User login
Diabetic dyslipidemia with eruptive xanthoma
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
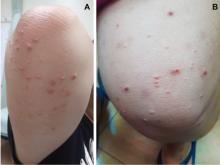
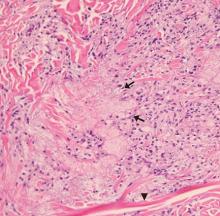
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
Weight loss surgery linked to lower CV event risk in diabetes
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
AT THE ESC CONGRESS 2019
Key clinical point: Bariatric surgery may reduce the risk of cardiovascular events in people with type 2 diabetes.
Major finding: Bariatric surgery is associated with a 39% reduction in risk of major cardiovascular events.
Study details: Retrospective cohort study in 13,722 individuals with type 2 diabetes and obesity.
Disclosures: The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
Source: Aminian A et al. JAMA 2019, September 2. DOI: 10.1001/jama.2019.14231.
Mediterranean diet tied to improved cognition in type 2 diabetes
People with type 2 diabetes whose diet followed a “Mediterranean” pattern – high in vegetables, legumes, fish, and unsaturated fats – saw global cognitive improvements over a 2-year period, compared with individuals with different eating patterns, even if the latter incorporated healthy dietary features. In addition, effective glycemic control seemed to have a role in sustaining the benefits associated with the Mediterranean-type diet.
Adults without type 2 diabetes, meanwhile, did not see the cognitive improvements associated with a Mediterranean diet, suggesting that the pathways linking diet to cognition may be different for individuals with and without diabetes, according to Josiemer Mattei, PhD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The investigators used data from the Boston Puerto Rican Health Study, a longitudinal cohort of about 1,499 adults aged 45-75 years who lived in Boston and identified as Puerto Rican, for their research, which was published in Diabetes Care.
At baseline, participants were administered a questionnaire to capture their eating patterns. Four diet-quality scores – Mediterranean Diet Score, Healthy Eating Index, Alternate Healthy Eating Index, and DASH (Dietary Approaches to Stop Hypertension) were analyzed. The participants were also screened for diabetes, and nearly 40% of them were found to have type 2 diabetes at baseline (74% uncontrolled). They underwent a battery of cognitive tests, including the Mini-Mental State Exam and tests for verbal fluency, executive function, word recognition, and figure copying. The study endpoints included 2-year change in global cognitive function as well as executive and memory function. At 2 years, data was available for 913 participants.
Among participants with type 2 diabetes, greater adherence to a Mediterranean-style diet was significantly associated with a higher positive change at the 2-year follow-up in global cognitive function score (0.027 [SD, 0.011]; P = .016), the Mini-Mental State Exam, and other individual tests. The association was significant for those who were under glycemic control at baseline and who remained stable or improved over 2 years, but not for those with poor or worsening glycemic control.
“The Mediterranean diet explained as much or more of the variability in predicting changes in cognitive function in our study as did age, especially for participants with type 2 diabetes under glycemic control. ... This dietary pattern may provide more cognitive benefits [in this patient group] than other modifiable and nonmodifiable factors,” the authors wrote in their analysis. They stressed that a Mediterranean dietary pattern can be realized through foods and dishes that are already standard in many Puerto Rican households.
In participants who did not have diabetes, improvement in memory function measures was seen in association with a Mediterranean diet, but also with adherence to other eating patterns that are deemed healthy. That suggests that for this subgroup, any evidence-based healthy diet – not just the Mediterranean diet – may have some benefits for memory function.
“Dietary recommendations for cognitive health may need to be tailored for individuals with versus without type 2 diabetes,” the authors concluded.
Dr. Mattei and colleagues acknowledged as a limitation of their study its observational design.
The study received funding from the National Heart, Lung, and Blood Institute; the National Institute on Aging; and Harvard University. The authors reported no financial conflicts of interest.
SOURCE: Mattei et al. Diabetes Care. 2019;42(8):1372-9.
People with type 2 diabetes whose diet followed a “Mediterranean” pattern – high in vegetables, legumes, fish, and unsaturated fats – saw global cognitive improvements over a 2-year period, compared with individuals with different eating patterns, even if the latter incorporated healthy dietary features. In addition, effective glycemic control seemed to have a role in sustaining the benefits associated with the Mediterranean-type diet.
Adults without type 2 diabetes, meanwhile, did not see the cognitive improvements associated with a Mediterranean diet, suggesting that the pathways linking diet to cognition may be different for individuals with and without diabetes, according to Josiemer Mattei, PhD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The investigators used data from the Boston Puerto Rican Health Study, a longitudinal cohort of about 1,499 adults aged 45-75 years who lived in Boston and identified as Puerto Rican, for their research, which was published in Diabetes Care.
At baseline, participants were administered a questionnaire to capture their eating patterns. Four diet-quality scores – Mediterranean Diet Score, Healthy Eating Index, Alternate Healthy Eating Index, and DASH (Dietary Approaches to Stop Hypertension) were analyzed. The participants were also screened for diabetes, and nearly 40% of them were found to have type 2 diabetes at baseline (74% uncontrolled). They underwent a battery of cognitive tests, including the Mini-Mental State Exam and tests for verbal fluency, executive function, word recognition, and figure copying. The study endpoints included 2-year change in global cognitive function as well as executive and memory function. At 2 years, data was available for 913 participants.
Among participants with type 2 diabetes, greater adherence to a Mediterranean-style diet was significantly associated with a higher positive change at the 2-year follow-up in global cognitive function score (0.027 [SD, 0.011]; P = .016), the Mini-Mental State Exam, and other individual tests. The association was significant for those who were under glycemic control at baseline and who remained stable or improved over 2 years, but not for those with poor or worsening glycemic control.
“The Mediterranean diet explained as much or more of the variability in predicting changes in cognitive function in our study as did age, especially for participants with type 2 diabetes under glycemic control. ... This dietary pattern may provide more cognitive benefits [in this patient group] than other modifiable and nonmodifiable factors,” the authors wrote in their analysis. They stressed that a Mediterranean dietary pattern can be realized through foods and dishes that are already standard in many Puerto Rican households.
In participants who did not have diabetes, improvement in memory function measures was seen in association with a Mediterranean diet, but also with adherence to other eating patterns that are deemed healthy. That suggests that for this subgroup, any evidence-based healthy diet – not just the Mediterranean diet – may have some benefits for memory function.
