User login
For MD-IQ use only
Should RAAS blockade therapy be continued in patients with advanced renal disease?
Evidence summary
Mixed results, Yes, but no evidence of harm in continuing RAAS therapy
A 2014 cohort study assessed the effect of treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) on all-cause mortality in US veterans (N = 141,413) with non-dialysis chronic kidney disease (CKD)—defined as either a stable estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a stable eGFR ≥ 60 mL/min/1.73 m2 and an elevated urine microalbumin measurement.1 In an intention-to-treat analysis, ACEI/ARB treatment was associated with a significantly decreased risk for all-cause mortality (hazard ratio [HR] = 0.81; 95% CI, 0.78-0.84).
A 2018 meta-analysis analyzed data from 9 RCTs comparing RAAS blockade therapy to placebo or alternative antihypertensive agents in patients with non-dialysis CKD stages 3 to 5.2 Although the meta-analysis authors focused on patients with comorbid diabetes and non-dialysis CKD (N = 9797), some included studies had a mixed population (ie, only a subset of patients had diabetes). This, among other variances in characteristics, participants, interventions, and endpoints, resulted in different numbers of participants included in the data extraction and analysis of outcomes. Overall, there was no difference between the RAAS group and the control group in terms of all-cause mortality (N = 5309; risk ratio [RR] = 0.97; 95% CI, 0.85-1.10), cardiovascular mortality (N = 3748; RR = 1.03; 95% CI, 0.75-1.41), or adverse events (N = 1822; RR = 1.05; 95% CI, 0.89-1.25). Compared to the control group, the RAAS group was less likely to experience a nonfatal cardiovascular event (N = 6138; RR = 0.90; 95% CI, 0.81-1.00). For the composite endpoint of need for renal replacement therapy/doubling of serum creatinine, RAAS therapy was associated with reduced risk in both the overall population (N = 5202; RR = 0.81; 95% CI, 0.70-0.92) and in patients with comorbid diabetes (N = 3314; RR = 0.78; 95% CI, 0.67-0.90).
A 2022 open-label trial (STOP ACEi) randomly assigned 411 patients with stage 4 or 5 CKD to either continue (N = 205) or discontinue (N = 206) RAAS inhibitor therapy.3 The primary outcome measure was eGFR at 3 years. The difference in the rate of decline in eGFR between groups was –0.7% (95% CI, –2.5 to 1.0; P = .42), favoring the group that continued therapy.
Recommendations from others
After reviewing data from multiple clinical trials, the authors of the 2018 report from the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF–KDOQI) concluded that the decision to continue or stop RAAS therapy in patients with advanced CKD should be individualized.4 Criteria that should be considered in the decision-making process include the presence or absence of large acute declines in eGFR (> 20% in the absence of a significant decrease in proteinuria), hypotension, or acute kidney injury with significant risk for worsening.
In 2021, the Renal Association and the Association of British Clinical Diabetologists published updated clinical practice guidelines for the management of hypertension and RAAS blockade in adults with diabetic kidney disease.5 Collective data indicated that, although outcomes varied based on type of diabetes (1 vs 2) and degree of proteinuria, blockade therapy overall led to improved outcomes; this was hypothesized to be due to the effects of reduced blood pressure. However, discontinuation of RAAS blockade therapy may be warranted when the patient (1) has a potassium level > 5 mmol/L pretreatment or ≥ 6 mmol/L with treatment, (2) demonstrates a decrease in eGFR > 25% or an increase in serum creatinine > 30% upon initiation of blockade, without another cause of renal deterioration, (3) is pregnant, or (4) has an acute illness with fluid depletion (in which case, RAAS therapy can be restarted 24 to 48 hours after recovery).
Editor’s takeaway
Evidence supports continuation of RAAS blockade, particularly in patients with significant comorbidities (diabetes and cardiovascular disease). Study data indicate continuation is either beneficial or neutral to further morbidity. The only caveat is that these patients should have their renal function and potassium level continuously monitored. The evidence should provide reassurance to patients and physicians that continuation is the correct course of action.
1. Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650-658. doi: 10.1016/j.jacc.2013.10.050
2. Nistor I, De Sutter J, Drechsler C, et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:12-22. doi: 10.1093/ndt/gfx072
3. Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021-2032. doi: 10.1056/NEJMoa2210639
4. Weir MR, Lakkis JI, Jaar B, et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF–KDOQI controversies report. Am J Kidney Dis. 2018;72:873-884. doi: 10.1053/j.ajkd.2018.06.010
5. Banerjee D, Winocour P, Chowdhury TA, et al. Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrol. 2022;23:9. doi: 10.1186/s12882-021-02587-5
Evidence summary
Mixed results, Yes, but no evidence of harm in continuing RAAS therapy
A 2014 cohort study assessed the effect of treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) on all-cause mortality in US veterans (N = 141,413) with non-dialysis chronic kidney disease (CKD)—defined as either a stable estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a stable eGFR ≥ 60 mL/min/1.73 m2 and an elevated urine microalbumin measurement.1 In an intention-to-treat analysis, ACEI/ARB treatment was associated with a significantly decreased risk for all-cause mortality (hazard ratio [HR] = 0.81; 95% CI, 0.78-0.84).
A 2018 meta-analysis analyzed data from 9 RCTs comparing RAAS blockade therapy to placebo or alternative antihypertensive agents in patients with non-dialysis CKD stages 3 to 5.2 Although the meta-analysis authors focused on patients with comorbid diabetes and non-dialysis CKD (N = 9797), some included studies had a mixed population (ie, only a subset of patients had diabetes). This, among other variances in characteristics, participants, interventions, and endpoints, resulted in different numbers of participants included in the data extraction and analysis of outcomes. Overall, there was no difference between the RAAS group and the control group in terms of all-cause mortality (N = 5309; risk ratio [RR] = 0.97; 95% CI, 0.85-1.10), cardiovascular mortality (N = 3748; RR = 1.03; 95% CI, 0.75-1.41), or adverse events (N = 1822; RR = 1.05; 95% CI, 0.89-1.25). Compared to the control group, the RAAS group was less likely to experience a nonfatal cardiovascular event (N = 6138; RR = 0.90; 95% CI, 0.81-1.00). For the composite endpoint of need for renal replacement therapy/doubling of serum creatinine, RAAS therapy was associated with reduced risk in both the overall population (N = 5202; RR = 0.81; 95% CI, 0.70-0.92) and in patients with comorbid diabetes (N = 3314; RR = 0.78; 95% CI, 0.67-0.90).
A 2022 open-label trial (STOP ACEi) randomly assigned 411 patients with stage 4 or 5 CKD to either continue (N = 205) or discontinue (N = 206) RAAS inhibitor therapy.3 The primary outcome measure was eGFR at 3 years. The difference in the rate of decline in eGFR between groups was –0.7% (95% CI, –2.5 to 1.0; P = .42), favoring the group that continued therapy.
