User login
For MD-IQ use only
FDA approves Recarbrio for cUTI, cIAI treatment in adults
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
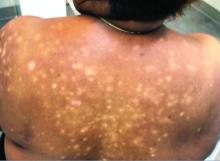
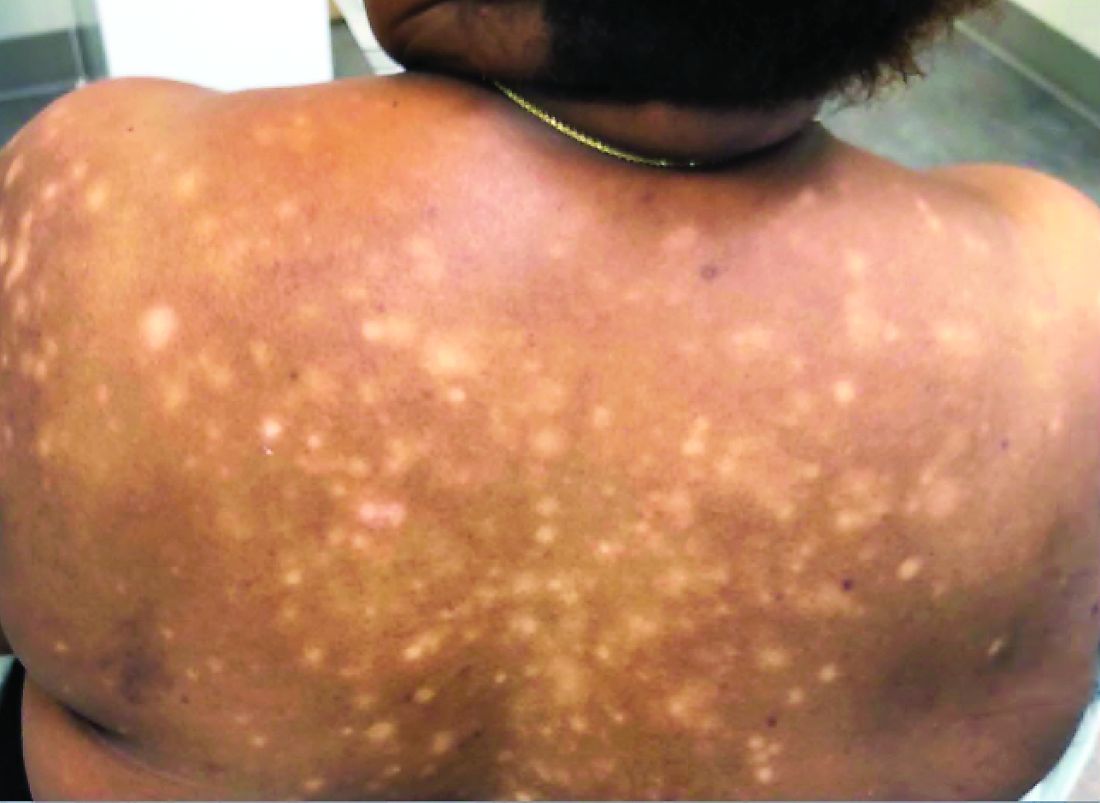
A 56-year-old black woman presented with asymptomatic hypopigmented macules on her back, chest, face, and lateral arms
They often coalesce near the midline, and occasionally extend beyond the trunk to the arms, legs, head, or neck. PMH is more frequently diagnosed among black individuals, although it affects all races and ethnicities. The natural history of PMH can vary from stable and progressive disease, and may resolve spontaneously after a few years. The pathogenesis of PMH remains unknown. It has been proposed that the hypopigmentation is caused by decreased melanin production and altered melanosome dispersal in reaction to Propionibacterium acnes.
PMH must be distinguished from some of its clinical mimickers, including vitiligo, hypopigmented mycosis fungoides, tinea versicolor, and pityriasis alba. Potassium hydroxide preparations can be performed in the office to evaluate for tinea versicolor. An additional tool to aid in diagnosis is the use of a Wood’s light. The lesions of PMH characteristically show punctiform orange-red follicular fluorescence when exposed to a Wood’s light, indicating the presence of a porphyrin-producing organism, presumably P. acnes. A skin biopsy is necessary to rule out hypopigmented mycosis fungoides.
Skin biopsy of PMH typically reveals decreased melanin with a normal number of melanocytes. In our patient, a punch biopsy of the right lateral arm demonstrated minimally decreased density of epidermal melanocytes with dermal pigment incontinence. SOX10 immunohistochemical staining demonstrated scattered melanocytes in the epidermis. Preserved melanin within keratinocytes was noted.
In our patient, there was significant spread to the face, which is highly unusual and has only been documented in a few case series. There are no standard recommendations for definitive treatment of PMH. Topical antimicrobial therapies, such as clindamycin solution and benzoyl peroxide gel, have been beneficial in some studies. Tetracyclines, narrow-band ultraviolet B phototherapy, and even isotretinoin have had some reported success.
This case and photo were submitted by Mr. Franzetti, Dr. Rush, and Dr. Shalin of the University of Arkansas for Medical Sciences, Little Rock.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
They often coalesce near the midline, and occasionally extend beyond the trunk to the arms, legs, head, or neck. PMH is more frequently diagnosed among black individuals, although it affects all races and ethnicities. The natural history of PMH can vary from stable and progressive disease, and may resolve spontaneously after a few years. The pathogenesis of PMH remains unknown. It has been proposed that the hypopigmentation is caused by decreased melanin production and altered melanosome dispersal in reaction to Propionibacterium acnes.
PMH must be distinguished from some of its clinical mimickers, including vitiligo, hypopigmented mycosis fungoides, tinea versicolor, and pityriasis alba. Potassium hydroxide preparations can be performed in the office to evaluate for tinea versicolor. An additional tool to aid in diagnosis is the use of a Wood’s light. The lesions of PMH characteristically show punctiform orange-red follicular fluorescence when exposed to a Wood’s light, indicating the presence of a porphyrin-producing organism, presumably P. acnes. A skin biopsy is necessary to rule out hypopigmented mycosis fungoides.
Skin biopsy of PMH typically reveals decreased melanin with a normal number of melanocytes. In our patient, a punch biopsy of the right lateral arm demonstrated minimally decreased density of epidermal melanocytes with dermal pigment incontinence. SOX10 immunohistochemical staining demonstrated scattered melanocytes in the epidermis. Preserved melanin within keratinocytes was noted.
In our patient, there was significant spread to the face, which is highly unusual and has only been documented in a few case series. There are no standard recommendations for definitive treatment of PMH. Topical antimicrobial therapies, such as clindamycin solution and benzoyl peroxide gel, have been beneficial in some studies. Tetracyclines, narrow-band ultraviolet B phototherapy, and even isotretinoin have had some reported success.
This case and photo were submitted by Mr. Franzetti, Dr. Rush, and Dr. Shalin of the University of Arkansas for Medical Sciences, Little Rock.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
They often coalesce near the midline, and occasionally extend beyond the trunk to the arms, legs, head, or neck. PMH is more frequently diagnosed among black individuals, although it affects all races and ethnicities. The natural history of PMH can vary from stable and progressive disease, and may resolve spontaneously after a few years. The pathogenesis of PMH remains unknown. It has been proposed that the hypopigmentation is caused by decreased melanin production and altered melanosome dispersal in reaction to Propionibacterium acnes.
PMH must be distinguished from some of its clinical mimickers, including vitiligo, hypopigmented mycosis fungoides, tinea versicolor, and pityriasis alba. Potassium hydroxide preparations can be performed in the office to evaluate for tinea versicolor. An additional tool to aid in diagnosis is the use of a Wood’s light. The lesions of PMH characteristically show punctiform orange-red follicular fluorescence when exposed to a Wood’s light, indicating the presence of a porphyrin-producing organism, presumably P. acnes. A skin biopsy is necessary to rule out hypopigmented mycosis fungoides.
Skin biopsy of PMH typically reveals decreased melanin with a normal number of melanocytes. In our patient, a punch biopsy of the right lateral arm demonstrated minimally decreased density of epidermal melanocytes with dermal pigment incontinence. SOX10 immunohistochemical staining demonstrated scattered melanocytes in the epidermis. Preserved melanin within keratinocytes was noted.
In our patient, there was significant spread to the face, which is highly unusual and has only been documented in a few case series. There are no standard recommendations for definitive treatment of PMH. Topical antimicrobial therapies, such as clindamycin solution and benzoyl peroxide gel, have been beneficial in some studies. Tetracyclines, narrow-band ultraviolet B phototherapy, and even isotretinoin have had some reported success.
This case and photo were submitted by Mr. Franzetti, Dr. Rush, and Dr. Shalin of the University of Arkansas for Medical Sciences, Little Rock.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Recertification: The FPHM option
ABIM now offers increased flexibility
Everyone always told me that my time in residency would fly by, and the 3 years of internal medicine training really did seem to pass in just a few moments. Before I knew it, I had passed my internal medicine boards and practiced hospital medicine at an academic medical center.
One day last fall, I received notice from the American Board of Internal Medicine that it was time to recertify. I was surprised – had it already been 10 years? What did I have to do to maintain my certification?
As I investigated what it would take to maintain certification, I discovered that the recertification process provided more flexibility, compared with original board certification. I now had the option to recertify in internal medicine with a Focused Practice in Hospital Medicine (FPHM). Beginning in 2014, ABIM offered hospitalists, or internists whose clinical practice is mainly in the inpatient setting, the option to recertify in internal medicine, but with the designation that highlighted their clinical practice in the inpatient setting.
The first step in recertification for me was deciding to recertify with the focus in hospital medicine or maintain the traditional internal medicine certification. I talked with several colleagues who are also practicing hospitalists and weighed their reasons for opting for FPHM. Ultimately, my decision to pursue a recertification with a focus in hospital medicine relied on three factors: First, my clinical practice since completing residency was exclusively in the inpatient setting. Day in and day out, I care for patients who are acutely ill and require inpatient medical care. Second, I wanted my board certification to reflect what I consider to be my area of clinical expertise, which is inpatient adult medicine. Pursuing the FPHM would provide that recognition. Finally, I wanted to study and be tested on topics that I could utilize in my day-to-day practice. Because I exclusively practiced hospital medicine since graduation, areas of clinical internal medicine that I did not frequently encounter in my daily practice became less accessible in my knowledge base.
The next step then was to enter the FPHM Maintenance of Certification (MOC) program.
The ABIM requires two attestations to verify that I met the requirements to be a hospitalist. First was a self-attestation confirming at least 3 years of unsupervised inpatient care practice experience, and meeting patient encounter thresholds in the inpatient setting. The second attestation was from a “Senior Hospital Officer” confirming the information in the self-attestation was accurate.
Once entered into the program and having an unrestricted medical license to practice, I had to complete the remaining requirements of earning MOC points and then passing a knowledge-based assessment. I had to accumulate a total of 100 MOC points in the past 5 years, which I succeeded in doing through participating in quality improvement projects, recording CME credits, studying for the exam, and even taking the exam. I could track my point totals through the ABIM Physician Portal, which updated my point tally automatically for activities that counted toward MOC, such as attending SHM’s annual conference.