“Dietary recommendations for cognitive health may need to be tailored for individuals with versus without type 2 diabetes,” the authors concluded.
Dr. Mattei and colleagues acknowledged as a limitation of their study its observational design.
The study received funding from the National Heart, Lung, and Blood Institute; the National Institute on Aging; and Harvard University. The authors reported no financial conflicts of interest.
SOURCE: Mattei et al. Diabetes Care. 2019;42(8):1372-9.
People with type 2 diabetes whose diet followed a “Mediterranean” pattern – high in vegetables, legumes, fish, and unsaturated fats – saw global cognitive improvements over a 2-year period, compared with individuals with different eating patterns, even if the latter incorporated healthy dietary features. In addition, effective glycemic control seemed to have a role in sustaining the benefits associated with the Mediterranean-type diet.
Adults without type 2 diabetes, meanwhile, did not see the cognitive improvements associated with a Mediterranean diet, suggesting that the pathways linking diet to cognition may be different for individuals with and without diabetes, according to Josiemer Mattei, PhD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The investigators used data from the Boston Puerto Rican Health Study, a longitudinal cohort of about 1,499 adults aged 45-75 years who lived in Boston and identified as Puerto Rican, for their research, which was published in Diabetes Care.
At baseline, participants were administered a questionnaire to capture their eating patterns. Four diet-quality scores – Mediterranean Diet Score, Healthy Eating Index, Alternate Healthy Eating Index, and DASH (Dietary Approaches to Stop Hypertension) were analyzed. The participants were also screened for diabetes, and nearly 40% of them were found to have type 2 diabetes at baseline (74% uncontrolled). They underwent a battery of cognitive tests, including the Mini-Mental State Exam and tests for verbal fluency, executive function, word recognition, and figure copying. The study endpoints included 2-year change in global cognitive function as well as executive and memory function. At 2 years, data was available for 913 participants.
Among participants with type 2 diabetes, greater adherence to a Mediterranean-style diet was significantly associated with a higher positive change at the 2-year follow-up in global cognitive function score (0.027 [SD, 0.011]; P = .016), the Mini-Mental State Exam, and other individual tests. The association was significant for those who were under glycemic control at baseline and who remained stable or improved over 2 years, but not for those with poor or worsening glycemic control.
“The Mediterranean diet explained as much or more of the variability in predicting changes in cognitive function in our study as did age, especially for participants with type 2 diabetes under glycemic control. ... This dietary pattern may provide more cognitive benefits [in this patient group] than other modifiable and nonmodifiable factors,” the authors wrote in their analysis. They stressed that a Mediterranean dietary pattern can be realized through foods and dishes that are already standard in many Puerto Rican households.
In participants who did not have diabetes, improvement in memory function measures was seen in association with a Mediterranean diet, but also with adherence to other eating patterns that are deemed healthy. That suggests that for this subgroup, any evidence-based healthy diet – not just the Mediterranean diet – may have some benefits for memory function.
“Dietary recommendations for cognitive health may need to be tailored for individuals with versus without type 2 diabetes,” the authors concluded.
Dr. Mattei and colleagues acknowledged as a limitation of their study its observational design.
The study received funding from the National Heart, Lung, and Blood Institute; the National Institute on Aging; and Harvard University. The authors reported no financial conflicts of interest.
SOURCE: Mattei et al. Diabetes Care. 2019;42(8):1372-9.
FROM DIABETES CARE
NAFLD unchecked is a ‘harbinger of deadly dysmetabolism’
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
EXPERT ANALYSIS FROM MEDS 2019
Beyond C. difficile: The future of fecal microbial transplantation
SAN DIEGO – Two leading figures in microbiome research took time during the annual Digestive Disease Week to share their perspective with members of the press. Identifying key research presented at the meeting and painting a broader picture of trends and challenges in research on the interplay with the microbiota with gut health, Colleen Kelly, MD, of Brown University, Providence, R.I., cochair and principal investigator, AGA FMT Registry Steering Committee, led off the round table with a discussion of human research on fecal microbial transplantation (FMT) and obesity.
Purna Kashyap, MBBS, of the Mayo Clinic, Rochester, Minn., member, AGA Center for Gut Microbiome Research and Education Scientific Advisory Board, delved into the potential for donor microbiota transplant to address small bowel disorders, such as small intestinal bacterial overgrowth, and provided commentary regarding the potential – and limitations – of using FMT in functional bowel disorders such as irritable bowel syndrome (IBS).
Obesity
Dr. Kelly noted that two abstracts at the conference presented data about FMT in obesity. The first study was presented by Jessica Allegretti, MD, a gastroenterologist at Brigham and Women’s Hospital, Boston; the second study was presented by Elaine Yu, MD, an endocrinologist at Massachusetts General Hospital, Boston.
The two studies shared some similarities, but had some differences, said Dr. Kelly. “They both used lean donor encapsulated FMT ... and they both were placebo controlled, using placebo capsules.” The first study looked at metabolically healthy obese patients, while the second study included patients with mild to moderate insulin resistance.
Dr. Allegretti’s work looked at the effect that FMT from lean donors had on levels of a satiety peptide, glucagonlike peptide–1 (GLP-1), while also looking at changes in weight and microbiota, as well as safety. Patients received an initial 30-capsule dose as well as two later doses of 12 capsules each. The 22-patient study, in which individuals were randomized 1:1 to FMT or placebo capsules, didn’t show statistically significant changes in GLP-1 levels or body mass index with FMT over the 12-week study period. “But they were able to show engraftment, which I think is an important thing that you do wonder about – over this period of time, the bacteria that came from the lean donor actually engrafted into the recipient and affected the diversity. The recipient became more similar to the donor,” said Dr. Kelly.
There were some clues among the findings that engraftment was effecting metabolic change in the recipient, she said, including differences in bile acid conversion among gut bacteria; also, lower levels of the primary bile acid taurocholic acid in recipients after FMT. “So it was a negative study in what she was looking for, but an example of these smaller studies kind of pushing the field along.”
In discussing the study presented by Dr. Yu that examined lean donor FMT in individuals with insulin resistance, Dr. Kelly said, “It was actually pretty sophisticated.” By using hyperinsulinemic euglycemic clamping, the investigators were able to measure insulin sensitivity based on glucose load. In this study of 24 individuals randomized 1:1 to receive FMT or placebo capsules, recipients received weekly doses over a period of 6 weeks. Here again, though Dr. Yu and colleagues again found engraftment, “they did not find the big changes in metabolic parameters that they hoped that they would,” said Dr. Kelly.