Recommendations from others
After reviewing data from multiple clinical trials, the authors of the 2018 report from the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF–KDOQI) concluded that the decision to continue or stop RAAS therapy in patients with advanced CKD should be individualized.4 Criteria that should be considered in the decision-making process include the presence or absence of large acute declines in eGFR (> 20% in the absence of a significant decrease in proteinuria), hypotension, or acute kidney injury with significant risk for worsening.
In 2021, the Renal Association and the Association of British Clinical Diabetologists published updated clinical practice guidelines for the management of hypertension and RAAS blockade in adults with diabetic kidney disease.5 Collective data indicated that, although outcomes varied based on type of diabetes (1 vs 2) and degree of proteinuria, blockade therapy overall led to improved outcomes; this was hypothesized to be due to the effects of reduced blood pressure. However, discontinuation of RAAS blockade therapy may be warranted when the patient (1) has a potassium level > 5 mmol/L pretreatment or ≥ 6 mmol/L with treatment, (2) demonstrates a decrease in eGFR > 25% or an increase in serum creatinine > 30% upon initiation of blockade, without another cause of renal deterioration, (3) is pregnant, or (4) has an acute illness with fluid depletion (in which case, RAAS therapy can be restarted 24 to 48 hours after recovery).
Editor’s takeaway
Evidence supports continuation of RAAS blockade, particularly in patients with significant comorbidities (diabetes and cardiovascular disease). Study data indicate continuation is either beneficial or neutral to further morbidity. The only caveat is that these patients should have their renal function and potassium level continuously monitored. The evidence should provide reassurance to patients and physicians that continuation is the correct course of action.
Evidence summary
Mixed results, Yes, but no evidence of harm in continuing RAAS therapy
A 2014 cohort study assessed the effect of treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) on all-cause mortality in US veterans (N = 141,413) with non-dialysis chronic kidney disease (CKD)—defined as either a stable estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a stable eGFR ≥ 60 mL/min/1.73 m2 and an elevated urine microalbumin measurement.1 In an intention-to-treat analysis, ACEI/ARB treatment was associated with a significantly decreased risk for all-cause mortality (hazard ratio [HR] = 0.81; 95% CI, 0.78-0.84).
A 2018 meta-analysis analyzed data from 9 RCTs comparing RAAS blockade therapy to placebo or alternative antihypertensive agents in patients with non-dialysis CKD stages 3 to 5.2 Although the meta-analysis authors focused on patients with comorbid diabetes and non-dialysis CKD (N = 9797), some included studies had a mixed population (ie, only a subset of patients had diabetes). This, among other variances in characteristics, participants, interventions, and endpoints, resulted in different numbers of participants included in the data extraction and analysis of outcomes. Overall, there was no difference between the RAAS group and the control group in terms of all-cause mortality (N = 5309; risk ratio [RR] = 0.97; 95% CI, 0.85-1.10), cardiovascular mortality (N = 3748; RR = 1.03; 95% CI, 0.75-1.41), or adverse events (N = 1822; RR = 1.05; 95% CI, 0.89-1.25). Compared to the control group, the RAAS group was less likely to experience a nonfatal cardiovascular event (N = 6138; RR = 0.90; 95% CI, 0.81-1.00). For the composite endpoint of need for renal replacement therapy/doubling of serum creatinine, RAAS therapy was associated with reduced risk in both the overall population (N = 5202; RR = 0.81; 95% CI, 0.70-0.92) and in patients with comorbid diabetes (N = 3314; RR = 0.78; 95% CI, 0.67-0.90).
A 2022 open-label trial (STOP ACEi) randomly assigned 411 patients with stage 4 or 5 CKD to either continue (N = 205) or discontinue (N = 206) RAAS inhibitor therapy.3 The primary outcome measure was eGFR at 3 years. The difference in the rate of decline in eGFR between groups was –0.7% (95% CI, –2.5 to 1.0; P = .42), favoring the group that continued therapy.
Recommendations from others
After reviewing data from multiple clinical trials, the authors of the 2018 report from the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF–KDOQI) concluded that the decision to continue or stop RAAS therapy in patients with advanced CKD should be individualized.4 Criteria that should be considered in the decision-making process include the presence or absence of large acute declines in eGFR (> 20% in the absence of a significant decrease in proteinuria), hypotension, or acute kidney injury with significant risk for worsening.
In 2021, the Renal Association and the Association of British Clinical Diabetologists published updated clinical practice guidelines for the management of hypertension and RAAS blockade in adults with diabetic kidney disease.5 Collective data indicated that, although outcomes varied based on type of diabetes (1 vs 2) and degree of proteinuria, blockade therapy overall led to improved outcomes; this was hypothesized to be due to the effects of reduced blood pressure. However, discontinuation of RAAS blockade therapy may be warranted when the patient (1) has a potassium level > 5 mmol/L pretreatment or ≥ 6 mmol/L with treatment, (2) demonstrates a decrease in eGFR > 25% or an increase in serum creatinine > 30% upon initiation of blockade, without another cause of renal deterioration, (3) is pregnant, or (4) has an acute illness with fluid depletion (in which case, RAAS therapy can be restarted 24 to 48 hours after recovery).
Editor’s takeaway
Evidence supports continuation of RAAS blockade, particularly in patients with significant comorbidities (diabetes and cardiovascular disease). Study data indicate continuation is either beneficial or neutral to further morbidity. The only caveat is that these patients should have their renal function and potassium level continuously monitored. The evidence should provide reassurance to patients and physicians that continuation is the correct course of action.
1. Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650-658. doi: 10.1016/j.jacc.2013.10.050
2. Nistor I, De Sutter J, Drechsler C, et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:12-22. doi: 10.1093/ndt/gfx072
3. Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021-2032. doi: 10.1056/NEJMoa2210639
4. Weir MR, Lakkis JI, Jaar B, et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF–KDOQI controversies report. Am J Kidney Dis. 2018;72:873-884. doi: 10.1053/j.ajkd.2018.06.010
5. Banerjee D, Winocour P, Chowdhury TA, et al. Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrol. 2022;23:9. doi: 10.1186/s12882-021-02587-5
1. Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650-658. doi: 10.1016/j.jacc.2013.10.050
2. Nistor I, De Sutter J, Drechsler C, et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:12-22. doi: 10.1093/ndt/gfx072
3. Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021-2032. doi: 10.1056/NEJMoa2210639
4. Weir MR, Lakkis JI, Jaar B, et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF–KDOQI controversies report. Am J Kidney Dis. 2018;72:873-884. doi: 10.1053/j.ajkd.2018.06.010
5. Banerjee D, Winocour P, Chowdhury TA, et al. Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrol. 2022;23:9. doi: 10.1186/s12882-021-02587-5
EVIDENCE-BASED REVIEW:
PROBABLY. Renin-angiotensin- aldosterone system (RAAS) blockade therapy should be continued in most patients with advanced renal disease and comorbid conditions; however, individualized treatment is warranted as data on the benefits and harms in all-cause mortality, cardiovascular mortality, and risk for renal replacement therapy are inconclusive (strength of recommendation [SOR]: B, based on observational studies, systematic reviews, and meta-analyses of randomized controlled trials [RCTs]). Certain patient populations, such as patients with diabetes or those with cardiovascular risk or history, may benefit most from continued RAAS blockade therapy (SOR: A, based on systematic reviews and meta-analyses of RCTs).
