User login
Children and COVID: Vaccinations, new cases both rising
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.
the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
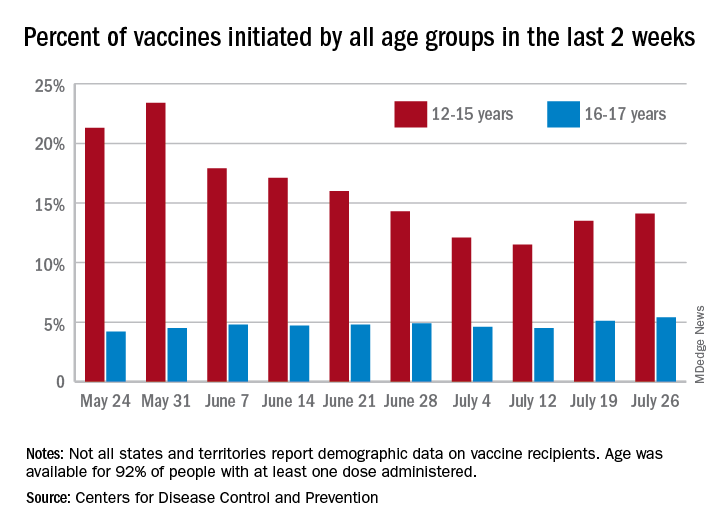
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
Nowhere to run and nowhere to hide
Not surprisingly, the pandemic has torn at the already fraying fabric of many families. Cooped up away from friends and the emotional relief valve of school, even children who had been relatively easy to manage in the past have posed disciplinary challenges beyond their parents’ abilities to cope.
In a recent study from the Parenting in Context Lab of the University of Michigan (“Child Discipline During the Covid-19 Pandemic,” Family Snapshots: Life During the Pandemic, American Academy of Pediatrics, June 8 2021) researchers found that one in six parents surveyed (n = 3,000 adults) admitted to spanking. Nearly half of the parents said that they had yelled at or threatened their children.
Five out of six parents reported using what the investigators described as less harsh “positive discipline measures.” Three-quarters of these parents used “explaining” as a strategy and nearly the same number used either time-outs or sent the children to their rooms.
Again, not surprisingly, parents who had experienced at least one adverse childhood experience (ACE) were more than twice as likely to spank. And parents who reported an episode of intimate partner violence (IPV) were more likely to resort to a harsh discipline strategy (yelling, threatening, or spanking).
Over my professional career I’ve spent a lot of time thinking about discipline and I have attempted to summarize my thoughts in a book titled, “How to Say No to Your Toddler” (Simon and Schuster, 2003), that has been published in four languages. Based on my observations, trying to explain to a misbehaving child the error of his ways is generally parental time not well spent. A well-structured time-out, preferably in a separate room with a door closed, is the most effective and safest discipline strategy.
However, as in all of my books, this advice on discipline was colored by the families in my practice and the audience for which I was writing, primarily middle class and upper middle class, reasonably affluent parents who buy books. These are usually folks who have homes in which children often have their own rooms, or where at least there are multiple rooms with doors – spaces to escape when tensions rise. Few of these parents have endured ACEs. Few have they experienced – nor have their children witnessed – IPV.
My advice that parents make only threats that can be safely carried, out such as time-out, and to always follow up on threats and promises, is valid regardless of a family’s socioeconomic situation. However, when it comes to choosing a consequence, my standard recommendation of a time-out can be difficult to follow for a family of six living in a three-room apartment, particularly during pandemic-dictated restrictions and lockdowns.
Of course there are alternatives to time-outs in a separate space, including an extended hug in a parental lap, but these responses require that the parents have been able to compose themselves well enough, and that they have the time. One of the important benefits of time-outs is that they can provide parents the time and space to reassess the situation and consider their role in the conflict. The bottom line is that a time-out is the safest and most effective form of discipline, but it requires space and a parent relatively unburdened of financial or emotional stress. Families without these luxuries are left with few alternatives other than physical or verbal abuse.
The AAP’s Family Snapshot concludes with the observation that “pediatricians and pediatric health care providers can continue to play an important role in supporting positive discipline strategies.” That is a difficult assignment even in prepandemic times, but for those of you working with families who lack the space and time to defuse disciplinary tensions, it is a heroic task.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Not surprisingly, the pandemic has torn at the already fraying fabric of many families. Cooped up away from friends and the emotional relief valve of school, even children who had been relatively easy to manage in the past have posed disciplinary challenges beyond their parents’ abilities to cope.
In a recent study from the Parenting in Context Lab of the University of Michigan (“Child Discipline During the Covid-19 Pandemic,” Family Snapshots: Life During the Pandemic, American Academy of Pediatrics, June 8 2021) researchers found that one in six parents surveyed (n = 3,000 adults) admitted to spanking. Nearly half of the parents said that they had yelled at or threatened their children.
Five out of six parents reported using what the investigators described as less harsh “positive discipline measures.” Three-quarters of these parents used “explaining” as a strategy and nearly the same number used either time-outs or sent the children to their rooms.
Again, not surprisingly, parents who had experienced at least one adverse childhood experience (ACE) were more than twice as likely to spank. And parents who reported an episode of intimate partner violence (IPV) were more likely to resort to a harsh discipline strategy (yelling, threatening, or spanking).
Over my professional career I’ve spent a lot of time thinking about discipline and I have attempted to summarize my thoughts in a book titled, “How to Say No to Your Toddler” (Simon and Schuster, 2003), that has been published in four languages. Based on my observations, trying to explain to a misbehaving child the error of his ways is generally parental time not well spent. A well-structured time-out, preferably in a separate room with a door closed, is the most effective and safest discipline strategy.
However, as in all of my books, this advice on discipline was colored by the families in my practice and the audience for which I was writing, primarily middle class and upper middle class, reasonably affluent parents who buy books. These are usually folks who have homes in which children often have their own rooms, or where at least there are multiple rooms with doors – spaces to escape when tensions rise. Few of these parents have endured ACEs. Few have they experienced – nor have their children witnessed – IPV.
My advice that parents make only threats that can be safely carried, out such as time-out, and to always follow up on threats and promises, is valid regardless of a family’s socioeconomic situation. However, when it comes to choosing a consequence, my standard recommendation of a time-out can be difficult to follow for a family of six living in a three-room apartment, particularly during pandemic-dictated restrictions and lockdowns.
Of course there are alternatives to time-outs in a separate space, including an extended hug in a parental lap, but these responses require that the parents have been able to compose themselves well enough, and that they have the time. One of the important benefits of time-outs is that they can provide parents the time and space to reassess the situation and consider their role in the conflict. The bottom line is that a time-out is the safest and most effective form of discipline, but it requires space and a parent relatively unburdened of financial or emotional stress. Families without these luxuries are left with few alternatives other than physical or verbal abuse.
The AAP’s Family Snapshot concludes with the observation that “pediatricians and pediatric health care providers can continue to play an important role in supporting positive discipline strategies.” That is a difficult assignment even in prepandemic times, but for those of you working with families who lack the space and time to defuse disciplinary tensions, it is a heroic task.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Not surprisingly, the pandemic has torn at the already fraying fabric of many families. Cooped up away from friends and the emotional relief valve of school, even children who had been relatively easy to manage in the past have posed disciplinary challenges beyond their parents’ abilities to cope.
In a recent study from the Parenting in Context Lab of the University of Michigan (“Child Discipline During the Covid-19 Pandemic,” Family Snapshots: Life During the Pandemic, American Academy of Pediatrics, June 8 2021) researchers found that one in six parents surveyed (n = 3,000 adults) admitted to spanking. Nearly half of the parents said that they had yelled at or threatened their children.
Five out of six parents reported using what the investigators described as less harsh “positive discipline measures.” Three-quarters of these parents used “explaining” as a strategy and nearly the same number used either time-outs or sent the children to their rooms.
Again, not surprisingly, parents who had experienced at least one adverse childhood experience (ACE) were more than twice as likely to spank. And parents who reported an episode of intimate partner violence (IPV) were more likely to resort to a harsh discipline strategy (yelling, threatening, or spanking).
Over my professional career I’ve spent a lot of time thinking about discipline and I have attempted to summarize my thoughts in a book titled, “How to Say No to Your Toddler” (Simon and Schuster, 2003), that has been published in four languages. Based on my observations, trying to explain to a misbehaving child the error of his ways is generally parental time not well spent. A well-structured time-out, preferably in a separate room with a door closed, is the most effective and safest discipline strategy.
However, as in all of my books, this advice on discipline was colored by the families in my practice and the audience for which I was writing, primarily middle class and upper middle class, reasonably affluent parents who buy books. These are usually folks who have homes in which children often have their own rooms, or where at least there are multiple rooms with doors – spaces to escape when tensions rise. Few of these parents have endured ACEs. Few have they experienced – nor have their children witnessed – IPV.