These two studies furthered previous work completed in the Netherlands examining lean donor FMT for individuals with metabolic syndrome. “Those [studies] did show both engraftment and some changes in insulin resistance,” but they were also small studies, noted Dr. Kelly. Dr. Kashyap pointed out that the earlier studies had shown in a subgroup analysis that response to FMT was limited to those patients who lacked microbial diversity pretransplant.
This makes some mechanistic sense when thinking about FMT’s greatest success to date: Treating Clostridioides difficile infection, a condition whose very hallmark is dysbiosis characterized by monospecies gut domination, noted Dr. Kashyap.
Added Dr. Kelly, “I think everyone’s hoping there’s going to be this pill that’s going to make us skinny, but I don’t think we’re going to find that with FMT and obesity. I do think these studies are important, because there’s so much animal data already, and we’re kind of like, ‘How much more can you do in mice?’ ” By translating this preclinical work into humans, the mechanisms of obesity and the role of the microbiome can be better understood.
IBS
Turning to functional bowel disorders, Dr. Kelly pointed out a new study examining FMT for IBS. “So far, the research has been pretty disappointing,” she noted. The study, presented by Prashant Singh, MBBS, a gastroenterology fellow at Beth Israel Deaconess Medical Center, Boston, examined FMT for patients with moderate to severe diarrhea-predominant IBS. The study had four arms, randomizing the 44 participants to FMT alone; pretreatment with metronidazole and ciprofloxacin or rifaximin, each followed by FMT; or placebo. “It really didn’t show any differences in their severity of IBS in any group; so no effect: a negative study,” said Dr. Kelly.
“It’s challenging. FMT is an appealing strategy because we don’t have to put a lot of thought process into it,” said Dr. Kashyap, but it’s no panacea. “We aren’t going to be able to treat everybody with FMT.
“The challenge with IBS is that if we look at all the compositional studies, the majority of them show that at least a big subset of patients with IBS already have a normal-appearing microbiome,” said Dr. Kashyap. In those patients, “It’s very hard to know what FMT is doing.” He noted that IBS subclassifications are made by pathophysiology, without regard to the intestinal microbiome, so these classifications may not be useful for determining who may benefit from FMT.
“Again, there’s always an opportunity to learn from these studies,” whether they’re positive or negative, as long as they’re well done, said Dr. Kashyap. “There’s always an opportunity to go back and see, was there a specific subgroup of patients who responded, where there might be one or more causes which might be more amenable” to FMT.
Small intestine
And most intestinal microbial research to date, noted Dr. Kelly, has focused on the colon. “Most of our knowledge is of fecal microbiota.” New techniques including double-balloon enteroscopy of the small bowel have promise to “provide completely new information about patterns of bacteria throughout the small bowel,” and of the role of small bowel bacteria in overall gut health, she said.
“The role of the small bowel has been ignored because of accessibility,” agreed Dr. Kashyap. There’s a current focus on research into enteroscopy and other techniques to sample small intestine microbiota, he said.
In a podium presentation, Eugene Chang, MD, Martin Boyer Professor in the University of Chicago’s department of medicine, gave a broad overview of how the small intestine microbiome modulates lipid regulation. This choice of topic for the Charles M. Mansbach Memorial Lecture shows that gastroenterologists are recognizing the importance of microbiome along the entire span of the gut, said Dr. Kashyap. Dr. Chang’s approach, he added, represents a departure in that “it’s not simply just looking at what’s present and what’s not, but seeing what’s functionally relevant to metabolism.”
“We always have known that the small intestine is the workhorse; that’s where everything happens” in terms of motility, absorption, and digestion, said Dr. Kashyap. “But because of our inability to reach it easily we’ve always chosen to ignore it; we always go after the low-hanging fruit.” Despite challenges, more microbiome research should be small-bowel focused. “Eventually, it’s no pain, no gain.”
Dr. Kashyap is on the advisory board of uBiome, and is an ad hoc advisory board member for Salix Pharmaceuticals. Dr. Kelly reported no conflicts of interest.
This story was updated on July 30, 2019.
SAN DIEGO – Two leading figures in microbiome research took time during the annual Digestive Disease Week to share their perspective with members of the press. Identifying key research presented at the meeting and painting a broader picture of trends and challenges in research on the interplay with the microbiota with gut health, Colleen Kelly, MD, of Brown University, Providence, R.I., cochair and principal investigator, AGA FMT Registry Steering Committee, led off the round table with a discussion of human research on fecal microbial transplantation (FMT) and obesity.
Purna Kashyap, MBBS, of the Mayo Clinic, Rochester, Minn., member, AGA Center for Gut Microbiome Research and Education Scientific Advisory Board, delved into the potential for donor microbiota transplant to address small bowel disorders, such as small intestinal bacterial overgrowth, and provided commentary regarding the potential – and limitations – of using FMT in functional bowel disorders such as irritable bowel syndrome (IBS).
Obesity
Dr. Kelly noted that two abstracts at the conference presented data about FMT in obesity. The first study was presented by Jessica Allegretti, MD, a gastroenterologist at Brigham and Women’s Hospital, Boston; the second study was presented by Elaine Yu, MD, an endocrinologist at Massachusetts General Hospital, Boston.
The two studies shared some similarities, but had some differences, said Dr. Kelly. “They both used lean donor encapsulated FMT ... and they both were placebo controlled, using placebo capsules.” The first study looked at metabolically healthy obese patients, while the second study included patients with mild to moderate insulin resistance.
Dr. Allegretti’s work looked at the effect that FMT from lean donors had on levels of a satiety peptide, glucagonlike peptide–1 (GLP-1), while also looking at changes in weight and microbiota, as well as safety. Patients received an initial 30-capsule dose as well as two later doses of 12 capsules each. The 22-patient study, in which individuals were randomized 1:1 to FMT or placebo capsules, didn’t show statistically significant changes in GLP-1 levels or body mass index with FMT over the 12-week study period. “But they were able to show engraftment, which I think is an important thing that you do wonder about – over this period of time, the bacteria that came from the lean donor actually engrafted into the recipient and affected the diversity. The recipient became more similar to the donor,” said Dr. Kelly.
