User login
The Impact of Primary Tumor Site on Survival in Mycosis Fungoides
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
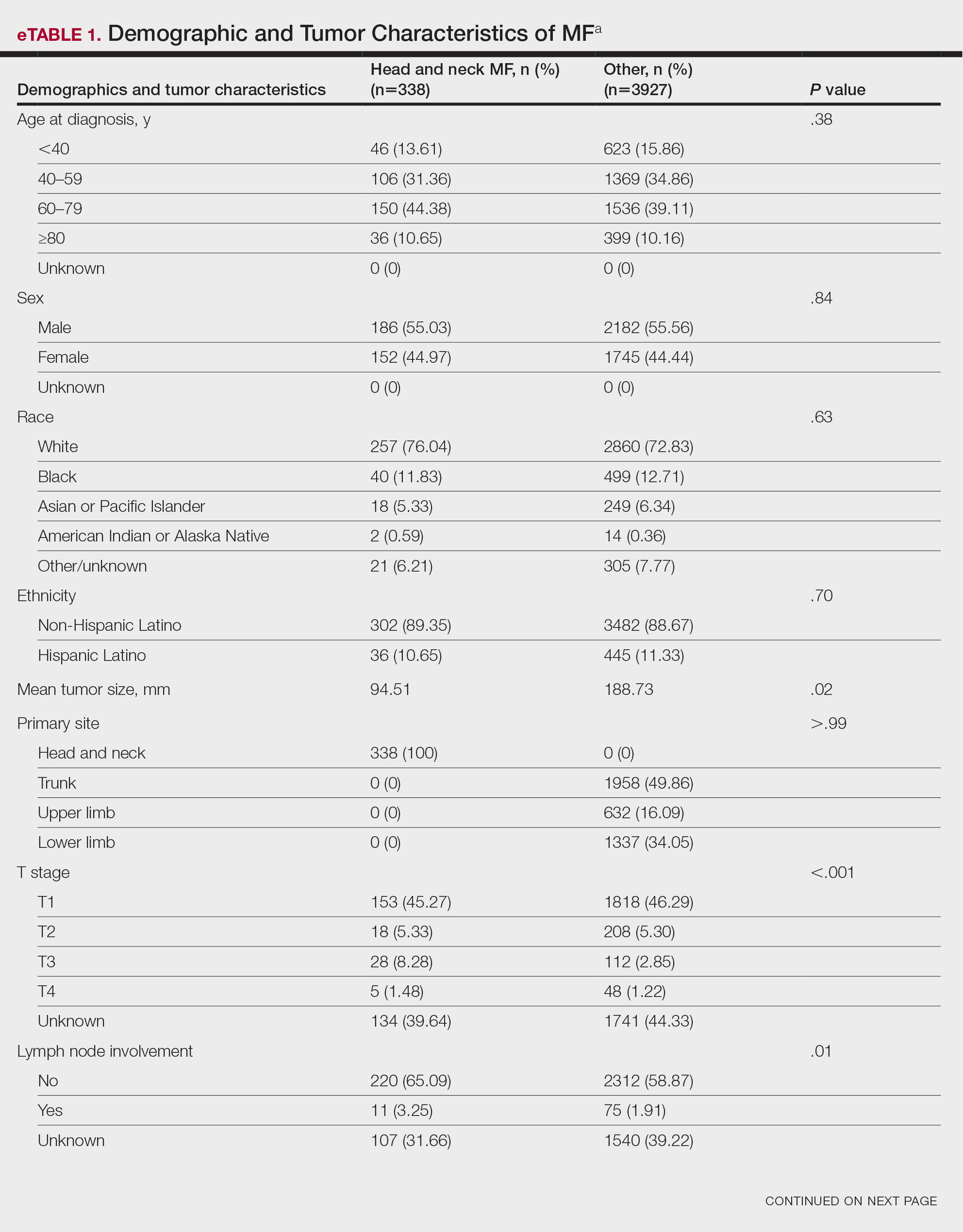