My advice that parents make only threats that can be safely carried, out such as time-out, and to always follow up on threats and promises, is valid regardless of a family’s socioeconomic situation. However, when it comes to choosing a consequence, my standard recommendation of a time-out can be difficult to follow for a family of six living in a three-room apartment, particularly during pandemic-dictated restrictions and lockdowns.
Of course there are alternatives to time-outs in a separate space, including an extended hug in a parental lap, but these responses require that the parents have been able to compose themselves well enough, and that they have the time. One of the important benefits of time-outs is that they can provide parents the time and space to reassess the situation and consider their role in the conflict. The bottom line is that a time-out is the safest and most effective form of discipline, but it requires space and a parent relatively unburdened of financial or emotional stress. Families without these luxuries are left with few alternatives other than physical or verbal abuse.
The AAP’s Family Snapshot concludes with the observation that “pediatricians and pediatric health care providers can continue to play an important role in supporting positive discipline strategies.” That is a difficult assignment even in prepandemic times, but for those of you working with families who lack the space and time to defuse disciplinary tensions, it is a heroic task.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Genetic testing for neurofibromatosis 1: An imperfect science
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
FROM SPD 2021
Childhood deprivation affects later executive function
Exposure to deprivation in early life was significantly associated with impaired executive functioning in children and adolescents, based on data from a systematic review and meta-analysis of 91 studies.
Previous research has shown connections between early-life adversity (ELA) and changes in psychological, cognitive, and neurobiological development, including increased risk of anxiety, depression, attention-deficit/hyperactivity disorder, conduct disorder, suicidality, and substance use disorder; however, research focusing on the associations between different types of ELA and specific processes is limited, wrote Dylan Johnson, MSc, of the University of Toronto and colleagues.
“We directly addressed this gap in the literature by examining the association between the type of ELA and executive functioning in children and youth,” they said.
In a study published in JAMA Pediatrics, the researchers identified 91 articles including 82 unique cohorts and 31,188 unique individuals aged 1-18 years.
The articles were selected from Embase, ERIC, MEDLINE, and PsycInfo databases and published up to Dec. 31, 2020. The primary outcomes were measures of the three domains of executive functioning: cognitive flexibility, inhibitory control, and working memory. To correct for small sample sizes in some studies, the researchers standardized their measures of association into Hedges g effect sizes.
Overall, the pooled estimates of the association of any childhood adversity with the three domains of executive functioning showed significant heterogeneity, with Hedges g effects of –0.49 for cognitive flexibility, –0.39 for inhibitory control, and –0.47 for working memory.
The researchers also examined a subsample of ELA–executive functioning associations in categories of early-life exposure to threat, compared with early-life deprivation, including 56 of the original 91 articles. In this analysis, significantly lower inhibitory control was associated with deprivation compared to threat (Hedges g –0.43 vs. –0.27). Similarly, significantly lower working memory was associated with deprivation, compared with threat (Hedges g –0.54 vs. Hedges g –0.28). For both inhibitory control and working memory, the association of adversity was not moderated by the age or sex of the study participants, study design, outcome quality, or selection quality, the researchers noted.
No significant difference in affect of exposure threat vs. deprivation was noted for the association with cognitive flexibility. The reason for this discrepancy remains unclear, the researchers said. “Some evidence suggests that individuals who grow up in unpredictable environments may have reduced inhibitory control but enhanced cognitive flexibility,” they noted.
However, the overall results suggest that exposure to deprivation may be associated with neurodevelopmental changes that support the development of executive functioning, they said.
The study findings were limited by several factors, including the substantial heterogeneity in the pooled estimates and the need to consider variation in study design, the researchers noted. In addition, the cross-sectional design of many studies prevented conclusions about causality between ELA and executive functioning, they said.
“Future research should explore the differences between threat and deprivation when emotionally salient executive functioning measures are used,” the researchers emphasized. “Threat experiences are often associated with alterations in emotional processing, and different findings may be observed when investigating emotionally salient executive functioning outcomes,” they concluded.
Prevention and intervention plans needed
“Although numerous studies have examined associations between ELA and executive functioning, the associations of threat and deprivation with specific executive functioning domains (e.g., cognitive flexibility, inhibitory control, and working memory) have not been explored comprehensively,” wrote Beth S. Slomine, PhD, and Nikeea Copeland-Linder, PhD, of the Kennedy Krieger Institute, Johns Hopkins University School, Baltimore, in an accompanying editorial.
The study is “critical and timely” because of the impact of the COVID-19 pandemic on children’s exposure to deprivation, the authors said. “Many children have experienced the death of family members or friends, food and housing insecurity owing to the economic recession, school closures, loss of critical support services, and increased isolation because of social distancing measures,” and these effects are even greater for children already living in poverty and those with developmental disabilities, they noted.
More resources are needed to develop and implement ELA prevention policies, as well as early intervention plans, the editorialists said.
“Early intervention programs have a great potential to reduce the risk of ELA and promote executive functioning development,” they said. “These programs, such as family support and preschool services, are viable solutions for children and their families,” they added. Although the pandemic prevented the use of many support services for children at risk, the adoption of telehealth technology means that “it is now more feasible for cognitive rehabilitation experts to implement the telehealth technology to train parents and school staff on how to assist with the delivery of interventions in real-world settings and how to promote executive functioning in daily life,” they noted.
Overall, the study findings highlight the urgency of identifying ELA and implementing strategies to reduce and prevent ELA, and to provide early intervention to mitigate the impact of ELA on executive function in children, the editorialists emphasized.
Data bring understanding, but barriers remain
“At this point, there are data demonstrating the significant impact that adverse childhood experiences have on health outcomes – from worsened mental health to an increased risk for cancer and diabetes,” said Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, in an interview.
“Physicians – myself included – tend to lump all these experiences together when thinking about future health outcomes,” Dr. Curran said. “However, there are evolving data that neurocognitive outcomes may be different based on the type of early-life adversity experienced. This meta-analysis examines the risk of different neurocognitive impact of threat versus deprivation types of adversity, which is important to pediatricians because it helps us to better understand the risks that our patients may experience,” she explained.
“The results of this meta-analysis were especially intriguing because I hadn’t previously considered the impact that different types of adversity had on neurocognitive development,” said Dr. Curran. “This study caused me to think about these experiences differently, and as I reflect on the patients I have cared for over the years, I can see the difference in their outcomes,” she said.
Many barriers persist in addressing the effects of early-life deprivation on executive function, Dr. Curran said.
“First are barriers around identification of these children and adolescents, who may not have regular contact with the medical system. Additionally, it’s important to provide resources for parents and caregivers – this includes creating a strong support network and providing education about the impact of these experiences,” she noted. “There are also barriers to identifying and connecting with what resources will help children at risk of poor neurodevelopmental outcomes,” she added.
“Now that we know that children who have experienced early-life deprivation are at increased risk of worsened neurodevelopmental outcomes, it will be important to understand what interventions can help improve their outcomes,” Dr. Curran said.
The study was supported by a Connaught New Researcher Award from the University of Toronto. The researchers had no financial conflicts to disclose.
Dr. Slomine disclosed book royalties from Cambridge University Press unrelated to this study. Dr. Curran had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Exposure to deprivation in early life was significantly associated with impaired executive functioning in children and adolescents, based on data from a systematic review and meta-analysis of 91 studies.
Previous research has shown connections between early-life adversity (ELA) and changes in psychological, cognitive, and neurobiological development, including increased risk of anxiety, depression, attention-deficit/hyperactivity disorder, conduct disorder, suicidality, and substance use disorder; however, research focusing on the associations between different types of ELA and specific processes is limited, wrote Dylan Johnson, MSc, of the University of Toronto and colleagues.
“We directly addressed this gap in the literature by examining the association between the type of ELA and executive functioning in children and youth,” they said.
In a study published in JAMA Pediatrics, the researchers identified 91 articles including 82 unique cohorts and 31,188 unique individuals aged 1-18 years.
The articles were selected from Embase, ERIC, MEDLINE, and PsycInfo databases and published up to Dec. 31, 2020. The primary outcomes were measures of the three domains of executive functioning: cognitive flexibility, inhibitory control, and working memory. To correct for small sample sizes in some studies, the researchers standardized their measures of association into Hedges g effect sizes.
Overall, the pooled estimates of the association of any childhood adversity with the three domains of executive functioning showed significant heterogeneity, with Hedges g effects of –0.49 for cognitive flexibility, –0.39 for inhibitory control, and –0.47 for working memory.