There were some clues among the findings that engraftment was effecting metabolic change in the recipient, she said, including differences in bile acid conversion among gut bacteria; also, lower levels of the primary bile acid taurocholic acid in recipients after FMT. “So it was a negative study in what she was looking for, but an example of these smaller studies kind of pushing the field along.”
In discussing the study presented by Dr. Yu that examined lean donor FMT in individuals with insulin resistance, Dr. Kelly said, “It was actually pretty sophisticated.” By using hyperinsulinemic euglycemic clamping, the investigators were able to measure insulin sensitivity based on glucose load. In this study of 24 individuals randomized 1:1 to receive FMT or placebo capsules, recipients received weekly doses over a period of 6 weeks. Here again, though Dr. Yu and colleagues again found engraftment, “they did not find the big changes in metabolic parameters that they hoped that they would,” said Dr. Kelly.
These two studies furthered previous work completed in the Netherlands examining lean donor FMT for individuals with metabolic syndrome. “Those [studies] did show both engraftment and some changes in insulin resistance,” but they were also small studies, noted Dr. Kelly. Dr. Kashyap pointed out that the earlier studies had shown in a subgroup analysis that response to FMT was limited to those patients who lacked microbial diversity pretransplant.
This makes some mechanistic sense when thinking about FMT’s greatest success to date: Treating Clostridioides difficile infection, a condition whose very hallmark is dysbiosis characterized by monospecies gut domination, noted Dr. Kashyap.
Added Dr. Kelly, “I think everyone’s hoping there’s going to be this pill that’s going to make us skinny, but I don’t think we’re going to find that with FMT and obesity. I do think these studies are important, because there’s so much animal data already, and we’re kind of like, ‘How much more can you do in mice?’ ” By translating this preclinical work into humans, the mechanisms of obesity and the role of the microbiome can be better understood.
IBS
Turning to functional bowel disorders, Dr. Kelly pointed out a new study examining FMT for IBS. “So far, the research has been pretty disappointing,” she noted. The study, presented by Prashant Singh, MBBS, a gastroenterology fellow at Beth Israel Deaconess Medical Center, Boston, examined FMT for patients with moderate to severe diarrhea-predominant IBS. The study had four arms, randomizing the 44 participants to FMT alone; pretreatment with metronidazole and ciprofloxacin or rifaximin, each followed by FMT; or placebo. “It really didn’t show any differences in their severity of IBS in any group; so no effect: a negative study,” said Dr. Kelly.
“It’s challenging. FMT is an appealing strategy because we don’t have to put a lot of thought process into it,” said Dr. Kashyap, but it’s no panacea. “We aren’t going to be able to treat everybody with FMT.
“The challenge with IBS is that if we look at all the compositional studies, the majority of them show that at least a big subset of patients with IBS already have a normal-appearing microbiome,” said Dr. Kashyap. In those patients, “It’s very hard to know what FMT is doing.” He noted that IBS subclassifications are made by pathophysiology, without regard to the intestinal microbiome, so these classifications may not be useful for determining who may benefit from FMT.
“Again, there’s always an opportunity to learn from these studies,” whether they’re positive or negative, as long as they’re well done, said Dr. Kashyap. “There’s always an opportunity to go back and see, was there a specific subgroup of patients who responded, where there might be one or more causes which might be more amenable” to FMT.
Small intestine
And most intestinal microbial research to date, noted Dr. Kelly, has focused on the colon. “Most of our knowledge is of fecal microbiota.” New techniques including double-balloon enteroscopy of the small bowel have promise to “provide completely new information about patterns of bacteria throughout the small bowel,” and of the role of small bowel bacteria in overall gut health, she said.
“The role of the small bowel has been ignored because of accessibility,” agreed Dr. Kashyap. There’s a current focus on research into enteroscopy and other techniques to sample small intestine microbiota, he said.
In a podium presentation, Eugene Chang, MD, Martin Boyer Professor in the University of Chicago’s department of medicine, gave a broad overview of how the small intestine microbiome modulates lipid regulation. This choice of topic for the Charles M. Mansbach Memorial Lecture shows that gastroenterologists are recognizing the importance of microbiome along the entire span of the gut, said Dr. Kashyap. Dr. Chang’s approach, he added, represents a departure in that “it’s not simply just looking at what’s present and what’s not, but seeing what’s functionally relevant to metabolism.”
“We always have known that the small intestine is the workhorse; that’s where everything happens” in terms of motility, absorption, and digestion, said Dr. Kashyap. “But because of our inability to reach it easily we’ve always chosen to ignore it; we always go after the low-hanging fruit.” Despite challenges, more microbiome research should be small-bowel focused. “Eventually, it’s no pain, no gain.”
Dr. Kashyap is on the advisory board of uBiome, and is an ad hoc advisory board member for Salix Pharmaceuticals. Dr. Kelly reported no conflicts of interest.
This story was updated on July 30, 2019.
SAN DIEGO – Two leading figures in microbiome research took time during the annual Digestive Disease Week to share their perspective with members of the press. Identifying key research presented at the meeting and painting a broader picture of trends and challenges in research on the interplay with the microbiota with gut health, Colleen Kelly, MD, of Brown University, Providence, R.I., cochair and principal investigator, AGA FMT Registry Steering Committee, led off the round table with a discussion of human research on fecal microbial transplantation (FMT) and obesity.
Purna Kashyap, MBBS, of the Mayo Clinic, Rochester, Minn., member, AGA Center for Gut Microbiome Research and Education Scientific Advisory Board, delved into the potential for donor microbiota transplant to address small bowel disorders, such as small intestinal bacterial overgrowth, and provided commentary regarding the potential – and limitations – of using FMT in functional bowel disorders such as irritable bowel syndrome (IBS).
Obesity
Dr. Kelly noted that two abstracts at the conference presented data about FMT in obesity. The first study was presented by Jessica Allegretti, MD, a gastroenterologist at Brigham and Women’s Hospital, Boston; the second study was presented by Elaine Yu, MD, an endocrinologist at Massachusetts General Hospital, Boston.
The two studies shared some similarities, but had some differences, said Dr. Kelly. “They both used lean donor encapsulated FMT ... and they both were placebo controlled, using placebo capsules.” The first study looked at metabolically healthy obese patients, while the second study included patients with mild to moderate insulin resistance.