Sports: An underutilized tool for patients with disabilities
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6
However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; kjones15@usf.edu
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; kjones15@usf.edu
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; kjones15@usf.edu
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
PRACTICE RECOMMENDATIONS
› Recommend physical activity as an adjunct to traditional medical management to maximize physical and psychosocial benefits in patients with intellectual/developmental disabilities. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Family violence after COVID: Understanding coercive relationships
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.
All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.
All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.
All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
85-year-old woman • insomnia • abdominal discomfort • urge to move at night • Dx?
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; hirohisa.fujikawa@gmail.com
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; hirohisa.fujikawa@gmail.com
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; hirohisa.fujikawa@gmail.com
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
TikTok offers to ‘balance your hormones’ are pure hokum
With more than 306 million views, #hormonebalance and #hormonebalancing are among the latest hacks to take over the social media platform TikTok, on which users post short videos. Influencers offer advice such as eating raw carrots for “happy hormones,” eating protein followed by fat for breakfast to regulate blood glucose, or taking vitamin B2 supplements for thyroid health.
Have you ever wondered if you were asleep during the lecture on “hormone balancing” in medical school? No, you weren’t. It was never a class for good reason, and you didn’t fail to read any such breakthrough studies in The New England Journal of Medicine either.
There are over 50 different hormones produced by humans and animals, regulating sleep, growth, metabolism and reproduction, among many other biological processes, so there is certainly no one-size-fits-all solution to ensure these are all working in perfect harmony.
When someone mentions “hormone balancing,” my mind wanders to the last time I took my car to have my tires rotated and balanced. If only it were as simple to balance hormones in real life. The best we can hope for is to get a specific hormone within the ideal physiologic range for that person’s age.
The term “hormone” can mean many things to different people. When a woman comes in with a hormone question, for example, it is often related to estrogen, followed by thyroid hormones. A wealth of misinformation exists in popular literature regarding these hormones alone.
Estrogen can be replaced, but not everyone needs it replaced. It depends on variables including age, underlying medical conditions, the time of day a test was drawn, and concomitant medications. Having low levels of a given hormone does not necessarily call for replacement either.
Insulin is another example of a hormone that can never completely be replaced in people with diabetes in a way that exactly mimics the normal physiologic release.
There are many lesser-known hormones that are measurable and replaceable but are also more difficult to reset to original manufacturer specifications.
A Google search for “hormone balancing” often sends you to “naturopaths” or “integrative medicine” practitioners, who often propose similar solutions to the TikTok influencers. Users are told that their hormones are out of whack and that restoring this “balance” can be achieved by purchasing whatever “natural products” or concoction they are selling.
These TikTok videos and online “experts” are the home-brewed versions of the strip-mall hormone specialists. TikTok videos claiming to help “balance hormones” typically don’t name a specific hormone either, or the end organs that each would have an impact on. Rather, they lump all hormones into a monolithic entity, implying that there is a single solution for all health problems. And personal testimonials extolling the benefits of a TikTok intervention don’t constitute proof of efficacy no matter how many “likes” they get. These influencers assume that viewers can “sense” their hormones are out of tune and no lab tests can convince them otherwise.
In these inflationary times, the cost of seeking medical care from conventional channels is increasingly prohibitive. It’s easy to understand the appeal of getting free advice from TikTok or some other Internet site. At best, following the advice will not have much impact; at worst, it could be harmful.
Don’t try this at home
There are some things that should never be tried at home, and do-it-yourself hormone replacement or remediation both fall under this umbrella.
Generally, the body does a good job of balancing its own hormones. Most patients don’t need to be worried if they’re in good health. If they’re in doubt, they should seek advice from a doctor, ideally an endocrinologist, but an ob.gyn. or general practitioner are also good options.
One of the first questions to ask a patient is “Which hormone are you worried about?” or “What health issue is it specifically that is bothering you?” Narrowing the focus to a single thing, if possible, will lead to a more efficient evaluation.
Often, patients arrive with multiple concerns written on little pieces of paper. These ubiquitous pieces of paper are the red flag for the flood of questions to follow.
Ordering the appropriate tests for the conditions they are concerned about can help put their minds at ease. If there are any specific deficiencies, or excesses in any hormones, then appropriate solutions can be discussed.
TikTok hormone-balancing solutions are simply the 21st-century version of the snake oil sold on late-night cable TV in the 1990s.
Needless to say, you should gently encourage your patients to stay away from these non–FDA-approved products, without making them feel stupid. Off-label use of hormones when these are not indicated is also to be avoided, unless a medical practitioner feels it is warranted.
Dr. de la Rosa is an endocrinologist in Englewood, Fla. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
With more than 306 million views, #hormonebalance and #hormonebalancing are among the latest hacks to take over the social media platform TikTok, on which users post short videos. Influencers offer advice such as eating raw carrots for “happy hormones,” eating protein followed by fat for breakfast to regulate blood glucose, or taking vitamin B2 supplements for thyroid health.
Have you ever wondered if you were asleep during the lecture on “hormone balancing” in medical school? No, you weren’t. It was never a class for good reason, and you didn’t fail to read any such breakthrough studies in The New England Journal of Medicine either.
There are over 50 different hormones produced by humans and animals, regulating sleep, growth, metabolism and reproduction, among many other biological processes, so there is certainly no one-size-fits-all solution to ensure these are all working in perfect harmony.
When someone mentions “hormone balancing,” my mind wanders to the last time I took my car to have my tires rotated and balanced. If only it were as simple to balance hormones in real life. The best we can hope for is to get a specific hormone within the ideal physiologic range for that person’s age.
The term “hormone” can mean many things to different people. When a woman comes in with a hormone question, for example, it is often related to estrogen, followed by thyroid hormones. A wealth of misinformation exists in popular literature regarding these hormones alone.
Estrogen can be replaced, but not everyone needs it replaced. It depends on variables including age, underlying medical conditions, the time of day a test was drawn, and concomitant medications. Having low levels of a given hormone does not necessarily call for replacement either.
Insulin is another example of a hormone that can never completely be replaced in people with diabetes in a way that exactly mimics the normal physiologic release.