The researchers also examined a subsample of ELA–executive functioning associations in categories of early-life exposure to threat, compared with early-life deprivation, including 56 of the original 91 articles. In this analysis, significantly lower inhibitory control was associated with deprivation compared to threat (Hedges g –0.43 vs. –0.27). Similarly, significantly lower working memory was associated with deprivation, compared with threat (Hedges g –0.54 vs. Hedges g –0.28). For both inhibitory control and working memory, the association of adversity was not moderated by the age or sex of the study participants, study design, outcome quality, or selection quality, the researchers noted.
No significant difference in affect of exposure threat vs. deprivation was noted for the association with cognitive flexibility. The reason for this discrepancy remains unclear, the researchers said. “Some evidence suggests that individuals who grow up in unpredictable environments may have reduced inhibitory control but enhanced cognitive flexibility,” they noted.
However, the overall results suggest that exposure to deprivation may be associated with neurodevelopmental changes that support the development of executive functioning, they said.
The study findings were limited by several factors, including the substantial heterogeneity in the pooled estimates and the need to consider variation in study design, the researchers noted. In addition, the cross-sectional design of many studies prevented conclusions about causality between ELA and executive functioning, they said.
“Future research should explore the differences between threat and deprivation when emotionally salient executive functioning measures are used,” the researchers emphasized. “Threat experiences are often associated with alterations in emotional processing, and different findings may be observed when investigating emotionally salient executive functioning outcomes,” they concluded.
Prevention and intervention plans needed
“Although numerous studies have examined associations between ELA and executive functioning, the associations of threat and deprivation with specific executive functioning domains (e.g., cognitive flexibility, inhibitory control, and working memory) have not been explored comprehensively,” wrote Beth S. Slomine, PhD, and Nikeea Copeland-Linder, PhD, of the Kennedy Krieger Institute, Johns Hopkins University School, Baltimore, in an accompanying editorial.
The study is “critical and timely” because of the impact of the COVID-19 pandemic on children’s exposure to deprivation, the authors said. “Many children have experienced the death of family members or friends, food and housing insecurity owing to the economic recession, school closures, loss of critical support services, and increased isolation because of social distancing measures,” and these effects are even greater for children already living in poverty and those with developmental disabilities, they noted.
More resources are needed to develop and implement ELA prevention policies, as well as early intervention plans, the editorialists said.
“Early intervention programs have a great potential to reduce the risk of ELA and promote executive functioning development,” they said. “These programs, such as family support and preschool services, are viable solutions for children and their families,” they added. Although the pandemic prevented the use of many support services for children at risk, the adoption of telehealth technology means that “it is now more feasible for cognitive rehabilitation experts to implement the telehealth technology to train parents and school staff on how to assist with the delivery of interventions in real-world settings and how to promote executive functioning in daily life,” they noted.
Overall, the study findings highlight the urgency of identifying ELA and implementing strategies to reduce and prevent ELA, and to provide early intervention to mitigate the impact of ELA on executive function in children, the editorialists emphasized.
Data bring understanding, but barriers remain
“At this point, there are data demonstrating the significant impact that adverse childhood experiences have on health outcomes – from worsened mental health to an increased risk for cancer and diabetes,” said Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, in an interview.
“Physicians – myself included – tend to lump all these experiences together when thinking about future health outcomes,” Dr. Curran said. “However, there are evolving data that neurocognitive outcomes may be different based on the type of early-life adversity experienced. This meta-analysis examines the risk of different neurocognitive impact of threat versus deprivation types of adversity, which is important to pediatricians because it helps us to better understand the risks that our patients may experience,” she explained.
“The results of this meta-analysis were especially intriguing because I hadn’t previously considered the impact that different types of adversity had on neurocognitive development,” said Dr. Curran. “This study caused me to think about these experiences differently, and as I reflect on the patients I have cared for over the years, I can see the difference in their outcomes,” she said.
Many barriers persist in addressing the effects of early-life deprivation on executive function, Dr. Curran said.
“First are barriers around identification of these children and adolescents, who may not have regular contact with the medical system. Additionally, it’s important to provide resources for parents and caregivers – this includes creating a strong support network and providing education about the impact of these experiences,” she noted. “There are also barriers to identifying and connecting with what resources will help children at risk of poor neurodevelopmental outcomes,” she added.
“Now that we know that children who have experienced early-life deprivation are at increased risk of worsened neurodevelopmental outcomes, it will be important to understand what interventions can help improve their outcomes,” Dr. Curran said.
The study was supported by a Connaught New Researcher Award from the University of Toronto. The researchers had no financial conflicts to disclose.
Dr. Slomine disclosed book royalties from Cambridge University Press unrelated to this study. Dr. Curran had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Exposure to deprivation in early life was significantly associated with impaired executive functioning in children and adolescents, based on data from a systematic review and meta-analysis of 91 studies.
Previous research has shown connections between early-life adversity (ELA) and changes in psychological, cognitive, and neurobiological development, including increased risk of anxiety, depression, attention-deficit/hyperactivity disorder, conduct disorder, suicidality, and substance use disorder; however, research focusing on the associations between different types of ELA and specific processes is limited, wrote Dylan Johnson, MSc, of the University of Toronto and colleagues.
“We directly addressed this gap in the literature by examining the association between the type of ELA and executive functioning in children and youth,” they said.
In a study published in JAMA Pediatrics, the researchers identified 91 articles including 82 unique cohorts and 31,188 unique individuals aged 1-18 years.
The articles were selected from Embase, ERIC, MEDLINE, and PsycInfo databases and published up to Dec. 31, 2020. The primary outcomes were measures of the three domains of executive functioning: cognitive flexibility, inhibitory control, and working memory. To correct for small sample sizes in some studies, the researchers standardized their measures of association into Hedges g effect sizes.
Overall, the pooled estimates of the association of any childhood adversity with the three domains of executive functioning showed significant heterogeneity, with Hedges g effects of –0.49 for cognitive flexibility, –0.39 for inhibitory control, and –0.47 for working memory.
The researchers also examined a subsample of ELA–executive functioning associations in categories of early-life exposure to threat, compared with early-life deprivation, including 56 of the original 91 articles. In this analysis, significantly lower inhibitory control was associated with deprivation compared to threat (Hedges g –0.43 vs. –0.27). Similarly, significantly lower working memory was associated with deprivation, compared with threat (Hedges g –0.54 vs. Hedges g –0.28). For both inhibitory control and working memory, the association of adversity was not moderated by the age or sex of the study participants, study design, outcome quality, or selection quality, the researchers noted.
No significant difference in affect of exposure threat vs. deprivation was noted for the association with cognitive flexibility. The reason for this discrepancy remains unclear, the researchers said. “Some evidence suggests that individuals who grow up in unpredictable environments may have reduced inhibitory control but enhanced cognitive flexibility,” they noted.
However, the overall results suggest that exposure to deprivation may be associated with neurodevelopmental changes that support the development of executive functioning, they said.
The study findings were limited by several factors, including the substantial heterogeneity in the pooled estimates and the need to consider variation in study design, the researchers noted. In addition, the cross-sectional design of many studies prevented conclusions about causality between ELA and executive functioning, they said.
“Future research should explore the differences between threat and deprivation when emotionally salient executive functioning measures are used,” the researchers emphasized. “Threat experiences are often associated with alterations in emotional processing, and different findings may be observed when investigating emotionally salient executive functioning outcomes,” they concluded.
Prevention and intervention plans needed
“Although numerous studies have examined associations between ELA and executive functioning, the associations of threat and deprivation with specific executive functioning domains (e.g., cognitive flexibility, inhibitory control, and working memory) have not been explored comprehensively,” wrote Beth S. Slomine, PhD, and Nikeea Copeland-Linder, PhD, of the Kennedy Krieger Institute, Johns Hopkins University School, Baltimore, in an accompanying editorial.
The study is “critical and timely” because of the impact of the COVID-19 pandemic on children’s exposure to deprivation, the authors said. “Many children have experienced the death of family members or friends, food and housing insecurity owing to the economic recession, school closures, loss of critical support services, and increased isolation because of social distancing measures,” and these effects are even greater for children already living in poverty and those with developmental disabilities, they noted.
More resources are needed to develop and implement ELA prevention policies, as well as early intervention plans, the editorialists said.
“Early intervention programs have a great potential to reduce the risk of ELA and promote executive functioning development,” they said. “These programs, such as family support and preschool services, are viable solutions for children and their families,” they added. Although the pandemic prevented the use of many support services for children at risk, the adoption of telehealth technology means that “it is now more feasible for cognitive rehabilitation experts to implement the telehealth technology to train parents and school staff on how to assist with the delivery of interventions in real-world settings and how to promote executive functioning in daily life,” they noted.
Overall, the study findings highlight the urgency of identifying ELA and implementing strategies to reduce and prevent ELA, and to provide early intervention to mitigate the impact of ELA on executive function in children, the editorialists emphasized.