The final component was to pass the knowledge assessment, the dreaded exam. In 2018, I had the option to take the 10-year FPHM exam or do a general internal medicine Knowledge Check-In. Beginning in 2020, candidates will be able to sit for either the 10-year Focused Practice in Hospital Medicine exam or begin the Hospital Medicine Knowledge Check-In pathway. I had already decided to pursue FPHM and began to prepare to sit for an exam. I scheduled my exam through the ABIM portal at a local testing center.
The exam was scheduled for a full day, consisting of four sections broken up by a lunch break and section breaks. Specifically, the 220 single best answer, multiple-choice exam covered diagnosis, testing, treatment decisions, epidemiology, and basic science content through patient scenarios that reflected the scope of practice of a hospitalist. The ABIM provided an exam blueprint that detailed the specific clinical topics and the likelihood that a question pertaining to that topic would show up on the exam. Content was described as high, medium, or low importance and the number of questions related to the content was 75% for high importance, no more than 25% for medium importance, and no questions for low-importance content. In addition, content was distributed in a way that was reflective of my clinical practice as a hospitalist: 63.5% inpatient and traditional care; 6.5% palliative care; 15% consultative comanagement; and 15% quality, safety, and clinical reasoning.
Beginning 6 months prior to my scheduled exam, I purchased two critical resources to guide my studying efforts: the SHM Spark Self-Assessment Tool and the American College of Physicians Medical Knowledge Self-Assessment Program to review subject matter content and also do practice questions.
The latest version of SHM’s program, Spark Edition 2, provides updated questions and resources tailored to the hospital medicine exams. I appreciated the ability to answer questions online, as well as on my phone so I could do questions on the go. Moreover, I was able to track which content areas were stronger or weaker for me, and focus attention on areas that needed more work. Importantly, the questions I answered using the Spark self-assessment tool closely aligned with the subject matter I encountered in the exam, as well as the clinical cases I encounter every day in my practice.
While the day-long exam was challenging, I was gratified to receive notice from the ABIM that I had successfully recertified in internal medicine with a Focused Practice in Hospital Medicine!
Dr. Tad-y is a hospitalist at the University of Colorado at Denver, Aurora, and associate vice chair of quality in the department of medicine at the University of Colorado.
ABIM now offers increased flexibility
ABIM now offers increased flexibility
Everyone always told me that my time in residency would fly by, and the 3 years of internal medicine training really did seem to pass in just a few moments. Before I knew it, I had passed my internal medicine boards and practiced hospital medicine at an academic medical center.
One day last fall, I received notice from the American Board of Internal Medicine that it was time to recertify. I was surprised – had it already been 10 years? What did I have to do to maintain my certification?
As I investigated what it would take to maintain certification, I discovered that the recertification process provided more flexibility, compared with original board certification. I now had the option to recertify in internal medicine with a Focused Practice in Hospital Medicine (FPHM). Beginning in 2014, ABIM offered hospitalists, or internists whose clinical practice is mainly in the inpatient setting, the option to recertify in internal medicine, but with the designation that highlighted their clinical practice in the inpatient setting.
The first step in recertification for me was deciding to recertify with the focus in hospital medicine or maintain the traditional internal medicine certification. I talked with several colleagues who are also practicing hospitalists and weighed their reasons for opting for FPHM. Ultimately, my decision to pursue a recertification with a focus in hospital medicine relied on three factors: First, my clinical practice since completing residency was exclusively in the inpatient setting. Day in and day out, I care for patients who are acutely ill and require inpatient medical care. Second, I wanted my board certification to reflect what I consider to be my area of clinical expertise, which is inpatient adult medicine. Pursuing the FPHM would provide that recognition. Finally, I wanted to study and be tested on topics that I could utilize in my day-to-day practice. Because I exclusively practiced hospital medicine since graduation, areas of clinical internal medicine that I did not frequently encounter in my daily practice became less accessible in my knowledge base.
The next step then was to enter the FPHM Maintenance of Certification (MOC) program.
The ABIM requires two attestations to verify that I met the requirements to be a hospitalist. First was a self-attestation confirming at least 3 years of unsupervised inpatient care practice experience, and meeting patient encounter thresholds in the inpatient setting. The second attestation was from a “Senior Hospital Officer” confirming the information in the self-attestation was accurate.
Once entered into the program and having an unrestricted medical license to practice, I had to complete the remaining requirements of earning MOC points and then passing a knowledge-based assessment. I had to accumulate a total of 100 MOC points in the past 5 years, which I succeeded in doing through participating in quality improvement projects, recording CME credits, studying for the exam, and even taking the exam. I could track my point totals through the ABIM Physician Portal, which updated my point tally automatically for activities that counted toward MOC, such as attending SHM’s annual conference.
The final component was to pass the knowledge assessment, the dreaded exam. In 2018, I had the option to take the 10-year FPHM exam or do a general internal medicine Knowledge Check-In. Beginning in 2020, candidates will be able to sit for either the 10-year Focused Practice in Hospital Medicine exam or begin the Hospital Medicine Knowledge Check-In pathway. I had already decided to pursue FPHM and began to prepare to sit for an exam. I scheduled my exam through the ABIM portal at a local testing center.
The exam was scheduled for a full day, consisting of four sections broken up by a lunch break and section breaks. Specifically, the 220 single best answer, multiple-choice exam covered diagnosis, testing, treatment decisions, epidemiology, and basic science content through patient scenarios that reflected the scope of practice of a hospitalist. The ABIM provided an exam blueprint that detailed the specific clinical topics and the likelihood that a question pertaining to that topic would show up on the exam. Content was described as high, medium, or low importance and the number of questions related to the content was 75% for high importance, no more than 25% for medium importance, and no questions for low-importance content. In addition, content was distributed in a way that was reflective of my clinical practice as a hospitalist: 63.5% inpatient and traditional care; 6.5% palliative care; 15% consultative comanagement; and 15% quality, safety, and clinical reasoning.
Beginning 6 months prior to my scheduled exam, I purchased two critical resources to guide my studying efforts: the SHM Spark Self-Assessment Tool and the American College of Physicians Medical Knowledge Self-Assessment Program to review subject matter content and also do practice questions.
The latest version of SHM’s program, Spark Edition 2, provides updated questions and resources tailored to the hospital medicine exams. I appreciated the ability to answer questions online, as well as on my phone so I could do questions on the go. Moreover, I was able to track which content areas were stronger or weaker for me, and focus attention on areas that needed more work. Importantly, the questions I answered using the Spark self-assessment tool closely aligned with the subject matter I encountered in the exam, as well as the clinical cases I encounter every day in my practice.
While the day-long exam was challenging, I was gratified to receive notice from the ABIM that I had successfully recertified in internal medicine with a Focused Practice in Hospital Medicine!
Dr. Tad-y is a hospitalist at the University of Colorado at Denver, Aurora, and associate vice chair of quality in the department of medicine at the University of Colorado.
Everyone always told me that my time in residency would fly by, and the 3 years of internal medicine training really did seem to pass in just a few moments. Before I knew it, I had passed my internal medicine boards and practiced hospital medicine at an academic medical center.
One day last fall, I received notice from the American Board of Internal Medicine that it was time to recertify. I was surprised – had it already been 10 years? What did I have to do to maintain my certification?
As I investigated what it would take to maintain certification, I discovered that the recertification process provided more flexibility, compared with original board certification. I now had the option to recertify in internal medicine with a Focused Practice in Hospital Medicine (FPHM). Beginning in 2014, ABIM offered hospitalists, or internists whose clinical practice is mainly in the inpatient setting, the option to recertify in internal medicine, but with the designation that highlighted their clinical practice in the inpatient setting.
The first step in recertification for me was deciding to recertify with the focus in hospital medicine or maintain the traditional internal medicine certification. I talked with several colleagues who are also practicing hospitalists and weighed their reasons for opting for FPHM. Ultimately, my decision to pursue a recertification with a focus in hospital medicine relied on three factors: First, my clinical practice since completing residency was exclusively in the inpatient setting. Day in and day out, I care for patients who are acutely ill and require inpatient medical care. Second, I wanted my board certification to reflect what I consider to be my area of clinical expertise, which is inpatient adult medicine. Pursuing the FPHM would provide that recognition. Finally, I wanted to study and be tested on topics that I could utilize in my day-to-day practice. Because I exclusively practiced hospital medicine since graduation, areas of clinical internal medicine that I did not frequently encounter in my daily practice became less accessible in my knowledge base.
The next step then was to enter the FPHM Maintenance of Certification (MOC) program.
The ABIM requires two attestations to verify that I met the requirements to be a hospitalist. First was a self-attestation confirming at least 3 years of unsupervised inpatient care practice experience, and meeting patient encounter thresholds in the inpatient setting. The second attestation was from a “Senior Hospital Officer” confirming the information in the self-attestation was accurate.
Once entered into the program and having an unrestricted medical license to practice, I had to complete the remaining requirements of earning MOC points and then passing a knowledge-based assessment. I had to accumulate a total of 100 MOC points in the past 5 years, which I succeeded in doing through participating in quality improvement projects, recording CME credits, studying for the exam, and even taking the exam. I could track my point totals through the ABIM Physician Portal, which updated my point tally automatically for activities that counted toward MOC, such as attending SHM’s annual conference.
The final component was to pass the knowledge assessment, the dreaded exam. In 2018, I had the option to take the 10-year FPHM exam or do a general internal medicine Knowledge Check-In. Beginning in 2020, candidates will be able to sit for either the 10-year Focused Practice in Hospital Medicine exam or begin the Hospital Medicine Knowledge Check-In pathway. I had already decided to pursue FPHM and began to prepare to sit for an exam. I scheduled my exam through the ABIM portal at a local testing center.
The exam was scheduled for a full day, consisting of four sections broken up by a lunch break and section breaks. Specifically, the 220 single best answer, multiple-choice exam covered diagnosis, testing, treatment decisions, epidemiology, and basic science content through patient scenarios that reflected the scope of practice of a hospitalist. The ABIM provided an exam blueprint that detailed the specific clinical topics and the likelihood that a question pertaining to that topic would show up on the exam. Content was described as high, medium, or low importance and the number of questions related to the content was 75% for high importance, no more than 25% for medium importance, and no questions for low-importance content. In addition, content was distributed in a way that was reflective of my clinical practice as a hospitalist: 63.5% inpatient and traditional care; 6.5% palliative care; 15% consultative comanagement; and 15% quality, safety, and clinical reasoning.
Beginning 6 months prior to my scheduled exam, I purchased two critical resources to guide my studying efforts: the SHM Spark Self-Assessment Tool and the American College of Physicians Medical Knowledge Self-Assessment Program to review subject matter content and also do practice questions.
The latest version of SHM’s program, Spark Edition 2, provides updated questions and resources tailored to the hospital medicine exams. I appreciated the ability to answer questions online, as well as on my phone so I could do questions on the go. Moreover, I was able to track which content areas were stronger or weaker for me, and focus attention on areas that needed more work. Importantly, the questions I answered using the Spark self-assessment tool closely aligned with the subject matter I encountered in the exam, as well as the clinical cases I encounter every day in my practice.