There are many lesser-known hormones that are measurable and replaceable but are also more difficult to reset to original manufacturer specifications.
A Google search for “hormone balancing” often sends you to “naturopaths” or “integrative medicine” practitioners, who often propose similar solutions to the TikTok influencers. Users are told that their hormones are out of whack and that restoring this “balance” can be achieved by purchasing whatever “natural products” or concoction they are selling.
These TikTok videos and online “experts” are the home-brewed versions of the strip-mall hormone specialists. TikTok videos claiming to help “balance hormones” typically don’t name a specific hormone either, or the end organs that each would have an impact on. Rather, they lump all hormones into a monolithic entity, implying that there is a single solution for all health problems. And personal testimonials extolling the benefits of a TikTok intervention don’t constitute proof of efficacy no matter how many “likes” they get. These influencers assume that viewers can “sense” their hormones are out of tune and no lab tests can convince them otherwise.
In these inflationary times, the cost of seeking medical care from conventional channels is increasingly prohibitive. It’s easy to understand the appeal of getting free advice from TikTok or some other Internet site. At best, following the advice will not have much impact; at worst, it could be harmful.
Don’t try this at home
There are some things that should never be tried at home, and do-it-yourself hormone replacement or remediation both fall under this umbrella.
Generally, the body does a good job of balancing its own hormones. Most patients don’t need to be worried if they’re in good health. If they’re in doubt, they should seek advice from a doctor, ideally an endocrinologist, but an ob.gyn. or general practitioner are also good options.
One of the first questions to ask a patient is “Which hormone are you worried about?” or “What health issue is it specifically that is bothering you?” Narrowing the focus to a single thing, if possible, will lead to a more efficient evaluation.
Often, patients arrive with multiple concerns written on little pieces of paper. These ubiquitous pieces of paper are the red flag for the flood of questions to follow.
Ordering the appropriate tests for the conditions they are concerned about can help put their minds at ease. If there are any specific deficiencies, or excesses in any hormones, then appropriate solutions can be discussed.
TikTok hormone-balancing solutions are simply the 21st-century version of the snake oil sold on late-night cable TV in the 1990s.
Needless to say, you should gently encourage your patients to stay away from these non–FDA-approved products, without making them feel stupid. Off-label use of hormones when these are not indicated is also to be avoided, unless a medical practitioner feels it is warranted.
Dr. de la Rosa is an endocrinologist in Englewood, Fla. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
With more than 306 million views, #hormonebalance and #hormonebalancing are among the latest hacks to take over the social media platform TikTok, on which users post short videos. Influencers offer advice such as eating raw carrots for “happy hormones,” eating protein followed by fat for breakfast to regulate blood glucose, or taking vitamin B2 supplements for thyroid health.
Have you ever wondered if you were asleep during the lecture on “hormone balancing” in medical school? No, you weren’t. It was never a class for good reason, and you didn’t fail to read any such breakthrough studies in The New England Journal of Medicine either.
There are over 50 different hormones produced by humans and animals, regulating sleep, growth, metabolism and reproduction, among many other biological processes, so there is certainly no one-size-fits-all solution to ensure these are all working in perfect harmony.
When someone mentions “hormone balancing,” my mind wanders to the last time I took my car to have my tires rotated and balanced. If only it were as simple to balance hormones in real life. The best we can hope for is to get a specific hormone within the ideal physiologic range for that person’s age.
The term “hormone” can mean many things to different people. When a woman comes in with a hormone question, for example, it is often related to estrogen, followed by thyroid hormones. A wealth of misinformation exists in popular literature regarding these hormones alone.
Estrogen can be replaced, but not everyone needs it replaced. It depends on variables including age, underlying medical conditions, the time of day a test was drawn, and concomitant medications. Having low levels of a given hormone does not necessarily call for replacement either.
Insulin is another example of a hormone that can never completely be replaced in people with diabetes in a way that exactly mimics the normal physiologic release.
There are many lesser-known hormones that are measurable and replaceable but are also more difficult to reset to original manufacturer specifications.
A Google search for “hormone balancing” often sends you to “naturopaths” or “integrative medicine” practitioners, who often propose similar solutions to the TikTok influencers. Users are told that their hormones are out of whack and that restoring this “balance” can be achieved by purchasing whatever “natural products” or concoction they are selling.
These TikTok videos and online “experts” are the home-brewed versions of the strip-mall hormone specialists. TikTok videos claiming to help “balance hormones” typically don’t name a specific hormone either, or the end organs that each would have an impact on. Rather, they lump all hormones into a monolithic entity, implying that there is a single solution for all health problems. And personal testimonials extolling the benefits of a TikTok intervention don’t constitute proof of efficacy no matter how many “likes” they get. These influencers assume that viewers can “sense” their hormones are out of tune and no lab tests can convince them otherwise.
In these inflationary times, the cost of seeking medical care from conventional channels is increasingly prohibitive. It’s easy to understand the appeal of getting free advice from TikTok or some other Internet site. At best, following the advice will not have much impact; at worst, it could be harmful.
Don’t try this at home
There are some things that should never be tried at home, and do-it-yourself hormone replacement or remediation both fall under this umbrella.
Generally, the body does a good job of balancing its own hormones. Most patients don’t need to be worried if they’re in good health. If they’re in doubt, they should seek advice from a doctor, ideally an endocrinologist, but an ob.gyn. or general practitioner are also good options.
One of the first questions to ask a patient is “Which hormone are you worried about?” or “What health issue is it specifically that is bothering you?” Narrowing the focus to a single thing, if possible, will lead to a more efficient evaluation.
Often, patients arrive with multiple concerns written on little pieces of paper. These ubiquitous pieces of paper are the red flag for the flood of questions to follow.
Ordering the appropriate tests for the conditions they are concerned about can help put their minds at ease. If there are any specific deficiencies, or excesses in any hormones, then appropriate solutions can be discussed.
TikTok hormone-balancing solutions are simply the 21st-century version of the snake oil sold on late-night cable TV in the 1990s.
Needless to say, you should gently encourage your patients to stay away from these non–FDA-approved products, without making them feel stupid. Off-label use of hormones when these are not indicated is also to be avoided, unless a medical practitioner feels it is warranted.
Dr. de la Rosa is an endocrinologist in Englewood, Fla. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Acute unilateral visual disturbance
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
One emergency is ending, and we’re ready for the next
I’ve always thought it was interesting that the first cases of COVID-19 were reported to the World Health Organization on December 31, 2019.1 How close we came to having COVID-20! On January 31, 2020, the US Department of Health and Human Services declared a national public health emergency due to COVID-19, and it’s been in effect ever since.
