User login
‘Round Face’: A Viral Term’s Real Diagnostic Implications
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
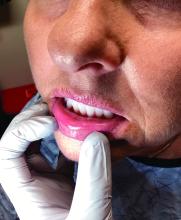
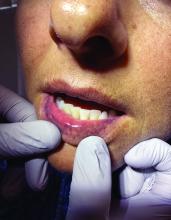
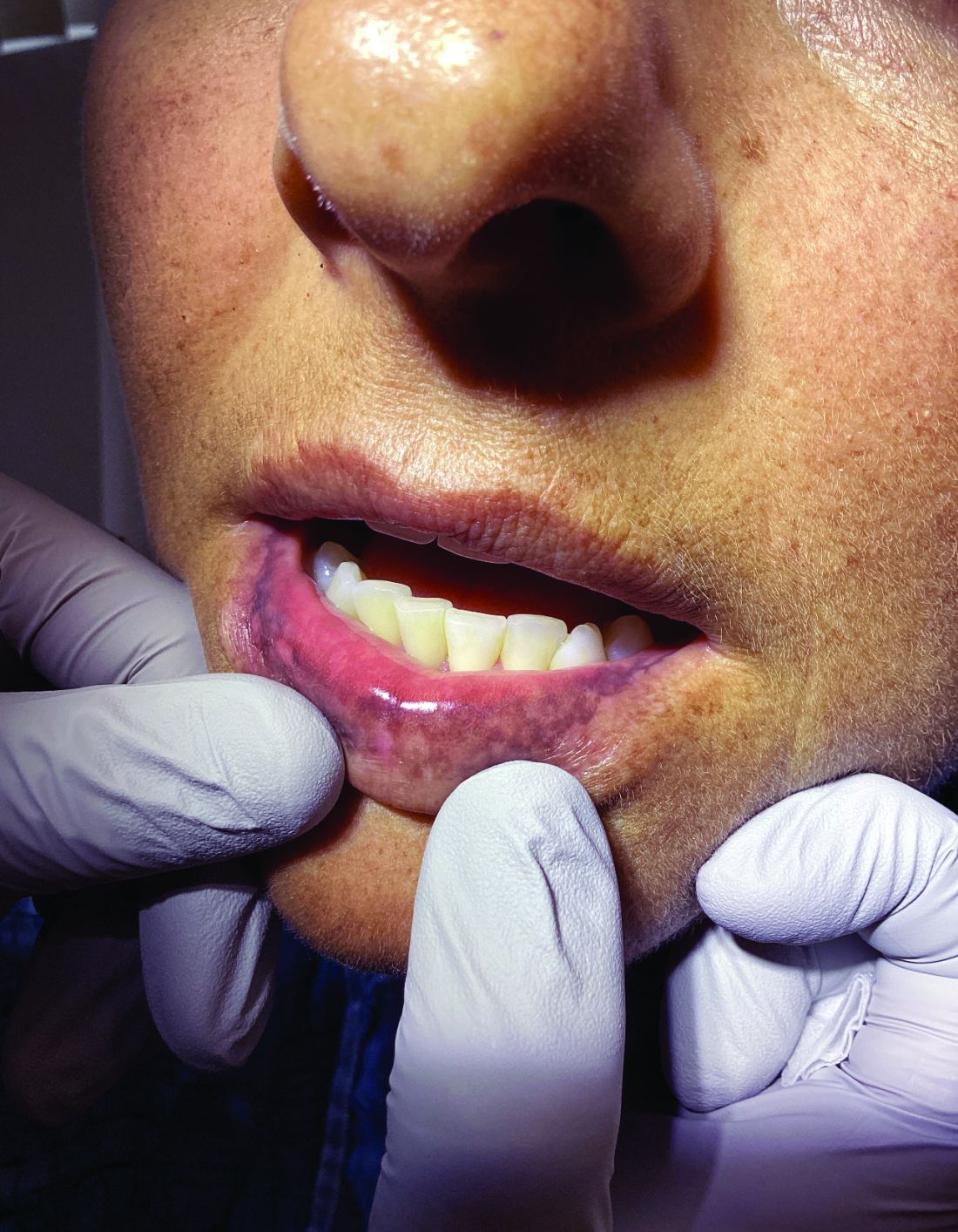
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
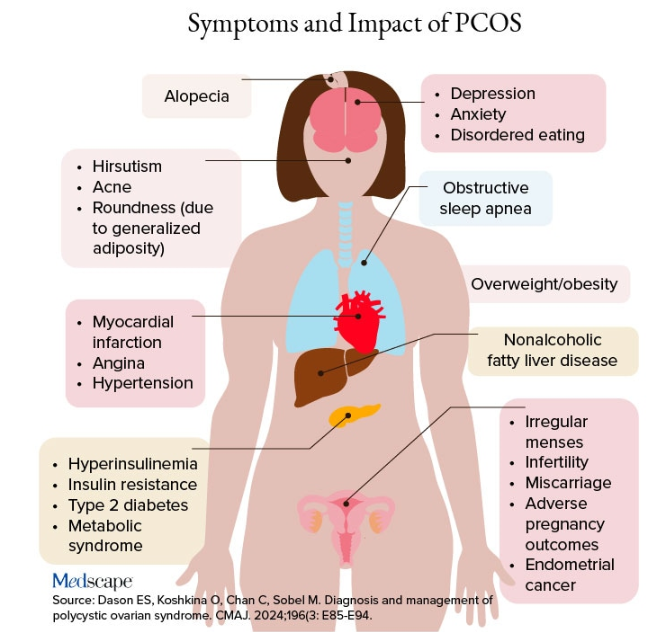
Additional symptoms of PCOS and its impact are found in the figure below.
PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
Update Coming for Thyroid Disease in Pregnancy Guidelines
CHICAGO — A preview of much-anticipated updates to guidelines on managing thyroid disease in pregnancy shows key changes to recommendations in the evolving field, ranging from consideration of the chance of spontaneous normalization of thyroid levels during pregnancy to a heightened emphasis on shared decision-making and the nuances can factor into personalized treatment.
The guidelines, expected to be published in early 2025, have not been updated since 2017, and with substantial advances and evidence from countless studies since then, the new guidelines were developed with a goal to start afresh, said ATA Thyroid and Pregnancy Guidelines Task Force cochair Tim IM Korevaar, MD, PhD, in presenting the final draft guidelines at the American Thyroid Association (ATA) 2024 Meeting.
“Obviously, we’re not going to ignore the 2017 guidelines, which have been a very good resource for us so far, but we really wanted to start from scratch and follow a ‘blank canvas’ approach in optimizing the evidence,” said Korevaar, an endocrinologist and obstetric internist with the Division of Pharmacology and Vascular Medicine & Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
The guidelines, developed through a collaborative effort involving a wide variety of related medical societies, involved 14 systematic literature reviews. While the pregnancy issues covered by the guidelines is extensive, key highlights include:
Management in Preconception
Beginning with preconception, a key change in the guidelines will be that patients with euthyroid thyroid peroxidase (TPO) antibodies, which can be indicative of thyroid dysfunction, routine treatment with levothyroxine is not recommended, based on new evidence from randomized trials of high-risk patients showing no clear benefit from the treatment.
“In these trials, and across analyses, there was absolutely no beneficial effect of levothyroxine in these patients [with euthyroid TPO antibody positivity],” he said.
With evidence showing, however, that TPO antibody positivity can lead to subclinical or overt hypothyroidism within 1 or 2 years, the guidelines will recommend that TPO antibody–positive patients do have thyroid stimulating hormone (TSH) levels tested every 3-6 months until pregnancy, and existing recommendations to test during pregnancy among those patients remain in place, Korevaar reported.
In terms of preconception subclinical hypothyroidism, the guidelines will emphasize the existing recommendation “to always strive to reassess” thyroid levels, and if subclinical hypothyroidism does persist, to treat with low-dose levothyroxine.
During Pregnancy
During pregnancy, the new proposed recommendations will reflect the important change that three key risk factors, including age over 30 years, having at least two prior pregnancies, and morbid obesity (body mass index [BMI] at least 40 kg/m2), previously considered a risk for thyroid dysfunction in pregnancy, should not, on their own, suggest the need for thyroid testing, based on low evidence of an increased risk in pregnancy.
Research on the issue includes a recent study from Korevaar’s team showing these factors to in fact have low predictability of thyroid dysfunction.
“We deemed that these risk differences weren’t really clinically meaningful (in predicting risk), and so we have removed to maternal age, BMI, and parity as risk factors for thyroid testing indications in pregnancy,” Korevaar said.
Factors considered a risk, resulting in recommended testing at presentation include a history of subclinical or clinical hypo- or hyperthyroidism, postpartum thyroiditis, known thyroid antibody positivity, symptoms of thyroid dysfunction or goiter, and other factors.
Treatment for Subclinical Hypothyroidism in Pregnancy
Whereas current guidelines recommend TPO antibody status in determining when to consider treatment for subclinical hypothyroidism, the new proposed guideline will instead recommend treatment based on the timing of the diagnosis of the subclinical hypothyroidism, with consideration of treatment during the first trimester, but not in the second or third trimester, based on newer evidence of the absolute risk for pregnancy complications and randomized trial data.
“The recommendations are now to no longer based on TPO antibody status, but instead according to the timing of the diagnosis of subclinical hypothyroidism,” Korevaar said.
Based on the collective data, “due to the low risk, we do not recommend for routine levothyroxine treatment in the second or third trimester groups with TSH levels under 10 mU/L now.”
“However, for subclinical hypothyroidism diagnosed in the first trimester, the recommendation would be that you can consider levothyroxine treatment,” he said.
While a clear indication for treatment in any trimester is the presence of overt hypothyroidism, or TSH levels over 10 mU/L, Korevaar underscored the importance of considering nuances of the recommendations that may warrant flexibility, for instance among patients with borderline TSH levels.
Spontaneous Normalization of Thyroid Levels in Pregnancy
Another new recommendation addresses the issue of spontaneous normalization of abnormal thyroid function during pregnancy, with several large studies showing a large proportion of subclinical hypothyroidism cases spontaneously revert to euthyroidism by the third trimester — despite no treatment having been provided.
Under the important proposed recommendation, retesting of subclinical hypothyroidism is suggested within 3 weeks.
“The data shows that a large proportion of patients spontaneously revert to euthyroidism,” Korevaar said.
“Upon identifying subclinical hypothyroidism in the first trimester, there will be essentially two options that clinicians can discuss with their patient — one would be to consider confirmatory tests in 3 weeks or to discuss the starting the lower dose levothyroxine in the first trimester,” he said.
In terms of overt hypothyroidism, likewise, if patients have a TSH levels below 6 mU/L in pregnancy, “you can either consider doing confirmatory testing within 3 weeks, or discussing with the patient starting levothyroxine treatment,” Korevaar added.
Overt Hyperthyroidism
For overt hyperthyroidism, no significant changes from current guidelines are being proposed, with the key exception of a heightened emphasis on the need for shared decision-making with patients, Korevaar said.
“We want to emphasize shared decision-making especially for women who have Graves’ disease prior to pregnancy, because the antithyroid treatment modalities, primarily methimazole (MMI) and propylthiouracil (PTU), have different advantages and disadvantages for an upcoming pregnancy,” he said.
“If you help a patient become involved in the decision-making process, that can also be very helpful in managing the disease and following-up on the pregnancy.”
Under the recommendations, PTU remains the preferred drug in overt hyperthyroidism, due to a more favorable profile in terms of potential birth defects vs MMI, with research showing a higher absolute risk of 3% vs 5%.
The guidelines further suggest the option of stopping the antithyroid medications upon a positive pregnancy test, with the exception of high-risk patients.
Korevaar noted that, if the treatment is stopped early in pregnancy, relapse is not likely to occur until after approximately 3 months, or 12 weeks, at which time, the high-risk teratogenic period, which is between week 5 and week 15, will have passed.
Current guidelines regarding whether to stop treatment in higher-risk hyperthyroid patients are recommended to remain unchanged.
Thyroid Nodules and Cancer
Recommendations regarding thyroid nodules and cancer during pregnancy are also expected to remain largely similar to those in the 2017 guidelines, with the exception of an emphasis on simply considering how the patient would normally be managed outside of pregnancy.
For instance, regarding the question of whether treatment can be withheld for 9 months during pregnancy. “A lot of times, the answer is yes,” Korevaar said.
Other topics that will be largely unchanged include issues of universal screening, definitions of normal and abnormal TSH and free T4 reference ranges and isolated hypothyroxinemia.
Steps Forward in Improving Updates, Readability
In addition to recommendation updates, the new guidelines are being revised to better reflect more recent evidence-based developments and user-friendliness.
“We have now made the step to a more systematic and replicable methodology to ensure for easier updates with a shorter interval,” Korevaar told this news organization.
“Furthermore, since 2006, the ATA guideline documents have followed a question-and-answer format, lacked recommendation tables and had none or only a few graphic illustrations,” he added.
“We are now further developing the typical outline of the guidelines to improve the readability and dissemination of the guideline document.”
Korevaar’s disclosures include lectureship fees from IBSA, Merck, and Berlin Chemie.
A version of this article first appeared on Medscape.com.
CHICAGO — A preview of much-anticipated updates to guidelines on managing thyroid disease in pregnancy shows key changes to recommendations in the evolving field, ranging from consideration of the chance of spontaneous normalization of thyroid levels during pregnancy to a heightened emphasis on shared decision-making and the nuances can factor into personalized treatment.
The guidelines, expected to be published in early 2025, have not been updated since 2017, and with substantial advances and evidence from countless studies since then, the new guidelines were developed with a goal to start afresh, said ATA Thyroid and Pregnancy Guidelines Task Force cochair Tim IM Korevaar, MD, PhD, in presenting the final draft guidelines at the American Thyroid Association (ATA) 2024 Meeting.
“Obviously, we’re not going to ignore the 2017 guidelines, which have been a very good resource for us so far, but we really wanted to start from scratch and follow a ‘blank canvas’ approach in optimizing the evidence,” said Korevaar, an endocrinologist and obstetric internist with the Division of Pharmacology and Vascular Medicine & Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
The guidelines, developed through a collaborative effort involving a wide variety of related medical societies, involved 14 systematic literature reviews. While the pregnancy issues covered by the guidelines is extensive, key highlights include:
Management in Preconception
Beginning with preconception, a key change in the guidelines will be that patients with euthyroid thyroid peroxidase (TPO) antibodies, which can be indicative of thyroid dysfunction, routine treatment with levothyroxine is not recommended, based on new evidence from randomized trials of high-risk patients showing no clear benefit from the treatment.
