User login
For MD-IQ use only
Infectious disease pop quiz: Clinical challenge #3 for the ObGyn
What are the major complications of pyelonephritis in pregnancy?
Continue to the answer...
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the major complications of pyelonephritis in pregnancy?
Continue to the answer...
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
What are the major complications of pyelonephritis in pregnancy?
Continue to the answer...
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
The third generation of therapeutic innovation and the future of psychopharmacology
The field of psychiatric therapeutics is now experiencing its third generation of progress. No sooner had the pace of innovation in psychiatry and psychopharmacology hit the doldrums a few years ago, following the dwindling of the second generation of progress, than the current third generation of new drug development in psychopharmacology was born.
That is, the first generation of discovery of psychiatric medications in the 1960s and 1970s ushered in the first known psychotropic drugs, such as the tricyclic antidepressants, as well as major and minor tranquilizers, such as chlorpromazine and benzodiazepines, only to fizzle out in the 1980s. By the 1990s, the second generation of innovation in psychopharmacology was in full swing, with the “new” serotonin selective reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors for depression, and the “atypical” antipsychotics for schizophrenia. However, soon after the turn of the century, pessimism for psychiatric therapeutics crept in again, and “big Pharma” abandoned their psychopharmacology programs in favor of other therapeutic areas. Surprisingly, the current “green shoots” of new ideas sprouting in our field today have not come from traditional big Pharma returning to psychiatry, but largely from small, innovative companies. These new entrepreneurial small pharmas and biotechs have found several new therapeutic targets. Furthermore, current innovation in psychopharmacology is increasingly following a paradigm shift away from DSM-5 disorders and instead to domains or symptoms of psychopathology that cut across numerous psychiatric conditions (transdiagnostic model).
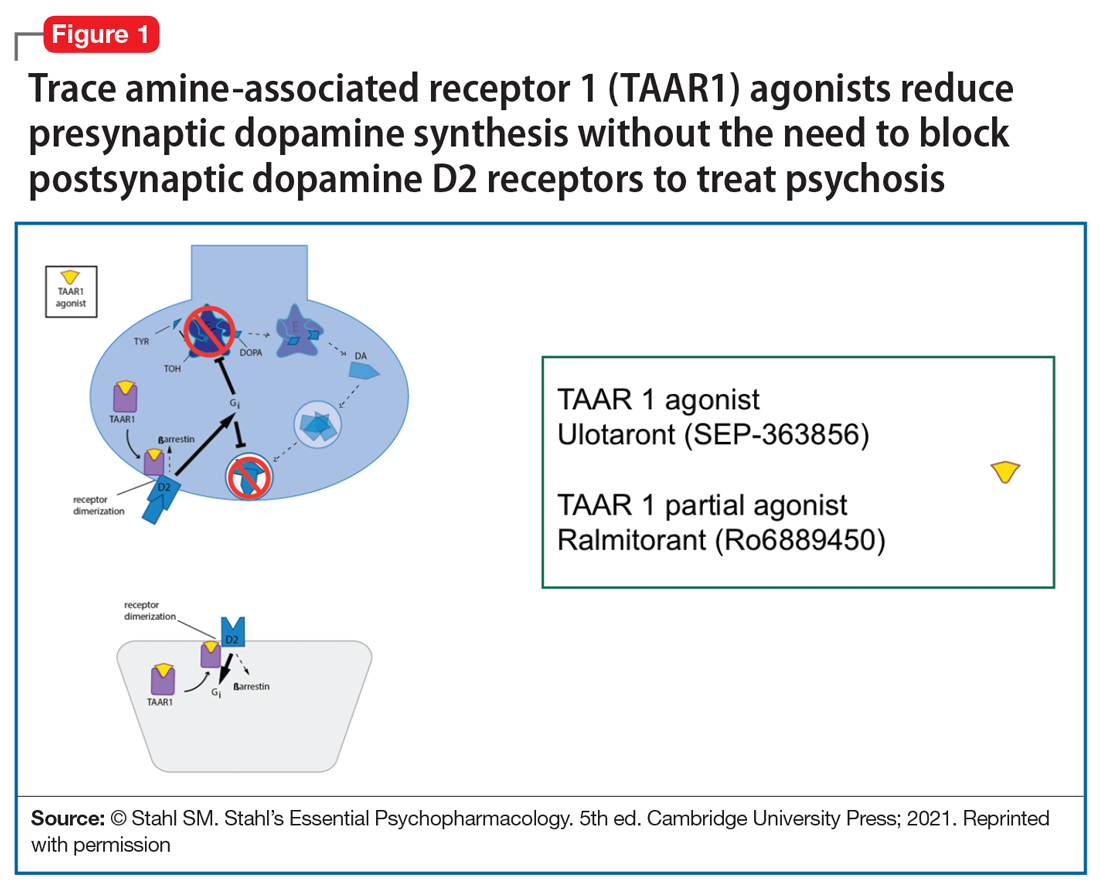
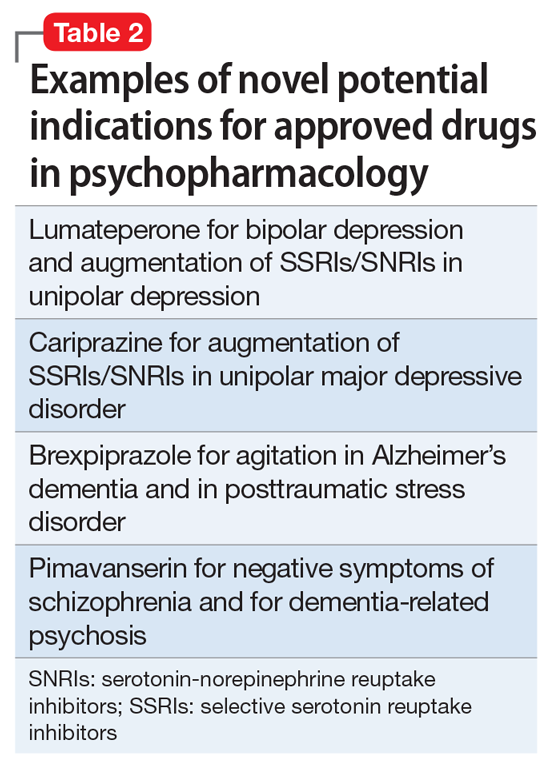
