User login
A guide to diagnosing and managing ascites in cirrhosis
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
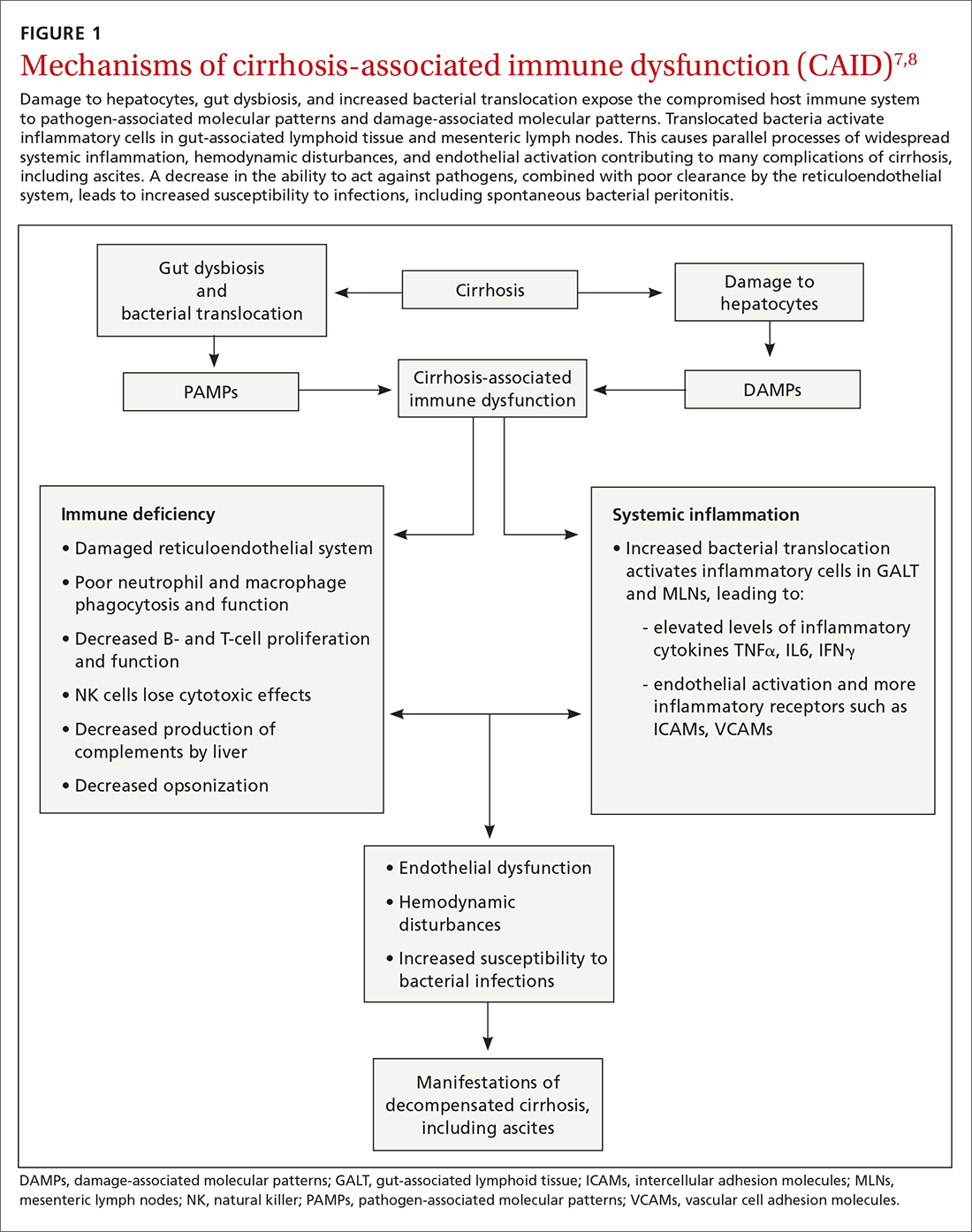
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).
History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
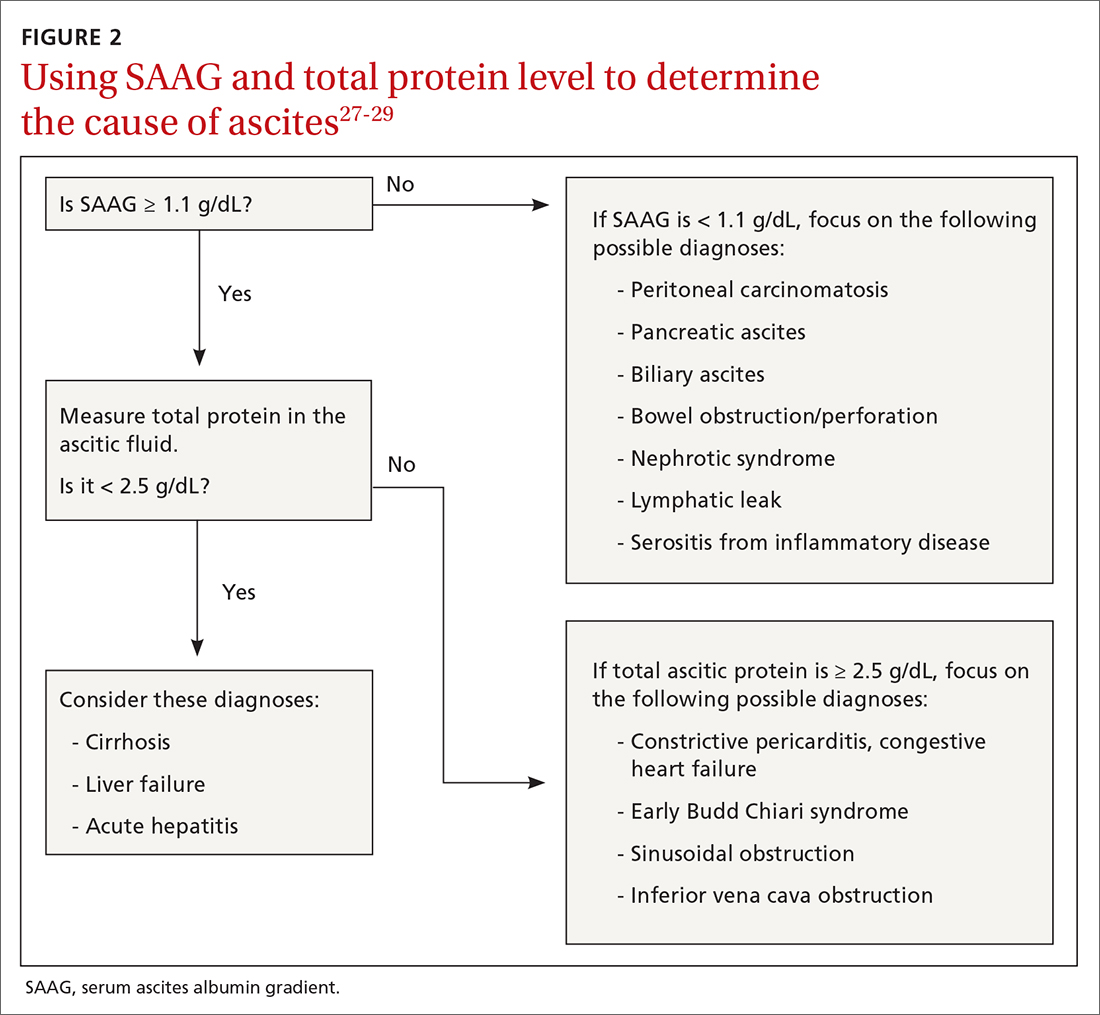
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; faisalm@ccf.org
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).
History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; faisalm@ccf.org
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).
History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; faisalm@ccf.org
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
PRACTICE RECOMMENDATIONS
› Calculate the serum ascites albumin gradient and measure the total ascites protein level to distinguish cirrhotic ascites from that caused by heart failure or other disorders. C
› Recommend sodium restriction of 4.9-6.9 g for patients with established ascites secondary to cirrhosis. C
› Avoid giving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs in cirrhosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vegetarians have better cholesterol levels, and more, than meat eaters
Vegetarians have more favorable levels of a number of biomarkers including cardiovascular-linked ones – total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein A and B – than meat eaters, according to results of the largest study of its kind to date.
Results of the cross-sectional, observational study of 178,000 participants were presented as an electronic poster at this year’s online European Congress on Obesity by Jirapitcha Boonpor of the Institute of Cardiovascular & Medical Sciences, University of Glasgow (Scotland).
“We found that the health benefits of becoming a vegetarian were independent of adiposity and other sociodemographic and lifestyle-related confounding factors,” senior author Carlos Celis-Morales, PhD, also from the University of Glasgow, said in an interview.