While the day-long exam was challenging, I was gratified to receive notice from the ABIM that I had successfully recertified in internal medicine with a Focused Practice in Hospital Medicine!
Dr. Tad-y is a hospitalist at the University of Colorado at Denver, Aurora, and associate vice chair of quality in the department of medicine at the University of Colorado.
Dealing with staffing shortfalls
Five options for covering unfilled positions
Being in stressful situations is part of being a hospitalist. During a hospitalist’s work shift, one of the key determinants of stress is adequate staffing. With use of survey data from 569 hospital medicine groups (HMGs) across the nation, one of the topics examined in the 2018 State of Hospital Medicine Report is how HMGs cope with unfilled hospitalist physician positions.
The survey presented five options for covering unfilled hospitalist physician positions: use of locum tenens, use of moonlighters, use of voluntary extra shifts by the HMG’s existing hospitalists, use of required extra shifts, and leaving some shifts uncovered. Recipients were instructed to select all options that applied, so totals exceeded 100%. The data is organized according to HMGs that serve adults only, children only, and both adults and children.
For all three types of HMGs, the most common tactic to fill coverage gaps is through voluntary extra shifts by existing clinicians, reportedly used by 70.3% of HMGs that cover adults only, 66.7% by those that cover children only, and 76.9% by those that cover both adults and children. Data for adults-only HMGs was further broken down by geographic region, academic status, teaching status, group size, and employment model. Among adults-only HMGs, there is a direct correlation between group size and having members voluntarily work extra shifts, with 91.1% of groups with 30 or more full-time equivalent positions employing this tactic.
For HMGs that cover adults only and those that cover children only, the second most common tactic is to use moonlighters (57.4% and 53.3% respectively), while use of moonlighters is the third most commonly employed surveyed tactic for HMGs that cover both adults and children (53.8%).
HMGs that serve both adults and children were much more likely to utilize locum tenens to cover unfilled positions (69.2%) than were groups that serve adults only (44.0%) or children only (26.7%). The variability in the use of locum tenens is likely because of the willingness and/or ability of the respective groups to afford this option because it is generally the most expensive option of those surveyed.
Requiring that members of the group work extra shifts is the least popular staffing method among adults-only HMGs (10.0%) and HMGs serving both children and adults (7.7%). This strategy is unpopular, especially when there is little advance warning. Surprisingly, 40.0% of HMGs that see children only require members to work extra shifts to cover unfilled slots. This could be because pediatric HMGs are often smaller, and it would be more difficult to absorb the work if the shift is left uncovered. In fact, many pediatric HMGs staff with only one clinician at a time, so there may be no option besides requiring someone else in the group to come in and work.Of the options surveyed, perhaps the most uncomfortable for those hospitalist physicians on duty is to leave some shifts uncovered. The rapid growth and development of the specialty of hospital medicine has made it difficult for HMGs to continuously hire qualified hospitalists fast enough to meet demand. The survey found 46.2% of HMGs that serve both adults and children and 31.4% of groups that serve adults only have employed the staffing model of going short-staffed for at least some shifts. HMGs serving children-only are much less likely to go short-staffed (20.0%).
I work with a large HMG that has more than 70 members, and when it has been short-staffed, it tries to ensure a full complement of evening and night staff as the top priority because these shifts are typically more stressful. Since we have more hospitalist capacity during the day to absorb the loss of a physician, we pull staff from their daytime rounding schedules to execute this strategy. While going short-staffed is not ideal, this option has worked for many groups out of sheer necessity.
Dr. Stephan is a hospitalist at Allina Health’s Abbott Northwestern Hospital in Minneapolis and is a member of the SHM Practice Analysis Committee.
Five options for covering unfilled positions
Five options for covering unfilled positions
Being in stressful situations is part of being a hospitalist. During a hospitalist’s work shift, one of the key determinants of stress is adequate staffing. With use of survey data from 569 hospital medicine groups (HMGs) across the nation, one of the topics examined in the 2018 State of Hospital Medicine Report is how HMGs cope with unfilled hospitalist physician positions.
The survey presented five options for covering unfilled hospitalist physician positions: use of locum tenens, use of moonlighters, use of voluntary extra shifts by the HMG’s existing hospitalists, use of required extra shifts, and leaving some shifts uncovered. Recipients were instructed to select all options that applied, so totals exceeded 100%. The data is organized according to HMGs that serve adults only, children only, and both adults and children.
For all three types of HMGs, the most common tactic to fill coverage gaps is through voluntary extra shifts by existing clinicians, reportedly used by 70.3% of HMGs that cover adults only, 66.7% by those that cover children only, and 76.9% by those that cover both adults and children. Data for adults-only HMGs was further broken down by geographic region, academic status, teaching status, group size, and employment model. Among adults-only HMGs, there is a direct correlation between group size and having members voluntarily work extra shifts, with 91.1% of groups with 30 or more full-time equivalent positions employing this tactic.
For HMGs that cover adults only and those that cover children only, the second most common tactic is to use moonlighters (57.4% and 53.3% respectively), while use of moonlighters is the third most commonly employed surveyed tactic for HMGs that cover both adults and children (53.8%).
HMGs that serve both adults and children were much more likely to utilize locum tenens to cover unfilled positions (69.2%) than were groups that serve adults only (44.0%) or children only (26.7%). The variability in the use of locum tenens is likely because of the willingness and/or ability of the respective groups to afford this option because it is generally the most expensive option of those surveyed.
Requiring that members of the group work extra shifts is the least popular staffing method among adults-only HMGs (10.0%) and HMGs serving both children and adults (7.7%). This strategy is unpopular, especially when there is little advance warning. Surprisingly, 40.0% of HMGs that see children only require members to work extra shifts to cover unfilled slots. This could be because pediatric HMGs are often smaller, and it would be more difficult to absorb the work if the shift is left uncovered. In fact, many pediatric HMGs staff with only one clinician at a time, so there may be no option besides requiring someone else in the group to come in and work.Of the options surveyed, perhaps the most uncomfortable for those hospitalist physicians on duty is to leave some shifts uncovered. The rapid growth and development of the specialty of hospital medicine has made it difficult for HMGs to continuously hire qualified hospitalists fast enough to meet demand. The survey found 46.2% of HMGs that serve both adults and children and 31.4% of groups that serve adults only have employed the staffing model of going short-staffed for at least some shifts. HMGs serving children-only are much less likely to go short-staffed (20.0%).
I work with a large HMG that has more than 70 members, and when it has been short-staffed, it tries to ensure a full complement of evening and night staff as the top priority because these shifts are typically more stressful. Since we have more hospitalist capacity during the day to absorb the loss of a physician, we pull staff from their daytime rounding schedules to execute this strategy. While going short-staffed is not ideal, this option has worked for many groups out of sheer necessity.
Dr. Stephan is a hospitalist at Allina Health’s Abbott Northwestern Hospital in Minneapolis and is a member of the SHM Practice Analysis Committee.
Being in stressful situations is part of being a hospitalist. During a hospitalist’s work shift, one of the key determinants of stress is adequate staffing. With use of survey data from 569 hospital medicine groups (HMGs) across the nation, one of the topics examined in the 2018 State of Hospital Medicine Report is how HMGs cope with unfilled hospitalist physician positions.
The survey presented five options for covering unfilled hospitalist physician positions: use of locum tenens, use of moonlighters, use of voluntary extra shifts by the HMG’s existing hospitalists, use of required extra shifts, and leaving some shifts uncovered. Recipients were instructed to select all options that applied, so totals exceeded 100%. The data is organized according to HMGs that serve adults only, children only, and both adults and children.
For all three types of HMGs, the most common tactic to fill coverage gaps is through voluntary extra shifts by existing clinicians, reportedly used by 70.3% of HMGs that cover adults only, 66.7% by those that cover children only, and 76.9% by those that cover both adults and children. Data for adults-only HMGs was further broken down by geographic region, academic status, teaching status, group size, and employment model. Among adults-only HMGs, there is a direct correlation between group size and having members voluntarily work extra shifts, with 91.1% of groups with 30 or more full-time equivalent positions employing this tactic.
For HMGs that cover adults only and those that cover children only, the second most common tactic is to use moonlighters (57.4% and 53.3% respectively), while use of moonlighters is the third most commonly employed surveyed tactic for HMGs that cover both adults and children (53.8%).
HMGs that serve both adults and children were much more likely to utilize locum tenens to cover unfilled positions (69.2%) than were groups that serve adults only (44.0%) or children only (26.7%). The variability in the use of locum tenens is likely because of the willingness and/or ability of the respective groups to afford this option because it is generally the most expensive option of those surveyed.
Requiring that members of the group work extra shifts is the least popular staffing method among adults-only HMGs (10.0%) and HMGs serving both children and adults (7.7%). This strategy is unpopular, especially when there is little advance warning. Surprisingly, 40.0% of HMGs that see children only require members to work extra shifts to cover unfilled slots. This could be because pediatric HMGs are often smaller, and it would be more difficult to absorb the work if the shift is left uncovered. In fact, many pediatric HMGs staff with only one clinician at a time, so there may be no option besides requiring someone else in the group to come in and work.Of the options surveyed, perhaps the most uncomfortable for those hospitalist physicians on duty is to leave some shifts uncovered. The rapid growth and development of the specialty of hospital medicine has made it difficult for HMGs to continuously hire qualified hospitalists fast enough to meet demand. The survey found 46.2% of HMGs that serve both adults and children and 31.4% of groups that serve adults only have employed the staffing model of going short-staffed for at least some shifts. HMGs serving children-only are much less likely to go short-staffed (20.0%).
I work with a large HMG that has more than 70 members, and when it has been short-staffed, it tries to ensure a full complement of evening and night staff as the top priority because these shifts are typically more stressful. Since we have more hospitalist capacity during the day to absorb the loss of a physician, we pull staff from their daytime rounding schedules to execute this strategy. While going short-staffed is not ideal, this option has worked for many groups out of sheer necessity.
Dr. Stephan is a hospitalist at Allina Health’s Abbott Northwestern Hospital in Minneapolis and is a member of the SHM Practice Analysis Committee.
What’s in the Water? Keeping Watch on Crypto
If it’s swimming season, it’s also Cryptosporidium season. The parasite, spread through feces of infected humans or animals, is the culprit in most disease outbreaks linked to water.
Between 2009 and 2017, Crypto-related outbreaks increased an average 13% per year, according to the CDC. In the 444 outbreaks reported, 7,465 people became sick, 287 people were hospitalized, and 1 person died. The CDC says these numbers are likely to be underestimates.
One-third of the outbreaks were in treated swimming water, including pools and water playgrounds. Smaller percentages were linked to contact with cattle, infected people in childcare settings, and raw milk or apple cider.
Crypto’s tough protective shell is the secret to its long life. It can survive for days even in chlorinated pools or on surfaces disinfected with chlorine bleach. Moreover, Cryptosporidium oocysts are immediately infectious upon excretion and are excreted in numbers multiple orders of magnitude higher than the human infectious dose (≤ 10 oocysts). Just a few germs can make someone sick—and there can be millions in a pool. Infection with Cryptosporidium can cause profuse, watery diarrhea that lasts for up to 3 weeks. It’s particularly dangerous for immunocompromised patients, leading to malnutrition and wasting.