“In these trials, and across analyses, there was absolutely no beneficial effect of levothyroxine in these patients [with euthyroid TPO antibody positivity],” he said.
With evidence showing, however, that TPO antibody positivity can lead to subclinical or overt hypothyroidism within 1 or 2 years, the guidelines will recommend that TPO antibody–positive patients do have thyroid stimulating hormone (TSH) levels tested every 3-6 months until pregnancy, and existing recommendations to test during pregnancy among those patients remain in place, Korevaar reported.
In terms of preconception subclinical hypothyroidism, the guidelines will emphasize the existing recommendation “to always strive to reassess” thyroid levels, and if subclinical hypothyroidism does persist, to treat with low-dose levothyroxine.
During Pregnancy
During pregnancy, the new proposed recommendations will reflect the important change that three key risk factors, including age over 30 years, having at least two prior pregnancies, and morbid obesity (body mass index [BMI] at least 40 kg/m2), previously considered a risk for thyroid dysfunction in pregnancy, should not, on their own, suggest the need for thyroid testing, based on low evidence of an increased risk in pregnancy.
Research on the issue includes a recent study from Korevaar’s team showing these factors to in fact have low predictability of thyroid dysfunction.
“We deemed that these risk differences weren’t really clinically meaningful (in predicting risk), and so we have removed to maternal age, BMI, and parity as risk factors for thyroid testing indications in pregnancy,” Korevaar said.
Factors considered a risk, resulting in recommended testing at presentation include a history of subclinical or clinical hypo- or hyperthyroidism, postpartum thyroiditis, known thyroid antibody positivity, symptoms of thyroid dysfunction or goiter, and other factors.
Treatment for Subclinical Hypothyroidism in Pregnancy
Whereas current guidelines recommend TPO antibody status in determining when to consider treatment for subclinical hypothyroidism, the new proposed guideline will instead recommend treatment based on the timing of the diagnosis of the subclinical hypothyroidism, with consideration of treatment during the first trimester, but not in the second or third trimester, based on newer evidence of the absolute risk for pregnancy complications and randomized trial data.
“The recommendations are now to no longer based on TPO antibody status, but instead according to the timing of the diagnosis of subclinical hypothyroidism,” Korevaar said.
Based on the collective data, “due to the low risk, we do not recommend for routine levothyroxine treatment in the second or third trimester groups with TSH levels under 10 mU/L now.”
“However, for subclinical hypothyroidism diagnosed in the first trimester, the recommendation would be that you can consider levothyroxine treatment,” he said.
While a clear indication for treatment in any trimester is the presence of overt hypothyroidism, or TSH levels over 10 mU/L, Korevaar underscored the importance of considering nuances of the recommendations that may warrant flexibility, for instance among patients with borderline TSH levels.
Spontaneous Normalization of Thyroid Levels in Pregnancy
Another new recommendation addresses the issue of spontaneous normalization of abnormal thyroid function during pregnancy, with several large studies showing a large proportion of subclinical hypothyroidism cases spontaneously revert to euthyroidism by the third trimester — despite no treatment having been provided.
Under the important proposed recommendation, retesting of subclinical hypothyroidism is suggested within 3 weeks.
“The data shows that a large proportion of patients spontaneously revert to euthyroidism,” Korevaar said.
“Upon identifying subclinical hypothyroidism in the first trimester, there will be essentially two options that clinicians can discuss with their patient — one would be to consider confirmatory tests in 3 weeks or to discuss the starting the lower dose levothyroxine in the first trimester,” he said.
In terms of overt hypothyroidism, likewise, if patients have a TSH levels below 6 mU/L in pregnancy, “you can either consider doing confirmatory testing within 3 weeks, or discussing with the patient starting levothyroxine treatment,” Korevaar added.
Overt Hyperthyroidism
For overt hyperthyroidism, no significant changes from current guidelines are being proposed, with the key exception of a heightened emphasis on the need for shared decision-making with patients, Korevaar said.
“We want to emphasize shared decision-making especially for women who have Graves’ disease prior to pregnancy, because the antithyroid treatment modalities, primarily methimazole (MMI) and propylthiouracil (PTU), have different advantages and disadvantages for an upcoming pregnancy,” he said.
“If you help a patient become involved in the decision-making process, that can also be very helpful in managing the disease and following-up on the pregnancy.”
Under the recommendations, PTU remains the preferred drug in overt hyperthyroidism, due to a more favorable profile in terms of potential birth defects vs MMI, with research showing a higher absolute risk of 3% vs 5%.
The guidelines further suggest the option of stopping the antithyroid medications upon a positive pregnancy test, with the exception of high-risk patients.
Korevaar noted that, if the treatment is stopped early in pregnancy, relapse is not likely to occur until after approximately 3 months, or 12 weeks, at which time, the high-risk teratogenic period, which is between week 5 and week 15, will have passed.
Current guidelines regarding whether to stop treatment in higher-risk hyperthyroid patients are recommended to remain unchanged.
Thyroid Nodules and Cancer
Recommendations regarding thyroid nodules and cancer during pregnancy are also expected to remain largely similar to those in the 2017 guidelines, with the exception of an emphasis on simply considering how the patient would normally be managed outside of pregnancy.
For instance, regarding the question of whether treatment can be withheld for 9 months during pregnancy. “A lot of times, the answer is yes,” Korevaar said.
Other topics that will be largely unchanged include issues of universal screening, definitions of normal and abnormal TSH and free T4 reference ranges and isolated hypothyroxinemia.
Steps Forward in Improving Updates, Readability
In addition to recommendation updates, the new guidelines are being revised to better reflect more recent evidence-based developments and user-friendliness.
“We have now made the step to a more systematic and replicable methodology to ensure for easier updates with a shorter interval,” Korevaar told this news organization.
“Furthermore, since 2006, the ATA guideline documents have followed a question-and-answer format, lacked recommendation tables and had none or only a few graphic illustrations,” he added.
“We are now further developing the typical outline of the guidelines to improve the readability and dissemination of the guideline document.”
Korevaar’s disclosures include lectureship fees from IBSA, Merck, and Berlin Chemie.
A version of this article first appeared on Medscape.com.
CHICAGO — A preview of much-anticipated updates to guidelines on managing thyroid disease in pregnancy shows key changes to recommendations in the evolving field, ranging from consideration of the chance of spontaneous normalization of thyroid levels during pregnancy to a heightened emphasis on shared decision-making and the nuances can factor into personalized treatment.
The guidelines, expected to be published in early 2025, have not been updated since 2017, and with substantial advances and evidence from countless studies since then, the new guidelines were developed with a goal to start afresh, said ATA Thyroid and Pregnancy Guidelines Task Force cochair Tim IM Korevaar, MD, PhD, in presenting the final draft guidelines at the American Thyroid Association (ATA) 2024 Meeting.
“Obviously, we’re not going to ignore the 2017 guidelines, which have been a very good resource for us so far, but we really wanted to start from scratch and follow a ‘blank canvas’ approach in optimizing the evidence,” said Korevaar, an endocrinologist and obstetric internist with the Division of Pharmacology and Vascular Medicine & Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
The guidelines, developed through a collaborative effort involving a wide variety of related medical societies, involved 14 systematic literature reviews. While the pregnancy issues covered by the guidelines is extensive, key highlights include:
Management in Preconception
Beginning with preconception, a key change in the guidelines will be that patients with euthyroid thyroid peroxidase (TPO) antibodies, which can be indicative of thyroid dysfunction, routine treatment with levothyroxine is not recommended, based on new evidence from randomized trials of high-risk patients showing no clear benefit from the treatment.
“In these trials, and across analyses, there was absolutely no beneficial effect of levothyroxine in these patients [with euthyroid TPO antibody positivity],” he said.
With evidence showing, however, that TPO antibody positivity can lead to subclinical or overt hypothyroidism within 1 or 2 years, the guidelines will recommend that TPO antibody–positive patients do have thyroid stimulating hormone (TSH) levels tested every 3-6 months until pregnancy, and existing recommendations to test during pregnancy among those patients remain in place, Korevaar reported.
In terms of preconception subclinical hypothyroidism, the guidelines will emphasize the existing recommendation “to always strive to reassess” thyroid levels, and if subclinical hypothyroidism does persist, to treat with low-dose levothyroxine.
During Pregnancy
During pregnancy, the new proposed recommendations will reflect the important change that three key risk factors, including age over 30 years, having at least two prior pregnancies, and morbid obesity (body mass index [BMI] at least 40 kg/m2), previously considered a risk for thyroid dysfunction in pregnancy, should not, on their own, suggest the need for thyroid testing, based on low evidence of an increased risk in pregnancy.
Research on the issue includes a recent study from Korevaar’s team showing these factors to in fact have low predictability of thyroid dysfunction.
“We deemed that these risk differences weren’t really clinically meaningful (in predicting risk), and so we have removed to maternal age, BMI, and parity as risk factors for thyroid testing indications in pregnancy,” Korevaar said.
Factors considered a risk, resulting in recommended testing at presentation include a history of subclinical or clinical hypo- or hyperthyroidism, postpartum thyroiditis, known thyroid antibody positivity, symptoms of thyroid dysfunction or goiter, and other factors.
Treatment for Subclinical Hypothyroidism in Pregnancy
Whereas current guidelines recommend TPO antibody status in determining when to consider treatment for subclinical hypothyroidism, the new proposed guideline will instead recommend treatment based on the timing of the diagnosis of the subclinical hypothyroidism, with consideration of treatment during the first trimester, but not in the second or third trimester, based on newer evidence of the absolute risk for pregnancy complications and randomized trial data.
“The recommendations are now to no longer based on TPO antibody status, but instead according to the timing of the diagnosis of subclinical hypothyroidism,” Korevaar said.
Based on the collective data, “due to the low risk, we do not recommend for routine levothyroxine treatment in the second or third trimester groups with TSH levels under 10 mU/L now.”
“However, for subclinical hypothyroidism diagnosed in the first trimester, the recommendation would be that you can consider levothyroxine treatment,” he said.
While a clear indication for treatment in any trimester is the presence of overt hypothyroidism, or TSH levels over 10 mU/L, Korevaar underscored the importance of considering nuances of the recommendations that may warrant flexibility, for instance among patients with borderline TSH levels.
Spontaneous Normalization of Thyroid Levels in Pregnancy
Another new recommendation addresses the issue of spontaneous normalization of abnormal thyroid function during pregnancy, with several large studies showing a large proportion of subclinical hypothyroidism cases spontaneously revert to euthyroidism by the third trimester — despite no treatment having been provided.
Under the important proposed recommendation, retesting of subclinical hypothyroidism is suggested within 3 weeks.
“The data shows that a large proportion of patients spontaneously revert to euthyroidism,” Korevaar said.
“Upon identifying subclinical hypothyroidism in the first trimester, there will be essentially two options that clinicians can discuss with their patient — one would be to consider confirmatory tests in 3 weeks or to discuss the starting the lower dose levothyroxine in the first trimester,” he said.
In terms of overt hypothyroidism, likewise, if patients have a TSH levels below 6 mU/L in pregnancy, “you can either consider doing confirmatory testing within 3 weeks, or discussing with the patient starting levothyroxine treatment,” Korevaar added.
Overt Hyperthyroidism
For overt hyperthyroidism, no significant changes from current guidelines are being proposed, with the key exception of a heightened emphasis on the need for shared decision-making with patients, Korevaar said.
“We want to emphasize shared decision-making especially for women who have Graves’ disease prior to pregnancy, because the antithyroid treatment modalities, primarily methimazole (MMI) and propylthiouracil (PTU), have different advantages and disadvantages for an upcoming pregnancy,” he said.
“If you help a patient become involved in the decision-making process, that can also be very helpful in managing the disease and following-up on the pregnancy.”
Under the recommendations, PTU remains the preferred drug in overt hyperthyroidism, due to a more favorable profile in terms of potential birth defects vs MMI, with research showing a higher absolute risk of 3% vs 5%.
The guidelines further suggest the option of stopping the antithyroid medications upon a positive pregnancy test, with the exception of high-risk patients.
Korevaar noted that, if the treatment is stopped early in pregnancy, relapse is not likely to occur until after approximately 3 months, or 12 weeks, at which time, the high-risk teratogenic period, which is between week 5 and week 15, will have passed.
Current guidelines regarding whether to stop treatment in higher-risk hyperthyroid patients are recommended to remain unchanged.
Thyroid Nodules and Cancer
Recommendations regarding thyroid nodules and cancer during pregnancy are also expected to remain largely similar to those in the 2017 guidelines, with the exception of an emphasis on simply considering how the patient would normally be managed outside of pregnancy.
For instance, regarding the question of whether treatment can be withheld for 9 months during pregnancy. “A lot of times, the answer is yes,” Korevaar said.
Other topics that will be largely unchanged include issues of universal screening, definitions of normal and abnormal TSH and free T4 reference ranges and isolated hypothyroxinemia.
Steps Forward in Improving Updates, Readability
In addition to recommendation updates, the new guidelines are being revised to better reflect more recent evidence-based developments and user-friendliness.
“We have now made the step to a more systematic and replicable methodology to ensure for easier updates with a shorter interval,” Korevaar told this news organization.
“Furthermore, since 2006, the ATA guideline documents have followed a question-and-answer format, lacked recommendation tables and had none or only a few graphic illustrations,” he added.
“We are now further developing the typical outline of the guidelines to improve the readability and dissemination of the guideline document.”
Korevaar’s disclosures include lectureship fees from IBSA, Merck, and Berlin Chemie.
A version of this article first appeared on Medscape.com.
FROM ATA 2024
Many Patients With Cancer Visit EDs Before Diagnosis
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CMAJ
ATA: Updates on Risk, Diagnosis, and Treatment of Thyroid Cancer
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
FROM ATA 2024
Thyroid Cancer Overdiagnosis Continues Despite Cautions
according to a recently published global study.