So, what are the new therapeutic mechanisms of this current third generation of innovation in psychopharmacology? Not all of these can be discussed here, but 2 examples of new approaches to psychosis deserve special mention because, for the first time in 70 years, they turn away from blocking postsynaptic dopamine D2 receptors to treat psychosis and instead stimulate receptors in other neurotransmitter systems that are linked to dopamine neurons in a network “upstream.” That is, trace amine-associated receptor 1 (TAAR1) agonists target the pre-synaptic dopamine neuron, where dopamine synthesis and release are too high in psychosis, and cause dopamine synthesis to be reduced so that blockade of postsynaptic dopamine receptors is no longer necessary (Table 1 and Figure 1).1 Similarly, muscarinic cholinergic 1 and 4 receptor agonists target excitatory cholinergic neurons upstream, and turn down their stimulation of dopamine neurons, thereby reducing dopamine release so that postsynaptic blockade of dopamine receptors is also not necessary to treat psychosis with this mechanism (Table 1 and Figure 2).1 A similar mechanism of reducing upstream stimulation of dopamine release by serotonin has led to demonstration of antipsychotic actions of blocking this stimulation at serotonin 2A receptors (Table 2), and multiple approaches to enhancing deficient glutamate actions upstream are also under investigation for the treatment of psychosis. 1
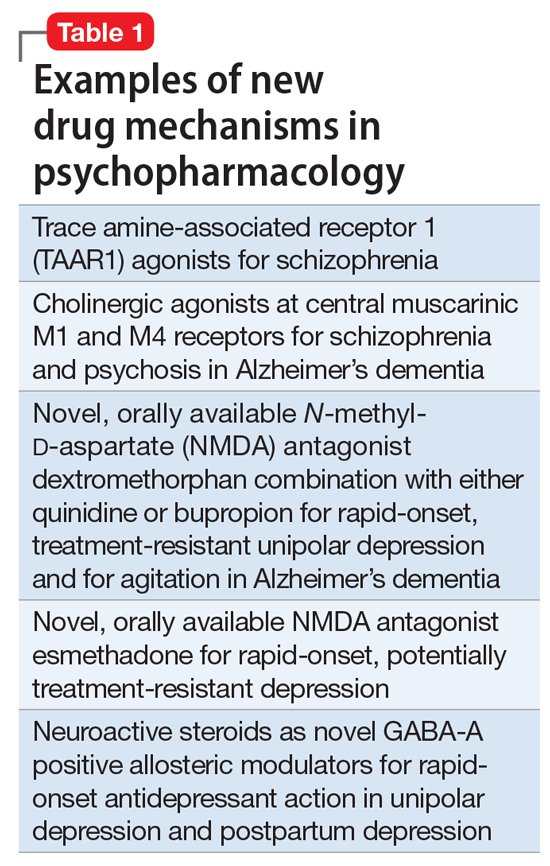

Another major area of innovation in psychopharmacology worthy of emphasis is the rapid induction of neurogenesis that is associated with rapid reduction in the symptoms of depression, even when many conventional treatments have failed. Blockade of N-methyl-
that may hypothetically drive rapid recovery from depression.1 Proof of this concept was first shown with intravenous ketamine, and then intranasal esketamine, and now the oral NMDA antagonists dextromethorphan (combined with either bupropion or quinidine) and esmethadone (Table 1).1 Interestingly, this same mechanism may lead to a novel treatment of agitation in Alzheimer’s dementia as well.1
Continue to: Yet another mechanism...
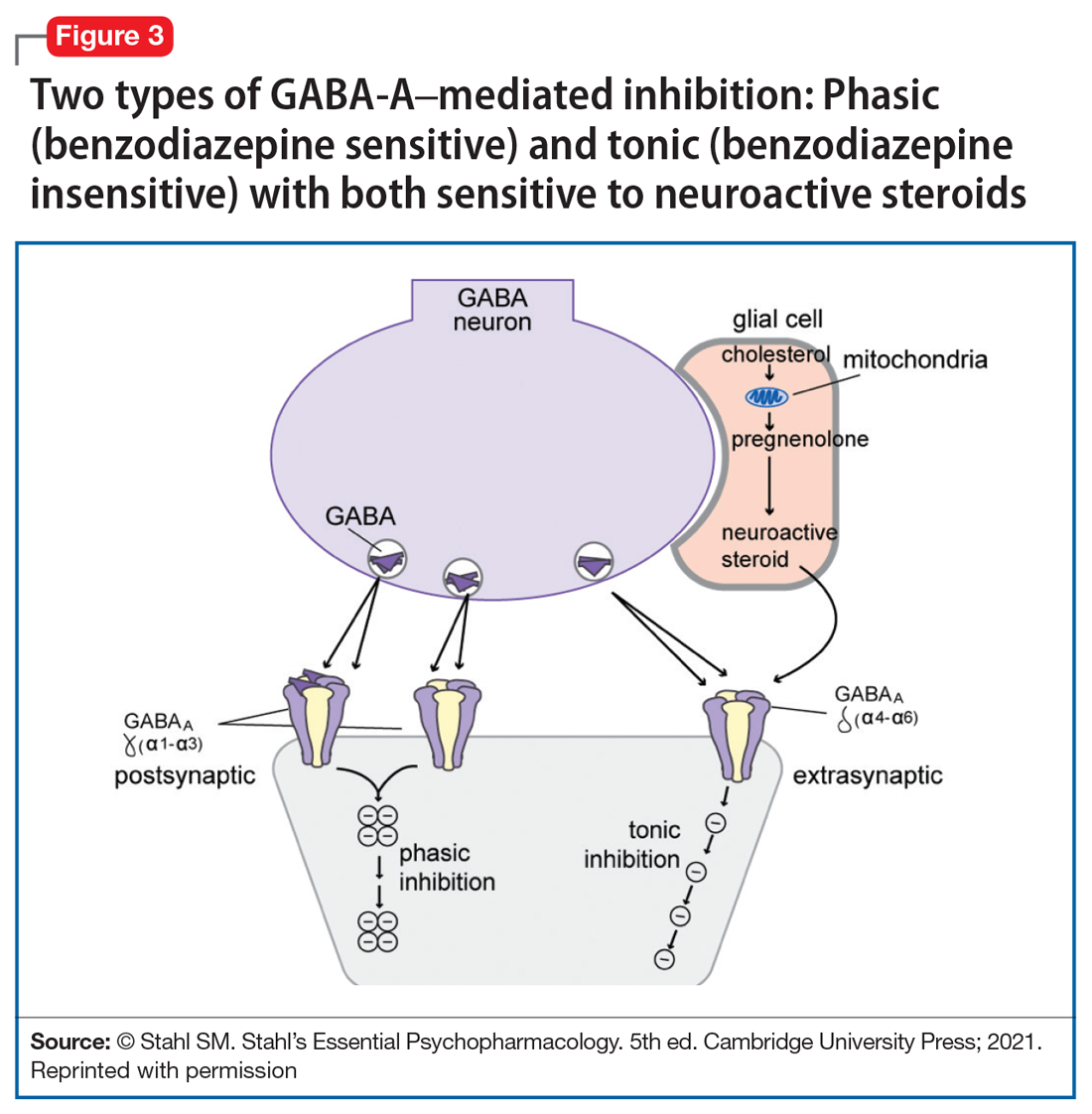
Yet another mechanism of potentially rapid onset antidepressant action is that of the novel agents known as neuroactive steroids that have a novel action at gamma aminobutyric acid A (GABA-A) receptors that are not sensitive to benzodiazepines (as well as those that are) (Table 1 and Figure 3).1 Finally, psychedelic drugs that target serotonin receptors such as psilocybin and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) seem to also have rapid onset of both neurogenesis and antidepressant action.