A national public health emergency allows the Department of Health and Human Services to access and designate funds to diagnose, treat, and prevent disease in response to the emergency. The declaration also facilitates the Centers for Disease Control and Prevention response to an infectious disease emergency. There are provisions for modifications to Medicare, Medicaid, and the Children’s Health Insurance Program so clinicians can continue seeing patients and be reimbursed for doing so, even in a situation in which the emergency disrupts usual reporting and documentation requirements. The declaration is essentially a shortcut through the typical bureaucracy that too often gums up the practice of medicine2; it allows for the rapid deployment of funds and personnel to a community affected by an emergency.
Unprecedented change. In the early days, plastic partitions were erected between patients in the hospital, and the scarce supply of N-95 masks was stored in paper bags and baked at low temperatures in ovens overnight.
My hospital enacted its incident command response procedures, just as we did the day our community experienced a mass shooting—except incident command stayed open for months. We had to adapt quickly. My office never closed to in-person visits; we decided that we took care of too many people who did not have other access to care to make closing practical. My practice partners and I spent a Friday afternoon in March 2020 writing policies. A policy for our residency practice. A policy for how to see patients who might have COVID. A policy for how to cover the residents and faculty when we inevitably got sick. A policy for how to do telehealth visits. By the following Monday, when the office reopened, we had already trained the staff on the new policies, and we were ready to implement them with our patients.
As COVID and our knowledge about it changed, we rewrote those policies dozens of times, and each time the staff retrained in a hurry. We all learned so much so quickly. So as the official public health emergency comes to an end, there are things that I think I will take from it, and things that I wish all of medicine could take from it too.
We adapted as a team. I will never forget the stress of the early days of the emergency, when the patient volume was overwhelming and the death rate was staggering. But shining through those dark times were wonderful moments of connection with the teams with which I worked. I think about the residents whose training shifted suddenly to full-time COVID, the nurses who learned new things every weekend for so many months, and everyone who went out on a limb to do the right thing.
We provided care without bureaucracy. I wish medicine could leave the bureaucracy behind along with the emergency. It was so much easier to practice medicine when we knew that the testing and treatment were covered, without “we’ll see” or “it depends on your insurance.” Telehealth is probably here to stay, thanks to widespread uptake by patients and clinicians alike during the pandemic. My wish is that we can make it as easy as possible to use going forward, instead of choosing to return to a more restricted and difficult path.3,4
Family physicians have much to be proud of. We can look back on the COVID-19 public health emergency as a time when we absorbed a huge amount of rapidly changing information and showed our adaptability to a frightening and uncertain environment. We are not returning to the office, as so many Americans are these days, because we never left the many settings where family physicians practice. We remained at work during the emergency and we took care of our patients.
When the next emergency is declared—whether it be national or local—we will once again be there for our patients.
1. CDC. CDC museum COVID-19 timeline. Updated March 15, 2023. Accessed March 28, 2023. www.cdc.gov/museum/timeline/covid19.html
2. US Department of Health and Human Services Administration for Strategic Preparedness & Response. A public health emer-gency declaration. Accessed March 28, 2023. https://aspr.hhs.gov/legal/PHE/Pages/Public-Health-Emergency-Declaration.aspx
3. US Department of Health and Human Services. Telehealth policy changes after the COVID-19 public health emergency. Updated February 16, 2023. Accessed March 28, 2023. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency/policy-changes-after-the-covid-19-public-health-emergency
4. Cox C, Kates J, Cubanski J, et al. The end of the COVID-19 public health emergency: details on health coverage and access. Kaiser Family Foundation. Published February 3, 2023. Accessed March 28, 2023. www.kff.org/policy-watch/the-end-of-the-covid-19-public-health-emergency-details-on-health-coverage-and-access/
I’ve always thought it was interesting that the first cases of COVID-19 were reported to the World Health Organization on December 31, 2019.1 How close we came to having COVID-20! On January 31, 2020, the US Department of Health and Human Services declared a national public health emergency due to COVID-19, and it’s been in effect ever since.
A national public health emergency allows the Department of Health and Human Services to access and designate funds to diagnose, treat, and prevent disease in response to the emergency. The declaration also facilitates the Centers for Disease Control and Prevention response to an infectious disease emergency. There are provisions for modifications to Medicare, Medicaid, and the Children’s Health Insurance Program so clinicians can continue seeing patients and be reimbursed for doing so, even in a situation in which the emergency disrupts usual reporting and documentation requirements. The declaration is essentially a shortcut through the typical bureaucracy that too often gums up the practice of medicine2; it allows for the rapid deployment of funds and personnel to a community affected by an emergency.
Unprecedented change. In the early days, plastic partitions were erected between patients in the hospital, and the scarce supply of N-95 masks was stored in paper bags and baked at low temperatures in ovens overnight.
My hospital enacted its incident command response procedures, just as we did the day our community experienced a mass shooting—except incident command stayed open for months. We had to adapt quickly. My office never closed to in-person visits; we decided that we took care of too many people who did not have other access to care to make closing practical. My practice partners and I spent a Friday afternoon in March 2020 writing policies. A policy for our residency practice. A policy for how to see patients who might have COVID. A policy for how to cover the residents and faculty when we inevitably got sick. A policy for how to do telehealth visits. By the following Monday, when the office reopened, we had already trained the staff on the new policies, and we were ready to implement them with our patients.
As COVID and our knowledge about it changed, we rewrote those policies dozens of times, and each time the staff retrained in a hurry. We all learned so much so quickly. So as the official public health emergency comes to an end, there are things that I think I will take from it, and things that I wish all of medicine could take from it too.
We adapted as a team. I will never forget the stress of the early days of the emergency, when the patient volume was overwhelming and the death rate was staggering. But shining through those dark times were wonderful moments of connection with the teams with which I worked. I think about the residents whose training shifted suddenly to full-time COVID, the nurses who learned new things every weekend for so many months, and everyone who went out on a limb to do the right thing.
We provided care without bureaucracy. I wish medicine could leave the bureaucracy behind along with the emergency. It was so much easier to practice medicine when we knew that the testing and treatment were covered, without “we’ll see” or “it depends on your insurance.” Telehealth is probably here to stay, thanks to widespread uptake by patients and clinicians alike during the pandemic. My wish is that we can make it as easy as possible to use going forward, instead of choosing to return to a more restricted and difficult path.3,4
Family physicians have much to be proud of. We can look back on the COVID-19 public health emergency as a time when we absorbed a huge amount of rapidly changing information and showed our adaptability to a frightening and uncertain environment. We are not returning to the office, as so many Americans are these days, because we never left the many settings where family physicians practice. We remained at work during the emergency and we took care of our patients.
When the next emergency is declared—whether it be national or local—we will once again be there for our patients.
I’ve always thought it was interesting that the first cases of COVID-19 were reported to the World Health Organization on December 31, 2019.1 How close we came to having COVID-20! On January 31, 2020, the US Department of Health and Human Services declared a national public health emergency due to COVID-19, and it’s been in effect ever since.