The proportion of thyroid cancer cases attributable to overdiagnosis globally was higher in women (78%) than in men (68%), with this rate varying substantially across countries, wrote Mengmeng Li, PhD, of the Sun Yat-sen University Cancer Center, Guangzhou, China, and coauthors in an October paper in The Lancet Diabetes & Endocrinology.
Overdiagnosis refers to the diagnosis of lesions that would not cause symptoms and that would not progress, if left alone.
Increased testing for thyroid cancer, fueled in large part by the expansion of imaging technologies and progressively more intense and disorganized scrutiny of the thyroid, led many people to be treated for often indolent lesions, exposing them to potential side effects as well as financial and emotional distress.
Li and coauthors estimate that more than 1.7 million people might have been overdiagnosed between 2013 and 2017 in 63 countries.
“Overdiagnosis clearly emerged in some high-resource countries with private-based health systems in which access to healthcare overrules regulatory controls (eg, in the USA) and in some high-quality public health systems with easy and broad access to thyroid gland diagnostic examinations (eg, in Canada),” Li and coauthors wrote. “Conversely, thyroid cancer is less commonly diagnosed in those countries in which access to diagnosis is guided by strong regulatory rules (eg, in Nordic countries).”
Their study drew from almost 40 years of research, including the latest available data from the World Health Organization’s International Agency for Research on Cancer’s (IARC’s) Global Cancer Observatory. Li and coauthors examined patterns in the time trends of thyroid cancer, mortality data, and trends in diagnosis of thyroid cancer before testing became common in many nations.
This approach is needed in estimating overdiagnosis, where it’s not possible to see what’s happening on a case-by-case level, Salvatore Vaccarella, PhD, a scientist at IARC’s Cancer Surveillance Branch, said in an interview.
Researchers can’t tell whether an individual’s detected early-stage cancers would have remained indolent for years or eventually would have put their life at risk, he said. Instead, the patterns emerge through larger studies of the reported cases of cancer like thyroid tumors and then looking at separate datasets on mortality.
“We can only see that as a big phenomenon when we look at population-based data,” Vaccarella said.
Persisting Problem
Recognition of the harms of overdiagnosis has resulted in some reduction of the incidence of thyroid cancer in the United States, Li and coauthors wrote. After adjusting for age, incidence has fallen from 19 per 100,000 women in 2013 to 16 per 100,000 women in 2017. The proportion of thyroid cancer attributed to overdiagnosis has dropped from 76% to 68% in the country.
The paper adds to the evidence suggesting that the rise in screening has not changed mortality rates for thyroid cancer. For example, Li and coauthors reported seeing “a small decrease in thyroid cancer mortality rates over time in some European countries, but this decline (less than 1 per 100,000 women) is marginal compared with the increases in incidence (reaching around 100 per 100,000 women).”
“Moreover, previous data show that the downward mortality trends had begun before the wide use of ultrasonography for early detection and that period and birth cohort effects have been declining, probably due to treatment advances and reduced prevalence of risk factors, such as the reduction in iodine deficiency,” they wrote.
In an interview, Amanda Davis, MD, of AnMed, a nonprofit health system based in Anderson, South Carolina, said the new paper from Li and Vaccarella provides further evidence for a cautious approach to thyroid nodules given concerns about overdiagnosis.
If early detection of cancer via discovery of thyroid nodules actually helped patients, mortality rates would have dropped with expansion of screening and the resulting diagnoses, said Davis, who is an associate program director at AnMed’s family medicine residency program and affiliate professor at the Medical University of South Carolina, Charleston.
In many cases, people learn they have thyroid lesions after being tested for other conditions such as ultrasound done on carotid arteries to check for stroke risk. The most common form of thyroid cancer is the papillary form. Papillary thyroid cancer tends to be slow growing, carries a low risk for distant metastasis, and in many cases poses little risk. Some small (< 1 cm) papillary thyroid cancers can be monitored with active surveillance as opposed to thyroid lobectomy.
“So just finding more nodules incidentally or through screening ultrasound and even finding more papillary cancers via these methods does not make people healthier or decrease mortality,” Davis said.
“So just finding more things and even finding more papillary cancers does not increase our ability to treat people and keep them alive longer,” Davis said.
The 5-year survival rate for thyroid cancer overall is 98.1% and varies from 99.9% for localized disease to 55.3% for distant disease, the US Preventive Services Task Force (USPSTF) said in a 2017 publication in JAMA. The task force that year gave a “D” rating on screening of asymptomatic people for thyroid cancer. That means there’s moderate certainty that screening for thyroid cancer in asymptomatic persons results in harms that outweigh the benefits. The decision to give this “D” rating meant this screening is not recommended. That’s still the panel’s view.
“You can think of it as a “D” for ‘don’t screen for thyroid cancer,’ ” in people who present no symptoms of this illness, John Wong, MD, the vice chair of the USPSTF, said in an interview.
In primary care, the challenge is assessing thyroid nodules detected when people undergo testing for another reason, such as an ultrasound of the carotid artery to check for stroke risk.
Thyroid nodules can be detected by ultrasonography in up to 68% of the general population, reported a study in American Family Physician. Nodules with suspicious features or ≥ 1 cm require fine needle aspiration. The Bethesda System for Reporting Thyroid Cytopathology can be used to classify samples, with molecular testing applied to guide treatment when fine needle aspiration yields an indeterminate result.
New Thinking on Thyroid Cancer
There’s been a shift in recent years in the approach to how physicians should proceed if certain kinds of thyroid cancer are detected, Cari M. Kitahara, PhD, of the National Cancer Institute noted in a comment accompanying the Li paper.
“Clinicians need to be judicious in the use of thyroid ultrasonography, the diagnostic follow-up of incidentally detected thyroid nodules, and determining the optimal course of treatment,” Kitahara wrote. “For low-risk and incidentally detected tumors, strong consideration should be given to less intensive treatment options (eg, lobectomy, delayed treatment, and active surveillance).”
The American Thyroid Association guidelines encourage de-escalation of treatment for low-risk papillary thyroid carcinoma up to 4 cm.
Physicians often need to make clear to patients how a diagnosis of low-risk papillary thyroid cancer differs from other oncology diagnoses, R. Michael Tuttle, MD, of Memorial Sloan Kettering Cancer Center, New York City, said in an interview.
“I’ll frequently say that everything you’ve ever learned about cancer, you need to forget,” Tuttle said.
Some patients will mistakenly think any cancer diagnosis is a likely death sentence, meaning they should rush to get aggressive treatment. Tuttle has been a leader for many years in efforts in advancing active surveillance as an option for certain people with low-risk thyroid cancer.
“I often start my consultation by saying: ‘We’re going to choose between two right answers here. One right answer is watching right. One right answer is going to surgery,’ ” Tuttle said.
Patients with low-risk thyroid cancer tend to fall into two camps, with maximalists likely to seek quick treatment and minimalists more inclined for surveillance if that’s an option for them, Tuttle said. As opinions have shifted within the medical community about approaches to low-risk thyroid cancer, there’s also been some growing awareness among the public about thyroid overdiagnosis.
“Ten or 15 years ago, people thought we were crazy” to consider active surveillance as an option for low-risk thyroid cancers,” Tuttle said. “Now we have swung, at least in some of the public opinion, to this recognition that every little speck of cancer doesn’t need to be immediately taken out of your body.”
Some patients express regret about having learned that they have low-risk thyroid cancer, Tuttle said.
“Over the last 5 years, it’s not uncommon for patients to ask me, ‘Is this one of those that needs to be treated now, or is this one of those that we wish we would have never found?’ Or people will say, ‘My doctor talked me into an ultrasound, I didn’t want it’ or ‘I had a car wreck, and I found this nodule and I wished I had never found it.’ ”
This study from Li and coauthors was funded by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Young Talents Program of Sun Yat-sen University Cancer Center, the Italian Association for Cancer Research, and the Italian Ministry of Health. Davis and Tuttle had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a recently published global study.
The proportion of thyroid cancer cases attributable to overdiagnosis globally was higher in women (78%) than in men (68%), with this rate varying substantially across countries, wrote Mengmeng Li, PhD, of the Sun Yat-sen University Cancer Center, Guangzhou, China, and coauthors in an October paper in The Lancet Diabetes & Endocrinology.
Overdiagnosis refers to the diagnosis of lesions that would not cause symptoms and that would not progress, if left alone.
Increased testing for thyroid cancer, fueled in large part by the expansion of imaging technologies and progressively more intense and disorganized scrutiny of the thyroid, led many people to be treated for often indolent lesions, exposing them to potential side effects as well as financial and emotional distress.
Li and coauthors estimate that more than 1.7 million people might have been overdiagnosed between 2013 and 2017 in 63 countries.
“Overdiagnosis clearly emerged in some high-resource countries with private-based health systems in which access to healthcare overrules regulatory controls (eg, in the USA) and in some high-quality public health systems with easy and broad access to thyroid gland diagnostic examinations (eg, in Canada),” Li and coauthors wrote. “Conversely, thyroid cancer is less commonly diagnosed in those countries in which access to diagnosis is guided by strong regulatory rules (eg, in Nordic countries).”
Their study drew from almost 40 years of research, including the latest available data from the World Health Organization’s International Agency for Research on Cancer’s (IARC’s) Global Cancer Observatory. Li and coauthors examined patterns in the time trends of thyroid cancer, mortality data, and trends in diagnosis of thyroid cancer before testing became common in many nations.
This approach is needed in estimating overdiagnosis, where it’s not possible to see what’s happening on a case-by-case level, Salvatore Vaccarella, PhD, a scientist at IARC’s Cancer Surveillance Branch, said in an interview.
Researchers can’t tell whether an individual’s detected early-stage cancers would have remained indolent for years or eventually would have put their life at risk, he said. Instead, the patterns emerge through larger studies of the reported cases of cancer like thyroid tumors and then looking at separate datasets on mortality.
“We can only see that as a big phenomenon when we look at population-based data,” Vaccarella said.
Persisting Problem
Recognition of the harms of overdiagnosis has resulted in some reduction of the incidence of thyroid cancer in the United States, Li and coauthors wrote. After adjusting for age, incidence has fallen from 19 per 100,000 women in 2013 to 16 per 100,000 women in 2017. The proportion of thyroid cancer attributed to overdiagnosis has dropped from 76% to 68% in the country.
The paper adds to the evidence suggesting that the rise in screening has not changed mortality rates for thyroid cancer. For example, Li and coauthors reported seeing “a small decrease in thyroid cancer mortality rates over time in some European countries, but this decline (less than 1 per 100,000 women) is marginal compared with the increases in incidence (reaching around 100 per 100,000 women).”
“Moreover, previous data show that the downward mortality trends had begun before the wide use of ultrasonography for early detection and that period and birth cohort effects have been declining, probably due to treatment advances and reduced prevalence of risk factors, such as the reduction in iodine deficiency,” they wrote.
In an interview, Amanda Davis, MD, of AnMed, a nonprofit health system based in Anderson, South Carolina, said the new paper from Li and Vaccarella provides further evidence for a cautious approach to thyroid nodules given concerns about overdiagnosis.
If early detection of cancer via discovery of thyroid nodules actually helped patients, mortality rates would have dropped with expansion of screening and the resulting diagnoses, said Davis, who is an associate program director at AnMed’s family medicine residency program and affiliate professor at the Medical University of South Carolina, Charleston.
In many cases, people learn they have thyroid lesions after being tested for other conditions such as ultrasound done on carotid arteries to check for stroke risk. The most common form of thyroid cancer is the papillary form. Papillary thyroid cancer tends to be slow growing, carries a low risk for distant metastasis, and in many cases poses little risk. Some small (< 1 cm) papillary thyroid cancers can be monitored with active surveillance as opposed to thyroid lobectomy.
“So just finding more nodules incidentally or through screening ultrasound and even finding more papillary cancers via these methods does not make people healthier or decrease mortality,” Davis said.
“So just finding more things and even finding more papillary cancers does not increase our ability to treat people and keep them alive longer,” Davis said.
The 5-year survival rate for thyroid cancer overall is 98.1% and varies from 99.9% for localized disease to 55.3% for distant disease, the US Preventive Services Task Force (USPSTF) said in a 2017 publication in JAMA. The task force that year gave a “D” rating on screening of asymptomatic people for thyroid cancer. That means there’s moderate certainty that screening for thyroid cancer in asymptomatic persons results in harms that outweigh the benefits. The decision to give this “D” rating meant this screening is not recommended. That’s still the panel’s view.
“You can think of it as a “D” for ‘don’t screen for thyroid cancer,’ ” in people who present no symptoms of this illness, John Wong, MD, the vice chair of the USPSTF, said in an interview.
In primary care, the challenge is assessing thyroid nodules detected when people undergo testing for another reason, such as an ultrasound of the carotid artery to check for stroke risk.
Thyroid nodules can be detected by ultrasonography in up to 68% of the general population, reported a study in American Family Physician. Nodules with suspicious features or ≥ 1 cm require fine needle aspiration. The Bethesda System for Reporting Thyroid Cytopathology can be used to classify samples, with molecular testing applied to guide treatment when fine needle aspiration yields an indeterminate result.
New Thinking on Thyroid Cancer
There’s been a shift in recent years in the approach to how physicians should proceed if certain kinds of thyroid cancer are detected, Cari M. Kitahara, PhD, of the National Cancer Institute noted in a comment accompanying the Li paper.
“Clinicians need to be judicious in the use of thyroid ultrasonography, the diagnostic follow-up of incidentally detected thyroid nodules, and determining the optimal course of treatment,” Kitahara wrote. “For low-risk and incidentally detected tumors, strong consideration should be given to less intensive treatment options (eg, lobectomy, delayed treatment, and active surveillance).”
The American Thyroid Association guidelines encourage de-escalation of treatment for low-risk papillary thyroid carcinoma up to 4 cm.
Physicians often need to make clear to patients how a diagnosis of low-risk papillary thyroid cancer differs from other oncology diagnoses, R. Michael Tuttle, MD, of Memorial Sloan Kettering Cancer Center, New York City, said in an interview.
“I’ll frequently say that everything you’ve ever learned about cancer, you need to forget,” Tuttle said.
Some patients will mistakenly think any cancer diagnosis is a likely death sentence, meaning they should rush to get aggressive treatment. Tuttle has been a leader for many years in efforts in advancing active surveillance as an option for certain people with low-risk thyroid cancer.
“I often start my consultation by saying: ‘We’re going to choose between two right answers here. One right answer is watching right. One right answer is going to surgery,’ ” Tuttle said.
Patients with low-risk thyroid cancer tend to fall into two camps, with maximalists likely to seek quick treatment and minimalists more inclined for surveillance if that’s an option for them, Tuttle said. As opinions have shifted within the medical community about approaches to low-risk thyroid cancer, there’s also been some growing awareness among the public about thyroid overdiagnosis.
“Ten or 15 years ago, people thought we were crazy” to consider active surveillance as an option for low-risk thyroid cancers,” Tuttle said. “Now we have swung, at least in some of the public opinion, to this recognition that every little speck of cancer doesn’t need to be immediately taken out of your body.”
Some patients express regret about having learned that they have low-risk thyroid cancer, Tuttle said.
“Over the last 5 years, it’s not uncommon for patients to ask me, ‘Is this one of those that needs to be treated now, or is this one of those that we wish we would have never found?’ Or people will say, ‘My doctor talked me into an ultrasound, I didn’t want it’ or ‘I had a car wreck, and I found this nodule and I wished I had never found it.’ ”
This study from Li and coauthors was funded by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Young Talents Program of Sun Yat-sen University Cancer Center, the Italian Association for Cancer Research, and the Italian Ministry of Health. Davis and Tuttle had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a recently published global study.
The proportion of thyroid cancer cases attributable to overdiagnosis globally was higher in women (78%) than in men (68%), with this rate varying substantially across countries, wrote Mengmeng Li, PhD, of the Sun Yat-sen University Cancer Center, Guangzhou, China, and coauthors in an October paper in The Lancet Diabetes & Endocrinology.
Overdiagnosis refers to the diagnosis of lesions that would not cause symptoms and that would not progress, if left alone.
Increased testing for thyroid cancer, fueled in large part by the expansion of imaging technologies and progressively more intense and disorganized scrutiny of the thyroid, led many people to be treated for often indolent lesions, exposing them to potential side effects as well as financial and emotional distress.
Li and coauthors estimate that more than 1.7 million people might have been overdiagnosed between 2013 and 2017 in 63 countries.
“Overdiagnosis clearly emerged in some high-resource countries with private-based health systems in which access to healthcare overrules regulatory controls (eg, in the USA) and in some high-quality public health systems with easy and broad access to thyroid gland diagnostic examinations (eg, in Canada),” Li and coauthors wrote. “Conversely, thyroid cancer is less commonly diagnosed in those countries in which access to diagnosis is guided by strong regulatory rules (eg, in Nordic countries).”
Their study drew from almost 40 years of research, including the latest available data from the World Health Organization’s International Agency for Research on Cancer’s (IARC’s) Global Cancer Observatory. Li and coauthors examined patterns in the time trends of thyroid cancer, mortality data, and trends in diagnosis of thyroid cancer before testing became common in many nations.
This approach is needed in estimating overdiagnosis, where it’s not possible to see what’s happening on a case-by-case level, Salvatore Vaccarella, PhD, a scientist at IARC’s Cancer Surveillance Branch, said in an interview.
Researchers can’t tell whether an individual’s detected early-stage cancers would have remained indolent for years or eventually would have put their life at risk, he said. Instead, the patterns emerge through larger studies of the reported cases of cancer like thyroid tumors and then looking at separate datasets on mortality.
“We can only see that as a big phenomenon when we look at population-based data,” Vaccarella said.
Persisting Problem
Recognition of the harms of overdiagnosis has resulted in some reduction of the incidence of thyroid cancer in the United States, Li and coauthors wrote. After adjusting for age, incidence has fallen from 19 per 100,000 women in 2013 to 16 per 100,000 women in 2017. The proportion of thyroid cancer attributed to overdiagnosis has dropped from 76% to 68% in the country.
The paper adds to the evidence suggesting that the rise in screening has not changed mortality rates for thyroid cancer. For example, Li and coauthors reported seeing “a small decrease in thyroid cancer mortality rates over time in some European countries, but this decline (less than 1 per 100,000 women) is marginal compared with the increases in incidence (reaching around 100 per 100,000 women).”
“Moreover, previous data show that the downward mortality trends had begun before the wide use of ultrasonography for early detection and that period and birth cohort effects have been declining, probably due to treatment advances and reduced prevalence of risk factors, such as the reduction in iodine deficiency,” they wrote.
In an interview, Amanda Davis, MD, of AnMed, a nonprofit health system based in Anderson, South Carolina, said the new paper from Li and Vaccarella provides further evidence for a cautious approach to thyroid nodules given concerns about overdiagnosis.
If early detection of cancer via discovery of thyroid nodules actually helped patients, mortality rates would have dropped with expansion of screening and the resulting diagnoses, said Davis, who is an associate program director at AnMed’s family medicine residency program and affiliate professor at the Medical University of South Carolina, Charleston.
In many cases, people learn they have thyroid lesions after being tested for other conditions such as ultrasound done on carotid arteries to check for stroke risk. The most common form of thyroid cancer is the papillary form. Papillary thyroid cancer tends to be slow growing, carries a low risk for distant metastasis, and in many cases poses little risk. Some small (< 1 cm) papillary thyroid cancers can be monitored with active surveillance as opposed to thyroid lobectomy.
“So just finding more nodules incidentally or through screening ultrasound and even finding more papillary cancers via these methods does not make people healthier or decrease mortality,” Davis said.
“So just finding more things and even finding more papillary cancers does not increase our ability to treat people and keep them alive longer,” Davis said.
The 5-year survival rate for thyroid cancer overall is 98.1% and varies from 99.9% for localized disease to 55.3% for distant disease, the US Preventive Services Task Force (USPSTF) said in a 2017 publication in JAMA. The task force that year gave a “D” rating on screening of asymptomatic people for thyroid cancer. That means there’s moderate certainty that screening for thyroid cancer in asymptomatic persons results in harms that outweigh the benefits. The decision to give this “D” rating meant this screening is not recommended. That’s still the panel’s view.
“You can think of it as a “D” for ‘don’t screen for thyroid cancer,’ ” in people who present no symptoms of this illness, John Wong, MD, the vice chair of the USPSTF, said in an interview.
In primary care, the challenge is assessing thyroid nodules detected when people undergo testing for another reason, such as an ultrasound of the carotid artery to check for stroke risk.
Thyroid nodules can be detected by ultrasonography in up to 68% of the general population, reported a study in American Family Physician. Nodules with suspicious features or ≥ 1 cm require fine needle aspiration. The Bethesda System for Reporting Thyroid Cytopathology can be used to classify samples, with molecular testing applied to guide treatment when fine needle aspiration yields an indeterminate result.
New Thinking on Thyroid Cancer
There’s been a shift in recent years in the approach to how physicians should proceed if certain kinds of thyroid cancer are detected, Cari M. Kitahara, PhD, of the National Cancer Institute noted in a comment accompanying the Li paper.
“Clinicians need to be judicious in the use of thyroid ultrasonography, the diagnostic follow-up of incidentally detected thyroid nodules, and determining the optimal course of treatment,” Kitahara wrote. “For low-risk and incidentally detected tumors, strong consideration should be given to less intensive treatment options (eg, lobectomy, delayed treatment, and active surveillance).”
The American Thyroid Association guidelines encourage de-escalation of treatment for low-risk papillary thyroid carcinoma up to 4 cm.
Physicians often need to make clear to patients how a diagnosis of low-risk papillary thyroid cancer differs from other oncology diagnoses, R. Michael Tuttle, MD, of Memorial Sloan Kettering Cancer Center, New York City, said in an interview.
“I’ll frequently say that everything you’ve ever learned about cancer, you need to forget,” Tuttle said.
Some patients will mistakenly think any cancer diagnosis is a likely death sentence, meaning they should rush to get aggressive treatment. Tuttle has been a leader for many years in efforts in advancing active surveillance as an option for certain people with low-risk thyroid cancer.
“I often start my consultation by saying: ‘We’re going to choose between two right answers here. One right answer is watching right. One right answer is going to surgery,’ ” Tuttle said.
Patients with low-risk thyroid cancer tend to fall into two camps, with maximalists likely to seek quick treatment and minimalists more inclined for surveillance if that’s an option for them, Tuttle said. As opinions have shifted within the medical community about approaches to low-risk thyroid cancer, there’s also been some growing awareness among the public about thyroid overdiagnosis.
“Ten or 15 years ago, people thought we were crazy” to consider active surveillance as an option for low-risk thyroid cancers,” Tuttle said. “Now we have swung, at least in some of the public opinion, to this recognition that every little speck of cancer doesn’t need to be immediately taken out of your body.”
Some patients express regret about having learned that they have low-risk thyroid cancer, Tuttle said.
“Over the last 5 years, it’s not uncommon for patients to ask me, ‘Is this one of those that needs to be treated now, or is this one of those that we wish we would have never found?’ Or people will say, ‘My doctor talked me into an ultrasound, I didn’t want it’ or ‘I had a car wreck, and I found this nodule and I wished I had never found it.’ ”
This study from Li and coauthors was funded by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Young Talents Program of Sun Yat-sen University Cancer Center, the Italian Association for Cancer Research, and the Italian Ministry of Health. Davis and Tuttle had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
A 51-year-old woman presented for a routine full body skin exam after vacationing in Hawaii.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Time to Stop Saying Thyroid Cancer Is a ‘Good’ Cancer
Papillary thyroid cancer is widely known as the “good cancer.” This term has been around for years and is used ubiquitously. Some think it’s “appropriate” because the cancer is highly treatable and has good survival rates. Yet, recent research, provider experiences, and patient feedback suggest the term should no longer be used.
Papillary is the most common type of thyroid cancer, comprising about 70%-80% of all thyroid cancers. It tends to grow slowly and “has a generally excellent outlook, even if there is spread to the lymph nodes,” according to the American Thyroid Association.
This “excellent outlook” can prompt a physician to call it a “good” cancer.
“There is often a perception that a patient is diagnosed, treated, and then once treatment is complete, gets to go back to their ‘normal’ lives,” said Fiona Schulte, PhD, RPsych, of the University of Calgary, Alberta, Canada.
“The surgery and other treatments thyroid patients may require are not benign and leave patients with many long-term challenging consequences,” she said in an interview. “For many, treatment is just the beginning of a long journey of dealing with multiple late effects.”
Misguided ‘Support’
“I do believe the doctor’s intention is to bring comfort to the patient by saying they have a very curable disease,” Miranda Fidler-Benaoudia, MD, of the University of Calgary, said in an interview. Fidler-Benaoudia is the principal author of a recent survey/interview study of early-onset thyroid cancer survivors, titled “No such thing as a good cancer.” Despite the doctor’s intention, her team found that “for the majority of individuals interviewed, the response was actually quite negative.”
“Specifically,” she said, “thyroid cancer patients felt that the use of the term ‘good cancer’ minimized their diagnosis and experience, often making them feel like their struggles with the diagnosis and its treatment were not justified. While they were indeed cancer patients, they did not feel they could claim to be one because their prognosis was very positive or they didn’t have more intensive therapies like radiotherapy or chemotherapy.”
These feelings were echoed in a recent Moffitt Center article. When Emma Stevens learned she had thyroid cancer at age 19, she said she heard the same statements repeatedly, including: “At least it’s only thyroid cancer.” “It’s the good cancer, and easy to deal with.”
“These are such weird things to say to me,” said Stevens, now 26. “I know they didn’t have any ill will and they couldn’t see how such statements could be upsetting. It’s been my goal to shed some light on how what they see as encouraging thoughts can upset someone like me.”
In an article on the appropriateness of the term “good cancer,” Reese W. Randle, MD, now at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and colleagues wrote, “Patients with papillary thyroid cancer commonly confront the perception that their malignancy is ‘good,’ but the favorable prognosis and treatability of the disease do not comprehensively represent their cancer fight.”
“The ‘good cancer’ perception is at the root of many mixed and confusing emotions,” they continued. “Clinicians emphasize optimistic outcomes, hoping to comfort, but they might inadvertently invalidate the impact thyroid cancer has on patients’ lives.”
Life-Altering
“Having a diagnosis of thyroid cancer, even with usually a very good prognosis, can be life-altering, said Caitlin P. McMullen, MD, a head and neck cancer specialist at Moffitt Cancer Center, Tampa, Florida.
Most papillary thyroid cancers are cured with surgery alone, sometimes followed by radioactive iodine, she said in an interview. “The surgery involves removing half (lobectomy), or sometimes all (total thyroidectomy), of the thyroid gland.” Patients with lymph node involvement have a longer surgery that includes lymph node removal.
Many patients must also remain on medication permanently to replace their thyroid hormone, she continued. And, after treatment is complete, “patients require regular follow up with bloodwork and imaging for many years to ensure the cancer does not return.”
“Repeated visits, medications, and testing can also result in financial toxicities and repeated disruptions for patients,” she added. “These downstream effects of a thyroid cancer diagnosis can significantly alter a patient’s life.”
Kaniksha Desai, MD, Endocrinology Quality Director at Stanford University School of Medicine, Palo Alto, California, said in an interview that thyroid cancer treatments carry some risks that shouldn’t be overlooked and may affect recovery for years. These include:
- Recurrent laryngeal nerve damage: Thyroid surgery can lead to vocal cord paralysis, affecting speech and swallowing.
- Hypoparathyroidism: Postsurgical damage to the parathyroid glands can cause long-term calcium regulation problems resulting in pain and emergency department visits as well as lifelong supplementation with calcium and vitamin D.
- Radioactive iodine (RAI) treatment: RAI can have side effects such as dry mouth, tear duct obstruction, salivary gland dysfunction, and an increased risk of secondary cancers.
- Psychosocial Impact: Being told they have cancer can create significant psychological distress for patients, including fear of recurrence, body image concerns, and anxiety, all of which persist even with a “good prognosis.”
Fidler-Benaoudia’s studies focused specifically on the psychosocial impact on younger patients. “Facing a cancer diagnosis at a young age really forces the person to hit the ‘pause button’ – they may need to take a break from school or work, and it may impact their relationships with their family and friends.”
“Even if their cancer has a very high survival rate, when a young person receives a cancer diagnosis they are often facing their own immortality for the first time, which can be very distressing,” she said. Many of her study participants also struggled to maintain appropriate thyroid hormone levels with medication, which left them feeling tired, losing hair or gaining weight. The surgery itself “can leave a substantial scar on the throat that is visible unless purposefully covered with clothing or accessories,” she noted. “We found that this scar impacted quite a few survivors’ body image.”
Awareness, Education
Two recent studies pointed to the need for clinicians to be aware of their patients’ reactions to a thyroid diagnosis. Susan C. Pitt, MD, associate professor of surgery and director of the endocrine surgery health services research program at the University of Michigan, Ann Arbor, and colleagues reviewed the literature on patient perception of receiving a thyroid diagnosis and found, “Fear and worry about cancer in general and the possibility for recurrence contribute to lasting psychological distress and decreased quality of life. Patients’ perceptions of their diagnosis and resulting emotional reactions influence treatment decision making and have the potential to contribute to decisions that may over-treat a low-risk thyroid cancer.”
In another recent study, Pitt and colleagues assessed fear of thyroid cancer in the general US population and found that close to half of 1136 respondents to an online survey had high levels of thyroid cancer-specific fear, particularly women and those under age 40. “Because disease-specific fear is associated with overtreatment, targeted education about the seriousness, incidence, and risk factors for developing thyroid cancer may decrease public fear and possibly overtreatment related to ‘scared decision-making,’” the authors concluded.
McMullen added, “Taking the time to educate the patient on the diagnosis, prognosis, and treatments can provide reassurance without being dismissive. Most patients are very receptive and understanding once things are explained thoroughly and their questions are answered. We find that factual information can be even more reassuring for patients than saying, ‘This is a good cancer.’”
Desai advised, “Clinicians should acknowledge the spectrum of experiences patients may have.” They should provide empathy and reassurance as well as personalized discussions regarding prognosis and treatment options. In addition, “they should focus on survivorship care by addressing both the long-term and short-term effects on health and lifestyle that can occur post treatment,” as well as the possible need for mental health support.
“I heard many times in residency that, ‘if you had to have cancer, have thyroid cancer,’ ” Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, and vice chair of the American Association of Clinical Endocrinology’s Disease State Networks, said in an interview.
“One should not want any cancer,” she said. “There are some very aggressive tumor markers in differentiated thyroid cancer that can have a worse prognosis. There are many aspects of thyroid cancer treatment that cause anxiety and a stress burden. Recovery varies from person to person.”
“There needs to be education across all sectors of healthcare, particularly in primary care,” she added. “I personally have medullary thyroid cancer that I found myself while fixing my ultrasound. There are many aspects to survivorship.”
Fidler-Benaoudia, Schulte, McMullen, and Desai declared no competing interests. Gupta is on the speaker bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie.
A version of this article first appeared on Medscape.com.
Papillary thyroid cancer is widely known as the “good cancer.” This term has been around for years and is used ubiquitously. Some think it’s “appropriate” because the cancer is highly treatable and has good survival rates. Yet, recent research, provider experiences, and patient feedback suggest the term should no longer be used.
Papillary is the most common type of thyroid cancer, comprising about 70%-80% of all thyroid cancers. It tends to grow slowly and “has a generally excellent outlook, even if there is spread to the lymph nodes,” according to the American Thyroid Association.
This “excellent outlook” can prompt a physician to call it a “good” cancer.
“There is often a perception that a patient is diagnosed, treated, and then once treatment is complete, gets to go back to their ‘normal’ lives,” said Fiona Schulte, PhD, RPsych, of the University of Calgary, Alberta, Canada.
“The surgery and other treatments thyroid patients may require are not benign and leave patients with many long-term challenging consequences,” she said in an interview. “For many, treatment is just the beginning of a long journey of dealing with multiple late effects.”
Misguided ‘Support’
“I do believe the doctor’s intention is to bring comfort to the patient by saying they have a very curable disease,” Miranda Fidler-Benaoudia, MD, of the University of Calgary, said in an interview. Fidler-Benaoudia is the principal author of a recent survey/interview study of early-onset thyroid cancer survivors, titled “No such thing as a good cancer.” Despite the doctor’s intention, her team found that “for the majority of individuals interviewed, the response was actually quite negative.”
“Specifically,” she said, “thyroid cancer patients felt that the use of the term ‘good cancer’ minimized their diagnosis and experience, often making them feel like their struggles with the diagnosis and its treatment were not justified. While they were indeed cancer patients, they did not feel they could claim to be one because their prognosis was very positive or they didn’t have more intensive therapies like radiotherapy or chemotherapy.”
These feelings were echoed in a recent Moffitt Center article. When Emma Stevens learned she had thyroid cancer at age 19, she said she heard the same statements repeatedly, including: “At least it’s only thyroid cancer.” “It’s the good cancer, and easy to deal with.”
“These are such weird things to say to me,” said Stevens, now 26. “I know they didn’t have any ill will and they couldn’t see how such statements could be upsetting. It’s been my goal to shed some light on how what they see as encouraging thoughts can upset someone like me.”
In an article on the appropriateness of the term “good cancer,” Reese W. Randle, MD, now at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and colleagues wrote, “Patients with papillary thyroid cancer commonly confront the perception that their malignancy is ‘good,’ but the favorable prognosis and treatability of the disease do not comprehensively represent their cancer fight.”
“The ‘good cancer’ perception is at the root of many mixed and confusing emotions,” they continued. “Clinicians emphasize optimistic outcomes, hoping to comfort, but they might inadvertently invalidate the impact thyroid cancer has on patients’ lives.”
Life-Altering
“Having a diagnosis of thyroid cancer, even with usually a very good prognosis, can be life-altering, said Caitlin P. McMullen, MD, a head and neck cancer specialist at Moffitt Cancer Center, Tampa, Florida.
Most papillary thyroid cancers are cured with surgery alone, sometimes followed by radioactive iodine, she said in an interview. “The surgery involves removing half (lobectomy), or sometimes all (total thyroidectomy), of the thyroid gland.” Patients with lymph node involvement have a longer surgery that includes lymph node removal.
Many patients must also remain on medication permanently to replace their thyroid hormone, she continued. And, after treatment is complete, “patients require regular follow up with bloodwork and imaging for many years to ensure the cancer does not return.”
“Repeated visits, medications, and testing can also result in financial toxicities and repeated disruptions for patients,” she added. “These downstream effects of a thyroid cancer diagnosis can significantly alter a patient’s life.”
Kaniksha Desai, MD, Endocrinology Quality Director at Stanford University School of Medicine, Palo Alto, California, said in an interview that thyroid cancer treatments carry some risks that shouldn’t be overlooked and may affect recovery for years. These include:
- Recurrent laryngeal nerve damage: Thyroid surgery can lead to vocal cord paralysis, affecting speech and swallowing.
- Hypoparathyroidism: Postsurgical damage to the parathyroid glands can cause long-term calcium regulation problems resulting in pain and emergency department visits as well as lifelong supplementation with calcium and vitamin D.
- Radioactive iodine (RAI) treatment: RAI can have side effects such as dry mouth, tear duct obstruction, salivary gland dysfunction, and an increased risk of secondary cancers.
- Psychosocial Impact: Being told they have cancer can create significant psychological distress for patients, including fear of recurrence, body image concerns, and anxiety, all of which persist even with a “good prognosis.”
Fidler-Benaoudia’s studies focused specifically on the psychosocial impact on younger patients. “Facing a cancer diagnosis at a young age really forces the person to hit the ‘pause button’ – they may need to take a break from school or work, and it may impact their relationships with their family and friends.”
“Even if their cancer has a very high survival rate, when a young person receives a cancer diagnosis they are often facing their own immortality for the first time, which can be very distressing,” she said. Many of her study participants also struggled to maintain appropriate thyroid hormone levels with medication, which left them feeling tired, losing hair or gaining weight. The surgery itself “can leave a substantial scar on the throat that is visible unless purposefully covered with clothing or accessories,” she noted. “We found that this scar impacted quite a few survivors’ body image.”
Awareness, Education
Two recent studies pointed to the need for clinicians to be aware of their patients’ reactions to a thyroid diagnosis. Susan C. Pitt, MD, associate professor of surgery and director of the endocrine surgery health services research program at the University of Michigan, Ann Arbor, and colleagues reviewed the literature on patient perception of receiving a thyroid diagnosis and found, “Fear and worry about cancer in general and the possibility for recurrence contribute to lasting psychological distress and decreased quality of life. Patients’ perceptions of their diagnosis and resulting emotional reactions influence treatment decision making and have the potential to contribute to decisions that may over-treat a low-risk thyroid cancer.”
In another recent study, Pitt and colleagues assessed fear of thyroid cancer in the general US population and found that close to half of 1136 respondents to an online survey had high levels of thyroid cancer-specific fear, particularly women and those under age 40. “Because disease-specific fear is associated with overtreatment, targeted education about the seriousness, incidence, and risk factors for developing thyroid cancer may decrease public fear and possibly overtreatment related to ‘scared decision-making,’” the authors concluded.
McMullen added, “Taking the time to educate the patient on the diagnosis, prognosis, and treatments can provide reassurance without being dismissive. Most patients are very receptive and understanding once things are explained thoroughly and their questions are answered. We find that factual information can be even more reassuring for patients than saying, ‘This is a good cancer.’”
Desai advised, “Clinicians should acknowledge the spectrum of experiences patients may have.” They should provide empathy and reassurance as well as personalized discussions regarding prognosis and treatment options. In addition, “they should focus on survivorship care by addressing both the long-term and short-term effects on health and lifestyle that can occur post treatment,” as well as the possible need for mental health support.
“I heard many times in residency that, ‘if you had to have cancer, have thyroid cancer,’ ” Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, and vice chair of the American Association of Clinical Endocrinology’s Disease State Networks, said in an interview.
“One should not want any cancer,” she said. “There are some very aggressive tumor markers in differentiated thyroid cancer that can have a worse prognosis. There are many aspects of thyroid cancer treatment that cause anxiety and a stress burden. Recovery varies from person to person.”
“There needs to be education across all sectors of healthcare, particularly in primary care,” she added. “I personally have medullary thyroid cancer that I found myself while fixing my ultrasound. There are many aspects to survivorship.”
Fidler-Benaoudia, Schulte, McMullen, and Desai declared no competing interests. Gupta is on the speaker bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie.
A version of this article first appeared on Medscape.com.
Papillary thyroid cancer is widely known as the “good cancer.” This term has been around for years and is used ubiquitously. Some think it’s “appropriate” because the cancer is highly treatable and has good survival rates. Yet, recent research, provider experiences, and patient feedback suggest the term should no longer be used.
Papillary is the most common type of thyroid cancer, comprising about 70%-80% of all thyroid cancers. It tends to grow slowly and “has a generally excellent outlook, even if there is spread to the lymph nodes,” according to the American Thyroid Association.
This “excellent outlook” can prompt a physician to call it a “good” cancer.
“There is often a perception that a patient is diagnosed, treated, and then once treatment is complete, gets to go back to their ‘normal’ lives,” said Fiona Schulte, PhD, RPsych, of the University of Calgary, Alberta, Canada.
“The surgery and other treatments thyroid patients may require are not benign and leave patients with many long-term challenging consequences,” she said in an interview. “For many, treatment is just the beginning of a long journey of dealing with multiple late effects.”
Misguided ‘Support’
“I do believe the doctor’s intention is to bring comfort to the patient by saying they have a very curable disease,” Miranda Fidler-Benaoudia, MD, of the University of Calgary, said in an interview. Fidler-Benaoudia is the principal author of a recent survey/interview study of early-onset thyroid cancer survivors, titled “No such thing as a good cancer.” Despite the doctor’s intention, her team found that “for the majority of individuals interviewed, the response was actually quite negative.”
“Specifically,” she said, “thyroid cancer patients felt that the use of the term ‘good cancer’ minimized their diagnosis and experience, often making them feel like their struggles with the diagnosis and its treatment were not justified. While they were indeed cancer patients, they did not feel they could claim to be one because their prognosis was very positive or they didn’t have more intensive therapies like radiotherapy or chemotherapy.”
These feelings were echoed in a recent Moffitt Center article. When Emma Stevens learned she had thyroid cancer at age 19, she said she heard the same statements repeatedly, including: “At least it’s only thyroid cancer.” “It’s the good cancer, and easy to deal with.”
“These are such weird things to say to me,” said Stevens, now 26. “I know they didn’t have any ill will and they couldn’t see how such statements could be upsetting. It’s been my goal to shed some light on how what they see as encouraging thoughts can upset someone like me.”
In an article on the appropriateness of the term “good cancer,” Reese W. Randle, MD, now at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and colleagues wrote, “Patients with papillary thyroid cancer commonly confront the perception that their malignancy is ‘good,’ but the favorable prognosis and treatability of the disease do not comprehensively represent their cancer fight.”
“The ‘good cancer’ perception is at the root of many mixed and confusing emotions,” they continued. “Clinicians emphasize optimistic outcomes, hoping to comfort, but they might inadvertently invalidate the impact thyroid cancer has on patients’ lives.”
Life-Altering
“Having a diagnosis of thyroid cancer, even with usually a very good prognosis, can be life-altering, said Caitlin P. McMullen, MD, a head and neck cancer specialist at Moffitt Cancer Center, Tampa, Florida.
Most papillary thyroid cancers are cured with surgery alone, sometimes followed by radioactive iodine, she said in an interview. “The surgery involves removing half (lobectomy), or sometimes all (total thyroidectomy), of the thyroid gland.” Patients with lymph node involvement have a longer surgery that includes lymph node removal.
Many patients must also remain on medication permanently to replace their thyroid hormone, she continued. And, after treatment is complete, “patients require regular follow up with bloodwork and imaging for many years to ensure the cancer does not return.”
“Repeated visits, medications, and testing can also result in financial toxicities and repeated disruptions for patients,” she added. “These downstream effects of a thyroid cancer diagnosis can significantly alter a patient’s life.”