The future of psychopharmacology is clearly going to be amazing.
1. Stahl SM. Stahl’s Essential Psychopharmacology. 5th ed. Cambridge University Press; 2021.
The field of psychiatric therapeutics is now experiencing its third generation of progress. No sooner had the pace of innovation in psychiatry and psychopharmacology hit the doldrums a few years ago, following the dwindling of the second generation of progress, than the current third generation of new drug development in psychopharmacology was born.
That is, the first generation of discovery of psychiatric medications in the 1960s and 1970s ushered in the first known psychotropic drugs, such as the tricyclic antidepressants, as well as major and minor tranquilizers, such as chlorpromazine and benzodiazepines, only to fizzle out in the 1980s. By the 1990s, the second generation of innovation in psychopharmacology was in full swing, with the “new” serotonin selective reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors for depression, and the “atypical” antipsychotics for schizophrenia. However, soon after the turn of the century, pessimism for psychiatric therapeutics crept in again, and “big Pharma” abandoned their psychopharmacology programs in favor of other therapeutic areas. Surprisingly, the current “green shoots” of new ideas sprouting in our field today have not come from traditional big Pharma returning to psychiatry, but largely from small, innovative companies. These new entrepreneurial small pharmas and biotechs have found several new therapeutic targets. Furthermore, current innovation in psychopharmacology is increasingly following a paradigm shift away from DSM-5 disorders and instead to domains or symptoms of psychopathology that cut across numerous psychiatric conditions (transdiagnostic model).
So, what are the new therapeutic mechanisms of this current third generation of innovation in psychopharmacology? Not all of these can be discussed here, but 2 examples of new approaches to psychosis deserve special mention because, for the first time in 70 years, they turn away from blocking postsynaptic dopamine D2 receptors to treat psychosis and instead stimulate receptors in other neurotransmitter systems that are linked to dopamine neurons in a network “upstream.” That is, trace amine-associated receptor 1 (TAAR1) agonists target the pre-synaptic dopamine neuron, where dopamine synthesis and release are too high in psychosis, and cause dopamine synthesis to be reduced so that blockade of postsynaptic dopamine receptors is no longer necessary (Table 1 and Figure 1).1 Similarly, muscarinic cholinergic 1 and 4 receptor agonists target excitatory cholinergic neurons upstream, and turn down their stimulation of dopamine neurons, thereby reducing dopamine release so that postsynaptic blockade of dopamine receptors is also not necessary to treat psychosis with this mechanism (Table 1 and Figure 2).1 A similar mechanism of reducing upstream stimulation of dopamine release by serotonin has led to demonstration of antipsychotic actions of blocking this stimulation at serotonin 2A receptors (Table 2), and multiple approaches to enhancing deficient glutamate actions upstream are also under investigation for the treatment of psychosis. 1



Another major area of innovation in psychopharmacology worthy of emphasis is the rapid induction of neurogenesis that is associated with rapid reduction in the symptoms of depression, even when many conventional treatments have failed. Blockade of N-methyl-
that may hypothetically drive rapid recovery from depression.1 Proof of this concept was first shown with intravenous ketamine, and then intranasal esketamine, and now the oral NMDA antagonists dextromethorphan (combined with either bupropion or quinidine) and esmethadone (Table 1).1 Interestingly, this same mechanism may lead to a novel treatment of agitation in Alzheimer’s dementia as well.1
Continue to: Yet another mechanism...
Yet another mechanism of potentially rapid onset antidepressant action is that of the novel agents known as neuroactive steroids that have a novel action at gamma aminobutyric acid A (GABA-A) receptors that are not sensitive to benzodiazepines (as well as those that are) (Table 1 and Figure 3).1 Finally, psychedelic drugs that target serotonin receptors such as psilocybin and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) seem to also have rapid onset of both neurogenesis and antidepressant action.


The future of psychopharmacology is clearly going to be amazing.
The field of psychiatric therapeutics is now experiencing its third generation of progress. No sooner had the pace of innovation in psychiatry and psychopharmacology hit the doldrums a few years ago, following the dwindling of the second generation of progress, than the current third generation of new drug development in psychopharmacology was born.