Dr. Allegretti’s work looked at the effect that FMT from lean donors had on levels of a satiety peptide, glucagonlike peptide–1 (GLP-1), while also looking at changes in weight and microbiota, as well as safety. Patients received an initial 30-capsule dose as well as two later doses of 12 capsules each. The 22-patient study, in which individuals were randomized 1:1 to FMT or placebo capsules, didn’t show statistically significant changes in GLP-1 levels or body mass index with FMT over the 12-week study period. “But they were able to show engraftment, which I think is an important thing that you do wonder about – over this period of time, the bacteria that came from the lean donor actually engrafted into the recipient and affected the diversity. The recipient became more similar to the donor,” said Dr. Kelly.
There were some clues among the findings that engraftment was effecting metabolic change in the recipient, she said, including differences in bile acid conversion among gut bacteria; also, lower levels of the primary bile acid taurocholic acid in recipients after FMT. “So it was a negative study in what she was looking for, but an example of these smaller studies kind of pushing the field along.”
In discussing the study presented by Dr. Yu that examined lean donor FMT in individuals with insulin resistance, Dr. Kelly said, “It was actually pretty sophisticated.” By using hyperinsulinemic euglycemic clamping, the investigators were able to measure insulin sensitivity based on glucose load. In this study of 24 individuals randomized 1:1 to receive FMT or placebo capsules, recipients received weekly doses over a period of 6 weeks. Here again, though Dr. Yu and colleagues again found engraftment, “they did not find the big changes in metabolic parameters that they hoped that they would,” said Dr. Kelly.
These two studies furthered previous work completed in the Netherlands examining lean donor FMT for individuals with metabolic syndrome. “Those [studies] did show both engraftment and some changes in insulin resistance,” but they were also small studies, noted Dr. Kelly. Dr. Kashyap pointed out that the earlier studies had shown in a subgroup analysis that response to FMT was limited to those patients who lacked microbial diversity pretransplant.
This makes some mechanistic sense when thinking about FMT’s greatest success to date: Treating Clostridioides difficile infection, a condition whose very hallmark is dysbiosis characterized by monospecies gut domination, noted Dr. Kashyap.
Added Dr. Kelly, “I think everyone’s hoping there’s going to be this pill that’s going to make us skinny, but I don’t think we’re going to find that with FMT and obesity. I do think these studies are important, because there’s so much animal data already, and we’re kind of like, ‘How much more can you do in mice?’ ” By translating this preclinical work into humans, the mechanisms of obesity and the role of the microbiome can be better understood.
IBS
Turning to functional bowel disorders, Dr. Kelly pointed out a new study examining FMT for IBS. “So far, the research has been pretty disappointing,” she noted. The study, presented by Prashant Singh, MBBS, a gastroenterology fellow at Beth Israel Deaconess Medical Center, Boston, examined FMT for patients with moderate to severe diarrhea-predominant IBS. The study had four arms, randomizing the 44 participants to FMT alone; pretreatment with metronidazole and ciprofloxacin or rifaximin, each followed by FMT; or placebo. “It really didn’t show any differences in their severity of IBS in any group; so no effect: a negative study,” said Dr. Kelly.
“It’s challenging. FMT is an appealing strategy because we don’t have to put a lot of thought process into it,” said Dr. Kashyap, but it’s no panacea. “We aren’t going to be able to treat everybody with FMT.
“The challenge with IBS is that if we look at all the compositional studies, the majority of them show that at least a big subset of patients with IBS already have a normal-appearing microbiome,” said Dr. Kashyap. In those patients, “It’s very hard to know what FMT is doing.” He noted that IBS subclassifications are made by pathophysiology, without regard to the intestinal microbiome, so these classifications may not be useful for determining who may benefit from FMT.
“Again, there’s always an opportunity to learn from these studies,” whether they’re positive or negative, as long as they’re well done, said Dr. Kashyap. “There’s always an opportunity to go back and see, was there a specific subgroup of patients who responded, where there might be one or more causes which might be more amenable” to FMT.
Small intestine
And most intestinal microbial research to date, noted Dr. Kelly, has focused on the colon. “Most of our knowledge is of fecal microbiota.” New techniques including double-balloon enteroscopy of the small bowel have promise to “provide completely new information about patterns of bacteria throughout the small bowel,” and of the role of small bowel bacteria in overall gut health, she said.
“The role of the small bowel has been ignored because of accessibility,” agreed Dr. Kashyap. There’s a current focus on research into enteroscopy and other techniques to sample small intestine microbiota, he said.
In a podium presentation, Eugene Chang, MD, Martin Boyer Professor in the University of Chicago’s department of medicine, gave a broad overview of how the small intestine microbiome modulates lipid regulation. This choice of topic for the Charles M. Mansbach Memorial Lecture shows that gastroenterologists are recognizing the importance of microbiome along the entire span of the gut, said Dr. Kashyap. Dr. Chang’s approach, he added, represents a departure in that “it’s not simply just looking at what’s present and what’s not, but seeing what’s functionally relevant to metabolism.”
“We always have known that the small intestine is the workhorse; that’s where everything happens” in terms of motility, absorption, and digestion, said Dr. Kashyap. “But because of our inability to reach it easily we’ve always chosen to ignore it; we always go after the low-hanging fruit.” Despite challenges, more microbiome research should be small-bowel focused. “Eventually, it’s no pain, no gain.”
Dr. Kashyap is on the advisory board of uBiome, and is an ad hoc advisory board member for Salix Pharmaceuticals. Dr. Kelly reported no conflicts of interest.
This story was updated on July 30, 2019.
EXPERT ANALYSIS FROM DDW 2019
Meeting just 2 of 7 ‘simple’ goals lowers HF risk
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
FROM JACC: HEART FAILURE
Plant-based foods could keep type 2 diabetes at bay
according to a new systematic review and meta-analysis of nine observational studies.
The findings aren’t conclusive, but the study authors wrote in JAMA Internal Medicine that they suggest that “plant-based dietary patterns were associated with lower risk of type 2 diabetes, even after adjustment for [body mass index].”
According to 2015 figures provided by the American Diabetes Association, about 29 million people in the United States have type 2 diabetes. Overall, diabetes contributes to hundreds of thousands of deaths each year, which makes it the seventh-leading cause of death in the nation.
For the new analysis, researchers led by Frank Qian, MPH, of Harvard T.H. Chan School of Public Health included nine studies that examined diet and type 2 diabetes. In all, the studies included 307,099 participants, and there were 23,544 cases of incident type 2 diabetes. They were conducted in five countries, including the United States, and tracked participants for 2-28 years; the studies were all published within the last 11 years. The mean ages of participants ranged from 36-65 years.
The meta-analysis linked higher consumption of plant-based foods to a 23% reduced risk of type 2 diabetes, compared with lower consumption (relative risk, 0.77; 95% confidence interval, 0.71-0.84; P = .07 for heterogeneity). The risk dipped even further (down to 30%) when the researchers analyzed four studies that focused on healthy plant-based foods, such as fruits and vegetables, instead of foods such as refined grains, starches, and sugars (RR, 0.70; 95% CI, 0.62-0.79).
The researchers suggested that plant-based diets may lower the risk of diabetes type 2 by limiting weight gain. They also noted various limitations to their analysis, such as the reliance on self-reports and the observational nature of the studies.
Still, “in general populations that do not practice strict vegetarian or vegan diets, replacing animal products with healthful plant-based foods is likely to exert a significant reduction in the risk of diabetes,” the authors wrote.
The study was funded by the National Institutes of Health, and one author received support from the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors reported no conflicts of interest withing the scope of this study.
SOURCE: Qian F et al. JAMA Internal Medicine. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2195.
according to a new systematic review and meta-analysis of nine observational studies.
The findings aren’t conclusive, but the study authors wrote in JAMA Internal Medicine that they suggest that “plant-based dietary patterns were associated with lower risk of type 2 diabetes, even after adjustment for [body mass index].”
According to 2015 figures provided by the American Diabetes Association, about 29 million people in the United States have type 2 diabetes. Overall, diabetes contributes to hundreds of thousands of deaths each year, which makes it the seventh-leading cause of death in the nation.
For the new analysis, researchers led by Frank Qian, MPH, of Harvard T.H. Chan School of Public Health included nine studies that examined diet and type 2 diabetes. In all, the studies included 307,099 participants, and there were 23,544 cases of incident type 2 diabetes. They were conducted in five countries, including the United States, and tracked participants for 2-28 years; the studies were all published within the last 11 years. The mean ages of participants ranged from 36-65 years.
The meta-analysis linked higher consumption of plant-based foods to a 23% reduced risk of type 2 diabetes, compared with lower consumption (relative risk, 0.77; 95% confidence interval, 0.71-0.84; P = .07 for heterogeneity). The risk dipped even further (down to 30%) when the researchers analyzed four studies that focused on healthy plant-based foods, such as fruits and vegetables, instead of foods such as refined grains, starches, and sugars (RR, 0.70; 95% CI, 0.62-0.79).
The researchers suggested that plant-based diets may lower the risk of diabetes type 2 by limiting weight gain. They also noted various limitations to their analysis, such as the reliance on self-reports and the observational nature of the studies.
Still, “in general populations that do not practice strict vegetarian or vegan diets, replacing animal products with healthful plant-based foods is likely to exert a significant reduction in the risk of diabetes,” the authors wrote.
The study was funded by the National Institutes of Health, and one author received support from the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors reported no conflicts of interest withing the scope of this study.
SOURCE: Qian F et al. JAMA Internal Medicine. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2195.
according to a new systematic review and meta-analysis of nine observational studies.
The findings aren’t conclusive, but the study authors wrote in JAMA Internal Medicine that they suggest that “plant-based dietary patterns were associated with lower risk of type 2 diabetes, even after adjustment for [body mass index].”
According to 2015 figures provided by the American Diabetes Association, about 29 million people in the United States have type 2 diabetes. Overall, diabetes contributes to hundreds of thousands of deaths each year, which makes it the seventh-leading cause of death in the nation.
For the new analysis, researchers led by Frank Qian, MPH, of Harvard T.H. Chan School of Public Health included nine studies that examined diet and type 2 diabetes. In all, the studies included 307,099 participants, and there were 23,544 cases of incident type 2 diabetes. They were conducted in five countries, including the United States, and tracked participants for 2-28 years; the studies were all published within the last 11 years. The mean ages of participants ranged from 36-65 years.
The meta-analysis linked higher consumption of plant-based foods to a 23% reduced risk of type 2 diabetes, compared with lower consumption (relative risk, 0.77; 95% confidence interval, 0.71-0.84; P = .07 for heterogeneity). The risk dipped even further (down to 30%) when the researchers analyzed four studies that focused on healthy plant-based foods, such as fruits and vegetables, instead of foods such as refined grains, starches, and sugars (RR, 0.70; 95% CI, 0.62-0.79).
The researchers suggested that plant-based diets may lower the risk of diabetes type 2 by limiting weight gain. They also noted various limitations to their analysis, such as the reliance on self-reports and the observational nature of the studies.
Still, “in general populations that do not practice strict vegetarian or vegan diets, replacing animal products with healthful plant-based foods is likely to exert a significant reduction in the risk of diabetes,” the authors wrote.
The study was funded by the National Institutes of Health, and one author received support from the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors reported no conflicts of interest withing the scope of this study.
SOURCE: Qian F et al. JAMA Internal Medicine. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2195.
FROM JAMA INTERNAL MEDICINE
Obesity tied to relapse in young patients with multiple sclerosis
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
, results of a recent large, single-center study show. The rate of switching to second-line disease-modifying therapy was consequently about 50% higher among the obese children in the study, which included a total of 453 pediatric patients.
The link between obesity and treatment response suggests that the management of these younger patients with MS could be improved through weight loss or body mass index (BMI)-adjusted dosing, according to Peter Huppke, MD, of Georg August University in Göttingen, Germany, and co-investigators.
“The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response,” Dr. Huppke and co-authors said in a report on their study, which was published online ahead of print July 15 in JAMA Neurology.
This is believed to be the first-ever study to find an association between BMI and treatment response in pediatric patients with MS, according to the authors, who said they also confirmed a link between obesity and MS.
Specifically, obesity increased MS susceptibility by two-fold as compared with healthy controls, a finding that they said adds to a small but growing body of evidence that high BMI is associated with increased risk of the disease in these younger individuals.
This retrospective study included 453 pediatric patients with MS treated at the Center for MS in Childhood and Adolescence in Göttingen, Germany between 1990 and 2016. About two-thirds were female and the mean age at MS diagnosis was about 14 years.
Of those patients, 126 (27.8%) were classified as obese based on a BMI greater than the 90th percentile, according to the report.
Dr. Huppke and co-investigators found that high BMI was linked to a significantly increased odds of pediatric MS, with odds ratios of 2.19 (95% CI, 1.5-3.1; P < 0.001) in girls and 2.14 (95% CI, 1.3-3.5; P = 0.003) in boys.
A total of 277 of these pediatric patients received a first-line disease-modifying therapy for 6 months or longer, including 249 treated with interferon beta and 51 treated with glatiramer.
Relapses were more common in obese patients, according to the report. with an annualized relapse rate of 1.29, compared to just 0.72 for those who were not overweight (P < 0.001).
Consequently, likelihood of receiving a second-line treatment was about 1.5 times higher in the obese or extremely obese patients, investigators said.
“A healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs,” they concluded in the report, adding that BMI-adjusted dosing may “increase the value” of first-line disease-modifying therapies.
Dr. Huppke reported disclosures related to Bayer Health Care, Merck Serono, and Novartis not associated with the current study.
SOURCE: Huppke B, et al. JAMA Neurol. 2019 Jul 15. doi: 10.1001/jamaneurol.2019.1997
FROM JAMA NEUROLOGY
Key clinical point: Obese children and adolescents with MS had about twice as many relapses on first-line treatment as compared with their non-obese counterparts.
Major finding: The annualized relapse rate was 1.29 for obese pediatric patients, compared to 0.72 for those who were not overweight (P < 0.001).
Study details: Retrospective study including 453 patients with pediatric MS treated at a center in Göttingen, Germany between 1990 and 2016.
Disclosures: The senior author reported disclosures related to Bayer Health Care, Merck Serono, and Novartis unrelated to the this study.
Source: Huppke B, et al. JAMA Neurol. 2019 Jul 15.
The costs and benefits of SGLT2 inhibitors & GLP-1 RAs
The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

BMI screening trigger for type 2 diabetes is unreliable for at-risk black, Hispanic adults
SAN FRANCISCO – according to findings presented at the annual scientific sessions of the American Diabetes Association.
The conclusions come from a review of 5,656 participants aged 45-84 years in the Multi-Ethnic Study of Atherosclerosis (MESA), an ongoing prospective cohort study. The participants had no signs of cardiovascular disease at enrollment.
The goal of the study reported at the meeting was to see if current ADA screening guidelines are appropriate across racial groups in an increasingly diverse United States. The guidelines recommend body mass index (BMI) as a trigger for screening for type 2 diabetes. The current advice is to screen any adult who has a BMI at or above 25 kg/m2 (23 kg/m2 for Asian Americans) and at least one risk factor for type 2 diabetes, such as hypertension, dyslipidemia, or physical inactivity.
Being black, Asian American, Hispanic, Pacific Islander, or American Indian is itself a risk factor, so “someone from a minority group just needs to meet the BMI criteria,” said lead investigator Luis Rodriguez, a PhD candidate in epidemiology at the University of California, San Francisco.
He and his colleagues focused on 2,383 white, 653 Chinese American, 1,459 black, and 1,161 Hispanic participants in MESA who did not have type 2 diabetes at baseline. Participants were in their early 60s at baseline, on average, and just more than half of them were women. They had five medical exams between 2000 and 2012. The investigators calculated the BMI at which each group hit a 10% risk of developing diabetes within the next 10 years.
In general, the lines crossed at a BMI of about 23 kg/m2 for Chinese Americans; 25 kg/m2 for black and Hispanic participants; and 27 kg/m2 for white participants, which is consistent with ADA advice. The guideline cut points are “appropriate for population-based screening where you may not know if the person in front of you has any diabetes risk factors,” Mr. Rodriguez said.
With known risk factors, however, BMI didn’t hold up. Black participants with one or more risk factor hit the 10% mark at a BMI of 24.7 kg/m2, Hispanics at 23.8 kg/m2, and Chinese Americans at 21.7 kg/m2, all of which are below the recommended cut-points.
“Almost 80%-90% of participants” in minority groups “who had one or more risk factors were above the 10% threshold, so if they have at least one factor, they should pretty much be screened for diabetes, regardless of BMI,” he said.
The 25-kg/m2 threshold worked for white participants; the investigators found that with one or more risk factors, white participants crossed the 10% line at a BMI of 26.2 kg/m2. In addition, white participants with no diabetes risk factors crossed the 10% mark at around a BMI of 30 kg/m2, which the investigators suggested as an appropriate screening trigger.
Participants were in their early 60s at baseline, on average, and just more than half of them were women. In all, 696 cases of type 2 diabetes were diagnosed over a median follow-up of about 9 years, which translated to an incidence rate of 11 cases per 1,000 person-years in white participants, 16 cases for Chinese Americans, 21 for black participants, and 22 for Hispanic participants. Well over half of the participants were overweight or obese at baseline, including about 80% of black and Hispanic participants.
The sample size reported in the abstract was smaller than that in the final presentation because the investigators did not adjust for diet in the final presentation, Mr. Rodriguez explained. Because some study participants did not report diet, by excluding diet from the model adjustment, the number of participants increased, as reflected in this text. Adjusting for diet did not alter the findings.
The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by a grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
SOURCE: Rodriguez L et al. ADA 2019, Abstract 115-OR.
SAN FRANCISCO – according to findings presented at the annual scientific sessions of the American Diabetes Association.
The conclusions come from a review of 5,656 participants aged 45-84 years in the Multi-Ethnic Study of Atherosclerosis (MESA), an ongoing prospective cohort study. The participants had no signs of cardiovascular disease at enrollment.
The goal of the study reported at the meeting was to see if current ADA screening guidelines are appropriate across racial groups in an increasingly diverse United States. The guidelines recommend body mass index (BMI) as a trigger for screening for type 2 diabetes. The current advice is to screen any adult who has a BMI at or above 25 kg/m2 (23 kg/m2 for Asian Americans) and at least one risk factor for type 2 diabetes, such as hypertension, dyslipidemia, or physical inactivity.