Total cholesterol and LDL cholesterol concentrations for vegetarians were 21% and 16.4% lower than in meat eaters. But some biomarkers considered beneficial – including vitamin D concentrations – were lower in vegetarians, while some considered unhealthy – including triglycerides and cystatin-C levels – were higher.
Vegetarian diets have recently become much more popular, but there is insufficient information about the health benefits. Prior reports of associations between biomarkers and a vegetarian diet were unclear, including evidence of any metabolic benefits, noted Dr. Celis-Morales.
Importantly, participants in the study had followed a vegetarian or meat-eater diet for at least 5 years before their biomarkers in blood and urine were assessed.
“If you modify your diet, then, 2 weeks later, you can see changes in some metabolic markers, but changes in markers of cardiovascular disease will take 5-10 years,” he explained.
No single biomarker can assess health
Asked to comment on the findings, John C. Mathers, PhD, noted that they clearly confirm the importance of not reading any biomarker result in isolation.
Health is complex and individual markers tell you just part of the story,” said Dr. Mathers of the Human Nutrition Research Centre, Newcastle (England) University.
He says a vegetarian diet can be nourishing but cautioned that “just because someone excludes meat from their diet does not mean necessarily that they will be eating a healthy diet.”
“Some of the biomarker differences seen in this work – such as the lower concentrations of total cholesterol and LDL cholesterol, GGT [gamma-glutamyl transferase], and ALT [alanine transaminase] – are indicators that the vegetarians were healthier than the meat eaters. However, other differences were less encouraging, including the lower concentrations of vitamin D and higher concentrations of triglycerides and cystatin-C.”
Also reflecting on the results, Jose Lara Gallegos, PhD, senior lecturer in human nutrition at Northumbria University, Newcastle upon Tyne, England, said they support previous evidence from large studies such as the European Prospective Investigation into Cancer and Nutrition (EPIC), which showed that a vegetarian diet is associated with a lower risk of heart disease.
“A vegetarian diet might also be associated with lower risk for liver diseases such as nonalcoholic fatty liver disease,” Dr. Gallegos said, but added that some levels of biomarkers considered to be “healthy” were lower in the vegetarians, and it is important to remember that strictly restricted diets might be associated with potential risks of nutritional inadequacies.
“Other, less restrictive dietary patterns, such as a Mediterranean diet, are also associated with ... health benefits,” he observed.
Large data sample from the UK Biobank study
“Specifically, we wanted to know if vegetarians were healthier because they are generally leaner and lead healthier lives, or whether their diet specifically was responsible for their improved metabolic and cardiovascular health,” Dr. Celis-Morales explained.
Data were included from 177,723 healthy participants from the UK Biobank study who were aged 37-73 years and had reported no major dietary changes over the last 5 years. In total, 4,111 participants were self-reported vegetarians who followed a diet without red meat, poultry, or fish, and 166,516 participants were meat eaters.
Nineteen biomarkers related to diabetes, hypertension, cardiovascular diseases, cancer, and liver and renal function were included, and the associations between vegetarian diet and biomarkers, compared with meat eaters, were examined.
To minimize confounding, the findings were adjusted for age, sex, deprivation, education, ethnicity, smoking, total sedentary time, type of physical activity, alcohol intake, body mass index, and waist circumference.
Compared with meat eaters, vegetarians had significantly lower concentrations of 14 biomarkers, including total cholesterol (21% lower); LDL (16% lower); lipoprotein A (1% lower), lipoprotein B (4% lower), and liver function markers (GGT: 354% lower, and ALT: 153% lower), IGF-1 (134% lower), urate (122% lower), total protein (29% lower), creatinine (607% lower), and C-reactive protein (10% lower).
However, the researchers found that, compared with meat eaters, vegetarians had significantly higher concentrations of some unhealthy biomarkers, including triglycerides (15% higher) and cystatin-C (4% higher), and lower levels of some beneficial biomarkers including high-density lipoprotein (HDL) cholesterol (5% lower), vitamin D (635% lower), and calcium (0.7% lower).
No associations were found for hemoglobin A1c, systolic blood pressure, and aminotransferase.
“Some biomarkers, for example urate, were very low in vegetarians, and this served to verify our results because we expected meat eaters to have higher levels of urate,” remarked Dr. Celis-Morales.
Diet commitment and cardiovascular outcomes
Many people, whether vegetarians or meat-eaters, follow short-term diets, for example, the Atkins or the 5:2 diet, and often lack continuity switching from one diet to the next, or back to regular eating.
“They are healthy, but they do not commit for long enough to make a difference to metabolic markers or potentially long-term health. In contrast, vegetarians are usually fully committed but the reasons behind this commitment might be a concern for the environment or animal welfare, for example,” Dr. Celis-Morales pointed out.
However, he added that many vegetarians replace the meat in their diet with unhealthy alternatives. “They often eat too much pasta or potatoes, or other high-energy food with low nutritional value.”
Having identified metabolic markers specific to long-term vegetarian diets, Dr. Celis-Morales wanted to know what happens to vegetarians’ long-term cardiovascular health. He analyzed and published these outcomes in a separate study published in December 2020.
“Over 9 years of follow-up, we have found that vegetarians have a lower risk in terms of myocardial infarction in the long-term, as well as other cardiovascular disease,” he reported.
Asked whether there was an optimum age or time in life to become a vegetarian to improve health, Dr. Celis-Morales explained that the healthier you are, the less likely you will reap the health benefits of dietary changes – for example to being a vegetarian.
“It is more likely that those people who have unhealthy lifestyle risk factors, such as smoking, and high consumption of high-energy foods or processed meat are more likely to see positive health effects,” he said.
Lifestyle changes to improve cardiovascular outcomes are usually more likely to be required at 40 or 50 years old than at younger ages. He also noted that metabolic markers tend to show clear improvement at around 3 months after adopting a particular diet but improvements in disease outcomes take a lot longer to become evident.
Dr. Celis-Morales and his team are currently conducting a further analysis to understand if the vegetarian diet is also associated with a lower risk of cancer, depression, and dementia, compared with meat-eaters.
Dr. Celis-Morales, Dr. Mathers, and Dr. Gallegos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vegetarians have more favorable levels of a number of biomarkers including cardiovascular-linked ones – total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein A and B – than meat eaters, according to results of the largest study of its kind to date.
Results of the cross-sectional, observational study of 178,000 participants were presented as an electronic poster at this year’s online European Congress on Obesity by Jirapitcha Boonpor of the Institute of Cardiovascular & Medical Sciences, University of Glasgow (Scotland).
“We found that the health benefits of becoming a vegetarian were independent of adiposity and other sociodemographic and lifestyle-related confounding factors,” senior author Carlos Celis-Morales, PhD, also from the University of Glasgow, said in an interview.
Total cholesterol and LDL cholesterol concentrations for vegetarians were 21% and 16.4% lower than in meat eaters. But some biomarkers considered beneficial – including vitamin D concentrations – were lower in vegetarians, while some considered unhealthy – including triglycerides and cystatin-C levels – were higher.
Vegetarian diets have recently become much more popular, but there is insufficient information about the health benefits. Prior reports of associations between biomarkers and a vegetarian diet were unclear, including evidence of any metabolic benefits, noted Dr. Celis-Morales.
Importantly, participants in the study had followed a vegetarian or meat-eater diet for at least 5 years before their biomarkers in blood and urine were assessed.
“If you modify your diet, then, 2 weeks later, you can see changes in some metabolic markers, but changes in markers of cardiovascular disease will take 5-10 years,” he explained.
No single biomarker can assess health
Asked to comment on the findings, John C. Mathers, PhD, noted that they clearly confirm the importance of not reading any biomarker result in isolation.
Health is complex and individual markers tell you just part of the story,” said Dr. Mathers of the Human Nutrition Research Centre, Newcastle (England) University.
He says a vegetarian diet can be nourishing but cautioned that “just because someone excludes meat from their diet does not mean necessarily that they will be eating a healthy diet.”
“Some of the biomarker differences seen in this work – such as the lower concentrations of total cholesterol and LDL cholesterol, GGT [gamma-glutamyl transferase], and ALT [alanine transaminase] – are indicators that the vegetarians were healthier than the meat eaters. However, other differences were less encouraging, including the lower concentrations of vitamin D and higher concentrations of triglycerides and cystatin-C.”
Also reflecting on the results, Jose Lara Gallegos, PhD, senior lecturer in human nutrition at Northumbria University, Newcastle upon Tyne, England, said they support previous evidence from large studies such as the European Prospective Investigation into Cancer and Nutrition (EPIC), which showed that a vegetarian diet is associated with a lower risk of heart disease.
“A vegetarian diet might also be associated with lower risk for liver diseases such as nonalcoholic fatty liver disease,” Dr. Gallegos said, but added that some levels of biomarkers considered to be “healthy” were lower in the vegetarians, and it is important to remember that strictly restricted diets might be associated with potential risks of nutritional inadequacies.
