User login
For MD-IQ use only
COVID-19 and Venous Thromboembolism Pharmacologic Thromboprophylaxis
The novel coronavirus SARS-CoV-2 and resulting viral syndrome (COVID-19) was first reported in China during December 2019 and within weeks emerged in the US.1 Since it is a rapidly evolving situation, clinicians must remain current on best practices—a challenging institutional responsibility. According to LitCovid, a curated literature hub for tracking scientific information on COVID-19, there are > 54,000 articles on the subject in PubMed. Among these include venous thromboembolism (VTE) prophylaxis guidance from 4 respected thrombosis organizations/societies and the US National Institutes of Health.1-5
Observations
COVID-19 predisposes patients with and without a history of cardiovascular disease to thrombotic complications, occurring in either the venous or arterial circulation system.2,6 Early observational studies suggest that thrombotic rates may be in excess of 20 to 30%; however, the use of prophylactic anticoagulation was inconsistent among studies that were rushed to publication.6
Autopsy data have demonstrated the presence of fibrin thrombi within distended small vessels and capillaries and extensive extracellular fibrin deposition.6 Investigators compared the characteristics of acute pulmonary embolism in 23 cases with COVID-19 but with no clinical signs of deep vein thrombosis with 100 controls without COVID-19.7 They observed that thrombotic lesions had a greater distribution in peripheral lung segments (ie, peripheral arteries) and were less extensive for those with COVID-19 vs without COVID-19 infection. Thus, experts currently hypothesize that COVID-19 has a distinct “pathomechanism.” As a unique phenotype, thrombotic events represent a combination of thromboembolic disease influenced by components of the Virchow triad (eg, acute illness and immobility) and in situ immunothrombosis, a local inflammatory response.6,7
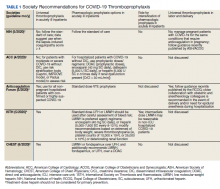
Well-established surgical and nonsurgical VTE thromboprophylaxis guidelines serve as the foundation for current COVID-19 thromboprophylaxis guidance.8,9 Condition specific guidance is extrapolated from small, retrospective observational studies or based on expert opinion, representing levels 2 and 3 evidence, respectively.1-5 Table 1 captures similarities and differences among COVID-19 VTE thromboprophylaxis recommendations which vary by time to publication and by society member expertise gained from practice in the field.
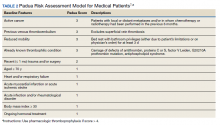
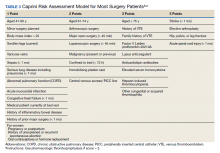
Three thrombosis societies recommend universal pharmacologic thromboprophylaxis for acutely ill COVID-19 patients who lack contraindications.3-5 Others recommend use of risk stratification scoring tools, such as the Padua risk assessment model (RAM) for medical patients or Caprini RAM for surgical patients, the disseminated intravascular coagulation (DIC) score, or the sepsis-induced coagulopathy score to determine therapeutic appropriateness (Tables 2 and 3).1,2 Since most patients hospitalized for COVID-19 will present with a pathognomonic pneumonia and an oxygen requirement, they will generally achieve a score of ≥ 4 when the Padua RAM is applied; thus, representing a clear indication for pharmacologic thromboprophylaxis.8,9 If the patient is pregnant, the Anticoagulation Forum recommends pharmacologic prophylaxis, consultation with an obstetrician, and use of obstetrical thromboprophylaxis guidelines.3,10,11
Most thrombosis experts prefer parenteral thromboprophylaxis, specifically low molecular-weight heparin (LMWH) or fondaparinux, for inpatients over use of direct oral anticoagulants (DOACs) in order to minimize the potential for drug interactions particularly when investigational antivirals are in use.4 Once-daily agents (eg, rivaroxaban, fondaparinux, and enoxaparin) are preferred over multiple daily doses to minimize staff contact with patients infected with COVID-19.4,5 Fondaparinux and DOACs should preferentially be used in patients with a recent history of heparin-induced thrombocytopenia with and without thrombosis (HIT/HITTS). Subcutaneous heparin is reserved for patients who are scheduled for invasive procedures or have reduced renal function (eg, creatinine clearance < 30 mL/min).1,3-5 In line with existing pharmacologic thromboprophylaxis guidance, standard prophylactic LMWH doses are recommended unless patients are obese (body mass index [BMI] > 30) or morbidly obese (BMI > 40) necessitating selection of intermediate doses.4
Since early non-US studies demonstrated high thrombotic risk without signaling a potential for harm from pharmacologic thromboprophylaxis, some organizations recommend empiric escalation of anticoagulation doses for critical illness.3,4,6 Thus, it may be reasonable to advance to either intermediate pharmacologic thromboprophylaxis dosing or therapeutic doses.3 However, observational studies question this aggressive practice unless a clear indication exists for intensification (ie, atrial fibrillation, known VTE).
A large multi-institutional registry study that included 400 subjects from 5 centers demonstrated a radiographically confirmed VTE rate of 4.8% and an arterial thrombosis rate of 2.8%.6 When limiting to the critically ill setting, VTE and arterial thrombosis occurred at slightly higher rates (7.6% and 5.6%, respectively). Patients also were at risk for nonvessel thrombotic complications (eg, CVVH circuit, central venous catheters, and arterial lines). Subsequently, the overall thrombotic complication rate was 9.5%. All thrombotic events except one arose in patients who were receiving standard doses of pharmacologic thromboprophylaxis. Unfortunately, D-dimer elevation at admission was not only predictive of thrombosis and death, but portended bleeding. The overall bleeding rate was 4.8%, with a major bleeding rate of 2.3%. In the context of observing thromboses at normally expected rates during critical illness in association with a significant bleeding risk, the authors recommended further investigation into the net clinical benefit.
Similarly, a National Institutes of Health funded, observational, single center US study evaluated 4,389 inpatients infected with COVID-19 and determined that therapeutic and prophylactic anticoagulation reduced inpatient mortality (adjusted hazard ratio [aHR], 0.53 and 0.50, respectively for the primary outcome) and intubation (aHR, 0.69 and 0.72, respectively) over no anticoagulation.12 Notably, use of inpatient therapeutic anticoagulation commonly represented a continuation of preadmission therapy or progressive COVID-19. A subanalysis demonstrated that timely use (eg, within 48 hours of admission) of prophylactic or therapeutic anticoagulation, resulted in no difference (P < .08) in the primary outcome. Bleeding rates were low overall: 3%, 1.7%, and 1.9% for therapeutic, prophylactic, and no anticoagulation groups, respectively. Furthermore, selection of DOACs seems to be associated with lower bleeding rates when compared with that of LMWH heparin (1.3% vs 2.6%, respectively). In those where site of bleeding could be ascertained, the most common sites were the gastrointestinal tract (50.7%) followed by mucocutaneous (19.4%), bronchopulmonary (14.9%), and intracranial (6%). In summary, prophylactic thromboprophylaxis doses seem to be associated with positive net clinical benefit.
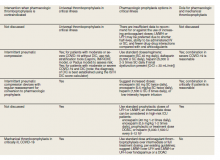
As of October 30, 2020, the US Department of Veterans Affairs (VA) had reported 75,156 COVID-19 cases and 3,961 deaths.13 Since the VA Pharmacy Benefits Management (PBM) does not disseminate nationally prepared anticoagulation order sets to the field, facility anticoagulation leads should be encouraged to develop local guidance-based policies to help standardize care and minimize further variations in practice, which would likely lack evidential support. Per the VA Tennessee Valley Healthcare System (TVHS)- Nashville/Murfreesboro anticoagulation policy, we limit the ordering of parenteral anticoagulation to Computerized Patient Record System (CPRS) order sets in order to provide decision support (eFigure 1, available at doi:10.12788/fp.0063). Other facilities have shown that embedded clinical decision support tools increase adherence to guideline VTE prophylaxis recommendations within the VA.14
In April 2020, the TVHS anticoagulation clinical pharmacy leads developed a COVID-19 specific order set based on review of societal guidance and the evolving, supportive literature summarized in this review with consideration of provider familiarity (eFigure 2, available at doi:10.12788/fp.0063)). Between April and June 2020, the COVID-19 order set content consistently evolved with publication of each COVID-19 thromboprophylaxis guideline.1-5
Since TVHS is a high-complexity facility, we elected to use universal pharmacologic thromboprophylaxis for patients with COVID-19. This construct bypasses the use of scoring tools (eg, RAM), although we use Padua and Caprini RAMS for medical and surgical patients, respectively, who are not diagnosed with COVID-19. The order set displays all acceptable guideline recommended options, delineated by location of care (eg, medical ward vs intensive care unit), prior history of heparin-induced thrombocytopenia, and renal function. Subsequently, all potential agents, doses, and dosing interval options are offered so that the provider autonomously determines how to individualize the clinical care. Since TVHS has only diagnosed 932 ambulatory/inpatient COVID-19 cases combined, our plans are to complete a future observational analysis to determine the effectiveness of the inpatient COVID-19 thromboprophylaxis order set for our internal customers.
Conclusions
The COVID-19 pandemic has resulted in arguably the most challenging medical climate in the evidence-based medicine era. Until high-quality randomized controlled trials are published, the medical community is, in a sense, operating within a crucible of crisis having to navigate therapeutic policy with little certainty. This principle holds true for thromboprophylaxis in patients with COVID-19 despite the numerous advancements in this field over the past decade.
A review of societal guidance shows there is universal agreement with regards to supporting standard doses of pharmacologicalprophylaxis in acutely ill patients either when universally applied or guided by a RAM as well as the use of universal thromboprophylaxis in critically ill patients. All societies discourage the use of antiplatelet therapy for arterial thrombosis prevention and advocate for mechanical compression in patients with contraindications to pharmacologic anticoagulation. Beyond this, divergence between guidance statements begins to appear. For example, societies do not currently agree on the role and approach for extended pharmacologic prophylaxis postdischarge. The differences between societal guidance speaks to the degree of uncertainty among leading experts, which is considered to be the logical outworking of the current level of evidence. Regardless, these guidance documents should be considered the best resource currently available.
The medical community is fortunate to have robust societies that have published guidance on thromboprophylaxis in patients with COVID-19. The novelty of COVID-19 precludes these societal guidance publications from being based on high-quality evidence, but at the very least, they provide insight into how leading experts in the field of thrombosis and hemostasis are currently navigating the therapeutic landscape.
While this paper provides a summary of the current guidance, evidence is evolving at an unprecedented pace. Facilities and anticoagulation leads should be actively and frequently evaluating literature and guidance to ensure their practices and policies remain current.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville/Murfreesboro.
1. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/whats-new/. Updated October 9, 2020. Accessed October 15, 2020.
2. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. doi:10.1016/j.jacc.2020.04.031
3. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72-81. doi:10.1007/s11239-020-02138-z
4. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. doi:10.1111/jth.14929
5. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143-1163. doi:10.1016/j.chest.2020.05.559
6. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520.
7. van Dam LF, Kroft LJM, van der Wal LI, et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease?. Thromb Res. 2020;193:86-89. doi:10.1016/j.thromres.2020.06.010
8. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S-e226S. doi:10.1378/chest.11-2296
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 May;141(5):1369]. Chest. 2012;141(suppl 2):e227S-e277S. doi:10.1378/chest.11-2297
10. ACOG Practice Bulletin No. 196 Summary: thromboembolism in pregnancy. Obstet Gynecol. 2018;132(1):243-248. doi:10.1097/AOG.0000000000002707
11. Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top Guideline. No. 37a. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf. Published April 2015. Accessed October 15, 2020.
12. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study [published online ahead of print, 2020 Aug 24]. J Am Coll Cardiol. 2020;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041
13. US Department of Veterans Affairs. Department of Veterans Affairs COVID-19 national summary. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary. Updated November 1, 2020. Accessed November 1, 2020.
14. George B, Gonzales S, Patel K, Petit S, Franck AJ, Bovio Franck J. Impact of a clinical decision-support tool on venous thromboembolism prophylaxis in acutely ill medical patients. J Pharm Technol. 2020;36(4):141-147. doi:10.1177/8755122520930288
The novel coronavirus SARS-CoV-2 and resulting viral syndrome (COVID-19) was first reported in China during December 2019 and within weeks emerged in the US.1 Since it is a rapidly evolving situation, clinicians must remain current on best practices—a challenging institutional responsibility. According to LitCovid, a curated literature hub for tracking scientific information on COVID-19, there are > 54,000 articles on the subject in PubMed. Among these include venous thromboembolism (VTE) prophylaxis guidance from 4 respected thrombosis organizations/societies and the US National Institutes of Health.1-5
Observations
COVID-19 predisposes patients with and without a history of cardiovascular disease to thrombotic complications, occurring in either the venous or arterial circulation system.2,6 Early observational studies suggest that thrombotic rates may be in excess of 20 to 30%; however, the use of prophylactic anticoagulation was inconsistent among studies that were rushed to publication.6
Autopsy data have demonstrated the presence of fibrin thrombi within distended small vessels and capillaries and extensive extracellular fibrin deposition.6 Investigators compared the characteristics of acute pulmonary embolism in 23 cases with COVID-19 but with no clinical signs of deep vein thrombosis with 100 controls without COVID-19.7 They observed that thrombotic lesions had a greater distribution in peripheral lung segments (ie, peripheral arteries) and were less extensive for those with COVID-19 vs without COVID-19 infection. Thus, experts currently hypothesize that COVID-19 has a distinct “pathomechanism.” As a unique phenotype, thrombotic events represent a combination of thromboembolic disease influenced by components of the Virchow triad (eg, acute illness and immobility) and in situ immunothrombosis, a local inflammatory response.6,7
Well-established surgical and nonsurgical VTE thromboprophylaxis guidelines serve as the foundation for current COVID-19 thromboprophylaxis guidance.8,9 Condition specific guidance is extrapolated from small, retrospective observational studies or based on expert opinion, representing levels 2 and 3 evidence, respectively.1-5 Table 1 captures similarities and differences among COVID-19 VTE thromboprophylaxis recommendations which vary by time to publication and by society member expertise gained from practice in the field.
Three thrombosis societies recommend universal pharmacologic thromboprophylaxis for acutely ill COVID-19 patients who lack contraindications.3-5 Others recommend use of risk stratification scoring tools, such as the Padua risk assessment model (RAM) for medical patients or Caprini RAM for surgical patients, the disseminated intravascular coagulation (DIC) score, or the sepsis-induced coagulopathy score to determine therapeutic appropriateness (Tables 2 and 3).1,2 Since most patients hospitalized for COVID-19 will present with a pathognomonic pneumonia and an oxygen requirement, they will generally achieve a score of ≥ 4 when the Padua RAM is applied; thus, representing a clear indication for pharmacologic thromboprophylaxis.8,9 If the patient is pregnant, the Anticoagulation Forum recommends pharmacologic prophylaxis, consultation with an obstetrician, and use of obstetrical thromboprophylaxis guidelines.3,10,11
Most thrombosis experts prefer parenteral thromboprophylaxis, specifically low molecular-weight heparin (LMWH) or fondaparinux, for inpatients over use of direct oral anticoagulants (DOACs) in order to minimize the potential for drug interactions particularly when investigational antivirals are in use.4 Once-daily agents (eg, rivaroxaban, fondaparinux, and enoxaparin) are preferred over multiple daily doses to minimize staff contact with patients infected with COVID-19.4,5 Fondaparinux and DOACs should preferentially be used in patients with a recent history of heparin-induced thrombocytopenia with and without thrombosis (HIT/HITTS). Subcutaneous heparin is reserved for patients who are scheduled for invasive procedures or have reduced renal function (eg, creatinine clearance < 30 mL/min).1,3-5 In line with existing pharmacologic thromboprophylaxis guidance, standard prophylactic LMWH doses are recommended unless patients are obese (body mass index [BMI] > 30) or morbidly obese (BMI > 40) necessitating selection of intermediate doses.4
Since early non-US studies demonstrated high thrombotic risk without signaling a potential for harm from pharmacologic thromboprophylaxis, some organizations recommend empiric escalation of anticoagulation doses for critical illness.3,4,6 Thus, it may be reasonable to advance to either intermediate pharmacologic thromboprophylaxis dosing or therapeutic doses.3 However, observational studies question this aggressive practice unless a clear indication exists for intensification (ie, atrial fibrillation, known VTE).
A large multi-institutional registry study that included 400 subjects from 5 centers demonstrated a radiographically confirmed VTE rate of 4.8% and an arterial thrombosis rate of 2.8%.6 When limiting to the critically ill setting, VTE and arterial thrombosis occurred at slightly higher rates (7.6% and 5.6%, respectively). Patients also were at risk for nonvessel thrombotic complications (eg, CVVH circuit, central venous catheters, and arterial lines). Subsequently, the overall thrombotic complication rate was 9.5%. All thrombotic events except one arose in patients who were receiving standard doses of pharmacologic thromboprophylaxis. Unfortunately, D-dimer elevation at admission was not only predictive of thrombosis and death, but portended bleeding. The overall bleeding rate was 4.8%, with a major bleeding rate of 2.3%. In the context of observing thromboses at normally expected rates during critical illness in association with a significant bleeding risk, the authors recommended further investigation into the net clinical benefit.
Similarly, a National Institutes of Health funded, observational, single center US study evaluated 4,389 inpatients infected with COVID-19 and determined that therapeutic and prophylactic anticoagulation reduced inpatient mortality (adjusted hazard ratio [aHR], 0.53 and 0.50, respectively for the primary outcome) and intubation (aHR, 0.69 and 0.72, respectively) over no anticoagulation.12 Notably, use of inpatient therapeutic anticoagulation commonly represented a continuation of preadmission therapy or progressive COVID-19. A subanalysis demonstrated that timely use (eg, within 48 hours of admission) of prophylactic or therapeutic anticoagulation, resulted in no difference (P < .08) in the primary outcome. Bleeding rates were low overall: 3%, 1.7%, and 1.9% for therapeutic, prophylactic, and no anticoagulation groups, respectively. Furthermore, selection of DOACs seems to be associated with lower bleeding rates when compared with that of LMWH heparin (1.3% vs 2.6%, respectively). In those where site of bleeding could be ascertained, the most common sites were the gastrointestinal tract (50.7%) followed by mucocutaneous (19.4%), bronchopulmonary (14.9%), and intracranial (6%). In summary, prophylactic thromboprophylaxis doses seem to be associated with positive net clinical benefit.
As of October 30, 2020, the US Department of Veterans Affairs (VA) had reported 75,156 COVID-19 cases and 3,961 deaths.13 Since the VA Pharmacy Benefits Management (PBM) does not disseminate nationally prepared anticoagulation order sets to the field, facility anticoagulation leads should be encouraged to develop local guidance-based policies to help standardize care and minimize further variations in practice, which would likely lack evidential support. Per the VA Tennessee Valley Healthcare System (TVHS)- Nashville/Murfreesboro anticoagulation policy, we limit the ordering of parenteral anticoagulation to Computerized Patient Record System (CPRS) order sets in order to provide decision support (eFigure 1, available at doi:10.12788/fp.0063). Other facilities have shown that embedded clinical decision support tools increase adherence to guideline VTE prophylaxis recommendations within the VA.14
In April 2020, the TVHS anticoagulation clinical pharmacy leads developed a COVID-19 specific order set based on review of societal guidance and the evolving, supportive literature summarized in this review with consideration of provider familiarity (eFigure 2, available at doi:10.12788/fp.0063)). Between April and June 2020, the COVID-19 order set content consistently evolved with publication of each COVID-19 thromboprophylaxis guideline.1-5
Since TVHS is a high-complexity facility, we elected to use universal pharmacologic thromboprophylaxis for patients with COVID-19. This construct bypasses the use of scoring tools (eg, RAM), although we use Padua and Caprini RAMS for medical and surgical patients, respectively, who are not diagnosed with COVID-19. The order set displays all acceptable guideline recommended options, delineated by location of care (eg, medical ward vs intensive care unit), prior history of heparin-induced thrombocytopenia, and renal function. Subsequently, all potential agents, doses, and dosing interval options are offered so that the provider autonomously determines how to individualize the clinical care. Since TVHS has only diagnosed 932 ambulatory/inpatient COVID-19 cases combined, our plans are to complete a future observational analysis to determine the effectiveness of the inpatient COVID-19 thromboprophylaxis order set for our internal customers.
Conclusions
The COVID-19 pandemic has resulted in arguably the most challenging medical climate in the evidence-based medicine era. Until high-quality randomized controlled trials are published, the medical community is, in a sense, operating within a crucible of crisis having to navigate therapeutic policy with little certainty. This principle holds true for thromboprophylaxis in patients with COVID-19 despite the numerous advancements in this field over the past decade.
A review of societal guidance shows there is universal agreement with regards to supporting standard doses of pharmacologicalprophylaxis in acutely ill patients either when universally applied or guided by a RAM as well as the use of universal thromboprophylaxis in critically ill patients. All societies discourage the use of antiplatelet therapy for arterial thrombosis prevention and advocate for mechanical compression in patients with contraindications to pharmacologic anticoagulation. Beyond this, divergence between guidance statements begins to appear. For example, societies do not currently agree on the role and approach for extended pharmacologic prophylaxis postdischarge. The differences between societal guidance speaks to the degree of uncertainty among leading experts, which is considered to be the logical outworking of the current level of evidence. Regardless, these guidance documents should be considered the best resource currently available.
The medical community is fortunate to have robust societies that have published guidance on thromboprophylaxis in patients with COVID-19. The novelty of COVID-19 precludes these societal guidance publications from being based on high-quality evidence, but at the very least, they provide insight into how leading experts in the field of thrombosis and hemostasis are currently navigating the therapeutic landscape.
While this paper provides a summary of the current guidance, evidence is evolving at an unprecedented pace. Facilities and anticoagulation leads should be actively and frequently evaluating literature and guidance to ensure their practices and policies remain current.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville/Murfreesboro.
The novel coronavirus SARS-CoV-2 and resulting viral syndrome (COVID-19) was first reported in China during December 2019 and within weeks emerged in the US.1 Since it is a rapidly evolving situation, clinicians must remain current on best practices—a challenging institutional responsibility. According to LitCovid, a curated literature hub for tracking scientific information on COVID-19, there are > 54,000 articles on the subject in PubMed. Among these include venous thromboembolism (VTE) prophylaxis guidance from 4 respected thrombosis organizations/societies and the US National Institutes of Health.1-5
Observations
COVID-19 predisposes patients with and without a history of cardiovascular disease to thrombotic complications, occurring in either the venous or arterial circulation system.2,6 Early observational studies suggest that thrombotic rates may be in excess of 20 to 30%; however, the use of prophylactic anticoagulation was inconsistent among studies that were rushed to publication.6
Autopsy data have demonstrated the presence of fibrin thrombi within distended small vessels and capillaries and extensive extracellular fibrin deposition.6 Investigators compared the characteristics of acute pulmonary embolism in 23 cases with COVID-19 but with no clinical signs of deep vein thrombosis with 100 controls without COVID-19.7 They observed that thrombotic lesions had a greater distribution in peripheral lung segments (ie, peripheral arteries) and were less extensive for those with COVID-19 vs without COVID-19 infection. Thus, experts currently hypothesize that COVID-19 has a distinct “pathomechanism.” As a unique phenotype, thrombotic events represent a combination of thromboembolic disease influenced by components of the Virchow triad (eg, acute illness and immobility) and in situ immunothrombosis, a local inflammatory response.6,7
Well-established surgical and nonsurgical VTE thromboprophylaxis guidelines serve as the foundation for current COVID-19 thromboprophylaxis guidance.8,9 Condition specific guidance is extrapolated from small, retrospective observational studies or based on expert opinion, representing levels 2 and 3 evidence, respectively.1-5 Table 1 captures similarities and differences among COVID-19 VTE thromboprophylaxis recommendations which vary by time to publication and by society member expertise gained from practice in the field.
Three thrombosis societies recommend universal pharmacologic thromboprophylaxis for acutely ill COVID-19 patients who lack contraindications.3-5 Others recommend use of risk stratification scoring tools, such as the Padua risk assessment model (RAM) for medical patients or Caprini RAM for surgical patients, the disseminated intravascular coagulation (DIC) score, or the sepsis-induced coagulopathy score to determine therapeutic appropriateness (Tables 2 and 3).1,2 Since most patients hospitalized for COVID-19 will present with a pathognomonic pneumonia and an oxygen requirement, they will generally achieve a score of ≥ 4 when the Padua RAM is applied; thus, representing a clear indication for pharmacologic thromboprophylaxis.8,9 If the patient is pregnant, the Anticoagulation Forum recommends pharmacologic prophylaxis, consultation with an obstetrician, and use of obstetrical thromboprophylaxis guidelines.3,10,11
Most thrombosis experts prefer parenteral thromboprophylaxis, specifically low molecular-weight heparin (LMWH) or fondaparinux, for inpatients over use of direct oral anticoagulants (DOACs) in order to minimize the potential for drug interactions particularly when investigational antivirals are in use.4 Once-daily agents (eg, rivaroxaban, fondaparinux, and enoxaparin) are preferred over multiple daily doses to minimize staff contact with patients infected with COVID-19.4,5 Fondaparinux and DOACs should preferentially be used in patients with a recent history of heparin-induced thrombocytopenia with and without thrombosis (HIT/HITTS). Subcutaneous heparin is reserved for patients who are scheduled for invasive procedures or have reduced renal function (eg, creatinine clearance < 30 mL/min).1,3-5 In line with existing pharmacologic thromboprophylaxis guidance, standard prophylactic LMWH doses are recommended unless patients are obese (body mass index [BMI] > 30) or morbidly obese (BMI > 40) necessitating selection of intermediate doses.4
Since early non-US studies demonstrated high thrombotic risk without signaling a potential for harm from pharmacologic thromboprophylaxis, some organizations recommend empiric escalation of anticoagulation doses for critical illness.3,4,6 Thus, it may be reasonable to advance to either intermediate pharmacologic thromboprophylaxis dosing or therapeutic doses.3 However, observational studies question this aggressive practice unless a clear indication exists for intensification (ie, atrial fibrillation, known VTE).
A large multi-institutional registry study that included 400 subjects from 5 centers demonstrated a radiographically confirmed VTE rate of 4.8% and an arterial thrombosis rate of 2.8%.6 When limiting to the critically ill setting, VTE and arterial thrombosis occurred at slightly higher rates (7.6% and 5.6%, respectively). Patients also were at risk for nonvessel thrombotic complications (eg, CVVH circuit, central venous catheters, and arterial lines). Subsequently, the overall thrombotic complication rate was 9.5%. All thrombotic events except one arose in patients who were receiving standard doses of pharmacologic thromboprophylaxis. Unfortunately, D-dimer elevation at admission was not only predictive of thrombosis and death, but portended bleeding. The overall bleeding rate was 4.8%, with a major bleeding rate of 2.3%. In the context of observing thromboses at normally expected rates during critical illness in association with a significant bleeding risk, the authors recommended further investigation into the net clinical benefit.
Similarly, a National Institutes of Health funded, observational, single center US study evaluated 4,389 inpatients infected with COVID-19 and determined that therapeutic and prophylactic anticoagulation reduced inpatient mortality (adjusted hazard ratio [aHR], 0.53 and 0.50, respectively for the primary outcome) and intubation (aHR, 0.69 and 0.72, respectively) over no anticoagulation.12 Notably, use of inpatient therapeutic anticoagulation commonly represented a continuation of preadmission therapy or progressive COVID-19. A subanalysis demonstrated that timely use (eg, within 48 hours of admission) of prophylactic or therapeutic anticoagulation, resulted in no difference (P < .08) in the primary outcome. Bleeding rates were low overall: 3%, 1.7%, and 1.9% for therapeutic, prophylactic, and no anticoagulation groups, respectively. Furthermore, selection of DOACs seems to be associated with lower bleeding rates when compared with that of LMWH heparin (1.3% vs 2.6%, respectively). In those where site of bleeding could be ascertained, the most common sites were the gastrointestinal tract (50.7%) followed by mucocutaneous (19.4%), bronchopulmonary (14.9%), and intracranial (6%). In summary, prophylactic thromboprophylaxis doses seem to be associated with positive net clinical benefit.
As of October 30, 2020, the US Department of Veterans Affairs (VA) had reported 75,156 COVID-19 cases and 3,961 deaths.13 Since the VA Pharmacy Benefits Management (PBM) does not disseminate nationally prepared anticoagulation order sets to the field, facility anticoagulation leads should be encouraged to develop local guidance-based policies to help standardize care and minimize further variations in practice, which would likely lack evidential support. Per the VA Tennessee Valley Healthcare System (TVHS)- Nashville/Murfreesboro anticoagulation policy, we limit the ordering of parenteral anticoagulation to Computerized Patient Record System (CPRS) order sets in order to provide decision support (eFigure 1, available at doi:10.12788/fp.0063). Other facilities have shown that embedded clinical decision support tools increase adherence to guideline VTE prophylaxis recommendations within the VA.14
In April 2020, the TVHS anticoagulation clinical pharmacy leads developed a COVID-19 specific order set based on review of societal guidance and the evolving, supportive literature summarized in this review with consideration of provider familiarity (eFigure 2, available at doi:10.12788/fp.0063)). Between April and June 2020, the COVID-19 order set content consistently evolved with publication of each COVID-19 thromboprophylaxis guideline.1-5
Since TVHS is a high-complexity facility, we elected to use universal pharmacologic thromboprophylaxis for patients with COVID-19. This construct bypasses the use of scoring tools (eg, RAM), although we use Padua and Caprini RAMS for medical and surgical patients, respectively, who are not diagnosed with COVID-19. The order set displays all acceptable guideline recommended options, delineated by location of care (eg, medical ward vs intensive care unit), prior history of heparin-induced thrombocytopenia, and renal function. Subsequently, all potential agents, doses, and dosing interval options are offered so that the provider autonomously determines how to individualize the clinical care. Since TVHS has only diagnosed 932 ambulatory/inpatient COVID-19 cases combined, our plans are to complete a future observational analysis to determine the effectiveness of the inpatient COVID-19 thromboprophylaxis order set for our internal customers.
Conclusions
The COVID-19 pandemic has resulted in arguably the most challenging medical climate in the evidence-based medicine era. Until high-quality randomized controlled trials are published, the medical community is, in a sense, operating within a crucible of crisis having to navigate therapeutic policy with little certainty. This principle holds true for thromboprophylaxis in patients with COVID-19 despite the numerous advancements in this field over the past decade.
A review of societal guidance shows there is universal agreement with regards to supporting standard doses of pharmacologicalprophylaxis in acutely ill patients either when universally applied or guided by a RAM as well as the use of universal thromboprophylaxis in critically ill patients. All societies discourage the use of antiplatelet therapy for arterial thrombosis prevention and advocate for mechanical compression in patients with contraindications to pharmacologic anticoagulation. Beyond this, divergence between guidance statements begins to appear. For example, societies do not currently agree on the role and approach for extended pharmacologic prophylaxis postdischarge. The differences between societal guidance speaks to the degree of uncertainty among leading experts, which is considered to be the logical outworking of the current level of evidence. Regardless, these guidance documents should be considered the best resource currently available.
The medical community is fortunate to have robust societies that have published guidance on thromboprophylaxis in patients with COVID-19. The novelty of COVID-19 precludes these societal guidance publications from being based on high-quality evidence, but at the very least, they provide insight into how leading experts in the field of thrombosis and hemostasis are currently navigating the therapeutic landscape.
While this paper provides a summary of the current guidance, evidence is evolving at an unprecedented pace. Facilities and anticoagulation leads should be actively and frequently evaluating literature and guidance to ensure their practices and policies remain current.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville/Murfreesboro.
1. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/whats-new/. Updated October 9, 2020. Accessed October 15, 2020.
2. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. doi:10.1016/j.jacc.2020.04.031
3. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72-81. doi:10.1007/s11239-020-02138-z
4. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. doi:10.1111/jth.14929
5. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143-1163. doi:10.1016/j.chest.2020.05.559
6. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520.
7. van Dam LF, Kroft LJM, van der Wal LI, et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease?. Thromb Res. 2020;193:86-89. doi:10.1016/j.thromres.2020.06.010
8. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S-e226S. doi:10.1378/chest.11-2296
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 May;141(5):1369]. Chest. 2012;141(suppl 2):e227S-e277S. doi:10.1378/chest.11-2297
10. ACOG Practice Bulletin No. 196 Summary: thromboembolism in pregnancy. Obstet Gynecol. 2018;132(1):243-248. doi:10.1097/AOG.0000000000002707
11. Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top Guideline. No. 37a. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf. Published April 2015. Accessed October 15, 2020.
12. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study [published online ahead of print, 2020 Aug 24]. J Am Coll Cardiol. 2020;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041
13. US Department of Veterans Affairs. Department of Veterans Affairs COVID-19 national summary. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary. Updated November 1, 2020. Accessed November 1, 2020.
14. George B, Gonzales S, Patel K, Petit S, Franck AJ, Bovio Franck J. Impact of a clinical decision-support tool on venous thromboembolism prophylaxis in acutely ill medical patients. J Pharm Technol. 2020;36(4):141-147. doi:10.1177/8755122520930288
1. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/whats-new/. Updated October 9, 2020. Accessed October 15, 2020.
2. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. doi:10.1016/j.jacc.2020.04.031
3. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72-81. doi:10.1007/s11239-020-02138-z
4. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. doi:10.1111/jth.14929
5. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143-1163. doi:10.1016/j.chest.2020.05.559
6. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520.
7. van Dam LF, Kroft LJM, van der Wal LI, et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease?. Thromb Res. 2020;193:86-89. doi:10.1016/j.thromres.2020.06.010
8. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S-e226S. doi:10.1378/chest.11-2296
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 May;141(5):1369]. Chest. 2012;141(suppl 2):e227S-e277S. doi:10.1378/chest.11-2297
10. ACOG Practice Bulletin No. 196 Summary: thromboembolism in pregnancy. Obstet Gynecol. 2018;132(1):243-248. doi:10.1097/AOG.0000000000002707
11. Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top Guideline. No. 37a. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf. Published April 2015. Accessed October 15, 2020.
12. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study [published online ahead of print, 2020 Aug 24]. J Am Coll Cardiol. 2020;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041
13. US Department of Veterans Affairs. Department of Veterans Affairs COVID-19 national summary. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary. Updated November 1, 2020. Accessed November 1, 2020.
14. George B, Gonzales S, Patel K, Petit S, Franck AJ, Bovio Franck J. Impact of a clinical decision-support tool on venous thromboembolism prophylaxis in acutely ill medical patients. J Pharm Technol. 2020;36(4):141-147. doi:10.1177/8755122520930288
Coaching in medicine: A perspective
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Poor and minority children with food allergies overlooked and in danger
As Emily Brown stood in a food pantry looking at her options, she felt alone. Up to that point, she had never struggled financially. But there she was, desperate to find safe food for her young daughter with food allergies. What she found was a jar of salsa and some potatoes.
“That was all that was available,” said Ms. Brown, who lives in Kansas City, Kansas. “It was just a desperate place.”
When she became a parent, Ms. Brown left her job for lack of child care that would accommodate her daughter’s allergies to peanuts, tree nuts, milk, eggs, wheat, and soy. When she and her husband then turned to a federal food assistance program, they found few allowable allergy substitutions. The closest allergy support group she could find was an hour away. She was almost always the only Black parent, and the only poor parent, there.
Ms. Brown called national food allergy advocacy organizations to ask for guidance to help poor families find safe food and medical resources, but she said she was told that wasn’t their focus. Support groups, fundraising activities, and advocacy efforts, plus clinical and research outreach, were targeted at wealthier – and White – families. Advertising rarely reflected families that looked like hers. She felt unseen.
“In many ways, food allergy is an invisible disease. The burden of the disease, the activities and energy it takes to avoid allergens, are mostly invisible to those not impacted,” Ms. Brown said. “Black and other minority patients often lack voice and visibility in the health care system. Add the additional burden of an invisible condition and you are in a really vulnerable position.”
An estimated 6 million children in the United States have food allergies, 40% of them with more than one. Though limited research has been done on race and class breakdowns, recent studies show that poor children and some groups of minority children not only have a higher incidence of food allergies than White children, but their families also have more difficulty accessing appropriate child care, safe food, medical care, and lifesaving medicine like epinephrine for them.
Black children are 7% more likely to have food allergies than white children, according to a 2020 study by Dr. Ruchi Gupta, MD, at Northwestern University, Chicago. To be sure, the study shows that Asian children are 24% more likely than White children to have food allergies. But Black and Hispanic children are disproportionately more likely to live in poor communities, to have asthma, and to suffer from systemic racism in the delivery of medical care.
“Many times, a mother is frank and says: ‘I have $20-$40 to buy groceries for the week, and if I buy these foods that you are telling me to buy, I will not be able to feed my entire family,’” said Carla Davis, MD, director of the food allergy program at Houston’s Texas Children’s Hospital. “If you are diagnosed with a food allergy and you don’t have disposable income or disposable time, there is really no way that you will be able to alter your diet in a way that your child is going to stay away from their allergen.”
Fed up with the lack of support, Ms. Brown founded the Food Equality Initiative advocacy organization in 2014. It offers an online marketplace to income-eligible families in Kansas and Missouri who, with a doctor’s note about the allergy, can order free allergy-safe food to fit their needs.
Nationwide, though, families’ needs far outstrip what her group can offer – and the problem has gotten worse amid the economic squeeze of the COVID pandemic. Job losses and business closures have exacerbated the barriers to finding and affording nutritious food, according to a report from Feeding America, an association of food banks.
Ms. Brown said her organization more than doubled its clientele in March through August, compared with the same period in 2019. And though it currently serves only Missouri and Kansas, she said the organization has been fielding an increasing number of calls from across the country since the pandemic began.
For low-income minorities, who live disproportionately in food deserts, fresh and allergy-friendly foods can be especially expensive and difficult to find in the best of times.
Food assistance programs are heavily weighted to prepackaged and processed foods, which often include the very ingredients that are problematic. Black children are more likely to be allergic to wheat and soy than White children, and both Black and Hispanic children are more likely to be allergic to corn, shellfish, and fish, according to a 2016 study.
Some programs allow few allergy substitutions. For example, the federal Special Supplemental Nutrition Program for Women, Infants, and Children allows only canned beans as a substitute for peanut butter. While nutritionally similar, beans are not as easy to pack for a kid’s lunch. Ms. Brown questions why WIC won’t allow a seed butter, such as sunflower butter, instead. She said they are nutritionally and functionally similar and are offered as allergy substitutions in other food programs.