Being black, Asian American, Hispanic, Pacific Islander, or American Indian is itself a risk factor, so “someone from a minority group just needs to meet the BMI criteria,” said lead investigator Luis Rodriguez, a PhD candidate in epidemiology at the University of California, San Francisco.
He and his colleagues focused on 2,383 white, 653 Chinese American, 1,459 black, and 1,161 Hispanic participants in MESA who did not have type 2 diabetes at baseline. Participants were in their early 60s at baseline, on average, and just more than half of them were women. They had five medical exams between 2000 and 2012. The investigators calculated the BMI at which each group hit a 10% risk of developing diabetes within the next 10 years.
In general, the lines crossed at a BMI of about 23 kg/m2 for Chinese Americans; 25 kg/m2 for black and Hispanic participants; and 27 kg/m2 for white participants, which is consistent with ADA advice. The guideline cut points are “appropriate for population-based screening where you may not know if the person in front of you has any diabetes risk factors,” Mr. Rodriguez said.
With known risk factors, however, BMI didn’t hold up. Black participants with one or more risk factor hit the 10% mark at a BMI of 24.7 kg/m2, Hispanics at 23.8 kg/m2, and Chinese Americans at 21.7 kg/m2, all of which are below the recommended cut-points.
“Almost 80%-90% of participants” in minority groups “who had one or more risk factors were above the 10% threshold, so if they have at least one factor, they should pretty much be screened for diabetes, regardless of BMI,” he said.
The 25-kg/m2 threshold worked for white participants; the investigators found that with one or more risk factors, white participants crossed the 10% line at a BMI of 26.2 kg/m2. In addition, white participants with no diabetes risk factors crossed the 10% mark at around a BMI of 30 kg/m2, which the investigators suggested as an appropriate screening trigger.
Participants were in their early 60s at baseline, on average, and just more than half of them were women. In all, 696 cases of type 2 diabetes were diagnosed over a median follow-up of about 9 years, which translated to an incidence rate of 11 cases per 1,000 person-years in white participants, 16 cases for Chinese Americans, 21 for black participants, and 22 for Hispanic participants. Well over half of the participants were overweight or obese at baseline, including about 80% of black and Hispanic participants.
The sample size reported in the abstract was smaller than that in the final presentation because the investigators did not adjust for diet in the final presentation, Mr. Rodriguez explained. Because some study participants did not report diet, by excluding diet from the model adjustment, the number of participants increased, as reflected in this text. Adjusting for diet did not alter the findings.
The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by a grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
SOURCE: Rodriguez L et al. ADA 2019, Abstract 115-OR.
SAN FRANCISCO – according to findings presented at the annual scientific sessions of the American Diabetes Association.
The conclusions come from a review of 5,656 participants aged 45-84 years in the Multi-Ethnic Study of Atherosclerosis (MESA), an ongoing prospective cohort study. The participants had no signs of cardiovascular disease at enrollment.
The goal of the study reported at the meeting was to see if current ADA screening guidelines are appropriate across racial groups in an increasingly diverse United States. The guidelines recommend body mass index (BMI) as a trigger for screening for type 2 diabetes. The current advice is to screen any adult who has a BMI at or above 25 kg/m2 (23 kg/m2 for Asian Americans) and at least one risk factor for type 2 diabetes, such as hypertension, dyslipidemia, or physical inactivity.
Being black, Asian American, Hispanic, Pacific Islander, or American Indian is itself a risk factor, so “someone from a minority group just needs to meet the BMI criteria,” said lead investigator Luis Rodriguez, a PhD candidate in epidemiology at the University of California, San Francisco.
He and his colleagues focused on 2,383 white, 653 Chinese American, 1,459 black, and 1,161 Hispanic participants in MESA who did not have type 2 diabetes at baseline. Participants were in their early 60s at baseline, on average, and just more than half of them were women. They had five medical exams between 2000 and 2012. The investigators calculated the BMI at which each group hit a 10% risk of developing diabetes within the next 10 years.
In general, the lines crossed at a BMI of about 23 kg/m2 for Chinese Americans; 25 kg/m2 for black and Hispanic participants; and 27 kg/m2 for white participants, which is consistent with ADA advice. The guideline cut points are “appropriate for population-based screening where you may not know if the person in front of you has any diabetes risk factors,” Mr. Rodriguez said.
With known risk factors, however, BMI didn’t hold up. Black participants with one or more risk factor hit the 10% mark at a BMI of 24.7 kg/m2, Hispanics at 23.8 kg/m2, and Chinese Americans at 21.7 kg/m2, all of which are below the recommended cut-points.
“Almost 80%-90% of participants” in minority groups “who had one or more risk factors were above the 10% threshold, so if they have at least one factor, they should pretty much be screened for diabetes, regardless of BMI,” he said.
The 25-kg/m2 threshold worked for white participants; the investigators found that with one or more risk factors, white participants crossed the 10% line at a BMI of 26.2 kg/m2. In addition, white participants with no diabetes risk factors crossed the 10% mark at around a BMI of 30 kg/m2, which the investigators suggested as an appropriate screening trigger.
Participants were in their early 60s at baseline, on average, and just more than half of them were women. In all, 696 cases of type 2 diabetes were diagnosed over a median follow-up of about 9 years, which translated to an incidence rate of 11 cases per 1,000 person-years in white participants, 16 cases for Chinese Americans, 21 for black participants, and 22 for Hispanic participants. Well over half of the participants were overweight or obese at baseline, including about 80% of black and Hispanic participants.
The sample size reported in the abstract was smaller than that in the final presentation because the investigators did not adjust for diet in the final presentation, Mr. Rodriguez explained. Because some study participants did not report diet, by excluding diet from the model adjustment, the number of participants increased, as reflected in this text. Adjusting for diet did not alter the findings.
The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by a grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
SOURCE: Rodriguez L et al. ADA 2019, Abstract 115-OR.
REPORTING FROM ADA 2019
Key clinical point:
Major finding: Black participants with one or more risk factors had a 10% or greater risk of type 2 diabetes at a BMI of 24.7 kg/m2; Hispanic participants had the same risk at 23.8 kg/m2; and Chinese Americans at 21.7 kg/mg2, all of which are below the BMI cut points recommended by the ADA.
Study details: Review of 5,656 participants in the Multi-Ethnic Study of Atherosclerosis .
Disclosures: The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
Source: Rodriguez L et al. ADA 2019, Abstract 115-OR.