“Other, less restrictive dietary patterns, such as a Mediterranean diet, are also associated with ... health benefits,” he observed.
Large data sample from the UK Biobank study
“Specifically, we wanted to know if vegetarians were healthier because they are generally leaner and lead healthier lives, or whether their diet specifically was responsible for their improved metabolic and cardiovascular health,” Dr. Celis-Morales explained.
Data were included from 177,723 healthy participants from the UK Biobank study who were aged 37-73 years and had reported no major dietary changes over the last 5 years. In total, 4,111 participants were self-reported vegetarians who followed a diet without red meat, poultry, or fish, and 166,516 participants were meat eaters.
Nineteen biomarkers related to diabetes, hypertension, cardiovascular diseases, cancer, and liver and renal function were included, and the associations between vegetarian diet and biomarkers, compared with meat eaters, were examined.
To minimize confounding, the findings were adjusted for age, sex, deprivation, education, ethnicity, smoking, total sedentary time, type of physical activity, alcohol intake, body mass index, and waist circumference.
Compared with meat eaters, vegetarians had significantly lower concentrations of 14 biomarkers, including total cholesterol (21% lower); LDL (16% lower); lipoprotein A (1% lower), lipoprotein B (4% lower), and liver function markers (GGT: 354% lower, and ALT: 153% lower), IGF-1 (134% lower), urate (122% lower), total protein (29% lower), creatinine (607% lower), and C-reactive protein (10% lower).
However, the researchers found that, compared with meat eaters, vegetarians had significantly higher concentrations of some unhealthy biomarkers, including triglycerides (15% higher) and cystatin-C (4% higher), and lower levels of some beneficial biomarkers including high-density lipoprotein (HDL) cholesterol (5% lower), vitamin D (635% lower), and calcium (0.7% lower).
No associations were found for hemoglobin A1c, systolic blood pressure, and aminotransferase.
“Some biomarkers, for example urate, were very low in vegetarians, and this served to verify our results because we expected meat eaters to have higher levels of urate,” remarked Dr. Celis-Morales.
Diet commitment and cardiovascular outcomes
Many people, whether vegetarians or meat-eaters, follow short-term diets, for example, the Atkins or the 5:2 diet, and often lack continuity switching from one diet to the next, or back to regular eating.
“They are healthy, but they do not commit for long enough to make a difference to metabolic markers or potentially long-term health. In contrast, vegetarians are usually fully committed but the reasons behind this commitment might be a concern for the environment or animal welfare, for example,” Dr. Celis-Morales pointed out.
However, he added that many vegetarians replace the meat in their diet with unhealthy alternatives. “They often eat too much pasta or potatoes, or other high-energy food with low nutritional value.”
Having identified metabolic markers specific to long-term vegetarian diets, Dr. Celis-Morales wanted to know what happens to vegetarians’ long-term cardiovascular health. He analyzed and published these outcomes in a separate study published in December 2020.
“Over 9 years of follow-up, we have found that vegetarians have a lower risk in terms of myocardial infarction in the long-term, as well as other cardiovascular disease,” he reported.
Asked whether there was an optimum age or time in life to become a vegetarian to improve health, Dr. Celis-Morales explained that the healthier you are, the less likely you will reap the health benefits of dietary changes – for example to being a vegetarian.
“It is more likely that those people who have unhealthy lifestyle risk factors, such as smoking, and high consumption of high-energy foods or processed meat are more likely to see positive health effects,” he said.
Lifestyle changes to improve cardiovascular outcomes are usually more likely to be required at 40 or 50 years old than at younger ages. He also noted that metabolic markers tend to show clear improvement at around 3 months after adopting a particular diet but improvements in disease outcomes take a lot longer to become evident.
Dr. Celis-Morales and his team are currently conducting a further analysis to understand if the vegetarian diet is also associated with a lower risk of cancer, depression, and dementia, compared with meat-eaters.
Dr. Celis-Morales, Dr. Mathers, and Dr. Gallegos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vegetarians have more favorable levels of a number of biomarkers including cardiovascular-linked ones – total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein A and B – than meat eaters, according to results of the largest study of its kind to date.
Results of the cross-sectional, observational study of 178,000 participants were presented as an electronic poster at this year’s online European Congress on Obesity by Jirapitcha Boonpor of the Institute of Cardiovascular & Medical Sciences, University of Glasgow (Scotland).
“We found that the health benefits of becoming a vegetarian were independent of adiposity and other sociodemographic and lifestyle-related confounding factors,” senior author Carlos Celis-Morales, PhD, also from the University of Glasgow, said in an interview.
Total cholesterol and LDL cholesterol concentrations for vegetarians were 21% and 16.4% lower than in meat eaters. But some biomarkers considered beneficial – including vitamin D concentrations – were lower in vegetarians, while some considered unhealthy – including triglycerides and cystatin-C levels – were higher.
Vegetarian diets have recently become much more popular, but there is insufficient information about the health benefits. Prior reports of associations between biomarkers and a vegetarian diet were unclear, including evidence of any metabolic benefits, noted Dr. Celis-Morales.
Importantly, participants in the study had followed a vegetarian or meat-eater diet for at least 5 years before their biomarkers in blood and urine were assessed.
“If you modify your diet, then, 2 weeks later, you can see changes in some metabolic markers, but changes in markers of cardiovascular disease will take 5-10 years,” he explained.
No single biomarker can assess health
Asked to comment on the findings, John C. Mathers, PhD, noted that they clearly confirm the importance of not reading any biomarker result in isolation.
Health is complex and individual markers tell you just part of the story,” said Dr. Mathers of the Human Nutrition Research Centre, Newcastle (England) University.
He says a vegetarian diet can be nourishing but cautioned that “just because someone excludes meat from their diet does not mean necessarily that they will be eating a healthy diet.”
“Some of the biomarker differences seen in this work – such as the lower concentrations of total cholesterol and LDL cholesterol, GGT [gamma-glutamyl transferase], and ALT [alanine transaminase] – are indicators that the vegetarians were healthier than the meat eaters. However, other differences were less encouraging, including the lower concentrations of vitamin D and higher concentrations of triglycerides and cystatin-C.”
Also reflecting on the results, Jose Lara Gallegos, PhD, senior lecturer in human nutrition at Northumbria University, Newcastle upon Tyne, England, said they support previous evidence from large studies such as the European Prospective Investigation into Cancer and Nutrition (EPIC), which showed that a vegetarian diet is associated with a lower risk of heart disease.
“A vegetarian diet might also be associated with lower risk for liver diseases such as nonalcoholic fatty liver disease,” Dr. Gallegos said, but added that some levels of biomarkers considered to be “healthy” were lower in the vegetarians, and it is important to remember that strictly restricted diets might be associated with potential risks of nutritional inadequacies.
“Other, less restrictive dietary patterns, such as a Mediterranean diet, are also associated with ... health benefits,” he observed.
Large data sample from the UK Biobank study
“Specifically, we wanted to know if vegetarians were healthier because they are generally leaner and lead healthier lives, or whether their diet specifically was responsible for their improved metabolic and cardiovascular health,” Dr. Celis-Morales explained.
Data were included from 177,723 healthy participants from the UK Biobank study who were aged 37-73 years and had reported no major dietary changes over the last 5 years. In total, 4,111 participants were self-reported vegetarians who followed a diet without red meat, poultry, or fish, and 166,516 participants were meat eaters.
Nineteen biomarkers related to diabetes, hypertension, cardiovascular diseases, cancer, and liver and renal function were included, and the associations between vegetarian diet and biomarkers, compared with meat eaters, were examined.
To minimize confounding, the findings were adjusted for age, sex, deprivation, education, ethnicity, smoking, total sedentary time, type of physical activity, alcohol intake, body mass index, and waist circumference.
Compared with meat eaters, vegetarians had significantly lower concentrations of 14 biomarkers, including total cholesterol (21% lower); LDL (16% lower); lipoprotein A (1% lower), lipoprotein B (4% lower), and liver function markers (GGT: 354% lower, and ALT: 153% lower), IGF-1 (134% lower), urate (122% lower), total protein (29% lower), creatinine (607% lower), and C-reactive protein (10% lower).
However, the researchers found that, compared with meat eaters, vegetarians had significantly higher concentrations of some unhealthy biomarkers, including triglycerides (15% higher) and cystatin-C (4% higher), and lower levels of some beneficial biomarkers including high-density lipoprotein (HDL) cholesterol (5% lower), vitamin D (635% lower), and calcium (0.7% lower).
No associations were found for hemoglobin A1c, systolic blood pressure, and aminotransferase.
“Some biomarkers, for example urate, were very low in vegetarians, and this served to verify our results because we expected meat eaters to have higher levels of urate,” remarked Dr. Celis-Morales.