A national public health emergency allows the Department of Health and Human Services to access and designate funds to diagnose, treat, and prevent disease in response to the emergency. The declaration also facilitates the Centers for Disease Control and Prevention response to an infectious disease emergency. There are provisions for modifications to Medicare, Medicaid, and the Children’s Health Insurance Program so clinicians can continue seeing patients and be reimbursed for doing so, even in a situation in which the emergency disrupts usual reporting and documentation requirements. The declaration is essentially a shortcut through the typical bureaucracy that too often gums up the practice of medicine2; it allows for the rapid deployment of funds and personnel to a community affected by an emergency.
Unprecedented change. In the early days, plastic partitions were erected between patients in the hospital, and the scarce supply of N-95 masks was stored in paper bags and baked at low temperatures in ovens overnight.
My hospital enacted its incident command response procedures, just as we did the day our community experienced a mass shooting—except incident command stayed open for months. We had to adapt quickly. My office never closed to in-person visits; we decided that we took care of too many people who did not have other access to care to make closing practical. My practice partners and I spent a Friday afternoon in March 2020 writing policies. A policy for our residency practice. A policy for how to see patients who might have COVID. A policy for how to cover the residents and faculty when we inevitably got sick. A policy for how to do telehealth visits. By the following Monday, when the office reopened, we had already trained the staff on the new policies, and we were ready to implement them with our patients.
As COVID and our knowledge about it changed, we rewrote those policies dozens of times, and each time the staff retrained in a hurry. We all learned so much so quickly. So as the official public health emergency comes to an end, there are things that I think I will take from it, and things that I wish all of medicine could take from it too.
We adapted as a team. I will never forget the stress of the early days of the emergency, when the patient volume was overwhelming and the death rate was staggering. But shining through those dark times were wonderful moments of connection with the teams with which I worked. I think about the residents whose training shifted suddenly to full-time COVID, the nurses who learned new things every weekend for so many months, and everyone who went out on a limb to do the right thing.
We provided care without bureaucracy. I wish medicine could leave the bureaucracy behind along with the emergency. It was so much easier to practice medicine when we knew that the testing and treatment were covered, without “we’ll see” or “it depends on your insurance.” Telehealth is probably here to stay, thanks to widespread uptake by patients and clinicians alike during the pandemic. My wish is that we can make it as easy as possible to use going forward, instead of choosing to return to a more restricted and difficult path.3,4
Family physicians have much to be proud of. We can look back on the COVID-19 public health emergency as a time when we absorbed a huge amount of rapidly changing information and showed our adaptability to a frightening and uncertain environment. We are not returning to the office, as so many Americans are these days, because we never left the many settings where family physicians practice. We remained at work during the emergency and we took care of our patients.
When the next emergency is declared—whether it be national or local—we will once again be there for our patients.
1. CDC. CDC museum COVID-19 timeline. Updated March 15, 2023. Accessed March 28, 2023. www.cdc.gov/museum/timeline/covid19.html
2. US Department of Health and Human Services Administration for Strategic Preparedness & Response. A public health emer-gency declaration. Accessed March 28, 2023. https://aspr.hhs.gov/legal/PHE/Pages/Public-Health-Emergency-Declaration.aspx
3. US Department of Health and Human Services. Telehealth policy changes after the COVID-19 public health emergency. Updated February 16, 2023. Accessed March 28, 2023. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency/policy-changes-after-the-covid-19-public-health-emergency
4. Cox C, Kates J, Cubanski J, et al. The end of the COVID-19 public health emergency: details on health coverage and access. Kaiser Family Foundation. Published February 3, 2023. Accessed March 28, 2023. www.kff.org/policy-watch/the-end-of-the-covid-19-public-health-emergency-details-on-health-coverage-and-access/
1. CDC. CDC museum COVID-19 timeline. Updated March 15, 2023. Accessed March 28, 2023. www.cdc.gov/museum/timeline/covid19.html
2. US Department of Health and Human Services Administration for Strategic Preparedness & Response. A public health emer-gency declaration. Accessed March 28, 2023. https://aspr.hhs.gov/legal/PHE/Pages/Public-Health-Emergency-Declaration.aspx
3. US Department of Health and Human Services. Telehealth policy changes after the COVID-19 public health emergency. Updated February 16, 2023. Accessed March 28, 2023. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency/policy-changes-after-the-covid-19-public-health-emergency
4. Cox C, Kates J, Cubanski J, et al. The end of the COVID-19 public health emergency: details on health coverage and access. Kaiser Family Foundation. Published February 3, 2023. Accessed March 28, 2023. www.kff.org/policy-watch/the-end-of-the-covid-19-public-health-emergency-details-on-health-coverage-and-access/
Hyperlipidemia management: A calibrated approach
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12
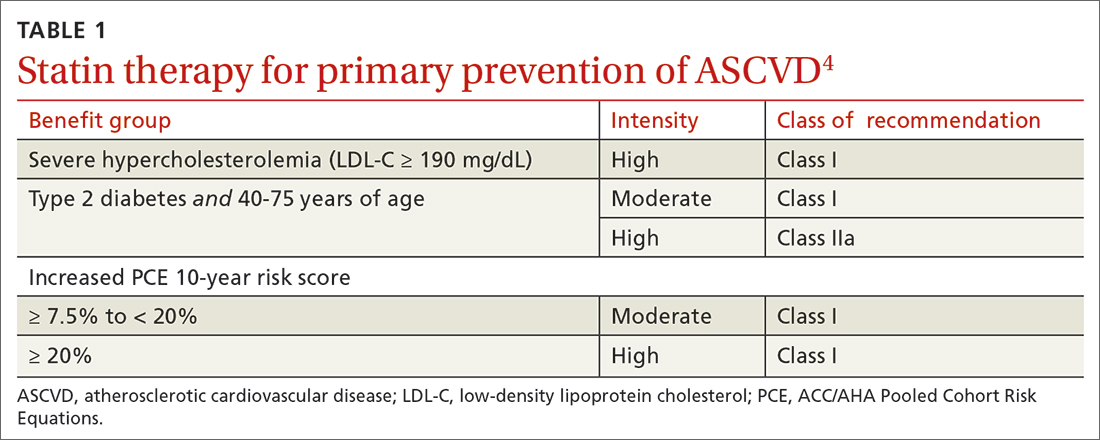
Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).