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.
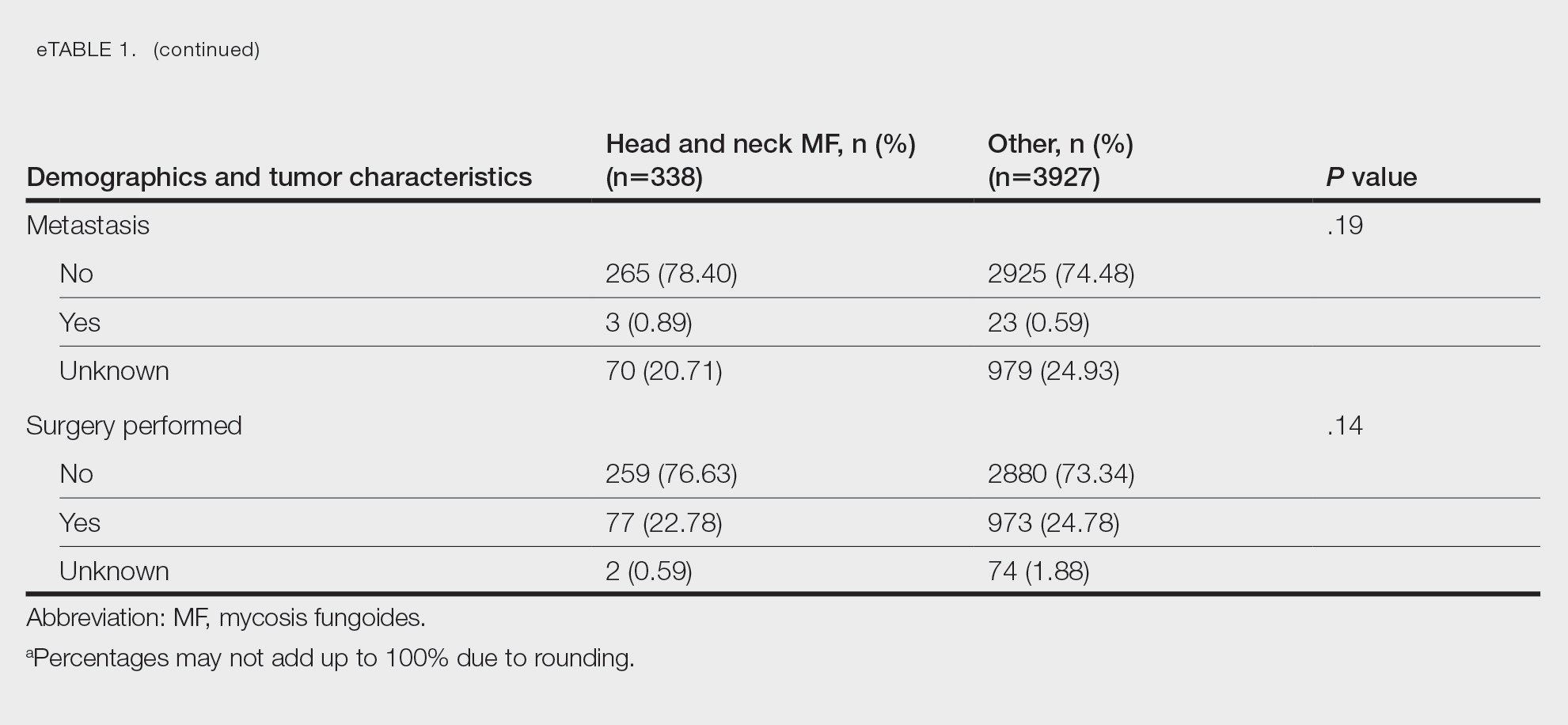
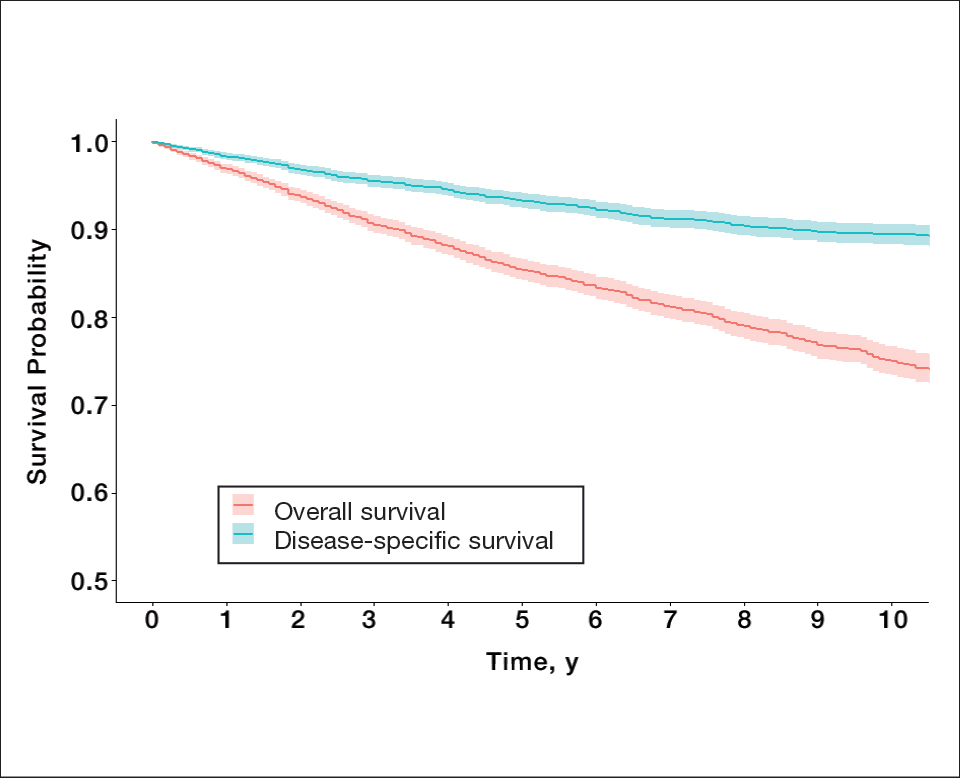
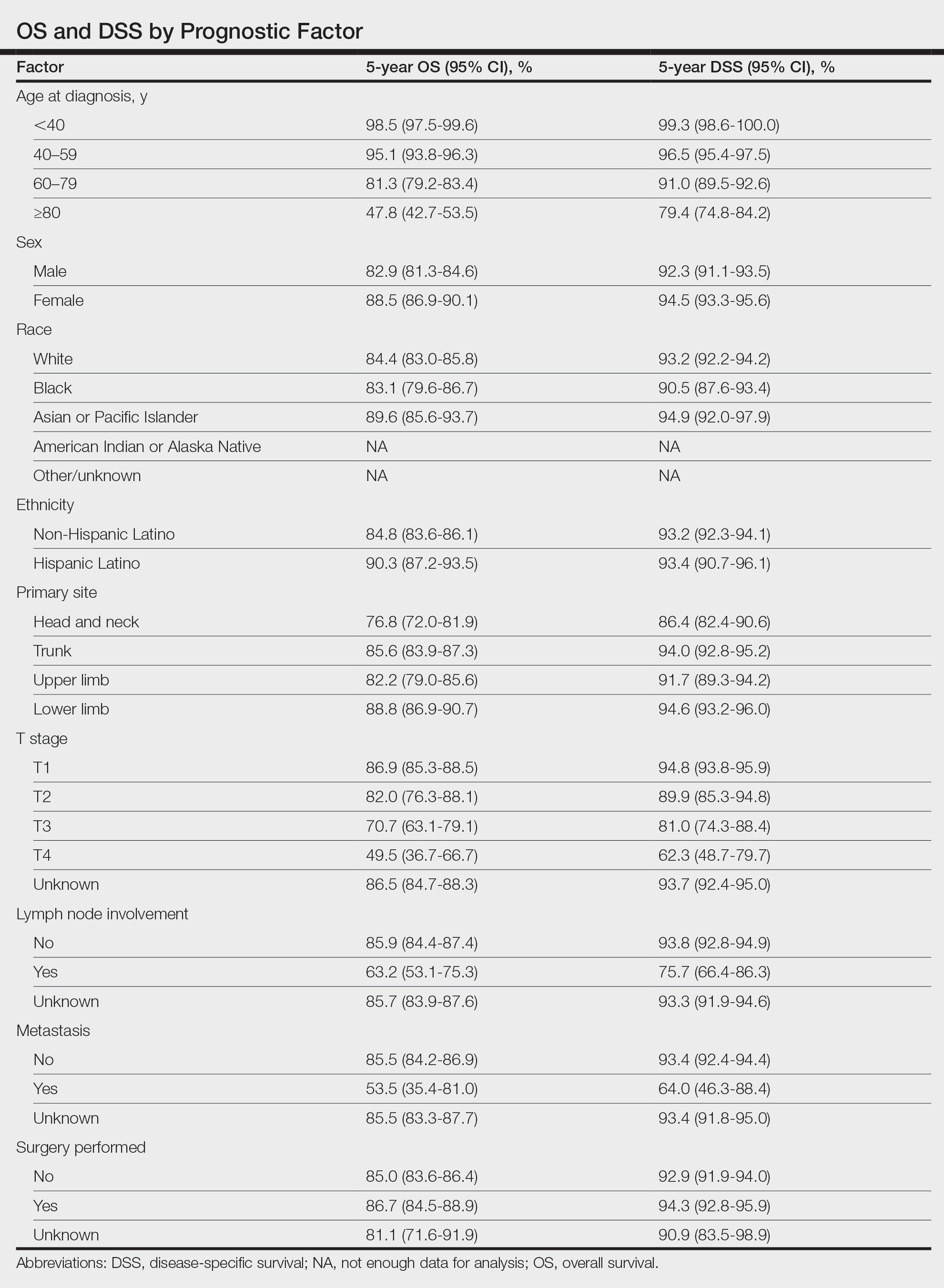