Diet commitment and cardiovascular outcomes
Many people, whether vegetarians or meat-eaters, follow short-term diets, for example, the Atkins or the 5:2 diet, and often lack continuity switching from one diet to the next, or back to regular eating.
“They are healthy, but they do not commit for long enough to make a difference to metabolic markers or potentially long-term health. In contrast, vegetarians are usually fully committed but the reasons behind this commitment might be a concern for the environment or animal welfare, for example,” Dr. Celis-Morales pointed out.
However, he added that many vegetarians replace the meat in their diet with unhealthy alternatives. “They often eat too much pasta or potatoes, or other high-energy food with low nutritional value.”
Having identified metabolic markers specific to long-term vegetarian diets, Dr. Celis-Morales wanted to know what happens to vegetarians’ long-term cardiovascular health. He analyzed and published these outcomes in a separate study published in December 2020.
“Over 9 years of follow-up, we have found that vegetarians have a lower risk in terms of myocardial infarction in the long-term, as well as other cardiovascular disease,” he reported.
Asked whether there was an optimum age or time in life to become a vegetarian to improve health, Dr. Celis-Morales explained that the healthier you are, the less likely you will reap the health benefits of dietary changes – for example to being a vegetarian.
“It is more likely that those people who have unhealthy lifestyle risk factors, such as smoking, and high consumption of high-energy foods or processed meat are more likely to see positive health effects,” he said.
Lifestyle changes to improve cardiovascular outcomes are usually more likely to be required at 40 or 50 years old than at younger ages. He also noted that metabolic markers tend to show clear improvement at around 3 months after adopting a particular diet but improvements in disease outcomes take a lot longer to become evident.
Dr. Celis-Morales and his team are currently conducting a further analysis to understand if the vegetarian diet is also associated with a lower risk of cancer, depression, and dementia, compared with meat-eaters.
Dr. Celis-Morales, Dr. Mathers, and Dr. Gallegos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A review of the latest USPSTF recommendations
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
How to help runners steer clear of injury
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.
This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.
Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.
Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; kartiksidhar@gmail.com
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.
This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.
Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.
Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; kartiksidhar@gmail.com
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.
This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.
Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.
Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; kartiksidhar@gmail.com
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
PRACTICE RECOMMENDATIONS
› Counsel runners to cross-train, replace shoes regularly, and use shoes with moderate-to-high (8-12 mm) heel-to-toe drop. C
› Don’t discourage running for exercise, as long as it is tolerated, in patients who have osteoarthritis. C
› Encourage moderation in running distance and intensity, especially in novice runners. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
High teen BMI linked to stroke risk in young adulthood
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
FROM STROKE
Two treatments show early promise for hypothalamic obesity
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
FROM ENDO 2021
Possible obesity effect detected in cancer death rates

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.
FROM JAMA NETWORK OPEN
A simple new definition for ‘metabolically healthy obesity’?
Scientists have proposed a simple new definition for “metabolically healthy obesity” to identify individuals who do not have an increased risk of cardiovascular disease (CVD) death and total mortality.
The team – led by Anika Zembic, MPH, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany – performed an assessment of anthropometric and metabolic risk factors as well as mortality data from two cohorts that “yielded a simple definition to categorize participants with obesity as metabolically healthy or unhealthy.”
They defined “metabolically healthy” as systolic blood pressure <130 mm Hg and no use of blood pressure-lowering medication; waist-to-hip ratio <0.95 (in women) and <1.03 (in men); and no prevalent type 2 diabetes.
Based on this new definition, 42% of participants in the third U.S. National Health and Nutrition Examination Survey (NHANES-III) and 19% of participants in the UK Biobank study had metabolically healthy obesity and did not have an increased risk for CVD mortality and total mortality compared with individuals with metabolically healthy normal weight.
“People with a phenotype defined as metabolically unhealthy using this definition had significantly higher hazard ratios for [CVD] mortality and total mortality irrespective of body mass index category, and people with phenotypes defined as having metabolically healthy obesity displayed no increased risk,” the researchers noted in their article, published May 7 in JAMA Network Open.
“Our new definition may be important not only to stratify risk of mortality in people with obesity, but also in people with overweight and normal weight,” they concluded.
Thirty different definitions of ‘metabolically healthy obesity’
“To date, there is no universally accepted standard for defining [metabolically healthy obesity] and more than 30 different definitions have been used to operationalize the phenotypes in studies,” which may explain the “continued unresolved debate” about outcomes in patients with metabolically unhealthy obesity, Ayana K. April-Sanders, PhD, and Carlos J. Rodriguez, MD, MPH, from Albert Einstein College of Medicine, New York, wrote in an accompanying commentary.
The current study, they noted, suggests that waist-to-hip ratio is a better measure of central adiposity than waist circumference, and that the effect of dyslipidemia on CVD mortality may be weaker among individuals with obesity.
However, the findings may not be generalizable to other CVD outcomes, they cautioned.
And importantly, some individuals with metabolically healthy obesity will likely transition to unhealthy obesity over time due to weight gain, aging, and lack of physical activity.
Therefore, “the present study provides a prototype of how that definition can be derived, but more rigorous tests and evidence using similar techniques are needed, particularly in prospective studies,” according to Dr. April-Sanders and Dr. Rodriguez.
They call for more research to establish a standardized definition of metabolically healthy obesity and then, using that definition, to determine the prevalence of healthy and unhealthy obesity and identify factors that preserve healthy obesity.
Definition developed from NHANES cohort, validated in UK biobank
Ms. Zembic and colleagues explained that previous definitions for metabolically healthy obesity were mainly based on the absence of either metabolic syndrome or insulin resistance, but some individuals with obesity but without metabolic disease still have increased risks of CVD mortality and total mortality.
To develop a more precise definition of metabolically healthy obesity, the researchers analyzed data from 12,341 individuals in the United States who participated in NHANES-III, conducted between 1988 and 1994. The individuals were a mean age of 42 and 51% were women, and they were followed for an average of 14.5 years.
The researchers validated this definition using data from 374,079 individuals in the population-based UK Biobank cohort who were assessed in 2006 to 2010. Those individuals were a mean age of 56 and 55% were women, and they were followed for a mean of 7.8 years.
The combination of systolic blood pressure and waist-to-hip ratio had the strongest association with CVD mortality and total mortality, and the prevalence of type 2 diabetes was also associated with greater risk.
Regardless of BMI, all groups of metabolically unhealthy individuals had increased risks of CVD mortality and total mortality.
The study and some of the researchers were supported by grants from the German Federal Ministry of Education and Research.
A version of this article first appeared on Medscape.com.
Scientists have proposed a simple new definition for “metabolically healthy obesity” to identify individuals who do not have an increased risk of cardiovascular disease (CVD) death and total mortality.
The team – led by Anika Zembic, MPH, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany – performed an assessment of anthropometric and metabolic risk factors as well as mortality data from two cohorts that “yielded a simple definition to categorize participants with obesity as metabolically healthy or unhealthy.”
They defined “metabolically healthy” as systolic blood pressure <130 mm Hg and no use of blood pressure-lowering medication; waist-to-hip ratio <0.95 (in women) and <1.03 (in men); and no prevalent type 2 diabetes.
Based on this new definition, 42% of participants in the third U.S. National Health and Nutrition Examination Survey (NHANES-III) and 19% of participants in the UK Biobank study had metabolically healthy obesity and did not have an increased risk for CVD mortality and total mortality compared with individuals with metabolically healthy normal weight.
“People with a phenotype defined as metabolically unhealthy using this definition had significantly higher hazard ratios for [CVD] mortality and total mortality irrespective of body mass index category, and people with phenotypes defined as having metabolically healthy obesity displayed no increased risk,” the researchers noted in their article, published May 7 in JAMA Network Open.
“Our new definition may be important not only to stratify risk of mortality in people with obesity, but also in people with overweight and normal weight,” they concluded.
Thirty different definitions of ‘metabolically healthy obesity’
“To date, there is no universally accepted standard for defining [metabolically healthy obesity] and more than 30 different definitions have been used to operationalize the phenotypes in studies,” which may explain the “continued unresolved debate” about outcomes in patients with metabolically unhealthy obesity, Ayana K. April-Sanders, PhD, and Carlos J. Rodriguez, MD, MPH, from Albert Einstein College of Medicine, New York, wrote in an accompanying commentary.
The current study, they noted, suggests that waist-to-hip ratio is a better measure of central adiposity than waist circumference, and that the effect of dyslipidemia on CVD mortality may be weaker among individuals with obesity.
However, the findings may not be generalizable to other CVD outcomes, they cautioned.
And importantly, some individuals with metabolically healthy obesity will likely transition to unhealthy obesity over time due to weight gain, aging, and lack of physical activity.