Data bring understanding, but barriers remain
“At this point, there are data demonstrating the significant impact that adverse childhood experiences have on health outcomes – from worsened mental health to an increased risk for cancer and diabetes,” said Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, in an interview.
“Physicians – myself included – tend to lump all these experiences together when thinking about future health outcomes,” Dr. Curran said. “However, there are evolving data that neurocognitive outcomes may be different based on the type of early-life adversity experienced. This meta-analysis examines the risk of different neurocognitive impact of threat versus deprivation types of adversity, which is important to pediatricians because it helps us to better understand the risks that our patients may experience,” she explained.
“The results of this meta-analysis were especially intriguing because I hadn’t previously considered the impact that different types of adversity had on neurocognitive development,” said Dr. Curran. “This study caused me to think about these experiences differently, and as I reflect on the patients I have cared for over the years, I can see the difference in their outcomes,” she said.
Many barriers persist in addressing the effects of early-life deprivation on executive function, Dr. Curran said.
“First are barriers around identification of these children and adolescents, who may not have regular contact with the medical system. Additionally, it’s important to provide resources for parents and caregivers – this includes creating a strong support network and providing education about the impact of these experiences,” she noted. “There are also barriers to identifying and connecting with what resources will help children at risk of poor neurodevelopmental outcomes,” she added.
“Now that we know that children who have experienced early-life deprivation are at increased risk of worsened neurodevelopmental outcomes, it will be important to understand what interventions can help improve their outcomes,” Dr. Curran said.
The study was supported by a Connaught New Researcher Award from the University of Toronto. The researchers had no financial conflicts to disclose.
Dr. Slomine disclosed book royalties from Cambridge University Press unrelated to this study. Dr. Curran had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM JAMA PEDIATRICS
No link between childhood vaccinations and allergies or asthma
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The first signs of elusive dysautonomia may appear on the skin
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
FROM SPD 2021
PHM virtual conference promises practical pearls, plus Dr. Fauci
The Pediatric Hospital Medicine annual conference, though virtual in 2021, promises to retain its role as the premier educational event for pediatric hospitalists and other clinicians involved in treating pediatric patients.
The “can’t-miss” session, on August 5, at 6:30 p.m. ET, is a one-on-one discussion between Anthony S. Fauci, MD, and Lee Savio Beers, MD, president of the American Academic of Pediatrics, according to members of the meeting planning committee.
In addition to the conversation between Dr. Beers and Dr. Fauci, this year’s meeting offers a mix of workshops with pointers and pearls to improve practice, keynote and plenary sessions to inform and inspire, and abstract presentations of new research. Three members of the PHM Planning Committee shared their insights on the hot topics, advice for new clinicians, and tips for making the most of this year’s meeting.
Workshops worth watching
“The keynote plenary sessions by Julie Silver, MD, on ‘Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion,’ and by Ilan Alhadeff, MD, on ‘Leading through Adversity’ should inspire even the least enthusiastic among us,” Mirna Giordano, MD, FHM, of Columbia University Medical Center, New York, said in an interview. A talk by Nathan T. Chomilo, MD, “will likely prompt reflection on how George Floyd’s death changed us, and how we practice medicine forever.” In addition, “PHM Stories are not to be missed, they are voices that speak loud and move mountains.”
The PHM Stories are concise, narrative talks with minimal use of slides; each PHM Stories session includes three distinct talks and a 15-minute question and answer session. PHM Stories sessions are scheduled for each day of the conference, and topics include “Practicing Medicine While Human: The Secrets Physicians Keep,” by Uchenna Ewulonu, MD; “Finding the Power of the Imposter: How I Learned to Be Exactly the Color I Am, Everywhere I Go,” by Alexandra Coria, MD; and “Purple Butterflies: A Reflection on Why I’m a Pediatric Hospitalist,” by Joanne Mendoza, MD.
“The PHM community has been through a lot in the aftermath of the pandemic,” said Dr. Giordano. “The mini-plenary session on the mental health needs of our patients, and clinical quick-hit sessions on verbal deescalation of the agitated patients and cardiac effects of COVID-19 will likely be not only very popular, but also useful in clinical endeavors. The workshop on how to navigate the adult issues in hospitalized patients will provide the Med-Peds pearls we all wish we heard earlier.”
Although a 75-minute workshop session may seem long, “the workshop choices will offer something for everyone’s taste: education, research, clinical topics, diversity, and advocacy,” Dr. Giordano said. “I suggest that attendees check in advance which sessions will be available after the meeting, so that they prioritize highly interactive sessions like workshops, and that they experience, even if virtual, small group/room gatherings and networking.” There will be time for fun, too, she emphasized, with social sessions “that we hope will break the screen monotony and bring smiles to everyone’s faces.”
For younger clinicians relatively new to practice, Dr. Giordano recommended several workshops for a wealth of advice and guidance, including “New Kids on the Block: Thriving in your First Faculty Position,” “Channeling Your Inner Coach: Techniques to Enhance Clinical Teaching & Feedback,” “Palliative Care Pearls for the Pediatric Hospitalist,” “Perioperative Medicine for Medically Complex Children: Case Studies in Programmatic Approaches,” “The Bare Necessities: Social Determinant of Health Screening for the Hospitalist,” and “Mentorship, Autonomy, and Supervising a PHM Fellow.”
Classic topics and new concepts
“We are so excited to be able to offer a full spectrum of offerings at this year’s virtual meeting,” Yemisi Jones, MD, FHM, of Cincinnati Children’s Hospital, said in an interview. “We are covering some classic topics that we can’t do without at PHM, such as clinical updates in the management of sick and well newborns; workshops on best practices for educators; as well as the latest in PHM scholarship.” Sessions include “timely topics such as equity for women in medicine with one of our plenary speakers, Julie Silver, MD, and new febrile infant guidelines,” she added.
In particular, the COVID-19 and mental health session will help address clinicians’ evolving understanding of the COVID-19 pandemic and its effects on hospitalized children, said Dr. Jones. “Attendees can expect practical, timely updates on the current state of the science and ways to improve their practice to provide the best care for our patients.”
Attendees will be able to maximize the virtual conference format by accessing archived recordings, including clinical quick hits, mini-plenaries, and PHM Stories, which can be viewed during the scheduled meeting time or after, Dr. Jones said. “Workshops and abstract presentations will involve real-time interaction with presenters, so would be highest yield to attend during the live meeting. We also encourage all participants to take full advantage of the platform and the various networking opportunities to engage with others in our PHM community.”
For residents and new fellows, Dr. Jones advised making the workshop, “A Whole New World: Tips and Tools to Soar Into Your First Year of Fellowship,” a priority. “For early-career faculty, the ‘New Kids on the Block: Thriving in your First Faculty Position workshop will be a valuable resource.”
Make the meeting content a priority
This year’s conference has an exceptional slate of plenary speakers, Michelle Marks, DO, SFHM, of the Cleveland Clinic said in an interview. In addition to the much-anticipated session on vaccinations, school guidelines, and other topics with Dr. Fauci and Dr. Beers, the sessions on leading through adversity and workforce diversity and inclusion are “important topics to the PHM community and to our greater communities as a whole.”
Dr. Marks also highlighted the value of the COVID-19 and mental health session, as the long-term impact of COVID-19 on mental health of children and adults continues to grab headlines. “From this session specifically, I hope the attendees will gain awareness of the special mental health needs for child during a global disaster like a pandemic, which can be generalized to other situations and gain skills and resources to help meet and advocate for children’s mental health needs.”
For clinicians attending the virtual conference, “The most important strategy is to schedule time off of clinical work for the virtual meeting if you can so you can focus on the content,” said Dr. Marks. “For the longer sessions, it would be very important to block time in your day to fully attend the session, attend in a private space if possible since there will be breakouts with discussion, have your camera on, and engage with the workshop group as much as possible. The virtual format can be challenging because of all the external distractions, so intentional focus is necessary,” to get the most out of the experience.
The mini-plenary session on “The New AAP Clinical Practice Guideline on the Evaluation and Management of Febrile Infants 8-60 Days Old,” is an important session for all attendees, Dr. Marks said. She also recommended the Clinical Quick Hits sessions for anyone seeking “a diverse array of practical knowledge which can be easily applied to everyday practice.” The Clinical Quick Hits are designed as 35-minute, rapid-fire presentations focused on clinical knowledge. Each of these presentations will focus on the latest updates or evolutions in clinical practice in one area. Some key topics include counseling parents when a child has an abnormal exam finding, assessing pelvic pain in adolescent girls, and preventing venous thromboembolism in the inpatient setting.
“I would also recommend that younger clinicians take in at least one or two workshops or sessions on nonclinical topics to see the breath of content at the meeting and to develop a niche interest for themselves outside of clinical work,” Dr. Marks noted.