The CDC has some advice for staying Crypto free:
- Don’t swim or let kids swim if anyone has diarrhea, and keep them home from daycare;
- Don’t swallow the water you swim in;
- Wash hands with soap and water after any contact with animals, especially animal feces (note: alcohol-based hand sanitizers are not effective against Crypto; hydrogen peroxide should be used in childcare settings to disinfect);
- Remove shoes worn in animal environments before going inside your home; and
- Drink only pasteurized milk or apple cider.
Although the numbers are still high, the CDC says testing has improved and might be helping with increased detection, especially since CryptoNet, the first US molecularly based surveillance system for a parasitic disease, was instituted. Based on DNA fingerprinting, it has already demonstrated that it can elucidate Cryptosporidium transmission chains in treated recreational water outbreaks, the CDC says, and has the potential to do the same for investigations in other Crypto outbreaks.
If it’s swimming season, it’s also Cryptosporidium season. The parasite, spread through feces of infected humans or animals, is the culprit in most disease outbreaks linked to water.
Between 2009 and 2017, Crypto-related outbreaks increased an average 13% per year, according to the CDC. In the 444 outbreaks reported, 7,465 people became sick, 287 people were hospitalized, and 1 person died. The CDC says these numbers are likely to be underestimates.
One-third of the outbreaks were in treated swimming water, including pools and water playgrounds. Smaller percentages were linked to contact with cattle, infected people in childcare settings, and raw milk or apple cider.
Crypto’s tough protective shell is the secret to its long life. It can survive for days even in chlorinated pools or on surfaces disinfected with chlorine bleach. Moreover, Cryptosporidium oocysts are immediately infectious upon excretion and are excreted in numbers multiple orders of magnitude higher than the human infectious dose (≤ 10 oocysts). Just a few germs can make someone sick—and there can be millions in a pool. Infection with Cryptosporidium can cause profuse, watery diarrhea that lasts for up to 3 weeks. It’s particularly dangerous for immunocompromised patients, leading to malnutrition and wasting.
The CDC has some advice for staying Crypto free:
- Don’t swim or let kids swim if anyone has diarrhea, and keep them home from daycare;
- Don’t swallow the water you swim in;
- Wash hands with soap and water after any contact with animals, especially animal feces (note: alcohol-based hand sanitizers are not effective against Crypto; hydrogen peroxide should be used in childcare settings to disinfect);
- Remove shoes worn in animal environments before going inside your home; and
- Drink only pasteurized milk or apple cider.
Although the numbers are still high, the CDC says testing has improved and might be helping with increased detection, especially since CryptoNet, the first US molecularly based surveillance system for a parasitic disease, was instituted. Based on DNA fingerprinting, it has already demonstrated that it can elucidate Cryptosporidium transmission chains in treated recreational water outbreaks, the CDC says, and has the potential to do the same for investigations in other Crypto outbreaks.
If it’s swimming season, it’s also Cryptosporidium season. The parasite, spread through feces of infected humans or animals, is the culprit in most disease outbreaks linked to water.
Between 2009 and 2017, Crypto-related outbreaks increased an average 13% per year, according to the CDC. In the 444 outbreaks reported, 7,465 people became sick, 287 people were hospitalized, and 1 person died. The CDC says these numbers are likely to be underestimates.
One-third of the outbreaks were in treated swimming water, including pools and water playgrounds. Smaller percentages were linked to contact with cattle, infected people in childcare settings, and raw milk or apple cider.
Crypto’s tough protective shell is the secret to its long life. It can survive for days even in chlorinated pools or on surfaces disinfected with chlorine bleach. Moreover, Cryptosporidium oocysts are immediately infectious upon excretion and are excreted in numbers multiple orders of magnitude higher than the human infectious dose (≤ 10 oocysts). Just a few germs can make someone sick—and there can be millions in a pool. Infection with Cryptosporidium can cause profuse, watery diarrhea that lasts for up to 3 weeks. It’s particularly dangerous for immunocompromised patients, leading to malnutrition and wasting.
The CDC has some advice for staying Crypto free:
- Don’t swim or let kids swim if anyone has diarrhea, and keep them home from daycare;
- Don’t swallow the water you swim in;
- Wash hands with soap and water after any contact with animals, especially animal feces (note: alcohol-based hand sanitizers are not effective against Crypto; hydrogen peroxide should be used in childcare settings to disinfect);
- Remove shoes worn in animal environments before going inside your home; and
- Drink only pasteurized milk or apple cider.
Although the numbers are still high, the CDC says testing has improved and might be helping with increased detection, especially since CryptoNet, the first US molecularly based surveillance system for a parasitic disease, was instituted. Based on DNA fingerprinting, it has already demonstrated that it can elucidate Cryptosporidium transmission chains in treated recreational water outbreaks, the CDC says, and has the potential to do the same for investigations in other Crypto outbreaks.
Adjustment for characteristics not used by Medicare reduces hospital variations in readmission rates
Clinical question: Can differences in hospital readmission rates be explained by patient characteristics not accounted for by Medicare?
Background: In its Pay for Performance program, Medicare ties payments to readmission rates but adjusts these rates only for limited patient characteristics. Hospitals serving higher-risk patients have received greater penalties. These programs may have the unintended consequence of penalizing hospitals that provide care to higher-risk patients.
Study design: Observational study.
Setting: Medicare admissions claims from 2013 through 2014 in 2,215 hospitals.
Synopsis: Using Medicare claims for admission and linked U.S. census data, the study assessed several clinical and social characteristics not currently used for risk adjustment. A sample of 1,169,014 index admissions among 1,003,664 unique beneficiaries was analyzed. The study compared rates with and without these additional adjustments.
Additional adjustments reduced overall variation in hospital readmission by 9.6%, changed rates upward or downward by 0.4%-0.7% for the 10% of hospitals most affected by the readjustments, and they would be expected to reduce penalties by 52%, 46%, and 41% for hospitals with the largest 1%, 5%, and 10% of penalty reductions, respectively.
Bottom line: Hospitals serving higher-risk patients may be penalized because of the patients they serve rather that the quality of care they provide.
Citation: Roberts ET et al. Assessment of the effect of adjustment for patient characteristics on hospital readmission rates: Implications for Pay for Performance. JAMA Intern Med. 2018;178(11)1498-1507.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Can differences in hospital readmission rates be explained by patient characteristics not accounted for by Medicare?
Background: In its Pay for Performance program, Medicare ties payments to readmission rates but adjusts these rates only for limited patient characteristics. Hospitals serving higher-risk patients have received greater penalties. These programs may have the unintended consequence of penalizing hospitals that provide care to higher-risk patients.
Study design: Observational study.
Setting: Medicare admissions claims from 2013 through 2014 in 2,215 hospitals.
Synopsis: Using Medicare claims for admission and linked U.S. census data, the study assessed several clinical and social characteristics not currently used for risk adjustment. A sample of 1,169,014 index admissions among 1,003,664 unique beneficiaries was analyzed. The study compared rates with and without these additional adjustments.
Additional adjustments reduced overall variation in hospital readmission by 9.6%, changed rates upward or downward by 0.4%-0.7% for the 10% of hospitals most affected by the readjustments, and they would be expected to reduce penalties by 52%, 46%, and 41% for hospitals with the largest 1%, 5%, and 10% of penalty reductions, respectively.
Bottom line: Hospitals serving higher-risk patients may be penalized because of the patients they serve rather that the quality of care they provide.
Citation: Roberts ET et al. Assessment of the effect of adjustment for patient characteristics on hospital readmission rates: Implications for Pay for Performance. JAMA Intern Med. 2018;178(11)1498-1507.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Can differences in hospital readmission rates be explained by patient characteristics not accounted for by Medicare?
Background: In its Pay for Performance program, Medicare ties payments to readmission rates but adjusts these rates only for limited patient characteristics. Hospitals serving higher-risk patients have received greater penalties. These programs may have the unintended consequence of penalizing hospitals that provide care to higher-risk patients.
Study design: Observational study.
Setting: Medicare admissions claims from 2013 through 2014 in 2,215 hospitals.
Synopsis: Using Medicare claims for admission and linked U.S. census data, the study assessed several clinical and social characteristics not currently used for risk adjustment. A sample of 1,169,014 index admissions among 1,003,664 unique beneficiaries was analyzed. The study compared rates with and without these additional adjustments.
Additional adjustments reduced overall variation in hospital readmission by 9.6%, changed rates upward or downward by 0.4%-0.7% for the 10% of hospitals most affected by the readjustments, and they would be expected to reduce penalties by 52%, 46%, and 41% for hospitals with the largest 1%, 5%, and 10% of penalty reductions, respectively.
Bottom line: Hospitals serving higher-risk patients may be penalized because of the patients they serve rather that the quality of care they provide.
Citation: Roberts ET et al. Assessment of the effect of adjustment for patient characteristics on hospital readmission rates: Implications for Pay for Performance. JAMA Intern Med. 2018;178(11)1498-1507.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinically Impressive Tophaceous Gout With Significant Bony Destruction
Gout is an in inflammatory condition that is generally characterized by red, hot, swollen, and painful joints. The disease is often associated with increased serum uric acid levels; which are considered elevated when they are > 6 mg/dL in women and > 7 mg/dL in men. When gout affects joints, the subchondral bone may be involved, leading to destructive, painful changes. This article presents the case of a patient diagnosed with tophaceous gout of the left second toe with bony erosive changes and calcified nodules noted on magnetic resonance images (MRI).
Case Presentation
A 70-year-old white male presented to the podiatry clinic for a left second-toe mass that was diagnosed as tophaceous gout after being seen by his primary care physician. The patient reported that the mass had slowly grown over the past 10 years. At presentation, he had a 0.2-cm ulcer on the dorsal aspect of the left second-toe mass. The patient stated that the ulcer had recently appeared with some exudate; however, there was no active drainage of material. The patient had a 20-year history of gout that was untreated with dietary modifications or medication. The patient also stated that although the left second-toe mass did not cause any pain on rest, it did cause pain with shoe gear and during ambulation. A community-based podiatrist had recommended amputation of the second toe and as a result the patient was seeking a second opinion at the US Department of Veterans Affairs (VA) Lebanon VA Medical Center (VAMC) in Pennsylvania. The patient had not had acute gouty attacks during the past 10 years.
The patient’s medical history was significant for uncontrolled gout, hyperlipidemia, coronary artery disease with a 4-vessel coronary artery bypass grafting, impaired fasting glucose, prostate cancer that was in remission, alcohol misuse (currently limited to ≤ 2 drinks per night), and 30-year history of cigarette smoking (quit 2 months prior to visit).1,2
At his first visit to the clinic, an examination revealed distinct evidence of bulging of the soft tissues of the second toe of the left foot with a dry sinus tract that was not malodorous (Figure 1). The left second toe was erythematous and edematous. A local increase in skin temperature was present on the second toe of the left foot compared with that of the contralateral foot and other toes. The dorsalis pedis and tibialis posterior pulses were easily palpated, and the capillary return was within normal limits. Palpation of the left second-toe plantar elicited mild tenderness. Crepitation was not present at the left second metatarsophalangeal joint (MPJ) nor at the interphalangeal joint. There was restricted range of motion at the left second MPJ compared with that of the right foot and no motion at the proximal interphalangeal joint. The movement at the left second metatarsophalangeal elicited tenderness. The mass on the left second toe was firm, nonpulsatile, oval-shaped, with a white pigmented consistency that measured 2 cm x 2.5 cm.
There were no deficits present on the neurologic examination, which was noncontributory. There also was no gross evidence of motor weakness. His initial temporal temperature was 98.2° F. The initial laboratory findings were uric acid, 9.5 mg/dL; fasting glucose, 117 g/dL; estimated glomerular filtration rate, 55 mL/min/1.73 m2; erythrocyte sedimentation rate, 6.5 mm/h; and white blood count, 6.6 K/uL.3,4-6
Diagnostic imaging included X-rays of the patient’s feet and a MRI of the left foot. The X-rays showed diffusely osteopenic bones with severe soft tissue swelling surrounding the second proximal interphalangeal joint. Also present was moderate soft tissue swelling at the level of the first metatarsophalangeal joint accompanied by extensive erosions at both of these joints, most pronounced at the second proximal interphalangeal joint. Also, there was narrowing at the first MPJ and the first interphalangeal joint. Erosive changes at the tarsometatarsal articulations and small lucencies within the navicular/midfoot joint were suggestive of additional gouty erosions. A small-to-moderate posterior calcaneal enthesophyte was present as well as a tiny calcaneal enthesophyte (Figure 2).
A MRI showed a destructive soft tissue mass, resulting in overhanging edges, with foci of calcifications centered about the proximal interphalangeal joint of the second toe, which is consistent with a calcified tophaceous gout nodule. The widest dimension of the mass measured 3.2 cm. There also was a less prominent calcified tophaceous gout nodule at the first MPJ. There were additional small punched-out lesions involving the bases of the first through fourth metatarsi and at the distal aspect of the first cuneiform in keeping with gouty arthropathy (Figure 3).4,7-10
The initial treatment plan presented to the patient was to amputate the left second toe. But the patient decided against amputation. Treatment guidelines for allopurinol are to titrate in 100-mg increments every 2 weeks until the serum uric acid levels are consistently < 6, tophi resolve, and the patient should be free of gout attacks.11 We initiated uric acid-lowering therapy with allopurinol at 50 mg/d for 7 days, increasing to 100 mg/d for 7 days, then to 200 mg/d for 10 days. The patient’s serum uric acid level was checked at 200 mg/d. Our patient could not tolerate the allopurinol and decided to discontinue treatment. After 1 year he started having severe pain and returned to have the toe amputated. The patient healed uneventfully.
Discussion
Tophaceous gout is characterized by collections of solid urate accompanied by chronic inflammatory and often destructive changes in the surrounding tissue brought on by periods of increased uric acid levels. Due to the patient’s 20-year history of untreated tophaceous gout, we saw the extent of bony and soft tissue destruction that this pathology created. This patient’s uric acid laboratory value of 9.5 mg/dL was well above the normal reference values of 2.6 to 7.2 mg/dL. The X-rays performed suggested that there was not only bony destruction, but also deformity.
The destruction to the surrounding soft tissues noted as advanced nonhealing wounds formed to the area of the tophi. The size of the second digit also was impressive, causing displacement of the other digits. As stated in the literature, tophaceous gout is usually painless as was the case in our patient. It is the combination of the relatively painless nature of this pathology accompanied by no treatment over many years that led to the patient’s level of deformity and tissue destruction.
Conclusion
We describe a common presentation of bone involvement secondary to significant tophaceous gout in the absence osteomyelitis. The goal of treatment was to maintain a functional foot free of major deformity, pain, or associated risk factors that could lead to a more significant surgical procedure, such as a proximal amputation.11 Given the destructive nature of this pathology, it is important to educate the patient, perform regular examinations, and start medications early to control uric acid levels. These measures will improve the patient’s prognosis and avoid severe sequelae.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155-175.
3. Choi H. Epidemiology of crystal arthropathy. Rheum Dis Clin North Am. 2006;32(2):255-273.
4. Nakayama DA, Barthelemy C, Carrera G, Lightfoot RW Jr, Wortmann RL. Tophaceous gout: a clinical and radiographic assessment. Arthritis Rheum. 1984;27(4):468-471.
5. Dalbeth N, Haskard DO. Pathophysiology of crystal-induced arthritis. In: Wortmann RL, Schumacher HR Jr, Becker MA, Ryan LM, eds. Crystal-induced Arthropathies. New York: Taylor & Francis; 2006.
6. Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum. 2010;62(5):1549-1556.
7. Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW. Tophaceous gout of the spine: MR imaging features. Clin Radiol. 2002;57(10):919-925.
8. Schumacher HR Jr, Becker MA, Edwards NL, et al. Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract. 2006;60(4):408-414.
9. McQueen FM, Doyle A, Dalbeth N. Imaging in the crystal arthropathies. Rheum Dis Clin North Am. 2014;40(2):231-249.
10. Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68(10):1609-1612.
11. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
Gout is an in inflammatory condition that is generally characterized by red, hot, swollen, and painful joints. The disease is often associated with increased serum uric acid levels; which are considered elevated when they are > 6 mg/dL in women and > 7 mg/dL in men. When gout affects joints, the subchondral bone may be involved, leading to destructive, painful changes. This article presents the case of a patient diagnosed with tophaceous gout of the left second toe with bony erosive changes and calcified nodules noted on magnetic resonance images (MRI).
Case Presentation
A 70-year-old white male presented to the podiatry clinic for a left second-toe mass that was diagnosed as tophaceous gout after being seen by his primary care physician. The patient reported that the mass had slowly grown over the past 10 years. At presentation, he had a 0.2-cm ulcer on the dorsal aspect of the left second-toe mass. The patient stated that the ulcer had recently appeared with some exudate; however, there was no active drainage of material. The patient had a 20-year history of gout that was untreated with dietary modifications or medication. The patient also stated that although the left second-toe mass did not cause any pain on rest, it did cause pain with shoe gear and during ambulation. A community-based podiatrist had recommended amputation of the second toe and as a result the patient was seeking a second opinion at the US Department of Veterans Affairs (VA) Lebanon VA Medical Center (VAMC) in Pennsylvania. The patient had not had acute gouty attacks during the past 10 years.
The patient’s medical history was significant for uncontrolled gout, hyperlipidemia, coronary artery disease with a 4-vessel coronary artery bypass grafting, impaired fasting glucose, prostate cancer that was in remission, alcohol misuse (currently limited to ≤ 2 drinks per night), and 30-year history of cigarette smoking (quit 2 months prior to visit).1,2
At his first visit to the clinic, an examination revealed distinct evidence of bulging of the soft tissues of the second toe of the left foot with a dry sinus tract that was not malodorous (Figure 1). The left second toe was erythematous and edematous. A local increase in skin temperature was present on the second toe of the left foot compared with that of the contralateral foot and other toes. The dorsalis pedis and tibialis posterior pulses were easily palpated, and the capillary return was within normal limits. Palpation of the left second-toe plantar elicited mild tenderness. Crepitation was not present at the left second metatarsophalangeal joint (MPJ) nor at the interphalangeal joint. There was restricted range of motion at the left second MPJ compared with that of the right foot and no motion at the proximal interphalangeal joint. The movement at the left second metatarsophalangeal elicited tenderness. The mass on the left second toe was firm, nonpulsatile, oval-shaped, with a white pigmented consistency that measured 2 cm x 2.5 cm.
There were no deficits present on the neurologic examination, which was noncontributory. There also was no gross evidence of motor weakness. His initial temporal temperature was 98.2° F. The initial laboratory findings were uric acid, 9.5 mg/dL; fasting glucose, 117 g/dL; estimated glomerular filtration rate, 55 mL/min/1.73 m2; erythrocyte sedimentation rate, 6.5 mm/h; and white blood count, 6.6 K/uL.3,4-6
Diagnostic imaging included X-rays of the patient’s feet and a MRI of the left foot. The X-rays showed diffusely osteopenic bones with severe soft tissue swelling surrounding the second proximal interphalangeal joint. Also present was moderate soft tissue swelling at the level of the first metatarsophalangeal joint accompanied by extensive erosions at both of these joints, most pronounced at the second proximal interphalangeal joint. Also, there was narrowing at the first MPJ and the first interphalangeal joint. Erosive changes at the tarsometatarsal articulations and small lucencies within the navicular/midfoot joint were suggestive of additional gouty erosions. A small-to-moderate posterior calcaneal enthesophyte was present as well as a tiny calcaneal enthesophyte (Figure 2).
A MRI showed a destructive soft tissue mass, resulting in overhanging edges, with foci of calcifications centered about the proximal interphalangeal joint of the second toe, which is consistent with a calcified tophaceous gout nodule. The widest dimension of the mass measured 3.2 cm. There also was a less prominent calcified tophaceous gout nodule at the first MPJ. There were additional small punched-out lesions involving the bases of the first through fourth metatarsi and at the distal aspect of the first cuneiform in keeping with gouty arthropathy (Figure 3).4,7-10
The initial treatment plan presented to the patient was to amputate the left second toe. But the patient decided against amputation. Treatment guidelines for allopurinol are to titrate in 100-mg increments every 2 weeks until the serum uric acid levels are consistently < 6, tophi resolve, and the patient should be free of gout attacks.11 We initiated uric acid-lowering therapy with allopurinol at 50 mg/d for 7 days, increasing to 100 mg/d for 7 days, then to 200 mg/d for 10 days. The patient’s serum uric acid level was checked at 200 mg/d. Our patient could not tolerate the allopurinol and decided to discontinue treatment. After 1 year he started having severe pain and returned to have the toe amputated. The patient healed uneventfully.
Discussion
Tophaceous gout is characterized by collections of solid urate accompanied by chronic inflammatory and often destructive changes in the surrounding tissue brought on by periods of increased uric acid levels. Due to the patient’s 20-year history of untreated tophaceous gout, we saw the extent of bony and soft tissue destruction that this pathology created. This patient’s uric acid laboratory value of 9.5 mg/dL was well above the normal reference values of 2.6 to 7.2 mg/dL. The X-rays performed suggested that there was not only bony destruction, but also deformity.
The destruction to the surrounding soft tissues noted as advanced nonhealing wounds formed to the area of the tophi. The size of the second digit also was impressive, causing displacement of the other digits. As stated in the literature, tophaceous gout is usually painless as was the case in our patient. It is the combination of the relatively painless nature of this pathology accompanied by no treatment over many years that led to the patient’s level of deformity and tissue destruction.
Conclusion
We describe a common presentation of bone involvement secondary to significant tophaceous gout in the absence osteomyelitis. The goal of treatment was to maintain a functional foot free of major deformity, pain, or associated risk factors that could lead to a more significant surgical procedure, such as a proximal amputation.11 Given the destructive nature of this pathology, it is important to educate the patient, perform regular examinations, and start medications early to control uric acid levels. These measures will improve the patient’s prognosis and avoid severe sequelae.
Gout is an in inflammatory condition that is generally characterized by red, hot, swollen, and painful joints. The disease is often associated with increased serum uric acid levels; which are considered elevated when they are > 6 mg/dL in women and > 7 mg/dL in men. When gout affects joints, the subchondral bone may be involved, leading to destructive, painful changes. This article presents the case of a patient diagnosed with tophaceous gout of the left second toe with bony erosive changes and calcified nodules noted on magnetic resonance images (MRI).
Case Presentation
A 70-year-old white male presented to the podiatry clinic for a left second-toe mass that was diagnosed as tophaceous gout after being seen by his primary care physician. The patient reported that the mass had slowly grown over the past 10 years. At presentation, he had a 0.2-cm ulcer on the dorsal aspect of the left second-toe mass. The patient stated that the ulcer had recently appeared with some exudate; however, there was no active drainage of material. The patient had a 20-year history of gout that was untreated with dietary modifications or medication. The patient also stated that although the left second-toe mass did not cause any pain on rest, it did cause pain with shoe gear and during ambulation. A community-based podiatrist had recommended amputation of the second toe and as a result the patient was seeking a second opinion at the US Department of Veterans Affairs (VA) Lebanon VA Medical Center (VAMC) in Pennsylvania. The patient had not had acute gouty attacks during the past 10 years.
The patient’s medical history was significant for uncontrolled gout, hyperlipidemia, coronary artery disease with a 4-vessel coronary artery bypass grafting, impaired fasting glucose, prostate cancer that was in remission, alcohol misuse (currently limited to ≤ 2 drinks per night), and 30-year history of cigarette smoking (quit 2 months prior to visit).1,2
At his first visit to the clinic, an examination revealed distinct evidence of bulging of the soft tissues of the second toe of the left foot with a dry sinus tract that was not malodorous (Figure 1). The left second toe was erythematous and edematous. A local increase in skin temperature was present on the second toe of the left foot compared with that of the contralateral foot and other toes. The dorsalis pedis and tibialis posterior pulses were easily palpated, and the capillary return was within normal limits. Palpation of the left second-toe plantar elicited mild tenderness. Crepitation was not present at the left second metatarsophalangeal joint (MPJ) nor at the interphalangeal joint. There was restricted range of motion at the left second MPJ compared with that of the right foot and no motion at the proximal interphalangeal joint. The movement at the left second metatarsophalangeal elicited tenderness. The mass on the left second toe was firm, nonpulsatile, oval-shaped, with a white pigmented consistency that measured 2 cm x 2.5 cm.
There were no deficits present on the neurologic examination, which was noncontributory. There also was no gross evidence of motor weakness. His initial temporal temperature was 98.2° F. The initial laboratory findings were uric acid, 9.5 mg/dL; fasting glucose, 117 g/dL; estimated glomerular filtration rate, 55 mL/min/1.73 m2; erythrocyte sedimentation rate, 6.5 mm/h; and white blood count, 6.6 K/uL.3,4-6
Diagnostic imaging included X-rays of the patient’s feet and a MRI of the left foot. The X-rays showed diffusely osteopenic bones with severe soft tissue swelling surrounding the second proximal interphalangeal joint. Also present was moderate soft tissue swelling at the level of the first metatarsophalangeal joint accompanied by extensive erosions at both of these joints, most pronounced at the second proximal interphalangeal joint. Also, there was narrowing at the first MPJ and the first interphalangeal joint. Erosive changes at the tarsometatarsal articulations and small lucencies within the navicular/midfoot joint were suggestive of additional gouty erosions. A small-to-moderate posterior calcaneal enthesophyte was present as well as a tiny calcaneal enthesophyte (Figure 2).
A MRI showed a destructive soft tissue mass, resulting in overhanging edges, with foci of calcifications centered about the proximal interphalangeal joint of the second toe, which is consistent with a calcified tophaceous gout nodule. The widest dimension of the mass measured 3.2 cm. There also was a less prominent calcified tophaceous gout nodule at the first MPJ. There were additional small punched-out lesions involving the bases of the first through fourth metatarsi and at the distal aspect of the first cuneiform in keeping with gouty arthropathy (Figure 3).4,7-10
The initial treatment plan presented to the patient was to amputate the left second toe. But the patient decided against amputation. Treatment guidelines for allopurinol are to titrate in 100-mg increments every 2 weeks until the serum uric acid levels are consistently < 6, tophi resolve, and the patient should be free of gout attacks.11 We initiated uric acid-lowering therapy with allopurinol at 50 mg/d for 7 days, increasing to 100 mg/d for 7 days, then to 200 mg/d for 10 days. The patient’s serum uric acid level was checked at 200 mg/d. Our patient could not tolerate the allopurinol and decided to discontinue treatment. After 1 year he started having severe pain and returned to have the toe amputated. The patient healed uneventfully.
Discussion
Tophaceous gout is characterized by collections of solid urate accompanied by chronic inflammatory and often destructive changes in the surrounding tissue brought on by periods of increased uric acid levels. Due to the patient’s 20-year history of untreated tophaceous gout, we saw the extent of bony and soft tissue destruction that this pathology created. This patient’s uric acid laboratory value of 9.5 mg/dL was well above the normal reference values of 2.6 to 7.2 mg/dL. The X-rays performed suggested that there was not only bony destruction, but also deformity.
The destruction to the surrounding soft tissues noted as advanced nonhealing wounds formed to the area of the tophi. The size of the second digit also was impressive, causing displacement of the other digits. As stated in the literature, tophaceous gout is usually painless as was the case in our patient. It is the combination of the relatively painless nature of this pathology accompanied by no treatment over many years that led to the patient’s level of deformity and tissue destruction.
Conclusion
We describe a common presentation of bone involvement secondary to significant tophaceous gout in the absence osteomyelitis. The goal of treatment was to maintain a functional foot free of major deformity, pain, or associated risk factors that could lead to a more significant surgical procedure, such as a proximal amputation.11 Given the destructive nature of this pathology, it is important to educate the patient, perform regular examinations, and start medications early to control uric acid levels. These measures will improve the patient’s prognosis and avoid severe sequelae.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155-175.
3. Choi H. Epidemiology of crystal arthropathy. Rheum Dis Clin North Am. 2006;32(2):255-273.
4. Nakayama DA, Barthelemy C, Carrera G, Lightfoot RW Jr, Wortmann RL. Tophaceous gout: a clinical and radiographic assessment. Arthritis Rheum. 1984;27(4):468-471.
5. Dalbeth N, Haskard DO. Pathophysiology of crystal-induced arthritis. In: Wortmann RL, Schumacher HR Jr, Becker MA, Ryan LM, eds. Crystal-induced Arthropathies. New York: Taylor & Francis; 2006.
6. Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum. 2010;62(5):1549-1556.
7. Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW. Tophaceous gout of the spine: MR imaging features. Clin Radiol. 2002;57(10):919-925.
8. Schumacher HR Jr, Becker MA, Edwards NL, et al. Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract. 2006;60(4):408-414.
9. McQueen FM, Doyle A, Dalbeth N. Imaging in the crystal arthropathies. Rheum Dis Clin North Am. 2014;40(2):231-249.
10. Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68(10):1609-1612.
11. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155-175.
3. Choi H. Epidemiology of crystal arthropathy. Rheum Dis Clin North Am. 2006;32(2):255-273.
4. Nakayama DA, Barthelemy C, Carrera G, Lightfoot RW Jr, Wortmann RL. Tophaceous gout: a clinical and radiographic assessment. Arthritis Rheum. 1984;27(4):468-471.
5. Dalbeth N, Haskard DO. Pathophysiology of crystal-induced arthritis. In: Wortmann RL, Schumacher HR Jr, Becker MA, Ryan LM, eds. Crystal-induced Arthropathies. New York: Taylor & Francis; 2006.
6. Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum. 2010;62(5):1549-1556.
7. Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW. Tophaceous gout of the spine: MR imaging features. Clin Radiol. 2002;57(10):919-925.
8. Schumacher HR Jr, Becker MA, Edwards NL, et al. Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract. 2006;60(4):408-414.
9. McQueen FM, Doyle A, Dalbeth N. Imaging in the crystal arthropathies. Rheum Dis Clin North Am. 2014;40(2):231-249.
10. Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68(10):1609-1612.
11. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
The Shot That Won the Revolutionary War and Is Still Reverberating
The disputes about those who decline to vaccinate their children for communicable infectious diseases, especially measles, have been in the headlines of late. Those refusals are often done in the name of “medical freedom.”1 Yet this is a much older debate for the military. It seems fitting in this month in which we celebrate the 243rd anniversary of the Declaration of Independence to reflect on the earliest history of the interaction between vaccinations and war in the US and what it tells us about the fight for religious and political freedom and individual liberty.
Go back in time with me to 1776, long before the Fourth of July was a day for barbecues and fireworks. We are in Boston, Philadelphia, and other important cities in colonial America. This time, concern was not about measles but the even more dreaded smallpox. In the first years of the Revolutionary War, General George Washington took command of a newly formed and named Continental Army. A catastrophic 90% of casualties in the Continental Army were from infectious diseases, with the lion’s share of these from smallpox, which at that time had a mortality rate of about 30%.2,3
Early efforts to introduce inoculation into the colonies had failed for many of the same reasons parents across the US today refuse immunization: fear and anxiety. When the renowned New England Puritan minister and scientist Cotton Mather attempted in 1721 to introduce variolation, his house was firebombed and his fellow clergy and physicians alleged that his efforts at inoculation were challenging God’s will to send a plague.3 Variolation was the now antiquated and then laborious process in which a previously unexposed individual was inoculated with material from the vesicle of someone infected with the disease.4,5 Variolation was practiced in parts of Africa and Asia and among wealthy Europeans but remained controversial in many colonies where few Americans had been exposed to smallpox or could afford the procedure.3
It is important to note that the use of variolation was practiced before Edward Jenner famously demonstrated that cowpox vaccine could provide immunity to smallpox in 1798. The majority of those inoculated would develop a mild case of smallpox that required a 5-week period of illness and recovery that provided lifelong immunity. However, during those 5 weeks, they remained a vector of disease for the uninoculated. Southern and New England colonies passed laws that prohibited variolation. Those anti-inoculation attitudes were the basis for the order given to the surgeons general of the Continental Army in 1776 that all inoculations of the troops were forbidden, despite the fact that perhaps only 25% of soldiers possessed any natural immunity.2,3
There was yet another reason that many colonial Americans opposed government-sponsored preventative care, and it was the same reason that they were fighting a war of independence: distrust and resentment of authority. The modern antivaccine movement voices similar fears and suspicions regarding public health campaigns and especially legislative efforts to mandate vaccinations or remove extant exemptions.
In 1775 in Boston, a smallpox outbreak occurred at the same time the Americans laid siege to the British troops occupying the city. Greater natural immunity to the scourge of smallpox either through exposure or variolation provided the British with a stronger defense than the mere city fortifications. There are even some suspicions that the British used the virus as a proto-biologic weapon.