Therefore, “the present study provides a prototype of how that definition can be derived, but more rigorous tests and evidence using similar techniques are needed, particularly in prospective studies,” according to Dr. April-Sanders and Dr. Rodriguez.
They call for more research to establish a standardized definition of metabolically healthy obesity and then, using that definition, to determine the prevalence of healthy and unhealthy obesity and identify factors that preserve healthy obesity.
Definition developed from NHANES cohort, validated in UK biobank
Ms. Zembic and colleagues explained that previous definitions for metabolically healthy obesity were mainly based on the absence of either metabolic syndrome or insulin resistance, but some individuals with obesity but without metabolic disease still have increased risks of CVD mortality and total mortality.
To develop a more precise definition of metabolically healthy obesity, the researchers analyzed data from 12,341 individuals in the United States who participated in NHANES-III, conducted between 1988 and 1994. The individuals were a mean age of 42 and 51% were women, and they were followed for an average of 14.5 years.
The researchers validated this definition using data from 374,079 individuals in the population-based UK Biobank cohort who were assessed in 2006 to 2010. Those individuals were a mean age of 56 and 55% were women, and they were followed for a mean of 7.8 years.
The combination of systolic blood pressure and waist-to-hip ratio had the strongest association with CVD mortality and total mortality, and the prevalence of type 2 diabetes was also associated with greater risk.
Regardless of BMI, all groups of metabolically unhealthy individuals had increased risks of CVD mortality and total mortality.
The study and some of the researchers were supported by grants from the German Federal Ministry of Education and Research.
A version of this article first appeared on Medscape.com.
Scientists have proposed a simple new definition for “metabolically healthy obesity” to identify individuals who do not have an increased risk of cardiovascular disease (CVD) death and total mortality.
The team – led by Anika Zembic, MPH, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany – performed an assessment of anthropometric and metabolic risk factors as well as mortality data from two cohorts that “yielded a simple definition to categorize participants with obesity as metabolically healthy or unhealthy.”
They defined “metabolically healthy” as systolic blood pressure <130 mm Hg and no use of blood pressure-lowering medication; waist-to-hip ratio <0.95 (in women) and <1.03 (in men); and no prevalent type 2 diabetes.
Based on this new definition, 42% of participants in the third U.S. National Health and Nutrition Examination Survey (NHANES-III) and 19% of participants in the UK Biobank study had metabolically healthy obesity and did not have an increased risk for CVD mortality and total mortality compared with individuals with metabolically healthy normal weight.
“People with a phenotype defined as metabolically unhealthy using this definition had significantly higher hazard ratios for [CVD] mortality and total mortality irrespective of body mass index category, and people with phenotypes defined as having metabolically healthy obesity displayed no increased risk,” the researchers noted in their article, published May 7 in JAMA Network Open.
“Our new definition may be important not only to stratify risk of mortality in people with obesity, but also in people with overweight and normal weight,” they concluded.
Thirty different definitions of ‘metabolically healthy obesity’
“To date, there is no universally accepted standard for defining [metabolically healthy obesity] and more than 30 different definitions have been used to operationalize the phenotypes in studies,” which may explain the “continued unresolved debate” about outcomes in patients with metabolically unhealthy obesity, Ayana K. April-Sanders, PhD, and Carlos J. Rodriguez, MD, MPH, from Albert Einstein College of Medicine, New York, wrote in an accompanying commentary.
The current study, they noted, suggests that waist-to-hip ratio is a better measure of central adiposity than waist circumference, and that the effect of dyslipidemia on CVD mortality may be weaker among individuals with obesity.
However, the findings may not be generalizable to other CVD outcomes, they cautioned.
And importantly, some individuals with metabolically healthy obesity will likely transition to unhealthy obesity over time due to weight gain, aging, and lack of physical activity.
Therefore, “the present study provides a prototype of how that definition can be derived, but more rigorous tests and evidence using similar techniques are needed, particularly in prospective studies,” according to Dr. April-Sanders and Dr. Rodriguez.
They call for more research to establish a standardized definition of metabolically healthy obesity and then, using that definition, to determine the prevalence of healthy and unhealthy obesity and identify factors that preserve healthy obesity.
Definition developed from NHANES cohort, validated in UK biobank
Ms. Zembic and colleagues explained that previous definitions for metabolically healthy obesity were mainly based on the absence of either metabolic syndrome or insulin resistance, but some individuals with obesity but without metabolic disease still have increased risks of CVD mortality and total mortality.
To develop a more precise definition of metabolically healthy obesity, the researchers analyzed data from 12,341 individuals in the United States who participated in NHANES-III, conducted between 1988 and 1994. The individuals were a mean age of 42 and 51% were women, and they were followed for an average of 14.5 years.
The researchers validated this definition using data from 374,079 individuals in the population-based UK Biobank cohort who were assessed in 2006 to 2010. Those individuals were a mean age of 56 and 55% were women, and they were followed for a mean of 7.8 years.
The combination of systolic blood pressure and waist-to-hip ratio had the strongest association with CVD mortality and total mortality, and the prevalence of type 2 diabetes was also associated with greater risk.
Regardless of BMI, all groups of metabolically unhealthy individuals had increased risks of CVD mortality and total mortality.
The study and some of the researchers were supported by grants from the German Federal Ministry of Education and Research.
A version of this article first appeared on Medscape.com.
High body fat tied to slowed breast maturation in girls with obesity
Girls in late stages of puberty who had elevated levels of body fat showed unusually high levels of several hormones that could contribute to an earlier age of menarche and also slow breast development, according to data from 90 girls who spanned a wide range of body fat in the first longitudinal study to examine links between fat volume, levels of reproductive hormones, and clinical manifestations of hormone action during puberty.
The results showed that girls with greater body fat had higher levels of follicle stimulating hormone, inhibin B, estrone, and certain male-like reproductive hormones, and that this pattern “is specifically tied to body fat,” said Natalie D. Shaw, MD, senior investigator for the study, reported at the annual meeting of the Endocrine Society.
“We found that total body fat is associated with the timing of menarche, as others have reported for body weight,” she noted. The new findings showed that every 1% rise in percent total body fat linked with a significant 3% rise in the likelihood of menarche, menstrual onset. In the new study the average age of menarche was 11.7 years among the overweight or obese girls and 12.8 years among those with normal weights.
But the study’s unique use of an average of about three serial ultrasound breast examinations of each subject during an average 4 years of follow-up also showed that higher levels of body fat linked with slowed breast development in later stages, specifically maturation from stage D to stages D/E and E.
For example, girls with 33% body fat spent an average of 8.2 months in stage D, which stretched to an average of 11.2 months among girls with 38% body fat, reported Madison T. Ortega, a researcher with the Pediatric Endocrinology Group of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C., who presented the report at the meeting.
Ultrasound shows what inspection can’t
Results from “several studies have shown earlier breast development in overweight and obese girls by inspection and palpation,” but the new findings from ultrasound examination provide more nuance about the structural breast changes actually occurring in these adolescents, said Dr. Shaw, who heads the Pediatric Endocrinology Group. The current study “was not designed to capture the onset of breast development,” and “it is possible that increased androgens or insulin resistance in girls with higher body fat interferes with normal breast development,” she explained in an interview.
“The authors showed that the timing and progress of early stages of puberty were not earlier in overweight or obese girls. Luteinizing hormone, the indicator of neuroendocrine pubertal onset, and timing of early stages of breast development were the same in all weight groups. The authors also discovered falsely advanced Turner breast stage designations with ultrasonography in some girls with obesity. This might suggest that prior findings in epidemiologic studies of an earlier start to puberty based mostly on breast development stages identified by self-reported inspection and, rarely, palpation, may have been biased by breast adipose tissue,” said Christine M. Burt Solorzano, MD, a pediatric endocrinologist at the University of Virginia in Charlottesville, who was not involved in the study.
“Development of increased follicle-stimulating hormone in late puberty suggests that pubertal tempo, not onset, may be increased in girls with obesity, and goes along with earlier menarche. Their finding of increased androgen levels during mid to late puberty with obesity are consistent with prior findings,” including work published Dr. Burt Solorzano and her associates, she noted. “Delayed timing of advanced breast morphology was unexpected and may reflect relatively lower levels of progesterone in girls with obesity,” a hormone necessary for later stages of breast maturation.
The findings “reinforce that early breast development in the setting of obesity may in fact reflect adipose tissue and not be a true representation of neuroendocrine precocious puberty,” Dr. Burt Solorzano said in an interview. The findings “also suggest that pubertal initiation may not happen earlier in girls with obesity, as has been thought, but rather the tempo of puberty may be more rapid, leading to earlier menarche.”
A possible step toward PCOS
The long-term clinical consequences of the hormonal state linked with overweight and obesity “are unknown,” said Dr. Shaw. However, she and her coworkers followed a few of their subjects with elevated testosterone levels during midpuberty, and several developed signs of early polycystic ovarian syndrome (PCOS) such as irregular menstrual cycles, acne, and hirsutism. “It may be possible to identify girls at high risk for PCOS before menarche,” she suggested.