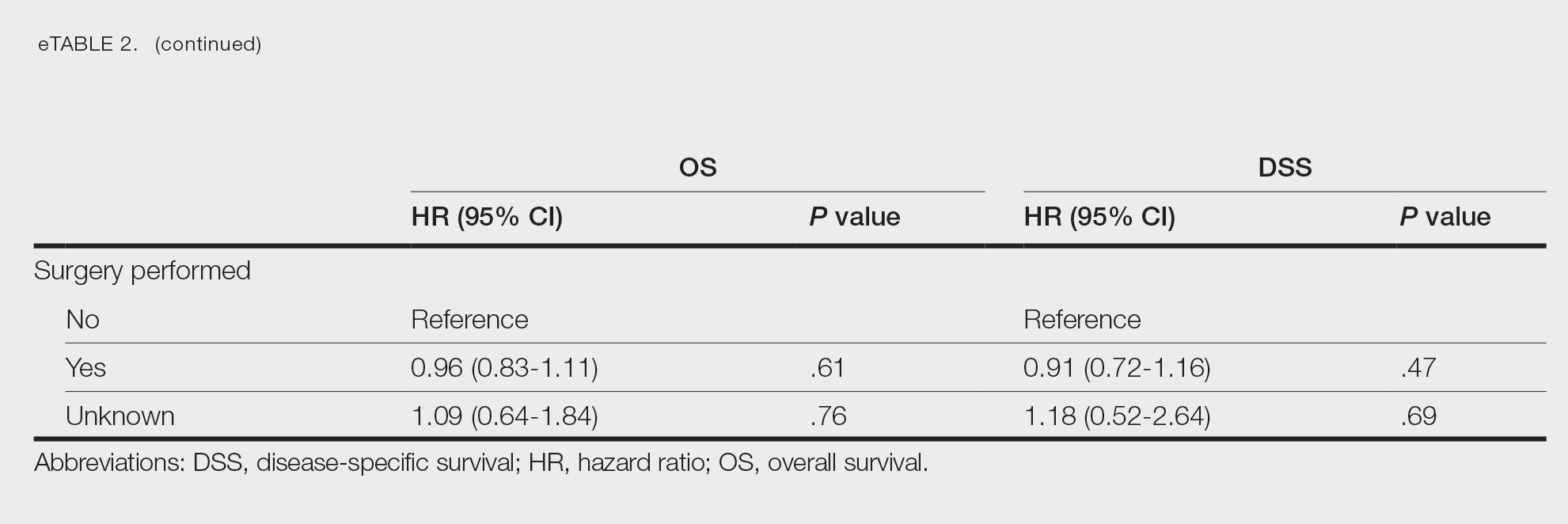
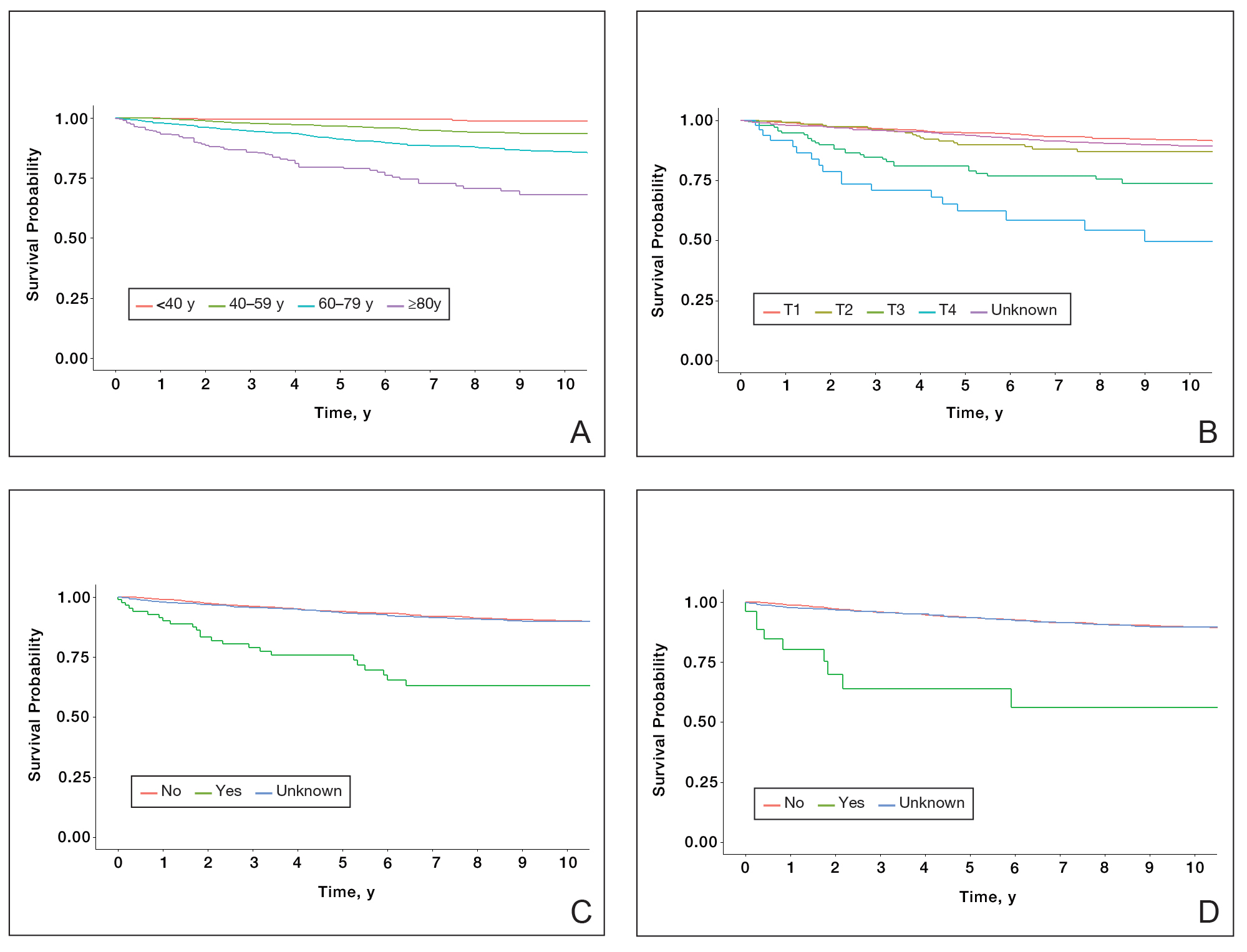
Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).
For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).
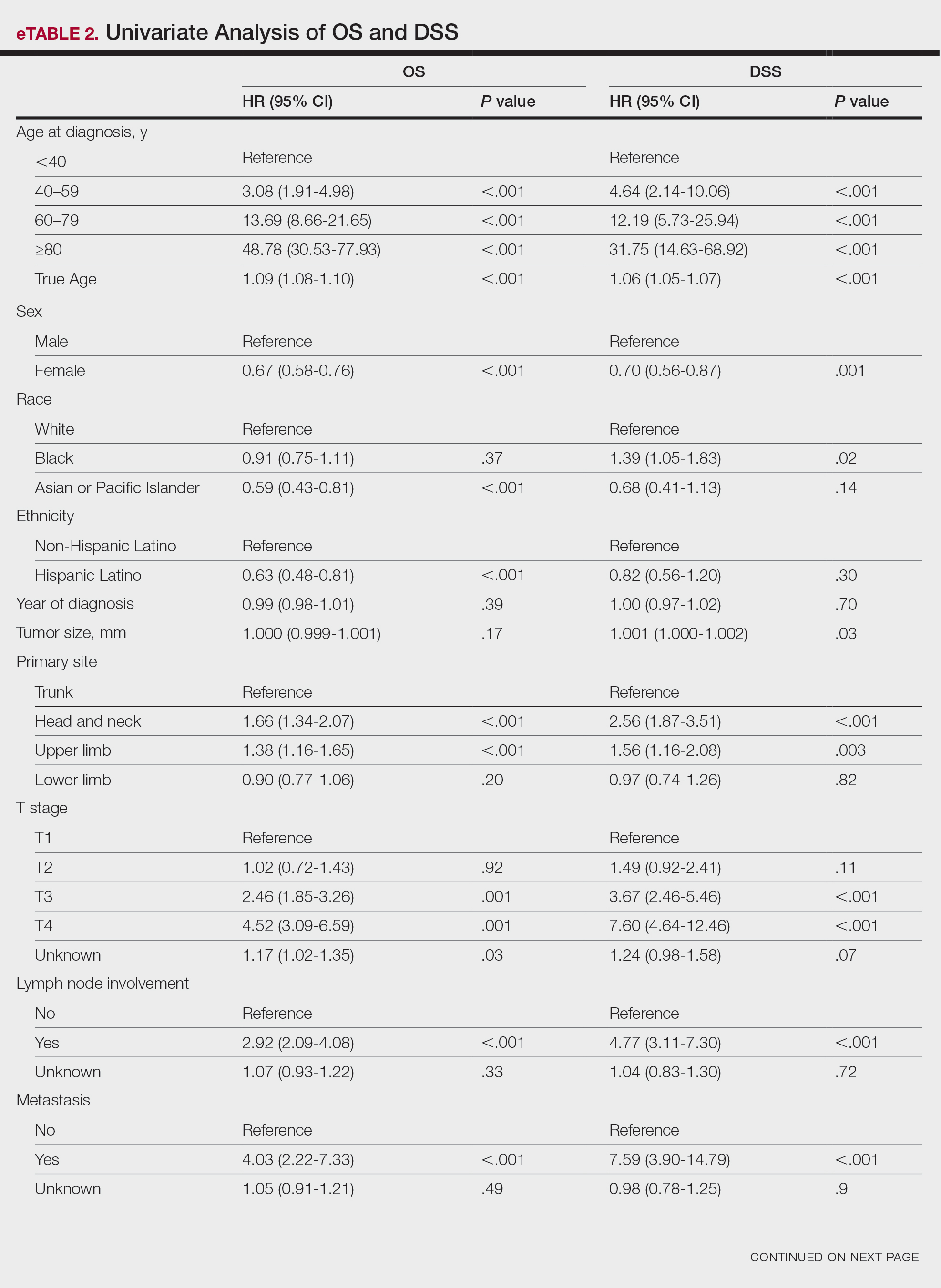
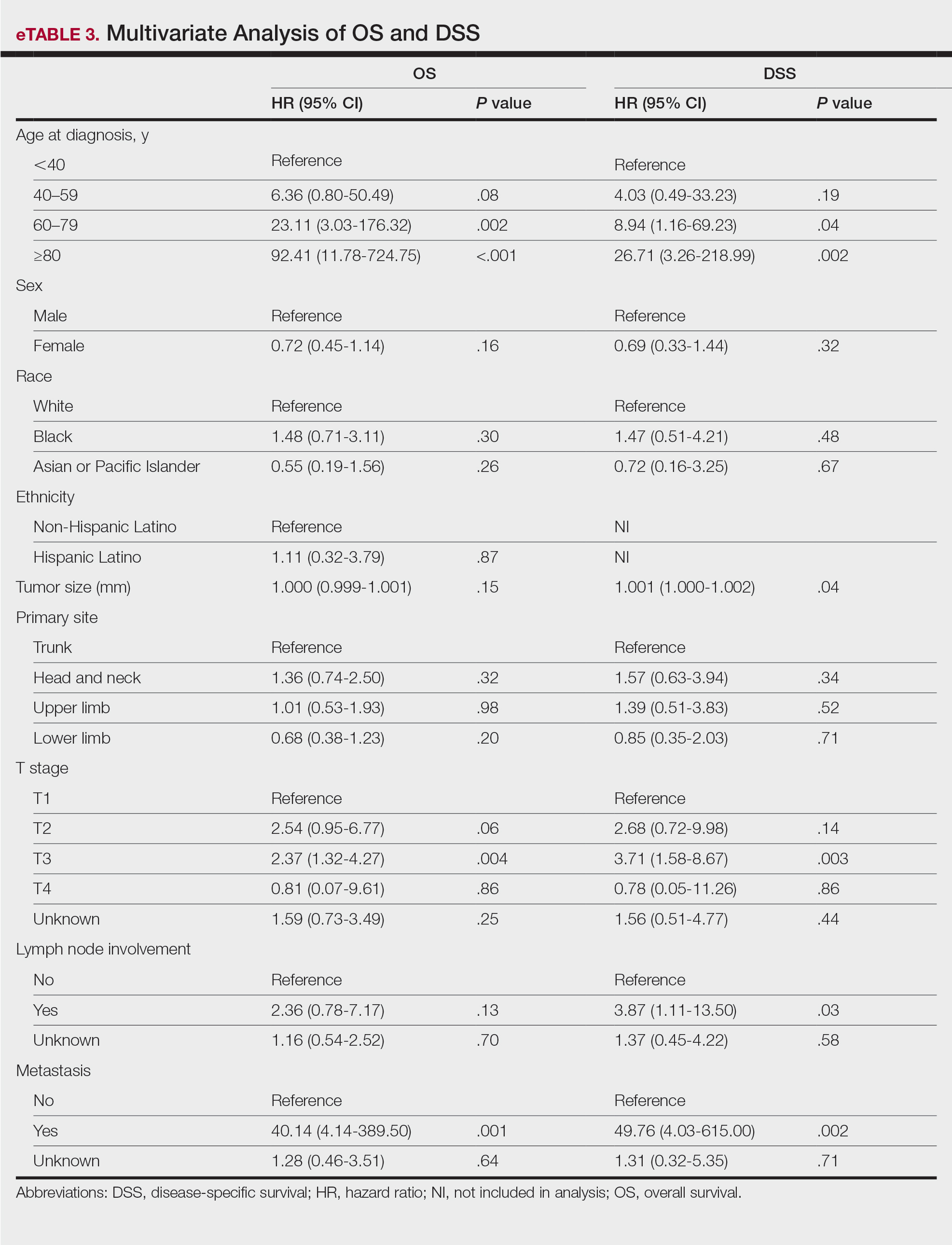
Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).
Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Practice Points
- Mycosis fungoides (MF) is the most common cutaneous T-cell lymphoma.
- Because MF is associated with diagnostic challenges due to its indolent course, data regarding primary tumor site as a prognostic factor are limited.
- Although MF originating from the head and neck region did not appear to influence survival, it was found that patients who were older or who had a larger tumor size at diagnosis, a higher T stage, lymph node involvement, or presence of metastasis had poorer survival overall and may benefit from additional counseling regarding their prognosis.
Virtual Reality Brings Relief to Hospitalized Patients With Cancer
suggests a new randomized controlled trial.
While both interventions brought some pain relief, VR therapy yielded greater, longer-lasting comfort, reported lead author Hunter Groninger, MD, of MedStar Health Research Institute, Hyattsville, Maryland, and colleagues.
“Investigators have explored immersive VR interventions in cancer populations for a variety of indications including anxiety, depression, fatigue, and procedure‐associated pain, particularly among patients with pediatric cancer and adult breast cancer,” the investigators wrote in Cancer. “Nevertheless, despite growing evidence supporting the efficacy of VR‐delivered interventions for analgesia, few data address its role to mitigate cancer‐related pain specifically.”
To address this knowledge gap, Dr. Groninger and colleagues enrolled 128 adult hospitalized patients with cancer of any kind, all of whom had moderate to severe pain (self-reported score at least 4 out of 10) within the past 24 hours.
Study Methods and Results
Patients were randomized to receive either 10 minutes of immersive VR distraction therapy or 10 minutes of two-dimensional guided imagery distraction therapy.
“[The VR therapy] provides noncompetitive experiences in which the user can move around and explore natural environments (e.g., beachscape, forest) from standing, seated, or fixed positions, including within a hospital bed or chair,” the investigators wrote. “We provided over‐the‐ear headphones to assure high sound quality for the experience in the virtual natural environment.”
The two-dimensional intervention, delivered via electronic tablet, featured a meditation with images of natural landscapes and instrumental background music.
“We chose this active control because it is readily available and reflects content similar to relaxation‐focused television channels that are increasingly common in hospital settings,” the investigators noted.
Compared with this more common approach, patients who received VR therapy had significantly greater immediate reduction in pain (mean change in pain score, –1.4 vs –0.7; P = .03). Twenty-four hours later, improvements in the VR group generally persisted, while pain level in the two-dimensional group returned almost to baseline (P = .004). In addition, patients in the VR group reported significantly greater improvements in general distress and pain bothersomeness.
“VR therapies may modulate the pain experience by reducing the level of attention paid to noxious stimuli, thereby suppressing transmission of painful sensations via pain processing pathways to the cerebral cortex, particularly with more active VR experiences compared to passive experiences,” the investigators wrote.
Downsides to Using VR
Although VR brought more benefit, participants in the VR group more often reported difficulty using the intervention compared with those who interacted with an electronic tablet.
Plus, one VR user described mild dizziness that resolved with pharmacologic intervention. Still, approximately 9 out of 10 participants in each group reported willingness to try the intervention again.
Future VR Research
“Virtual reality is a rapidly evolving technology with a wealth of potential patient‐facing applications,” the investigators wrote. “Future studies should explore repeated use, optimal dosing, and impact on VR therapy on opioid analgesic requirements as well as usability testing, VR content preferences and facilitators of analgesia, and barriers and facilitators to use in acute care settings.”
This study was supported by the American Cancer Society. The investigators disclosed no conflicts of interest.
suggests a new randomized controlled trial.
While both interventions brought some pain relief, VR therapy yielded greater, longer-lasting comfort, reported lead author Hunter Groninger, MD, of MedStar Health Research Institute, Hyattsville, Maryland, and colleagues.
“Investigators have explored immersive VR interventions in cancer populations for a variety of indications including anxiety, depression, fatigue, and procedure‐associated pain, particularly among patients with pediatric cancer and adult breast cancer,” the investigators wrote in Cancer. “Nevertheless, despite growing evidence supporting the efficacy of VR‐delivered interventions for analgesia, few data address its role to mitigate cancer‐related pain specifically.”
To address this knowledge gap, Dr. Groninger and colleagues enrolled 128 adult hospitalized patients with cancer of any kind, all of whom had moderate to severe pain (self-reported score at least 4 out of 10) within the past 24 hours.
Study Methods and Results
Patients were randomized to receive either 10 minutes of immersive VR distraction therapy or 10 minutes of two-dimensional guided imagery distraction therapy.
“[The VR therapy] provides noncompetitive experiences in which the user can move around and explore natural environments (e.g., beachscape, forest) from standing, seated, or fixed positions, including within a hospital bed or chair,” the investigators wrote. “We provided over‐the‐ear headphones to assure high sound quality for the experience in the virtual natural environment.”
The two-dimensional intervention, delivered via electronic tablet, featured a meditation with images of natural landscapes and instrumental background music.
“We chose this active control because it is readily available and reflects content similar to relaxation‐focused television channels that are increasingly common in hospital settings,” the investigators noted.
Compared with this more common approach, patients who received VR therapy had significantly greater immediate reduction in pain (mean change in pain score, –1.4 vs –0.7; P = .03). Twenty-four hours later, improvements in the VR group generally persisted, while pain level in the two-dimensional group returned almost to baseline (P = .004). In addition, patients in the VR group reported significantly greater improvements in general distress and pain bothersomeness.
“VR therapies may modulate the pain experience by reducing the level of attention paid to noxious stimuli, thereby suppressing transmission of painful sensations via pain processing pathways to the cerebral cortex, particularly with more active VR experiences compared to passive experiences,” the investigators wrote.
Downsides to Using VR
Although VR brought more benefit, participants in the VR group more often reported difficulty using the intervention compared with those who interacted with an electronic tablet.
Plus, one VR user described mild dizziness that resolved with pharmacologic intervention. Still, approximately 9 out of 10 participants in each group reported willingness to try the intervention again.
Future VR Research
“Virtual reality is a rapidly evolving technology with a wealth of potential patient‐facing applications,” the investigators wrote. “Future studies should explore repeated use, optimal dosing, and impact on VR therapy on opioid analgesic requirements as well as usability testing, VR content preferences and facilitators of analgesia, and barriers and facilitators to use in acute care settings.”
This study was supported by the American Cancer Society. The investigators disclosed no conflicts of interest.
suggests a new randomized controlled trial.