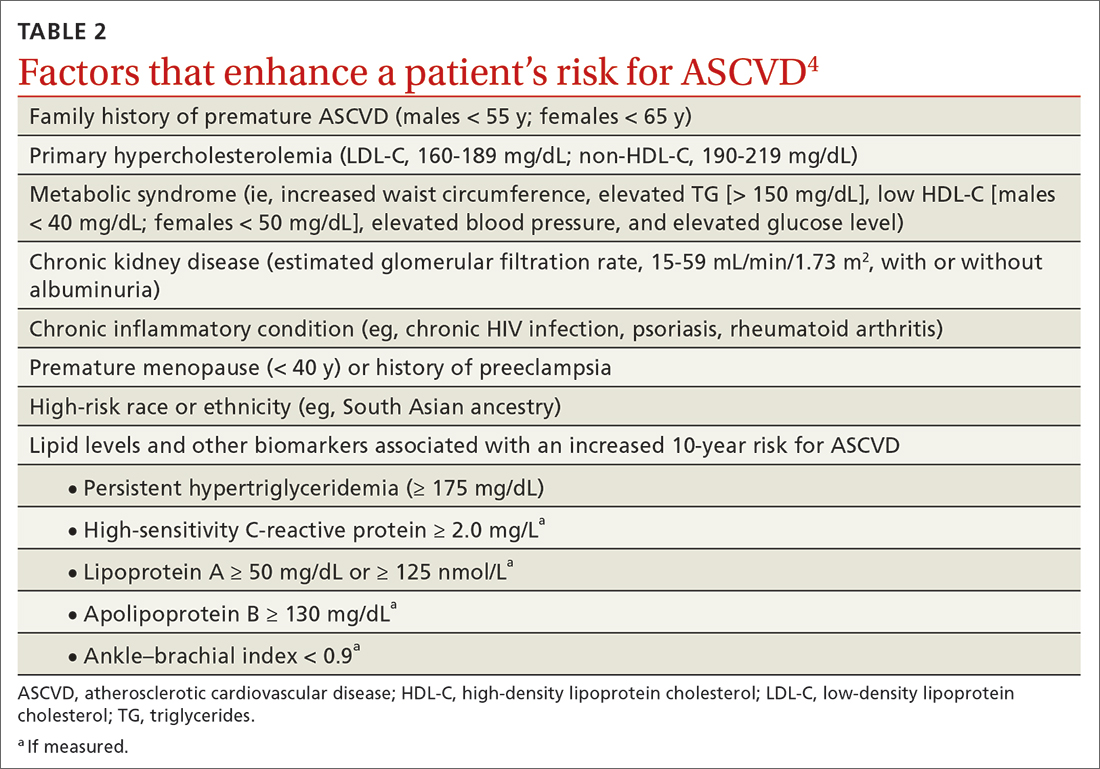
If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4
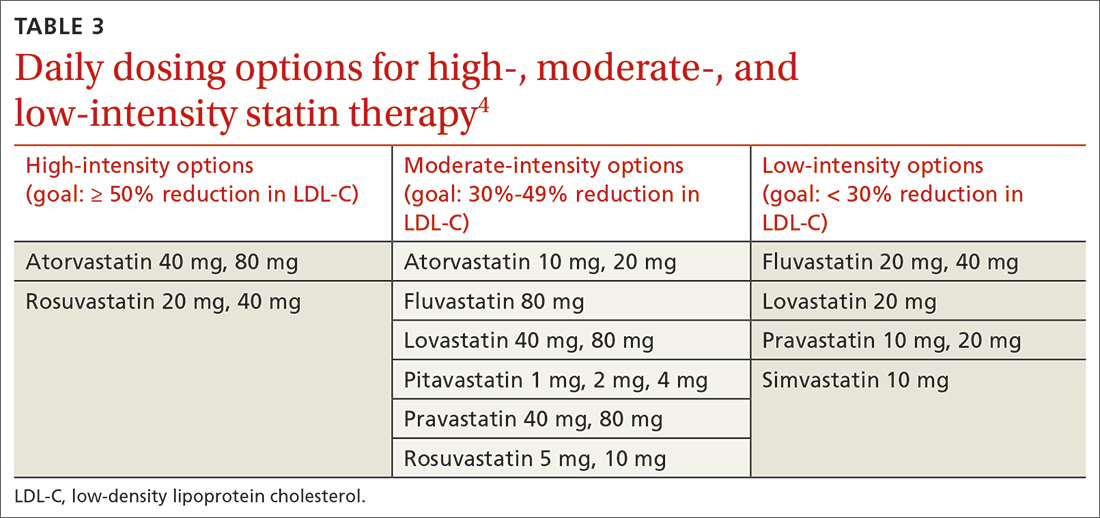
Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; firnhaberj@ecu.edu
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2

The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; firnhaberj@ecu.edu
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2

The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; firnhaberj@ecu.edu
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
PRACTICE RECOMMENDATIONS
› Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
› Consider the impact of ASCVD risk-enhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
› Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Study highlights potential skin cancer risk of UV nail polish dryers
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Clinical Practice Update: Alpha-gal syndrome often causes GI issues without anaphylaxis, skin changes
according to an American Gastroenterological Association clinical practice update.
Although the allergic response is best known for a combination of anaphylaxis, skin changes, and gastrointestinal symptoms that occurs within hours of consuming mammalian-derived food products, health care providers should know that many patients experience gastrointestinal distress in the absence of other clinical signs, lead author Sarah K. McGill, MD, MSc, of the University of North Carolina at Chapel Hill, and colleagues reported.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” the investigators wrote in Clinical Gastroenterology and Hepatology.
To this end, Dr. McGill and colleagues drafted the present clinical practice update, covering pathogenesis, clinical manifestations, diagnosis, and management.
“The allergy in alpha-gal syndrome is to galactose alpha-1,3-galactose, an oligosaccharide on the cells of all nonprimate mammals,” the investigators wrote. “Surprisingly, sensitization to alpha-gal, that is, the process by which human beings develop IgE antibodies to the sugar, is understood to occur after the bite of a tick or parasitic infection. In the United States, the Lone Star tick, an ectoparasite whose principal host is deer, is strongly implicated.”
Gastrointestinal focused clinical research is scarce, the investigators noted, citing two observational studies involving 375 patients positive for alpha-gal IgE. Almost half of these patients (40.7%) had gastrointestinal symptoms alone. Across the entire population, the most common gastrointestinal symptoms were abdominal pain (71%) and vomiting (22%). About three out of four patients reported improvement on an alpha-gal avoidance diet.
“Clinicians should consider alpha-gal syndrome in the differential diagnosis of patients with unexplained gastrointestinal symptoms of abdominal pain, diarrhea, nausea, and vomiting, particularly those who live or have lived in an alpha-gal–prevalent area,” the investigators wrote.
In the United States, these areas span the domain of the Lone Star tick, including most of the East Coast, the central Midwest, the South, and all of Texas. Overseas, alpha-gal syndrome has been reported in Japan, Australia, Western Europe, and South Africa.
Clinical suspicion should be increased in patients with a history of tick bite, engagement in outdoor activities, and awakening in the night with gastrointestinal distress (because of the delay between allergen ingestion and symptom onset). Workup should include serum testing for alpha-gal IgE antibodies, according to the update. Serum positivity alone, however, is not sufficient for diagnosis. Alpha-gal syndrome must be confirmed by symptom resolution or improvement upon adherence to an alpha-gal avoidance diet for at least a month.
“During this time, patients may want to avoid eating at restaurants, which can easily cross-contaminate food, and processed food, which may contain alpha-gal in additives,” Dr. McGill and colleagues wrote.
Patients with alpha-gal syndrome who accidentally consume alpha-gal should take 25-50 mg of diphenhydramine and ensure access to a self-injectable epinephrine if symptoms progress, particularly if respiratory compromise occurs, they added.
The coauthors are Jana G. Hasash, MD, and Thomas A. Platts-Mills, MD, PhD.
The investigators disclosed relationships with Olympus America, Exact Sciences, Guardant Health, Finch Therapeutics, and others.
according to an American Gastroenterological Association clinical practice update.
Although the allergic response is best known for a combination of anaphylaxis, skin changes, and gastrointestinal symptoms that occurs within hours of consuming mammalian-derived food products, health care providers should know that many patients experience gastrointestinal distress in the absence of other clinical signs, lead author Sarah K. McGill, MD, MSc, of the University of North Carolina at Chapel Hill, and colleagues reported.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” the investigators wrote in Clinical Gastroenterology and Hepatology.
To this end, Dr. McGill and colleagues drafted the present clinical practice update, covering pathogenesis, clinical manifestations, diagnosis, and management.
“The allergy in alpha-gal syndrome is to galactose alpha-1,3-galactose, an oligosaccharide on the cells of all nonprimate mammals,” the investigators wrote. “Surprisingly, sensitization to alpha-gal, that is, the process by which human beings develop IgE antibodies to the sugar, is understood to occur after the bite of a tick or parasitic infection. In the United States, the Lone Star tick, an ectoparasite whose principal host is deer, is strongly implicated.”
Gastrointestinal focused clinical research is scarce, the investigators noted, citing two observational studies involving 375 patients positive for alpha-gal IgE. Almost half of these patients (40.7%) had gastrointestinal symptoms alone. Across the entire population, the most common gastrointestinal symptoms were abdominal pain (71%) and vomiting (22%). About three out of four patients reported improvement on an alpha-gal avoidance diet.
“Clinicians should consider alpha-gal syndrome in the differential diagnosis of patients with unexplained gastrointestinal symptoms of abdominal pain, diarrhea, nausea, and vomiting, particularly those who live or have lived in an alpha-gal–prevalent area,” the investigators wrote.
In the United States, these areas span the domain of the Lone Star tick, including most of the East Coast, the central Midwest, the South, and all of Texas. Overseas, alpha-gal syndrome has been reported in Japan, Australia, Western Europe, and South Africa.
Clinical suspicion should be increased in patients with a history of tick bite, engagement in outdoor activities, and awakening in the night with gastrointestinal distress (because of the delay between allergen ingestion and symptom onset). Workup should include serum testing for alpha-gal IgE antibodies, according to the update. Serum positivity alone, however, is not sufficient for diagnosis. Alpha-gal syndrome must be confirmed by symptom resolution or improvement upon adherence to an alpha-gal avoidance diet for at least a month.
“During this time, patients may want to avoid eating at restaurants, which can easily cross-contaminate food, and processed food, which may contain alpha-gal in additives,” Dr. McGill and colleagues wrote.
Patients with alpha-gal syndrome who accidentally consume alpha-gal should take 25-50 mg of diphenhydramine and ensure access to a self-injectable epinephrine if symptoms progress, particularly if respiratory compromise occurs, they added.
The coauthors are Jana G. Hasash, MD, and Thomas A. Platts-Mills, MD, PhD.
The investigators disclosed relationships with Olympus America, Exact Sciences, Guardant Health, Finch Therapeutics, and others.
according to an American Gastroenterological Association clinical practice update.
Although the allergic response is best known for a combination of anaphylaxis, skin changes, and gastrointestinal symptoms that occurs within hours of consuming mammalian-derived food products, health care providers should know that many patients experience gastrointestinal distress in the absence of other clinical signs, lead author Sarah K. McGill, MD, MSc, of the University of North Carolina at Chapel Hill, and colleagues reported.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” the investigators wrote in Clinical Gastroenterology and Hepatology.
To this end, Dr. McGill and colleagues drafted the present clinical practice update, covering pathogenesis, clinical manifestations, diagnosis, and management.
“The allergy in alpha-gal syndrome is to galactose alpha-1,3-galactose, an oligosaccharide on the cells of all nonprimate mammals,” the investigators wrote. “Surprisingly, sensitization to alpha-gal, that is, the process by which human beings develop IgE antibodies to the sugar, is understood to occur after the bite of a tick or parasitic infection. In the United States, the Lone Star tick, an ectoparasite whose principal host is deer, is strongly implicated.”
Gastrointestinal focused clinical research is scarce, the investigators noted, citing two observational studies involving 375 patients positive for alpha-gal IgE. Almost half of these patients (40.7%) had gastrointestinal symptoms alone. Across the entire population, the most common gastrointestinal symptoms were abdominal pain (71%) and vomiting (22%). About three out of four patients reported improvement on an alpha-gal avoidance diet.
“Clinicians should consider alpha-gal syndrome in the differential diagnosis of patients with unexplained gastrointestinal symptoms of abdominal pain, diarrhea, nausea, and vomiting, particularly those who live or have lived in an alpha-gal–prevalent area,” the investigators wrote.
In the United States, these areas span the domain of the Lone Star tick, including most of the East Coast, the central Midwest, the South, and all of Texas. Overseas, alpha-gal syndrome has been reported in Japan, Australia, Western Europe, and South Africa.
Clinical suspicion should be increased in patients with a history of tick bite, engagement in outdoor activities, and awakening in the night with gastrointestinal distress (because of the delay between allergen ingestion and symptom onset). Workup should include serum testing for alpha-gal IgE antibodies, according to the update. Serum positivity alone, however, is not sufficient for diagnosis. Alpha-gal syndrome must be confirmed by symptom resolution or improvement upon adherence to an alpha-gal avoidance diet for at least a month.
“During this time, patients may want to avoid eating at restaurants, which can easily cross-contaminate food, and processed food, which may contain alpha-gal in additives,” Dr. McGill and colleagues wrote.
Patients with alpha-gal syndrome who accidentally consume alpha-gal should take 25-50 mg of diphenhydramine and ensure access to a self-injectable epinephrine if symptoms progress, particularly if respiratory compromise occurs, they added.
The coauthors are Jana G. Hasash, MD, and Thomas A. Platts-Mills, MD, PhD.
The investigators disclosed relationships with Olympus America, Exact Sciences, Guardant Health, Finch Therapeutics, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY