obviously, men aren’t going through the same endocrine changes that women are, but nonetheless, a lot of men do actually struggle with it,” said Ogrodniczuk, who is also the founder of HeadsUpGuys, a mental health resource for men.
“Understandably, most of the literature is around postpartum depression in women, not so much around men. The positive results seen here are interesting, especially in a country that is patriarchal and where there is not a lot of uptake of mental health interventions and services by men,” he said.
“The success rate of this psychosocial intervention is remarkable, so I am excited to see that the researchers have secured funding to expand the study and validate their results with a larger group of participants,” Simon B. Sherry, PhD, professor of psychology and neuroscience at Dalhousie University, Halifax, Nova Scotia, Canada, said in an interview.
“I am also encouraged by the inclusion of play-based activities in addition to cognitive behavioral therapy. Perhaps more than any other role we hold through life, the role of parent comes with copious societal and personal expectations, plus with all that pressure, transitioning into that role is hard for everyone, but especially for those with postpartum depression. Supporting parents and improving their mental well-being goes a long way toward raising mentally healthy kids,” said Sherry, who was not part of the study.
The study was funded by a grant from Grand Challenges Canada, an Academic Scholars Award from the Department of Psychiatry at the University of Toronto, and a Tier 2 Canada Research Chair from the Canadian Institutes of Health Research. Husain reported receiving grants from COMPASS Pathfinder, stock options from Mindset Pharma, and personal fees from Wake Network, outside the submitted work. He previously served as a trustee for the Pakistan Institute of Living and Learning. Ogrodniczuk and Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A psychosocial intervention designed to improve depressive symptoms and promote good parenting skills can be an effective way of treating male postpartum depression, according to new research.
In a study conducted in Pakistan, about 70% fathers with postpartum depression who received the intervention showed complete remission of their depressive symptoms and experienced enhanced relationships with their children and domestic partners.
Called Learning Through Play Plus Dads (LTP + Dads), the intervention, which can be delivered by community health workers, could improve paternal mental health and child development not only in Pakistan but also in other populations, the authors stated.
The results of the study were published on October 2, 2024, in JAMA Psychiatry.
Stigmatized and Understudied
“Pakistan is a patriarchal society with strict gender roles, and male mental health, particularly postpartum depression in new fathers, is stigmatized and understudied,” lead investigator Ishrat Husain, MD, a senior scientist at the Centre for Addiction and Mental Health and associate professor of psychiatry at the University of Toronto in Ontario, Canada, said in an interview.
“Historically, and rightly so, the focus has always been on the mother, but men also experience significant emotional challenges as they adapt to being a parent. Fathers are also in need of support,” said Husain.
Male postpartum depression is prevalent in all populations. Globally, about 10% fathers have postpartum depression. But in societies like Pakistan, rates of male postpartum depression have been reported to be as high as 23.5%.
The study included 357 fathers aged 18 years or older (mean age, 31.44 years) with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, diagnosis of major depressive episode and a child younger than 30 months.
They were randomly assigned either to receive treatment as usual (n = 186) or to participate in the LTP + Dads program (n = 171). LTP + Dads is a parenting and mental health initiative adapted from a similar program for Pakistani mothers. It combines parenting skills training, play therapy, and cognitive behavioral therapy. In this study, the initiative was delivered by community health workers in 12 group sessions over 4 months. Sessions took place weekly for the first 2 months and biweekly thereafter.
The researchers assessed changes in the 17-item Hamilton Depression Rating Scale (HDRS-17) score at 4 months and at 6 months. They also looked at anxiety symptoms; parenting stress; intimate partner violence; functioning; quality of life; and child social, emotional, and physical health outcomes.
Improved Child Development
There were significantly greater reductions in HDRS-17 scores in the LTP + Dads group than in the treatment as usual group at 4 months (group difference ratio [GDR], 0.66; P < .001) and at 6 months (GDR, 0.67; P < .001).
Similar results were seen for anxiety (GDR, 0.62; P < .001), parenting stress (GDR, −12.5; P < .001), intimate partner violence (GDR, 0.89; P = .05), disability (GDR, 0.77; P = .03), and health-related quality of life (GDR, 12.7; P < .001) at 4 months. The differences in depression and parenting stress were sustained at 6 months.
In addition, children of fathers who received the parenting intervention showed significantly greater improvements in social-emotional development scores (mean difference, −20.8; P < .001) at 6 months than children of those who received the treatment as usual.
“We believe that this program could also be successful in other countries, including Canada,” said Husain. “Canada is multicultural, and similar patterns of male postpartum depression probably exist here. We know that cultural and social pressures create barriers to seeking mental health support for men. Stigma and cultural beliefs often prevent new fathers from seeking the help they need. Programs like LTP + Dads can help men transition to their new role as fathers by giving them support to process their emotions,” he said.
Husain added that the program will be expanded throughout Pakistan to include about 4000 fathers and their partners.
‘Remarkable’ Success Rate
“Postpartum depression in men is still something that people are trying to understand,” John Ogrodniczuk, MD, professor of psychiatry and director of the psychotherapy program at The University of British Columbia, Vancouver, Canada, said in an interview. He did not participate in the study.
“Obviously, men aren’t going through the same endocrine changes that women are, but nonetheless, a lot of men do actually struggle with it,” said Ogrodniczuk, who is also the founder of HeadsUpGuys, a mental health resource for men.
“Understandably, most of the literature is around postpartum depression in women, not so much around men. The positive results seen here are interesting, especially in a country that is patriarchal and where there is not a lot of uptake of mental health interventions and services by men,” he said.
“The success rate of this psychosocial intervention is remarkable, so I am excited to see that the researchers have secured funding to expand the study and validate their results with a larger group of participants,” Simon B. Sherry, PhD, professor of psychology and neuroscience at Dalhousie University, Halifax, Nova Scotia, Canada, said in an interview.
“I am also encouraged by the inclusion of play-based activities in addition to cognitive behavioral therapy. Perhaps more than any other role we hold through life, the role of parent comes with copious societal and personal expectations, plus with all that pressure, transitioning into that role is hard for everyone, but especially for those with postpartum depression. Supporting parents and improving their mental well-being goes a long way toward raising mentally healthy kids,” said Sherry, who was not part of the study.
The study was funded by a grant from Grand Challenges Canada, an Academic Scholars Award from the Department of Psychiatry at the University of Toronto, and a Tier 2 Canada Research Chair from the Canadian Institutes of Health Research. Husain reported receiving grants from COMPASS Pathfinder, stock options from Mindset Pharma, and personal fees from Wake Network, outside the submitted work. He previously served as a trustee for the Pakistan Institute of Living and Learning. Ogrodniczuk and Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
How to Treat Cancer While Preserving Fertility
Thanks to the continuously improving treatment options for cancer, the number of cancer survivors is increasing, and a large proportion of survivors is confronted with the long-term effects of cancer treatment. Especially for young patients, the question of the impact of therapy on fertility arises.
Dose adjustment or modification of the treatment regimen can achieve a lot. But experts at the congress of the European Society for Medical Oncology (ESMO) 2024 noted that knowledge about newer treatment options like immunotherapies is still insufficient.
Therapy Selection
The question of preserving fertility must be considered when deciding on the appropriate treatment, said Matteo Lambertini, MD, PhD, medical oncology consultant at the University of Genoa in Genoa, Italy. “Preserving fertility is also an aim of cancer therapy,” he said.
Lambertini, who is also a member of the ESMO Guideline Group on fertility preservation in cancer patients, referred to the 2020 ESMO guidelines, which list the gonadotoxicity of a substance depending on the treatment regimen and the patient’s age.
Isabelle Demeestere, MD, PhD, director of the research lab for human reproduction at the Erasmus Hospital of the Free University of Brussels in Brussels, Belgium, pointed out the limitations of general guidelines. “Therapies change over time, and a classification must be updated regularly.”
Knowledge gaps related to well-known therapies and many novel options persist. “For many FDA-approved medications, there are either no fertility data or only preclinical data available,” she added.
Chemotherapies and Immunotherapies
Chemotherapies with alkylating or platinum-containing substances are known for their effects on oocytes, follicle maturation, and spermatogenesis, said Demeestere.
Chemotherapy is gonadotoxic and leads to a temporary decrease in sperm quality or temporary azoospermia in men.
These effects, however, can lead to permanent azoospermia and endocrine disorders, depending on the dose, duration, or combination with radiation, said Demeestere.
Cryopreservation of sperm should always be performed before starting treatment. For high-risk patients who are prepubertal, samples of testicular tissue are taken.
In women, chemotherapy affects primordial follicles and follicle maturation through DNA damage. This process results in severe or temporary amenorrhea, a temporary or permanent decrease in egg reserve, and ultimately premature egg insufficiency.
Novel immunotherapies also influence fertility, presumably through interactions of the immune system with the reproductive organs. But insufficient data are available, according to Lambertini, who emphasized that “these data are urgently needed, especially for young patients with cancer.”
In a mouse model, immune checkpoint inhibitors affected ovarian function, and the inflammatory reaction in humans can affect fertility. No long-term data are available for women yet, however, explained Demeestere. The effects of other therapeutics such as PARP, CDK4/6, or tyrosine kinase inhibitors, as well as monoclonal antibodies like trastuzumab, are only seen sporadically.
In the PENELOPE-B phase 3 study, the CDK4/6 inhibitor palbociclib did not affect ovarian function, even though the cyclin-dependent kinases play an important role in mitotic arrest, said Demeestere.
Adjusting the Regimen
In a PET-guided approach, Demeestere’s research team investigated the effects of dose reduction or adjustment of the treatment regimen of procarbazine and cyclophosphamide on the fertility of patients younger than 45 years with advanced Hodgkin lymphoma.
By regularly controlling tumor growth with PET, the treatment could be adjusted so that the effect on egg reserve or spermatogenesis was significantly reduced and loss of fertility could be prevented.
During the 5-year follow-up period, the ovarian function of participating women was assessed by the serum concentration of follicle-stimulating hormone (FSH), estradiol, and anti-Müllerian hormone (AMH) to evaluate egg reserve. In men, testicular function was assessed at the beginning of the study. At the end of treatment, sperm analysis and FSH and testosterone levels were checked.
Demeestere and colleagues demonstrated that dose reduction or altering the treatment regimen for patients who responded early to treatment (determined by PET-guided monitoring) reduced the risk for gonadotoxicity from 46% to 14.5%. That is, the risk was reduced by more than half.
FSH and AMH correlated with the patient’s age and the dose of the alkylating agent. In men, sperm parameters recovered after dose or agent adjustment compared with the unchanged treatment regimen.
Newer results from the PHERGain study in women with early human epidermal growth factor receptor 2–positive breast cancer also provided hope, according to Demeestere. Under PET-guided control, chemotherapy could be reduced.
More Data Needed
The new treatment options pose a challenge to preserving fertility during cancer treatment, said Demeestere.
For new targeted therapies, uniform recommendations cannot be issued because of the lack of data and varying treatment durations. Still, the new therapies are safer than chemotherapy.
The need to collect data on fertility and long-term effects in cancer survivors in clinical studies is also reflected in the literature, according to Demeestere. “There are more review articles on this topic than clinical studies.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Thanks to the continuously improving treatment options for cancer, the number of cancer survivors is increasing, and a large proportion of survivors is confronted with the long-term effects of cancer treatment. Especially for young patients, the question of the impact of therapy on fertility arises.
Dose adjustment or modification of the treatment regimen can achieve a lot. But experts at the congress of the European Society for Medical Oncology (ESMO) 2024 noted that knowledge about newer treatment options like immunotherapies is still insufficient.
Therapy Selection
The question of preserving fertility must be considered when deciding on the appropriate treatment, said Matteo Lambertini, MD, PhD, medical oncology consultant at the University of Genoa in Genoa, Italy. “Preserving fertility is also an aim of cancer therapy,” he said.
Lambertini, who is also a member of the ESMO Guideline Group on fertility preservation in cancer patients, referred to the 2020 ESMO guidelines, which list the gonadotoxicity of a substance depending on the treatment regimen and the patient’s age.
Isabelle Demeestere, MD, PhD, director of the research lab for human reproduction at the Erasmus Hospital of the Free University of Brussels in Brussels, Belgium, pointed out the limitations of general guidelines. “Therapies change over time, and a classification must be updated regularly.”
Knowledge gaps related to well-known therapies and many novel options persist. “For many FDA-approved medications, there are either no fertility data or only preclinical data available,” she added.
Chemotherapies and Immunotherapies
Chemotherapies with alkylating or platinum-containing substances are known for their effects on oocytes, follicle maturation, and spermatogenesis, said Demeestere.
Chemotherapy is gonadotoxic and leads to a temporary decrease in sperm quality or temporary azoospermia in men.
These effects, however, can lead to permanent azoospermia and endocrine disorders, depending on the dose, duration, or combination with radiation, said Demeestere.
Cryopreservation of sperm should always be performed before starting treatment. For high-risk patients who are prepubertal, samples of testicular tissue are taken.
In women, chemotherapy affects primordial follicles and follicle maturation through DNA damage. This process results in severe or temporary amenorrhea, a temporary or permanent decrease in egg reserve, and ultimately premature egg insufficiency.
Novel immunotherapies also influence fertility, presumably through interactions of the immune system with the reproductive organs. But insufficient data are available, according to Lambertini, who emphasized that “these data are urgently needed, especially for young patients with cancer.”
In a mouse model, immune checkpoint inhibitors affected ovarian function, and the inflammatory reaction in humans can affect fertility. No long-term data are available for women yet, however, explained Demeestere. The effects of other therapeutics such as PARP, CDK4/6, or tyrosine kinase inhibitors, as well as monoclonal antibodies like trastuzumab, are only seen sporadically.
In the PENELOPE-B phase 3 study, the CDK4/6 inhibitor palbociclib did not affect ovarian function, even though the cyclin-dependent kinases play an important role in mitotic arrest, said Demeestere.
Adjusting the Regimen
In a PET-guided approach, Demeestere’s research team investigated the effects of dose reduction or adjustment of the treatment regimen of procarbazine and cyclophosphamide on the fertility of patients younger than 45 years with advanced Hodgkin lymphoma.
By regularly controlling tumor growth with PET, the treatment could be adjusted so that the effect on egg reserve or spermatogenesis was significantly reduced and loss of fertility could be prevented.
During the 5-year follow-up period, the ovarian function of participating women was assessed by the serum concentration of follicle-stimulating hormone (FSH), estradiol, and anti-Müllerian hormone (AMH) to evaluate egg reserve. In men, testicular function was assessed at the beginning of the study. At the end of treatment, sperm analysis and FSH and testosterone levels were checked.
Demeestere and colleagues demonstrated that dose reduction or altering the treatment regimen for patients who responded early to treatment (determined by PET-guided monitoring) reduced the risk for gonadotoxicity from 46% to 14.5%. That is, the risk was reduced by more than half.
FSH and AMH correlated with the patient’s age and the dose of the alkylating agent. In men, sperm parameters recovered after dose or agent adjustment compared with the unchanged treatment regimen.
Newer results from the PHERGain study in women with early human epidermal growth factor receptor 2–positive breast cancer also provided hope, according to Demeestere. Under PET-guided control, chemotherapy could be reduced.
More Data Needed
The new treatment options pose a challenge to preserving fertility during cancer treatment, said Demeestere.
For new targeted therapies, uniform recommendations cannot be issued because of the lack of data and varying treatment durations. Still, the new therapies are safer than chemotherapy.
The need to collect data on fertility and long-term effects in cancer survivors in clinical studies is also reflected in the literature, according to Demeestere. “There are more review articles on this topic than clinical studies.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Thanks to the continuously improving treatment options for cancer, the number of cancer survivors is increasing, and a large proportion of survivors is confronted with the long-term effects of cancer treatment. Especially for young patients, the question of the impact of therapy on fertility arises.
Dose adjustment or modification of the treatment regimen can achieve a lot. But experts at the congress of the European Society for Medical Oncology (ESMO) 2024 noted that knowledge about newer treatment options like immunotherapies is still insufficient.
Therapy Selection
The question of preserving fertility must be considered when deciding on the appropriate treatment, said Matteo Lambertini, MD, PhD, medical oncology consultant at the University of Genoa in Genoa, Italy. “Preserving fertility is also an aim of cancer therapy,” he said.
Lambertini, who is also a member of the ESMO Guideline Group on fertility preservation in cancer patients, referred to the 2020 ESMO guidelines, which list the gonadotoxicity of a substance depending on the treatment regimen and the patient’s age.
Isabelle Demeestere, MD, PhD, director of the research lab for human reproduction at the Erasmus Hospital of the Free University of Brussels in Brussels, Belgium, pointed out the limitations of general guidelines. “Therapies change over time, and a classification must be updated regularly.”
Knowledge gaps related to well-known therapies and many novel options persist. “For many FDA-approved medications, there are either no fertility data or only preclinical data available,” she added.
Chemotherapies and Immunotherapies
Chemotherapies with alkylating or platinum-containing substances are known for their effects on oocytes, follicle maturation, and spermatogenesis, said Demeestere.
Chemotherapy is gonadotoxic and leads to a temporary decrease in sperm quality or temporary azoospermia in men.
These effects, however, can lead to permanent azoospermia and endocrine disorders, depending on the dose, duration, or combination with radiation, said Demeestere.
Cryopreservation of sperm should always be performed before starting treatment. For high-risk patients who are prepubertal, samples of testicular tissue are taken.
In women, chemotherapy affects primordial follicles and follicle maturation through DNA damage. This process results in severe or temporary amenorrhea, a temporary or permanent decrease in egg reserve, and ultimately premature egg insufficiency.
Novel immunotherapies also influence fertility, presumably through interactions of the immune system with the reproductive organs. But insufficient data are available, according to Lambertini, who emphasized that “these data are urgently needed, especially for young patients with cancer.”
In a mouse model, immune checkpoint inhibitors affected ovarian function, and the inflammatory reaction in humans can affect fertility. No long-term data are available for women yet, however, explained Demeestere. The effects of other therapeutics such as PARP, CDK4/6, or tyrosine kinase inhibitors, as well as monoclonal antibodies like trastuzumab, are only seen sporadically.
In the PENELOPE-B phase 3 study, the CDK4/6 inhibitor palbociclib did not affect ovarian function, even though the cyclin-dependent kinases play an important role in mitotic arrest, said Demeestere.
Adjusting the Regimen
In a PET-guided approach, Demeestere’s research team investigated the effects of dose reduction or adjustment of the treatment regimen of procarbazine and cyclophosphamide on the fertility of patients younger than 45 years with advanced Hodgkin lymphoma.
By regularly controlling tumor growth with PET, the treatment could be adjusted so that the effect on egg reserve or spermatogenesis was significantly reduced and loss of fertility could be prevented.
During the 5-year follow-up period, the ovarian function of participating women was assessed by the serum concentration of follicle-stimulating hormone (FSH), estradiol, and anti-Müllerian hormone (AMH) to evaluate egg reserve. In men, testicular function was assessed at the beginning of the study. At the end of treatment, sperm analysis and FSH and testosterone levels were checked.
Demeestere and colleagues demonstrated that dose reduction or altering the treatment regimen for patients who responded early to treatment (determined by PET-guided monitoring) reduced the risk for gonadotoxicity from 46% to 14.5%. That is, the risk was reduced by more than half.
FSH and AMH correlated with the patient’s age and the dose of the alkylating agent. In men, sperm parameters recovered after dose or agent adjustment compared with the unchanged treatment regimen.
Newer results from the PHERGain study in women with early human epidermal growth factor receptor 2–positive breast cancer also provided hope, according to Demeestere. Under PET-guided control, chemotherapy could be reduced.
More Data Needed
The new treatment options pose a challenge to preserving fertility during cancer treatment, said Demeestere.
For new targeted therapies, uniform recommendations cannot be issued because of the lack of data and varying treatment durations. Still, the new therapies are safer than chemotherapy.
The need to collect data on fertility and long-term effects in cancer survivors in clinical studies is also reflected in the literature, according to Demeestere. “There are more review articles on this topic than clinical studies.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.