User login
Survival similar with hearts donated after circulatory or brain death
in the first randomized trial comparing the two approaches.
“This randomized trial showing recipient survival with DCD to be similar to DBD should lead to DCD becoming the standard of care alongside DBD,” lead author Jacob Schroder, MD, surgical director, heart transplantation program, Duke University Medical Center, Durham, N.C., said in an interview.
“This should enable many more heart transplants to take place and for us to be able to cast the net further and wider for donors,” he said.
The trial was published online in the New England Journal of Medicine.
Dr. Schroder estimated that only around one-fifth of the 120 U.S. heart transplant centers currently carry out DCD transplants, but he is hopeful that the publication of this study will encourage more transplant centers to do these DCD procedures.
“The problem is there are many low-volume heart transplant centers, which may not be keen to do DCD transplants as they are a bit more complicated and expensive than DBD heart transplants,” he said. “But we need to look at the big picture of how many lives can be saved by increasing the number of heart transplant procedures and the money saved by getting more patients off the waiting list.”
The authors explain that heart transplantation has traditionally been limited to the use of hearts obtained from donors after brain death, which allows in situ assessment of cardiac function and of the suitability for transplantation of the donor allograft before surgical procurement.
But because the need for heart transplants far exceeds the availability of suitable donors, the use of DCD hearts has been investigated and this approach is now being pursued in many countries. In the DCD approach, the heart will have stopped beating in the donor, and perfusion techniques are used to restart the organ.
There are two different approaches to restarting the heart in DCD. The first approach involves the heart being removed from the donor and reanimated, preserved, assessed, and transported with the use of a portable extracorporeal perfusion and preservation system (Organ Care System, TransMedics). The second involves restarting the heart in the donor’s body for evaluation before removal and transportation under the traditional cold storage method used for donations after brain death.
The current trial was designed to compare clinical outcomes in patients who had received a heart from a circulatory death donor using the portable extracorporeal perfusion method for DCD transplantation, with outcomes from the traditional method of heart transplantation using organs donated after brain death.
For the randomized, noninferiority trial, adult candidates for heart transplantation were assigned to receive a heart after the circulatory death of the donor or a heart from a donor after brain death if that heart was available first (circulatory-death group) or to receive only a heart that had been preserved with the use of traditional cold storage after the brain death of the donor (brain-death group).
The primary end point was the risk-adjusted survival at 6 months in the as-treated circulatory-death group, as compared with the brain-death group. The primary safety end point was serious adverse events associated with the heart graft at 30 days after transplantation.
A total of 180 patients underwent transplantation, 90 of whom received a heart donated after circulatory death and 90 who received a heart donated after brain death. A total of 166 transplant recipients were included in the as-treated primary analysis (80 who received a heart from a circulatory-death donor and 86 who received a heart from a brain-death donor).
The risk-adjusted 6-month survival in the as-treated population was 94% among recipients of a heart from a circulatory-death donor, as compared with 90% among recipients of a heart from a brain-death donor (P < .001 for noninferiority).
There were no substantial between-group differences in the mean per-patient number of serious adverse events associated with the heart graft at 30 days after transplantation.
Of 101 hearts from circulatory-death donors that were preserved with the use of the perfusion system, 90 were successfully transplanted according to the criteria for lactate trend and overall contractility of the donor heart, which resulted in overall utilization percentage of 89%.
More patients who received a heart from a circulatory-death donor had moderate or severe primary graft dysfunction (22%) than those who received a heart from a brain-death donor (10%). However, graft failure that resulted in retransplantation occurred in two (2.3%) patients who received a heart from a brain-death donor versus zero patients who received a heart from a circulatory-death donor.
The researchers note that the higher incidence of primary graft dysfunction in the circulatory-death group is expected, given the period of warm ischemia that occurs in this approach. But they point out that this did not affect patient or graft survival at 30 days or 1 year.
“Primary graft dysfunction is when the heart doesn’t fully work immediately after transplant and some mechanical support is needed,” Dr. Schroder commented to this news organization. “This occurred more often in the DCD group, but this mechanical support is only temporary, and generally only needed for a day or two.
“It looks like it might take the heart a little longer to start fully functioning after DCD, but our results show this doesn’t seem to affect recipient survival.”
He added: “We’ve started to become more comfortable with DCD. Sometimes it may take a little longer to get the heart working properly on its own, but the rate of mechanical support is now much lower than when we first started doing these procedures. And cardiac MRI on the recipient patients before discharge have shown that the DCD hearts are not more damaged than those from DBD donors.”
The authors also report that there were six donor hearts in the DCD group for which there were protocol deviations of functional warm ischemic time greater than 30 minutes or continuously rising lactate levels and these hearts did not show primary graft dysfunction.
On this observation, Dr. Schroder said: “I think we need to do more work on understanding the ischemic time limits. The current 30 minutes time limit was estimated in animal studies. We need to look more closely at data from actual DCD transplants. While 30 minutes may be too long for a heart from an older donor, the heart from a younger donor may be fine for a longer period of ischemic time as it will be healthier.”
“Exciting” results
In an editorial, Nancy K. Sweitzer, MD, PhD, vice chair of clinical research, department of medicine, and director of clinical research, division of cardiology, Washington University in St. Louis, describes the results of the current study as “exciting,” adding that, “They clearly show the feasibility and safety of transplantation of hearts from circulatory-death donors.”
However, Dr. Sweitzer points out that the sickest patients in the study – those who were United Network for Organ Sharing (UNOS) status 1 and 2 – were more likely to receive a DBD heart and the more stable patients (UNOS 3-6) were more likely to receive a DCD heart.
“This imbalance undoubtedly contributed to the success of the trial in meeting its noninferiority end point. Whether transplantation of hearts from circulatory-death donors is truly safe in our sickest patients with heart failure is not clear,” she says.
However, she concludes, “Although caution and continuous evaluation of data are warranted, the increased use of hearts from circulatory-death donors appears to be safe in the hands of experienced transplantation teams and will launch an exciting phase of learning and improvement.”
“A safely expanded pool of heart donors has the potential to increase fairness and equity in heart transplantation, allowing more persons with heart failure to have access to this lifesaving therapy,” she adds. “Organ donors and transplantation teams will save increasing numbers of lives with this most precious gift.”
The current study was supported by TransMedics. Dr. Schroder reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in the first randomized trial comparing the two approaches.
“This randomized trial showing recipient survival with DCD to be similar to DBD should lead to DCD becoming the standard of care alongside DBD,” lead author Jacob Schroder, MD, surgical director, heart transplantation program, Duke University Medical Center, Durham, N.C., said in an interview.
“This should enable many more heart transplants to take place and for us to be able to cast the net further and wider for donors,” he said.
The trial was published online in the New England Journal of Medicine.
Dr. Schroder estimated that only around one-fifth of the 120 U.S. heart transplant centers currently carry out DCD transplants, but he is hopeful that the publication of this study will encourage more transplant centers to do these DCD procedures.
“The problem is there are many low-volume heart transplant centers, which may not be keen to do DCD transplants as they are a bit more complicated and expensive than DBD heart transplants,” he said. “But we need to look at the big picture of how many lives can be saved by increasing the number of heart transplant procedures and the money saved by getting more patients off the waiting list.”
The authors explain that heart transplantation has traditionally been limited to the use of hearts obtained from donors after brain death, which allows in situ assessment of cardiac function and of the suitability for transplantation of the donor allograft before surgical procurement.
But because the need for heart transplants far exceeds the availability of suitable donors, the use of DCD hearts has been investigated and this approach is now being pursued in many countries. In the DCD approach, the heart will have stopped beating in the donor, and perfusion techniques are used to restart the organ.
There are two different approaches to restarting the heart in DCD. The first approach involves the heart being removed from the donor and reanimated, preserved, assessed, and transported with the use of a portable extracorporeal perfusion and preservation system (Organ Care System, TransMedics). The second involves restarting the heart in the donor’s body for evaluation before removal and transportation under the traditional cold storage method used for donations after brain death.
The current trial was designed to compare clinical outcomes in patients who had received a heart from a circulatory death donor using the portable extracorporeal perfusion method for DCD transplantation, with outcomes from the traditional method of heart transplantation using organs donated after brain death.
For the randomized, noninferiority trial, adult candidates for heart transplantation were assigned to receive a heart after the circulatory death of the donor or a heart from a donor after brain death if that heart was available first (circulatory-death group) or to receive only a heart that had been preserved with the use of traditional cold storage after the brain death of the donor (brain-death group).
The primary end point was the risk-adjusted survival at 6 months in the as-treated circulatory-death group, as compared with the brain-death group. The primary safety end point was serious adverse events associated with the heart graft at 30 days after transplantation.
A total of 180 patients underwent transplantation, 90 of whom received a heart donated after circulatory death and 90 who received a heart donated after brain death. A total of 166 transplant recipients were included in the as-treated primary analysis (80 who received a heart from a circulatory-death donor and 86 who received a heart from a brain-death donor).
The risk-adjusted 6-month survival in the as-treated population was 94% among recipients of a heart from a circulatory-death donor, as compared with 90% among recipients of a heart from a brain-death donor (P < .001 for noninferiority).
There were no substantial between-group differences in the mean per-patient number of serious adverse events associated with the heart graft at 30 days after transplantation.
Of 101 hearts from circulatory-death donors that were preserved with the use of the perfusion system, 90 were successfully transplanted according to the criteria for lactate trend and overall contractility of the donor heart, which resulted in overall utilization percentage of 89%.
More patients who received a heart from a circulatory-death donor had moderate or severe primary graft dysfunction (22%) than those who received a heart from a brain-death donor (10%). However, graft failure that resulted in retransplantation occurred in two (2.3%) patients who received a heart from a brain-death donor versus zero patients who received a heart from a circulatory-death donor.
The researchers note that the higher incidence of primary graft dysfunction in the circulatory-death group is expected, given the period of warm ischemia that occurs in this approach. But they point out that this did not affect patient or graft survival at 30 days or 1 year.
“Primary graft dysfunction is when the heart doesn’t fully work immediately after transplant and some mechanical support is needed,” Dr. Schroder commented to this news organization. “This occurred more often in the DCD group, but this mechanical support is only temporary, and generally only needed for a day or two.
“It looks like it might take the heart a little longer to start fully functioning after DCD, but our results show this doesn’t seem to affect recipient survival.”
He added: “We’ve started to become more comfortable with DCD. Sometimes it may take a little longer to get the heart working properly on its own, but the rate of mechanical support is now much lower than when we first started doing these procedures. And cardiac MRI on the recipient patients before discharge have shown that the DCD hearts are not more damaged than those from DBD donors.”
The authors also report that there were six donor hearts in the DCD group for which there were protocol deviations of functional warm ischemic time greater than 30 minutes or continuously rising lactate levels and these hearts did not show primary graft dysfunction.