Dr. Burt Solorzano agreed that delayed breast development in girls with high levels of body fat may reflect inadequate progesterone production, which when coupled with an obesity-related excess level of androgens could put girls at risk for chronic anovulation and later PCOS.
“Weight management during childhood and early puberty may mitigate the adverse effects of obesity on pubertal progression and avoid some of the lifetime complications related to early menarche,” Dr. Burt Solorzano said.
The Body Weight and Puberty Study enrolled 36 girls who were overweight or obese and 54 girls with normal weight. They averaged 11 years of age, with a range of 8.2-14.7 years. Average percent body fat was 41% among the overweight or obese girls and 27% among those with normal weight. The results reported by Ms. Ortega also appeared in a report published Feb 22, 2021 (J Clin Endocrinol Metab. doi: 10.1210/clinem/dgab092).
Dr. Shaw, Ms. Ortega, and Dr. Burt Solorzano had no disclosures.
Girls in late stages of puberty who had elevated levels of body fat showed unusually high levels of several hormones that could contribute to an earlier age of menarche and also slow breast development, according to data from 90 girls who spanned a wide range of body fat in the first longitudinal study to examine links between fat volume, levels of reproductive hormones, and clinical manifestations of hormone action during puberty.
The results showed that girls with greater body fat had higher levels of follicle stimulating hormone, inhibin B, estrone, and certain male-like reproductive hormones, and that this pattern “is specifically tied to body fat,” said Natalie D. Shaw, MD, senior investigator for the study, reported at the annual meeting of the Endocrine Society.
“We found that total body fat is associated with the timing of menarche, as others have reported for body weight,” she noted. The new findings showed that every 1% rise in percent total body fat linked with a significant 3% rise in the likelihood of menarche, menstrual onset. In the new study the average age of menarche was 11.7 years among the overweight or obese girls and 12.8 years among those with normal weights.
But the study’s unique use of an average of about three serial ultrasound breast examinations of each subject during an average 4 years of follow-up also showed that higher levels of body fat linked with slowed breast development in later stages, specifically maturation from stage D to stages D/E and E.
For example, girls with 33% body fat spent an average of 8.2 months in stage D, which stretched to an average of 11.2 months among girls with 38% body fat, reported Madison T. Ortega, a researcher with the Pediatric Endocrinology Group of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C., who presented the report at the meeting.
Ultrasound shows what inspection can’t
Results from “several studies have shown earlier breast development in overweight and obese girls by inspection and palpation,” but the new findings from ultrasound examination provide more nuance about the structural breast changes actually occurring in these adolescents, said Dr. Shaw, who heads the Pediatric Endocrinology Group. The current study “was not designed to capture the onset of breast development,” and “it is possible that increased androgens or insulin resistance in girls with higher body fat interferes with normal breast development,” she explained in an interview.
“The authors showed that the timing and progress of early stages of puberty were not earlier in overweight or obese girls. Luteinizing hormone, the indicator of neuroendocrine pubertal onset, and timing of early stages of breast development were the same in all weight groups. The authors also discovered falsely advanced Turner breast stage designations with ultrasonography in some girls with obesity. This might suggest that prior findings in epidemiologic studies of an earlier start to puberty based mostly on breast development stages identified by self-reported inspection and, rarely, palpation, may have been biased by breast adipose tissue,” said Christine M. Burt Solorzano, MD, a pediatric endocrinologist at the University of Virginia in Charlottesville, who was not involved in the study.
“Development of increased follicle-stimulating hormone in late puberty suggests that pubertal tempo, not onset, may be increased in girls with obesity, and goes along with earlier menarche. Their finding of increased androgen levels during mid to late puberty with obesity are consistent with prior findings,” including work published Dr. Burt Solorzano and her associates, she noted. “Delayed timing of advanced breast morphology was unexpected and may reflect relatively lower levels of progesterone in girls with obesity,” a hormone necessary for later stages of breast maturation.
The findings “reinforce that early breast development in the setting of obesity may in fact reflect adipose tissue and not be a true representation of neuroendocrine precocious puberty,” Dr. Burt Solorzano said in an interview. The findings “also suggest that pubertal initiation may not happen earlier in girls with obesity, as has been thought, but rather the tempo of puberty may be more rapid, leading to earlier menarche.”
A possible step toward PCOS
The long-term clinical consequences of the hormonal state linked with overweight and obesity “are unknown,” said Dr. Shaw. However, she and her coworkers followed a few of their subjects with elevated testosterone levels during midpuberty, and several developed signs of early polycystic ovarian syndrome (PCOS) such as irregular menstrual cycles, acne, and hirsutism. “It may be possible to identify girls at high risk for PCOS before menarche,” she suggested.
Dr. Burt Solorzano agreed that delayed breast development in girls with high levels of body fat may reflect inadequate progesterone production, which when coupled with an obesity-related excess level of androgens could put girls at risk for chronic anovulation and later PCOS.
“Weight management during childhood and early puberty may mitigate the adverse effects of obesity on pubertal progression and avoid some of the lifetime complications related to early menarche,” Dr. Burt Solorzano said.
The Body Weight and Puberty Study enrolled 36 girls who were overweight or obese and 54 girls with normal weight. They averaged 11 years of age, with a range of 8.2-14.7 years. Average percent body fat was 41% among the overweight or obese girls and 27% among those with normal weight. The results reported by Ms. Ortega also appeared in a report published Feb 22, 2021 (J Clin Endocrinol Metab. doi: 10.1210/clinem/dgab092).
Dr. Shaw, Ms. Ortega, and Dr. Burt Solorzano had no disclosures.
Girls in late stages of puberty who had elevated levels of body fat showed unusually high levels of several hormones that could contribute to an earlier age of menarche and also slow breast development, according to data from 90 girls who spanned a wide range of body fat in the first longitudinal study to examine links between fat volume, levels of reproductive hormones, and clinical manifestations of hormone action during puberty.
The results showed that girls with greater body fat had higher levels of follicle stimulating hormone, inhibin B, estrone, and certain male-like reproductive hormones, and that this pattern “is specifically tied to body fat,” said Natalie D. Shaw, MD, senior investigator for the study, reported at the annual meeting of the Endocrine Society.
“We found that total body fat is associated with the timing of menarche, as others have reported for body weight,” she noted. The new findings showed that every 1% rise in percent total body fat linked with a significant 3% rise in the likelihood of menarche, menstrual onset. In the new study the average age of menarche was 11.7 years among the overweight or obese girls and 12.8 years among those with normal weights.
But the study’s unique use of an average of about three serial ultrasound breast examinations of each subject during an average 4 years of follow-up also showed that higher levels of body fat linked with slowed breast development in later stages, specifically maturation from stage D to stages D/E and E.
For example, girls with 33% body fat spent an average of 8.2 months in stage D, which stretched to an average of 11.2 months among girls with 38% body fat, reported Madison T. Ortega, a researcher with the Pediatric Endocrinology Group of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C., who presented the report at the meeting.
Ultrasound shows what inspection can’t
Results from “several studies have shown earlier breast development in overweight and obese girls by inspection and palpation,” but the new findings from ultrasound examination provide more nuance about the structural breast changes actually occurring in these adolescents, said Dr. Shaw, who heads the Pediatric Endocrinology Group. The current study “was not designed to capture the onset of breast development,” and “it is possible that increased androgens or insulin resistance in girls with higher body fat interferes with normal breast development,” she explained in an interview.
“The authors showed that the timing and progress of early stages of puberty were not earlier in overweight or obese girls. Luteinizing hormone, the indicator of neuroendocrine pubertal onset, and timing of early stages of breast development were the same in all weight groups. The authors also discovered falsely advanced Turner breast stage designations with ultrasonography in some girls with obesity. This might suggest that prior findings in epidemiologic studies of an earlier start to puberty based mostly on breast development stages identified by self-reported inspection and, rarely, palpation, may have been biased by breast adipose tissue,” said Christine M. Burt Solorzano, MD, a pediatric endocrinologist at the University of Virginia in Charlottesville, who was not involved in the study.
“Development of increased follicle-stimulating hormone in late puberty suggests that pubertal tempo, not onset, may be increased in girls with obesity, and goes along with earlier menarche. Their finding of increased androgen levels during mid to late puberty with obesity are consistent with prior findings,” including work published Dr. Burt Solorzano and her associates, she noted. “Delayed timing of advanced breast morphology was unexpected and may reflect relatively lower levels of progesterone in girls with obesity,” a hormone necessary for later stages of breast maturation.