Kaniksha Desai, MD, Endocrinology Quality Director at Stanford University School of Medicine, Palo Alto, California, said in an interview that thyroid cancer treatments carry some risks that shouldn’t be overlooked and may affect recovery for years. These include:
- Recurrent laryngeal nerve damage: Thyroid surgery can lead to vocal cord paralysis, affecting speech and swallowing.
- Hypoparathyroidism: Postsurgical damage to the parathyroid glands can cause long-term calcium regulation problems resulting in pain and emergency department visits as well as lifelong supplementation with calcium and vitamin D.
- Radioactive iodine (RAI) treatment: RAI can have side effects such as dry mouth, tear duct obstruction, salivary gland dysfunction, and an increased risk of secondary cancers.
- Psychosocial Impact: Being told they have cancer can create significant psychological distress for patients, including fear of recurrence, body image concerns, and anxiety, all of which persist even with a “good prognosis.”
Fidler-Benaoudia’s studies focused specifically on the psychosocial impact on younger patients. “Facing a cancer diagnosis at a young age really forces the person to hit the ‘pause button’ – they may need to take a break from school or work, and it may impact their relationships with their family and friends.”
“Even if their cancer has a very high survival rate, when a young person receives a cancer diagnosis they are often facing their own immortality for the first time, which can be very distressing,” she said. Many of her study participants also struggled to maintain appropriate thyroid hormone levels with medication, which left them feeling tired, losing hair or gaining weight. The surgery itself “can leave a substantial scar on the throat that is visible unless purposefully covered with clothing or accessories,” she noted. “We found that this scar impacted quite a few survivors’ body image.”
Awareness, Education
Two recent studies pointed to the need for clinicians to be aware of their patients’ reactions to a thyroid diagnosis. Susan C. Pitt, MD, associate professor of surgery and director of the endocrine surgery health services research program at the University of Michigan, Ann Arbor, and colleagues reviewed the literature on patient perception of receiving a thyroid diagnosis and found, “Fear and worry about cancer in general and the possibility for recurrence contribute to lasting psychological distress and decreased quality of life. Patients’ perceptions of their diagnosis and resulting emotional reactions influence treatment decision making and have the potential to contribute to decisions that may over-treat a low-risk thyroid cancer.”
In another recent study, Pitt and colleagues assessed fear of thyroid cancer in the general US population and found that close to half of 1136 respondents to an online survey had high levels of thyroid cancer-specific fear, particularly women and those under age 40. “Because disease-specific fear is associated with overtreatment, targeted education about the seriousness, incidence, and risk factors for developing thyroid cancer may decrease public fear and possibly overtreatment related to ‘scared decision-making,’” the authors concluded.
McMullen added, “Taking the time to educate the patient on the diagnosis, prognosis, and treatments can provide reassurance without being dismissive. Most patients are very receptive and understanding once things are explained thoroughly and their questions are answered. We find that factual information can be even more reassuring for patients than saying, ‘This is a good cancer.’”
Desai advised, “Clinicians should acknowledge the spectrum of experiences patients may have.” They should provide empathy and reassurance as well as personalized discussions regarding prognosis and treatment options. In addition, “they should focus on survivorship care by addressing both the long-term and short-term effects on health and lifestyle that can occur post treatment,” as well as the possible need for mental health support.
“I heard many times in residency that, ‘if you had to have cancer, have thyroid cancer,’ ” Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, and vice chair of the American Association of Clinical Endocrinology’s Disease State Networks, said in an interview.
“One should not want any cancer,” she said. “There are some very aggressive tumor markers in differentiated thyroid cancer that can have a worse prognosis. There are many aspects of thyroid cancer treatment that cause anxiety and a stress burden. Recovery varies from person to person.”
“There needs to be education across all sectors of healthcare, particularly in primary care,” she added. “I personally have medullary thyroid cancer that I found myself while fixing my ultrasound. There are many aspects to survivorship.”
Fidler-Benaoudia, Schulte, McMullen, and Desai declared no competing interests. Gupta is on the speaker bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie.
A version of this article first appeared on Medscape.com.
Hypothyroidism Treatment Does Not Affect Cognitive Decline in Menopausal Women
TOPLINE:
Women with hypothyroidism treated with levothyroxine show no significant cognitive decline across the menopausal transition compared with those without thyroid disease.
METHODOLOGY:
- Levothyroxine, the primary treatment for hypothyroidism, has been linked to perceived cognitive deficits, yet it is unclear whether this is due to the underlying condition being inadequately treated or other factors.
- Using data collected from the Study of Women’s Health Across the Nation, which encompasses five ethnic/racial groups from seven centers across the United States, researchers compared cognitive function over time between women with hypothyroidism treated with levothyroxine and those without thyroid disease.
- Participants underwent cognitive testing across three domains — processing speed, working memory, and episodic memory — which were assessed over a mean follow-up of 13 years.
- Further analyses assessed the impact of abnormal levels of thyroid-stimulating hormone on cognitive outcomes.
TAKEAWAY:
- Of 2033 women included, 227 (mean age, 49.8 years) had levothyroxine-treated hypothyroidism and 1806 (mean age, 50.0 years) did not have thyroid disease; the proportion of women with premenopausal or early perimenopausal status at baseline was higher in the hypothyroidism group (54.2% vs 49.8%; P = .010).
- At baseline, levothyroxine-treated women had higher scores for processing speed (mean score, 56.5 vs 54.4; P = .006) and working memory (mean score, 6.8 vs 6.4; P = .018) than those without thyroid disease; however, no difference in episodic memory was observed between the groups.
- Over the study period, there was no significant difference in cognitive decline between the groups.
- There was no significant effect of levothyroxine-treated hypothyroidism on working memory or episodic memory, although an annual decline in processing speed was observed (P < .001).
- Sensitivity analyses determined that abnormal levels of thyroid-stimulating hormone did not affect cognitive outcomes in women with hypothyroidism.
IN PRACTICE:
When cognitive decline is observed in these patients, the authors advised that “clinicians should resist anchoring on inadequate treatment of hypothyroidism as the cause of these symptoms and may investigate other disease processes (eg, iron deficiency, B12 deficiency, sleep apnea, celiac disease).”
SOURCE:
The study, led by Matthew D. Ettleson, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, was published online in Thyroid.
LIMITATIONS:
The cognitive assessments in the study were not designed to provide a thorough evaluation of all aspects of cognitive function. The study may not have been adequately powered to detect small effects of levothyroxine-treated hypothyroidism on cognitive outcomes. The higher levels of education attained by the study population may have acted as a protective factor against cognitive decline, potentially biasing the results.
DISCLOSURES:
The Study of Women’s Health Across the Nation was supported by grants from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with hypothyroidism treated with levothyroxine show no significant cognitive decline across the menopausal transition compared with those without thyroid disease.
METHODOLOGY:
- Levothyroxine, the primary treatment for hypothyroidism, has been linked to perceived cognitive deficits, yet it is unclear whether this is due to the underlying condition being inadequately treated or other factors.
- Using data collected from the Study of Women’s Health Across the Nation, which encompasses five ethnic/racial groups from seven centers across the United States, researchers compared cognitive function over time between women with hypothyroidism treated with levothyroxine and those without thyroid disease.
- Participants underwent cognitive testing across three domains — processing speed, working memory, and episodic memory — which were assessed over a mean follow-up of 13 years.
- Further analyses assessed the impact of abnormal levels of thyroid-stimulating hormone on cognitive outcomes.
TAKEAWAY:
- Of 2033 women included, 227 (mean age, 49.8 years) had levothyroxine-treated hypothyroidism and 1806 (mean age, 50.0 years) did not have thyroid disease; the proportion of women with premenopausal or early perimenopausal status at baseline was higher in the hypothyroidism group (54.2% vs 49.8%; P = .010).
- At baseline, levothyroxine-treated women had higher scores for processing speed (mean score, 56.5 vs 54.4; P = .006) and working memory (mean score, 6.8 vs 6.4; P = .018) than those without thyroid disease; however, no difference in episodic memory was observed between the groups.
- Over the study period, there was no significant difference in cognitive decline between the groups.
- There was no significant effect of levothyroxine-treated hypothyroidism on working memory or episodic memory, although an annual decline in processing speed was observed (P < .001).
- Sensitivity analyses determined that abnormal levels of thyroid-stimulating hormone did not affect cognitive outcomes in women with hypothyroidism.
IN PRACTICE:
When cognitive decline is observed in these patients, the authors advised that “clinicians should resist anchoring on inadequate treatment of hypothyroidism as the cause of these symptoms and may investigate other disease processes (eg, iron deficiency, B12 deficiency, sleep apnea, celiac disease).”
SOURCE:
The study, led by Matthew D. Ettleson, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, was published online in Thyroid.
LIMITATIONS:
The cognitive assessments in the study were not designed to provide a thorough evaluation of all aspects of cognitive function. The study may not have been adequately powered to detect small effects of levothyroxine-treated hypothyroidism on cognitive outcomes. The higher levels of education attained by the study population may have acted as a protective factor against cognitive decline, potentially biasing the results.
DISCLOSURES:
The Study of Women’s Health Across the Nation was supported by grants from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with hypothyroidism treated with levothyroxine show no significant cognitive decline across the menopausal transition compared with those without thyroid disease.
METHODOLOGY:
- Levothyroxine, the primary treatment for hypothyroidism, has been linked to perceived cognitive deficits, yet it is unclear whether this is due to the underlying condition being inadequately treated or other factors.
- Using data collected from the Study of Women’s Health Across the Nation, which encompasses five ethnic/racial groups from seven centers across the United States, researchers compared cognitive function over time between women with hypothyroidism treated with levothyroxine and those without thyroid disease.
- Participants underwent cognitive testing across three domains — processing speed, working memory, and episodic memory — which were assessed over a mean follow-up of 13 years.
- Further analyses assessed the impact of abnormal levels of thyroid-stimulating hormone on cognitive outcomes.
TAKEAWAY:
- Of 2033 women included, 227 (mean age, 49.8 years) had levothyroxine-treated hypothyroidism and 1806 (mean age, 50.0 years) did not have thyroid disease; the proportion of women with premenopausal or early perimenopausal status at baseline was higher in the hypothyroidism group (54.2% vs 49.8%; P = .010).
- At baseline, levothyroxine-treated women had higher scores for processing speed (mean score, 56.5 vs 54.4; P = .006) and working memory (mean score, 6.8 vs 6.4; P = .018) than those without thyroid disease; however, no difference in episodic memory was observed between the groups.
- Over the study period, there was no significant difference in cognitive decline between the groups.
- There was no significant effect of levothyroxine-treated hypothyroidism on working memory or episodic memory, although an annual decline in processing speed was observed (P < .001).
- Sensitivity analyses determined that abnormal levels of thyroid-stimulating hormone did not affect cognitive outcomes in women with hypothyroidism.
IN PRACTICE:
When cognitive decline is observed in these patients, the authors advised that “clinicians should resist anchoring on inadequate treatment of hypothyroidism as the cause of these symptoms and may investigate other disease processes (eg, iron deficiency, B12 deficiency, sleep apnea, celiac disease).”
SOURCE:
The study, led by Matthew D. Ettleson, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, was published online in Thyroid.
LIMITATIONS:
The cognitive assessments in the study were not designed to provide a thorough evaluation of all aspects of cognitive function. The study may not have been adequately powered to detect small effects of levothyroxine-treated hypothyroidism on cognitive outcomes. The higher levels of education attained by the study population may have acted as a protective factor against cognitive decline, potentially biasing the results.
DISCLOSURES:
The Study of Women’s Health Across the Nation was supported by grants from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Cannabis Linked to Bulging Eyes in Graves’ Disease
TOPLINE:
Among patients with autoimmune hyperthyroidism, those who use cannabis are 1.9 times more likely to develop exophthalmos — eyes that appear to bulge from the face — within 1 year of diagnosis, than those who do not use the drug. However, the added risk may wane over time.
METHODOLOGY:
- Researchers analyzed data from TriNetX, an electronic health record platform, for more than 36,000 patients with autoimmune hyperthyroidism between 2003 and 2023.
- The dataset included cannabis users (n = 783), nicotine users (n = 17,310), and control individuals (n = 18,093) who did not use either substance.
- Primary outcomes included presentations of thyroid eye disease (TED) and the use of treatments for the condition, such as teprotumumab, steroids, eyelid retraction repair, tarsorrhaphy, strabismus surgery, or orbital decompression.
- The investigators used propensity matching to control for characteristics such as age, sex, race, and prior thyroidectomy or radio ablation.
TAKEAWAY:
- The incidence of exophthalmos at 1 year was 4.1% among nicotine users, 4.1% among cannabis users, and 2.2% among controls.
- Cannabis users were 1.9 times more likely than controls to develop exophthalmos within 1 year (P = .03).
- At 2 years, the researchers identified a trend toward more TED in cannabis users than in controls, but the difference was no longer statistically significant.
- Cannabis users were about 2.5 times more likely than controls to be prescribed steroids throughout the 2-year follow-up period.
IN PRACTICE:
“These findings altogether suggest that cannabis usage may be associated with earlier progression or increased short-term severity of TED symptoms,” the authors of the study wrote. The mechanisms may be like those for cigarette smoking and could include inflammation and vascular congestion, they added.
SOURCE:
The study was conducted by Amanda M. Zong and Anne Barmettler, MD, with Albert Einstein College of Medicine in New York City. It was published online in Ophthalmic Plastic and Reconstructive Surgery.
LIMITATIONS:
The number of cannabis users was relatively small and included only patients who had received a diagnosis of a cannabis-usage disorder prior to the diagnosis of autoimmune hyperthyroidism, the researchers noted. TED lacks a specific International Classification of Diseases–10 code, which necessitated the use of indirect measures. “Furthermore, the mode of administration, duration, and frequency of cannabis and nicotine usage were not available in the dataset used, limiting analysis of degree of association and modifiable risk,” they wrote.
DISCLOSURES:
The researchers disclosed no relevant financial relationships.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with autoimmune hyperthyroidism, those who use cannabis are 1.9 times more likely to develop exophthalmos — eyes that appear to bulge from the face — within 1 year of diagnosis, than those who do not use the drug. However, the added risk may wane over time.
METHODOLOGY:
- Researchers analyzed data from TriNetX, an electronic health record platform, for more than 36,000 patients with autoimmune hyperthyroidism between 2003 and 2023.
- The dataset included cannabis users (n = 783), nicotine users (n = 17,310), and control individuals (n = 18,093) who did not use either substance.
- Primary outcomes included presentations of thyroid eye disease (TED) and the use of treatments for the condition, such as teprotumumab, steroids, eyelid retraction repair, tarsorrhaphy, strabismus surgery, or orbital decompression.
- The investigators used propensity matching to control for characteristics such as age, sex, race, and prior thyroidectomy or radio ablation.
TAKEAWAY:
- The incidence of exophthalmos at 1 year was 4.1% among nicotine users, 4.1% among cannabis users, and 2.2% among controls.
- Cannabis users were 1.9 times more likely than controls to develop exophthalmos within 1 year (P = .03).
- At 2 years, the researchers identified a trend toward more TED in cannabis users than in controls, but the difference was no longer statistically significant.
- Cannabis users were about 2.5 times more likely than controls to be prescribed steroids throughout the 2-year follow-up period.
IN PRACTICE:
“These findings altogether suggest that cannabis usage may be associated with earlier progression or increased short-term severity of TED symptoms,” the authors of the study wrote. The mechanisms may be like those for cigarette smoking and could include inflammation and vascular congestion, they added.
SOURCE:
The study was conducted by Amanda M. Zong and Anne Barmettler, MD, with Albert Einstein College of Medicine in New York City. It was published online in Ophthalmic Plastic and Reconstructive Surgery.
LIMITATIONS:
The number of cannabis users was relatively small and included only patients who had received a diagnosis of a cannabis-usage disorder prior to the diagnosis of autoimmune hyperthyroidism, the researchers noted. TED lacks a specific International Classification of Diseases–10 code, which necessitated the use of indirect measures. “Furthermore, the mode of administration, duration, and frequency of cannabis and nicotine usage were not available in the dataset used, limiting analysis of degree of association and modifiable risk,” they wrote.
DISCLOSURES:
The researchers disclosed no relevant financial relationships.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with autoimmune hyperthyroidism, those who use cannabis are 1.9 times more likely to develop exophthalmos — eyes that appear to bulge from the face — within 1 year of diagnosis, than those who do not use the drug. However, the added risk may wane over time.
METHODOLOGY:
- Researchers analyzed data from TriNetX, an electronic health record platform, for more than 36,000 patients with autoimmune hyperthyroidism between 2003 and 2023.
- The dataset included cannabis users (n = 783), nicotine users (n = 17,310), and control individuals (n = 18,093) who did not use either substance.
- Primary outcomes included presentations of thyroid eye disease (TED) and the use of treatments for the condition, such as teprotumumab, steroids, eyelid retraction repair, tarsorrhaphy, strabismus surgery, or orbital decompression.
- The investigators used propensity matching to control for characteristics such as age, sex, race, and prior thyroidectomy or radio ablation.
TAKEAWAY:
- The incidence of exophthalmos at 1 year was 4.1% among nicotine users, 4.1% among cannabis users, and 2.2% among controls.
- Cannabis users were 1.9 times more likely than controls to develop exophthalmos within 1 year (P = .03).
- At 2 years, the researchers identified a trend toward more TED in cannabis users than in controls, but the difference was no longer statistically significant.
- Cannabis users were about 2.5 times more likely than controls to be prescribed steroids throughout the 2-year follow-up period.
IN PRACTICE:
“These findings altogether suggest that cannabis usage may be associated with earlier progression or increased short-term severity of TED symptoms,” the authors of the study wrote. The mechanisms may be like those for cigarette smoking and could include inflammation and vascular congestion, they added.
SOURCE:
The study was conducted by Amanda M. Zong and Anne Barmettler, MD, with Albert Einstein College of Medicine in New York City. It was published online in Ophthalmic Plastic and Reconstructive Surgery.
LIMITATIONS:
The number of cannabis users was relatively small and included only patients who had received a diagnosis of a cannabis-usage disorder prior to the diagnosis of autoimmune hyperthyroidism, the researchers noted. TED lacks a specific International Classification of Diseases–10 code, which necessitated the use of indirect measures. “Furthermore, the mode of administration, duration, and frequency of cannabis and nicotine usage were not available in the dataset used, limiting analysis of degree of association and modifiable risk,” they wrote.
DISCLOSURES:
The researchers disclosed no relevant financial relationships.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Abnormal TSH: Forget it or Fret it?
If you’re like most primary care clinicians, your email inbox is flooded with messages from patients with questions about lab results. A common query: Should I be worried about an abnormal value on a test of thyroid-stimulating hormone (TSH)?
For guidance, this news organization spoke with Angela Leung, MD, associate professor of medicine in the Division of Endocrinology, Diabetes & Metabolism at the UCLA David Geffen School of Medicine and an endocrinologist at UCLA and the VA Greater Los Angeles Healthcare System, and Karen Tsai, MD, assistant clinical professor of endocrinology at City of Hope Comprehensive Cancer Center in Duarte, California. The following interview has been edited for length and clarity.
Question: Why do you usually start by measuring TSH levels?
Dr. Leung: We need to measure the thyroid status in a way that integrates more information about the long-term thyroid status and not small changes in thyroid hormone levels. TSH is made by the pituitary gland in the brain, which integrates information about the signals of high and low levels from each of the different thyroid hormones.
Now we can measure the actual thyroid hormones — primarily we’re talking about T3 and T4 — but if we do that, we are relying on a single snapshot in the bloodstream at that moment. The levels might change throughout the day in response to ongoing metabolism and outside stresses. So we usually start by measuring the TSH level, which is a good representation of the compilation of all those things over the past 30 days or so.
Question: How do you describe a low TSH result to patients?
Dr. Leung: Whenever we encounter a low TSH level, we want to repeat the test because it is a dynamic test, and it can change in response to several factors. If it is indeed low, we’re thinking that perhaps there’s a little bit of extra thyroid hormone in the body. It can be either temporary or more chronic, but that higher amount of thyroid hormone is telling the pituitary gland in the brain to start making less. So TSH levels go low when we need less thyroid hormone.
Question: What are some of the reasons for a low TSH level?
Dr. Leung: One of the most common situations for a temporarily low TSH level I see is what we call nonthyroidal illness, like a common cold or just being under the weather. Other things that can artifactually lower the TSH level could be the use of steroids, such as prednisone for asthma or some sort of a rheumatologic condition. Also, the TSH level could be low if a person has been recently exposed to very high amounts of iodine, such as iodinated contrast needed for a CT scan.
If the TSH level remains persistently low, usually in the presence of high thyroid hormone (T3 and/or T4) levels, the most common reason for hyperthyroidism is Graves disease, in which there are autoantibodies — measurable in the blood — that can stimulate the thyroid gland in the neck to make extra thyroid hormone.
Question: And what does an elevated TSH level mean?
Dr. Leung: Again, we want to confirm that it is elevated. We need at least two tests to confirm a high TSH level. A persistently elevated TSH level is a signal there might be low thyroid hormone levels in the body, which could be transient or more longer lasting.
Question: What are some of the most common causes of an elevated TSH level?
Dr. Leung: If the TSH level is confirmed high and the thyroid hormone levels are low, the most common cause of hypothyroidism here in the United States is Hashimoto thyroiditis.
Globally, iodine deficiency is the most common reason for hypothyroidism and may be a problem in parts of the globe where there are endemically low iodine levels in soil, crops, and the food supply like not having enough iodized salt. The thyroid is reliant on having enough iodine as a micronutrient to make thyroid hormone. If it doesn’t, the thyroid really can’t make thyroid hormone. It’s important to also remember, though, that having too much iodine can result in hypo- or hyperthyroidism.
Dr. Tsai: I take a glance at their medication list. Some of the patients are on methimazole or levothyroxine, and those medications should be adjusted first to normalize the TSH level. Other medications like lithium and amiodarone can also cause elevated TSH levels. We are also seeing a lot of patients on cancer therapies, such as tyrosine kinase inhibitors or immunotherapy, that can cause an elevated TSH level.
Question: If the repeat TSH test shows that TSH levels are still elevated, what comes next in your workup?
Dr. Tsai: If there’s not a real clear-cut diagnosis, I’ll order the thyroid peroxidase antibody and the thyroglobulin antibody, although thyroid peroxidase antibody, which is indicative of autoimmune thyroid disease, alone is usually sufficient to make that diagnosis.
Question: Should clinicians follow thyroid antibodies over time?
Dr. Tsai: I usually don’t repeat the antibody tests. In those circumstances where patients who were diagnosed 50-60 years ago and perhaps it is unknown if they had the thyroid antibodies measured at the time and now they’re saying, “Do I actually have Hashimoto’s?” or “Do I really need to continue this for the rest of my life?” I do repeat antibody tests to help gauge if the patient’s levothyroxine can be stopped.
Question: How important is it to follow T4 or T3 levels?
Dr. Tsai: T4 and T3 levels can help differentiate overt thyroid dysfunction — where T3 and/or T4 levels will be abnormal — from subclinical thyroid dysfunction — where T3 and T4 levels would be normal. In general, although we do not fully appreciate the best metric to monitor hypo- or hyperthyroidism, because some patients with a normal TSH level still may have symptoms of thyroid dysfunction, these peripheral thyroid hormone levels are usually the most helpful at the time of initial diagnosis.
Question: What are your criteria for initiating treatment for hypothyroidism?
Dr. Tsai: If the TSH level > 10 mIU/L, I recommend levothyroxine hormone replacement. A lot of published data support clinical benefit in this group.
There is a gray area in those patients who have a TSH level higher than the upper limit of the reference range but less than 10. If the patient doesn’t have overt hypothyroid symptoms, I discuss the findings with the patient but don’t really feel eager to treat. I recommend checking the levels again in 6 months to see where that TSH goes, and if it worsens or becomes greater than 10 mIU/L, I then recommend levothyroxine hormone replacement.
It is also important to note that a TSH level of 5-7 may be an acceptable range for older patients, and they do not require levothyroxine.
The other category is patients whose TSH level is greater than the upper limit of the normal reference range but less than 10 and with overt hypothyroid symptoms such as fatigue, unintentional weight gain, constipation, or cold intolerance. In these patients, it is worthwhile to try a low dose of levothyroxine (25-50 mcg/d) and repeat TSH and free T4 tests in 6-8 weeks and see if the TSH level normalizes.
Dr. Leung: When you look at subclinical hypothyroidism, the situation of an isolated high TSH level in the setting of normal T4 levels, if the TSH level is mildly elevated in the 5-7 mIU/L range, there’s a 60% chance that it will normalize within 6 months.
Going back to Karen’s point, a lot of people are started and maintained on low doses of thyroid hormone forever and ever. A recent study on levothyroxine use found half of the prescriptions were unnecessary.
Question: In an era where many patients obtain much of their health information from TikTok, what’s your approach with patients with a normal TSH level who feel that they should have more testing or start treatment?
Dr. Tsai: Fatigue is one of the common referrals we get into our endocrinology practice, and everyone is convinced that their thyroid is the culprit. It is important to note, however, that fatigue can be due to different diseases such as anemia, depression, sleep disorders, or a recent viral illness.
TSH tests are readily available and cheap. I don’t mind ordering the lab test again if it helps give the patient some reassurance. I also find that patients are relieved once they hear from their endocrinologist that their thyroid is unlikely to be the cause of their fatigue.
Some other endocrine causes we may consider additionally working up include adrenal insufficiency, vitamin D deficiency, and diabetes. A comprehensive metabolic panel and complete blood count is part of my workup to rule out any gross electrolyte abnormalities or any new diagnosis of anemia, liver disease, or chronic kidney disease.
Question: What are your criteria for recommending that someone see an endocrinologist?
Dr. Tsai: Our primary care colleagues can do a workup and interpretation of thyroid function tests in most cases. In the situations where the thyroid function test results are discordant (ie, elevated TSH and elevated free T4 levels or low TSH and low free T4 levels) or difficult to interpret, it would be appropriate to refer the patient to an endocrinologist.
One of the common referrals that we do get from the community is a patient’s thyroid function tests going from hyperthyroid to hypothyroid without a clear explanation or the patient is suboptimally controlled with levothyroxine or methimazole. In those circumstances, it would be worthwhile to send to an endocrinologist try to discern an underlying cause or for optimization of medication.
Dr. Leung and Dr. Tsai had no financial disclosures.
A version of this article appeared on Medscape.com.
If you’re like most primary care clinicians, your email inbox is flooded with messages from patients with questions about lab results. A common query: Should I be worried about an abnormal value on a test of thyroid-stimulating hormone (TSH)?
For guidance, this news organization spoke with Angela Leung, MD, associate professor of medicine in the Division of Endocrinology, Diabetes & Metabolism at the UCLA David Geffen School of Medicine and an endocrinologist at UCLA and the VA Greater Los Angeles Healthcare System, and Karen Tsai, MD, assistant clinical professor of endocrinology at City of Hope Comprehensive Cancer Center in Duarte, California. The following interview has been edited for length and clarity.
Question: Why do you usually start by measuring TSH levels?
Dr. Leung: We need to measure the thyroid status in a way that integrates more information about the long-term thyroid status and not small changes in thyroid hormone levels. TSH is made by the pituitary gland in the brain, which integrates information about the signals of high and low levels from each of the different thyroid hormones.
Now we can measure the actual thyroid hormones — primarily we’re talking about T3 and T4 — but if we do that, we are relying on a single snapshot in the bloodstream at that moment. The levels might change throughout the day in response to ongoing metabolism and outside stresses. So we usually start by measuring the TSH level, which is a good representation of the compilation of all those things over the past 30 days or so.
Question: How do you describe a low TSH result to patients?
Dr. Leung: Whenever we encounter a low TSH level, we want to repeat the test because it is a dynamic test, and it can change in response to several factors. If it is indeed low, we’re thinking that perhaps there’s a little bit of extra thyroid hormone in the body. It can be either temporary or more chronic, but that higher amount of thyroid hormone is telling the pituitary gland in the brain to start making less. So TSH levels go low when we need less thyroid hormone.
Question: What are some of the reasons for a low TSH level?
Dr. Leung: One of the most common situations for a temporarily low TSH level I see is what we call nonthyroidal illness, like a common cold or just being under the weather. Other things that can artifactually lower the TSH level could be the use of steroids, such as prednisone for asthma or some sort of a rheumatologic condition. Also, the TSH level could be low if a person has been recently exposed to very high amounts of iodine, such as iodinated contrast needed for a CT scan.
If the TSH level remains persistently low, usually in the presence of high thyroid hormone (T3 and/or T4) levels, the most common reason for hyperthyroidism is Graves disease, in which there are autoantibodies — measurable in the blood — that can stimulate the thyroid gland in the neck to make extra thyroid hormone.
Question: And what does an elevated TSH level mean?
Dr. Leung: Again, we want to confirm that it is elevated. We need at least two tests to confirm a high TSH level. A persistently elevated TSH level is a signal there might be low thyroid hormone levels in the body, which could be transient or more longer lasting.
Question: What are some of the most common causes of an elevated TSH level?
Dr. Leung: If the TSH level is confirmed high and the thyroid hormone levels are low, the most common cause of hypothyroidism here in the United States is Hashimoto thyroiditis.
Globally, iodine deficiency is the most common reason for hypothyroidism and may be a problem in parts of the globe where there are endemically low iodine levels in soil, crops, and the food supply like not having enough iodized salt. The thyroid is reliant on having enough iodine as a micronutrient to make thyroid hormone. If it doesn’t, the thyroid really can’t make thyroid hormone. It’s important to also remember, though, that having too much iodine can result in hypo- or hyperthyroidism.
Dr. Tsai: I take a glance at their medication list. Some of the patients are on methimazole or levothyroxine, and those medications should be adjusted first to normalize the TSH level. Other medications like lithium and amiodarone can also cause elevated TSH levels. We are also seeing a lot of patients on cancer therapies, such as tyrosine kinase inhibitors or immunotherapy, that can cause an elevated TSH level.
Question: If the repeat TSH test shows that TSH levels are still elevated, what comes next in your workup?
Dr. Tsai: If there’s not a real clear-cut diagnosis, I’ll order the thyroid peroxidase antibody and the thyroglobulin antibody, although thyroid peroxidase antibody, which is indicative of autoimmune thyroid disease, alone is usually sufficient to make that diagnosis.
Question: Should clinicians follow thyroid antibodies over time?
Dr. Tsai: I usually don’t repeat the antibody tests. In those circumstances where patients who were diagnosed 50-60 years ago and perhaps it is unknown if they had the thyroid antibodies measured at the time and now they’re saying, “Do I actually have Hashimoto’s?” or “Do I really need to continue this for the rest of my life?” I do repeat antibody tests to help gauge if the patient’s levothyroxine can be stopped.
Question: How important is it to follow T4 or T3 levels?
Dr. Tsai: T4 and T3 levels can help differentiate overt thyroid dysfunction — where T3 and/or T4 levels will be abnormal — from subclinical thyroid dysfunction — where T3 and T4 levels would be normal. In general, although we do not fully appreciate the best metric to monitor hypo- or hyperthyroidism, because some patients with a normal TSH level still may have symptoms of thyroid dysfunction, these peripheral thyroid hormone levels are usually the most helpful at the time of initial diagnosis.
Question: What are your criteria for initiating treatment for hypothyroidism?
Dr. Tsai: If the TSH level > 10 mIU/L, I recommend levothyroxine hormone replacement. A lot of published data support clinical benefit in this group.
There is a gray area in those patients who have a TSH level higher than the upper limit of the reference range but less than 10. If the patient doesn’t have overt hypothyroid symptoms, I discuss the findings with the patient but don’t really feel eager to treat. I recommend checking the levels again in 6 months to see where that TSH goes, and if it worsens or becomes greater than 10 mIU/L, I then recommend levothyroxine hormone replacement.
It is also important to note that a TSH level of 5-7 may be an acceptable range for older patients, and they do not require levothyroxine.
The other category is patients whose TSH level is greater than the upper limit of the normal reference range but less than 10 and with overt hypothyroid symptoms such as fatigue, unintentional weight gain, constipation, or cold intolerance. In these patients, it is worthwhile to try a low dose of levothyroxine (25-50 mcg/d) and repeat TSH and free T4 tests in 6-8 weeks and see if the TSH level normalizes.
Dr. Leung: When you look at subclinical hypothyroidism, the situation of an isolated high TSH level in the setting of normal T4 levels, if the TSH level is mildly elevated in the 5-7 mIU/L range, there’s a 60% chance that it will normalize within 6 months.
Going back to Karen’s point, a lot of people are started and maintained on low doses of thyroid hormone forever and ever. A recent study on levothyroxine use found half of the prescriptions were unnecessary.
Question: In an era where many patients obtain much of their health information from TikTok, what’s your approach with patients with a normal TSH level who feel that they should have more testing or start treatment?
Dr. Tsai: Fatigue is one of the common referrals we get into our endocrinology practice, and everyone is convinced that their thyroid is the culprit. It is important to note, however, that fatigue can be due to different diseases such as anemia, depression, sleep disorders, or a recent viral illness.
TSH tests are readily available and cheap. I don’t mind ordering the lab test again if it helps give the patient some reassurance. I also find that patients are relieved once they hear from their endocrinologist that their thyroid is unlikely to be the cause of their fatigue.
Some other endocrine causes we may consider additionally working up include adrenal insufficiency, vitamin D deficiency, and diabetes. A comprehensive metabolic panel and complete blood count is part of my workup to rule out any gross electrolyte abnormalities or any new diagnosis of anemia, liver disease, or chronic kidney disease.
Question: What are your criteria for recommending that someone see an endocrinologist?
Dr. Tsai: Our primary care colleagues can do a workup and interpretation of thyroid function tests in most cases. In the situations where the thyroid function test results are discordant (ie, elevated TSH and elevated free T4 levels or low TSH and low free T4 levels) or difficult to interpret, it would be appropriate to refer the patient to an endocrinologist.
One of the common referrals that we do get from the community is a patient’s thyroid function tests going from hyperthyroid to hypothyroid without a clear explanation or the patient is suboptimally controlled with levothyroxine or methimazole. In those circumstances, it would be worthwhile to send to an endocrinologist try to discern an underlying cause or for optimization of medication.
Dr. Leung and Dr. Tsai had no financial disclosures.
A version of this article appeared on Medscape.com.
If you’re like most primary care clinicians, your email inbox is flooded with messages from patients with questions about lab results. A common query: Should I be worried about an abnormal value on a test of thyroid-stimulating hormone (TSH)?
For guidance, this news organization spoke with Angela Leung, MD, associate professor of medicine in the Division of Endocrinology, Diabetes & Metabolism at the UCLA David Geffen School of Medicine and an endocrinologist at UCLA and the VA Greater Los Angeles Healthcare System, and Karen Tsai, MD, assistant clinical professor of endocrinology at City of Hope Comprehensive Cancer Center in Duarte, California. The following interview has been edited for length and clarity.
Question: Why do you usually start by measuring TSH levels?
Dr. Leung: We need to measure the thyroid status in a way that integrates more information about the long-term thyroid status and not small changes in thyroid hormone levels. TSH is made by the pituitary gland in the brain, which integrates information about the signals of high and low levels from each of the different thyroid hormones.
Now we can measure the actual thyroid hormones — primarily we’re talking about T3 and T4 — but if we do that, we are relying on a single snapshot in the bloodstream at that moment. The levels might change throughout the day in response to ongoing metabolism and outside stresses. So we usually start by measuring the TSH level, which is a good representation of the compilation of all those things over the past 30 days or so.
Question: How do you describe a low TSH result to patients?
Dr. Leung: Whenever we encounter a low TSH level, we want to repeat the test because it is a dynamic test, and it can change in response to several factors. If it is indeed low, we’re thinking that perhaps there’s a little bit of extra thyroid hormone in the body. It can be either temporary or more chronic, but that higher amount of thyroid hormone is telling the pituitary gland in the brain to start making less. So TSH levels go low when we need less thyroid hormone.
Question: What are some of the reasons for a low TSH level?
Dr. Leung: One of the most common situations for a temporarily low TSH level I see is what we call nonthyroidal illness, like a common cold or just being under the weather. Other things that can artifactually lower the TSH level could be the use of steroids, such as prednisone for asthma or some sort of a rheumatologic condition. Also, the TSH level could be low if a person has been recently exposed to very high amounts of iodine, such as iodinated contrast needed for a CT scan.
If the TSH level remains persistently low, usually in the presence of high thyroid hormone (T3 and/or T4) levels, the most common reason for hyperthyroidism is Graves disease, in which there are autoantibodies — measurable in the blood — that can stimulate the thyroid gland in the neck to make extra thyroid hormone.
Question: And what does an elevated TSH level mean?
Dr. Leung: Again, we want to confirm that it is elevated. We need at least two tests to confirm a high TSH level. A persistently elevated TSH level is a signal there might be low thyroid hormone levels in the body, which could be transient or more longer lasting.
Question: What are some of the most common causes of an elevated TSH level?
Dr. Leung: If the TSH level is confirmed high and the thyroid hormone levels are low, the most common cause of hypothyroidism here in the United States is Hashimoto thyroiditis.
Globally, iodine deficiency is the most common reason for hypothyroidism and may be a problem in parts of the globe where there are endemically low iodine levels in soil, crops, and the food supply like not having enough iodized salt. The thyroid is reliant on having enough iodine as a micronutrient to make thyroid hormone. If it doesn’t, the thyroid really can’t make thyroid hormone. It’s important to also remember, though, that having too much iodine can result in hypo- or hyperthyroidism.
Dr. Tsai: I take a glance at their medication list. Some of the patients are on methimazole or levothyroxine, and those medications should be adjusted first to normalize the TSH level. Other medications like lithium and amiodarone can also cause elevated TSH levels. We are also seeing a lot of patients on cancer therapies, such as tyrosine kinase inhibitors or immunotherapy, that can cause an elevated TSH level.
Question: If the repeat TSH test shows that TSH levels are still elevated, what comes next in your workup?
Dr. Tsai: If there’s not a real clear-cut diagnosis, I’ll order the thyroid peroxidase antibody and the thyroglobulin antibody, although thyroid peroxidase antibody, which is indicative of autoimmune thyroid disease, alone is usually sufficient to make that diagnosis.
Question: Should clinicians follow thyroid antibodies over time?
Dr. Tsai: I usually don’t repeat the antibody tests. In those circumstances where patients who were diagnosed 50-60 years ago and perhaps it is unknown if they had the thyroid antibodies measured at the time and now they’re saying, “Do I actually have Hashimoto’s?” or “Do I really need to continue this for the rest of my life?” I do repeat antibody tests to help gauge if the patient’s levothyroxine can be stopped.
Question: How important is it to follow T4 or T3 levels?
Dr. Tsai: T4 and T3 levels can help differentiate overt thyroid dysfunction — where T3 and/or T4 levels will be abnormal — from subclinical thyroid dysfunction — where T3 and T4 levels would be normal. In general, although we do not fully appreciate the best metric to monitor hypo- or hyperthyroidism, because some patients with a normal TSH level still may have symptoms of thyroid dysfunction, these peripheral thyroid hormone levels are usually the most helpful at the time of initial diagnosis.
Question: What are your criteria for initiating treatment for hypothyroidism?
Dr. Tsai: If the TSH level > 10 mIU/L, I recommend levothyroxine hormone replacement. A lot of published data support clinical benefit in this group.
There is a gray area in those patients who have a TSH level higher than the upper limit of the reference range but less than 10. If the patient doesn’t have overt hypothyroid symptoms, I discuss the findings with the patient but don’t really feel eager to treat. I recommend checking the levels again in 6 months to see where that TSH goes, and if it worsens or becomes greater than 10 mIU/L, I then recommend levothyroxine hormone replacement.
It is also important to note that a TSH level of 5-7 may be an acceptable range for older patients, and they do not require levothyroxine.
The other category is patients whose TSH level is greater than the upper limit of the normal reference range but less than 10 and with overt hypothyroid symptoms such as fatigue, unintentional weight gain, constipation, or cold intolerance. In these patients, it is worthwhile to try a low dose of levothyroxine (25-50 mcg/d) and repeat TSH and free T4 tests in 6-8 weeks and see if the TSH level normalizes.
Dr. Leung: When you look at subclinical hypothyroidism, the situation of an isolated high TSH level in the setting of normal T4 levels, if the TSH level is mildly elevated in the 5-7 mIU/L range, there’s a 60% chance that it will normalize within 6 months.
Going back to Karen’s point, a lot of people are started and maintained on low doses of thyroid hormone forever and ever. A recent study on levothyroxine use found half of the prescriptions were unnecessary.
Question: In an era where many patients obtain much of their health information from TikTok, what’s your approach with patients with a normal TSH level who feel that they should have more testing or start treatment?
Dr. Tsai: Fatigue is one of the common referrals we get into our endocrinology practice, and everyone is convinced that their thyroid is the culprit. It is important to note, however, that fatigue can be due to different diseases such as anemia, depression, sleep disorders, or a recent viral illness.
TSH tests are readily available and cheap. I don’t mind ordering the lab test again if it helps give the patient some reassurance. I also find that patients are relieved once they hear from their endocrinologist that their thyroid is unlikely to be the cause of their fatigue.
Some other endocrine causes we may consider additionally working up include adrenal insufficiency, vitamin D deficiency, and diabetes. A comprehensive metabolic panel and complete blood count is part of my workup to rule out any gross electrolyte abnormalities or any new diagnosis of anemia, liver disease, or chronic kidney disease.
Question: What are your criteria for recommending that someone see an endocrinologist?
Dr. Tsai: Our primary care colleagues can do a workup and interpretation of thyroid function tests in most cases. In the situations where the thyroid function test results are discordant (ie, elevated TSH and elevated free T4 levels or low TSH and low free T4 levels) or difficult to interpret, it would be appropriate to refer the patient to an endocrinologist.
One of the common referrals that we do get from the community is a patient’s thyroid function tests going from hyperthyroid to hypothyroid without a clear explanation or the patient is suboptimally controlled with levothyroxine or methimazole. In those circumstances, it would be worthwhile to send to an endocrinologist try to discern an underlying cause or for optimization of medication.
Dr. Leung and Dr. Tsai had no financial disclosures.
A version of this article appeared on Medscape.com.