On this observation, Dr. Schroder said: “I think we need to do more work on understanding the ischemic time limits. The current 30 minutes time limit was estimated in animal studies. We need to look more closely at data from actual DCD transplants. While 30 minutes may be too long for a heart from an older donor, the heart from a younger donor may be fine for a longer period of ischemic time as it will be healthier.”
“Exciting” results
In an editorial, Nancy K. Sweitzer, MD, PhD, vice chair of clinical research, department of medicine, and director of clinical research, division of cardiology, Washington University in St. Louis, describes the results of the current study as “exciting,” adding that, “They clearly show the feasibility and safety of transplantation of hearts from circulatory-death donors.”
However, Dr. Sweitzer points out that the sickest patients in the study – those who were United Network for Organ Sharing (UNOS) status 1 and 2 – were more likely to receive a DBD heart and the more stable patients (UNOS 3-6) were more likely to receive a DCD heart.
“This imbalance undoubtedly contributed to the success of the trial in meeting its noninferiority end point. Whether transplantation of hearts from circulatory-death donors is truly safe in our sickest patients with heart failure is not clear,” she says.
However, she concludes, “Although caution and continuous evaluation of data are warranted, the increased use of hearts from circulatory-death donors appears to be safe in the hands of experienced transplantation teams and will launch an exciting phase of learning and improvement.”
“A safely expanded pool of heart donors has the potential to increase fairness and equity in heart transplantation, allowing more persons with heart failure to have access to this lifesaving therapy,” she adds. “Organ donors and transplantation teams will save increasing numbers of lives with this most precious gift.”
The current study was supported by TransMedics. Dr. Schroder reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in the first randomized trial comparing the two approaches.
“This randomized trial showing recipient survival with DCD to be similar to DBD should lead to DCD becoming the standard of care alongside DBD,” lead author Jacob Schroder, MD, surgical director, heart transplantation program, Duke University Medical Center, Durham, N.C., said in an interview.
“This should enable many more heart transplants to take place and for us to be able to cast the net further and wider for donors,” he said.
The trial was published online in the New England Journal of Medicine.
Dr. Schroder estimated that only around one-fifth of the 120 U.S. heart transplant centers currently carry out DCD transplants, but he is hopeful that the publication of this study will encourage more transplant centers to do these DCD procedures.
“The problem is there are many low-volume heart transplant centers, which may not be keen to do DCD transplants as they are a bit more complicated and expensive than DBD heart transplants,” he said. “But we need to look at the big picture of how many lives can be saved by increasing the number of heart transplant procedures and the money saved by getting more patients off the waiting list.”
The authors explain that heart transplantation has traditionally been limited to the use of hearts obtained from donors after brain death, which allows in situ assessment of cardiac function and of the suitability for transplantation of the donor allograft before surgical procurement.
But because the need for heart transplants far exceeds the availability of suitable donors, the use of DCD hearts has been investigated and this approach is now being pursued in many countries. In the DCD approach, the heart will have stopped beating in the donor, and perfusion techniques are used to restart the organ.
There are two different approaches to restarting the heart in DCD. The first approach involves the heart being removed from the donor and reanimated, preserved, assessed, and transported with the use of a portable extracorporeal perfusion and preservation system (Organ Care System, TransMedics). The second involves restarting the heart in the donor’s body for evaluation before removal and transportation under the traditional cold storage method used for donations after brain death.
The current trial was designed to compare clinical outcomes in patients who had received a heart from a circulatory death donor using the portable extracorporeal perfusion method for DCD transplantation, with outcomes from the traditional method of heart transplantation using organs donated after brain death.
For the randomized, noninferiority trial, adult candidates for heart transplantation were assigned to receive a heart after the circulatory death of the donor or a heart from a donor after brain death if that heart was available first (circulatory-death group) or to receive only a heart that had been preserved with the use of traditional cold storage after the brain death of the donor (brain-death group).
The primary end point was the risk-adjusted survival at 6 months in the as-treated circulatory-death group, as compared with the brain-death group. The primary safety end point was serious adverse events associated with the heart graft at 30 days after transplantation.
A total of 180 patients underwent transplantation, 90 of whom received a heart donated after circulatory death and 90 who received a heart donated after brain death. A total of 166 transplant recipients were included in the as-treated primary analysis (80 who received a heart from a circulatory-death donor and 86 who received a heart from a brain-death donor).
The risk-adjusted 6-month survival in the as-treated population was 94% among recipients of a heart from a circulatory-death donor, as compared with 90% among recipients of a heart from a brain-death donor (P < .001 for noninferiority).
There were no substantial between-group differences in the mean per-patient number of serious adverse events associated with the heart graft at 30 days after transplantation.
Of 101 hearts from circulatory-death donors that were preserved with the use of the perfusion system, 90 were successfully transplanted according to the criteria for lactate trend and overall contractility of the donor heart, which resulted in overall utilization percentage of 89%.
More patients who received a heart from a circulatory-death donor had moderate or severe primary graft dysfunction (22%) than those who received a heart from a brain-death donor (10%). However, graft failure that resulted in retransplantation occurred in two (2.3%) patients who received a heart from a brain-death donor versus zero patients who received a heart from a circulatory-death donor.
The researchers note that the higher incidence of primary graft dysfunction in the circulatory-death group is expected, given the period of warm ischemia that occurs in this approach. But they point out that this did not affect patient or graft survival at 30 days or 1 year.
“Primary graft dysfunction is when the heart doesn’t fully work immediately after transplant and some mechanical support is needed,” Dr. Schroder commented to this news organization. “This occurred more often in the DCD group, but this mechanical support is only temporary, and generally only needed for a day or two.
“It looks like it might take the heart a little longer to start fully functioning after DCD, but our results show this doesn’t seem to affect recipient survival.”
He added: “We’ve started to become more comfortable with DCD. Sometimes it may take a little longer to get the heart working properly on its own, but the rate of mechanical support is now much lower than when we first started doing these procedures. And cardiac MRI on the recipient patients before discharge have shown that the DCD hearts are not more damaged than those from DBD donors.”
The authors also report that there were six donor hearts in the DCD group for which there were protocol deviations of functional warm ischemic time greater than 30 minutes or continuously rising lactate levels and these hearts did not show primary graft dysfunction.
On this observation, Dr. Schroder said: “I think we need to do more work on understanding the ischemic time limits. The current 30 minutes time limit was estimated in animal studies. We need to look more closely at data from actual DCD transplants. While 30 minutes may be too long for a heart from an older donor, the heart from a younger donor may be fine for a longer period of ischemic time as it will be healthier.”
“Exciting” results
In an editorial, Nancy K. Sweitzer, MD, PhD, vice chair of clinical research, department of medicine, and director of clinical research, division of cardiology, Washington University in St. Louis, describes the results of the current study as “exciting,” adding that, “They clearly show the feasibility and safety of transplantation of hearts from circulatory-death donors.”
However, Dr. Sweitzer points out that the sickest patients in the study – those who were United Network for Organ Sharing (UNOS) status 1 and 2 – were more likely to receive a DBD heart and the more stable patients (UNOS 3-6) were more likely to receive a DCD heart.