Nonclinical sessions at PHM 2021 include workshops on a pilot for a comprehensive LGBTQ+ curriculum, using media tools for public health messaging, and practicing health literacy.
To register for the Pediatric Hospital Medicine 2021 virtual conference, visit https://apaevents.regfox.com/phm21-virtual-conference.
Dr. Giordano, Dr. Jones, and Dr. Marks are members of the PHM conference planning committee and had no relevant financial conflicts to disclose.
The Pediatric Hospital Medicine annual conference, though virtual in 2021, promises to retain its role as the premier educational event for pediatric hospitalists and other clinicians involved in treating pediatric patients.
The “can’t-miss” session, on August 5, at 6:30 p.m. ET, is a one-on-one discussion between Anthony S. Fauci, MD, and Lee Savio Beers, MD, president of the American Academic of Pediatrics, according to members of the meeting planning committee.
In addition to the conversation between Dr. Beers and Dr. Fauci, this year’s meeting offers a mix of workshops with pointers and pearls to improve practice, keynote and plenary sessions to inform and inspire, and abstract presentations of new research. Three members of the PHM Planning Committee shared their insights on the hot topics, advice for new clinicians, and tips for making the most of this year’s meeting.
Workshops worth watching
“The keynote plenary sessions by Julie Silver, MD, on ‘Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion,’ and by Ilan Alhadeff, MD, on ‘Leading through Adversity’ should inspire even the least enthusiastic among us,” Mirna Giordano, MD, FHM, of Columbia University Medical Center, New York, said in an interview. A talk by Nathan T. Chomilo, MD, “will likely prompt reflection on how George Floyd’s death changed us, and how we practice medicine forever.” In addition, “PHM Stories are not to be missed, they are voices that speak loud and move mountains.”
The PHM Stories are concise, narrative talks with minimal use of slides; each PHM Stories session includes three distinct talks and a 15-minute question and answer session. PHM Stories sessions are scheduled for each day of the conference, and topics include “Practicing Medicine While Human: The Secrets Physicians Keep,” by Uchenna Ewulonu, MD; “Finding the Power of the Imposter: How I Learned to Be Exactly the Color I Am, Everywhere I Go,” by Alexandra Coria, MD; and “Purple Butterflies: A Reflection on Why I’m a Pediatric Hospitalist,” by Joanne Mendoza, MD.
“The PHM community has been through a lot in the aftermath of the pandemic,” said Dr. Giordano. “The mini-plenary session on the mental health needs of our patients, and clinical quick-hit sessions on verbal deescalation of the agitated patients and cardiac effects of COVID-19 will likely be not only very popular, but also useful in clinical endeavors. The workshop on how to navigate the adult issues in hospitalized patients will provide the Med-Peds pearls we all wish we heard earlier.”
Although a 75-minute workshop session may seem long, “the workshop choices will offer something for everyone’s taste: education, research, clinical topics, diversity, and advocacy,” Dr. Giordano said. “I suggest that attendees check in advance which sessions will be available after the meeting, so that they prioritize highly interactive sessions like workshops, and that they experience, even if virtual, small group/room gatherings and networking.” There will be time for fun, too, she emphasized, with social sessions “that we hope will break the screen monotony and bring smiles to everyone’s faces.”
For younger clinicians relatively new to practice, Dr. Giordano recommended several workshops for a wealth of advice and guidance, including “New Kids on the Block: Thriving in your First Faculty Position,” “Channeling Your Inner Coach: Techniques to Enhance Clinical Teaching & Feedback,” “Palliative Care Pearls for the Pediatric Hospitalist,” “Perioperative Medicine for Medically Complex Children: Case Studies in Programmatic Approaches,” “The Bare Necessities: Social Determinant of Health Screening for the Hospitalist,” and “Mentorship, Autonomy, and Supervising a PHM Fellow.”
Classic topics and new concepts
“We are so excited to be able to offer a full spectrum of offerings at this year’s virtual meeting,” Yemisi Jones, MD, FHM, of Cincinnati Children’s Hospital, said in an interview. “We are covering some classic topics that we can’t do without at PHM, such as clinical updates in the management of sick and well newborns; workshops on best practices for educators; as well as the latest in PHM scholarship.” Sessions include “timely topics such as equity for women in medicine with one of our plenary speakers, Julie Silver, MD, and new febrile infant guidelines,” she added.
In particular, the COVID-19 and mental health session will help address clinicians’ evolving understanding of the COVID-19 pandemic and its effects on hospitalized children, said Dr. Jones. “Attendees can expect practical, timely updates on the current state of the science and ways to improve their practice to provide the best care for our patients.”
Attendees will be able to maximize the virtual conference format by accessing archived recordings, including clinical quick hits, mini-plenaries, and PHM Stories, which can be viewed during the scheduled meeting time or after, Dr. Jones said. “Workshops and abstract presentations will involve real-time interaction with presenters, so would be highest yield to attend during the live meeting. We also encourage all participants to take full advantage of the platform and the various networking opportunities to engage with others in our PHM community.”
For residents and new fellows, Dr. Jones advised making the workshop, “A Whole New World: Tips and Tools to Soar Into Your First Year of Fellowship,” a priority. “For early-career faculty, the ‘New Kids on the Block: Thriving in your First Faculty Position workshop will be a valuable resource.”
Make the meeting content a priority
This year’s conference has an exceptional slate of plenary speakers, Michelle Marks, DO, SFHM, of the Cleveland Clinic said in an interview. In addition to the much-anticipated session on vaccinations, school guidelines, and other topics with Dr. Fauci and Dr. Beers, the sessions on leading through adversity and workforce diversity and inclusion are “important topics to the PHM community and to our greater communities as a whole.”
Dr. Marks also highlighted the value of the COVID-19 and mental health session, as the long-term impact of COVID-19 on mental health of children and adults continues to grab headlines. “From this session specifically, I hope the attendees will gain awareness of the special mental health needs for child during a global disaster like a pandemic, which can be generalized to other situations and gain skills and resources to help meet and advocate for children’s mental health needs.”
For clinicians attending the virtual conference, “The most important strategy is to schedule time off of clinical work for the virtual meeting if you can so you can focus on the content,” said Dr. Marks. “For the longer sessions, it would be very important to block time in your day to fully attend the session, attend in a private space if possible since there will be breakouts with discussion, have your camera on, and engage with the workshop group as much as possible. The virtual format can be challenging because of all the external distractions, so intentional focus is necessary,” to get the most out of the experience.
The mini-plenary session on “The New AAP Clinical Practice Guideline on the Evaluation and Management of Febrile Infants 8-60 Days Old,” is an important session for all attendees, Dr. Marks said. She also recommended the Clinical Quick Hits sessions for anyone seeking “a diverse array of practical knowledge which can be easily applied to everyday practice.” The Clinical Quick Hits are designed as 35-minute, rapid-fire presentations focused on clinical knowledge. Each of these presentations will focus on the latest updates or evolutions in clinical practice in one area. Some key topics include counseling parents when a child has an abnormal exam finding, assessing pelvic pain in adolescent girls, and preventing venous thromboembolism in the inpatient setting.
“I would also recommend that younger clinicians take in at least one or two workshops or sessions on nonclinical topics to see the breath of content at the meeting and to develop a niche interest for themselves outside of clinical work,” Dr. Marks noted.
Nonclinical sessions at PHM 2021 include workshops on a pilot for a comprehensive LGBTQ+ curriculum, using media tools for public health messaging, and practicing health literacy.
To register for the Pediatric Hospital Medicine 2021 virtual conference, visit https://apaevents.regfox.com/phm21-virtual-conference.
Dr. Giordano, Dr. Jones, and Dr. Marks are members of the PHM conference planning committee and had no relevant financial conflicts to disclose.
The Pediatric Hospital Medicine annual conference, though virtual in 2021, promises to retain its role as the premier educational event for pediatric hospitalists and other clinicians involved in treating pediatric patients.
The “can’t-miss” session, on August 5, at 6:30 p.m. ET, is a one-on-one discussion between Anthony S. Fauci, MD, and Lee Savio Beers, MD, president of the American Academic of Pediatrics, according to members of the meeting planning committee.
In addition to the conversation between Dr. Beers and Dr. Fauci, this year’s meeting offers a mix of workshops with pointers and pearls to improve practice, keynote and plenary sessions to inform and inspire, and abstract presentations of new research. Three members of the PHM Planning Committee shared their insights on the hot topics, advice for new clinicians, and tips for making the most of this year’s meeting.
Workshops worth watching
“The keynote plenary sessions by Julie Silver, MD, on ‘Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion,’ and by Ilan Alhadeff, MD, on ‘Leading through Adversity’ should inspire even the least enthusiastic among us,” Mirna Giordano, MD, FHM, of Columbia University Medical Center, New York, said in an interview. A talk by Nathan T. Chomilo, MD, “will likely prompt reflection on how George Floyd’s death changed us, and how we practice medicine forever.” In addition, “PHM Stories are not to be missed, they are voices that speak loud and move mountains.”
The PHM Stories are concise, narrative talks with minimal use of slides; each PHM Stories session includes three distinct talks and a 15-minute question and answer session. PHM Stories sessions are scheduled for each day of the conference, and topics include “Practicing Medicine While Human: The Secrets Physicians Keep,” by Uchenna Ewulonu, MD; “Finding the Power of the Imposter: How I Learned to Be Exactly the Color I Am, Everywhere I Go,” by Alexandra Coria, MD; and “Purple Butterflies: A Reflection on Why I’m a Pediatric Hospitalist,” by Joanne Mendoza, MD.
“The PHM community has been through a lot in the aftermath of the pandemic,” said Dr. Giordano. “The mini-plenary session on the mental health needs of our patients, and clinical quick-hit sessions on verbal deescalation of the agitated patients and cardiac effects of COVID-19 will likely be not only very popular, but also useful in clinical endeavors. The workshop on how to navigate the adult issues in hospitalized patients will provide the Med-Peds pearls we all wish we heard earlier.”
Although a 75-minute workshop session may seem long, “the workshop choices will offer something for everyone’s taste: education, research, clinical topics, diversity, and advocacy,” Dr. Giordano said. “I suggest that attendees check in advance which sessions will be available after the meeting, so that they prioritize highly interactive sessions like workshops, and that they experience, even if virtual, small group/room gatherings and networking.” There will be time for fun, too, she emphasized, with social sessions “that we hope will break the screen monotony and bring smiles to everyone’s faces.”
For younger clinicians relatively new to practice, Dr. Giordano recommended several workshops for a wealth of advice and guidance, including “New Kids on the Block: Thriving in your First Faculty Position,” “Channeling Your Inner Coach: Techniques to Enhance Clinical Teaching & Feedback,” “Palliative Care Pearls for the Pediatric Hospitalist,” “Perioperative Medicine for Medically Complex Children: Case Studies in Programmatic Approaches,” “The Bare Necessities: Social Determinant of Health Screening for the Hospitalist,” and “Mentorship, Autonomy, and Supervising a PHM Fellow.”
Classic topics and new concepts
“We are so excited to be able to offer a full spectrum of offerings at this year’s virtual meeting,” Yemisi Jones, MD, FHM, of Cincinnati Children’s Hospital, said in an interview. “We are covering some classic topics that we can’t do without at PHM, such as clinical updates in the management of sick and well newborns; workshops on best practices for educators; as well as the latest in PHM scholarship.” Sessions include “timely topics such as equity for women in medicine with one of our plenary speakers, Julie Silver, MD, and new febrile infant guidelines,” she added.
In particular, the COVID-19 and mental health session will help address clinicians’ evolving understanding of the COVID-19 pandemic and its effects on hospitalized children, said Dr. Jones. “Attendees can expect practical, timely updates on the current state of the science and ways to improve their practice to provide the best care for our patients.”
Attendees will be able to maximize the virtual conference format by accessing archived recordings, including clinical quick hits, mini-plenaries, and PHM Stories, which can be viewed during the scheduled meeting time or after, Dr. Jones said. “Workshops and abstract presentations will involve real-time interaction with presenters, so would be highest yield to attend during the live meeting. We also encourage all participants to take full advantage of the platform and the various networking opportunities to engage with others in our PHM community.”
For residents and new fellows, Dr. Jones advised making the workshop, “A Whole New World: Tips and Tools to Soar Into Your First Year of Fellowship,” a priority. “For early-career faculty, the ‘New Kids on the Block: Thriving in your First Faculty Position workshop will be a valuable resource.”
Make the meeting content a priority
This year’s conference has an exceptional slate of plenary speakers, Michelle Marks, DO, SFHM, of the Cleveland Clinic said in an interview. In addition to the much-anticipated session on vaccinations, school guidelines, and other topics with Dr. Fauci and Dr. Beers, the sessions on leading through adversity and workforce diversity and inclusion are “important topics to the PHM community and to our greater communities as a whole.”
Dr. Marks also highlighted the value of the COVID-19 and mental health session, as the long-term impact of COVID-19 on mental health of children and adults continues to grab headlines. “From this session specifically, I hope the attendees will gain awareness of the special mental health needs for child during a global disaster like a pandemic, which can be generalized to other situations and gain skills and resources to help meet and advocate for children’s mental health needs.”
For clinicians attending the virtual conference, “The most important strategy is to schedule time off of clinical work for the virtual meeting if you can so you can focus on the content,” said Dr. Marks. “For the longer sessions, it would be very important to block time in your day to fully attend the session, attend in a private space if possible since there will be breakouts with discussion, have your camera on, and engage with the workshop group as much as possible. The virtual format can be challenging because of all the external distractions, so intentional focus is necessary,” to get the most out of the experience.
The mini-plenary session on “The New AAP Clinical Practice Guideline on the Evaluation and Management of Febrile Infants 8-60 Days Old,” is an important session for all attendees, Dr. Marks said. She also recommended the Clinical Quick Hits sessions for anyone seeking “a diverse array of practical knowledge which can be easily applied to everyday practice.” The Clinical Quick Hits are designed as 35-minute, rapid-fire presentations focused on clinical knowledge. Each of these presentations will focus on the latest updates or evolutions in clinical practice in one area. Some key topics include counseling parents when a child has an abnormal exam finding, assessing pelvic pain in adolescent girls, and preventing venous thromboembolism in the inpatient setting.
“I would also recommend that younger clinicians take in at least one or two workshops or sessions on nonclinical topics to see the breath of content at the meeting and to develop a niche interest for themselves outside of clinical work,” Dr. Marks noted.
Nonclinical sessions at PHM 2021 include workshops on a pilot for a comprehensive LGBTQ+ curriculum, using media tools for public health messaging, and practicing health literacy.
To register for the Pediatric Hospital Medicine 2021 virtual conference, visit https://apaevents.regfox.com/phm21-virtual-conference.
Dr. Giordano, Dr. Jones, and Dr. Marks are members of the PHM conference planning committee and had no relevant financial conflicts to disclose.
FDA okays extended-release exenatide for children with T2D
the agency announced July 22.
Previously approved in adults, the injectable is now the second glucagonlike peptide-1 receptor agonist approved for use in pediatric type 2 diabetes, after liraglutide (Victoza, Novo Nordisk) in 2019, and the first with once-weekly administration.
The two extended-release Bydureon products – which differ in delivery device and mixing procedure – are now indicated for use in addition to diet and exercise to improve glycemic control in pediatric patients 10 years of age or older with type 2 diabetes.
Exenatide extended release is not recommended as first-line treatment following diet and exercise.
The approval was based on a 24-week, double-blind, placebo-controlled study in 82 children with type 2 diabetes aged 10 and older. They were randomized to 2 mg once-weekly exenatide extended release or placebo. At week 24, hemoglobin A1c in those randomized to the drug had dropped by 0.25 percentage points, compared with a 0.45 percentage point increase in the placebo group.
Side effects were similar to those seen in adults, including injection site reactions, headaches, and gastrointestinal discomfort.
Currently, metformin is the only oral medication approved for treating pediatric type 2 diabetes, while the injectables also include insulin in addition to the two GLP-1 receptor agonists. During a symposium held in June 2021 at the annual scientific sessions of the American Diabetes Association, speakers expressed alarm about the rise in youth developing type 2 diabetes, noting that the condition typically progresses more rapidly and is less likely to respond well to metformin, compared with adults.
But, the panelists were also optimistic about extended-release exenatide as well as several other therapies for pediatric patients with type 2 diabetes in ongoing phase 3 trials, including the sodium-glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin, and the dipeptidyl peptidase–4 inhibitors alogliptin and linagliptin. Results are expected in the next 1-2 years.
the agency announced July 22.
Previously approved in adults, the injectable is now the second glucagonlike peptide-1 receptor agonist approved for use in pediatric type 2 diabetes, after liraglutide (Victoza, Novo Nordisk) in 2019, and the first with once-weekly administration.
The two extended-release Bydureon products – which differ in delivery device and mixing procedure – are now indicated for use in addition to diet and exercise to improve glycemic control in pediatric patients 10 years of age or older with type 2 diabetes.
Exenatide extended release is not recommended as first-line treatment following diet and exercise.
The approval was based on a 24-week, double-blind, placebo-controlled study in 82 children with type 2 diabetes aged 10 and older. They were randomized to 2 mg once-weekly exenatide extended release or placebo. At week 24, hemoglobin A1c in those randomized to the drug had dropped by 0.25 percentage points, compared with a 0.45 percentage point increase in the placebo group.
Side effects were similar to those seen in adults, including injection site reactions, headaches, and gastrointestinal discomfort.
Currently, metformin is the only oral medication approved for treating pediatric type 2 diabetes, while the injectables also include insulin in addition to the two GLP-1 receptor agonists. During a symposium held in June 2021 at the annual scientific sessions of the American Diabetes Association, speakers expressed alarm about the rise in youth developing type 2 diabetes, noting that the condition typically progresses more rapidly and is less likely to respond well to metformin, compared with adults.
But, the panelists were also optimistic about extended-release exenatide as well as several other therapies for pediatric patients with type 2 diabetes in ongoing phase 3 trials, including the sodium-glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin, and the dipeptidyl peptidase–4 inhibitors alogliptin and linagliptin. Results are expected in the next 1-2 years.
the agency announced July 22.
Previously approved in adults, the injectable is now the second glucagonlike peptide-1 receptor agonist approved for use in pediatric type 2 diabetes, after liraglutide (Victoza, Novo Nordisk) in 2019, and the first with once-weekly administration.
The two extended-release Bydureon products – which differ in delivery device and mixing procedure – are now indicated for use in addition to diet and exercise to improve glycemic control in pediatric patients 10 years of age or older with type 2 diabetes.
Exenatide extended release is not recommended as first-line treatment following diet and exercise.
The approval was based on a 24-week, double-blind, placebo-controlled study in 82 children with type 2 diabetes aged 10 and older. They were randomized to 2 mg once-weekly exenatide extended release or placebo. At week 24, hemoglobin A1c in those randomized to the drug had dropped by 0.25 percentage points, compared with a 0.45 percentage point increase in the placebo group.
Side effects were similar to those seen in adults, including injection site reactions, headaches, and gastrointestinal discomfort.
Currently, metformin is the only oral medication approved for treating pediatric type 2 diabetes, while the injectables also include insulin in addition to the two GLP-1 receptor agonists. During a symposium held in June 2021 at the annual scientific sessions of the American Diabetes Association, speakers expressed alarm about the rise in youth developing type 2 diabetes, noting that the condition typically progresses more rapidly and is less likely to respond well to metformin, compared with adults.
But, the panelists were also optimistic about extended-release exenatide as well as several other therapies for pediatric patients with type 2 diabetes in ongoing phase 3 trials, including the sodium-glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin, and the dipeptidyl peptidase–4 inhibitors alogliptin and linagliptin. Results are expected in the next 1-2 years.
Autoinflammatory diseases ‘not so rare after all,’ expert says
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
FROM SPD 2021
Expert proposes rethinking the classification of SJS/TEN
In the opinion of Neil H. Shear, MD, a stepwise approach is the best way to diagnose possible drug-induced skin disease and determine the root cause.
“Often, we need to think of more than one cause,” he said during the annual meeting of the Society for Pediatric Dermatology. “It could be drug X. It could be drug Y. It could be contrast media. We must think broadly and pay special attention to skin of color, overlapping syndromes, and the changing diagnostic assessment over time.”
His suggested diagnostic triangle includes appearance of the rash or lesion(s), systemic impact, and histology. “The first is the appearance,” said Dr. Shear, professor emeritus of dermatology, clinical pharmacology and toxicology, and medicine at the University of Toronto. “Is it exanthem? Is it blistering? Don’t just say drug ‘rash.’ That doesn’t work. You need to know if there are systemic features, and sometimes histologic information can change your approach or diagnosis, but not as often as one might think,” he said, noting that, in his view, the two main factors are appearance and systemic impact.
The presence of fever is a hallmark of systemic problems, he continued, “so if you see fever, you know you’re probably going to be dealing with a complex reaction, so we need to know the morphology.” Consider whether it is simple exanthem (a mild, uncomplicated rash) or complex exanthem (drug rash with eosinophilia and systemic symptoms or fever, malaise, and adenopathy).
As for other morphologies, urticarial lesions could be urticaria or a serum sickness-like reaction, pustular lesions could be acneiform or acute generalized exanthematous pustulosis, while
Dr. Shear considers SJS/TEN as a spectrum of blistering disease, “because there’s not a single diagnosis,” he said. “There’s a spectrum, if you will, depending on how advanced people are in their disease.” He coauthored a 1991 report describing eight cases of mycoplasma and Stevens-Johnson syndrome. “I was surprised at how long that stood up as about the only paper in that area,” he said. “But there’s much more happening now with a proliferation of terms,” he added, referring to MIRM (Mycoplasma pneumonia–induced rash and mucositis), RIME (reactive infectious mucocutaneous eruption); and Fuchs syndrome, or SJS without skin lesions.
What was not appreciated in the early classification of SJS, he continued, was a “side basket” of bullous erythema multiforme. “We didn’t know what to call it,” he said. “At one point we called it bullous erythema multiforme. At another point we called it erythema multiforme major. We just didn’t know what it was.”
The appearance and systemic effects of SJS comprise what he termed SJS type 2 – or the early stages of TEN. Taken together, he refers to these two conditions as TEN Spectrum, or TENS. “One of the traps is that TENS can look like varicella, and vice versa, especially in very dark brown or black skin,” Dr. Shear said. “You have to be careful. A biopsy might be worthwhile. Acute lupus has the pathology of TENS but the patients are not as systemically ill as true TENS.”
In 2011, Japanese researchers reported on 38 cases of SJS associated with M. pneumoniae, and 78 cases of drug-induced SJS. They found that 66% of adult patients with M. pneumoniae–associated SJS developed mucocutaneous lesions and fever/respiratory symptoms on the same day, mostly shortness of breath and cough. In contrast, most of the patients aged under 20 years developed fever/respiratory symptoms before mucocutaneous involvement.
“The big clinical differentiator between drug-induced SJS and mycoplasma-induced SJS was respiratory disorder,” said Dr. Shear, who was not affiliated with the study. “That means you’re probably looking at something that’s mycoplasma related [when respiratory problems are present]. Even if you can’t prove it’s mycoplasma related, that probably needs to be the target of your therapy. The idea ... is to make sure it’s clear at the end. One, so they get better, and two, so that we’re not giving drugs needlessly when it was really mycoplasma.”
Noting that HLA-B*15:02 is a marker for carbamazepine-induced SJS and TEN, he said, “a positive HLA test can support the diagnosis, confirm the suspected offending drug, and is valuable for familial genetic counseling.”
As for treatment of SJS, TEN, and other cytotoxic T-lymphocyte–mediated severe cutaneous adverse reactions, a randomized Japanese clinical trial evaluating prednisolone 1-1.5 mg/kg/day IV versus etanercept 25-50 mg subcutaneously twice per week in 96 patients with SJS-TEN found that etanercept decreased the mortality rate by 8.3%. In addition, etanercept reduced skin healing time, when compared with prednisolone (a median of 14 vs. 19 days, respectively; P = .010), and was associated with a lower incidence of GI hemorrhage (2.6% vs. 18.2%, respectively; P = .03).
Dr. Shear said that he would like to see better therapeutics for severe, complex patients. “After leaving the hospital, people with SJS or people with TEN need to have ongoing care, consultation, and explanation so they and their families know what drugs are safe in the future.”
Dr. Shear disclosed that he has been a consultant to AbbVie, Amgen, Bausch Medicine, Novartis, Sanofi-Genzyme, UCB, LEO Pharma, Otsuka, Janssen, Alpha Laboratories, Lilly, ChemoCentryx, Vivoryon, Galderma, Innovaderm, Chromocell, and Kyowa Kirin.
In the opinion of Neil H. Shear, MD, a stepwise approach is the best way to diagnose possible drug-induced skin disease and determine the root cause.
“Often, we need to think of more than one cause,” he said during the annual meeting of the Society for Pediatric Dermatology. “It could be drug X. It could be drug Y. It could be contrast media. We must think broadly and pay special attention to skin of color, overlapping syndromes, and the changing diagnostic assessment over time.”
His suggested diagnostic triangle includes appearance of the rash or lesion(s), systemic impact, and histology. “The first is the appearance,” said Dr. Shear, professor emeritus of dermatology, clinical pharmacology and toxicology, and medicine at the University of Toronto. “Is it exanthem? Is it blistering? Don’t just say drug ‘rash.’ That doesn’t work. You need to know if there are systemic features, and sometimes histologic information can change your approach or diagnosis, but not as often as one might think,” he said, noting that, in his view, the two main factors are appearance and systemic impact.
The presence of fever is a hallmark of systemic problems, he continued, “so if you see fever, you know you’re probably going to be dealing with a complex reaction, so we need to know the morphology.” Consider whether it is simple exanthem (a mild, uncomplicated rash) or complex exanthem (drug rash with eosinophilia and systemic symptoms or fever, malaise, and adenopathy).