Making matters worse, low-income households pay more than twice as much as higher-income families for the emergency medical care their children receive for their allergies, according to a 2016 study by Dr. Gupta. The kids often arrive at the hospital in more distress because they lack safe food and allergy medications – and because asthma, which disproportionately hits Black and Puerto Rican children and low-income communities, complicates allergic reactions.
“So, in these vulnerable populations, it’s like a double whammy, and we see that reflected in the data,” said Lakiea Wright-Bello, MD, a medical director in specialty diagnostics at Thermo Fisher Scientific and an allergist at Brigham and Women’s Hospital in Boston.
Thomas and Dina Silvera, who are Black and Latina, lived this horror firsthand. After their 3-year-old son, Elijah-Alavi, died as a result of a dairy allergy when fed a grilled cheese instead of his allergen-free food at his preschool, they launched the Elijah-Alavi Foundation to address the dearth of information about food allergies and the critical lack of culturally sensitive medical care in low-income communities.
“We started it for a cause, not because we wanted to, but because we had to,” said Thomas Silvera. “Our main focus is to bring to underserved communities – especially communities of color – this information at no cost to them.”
Recently, other advocacy groups, including Food Allergy Research & Education, a national advocacy organization, also have started to turn their attention to a lack of access and support in poor and minority communities. When Lisa Gable, who is White, took over at the group known as FARE in 2018, she began to diversify the organization internally and to make it more inclusive.
“There wasn’t a big tent when I walked in the door,” said Ms. Gable. “What we have been focused on doing is trying to find partners and relationships that will allow us to diversify those engaged in the community, because it has not been a diverse community.”
FARE has funded research into the cost of food allergies. It is also expanding its patient registry, which collects data for research, as well as its clinical network of medical institutions to include more diverse communities.
Dr. Gupta is now leading one of the first studies funded by the National Institutes of Health to investigate food allergy in children by race and ethnicity. It looks at all aspects of food allergies, including family life, management, access to care, and genetics.
“That’s a big deal,” said Dr. Gupta. “Because if we really want to improve food allergy management, care and understanding, we really need to understand how it impacts different groups. And that hasn’t been done.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
As Emily Brown stood in a food pantry looking at her options, she felt alone. Up to that point, she had never struggled financially. But there she was, desperate to find safe food for her young daughter with food allergies. What she found was a jar of salsa and some potatoes.
“That was all that was available,” said Ms. Brown, who lives in Kansas City, Kansas. “It was just a desperate place.”
When she became a parent, Ms. Brown left her job for lack of child care that would accommodate her daughter’s allergies to peanuts, tree nuts, milk, eggs, wheat, and soy. When she and her husband then turned to a federal food assistance program, they found few allowable allergy substitutions. The closest allergy support group she could find was an hour away. She was almost always the only Black parent, and the only poor parent, there.
Ms. Brown called national food allergy advocacy organizations to ask for guidance to help poor families find safe food and medical resources, but she said she was told that wasn’t their focus. Support groups, fundraising activities, and advocacy efforts, plus clinical and research outreach, were targeted at wealthier – and White – families. Advertising rarely reflected families that looked like hers. She felt unseen.
“In many ways, food allergy is an invisible disease. The burden of the disease, the activities and energy it takes to avoid allergens, are mostly invisible to those not impacted,” Ms. Brown said. “Black and other minority patients often lack voice and visibility in the health care system. Add the additional burden of an invisible condition and you are in a really vulnerable position.”
An estimated 6 million children in the United States have food allergies, 40% of them with more than one. Though limited research has been done on race and class breakdowns, recent studies show that poor children and some groups of minority children not only have a higher incidence of food allergies than White children, but their families also have more difficulty accessing appropriate child care, safe food, medical care, and lifesaving medicine like epinephrine for them.
Black children are 7% more likely to have food allergies than white children, according to a 2020 study by Dr. Ruchi Gupta, MD, at Northwestern University, Chicago. To be sure, the study shows that Asian children are 24% more likely than White children to have food allergies. But Black and Hispanic children are disproportionately more likely to live in poor communities, to have asthma, and to suffer from systemic racism in the delivery of medical care.
“Many times, a mother is frank and says: ‘I have $20-$40 to buy groceries for the week, and if I buy these foods that you are telling me to buy, I will not be able to feed my entire family,’” said Carla Davis, MD, director of the food allergy program at Houston’s Texas Children’s Hospital. “If you are diagnosed with a food allergy and you don’t have disposable income or disposable time, there is really no way that you will be able to alter your diet in a way that your child is going to stay away from their allergen.”
Fed up with the lack of support, Ms. Brown founded the Food Equality Initiative advocacy organization in 2014. It offers an online marketplace to income-eligible families in Kansas and Missouri who, with a doctor’s note about the allergy, can order free allergy-safe food to fit their needs.
Nationwide, though, families’ needs far outstrip what her group can offer – and the problem has gotten worse amid the economic squeeze of the COVID pandemic. Job losses and business closures have exacerbated the barriers to finding and affording nutritious food, according to a report from Feeding America, an association of food banks.
Ms. Brown said her organization more than doubled its clientele in March through August, compared with the same period in 2019. And though it currently serves only Missouri and Kansas, she said the organization has been fielding an increasing number of calls from across the country since the pandemic began.
For low-income minorities, who live disproportionately in food deserts, fresh and allergy-friendly foods can be especially expensive and difficult to find in the best of times.
Food assistance programs are heavily weighted to prepackaged and processed foods, which often include the very ingredients that are problematic. Black children are more likely to be allergic to wheat and soy than White children, and both Black and Hispanic children are more likely to be allergic to corn, shellfish, and fish, according to a 2016 study.
Some programs allow few allergy substitutions. For example, the federal Special Supplemental Nutrition Program for Women, Infants, and Children allows only canned beans as a substitute for peanut butter. While nutritionally similar, beans are not as easy to pack for a kid’s lunch. Ms. Brown questions why WIC won’t allow a seed butter, such as sunflower butter, instead. She said they are nutritionally and functionally similar and are offered as allergy substitutions in other food programs.
Making matters worse, low-income households pay more than twice as much as higher-income families for the emergency medical care their children receive for their allergies, according to a 2016 study by Dr. Gupta. The kids often arrive at the hospital in more distress because they lack safe food and allergy medications – and because asthma, which disproportionately hits Black and Puerto Rican children and low-income communities, complicates allergic reactions.
“So, in these vulnerable populations, it’s like a double whammy, and we see that reflected in the data,” said Lakiea Wright-Bello, MD, a medical director in specialty diagnostics at Thermo Fisher Scientific and an allergist at Brigham and Women’s Hospital in Boston.
Thomas and Dina Silvera, who are Black and Latina, lived this horror firsthand. After their 3-year-old son, Elijah-Alavi, died as a result of a dairy allergy when fed a grilled cheese instead of his allergen-free food at his preschool, they launched the Elijah-Alavi Foundation to address the dearth of information about food allergies and the critical lack of culturally sensitive medical care in low-income communities.
“We started it for a cause, not because we wanted to, but because we had to,” said Thomas Silvera. “Our main focus is to bring to underserved communities – especially communities of color – this information at no cost to them.”
Recently, other advocacy groups, including Food Allergy Research & Education, a national advocacy organization, also have started to turn their attention to a lack of access and support in poor and minority communities. When Lisa Gable, who is White, took over at the group known as FARE in 2018, she began to diversify the organization internally and to make it more inclusive.
“There wasn’t a big tent when I walked in the door,” said Ms. Gable. “What we have been focused on doing is trying to find partners and relationships that will allow us to diversify those engaged in the community, because it has not been a diverse community.”
FARE has funded research into the cost of food allergies. It is also expanding its patient registry, which collects data for research, as well as its clinical network of medical institutions to include more diverse communities.
Dr. Gupta is now leading one of the first studies funded by the National Institutes of Health to investigate food allergy in children by race and ethnicity. It looks at all aspects of food allergies, including family life, management, access to care, and genetics.
“That’s a big deal,” said Dr. Gupta. “Because if we really want to improve food allergy management, care and understanding, we really need to understand how it impacts different groups. And that hasn’t been done.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
As Emily Brown stood in a food pantry looking at her options, she felt alone. Up to that point, she had never struggled financially. But there she was, desperate to find safe food for her young daughter with food allergies. What she found was a jar of salsa and some potatoes.
“That was all that was available,” said Ms. Brown, who lives in Kansas City, Kansas. “It was just a desperate place.”
When she became a parent, Ms. Brown left her job for lack of child care that would accommodate her daughter’s allergies to peanuts, tree nuts, milk, eggs, wheat, and soy. When she and her husband then turned to a federal food assistance program, they found few allowable allergy substitutions. The closest allergy support group she could find was an hour away. She was almost always the only Black parent, and the only poor parent, there.
Ms. Brown called national food allergy advocacy organizations to ask for guidance to help poor families find safe food and medical resources, but she said she was told that wasn’t their focus. Support groups, fundraising activities, and advocacy efforts, plus clinical and research outreach, were targeted at wealthier – and White – families. Advertising rarely reflected families that looked like hers. She felt unseen.
“In many ways, food allergy is an invisible disease. The burden of the disease, the activities and energy it takes to avoid allergens, are mostly invisible to those not impacted,” Ms. Brown said. “Black and other minority patients often lack voice and visibility in the health care system. Add the additional burden of an invisible condition and you are in a really vulnerable position.”
An estimated 6 million children in the United States have food allergies, 40% of them with more than one. Though limited research has been done on race and class breakdowns, recent studies show that poor children and some groups of minority children not only have a higher incidence of food allergies than White children, but their families also have more difficulty accessing appropriate child care, safe food, medical care, and lifesaving medicine like epinephrine for them.
Black children are 7% more likely to have food allergies than white children, according to a 2020 study by Dr. Ruchi Gupta, MD, at Northwestern University, Chicago. To be sure, the study shows that Asian children are 24% more likely than White children to have food allergies. But Black and Hispanic children are disproportionately more likely to live in poor communities, to have asthma, and to suffer from systemic racism in the delivery of medical care.
“Many times, a mother is frank and says: ‘I have $20-$40 to buy groceries for the week, and if I buy these foods that you are telling me to buy, I will not be able to feed my entire family,’” said Carla Davis, MD, director of the food allergy program at Houston’s Texas Children’s Hospital. “If you are diagnosed with a food allergy and you don’t have disposable income or disposable time, there is really no way that you will be able to alter your diet in a way that your child is going to stay away from their allergen.”
Fed up with the lack of support, Ms. Brown founded the Food Equality Initiative advocacy organization in 2014. It offers an online marketplace to income-eligible families in Kansas and Missouri who, with a doctor’s note about the allergy, can order free allergy-safe food to fit their needs.
Nationwide, though, families’ needs far outstrip what her group can offer – and the problem has gotten worse amid the economic squeeze of the COVID pandemic. Job losses and business closures have exacerbated the barriers to finding and affording nutritious food, according to a report from Feeding America, an association of food banks.
Ms. Brown said her organization more than doubled its clientele in March through August, compared with the same period in 2019. And though it currently serves only Missouri and Kansas, she said the organization has been fielding an increasing number of calls from across the country since the pandemic began.
For low-income minorities, who live disproportionately in food deserts, fresh and allergy-friendly foods can be especially expensive and difficult to find in the best of times.
Food assistance programs are heavily weighted to prepackaged and processed foods, which often include the very ingredients that are problematic. Black children are more likely to be allergic to wheat and soy than White children, and both Black and Hispanic children are more likely to be allergic to corn, shellfish, and fish, according to a 2016 study.
Some programs allow few allergy substitutions. For example, the federal Special Supplemental Nutrition Program for Women, Infants, and Children allows only canned beans as a substitute for peanut butter. While nutritionally similar, beans are not as easy to pack for a kid’s lunch. Ms. Brown questions why WIC won’t allow a seed butter, such as sunflower butter, instead. She said they are nutritionally and functionally similar and are offered as allergy substitutions in other food programs.
Making matters worse, low-income households pay more than twice as much as higher-income families for the emergency medical care their children receive for their allergies, according to a 2016 study by Dr. Gupta. The kids often arrive at the hospital in more distress because they lack safe food and allergy medications – and because asthma, which disproportionately hits Black and Puerto Rican children and low-income communities, complicates allergic reactions.
“So, in these vulnerable populations, it’s like a double whammy, and we see that reflected in the data,” said Lakiea Wright-Bello, MD, a medical director in specialty diagnostics at Thermo Fisher Scientific and an allergist at Brigham and Women’s Hospital in Boston.
Thomas and Dina Silvera, who are Black and Latina, lived this horror firsthand. After their 3-year-old son, Elijah-Alavi, died as a result of a dairy allergy when fed a grilled cheese instead of his allergen-free food at his preschool, they launched the Elijah-Alavi Foundation to address the dearth of information about food allergies and the critical lack of culturally sensitive medical care in low-income communities.
“We started it for a cause, not because we wanted to, but because we had to,” said Thomas Silvera. “Our main focus is to bring to underserved communities – especially communities of color – this information at no cost to them.”
Recently, other advocacy groups, including Food Allergy Research & Education, a national advocacy organization, also have started to turn their attention to a lack of access and support in poor and minority communities. When Lisa Gable, who is White, took over at the group known as FARE in 2018, she began to diversify the organization internally and to make it more inclusive.
“There wasn’t a big tent when I walked in the door,” said Ms. Gable. “What we have been focused on doing is trying to find partners and relationships that will allow us to diversify those engaged in the community, because it has not been a diverse community.”
FARE has funded research into the cost of food allergies. It is also expanding its patient registry, which collects data for research, as well as its clinical network of medical institutions to include more diverse communities.
Dr. Gupta is now leading one of the first studies funded by the National Institutes of Health to investigate food allergy in children by race and ethnicity. It looks at all aspects of food allergies, including family life, management, access to care, and genetics.
“That’s a big deal,” said Dr. Gupta. “Because if we really want to improve food allergy management, care and understanding, we really need to understand how it impacts different groups. And that hasn’t been done.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Primary care workforce expanding, but mostly in cities
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Burnout risk may be exacerbated by COVID crisis
New kinds of job stress multiply in unusual times
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
New kinds of job stress multiply in unusual times
New kinds of job stress multiply in unusual times
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
Tylosis in a Patient With Howel-Evans Syndrome: Management With Acitretin
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.
There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.
There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.
There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
Practice Points
- Keratoderma can be especially painful for patients and can have a great impact on their quality of life. For these patients, acitretin should be considered when topical therapies have failed.
- Howel-Evans syndrome is an autosomal-dominant condition that predominantly presents with plantar keratoderma and has a high risk for esophageal cancer.
Medicare fines half of hospitals for readmitting too many patients
Nearly half the nation’s hospitals, many of which are still wrestling with the financial fallout of the unexpected coronavirus, will get lower payments for all Medicare patients because of their history of readmitting patients, federal records show.
The penalties are the ninth annual round of the Hospital Readmissions Reduction Program created as part of the Affordable Care Act’s broader effort to improve quality and lower costs. The latest penalties are calculated using each hospital case history between July 2016 and June 2019, so the flood of coronavirus patients that have swamped hospitals this year were not included.
The Centers for Medicare & Medicaid Services announced in September it may suspend the penalty program in the future if the chaos surrounding the pandemic, including the spring’s moratorium on elective surgeries, makes it too difficult to assess hospital performance.
For this year, the penalties remain in effect. Retroactive to the federal fiscal year that began Oct. 1, Medicare will lower a year’s worth of payments to 2,545 hospitals, the data show. The average reduction is 0.69%, with 613 hospitals receiving a penalty of 1% or more.
Out of 5,267 hospitals in the country, Congress has exempted 2,176 from the threat of penalties, either because they are critical access hospitals – defined as the only inpatient facility in an area – or hospitals that specialize in psychiatric patients, children, veterans, rehabilitation or long-term care. Of the 3,080 hospitals CMS evaluated, 83% received a penalty.
The number and severity of penalties were comparable to those of recent years, although the number of hospitals receiving the maximum penalty of 3% dropped from 56 to 39. Because the penalties are applied to new admission payments, the total dollar amount each hospital will lose will not be known until after the fiscal year ends on July 30.
“It’s unfortunate that hospitals will face readmission penalties in fiscal year 2021,” said Akin Demehin, director of policy at the American Hospital Association. “Given the financial strain that hospitals are under, every dollar counts, and the impact of any penalty is significant.”
The penalties are based on readmissions of Medicare patients who initially came to the hospital with diagnoses of congestive heart failure, heart attack, pneumonia, chronic obstructive pulmonary disease, hip or knee replacement, or coronary artery bypass graft surgery. Medicare counts as a readmission any of those patients who ended up back in any hospital within 30 days of discharge, except for planned returns like a second phase of surgery.
A hospital will be penalized if its readmission rate is higher than expected given the national trends in any one of those categories.
The industry has disapproved of the program since its inception, complaining the measures aren’t precise and it unfairly punishes hospitals that treat low-income patients, who often don’t have the resources to ensure their recoveries are successful.
Michael Millenson, a health quality consultant who focuses on patient safety, said the penalties are a useful but imperfect mechanism to push hospitals to improve their care. The designers of the penalty system envisioned it as a way to neutralize the economic benefit hospitals get from readmitted patients under Medicare’s fee-for-service payment model, as they are otherwise paid for two stays instead of just one.
“Every industry complains the penalties are too harsh,” he said. “if you’re going to tell me we don’t need any economic incentives to do the right thing because we’re always doing the right thing – that’s not true.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Nearly half the nation’s hospitals, many of which are still wrestling with the financial fallout of the unexpected coronavirus, will get lower payments for all Medicare patients because of their history of readmitting patients, federal records show.
The penalties are the ninth annual round of the Hospital Readmissions Reduction Program created as part of the Affordable Care Act’s broader effort to improve quality and lower costs. The latest penalties are calculated using each hospital case history between July 2016 and June 2019, so the flood of coronavirus patients that have swamped hospitals this year were not included.
The Centers for Medicare & Medicaid Services announced in September it may suspend the penalty program in the future if the chaos surrounding the pandemic, including the spring’s moratorium on elective surgeries, makes it too difficult to assess hospital performance.
For this year, the penalties remain in effect. Retroactive to the federal fiscal year that began Oct. 1, Medicare will lower a year’s worth of payments to 2,545 hospitals, the data show. The average reduction is 0.69%, with 613 hospitals receiving a penalty of 1% or more.
Out of 5,267 hospitals in the country, Congress has exempted 2,176 from the threat of penalties, either because they are critical access hospitals – defined as the only inpatient facility in an area – or hospitals that specialize in psychiatric patients, children, veterans, rehabilitation or long-term care. Of the 3,080 hospitals CMS evaluated, 83% received a penalty.
The number and severity of penalties were comparable to those of recent years, although the number of hospitals receiving the maximum penalty of 3% dropped from 56 to 39. Because the penalties are applied to new admission payments, the total dollar amount each hospital will lose will not be known until after the fiscal year ends on July 30.
“It’s unfortunate that hospitals will face readmission penalties in fiscal year 2021,” said Akin Demehin, director of policy at the American Hospital Association. “Given the financial strain that hospitals are under, every dollar counts, and the impact of any penalty is significant.”
The penalties are based on readmissions of Medicare patients who initially came to the hospital with diagnoses of congestive heart failure, heart attack, pneumonia, chronic obstructive pulmonary disease, hip or knee replacement, or coronary artery bypass graft surgery. Medicare counts as a readmission any of those patients who ended up back in any hospital within 30 days of discharge, except for planned returns like a second phase of surgery.
A hospital will be penalized if its readmission rate is higher than expected given the national trends in any one of those categories.
The industry has disapproved of the program since its inception, complaining the measures aren’t precise and it unfairly punishes hospitals that treat low-income patients, who often don’t have the resources to ensure their recoveries are successful.
Michael Millenson, a health quality consultant who focuses on patient safety, said the penalties are a useful but imperfect mechanism to push hospitals to improve their care. The designers of the penalty system envisioned it as a way to neutralize the economic benefit hospitals get from readmitted patients under Medicare’s fee-for-service payment model, as they are otherwise paid for two stays instead of just one.
“Every industry complains the penalties are too harsh,” he said. “if you’re going to tell me we don’t need any economic incentives to do the right thing because we’re always doing the right thing – that’s not true.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Nearly half the nation’s hospitals, many of which are still wrestling with the financial fallout of the unexpected coronavirus, will get lower payments for all Medicare patients because of their history of readmitting patients, federal records show.
The penalties are the ninth annual round of the Hospital Readmissions Reduction Program created as part of the Affordable Care Act’s broader effort to improve quality and lower costs. The latest penalties are calculated using each hospital case history between July 2016 and June 2019, so the flood of coronavirus patients that have swamped hospitals this year were not included.
The Centers for Medicare & Medicaid Services announced in September it may suspend the penalty program in the future if the chaos surrounding the pandemic, including the spring’s moratorium on elective surgeries, makes it too difficult to assess hospital performance.
For this year, the penalties remain in effect. Retroactive to the federal fiscal year that began Oct. 1, Medicare will lower a year’s worth of payments to 2,545 hospitals, the data show. The average reduction is 0.69%, with 613 hospitals receiving a penalty of 1% or more.
Out of 5,267 hospitals in the country, Congress has exempted 2,176 from the threat of penalties, either because they are critical access hospitals – defined as the only inpatient facility in an area – or hospitals that specialize in psychiatric patients, children, veterans, rehabilitation or long-term care. Of the 3,080 hospitals CMS evaluated, 83% received a penalty.
The number and severity of penalties were comparable to those of recent years, although the number of hospitals receiving the maximum penalty of 3% dropped from 56 to 39. Because the penalties are applied to new admission payments, the total dollar amount each hospital will lose will not be known until after the fiscal year ends on July 30.
“It’s unfortunate that hospitals will face readmission penalties in fiscal year 2021,” said Akin Demehin, director of policy at the American Hospital Association. “Given the financial strain that hospitals are under, every dollar counts, and the impact of any penalty is significant.”
The penalties are based on readmissions of Medicare patients who initially came to the hospital with diagnoses of congestive heart failure, heart attack, pneumonia, chronic obstructive pulmonary disease, hip or knee replacement, or coronary artery bypass graft surgery. Medicare counts as a readmission any of those patients who ended up back in any hospital within 30 days of discharge, except for planned returns like a second phase of surgery.
A hospital will be penalized if its readmission rate is higher than expected given the national trends in any one of those categories.
The industry has disapproved of the program since its inception, complaining the measures aren’t precise and it unfairly punishes hospitals that treat low-income patients, who often don’t have the resources to ensure their recoveries are successful.
Michael Millenson, a health quality consultant who focuses on patient safety, said the penalties are a useful but imperfect mechanism to push hospitals to improve their care. The designers of the penalty system envisioned it as a way to neutralize the economic benefit hospitals get from readmitted patients under Medicare’s fee-for-service payment model, as they are otherwise paid for two stays instead of just one.
“Every industry complains the penalties are too harsh,” he said. “if you’re going to tell me we don’t need any economic incentives to do the right thing because we’re always doing the right thing – that’s not true.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Nivolumab Use for First-Line Management of Hepatocellular Carcinoma: Results of a Real-World Cohort of Patients
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
Preemptive CMV monitoring beats prophylaxis post liver transplant
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
FROM IDWEEK 2020
Health sector has spent $464 million on lobbying in 2020
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.