“This imbalance undoubtedly contributed to the success of the trial in meeting its noninferiority end point. Whether transplantation of hearts from circulatory-death donors is truly safe in our sickest patients with heart failure is not clear,” she says.
However, she concludes, “Although caution and continuous evaluation of data are warranted, the increased use of hearts from circulatory-death donors appears to be safe in the hands of experienced transplantation teams and will launch an exciting phase of learning and improvement.”
“A safely expanded pool of heart donors has the potential to increase fairness and equity in heart transplantation, allowing more persons with heart failure to have access to this lifesaving therapy,” she adds. “Organ donors and transplantation teams will save increasing numbers of lives with this most precious gift.”
The current study was supported by TransMedics. Dr. Schroder reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Transplant centers often skip the top spot on the kidney waitlist
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
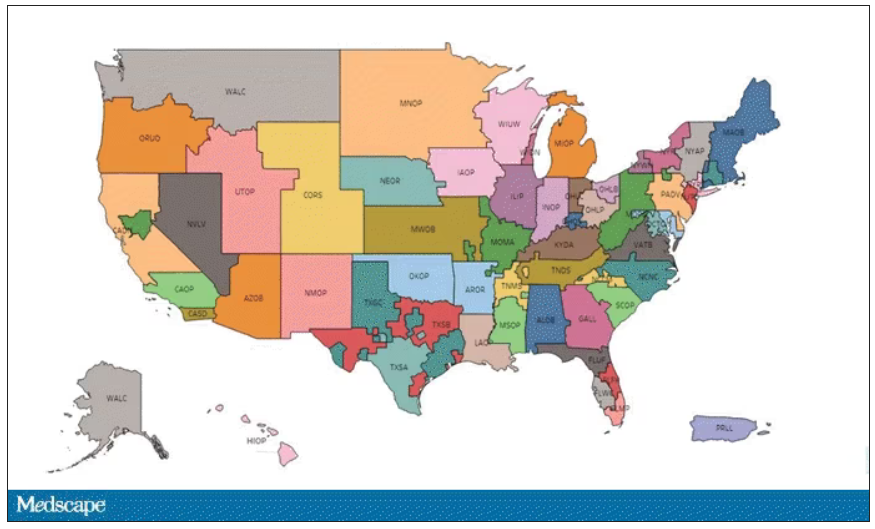
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
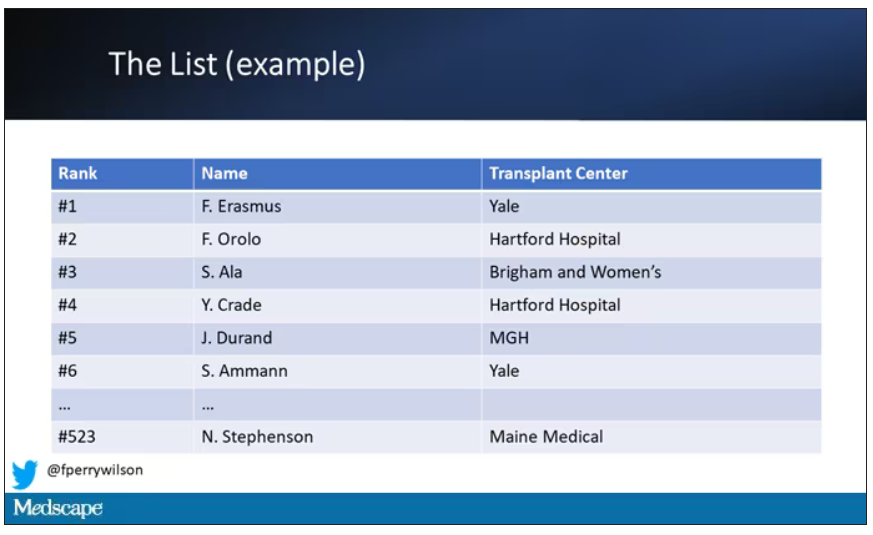
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
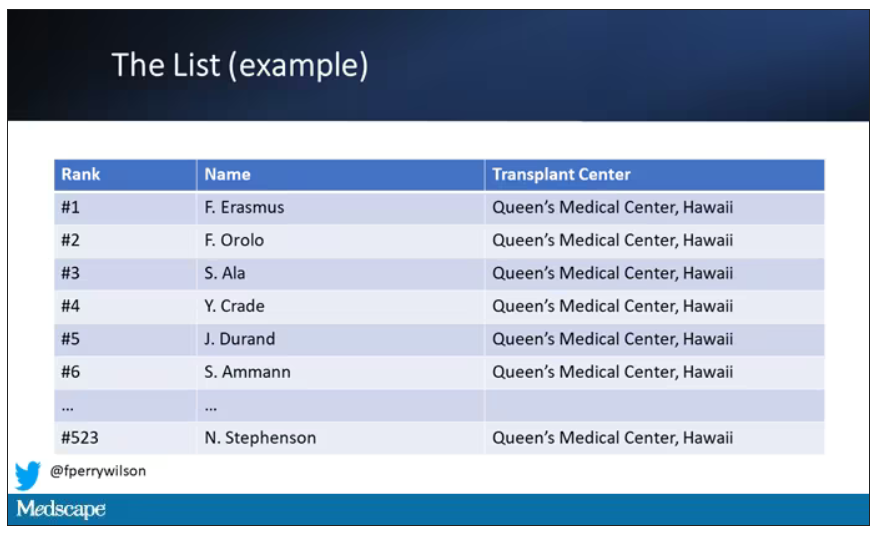
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
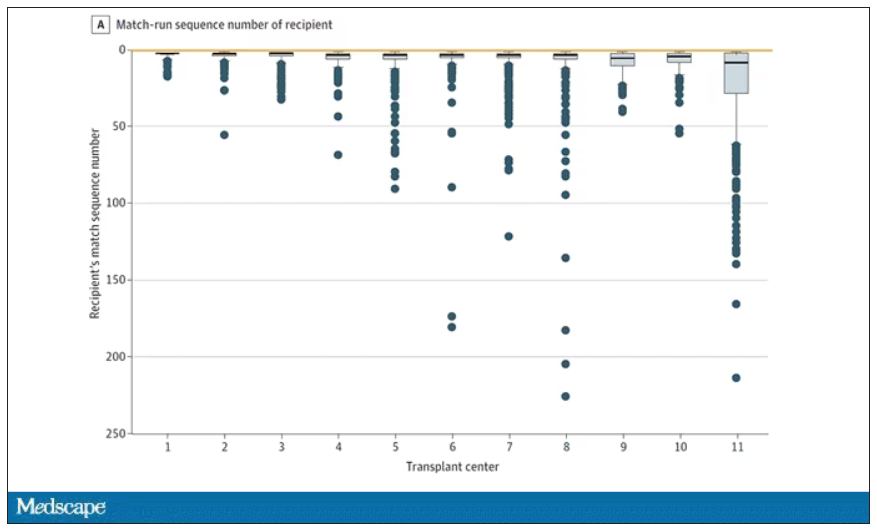
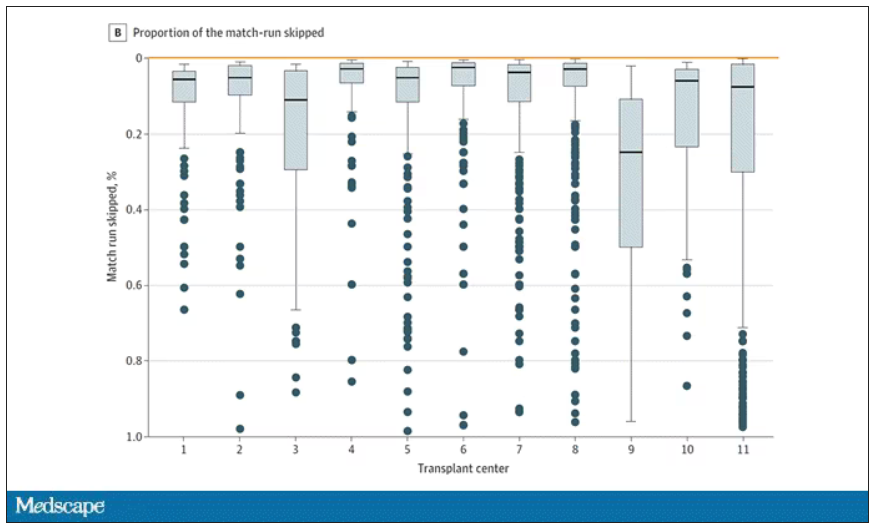
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Phase 3 trial: Maribavir yields post-transplant benefits
Overall mortality in the 109 patients from these subcohorts from SOLSTICE was lower, compared with mortality reported for similar populations treated with conventional therapies used to treat relapsed or refractory (R/R) CMV, according to findings presented in April at the annual meeting of the European Society for Bone and Marrow Transplantation.
“These results, in addition to the superior efficacy in CMV clearance observed for maribavir in SOLSTICE provide supportive evidence of the potential for the long-term benefit of maribavir treatment for post-transplant CMV infection,” Ishan Hirji, of Takeda Development Center Americas, and colleagues reported during a poster session at the meeting.
A retrospective chart review of the 41 hematopoietic stem cell transplant (HSCT) patients and 68 solid organ transplant (SOT) patients randomized to receive maribavir showed an overall mortality rate of 15.6% at 52 weeks after initiation of treatment with the antiviral agent. Among the HSCT patients, 14 deaths occurred (34.1%), with 8 occurring during the study periods and 6 occurring during follow-up. Among the SOT patients, three deaths occurred (4.4%), all during follow-up chart review.
Causes of death included underlying disease relapse in four patients, infection other than CMV in six patients, and one case each of CMV-related factors, transplant-related factors, acute lymphoblastic leukemia, and septic shock. Causes of death in the SOT patients included one case each of CMV-related factors, anemia, and renal failure.
“No patients had new graft loss or retransplantation during the chart review period,” the investigators noted.
The findings are notable as CMV infection occurs in 30%-70% of HSCT recipients and 16%-56% of SOT recipients and can lead to complications, including transplant failure and death. Reported 1-year mortality rates following standard therapies for CMV range from 31% to 50%, they explained.
Patients in the SOLSTICE trial received 8 weeks of treatment and were followed for 12 additional weeks. CMV clearance at the end of treatment was 55.7% in the maribavir treatment arm versus 23.9% in a control group of patients treated with investigator choice of therapy. As reported by this news organization, the findings formed the basis for U.S. Food and Drug Administration approval of maribavir in November 2021.
The current analysis included a chart review period that started 1 day after the SOLSTICE trial period and continued for 32 additional weeks.
These long-term follow-up data confirm the benefits of maribavir for the treatment of post-transplant CMV, according to the investigators, and findings from a separate study reported at the ESBMT meeting underscore the importance of the durable benefits observed with maribavir treatment.
For that retrospective study, Maria Laura Fox, of Vall d’Hebron Institute of Oncology, Barcelona, and colleagues pooled de-identified data from 250 adult HSCT recipients with R/R CMV who were treated with agents other than maribavir at transplant centers in the United States or Europe. They aimed to “generate real-world evidence on the burden of CMV infection/disease in HSCT recipients who had refractory/resistant CMV or were intolerant to current treatments.”
Nearly 92% of patients received two or more therapies to treat CMV, and 92.2% discontinued treatment or had one or more therapy dose changes or discontinuation, and 42 patients failed to achieve clearance of the CMV index episode.
CMV recurred in 35.2% of patients, and graft failure occurred in 4% of patients, the investigators reported.
All-cause mortality was 56.0%, and mortality at 1 year after identification of R/R disease or treatment intolerance was 45.2%, they noted, adding that the study results “highlight the real-world complexities and high burden of CMV infection for HSCT recipients.”
“With available anti-CMV agents [excluding maribavir], a notable proportion of patients failed to achieve viremia clearance once developing RRI [resistant, refractory, or intolerant] CMV and/or experienced recurrence, and were at risk of adverse outcomes, including myelosuppression and mortality. There is a need for therapies that achieve and maintain CMV clearance with improved safety profiles,” they concluded.
Both studies were funded by Takeda Development Center Americas, the maker of Levtencity. Ms. Hirji is an employee of Takeda and reported stock ownership. Ms. Fox reported relationships with Sierra Oncology, GlaxoSmithKline, Bristol Myers Squibb, Novartis, and AbbVie.
Overall mortality in the 109 patients from these subcohorts from SOLSTICE was lower, compared with mortality reported for similar populations treated with conventional therapies used to treat relapsed or refractory (R/R) CMV, according to findings presented in April at the annual meeting of the European Society for Bone and Marrow Transplantation.
“These results, in addition to the superior efficacy in CMV clearance observed for maribavir in SOLSTICE provide supportive evidence of the potential for the long-term benefit of maribavir treatment for post-transplant CMV infection,” Ishan Hirji, of Takeda Development Center Americas, and colleagues reported during a poster session at the meeting.
A retrospective chart review of the 41 hematopoietic stem cell transplant (HSCT) patients and 68 solid organ transplant (SOT) patients randomized to receive maribavir showed an overall mortality rate of 15.6% at 52 weeks after initiation of treatment with the antiviral agent. Among the HSCT patients, 14 deaths occurred (34.1%), with 8 occurring during the study periods and 6 occurring during follow-up. Among the SOT patients, three deaths occurred (4.4%), all during follow-up chart review.
Causes of death included underlying disease relapse in four patients, infection other than CMV in six patients, and one case each of CMV-related factors, transplant-related factors, acute lymphoblastic leukemia, and septic shock. Causes of death in the SOT patients included one case each of CMV-related factors, anemia, and renal failure.
“No patients had new graft loss or retransplantation during the chart review period,” the investigators noted.
The findings are notable as CMV infection occurs in 30%-70% of HSCT recipients and 16%-56% of SOT recipients and can lead to complications, including transplant failure and death. Reported 1-year mortality rates following standard therapies for CMV range from 31% to 50%, they explained.
Patients in the SOLSTICE trial received 8 weeks of treatment and were followed for 12 additional weeks. CMV clearance at the end of treatment was 55.7% in the maribavir treatment arm versus 23.9% in a control group of patients treated with investigator choice of therapy. As reported by this news organization, the findings formed the basis for U.S. Food and Drug Administration approval of maribavir in November 2021.
The current analysis included a chart review period that started 1 day after the SOLSTICE trial period and continued for 32 additional weeks.
These long-term follow-up data confirm the benefits of maribavir for the treatment of post-transplant CMV, according to the investigators, and findings from a separate study reported at the ESBMT meeting underscore the importance of the durable benefits observed with maribavir treatment.
For that retrospective study, Maria Laura Fox, of Vall d’Hebron Institute of Oncology, Barcelona, and colleagues pooled de-identified data from 250 adult HSCT recipients with R/R CMV who were treated with agents other than maribavir at transplant centers in the United States or Europe. They aimed to “generate real-world evidence on the burden of CMV infection/disease in HSCT recipients who had refractory/resistant CMV or were intolerant to current treatments.”