As for other morphologies, urticarial lesions could be urticaria or a serum sickness-like reaction, pustular lesions could be acneiform or acute generalized exanthematous pustulosis, while
Dr. Shear considers SJS/TEN as a spectrum of blistering disease, “because there’s not a single diagnosis,” he said. “There’s a spectrum, if you will, depending on how advanced people are in their disease.” He coauthored a 1991 report describing eight cases of mycoplasma and Stevens-Johnson syndrome. “I was surprised at how long that stood up as about the only paper in that area,” he said. “But there’s much more happening now with a proliferation of terms,” he added, referring to MIRM (Mycoplasma pneumonia–induced rash and mucositis), RIME (reactive infectious mucocutaneous eruption); and Fuchs syndrome, or SJS without skin lesions.
What was not appreciated in the early classification of SJS, he continued, was a “side basket” of bullous erythema multiforme. “We didn’t know what to call it,” he said. “At one point we called it bullous erythema multiforme. At another point we called it erythema multiforme major. We just didn’t know what it was.”
The appearance and systemic effects of SJS comprise what he termed SJS type 2 – or the early stages of TEN. Taken together, he refers to these two conditions as TEN Spectrum, or TENS. “One of the traps is that TENS can look like varicella, and vice versa, especially in very dark brown or black skin,” Dr. Shear said. “You have to be careful. A biopsy might be worthwhile. Acute lupus has the pathology of TENS but the patients are not as systemically ill as true TENS.”
In 2011, Japanese researchers reported on 38 cases of SJS associated with M. pneumoniae, and 78 cases of drug-induced SJS. They found that 66% of adult patients with M. pneumoniae–associated SJS developed mucocutaneous lesions and fever/respiratory symptoms on the same day, mostly shortness of breath and cough. In contrast, most of the patients aged under 20 years developed fever/respiratory symptoms before mucocutaneous involvement.
“The big clinical differentiator between drug-induced SJS and mycoplasma-induced SJS was respiratory disorder,” said Dr. Shear, who was not affiliated with the study. “That means you’re probably looking at something that’s mycoplasma related [when respiratory problems are present]. Even if you can’t prove it’s mycoplasma related, that probably needs to be the target of your therapy. The idea ... is to make sure it’s clear at the end. One, so they get better, and two, so that we’re not giving drugs needlessly when it was really mycoplasma.”
Noting that HLA-B*15:02 is a marker for carbamazepine-induced SJS and TEN, he said, “a positive HLA test can support the diagnosis, confirm the suspected offending drug, and is valuable for familial genetic counseling.”
As for treatment of SJS, TEN, and other cytotoxic T-lymphocyte–mediated severe cutaneous adverse reactions, a randomized Japanese clinical trial evaluating prednisolone 1-1.5 mg/kg/day IV versus etanercept 25-50 mg subcutaneously twice per week in 96 patients with SJS-TEN found that etanercept decreased the mortality rate by 8.3%. In addition, etanercept reduced skin healing time, when compared with prednisolone (a median of 14 vs. 19 days, respectively; P = .010), and was associated with a lower incidence of GI hemorrhage (2.6% vs. 18.2%, respectively; P = .03).
Dr. Shear said that he would like to see better therapeutics for severe, complex patients. “After leaving the hospital, people with SJS or people with TEN need to have ongoing care, consultation, and explanation so they and their families know what drugs are safe in the future.”
Dr. Shear disclosed that he has been a consultant to AbbVie, Amgen, Bausch Medicine, Novartis, Sanofi-Genzyme, UCB, LEO Pharma, Otsuka, Janssen, Alpha Laboratories, Lilly, ChemoCentryx, Vivoryon, Galderma, Innovaderm, Chromocell, and Kyowa Kirin.
In the opinion of Neil H. Shear, MD, a stepwise approach is the best way to diagnose possible drug-induced skin disease and determine the root cause.
“Often, we need to think of more than one cause,” he said during the annual meeting of the Society for Pediatric Dermatology. “It could be drug X. It could be drug Y. It could be contrast media. We must think broadly and pay special attention to skin of color, overlapping syndromes, and the changing diagnostic assessment over time.”
His suggested diagnostic triangle includes appearance of the rash or lesion(s), systemic impact, and histology. “The first is the appearance,” said Dr. Shear, professor emeritus of dermatology, clinical pharmacology and toxicology, and medicine at the University of Toronto. “Is it exanthem? Is it blistering? Don’t just say drug ‘rash.’ That doesn’t work. You need to know if there are systemic features, and sometimes histologic information can change your approach or diagnosis, but not as often as one might think,” he said, noting that, in his view, the two main factors are appearance and systemic impact.
The presence of fever is a hallmark of systemic problems, he continued, “so if you see fever, you know you’re probably going to be dealing with a complex reaction, so we need to know the morphology.” Consider whether it is simple exanthem (a mild, uncomplicated rash) or complex exanthem (drug rash with eosinophilia and systemic symptoms or fever, malaise, and adenopathy).
As for other morphologies, urticarial lesions could be urticaria or a serum sickness-like reaction, pustular lesions could be acneiform or acute generalized exanthematous pustulosis, while
Dr. Shear considers SJS/TEN as a spectrum of blistering disease, “because there’s not a single diagnosis,” he said. “There’s a spectrum, if you will, depending on how advanced people are in their disease.” He coauthored a 1991 report describing eight cases of mycoplasma and Stevens-Johnson syndrome. “I was surprised at how long that stood up as about the only paper in that area,” he said. “But there’s much more happening now with a proliferation of terms,” he added, referring to MIRM (Mycoplasma pneumonia–induced rash and mucositis), RIME (reactive infectious mucocutaneous eruption); and Fuchs syndrome, or SJS without skin lesions.
What was not appreciated in the early classification of SJS, he continued, was a “side basket” of bullous erythema multiforme. “We didn’t know what to call it,” he said. “At one point we called it bullous erythema multiforme. At another point we called it erythema multiforme major. We just didn’t know what it was.”
The appearance and systemic effects of SJS comprise what he termed SJS type 2 – or the early stages of TEN. Taken together, he refers to these two conditions as TEN Spectrum, or TENS. “One of the traps is that TENS can look like varicella, and vice versa, especially in very dark brown or black skin,” Dr. Shear said. “You have to be careful. A biopsy might be worthwhile. Acute lupus has the pathology of TENS but the patients are not as systemically ill as true TENS.”
In 2011, Japanese researchers reported on 38 cases of SJS associated with M. pneumoniae, and 78 cases of drug-induced SJS. They found that 66% of adult patients with M. pneumoniae–associated SJS developed mucocutaneous lesions and fever/respiratory symptoms on the same day, mostly shortness of breath and cough. In contrast, most of the patients aged under 20 years developed fever/respiratory symptoms before mucocutaneous involvement.
“The big clinical differentiator between drug-induced SJS and mycoplasma-induced SJS was respiratory disorder,” said Dr. Shear, who was not affiliated with the study. “That means you’re probably looking at something that’s mycoplasma related [when respiratory problems are present]. Even if you can’t prove it’s mycoplasma related, that probably needs to be the target of your therapy. The idea ... is to make sure it’s clear at the end. One, so they get better, and two, so that we’re not giving drugs needlessly when it was really mycoplasma.”
Noting that HLA-B*15:02 is a marker for carbamazepine-induced SJS and TEN, he said, “a positive HLA test can support the diagnosis, confirm the suspected offending drug, and is valuable for familial genetic counseling.”
As for treatment of SJS, TEN, and other cytotoxic T-lymphocyte–mediated severe cutaneous adverse reactions, a randomized Japanese clinical trial evaluating prednisolone 1-1.5 mg/kg/day IV versus etanercept 25-50 mg subcutaneously twice per week in 96 patients with SJS-TEN found that etanercept decreased the mortality rate by 8.3%. In addition, etanercept reduced skin healing time, when compared with prednisolone (a median of 14 vs. 19 days, respectively; P = .010), and was associated with a lower incidence of GI hemorrhage (2.6% vs. 18.2%, respectively; P = .03).
Dr. Shear said that he would like to see better therapeutics for severe, complex patients. “After leaving the hospital, people with SJS or people with TEN need to have ongoing care, consultation, and explanation so they and their families know what drugs are safe in the future.”
Dr. Shear disclosed that he has been a consultant to AbbVie, Amgen, Bausch Medicine, Novartis, Sanofi-Genzyme, UCB, LEO Pharma, Otsuka, Janssen, Alpha Laboratories, Lilly, ChemoCentryx, Vivoryon, Galderma, Innovaderm, Chromocell, and Kyowa Kirin.
FROM SPD 2021