General Washington had initially been against inoculation until he realized that without it the British might win the war. This possibility presented him with a momentous decision: inoculate despite widespread anxiety that variolation would spread the disease or risk the virus ravaging the fighting force. Perhaps the most compelling reason to variolate was that new recruits refused to sign up, fearing not that they would die in battle but of smallpox. In 1777, Washington mandated variolation of the nonimmune troops and new recruits, making it the first large-scale military preventative care measure in history.
Recapitulating an ethical dilemma that still rages in the military nearly 3 centuries later, for British soldiers, inoculation was voluntary not compulsory as for the Americans. There was so much opposition to Washington’s order that communications with surgeons were secret, and commanding officers had to oversee the inoculations.2,3
Washington’s policy not only contributed mightily to the American victory in the war, but also set the precedent for compulsory vaccination in the US military for the next 3 centuries. Currently, regulations require that service members be vaccinated for multiple infectious diseases. Of interest, this mandatory vaccination program has led to no reported cases of measles among military families to date, in part because of federal regulations requiring families of those service members to be vaccinated.6
Ironically, once General Washington made the decision for mass inoculation, he encountered little actual resistance among the troops. However, throughout military history some service members have objected to compulsory vaccination on medical, religious, and personal grounds. In United States v Chadwell, a military court ruled against 2 Marine Corps members who refused vaccination for smallpox, typhoid, paratyphoid, and influenza, citing religious grounds. The court opined that the military orders that ensure the health and safety of the armed forces and thereby that of the public override personal religious beliefs.7
The paradox of liberty—the liberty first won in the Revolutionary War—is that in a pluralistic representative democracy like ours to secure the freedom for all, some, such as the military, must relinquish the very choice to refuse. Their sacrifices grant liberty to others. On June 6, we commemorated the seventy-fifth anniversary of D-Day, remembering how great the cost of that eternal vigilance, which the patriot Thomas Paine said was the price of liberty. On Memorial Day, we remember all those men and women who died in the service of their country. And while they gave up the most precious gift, we must never forget that every person in uniform also surrenders many other significant personal freedoms so that their fellow civilians may exercise them.
The question General Washington faced is one that public health authorities and our legislators again confront. When should the freedom to refuse, which was won with the blood of many valiant heroes and has been defended since 1776, be curtailed for the greater good? We are the one nation in history that has made the defense of self-determination its highest value and in so doing, its greatest challenge.
1. Sun LH. Senate panel warns of dangers of ant-vaccine movement. https://www.washingtonpost.com/health/2019/03/05/combat-anti-vaxxers-us-needs-national-campaign-top-washington-state-official-says/?utm_term=.9a4201be0ed1. Published March 5, 2019. Accessed June 9, 2019.
2. Filsinger AL, Dwek R. George Washington and the first mass military inoculation. http://www.loc.gov/rr/scitech/GW&smallpoxinoculation.html. Published February 12, 2009. Accessed June 10, 2019.
3. Fenn EA. Pox Americana. New York: Hill and Wang; 2001.
4. Steadman’s Medical Dictionary. 28th edition. Philadelphia, PA: Lippincott, Williams & Wilkins; 2006.
5. Artenstein AW, Opal JM, Opal SM, Tramont EC, Georges P, Russell PK. History of U.S. military contributions to the study of vaccines and infectious diseases. Mil Med. 2005;170(suppl 4):3-11.
6. Jowers K. So far, no measles cases at military medical facilities—but officials are watching. https://www.militarytimes.com/pay-benefits/2019/04/19/so-far-no-measles-cases-at-military-medical-facilities-but-officials-are-watching/. Published April 19, 2019. Accessed June 9, 2019.
7. Cole JP, Swendiman KS. Mandatory vaccinations: precedent and current laws. https://fas.org/sgp/crs/misc/RS21414.pdf. Published May 21, 2014. Accessed June 10, 2019.
The disputes about those who decline to vaccinate their children for communicable infectious diseases, especially measles, have been in the headlines of late. Those refusals are often done in the name of “medical freedom.”1 Yet this is a much older debate for the military. It seems fitting in this month in which we celebrate the 243rd anniversary of the Declaration of Independence to reflect on the earliest history of the interaction between vaccinations and war in the US and what it tells us about the fight for religious and political freedom and individual liberty.
Go back in time with me to 1776, long before the Fourth of July was a day for barbecues and fireworks. We are in Boston, Philadelphia, and other important cities in colonial America. This time, concern was not about measles but the even more dreaded smallpox. In the first years of the Revolutionary War, General George Washington took command of a newly formed and named Continental Army. A catastrophic 90% of casualties in the Continental Army were from infectious diseases, with the lion’s share of these from smallpox, which at that time had a mortality rate of about 30%.2,3
Early efforts to introduce inoculation into the colonies had failed for many of the same reasons parents across the US today refuse immunization: fear and anxiety. When the renowned New England Puritan minister and scientist Cotton Mather attempted in 1721 to introduce variolation, his house was firebombed and his fellow clergy and physicians alleged that his efforts at inoculation were challenging God’s will to send a plague.3 Variolation was the now antiquated and then laborious process in which a previously unexposed individual was inoculated with material from the vesicle of someone infected with the disease.4,5 Variolation was practiced in parts of Africa and Asia and among wealthy Europeans but remained controversial in many colonies where few Americans had been exposed to smallpox or could afford the procedure.3
It is important to note that the use of variolation was practiced before Edward Jenner famously demonstrated that cowpox vaccine could provide immunity to smallpox in 1798. The majority of those inoculated would develop a mild case of smallpox that required a 5-week period of illness and recovery that provided lifelong immunity. However, during those 5 weeks, they remained a vector of disease for the uninoculated. Southern and New England colonies passed laws that prohibited variolation. Those anti-inoculation attitudes were the basis for the order given to the surgeons general of the Continental Army in 1776 that all inoculations of the troops were forbidden, despite the fact that perhaps only 25% of soldiers possessed any natural immunity.2,3
There was yet another reason that many colonial Americans opposed government-sponsored preventative care, and it was the same reason that they were fighting a war of independence: distrust and resentment of authority. The modern antivaccine movement voices similar fears and suspicions regarding public health campaigns and especially legislative efforts to mandate vaccinations or remove extant exemptions.
In 1775 in Boston, a smallpox outbreak occurred at the same time the Americans laid siege to the British troops occupying the city. Greater natural immunity to the scourge of smallpox either through exposure or variolation provided the British with a stronger defense than the mere city fortifications. There are even some suspicions that the British used the virus as a proto-biologic weapon.
General Washington had initially been against inoculation until he realized that without it the British might win the war. This possibility presented him with a momentous decision: inoculate despite widespread anxiety that variolation would spread the disease or risk the virus ravaging the fighting force. Perhaps the most compelling reason to variolate was that new recruits refused to sign up, fearing not that they would die in battle but of smallpox. In 1777, Washington mandated variolation of the nonimmune troops and new recruits, making it the first large-scale military preventative care measure in history.
Recapitulating an ethical dilemma that still rages in the military nearly 3 centuries later, for British soldiers, inoculation was voluntary not compulsory as for the Americans. There was so much opposition to Washington’s order that communications with surgeons were secret, and commanding officers had to oversee the inoculations.2,3
Washington’s policy not only contributed mightily to the American victory in the war, but also set the precedent for compulsory vaccination in the US military for the next 3 centuries. Currently, regulations require that service members be vaccinated for multiple infectious diseases. Of interest, this mandatory vaccination program has led to no reported cases of measles among military families to date, in part because of federal regulations requiring families of those service members to be vaccinated.6
Ironically, once General Washington made the decision for mass inoculation, he encountered little actual resistance among the troops. However, throughout military history some service members have objected to compulsory vaccination on medical, religious, and personal grounds. In United States v Chadwell, a military court ruled against 2 Marine Corps members who refused vaccination for smallpox, typhoid, paratyphoid, and influenza, citing religious grounds. The court opined that the military orders that ensure the health and safety of the armed forces and thereby that of the public override personal religious beliefs.7
The paradox of liberty—the liberty first won in the Revolutionary War—is that in a pluralistic representative democracy like ours to secure the freedom for all, some, such as the military, must relinquish the very choice to refuse. Their sacrifices grant liberty to others. On June 6, we commemorated the seventy-fifth anniversary of D-Day, remembering how great the cost of that eternal vigilance, which the patriot Thomas Paine said was the price of liberty. On Memorial Day, we remember all those men and women who died in the service of their country. And while they gave up the most precious gift, we must never forget that every person in uniform also surrenders many other significant personal freedoms so that their fellow civilians may exercise them.
The question General Washington faced is one that public health authorities and our legislators again confront. When should the freedom to refuse, which was won with the blood of many valiant heroes and has been defended since 1776, be curtailed for the greater good? We are the one nation in history that has made the defense of self-determination its highest value and in so doing, its greatest challenge.
The disputes about those who decline to vaccinate their children for communicable infectious diseases, especially measles, have been in the headlines of late. Those refusals are often done in the name of “medical freedom.”1 Yet this is a much older debate for the military. It seems fitting in this month in which we celebrate the 243rd anniversary of the Declaration of Independence to reflect on the earliest history of the interaction between vaccinations and war in the US and what it tells us about the fight for religious and political freedom and individual liberty.
Go back in time with me to 1776, long before the Fourth of July was a day for barbecues and fireworks. We are in Boston, Philadelphia, and other important cities in colonial America. This time, concern was not about measles but the even more dreaded smallpox. In the first years of the Revolutionary War, General George Washington took command of a newly formed and named Continental Army. A catastrophic 90% of casualties in the Continental Army were from infectious diseases, with the lion’s share of these from smallpox, which at that time had a mortality rate of about 30%.2,3
Early efforts to introduce inoculation into the colonies had failed for many of the same reasons parents across the US today refuse immunization: fear and anxiety. When the renowned New England Puritan minister and scientist Cotton Mather attempted in 1721 to introduce variolation, his house was firebombed and his fellow clergy and physicians alleged that his efforts at inoculation were challenging God’s will to send a plague.3 Variolation was the now antiquated and then laborious process in which a previously unexposed individual was inoculated with material from the vesicle of someone infected with the disease.4,5 Variolation was practiced in parts of Africa and Asia and among wealthy Europeans but remained controversial in many colonies where few Americans had been exposed to smallpox or could afford the procedure.3
It is important to note that the use of variolation was practiced before Edward Jenner famously demonstrated that cowpox vaccine could provide immunity to smallpox in 1798. The majority of those inoculated would develop a mild case of smallpox that required a 5-week period of illness and recovery that provided lifelong immunity. However, during those 5 weeks, they remained a vector of disease for the uninoculated. Southern and New England colonies passed laws that prohibited variolation. Those anti-inoculation attitudes were the basis for the order given to the surgeons general of the Continental Army in 1776 that all inoculations of the troops were forbidden, despite the fact that perhaps only 25% of soldiers possessed any natural immunity.2,3
There was yet another reason that many colonial Americans opposed government-sponsored preventative care, and it was the same reason that they were fighting a war of independence: distrust and resentment of authority. The modern antivaccine movement voices similar fears and suspicions regarding public health campaigns and especially legislative efforts to mandate vaccinations or remove extant exemptions.