Nearly 92% of patients received two or more therapies to treat CMV, and 92.2% discontinued treatment or had one or more therapy dose changes or discontinuation, and 42 patients failed to achieve clearance of the CMV index episode.
CMV recurred in 35.2% of patients, and graft failure occurred in 4% of patients, the investigators reported.
All-cause mortality was 56.0%, and mortality at 1 year after identification of R/R disease or treatment intolerance was 45.2%, they noted, adding that the study results “highlight the real-world complexities and high burden of CMV infection for HSCT recipients.”
“With available anti-CMV agents [excluding maribavir], a notable proportion of patients failed to achieve viremia clearance once developing RRI [resistant, refractory, or intolerant] CMV and/or experienced recurrence, and were at risk of adverse outcomes, including myelosuppression and mortality. There is a need for therapies that achieve and maintain CMV clearance with improved safety profiles,” they concluded.
Both studies were funded by Takeda Development Center Americas, the maker of Levtencity. Ms. Hirji is an employee of Takeda and reported stock ownership. Ms. Fox reported relationships with Sierra Oncology, GlaxoSmithKline, Bristol Myers Squibb, Novartis, and AbbVie.
Overall mortality in the 109 patients from these subcohorts from SOLSTICE was lower, compared with mortality reported for similar populations treated with conventional therapies used to treat relapsed or refractory (R/R) CMV, according to findings presented in April at the annual meeting of the European Society for Bone and Marrow Transplantation.
“These results, in addition to the superior efficacy in CMV clearance observed for maribavir in SOLSTICE provide supportive evidence of the potential for the long-term benefit of maribavir treatment for post-transplant CMV infection,” Ishan Hirji, of Takeda Development Center Americas, and colleagues reported during a poster session at the meeting.
A retrospective chart review of the 41 hematopoietic stem cell transplant (HSCT) patients and 68 solid organ transplant (SOT) patients randomized to receive maribavir showed an overall mortality rate of 15.6% at 52 weeks after initiation of treatment with the antiviral agent. Among the HSCT patients, 14 deaths occurred (34.1%), with 8 occurring during the study periods and 6 occurring during follow-up. Among the SOT patients, three deaths occurred (4.4%), all during follow-up chart review.
Causes of death included underlying disease relapse in four patients, infection other than CMV in six patients, and one case each of CMV-related factors, transplant-related factors, acute lymphoblastic leukemia, and septic shock. Causes of death in the SOT patients included one case each of CMV-related factors, anemia, and renal failure.
“No patients had new graft loss or retransplantation during the chart review period,” the investigators noted.
The findings are notable as CMV infection occurs in 30%-70% of HSCT recipients and 16%-56% of SOT recipients and can lead to complications, including transplant failure and death. Reported 1-year mortality rates following standard therapies for CMV range from 31% to 50%, they explained.
Patients in the SOLSTICE trial received 8 weeks of treatment and were followed for 12 additional weeks. CMV clearance at the end of treatment was 55.7% in the maribavir treatment arm versus 23.9% in a control group of patients treated with investigator choice of therapy. As reported by this news organization, the findings formed the basis for U.S. Food and Drug Administration approval of maribavir in November 2021.
The current analysis included a chart review period that started 1 day after the SOLSTICE trial period and continued for 32 additional weeks.
These long-term follow-up data confirm the benefits of maribavir for the treatment of post-transplant CMV, according to the investigators, and findings from a separate study reported at the ESBMT meeting underscore the importance of the durable benefits observed with maribavir treatment.
For that retrospective study, Maria Laura Fox, of Vall d’Hebron Institute of Oncology, Barcelona, and colleagues pooled de-identified data from 250 adult HSCT recipients with R/R CMV who were treated with agents other than maribavir at transplant centers in the United States or Europe. They aimed to “generate real-world evidence on the burden of CMV infection/disease in HSCT recipients who had refractory/resistant CMV or were intolerant to current treatments.”
Nearly 92% of patients received two or more therapies to treat CMV, and 92.2% discontinued treatment or had one or more therapy dose changes or discontinuation, and 42 patients failed to achieve clearance of the CMV index episode.
CMV recurred in 35.2% of patients, and graft failure occurred in 4% of patients, the investigators reported.
All-cause mortality was 56.0%, and mortality at 1 year after identification of R/R disease or treatment intolerance was 45.2%, they noted, adding that the study results “highlight the real-world complexities and high burden of CMV infection for HSCT recipients.”
“With available anti-CMV agents [excluding maribavir], a notable proportion of patients failed to achieve viremia clearance once developing RRI [resistant, refractory, or intolerant] CMV and/or experienced recurrence, and were at risk of adverse outcomes, including myelosuppression and mortality. There is a need for therapies that achieve and maintain CMV clearance with improved safety profiles,” they concluded.
Both studies were funded by Takeda Development Center Americas, the maker of Levtencity. Ms. Hirji is an employee of Takeda and reported stock ownership. Ms. Fox reported relationships with Sierra Oncology, GlaxoSmithKline, Bristol Myers Squibb, Novartis, and AbbVie.
FROM ESBMT 2023
Experts debate reducing ASCT for multiple myeloma
NEW YORK –
Hematologist-oncologists whose top priority is ensuring that patients have the best chance of progression-free survival (PFS) will continue to choose ASCT as a best practice, argued Amrita Krishnan, MD, hematologist at the Judy and Bernard Briskin Center for Multiple Myeloma Research, City of Hope Comprehensive Cancer Center, Duarte, Calif.
A differing perspective was presented by C. Ola Landgren, MD, PhD, hematologist at the Sylvester Comprehensive Cancer Center at the University of Miami. Dr. Landgren cited evidence that, for newly diagnosed MM patients treated successfully with modern combination therapies, ASCT is not a mandatory treatment step before starting maintenance therapy.
Making a case for ASCT as the SoC, Dr. Krishnan noted, “based on the DETERMINATION trial [DT], there is far superior rate of PFS with patients who get ASCT up front, compared patients who got only conventional chemotherapy with lenalidomide, bortezomib, and dexamethasone [RVd]. PFS is the endpoint we look for in our treatment regimens.
“If you don’t use ASCT up front, you may lose the opportunity at later relapse. This is not to say that transplant is the only tool at our disposal. It is just an indispensable one. The GRIFFIN trial [GT] has shown us that robust combinations of drugs [both RVd and dexamethasone +RVd] can improve patient outcomes both before and after ASCT,” Dr. Krishnan concluded.
In his presentation, Dr. Landgren stated that, in the DT, while PFS is prolonged by the addition of ASCT to RVd, adding ASCT did not significantly increase overall survival (OS) rates. He added that treatment-related AEs of grade 3+ occurred in only 78.2% of patients on RVd versus 94.2% of RVd + ASCT patients.
“ASCT should not be the SoC frontline treatment in MM because it does not prolong OS. The IFM trial and the DT both show that there is no difference in OS between drug combination therapy followed by transplant and maintenance versus combination therapy alone, followed by transplant and maintenance. Furthermore, patients who get ASCT have higher risk of developing secondary malignancies, worse quality of life, and higher long-term morbidity with other conditions,” Dr. Landgren said.
He cited the MAIA trial administered daratumumab and lenalidomide plus dexamethasone (DRd) to patients who were too old or too frail to qualify for ASCT. Over half of patients in the DRd arm of MAIA had an estimated progression-free survival rate at 60 months.
“Furthermore, GT and the MANHATTAN clinical trials showed that we can safely add CD38-targeted monoclonal antibodies to standard combination therapies [lenalidomide, bortezomib, and dexamethasone (KRd)], resulting in higher rates of minimal-residual-disease (MRD) negativity. That means modern four-drug combination therapies [DR-RVd and DR-KRd] will allow more [and more newly diagnosed] MM patients to achieve MRD negativity in the absence of ASCT,” Dr. Landgren concluded.
Asked to comment on the two viewpoints, Joshua Richter, MD, director of myeloma treatment at the Blavatnik Family Center at Chelsea Mount Sinai, New York, said: “With some patients, we can get similar outcomes, whether or not we do a transplant. Doctors need to be better at choosing who really needs ASCT. Older people with standard-risk disease or people who achieve MRD-negative status after pharmacological treatment might not need to receive a transplant as much as those who have bulk disease or high-risk cytogenetics.
“Although ASCT might not be the best frontline option for everyone, collecting cells from most patients and storing them has many advantages. It allows us to do have the option of ASCT in later lines of therapy. In some patients with low blood counts, we can use stored cells to reboot their marrow and make them eligible for trials of promising new drugs,” Dr. Richter said.
Dr. Krishnan disclosed relationships with Takeda, Amgen, GlaxoSmithKline, Bristol-Myers Squibb, Sanofi, Pfizer, Adaptive, Regeneron, Janssen, AstraZeneca, Artiva, and Sutro. Dr. Landgren reported ties with Amgen, BMS, Celgene, Janssen, Takedam Glenmark, Juno, Pfizer, Merck, and others. Dr. Richter disclosed relationships with Janssen, BMS, and Takeda.
NEW YORK –
Hematologist-oncologists whose top priority is ensuring that patients have the best chance of progression-free survival (PFS) will continue to choose ASCT as a best practice, argued Amrita Krishnan, MD, hematologist at the Judy and Bernard Briskin Center for Multiple Myeloma Research, City of Hope Comprehensive Cancer Center, Duarte, Calif.
A differing perspective was presented by C. Ola Landgren, MD, PhD, hematologist at the Sylvester Comprehensive Cancer Center at the University of Miami. Dr. Landgren cited evidence that, for newly diagnosed MM patients treated successfully with modern combination therapies, ASCT is not a mandatory treatment step before starting maintenance therapy.
Making a case for ASCT as the SoC, Dr. Krishnan noted, “based on the DETERMINATION trial [DT], there is far superior rate of PFS with patients who get ASCT up front, compared patients who got only conventional chemotherapy with lenalidomide, bortezomib, and dexamethasone [RVd]. PFS is the endpoint we look for in our treatment regimens.
“If you don’t use ASCT up front, you may lose the opportunity at later relapse. This is not to say that transplant is the only tool at our disposal. It is just an indispensable one. The GRIFFIN trial [GT] has shown us that robust combinations of drugs [both RVd and dexamethasone +RVd] can improve patient outcomes both before and after ASCT,” Dr. Krishnan concluded.
In his presentation, Dr. Landgren stated that, in the DT, while PFS is prolonged by the addition of ASCT to RVd, adding ASCT did not significantly increase overall survival (OS) rates. He added that treatment-related AEs of grade 3+ occurred in only 78.2% of patients on RVd versus 94.2% of RVd + ASCT patients.
“ASCT should not be the SoC frontline treatment in MM because it does not prolong OS. The IFM trial and the DT both show that there is no difference in OS between drug combination therapy followed by transplant and maintenance versus combination therapy alone, followed by transplant and maintenance. Furthermore, patients who get ASCT have higher risk of developing secondary malignancies, worse quality of life, and higher long-term morbidity with other conditions,” Dr. Landgren said.
He cited the MAIA trial administered daratumumab and lenalidomide plus dexamethasone (DRd) to patients who were too old or too frail to qualify for ASCT. Over half of patients in the DRd arm of MAIA had an estimated progression-free survival rate at 60 months.
“Furthermore, GT and the MANHATTAN clinical trials showed that we can safely add CD38-targeted monoclonal antibodies to standard combination therapies [lenalidomide, bortezomib, and dexamethasone (KRd)], resulting in higher rates of minimal-residual-disease (MRD) negativity. That means modern four-drug combination therapies [DR-RVd and DR-KRd] will allow more [and more newly diagnosed] MM patients to achieve MRD negativity in the absence of ASCT,” Dr. Landgren concluded.
Asked to comment on the two viewpoints, Joshua Richter, MD, director of myeloma treatment at the Blavatnik Family Center at Chelsea Mount Sinai, New York, said: “With some patients, we can get similar outcomes, whether or not we do a transplant. Doctors need to be better at choosing who really needs ASCT. Older people with standard-risk disease or people who achieve MRD-negative status after pharmacological treatment might not need to receive a transplant as much as those who have bulk disease or high-risk cytogenetics.
“Although ASCT might not be the best frontline option for everyone, collecting cells from most patients and storing them has many advantages. It allows us to do have the option of ASCT in later lines of therapy. In some patients with low blood counts, we can use stored cells to reboot their marrow and make them eligible for trials of promising new drugs,” Dr. Richter said.
Dr. Krishnan disclosed relationships with Takeda, Amgen, GlaxoSmithKline, Bristol-Myers Squibb, Sanofi, Pfizer, Adaptive, Regeneron, Janssen, AstraZeneca, Artiva, and Sutro. Dr. Landgren reported ties with Amgen, BMS, Celgene, Janssen, Takedam Glenmark, Juno, Pfizer, Merck, and others. Dr. Richter disclosed relationships with Janssen, BMS, and Takeda.
NEW YORK –
Hematologist-oncologists whose top priority is ensuring that patients have the best chance of progression-free survival (PFS) will continue to choose ASCT as a best practice, argued Amrita Krishnan, MD, hematologist at the Judy and Bernard Briskin Center for Multiple Myeloma Research, City of Hope Comprehensive Cancer Center, Duarte, Calif.
A differing perspective was presented by C. Ola Landgren, MD, PhD, hematologist at the Sylvester Comprehensive Cancer Center at the University of Miami. Dr. Landgren cited evidence that, for newly diagnosed MM patients treated successfully with modern combination therapies, ASCT is not a mandatory treatment step before starting maintenance therapy.
Making a case for ASCT as the SoC, Dr. Krishnan noted, “based on the DETERMINATION trial [DT], there is far superior rate of PFS with patients who get ASCT up front, compared patients who got only conventional chemotherapy with lenalidomide, bortezomib, and dexamethasone [RVd]. PFS is the endpoint we look for in our treatment regimens.
“If you don’t use ASCT up front, you may lose the opportunity at later relapse. This is not to say that transplant is the only tool at our disposal. It is just an indispensable one. The GRIFFIN trial [GT] has shown us that robust combinations of drugs [both RVd and dexamethasone +RVd] can improve patient outcomes both before and after ASCT,” Dr. Krishnan concluded.
In his presentation, Dr. Landgren stated that, in the DT, while PFS is prolonged by the addition of ASCT to RVd, adding ASCT did not significantly increase overall survival (OS) rates. He added that treatment-related AEs of grade 3+ occurred in only 78.2% of patients on RVd versus 94.2% of RVd + ASCT patients.
“ASCT should not be the SoC frontline treatment in MM because it does not prolong OS. The IFM trial and the DT both show that there is no difference in OS between drug combination therapy followed by transplant and maintenance versus combination therapy alone, followed by transplant and maintenance. Furthermore, patients who get ASCT have higher risk of developing secondary malignancies, worse quality of life, and higher long-term morbidity with other conditions,” Dr. Landgren said.
He cited the MAIA trial administered daratumumab and lenalidomide plus dexamethasone (DRd) to patients who were too old or too frail to qualify for ASCT. Over half of patients in the DRd arm of MAIA had an estimated progression-free survival rate at 60 months.
“Furthermore, GT and the MANHATTAN clinical trials showed that we can safely add CD38-targeted monoclonal antibodies to standard combination therapies [lenalidomide, bortezomib, and dexamethasone (KRd)], resulting in higher rates of minimal-residual-disease (MRD) negativity. That means modern four-drug combination therapies [DR-RVd and DR-KRd] will allow more [and more newly diagnosed] MM patients to achieve MRD negativity in the absence of ASCT,” Dr. Landgren concluded.
Asked to comment on the two viewpoints, Joshua Richter, MD, director of myeloma treatment at the Blavatnik Family Center at Chelsea Mount Sinai, New York, said: “With some patients, we can get similar outcomes, whether or not we do a transplant. Doctors need to be better at choosing who really needs ASCT. Older people with standard-risk disease or people who achieve MRD-negative status after pharmacological treatment might not need to receive a transplant as much as those who have bulk disease or high-risk cytogenetics.
“Although ASCT might not be the best frontline option for everyone, collecting cells from most patients and storing them has many advantages. It allows us to do have the option of ASCT in later lines of therapy. In some patients with low blood counts, we can use stored cells to reboot their marrow and make them eligible for trials of promising new drugs,” Dr. Richter said.
Dr. Krishnan disclosed relationships with Takeda, Amgen, GlaxoSmithKline, Bristol-Myers Squibb, Sanofi, Pfizer, Adaptive, Regeneron, Janssen, AstraZeneca, Artiva, and Sutro. Dr. Landgren reported ties with Amgen, BMS, Celgene, Janssen, Takedam Glenmark, Juno, Pfizer, Merck, and others. Dr. Richter disclosed relationships with Janssen, BMS, and Takeda.
AT 2023 GREAT DEBATES AND UPDATES HEMATOLOGIC MALIGNANCIES CONFERENCE
Motixafortide may improve MM outcomes
Motixafortide, a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity , appears to increase the number of stem cells that can be harvested from transplant candidates, thereby increasing the likelihood of successful transplant, the authors reported.
An application for a new drug approval is currently under review by the Food and Drug Administration.
In the prospective, international, phase 3 GENESIS clinical trial , motixafortide plus granulocyte colony-stimulating factor (G-CSF) – the standard therapy for mobilizing stem cells – significantly increased the number stem cells harvested, when compared with standard therapy plus placebo. After one collection procedure, the combination approach allowed for harvesting of an optimal number of cells in 88% versus 9% of patients who received G-CSF plus placebo. After two collections, optimal collection occurred in 92% versus 26% of patients in the groups, respectively, first author Zachary D. Crees, MD, and colleagues found.
Motixafortide plus G-CSF was also associated with a tenfold increase in the number of primitive stem cells that could be collected. These stem cells are particularly effective for reconstituting red blood cells, white blood cells, and platelets, which all are important for patients’ recovery, they noted.
Stem cells mobilized by motixafortide were also associated with increased expression of genes and genetic pathways involved in self-renewal and regeneration, which are also of benefit for increasing the effectiveness of stem cell transplantation.
The findings were published in Nature Medicine.
“Stem cell transplantation is central to the treatment of multiple myeloma, but some patients don’t see as much benefit because standard therapies can’t harvest enough stem cells for the transplant to be effective, senior author John F. DiPersio, MD, PhD, stated in a news release . “This study suggests motixafortide works extremely well in combination with [G-CSF] in mobilizing stem cells in patients with multiple myeloma.
“The study also found that the combination worked rapidly and was generally well tolerated by patients,” added Dr. DiPersio, the Virginia E. & Sam J. Goldman Professor of Medicine at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University.
Dr. DiPersio is the lead author of another study investigating therapies beyond stem cell transplants. He and his colleagues recently reported the first comprehensive genomic and protein-based analysis of bone marrow samples from patients with multiple myeloma in an effort to identify targets for immunotherapies.
That study, published online in Cancer Research, identified 53 genes that could be targets, including 38 that are responsible for creating abnormal proteins on the surface of multiple myeloma cells; 11 of the 38 had not been previously identified as potential targets.
Dr. DiPersio and Dr. Crees, an assistant professor of medicine and the assistant clinical director of the Washington University Center for Gene and Cellular Immunotherapy, are also evaluating motixafortide’s potential for mobilizing stem cells to support “the genetic correction of the inherited disease sickle cell anemia.”
“This work is of particular importance because patients with sickle cell disease can’t be treated with G-CSF … due to dangerous side effects,” the news release stated. “The hope is that development of a novel, effective, and well-tolerated stem cell mobilizing regimen for a viral-based gene therapy approach using CRISPR-based gene editing will lead to improved outcomes for patients with sickle cell disease.”
The study published in Nature Medicine was supported by the National Institutes of Health and BioLineRx, which makes motixafortide. The study published in Cancer Research was supported by the Paula C. And Rodger O. Riney Blood Cancer Research Fund and the National Cancer Institute.
Dr. Crees reported research funding from BioLineRx. Dr. DiPersio reported relationships with Magenta Therapeutics, WUGEN, Incyte, RiverVest Venture Partners, Cellworks Group, Amphivena Therapeutics, NeoImmune Tech, Macrogenics, and BioLineRx.
Correction, 4/26/23: The headline on an earlier version of this article mischaracterized the study findings.
Motixafortide, a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity , appears to increase the number of stem cells that can be harvested from transplant candidates, thereby increasing the likelihood of successful transplant, the authors reported.
An application for a new drug approval is currently under review by the Food and Drug Administration.
In the prospective, international, phase 3 GENESIS clinical trial , motixafortide plus granulocyte colony-stimulating factor (G-CSF) – the standard therapy for mobilizing stem cells – significantly increased the number stem cells harvested, when compared with standard therapy plus placebo. After one collection procedure, the combination approach allowed for harvesting of an optimal number of cells in 88% versus 9% of patients who received G-CSF plus placebo. After two collections, optimal collection occurred in 92% versus 26% of patients in the groups, respectively, first author Zachary D. Crees, MD, and colleagues found.
Motixafortide plus G-CSF was also associated with a tenfold increase in the number of primitive stem cells that could be collected. These stem cells are particularly effective for reconstituting red blood cells, white blood cells, and platelets, which all are important for patients’ recovery, they noted.
Stem cells mobilized by motixafortide were also associated with increased expression of genes and genetic pathways involved in self-renewal and regeneration, which are also of benefit for increasing the effectiveness of stem cell transplantation.
The findings were published in Nature Medicine.
“Stem cell transplantation is central to the treatment of multiple myeloma, but some patients don’t see as much benefit because standard therapies can’t harvest enough stem cells for the transplant to be effective, senior author John F. DiPersio, MD, PhD, stated in a news release . “This study suggests motixafortide works extremely well in combination with [G-CSF] in mobilizing stem cells in patients with multiple myeloma.
“The study also found that the combination worked rapidly and was generally well tolerated by patients,” added Dr. DiPersio, the Virginia E. & Sam J. Goldman Professor of Medicine at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University.
Dr. DiPersio is the lead author of another study investigating therapies beyond stem cell transplants. He and his colleagues recently reported the first comprehensive genomic and protein-based analysis of bone marrow samples from patients with multiple myeloma in an effort to identify targets for immunotherapies.
That study, published online in Cancer Research, identified 53 genes that could be targets, including 38 that are responsible for creating abnormal proteins on the surface of multiple myeloma cells; 11 of the 38 had not been previously identified as potential targets.
Dr. DiPersio and Dr. Crees, an assistant professor of medicine and the assistant clinical director of the Washington University Center for Gene and Cellular Immunotherapy, are also evaluating motixafortide’s potential for mobilizing stem cells to support “the genetic correction of the inherited disease sickle cell anemia.”
“This work is of particular importance because patients with sickle cell disease can’t be treated with G-CSF … due to dangerous side effects,” the news release stated. “The hope is that development of a novel, effective, and well-tolerated stem cell mobilizing regimen for a viral-based gene therapy approach using CRISPR-based gene editing will lead to improved outcomes for patients with sickle cell disease.”
The study published in Nature Medicine was supported by the National Institutes of Health and BioLineRx, which makes motixafortide. The study published in Cancer Research was supported by the Paula C. And Rodger O. Riney Blood Cancer Research Fund and the National Cancer Institute.
Dr. Crees reported research funding from BioLineRx. Dr. DiPersio reported relationships with Magenta Therapeutics, WUGEN, Incyte, RiverVest Venture Partners, Cellworks Group, Amphivena Therapeutics, NeoImmune Tech, Macrogenics, and BioLineRx.
Correction, 4/26/23: The headline on an earlier version of this article mischaracterized the study findings.
Motixafortide, a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity , appears to increase the number of stem cells that can be harvested from transplant candidates, thereby increasing the likelihood of successful transplant, the authors reported.
An application for a new drug approval is currently under review by the Food and Drug Administration.
In the prospective, international, phase 3 GENESIS clinical trial , motixafortide plus granulocyte colony-stimulating factor (G-CSF) – the standard therapy for mobilizing stem cells – significantly increased the number stem cells harvested, when compared with standard therapy plus placebo. After one collection procedure, the combination approach allowed for harvesting of an optimal number of cells in 88% versus 9% of patients who received G-CSF plus placebo. After two collections, optimal collection occurred in 92% versus 26% of patients in the groups, respectively, first author Zachary D. Crees, MD, and colleagues found.
Motixafortide plus G-CSF was also associated with a tenfold increase in the number of primitive stem cells that could be collected. These stem cells are particularly effective for reconstituting red blood cells, white blood cells, and platelets, which all are important for patients’ recovery, they noted.
Stem cells mobilized by motixafortide were also associated with increased expression of genes and genetic pathways involved in self-renewal and regeneration, which are also of benefit for increasing the effectiveness of stem cell transplantation.
The findings were published in Nature Medicine.
“Stem cell transplantation is central to the treatment of multiple myeloma, but some patients don’t see as much benefit because standard therapies can’t harvest enough stem cells for the transplant to be effective, senior author John F. DiPersio, MD, PhD, stated in a news release . “This study suggests motixafortide works extremely well in combination with [G-CSF] in mobilizing stem cells in patients with multiple myeloma.
“The study also found that the combination worked rapidly and was generally well tolerated by patients,” added Dr. DiPersio, the Virginia E. & Sam J. Goldman Professor of Medicine at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University.
Dr. DiPersio is the lead author of another study investigating therapies beyond stem cell transplants. He and his colleagues recently reported the first comprehensive genomic and protein-based analysis of bone marrow samples from patients with multiple myeloma in an effort to identify targets for immunotherapies.
That study, published online in Cancer Research, identified 53 genes that could be targets, including 38 that are responsible for creating abnormal proteins on the surface of multiple myeloma cells; 11 of the 38 had not been previously identified as potential targets.
Dr. DiPersio and Dr. Crees, an assistant professor of medicine and the assistant clinical director of the Washington University Center for Gene and Cellular Immunotherapy, are also evaluating motixafortide’s potential for mobilizing stem cells to support “the genetic correction of the inherited disease sickle cell anemia.”
“This work is of particular importance because patients with sickle cell disease can’t be treated with G-CSF … due to dangerous side effects,” the news release stated. “The hope is that development of a novel, effective, and well-tolerated stem cell mobilizing regimen for a viral-based gene therapy approach using CRISPR-based gene editing will lead to improved outcomes for patients with sickle cell disease.”
The study published in Nature Medicine was supported by the National Institutes of Health and BioLineRx, which makes motixafortide. The study published in Cancer Research was supported by the Paula C. And Rodger O. Riney Blood Cancer Research Fund and the National Cancer Institute.
Dr. Crees reported research funding from BioLineRx. Dr. DiPersio reported relationships with Magenta Therapeutics, WUGEN, Incyte, RiverVest Venture Partners, Cellworks Group, Amphivena Therapeutics, NeoImmune Tech, Macrogenics, and BioLineRx.
Correction, 4/26/23: The headline on an earlier version of this article mischaracterized the study findings.
FROM NATURE MEDICINE
FDA OKs stem cell therapy for blood cancer patients to reduce infection risks
Omidubicel is made from umbilical cord donor stem cells that are processed with nicotinamide, a form of vitamin B3, to enhance and expand the number of progenitor cells, the product’s maker, Jerusalem-based Gamida Cell, explained in a press announcement.
The FDA approval was based on phase 3 testing that pitted the use of omidubicel in 62 patients against standard unmanipulated cord blood transplants in 63 patients following myeloablative conditioning.
The median time to neutrophil recovery was 12 days in the omidubicel group, compared with 22 days with standard care. Overall, 87% of patients who received omidubicel achieved neutrophil recovery versus 83% of patients with standard transplants.
The incidence of grade 2/3 bacterial or grade 3 fungal infections 100 days following transplant was 39% with omidubicel versus 60% with standard transplants.
The FDA’s “approval is an important advance in cell therapy treatment in patients with blood cancers. Hastening the return of the body’s white blood cells can reduce the possibility of serious or overwhelming infection associated with stem cell transplantation,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an agency press release.
Abbey Jenkins, president and CEO of Gamida, called the approval “a major advancement in the treatment of patients with hematologic malignancies that we believe may increase access to stem cell transplant and help improve patient outcomes.”
The most common grade 3–5 adverse reactions in the approval study were pain (33%), mucosal inflammation (31%), hypertension (25%), and gastrointestinal toxicity (19%).
Adverse events are consistent with allogeneic hematopoietic stem cell transplantation. Among 117 patients who received omidubicel for any indication, infusion reactions occurred in 47% of patients, acute graft-versus-host disease occurred in 58%, chronic GVHD occurred in 35%, and graft failure occurred in 3%. Labeling includes a boxed warning of the possibilities. There is also a small risk of infections and malignancies from donor blood.
Omidubicel is manufactured in Gamida’s facility in Kiryat Gat, Israel. It is available for order now and is expected to be delivered to transplant centers within 30 days after the start of manufacturing, the company said.
A version of this article originally appeared on Medscape.com.
Omidubicel is made from umbilical cord donor stem cells that are processed with nicotinamide, a form of vitamin B3, to enhance and expand the number of progenitor cells, the product’s maker, Jerusalem-based Gamida Cell, explained in a press announcement.
The FDA approval was based on phase 3 testing that pitted the use of omidubicel in 62 patients against standard unmanipulated cord blood transplants in 63 patients following myeloablative conditioning.
The median time to neutrophil recovery was 12 days in the omidubicel group, compared with 22 days with standard care. Overall, 87% of patients who received omidubicel achieved neutrophil recovery versus 83% of patients with standard transplants.
The incidence of grade 2/3 bacterial or grade 3 fungal infections 100 days following transplant was 39% with omidubicel versus 60% with standard transplants.
The FDA’s “approval is an important advance in cell therapy treatment in patients with blood cancers. Hastening the return of the body’s white blood cells can reduce the possibility of serious or overwhelming infection associated with stem cell transplantation,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an agency press release.
Abbey Jenkins, president and CEO of Gamida, called the approval “a major advancement in the treatment of patients with hematologic malignancies that we believe may increase access to stem cell transplant and help improve patient outcomes.”
The most common grade 3–5 adverse reactions in the approval study were pain (33%), mucosal inflammation (31%), hypertension (25%), and gastrointestinal toxicity (19%).
Adverse events are consistent with allogeneic hematopoietic stem cell transplantation. Among 117 patients who received omidubicel for any indication, infusion reactions occurred in 47% of patients, acute graft-versus-host disease occurred in 58%, chronic GVHD occurred in 35%, and graft failure occurred in 3%. Labeling includes a boxed warning of the possibilities. There is also a small risk of infections and malignancies from donor blood.
Omidubicel is manufactured in Gamida’s facility in Kiryat Gat, Israel. It is available for order now and is expected to be delivered to transplant centers within 30 days after the start of manufacturing, the company said.
A version of this article originally appeared on Medscape.com.
Omidubicel is made from umbilical cord donor stem cells that are processed with nicotinamide, a form of vitamin B3, to enhance and expand the number of progenitor cells, the product’s maker, Jerusalem-based Gamida Cell, explained in a press announcement.
The FDA approval was based on phase 3 testing that pitted the use of omidubicel in 62 patients against standard unmanipulated cord blood transplants in 63 patients following myeloablative conditioning.
The median time to neutrophil recovery was 12 days in the omidubicel group, compared with 22 days with standard care. Overall, 87% of patients who received omidubicel achieved neutrophil recovery versus 83% of patients with standard transplants.
The incidence of grade 2/3 bacterial or grade 3 fungal infections 100 days following transplant was 39% with omidubicel versus 60% with standard transplants.
The FDA’s “approval is an important advance in cell therapy treatment in patients with blood cancers. Hastening the return of the body’s white blood cells can reduce the possibility of serious or overwhelming infection associated with stem cell transplantation,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an agency press release.
Abbey Jenkins, president and CEO of Gamida, called the approval “a major advancement in the treatment of patients with hematologic malignancies that we believe may increase access to stem cell transplant and help improve patient outcomes.”
The most common grade 3–5 adverse reactions in the approval study were pain (33%), mucosal inflammation (31%), hypertension (25%), and gastrointestinal toxicity (19%).
Adverse events are consistent with allogeneic hematopoietic stem cell transplantation. Among 117 patients who received omidubicel for any indication, infusion reactions occurred in 47% of patients, acute graft-versus-host disease occurred in 58%, chronic GVHD occurred in 35%, and graft failure occurred in 3%. Labeling includes a boxed warning of the possibilities. There is also a small risk of infections and malignancies from donor blood.
Omidubicel is manufactured in Gamida’s facility in Kiryat Gat, Israel. It is available for order now and is expected to be delivered to transplant centers within 30 days after the start of manufacturing, the company said.
A version of this article originally appeared on Medscape.com.
Red-cell donor’s sex does not affect transfusion survival
In a randomized clinical trial with almost 9,000 patients, the adjusted hazard ratio of death among recipients of female donors’ blood, compared with recipients of male donors’ blood, was 0.98. The data contradict the finding of previous observational studies that donor sex is associated with recipient outcomes.
“The key finding was that we actually had a null result,” study author Dean Fergusson, MD, PhD, senior scientist at the Ottawa Hospital Research Institute, said in an interview. “We went in thinking that male donor blood would confer a benefit over female donor blood, and we found that there’s absolutely no difference between the donor sexes on recipient outcomes – mortality and other major secondary outcomes,” Dr. Fergusson added.
The study was published in the New England Journal of Medicine.
Differences ‘don’t matter’
A 2015 article from the National Heart, Lung, and Blood Institute identified a potential effect of donor sex on transfusion recipient survival. Since then, several observational studies have suggested that donor sex may influence survival after transfusion. This research includes two large studies, one from Canada and one from the Netherlands, that reported a heightened risk of death among recipients of red-cell units from female donors or donors who had been pregnant. Other studies, however, yielded conflicting results.
“The rationale was that female blood, because of biochemical properties, different hormones, exposure to babies and other males, all led to a different product, if you will, and these subtle changes could affect the blood product in terms of shelf life and potency,” said Dr. Fergusson. “That itself would have downstream effects on the recipient.”
The current double-blind study included 8,719 patients who received transfusions from September 2018 to December 2020 at three academic medical centers in Canada. Of this group, 5,190 received male donor blood, and 3,529 received blood from female donors.
The researchers randomly assigned patients in a 60:40 ratio to male and female donor groups. Data collection and follow-up were performed by the Ottawa Hospital Data Warehouse, Canadian Blood Services, and ICES, an independent research institute. Patient characteristics were similar in both trial groups at baseline.
After an average follow-up of 11.2 months, with a maximum follow-up of 29 months, 1,141 patients in the female donor group and 1,712 in the male donor group died. The study found no statistically significant difference in overall survival between the two groups. The unadjusted HR for death, with the male group as the reference, was 0.97, and the adjusted HR was 0.98. The rates of overall survival were 58% and 56.1% in the female and male donor groups, respectively.
The study did not prove that differences in outcome based on donor sex do not exist, said Dr. Fergusson. “But those differences really don’t matter in the recipient.”
The design of the trial itself was unique, Dr. Fergusson said. After patients consented to participate and underwent randomization, the study used routinely collected data from the participating hospitals’ electronic medical records rather than collect data anew for each patient. “That had a profound effect on the efficiency of the trial. We did this trial for a cost of less than $300,000, and typically it would cost $9 million by using high-quality electronic health data.”
The study also evaluated several secondary outcomes. Recipients of female donor blood had twice the incidence of MRSA infection. In addition, an unadjusted subgroup analysis suggested a 10% lower risk of death among male patients assigned to the female donor group, compared with those assigned to the male donor group.
The risk of death was almost three times higher among patients in the female donor group who received units from donors aged 20-29.9 years (HR, 2.93). “The inconsistency of the point estimates across groups and the multiplicity of analyses increase the risk that those findings were due to chance,” according to the authors.
Big data
Commenting on the study, Jeannie Callum, MD, professor and director of transfusion medicine at Queen’s University, Kingston, Ont., said that the use of routinely collected data from the participating hospitals’ electronic medical records was “one of the really great things about this paper.”
This use of Big Data “allows you to do a trial like this with almost 9,000 patients without spending millions and millions of dollars to have people go through charts and record data,” she added.
Dr. Callum also pointed out some of the trial’s limitations. “One of the things that kind of detracts from the study in my mind is that they randomized everybody that was getting a transfusion, but outpatients getting a transfusion have a very low mortality rate. So, you have a group of patients that are never going to have that endpoint being included in the study, and that might’ve diluted the findings.”
About 11.4% of participants received blood from a donor group other than the one to which they had been assigned, and this factor may further dilute the findings, said Dr. Callum. “That’s a difficult thing to avoid.” She noted that a trial in which she is collaborating, called Sex Matters, may answer some of these questions about the use of female versus male donor blood.
The investigators also noted that the findings may not be generalizable to other countries. “Just because we didn’t find something in Canada with our blood production system doesn’t mean that the United States might not find it different, because how they manufacture their red blood cells for transfusion is different than how we do them in Canada,” said Dr. Callum.
Nonetheless, this study shows the potential of using Big Data in medicine. “This is the future of large randomized clinical trials to quickly answer questions,” said Dr. Callum. “In the United States, Canada, and other countries that have these large electronic medical records systems, this kind of trial would be able to be done in other centers.”
The study was funded by the Canadian Institutes of Health Research. Dr. Fergusson and Dr. Callum disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial with almost 9,000 patients, the adjusted hazard ratio of death among recipients of female donors’ blood, compared with recipients of male donors’ blood, was 0.98. The data contradict the finding of previous observational studies that donor sex is associated with recipient outcomes.
“The key finding was that we actually had a null result,” study author Dean Fergusson, MD, PhD, senior scientist at the Ottawa Hospital Research Institute, said in an interview. “We went in thinking that male donor blood would confer a benefit over female donor blood, and we found that there’s absolutely no difference between the donor sexes on recipient outcomes – mortality and other major secondary outcomes,” Dr. Fergusson added.
The study was published in the New England Journal of Medicine.
Differences ‘don’t matter’
A 2015 article from the National Heart, Lung, and Blood Institute identified a potential effect of donor sex on transfusion recipient survival. Since then, several observational studies have suggested that donor sex may influence survival after transfusion. This research includes two large studies, one from Canada and one from the Netherlands, that reported a heightened risk of death among recipients of red-cell units from female donors or donors who had been pregnant. Other studies, however, yielded conflicting results.
“The rationale was that female blood, because of biochemical properties, different hormones, exposure to babies and other males, all led to a different product, if you will, and these subtle changes could affect the blood product in terms of shelf life and potency,” said Dr. Fergusson. “That itself would have downstream effects on the recipient.”
The current double-blind study included 8,719 patients who received transfusions from September 2018 to December 2020 at three academic medical centers in Canada. Of this group, 5,190 received male donor blood, and 3,529 received blood from female donors.
The researchers randomly assigned patients in a 60:40 ratio to male and female donor groups. Data collection and follow-up were performed by the Ottawa Hospital Data Warehouse, Canadian Blood Services, and ICES, an independent research institute. Patient characteristics were similar in both trial groups at baseline.
After an average follow-up of 11.2 months, with a maximum follow-up of 29 months, 1,141 patients in the female donor group and 1,712 in the male donor group died. The study found no statistically significant difference in overall survival between the two groups. The unadjusted HR for death, with the male group as the reference, was 0.97, and the adjusted HR was 0.98. The rates of overall survival were 58% and 56.1% in the female and male donor groups, respectively.
The study did not prove that differences in outcome based on donor sex do not exist, said Dr. Fergusson. “But those differences really don’t matter in the recipient.”
The design of the trial itself was unique, Dr. Fergusson said. After patients consented to participate and underwent randomization, the study used routinely collected data from the participating hospitals’ electronic medical records rather than collect data anew for each patient. “That had a profound effect on the efficiency of the trial. We did this trial for a cost of less than $300,000, and typically it would cost $9 million by using high-quality electronic health data.”
The study also evaluated several secondary outcomes. Recipients of female donor blood had twice the incidence of MRSA infection. In addition, an unadjusted subgroup analysis suggested a 10% lower risk of death among male patients assigned to the female donor group, compared with those assigned to the male donor group.
The risk of death was almost three times higher among patients in the female donor group who received units from donors aged 20-29.9 years (HR, 2.93). “The inconsistency of the point estimates across groups and the multiplicity of analyses increase the risk that those findings were due to chance,” according to the authors.
Big data
Commenting on the study, Jeannie Callum, MD, professor and director of transfusion medicine at Queen’s University, Kingston, Ont., said that the use of routinely collected data from the participating hospitals’ electronic medical records was “one of the really great things about this paper.”
This use of Big Data “allows you to do a trial like this with almost 9,000 patients without spending millions and millions of dollars to have people go through charts and record data,” she added.
Dr. Callum also pointed out some of the trial’s limitations. “One of the things that kind of detracts from the study in my mind is that they randomized everybody that was getting a transfusion, but outpatients getting a transfusion have a very low mortality rate. So, you have a group of patients that are never going to have that endpoint being included in the study, and that might’ve diluted the findings.”
About 11.4% of participants received blood from a donor group other than the one to which they had been assigned, and this factor may further dilute the findings, said Dr. Callum. “That’s a difficult thing to avoid.” She noted that a trial in which she is collaborating, called Sex Matters, may answer some of these questions about the use of female versus male donor blood.
The investigators also noted that the findings may not be generalizable to other countries. “Just because we didn’t find something in Canada with our blood production system doesn’t mean that the United States might not find it different, because how they manufacture their red blood cells for transfusion is different than how we do them in Canada,” said Dr. Callum.
Nonetheless, this study shows the potential of using Big Data in medicine. “This is the future of large randomized clinical trials to quickly answer questions,” said Dr. Callum. “In the United States, Canada, and other countries that have these large electronic medical records systems, this kind of trial would be able to be done in other centers.”
The study was funded by the Canadian Institutes of Health Research. Dr. Fergusson and Dr. Callum disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial with almost 9,000 patients, the adjusted hazard ratio of death among recipients of female donors’ blood, compared with recipients of male donors’ blood, was 0.98. The data contradict the finding of previous observational studies that donor sex is associated with recipient outcomes.
“The key finding was that we actually had a null result,” study author Dean Fergusson, MD, PhD, senior scientist at the Ottawa Hospital Research Institute, said in an interview. “We went in thinking that male donor blood would confer a benefit over female donor blood, and we found that there’s absolutely no difference between the donor sexes on recipient outcomes – mortality and other major secondary outcomes,” Dr. Fergusson added.
The study was published in the New England Journal of Medicine.
Differences ‘don’t matter’
A 2015 article from the National Heart, Lung, and Blood Institute identified a potential effect of donor sex on transfusion recipient survival. Since then, several observational studies have suggested that donor sex may influence survival after transfusion. This research includes two large studies, one from Canada and one from the Netherlands, that reported a heightened risk of death among recipients of red-cell units from female donors or donors who had been pregnant. Other studies, however, yielded conflicting results.
“The rationale was that female blood, because of biochemical properties, different hormones, exposure to babies and other males, all led to a different product, if you will, and these subtle changes could affect the blood product in terms of shelf life and potency,” said Dr. Fergusson. “That itself would have downstream effects on the recipient.”
The current double-blind study included 8,719 patients who received transfusions from September 2018 to December 2020 at three academic medical centers in Canada. Of this group, 5,190 received male donor blood, and 3,529 received blood from female donors.
The researchers randomly assigned patients in a 60:40 ratio to male and female donor groups. Data collection and follow-up were performed by the Ottawa Hospital Data Warehouse, Canadian Blood Services, and ICES, an independent research institute. Patient characteristics were similar in both trial groups at baseline.
After an average follow-up of 11.2 months, with a maximum follow-up of 29 months, 1,141 patients in the female donor group and 1,712 in the male donor group died. The study found no statistically significant difference in overall survival between the two groups. The unadjusted HR for death, with the male group as the reference, was 0.97, and the adjusted HR was 0.98. The rates of overall survival were 58% and 56.1% in the female and male donor groups, respectively.
The study did not prove that differences in outcome based on donor sex do not exist, said Dr. Fergusson. “But those differences really don’t matter in the recipient.”
The design of the trial itself was unique, Dr. Fergusson said. After patients consented to participate and underwent randomization, the study used routinely collected data from the participating hospitals’ electronic medical records rather than collect data anew for each patient. “That had a profound effect on the efficiency of the trial. We did this trial for a cost of less than $300,000, and typically it would cost $9 million by using high-quality electronic health data.”
The study also evaluated several secondary outcomes. Recipients of female donor blood had twice the incidence of MRSA infection. In addition, an unadjusted subgroup analysis suggested a 10% lower risk of death among male patients assigned to the female donor group, compared with those assigned to the male donor group.
The risk of death was almost three times higher among patients in the female donor group who received units from donors aged 20-29.9 years (HR, 2.93). “The inconsistency of the point estimates across groups and the multiplicity of analyses increase the risk that those findings were due to chance,” according to the authors.
Big data
Commenting on the study, Jeannie Callum, MD, professor and director of transfusion medicine at Queen’s University, Kingston, Ont., said that the use of routinely collected data from the participating hospitals’ electronic medical records was “one of the really great things about this paper.”
This use of Big Data “allows you to do a trial like this with almost 9,000 patients without spending millions and millions of dollars to have people go through charts and record data,” she added.
Dr. Callum also pointed out some of the trial’s limitations. “One of the things that kind of detracts from the study in my mind is that they randomized everybody that was getting a transfusion, but outpatients getting a transfusion have a very low mortality rate. So, you have a group of patients that are never going to have that endpoint being included in the study, and that might’ve diluted the findings.”
About 11.4% of participants received blood from a donor group other than the one to which they had been assigned, and this factor may further dilute the findings, said Dr. Callum. “That’s a difficult thing to avoid.” She noted that a trial in which she is collaborating, called Sex Matters, may answer some of these questions about the use of female versus male donor blood.
The investigators also noted that the findings may not be generalizable to other countries. “Just because we didn’t find something in Canada with our blood production system doesn’t mean that the United States might not find it different, because how they manufacture their red blood cells for transfusion is different than how we do them in Canada,” said Dr. Callum.
Nonetheless, this study shows the potential of using Big Data in medicine. “This is the future of large randomized clinical trials to quickly answer questions,” said Dr. Callum. “In the United States, Canada, and other countries that have these large electronic medical records systems, this kind of trial would be able to be done in other centers.”
The study was funded by the Canadian Institutes of Health Research. Dr. Fergusson and Dr. Callum disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Financial navigators saved about $2,500 per cancer patient
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
Living kidney donors should receive money for their costs of donating
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
Erythema extent predicts death in cutaneous GVHD
“There is value in collecting erythema serially over time as a continuous variable on a scale of 0%-100%” to identify high-risk patients for prophylactic and preemptive treatment, say investigators led by dermatologist Emily Baumrin, MD, director of the GVHD clinic at the University of Pennsylvania, Philadelphia.
They report a study of more than 300 patients with ccGVHD, which found that the extent of skin erythema strongly predicted the risk for death from GVHD.
Of the 267 patients with cutaneous GVHD at baseline, 103 patients died, the majority without a relapse of their blood cancer.
With additional research, erythema body surface area (BSA) should be “introduced as an outcome measure in clinical practice and trials,” they conclude.
At the moment, the NIH Skin Score is commonly used for risk assessment in cutaneous GVHD, but the researchers found that erythema BSA out-predicts this score.
The investigators explain that the NIH Skin Score does incorporate erythema surface area, but it does so as a categorical variable, not a continuous variable. Among other additional factors, it also includes assessments of skin sclerosis, which the investigators found was not associated with GVHD mortality.
Overall, the composite score waters down the weight given to erythema BSA because the score is “driven by stable sclerotic features, and erythema changes are missed,” they explain.
The study was published online in JAMA Dermatology.
Study details
The study included 469 patients with chronic GVHD (cGVHD), of whom 267 (57%) had cutaneous cGVHD at enrollment and 89 (19%) developed skin involvement subsequently.
All of the patients were on systemic immunosuppression for GVHD after allogeneic stem cell transplants for various blood cancers.
They were enrolled from 2007 through 2012 at nine U.S. medical centers – all members of the Chronic Graft Versus Host Disease Consortium – and they were followed until 2018.
Erythema BSA and NIH Skin Score were assessed at baseline and then every 3-6 months. Erythema was the first manifestation of skin involvement in the majority of patients, with a median surface area involvement of 11% at baseline.
The study team found that the extent of erythema at first follow-up visit was associated with both nonrelapse mortality (hazard ratio, 1.33 per 10% BSA increase; P < .001) and overall survival (HR, 1.28 per 10% BSA increase; P < .001), whereas extent of sclerotic skin involvement was not associated with either.
Participants in the study were predominantly White. The investigators note that “BSA assessments of erythema may be less reliable in patients with darker skin.”
The work was funded by the Department of Veterans Affairs and the National Institutes of Health. Dr. Baumrin had no disclosures; one coauthor is an employee of CorEvitas, and two others reported grants/adviser fees from several companies, including Janssen, Mallinckrodt, and Pfizer.
A version of this article first appeared on Medscape.com.
“There is value in collecting erythema serially over time as a continuous variable on a scale of 0%-100%” to identify high-risk patients for prophylactic and preemptive treatment, say investigators led by dermatologist Emily Baumrin, MD, director of the GVHD clinic at the University of Pennsylvania, Philadelphia.
They report a study of more than 300 patients with ccGVHD, which found that the extent of skin erythema strongly predicted the risk for death from GVHD.
Of the 267 patients with cutaneous GVHD at baseline, 103 patients died, the majority without a relapse of their blood cancer.
With additional research, erythema body surface area (BSA) should be “introduced as an outcome measure in clinical practice and trials,” they conclude.
At the moment, the NIH Skin Score is commonly used for risk assessment in cutaneous GVHD, but the researchers found that erythema BSA out-predicts this score.
The investigators explain that the NIH Skin Score does incorporate erythema surface area, but it does so as a categorical variable, not a continuous variable. Among other additional factors, it also includes assessments of skin sclerosis, which the investigators found was not associated with GVHD mortality.
Overall, the composite score waters down the weight given to erythema BSA because the score is “driven by stable sclerotic features, and erythema changes are missed,” they explain.
The study was published online in JAMA Dermatology.
Study details
The study included 469 patients with chronic GVHD (cGVHD), of whom 267 (57%) had cutaneous cGVHD at enrollment and 89 (19%) developed skin involvement subsequently.
All of the patients were on systemic immunosuppression for GVHD after allogeneic stem cell transplants for various blood cancers.
They were enrolled from 2007 through 2012 at nine U.S. medical centers – all members of the Chronic Graft Versus Host Disease Consortium – and they were followed until 2018.
Erythema BSA and NIH Skin Score were assessed at baseline and then every 3-6 months. Erythema was the first manifestation of skin involvement in the majority of patients, with a median surface area involvement of 11% at baseline.
The study team found that the extent of erythema at first follow-up visit was associated with both nonrelapse mortality (hazard ratio, 1.33 per 10% BSA increase; P < .001) and overall survival (HR, 1.28 per 10% BSA increase; P < .001), whereas extent of sclerotic skin involvement was not associated with either.
Participants in the study were predominantly White. The investigators note that “BSA assessments of erythema may be less reliable in patients with darker skin.”
The work was funded by the Department of Veterans Affairs and the National Institutes of Health. Dr. Baumrin had no disclosures; one coauthor is an employee of CorEvitas, and two others reported grants/adviser fees from several companies, including Janssen, Mallinckrodt, and Pfizer.
A version of this article first appeared on Medscape.com.
“There is value in collecting erythema serially over time as a continuous variable on a scale of 0%-100%” to identify high-risk patients for prophylactic and preemptive treatment, say investigators led by dermatologist Emily Baumrin, MD, director of the GVHD clinic at the University of Pennsylvania, Philadelphia.
They report a study of more than 300 patients with ccGVHD, which found that the extent of skin erythema strongly predicted the risk for death from GVHD.
Of the 267 patients with cutaneous GVHD at baseline, 103 patients died, the majority without a relapse of their blood cancer.
With additional research, erythema body surface area (BSA) should be “introduced as an outcome measure in clinical practice and trials,” they conclude.
At the moment, the NIH Skin Score is commonly used for risk assessment in cutaneous GVHD, but the researchers found that erythema BSA out-predicts this score.
The investigators explain that the NIH Skin Score does incorporate erythema surface area, but it does so as a categorical variable, not a continuous variable. Among other additional factors, it also includes assessments of skin sclerosis, which the investigators found was not associated with GVHD mortality.
Overall, the composite score waters down the weight given to erythema BSA because the score is “driven by stable sclerotic features, and erythema changes are missed,” they explain.
The study was published online in JAMA Dermatology.
Study details
The study included 469 patients with chronic GVHD (cGVHD), of whom 267 (57%) had cutaneous cGVHD at enrollment and 89 (19%) developed skin involvement subsequently.
All of the patients were on systemic immunosuppression for GVHD after allogeneic stem cell transplants for various blood cancers.
They were enrolled from 2007 through 2012 at nine U.S. medical centers – all members of the Chronic Graft Versus Host Disease Consortium – and they were followed until 2018.
Erythema BSA and NIH Skin Score were assessed at baseline and then every 3-6 months. Erythema was the first manifestation of skin involvement in the majority of patients, with a median surface area involvement of 11% at baseline.
The study team found that the extent of erythema at first follow-up visit was associated with both nonrelapse mortality (hazard ratio, 1.33 per 10% BSA increase; P < .001) and overall survival (HR, 1.28 per 10% BSA increase; P < .001), whereas extent of sclerotic skin involvement was not associated with either.
Participants in the study were predominantly White. The investigators note that “BSA assessments of erythema may be less reliable in patients with darker skin.”
The work was funded by the Department of Veterans Affairs and the National Institutes of Health. Dr. Baumrin had no disclosures; one coauthor is an employee of CorEvitas, and two others reported grants/adviser fees from several companies, including Janssen, Mallinckrodt, and Pfizer.
A version of this article first appeared on Medscape.com.