The findings “reinforce that early breast development in the setting of obesity may in fact reflect adipose tissue and not be a true representation of neuroendocrine precocious puberty,” Dr. Burt Solorzano said in an interview. The findings “also suggest that pubertal initiation may not happen earlier in girls with obesity, as has been thought, but rather the tempo of puberty may be more rapid, leading to earlier menarche.”
A possible step toward PCOS
The long-term clinical consequences of the hormonal state linked with overweight and obesity “are unknown,” said Dr. Shaw. However, she and her coworkers followed a few of their subjects with elevated testosterone levels during midpuberty, and several developed signs of early polycystic ovarian syndrome (PCOS) such as irregular menstrual cycles, acne, and hirsutism. “It may be possible to identify girls at high risk for PCOS before menarche,” she suggested.
Dr. Burt Solorzano agreed that delayed breast development in girls with high levels of body fat may reflect inadequate progesterone production, which when coupled with an obesity-related excess level of androgens could put girls at risk for chronic anovulation and later PCOS.
“Weight management during childhood and early puberty may mitigate the adverse effects of obesity on pubertal progression and avoid some of the lifetime complications related to early menarche,” Dr. Burt Solorzano said.
The Body Weight and Puberty Study enrolled 36 girls who were overweight or obese and 54 girls with normal weight. They averaged 11 years of age, with a range of 8.2-14.7 years. Average percent body fat was 41% among the overweight or obese girls and 27% among those with normal weight. The results reported by Ms. Ortega also appeared in a report published Feb 22, 2021 (J Clin Endocrinol Metab. doi: 10.1210/clinem/dgab092).
Dr. Shaw, Ms. Ortega, and Dr. Burt Solorzano had no disclosures.
FROM ENDO 2021
Exercise plus liraglutide better for maintaining weight loss than either strategy alone
For persons with obesity who lost a substantial amount of weight on a low-calorie diet, the combination of exercise and medication significantly improved weight-loss maintenance, and more so than either strategy alone, according to results of a randomized, head-to-head trial.
A year after starting moderate to vigorous exercise coupled with liraglutide treatment, study participants had a weight loss 9.5 kg more than those who received placebo and usual activity, study results show.
Reductions in both weight and fat loss seen with exercise and liraglutide was roughly twice as much as what was achieved at 1 year with the strategies of liraglutide or exercise alone, according to authors of the study, which appears in the New England Journal of Medicine .
Although the findings may not apply to those who can’t or won’t perform moderate to vigorous exercise, the intervention in this study was nevertheless feasible in this group of persons with obesity who had a very low level of fitness, according to the authors.
Hope for healthy weight loss maintenance
Investigator Signe S. Torekov, PhD, said in an interview that these results provide hope that more-intensive exercise regimens, with or without medication, can be useful and well accepted among individuals struggling with obesity.
“When we started our study, we were told, ‘you are never going to have people with obesity exercising that much, and for that long’ – but people were actually very happy about the exercise,” said Dr. Torekov, a professor in the department of biomedical sciences at the University of Copenhagen.
“If you actually set up a program where people are monitored and you have a feedback system, then exercise is an excellent component in obesity treatment that should be much more actively used – not only for its weight-lowering component, but also for improving health and quality of life,” she said in an interview.
Weight-management specialist John D. Clark, MD, PhD, said results of this study can be used to help inform patients about how successful different strategies incorporating exercise and medication may be following initial weight loss.
“When patients plateau on a consistent, calorie-restricted dietary plan, we can educate them and manage expectations about what options may be available to them after their initial weight loss,” said Dr. Clark, of the University of Texas, Dallas.
“If the patient’s goal specifically is weight loss at all costs, then I may suggest, ‘let’s consider liraglutide or liraglutide in combination with exercise,’ ” he said in an interview. “Exercise improves body composition, even if it may not on its own be as successful in the next phase of their weight-loss journey, as shown in this study.”
Obesity and weight-loss challenges
Although it’s not uncommon for obese patients to lose a large amount of weight, keeping the weight off is frequently a challenge unless the patient follows a structured weight maintenance program, according to Dr. Torekov and coauthors.
The rapid weight regain seen in many obese patients could be a result of reductions in total energy expenditure or increased appetite. Exercise is one strategy to sustain weight loss, though according to the authors, very few studies have looked at exercise in isolation to quantify its contribution to maintenance.
Accordingly, the present study sought to determine whether exercise, medication, or the combination thereof works best to keep weight off.
The study incorporated liraglutide, a GLP-1 receptor agonist indicated for chronic weight management, along with a reduced-calorie diet and increased physical activity, in adults with elevated body mass index and at least one weight-related comorbidity.
The investigator-initiated phase 3 trial included 215 adults with a body mass index of 32-43. Individuals with type 2 diabetes were excluded. All participants followed an 8-week, low-calorie diet comprising 800 calories per day.
Participants who lost 5% or more of their body weight were then randomized to 1 year of exercise plus liraglutide, exercise plus placebo, usual activity plus liraglutide, or usual activity plus placebo.
The exercise program – which was structured but flexible, according to investigators – included group exercise sessions that incorporated 30 minutes of indoor cycling and 15 minutes of circuit training 2 days each week. Participants wore heart rate monitors during exercise to make sure they reached targets for moderate to vigorous intensity.
Instructors trained in exercise physiology planned and monitored individualized exercise programs for each participant in the exercise-medication or exercise-only arms of the study.
Participants in all groups attended 12 one-on-one consultations where body weight was measured and dietetic support was provided.
Weight loss with exercise and medication
Out of 215 individuals enrolled in the study, 195 lost at least 5% of body weight and continued on to the randomized portion, the investigators reported. During the diet phase, they lost a mean of 13.1 kg, translating into a 12% mean reduction in body weight.
The mean frequency of exercise was 2.4 times per week in the exercise-plus-medication group and 2.5 times per week in the exercise-only group. About one-third of the exercise took place in the group sessions, and there was no difference in relative intensity between group and individual exercise regimens, the investigators said.
Individuals in the exercise plus medication group continued to lose more weight, such that, at the end of 1 year, the weight loss decreased even further, by a mean of –3.4 kg. By contrast, weight increased by a mean of 6.1 kg for the placebo group, adding up to a treatment difference of –9.5 kg (95% confidence interval, –13.1 to –5.9; P < .001), according to the report.
That treatment effect was also seen, but more muted, in the exercise- and liraglutide-only groups, at –4.1 kg and –6.8 kg, respectively.
A significant treatment effect was observed for exercise plus liraglutide, compared with exercise alone, at –5.4 kg (P = .004), while the treatment effect for the combination versus liraglutide alone was not significant at –2.7 kg (P = .13), the data show.
Body-fat reduction at 52 weeks was –3.9 percentage points for exercise plus liraglutide as compared with placebo, or roughly twice the reductions seen in the exercise- and liraglutide-alone groups, the investigators said, adding that the combination preserved lean mass.
Reductions in hemoglobin A1c, which are generally thought to reduce diabetes risk, were reduced in both the liraglutide and liraglutide-exercise combination group, according to their report.
The research was supported in part by grants from the Novo Nordisk Foundation.
For persons with obesity who lost a substantial amount of weight on a low-calorie diet, the combination of exercise and medication significantly improved weight-loss maintenance, and more so than either strategy alone, according to results of a randomized, head-to-head trial.
A year after starting moderate to vigorous exercise coupled with liraglutide treatment, study participants had a weight loss 9.5 kg more than those who received placebo and usual activity, study results show.
Reductions in both weight and fat loss seen with exercise and liraglutide was roughly twice as much as what was achieved at 1 year with the strategies of liraglutide or exercise alone, according to authors of the study, which appears in the New England Journal of Medicine .
Although the findings may not apply to those who can’t or won’t perform moderate to vigorous exercise, the intervention in this study was nevertheless feasible in this group of persons with obesity who had a very low level of fitness, according to the authors.
Hope for healthy weight loss maintenance
Investigator Signe S. Torekov, PhD, said in an interview that these results provide hope that more-intensive exercise regimens, with or without medication, can be useful and well accepted among individuals struggling with obesity.
“When we started our study, we were told, ‘you are never going to have people with obesity exercising that much, and for that long’ – but people were actually very happy about the exercise,” said Dr. Torekov, a professor in the department of biomedical sciences at the University of Copenhagen.
“If you actually set up a program where people are monitored and you have a feedback system, then exercise is an excellent component in obesity treatment that should be much more actively used – not only for its weight-lowering component, but also for improving health and quality of life,” she said in an interview.
Weight-management specialist John D. Clark, MD, PhD, said results of this study can be used to help inform patients about how successful different strategies incorporating exercise and medication may be following initial weight loss.
“When patients plateau on a consistent, calorie-restricted dietary plan, we can educate them and manage expectations about what options may be available to them after their initial weight loss,” said Dr. Clark, of the University of Texas, Dallas.