That is, the first generation of discovery of psychiatric medications in the 1960s and 1970s ushered in the first known psychotropic drugs, such as the tricyclic antidepressants, as well as major and minor tranquilizers, such as chlorpromazine and benzodiazepines, only to fizzle out in the 1980s. By the 1990s, the second generation of innovation in psychopharmacology was in full swing, with the “new” serotonin selective reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors for depression, and the “atypical” antipsychotics for schizophrenia. However, soon after the turn of the century, pessimism for psychiatric therapeutics crept in again, and “big Pharma” abandoned their psychopharmacology programs in favor of other therapeutic areas. Surprisingly, the current “green shoots” of new ideas sprouting in our field today have not come from traditional big Pharma returning to psychiatry, but largely from small, innovative companies. These new entrepreneurial small pharmas and biotechs have found several new therapeutic targets. Furthermore, current innovation in psychopharmacology is increasingly following a paradigm shift away from DSM-5 disorders and instead to domains or symptoms of psychopathology that cut across numerous psychiatric conditions (transdiagnostic model).
So, what are the new therapeutic mechanisms of this current third generation of innovation in psychopharmacology? Not all of these can be discussed here, but 2 examples of new approaches to psychosis deserve special mention because, for the first time in 70 years, they turn away from blocking postsynaptic dopamine D2 receptors to treat psychosis and instead stimulate receptors in other neurotransmitter systems that are linked to dopamine neurons in a network “upstream.” That is, trace amine-associated receptor 1 (TAAR1) agonists target the pre-synaptic dopamine neuron, where dopamine synthesis and release are too high in psychosis, and cause dopamine synthesis to be reduced so that blockade of postsynaptic dopamine receptors is no longer necessary (Table 1 and Figure 1).1 Similarly, muscarinic cholinergic 1 and 4 receptor agonists target excitatory cholinergic neurons upstream, and turn down their stimulation of dopamine neurons, thereby reducing dopamine release so that postsynaptic blockade of dopamine receptors is also not necessary to treat psychosis with this mechanism (Table 1 and Figure 2).1 A similar mechanism of reducing upstream stimulation of dopamine release by serotonin has led to demonstration of antipsychotic actions of blocking this stimulation at serotonin 2A receptors (Table 2), and multiple approaches to enhancing deficient glutamate actions upstream are also under investigation for the treatment of psychosis. 1



Another major area of innovation in psychopharmacology worthy of emphasis is the rapid induction of neurogenesis that is associated with rapid reduction in the symptoms of depression, even when many conventional treatments have failed. Blockade of N-methyl-
that may hypothetically drive rapid recovery from depression.1 Proof of this concept was first shown with intravenous ketamine, and then intranasal esketamine, and now the oral NMDA antagonists dextromethorphan (combined with either bupropion or quinidine) and esmethadone (Table 1).1 Interestingly, this same mechanism may lead to a novel treatment of agitation in Alzheimer’s dementia as well.1
Continue to: Yet another mechanism...
Yet another mechanism of potentially rapid onset antidepressant action is that of the novel agents known as neuroactive steroids that have a novel action at gamma aminobutyric acid A (GABA-A) receptors that are not sensitive to benzodiazepines (as well as those that are) (Table 1 and Figure 3).1 Finally, psychedelic drugs that target serotonin receptors such as psilocybin and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) seem to also have rapid onset of both neurogenesis and antidepressant action.


The future of psychopharmacology is clearly going to be amazing.
1. Stahl SM. Stahl’s Essential Psychopharmacology. 5th ed. Cambridge University Press; 2021.
1. Stahl SM. Stahl’s Essential Psychopharmacology. 5th ed. Cambridge University Press; 2021.
Did prior authorization refusals lead to this patient’s death?
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Does vitamin D benefit only those who are deficient?
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
FDA approves first drug for treatment of resistant cytomegalovirus infection
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
Malpractice case: What really killed this patient? Experts disagree
A patient with many comorbidities undergoing surgery presents a number of challenges to the healthcare team. This case highlights why solid preparation for the pre-and post-op care of such patients is so important.
A 56-year-old morbidly obese man with a history of hypertension, diabetes, sleep apnea, and elevated cholesterol presented to an ambulatory surgery center for knee arthroscopy. Following a brief pre-op assessment, his airway was rated a III using both the American Society of Anesthesiologists (ASA) and Mallampati classification systems. It was decided to use a laryngeal mask airway (LMA) with 100 µg of fentanyl and 2 mgmidazolam, followed by inhalation anesthesia.
After the procedure, the LMA was removed and the patient was moved to the post-anesthesia care unit (PACU). The patient was unresponsive for about 20 minutes and exhibited signs of respiratory distress. Efforts were made to open the airway with jaw thrusts and nasal trumpet. The anesthesiologist determined that the patient was suffering from congestive heart failure, aspiration, or pulmonary edema.
The anesthesiologist administered 40 µg of naloxone. The patient began to awaken but had oxygen saturation readings in the high 70s. The patient was encouraged to take slow, deep breaths. Rhonchi were heard, and the patient complained of shortness of breath. The ECG reading was unchanged from the pre-op test.
Thirty minutes after the first dose, a second dose of 40 µg naloxone was administered with no improvement. Oxygen saturation remained between 79% and 88%. Albuterol was given with little effect. The patient’s respiration rate was 44.
The patient was reintubated. Copious pink, frothy fluid was suctioned from the endotracheal tube. The patient received propofol, urosemide, and paralytic agents with the code team present to assist. The patient’s heart rate continued to decline to about 45 beats/min. The patient was transferred to a hospital emergency department.
Upon arrival in the emergency department, the patient was in asystolic arrest. Attempts to place a transvenous pacer were unsuccessful. The nasogastric tube returned 400 cc of brown coffee-grounds gastric fluid. After 30 minutes of CPR, the patient was pronounced dead.
The autopsy report noted no apparent airway obstruction, so the pathologist determined that the cause of death was flash pulmonary edema. Negative pressure pulmonary edema is a form of flash pulmonary edema caused by forceful inspiratory efforts made against a blocked airway. Toxic levels of ropivacaine were found in the patient’s blood. The pathologist noted hypertrophic cardiomyopathy and a grossly enlarged heart.