While both interventions brought some pain relief, VR therapy yielded greater, longer-lasting comfort, reported lead author Hunter Groninger, MD, of MedStar Health Research Institute, Hyattsville, Maryland, and colleagues.
“Investigators have explored immersive VR interventions in cancer populations for a variety of indications including anxiety, depression, fatigue, and procedure‐associated pain, particularly among patients with pediatric cancer and adult breast cancer,” the investigators wrote in Cancer. “Nevertheless, despite growing evidence supporting the efficacy of VR‐delivered interventions for analgesia, few data address its role to mitigate cancer‐related pain specifically.”
To address this knowledge gap, Dr. Groninger and colleagues enrolled 128 adult hospitalized patients with cancer of any kind, all of whom had moderate to severe pain (self-reported score at least 4 out of 10) within the past 24 hours.
Study Methods and Results
Patients were randomized to receive either 10 minutes of immersive VR distraction therapy or 10 minutes of two-dimensional guided imagery distraction therapy.
“[The VR therapy] provides noncompetitive experiences in which the user can move around and explore natural environments (e.g., beachscape, forest) from standing, seated, or fixed positions, including within a hospital bed or chair,” the investigators wrote. “We provided over‐the‐ear headphones to assure high sound quality for the experience in the virtual natural environment.”
The two-dimensional intervention, delivered via electronic tablet, featured a meditation with images of natural landscapes and instrumental background music.
“We chose this active control because it is readily available and reflects content similar to relaxation‐focused television channels that are increasingly common in hospital settings,” the investigators noted.
Compared with this more common approach, patients who received VR therapy had significantly greater immediate reduction in pain (mean change in pain score, –1.4 vs –0.7; P = .03). Twenty-four hours later, improvements in the VR group generally persisted, while pain level in the two-dimensional group returned almost to baseline (P = .004). In addition, patients in the VR group reported significantly greater improvements in general distress and pain bothersomeness.
“VR therapies may modulate the pain experience by reducing the level of attention paid to noxious stimuli, thereby suppressing transmission of painful sensations via pain processing pathways to the cerebral cortex, particularly with more active VR experiences compared to passive experiences,” the investigators wrote.
Downsides to Using VR
Although VR brought more benefit, participants in the VR group more often reported difficulty using the intervention compared with those who interacted with an electronic tablet.
Plus, one VR user described mild dizziness that resolved with pharmacologic intervention. Still, approximately 9 out of 10 participants in each group reported willingness to try the intervention again.
Future VR Research
“Virtual reality is a rapidly evolving technology with a wealth of potential patient‐facing applications,” the investigators wrote. “Future studies should explore repeated use, optimal dosing, and impact on VR therapy on opioid analgesic requirements as well as usability testing, VR content preferences and facilitators of analgesia, and barriers and facilitators to use in acute care settings.”
This study was supported by the American Cancer Society. The investigators disclosed no conflicts of interest.
FROM CANCER
Should Opioids Be Used for Chronic Cancer Pain?
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
FROM CANCER
A Banned Chemical That Is Still Causing Cancer
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Active Surveillance for Cancer Doesn’t Increase Malpractice Risk
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
Florida Legislature Passes Free Skin Cancer Screening Requirement
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
Few Childhood Cancer Survivors Get Recommended Screenings
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
AI in Clinical Dermatology: Consider Limitations, Current Issues
SAN DIEGO — Just a day before the annual meeting of the American Academy of Dermatology (AAD) began, .
Not least of the problems among the 41 apps evaluated, the majority offered no supporting evidence, no information about whether the app performance had been validated, and no information about how user privacy would be managed, reported Shannon Wongvibulsin, MD, PhD, a resident in the dermatology program at the University of California, Los Angeles, and her coauthors.
The findings from this report were also summarized in a poster at the AAD meeting, and the major themes were reiterated in several AAD symposia devoted to AI at the meeting. Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York City, was one of those who weighed in on the future of AI. Although she was the senior author of the report, she did not address the report or poster directly, but her presentation on the practical aspects of incorporating AI into dermatology practice revisited several of its themes.
Of the different themes, perhaps the most important were the concept that the source of AI data matters and the point that practicing clinicians should be familiar with the data source.
To date, “there is not much transparency in what data AI models are using,” Dr. Rotemberg said at the meeting. Based on the expectation that dermatologists will be purchasing rather than developing their own AI-based systems, she reiterated more than once that “transparency of data is critical,” even if vendors are often reluctant to reveal how their proprietary systems have been developed.
Few Dermatology Apps Are Vetted for Accuracy
In the poster and in the more detailed JAMA Dermatology paper, Dr. Wongvibulsin and her coinvestigators evaluated direct-to-consumer downloadable apps that claim to help with the assessment and management of skin conditions. Very few provided any supporting evidence of accuracy or even information about how they functioned.
The 41 apps were drawn from more than 300 apps; the others were excluded for failing to meet such criteria as failing to employ AI, not being available in English, or not addressing clinical management of dermatologic diseases. Dr. Wongvibulsin pointed out that none of the apps had been granted regulatory approval even though only two provided a disclaimer to that effect.
In all, just 5 of the 41 provided supporting evidence from a peer-reviewed journal, and less than 40% were created with any input from a dermatologist, Dr. Wongvibulsin reported. The result is that the utility and accuracy of these apps were, for the most part, difficult to judge.
“At a minimum, app developers should provide details on what AI algorithms are used, what data sets were used for training, testing, and validation, whether there was any clinician input, whether there are any supporting publications, how user-submitted images are used, and if there are any measures used to ensure data privacy,” Dr. Wongvibulsin wrote in the poster.
For AI-based apps or systems designed for use by dermatologists, Dr. Rotemberg made similar assertions in her overview of what clinicians should be considering for proprietary AI systems, whether to help with diagnosis or improve office efficiency.
Only One Dermatology App Cleared By the FDA
Currently, the only FDA-cleared app for dermatologic use is the DermaSensor, an AI-driven device. It was cleared for use in January 2024 for the evaluation of skin lesions “suggestive” of melanoma, basal cell carcinoma, and/or squamous cell carcinoma in patients aged ≥ 40 years “to assist health care providers in determining whether to refer a patient to a dermatologist,” according to an FDA announcement.
Using elastic scattering spectroscopy to analyze light reflecting off the skin to detect malignancy, the manufacturer’s promotional material claims a 96% sensitivity and a 97% specificity.
While Dr. Rotemberg did not comment on these claims, she cautioned that AI models differ with regards to how they were trained and the relative heterogeneity of the training dataset defined by types of patients, types of skin, and types of AI learning processes. All of these variables are relevant in whether the AI will perform in a given clinical setting at the level it performed during development.
“The most accurate models employ narrow datasets, but these do not necessarily mimic what we see in practice,” she said.
In addition, even when an AI-based system is working for a given task, it must be monitored over time. Dr. Rotemberg warned about the potential for “data drift,” which describes the slow evolution in how diseases present, their prevalence by age, or other factors that might affect AI performance. She explained that repeated validation is needed to ensure that the AI-based models remain as accurate over time as they were when first used.
Many of these concepts were explored in a consensus statement from the International Skin Imaging Collaboration AI Working Group, published in JAMA Dermatology in December 2021. The statement, of which Dr. Rotemberg was a coauthor, provided recommendations for the principles of AI algorithm development specific to dermatologic considerations.
At the AAD symposium, Dr. Rotemberg asked the audience for suggestions about the needs they hoped AI might address for in office care or efficiency. Their responses included generating prior authorizations for prescriptions, triaging email for importance, and helping to improve efficiency for common front desk tasks. She liked all of these suggestions, but she warned that as powerful as it can be, AI is not likely to provide technology that will fit seamlessly into workflows without adjustment.
“Our current systems do not allow human integration of AI models,” Dr. Rotemberg said. Rather than counting on AI to adapt to current practices, she cautioned that “we may have to redesign our entire structure to actually be able to accommodate AI-based” systems. The risk for users is tasks that become more challenging before they become easier.
AI Should Not Be a Black Box
AI is promising, but it is not magic, according to other investigators, including Tofunmi A. Omiye, PhD, a postdoctoral scholar in dermatology at Stanford University, California. First author of a recent review of AI in dermatology published in Frontiers in Medicine, Dr. Omiye agreed that clinicians who want to employ AI should be able to understand basic principles if they want the technology to perform as expected.
“I totally agree that physicians should at least have a basic understanding of the data sources for training AI models as we have found that to be important to the performance of these models in the clinical setting,” he told this news organization.
“Beyond understanding the data sources, I believe physicians can also try to have a comprehensive understanding of what AI means, its training process, and evaluation as this will help them to evaluate its utility in their practice,” he added. He also reinforced the relevance of data drift.
“Concepts like distribution shift — where models perform less well over time due to changes in the patient population — are also important to keep in mind,” Dr. Omiye said.
Dr. Wongvibulsin, Dr. Rotemberg, and Dr. Omiye reported no potential financial conflicts of interest relevant to this topic.
A version of this article appeared on Medscape.com .
SAN DIEGO — Just a day before the annual meeting of the American Academy of Dermatology (AAD) began, .
Not least of the problems among the 41 apps evaluated, the majority offered no supporting evidence, no information about whether the app performance had been validated, and no information about how user privacy would be managed, reported Shannon Wongvibulsin, MD, PhD, a resident in the dermatology program at the University of California, Los Angeles, and her coauthors.
The findings from this report were also summarized in a poster at the AAD meeting, and the major themes were reiterated in several AAD symposia devoted to AI at the meeting. Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York City, was one of those who weighed in on the future of AI. Although she was the senior author of the report, she did not address the report or poster directly, but her presentation on the practical aspects of incorporating AI into dermatology practice revisited several of its themes.
Of the different themes, perhaps the most important were the concept that the source of AI data matters and the point that practicing clinicians should be familiar with the data source.
To date, “there is not much transparency in what data AI models are using,” Dr. Rotemberg said at the meeting. Based on the expectation that dermatologists will be purchasing rather than developing their own AI-based systems, she reiterated more than once that “transparency of data is critical,” even if vendors are often reluctant to reveal how their proprietary systems have been developed.
Few Dermatology Apps Are Vetted for Accuracy
In the poster and in the more detailed JAMA Dermatology paper, Dr. Wongvibulsin and her coinvestigators evaluated direct-to-consumer downloadable apps that claim to help with the assessment and management of skin conditions. Very few provided any supporting evidence of accuracy or even information about how they functioned.
The 41 apps were drawn from more than 300 apps; the others were excluded for failing to meet such criteria as failing to employ AI, not being available in English, or not addressing clinical management of dermatologic diseases. Dr. Wongvibulsin pointed out that none of the apps had been granted regulatory approval even though only two provided a disclaimer to that effect.
In all, just 5 of the 41 provided supporting evidence from a peer-reviewed journal, and less than 40% were created with any input from a dermatologist, Dr. Wongvibulsin reported. The result is that the utility and accuracy of these apps were, for the most part, difficult to judge.
“At a minimum, app developers should provide details on what AI algorithms are used, what data sets were used for training, testing, and validation, whether there was any clinician input, whether there are any supporting publications, how user-submitted images are used, and if there are any measures used to ensure data privacy,” Dr. Wongvibulsin wrote in the poster.
For AI-based apps or systems designed for use by dermatologists, Dr. Rotemberg made similar assertions in her overview of what clinicians should be considering for proprietary AI systems, whether to help with diagnosis or improve office efficiency.
Only One Dermatology App Cleared By the FDA
Currently, the only FDA-cleared app for dermatologic use is the DermaSensor, an AI-driven device. It was cleared for use in January 2024 for the evaluation of skin lesions “suggestive” of melanoma, basal cell carcinoma, and/or squamous cell carcinoma in patients aged ≥ 40 years “to assist health care providers in determining whether to refer a patient to a dermatologist,” according to an FDA announcement.
Using elastic scattering spectroscopy to analyze light reflecting off the skin to detect malignancy, the manufacturer’s promotional material claims a 96% sensitivity and a 97% specificity.
While Dr. Rotemberg did not comment on these claims, she cautioned that AI models differ with regards to how they were trained and the relative heterogeneity of the training dataset defined by types of patients, types of skin, and types of AI learning processes. All of these variables are relevant in whether the AI will perform in a given clinical setting at the level it performed during development.
“The most accurate models employ narrow datasets, but these do not necessarily mimic what we see in practice,” she said.
In addition, even when an AI-based system is working for a given task, it must be monitored over time. Dr. Rotemberg warned about the potential for “data drift,” which describes the slow evolution in how diseases present, their prevalence by age, or other factors that might affect AI performance. She explained that repeated validation is needed to ensure that the AI-based models remain as accurate over time as they were when first used.
Many of these concepts were explored in a consensus statement from the International Skin Imaging Collaboration AI Working Group, published in JAMA Dermatology in December 2021. The statement, of which Dr. Rotemberg was a coauthor, provided recommendations for the principles of AI algorithm development specific to dermatologic considerations.
At the AAD symposium, Dr. Rotemberg asked the audience for suggestions about the needs they hoped AI might address for in office care or efficiency. Their responses included generating prior authorizations for prescriptions, triaging email for importance, and helping to improve efficiency for common front desk tasks. She liked all of these suggestions, but she warned that as powerful as it can be, AI is not likely to provide technology that will fit seamlessly into workflows without adjustment.
“Our current systems do not allow human integration of AI models,” Dr. Rotemberg said. Rather than counting on AI to adapt to current practices, she cautioned that “we may have to redesign our entire structure to actually be able to accommodate AI-based” systems. The risk for users is tasks that become more challenging before they become easier.
AI Should Not Be a Black Box
AI is promising, but it is not magic, according to other investigators, including Tofunmi A. Omiye, PhD, a postdoctoral scholar in dermatology at Stanford University, California. First author of a recent review of AI in dermatology published in Frontiers in Medicine, Dr. Omiye agreed that clinicians who want to employ AI should be able to understand basic principles if they want the technology to perform as expected.
“I totally agree that physicians should at least have a basic understanding of the data sources for training AI models as we have found that to be important to the performance of these models in the clinical setting,” he told this news organization.
“Beyond understanding the data sources, I believe physicians can also try to have a comprehensive understanding of what AI means, its training process, and evaluation as this will help them to evaluate its utility in their practice,” he added. He also reinforced the relevance of data drift.
“Concepts like distribution shift — where models perform less well over time due to changes in the patient population — are also important to keep in mind,” Dr. Omiye said.
Dr. Wongvibulsin, Dr. Rotemberg, and Dr. Omiye reported no potential financial conflicts of interest relevant to this topic.
A version of this article appeared on Medscape.com .
SAN DIEGO — Just a day before the annual meeting of the American Academy of Dermatology (AAD) began, .
Not least of the problems among the 41 apps evaluated, the majority offered no supporting evidence, no information about whether the app performance had been validated, and no information about how user privacy would be managed, reported Shannon Wongvibulsin, MD, PhD, a resident in the dermatology program at the University of California, Los Angeles, and her coauthors.
The findings from this report were also summarized in a poster at the AAD meeting, and the major themes were reiterated in several AAD symposia devoted to AI at the meeting. Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York City, was one of those who weighed in on the future of AI. Although she was the senior author of the report, she did not address the report or poster directly, but her presentation on the practical aspects of incorporating AI into dermatology practice revisited several of its themes.
Of the different themes, perhaps the most important were the concept that the source of AI data matters and the point that practicing clinicians should be familiar with the data source.
To date, “there is not much transparency in what data AI models are using,” Dr. Rotemberg said at the meeting. Based on the expectation that dermatologists will be purchasing rather than developing their own AI-based systems, she reiterated more than once that “transparency of data is critical,” even if vendors are often reluctant to reveal how their proprietary systems have been developed.
Few Dermatology Apps Are Vetted for Accuracy
In the poster and in the more detailed JAMA Dermatology paper, Dr. Wongvibulsin and her coinvestigators evaluated direct-to-consumer downloadable apps that claim to help with the assessment and management of skin conditions. Very few provided any supporting evidence of accuracy or even information about how they functioned.
The 41 apps were drawn from more than 300 apps; the others were excluded for failing to meet such criteria as failing to employ AI, not being available in English, or not addressing clinical management of dermatologic diseases. Dr. Wongvibulsin pointed out that none of the apps had been granted regulatory approval even though only two provided a disclaimer to that effect.
In all, just 5 of the 41 provided supporting evidence from a peer-reviewed journal, and less than 40% were created with any input from a dermatologist, Dr. Wongvibulsin reported. The result is that the utility and accuracy of these apps were, for the most part, difficult to judge.
“At a minimum, app developers should provide details on what AI algorithms are used, what data sets were used for training, testing, and validation, whether there was any clinician input, whether there are any supporting publications, how user-submitted images are used, and if there are any measures used to ensure data privacy,” Dr. Wongvibulsin wrote in the poster.
For AI-based apps or systems designed for use by dermatologists, Dr. Rotemberg made similar assertions in her overview of what clinicians should be considering for proprietary AI systems, whether to help with diagnosis or improve office efficiency.
Only One Dermatology App Cleared By the FDA
Currently, the only FDA-cleared app for dermatologic use is the DermaSensor, an AI-driven device. It was cleared for use in January 2024 for the evaluation of skin lesions “suggestive” of melanoma, basal cell carcinoma, and/or squamous cell carcinoma in patients aged ≥ 40 years “to assist health care providers in determining whether to refer a patient to a dermatologist,” according to an FDA announcement.
Using elastic scattering spectroscopy to analyze light reflecting off the skin to detect malignancy, the manufacturer’s promotional material claims a 96% sensitivity and a 97% specificity.
While Dr. Rotemberg did not comment on these claims, she cautioned that AI models differ with regards to how they were trained and the relative heterogeneity of the training dataset defined by types of patients, types of skin, and types of AI learning processes. All of these variables are relevant in whether the AI will perform in a given clinical setting at the level it performed during development.
“The most accurate models employ narrow datasets, but these do not necessarily mimic what we see in practice,” she said.
In addition, even when an AI-based system is working for a given task, it must be monitored over time. Dr. Rotemberg warned about the potential for “data drift,” which describes the slow evolution in how diseases present, their prevalence by age, or other factors that might affect AI performance. She explained that repeated validation is needed to ensure that the AI-based models remain as accurate over time as they were when first used.
Many of these concepts were explored in a consensus statement from the International Skin Imaging Collaboration AI Working Group, published in JAMA Dermatology in December 2021. The statement, of which Dr. Rotemberg was a coauthor, provided recommendations for the principles of AI algorithm development specific to dermatologic considerations.
At the AAD symposium, Dr. Rotemberg asked the audience for suggestions about the needs they hoped AI might address for in office care or efficiency. Their responses included generating prior authorizations for prescriptions, triaging email for importance, and helping to improve efficiency for common front desk tasks. She liked all of these suggestions, but she warned that as powerful as it can be, AI is not likely to provide technology that will fit seamlessly into workflows without adjustment.
“Our current systems do not allow human integration of AI models,” Dr. Rotemberg said. Rather than counting on AI to adapt to current practices, she cautioned that “we may have to redesign our entire structure to actually be able to accommodate AI-based” systems. The risk for users is tasks that become more challenging before they become easier.
AI Should Not Be a Black Box
AI is promising, but it is not magic, according to other investigators, including Tofunmi A. Omiye, PhD, a postdoctoral scholar in dermatology at Stanford University, California. First author of a recent review of AI in dermatology published in Frontiers in Medicine, Dr. Omiye agreed that clinicians who want to employ AI should be able to understand basic principles if they want the technology to perform as expected.
“I totally agree that physicians should at least have a basic understanding of the data sources for training AI models as we have found that to be important to the performance of these models in the clinical setting,” he told this news organization.
“Beyond understanding the data sources, I believe physicians can also try to have a comprehensive understanding of what AI means, its training process, and evaluation as this will help them to evaluate its utility in their practice,” he added. He also reinforced the relevance of data drift.
“Concepts like distribution shift — where models perform less well over time due to changes in the patient population — are also important to keep in mind,” Dr. Omiye said.
Dr. Wongvibulsin, Dr. Rotemberg, and Dr. Omiye reported no potential financial conflicts of interest relevant to this topic.
A version of this article appeared on Medscape.com .
FROM AAD 2024
Most Cancer Trial Centers Located Closer to White, Affluent Populations
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
FROM JAMA ONCOLOGY
New Drug Approvals Are the Wrong Metric for Cancer Policy
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.