In 1775 in Boston, a smallpox outbreak occurred at the same time the Americans laid siege to the British troops occupying the city. Greater natural immunity to the scourge of smallpox either through exposure or variolation provided the British with a stronger defense than the mere city fortifications. There are even some suspicions that the British used the virus as a proto-biologic weapon.
General Washington had initially been against inoculation until he realized that without it the British might win the war. This possibility presented him with a momentous decision: inoculate despite widespread anxiety that variolation would spread the disease or risk the virus ravaging the fighting force. Perhaps the most compelling reason to variolate was that new recruits refused to sign up, fearing not that they would die in battle but of smallpox. In 1777, Washington mandated variolation of the nonimmune troops and new recruits, making it the first large-scale military preventative care measure in history.
Recapitulating an ethical dilemma that still rages in the military nearly 3 centuries later, for British soldiers, inoculation was voluntary not compulsory as for the Americans. There was so much opposition to Washington’s order that communications with surgeons were secret, and commanding officers had to oversee the inoculations.2,3
Washington’s policy not only contributed mightily to the American victory in the war, but also set the precedent for compulsory vaccination in the US military for the next 3 centuries. Currently, regulations require that service members be vaccinated for multiple infectious diseases. Of interest, this mandatory vaccination program has led to no reported cases of measles among military families to date, in part because of federal regulations requiring families of those service members to be vaccinated.6
Ironically, once General Washington made the decision for mass inoculation, he encountered little actual resistance among the troops. However, throughout military history some service members have objected to compulsory vaccination on medical, religious, and personal grounds. In United States v Chadwell, a military court ruled against 2 Marine Corps members who refused vaccination for smallpox, typhoid, paratyphoid, and influenza, citing religious grounds. The court opined that the military orders that ensure the health and safety of the armed forces and thereby that of the public override personal religious beliefs.7
The paradox of liberty—the liberty first won in the Revolutionary War—is that in a pluralistic representative democracy like ours to secure the freedom for all, some, such as the military, must relinquish the very choice to refuse. Their sacrifices grant liberty to others. On June 6, we commemorated the seventy-fifth anniversary of D-Day, remembering how great the cost of that eternal vigilance, which the patriot Thomas Paine said was the price of liberty. On Memorial Day, we remember all those men and women who died in the service of their country. And while they gave up the most precious gift, we must never forget that every person in uniform also surrenders many other significant personal freedoms so that their fellow civilians may exercise them.
The question General Washington faced is one that public health authorities and our legislators again confront. When should the freedom to refuse, which was won with the blood of many valiant heroes and has been defended since 1776, be curtailed for the greater good? We are the one nation in history that has made the defense of self-determination its highest value and in so doing, its greatest challenge.
1. Sun LH. Senate panel warns of dangers of ant-vaccine movement. https://www.washingtonpost.com/health/2019/03/05/combat-anti-vaxxers-us-needs-national-campaign-top-washington-state-official-says/?utm_term=.9a4201be0ed1. Published March 5, 2019. Accessed June 9, 2019.
2. Filsinger AL, Dwek R. George Washington and the first mass military inoculation. http://www.loc.gov/rr/scitech/GW&smallpoxinoculation.html. Published February 12, 2009. Accessed June 10, 2019.
3. Fenn EA. Pox Americana. New York: Hill and Wang; 2001.
4. Steadman’s Medical Dictionary. 28th edition. Philadelphia, PA: Lippincott, Williams & Wilkins; 2006.
5. Artenstein AW, Opal JM, Opal SM, Tramont EC, Georges P, Russell PK. History of U.S. military contributions to the study of vaccines and infectious diseases. Mil Med. 2005;170(suppl 4):3-11.
6. Jowers K. So far, no measles cases at military medical facilities—but officials are watching. https://www.militarytimes.com/pay-benefits/2019/04/19/so-far-no-measles-cases-at-military-medical-facilities-but-officials-are-watching/. Published April 19, 2019. Accessed June 9, 2019.
7. Cole JP, Swendiman KS. Mandatory vaccinations: precedent and current laws. https://fas.org/sgp/crs/misc/RS21414.pdf. Published May 21, 2014. Accessed June 10, 2019.
1. Sun LH. Senate panel warns of dangers of ant-vaccine movement. https://www.washingtonpost.com/health/2019/03/05/combat-anti-vaxxers-us-needs-national-campaign-top-washington-state-official-says/?utm_term=.9a4201be0ed1. Published March 5, 2019. Accessed June 9, 2019.
2. Filsinger AL, Dwek R. George Washington and the first mass military inoculation. http://www.loc.gov/rr/scitech/GW&smallpoxinoculation.html. Published February 12, 2009. Accessed June 10, 2019.
3. Fenn EA. Pox Americana. New York: Hill and Wang; 2001.
4. Steadman’s Medical Dictionary. 28th edition. Philadelphia, PA: Lippincott, Williams & Wilkins; 2006.
5. Artenstein AW, Opal JM, Opal SM, Tramont EC, Georges P, Russell PK. History of U.S. military contributions to the study of vaccines and infectious diseases. Mil Med. 2005;170(suppl 4):3-11.
6. Jowers K. So far, no measles cases at military medical facilities—but officials are watching. https://www.militarytimes.com/pay-benefits/2019/04/19/so-far-no-measles-cases-at-military-medical-facilities-but-officials-are-watching/. Published April 19, 2019. Accessed June 9, 2019.
7. Cole JP, Swendiman KS. Mandatory vaccinations: precedent and current laws. https://fas.org/sgp/crs/misc/RS21414.pdf. Published May 21, 2014. Accessed June 10, 2019.
Polypharmacy: When might it make sense?
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
Wellness seminars won’t fix burnout
“Burnout” has been defined as long-term, unresolvable job stress that leads to exhaustion, depression, and in some tragic circumstances, suicide. One of our lead articles this month concerns an attempt to place a financial cost on physician burnout. More important, I think, is the toll burnout takes on an individual, their family, and their patients. In my role as Chief Clinical Officer of the University of Michigan Medical Group (our faculty and other clinical providers), I struggle to balance productivity demands with the increasing damage such demands are doing to our clinicians. Few primary care physicians at Michigan Medicine work full-time as clinicians (defined as 32 hours patient facing time per week for 46 weeks). Almost all request part-time status if they do not have protected, grant-funded time. They simply cannot keep up with the documentation required in our electronic health record, combined with our “patient-friendly” access via the electronic portal. One-third of the private practice group I helped build was part-time when I left in 2012, and it is not unusual to hear complaints about burnout from my ex-partners.
Let’s be clear, burnout is not going to be solved by increasing the resilience of our physicians or sending us to wellness seminars. That approach is a direct blame-the-victim paradigm. Physicians are burned out because of the constant assault on the core reasons we entered medicine – to help people (this assault has been termed “moral injury”). BPAs (best practice alerts), coding requirements, inbox demands, prior authorizations (see the practice management section of this issue), electronic-order entry, and most other practice enhancement tools rely on the willingness of physicians to sacrifice more time and energy and sit in front of a computer screen.
Salvation of our health care system will not come from mass retirements (although that is happening), concierge practices, part-time status, or other individual responses to this crisis. We will need a fundamental reorganization of our practice, where we (physicians) reduce our work to activities for which we trained combined with a shift of nonphysician work to others; better technology, virtual visits, and ancillary personnel. Patient expectations must be realistic and legal protections need strengthening. The politics of health care has focused on funds flow and ideology. We need a stronger voice that articulates the daily microaggressions that we each endure as we try to live Oslerian physician ideals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Burnout” has been defined as long-term, unresolvable job stress that leads to exhaustion, depression, and in some tragic circumstances, suicide. One of our lead articles this month concerns an attempt to place a financial cost on physician burnout. More important, I think, is the toll burnout takes on an individual, their family, and their patients. In my role as Chief Clinical Officer of the University of Michigan Medical Group (our faculty and other clinical providers), I struggle to balance productivity demands with the increasing damage such demands are doing to our clinicians. Few primary care physicians at Michigan Medicine work full-time as clinicians (defined as 32 hours patient facing time per week for 46 weeks). Almost all request part-time status if they do not have protected, grant-funded time. They simply cannot keep up with the documentation required in our electronic health record, combined with our “patient-friendly” access via the electronic portal. One-third of the private practice group I helped build was part-time when I left in 2012, and it is not unusual to hear complaints about burnout from my ex-partners.
Let’s be clear, burnout is not going to be solved by increasing the resilience of our physicians or sending us to wellness seminars. That approach is a direct blame-the-victim paradigm. Physicians are burned out because of the constant assault on the core reasons we entered medicine – to help people (this assault has been termed “moral injury”). BPAs (best practice alerts), coding requirements, inbox demands, prior authorizations (see the practice management section of this issue), electronic-order entry, and most other practice enhancement tools rely on the willingness of physicians to sacrifice more time and energy and sit in front of a computer screen.
Salvation of our health care system will not come from mass retirements (although that is happening), concierge practices, part-time status, or other individual responses to this crisis. We will need a fundamental reorganization of our practice, where we (physicians) reduce our work to activities for which we trained combined with a shift of nonphysician work to others; better technology, virtual visits, and ancillary personnel. Patient expectations must be realistic and legal protections need strengthening. The politics of health care has focused on funds flow and ideology. We need a stronger voice that articulates the daily microaggressions that we each endure as we try to live Oslerian physician ideals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Burnout” has been defined as long-term, unresolvable job stress that leads to exhaustion, depression, and in some tragic circumstances, suicide. One of our lead articles this month concerns an attempt to place a financial cost on physician burnout. More important, I think, is the toll burnout takes on an individual, their family, and their patients. In my role as Chief Clinical Officer of the University of Michigan Medical Group (our faculty and other clinical providers), I struggle to balance productivity demands with the increasing damage such demands are doing to our clinicians. Few primary care physicians at Michigan Medicine work full-time as clinicians (defined as 32 hours patient facing time per week for 46 weeks). Almost all request part-time status if they do not have protected, grant-funded time. They simply cannot keep up with the documentation required in our electronic health record, combined with our “patient-friendly” access via the electronic portal. One-third of the private practice group I helped build was part-time when I left in 2012, and it is not unusual to hear complaints about burnout from my ex-partners.
Let’s be clear, burnout is not going to be solved by increasing the resilience of our physicians or sending us to wellness seminars. That approach is a direct blame-the-victim paradigm. Physicians are burned out because of the constant assault on the core reasons we entered medicine – to help people (this assault has been termed “moral injury”). BPAs (best practice alerts), coding requirements, inbox demands, prior authorizations (see the practice management section of this issue), electronic-order entry, and most other practice enhancement tools rely on the willingness of physicians to sacrifice more time and energy and sit in front of a computer screen.
Salvation of our health care system will not come from mass retirements (although that is happening), concierge practices, part-time status, or other individual responses to this crisis. We will need a fundamental reorganization of our practice, where we (physicians) reduce our work to activities for which we trained combined with a shift of nonphysician work to others; better technology, virtual visits, and ancillary personnel. Patient expectations must be realistic and legal protections need strengthening. The politics of health care has focused on funds flow and ideology. We need a stronger voice that articulates the daily microaggressions that we each endure as we try to live Oslerian physician ideals.
John I. Allen, MD, MBA, AGAF
Editor in Chief