“If the patient’s goal specifically is weight loss at all costs, then I may suggest, ‘let’s consider liraglutide or liraglutide in combination with exercise,’ ” he said in an interview. “Exercise improves body composition, even if it may not on its own be as successful in the next phase of their weight-loss journey, as shown in this study.”
Obesity and weight-loss challenges
Although it’s not uncommon for obese patients to lose a large amount of weight, keeping the weight off is frequently a challenge unless the patient follows a structured weight maintenance program, according to Dr. Torekov and coauthors.
The rapid weight regain seen in many obese patients could be a result of reductions in total energy expenditure or increased appetite. Exercise is one strategy to sustain weight loss, though according to the authors, very few studies have looked at exercise in isolation to quantify its contribution to maintenance.
Accordingly, the present study sought to determine whether exercise, medication, or the combination thereof works best to keep weight off.
The study incorporated liraglutide, a GLP-1 receptor agonist indicated for chronic weight management, along with a reduced-calorie diet and increased physical activity, in adults with elevated body mass index and at least one weight-related comorbidity.
The investigator-initiated phase 3 trial included 215 adults with a body mass index of 32-43. Individuals with type 2 diabetes were excluded. All participants followed an 8-week, low-calorie diet comprising 800 calories per day.
Participants who lost 5% or more of their body weight were then randomized to 1 year of exercise plus liraglutide, exercise plus placebo, usual activity plus liraglutide, or usual activity plus placebo.
The exercise program – which was structured but flexible, according to investigators – included group exercise sessions that incorporated 30 minutes of indoor cycling and 15 minutes of circuit training 2 days each week. Participants wore heart rate monitors during exercise to make sure they reached targets for moderate to vigorous intensity.
Instructors trained in exercise physiology planned and monitored individualized exercise programs for each participant in the exercise-medication or exercise-only arms of the study.
Participants in all groups attended 12 one-on-one consultations where body weight was measured and dietetic support was provided.
Weight loss with exercise and medication
Out of 215 individuals enrolled in the study, 195 lost at least 5% of body weight and continued on to the randomized portion, the investigators reported. During the diet phase, they lost a mean of 13.1 kg, translating into a 12% mean reduction in body weight.
The mean frequency of exercise was 2.4 times per week in the exercise-plus-medication group and 2.5 times per week in the exercise-only group. About one-third of the exercise took place in the group sessions, and there was no difference in relative intensity between group and individual exercise regimens, the investigators said.
Individuals in the exercise plus medication group continued to lose more weight, such that, at the end of 1 year, the weight loss decreased even further, by a mean of –3.4 kg. By contrast, weight increased by a mean of 6.1 kg for the placebo group, adding up to a treatment difference of –9.5 kg (95% confidence interval, –13.1 to –5.9; P < .001), according to the report.
That treatment effect was also seen, but more muted, in the exercise- and liraglutide-only groups, at –4.1 kg and –6.8 kg, respectively.
A significant treatment effect was observed for exercise plus liraglutide, compared with exercise alone, at –5.4 kg (P = .004), while the treatment effect for the combination versus liraglutide alone was not significant at –2.7 kg (P = .13), the data show.
Body-fat reduction at 52 weeks was –3.9 percentage points for exercise plus liraglutide as compared with placebo, or roughly twice the reductions seen in the exercise- and liraglutide-alone groups, the investigators said, adding that the combination preserved lean mass.
Reductions in hemoglobin A1c, which are generally thought to reduce diabetes risk, were reduced in both the liraglutide and liraglutide-exercise combination group, according to their report.
The research was supported in part by grants from the Novo Nordisk Foundation.
For persons with obesity who lost a substantial amount of weight on a low-calorie diet, the combination of exercise and medication significantly improved weight-loss maintenance, and more so than either strategy alone, according to results of a randomized, head-to-head trial.
A year after starting moderate to vigorous exercise coupled with liraglutide treatment, study participants had a weight loss 9.5 kg more than those who received placebo and usual activity, study results show.
Reductions in both weight and fat loss seen with exercise and liraglutide was roughly twice as much as what was achieved at 1 year with the strategies of liraglutide or exercise alone, according to authors of the study, which appears in the New England Journal of Medicine .
Although the findings may not apply to those who can’t or won’t perform moderate to vigorous exercise, the intervention in this study was nevertheless feasible in this group of persons with obesity who had a very low level of fitness, according to the authors.
Hope for healthy weight loss maintenance
Investigator Signe S. Torekov, PhD, said in an interview that these results provide hope that more-intensive exercise regimens, with or without medication, can be useful and well accepted among individuals struggling with obesity.
“When we started our study, we were told, ‘you are never going to have people with obesity exercising that much, and for that long’ – but people were actually very happy about the exercise,” said Dr. Torekov, a professor in the department of biomedical sciences at the University of Copenhagen.
“If you actually set up a program where people are monitored and you have a feedback system, then exercise is an excellent component in obesity treatment that should be much more actively used – not only for its weight-lowering component, but also for improving health and quality of life,” she said in an interview.
Weight-management specialist John D. Clark, MD, PhD, said results of this study can be used to help inform patients about how successful different strategies incorporating exercise and medication may be following initial weight loss.
“When patients plateau on a consistent, calorie-restricted dietary plan, we can educate them and manage expectations about what options may be available to them after their initial weight loss,” said Dr. Clark, of the University of Texas, Dallas.
“If the patient’s goal specifically is weight loss at all costs, then I may suggest, ‘let’s consider liraglutide or liraglutide in combination with exercise,’ ” he said in an interview. “Exercise improves body composition, even if it may not on its own be as successful in the next phase of their weight-loss journey, as shown in this study.”
Obesity and weight-loss challenges
Although it’s not uncommon for obese patients to lose a large amount of weight, keeping the weight off is frequently a challenge unless the patient follows a structured weight maintenance program, according to Dr. Torekov and coauthors.
The rapid weight regain seen in many obese patients could be a result of reductions in total energy expenditure or increased appetite. Exercise is one strategy to sustain weight loss, though according to the authors, very few studies have looked at exercise in isolation to quantify its contribution to maintenance.
Accordingly, the present study sought to determine whether exercise, medication, or the combination thereof works best to keep weight off.
The study incorporated liraglutide, a GLP-1 receptor agonist indicated for chronic weight management, along with a reduced-calorie diet and increased physical activity, in adults with elevated body mass index and at least one weight-related comorbidity.
The investigator-initiated phase 3 trial included 215 adults with a body mass index of 32-43. Individuals with type 2 diabetes were excluded. All participants followed an 8-week, low-calorie diet comprising 800 calories per day.
Participants who lost 5% or more of their body weight were then randomized to 1 year of exercise plus liraglutide, exercise plus placebo, usual activity plus liraglutide, or usual activity plus placebo.
The exercise program – which was structured but flexible, according to investigators – included group exercise sessions that incorporated 30 minutes of indoor cycling and 15 minutes of circuit training 2 days each week. Participants wore heart rate monitors during exercise to make sure they reached targets for moderate to vigorous intensity.
Instructors trained in exercise physiology planned and monitored individualized exercise programs for each participant in the exercise-medication or exercise-only arms of the study.
Participants in all groups attended 12 one-on-one consultations where body weight was measured and dietetic support was provided.
Weight loss with exercise and medication
Out of 215 individuals enrolled in the study, 195 lost at least 5% of body weight and continued on to the randomized portion, the investigators reported. During the diet phase, they lost a mean of 13.1 kg, translating into a 12% mean reduction in body weight.
The mean frequency of exercise was 2.4 times per week in the exercise-plus-medication group and 2.5 times per week in the exercise-only group. About one-third of the exercise took place in the group sessions, and there was no difference in relative intensity between group and individual exercise regimens, the investigators said.
Individuals in the exercise plus medication group continued to lose more weight, such that, at the end of 1 year, the weight loss decreased even further, by a mean of –3.4 kg. By contrast, weight increased by a mean of 6.1 kg for the placebo group, adding up to a treatment difference of –9.5 kg (95% confidence interval, –13.1 to –5.9; P < .001), according to the report.
That treatment effect was also seen, but more muted, in the exercise- and liraglutide-only groups, at –4.1 kg and –6.8 kg, respectively.
A significant treatment effect was observed for exercise plus liraglutide, compared with exercise alone, at –5.4 kg (P = .004), while the treatment effect for the combination versus liraglutide alone was not significant at –2.7 kg (P = .13), the data show.
Body-fat reduction at 52 weeks was –3.9 percentage points for exercise plus liraglutide as compared with placebo, or roughly twice the reductions seen in the exercise- and liraglutide-alone groups, the investigators said, adding that the combination preserved lean mass.
Reductions in hemoglobin A1c, which are generally thought to reduce diabetes risk, were reduced in both the liraglutide and liraglutide-exercise combination group, according to their report.
The research was supported in part by grants from the Novo Nordisk Foundation.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE