User login
For MD-IQ use only
Passing the torch
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me (bryson.katona@pennmedicine.upenn.edu), incoming editor in chief Vijaya Rao (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me (bryson.katona@pennmedicine.upenn.edu), incoming editor in chief Vijaya Rao (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me (bryson.katona@pennmedicine.upenn.edu), incoming editor in chief Vijaya Rao (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Short Takes
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Improving Comorbidities With Psoriasis Treatment
Psoriasis is a common immune-mediated inflammatory skin disorder that affects up to 3.2% of adults in the United States.1 It is a TH1, TH17, and TH22 inflammatory disease resulting in increased levels of cytokines in the skin, including IFN-γ, tumor necrosis factor (TNF), IL-17, and IL-22. Dendritic antigen-presenting cells also are increased in the skin of patients with psoriasis resulting in increased levels of IL-23.2 Although skin disease often is its most prominent and sometimes its only documented manifestation, an understanding of psoriasis as a chronic multisystem inflammatory disorder is essential to optimize outcomes.1,3 Multiple comorbidities that may affect treatment selection often are associated with psoriasis, including psoriatic arthritis, cardiovascular disease, depression, obesity, metabolic syndrome, cardiovascular disease (CVD), cerebrovascular disease, and peripheral vascular disease.
As with other immune-mediated inflammatory diseases, it has been hypothesized that psoriasis may influence comorbidities through shared genetic risks, environmental factors, and pathogenic factors or inflammatory pathways.2-4 For example, emerging evidence suggests that comorbidities such as metabolic syndrome may be related to the chronic inflammation that accompanies psoriasis, a finding that has important clinical implications.3
The interplay and dependence or interdependence of psoriasis and its comorbidities is complex, and it is an area deserving of vigorous research.1 At present, observational and epidemiological data such as the present case suggest that effective treatment of psoriasis could lead to benefits “beyond the skin” and potentially even prevent future disease-associated comorbidity.1-3
Metabolic Comorbidities and Psoriasis Treatment
Although the prevalence of CVD and CVD risk factors is increased in patients with psoriasis, studies suggest that the suppression of systemic inflammation that accompanies adequate psoriasis treatment, particularly in patients with moderate to severe disease, may decrease the risk for cardiovascular comorbidities.5 For example, a number of studies have found treatment of psoriasis with methotrexate may decrease the risk for cardiovascular events, including ischemic heart disease, stroke, and cardiovascular death.6-10 Low-dose methotrexate has been shown to be particularly advantageous for decreasing CVD in patients with psoriasis.5,8
Tumor necrosis factor α inhibitors, which are frequently used for moderate to severe plaque psoriasis, also may notably decrease cardiovascular risk.5 One study showed a significant decrease in the risk for myocardial infarction in patients with psoriasis who were treated with TNF-α inhibitors (hazard ratio, 0.50; 95% CI, 0.32-0.79)11; other studies have confirmed this benefit.12-17 Moreover, the reduction in cardiovascular events may be greater with TNF-α inhibitors than with methotrexate when the former is used for psoriasis treatment, with longer duration of TNF-α inhibition leading to greater risk reduction.18,19
In patients with severe psoriasis, treatment with TNF-α inhibitors has been associated with improvements in subclinical CVD (abnormalities in echocardiogram), improved coronary microvascular function (determined by transthoracic Doppler echocardiography), and reduced progression in coronary artery disease (assessed by coronary computed tomography).20-22 Improvement in endothelial function (brachial artery reactivity) and carotid arterial stiffness also has been reported following 6 months of treatment with adalimumab for moderate to severe psoriasis.21
Data concerning potential cardiovascular risk reduction with treatment of psoriasis utilizing newer agents are continuing to emerge. To date, no increase in the incidence of major adverse cardiovascular events has been shown in patients with psoriasis treated with anti–IL-17 agents, such as secukinumab; however, additional long-term studies are needed.18,23-25
Apremilast, an oral phosphodiesterase 4 inhibitor, is another addition to the psoriasis armamentarium.26 No increase in the risk for major cardiac events has been shown in randomized controlled trials of patients with psoriasis receiving apremilast for up to 156 weeks.27,28 As with secukinumab, additional long-term, large-scale studies are needed to determine the effects of apremilast on cardiovascular risk in patients with psoriasis.5
Other Comorbidities
Effective treatment of psoriasis also appears to benefit various other comorbidities. Numerous studies have shown an increased incidence of depression in patients with psoriasis vs controls and a concurrent improvement in psychiatric symptoms with psoriasis disease control.1 For instance, a multicenter, randomized, open-label study of 352 patients with psoriasis showed treatment with etanercept, a TNF inhibitor, significantly improved scores for concomitant depression and anxiety (P<.05).29 Similarly, a double-blind, randomized clinical trial of patients with psoriasis found significant improvement in depression at 12 weeks in patients treated with adalimumab vs placebo (P<.001).30 Likewise, a multicenter phase 3 trial of more than 600 psoriatic patients showed improved Beck depression inventory and Hamilton depression rating scale scores at 12 weeks in patients with psoriasis treated with etanercept compared to placebo.31
A much larger analysis of 7490 patients with psoriasis compared the rates of depression among patients receiving biologic therapy, phototherapy, and conventional systemic therapy. The greatest impact on depression symptoms was seen with biologic therapy (incidence rate, 3.01/100 patient-years), followed by conventional systemic therapy (5.70/100 patient-years), and phototherapy (5.85/100 patient-years).32
Uveitis, or inflammation of the middle layer of the eye (the uvea), frequently is seen in patients with psoriasis. In a cohort study of 60,000 patients with mild psoriasis and more than 7000 patients with severe psoriasis, the incidence of uveitis in patients was significantly increased in both patients with severe disease and those with mild disease (P<.001 for both).33 In a case series of 8 patients with concomitant psoriasis and uveitis, 4 patients were treated with infliximab and 4 with adalimumab; 7 patients treated achieved remission of their uveitis.34
Role of the TNF-α Blockade in Sickle Cell Disease
Presently, no reported human studies have shown TNF-α blockade as a possible treatment of sickle cell disease.35 However, increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and to the severity of sickle cell disease due to their integral role in the development of vascular wall dysfunction and ischemia.35,36 Studies have shown that TNF-α is released in homozygous sickle cell anemia (HbSS) disease and impedes blood flow during sickle cell crisis, resulting in worsening ischemia and painful infarction.35,36 Moreover, cytokine analysis has shown significantly (P<.05) elevated levels of TNF-α during sickle cell crises and at baseline in patients with HbSS vs healthy controls, suggesting a possible role of TNF-α in the pathogenesis of sickle cell crisis.36
The case patient reported a 50% reduction in pain level and the use of pain medications that overlapped with the initiation of adalimumab for treatment of her psoriasis. Moreover, although radiographs showed possible psoriatic changes of the distal metatarsal row, she described sickle cell pain and pain crises that were uncharacteristic of psoriatic arthralgia.35 Although these findings are observational in nature and limited to one patient, they do suggest an interesting hypothesis. If a common inflammatory mediator is the culprit, it is possible that TNF-α inhibitors could be the preferred treatment option for patients with psoriasis and comorbid HbSS or HbSC disease. Further studies are needed to analyze the role of TNF-α inhibition in sickle cell disease.
Bottom Line
Psoriasis may influence comorbidities through shared genetic risks, environmental factors, or inflammatory pathways. Improvement in metabolic and other comorbidities have been shown with the effective treatment of psoriasis. The case described here showed improvement in sickle cell crises and pain with treatment of psoriasis with adalimumab. Tumor necrosis factor inhibitors may be an optimal choice for patients with both psoriasis and sickle cell disease.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
- Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
- Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
- Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
- Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
- Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
- Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
- Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
- Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
- Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
- Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
- Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
- Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
- Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Psoriasis is a common immune-mediated inflammatory skin disorder that affects up to 3.2% of adults in the United States.1 It is a TH1, TH17, and TH22 inflammatory disease resulting in increased levels of cytokines in the skin, including IFN-γ, tumor necrosis factor (TNF), IL-17, and IL-22. Dendritic antigen-presenting cells also are increased in the skin of patients with psoriasis resulting in increased levels of IL-23.2 Although skin disease often is its most prominent and sometimes its only documented manifestation, an understanding of psoriasis as a chronic multisystem inflammatory disorder is essential to optimize outcomes.1,3 Multiple comorbidities that may affect treatment selection often are associated with psoriasis, including psoriatic arthritis, cardiovascular disease, depression, obesity, metabolic syndrome, cardiovascular disease (CVD), cerebrovascular disease, and peripheral vascular disease.
As with other immune-mediated inflammatory diseases, it has been hypothesized that psoriasis may influence comorbidities through shared genetic risks, environmental factors, and pathogenic factors or inflammatory pathways.2-4 For example, emerging evidence suggests that comorbidities such as metabolic syndrome may be related to the chronic inflammation that accompanies psoriasis, a finding that has important clinical implications.3
The interplay and dependence or interdependence of psoriasis and its comorbidities is complex, and it is an area deserving of vigorous research.1 At present, observational and epidemiological data such as the present case suggest that effective treatment of psoriasis could lead to benefits “beyond the skin” and potentially even prevent future disease-associated comorbidity.1-3
Metabolic Comorbidities and Psoriasis Treatment
Although the prevalence of CVD and CVD risk factors is increased in patients with psoriasis, studies suggest that the suppression of systemic inflammation that accompanies adequate psoriasis treatment, particularly in patients with moderate to severe disease, may decrease the risk for cardiovascular comorbidities.5 For example, a number of studies have found treatment of psoriasis with methotrexate may decrease the risk for cardiovascular events, including ischemic heart disease, stroke, and cardiovascular death.6-10 Low-dose methotrexate has been shown to be particularly advantageous for decreasing CVD in patients with psoriasis.5,8
Tumor necrosis factor α inhibitors, which are frequently used for moderate to severe plaque psoriasis, also may notably decrease cardiovascular risk.5 One study showed a significant decrease in the risk for myocardial infarction in patients with psoriasis who were treated with TNF-α inhibitors (hazard ratio, 0.50; 95% CI, 0.32-0.79)11; other studies have confirmed this benefit.12-17 Moreover, the reduction in cardiovascular events may be greater with TNF-α inhibitors than with methotrexate when the former is used for psoriasis treatment, with longer duration of TNF-α inhibition leading to greater risk reduction.18,19
In patients with severe psoriasis, treatment with TNF-α inhibitors has been associated with improvements in subclinical CVD (abnormalities in echocardiogram), improved coronary microvascular function (determined by transthoracic Doppler echocardiography), and reduced progression in coronary artery disease (assessed by coronary computed tomography).20-22 Improvement in endothelial function (brachial artery reactivity) and carotid arterial stiffness also has been reported following 6 months of treatment with adalimumab for moderate to severe psoriasis.21
Data concerning potential cardiovascular risk reduction with treatment of psoriasis utilizing newer agents are continuing to emerge. To date, no increase in the incidence of major adverse cardiovascular events has been shown in patients with psoriasis treated with anti–IL-17 agents, such as secukinumab; however, additional long-term studies are needed.18,23-25
Apremilast, an oral phosphodiesterase 4 inhibitor, is another addition to the psoriasis armamentarium.26 No increase in the risk for major cardiac events has been shown in randomized controlled trials of patients with psoriasis receiving apremilast for up to 156 weeks.27,28 As with secukinumab, additional long-term, large-scale studies are needed to determine the effects of apremilast on cardiovascular risk in patients with psoriasis.5
Other Comorbidities
Effective treatment of psoriasis also appears to benefit various other comorbidities. Numerous studies have shown an increased incidence of depression in patients with psoriasis vs controls and a concurrent improvement in psychiatric symptoms with psoriasis disease control.1 For instance, a multicenter, randomized, open-label study of 352 patients with psoriasis showed treatment with etanercept, a TNF inhibitor, significantly improved scores for concomitant depression and anxiety (P<.05).29 Similarly, a double-blind, randomized clinical trial of patients with psoriasis found significant improvement in depression at 12 weeks in patients treated with adalimumab vs placebo (P<.001).30 Likewise, a multicenter phase 3 trial of more than 600 psoriatic patients showed improved Beck depression inventory and Hamilton depression rating scale scores at 12 weeks in patients with psoriasis treated with etanercept compared to placebo.31
A much larger analysis of 7490 patients with psoriasis compared the rates of depression among patients receiving biologic therapy, phototherapy, and conventional systemic therapy. The greatest impact on depression symptoms was seen with biologic therapy (incidence rate, 3.01/100 patient-years), followed by conventional systemic therapy (5.70/100 patient-years), and phototherapy (5.85/100 patient-years).32
Uveitis, or inflammation of the middle layer of the eye (the uvea), frequently is seen in patients with psoriasis. In a cohort study of 60,000 patients with mild psoriasis and more than 7000 patients with severe psoriasis, the incidence of uveitis in patients was significantly increased in both patients with severe disease and those with mild disease (P<.001 for both).33 In a case series of 8 patients with concomitant psoriasis and uveitis, 4 patients were treated with infliximab and 4 with adalimumab; 7 patients treated achieved remission of their uveitis.34
Role of the TNF-α Blockade in Sickle Cell Disease
Presently, no reported human studies have shown TNF-α blockade as a possible treatment of sickle cell disease.35 However, increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and to the severity of sickle cell disease due to their integral role in the development of vascular wall dysfunction and ischemia.35,36 Studies have shown that TNF-α is released in homozygous sickle cell anemia (HbSS) disease and impedes blood flow during sickle cell crisis, resulting in worsening ischemia and painful infarction.35,36 Moreover, cytokine analysis has shown significantly (P<.05) elevated levels of TNF-α during sickle cell crises and at baseline in patients with HbSS vs healthy controls, suggesting a possible role of TNF-α in the pathogenesis of sickle cell crisis.36
The case patient reported a 50% reduction in pain level and the use of pain medications that overlapped with the initiation of adalimumab for treatment of her psoriasis. Moreover, although radiographs showed possible psoriatic changes of the distal metatarsal row, she described sickle cell pain and pain crises that were uncharacteristic of psoriatic arthralgia.35 Although these findings are observational in nature and limited to one patient, they do suggest an interesting hypothesis. If a common inflammatory mediator is the culprit, it is possible that TNF-α inhibitors could be the preferred treatment option for patients with psoriasis and comorbid HbSS or HbSC disease. Further studies are needed to analyze the role of TNF-α inhibition in sickle cell disease.
Bottom Line
Psoriasis may influence comorbidities through shared genetic risks, environmental factors, or inflammatory pathways. Improvement in metabolic and other comorbidities have been shown with the effective treatment of psoriasis. The case described here showed improvement in sickle cell crises and pain with treatment of psoriasis with adalimumab. Tumor necrosis factor inhibitors may be an optimal choice for patients with both psoriasis and sickle cell disease.
Psoriasis is a common immune-mediated inflammatory skin disorder that affects up to 3.2% of adults in the United States.1 It is a TH1, TH17, and TH22 inflammatory disease resulting in increased levels of cytokines in the skin, including IFN-γ, tumor necrosis factor (TNF), IL-17, and IL-22. Dendritic antigen-presenting cells also are increased in the skin of patients with psoriasis resulting in increased levels of IL-23.2 Although skin disease often is its most prominent and sometimes its only documented manifestation, an understanding of psoriasis as a chronic multisystem inflammatory disorder is essential to optimize outcomes.1,3 Multiple comorbidities that may affect treatment selection often are associated with psoriasis, including psoriatic arthritis, cardiovascular disease, depression, obesity, metabolic syndrome, cardiovascular disease (CVD), cerebrovascular disease, and peripheral vascular disease.
As with other immune-mediated inflammatory diseases, it has been hypothesized that psoriasis may influence comorbidities through shared genetic risks, environmental factors, and pathogenic factors or inflammatory pathways.2-4 For example, emerging evidence suggests that comorbidities such as metabolic syndrome may be related to the chronic inflammation that accompanies psoriasis, a finding that has important clinical implications.3
The interplay and dependence or interdependence of psoriasis and its comorbidities is complex, and it is an area deserving of vigorous research.1 At present, observational and epidemiological data such as the present case suggest that effective treatment of psoriasis could lead to benefits “beyond the skin” and potentially even prevent future disease-associated comorbidity.1-3
Metabolic Comorbidities and Psoriasis Treatment
Although the prevalence of CVD and CVD risk factors is increased in patients with psoriasis, studies suggest that the suppression of systemic inflammation that accompanies adequate psoriasis treatment, particularly in patients with moderate to severe disease, may decrease the risk for cardiovascular comorbidities.5 For example, a number of studies have found treatment of psoriasis with methotrexate may decrease the risk for cardiovascular events, including ischemic heart disease, stroke, and cardiovascular death.6-10 Low-dose methotrexate has been shown to be particularly advantageous for decreasing CVD in patients with psoriasis.5,8
Tumor necrosis factor α inhibitors, which are frequently used for moderate to severe plaque psoriasis, also may notably decrease cardiovascular risk.5 One study showed a significant decrease in the risk for myocardial infarction in patients with psoriasis who were treated with TNF-α inhibitors (hazard ratio, 0.50; 95% CI, 0.32-0.79)11; other studies have confirmed this benefit.12-17 Moreover, the reduction in cardiovascular events may be greater with TNF-α inhibitors than with methotrexate when the former is used for psoriasis treatment, with longer duration of TNF-α inhibition leading to greater risk reduction.18,19
In patients with severe psoriasis, treatment with TNF-α inhibitors has been associated with improvements in subclinical CVD (abnormalities in echocardiogram), improved coronary microvascular function (determined by transthoracic Doppler echocardiography), and reduced progression in coronary artery disease (assessed by coronary computed tomography).20-22 Improvement in endothelial function (brachial artery reactivity) and carotid arterial stiffness also has been reported following 6 months of treatment with adalimumab for moderate to severe psoriasis.21
Data concerning potential cardiovascular risk reduction with treatment of psoriasis utilizing newer agents are continuing to emerge. To date, no increase in the incidence of major adverse cardiovascular events has been shown in patients with psoriasis treated with anti–IL-17 agents, such as secukinumab; however, additional long-term studies are needed.18,23-25
Apremilast, an oral phosphodiesterase 4 inhibitor, is another addition to the psoriasis armamentarium.26 No increase in the risk for major cardiac events has been shown in randomized controlled trials of patients with psoriasis receiving apremilast for up to 156 weeks.27,28 As with secukinumab, additional long-term, large-scale studies are needed to determine the effects of apremilast on cardiovascular risk in patients with psoriasis.5
Other Comorbidities
Effective treatment of psoriasis also appears to benefit various other comorbidities. Numerous studies have shown an increased incidence of depression in patients with psoriasis vs controls and a concurrent improvement in psychiatric symptoms with psoriasis disease control.1 For instance, a multicenter, randomized, open-label study of 352 patients with psoriasis showed treatment with etanercept, a TNF inhibitor, significantly improved scores for concomitant depression and anxiety (P<.05).29 Similarly, a double-blind, randomized clinical trial of patients with psoriasis found significant improvement in depression at 12 weeks in patients treated with adalimumab vs placebo (P<.001).30 Likewise, a multicenter phase 3 trial of more than 600 psoriatic patients showed improved Beck depression inventory and Hamilton depression rating scale scores at 12 weeks in patients with psoriasis treated with etanercept compared to placebo.31
A much larger analysis of 7490 patients with psoriasis compared the rates of depression among patients receiving biologic therapy, phototherapy, and conventional systemic therapy. The greatest impact on depression symptoms was seen with biologic therapy (incidence rate, 3.01/100 patient-years), followed by conventional systemic therapy (5.70/100 patient-years), and phototherapy (5.85/100 patient-years).32
Uveitis, or inflammation of the middle layer of the eye (the uvea), frequently is seen in patients with psoriasis. In a cohort study of 60,000 patients with mild psoriasis and more than 7000 patients with severe psoriasis, the incidence of uveitis in patients was significantly increased in both patients with severe disease and those with mild disease (P<.001 for both).33 In a case series of 8 patients with concomitant psoriasis and uveitis, 4 patients were treated with infliximab and 4 with adalimumab; 7 patients treated achieved remission of their uveitis.34
Role of the TNF-α Blockade in Sickle Cell Disease
Presently, no reported human studies have shown TNF-α blockade as a possible treatment of sickle cell disease.35 However, increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and to the severity of sickle cell disease due to their integral role in the development of vascular wall dysfunction and ischemia.35,36 Studies have shown that TNF-α is released in homozygous sickle cell anemia (HbSS) disease and impedes blood flow during sickle cell crisis, resulting in worsening ischemia and painful infarction.35,36 Moreover, cytokine analysis has shown significantly (P<.05) elevated levels of TNF-α during sickle cell crises and at baseline in patients with HbSS vs healthy controls, suggesting a possible role of TNF-α in the pathogenesis of sickle cell crisis.36
The case patient reported a 50% reduction in pain level and the use of pain medications that overlapped with the initiation of adalimumab for treatment of her psoriasis. Moreover, although radiographs showed possible psoriatic changes of the distal metatarsal row, she described sickle cell pain and pain crises that were uncharacteristic of psoriatic arthralgia.35 Although these findings are observational in nature and limited to one patient, they do suggest an interesting hypothesis. If a common inflammatory mediator is the culprit, it is possible that TNF-α inhibitors could be the preferred treatment option for patients with psoriasis and comorbid HbSS or HbSC disease. Further studies are needed to analyze the role of TNF-α inhibition in sickle cell disease.
Bottom Line
Psoriasis may influence comorbidities through shared genetic risks, environmental factors, or inflammatory pathways. Improvement in metabolic and other comorbidities have been shown with the effective treatment of psoriasis. The case described here showed improvement in sickle cell crises and pain with treatment of psoriasis with adalimumab. Tumor necrosis factor inhibitors may be an optimal choice for patients with both psoriasis and sickle cell disease.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
- Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
- Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
- Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
- Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
- Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
- Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
- Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
- Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
- Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
- Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
- Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
- Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
- Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
- Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
- Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
- Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
- Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
- Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
- Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
- Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
- Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
- Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
- Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
- Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
- Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
- Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
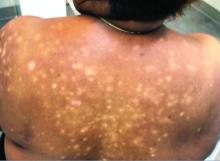
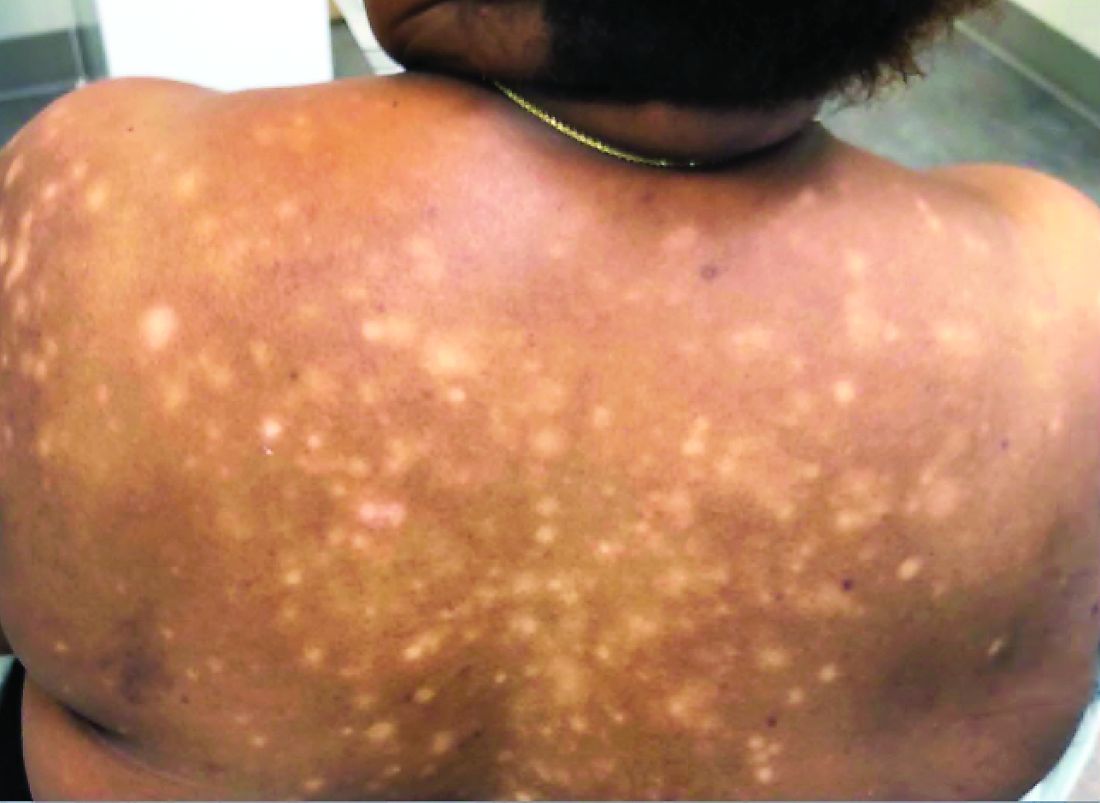
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area). The patient’s medical history was positive for sickle cell disease, specifically hemoglobin SC disease (HbSC). She reported chronic dull arthralgia in the ankles that was worse at night. She was being treated by hematology with ibuprofen and ketorolac. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. At the current presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on its potential for improvement in HbSC symptoms as well as psoriasis.
Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and she reported a decrease in her HbSC pain from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. She also reported decreased use of pain medication with rare sickle cell pain crises following initiation of adalimumab.
This case was adapted from Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease. Cutis. 2019;103:93-94.
Keeping up to date at the Florida Society of Rheumatology annual meeting
The Florida Society of Rheumatology is an excellent state conference that is very well attended because of the comprehensive clinical topics covered by esteemed faculty. Every year, there is a balance of patient care lectures and updates in advocacy, billing, and coding. Clinicians need a combination of both arenas to be successful with the day-to-day practice of rheumatology and to render evidenced-based patient care. This year, the FSR certainly delivered on this mission as reflected by articles on these presentations published at MDedge Rheumatology. An added focus this year was how to leverage technology in a rheumatology practice to capture patient-reported outcomes (PROs) to better understand issues affecting our patient population and improve therapy plans where indicated.
In his lecture on digital PROs, Jeffrey Curtis, MD, explained the difference between active capture of data through tools such as the Routine Assessment of Patient Index Data 3 (RAPID3) or Health Assessment Questionnaire (HAQ) and passive capture through wearable devices such as a Fitbit or Apple watch. A key point for the audience was that this information improves clinical care and improves medical decision making, and thus all rheumatologists should consider using these tools in practice. Dr. Curtis, William J. Koopman Endowed Professor in Rheumatology and Immunology and director of the UAB Arthritis Clinical Intervention Program at the University of Alabama at Birmingham, is well aware of the practical concerns that face clinicians, namely that this is time consuming. He suggests to keep it short and find a tool that works for you in your practice to understand how your patients are progressing on a treatment regimen. He was clear that “data for the sake of data is not compelling for patients [or clinicians].” The ideal is not to paralyze your practice and drown in patient questionnaires but rather to empower patients to report using standardized tools so we can effect change that will help us to treat rheumatic diseases.
An important point mentioned during this lecture was to keep in mind that, if a patient appears to be a “nonresponder” on RAPID3, for example, it is important to understand whether the patient has a confounding comorbidity, such as fibromyalgia, that may account for the limited improvement.
Michelle Petri, MD, gave two excellent talks at FSR this year. Her lectures are packed with excellent pearls about treating patients with systemic lupus erythematosus. Interestingly, she said to never underestimate the prognostic factor of a low C3. This can indicate a worse clinical course is ahead. In addition, she reminds us as clinicians to protect the kidneys of our lupus patients who have renal disease by avoiding common toxins such as NSAIDs and CT contrast. Of course, she reminds us to use the lowest dose of steroids possible during flares, as prednisone is directly or indirectly responsible for 80% of organ damage over 15 years. She reminds us that lupus patients do not die of lupus. They have a 2.66-fold higher risk of cardiovascular events than the general public. In addition to maintaining lupus patients on hydroxychloroquine, Dr. Petri, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore, noted that vitamin D can have cardiovascular and hematologic benefits along with reducing thrombosis in some clinical studies. Low vitamin D was significantly associated with deep venous thrombosis.
In his lecture, Leonard Calabrese, DO, made a compelling argument for the rheumatologists in the audience to call the local oncologists with whom they work. We need to discuss and collaborate on the care of patients experiencing immune-mediated adverse events from exposure to checkpoint inhibitors used to treat malignancy. There is a limited mechanistic understanding of these adverse events, but as rheumatologists we need to get involved and help these patients. We are the experts in managing these newly emerging autoimmune events. We can help to create the best possible therapeutic interventions to help our oncology colleagues with these challenging cases, Dr. Calabrese, professor of medicine and chair of clinical immunology at the Cleveland Clinic, said.
Besides paying our dues to be members of the FSR, it is important for us as rheumatologists to get involved at the state legislature and national level to bring about change for our practices and patients. Currently, the climate can be hostile for reimbursement and for our patients to get the therapies they need. In another presentation, Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee, described recent successes at the national level, and he also discussed how we can have our voices heard at the state and national level to protect our profession and the people who rely on our expertise. The FSR and other state rheumatology organizations, as well as the ACR, need our support to continue to be the collective voice for what is right for clinicians and patients alike.
Dr. Oberstein is a practicing rheumatologist at the University of Miami Health System and is senior medical director of musculoskeletal at Modernizing Medicine in Boca Raton, Fla. She has no relevant disclosures to report.
The Florida Society of Rheumatology is an excellent state conference that is very well attended because of the comprehensive clinical topics covered by esteemed faculty. Every year, there is a balance of patient care lectures and updates in advocacy, billing, and coding. Clinicians need a combination of both arenas to be successful with the day-to-day practice of rheumatology and to render evidenced-based patient care. This year, the FSR certainly delivered on this mission as reflected by articles on these presentations published at MDedge Rheumatology. An added focus this year was how to leverage technology in a rheumatology practice to capture patient-reported outcomes (PROs) to better understand issues affecting our patient population and improve therapy plans where indicated.
In his lecture on digital PROs, Jeffrey Curtis, MD, explained the difference between active capture of data through tools such as the Routine Assessment of Patient Index Data 3 (RAPID3) or Health Assessment Questionnaire (HAQ) and passive capture through wearable devices such as a Fitbit or Apple watch. A key point for the audience was that this information improves clinical care and improves medical decision making, and thus all rheumatologists should consider using these tools in practice. Dr. Curtis, William J. Koopman Endowed Professor in Rheumatology and Immunology and director of the UAB Arthritis Clinical Intervention Program at the University of Alabama at Birmingham, is well aware of the practical concerns that face clinicians, namely that this is time consuming. He suggests to keep it short and find a tool that works for you in your practice to understand how your patients are progressing on a treatment regimen. He was clear that “data for the sake of data is not compelling for patients [or clinicians].” The ideal is not to paralyze your practice and drown in patient questionnaires but rather to empower patients to report using standardized tools so we can effect change that will help us to treat rheumatic diseases.
An important point mentioned during this lecture was to keep in mind that, if a patient appears to be a “nonresponder” on RAPID3, for example, it is important to understand whether the patient has a confounding comorbidity, such as fibromyalgia, that may account for the limited improvement.
Michelle Petri, MD, gave two excellent talks at FSR this year. Her lectures are packed with excellent pearls about treating patients with systemic lupus erythematosus. Interestingly, she said to never underestimate the prognostic factor of a low C3. This can indicate a worse clinical course is ahead. In addition, she reminds us as clinicians to protect the kidneys of our lupus patients who have renal disease by avoiding common toxins such as NSAIDs and CT contrast. Of course, she reminds us to use the lowest dose of steroids possible during flares, as prednisone is directly or indirectly responsible for 80% of organ damage over 15 years. She reminds us that lupus patients do not die of lupus. They have a 2.66-fold higher risk of cardiovascular events than the general public. In addition to maintaining lupus patients on hydroxychloroquine, Dr. Petri, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore, noted that vitamin D can have cardiovascular and hematologic benefits along with reducing thrombosis in some clinical studies. Low vitamin D was significantly associated with deep venous thrombosis.
In his lecture, Leonard Calabrese, DO, made a compelling argument for the rheumatologists in the audience to call the local oncologists with whom they work. We need to discuss and collaborate on the care of patients experiencing immune-mediated adverse events from exposure to checkpoint inhibitors used to treat malignancy. There is a limited mechanistic understanding of these adverse events, but as rheumatologists we need to get involved and help these patients. We are the experts in managing these newly emerging autoimmune events. We can help to create the best possible therapeutic interventions to help our oncology colleagues with these challenging cases, Dr. Calabrese, professor of medicine and chair of clinical immunology at the Cleveland Clinic, said.
Besides paying our dues to be members of the FSR, it is important for us as rheumatologists to get involved at the state legislature and national level to bring about change for our practices and patients. Currently, the climate can be hostile for reimbursement and for our patients to get the therapies they need. In another presentation, Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee, described recent successes at the national level, and he also discussed how we can have our voices heard at the state and national level to protect our profession and the people who rely on our expertise. The FSR and other state rheumatology organizations, as well as the ACR, need our support to continue to be the collective voice for what is right for clinicians and patients alike.
Dr. Oberstein is a practicing rheumatologist at the University of Miami Health System and is senior medical director of musculoskeletal at Modernizing Medicine in Boca Raton, Fla. She has no relevant disclosures to report.
The Florida Society of Rheumatology is an excellent state conference that is very well attended because of the comprehensive clinical topics covered by esteemed faculty. Every year, there is a balance of patient care lectures and updates in advocacy, billing, and coding. Clinicians need a combination of both arenas to be successful with the day-to-day practice of rheumatology and to render evidenced-based patient care. This year, the FSR certainly delivered on this mission as reflected by articles on these presentations published at MDedge Rheumatology. An added focus this year was how to leverage technology in a rheumatology practice to capture patient-reported outcomes (PROs) to better understand issues affecting our patient population and improve therapy plans where indicated.
In his lecture on digital PROs, Jeffrey Curtis, MD, explained the difference between active capture of data through tools such as the Routine Assessment of Patient Index Data 3 (RAPID3) or Health Assessment Questionnaire (HAQ) and passive capture through wearable devices such as a Fitbit or Apple watch. A key point for the audience was that this information improves clinical care and improves medical decision making, and thus all rheumatologists should consider using these tools in practice. Dr. Curtis, William J. Koopman Endowed Professor in Rheumatology and Immunology and director of the UAB Arthritis Clinical Intervention Program at the University of Alabama at Birmingham, is well aware of the practical concerns that face clinicians, namely that this is time consuming. He suggests to keep it short and find a tool that works for you in your practice to understand how your patients are progressing on a treatment regimen. He was clear that “data for the sake of data is not compelling for patients [or clinicians].” The ideal is not to paralyze your practice and drown in patient questionnaires but rather to empower patients to report using standardized tools so we can effect change that will help us to treat rheumatic diseases.
An important point mentioned during this lecture was to keep in mind that, if a patient appears to be a “nonresponder” on RAPID3, for example, it is important to understand whether the patient has a confounding comorbidity, such as fibromyalgia, that may account for the limited improvement.
Michelle Petri, MD, gave two excellent talks at FSR this year. Her lectures are packed with excellent pearls about treating patients with systemic lupus erythematosus. Interestingly, she said to never underestimate the prognostic factor of a low C3. This can indicate a worse clinical course is ahead. In addition, she reminds us as clinicians to protect the kidneys of our lupus patients who have renal disease by avoiding common toxins such as NSAIDs and CT contrast. Of course, she reminds us to use the lowest dose of steroids possible during flares, as prednisone is directly or indirectly responsible for 80% of organ damage over 15 years. She reminds us that lupus patients do not die of lupus. They have a 2.66-fold higher risk of cardiovascular events than the general public. In addition to maintaining lupus patients on hydroxychloroquine, Dr. Petri, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore, noted that vitamin D can have cardiovascular and hematologic benefits along with reducing thrombosis in some clinical studies. Low vitamin D was significantly associated with deep venous thrombosis.
In his lecture, Leonard Calabrese, DO, made a compelling argument for the rheumatologists in the audience to call the local oncologists with whom they work. We need to discuss and collaborate on the care of patients experiencing immune-mediated adverse events from exposure to checkpoint inhibitors used to treat malignancy. There is a limited mechanistic understanding of these adverse events, but as rheumatologists we need to get involved and help these patients. We are the experts in managing these newly emerging autoimmune events. We can help to create the best possible therapeutic interventions to help our oncology colleagues with these challenging cases, Dr. Calabrese, professor of medicine and chair of clinical immunology at the Cleveland Clinic, said.
Besides paying our dues to be members of the FSR, it is important for us as rheumatologists to get involved at the state legislature and national level to bring about change for our practices and patients. Currently, the climate can be hostile for reimbursement and for our patients to get the therapies they need. In another presentation, Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee, described recent successes at the national level, and he also discussed how we can have our voices heard at the state and national level to protect our profession and the people who rely on our expertise. The FSR and other state rheumatology organizations, as well as the ACR, need our support to continue to be the collective voice for what is right for clinicians and patients alike.
Dr. Oberstein is a practicing rheumatologist at the University of Miami Health System and is senior medical director of musculoskeletal at Modernizing Medicine in Boca Raton, Fla. She has no relevant disclosures to report.
When Flu Goes to Work
When influenza season arrives, conventional morbidity and mortality statistics, health care encounters, and laboratory data might not “fully reflect the disruption caused to the social and economic life of the community,” say CDC researchers. That is the reason that the National Institute for Occupational Safety and Health (NIOSH) monitors health-related workplace absenteeism every month, and why the World Health Organization (WHO) uses those data to help determine the impact of influenza season worldwide.
The workplace is a prime area for transmission—people share workspace and equipment and interact with one another closely. Estimates of influenza attack rates for working-aged adults can be as high as 14.3% in a given influenza season, the CDC says.
According to NIOSH, absenteeism rose sharply in the 2017-2018 season, to a level significantly higher than that of the average during the previous 5 seasons. In October, 1.7% of workers were absent due to health issues. That figure began climbing in November, peaking in January at 3.0%, significantly exceeding the epidemic threshold. Absenteeism declined steadily after that to a low of 1.4%, then began rising again in August and September.
Male workers, people aged 45 to 64 years, and those working in certain occupations (including management, business, and repair services) were more likely to be out.
Regional absenteeism peaks corresponded to concurrent peaks in influenza-like illness (ILI) activity in those regions. The researchers say this is in line with a longtime recognition that health-related workplace absenteeism correlates well with the presence of ILI, which is why absenteeism data are used as a nonspecific indicator of ILI in the community. During the 2009-2010 influenza A (H1N1) pandemic, for instance, peak workplace absenteeism correlated with the highest occurrence of both ILI and influenza-positive laboratory tests, according to NIOSH.
The associations between ILI, absenteeism, and demographic characteristics are complex, the researchers say, and mediated by factors such as vaccination coverage and access to paid sick leave.
The usual recommendations—vaccination, covering coughs and sneezes, handwashing, and routinely cleaning frequently touched surfaces—are the most effective ways to prevent transmission, the researchers note. During a pandemic, other measures may be needed, such as “social distancing” in workplaces.
NIOSH makes absenteeism surveillance results available (https://www.cdc.gov/niosh/topics/absences/default.html). The researchers suggest that employers might wish to consult them when developing prevention messages.
When influenza season arrives, conventional morbidity and mortality statistics, health care encounters, and laboratory data might not “fully reflect the disruption caused to the social and economic life of the community,” say CDC researchers. That is the reason that the National Institute for Occupational Safety and Health (NIOSH) monitors health-related workplace absenteeism every month, and why the World Health Organization (WHO) uses those data to help determine the impact of influenza season worldwide.
The workplace is a prime area for transmission—people share workspace and equipment and interact with one another closely. Estimates of influenza attack rates for working-aged adults can be as high as 14.3% in a given influenza season, the CDC says.
According to NIOSH, absenteeism rose sharply in the 2017-2018 season, to a level significantly higher than that of the average during the previous 5 seasons. In October, 1.7% of workers were absent due to health issues. That figure began climbing in November, peaking in January at 3.0%, significantly exceeding the epidemic threshold. Absenteeism declined steadily after that to a low of 1.4%, then began rising again in August and September.
Male workers, people aged 45 to 64 years, and those working in certain occupations (including management, business, and repair services) were more likely to be out.
Regional absenteeism peaks corresponded to concurrent peaks in influenza-like illness (ILI) activity in those regions. The researchers say this is in line with a longtime recognition that health-related workplace absenteeism correlates well with the presence of ILI, which is why absenteeism data are used as a nonspecific indicator of ILI in the community. During the 2009-2010 influenza A (H1N1) pandemic, for instance, peak workplace absenteeism correlated with the highest occurrence of both ILI and influenza-positive laboratory tests, according to NIOSH.
The associations between ILI, absenteeism, and demographic characteristics are complex, the researchers say, and mediated by factors such as vaccination coverage and access to paid sick leave.
The usual recommendations—vaccination, covering coughs and sneezes, handwashing, and routinely cleaning frequently touched surfaces—are the most effective ways to prevent transmission, the researchers note. During a pandemic, other measures may be needed, such as “social distancing” in workplaces.
NIOSH makes absenteeism surveillance results available (https://www.cdc.gov/niosh/topics/absences/default.html). The researchers suggest that employers might wish to consult them when developing prevention messages.
When influenza season arrives, conventional morbidity and mortality statistics, health care encounters, and laboratory data might not “fully reflect the disruption caused to the social and economic life of the community,” say CDC researchers. That is the reason that the National Institute for Occupational Safety and Health (NIOSH) monitors health-related workplace absenteeism every month, and why the World Health Organization (WHO) uses those data to help determine the impact of influenza season worldwide.
The workplace is a prime area for transmission—people share workspace and equipment and interact with one another closely. Estimates of influenza attack rates for working-aged adults can be as high as 14.3% in a given influenza season, the CDC says.
According to NIOSH, absenteeism rose sharply in the 2017-2018 season, to a level significantly higher than that of the average during the previous 5 seasons. In October, 1.7% of workers were absent due to health issues. That figure began climbing in November, peaking in January at 3.0%, significantly exceeding the epidemic threshold. Absenteeism declined steadily after that to a low of 1.4%, then began rising again in August and September.
Male workers, people aged 45 to 64 years, and those working in certain occupations (including management, business, and repair services) were more likely to be out.
Regional absenteeism peaks corresponded to concurrent peaks in influenza-like illness (ILI) activity in those regions. The researchers say this is in line with a longtime recognition that health-related workplace absenteeism correlates well with the presence of ILI, which is why absenteeism data are used as a nonspecific indicator of ILI in the community. During the 2009-2010 influenza A (H1N1) pandemic, for instance, peak workplace absenteeism correlated with the highest occurrence of both ILI and influenza-positive laboratory tests, according to NIOSH.
The associations between ILI, absenteeism, and demographic characteristics are complex, the researchers say, and mediated by factors such as vaccination coverage and access to paid sick leave.
The usual recommendations—vaccination, covering coughs and sneezes, handwashing, and routinely cleaning frequently touched surfaces—are the most effective ways to prevent transmission, the researchers note. During a pandemic, other measures may be needed, such as “social distancing” in workplaces.
NIOSH makes absenteeism surveillance results available (https://www.cdc.gov/niosh/topics/absences/default.html). The researchers suggest that employers might wish to consult them when developing prevention messages.
More awareness needed of compensation in autism
Understanding benefits, costs of strategies could guide diagnoses
Individuals with undiagnosed autism spectrum disorders and those with a formal diagnosis employ similar compensation behaviors to manage social and cognitive difficulties, and undiagnosed individuals may go unrecognized, data from an online survey of 136 adults suggest.
Unlike other adaptive behaviors in psychiatry, “autistic compensators, despite apparent lack of observable autistic behavior, continue being autistic at the neurocognitive level,” wrote Lucy Anne Livingston of Kings College London, and colleagues. “Because autism spectrum disorder is diagnosed by behavior alone, compensators might not receive a diagnosis and support until later in life, if at all. “
The researchers compared compensation behaviors in adults with and without an autism diagnosis. They recruited adults aged 18 years and older through an online advertisement distributed through social media and the U.K. National Autistic Society. Participants completed an online survey during Oct. 19, 2017–Jan. 2, 2018. The study was published in the Lancet Psychiatry.
preplanning social niceties (e.g., asking others questions about themselves), and switching between social rules,” the researchers wrote.
The study population included 58 individuals with a clinical diagnosis of autism, 19 of whom self-identified as autistic but were not formally diagnosed, and 59 of whom reported social difficulties but had no formal diagnosis and did not self-identify as autistic. The average age of the three groups was 36 years, 40 years, and 34 years, respectively, and the average age at diagnosis for the diagnosed group was 30 years. Most of the individuals in each group were women (64%, 47%, and 86%, respectively). Responses were examined using a thematic analysis and thematic map.
In general, participants reported that compensation was a cognitively taxing process that served as a secondary route for social interaction because the primary route was unavailable, but that compensation strategies fell short in certain situations, such as unexpected turns of conversation. Overall, 38% of the respondents said their compensation strategies were “extremely successful” and 56% reported “somewhat successful.” However, 12% also reported their strategies were “extremely tiring,” and 36% reported “somewhat tiring.”
Factors affecting the participants’ abilities to compensate included environmental and sensory factors such as bright lights, loud noise, and large groups with unstructured social settings, such as parties. Also, transition to living independently as an adult led to problems, because compensation had allowed individuals to grow up appearing normal but lacking in additional support and strategies, the researchers noted.
Factors that contributed to successful interactions included similar interests with an interaction partner, and motivation to develop meaningful relationships. Participants also said they viewed compensation as a way to avoid ostracism and bullying. In addition, “fitting neurotypical peoples’ interaction style (e.g., eye contact or small talk) was viewed as vital for achieving life goals (e.g., independence and employment),” the researchers wrote.
In an accompanying editorial, Julia Parish-Morris, PhD, suggested the observation made by Ms. Livingston, a researcher at the Institute of Psychiatry, Psychology and Neuroscience at the college, and associates that compensation also occurs in people who do not have autism suggests that compensation might be a “general social lubricant that facilitates community living and is therefore a potentially useful tool.”
“In other words, perhaps raising awareness about compensation in autism spectrum disorder is an important first step toward eliminating the need for it,” wrote Dr. Parish-Morris, of the Center for Autism Research at Children’s Hospital of Philadelphia (Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366[19]30294-9).
The study data were limited by the prevalence of female, well-educated, late-diagnosed individuals in the study population, which might limit the generalizability of the findings, and the lack of data on subconscious compensation because of the use of self-reports, the researchers noted.
However, “Given the individual differences found in this study, we tentatively suggest that clinicians take an individualized approach when assessing and discussing compensatory strategies with people with autism,” they said. “It will be important to establish which compensatory strategies are most beneficial, and how their success might be maximized with changes to external environments irrespective of clinical intervention.”
The researchers had no financial interests to disclose.
SOURCE: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi. org/10.1016/S2215-0366(19)30224-X.
Understanding benefits, costs of strategies could guide diagnoses
Understanding benefits, costs of strategies could guide diagnoses
Individuals with undiagnosed autism spectrum disorders and those with a formal diagnosis employ similar compensation behaviors to manage social and cognitive difficulties, and undiagnosed individuals may go unrecognized, data from an online survey of 136 adults suggest.
Unlike other adaptive behaviors in psychiatry, “autistic compensators, despite apparent lack of observable autistic behavior, continue being autistic at the neurocognitive level,” wrote Lucy Anne Livingston of Kings College London, and colleagues. “Because autism spectrum disorder is diagnosed by behavior alone, compensators might not receive a diagnosis and support until later in life, if at all. “
The researchers compared compensation behaviors in adults with and without an autism diagnosis. They recruited adults aged 18 years and older through an online advertisement distributed through social media and the U.K. National Autistic Society. Participants completed an online survey during Oct. 19, 2017–Jan. 2, 2018. The study was published in the Lancet Psychiatry.
preplanning social niceties (e.g., asking others questions about themselves), and switching between social rules,” the researchers wrote.
The study population included 58 individuals with a clinical diagnosis of autism, 19 of whom self-identified as autistic but were not formally diagnosed, and 59 of whom reported social difficulties but had no formal diagnosis and did not self-identify as autistic. The average age of the three groups was 36 years, 40 years, and 34 years, respectively, and the average age at diagnosis for the diagnosed group was 30 years. Most of the individuals in each group were women (64%, 47%, and 86%, respectively). Responses were examined using a thematic analysis and thematic map.
In general, participants reported that compensation was a cognitively taxing process that served as a secondary route for social interaction because the primary route was unavailable, but that compensation strategies fell short in certain situations, such as unexpected turns of conversation. Overall, 38% of the respondents said their compensation strategies were “extremely successful” and 56% reported “somewhat successful.” However, 12% also reported their strategies were “extremely tiring,” and 36% reported “somewhat tiring.”
Factors affecting the participants’ abilities to compensate included environmental and sensory factors such as bright lights, loud noise, and large groups with unstructured social settings, such as parties. Also, transition to living independently as an adult led to problems, because compensation had allowed individuals to grow up appearing normal but lacking in additional support and strategies, the researchers noted.
Factors that contributed to successful interactions included similar interests with an interaction partner, and motivation to develop meaningful relationships. Participants also said they viewed compensation as a way to avoid ostracism and bullying. In addition, “fitting neurotypical peoples’ interaction style (e.g., eye contact or small talk) was viewed as vital for achieving life goals (e.g., independence and employment),” the researchers wrote.
In an accompanying editorial, Julia Parish-Morris, PhD, suggested the observation made by Ms. Livingston, a researcher at the Institute of Psychiatry, Psychology and Neuroscience at the college, and associates that compensation also occurs in people who do not have autism suggests that compensation might be a “general social lubricant that facilitates community living and is therefore a potentially useful tool.”
“In other words, perhaps raising awareness about compensation in autism spectrum disorder is an important first step toward eliminating the need for it,” wrote Dr. Parish-Morris, of the Center for Autism Research at Children’s Hospital of Philadelphia (Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366[19]30294-9).
The study data were limited by the prevalence of female, well-educated, late-diagnosed individuals in the study population, which might limit the generalizability of the findings, and the lack of data on subconscious compensation because of the use of self-reports, the researchers noted.
However, “Given the individual differences found in this study, we tentatively suggest that clinicians take an individualized approach when assessing and discussing compensatory strategies with people with autism,” they said. “It will be important to establish which compensatory strategies are most beneficial, and how their success might be maximized with changes to external environments irrespective of clinical intervention.”
The researchers had no financial interests to disclose.
SOURCE: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi. org/10.1016/S2215-0366(19)30224-X.
Individuals with undiagnosed autism spectrum disorders and those with a formal diagnosis employ similar compensation behaviors to manage social and cognitive difficulties, and undiagnosed individuals may go unrecognized, data from an online survey of 136 adults suggest.
Unlike other adaptive behaviors in psychiatry, “autistic compensators, despite apparent lack of observable autistic behavior, continue being autistic at the neurocognitive level,” wrote Lucy Anne Livingston of Kings College London, and colleagues. “Because autism spectrum disorder is diagnosed by behavior alone, compensators might not receive a diagnosis and support until later in life, if at all. “
The researchers compared compensation behaviors in adults with and without an autism diagnosis. They recruited adults aged 18 years and older through an online advertisement distributed through social media and the U.K. National Autistic Society. Participants completed an online survey during Oct. 19, 2017–Jan. 2, 2018. The study was published in the Lancet Psychiatry.
preplanning social niceties (e.g., asking others questions about themselves), and switching between social rules,” the researchers wrote.
The study population included 58 individuals with a clinical diagnosis of autism, 19 of whom self-identified as autistic but were not formally diagnosed, and 59 of whom reported social difficulties but had no formal diagnosis and did not self-identify as autistic. The average age of the three groups was 36 years, 40 years, and 34 years, respectively, and the average age at diagnosis for the diagnosed group was 30 years. Most of the individuals in each group were women (64%, 47%, and 86%, respectively). Responses were examined using a thematic analysis and thematic map.
In general, participants reported that compensation was a cognitively taxing process that served as a secondary route for social interaction because the primary route was unavailable, but that compensation strategies fell short in certain situations, such as unexpected turns of conversation. Overall, 38% of the respondents said their compensation strategies were “extremely successful” and 56% reported “somewhat successful.” However, 12% also reported their strategies were “extremely tiring,” and 36% reported “somewhat tiring.”
Factors affecting the participants’ abilities to compensate included environmental and sensory factors such as bright lights, loud noise, and large groups with unstructured social settings, such as parties. Also, transition to living independently as an adult led to problems, because compensation had allowed individuals to grow up appearing normal but lacking in additional support and strategies, the researchers noted.
Factors that contributed to successful interactions included similar interests with an interaction partner, and motivation to develop meaningful relationships. Participants also said they viewed compensation as a way to avoid ostracism and bullying. In addition, “fitting neurotypical peoples’ interaction style (e.g., eye contact or small talk) was viewed as vital for achieving life goals (e.g., independence and employment),” the researchers wrote.
In an accompanying editorial, Julia Parish-Morris, PhD, suggested the observation made by Ms. Livingston, a researcher at the Institute of Psychiatry, Psychology and Neuroscience at the college, and associates that compensation also occurs in people who do not have autism suggests that compensation might be a “general social lubricant that facilitates community living and is therefore a potentially useful tool.”
“In other words, perhaps raising awareness about compensation in autism spectrum disorder is an important first step toward eliminating the need for it,” wrote Dr. Parish-Morris, of the Center for Autism Research at Children’s Hospital of Philadelphia (Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366[19]30294-9).
The study data were limited by the prevalence of female, well-educated, late-diagnosed individuals in the study population, which might limit the generalizability of the findings, and the lack of data on subconscious compensation because of the use of self-reports, the researchers noted.
However, “Given the individual differences found in this study, we tentatively suggest that clinicians take an individualized approach when assessing and discussing compensatory strategies with people with autism,” they said. “It will be important to establish which compensatory strategies are most beneficial, and how their success might be maximized with changes to external environments irrespective of clinical intervention.”
The researchers had no financial interests to disclose.
SOURCE: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi. org/10.1016/S2215-0366(19)30224-X.
FROM THE LANCET PSYCHIATRY
Key clinical point: Individuals with social difficulties use similar compensation strategies to manage social situations whether or not they have an autism diagnosis.
Major finding: A total of 38% of respondents said their compensation behaviors were “extremely successful,” but 12% also reported those strategies were “extremely tiring.”
Study details: The data come from 136 adults who responded to an online survey; 58 diagnosed with autism, 19 self-identified, and 59 reported social difficulties without self-identification or diagnosis.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366(19)30224-X.
Scrotal Ulceration: A Complication of Hyperthermic Intraperitoneal Chemotherapy and Subsequent Treatment With Dimethyl Sulfoxide
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
Practice Points
- Scrotal ulceration following hyperthermic intraperitoneal chemotherapy has been reported only a few times in the literature and is likely underreported. The presentation in all reported cases was similar, with a delay in symptom onset of weeks to months, involvement of the anterior scrotum, and pain.
- Dimethyl sulfoxide, used in other vesicant reactions, may have a role in mitigating tissue damage. Alternatively, methods to prevent sequestration of vesicants in the potential space of the tunica vaginalis layers can be employed.
FDA approves Recarbrio for cUTI, cIAI treatment in adults
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
A 56-year-old black woman presented with asymptomatic hypopigmented macules on her back, chest, face, and lateral arms
They often coalesce near the midline, and occasionally extend beyond the trunk to the arms, legs, head, or neck. PMH is more frequently diagnosed among black individuals, although it affects all races and ethnicities. The natural history of PMH can vary from stable and progressive disease, and may resolve spontaneously after a few years. The pathogenesis of PMH remains unknown. It has been proposed that the hypopigmentation is caused by decreased melanin production and altered melanosome dispersal in reaction to Propionibacterium acnes.
PMH must be distinguished from some of its clinical mimickers, including vitiligo, hypopigmented mycosis fungoides, tinea versicolor, and pityriasis alba. Potassium hydroxide preparations can be performed in the office to evaluate for tinea versicolor. An additional tool to aid in diagnosis is the use of a Wood’s light. The lesions of PMH characteristically show punctiform orange-red follicular fluorescence when exposed to a Wood’s light, indicating the presence of a porphyrin-producing organism, presumably P. acnes. A skin biopsy is necessary to rule out hypopigmented mycosis fungoides.
Skin biopsy of PMH typically reveals decreased melanin with a normal number of melanocytes. In our patient, a punch biopsy of the right lateral arm demonstrated minimally decreased density of epidermal melanocytes with dermal pigment incontinence. SOX10 immunohistochemical staining demonstrated scattered melanocytes in the epidermis. Preserved melanin within keratinocytes was noted.
In our patient, there was significant spread to the face, which is highly unusual and has only been documented in a few case series. There are no standard recommendations for definitive treatment of PMH. Topical antimicrobial therapies, such as clindamycin solution and benzoyl peroxide gel, have been beneficial in some studies. Tetracyclines, narrow-band ultraviolet B phototherapy, and even isotretinoin have had some reported success.
This case and photo were submitted by Mr. Franzetti, Dr. Rush, and Dr. Shalin of the University of Arkansas for Medical Sciences, Little Rock.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
They often coalesce near the midline, and occasionally extend beyond the trunk to the arms, legs, head, or neck. PMH is more frequently diagnosed among black individuals, although it affects all races and ethnicities. The natural history of PMH can vary from stable and progressive disease, and may resolve spontaneously after a few years. The pathogenesis of PMH remains unknown. It has been proposed that the hypopigmentation is caused by decreased melanin production and altered melanosome dispersal in reaction to Propionibacterium acnes.
PMH must be distinguished from some of its clinical mimickers, including vitiligo, hypopigmented mycosis fungoides, tinea versicolor, and pityriasis alba. Potassium hydroxide preparations can be performed in the office to evaluate for tinea versicolor. An additional tool to aid in diagnosis is the use of a Wood’s light. The lesions of PMH characteristically show punctiform orange-red follicular fluorescence when exposed to a Wood’s light, indicating the presence of a porphyrin-producing organism, presumably P. acnes. A skin biopsy is necessary to rule out hypopigmented mycosis fungoides.
Skin biopsy of PMH typically reveals decreased melanin with a normal number of melanocytes. In our patient, a punch biopsy of the right lateral arm demonstrated minimally decreased density of epidermal melanocytes with dermal pigment incontinence. SOX10 immunohistochemical staining demonstrated scattered melanocytes in the epidermis. Preserved melanin within keratinocytes was noted.
In our patient, there was significant spread to the face, which is highly unusual and has only been documented in a few case series. There are no standard recommendations for definitive treatment of PMH. Topical antimicrobial therapies, such as clindamycin solution and benzoyl peroxide gel, have been beneficial in some studies. Tetracyclines, narrow-band ultraviolet B phototherapy, and even isotretinoin have had some reported success.
This case and photo were submitted by Mr. Franzetti, Dr. Rush, and Dr. Shalin of the University of Arkansas for Medical Sciences, Little Rock.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
They often coalesce near the midline, and occasionally extend beyond the trunk to the arms, legs, head, or neck. PMH is more frequently diagnosed among black individuals, although it affects all races and ethnicities. The natural history of PMH can vary from stable and progressive disease, and may resolve spontaneously after a few years. The pathogenesis of PMH remains unknown. It has been proposed that the hypopigmentation is caused by decreased melanin production and altered melanosome dispersal in reaction to Propionibacterium acnes.
PMH must be distinguished from some of its clinical mimickers, including vitiligo, hypopigmented mycosis fungoides, tinea versicolor, and pityriasis alba. Potassium hydroxide preparations can be performed in the office to evaluate for tinea versicolor. An additional tool to aid in diagnosis is the use of a Wood’s light. The lesions of PMH characteristically show punctiform orange-red follicular fluorescence when exposed to a Wood’s light, indicating the presence of a porphyrin-producing organism, presumably P. acnes. A skin biopsy is necessary to rule out hypopigmented mycosis fungoides.
Skin biopsy of PMH typically reveals decreased melanin with a normal number of melanocytes. In our patient, a punch biopsy of the right lateral arm demonstrated minimally decreased density of epidermal melanocytes with dermal pigment incontinence. SOX10 immunohistochemical staining demonstrated scattered melanocytes in the epidermis. Preserved melanin within keratinocytes was noted.
In our patient, there was significant spread to the face, which is highly unusual and has only been documented in a few case series. There are no standard recommendations for definitive treatment of PMH. Topical antimicrobial therapies, such as clindamycin solution and benzoyl peroxide gel, have been beneficial in some studies. Tetracyclines, narrow-band ultraviolet B phototherapy, and even isotretinoin have had some reported success.
This case and photo were submitted by Mr. Franzetti, Dr. Rush, and Dr. Shalin of the University of Arkansas for Medical Sciences, Little Rock.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Recertification: The FPHM option
ABIM now offers increased flexibility
Everyone always told me that my time in residency would fly by, and the 3 years of internal medicine training really did seem to pass in just a few moments. Before I knew it, I had passed my internal medicine boards and practiced hospital medicine at an academic medical center.
One day last fall, I received notice from the American Board of Internal Medicine that it was time to recertify. I was surprised – had it already been 10 years? What did I have to do to maintain my certification?
As I investigated what it would take to maintain certification, I discovered that the recertification process provided more flexibility, compared with original board certification. I now had the option to recertify in internal medicine with a Focused Practice in Hospital Medicine (FPHM). Beginning in 2014, ABIM offered hospitalists, or internists whose clinical practice is mainly in the inpatient setting, the option to recertify in internal medicine, but with the designation that highlighted their clinical practice in the inpatient setting.
The first step in recertification for me was deciding to recertify with the focus in hospital medicine or maintain the traditional internal medicine certification. I talked with several colleagues who are also practicing hospitalists and weighed their reasons for opting for FPHM. Ultimately, my decision to pursue a recertification with a focus in hospital medicine relied on three factors: First, my clinical practice since completing residency was exclusively in the inpatient setting. Day in and day out, I care for patients who are acutely ill and require inpatient medical care. Second, I wanted my board certification to reflect what I consider to be my area of clinical expertise, which is inpatient adult medicine. Pursuing the FPHM would provide that recognition. Finally, I wanted to study and be tested on topics that I could utilize in my day-to-day practice. Because I exclusively practiced hospital medicine since graduation, areas of clinical internal medicine that I did not frequently encounter in my daily practice became less accessible in my knowledge base.
The next step then was to enter the FPHM Maintenance of Certification (MOC) program.
The ABIM requires two attestations to verify that I met the requirements to be a hospitalist. First was a self-attestation confirming at least 3 years of unsupervised inpatient care practice experience, and meeting patient encounter thresholds in the inpatient setting. The second attestation was from a “Senior Hospital Officer” confirming the information in the self-attestation was accurate.
Once entered into the program and having an unrestricted medical license to practice, I had to complete the remaining requirements of earning MOC points and then passing a knowledge-based assessment. I had to accumulate a total of 100 MOC points in the past 5 years, which I succeeded in doing through participating in quality improvement projects, recording CME credits, studying for the exam, and even taking the exam. I could track my point totals through the ABIM Physician Portal, which updated my point tally automatically for activities that counted toward MOC, such as attending SHM’s annual conference.
The final component was to pass the knowledge assessment, the dreaded exam. In 2018, I had the option to take the 10-year FPHM exam or do a general internal medicine Knowledge Check-In. Beginning in 2020, candidates will be able to sit for either the 10-year Focused Practice in Hospital Medicine exam or begin the Hospital Medicine Knowledge Check-In pathway. I had already decided to pursue FPHM and began to prepare to sit for an exam. I scheduled my exam through the ABIM portal at a local testing center.
The exam was scheduled for a full day, consisting of four sections broken up by a lunch break and section breaks. Specifically, the 220 single best answer, multiple-choice exam covered diagnosis, testing, treatment decisions, epidemiology, and basic science content through patient scenarios that reflected the scope of practice of a hospitalist. The ABIM provided an exam blueprint that detailed the specific clinical topics and the likelihood that a question pertaining to that topic would show up on the exam. Content was described as high, medium, or low importance and the number of questions related to the content was 75% for high importance, no more than 25% for medium importance, and no questions for low-importance content. In addition, content was distributed in a way that was reflective of my clinical practice as a hospitalist: 63.5% inpatient and traditional care; 6.5% palliative care; 15% consultative comanagement; and 15% quality, safety, and clinical reasoning.
Beginning 6 months prior to my scheduled exam, I purchased two critical resources to guide my studying efforts: the SHM Spark Self-Assessment Tool and the American College of Physicians Medical Knowledge Self-Assessment Program to review subject matter content and also do practice questions.
The latest version of SHM’s program, Spark Edition 2, provides updated questions and resources tailored to the hospital medicine exams. I appreciated the ability to answer questions online, as well as on my phone so I could do questions on the go. Moreover, I was able to track which content areas were stronger or weaker for me, and focus attention on areas that needed more work. Importantly, the questions I answered using the Spark self-assessment tool closely aligned with the subject matter I encountered in the exam, as well as the clinical cases I encounter every day in my practice.
While the day-long exam was challenging, I was gratified to receive notice from the ABIM that I had successfully recertified in internal medicine with a Focused Practice in Hospital Medicine!
Dr. Tad-y is a hospitalist at the University of Colorado at Denver, Aurora, and associate vice chair of quality in the department of medicine at the University of Colorado.
ABIM now offers increased flexibility
ABIM now offers increased flexibility
Everyone always told me that my time in residency would fly by, and the 3 years of internal medicine training really did seem to pass in just a few moments. Before I knew it, I had passed my internal medicine boards and practiced hospital medicine at an academic medical center.
One day last fall, I received notice from the American Board of Internal Medicine that it was time to recertify. I was surprised – had it already been 10 years? What did I have to do to maintain my certification?
As I investigated what it would take to maintain certification, I discovered that the recertification process provided more flexibility, compared with original board certification. I now had the option to recertify in internal medicine with a Focused Practice in Hospital Medicine (FPHM). Beginning in 2014, ABIM offered hospitalists, or internists whose clinical practice is mainly in the inpatient setting, the option to recertify in internal medicine, but with the designation that highlighted their clinical practice in the inpatient setting.
The first step in recertification for me was deciding to recertify with the focus in hospital medicine or maintain the traditional internal medicine certification. I talked with several colleagues who are also practicing hospitalists and weighed their reasons for opting for FPHM. Ultimately, my decision to pursue a recertification with a focus in hospital medicine relied on three factors: First, my clinical practice since completing residency was exclusively in the inpatient setting. Day in and day out, I care for patients who are acutely ill and require inpatient medical care. Second, I wanted my board certification to reflect what I consider to be my area of clinical expertise, which is inpatient adult medicine. Pursuing the FPHM would provide that recognition. Finally, I wanted to study and be tested on topics that I could utilize in my day-to-day practice. Because I exclusively practiced hospital medicine since graduation, areas of clinical internal medicine that I did not frequently encounter in my daily practice became less accessible in my knowledge base.
The next step then was to enter the FPHM Maintenance of Certification (MOC) program.
The ABIM requires two attestations to verify that I met the requirements to be a hospitalist. First was a self-attestation confirming at least 3 years of unsupervised inpatient care practice experience, and meeting patient encounter thresholds in the inpatient setting. The second attestation was from a “Senior Hospital Officer” confirming the information in the self-attestation was accurate.
Once entered into the program and having an unrestricted medical license to practice, I had to complete the remaining requirements of earning MOC points and then passing a knowledge-based assessment. I had to accumulate a total of 100 MOC points in the past 5 years, which I succeeded in doing through participating in quality improvement projects, recording CME credits, studying for the exam, and even taking the exam. I could track my point totals through the ABIM Physician Portal, which updated my point tally automatically for activities that counted toward MOC, such as attending SHM’s annual conference.
The final component was to pass the knowledge assessment, the dreaded exam. In 2018, I had the option to take the 10-year FPHM exam or do a general internal medicine Knowledge Check-In. Beginning in 2020, candidates will be able to sit for either the 10-year Focused Practice in Hospital Medicine exam or begin the Hospital Medicine Knowledge Check-In pathway. I had already decided to pursue FPHM and began to prepare to sit for an exam. I scheduled my exam through the ABIM portal at a local testing center.
The exam was scheduled for a full day, consisting of four sections broken up by a lunch break and section breaks. Specifically, the 220 single best answer, multiple-choice exam covered diagnosis, testing, treatment decisions, epidemiology, and basic science content through patient scenarios that reflected the scope of practice of a hospitalist. The ABIM provided an exam blueprint that detailed the specific clinical topics and the likelihood that a question pertaining to that topic would show up on the exam. Content was described as high, medium, or low importance and the number of questions related to the content was 75% for high importance, no more than 25% for medium importance, and no questions for low-importance content. In addition, content was distributed in a way that was reflective of my clinical practice as a hospitalist: 63.5% inpatient and traditional care; 6.5% palliative care; 15% consultative comanagement; and 15% quality, safety, and clinical reasoning.
Beginning 6 months prior to my scheduled exam, I purchased two critical resources to guide my studying efforts: the SHM Spark Self-Assessment Tool and the American College of Physicians Medical Knowledge Self-Assessment Program to review subject matter content and also do practice questions.
The latest version of SHM’s program, Spark Edition 2, provides updated questions and resources tailored to the hospital medicine exams. I appreciated the ability to answer questions online, as well as on my phone so I could do questions on the go. Moreover, I was able to track which content areas were stronger or weaker for me, and focus attention on areas that needed more work. Importantly, the questions I answered using the Spark self-assessment tool closely aligned with the subject matter I encountered in the exam, as well as the clinical cases I encounter every day in my practice.
While the day-long exam was challenging, I was gratified to receive notice from the ABIM that I had successfully recertified in internal medicine with a Focused Practice in Hospital Medicine!
Dr. Tad-y is a hospitalist at the University of Colorado at Denver, Aurora, and associate vice chair of quality in the department of medicine at the University of Colorado.
Everyone always told me that my time in residency would fly by, and the 3 years of internal medicine training really did seem to pass in just a few moments. Before I knew it, I had passed my internal medicine boards and practiced hospital medicine at an academic medical center.
One day last fall, I received notice from the American Board of Internal Medicine that it was time to recertify. I was surprised – had it already been 10 years? What did I have to do to maintain my certification?
As I investigated what it would take to maintain certification, I discovered that the recertification process provided more flexibility, compared with original board certification. I now had the option to recertify in internal medicine with a Focused Practice in Hospital Medicine (FPHM). Beginning in 2014, ABIM offered hospitalists, or internists whose clinical practice is mainly in the inpatient setting, the option to recertify in internal medicine, but with the designation that highlighted their clinical practice in the inpatient setting.
The first step in recertification for me was deciding to recertify with the focus in hospital medicine or maintain the traditional internal medicine certification. I talked with several colleagues who are also practicing hospitalists and weighed their reasons for opting for FPHM. Ultimately, my decision to pursue a recertification with a focus in hospital medicine relied on three factors: First, my clinical practice since completing residency was exclusively in the inpatient setting. Day in and day out, I care for patients who are acutely ill and require inpatient medical care. Second, I wanted my board certification to reflect what I consider to be my area of clinical expertise, which is inpatient adult medicine. Pursuing the FPHM would provide that recognition. Finally, I wanted to study and be tested on topics that I could utilize in my day-to-day practice. Because I exclusively practiced hospital medicine since graduation, areas of clinical internal medicine that I did not frequently encounter in my daily practice became less accessible in my knowledge base.
The next step then was to enter the FPHM Maintenance of Certification (MOC) program.
The ABIM requires two attestations to verify that I met the requirements to be a hospitalist. First was a self-attestation confirming at least 3 years of unsupervised inpatient care practice experience, and meeting patient encounter thresholds in the inpatient setting. The second attestation was from a “Senior Hospital Officer” confirming the information in the self-attestation was accurate.
Once entered into the program and having an unrestricted medical license to practice, I had to complete the remaining requirements of earning MOC points and then passing a knowledge-based assessment. I had to accumulate a total of 100 MOC points in the past 5 years, which I succeeded in doing through participating in quality improvement projects, recording CME credits, studying for the exam, and even taking the exam. I could track my point totals through the ABIM Physician Portal, which updated my point tally automatically for activities that counted toward MOC, such as attending SHM’s annual conference.
The final component was to pass the knowledge assessment, the dreaded exam. In 2018, I had the option to take the 10-year FPHM exam or do a general internal medicine Knowledge Check-In. Beginning in 2020, candidates will be able to sit for either the 10-year Focused Practice in Hospital Medicine exam or begin the Hospital Medicine Knowledge Check-In pathway. I had already decided to pursue FPHM and began to prepare to sit for an exam. I scheduled my exam through the ABIM portal at a local testing center.
The exam was scheduled for a full day, consisting of four sections broken up by a lunch break and section breaks. Specifically, the 220 single best answer, multiple-choice exam covered diagnosis, testing, treatment decisions, epidemiology, and basic science content through patient scenarios that reflected the scope of practice of a hospitalist. The ABIM provided an exam blueprint that detailed the specific clinical topics and the likelihood that a question pertaining to that topic would show up on the exam. Content was described as high, medium, or low importance and the number of questions related to the content was 75% for high importance, no more than 25% for medium importance, and no questions for low-importance content. In addition, content was distributed in a way that was reflective of my clinical practice as a hospitalist: 63.5% inpatient and traditional care; 6.5% palliative care; 15% consultative comanagement; and 15% quality, safety, and clinical reasoning.
Beginning 6 months prior to my scheduled exam, I purchased two critical resources to guide my studying efforts: the SHM Spark Self-Assessment Tool and the American College of Physicians Medical Knowledge Self-Assessment Program to review subject matter content and also do practice questions.
The latest version of SHM’s program, Spark Edition 2, provides updated questions and resources tailored to the hospital medicine exams. I appreciated the ability to answer questions online, as well as on my phone so I could do questions on the go. Moreover, I was able to track which content areas were stronger or weaker for me, and focus attention on areas that needed more work. Importantly, the questions I answered using the Spark self-assessment tool closely aligned with the subject matter I encountered in the exam, as well as the clinical cases I encounter every day in my practice.
While the day-long exam was challenging, I was gratified to receive notice from the ABIM that I had successfully recertified in internal medicine with a Focused Practice in Hospital Medicine!
Dr. Tad-y is a hospitalist at the University of Colorado at Denver, Aurora, and associate vice chair of quality in the department of medicine at the University of Colorado.