The patient’s family filed a claim after his death. The plaintiffs argued that the LMA was removed too soon for a patient with sleep apnea and a class III Mallampati score. They raised questions about the high levels of ropivacaine and wondered whether it contributed to bradycardia. They claimed that the reintubation took too long, resulting in high end-tidal CO2. They also noted inconsistent documentation between PACU nurses and the anesthesiologist.
Some defense experts were supportive of the care, stating that the cause of death was probably from a fatal arrhythmia due to hypotension and an enlarged heart. The defense experts questioned whether undiagnosed pulmonary hypertension would explain the failure to respond to furosemide. It was noted that both of the patient’s parents had died suddenly following surgeries. The assumed cause of their deaths was coronary artery disease. This case settled.
How the claim may have been prevented: Dr. Feldman’s tips
Prevent adverse events by managing clinical decisions based on the individual patient’s needs. The history of sleep apnea and a rating of a Mallampati class III airway in this ASA III patient indicated a high risk for a difficult intubation. Consideration should have been given to performing the procedure in a hospital rather than in an ambulatory surgery center. The overall goal is to maintain a secure airway until the patient is able to maintain it on their own.
Preclude malpractice claims by having good communication with patients. Unfortunately, anesthesiologists don’t typically have an opportunity to develop a relationship with patients, but for patients at high risk, like this one, mandatory visits or calls to an anesthesiology-run pre-op clinic or ambulatory surgery center would give the anesthesiologist the opportunity to have a lengthy and informative discussion about risks, benefits, and alternatives. In addition, it would give the anesthesiologist time to discuss risks with both the surgeon and the patient.
Prevail in lawsuits by fully documenting the preoperative anesthesia assessment. There were questions about inconsistencies in documentation between the PACU nurses and anesthesiologists. Frequent huddles between the PACU staff (including nurses and physicians) may lead not only to more coordinated care but also to more consistent documentation, which will show that the care team acted together in caring for the patient.
A version of this article first appeared on Medscape.com.
A patient with many comorbidities undergoing surgery presents a number of challenges to the healthcare team. This case highlights why solid preparation for the pre-and post-op care of such patients is so important.
A 56-year-old morbidly obese man with a history of hypertension, diabetes, sleep apnea, and elevated cholesterol presented to an ambulatory surgery center for knee arthroscopy. Following a brief pre-op assessment, his airway was rated a III using both the American Society of Anesthesiologists (ASA) and Mallampati classification systems. It was decided to use a laryngeal mask airway (LMA) with 100 µg of fentanyl and 2 mgmidazolam, followed by inhalation anesthesia.
After the procedure, the LMA was removed and the patient was moved to the post-anesthesia care unit (PACU). The patient was unresponsive for about 20 minutes and exhibited signs of respiratory distress. Efforts were made to open the airway with jaw thrusts and nasal trumpet. The anesthesiologist determined that the patient was suffering from congestive heart failure, aspiration, or pulmonary edema.
The anesthesiologist administered 40 µg of naloxone. The patient began to awaken but had oxygen saturation readings in the high 70s. The patient was encouraged to take slow, deep breaths. Rhonchi were heard, and the patient complained of shortness of breath. The ECG reading was unchanged from the pre-op test.
Thirty minutes after the first dose, a second dose of 40 µg naloxone was administered with no improvement. Oxygen saturation remained between 79% and 88%. Albuterol was given with little effect. The patient’s respiration rate was 44.
The patient was reintubated. Copious pink, frothy fluid was suctioned from the endotracheal tube. The patient received propofol, urosemide, and paralytic agents with the code team present to assist. The patient’s heart rate continued to decline to about 45 beats/min. The patient was transferred to a hospital emergency department.
Upon arrival in the emergency department, the patient was in asystolic arrest. Attempts to place a transvenous pacer were unsuccessful. The nasogastric tube returned 400 cc of brown coffee-grounds gastric fluid. After 30 minutes of CPR, the patient was pronounced dead.
The autopsy report noted no apparent airway obstruction, so the pathologist determined that the cause of death was flash pulmonary edema. Negative pressure pulmonary edema is a form of flash pulmonary edema caused by forceful inspiratory efforts made against a blocked airway. Toxic levels of ropivacaine were found in the patient’s blood. The pathologist noted hypertrophic cardiomyopathy and a grossly enlarged heart.
The patient’s family filed a claim after his death. The plaintiffs argued that the LMA was removed too soon for a patient with sleep apnea and a class III Mallampati score. They raised questions about the high levels of ropivacaine and wondered whether it contributed to bradycardia. They claimed that the reintubation took too long, resulting in high end-tidal CO2. They also noted inconsistent documentation between PACU nurses and the anesthesiologist.
Some defense experts were supportive of the care, stating that the cause of death was probably from a fatal arrhythmia due to hypotension and an enlarged heart. The defense experts questioned whether undiagnosed pulmonary hypertension would explain the failure to respond to furosemide. It was noted that both of the patient’s parents had died suddenly following surgeries. The assumed cause of their deaths was coronary artery disease. This case settled.
How the claim may have been prevented: Dr. Feldman’s tips
Prevent adverse events by managing clinical decisions based on the individual patient’s needs. The history of sleep apnea and a rating of a Mallampati class III airway in this ASA III patient indicated a high risk for a difficult intubation. Consideration should have been given to performing the procedure in a hospital rather than in an ambulatory surgery center. The overall goal is to maintain a secure airway until the patient is able to maintain it on their own.
Preclude malpractice claims by having good communication with patients. Unfortunately, anesthesiologists don’t typically have an opportunity to develop a relationship with patients, but for patients at high risk, like this one, mandatory visits or calls to an anesthesiology-run pre-op clinic or ambulatory surgery center would give the anesthesiologist the opportunity to have a lengthy and informative discussion about risks, benefits, and alternatives. In addition, it would give the anesthesiologist time to discuss risks with both the surgeon and the patient.
Prevail in lawsuits by fully documenting the preoperative anesthesia assessment. There were questions about inconsistencies in documentation between the PACU nurses and anesthesiologists. Frequent huddles between the PACU staff (including nurses and physicians) may lead not only to more coordinated care but also to more consistent documentation, which will show that the care team acted together in caring for the patient.
A version of this article first appeared on Medscape.com.
A patient with many comorbidities undergoing surgery presents a number of challenges to the healthcare team. This case highlights why solid preparation for the pre-and post-op care of such patients is so important.
A 56-year-old morbidly obese man with a history of hypertension, diabetes, sleep apnea, and elevated cholesterol presented to an ambulatory surgery center for knee arthroscopy. Following a brief pre-op assessment, his airway was rated a III using both the American Society of Anesthesiologists (ASA) and Mallampati classification systems. It was decided to use a laryngeal mask airway (LMA) with 100 µg of fentanyl and 2 mgmidazolam, followed by inhalation anesthesia.
After the procedure, the LMA was removed and the patient was moved to the post-anesthesia care unit (PACU). The patient was unresponsive for about 20 minutes and exhibited signs of respiratory distress. Efforts were made to open the airway with jaw thrusts and nasal trumpet. The anesthesiologist determined that the patient was suffering from congestive heart failure, aspiration, or pulmonary edema.
The anesthesiologist administered 40 µg of naloxone. The patient began to awaken but had oxygen saturation readings in the high 70s. The patient was encouraged to take slow, deep breaths. Rhonchi were heard, and the patient complained of shortness of breath. The ECG reading was unchanged from the pre-op test.
Thirty minutes after the first dose, a second dose of 40 µg naloxone was administered with no improvement. Oxygen saturation remained between 79% and 88%. Albuterol was given with little effect. The patient’s respiration rate was 44.
The patient was reintubated. Copious pink, frothy fluid was suctioned from the endotracheal tube. The patient received propofol, urosemide, and paralytic agents with the code team present to assist. The patient’s heart rate continued to decline to about 45 beats/min. The patient was transferred to a hospital emergency department.
Upon arrival in the emergency department, the patient was in asystolic arrest. Attempts to place a transvenous pacer were unsuccessful. The nasogastric tube returned 400 cc of brown coffee-grounds gastric fluid. After 30 minutes of CPR, the patient was pronounced dead.
The autopsy report noted no apparent airway obstruction, so the pathologist determined that the cause of death was flash pulmonary edema. Negative pressure pulmonary edema is a form of flash pulmonary edema caused by forceful inspiratory efforts made against a blocked airway. Toxic levels of ropivacaine were found in the patient’s blood. The pathologist noted hypertrophic cardiomyopathy and a grossly enlarged heart.
The patient’s family filed a claim after his death. The plaintiffs argued that the LMA was removed too soon for a patient with sleep apnea and a class III Mallampati score. They raised questions about the high levels of ropivacaine and wondered whether it contributed to bradycardia. They claimed that the reintubation took too long, resulting in high end-tidal CO2. They also noted inconsistent documentation between PACU nurses and the anesthesiologist.
Some defense experts were supportive of the care, stating that the cause of death was probably from a fatal arrhythmia due to hypotension and an enlarged heart. The defense experts questioned whether undiagnosed pulmonary hypertension would explain the failure to respond to furosemide. It was noted that both of the patient’s parents had died suddenly following surgeries. The assumed cause of their deaths was coronary artery disease. This case settled.
How the claim may have been prevented: Dr. Feldman’s tips
Prevent adverse events by managing clinical decisions based on the individual patient’s needs. The history of sleep apnea and a rating of a Mallampati class III airway in this ASA III patient indicated a high risk for a difficult intubation. Consideration should have been given to performing the procedure in a hospital rather than in an ambulatory surgery center. The overall goal is to maintain a secure airway until the patient is able to maintain it on their own.
Preclude malpractice claims by having good communication with patients. Unfortunately, anesthesiologists don’t typically have an opportunity to develop a relationship with patients, but for patients at high risk, like this one, mandatory visits or calls to an anesthesiology-run pre-op clinic or ambulatory surgery center would give the anesthesiologist the opportunity to have a lengthy and informative discussion about risks, benefits, and alternatives. In addition, it would give the anesthesiologist time to discuss risks with both the surgeon and the patient.
Prevail in lawsuits by fully documenting the preoperative anesthesia assessment. There were questions about inconsistencies in documentation between the PACU nurses and anesthesiologists. Frequent huddles between the PACU staff (including nurses and physicians) may lead not only to more coordinated care but also to more consistent documentation, which will show that the care team acted together in caring for the patient.
A version of this article first appeared on Medscape.com.
Vaping: Understand the risks
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
Certain opioids hold promise for treating itch
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Psychiatrist’s killer gets life in prison
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
TikTok trends: Scalp popping, EpiPen tutorial, and plant juice
With the holidays just around the corner (how did that happen?), it’s a good time to remind yourself of the things you’re grateful for.
Perhaps you’re grateful for spending chilly evenings under a warm blanket binge-watching your favorite shows or being able to safely gather with loved ones. If you’re William Shatner, maybe you’re grateful for that quick trip to space (because apparently, that’s a thing now) and the poetic tweets it induced. Down here on earth, TikTok has surpassed 1 billion users, and while we’re not grateful, necessarily, we are entertained.
Here are the latest ugly, good, and bad TikToks that have been trending lately.
The Ugly: Scalp popping
Warning: Don’t watch this if you’re easily freaked out by weird body sounds. It’s like cracking your knuckles but way, way worse.
This TikTok from @asmr.barber has 1.7 million likes, and lots of people are trying it out for themselves. The viral video features the (disturbed) art of scalp popping, also known as hair cracking. It features what is assumed to be some sort of barber or professional (here’s hoping) twisting a client’s hair around his fingers and then yanking, creating an audible popping sound. Many are posting their own hair-cracking attempts on the platform. It’s unclear if this is supposed to feel good or just be grossly satisfying, though some users claim it helps with migraines.
But it turns out this might be more than kind of gross; it can be dangerous, too.
Anthony Youn, MD, a board-certified plastic surgeon, comments on the trend with concern: “What the hell is going on here?” Not something you want to hear from a doctor. Dr. Youn explained that the popping sound comes from the galea aponeurotica, a fibrous sheet of connective tissue under your scalp, being pulled off the skull.
In a comment, Dr. Youn continued to warn people of replicating this trend: “It can tear the inside of the scalp, which can bleed a ton on the inside. Think boxer or MMA fighter with scalp hematoma.”
Let’s keep our scalps attached to our skulls, people. If I never have to hear that sound again, I’ll be eternally grateful.
The Good: Doctor demonstrates correct EpiPen use
This reaction TikTok from medical student Mutahir Farhan (aka @madmedicine) has over 252,000 likes and hundreds of comments. In it, Ms. Farhan watches a video of a young woman attempting to administer an EpiPen to her friend, with the caption “How NOT to use an EpiPen” over it (in bright red, of course).
The woman in the video is using the wrong end of the EpiPen against her friend’s leg, so it isn’t working. When she uses her thumb to press down and help, her thumb is actually pressed against the needle end and the EpiPen sticks her instead of her friend. Ouch!
Ms. Farhan goes on to explain the anatomy of the EpiPen and shows his audience of 1.1 million followers where to inject it.
“You gotta remember that the orange tip is where the needle comes out. Otherwise, you’re going to end up stabbing yourself with epinephrine, like that girl in the video,” Ms. Farhan says. He goes on to instruct the important, but often overlooked, follow-up: “After you stab someone with epinephrine, call 911 or go to the ER, so that we can make sure they’re actually okay and good to go.”
The Bad: Liquid chlorophyll
Here is another one of those tricky trends that are so widespread and popular that it’s hard to find exactly where it originated from. A video from @lenamaiah has over 5 million views and 800,000 likes, which even by TikTok standards, is a lot. TikTok is rife with similar videos, which feature drops of liquid chlorophyll being added to water and smoothies.
The pretty emerald hue is mesmerizing and it’s hard to resist trying it out when it’s being peddled by seemingly every pretty, smooth-skinned pseudo-model on the platform. In this video, Lena says drinking a glass of water with a few drops of chlorophyll can reduce inflammation, get rid of eye bags, boost your vitamin levels, reduce free radical damage, detoxify your system, and file your taxes. Okay, I made that last one up, but it follows, doesn’t it? This stuff sounds pretty good. Maybe too good.
Chlorophyll, if you skipped biology class (somehow, I doubt you did), is what makes plants green. Medscape has a detailed explanation of chlorophyll, but all you really need to know is that it’s the secret to that cool thing plants do: photosynthesis, or turning sunlight into energy. Scientists have been trying to find uses for it in people since the 1940s. Unfortunately, studies never found much that it can do for us, aside from being kind of deodorizing. So, while it’s been historically marketed as toothpaste and deodorant, the new TikTok claims of it being a cure-all or the next big skincare supplement are not widely substantiated by scientific studies. The only real evidence of it being effective is word of mouth from those who claim to like the way they look or feel since taking it, which isn’t enough for doctors to recommend it.
TikTok’s resident dermatologist, Muneeb Shah, DO, stitched a TikTok from another user, with his captions explaining, “[There’s] no scientific evidence for liquid chlorophyll [helping] rosacea or acne.”
His advice: “Chlorophyll is great, but just eat more veggies.”
A version of this article first appeared on Medscape.com.
With the holidays just around the corner (how did that happen?), it’s a good time to remind yourself of the things you’re grateful for.
Perhaps you’re grateful for spending chilly evenings under a warm blanket binge-watching your favorite shows or being able to safely gather with loved ones. If you’re William Shatner, maybe you’re grateful for that quick trip to space (because apparently, that’s a thing now) and the poetic tweets it induced. Down here on earth, TikTok has surpassed 1 billion users, and while we’re not grateful, necessarily, we are entertained.
Here are the latest ugly, good, and bad TikToks that have been trending lately.
The Ugly: Scalp popping
Warning: Don’t watch this if you’re easily freaked out by weird body sounds. It’s like cracking your knuckles but way, way worse.
This TikTok from @asmr.barber has 1.7 million likes, and lots of people are trying it out for themselves. The viral video features the (disturbed) art of scalp popping, also known as hair cracking. It features what is assumed to be some sort of barber or professional (here’s hoping) twisting a client’s hair around his fingers and then yanking, creating an audible popping sound. Many are posting their own hair-cracking attempts on the platform. It’s unclear if this is supposed to feel good or just be grossly satisfying, though some users claim it helps with migraines.
But it turns out this might be more than kind of gross; it can be dangerous, too.
Anthony Youn, MD, a board-certified plastic surgeon, comments on the trend with concern: “What the hell is going on here?” Not something you want to hear from a doctor. Dr. Youn explained that the popping sound comes from the galea aponeurotica, a fibrous sheet of connective tissue under your scalp, being pulled off the skull.
In a comment, Dr. Youn continued to warn people of replicating this trend: “It can tear the inside of the scalp, which can bleed a ton on the inside. Think boxer or MMA fighter with scalp hematoma.”
Let’s keep our scalps attached to our skulls, people. If I never have to hear that sound again, I’ll be eternally grateful.
The Good: Doctor demonstrates correct EpiPen use
This reaction TikTok from medical student Mutahir Farhan (aka @madmedicine) has over 252,000 likes and hundreds of comments. In it, Ms. Farhan watches a video of a young woman attempting to administer an EpiPen to her friend, with the caption “How NOT to use an EpiPen” over it (in bright red, of course).
The woman in the video is using the wrong end of the EpiPen against her friend’s leg, so it isn’t working. When she uses her thumb to press down and help, her thumb is actually pressed against the needle end and the EpiPen sticks her instead of her friend. Ouch!
Ms. Farhan goes on to explain the anatomy of the EpiPen and shows his audience of 1.1 million followers where to inject it.
“You gotta remember that the orange tip is where the needle comes out. Otherwise, you’re going to end up stabbing yourself with epinephrine, like that girl in the video,” Ms. Farhan says. He goes on to instruct the important, but often overlooked, follow-up: “After you stab someone with epinephrine, call 911 or go to the ER, so that we can make sure they’re actually okay and good to go.”
The Bad: Liquid chlorophyll
Here is another one of those tricky trends that are so widespread and popular that it’s hard to find exactly where it originated from. A video from @lenamaiah has over 5 million views and 800,000 likes, which even by TikTok standards, is a lot. TikTok is rife with similar videos, which feature drops of liquid chlorophyll being added to water and smoothies.
The pretty emerald hue is mesmerizing and it’s hard to resist trying it out when it’s being peddled by seemingly every pretty, smooth-skinned pseudo-model on the platform. In this video, Lena says drinking a glass of water with a few drops of chlorophyll can reduce inflammation, get rid of eye bags, boost your vitamin levels, reduce free radical damage, detoxify your system, and file your taxes. Okay, I made that last one up, but it follows, doesn’t it? This stuff sounds pretty good. Maybe too good.
Chlorophyll, if you skipped biology class (somehow, I doubt you did), is what makes plants green. Medscape has a detailed explanation of chlorophyll, but all you really need to know is that it’s the secret to that cool thing plants do: photosynthesis, or turning sunlight into energy. Scientists have been trying to find uses for it in people since the 1940s. Unfortunately, studies never found much that it can do for us, aside from being kind of deodorizing. So, while it’s been historically marketed as toothpaste and deodorant, the new TikTok claims of it being a cure-all or the next big skincare supplement are not widely substantiated by scientific studies. The only real evidence of it being effective is word of mouth from those who claim to like the way they look or feel since taking it, which isn’t enough for doctors to recommend it.
TikTok’s resident dermatologist, Muneeb Shah, DO, stitched a TikTok from another user, with his captions explaining, “[There’s] no scientific evidence for liquid chlorophyll [helping] rosacea or acne.”
His advice: “Chlorophyll is great, but just eat more veggies.”
A version of this article first appeared on Medscape.com.
With the holidays just around the corner (how did that happen?), it’s a good time to remind yourself of the things you’re grateful for.
Perhaps you’re grateful for spending chilly evenings under a warm blanket binge-watching your favorite shows or being able to safely gather with loved ones. If you’re William Shatner, maybe you’re grateful for that quick trip to space (because apparently, that’s a thing now) and the poetic tweets it induced. Down here on earth, TikTok has surpassed 1 billion users, and while we’re not grateful, necessarily, we are entertained.
Here are the latest ugly, good, and bad TikToks that have been trending lately.
The Ugly: Scalp popping
Warning: Don’t watch this if you’re easily freaked out by weird body sounds. It’s like cracking your knuckles but way, way worse.
This TikTok from @asmr.barber has 1.7 million likes, and lots of people are trying it out for themselves. The viral video features the (disturbed) art of scalp popping, also known as hair cracking. It features what is assumed to be some sort of barber or professional (here’s hoping) twisting a client’s hair around his fingers and then yanking, creating an audible popping sound. Many are posting their own hair-cracking attempts on the platform. It’s unclear if this is supposed to feel good or just be grossly satisfying, though some users claim it helps with migraines.
But it turns out this might be more than kind of gross; it can be dangerous, too.
Anthony Youn, MD, a board-certified plastic surgeon, comments on the trend with concern: “What the hell is going on here?” Not something you want to hear from a doctor. Dr. Youn explained that the popping sound comes from the galea aponeurotica, a fibrous sheet of connective tissue under your scalp, being pulled off the skull.
In a comment, Dr. Youn continued to warn people of replicating this trend: “It can tear the inside of the scalp, which can bleed a ton on the inside. Think boxer or MMA fighter with scalp hematoma.”
Let’s keep our scalps attached to our skulls, people. If I never have to hear that sound again, I’ll be eternally grateful.
The Good: Doctor demonstrates correct EpiPen use
This reaction TikTok from medical student Mutahir Farhan (aka @madmedicine) has over 252,000 likes and hundreds of comments. In it, Ms. Farhan watches a video of a young woman attempting to administer an EpiPen to her friend, with the caption “How NOT to use an EpiPen” over it (in bright red, of course).
The woman in the video is using the wrong end of the EpiPen against her friend’s leg, so it isn’t working. When she uses her thumb to press down and help, her thumb is actually pressed against the needle end and the EpiPen sticks her instead of her friend. Ouch!
Ms. Farhan goes on to explain the anatomy of the EpiPen and shows his audience of 1.1 million followers where to inject it.
“You gotta remember that the orange tip is where the needle comes out. Otherwise, you’re going to end up stabbing yourself with epinephrine, like that girl in the video,” Ms. Farhan says. He goes on to instruct the important, but often overlooked, follow-up: “After you stab someone with epinephrine, call 911 or go to the ER, so that we can make sure they’re actually okay and good to go.”
The Bad: Liquid chlorophyll
Here is another one of those tricky trends that are so widespread and popular that it’s hard to find exactly where it originated from. A video from @lenamaiah has over 5 million views and 800,000 likes, which even by TikTok standards, is a lot. TikTok is rife with similar videos, which feature drops of liquid chlorophyll being added to water and smoothies.
The pretty emerald hue is mesmerizing and it’s hard to resist trying it out when it’s being peddled by seemingly every pretty, smooth-skinned pseudo-model on the platform. In this video, Lena says drinking a glass of water with a few drops of chlorophyll can reduce inflammation, get rid of eye bags, boost your vitamin levels, reduce free radical damage, detoxify your system, and file your taxes. Okay, I made that last one up, but it follows, doesn’t it? This stuff sounds pretty good. Maybe too good.
Chlorophyll, if you skipped biology class (somehow, I doubt you did), is what makes plants green. Medscape has a detailed explanation of chlorophyll, but all you really need to know is that it’s the secret to that cool thing plants do: photosynthesis, or turning sunlight into energy. Scientists have been trying to find uses for it in people since the 1940s. Unfortunately, studies never found much that it can do for us, aside from being kind of deodorizing. So, while it’s been historically marketed as toothpaste and deodorant, the new TikTok claims of it being a cure-all or the next big skincare supplement are not widely substantiated by scientific studies. The only real evidence of it being effective is word of mouth from those who claim to like the way they look or feel since taking it, which isn’t enough for doctors to recommend it.
TikTok’s resident dermatologist, Muneeb Shah, DO, stitched a TikTok from another user, with his captions explaining, “[There’s] no scientific evidence for liquid chlorophyll [helping] rosacea or acne.”
His advice: “Chlorophyll is great, but just eat more veggies.”
A version of this article first appeared on Medscape.com.




